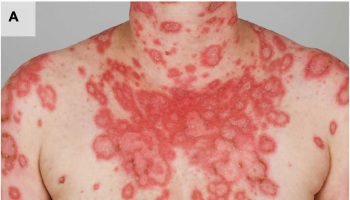
What is acute radiation sickness
Acute Radiation Sickness sometimes known as acute radiation syndrome, radiation poisoning or radiation toxicity, is an acute illness caused by irradiation >1 Gy (more than 1 Gray or 100 rads) total dose, delivered acutely (over a short period of time) at a relatively high-dose rate to the entire body (or most of the body ~ 60%) by a high dose of penetrating radiation in a very short period of time (usually a matter of minutes) 1. The major cause of acute radiation sickness is depletion of immature parenchymal stem cells in specific tissues. Examples of people who suffered from acute radiation sickness are the survivors of the Hiroshima and Nagasaki atomic bombs, the firefighters that first responded after the Chernobyl Nuclear Power Plant event in 1986, and some unintentional exposures to sterilization irradiators. The amount of radiation that a person’s body absorbs is called the radiation dose.
The absorbed dose of radiation is measured in a unit called a gray (Gy). Diagnostic tests that use radiation, such as an X-ray, result in a small dose of radiation — typically well below 0.1 Gy — focused on a few organs or small amount of tissue.
One gray (1 Gy) is the absorption of one joule of energy, in the form of ionizing radiation, per kilogram of matter. To put that into perspective, a 1 Gy exposure is equivalent to having 200,000 dental X-rays or 4.347 million hours of average background radiation (496 years worth of background radiation).
Signs and symptoms of radiation sickness usually appear when the entire body receives an absorbed dose of at least 1 Gy. Doses greater than 10 Gy to the whole body are generally not treatable and usually lead to death within two days to two weeks, depending on the dose and duration of the exposure.
People are constantly exposed to low levels of naturally occurring radiation called background radiation. Background radiation comes from cosmic radiation and from radioactive elements in the air, water, and ground. Cosmic radiation is concentrated at the poles by the earth’s magnetic field and is attenuated by the atmosphere. Thus, exposure is greater for people living at high latitudes, at high altitudes, or both and during airplane flights. Terrestrial sources of external radiation exposure are primarily due to the presence of radioactive elements with half-lives comparable to the age of the earth (~4.5 billion years). In particular, uranium (238U) and thorium (232Th) along with several dozen of their radioactive progeny and a radioactive isotope of potassium (40K) are present in many rocks and minerals. Small quantities of these radionuclides are in the food, water, and air and thus contribute to internal exposure as these radionuclides are invariably incorporated into the body. The majority of the dose from internally incorporated radionuclides is from radioisotopes of carbon (14C) and potassium (40K), and because these and other elements (stable and radioactive forms) are constantly replenished in the body by ingestion and inhalation, there are approximately 7000 atoms undergoing radioactive decay each second.
Internal exposure from the inhalation of radioactive isotopes of the noble gas radon (222Rn and 220Rn), which are also formed from the Uranium (238U) decay series, accounts for the largest portion (73%) of the US population’s average per capita naturally occurring radiation dose. Cosmic radiation accounts for 11%, radioactive elements in the body for 9%, and external terrestrial radiation for 7%. In the US, people receive an average effective dose of about 3 millisieverts (mSv)/yr from natural sources (range ~0.5 to 20 mSv/yr). However, in some parts of the world, people receive > 50 mSv/yr. The doses from natural background radiation are far too low to cause radiation injuries; they may result in a small increase in the risk of cancer, although some experts think there may be no increased risk.
Radiation exposure
Radiation exposure is expressed in several ways to account for the different levels of harm caused by different forms of radiation and the different sensitivity of body tissues.
Absorbed Dose
Radiation exposure is measured in an international (SI) unit called the gray (Gy). The radiation exposure is equivalent to the energy “deposited” in a kilogram of a substance by the radiation. Exposure is also referred to as absorbed dose. The important concept is that exposure is measured by what radiation does to substances, not anything particular about the radiation itself. This allows scientists to unify the measurement of different types of radiation (i.e., particles and wave) by measuring what they do to materials.
The gray (Gy) is a large unit and for normal radiation protection levels a series of prefixes are used:
- nanogray (nGy) is one thousand millionth of a gray (1/1,000,000,000)
- microgray (µGy) is one millionth of a gray (1/1,000,000)
- milligray (mGy) is one thousandth of a gray (1/1,000)
Equivalent dose
Often scientists are interested in the effect of radiation exposure on human tissue. Enter a quantity called equivalent dose, which relates the absorbed dose in human tissue to the effective biological damage of the radiation. Not all radiation has the same biological effect, even for the same amount of absorbed dose. Equivalent dose is measured in an international (SI) unit called the sievert (Sv). Like the gray (Gy), the sievert (Sv) is a large unit and for normal radiation protection levels a series of prefixes are used:
- nanosievert (nSv) is one thousand millionth of a sievert (1/1,000,000,000)
- microsievert (µSv) is one millionth of a sievert (1/1,000,000)
- millisievert (mSv) is one thousandth of a sievert (1/1,000)
To determine equivalent dose (Sv), you multiply absorbed dose (Gy) by a radiation weighting factor (WR) that is unique to the type of radiation. The radiation weighting factor (WR) takes into account that some kinds of radiation are inherently more dangerous to biological tissue, even if their “energy deposition” levels are the same.
For x-rays and gamma rays and electrons absorbed by human tissue, the radiation weighting factor (WR) is 1. For alpha particles (α, α2+, He2+) the radiation weighting factor (WR) is 20. To compute sieverts (Sv) from grays (Gy), simply multiply by the radiation weighting factor (WR). This is obviously a simplification. The radiation weighting factor WR approximates what otherwise would be very complicated computations. The values for the radiation weighting factor (WR) change periodically as new research refines the approximations.
Effective dose
The probability of a harmful effect from radiation exposure depends on what part or parts of the body are exposed. Some organs are more sensitive to radiation than others. A tissue weighting factor (WT) is used to take this into account. When an equivalent dose to an organ is multiplied by the tissue weighting factor for that organ the result is the effective dose to that organ. The unit of effective dose is the sievert (Sv).
If more than one organ is exposed then the effective dose, E, is the sum of the effective doses to all exposed organs.
| Tissue | Tissue weighting factor (WT) | Sum of Tissue weighting factors |
|---|---|---|
| Bone-marrow (red), colon, lung, stomach, breast, remaining tissues* | 0.12 | 0.72 |
| Gonads | 0.08 | 0.08 |
| Bladder, esophagus, liver, thyroid | 0.04 | 0.16 |
| Bone surface, brain, salivary glands, skin | 0.01 | 0.04 |
| Total | 1 |
Footnotes: * Remaining tissues: adrenal glands, extrathoracic region, gall bladder, heart, kidneys, lymphatic nodes, muscle, oral mucosa, pancreas, prostate, small intestine, spleen, thymus, uterus/cervix.
What is ionizing radiation?
The process in which an electron is given enough energy to break away from an atom is called ionization. This process results in the formation of two charged particles or ions: the molecule with a net positive charge, and the free electron with a negative charge.
Ionizing radiation consists of electromagnetic waves or atomic particles with the capacity to strike an electron with sufficient force to strip it from its atom, thus creating an ion.
Ionizing radiation is the energy produced from natural or artificial sources. It has more energy than non-ionizing radiation, enough to cause chemical changes by breaking chemical bonds. This effect can cause damage to living tissue.
X-ray and gamma ray radiation, which are at the upper end of electromagnetic spectrum, have very high frequencies (in the range of 100 billion billion hertz) and very short wavelengths (1 million millionth of a metre). Radiation in this range has high energy. It has enough energy to strip electrons from an atom or, in the case of very high-energy radiation, break up the nucleus of the atom.
Each ionization releases energy that is absorbed by material surrounding the ionized atom. Ionizing radiation deposits a large amount of energy into a small area. In fact, the energy from one ionization is more than enough energy to disrupt the chemical bond between two carbon atoms.
There are three main kinds of ionizing radiation:
- Alpha particles, which include two protons and two neutrons
- Beta particles, which are essentially electrons
- Gamma rays and X-rays, which are pure energy (photons).
Alpha particles and beta particles are not part of the electromagnetic spectrum; they are energetic particles as opposed to pure energy bundles (photons).
Health effects of ionizing radiation
The fetus and children are more sensitive to ionizing radiation exposure than adults. An absorbed dose to the fetus of 100 – 500 millisievert can cause developmental problems such as malformation or reduced IQ.
It is well known that high doses of ionizing radiation can cause harm, but there is continuing scientific uncertainty about effects at low doses. At levels of dose routinely encountered by members of the public and most present-day radiation workers, there is little or no epidemiological evidence of health effects. Radiation protection standards recognize that it is not possible to eliminate all radiation exposure, but they do provide for a system of control to avoid unnecessary exposure and to keep doses in the low dose range.
Is there more than one kind of ionizing radiation?
