21 hydroxylase deficiency
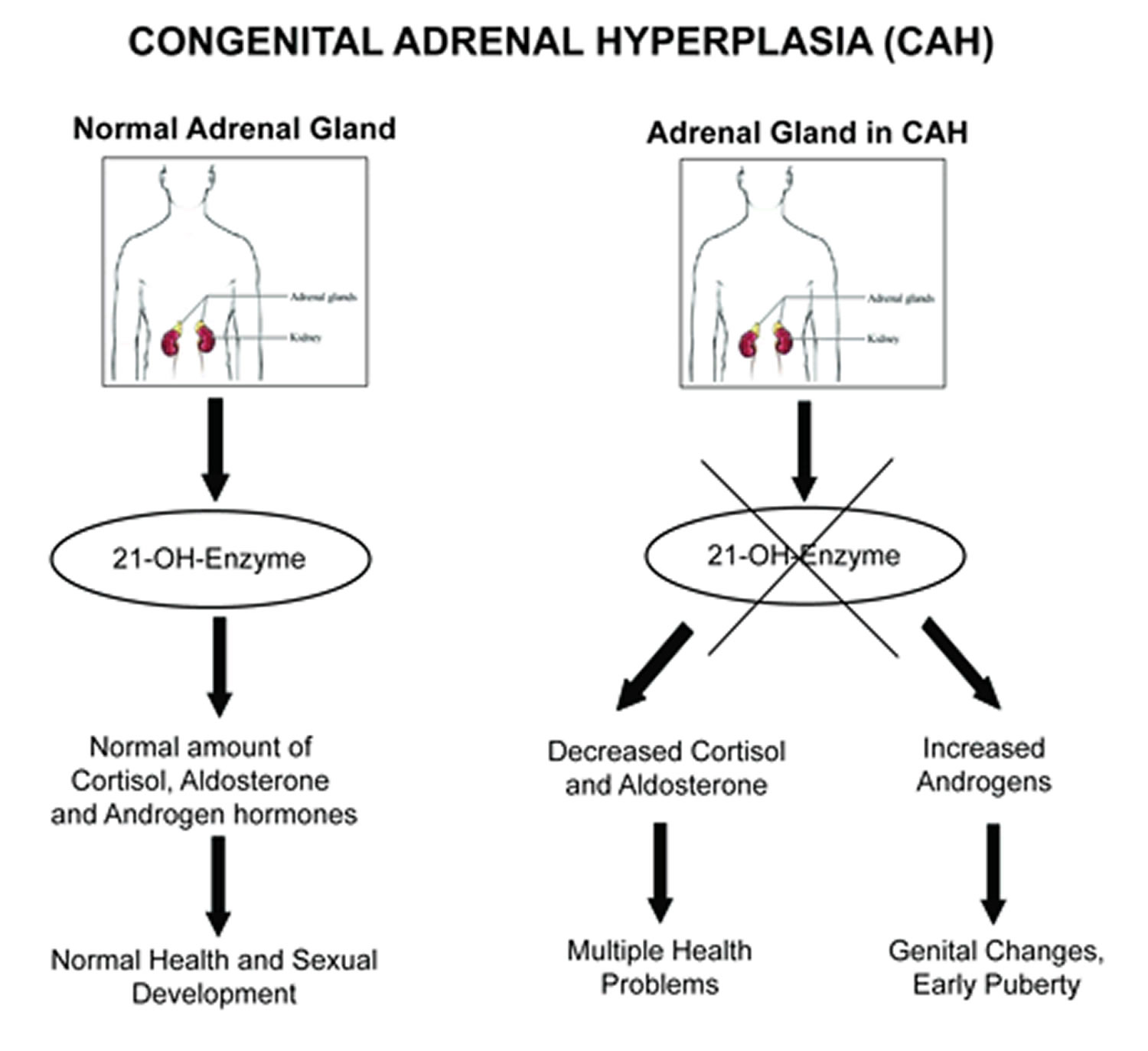
21-hydroxylase deficiency is an inherited disorder that affects the adrenal glands and is the most common cause of congenital adrenal hyperplasia (also known as congenital adrenal hyperplasia 1) 1. The adrenal glands are located on top of the kidneys and produce a variety of hormones that regulate many essential functions in the body. In people with 21-hydroxylase deficiency, the adrenal glands produce excess androgens, which are male sex hormones.
21-hydroxylase deficiency is one of a group of disorders known as congenital adrenal hyperplasias that impair hormone production and disrupt sexual development. 21-hydroxylase deficiency is responsible for about 95 percent of all cases of congenital adrenal hyperplasia.
