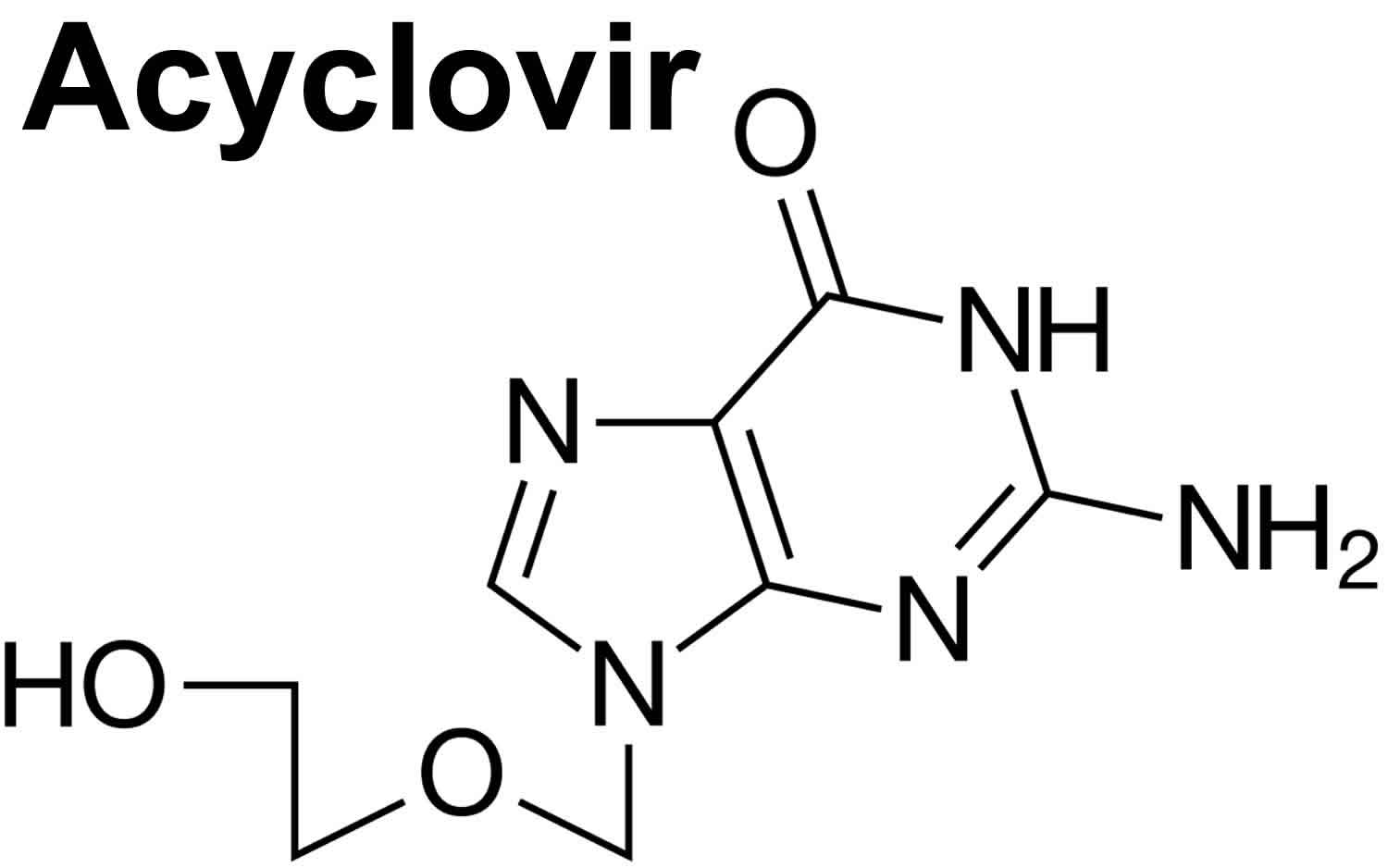
What is acyclovir
Acyclovir is an antiviral prescription medicine approved by the U.S. Food and Drug Administration (FDA) to treat and prevent reoccurrence of herpes simplex virus (HSV) infections (including genital herpes) and to treat varicella zoster virus (VZV) diseases (including chicken pox and shingles). Acyclovir is approved in different formulations and strengths for use in specific populations, including in people who are immunocompromised (have a weakened immune system). Acyclovir slows the growth and spread of the herpes virus in the body. Acyclovir will not cure herpes, but it can lessen the symptoms of the infection. Acyclovir will not cure genital herpes and may not stop the spread of genital herpes to other people. Acyclovir is used to treat infections caused by herpes viruses, such as genital herpes, cold sores, shingles, and chicken pox, as well as varicella (chickenpox), and cytomegalovirus (CMV). Acyclovir is used to decrease pain and speed the healing of sores or blisters in people who have chickenpox (varicella), shingles (herpes zoster, a rash that can occur in people who have had chickenpox in the past), and first-time or repeat outbreaks of genital herpes (a herpes virus infection that causes sores to form around the genitals and rectum from time to time). Acyclovir is also sometimes used to prevent outbreaks of genital herpes in people who are infected with the virus. Acyclovir is in a class of antiviral medications called synthetic nucleoside analogues. Acyclovir works by stopping the spread of the herpes virus in the body.