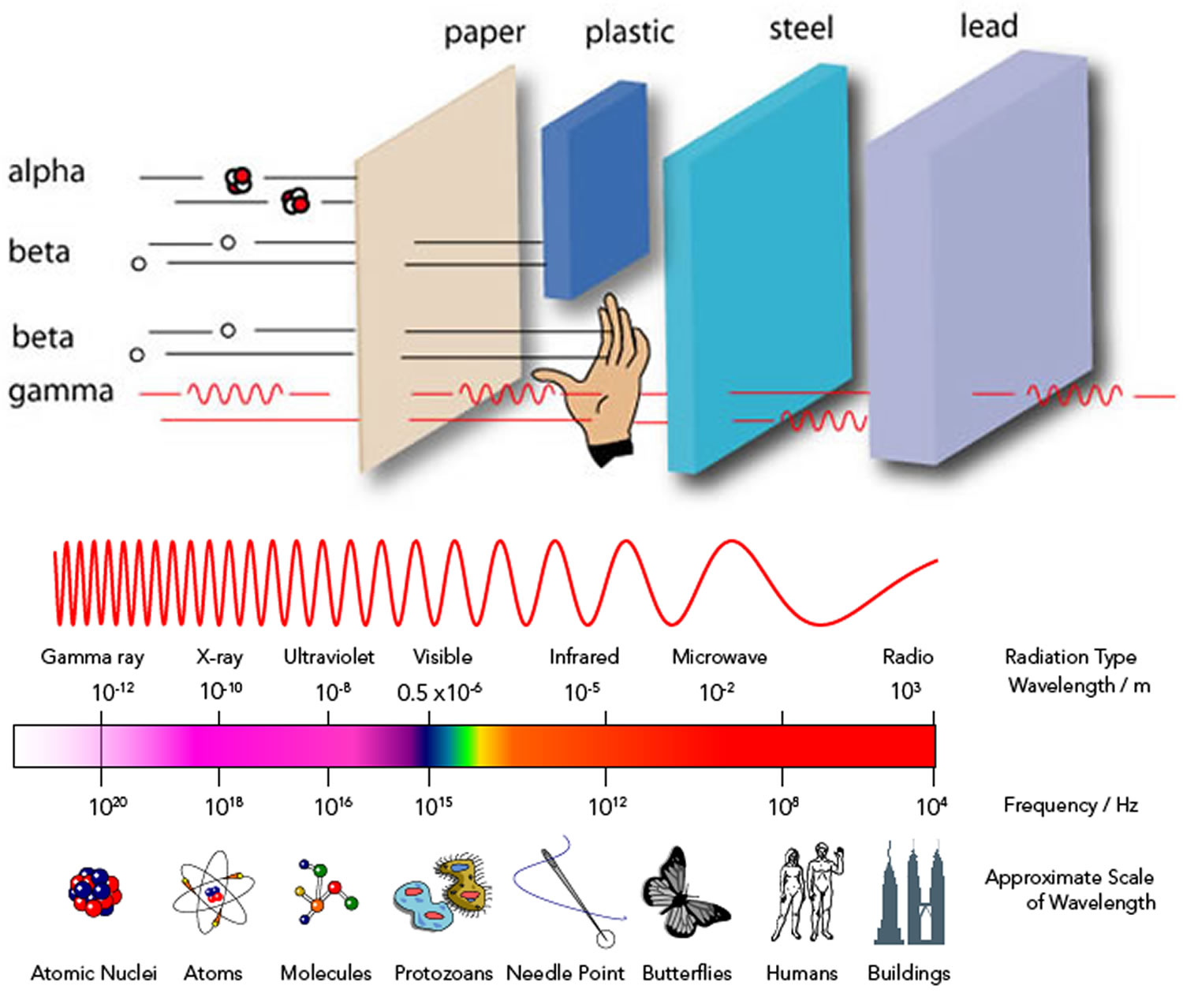
Yes. In addition to X-rays, there are three main kinds of ionizing radiation. They are called alpha (α), beta (β) and gamma (ɣ) radiation. Alpha particles (helium nuclei consisting of two protons and two neutrons) may be stopped completely by a sheet of paper, beta particles (high-speed electrons) can be stopped by perspex, while gamma rays (like X-rays, but with a shorter wavelength) may need lead or concrete to efficiently stop them – but can be stopped by any material providing there is enough of it. Other less common types of ionizing radiation also exist.
What is the difference between radiation and radioactivity?
Radiation can be described as energy or particles from a source that travel through space or other mediums. Light, heat, and the microwaves and radio waves used for wireless communications are all forms of radiation. Radiation includes particles and electromagnetic waves that are emitted by some materials and carry energy.
Radioactivity is the property of some unstable atoms (radionuclides) to spontaneously emit nuclear radiation, usually alpha particles or beta particles often accompanied by gamma-rays. This radiation is emitted when the nucleus undergoes radioactive decay and is converted into a different isotope which may, according to its number of neutrons and protons, be either radioactive (unstable) or non-radioactive (stable). This “daughter” nucleus will usually be of a different chemical element to the original isotope.
Atoms found in nature are either stable or unstable. An atom is stable if the forces among the particles that makeup the nucleus are balanced. An atom is unstable (radioactive) if these forces are unbalanced; if the nucleus has an excess of internal energy. Instability of an atom’s nucleus may result from an excess of either neutrons or protons. A radioactive atom will attempt to reach stability by ejecting nucleons (protons or neutrons), as well as other particles, or by releasing energy in other forms.
A radioactive atom is unstable because it contains extra energy or an unbalanced number of particles, in its nucleus. When this atom ‘decays’ to a more stable atom, it releases the extra energy and/or particles as ionizing radiation.
How is ionizing radiation different from other types of radiation?
Ionizing radiation can eject electrons out of atoms (thereby ionizing them), either by direct interaction with the atoms or by other methods. Alpha and beta particles, as well as X-rays and gamma rays, are examples of directly-ionizing radiation, while neutrons cause ionisation by indirect processes.
What is radiation?
Radiation can be described as energy or particles from a source that travel through space or other mediums. Light, heat, and the microwaves and radio waves used for wireless communications are all forms of radiation.
Radiation includes particles and electromagnetic waves that are emitted by some materials and carry energy. The kind of radiation discussed below is called ionizing radiation because it can produce charged particles (or ions) in matter. X-rays, gamma-rays, alpha particles, beta particles and neutrons are all examples of ionizing radiation.
Radiation activity is measured in an international (SI) unit called a becquerel (Bq). The becquerel counts how many particles or photons (in the case of wave radiation) are emitted per second by a source. The device used for measurement is often the familiar Geiger counter. If you put a Geiger counter over a gram of substance and count 3 clicks per second, the radioactivity of that substance would be 3 becquerel.
Types of radiation
Radiation includes:
- High-energy electromagnetic waves (x-rays, gamma rays). Gamma radiation and x-rays are electromagnetic radiation (ie, photons) of very short wavelength that can penetrate deeply into tissue (many centimeters). While some photons deposit all their energy in the body, other photons of the same energy may only deposit a fraction of their energy and others may pass completely through the body without interacting. The key difference between gamma rays and X-rays is how they are produced. Gamma rays originate from the settling process of an excited nucleus of a radionuclide after it undergoes radioactive decay whereas X-rays are produced when electrons strike a target or when electrons rearrange within an atom.
- Particles (alpha particles, beta particles, neutrons).
Alpha particles and beta particles are not part of the electromagnetic spectrum; they are energetic particles as opposed to pure energy bundles (photons). Because of these characteristics, alpha and beta particles cause the most damage when the radioactive atoms that emit them are within the body (internal contamination) or, in the case of beta-emitters, directly on the body; only tissue in close proximity to the radionuclide is affected. Gamma rays and x-rays can cause damage distant from their source and are typically responsible for acute radiation sickness. Acute Radiation Syndrome can be caused by a sufficient dose of some internally deposited radionuclides that are widely distributed in tissues and organs and have a high specific activity. For example, polonium-210 (Po-210) has a specific activity of 166 terabecquerels per gm (TBq/g) and 1 mcg (size of a grain of salt) of Po-210 delivers a whole body dose of 50 Sv (~20 times the median lethal dose).
Alpha particles
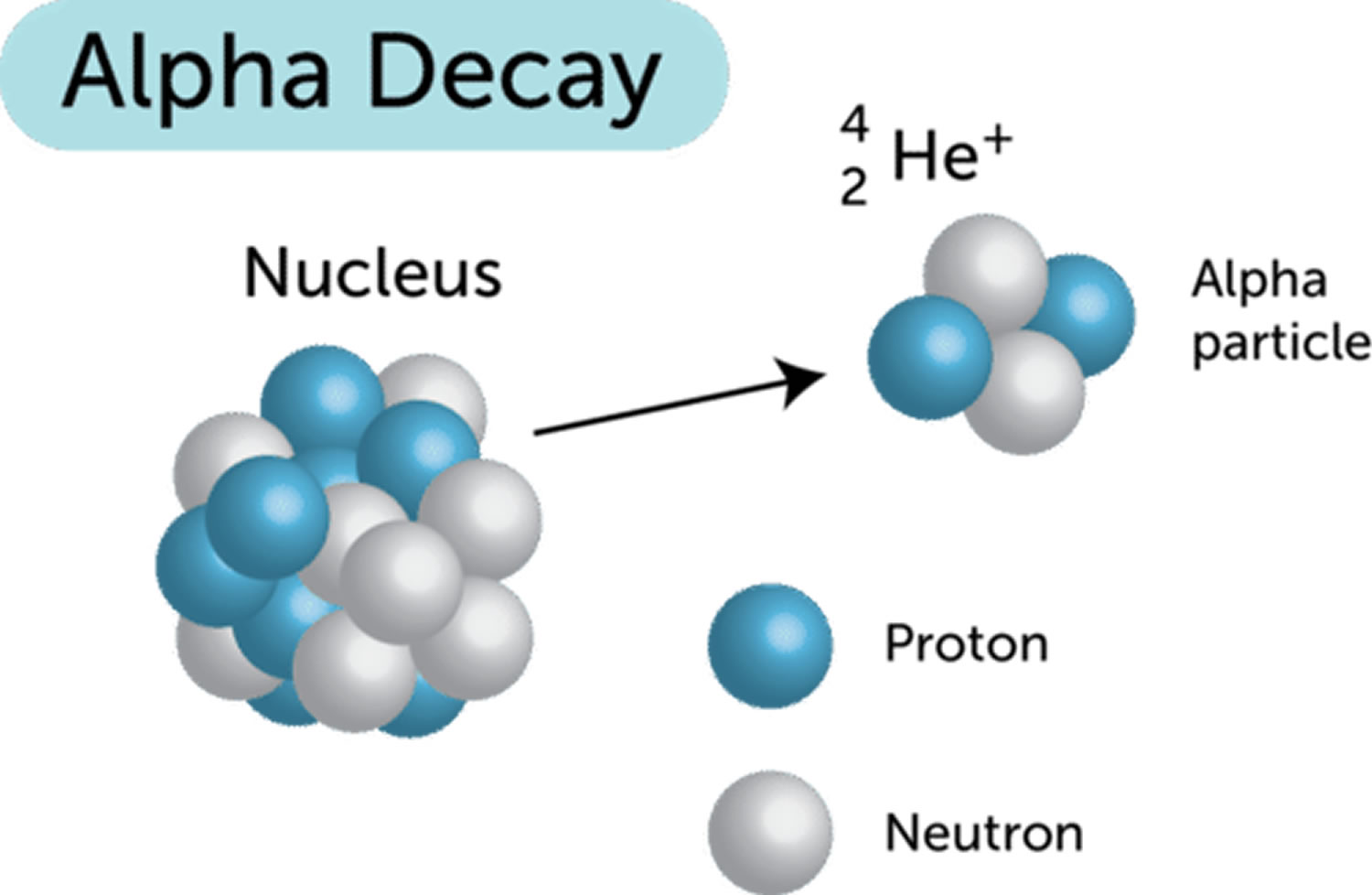
Alpha particles (α, α2+, He2+) also called alpha rays or alpha radiation, are composite particles consisting of two protons and two neutrons tightly bound together into a particle identical to a helium-4 nucleus (Figure 1). Alpha particles (α, α2+, He2+) are emitted from the nucleus of some radionuclides with high atomic numbers (eg, plutonium, radium, uranium) during a form of radioactive decay, called alpha-decay. An alpha-particle is identical to the nucleus of a normal (atomic mass four) helium atom i.e. a doubly ionized helium atom. Many alpha emitters occur naturally in the environment. For example, alpha particles are given off by radionuclides such as uranium-238, radium-226, and other members of the naturally occurring uranium, thorium and actinium decay series which are present in varying amounts in nearly all rocks, soils, and water. Artificially produced sources of alpha particles include the radioisotopes of elements such as plutonium, americium, curium and californium. These are generally produced in a nuclear reactor through the absorption of neutrons by various uranium radioisotopes.