There are three types of 21-hydroxylase deficiency. Two types are classic forms, known as the salt-wasting and simple virilizing types. The third type is called the non-classic type. The salt-wasting type is the most severe, the simple virilizing type is less severe, and the non-classic type is the least severe form.
Males and females with either classic form of 21-hydroxylase deficiency tend to have an early growth spurt, but their final adult height is usually shorter than others in their family. Additionally, affected individuals may have a reduced ability to have biological children (decreased fertility). Females may also develop excessive body hair growth (hirsutism), male pattern baldness, and irregular menstruation.
Approximately 75 percent of individuals with classic 21-hydroxylase deficiency have the salt-wasting type 2. Hormone production is extremely low in this form of the disorder. Affected individuals lose large amounts of sodium in their urine, which can be life-threatening in early infancy. Babies with the salt-wasting type can experience poor feeding, weight loss, dehydration, and vomiting. Individuals with the simple virilizing form do not experience salt loss.
In both the salt-wasting and simple virilizing forms of this disorder, females typically have external genitalia that do not look clearly male or female (ambiguous genitalia). Males usually have normal genitalia, but the testes may be small.
Nonclassic congenital adrenal hyperplasia has a prevalence of approximately 1 in 1000 in general population but occurs most frequently in specific ethnic groups such as Ashkenazi Jews and Hispanics.
Females with the non-classic type of 21-hydroxylase deficiency have normal female genitalia. As affected females get older, they may experience hirsutism, male pattern baldness, irregular menstruation, and decreased fertility. Males with the non-classic type may have early beard growth and small testes. Some individuals with this type of 21-hydroxylase deficiency have no symptoms of the disorder.
The classic forms of 21-hydroxylase deficiency occur in 1 in 15,000 newborns in the US 3. The prevalence is higher is other parts of the world. The prevalence of the non-classic form of 21-hydroxylase deficiency is estimated to be 1 in 1,000 individuals. The prevalence of both classic and non-classic forms varies among different ethnic populations.
Glucocorticoid and mineralocorticoid replacement are the mainstays of treatment 4.
Prenatal diagnosis and treatment of affected females are very important, to minimize genital virilization. Because 21-hydroxylase deficiency is often undiagnosed in affected males until they have severe adrenal insufficiency, all US states and many other countries have instituted newborn screening programs that measure 17-hydroxyprogesterone concentration. Newborn screening can detect almost all infants with classic congenital adrenal hyperplasia and some infants with nonclassic congenital adrenal hyperplasia. Although false-negative results are uncommon, false-positive results are usually seen in premature infants; therefore, serial measurements of 17-hydroxyprogesterone are advised for premature infants. A positive newborn screening test for congenital adrenal hyperplasia must be confirmed by a second plasma sample (17-hydroxyprogesterone), and serum electrolytes should be measured.
21-hydroxylase deficiency key points:
- Both molecular genetic testing of the fetus and prenatal treatment of mothers with dexamethasone are available.
- Dexamethasone must be given before the seventh to eighth week of gestation to suppress the fetal pituitary-adrenal axis before virilization occurs.
- In a patient with ambiguous genitalia, the diagnosis is not difficult; however, in affected males with no symptoms, newborn screening may be lifesaving. Without it, the diagnosis can be missed until the patient is in acute adrenal crisis.
- The objective of treatment of congenital adrenal hyperplasia is to prevent adrenal crisis and virilization and to achieve normal growth, pubertal development, sexual function, and fertility. Both male and female patients are fertile but have reduced fertility rates. This consequence is due to biologic, psychological, social, and sexual factors.
- The prevalence of metabolic abnormalities such as obesity, insulin resistance, dyslipidemia, and polycystic ovarian syndrome has been reported to be high due to the diseases themselves or glucocorticoid treatment.