Acyclovir was approved for use in herpes virus infections in the United States in 1982, and is still widely used in treatment and prophylaxis of genital and mucocutaneous herpes simplex infection with almost 5 million prescriptions filled yearly. Acyclovir is available as capsules of 200 mg, tablets of 400 and 800 mg, oral suspensions, creams, ointments, and parenteral preparations in several generic forms, as well as under the brand name of Zovirax. The typical recommended oral dose in adults for genital or oral herpes simplex is 200 to 800 mg three to five times daily for 5 to 10 days; the usual prophylactic dose is 400 mg twice daily. The typical intravenous doses for severe infections is 5 to 10 mg/kg every 8 hours for 5 to 10 days. Side effects are uncommon with oral formulations, but can include myalgias, rash, temors, lethargy and confusion. Rare side effects include bone marrow toxicity and Stevens Johnson syndrome.
Acyclovir comes as a tablet, a capsule, and a suspension (liquid) to take by mouth. It is usually taken with or without food two to five times a day for 5 to 10 days, starting as soon as possible after your symptoms begin. When acyclovir is used to prevent outbreaks of genital herpes, it is usually taken two to five times a day for up to 12 months. Take acyclovir at around the same times every day. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Take acyclovir exactly as directed. Do not take more or less of it or take it more often or for a longer time than prescribed by your doctor.
Shake the liquid well before each use to mix the medication evenly.
Your symptoms should improve during your treatment with acyclovir. Call your doctor if your symptoms do not improve or if they get worse.
Take acyclovir until you finish the prescription, even if you feel better. If you stop taking acyclovir too soon or skip doses, your infection may not be completely treated or may become more difficult to treat.
Is acyclovir an antibiotic?
No. Acyclovir is an antiviral drug.
How long does it take for acyclovir to work?
Shingles (Herpes Zoster): There are no data on treatment initiated more than 72 hours after onset of the zoster rash. Patients should be advised to initiate treatment as soon as possible after a diagnosis of shingles.
Chickenpox: Chickenpox in otherwise healthy children is usually a self-limited disease of mild to moderate severity. Adolescents and adults tend to have more severe disease. Treatment was initiated within 24 hours of the typical chickenpox rash in the controlled studies, and there is no information regarding the effects of treatment begun later in the disease course.
Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV).
The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by herpes simplex virus and varicella-zoster virus. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In vitro, acyclovir triphosphate stops replication of herpes viral DNA. This is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase. The greater antiviral activity of acyclovir against herpes simplex virus compared with varicella-zoster virus is due to its more efficient phosphorylation by the viral thymidine kinase.
Acyclovir precautions
- Safety and efficacy of oral acyclovir formulations have not been established in patients younger than 2 years.
- Safety and efficacy of buccal acyclovir tablets have not been established in pediatric patients.
- Drink plenty of fluids while you are taking acyclovir.
Before taking acyclovir:
- tell your doctor and pharmacist if you are allergic to acyclovir, valacyclovir (Valtrex), any other medications, or any of the ingredients in acyclovir. Ask your pharmacist for a list of the ingredients.
- tell your doctor and pharmacist what prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking or plan to take. Be sure to mention any of the following: amphotericin B (Fungizone); aminoglycoside antibiotics such as amikacin (Amikin), gentamicin (Garamycin), kanamycin (Kantrex), neomycin (Nes-RX, Neo-Fradin), paramomycin (Humatin), streptomycin, and tobramycin (Tobi, Nebcin); aspirin and other nonsteroidal anti-inflammatory drugs such as ibuprofen (Advil, Motrin), and naproxen (Aleve, Naprosyn); cyclosporine (Neoral, Sandimmune); medications to treat HIV or AIDS such as zidovudine (Retrovir, AZT); pentamidine (NebuPent); probenecid (Benemid); sulfonamides such as sulfamethoxazole and trimethoprim (Bactrim); tacrolimus (Prograf); and vancomycin. Many other medications may also interact with acyclovir, so be sure to tell your doctor about all the medications you are taking, even those that do not appear on this list. Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if there is a possibility you may be dehydrated from a recent illness or activity, or if you have or have ever had problems with your immune system; human immunodeficiency virus infection (HIV); acquired immunodeficiency syndrome (AIDS); or kidney disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking acyclovir, call your doctor.
if you are taking acyclovir to treat genital herpes, you should know that genital herpes can be spread through sexual contact even if you don’t have blisters or other symptoms and possibly even if you are taking acyclovir. Talk to your doctor about ways to stop the spread of genital herpes and about whether your partner(s) should receive treatment.