Alpha particles (α, α2+, He2+) are relatively slow and heavy compared with other forms of nuclear radiation. Alpha particles (α, α2+, He2+) travel at 5 to 7 % of the speed of light or 20,000,000 meters per second and has a mass approximately equivalent to 4 protons.
Alpha particles, because they are highly ionizing, are unable to penetrate very far through matter and are brought to rest by a few centimeters of air or less than a tenth of a millimeter of biological tissue; alpha particles cannot penetrate skin beyond a shallow depth (< 0.1 mm).
Health effects of exposure to alpha particles
Alpha particles are highly ionizing because of their double positive charge, large mass (compared to a beta particle) and because they are relatively slow. They can cause multiple ionisations within a very small distance. This gives them the potential to do much more biological damage for the same amount of deposited energy.
Alpha particles can’t penetrate the normal layer of dead cells on the outside of our skin but can damage the cornea of the eye. Alpha-particle radiation is normally only a safety concern if the radioactive decay occurs from an atom that is already inside the body or a cell. Alpha-particle emitters are particularly dangerous if inhaled, ingested, or if they enter a wound.
Some uses of alpha particles
Despite alpha particles low penetrating power, they still provide a range of useful applications such as:
- smoke detectors – americium-241 is commonly used in ionising smoke detectors. Smoke that enters the detector reduces the amount of alpha particles that are detected and triggers the alarm
- static eliminators typically use alpha particles from polonium-210 to remove static charges from equipment
- radioisotope thermoelectric generators use alpha particle decay from plutonium-238 to generate heat which is converted to electricity, commonly used in space probes
- some alpha emitters are being investigated for their potential use in unsealed source radiotherapy to treat cancer.
Figure 1. Alpha particles
Beta particles
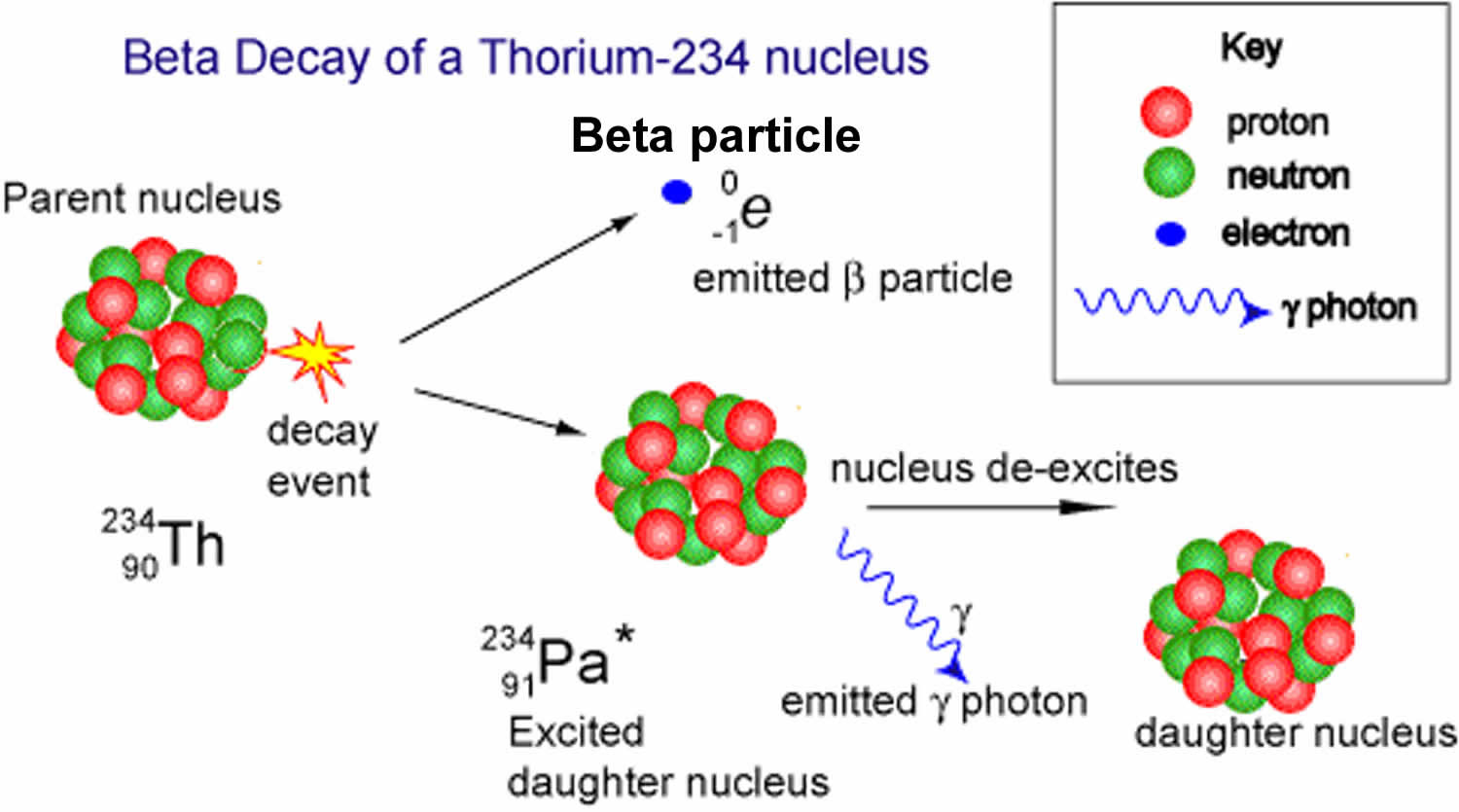
Beta particles (β) are high energy, high speed electrons (β-) or positrons (β+) that are ejected from the nucleus by some radionuclides (eg, cesium-137, iodine-131) during a form of radioactive decay called beta-decay. Beta-decay normally occurs in nuclei that have too many neutrons to achieve stability.
Many beta emitters occur naturally in the radioisotopes found in the natural radioactive decay chains of uranium, thorium and actinium. Examples include lead-210, bismuth-214 and thallium-206. Beta emitters are also commonly found in the radioactive products of nuclear fission. Examples include strontium-90, caesium-137 and tritium.
Beta particles (β) light mass means that they lose energy quickly through interaction with matter and have a haphazard path as they move through air or other materials.
Beta particles are much less ionizing than alpha particles and generally do less damage for a given amount of energy deposition. They typically have ranges of tens of centimetres in air (energy dependent) and a few millimetres in materials.
Beta particles (high-energy electrons) can penetrate more deeply into skin (1 to 2 cm) and cause both epithelial and subepithelial damage.
Beta particles have a mass which is half of one thousandth of the mass of a proton and carry either a single negative (electron) or positive (positron) charge. As they have a small mass and can be released with high energy, they can reach relativistic speeds (close to the speed of light).
Alpha particles and beta particles are not part of the electromagnetic spectrum; they are energetic particles as opposed to pure energy bundles (photons).
Health effects of exposure to beta particles
Beta-particles [high speed electrons (β-) or positrons (β+)], being less ionizing than alpha-particles (α, α2+, He2+), can travel through many centimeters or even meters in air and through millimeters of skin or tissue. Sufficient intensity of beta-radiation can cause burns, rather like severe sunburn. If beta-emitting radionuclides are inhaled or ingested, they can also do damage to internal cells and organs.
Some uses of beta particles
The medium penetrating power of beta particles provides a range of useful applications which include:
- thickness detectors for the quality control of thin materials i.e. paper
- treatment of eye and bone cancers, strontium-90 or strontium-89 are commonly used
- Tritium is used in some phosphorescent lighting typically for emergency lighting as it requires no power
- Fluorine-18 is commonly used as a tracer for positron emission tomography (PET).
Figure 2. Beta particles
Neutrons
Neutrons are electrically neutral particles emitted by a few radionuclides (eg, californium-252) and produced in nuclear fission reactions (eg, in nuclear reactors); their depth of tissue penetration varies from a few millimeters to several tens of centimeters, depending on their energy. Neutrons collide with the nuclei of stable atoms, resulting in emission of energetic protons, alpha and beta particles, and gamma radiation.
Gamma radiation
A gamma ray (g) is a packet of electromagnetic energy (photon) emitted by the nucleus of some radionuclides following radioactive decay. Gamma photons are the most energetic photons in the electromagnetic spectrum. Gamma radiation is released from many of the radioisotopes found in the natural radiation decay series of uranium, thorium and actinium as well as being emitted by the naturally occurring radioisotopes potassium-40 and carbon-14. These are found in all rocks and soil and even in our food and water. Artificial sources of gamma radiation are produced in fission in nuclear reactors, high energy physics experiments, nuclear explosions and accidents.
Scientists measure the energy of photons in electron volts (eV). X-ray photons have energies in the range 100 eV to 100,000 eV (or 100 keV). Gamma-ray photons generally have energies greater than 100 keV. For comparison, ultraviolet radiation has energy that falls in the range from a few electron volts to about 100 eV and does not have enough energy to be classified as ionising radiation. The high energy of gamma rays enables them to pass through many kinds of materials, including human tissue. Very dense materials, such as lead, are commonly used as shielding to slow or stop gamma rays.
Gamma rays are a form of electromagnetic radiation that are the similar to X-rays, distinguished only by the fact that they are emitted from an excited nucleus following radioactive decay. Electromagnetic radiation can be described in terms of a stream of photons, which are massless particles each traveling in a wave-like pattern and moving at the speed of light. Each photon contains a certain amount (or bundle) of energy, and all electromagnetic radiation consists of these photons. Gamma-ray photons have the highest energy in the electromagnetic radiation spectrum and their waves have the shortest wavelength. The high energy of gamma rays enables them to pass through many kinds of materials, including human tissue. Very dense materials, such as lead, are commonly used as shielding to slow or stop gamma rays.
Health effects of exposure to gamma radiation
Gamma radiation is highly penetrating and interacts with matter through ionisation via three processes; photoelectric effect, Compton scattering or pair production. Due to their high penetration power, the impact of gamma radiation can occur throughout a body, they are however less ionizing than alpha particles. Gamma radiation is considered an external hazard with regards to radiation protection.
Similar to all exposure to ionizing radiation, high exposures can cause direct acute effects through immediate damage to cells. Low levels of exposure carry a stochastic health risk where the probability of cancer induction rises with increased exposure.