Figure 1. 21-hydroxylase deficiency
21 hydroxylase deficiency causes
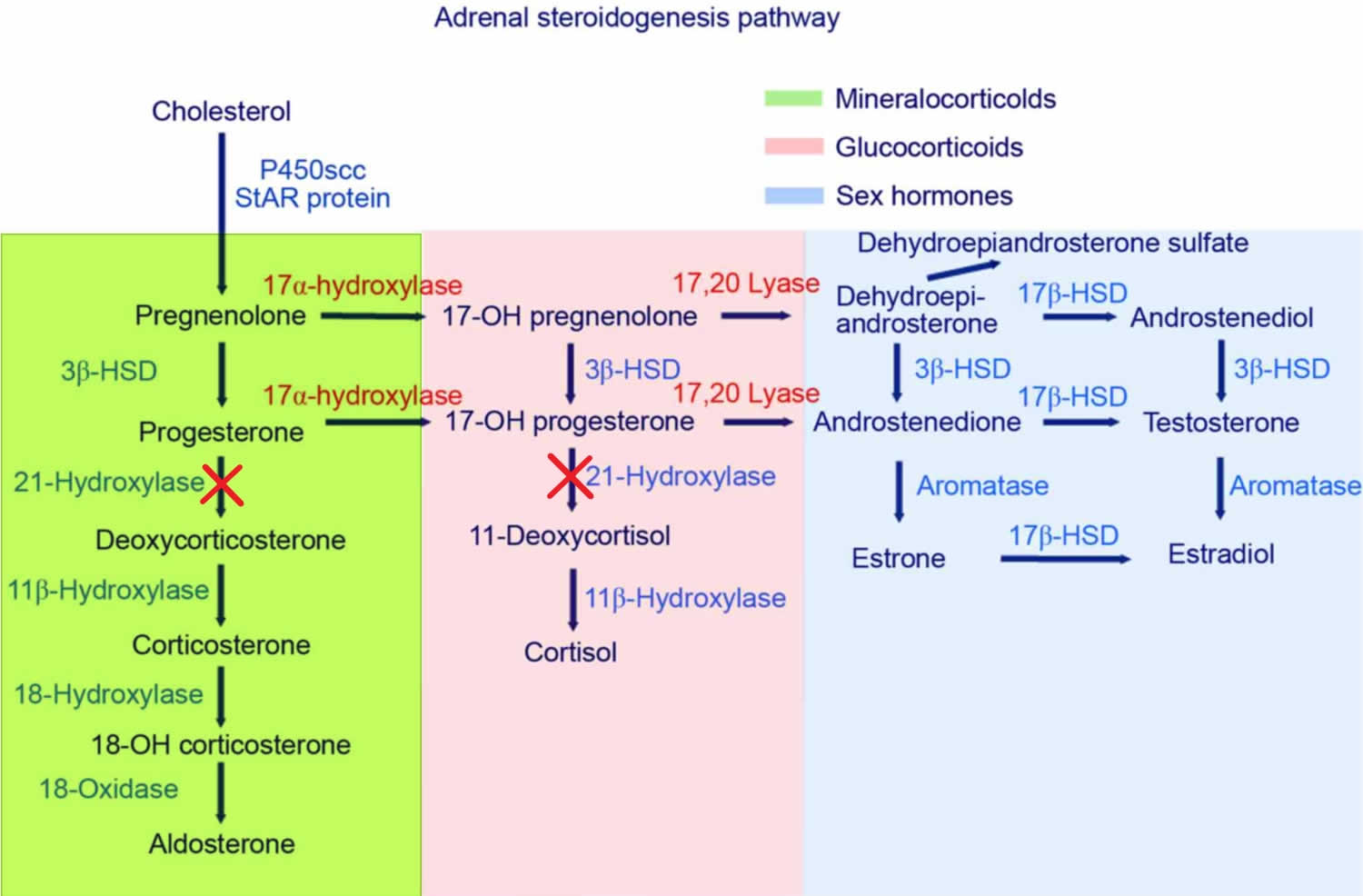
Mutations in the CYP21A2 gene cause 21-hydroxylase deficiency. The CYP21A2 gene provides instructions for making an enzyme called 21-hydroxylase. This enzyme is found in the adrenal glands, where it plays a role in producing hormones called cortisol and aldosterone. Cortisol has numerous functions, such as maintaining blood sugar levels, protecting the body from stress, and suppressing inflammation. Aldosterone is sometimes called the salt-retaining hormone because it regulates the amount of salt retained by the kidneys. The retention of salt affects fluid levels in the body and blood pressure.
21-hydroxylase deficiency is caused by a shortage (deficiency) of the 21-hydroxylase enzyme. When 21-hydroxylase is lacking, substances that are usually used to form cortisol and aldosterone instead build up in the adrenal glands and are converted to androgens. The excess production of androgens leads to abnormalities of sexual development in people with 21-hydroxylase deficiency. A lack of aldosterone production contributes to the salt loss in people with the salt-wasting form of this condition.
The amount of functional 21-hydroxylase enzyme determines the severity of the disorder. Individuals with the salt-wasting type have CYP21A2 mutations that result in a completely nonfunctional enzyme. People with the simple virilizing type of this condition have CYP21A2 gene mutations that allow the production of low levels of functional enzyme. Individuals with the non-classic type of this disorder have CYP21A2 mutations that result in the production of reduced amounts of the enzyme, but more enzyme than either of the other types.
