Alzheimer’s disease
Alzheimer’s disease is a progressive neurodegenerative brain disease and is the most common cause of a progressive dementia in older people characterized by the accumulation of toxic, misfolded beta-amyloid proteins that form plaques in the brain. Alzheimer’s disease diagnosis requires that beta-amyloid plaques accumulate to a specific level that is likely to cause symptoms including dementia. Dementia describes a group of symptoms affecting memory, thinking and social abilities severely enough to interfere with your daily life. The National Institutes of Health (NIH) defines dementia as “a loss of thinking, remembering, and reasoning skills that interferes with a person’s daily life and activities.” Other forms include frontotemporal degeneration, dementia with Lewy bodies, and vascular and mixed dementias. Dementia isn’t a specific disease, but several different diseases may cause dementia. Though dementia generally involves memory loss, memory loss has different causes. Having memory loss alone doesn’t mean you have dementia.
Alzheimer’s disease is a progressive disorder that causes brain cells to waste away (degenerate) and die. It first involves the parts of the brain that control thought, memory and language. Memory problems are typically one of the first warning signs of cognitive loss, though initial symptoms may vary from person to person. A decline in other aspects of thinking, such as finding the right words, vision/spatial issues, and impaired reasoning or judgment, may also signal the very early stages of Alzheimer’s disease. Alzheimer’s disease cause a continuous decline in thinking, behavioral and social skills that disrupts a person’s ability to function independently. People with Alzheimer’s disease may have trouble remembering things that happened recently or names of people they know. As Alzheimer’s disease progresses, a person with Alzheimer’s disease will develop severe memory impairment and lose the ability to carry out everyday tasks like driving a car, cooking a meal, or paying bills. They may ask the same questions over and over, get lost easily, lose things or put them in odd places, and find even simple things confusing. As the disease progresses, some people become worried, angry, or violent. A related problem, mild cognitive impairment (MCI) causes more memory problems than normal for people of the same age. Many, but not all, people with mild cognitive impairment (MCI) will develop Alzheimer’s disease.
In Alzheimer’s disease, over time, symptoms get worse. People may not recognize family members. They may have trouble speaking, reading or writing. They may forget how to brush their teeth or comb their hair. Later on, they may become anxious or aggressive, or wander away from home. Eventually, they need total care. This can cause great stress for family members who must care for them.
The most common type of Alzheimer’s disease usually begins after age 65 (late-onset Alzheimer’s disease). Alzheimer’s disease usually begins after age 60. However, Alzheimer’s disease is not a normal part of aging, though the risk goes up as you get older. Your risk is also higher if a family member has had the disease. Furthermore, younger-onset also known as early-onset Alzheimer’s disease affects people younger than age 65. Up to 5 percent (1 in every 20 people) of the more than 6 million Americans with Alzheimer’s disease have early- or young-onset Alzheimer’s disease.
There is currently no known cure for Alzheimer’s disease. No treatment can stop Alzheimer’s disease. However, some drugs may help keep symptoms from getting worse for a limited time.
Current Alzheimer’s disease medications may temporarily improve symptoms or slow the rate of decline. These treatments can sometimes help people with Alzheimer’s disease maximize function and maintain independence for a time. Different programs and services can help support people with Alzheimer’s disease and their caregivers.
There is no treatment that cures Alzheimer’s disease or alters the disease process in the brain. In advanced stages of the disease, complications from severe loss of brain function — such as dehydration, malnutrition or infection — result in death.
A number of conditions, including treatable conditions, can result in memory loss or other dementia symptoms. If you are concerned about your memory or other thinking skills, talk to your doctor for a thorough assessment and diagnosis.
If you are concerned about thinking skills you observe in a family member or friend, talk about your concerns and ask about going together to a doctor’s appointment.
Alzheimer’s disease cause
Scientists do not yet fully understand what causes Alzheimer’s disease. There probably is not one single cause, but several factors that affect each person differently. Scientists believe that for most people, Alzheimer’s disease is caused by a combination of genetic, lifestyle and environmental factors that affect the brain over time.
- Age is the best known risk factor for Alzheimer’s disease. As you age, biological processes can become dysfunctional and contribute to Alzheimer’s. The risk of Alzheimer’s disease and other types of dementia increases with age, affecting an estimated 1 in 14 people over the age of 65 and 1 in every 6 people over the age of 80.
- Inflammation is a part of your normal immune response and helps prevent infection. Inflammation can be both protective and harmful. But chronic inflammation can damage your neurons. When you are young, inflammation ramps up and then subsides quickly. But as you get older, it takes longer to reset, meaning you stay inflamed even when your body is healthy.
- Family history—researchers believe that genetics may play a role in developing Alzheimer’s disease. Familial Alzheimer’s disease also known as early-onset Alzheimer’s disease is a very rare form of the disease caused by a gene mutation inherited from a parent. It involves single gene mutations on chromosomes 1, 14, or 21. There are no genetic “causes” of the more common late-onset form of Alzheimer’s. Though genes such as APOE4 (apolipoprotein E4) can increase a person’s risk of developing Alzheimer’s disease, they don’t mean you will get it. Epigenetic factors, which influence how much or how little your genes are expressed, can also contribute by turning up “bad” genes or turning down “good” ones.
- Mitochondrial dysfunction. Your brain accounts for about 2% of your body weight but use 20% of your body’s energy. This energy comes from glucose, and brain’s ability to process and use it can become less efficient with age, which starves your neurons. Some of this can stem from dysfunction in your mitochondria, which are the engines that power your cells. Diabetes exacerbates this problem, which is why it is a major risk factor for Alzheimer’s disease.