Geriatric
Agitation, confusion, dizziness, and drowsiness may be especially likely to occur in elderly patients who are usually more sensitive than younger adults to the central nervous system effects of acyclovir.
Pregnancy
Pregnancy Category B: Animal studies have revealed no evidence of harm to the fetus, however, there are no adequate studies in pregnant women OR animal studies have shown an adverse effect, but adequate studies in pregnant women have failed to demonstrate a risk to the fetus.
Breastfeeding
Studies in women suggest that this medication poses minimal risk to the infant when used during breastfeeding.
Drug Interactions
Although certain medicines should not be used together at all, in other cases two different medicines may be used together even if an interaction might occur. In these cases, your doctor may want to change the dose, or other precautions may be necessary. When you are taking this medicine, it is especially important that your healthcare professional know if you are taking any of the medicines listed below. The following interactions have been selected on the basis of their potential significance and are not necessarily all-inclusive.
Using this medicine with any of the following medicines is usually not recommended, but may be required in some cases. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Foscarnet
- Tolvaptan
Using this medicine with any of the following medicines may cause an increased risk of certain side effects, but using both drugs may be the best treatment for you. If both medicines are prescribed together, your doctor may change the dose or how often you use one or both of the medicines.
- Fosphenytoin
- Phenytoin
- Valproic Acid
Other Interactions
Certain medicines should not be used at or around the time of eating food or eating certain types of food since interactions may occur. Using alcohol or tobacco with certain medicines may also cause interactions to occur. Discuss with your healthcare professional the use of your medicine with food, alcohol, or tobacco.
Other Medical Problems
The presence of other medical problems may affect the use of this medicine. Make sure you tell your doctor if you have any other medical problems, especially:
- Dehydration or
- Kidney disease—Dehydration or kidney disease may increase blood levels of acyclovir, increasing the chance of side effects.
- Nervous system problems—Acyclovir may make these problems worse.
Acyclovir uses
Acyclovir is used to decrease pain and speed the healing of sores or blisters in people who have chickenpox (varicella), shingles (herpes zoster, a rash that can occur in people who have had chickenpox in the past), and first-time or repeat outbreaks of genital herpes (a herpes virus infection that causes sores to form around the genitals and rectum from time to time). Acyclovir is also sometimes used to prevent outbreaks of genital herpes in people who are infected with the virus. Acyclovir is in a class of antiviral medications called synthetic nucleoside analogues. Acyclovir works by stopping the spread of the herpes virus in the body.
Acyclovir will not cure herpes, but it can lessen the symptoms of the infection. Acyclovir will not cure genital herpes and may not stop the spread of genital herpes to other people.
Acyclovir is also sometimes used to treat eczema herpeticum (a skin infection caused by the herpes virus) to treat and prevent herpes infections of the skin, eyes, nose, and mouth in patients with human immunodeficiency virus (HIV), and to treat oral hairy leukoplakia (condition that causes hairy white or gray-colored patches on the tongue or inside of the cheek).
Acyclovir can also be used off-label to prevent and treat varicella zoster virus diseases (including chicken pox and shingles) in people with HIV. Off-label use refers to use of an FDA-approved medicine in a manner different from that described on the medicine label. Good medical practice and the best interests of a patient sometimes require that a medicine be used off-label.
Acyclovir may be prescribed for other uses; ask your doctor or pharmacist for more information.
Acyclovir dosage
The amount of acyclovir that you take depends on the strength of acyclovir. Also, the number of doses you take each day, the time allowed between doses, and the length of time you take the medicine depend on the medical problem for which you are using the medicine.
For oral acyclovir dosage forms (capsules, oral suspension, or tablets):
- For treatment of genital herpes:
- Adults and children 12 years of age and older—200 milligrams (mg) five times a day for ten days.
- Children up to 12 years of age—Use and dose must be determined by the doctor.
- For prevention of recurrent outbreaks of genital herpes infections:
- Adults and children 12 years of age and older—200 to 400 mg two to five times a day for five days or up to twelve months, depending on how often your outbreaks of infection occur.
- Children up to 12 years of age—Use and dose must be determined by the doctor.
- For treatment of chickenpox:
- Adults and children who weigh over 88 pounds (40 kilograms)—800 mg four times a day for five days.
- Children 2 years of age and older and weighing 88 pounds (40 kilograms) or less—Dose is based on body weight and must be determined by the doctor. The usual dose is 20 mg per kilogram (kg) of body weight, up to 800 mg, four times a day for five days.
- Children up to 2 years of age—Use and dose must be determined by the doctor.
- For treatment of shingles:
- Adults and children 12 years of age and older—800 mg five times a day for seven to ten days.
- Children up to 12 years of age—Use and dose must be determined by the doctor.