21-hydroxylase deficiency inheritance pattern
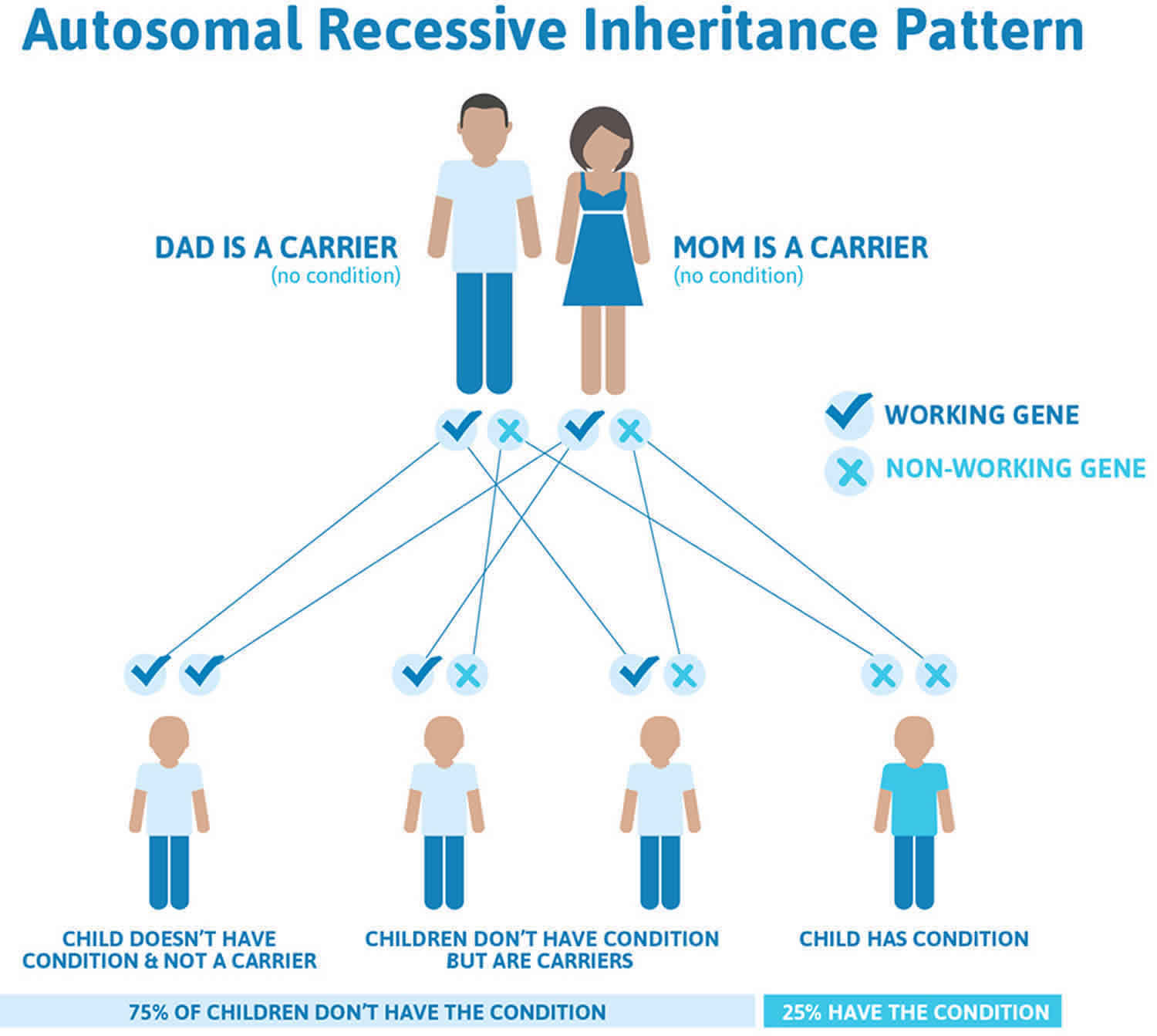
21-hydroxylase deficiency is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
It is rare to see any history of autosomal recessive conditions within a family because if someone is a carrier for one of these conditions, they would have to have a child with someone who is also a carrier for the same condition. Autosomal recessive conditions are individually pretty rare, so the chance that you and your partner are carriers for the same recessive genetic condition are likely low. Even if both partners are a carrier for the same condition, there is only a 25% chance that they will both pass down the non-working copy of the gene to the baby, thus causing a genetic condition. This chance is the same with each pregnancy, no matter how many children they have with or without the condition.
- If both partners are carriers of the same abnormal gene, they may pass on either their normal gene or their abnormal gene to their child. This occurs randomly.
- Each child of parents who both carry the same abnormal gene therefore has a 25% (1 in 4) chance of inheriting a abnormal gene from both parents and being affected by the condition.