Some uses of gamma ray emitters
Gamma emitting radionuclides are the most widely used radiation sources. The penetrating power of gamma rays has many applications. However, while gamma rays penetrate many materials, this does not make them radioactive. The three radionuclides that are by far the most useful are cobalt-60, caesium-137, technetium-99m and americium-241.
Uses of cobalt-60:
- sterilization of medical equipment in hospitals
- pasteurization, via irradiation, of certain foodstuffs
- leveling or thickness gauges (i.e. food packaging, steel mills)
- industrial radiography.
Uses of caesium-137:
- measurement and control of the flow of liquids in industrial processes
- investigation of subterranean strata (i.e. oil, coal, gas and other mineralization)
- measurement of soil moisture-density at construction sites
- leveling gauges for packaging of food, drugs and other products.
Uses of technetium-99m:
- Tc-99m is the most widely used radioactive isotope for medical diagnostic studies
- different chemical forms are used for brain, bone, liver, spleen and kidney imaging. It is also used for blood flow studies.
Uses of americium-241:
- smoke detectors for households
- fluid leveling and density gauges
- thickness gauges for thin materials (i.e. paper, foil, glass)
- aircraft fuel gauges
- when mixed with beryllium, americium-241 produces a 241AmBe neutron source with uses in well logging, neutron radiography and tomography.
Figure 3. Gamma radiation
X-rays
An X-ray is a packet of electromagnetic energy (photon) that originate from the electron cloud of an atom. This is generally caused by energy changes in an electron, which moves from a higher energy level to a lower one, causing the excess energy to be released. X-rays are similar to gamma rays however the main difference is the way they are produced, X-rays are produced by electrons external to the nucleus. Traditionally X-rays had longer-wavelengths and lower energy than gamma rays but this is obsolete with modern X-ray production methods.
X-rays are commonly produced in X-ray tubes by accelerating electrons through a potential difference (a voltage drop) and directing them onto a target material (i.e. tungsten). The incoming electrons release X-rays as they slowdown in the target (braking radiation or bremsstrahlung). The X-ray photons produced in this manner range in energy from near zero up to the energy of the electrons. An incoming electron may also collide with an atom in the target, kicking out an electron and leaving a vacancy in one of the atom’s electron shells. Another electron may fill the vacancy and in so doing release an X-ray photon of a specific energy (a characteristic X-ray).
X-rays can also be produced by a synchrotron. A synchrotron is a device that accelerates electrons in an evacuated ring (often several tens of metres in diameter), steering them with magnets. Manipulating the electron beam in a controlled way with the magnets can produce intense beams of X-rays.
X-ray photons are highly energetic and have enough energy to break up molecules and hence damage living cells. When X-rays hit a material some are absorbed and others pass through. Generally, the higher the energy the more X-rays will pass through (see Table 1). It is this penetrating power that allows doctors to take internal images of the human body or objects. X-rays cannot be steered by electric and magnetic fields like alphas, betas or other charged particles.
Table 1. Properties of X-rays
| Energy carried by each photon (γ) | Frequency of electromagnetic wave (Hz) | Wavelength (picometer, 1 picometer = 10-12m) | Thickness of material to halve number of photons (half value thickness) (mm) | ||||
|---|---|---|---|---|---|---|---|
| in electron-volts (eV) | in joules (J) | Concrete | Lead | Human tissue | Aluminium | ||
| 1keV | 1.602 X 10-16 | 2.418 X 1017 | 1240 | 0.0009 | 0.00012 | 0.0018 | 0.0022 |
| 10keV | 1.602 X 10-15 | 2.418 X 1018 | 124 | 0.147 | 0.047 | 1.22 | 0.098 |
| 100keV | 1.602 X 10-14 | 2.418 X 1019 | 12.4 | 17.3 | 0.11 | 38.6 | 15.1 |
| 1MeV | 1.602 X 10-13 | 2.418 X 1020 | 1.24 | 46.4 | 8.6 | 93.3 | 41.8 |
| 10MeV | 1.602 X 10-12 | 2.418 X 1021 | 0.124 | 132 | 12.3 | 298 | 111 |
Health effects of exposure to X-rays
X-rays are highly penetrating and interact with matter through ionization via three processes, photoelectric effect, Compton scattering or pair production. Due to their high penetration power the impact of X-rays can occur throughout a body, they are however less ionizing than alpha particles. X-rays are considered an external hazard with regards to radiation protection.
Similar to all exposure to ionizing radiation high exposures can cause direct acute effects through immediate damage to cells. Low levels of exposure carry a stochastic health risk where the probability of cancer induction increases with increased exposure.
Some uses of X-rays
X-rays have a large range of uses for medical, industrial and research purposes. Diagnostic medical X-rays are the most likely way you will encounter X-rays. Radiotherapy is another example of the medical use of X-rays to treat cancer.
On average, each person receives an effective dose of about 1.7 mSv per year from medical procedures, including about 1.1 mSv from computed tomography (CT) scans. This is similar to the dose everyone receives from background radiation that is and always has been in our environment.
Industrial and research uses of X-rays include X-ray crystallography and fluoroscopy which are commonly used for the quality control of materials (i.e. metal quality) and investigating the properties of materials. Industrial radiography can use X-ray or gamma sources for analysis to look for cracks in buildings, structures or pressure vessels.
X-rays are also used in security processes for baggage/container screening at airports and ports.
Measurement of radiation
Conventional units of measurement include the roentgen, rad, and rem. The roentgen (R) is a unit of exposure measuring the ionizing ability of x-rays or gamma radiation in air. The radiation absorbed dose (rad) is the amount of that radiation energy absorbed per unit of mass. Because biologic damage per rad varies with radiation type (eg, it is higher for neutrons than for x-rays or gamma radiation), the dose in rad is corrected by a quality factor; the resulting equivalent dose unit is the roentgen equivalent in man (rem). Outside the US and in the scientific literature, SI (International System) units are used, in which the rad is replaced by the gray (Gy) and the rem by the sievert (Sv); 1 Gy = 100 rad and 1 Sv = 100 rem. The rad and rem (and hence Gy and Sv) are essentially equal (ie, the quality factor equals 1) when describing x-rays or gamma or beta radiation.
The amount (quantity) of radioactivity is expressed in terms of the number of nuclear disintegrations (transformations) per second. The becquerel (Bq) is the SI unit of radioactivity; one Bq is 1 disintegration per second (dps). In the US system, one curie is 37 billion Bq.
Sources of high-dose radiation
Possible sources of high-dose radiation include the following:
- An accident at a nuclear industrial facility
- An attack on a nuclear industrial facility
- Detonation of a small radioactive device
- Detonation of a conventional explosive device that disperses radioactive material (dirty bomb)
- Detonation of a standard nuclear weapon
Radiation sickness occurs when high-energy radiation damages or destroys certain cells in your body. Regions of the body most vulnerable to high-energy radiation are cells in the lining of your intestinal tract, including your stomach, and the blood cell-producing cells of bone marrow.
People exposed to radiation will get Acute Radiation Syndrome only if:
- The radiation dose must be large (i.e., greater than 0.7 Gray (Gy) or 70 rads) 2.
- Mild symptoms may be observed with doses as low as 0.3 Gy or 30 rads.
- The dose usually must be external (i.e., the source of radiation is outside of the patient’s body).
- Radioactive materials deposited inside the body have produced some acute radiation sickness effects only in extremely rare cases.
- The radiation must be penetrating (i.e., able to reach the internal organs).
- High energy X-rays, gamma rays, and neutrons are penetrating radiations.
- The entire body (or a significant portion of it) must have received the dose 3.
- Most radiation injuries are local, frequently involving the hands, and these local injuries seldom cause classical signs of acute radiation sickness.
- The dose must have been delivered in a short time (usually a matter of minutes).
- Fractionated doses are often used in radiation therapy. These large total doses are delivered in small daily amounts over a period of time. Fractionated doses are less effective at inducing acute radiation sickness than a single dose of the same magnitude.
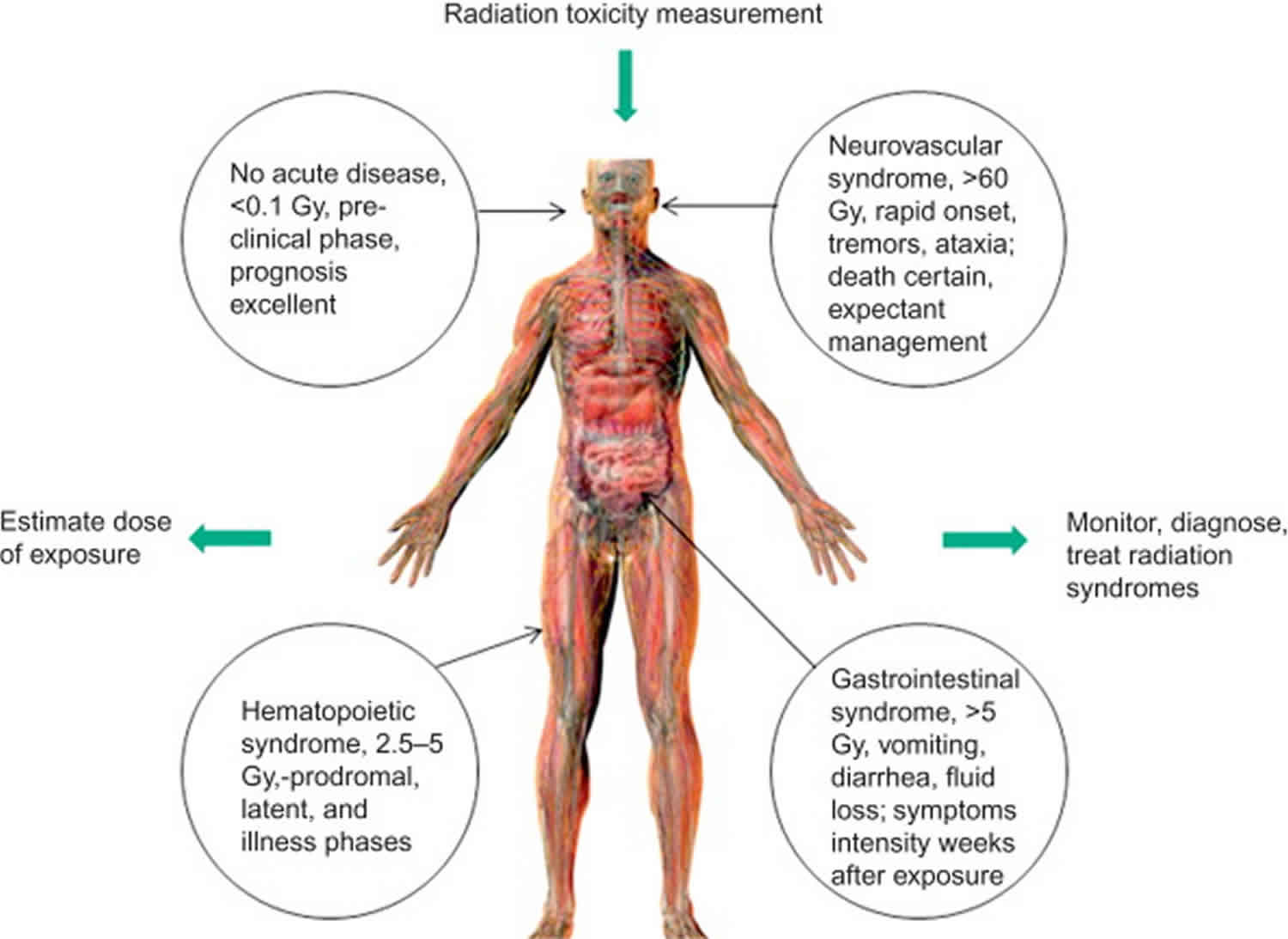
Such irradiation injury initially affects all organs to some extent, but the timing and extent of the injury manifestations depend upon the type, rate, and dose of radiation received 4. The percentage of the body that is injured, the dose homogeneity, and the intrinsic radiosensitivity of the exposed individual also influence manifestations. Different ranges of whole-body doses produce different manifestations of injury. The three main ranges that produce the most characteristic manifestations are referred to as the hematological, gastrointestinal, and neurovascular syndromes. These syndromes are, as a rule, produced only with whole-body or near whole-body irradiation by photon or mixed photon/neutron radiation. High-dose injuries to smaller percentages of the body produce local injury effects, but may not cause Acute Radiation Syndrome 5.