For injection acyclovir dosage form:
- For treatment of herpes of the brain, genitals, or mucous membranes, or for the treatment of shingles:
- Adults and children 12 years of age and older—Dose is based on body weight and must be determined by the doctor. The usual dose is 5 to 10 mg of acyclovir per kg (2.3 to 4.5 mg per pound) of body weight, injected slowly into a vein over at least a one-hour period, and repeated every eight hours for five to ten days.
- Children up to 12 years of age—Dose is based on body weight and must be determined by the doctor. The usual dose is 10 mg to 20 mg of acyclovir per kg (4.5 mg to 9.1 mg per pound) of body weight, injected slowly into a vein over at least a one-hour period and repeated every eight hours for seven to ten days.
- For treatment of widespread herpes virus infection in newborns:
- Infants from birth to 3 months of age—Dose is based on body weight and must be determined by the doctor. The usual dose is 10 mg of acyclovir per kg (4.5 mg per pound) of body weight, injected slowly into a vein over at least a one-hour period and repeated every eight hours for ten days.
Adult Dose for Cold Sores
Use: For the treatment of herpes simplex labialis (cold sores).
Immunocompetent host:
- Apply 50 mg (1 buccal tablet) as a single-dose to the upper gum region (canine fossa)
Comments:
- Tablet should be applied within 1 hour after the onset of prodromal symptoms and before the appearance of any signs of herpes labialis lesions.
- Tablet should be applied on the same side of the mouth as the herpes labialis symptoms.
- Use of buccal tablets has not been studied in immunocompromised subjects.
Concomitant HIV infection:
- Oral tablets: 400 mg orally 3 times a day for 5 to 10 days
Comment: Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Adult Dose for Herpes Simplex – Mucocutaneous/Immunocompetent Host
Use: For the initial treatment and recurrent episodes of mucosal and cutaneous herpes simplex (HSV-1 and HSV-2).
Treatment of First Episode of Genital Herpes:
- 200 mg orally every 4 hours 5 times a day for 10 days (manufacturer dosing)
- 400 mg orally 3 times a day for 5 to 10 days (CDC recommendation)
Severe Disease or Complications Requiring Hospitalization:
- 5 mg/kg IV every 8 hours for 5 days (manufacturer dosing)
- 5 to 10 mg/kg IV every 8 hours for 2 to 7 days or until clinical improvement is observed, followed by oral antiviral therapy to complete at least 10 days of total therapy (Centers for Disease Control and Prevention (CDC) recommendation)
Episodic (Intermittent) Therapy: Effective treatment requires therapy initiation within 1 day of lesion onset or during the prodrome preceding an episode/recurrence
- 200 mg orally every 4 hours 5 times a day for 5 days (manufacturer dosing)
- 400 mg orally 3 times a day for 5 days OR 800 mg orally 2 times a day for 5 days OR 800 mg orally 3 times a day for 2 days (CDC recommendations)
Comments:
- All patients with newly acquired genital herpes should receive antiviral therapy as first episodes can cause a prolonged clinical illness, even among persons with mild clinical manifestations initially; therapy should be initiated at the earliest sign or symptom of primary infection.
- IV therapy is indicated for patients with severe infection.
- Centers for Disease Control and Prevention (CDC) sexually transmitted disease treatment Guidelines may be consulted for additional guidance.
Adult Dose for Herpes Simplex – HIV infected Host
Use: For the treatment of initial and recurrent mucosal and cutaneous herpes simplex (HSV-1 and HSV-2) in immunocompromised patients.
Concomitant HIV infection:
- Treatment of First Episode of Genital Herpes: 400 mg orally 3 times a day for 5 to 10 days (guideline recommendation)
- Duration of therapy: 5 to 10 days
Severe Disease:
- 5 mg/kg IV every 8 hours after lesions begin to regress, may change to oral therapy; continue treatment until lesions have completely healed (guideline recommendation)
Episodic (Intermittent) Therapy: Effective treatment requires therapy initiation within 1 day of lesion onset or during the prodrome preceding an episode/recurrence
- 400 mg orally 3 times a day for 5 to 14 days
Comments:
- Immunocompromised patients can have prolonged or severe episodes of genital, perianal, or oral herpes.
- Clinical manifestations of genital herpes may worsen during immune reconstitution early after initiation of antiretroviral therapy.
- Suppressive or episodic therapy with oral antiviral agents is effective in decreasing the clinical manifestations of HSV in persons with HIV infection.
- Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Adult Dose for Herpes Simplex Encephalitis
Use: For the treatment of HSV encephalitis.
- 10 mg/kg IV every 8 hours
- Duration of therapy: 10 days (manufacturer); 21 days (CDC)
Comments:
- The Center for Disease Control and Prevention (CDC) recommends 21 days of IV therapy to treat HSV encephalitis.
Adult Dose for Shingles
Use: For the acute treatment of herpes zoster (shingles).