- This also means that there is a 75% ( 3 in 4) chance that a child will not be affected by the condition. This chance remains the same in every pregnancy and is the same for boys or girls.
- There is also a 50% (2 in 4) chance that the child will inherit just one copy of the abnormal gene from a parent. If this happens, then they will be healthy carriers like their parents.
- Lastly, there is a 25% (1 in 4) chance that the child will inherit both normal copies of the gene. In this case the child will not have the condition, and will not be a carrier.
These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
Figure 1 illustrates autosomal recessive inheritance. The example below shows what happens when both dad and mum is a carrier of the abnormal gene, there is only a 25% chance that they will both pass down the abnormal gene to the baby, thus causing a genetic condition.
Figure 1. 21-hydroxylase deficiency autosomal recessive inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
21 hydroxylase deficiency symptoms
The symptoms of 21-hydroxylase deficiency may be different from person to person. Some people may be more severely affected than others, even people who have the same form. Not everyone with 21-hydroxylase deficiency will have the same symptoms, and some may have few or no symptoms.
There are three forms of 21-hydroxylase deficiency: the classic salt wasting form, the simple virilizing form, and the non-classic form. Most patients with 21-hydroxylase deficiency will have the classic salt-wasting form or the simple virilizing form 5.
Classic 21-hydroxylase deficiency
Salt-wasting (severe form, with a defect in cortisol and aldosterone biosynthesis)
Approximately 75% of patients with classic 21-hydroxylase deficiency have the salt-wasting type. It is the most severe form of the disease, and it is most often associated with large gene deletions or intron mutations that result in no enzyme activity.
Infants with the severe classic salt wasting form develop symptoms within the first few weeks of life. These include:
- Salt wasting crisis
- low sodium levels (hyponatremia)
- high potassium levels (hyperkalemia)
- high levels of renin in the blood (hyperreninemia)
- low blood volume (hypovolemic shock)
- Ambiguous genitalia in female newborns babies (genitalia that is not typical female nor male appearing), with normal internal feminine reproductive organs (ovaries, uterus, and fallopian tubes); male babies usually have normal genitalia but may have small testes and an enlarged penis.
Salt wasting crises can be life-threatening and require immediate treatment.
The biochemical and clinical abnormalities of the classic form manifest both prenatally and postnatally. Physicians recognize the condition in infant females in the neonatal period because of ambiguous genitalia compared with that of infant male. Males have normal genitalia and, in the case of the salt-wasting form, they can present with nonspecific symptoms like vomiting, dehydration, and poor feeding at ages 1 to 3 weeks. Hence, the diagnosis in boys can be delayed or missed.
Since aldosterone regulates sodium homeostasis, untreated patients will have excessive renal sodium excretion resulting in hypovolemia and hyperreninemia. These patients cannot excrete potassium efficiently and are prone to hyperkalemia, especially in infancy. In addition, accumulated steroid precursors may directly antagonize the mineralocorticoid receptor and exacerbate mineralocorticoid deficiency, particularly in untreated patients. Progesterone is well known to have anti-mineralcorticoid effects.
Cortisol deficiency contributes to poor cardiac function, poor vascular response to catecholamine, a decreased glomerular filtration rate, and increased secretion of antidiuretic hormone. Cortisol and aldosterone deficiency together cause hyponatremic dehydration and shock in inadequately treated patients.
Since high levels of glucocorticoids are needed for normal development of the adrenal medulla, as well as, for expression of the enzymes required to synthesize catecholamines, patients with salt-wasting type may also have catecholamine deficiency, further increasing the shock.
Females are exposed to high systemic levels of adrenal androgens from week 7 of gestation. Thus, they have ambiguous genitalia at birth: a large clitoris, rugated and potentially fused labia majora, and a common urogenital sinus instead of separate urethra and vagina. The uterus, fallopian tubes, and ovaries are normally formed, but there is no development of Wolffian ducts.
Postnatally, in untreated or inadequately treated patients, long-term exposure to high sex hormones promotes rapid somatic growth and advanced bone age. Linear growth is affected, even with close therapeutic monitoring. Pubic and axillary hair may develop early. Clitoral growth may continue in girls. Young boys may have penile growth despite having small testes. Long-term exposure to androgens may activate the hypothalamic-pituitary-gonadal axis, causing centrally mediated precocious puberty.
Girls may present with oligomenorrhea or amenorrhea in adolescence. As surgical, medical, psychological treatments have improved, more women with 21-hydroxylase deficiency, have completed pregnancies and giving birth.
The prevalence of testicular adrenal rests in boys with classic congenital adrenal hyperplasia aged 2 to 18 years varies from 21% to 28%. These so-called testicular adrenal rest tumors are benign, often related to suboptimal therapy, and usually, a decrease in size after optimization of glucocorticoid therapy. Testicular masses in boys with classic congenital adrenal hyperplasia are usually bilateral and smaller than 2 cm in diameter and therefore not palpable but detectable by ultrasound.