The three classic Acute Radiation Sickness
- Bone marrow syndrome (sometimes referred to as hematopoietic syndrome): the full syndrome will usually occur with a dose greater than approximately 0.7 Gy (70 rads) although mild symptoms may occur as low as 0.3 Gy or 30 rads 6. The survival rate of patients with this syndrome decreases with increasing dose. The primary cause of death is the destruction of the bone marrow, resulting in infection and hemorrhage.
- Gastrointestinal (GI) syndrome: the full syndrome will usually occur with a dose greater than approximately 10 Gy (1000 rads) although some symptoms may occur as low as 6 Gy or 600 rads. Survival is extremely unlikely with this syndrome. Destructive and irreparable changes in the GI tract and bone marrow usually cause infection, dehydration, and electrolyte imbalance. Death usually occurs within 2 weeks.
- Cardiovascular (CV)/ Central Nervous System (CNS) syndrome: the full syndrome will usually occur with a dose greater than approximately 50 Gy (5000 rads) although some symptoms may occur as low as 20 Gy or 2000 rads. Death occurs within 3 days. Death likely is due to collapse of the circulatory system as well as increased pressure in the confining cranial vault as the result of increased fluid content caused by edema, vasculitis, and meningitis.
The four stages of Acute Radiation Sickness
- Prodromal stage (N-V-D stage): The classic symptoms for this stage are nausea, vomiting, as well as anorexia and possibly diarrhea (depending on dose), which occur from minutes to days following exposure. The symptoms may last (episodically) for minutes up to several days.
- Latent stage: In this stage, the patient looks and feels generally healthy for a few hours or even up to a few weeks.
- Manifest illness stage: In this stage, the symptoms depend on the specific syndrome (see Table 1) and last from hours up to several months.
- Recovery or death: Most patients who do not recover will die within several months of exposure. The recovery process lasts from several weeks up to two years.
These stages are described in more detail in Table 2.
Table 2. Acute radiation sickness stages
| Syndrome | Signs and symptoms | |||
|---|---|---|---|---|
| Prodromal phase first 48 hoursb | Latent phase lasts up to a month | Manifest illness phase | Final outcome: survival or death | |
| Gastrointestinal Occurs at doses between 5 Gy and 12 Gy | Nausea, vomiting, diarrhea, anorexia, hemorrhage, weakness through denuded areas Loss of absorptive capacity Increased intensity 4–8 hours | Tiredness and anorexia | Vomiting and fever; progression of bloody diarrhea to shock and death or treatment | Radiation 8–30 Gy dose range cause death from gastrointestinal syndrome |
| Hematologic High dose between 2–3 Gy and 8 Gy. Low dose (<2 Gy) radiation | Often asymptomatic Some fatigue, fever, and bacteremia | Lymphopenia Granulocytopenia Thrombocytopenia | Neutropenia (ANC < 0.5)c Fever, sepsis, hemorrhage, purpura, electrolyte disturbances, and epilation | Agranulocytosis irresponsive to GM-CSF after first cell cycle |
| Central nervous system | No specific signs and symptoms Unspecific fatigue, malaise, anorexia, and drowsiness Not consistently correlated to exposed dose | Latency up to a month Asymptomatic phase except for tiredness and weakness | Headache Impaired cognition, disorientation, seizure, tremor, ataxia Grand mal seizures | Irreversible brain damage secondary to continuous cramps |
| Pulmonary dysfunction | Acute radiation pneumonitis Cough, shortness of breath ALId with inflammatory coagulation activation | Pulmonary edema Pneumonitis | ARDSe Intubation and mechanical ventilation Severe pneumonia Lung fibrosis after 14–30 days from first exposure | Absolute respiratory insufficiency Severely reduced oxygen transport capacity |
Footnotes:
a Induced by a radiation dose of ≥1 Gy;
b onset within the first hour of explosive bloody diarrhea signals a fatal outcome. Appearance during the first 2–3 hours indicates a high dose. Onset between 6–12 hours and termination within 24 hours suggest a sublethal (1–2 Gy) dose. Gastrointestinal symptoms must be documented at the initial and each subsequent examination, and differentiated from a normal stress/anxiety response;
c growth factors should be started promptly and continued until absolute neutrophil count (ANC) >1000;
d infiltrates on chest film and moderately reduced oxygen transport (PaO2/FiO2 < 300 mmHg);
e confluent infiltrates on chest film and severely reduced oxygen transport capacity (PaO2/FiO2 < 200 mmHg).
Abbreviations: ALI = acute lung insufficiency; ANC = absolute neutrophil count; ARDS = acute respiratory distress syndrome; FiO2 = fraction of inspired oxygen in a gas mixture; GM-CSF = granulocyte-macrophage colony-stimulating factor; PaO2 = partial pressure of oxygen in the blood.
[Source 1 ]The most sensitive cells to acute radiation effect are in bone marrow. However, an overlooked fact is that there are other important replicative cells, namely the fixed tissue macrophages in tissue and vital organs. Depending on the absorbed radioactive dose, symptoms appear within hours to weeks, following a predictable clinical course. The prodromal phase of Acute Radiation Syndrome usually occurs in the first 48 hours, but may develop up to 6 days after exposure 7. The latent phase is a short period characterized by improvement of symptoms as the person appears to have recovered. Unfortunately, this effect is transient, lasting for several days to a month.
Symptoms of manifest illness then appear and may last for weeks. This stage is characterized by intense immunosuppression and is the most difficult to manage. If a person survives this stage, recovery is likely. Individuals exposed to a supralethal dose of radiation deteriorate over a period of hours, resulting in early death 5.
Symptoms of acute, high-dose radiation are dependent on the absorbed dose. They may appear within hours to days and follow a somewhat predictable course 8. Early symptoms resulting from an acute whole-body exposure constitute the prodromal radiation sickness. Virtually all individuals receiving a dose of 10–20 Gy develop prodromal signs and symptoms within 1–72 hours after exposure 7. The initial clinical picture is most often dominated by gastrointestinal signs and symptoms (Table 1) primarily resulting from central nervous system manifestations due to the location of the control center of anorexia, nausea, and vomiting in the brain. Central nervous system dysfunction may be evident early on by changes in the electroencephalography, even at much lower doses. In later phases, the symptoms gradually merge into loss of consciousness, hypotension, and death (components of the cerebrovascular syndrome that is characterized by neurologic failure and cardiovascular collapse) before toxicity to other organ systems (such as the gastrointestinal and hematologic systems) can develop.
Death occurs within a few days after exposure to 10–20 Gy, in absence of treatment 4. A rapid, severe prodromal response is the harbinger of a poor clinical outcome that is complicated by severe leukoneutropenia, thrombocytopenia, and anemia with reticulocytopenia, accompanied by hemorrhage, infection, and death. At lower doses (2–10 Gy), it is difficult to establish a prognosis based on the prodromal phase. The prodromal phase is followed by a phase of manifest illness where syndromes specific to various organ systems emerge. Four major organ subsystems are known to be of critical significance in the development of Acute Radiation Syndrome: the gastrointestinal system, neurovascular system, hematologic system, and pulmonary system. Evaluation of system-specific signs and symptoms is required for triage of victims, selection of therapy, and determination of prognosis 9.
Cutaneous Radiation Syndrome
The concept of cutaneous radiation syndrome was introduced in recent years to describe the complex pathological syndrome that results from acute radiation exposure to the skin.
Acute Radiation Syndrome usually will be accompanied by some skin damage. It is also possible to receive a damaging dose to the skin without symptoms of acute radiation sickness, especially with acute exposures to beta radiation or X-rays. Sometimes this occurs when radioactive materials contaminate a patient’s skin or clothes.
When the basal cell layer of the skin is damaged by radiation, inflammation, erythema, and dry or moist desquamation can occur. Also, hair follicles may be damaged, causing epilation. Within a few hours after irradiation, a transient and inconsistent erythema (associated with itching) can occur. Then, a latent phase may occur and last from a few days up to several weeks, when intense reddening, blistering, and ulceration of the irradiated site are visible.
In most cases, healing occurs by regenerative means; however, very large skin doses can cause permanent hair loss, damaged sebaceous and sweat glands, atrophy, fibrosis, decreased or increased skin pigmentation, and ulceration or necrosis of the exposed tissue.
Radiation poisoning symptoms
The severity of signs and symptoms of radiation sickness depends on how much radiation you’ve absorbed. How much you absorb depends on the strength of the radiated energy, the time of your exposures, and the distance between you and the source of radiation.
Radiation sickness signs and symptoms are also affected by the type of exposure — such as total or partial body. The severity of radiation sickness also depends on how sensitive the affected tissue is. For instance, the gastrointestinal system and bone marrow are highly sensitive to radiation.
The initial signs and symptoms of treatable radiation sickness are usually nausea and vomiting. The amount of time between exposure and when these symptoms develop is an indicator of how much radiation a person has absorbed.
After the first round of signs and symptoms, a person with radiation sickness may have a brief period with no apparent illness, followed by the onset of new, more-serious symptoms.
In general, the greater your radiation exposure, the more rapid and more severe your symptoms will be.
Symptoms of acute radiation sickness may include nausea, vomiting, headache, and diarrhea.
- These symptoms start within minutes to days after the exposure, can last for minutes up to several days, and may come and go.