- 800 mg orally every 4 hours 5 times a day for 7 to 10 days
Immunocompromised host:
- 10 mg/kg IV every 8 hours for 7 days
Concomitant HIV infection:
- Localized Dermatomal: 800 mg orally 5 times a day for 7 to 10 days (alternative therapy; oral valacyclovir or famciclovir are preferred therapy)
- Extensive Cutaneous Lesion or Visceral Involvement: 10 to 15 mg/kg IV every 8 hours until clinical improvement (i.e. no new vesicle formation or improvement of signs and symptoms of visceral disease), then switch to oral therapy
- Duration of therapy: 7 to 14-day course (oral plus IV)
Comments:
- Treatment should be initiated as soon as possible after a diagnosis of herpes zoster; parenteral dosing is based on ideal body weight (IBW).
- Oral acyclovir therapy should be considered an alternative therapy to treat acute localized dermatomal herpes zoster in HIV-infected adults according to the Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents; IV acyclovir is preferred therapy with extensive cutaneous lesion or visceral involvement.
Adult Dose for Chickenpox
Use: For the treatment of chickenpox (varicella).
- Immunocompetent Host: 800 mg orally 4 times a day for 5 days
- Immunocompromised Host: 10 mg/kg IV every 8 hours for 7 days
HIV-Infected Adults:
- Uncomplicated course: 800 mg orally 5 times a day for 5 to 7 days (alternative therapy; oral valacyclovir or famciclovir are preferred therapy)
- Severe or complicated course: 10 to 15 mg/kg IV every 8 hours for 7 to 10 days; may switch to oral therapy after defervescence if no evidence of visceral involvement
Comments:
- Therapy should be initiated at the earliest sign or symptom of chickenpox; there is no information of efficacy when initiated more than 24 hours after onset of symptoms.
- According to the Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents, oral acyclovir therapy should be considered alternative therapy for the treatment of uncomplicated cases of chickenpox; IV acyclovir is the preferred therapy for severe or complicated cases.
Adult Dose for Herpes Simplex – Suppression
Use: For secondary prophylaxis and treatment of recurrent herpes simplex virus (HSV) disease.
Daily Suppressive Therapy for Recurrent Disease: 400 mg orally 2 times a day
- Alternative regimens from 200 mg orally 3 times a day to 200 mg orally 5 times a day have been used
Concomitant HIV infection: 400 to 800 mg orally 2 to 3 times a day
Comments:
- Suppressive therapy has been shown to reduce the frequency of recurrences by 70% to 80% in patients who have frequent recurrences.
- The frequency of recurrences has been shown to decrease over time and therefore continued therapy should be reevaluated at least annually.
- Experience has shown immunocompromised persons (i.e. hematopoietic stem-cell recipients) who received daily suppressive antiviral therapy were less likely to develop drug-resistant
- Herpes simplex virus (HSV) compared with those receiving episodic therapy; however, resistance is possible and should be suspected and investigated if lesions persist or recur.
- CDC STD Treatment Guidelines and the Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Adult Dose for Herpes Zoster – Prophylaxis
Use: For HIV-infected person who has had close contact with a person who has active varicella or herpes zoster and is susceptible to the virus (e.g. no history of vaccination or either condition, or is known to be seronegative).
HIV-Infected Adults (guideline dosing):
- Post-Exposure Prophylaxis: 800 mg orally 5 times a day for 5 to 7 days; begin 7 to 10 days after exposure
Comments:
- Varicella-zoster immune globulin is the preferred therapy for postexposure prophylaxis; oral antiviral therapy may be used when passive immunization is not possible; if antiviral therapy is used, varicella vaccines should not be given for at least 72 hours following last dose.
- Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Adult Dose for Varicella-Zoster – Prophylaxis
Use: For HIV-infected person who has had close contact with a person who has active varicella or herpes zoster and is susceptible to the virus (e.g. no history of vaccination or either condition, or is known to be seronegative).
HIV-Infected Adults (guideline dosing):
- Post-Exposure Prophylaxis: 800 mg orally 5 times a day for 5 to 7 days; begin 7 to 10 days after exposure
Comments:
- Varicella-zoster immune globulin is the preferred therapy for postexposure prophylaxis; oral antiviral therapy may be used when passive immunization is not possible; if antiviral therapy is used, varicella vaccines should not be given for at least 72 hours following last dose.
- Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Pediatric Dose for Herpes Simplex – Congenital
Use: For known or suspected neonatal herpes simplex virus (HSV).
Neonatal Herpes:
- Birth to 3 months: 10 mg/kg IV every 8 hours for 10 days (manufacturer dosing)
- Birth to 3 months: 20 mg/kg IV every 8 hours (CDC recommendation)
- Duration of therapy: Disease limited to the skin and mucous membranes: 14 days; Disseminated disease or disease involving the CNS: 21 days
Follow with oral suppressive therapy: 300 mg/m2 orally 3 times a day for 6 months
Comments:
- Neonates born to women who acquire HSV near term should be treated due to high risk of infection; infants exposed to HSV during birth should be followed by a pediatric infectious-disease specialist.