Simple Virilizing (normal aldosterone biosynthesis)
Approximately 25% of patients with classic 21-hydroxylase deficiency present with simple virilization without salt wasting. The simple virilizing form most commonly results from point mutations that lead to amino acid substitution, causing low but detectable enzyme activity resulting in adequate aldosterone secretion, but decreased levels of cortisol.
Females present at birth with ambiguous genitalia. Without newborn screening, affected boys are diagnosed in childhood when signs of androgen excess develop. Later diagnosis is associated with greater difficulty in achieving hormonal control, and short stature.
Infants with the classic simple virilizing form may have:
- Ambiguous external genitalia in female babies with normal internal reproductive organs; males are born with normal genitalia and may have small testes and an enlarged penis
Later in life both males and females with both classic forms of 21-hydroxylase deficiency may have:
- Puberty starting in childhood (precocious puberty)
- Excessive hair growth
- Acne
- Shorter than average adult height
- Reduced fertility
- Irregular periods (females)
- Testicular enlargement and testicular tumors (males)
Non-classic 21-hydroxylase deficiency (mild form)
The nonclassic or late-onset form is more common, occurring in 0.1% to 0.2% in the general white population and 1% to 2% among Ashkenazi Jews. Females with the nonclassic form may be compound heterozygotes with a classic mutation and variant allele or heterozygotes with two variant alleles, allowing 20% to 60% of normal enzymatic activity.
Compound heterozygote females have a less severe phenotype, and clinical presentation varies. Females may present at any age but usually not younger than 6 months. Heterozygote females may have mild biochemical abnormalities but no clinically important endocrine disorder.
Patients with nonclassic form, have normal levels of cortisol and aldosterone at the expense of mild to moderate overproduction of sex hormones precursors. Newborn screening can detect nonclassic cases, but most are missed because of relatively low baseline levels of 17 hydroxyprogesterone.
Hirsutism is the single most common symptom at presentation, followed by oligomenorrhea and acne. Thus, nonclassic 21-hydroxylase deficiency and polycystic ovarian syndrome may present in similar ways.
Females with the non-classic type of 21-hydroxylase deficiency have normal female genitalia, but when they get older, symptoms may include excessive hair growth (hirsutism), male pattern baldness, irregular periods and reduced fertility. Males with the non-classic type may have early beard growth, an enlarged penis, and small testes. The non-classical form is not considered a rare disease and some people with this form of 21-hydroxylase deficiency may not experience any signs or symptoms.
21 hydroxylase deficiency complications
Complications of congenital adrenal hyperplasia are common. If the patient does not get enough glucocorticoids, he or she can develop adrenal insufficiency and further virilization in the virilizing forms. If a patient receives excessive glucocorticoids, he or she can develop growth failure, obesity, striae, hypertension, hyperglycemia, and cataracts.
The complications of excess mineralocorticoid administration include hypertension and hypokalemia.
Aldosterone deficiency may lead to salt wasting with consequent failure to thrive, hypovolemia, and shock.
21 hydroxylase deficiency diagnosis
Babies born in the USA are screened at birth through newborn screening for the classic salt wasting and simple virilizing forms of 21-hydroxylase deficiency. For babies that test positive on the newborn screen for this disorder, additional biochemical and genetic testing is done to confirm the diagnosis 6. The less severe, non-classical form of 21-hydroxylase deficiency is diagnosed based on the clinical symptoms, biochemical testing to look for excess hormone production. Genetic testing may also be helpful to determine the type and severity of 21-hydroxylase deficiency 7.
Positive Newborn Screen
Newborn screening for congenital adrenal hyperplasia is routinely performed in all 50 US states and at least 40 other countries 8.
- A positive newborn screening test for congenital adrenal hyperplasia must be confirmed by a second plasma sample (17-hydroxyprogesterone), and serum electrolytes should be measured.
- After the confirmatory blood sample is obtained, treatment doses of glucocorticoid and mineralocorticoid should be initiated in all infants in whom congenital adrenal hyperplasia is a consideration, to prevent the potentially life-threatening manifestations of an adrenal crisis.
- If the physician chooses not to initiate treatment while awaiting confirmatory steroid hormone measurements, serum electrolytes should be measured daily.
- A pediatric endocrinologist should manage these patients.