- If you have these symptoms after a radiation emergency, seek medical attention as soon as emergency officials determine it is safe to do so.
After the initial symptoms, a person usually looks and feels healthy for a period of time, after which he or she will become sick again with variable symptoms and severity that vary depending on the radiation dose that he or she received.
- These symptoms include loss of appetite, fatigue, fever, nausea, vomiting, diarrhea, and possibly even seizures and coma.
- This seriously ill stage may last from a few hours up to several months.
- People who receive a high radiation dose also can have skin damage. This damage can start to show within a few hours after exposure or it may be delayed for several days. It can include swelling, itching, and redness of the skin (like a bad sunburn) or may be more severe and include blisters or ulcers.
- The skin may heal for a short time, followed by the return of swelling, itching, and redness days or weeks later.
- Complete healing of the skin may take from several weeks up to a few years.
- The time for skin to heal depends on the radiation dose the person’s skin received.
- People who receive a high radiation dose to all or part of the body also may experience temporary hair loss. It may take several weeks for the hair to grow back.
People with acute radiation sickness typically also have some skin damage. This damage can start to show within a few hours after exposure and can include swelling, itching, and redness of the skin (like a bad sunburn).
There also can be hair loss. As with the other symptoms, the skin may heal for a short time, followed by the return of swelling, itching, and redness days or weeks later. Complete healing of the skin may take from several weeks up to a few years radiation sickness depending on the radiation dose the person’s skin received.
Table 3. Signs and symptoms of radiation sickness
| Signs and symptoms of radiation sickness | Mild exposure (1-2 Gy) | Moderate exposure (2-6 Gy) | Severe exposure (6-9 Gy) | Very severe exposure (10 Gy or higher) |
|---|---|---|---|---|
| Nausea and vomiting | Within 6 hours | Within 2 hours | Within 1 hour | Within 10 minutes |
| Diarrhea | — | Within 8 hours | Within 3 hours | Within 1 hour |
| Headache | — | Within 24 hours | Within 4 hours | Within 2 hours |
| Fever | — | Within 3 hours | Within 1 hour | Within 1 hour |
| Dizziness and disorientation | — | — | Within 1 week | Immediate |
| Weakness, fatigue | Within 4 weeks | Within 1-4 weeks | Within 1 week | Immediate |
| Hair loss, bloody vomit and stools, infections, poor wound healing, low blood pressure | — | Within 1-4 weeks | Within 1 week | Immediate |
Radiation poisoning prevention
In the event of a radiation emergency, stay tuned to your radio or television to hear what protective actions local, state and federal authorities recommend. Recommended actions will depend on the situation, but you will be told to either stay in place or evacuate your area.
Shelter in place
If you’re advised to stay where you are, whether you’re at home or work or elsewhere, do the following:
- Close and lock all doors and windows.
- Turn off fans, air conditioners and heating units that bring air in from outside.
- Close fireplace dampers.
- Bring pets indoors.
- Move to an inner room or basement.
- Stay tuned to your emergency response network or local news.
Evacuate
If you’re advised to evacuate, follow the instructions provided by your local authorities. Try to stay calm and move quickly and in an orderly manner. In addition, travel lightly, but take supplies, including:
- Flashlight
- Portable radio
- Batteries
- First-aid kit
- Necessary medicines
- Sealed food, such as canned foods, and bottled water
- Manual can opener
- Cash and credit cards
- Extra clothes
Be aware that most emergency vehicles and shelters won’t accept pets. Take them only if you’re driving your own vehicle and going someplace other than a shelter.
Acute Radiation Sickness Diagnosis
The diagnosis of Acute Radiation Sickness can be difficult to make because acute radiation sickness causes no unique disease. Also, depending on the dose, the prodromal stage may not occur for hours or days after exposure, or the patient may already be in the latent stage by the time they receive treatment, in which case the patient may appear and feel well when first assessed.
Information important for determining an absorbed radiation dose includes:
- Known exposure. Details about distance from the source of radiation and duration of exposure can help provide a rough estimate of the severity of radiation sickness.
- Vomiting and other symptoms. The time between radiation exposure and the onset of vomiting is a fairly accurate screening tool to estimate absorbed radiation dose. The shorter the time before the onset of this sign, the higher the dose. The severity and timing of other signs and symptoms also may help medical personnel determine the absorbed dose.
- Blood tests. Frequent blood tests over several days enable medical personnel to look for drops in disease-fighting white blood cells and abnormal changes in the DNA of blood cells. These factors indicate the degree of bone marrow damage, which is determined by the level of an absorbed dose.
- Dosimeter. A device called a dosimeter can measure the absorbed dose of radiation but only if it was exposed to the same radiation event as the affected person.
- Survey meter. A device such as a Geiger counter can be used to survey people to determine the body location of radioactive particles.
- Type of radiation. A part of the larger emergency response to a radioactive accident or attack would include identifying the type of radiation exposure. This information would guide some decisions for treating people with radiation sickness.
If a patient received more than 0.05 Gy (5 rads) and three or four complete blood counts (CBCs) are taken within 8 to 12 hours of the exposure, a quick estimate of the dose can be made. If these initial blood counts are not taken, the dose can still be estimated by using complete blood count (CBC) results over the first few days. It would be best to have radiation dosimetrists conduct the dose assessment, if possible.
If a patient is known to have been or suspected of having been exposed to a large radiation dose, draw blood for complete blood count (CBC) analysis with special attention to the lymphocyte count, every 2 to 3 hours during the first 8 hours after exposure (and every 4 to 6 hours for the next 2 days). Observe the patient during this time for symptoms and consult with radiation experts before ruling out acute radiation sickness.
If no radiation exposure is initially suspected, you may consider acute radiation sickness in the differential diagnosis if a history exists of nausea and vomiting that is unexplained by other causes. Other indications are bleeding, epilation, or white blood count (WBC) and platelet counts abnormally low a few days or weeks after unexplained nausea and vomiting. Again, consider complete blood count (CBC) and chromosome analysis and consultation with radiation experts to confirm diagnosis.
Radiation poisoning treatment
Treatment of acute radiation sickness
- Treatment of acute radiation sickness focuses on reducing and treating infections, maintaining hydration, and treating injuries and burns. Some patients may benefit from treatments that help the bone marrow recover its function.
- The lower the radiation dose, the more likely it is that the person will recover from acute radiation sickness.
- The cause of death in most cases is the destruction of the person’s bone marrow, which results in infections and internal bleeding.
- For survivors of acute radiation sickness, the recovery process may last from several weeks up to 2 years.
- Cutaneous Radiation Injury happens when exposure to a large dose of radiation causes injury to the skin. A doctor will suspect the presence of a Cutaneous Radiation Injury when a skin burn develops in a person who was not exposed to a source of heat, electrical current, or chemicals.
Decontamination
Decontamination involves removing external radioactive particles. Removing clothing and shoes eliminates about 90 percent of external contamination. Gently washing with water and soap removes additional radiation particles from the skin.
Decontamination prevents radioactive materials from spreading more. It also lowers the risk of internal contamination from inhalation, ingestion or open wounds.
Initial Treatment and Diagnostic Evaluation
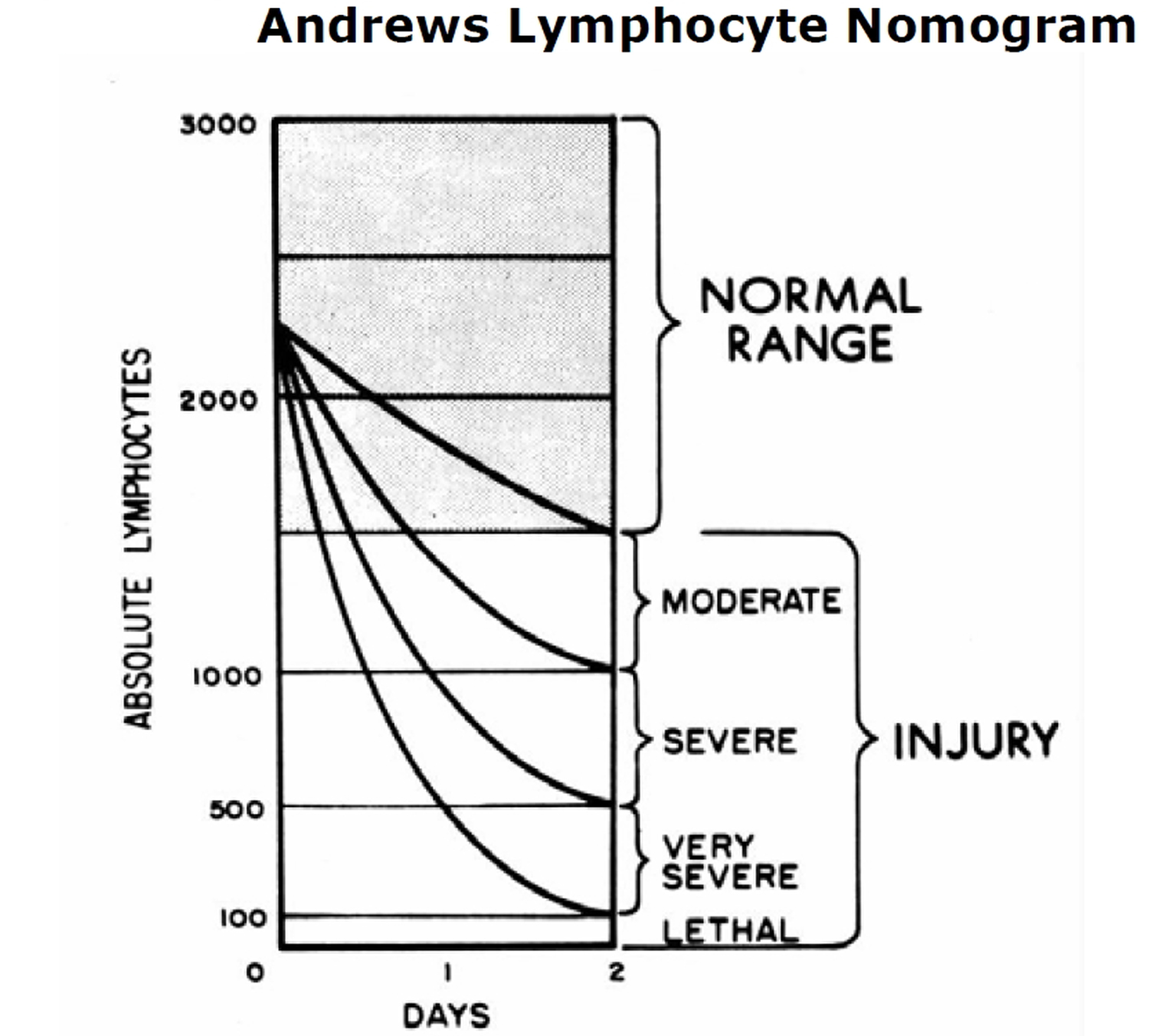
Treat vomiting 11 and repeat complete blood count (CBC) analysis with special attention to the lymphocyte count every 2 to 3 hours for the first 8 to 12 hours after exposure (and every 4 to 6 hours for the following 2 or 3 days). Sequential changes in absolute lymphocyte counts over time are demonstrated below in the Andrews Lymphocyte Nomogram (see Figure 4). Precisely record all clinical symptoms, particularly nausea, vomiting, diarrhea, and itching, reddening or blistering of the skin. Be sure to include time of onset.