- For neonatal HSV with CNS involvement, confirm virus is absent from cerebrospinal fluid prior to stopping therapy; CSF HSV DNA PCR should be performed on days 19 and 21 and repeated as needed.
- Following IV treatment, oral prophylaxis for 6 months should be considered in those with CNS or skin, eyes, and mouth disease as it may be associated with superior neurodevelopmental outcome and prevent cutaneous recurrences.
- CDC STD Treatment Guidelines and the Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Exposed and HIV-Infected Children may be consulted for additional guidance.
Pediatric Dose for Herpes Simplex – Mucocutaneous/Immunocompetent Host
Use: For the treatment of first episode or recurrence of mucosal and cutaneous herpes simplex (HSV-1 and HSV-2).
Treatment of First Episode of Genital Herpes:
Less than 12 years: 40 to 80 mg/kg/day orally in divided doses 3 to 4 times a day for 5 to 10 days
- Maximum dose: 1000 mg/day
12 years or older: 200 mg orally every 4 hours 5 times a day OR 400 mg orally 3 times a day
Duration of therapy: 7 to 10 days
Severe Disease or Complications Requiring Hospitalization:
- Less than 12 years: 10 mg/kg IV every 8 hours for 7 days
- 12 years or older: 5 mg/kg IV every 8 hours for 7 days
Recurrence of Genital HSV Infection:
- Less than 12 years: 20 to 25 mg/kg orally twice a day; Maximum dose: 400 mg
- 12 years or older: 200 mg orally 5 times a day for 5 days OR 800 mg orally 2 times a day for 5 days OR 800 mg orally 3 times a day for 2 days
Comments:
- All patients with newly acquired genital herpes should receive antiviral therapy as first episodes can cause a prolonged clinical illness, even among persons with mild clinical manifestations initially; therapy should be initiated at the earliest sign or symptom of primary infection; IV therapy is indicated for patients with severe infection.
Pediatric Dose for Herpes Simplex Encephalitis
Use: For the treatment of Herpes Simplex Encephalitis
- 3 months to 12 years old: 10 to 20 mg/kg IV every 8 hours
- 12 years or older: 10 mg/kg IV every 8 hours
Duration of therapy: 10 days (manufacturer); 21 days (CDC)
Comments:
- The Center for Disease Control and Prevention (CDC) recommends 21 days of IV therapy to treat HSV encephalitis.
- Acyclovir is the drug of choice for local and disseminated herpes simplex infection in infants and children.
- CDC STD Treatment Guidelines and the Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Exposed and HIV-Infected Children may be consulted for additional guidance.
Pediatric Dose for Herpes Simplex – Mucocutaneous/Immunocompromised Host
Use: For the treatment of initial and recurrent mucosal and cutaneous herpes simplex (HSV-1 and HSV-2) in immunocompromised patients
- Less than 12 years: 10 mg/kg IV every 8 hours for 7 days (manufacturer dosing)
- 12 years or older: 5 mg/kg IV every 8 hours for 7 days (manufacturer dosing)
Concomitant HIV infection (guideline dosing):
- Mild Symptomatic Gingivostomatitis: 20 mg/kg orally 4 times a day for 7 to 10 days
- Maximum dose: 400 mg
- Moderate to Severe Gingivostomatitis: 5 to 10 mg/kg IV 3 times a day
- May switch to oral therapy after lesions have begun to regress; treat until lesions have completely healed
Comments:
- Acyclovir is the drug of choice for local and disseminated herpes simplex in HIV-infected and exposed infants and children; children with severe immunosuppression and moderate to severe lesions should be treated initially with IV therapy and may require longer therapy.
- Immunocompromised patients may have prolonged or severe episodes; clinical manifestations of genital herpes may worsen during immune reconstitution early after initiation of antiretroviral therapy.
- CDC STD Treatment Guidelines and the Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Exposed and HIV-Infected Children may be consulted for additional guidance.
Pediatric Dose for Herpes Zoster
Use: For the acute treatment of herpes zoster (shingles).