The screening workup should include:
- Newborn screening programs check for 21-hydroxylase deficiency
- 17-hydroxyprogesterone will be very high (usually greater than 1000 ng/dL) in a patient with the classic form
- Hyperkalemia, hyponatremia, low aldosterone, and high plasma renin activity (PRA), particularly the ratio of plasma renin activity to aldosterone, are markers of impaired mineralocorticoid synthesis
- An ACTH stimulation test should be performed to evaluate adrenal function and differentiate among the various potential enzymatic defects. Administration of 0.25 mg of cosyntropin (a synthetic ACTH) provides a pharmacologic stimulus to the adrenal glands, maximizing hormone secretion.
- A full adrenal profile, including measurement of 17-OHP, cortisol, deoxycorticosterone, 11-deoxycortisol, 17-hydroxypregnenolone, dehydroepiandrosterone (DHEA), and androstenedione, should be obtained immediately before and 60 minutes after cosyntropin administration.
- Nomograms are available for interpreting the results
- In an infant with ambiguous genitalia, do karyotype to establish the chromosomal sex
- The pelvic ultrasound should be done to check for uterus or associated renal anomalies
- A bone age study is helpful in patients with precocious pubic hair
- In patients with signs of acute adrenal failure, CT of the adrenal glands can be done to exclude adrenal hemorrhage
- Urogenitography for defining the anatomy of the internal genitalia
21 hydroxylase deficiency treatment
Treatment for 21-hydroxylase deficiency depends on the severity of symptoms and the form of the condition. The goals of treatment are to manage to symptoms. Infants identified at birth with 21-hydroxylase deficiency are treated with hormones and steroids to prevent a salt-wasting crisis. In childhood and adulthood, other medications may be used to improve growth and fertility. Males should be monitored for the growth of testicular adrenal rest tumors, a benign tumor that can cause infertility. In some cases, females with ambiguous genitalia may be offered surgical correction. Some people with this condition have psychological issues and may benefit from therapy 9.
Acute adrenal crisis
- Is a medical emergency
- Initial management should be fluid resuscitation: Intravenous (IV) bolus of isotonic sodium chloride solution (20 mL/kg). Repeated boluses may be needed.
- Administer dextrose if the patient is hypoglycemic and must be rehydrated with fluid after the bolus dose to prevent hypoglycemia
- Stress doses of hydrocortisone (100 mg/m2 per day) are vital in the management and should be given as early as possible, concomitant with IV fluid treatment.
- Central access and vasopressors, along with higher glucose concentrations, may be required in profoundly ill patients.
- Life-threatening hyperkalemia may require additional therapy with potassium-lowering resin, IV calcium, insulin, and bicarbonate.
Long-term management
The goal of therapy is to reduce excessive androgen secretion by replacing the deficient hormones. Proper treatment prevents adrenal crisis and virilization, allowing normal growth and development, normal pubertal development, sexual function, and fertility.
Glucocorticoids
- Cortisol replacement: Oral hydrocortisone, in three divided doses of 10 to 20 mg/m2 per day.
- Patients with classic 21-hydroxylase deficiency require long-term glucocorticoid treatment to inhibit excessive secretion of CRH and ACTH and reduce the abnormally high serum concentrations of adrenal androgens.
- Hydrocortisone is the treatment of choice because of its short half-life and minimal growth suppressive effect.
- The efficacy of treatment is best assessed by monitoring ACTH, 17-OHP, DHEA, and androstenedione. A target 17-OHP range of 500 to 1000 ng/dL, although still higher than normal, helps to avoid the adverse effects of overtreatment. Children also should have an annual bone age radiograph and careful monitoring of linear growth
- Older children and adolescents, where growth is complete, may be treated with prednisone (5 to 7.5 mg daily in 2 divided doses ) or once-daily dexamethasone (0.25 to 0.5 mg).
Mineralocorticoids
- Infants born with the salt-wasting form of 21 hydroxylase deficiency require replacement with mineralocorticoids. Fludrocortisone (usually 0.1 to 0.2 mg, but occasionally patients require up to 0.4 mg per day) and sodium chloride (1 to 2 g, each gram of sodium chloride contains 17 mEq of sodium)
- The sodium content of human milk or most infant formulas are about eight mEq/L, and is insufficient to compensate for sodium loses in these infants.
- Plasma Renin activity levels may be used to monitor the effectiveness of mineralocorticoid and sodium replacement. Hypotension, hyperkalemia, and elevated renin levels suggest the need to increase the dose, whereas hypertension, tachycardia, and suppressed plasma renin activity production are clinical signs of overtreatment.
- Excessive increases in fludrocortisone dosage also may retard growth.
Surgical care
Infants with ambiguous genitalia require surgical evaluation and, if needed, plans for corrective surgery. Risks and benefits of surgery should be fully discussed with parents of affected females.