Figure 4. Andrews Lymphocyte Nomogram
 Note and record areas of erythema. If possible, take color photographs of suspected radiation skin damage. Consider tissue, blood typing, and initiating viral prophylaxis.
Note and record areas of erythema. If possible, take color photographs of suspected radiation skin damage. Consider tissue, blood typing, and initiating viral prophylaxis.
After consultation, begin the following treatment (as indicated):
- supportive care in a clean environment (if available, the use of a burn unit may be quite effective)
- prevention and treatment of infections
- stimulation of hematopoiesis by use of growth factors
- stem cell transfusions or platelet transfusions (if platelet count is too low)
- psychological support
- careful observation for erythema (document locations), hair loss, skin injury, mucositis, parotitis, weight loss, or fever
- confirmation of initial dose estimate using chromosome aberration cytogenetic bioassay when possible. Although resource intensive, this is the best method of dose assessment following acute exposures.
- consultation with experts in radiation accident management
Treatment for damaged bone marrow
Management of the hematologic syndrome, as a component of acute radiation sickness, requires understanding of its manifestations and implementation of clinical biodosimetry to provide appropriate therapeutic support. Hematopoietic growth factors may be of value if administered early as a component of supportive care. A protein called granulocyte colony-stimulating factor (G-CSF), which promotes the growth of white blood cells, may counter the effect of radiation sickness on bone marrow. Treatment with this protein-based medication, which includes filgrastim (Neupogen), sargramostim (Leukine) and pegfilgrastim (Neulasta), may increase white blood cell production and help prevent subsequent infections.
If you have severe damage to bone marrow, you may also receive transfusions of red blood cells or blood platelets.
Planning for urgent Hematopoietic Stem Cell Transplantation for those with intermediate- to high-dose radiation (4–10 Gy) may be required (Table 4), although the use of Hematopoietic Stem Cell Transplantation is controversial as outcomes after radiation accidents have been poor 12. Establishing contingency plans for triage, assessment, supportive care, and treatment with defined eligibilities, treatment plans, and incorporated data collection to assess results and plan further improvements in care is imperative for the effective management of large scale radiation accidents 9. The hematology/oncology community is most suited to participate in such contingency planning 13.
Table 4. Candidates for bone marrow transplantation
| First step | |
| Prompt growth factor intervention | In the case of aplasia in relation to acute radiation sickness, emergency HSCT is not necessary. G-CSF/GM-CSF promotes hematological reconstruction and should be evaluated after prompt administration and after 14 days of high dose GM-CSF administration |
| Second step | |
| Final evaluation for candidates for HSCT | HSCT should only be implemented after residual hematopoiesis and only considered if severe aplasia persists after long G-CSF/GM-CSF high-dose treatment |
Abbreviations: G-CSF = granulocyte colony-stimulating factor; GM-CSF = granulocyte-macrophage colony-stimulating factor; HSCT = hematopoietic stem cell transplantation.
[Source 14, 15 ]Survival requires hematologic recovery
Management of patients with Acute Radiation Sickness includes early use of hematopoietic cytokines, antimicrobials, and transfusion support. Recommendations based on radiation dose and physiologic response is made for treatment of the hematologic syndrome, and therapy includes treatment with hematopoietic cytokines, blood transfusion, and, in selected cases, Hematopoietic Stem Cell Transplantation 16.
Additional medical management based on the evolution of clinical signs and symptoms includes the use of antimicrobial agents (quinolones, antiviral therapy, and antifungal agents), antiemetic agents, and analgesic agents. Because of the strong psychological impact of possible radiation exposure, psychosocial support is required for those exposed, regardless of the dose.
The prevention and management of infection is the mainstay of therapy. There is a quantitative relationship between the degree of neutropenia and the increased risk of infectious complications 16. Antibiotic prophylaxis should only be considered in afebrile patients at the highest risk for infection. These patients have profound duration of more than 7 days neutropenia (eg, measured in whole blood as absolute neutrophil count <500/mL). Although the degree of neutropenia is the greatest risk factor for developing infection, other factors influence treatment choice and outcome. Such factors include duration of neutropenia, bactericidal functionality of surviving neutrophils, alteration of physical defense barriers, the patient’s endogenous microflora, and organisms endemic to the hospital and community.19 As the duration of neutropenia increases, the risk of secondary infections such as invasive mycoses also increases 17.
It is for these reasons that adjuvant therapy such as the cytokines sargramostim (GM-CSF [Leukine®; Immunex, Seattle, WA]) and filgrastim (G-CSF [Neupogen®; Amgen, Inc, Thousand Oaks, CA]) will prove invaluable in the treatment of the severely irradiated person, although only sparse data is available 16. The treatment recommendations on irradiation-associated aplasia are based on the known effect of CSF’s (colony stimulating factor) beneficial effect on recovery of neutrophils in oncology and hematology patients 18 and in recipients of bone marrow transplantation 19, on their seemingly positive role on hematological recovery in a small number of radiation accident victims 20 and, most importantly, on the improved survival and positive effects on neutrophils in a number of well-conducted studies of animals exposed to radiation 21.
Treatment with growth factors
Cytokines include GM-CSF, macrophage CSF, G-CSF, stem cell factor, and interleukin series (interleukin-1 to interleukin-16). GM-CSF and G-CSF have been available since 1997 for the treatment of radiation myelosuppression. G-CSF, also known as filgrastim, is administered in a dose of 100–200 mcg/m2/day and GM-CSF, also known as sargramostim, is administered in a dose of 200–400 mcg/m2/day. Both are given intravenously or subcutaneously. Both drugs should be initiated promptly upon diagnosis of significant bone marrow damage and continued until recovery of neutrophil counts is sustained above 800/mm3 15.
G-CSF and GM-CSF are currently in widespread clinical use for the treatment of acute neutropenic conditions, and in turn are used in the management of infections following radiochemotherapy of cancer patients. Both of these agents are potent but selective stimulators of granulopoietic arm of the hematologic system, and serve not only to increase blood neutrophil counts but also to enhance the maturation and function of these vital cells. Both agents have high therapeutic ratios, minimal nonperformance side adverse effects, can be administered and monitored with relative ease, and can effectively serve to minimize the risk of infection resulting from a radiation-compromised lymphohematopoietic system.
G-CSF and GM-CSF are potent stimulators of hematopoiesis and effective in reducing duration and degree of neutropenia. An additional benefit of CSFs is their ability to increase functional capacity of neutrophil and thereby contribute to the prevention of infection in an active role as cellular host. They constitute a remarkable advance in the treatment of neutropenia and are, thus, powerful tools for oncologists in the clinical management of cancer patients. The drugs act upon uncommitted stem cell populations within bone marrow to increase mitotic rate, accelerate repopulation, differentiate daughter cells to become committed stem cells, speed the maturation process, and improve the function of existing granulocytes. In this way, they improve the immune function of existing cells while speeding the recovery of stem cell populations, thus reducing the extent and duration of the white cell nadir and total compromise of the immune system.
Studies of cancer patients suffering from neutropenia consistently show that use of these cytokines reduces infection rates, admissions, and days hospitalized 22. GM-CSF and G-CSF have both been used for treatment of ARS because their effect on granulocytes and myeloid stem cell lines do not come at the expense of other marrow cell lines. In order to achieve maximum clinical response, G-CSF or GM-CSF should be started as soon as possible after exposure 5. This provides the opportunity for maximum recovery. Cytokine administration should continue, with daily consecutive injections, to reach the desired effect of an absolute neutrophil count of 1000/μL after the absolute neutrophil count nadir.
Table 5. Comparison of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF)
| GM-CSF | G-CSF | |
|---|---|---|
| Upregulatinga | Effect on monocytes, tissue macrophages, and granulocytes | Only effect on granulocytes |
| Adverse effects | Fever, nausea, fatigue, headache, bone pain, myalgia | Medullary bone pain observed shortly after initiation of G-CSF treatment |
| Dosing | 200–400 mg/m2/day | 5 μg/kg body weight |
| Initiation | Promptly when significant radiation dose is suspected | Either promptly when significant radiation dose is suspected or when neutrocytes <0.5 × 109 |
| Stopping criteria | Neutrocytes increasing, eg, >1.0 × 109 ANCb | Neutrocytes increasing, eg, >1.0 × 109 ANC |
| Route of administration | Subcutaneous/Infusion/Inhalation | Subcutaneous/Infusion |
Footnotes:
a Includes both quantitative (increased number) and qualitative (improved maturation and function) related variables effects;
b stopping criteria should be based on the response of macrophage activation, however, this variable cannot be measured, therefore the stopping criteria of G-CSF is applied.
Abbreviation: ANC = absolute neutrophil count.
[Source 1 ]Prophylactic intervention
A number of papers document that a prompt initiation of treatment with growth factors implies an optimal outcome after radiation exposure 16, as the tissue macrophages will be transformed into fully matured immunocompetent dendritic cells in 7–10 days. This implies that preemptive intervention, or intervention initiated as early as possible after the radiation exposure, is the preferred intervention after an exposure of a high radiation dose, eg, whole-body exposure to a radiation dose of 2–3 Gy, at which time the number and function of the peripheral monocytes and neutrocytes are not yet affected. Treatment of Acute Radiation Syndrome with GM-CSF ensures both quantitative and qualitative effect on all effector cells, ie, monocytes, tissue-bound macrophages, and neutrocytes (Figure 1), as G-CSF does not stimulate local maturation of resting and immunoincompetent macrophages. In organs and tissues, these cells will mediate their front defense bastion in relation to the immune defense. The activated macrophages orchestrate the overall actions and recruitment of systemic host cells like the lymphocytic cells and neutrophils, ensuring and maintaining a normal host barrier function in respect to endogenous and external biological agents in the hypoplastic or aplastic ARS patient. Macrophages are found in all human tissues, not only in bones, skin, and mucosa (eg, of the gut, eye, peritoneum, and meninges), but also in all organs including lungs, kidneys, heart, central nervous system, pericardium, pleura, and liver. Only GM-CSF, and not C-CSF, activates macrophages by production from location-specific tissue cells. Therefore, in the sealed-off compartment of the lungs, activation of the macrophages can only be achieved by stimulation of GM-CSF, either by local production or by inhalation.