Immunocompetent Host:
- Parenteral:
- Less than 1 year: 10 mg/kg IV every 8 hours for 7 to 10 days
- 1 year or older: 500 mg/m2 IV every 8 hours for 7 to 10 days
- Oral:
- 12 years or older: 800 mg orally 5 times a day for 5 to 7 days
Immunocompromised Host: 10 mg/kg IV every 8 hours for 7 to 10 days
HIV-exposed and HIV-Infected Children:
- Uncomplicated Zoster: 20 mg/kg orally 4 times a day for 7 to 10 days; Maximum dose: 800 mg
- Severe immunosuppression (CDC immunologic category 3), trigeminal or sacral nerve involvement, extensive multidermatomal, or disseminated zoster:
10 mg/kg IV every 8 hours until cutaneous lesions and visceral disease are clearly resolving; then may switch to oral therapy to complete a 10 to 14-day course
HIV-Infected Adolescents:
- Localized Dermatomal: 800 mg orally 5 times a day for 7 to 10 days (alternative therapy; oral valacyclovir or famciclovir are preferred therapy)
- Extensive Cutaneous Lesion or Visceral Involvement: 10 to 15 mg/kg IV every 8 hours until clinical improvement (i.e. no new vesicle formation or improvement of signs and symptoms of visceral disease), then switch to oral therapy
Duration of therapy: 7 to 14-day course (oral plus IV)
Comments:
- Acyclovir is the oral drug of choice for treating herpes zoster in HIV-infected children; it should be given for 7 to 10 days, although longer durations should be considered if lesions are slow to resolve.
- Initial IV therapy is recommended in children with more severe immunosuppression.
- According to the Guidelines for the Prevention and Treatment of Opportunistic Infections, oral acyclovir therapy in adolescents should be considered alternative therapy for the treatment of uncomplicated cases of herpes zoster; IV acyclovir is preferred therapy for extensive cutaneous lesion or visceral involvement.
- Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Exposed and HIV-Infected Children and HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Pediatric Dose for Varicella-Zoster
Use: For the treatment of chickenpox (varicella).
Immunocompetent host:
- 2 years or older (40 kg or less): 20 mg/kg orally 4 times a day for 5 days
- 2 years or older (over 40 kg): 800 mg orally 4 times a day for 5 days
- Maximum doses: Single: 800 mg; Daily: 3200 mg/day
Immunocompromised host:
- Less than 1 year: 10 mg/kg IV 3 times a day for 7 to 10 days
- 1 year or older: 500 mg/m2 IV 3 times a day for 7 to 10 days
HIV-exposed and HIV-infected Children:
- Mild disease with no or moderate immune suppression (CDC immunologic category 1 and 2): 20 mg/kg orally 4 times a day for 7 to 10 days and until no new lesions for 48 hours
- Maximum dose: 800 mg
- Severe immune suppression (CDC immunologic category 3): 10 mg/kg or 500 mg/m2 IV every 8 hours for 7 to 10 days and until no new lesions for 48 hours
HIV-Infected Adolescents:
- Uncomplicated course: 800 mg orally 5 times a day for 5 to 7 days (alternative therapy; oral valacyclovir or famciclovir are preferred therapy)
- Severe or complicated course: 10 to 15 mg/kg IV every 8 hours for 7 to 10 days; may switch to oral therapy after defervescence if no evidence of visceral involvement
Comments:
- Therapy should be initiated at the earliest sign of chickenpox, no later than 24 hours after onset of rash.
- In children 1 year or older, body surface area may be used for dosing instead of body weight.
- Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Exposed and HIV-Infected Children and HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Pediatric Dose for Herpes Simplex – Suppression
Use: For the secondary prophylaxis of recurrent HSV disease.
Neonatal period (less than 1 year): 300 mg/m2 orally 3 times a day for 6 months
Secondary Prophylaxis in HIV-Exposed and HIV-infected Children:
- 20 mg/kg orally twice a day
- Maximum dose: 800 mg
Comments:
- Suppressive therapy following treatment of neonatal HSV disease involving the CNS or skin, eyes, and mouth may prevent cutaneous recurrences and possibly provide superior neurodevelopmental outcomes.
- Beyond the neonatal period, recurrent HSV episodes can be treated successfully and chronic prophylaxis is generally not warranted; however, it may be considered for children with severe and recurrent mucocutaneous (oral or genital) disease.
- Secondary prophylaxis should be re-evaluated periodically (at least annually) as the frequency and severity of infection changes over time.
- Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Exposed and HIV-Infected Children and HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Pediatric Dose for Herpes Simplex Labialis
Use: For the treatment of recurrent herpes simplex labialis (cold sores).
Concomitant HIV infection:
- 20 mg/kg orally 4 times a day for 5 days
- Maximum dose: 400 mg
Adolescents: 400 mg orally 3 times a day for 5 to 10 days
Comments:
- The safety and efficacy of buccal tablets in pediatric patients has not been evaluated.
- Use of buccal tablets in younger children may present a choking risk.
- Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Pediatric Dose for Herpes Zoster – Prophylaxis
Use: For HIV-infected person who has had close contact with a person who has active varicella or herpes zoster and is susceptible to the virus (e.g. no history of vaccination or either condition, or is known to be seronegative).
HIV-Infected Children or Adolescents (guideline dosing):
- Post-exposure Prophylaxis in HIV-Infected Children or Adolescents:
- 20 mg/kg orally 4 times a day (maximum dose 800 mg) for 7 days beginning 7 to 10 days after exposure
Comments:
- Varicella-zoster immune globulin is the preferred therapy for postexposure prophylaxis; oral antiviral therapy may be used when passive immunization is not possible; if antiviral therapy is used, varicella vaccines should not be given for at least 72 hours following last dose.
- Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Exposed and HIV-Infected Children or HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Pediatric Dose for Varicella-Zoster – Prophylaxis
Use: For HIV-infected person who has had close contact with a person who has active varicella or herpes zoster and is susceptible to the virus (e.g. no history of vaccination or either condition, or is known to be seronegative).
HIV-Infected Children or Adolescents (guideline dosing):
- Post-exposure Prophylaxis in HIV-Infected Children or Adolescents:
- 20 mg/kg orally 4 times a day (maximum dose 800 mg) for 7 days beginning 7 to 10 days after exposure
Comments:
- Varicella-zoster immune globulin is the preferred therapy for postexposure prophylaxis; oral antiviral therapy may be used when passive immunization is not possible; if antiviral therapy is used, varicella vaccines should not be given for at least 72 hours following last dose.
- Guidelines for the Prevention and Treatment of Opportunistic Infections Among HIV- Exposed and HIV-Infected Children or HIV- Infected Adults and Adolescents may be consulted for additional guidance.
Renal Dose Adjustments
Oral:
For CrCl 0 to 10 mL/min/1.73 m²:
- If normal dose is 200 mg orally every 4 hours 5 times a day: Reduce dose to 200 mg orally every 12 hours
- If normal dose is 400 mg orally every 12 hours: Reduce dose to 200 mg orally every 12 hours
- If normal dose is 800 mg orally every 4 hours 5 times a day: Reduce dose to 800 mg orally every 12 hours
For CrCl 10 to 25 mL/min/1.73 m²:
- If normal dose is 800 mg orally every 4 hours 5 times a day: Reduce dose to 800 mg orally every 8 hours
IV:
- For CrCl 0 to 10 mL/min/1.73 m²: Give 50% of dose every 24 hours
- For CrCl 10 to 25 mL/min/1.73 m²: Give 100% of dose every 24 hours
- For CrCl 25 to 50 mL/min/1.73 m²: Give 100% of dose every 12 hours
- For CrCl greater than 50 mL/min/1.73 m²: Give 100% of dose every 8 hours
Liver Dose Adjustments
- No adjustment recommended
Dose Adjustments
- Obese patients should be dosed at the recommended doses using Ideal Body Weight (IBW)
- Elderly patients are more likely to have reduced renal function and require dose reduction.
What should I do if I forget a dose?
Take the missed dose as soon as you remember it and take any remaining doses for that day at evenly spaced intervals. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for a missed one.
Acyclovir side effects
Acyclovir may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- upset stomach
- vomiting
- diarrhea
- dizziness
- tiredness
- agitation
- pain, especially in the joints
- hair loss
- changes in vision
Some side effects can be serious. If you experience any of the following symptoms, call your doctor immediately:
- hives
- rash or blisters
- itching
- difficulty breathing or swallowing
- swelling of the face, throat, tongue, lips, eyes, hands, feet, ankles, or lower legs
- hoarseness
- fast heartbeat
- weakness
- pale skin
- difficulty sleeping
- fever, sore throat, chills, cough, and other signs of infection
- unusual bruising or bleeding
- blood in the urine
- stomach pain or cramps
- bloody diarrhea
- decreased urination
- headache
- hallucinations (seeing things or hearing voices that do not exist)
- confusion
- aggressive behavior
- difficulty speaking
- numbness, burning, or tingling in the arms or legs
- temporary inability to move parts of your body
- shaking of a part of your body that you cannot control
- seizures
- loss of consciousness
Acyclovir may cause other side effects. Call your doctor if you have any unusual problems while you are taking acyclovir.
Acyclovir overdose
Overdoses involving ingestion of up to 100 capsules (20 g) have been reported. Adverse events that have been reported in association with overdosage include agitation, coma, seizures, and lethargy. Precipitation of acyclovir in renal tubules may occur when the solubility (2.5 mg/mL) is exceeded in the intratubular fluid. Overdosage has been reported following bolus injections or inappropriately high doses and in patients whose fluid and electrolyte balance were not properly monitored. This has resulted in elevated BUN and serum creatinine and subsequent renal failure. In the event of acute renal failure and anuria, the patient may benefit from hemodialysis until renal function is restored.
Symptoms of acyclovir overdose may include:
- agitation
- seizures
- extreme tiredness
- loss of consciousness
- swelling of the hands, feet, ankles, or lower legs
- decreased urination
In case of emergency/acyclovir overdose
In case of overdose, call the poison control helpline at 1-800-222-1222. Information is also available online at https://www.poisonhelp.org/help. If the victim has collapsed, had a seizure, has trouble breathing, or can’t be awakened, immediately call your local emergency services number.