Significantly virilized females usually undergo surgery before 1 year of age. If there is severe clitoromegaly, the clitoris is reduced, with partial excision of the corporal bodies and preservation of the neurovascular bundle. Vaginoplasty and correction of the urogenital sinus usually are performed at the time of clitoral surgery. Revision in adolescence is often necessary.
Bilateral adrenalectomy for congenital adrenal hyperplasia is controversial. May be considered only in select cases that have failed medical therapy, especially in rare cases of adult females with salt-wasting congenital adrenal hyperplasia and infertility. The risk for noncompliance must be considered before surgery.
21 hydroxylase deficiency prognosis
The long-term outlook for people with 21-hydroxylase deficiency is dependent on the severity of the symptoms, the response to medications and the presence of any other medical conditions. In general, with early diagnosis and continuous lifetime treatment, the long-term outlook for people with this disorder is good. Long term complications of this condition may include fertility and mental health issues 9.
Children who have congenital adrenal hyperplasia often are tall in early childhood, but ultimately are short in adulthood. Recent data suggest that patients born with congenital adrenal hyperplasia are about 10 cm shorter than their parentally based targets. Advanced bone age and central precocious puberty due to androgen excess causing early epiphyseal fusion are the primary factors. In addition, treatment of congenital adrenal hyperplasia with glucocorticoids can suppress growth and diminish final height. Experimental treatment with growth hormone and luteinizing hormone-releasing hormone analog (to hold off puberty) are reported to lead to an average height gain of 7.3 cm 10.
The influence of prenatal sex steroid exposure on personality is controversial. More consistent evidence regarding the effects of androgens comes from gendered play activities of young children.
Most children with congenital adrenal hyperplasia manifest normal neuropsychological development. Moreover, despite a tendency toward male gender role behavior and homoerotic fantasy, most girls with congenital adrenal hyperplasia identify as females and exhibit a heterosexual preference. Both male and female patients are fertile but have reduced fertility rates. This consequence is due to biologic, psychological, social, and sexual factors.
Bone density is reported to be normal in most patients. The prevalence of metabolic abnormalities such as obesity, insulin resistance, dyslipidemia, and polycystic ovarian syndrome has been reported to be high due to the diseases themselves or glucocorticoid treatment
This disease and treatment complications and long-term consequences are challenging for practitioners. Multiple subspecialty professionals should be involved in management. Gene therapy shows potential for a congenital adrenal hyperplasia cure.
References- Burdea L, Mendez MD. 21 Hydroxylase Deficiency. [Updated 2019 Jun 16]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493164
- Daae E, Feragen KB, Nermoen I, Falhammar H. Psychological adjustment, quality of life, and self-perceptions of reproductive health in males with congenital adrenal hyperplasia: a systematic review. Endocrine. 2018 Oct;62(1):3-13.
- 21-hydroxylase deficiency. https://ghr.nlm.nih.gov/condition/21-hydroxylase-deficiency
- Nasir H, Ali SI, Haque N, Grebe SK, Kirmani S. Compound heterozygosity for a whole gene deletion and p.R124C mutation in CYP21A2 causing nonclassic congenital adrenal hyperplasia. Ann Pediatr Endocrinol Metab. 2018 Sep;23(3):158-161.
- Parsa AA, New MI. Steroid 21-hydroxylase deficiency in congenital adrenal hyperplasia. Jl Steroid Biochem Mol Biol. Jan 2017; 165(pt A):2-11. https://www.ncbi.nlm.nih.gov/pubmed/27380651
- Nimkarn S, Gangishetti PK, Yau M, et al. 21-Hydroxylase-Deficient Congenital Adrenal Hyperplasia. 2002 Feb 26 [Updated 2016 Feb 4]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1171
- Concolino P, Costella A. Congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency: A comprehensive focus on 233 pathogenic variants of CYP21A2 gene. Mol Diagn Ther. Jun 2018; 22(3):261-280. https://www.ncbi.nlm.nih.gov/pubmed/29450859
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, Meyer-Bahlburg HFL, Miller WL, Murad MH, Oberfield SE, White PC. Congenital Adrenal Hyperplasia Due to Steroid 21-Hydroxylase Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018 Nov 01;103(11):4043-4088.
- Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP et al.. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An Endocrine Society clinical practice guideline. Jl Clin Endo Metab. Nov 2018; 103(11):4043-4088. https://www.ncbi.nlm.nih.gov/pubmed/30272171
- Bachelot A, Grouthier V, Courtillot C, Dulon J, Touraine P. MANAGEMENT OF ENDOCRINE DISEASE: Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: update on the management of adult patients and prenatal treatment. Eur. J. Endocrinol. 2017 Apr;176(4):R167-R181.