Local pulmonary host defense and Acute Radiation Sickness
There has recently been a discussion whether recombinant proteins should be inhaled or administered systemically in order to achieve a pulmonary effect by reaching the alveolar receptors 23. The question is then, which is the preferred route when intervening with GM-CSF?
In bone marrow, stem cells, and all organs, macrophage activity is enhanced by the systemic administration of GM-CSF dosing. In a radiation disaster, the lungs receive a comparable dose of radiation as the rest of the body, but as the lungs depend solely on local endogenous GM-CSF expression by alveolar macrophages, the endogenous GMCSF produced from systemic tissues does not penetrate across the alveolar capillary barrier.
It has been documented that inhaled GM-CSF increases the number and function of phagocytic cells from bronchoalveolar lavage, but only a sparse and transient increase in the number of myeloid cells in circulation.34 When administered intravenously, however, there is only a limited response in alveolar cellularity. It follows that pulmonary innate host defense is separated from the systemic defense system in respect to GM- CSF, and that biologicsa does not penetrate from systemic circulation to the alveolar space 24. It has been documented that when administered intravenously, only 2% of smaller molecules such as recombinant antitrypsin reach the alveolar space 25. In larger molecules, such as recombinant activated protein C, there is no effect when administered intravenously,50 however when inhaled, activated protein C achieves the expected effect in the alveolus with no adverse effects 26.
An important point is whether the inhaled drug reaches the GM-CSF receptors of alveolar macrophages in the peripheral airways. By using a micropump nebulizer, sufficiently small respirable aerosol particles with a size of <2.5 μm are produced which means that a high degree of peripheral lung deposition is obtained 27.
It has been thoroughly documented that there are no known adverse effects in relation to administering inhaled GM-CSF, even when administered in very high doses 28.
Inhaled GM-CSF in antiradiation intervention in Acute Radiation Sickness
Inhaled GM-CSF in antiradiation intervention maintains lung host defense and prevents severe pneumonia with endogenous microbiological agents such as viruses, bacteria, and fungi. Inhaled GM-CSF should be administered promptly and concomitantly with systemic intervention in the antiradiation therapy regime. The initial dose should be <300 μg/day up to 300 μg/m2 daily depending on the response 15. The inhaled drug is effective and has no adverse effect even in the very high dose range, making it highly recommendable in Acute Radiation Syndrome 20.
Treatment for internal contamination
Some treatments may reduce damage to internal organs caused by radioactive particles. Medical personnel would use these treatments only if you’ve been exposed to a specific type of radiation. These treatments include the following:
- Potassium iodide (Thyroshield, Iosat). This is a nonradioactive form of iodine. Because iodine is essential for proper thyroid function, the thyroid becomes a “destination” for iodine in the body. If you have internal contamination with radioactive iodine (radioiodine), your thyroid will absorb radioiodine just as it would other forms of iodine. Treatment with potassium iodide may fill “vacancies” in the thyroid and prevent absorption of radioiodine. The radioiodine is eventually cleared from the body in urine. Potassium iodide isn’t a cure-all and is most effective if taken within a day of exposure.
- Prussian blue (Radiogardase). This type of dye binds to particles of radioactive elements known as cesium and thallium. The radioactive particles are then excreted in feces. This treatment speeds up the elimination of the radioactive particles and reduces the amount of radiation cells may absorb.
- Diethylenetriamine pentaacetic acid (DTPA). This substance binds to metals. DTPA binds to particles of the radioactive elements plutonium, americium and curium. The radioactive particles pass out of the body in urine, thereby reducing the amount of radiation absorbed.
Supportive treatment
If you have radiation sickness, you may receive additional medications or interventions to treat:
- Bacterial infections
- Headache
- Fever
- Diarrhea
- Nausea and vomiting
- Dehydration
- Burns
- Sores or ulcers
End-of-life care
A person who has absorbed very large doses of radiation has little chance of recovery. Depending on the severity of illness, death can occur within two days or two weeks. People with a lethal radiation dose will receive medications to control pain, nausea, vomiting and diarrhea. They may also benefit from psychological or pastoral care.
References- Heslet L, Bay C, Nepper-Christensen S. Acute radiation syndrome (ARS) – treatment of the reduced host defense. International Journal of General Medicine. 2012;5:105-115. doi:10.2147/IJGM.S22177. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3273373/
- Berger ME, O’Hare FM Jr, Ricks RC, editors. The Medical Basis for Radiation Accident Preparedness: The Clinical Care of Victims. REAC/TS Conference on the Medical Basis for Radiation Accident Preparedness. New York: Parthenon Publishing; 2002.
- Jarrett DG. Medical Management of Radiological Casualties Handbook, 1st ed. Bethesda, Maryland: Armed Forces Radiobiology Research Institute (AFRRI); 1999.
- Anno GH, Baum SJ, Withers HR, Young RW. Symptomatology of acute radiation effects in humans after exposure to doses of 0.5–30 Gy. Health Phys. 1989;56(6):821–838. https://www.ncbi.nlm.nih.gov/pubmed/2722506
- Mettler FA, Jr, Gus’kova AK, Gusev I. Health effects in those with acute radiation sickness from the Chernobyl accident. Health Phys. 2007;93(5):462–469. https://www.ncbi.nlm.nih.gov/pubmed/18049222
- LaTorre TE. Primer of Medical Radiobiology, 2nd ed. Chicago: Year Book Medical Publishers, Inc.; 1989.
- Hall EJ. Acute effects of total-body irradiation. In: Hall EJ, editor. Radiobiology for the Radiologist. 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. pp. 117–128.
- Schull WJ. Effects of Atomic Radiation. New York, NY: Wiley-Liss; 1995.
- Weisdorf D, Chao N, Waselenko JK, et al. Acute radiation injury: contingency planning for triage, supportive care, and transplantation. Biol Blood Marrow Transplant. 2006;12(6):672–682. https://www.ncbi.nlm.nih.gov/pubmed/16737941
- Radiation Exposure and Contamination. https://www.merckmanuals.com/professional/injuries-poisoning/radiation-exposure-and-contamination/radiation-exposure-and-contamination
- National Council on Radiation Protection and Measurements (NCRP). Management of Terrorist Events Involving Radioactive Material, NCRP Report No. 138. Bethesda, Maryland: NCRP; 2001.
- Baranov A, Gale RP, Guskova A, et al. Bone marrow transplantation after the Chernobyl nuclear accident. N Engl J Med. 1989;321(4):205–212. https://www.ncbi.nlm.nih.gov/pubmed/2664512
- Fliedner TM. Nuclear terrorism: the role of hematology in coping with its health consequences. Curr Opin Hematol. 2006;13(6):436–444. https://www.ncbi.nlm.nih.gov/pubmed/17053455
- Gourmelon P, Benderitter M, Bertho JM, Huet C, Gorin NC, De Revel P. European consensus on the medical management of acute radiation sickness and analysis of the radiation accidents in Belgium and Senegal. Health Phys. 2010;98(6):825–832. https://www.ncbi.nlm.nih.gov/pubmed/20445389
- Waselenko JK, MacVittie TJ, Blakely WF, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140(12):1037–1051. https://www.ncbi.nlm.nih.gov/pubmed/15197022
- Gourmelon P, Benderitter M, Bertho JM, Huet C, Gorin NC, De Revel P. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys. 2010;98(6):825–832. https://www.ncbi.nlm.nih.gov/pubmed/20445389
- Fliedner TM, Andrews GA, Cronkite EP, Bond VP. Early and late cytologic effects of whole body irradiation on human marrow. Blood. 1964;23:471–487. http://www.bloodjournal.org/content/23/4/471.long
- Gurion R, Gafter-Gvili A, Paul M, et al. Hematopoietic growth factors in aplastic anemia patients treated with immunosuppressive therapy-systematic review and meta-analysis. Haematologica. 2009;94(5):712–719. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2675684/
- Nemunaitis J, Rabinowe SN, Singer JW, et al. Recombinant granulocyte-macrophage colony-stimulating factor after autologous bone marrow transplantation for lymphoid cancer. N Engl J Med. 1991;324(25):1773–1778. http://www.nejm.org/doi/full/10.1056/NEJM199106203242504
- Liu Q, Jiang B, Jiang LP, et al. Clinical report of three cases of acute radiation sickness from a (60)Co radiation accident in Henan Province in China. J Radiat Res (Tokyo) 2008;49(1):63–69. https://www.ncbi.nlm.nih.gov/pubmed/18187937
- Mayer P, Schütze E, Lam C, Kricek F, Liehl E. Recombinant murine granulocyte-macrophage colony-stimulating factor augments neutrophil recovery and enhances resistance to infections in myelosuppressed mice. J Infect Dis. 1991;163(3):584–590. https://www.ncbi.nlm.nih.gov/pubmed/1995731
- Anderson PM, Markovic SN, Sloan JA, et al. Aerosol granulocyte macrophage-colony stimulating factor: a low toxicity, lung-specific biological therapy in patients with lung metastases. Clin Cancer Res. 1999;5(9):2316–2323. https://www.ncbi.nlm.nih.gov/pubmed/10499599
- Heslet L. Look on the “air side” in pneumonia. Crit Care Med. 2009;37(2):774–775. https://www.ncbi.nlm.nih.gov/pubmed/19325384
- Rose RM, Kobzik L, Dushay K, et al. The effect of aerosolized recombinant human granulocyte macrophage colony-stimulating factor on lung leukocytes in nonhuman primates. Am Rev Respir Dis. 1992;146(5 Pt 1):1279–1286. https://www.ncbi.nlm.nih.gov/pubmed/1443885
- Brand P, Beckmann H, Maas Enriquez M, et al. Peripheral deposition of alpha1-protease inhibitor using commercial inhalation devices. Eur Respir J. 2003;22(2):263–267. https://www.ncbi.nlm.nih.gov/pubmed/12952258
- Waerhaug K, Kuzkov VV, Kuklin VN, et al. Inhaled aerosolised recombinant human activated protein C ameliorates endotoxin-induced lung injury in anaesthetised sheep. Crit Care. 2009;13(2):R51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2689497/
- Luisetti M, Kroneberg P, Suzuki T, et al. Physical properties, lung deposition modeling, and bioactivity of recombinant GM-CSF aerosolised with a highly efficient nebulizer. Pulm Pharmacol Ther. 2011;24(1):123–127. https://www.ncbi.nlm.nih.gov/pubmed/20728558
- Anderson PM, Markovic SN, Sloan JA, et al. Aerosol granulocyte macrophage-colony stimulating factor: a low toxicity, lung-specific biological therapy in patients with lung metastases. Clin Cancer Res. 1999;5(9):2316–2323. http://clincancerres.aacrjournals.org/content/5/9/2316.long