- Oxidation. During our lives, we are exposed to various things in the environment that can damage our DNA and proteins. Free radicals, the sun, pollution—these all contribute to oxidation and cellular stress. Our ability to repair such cellular damage wanes as we get older.
- Changes in the brain can begin years before the first symptoms appear.
- Researchers are studying whether education, diet, and environment play a role in developing Alzheimer’s disease.
- Scientists are finding more evidence that some of the risk factors for heart disease and stroke, such as high blood pressure and high cholesterol may also increase the risk of Alzheimer’s disease. Damage to the body’s blood vessel network can limit the brain’s supply of oxygen and vital nutrients needed for cells to work properly. Neurons are particularly vulnerable due to their significant energy needs. High blood pressure and cardiovascular disease are major risk factors for Alzheimer’s because of their negative impact on your vasculature.
- There is growing evidence that physical, mental, and social activities may reduce the risk of Alzheimer’s disease.
Less than 1 percent of the time, Alzheimer’s disease is caused by specific genetic changes that virtually guarantee a person will develop the disease. These rare occurrences usually result in disease onset in middle age.
The exact causes of Alzheimer’s disease aren’t fully understood, but at its core are problems with brain proteins that fail to function normally, disrupt the work of brain cells (neurons) and unleash a series of toxic events. Neurons are damaged, lose connections to each other and eventually die.
The damage most often starts in the region of the brain that controls memory, but the process begins years before the first symptoms. The loss of neurons spreads in a somewhat predictable pattern to other regions of the brains. By the late stage of the disease, the brain has shrunk significantly.
Researchers are focused on the role of two proteins:
- Plaques. Beta-amyloid is a leftover fragment of a larger protein. When these fragments cluster together, they appear to have a toxic effect on neurons and to disrupt cell-to-cell communication. These clusters form larger deposits called amyloid plaques, which also include other cellular debris.
- Tangles. Tau proteins play a part in a neuron’s internal support and transport system to carry nutrients and other essential materials. In Alzheimer’s disease, tau proteins change shape and organize themselves into structures called neurofibrillary tangles. The tangles disrupt the transport system and are toxic to cells.
Proteins are critical to your survival, as they carry out most functions in your cells. Proteostasis is the “quality control” system for your proteins, which is responsible for their correct formation and for recycling them after they are no longer needed. Both sides of this system are more likely to malfunction as you get older, resulting in an excess of toxic, misfolded proteins such as beta-amyloid and tau proteins, respectively, accumulate into plaques and tangles. Other proteins that can be involved in Alzheimer’s include alpha-synuclein (a component of Lewy bodies), and TDP-43.
Although it’s not known exactly what causes this process to begin, scientists now know that it begins many years before symptoms appear. As brain cells become affected, there’s also a decrease in chemical messengers (called neurotransmitters) involved in sending messages, or signals, between brain cells. Levels of one neurotransmitter, acetylcholine (ACh), are particularly low in the brains of people with Alzheimer’s disease.
Over time, different areas of the brain shrink. The first areas usually affected are responsible for memories. In more unusual forms of Alzheimer’s disease, different areas of the brain are affected. The first symptoms may be problems with vision or language rather than memory.
Risk factors for developing Alzheimer’s disease
Although it’s still unknown what triggers Alzheimer’s disease, several factors are known to increase your risk of developing the disease.
Age
Increasing age is the greatest known risk factor for Alzheimer’s disease. Alzheimer’s is not a part of normal aging, but as you grow older the likelihood of developing Alzheimer’s disease increases. The likelihood of developing Alzheimer’s disease doubles every 5 years after you reach 65.
Around 1 in 20 people under 65 develop the early- or young-onset Alzheimer’s disease. This is early- or young-onset Alzheimer’s disease can affect people from around the age of 40.
One study, for example, found that annually there were two new diagnoses per 1,000 people ages 65 to 74, 11 new diagnoses per 1,000 people ages 75 to 84, and 37 new diagnoses per 1,000 people age 85 and older.
Young-onset Alzheimer’s disease
A very small percentage of people who develop Alzheimer’s disease have the young-onset type. Signs and symptoms of this type usually appear between ages 30 and 60 years. This type of Alzheimer’s disease is very strongly linked to your genes.
Scientists have identified three genes in which mutations cause early-onset Alzheimer’s disease. If you inherit one of these mutated genes from either parent, you will probably have Alzheimer’s symptoms before age 65. The genes involved are:
- Amyloid precursor protein (APP)
- Presenilin 1 (PSEN1)
- Presenilin 2 (PSEN2)
Mutations of these genes cause the production of excessive amounts of a toxic protein fragment called amyloid-beta peptide. This peptide can build up in the brain to form clumps called amyloid plaques, which are characteristic of Alzheimer’s disease. A buildup of toxic amyloid-beta peptide and amyloid plaques may lead to the death of nerve cells and the progressive signs and symptoms of this disorder.
As amyloid plaques collect in the brain, tau proteins malfunction and stick together to form neurofibrillary tangles. These tangles are associated with the abnormal brain functions seen in Alzheimer’s disease.
However, some people who have early-onset Alzheimer’s don’t have mutations in these three genes. That suggests that some early-onset forms of Alzheimer’s disease are linked to other genetic mutations or other factors that haven’t been identified yet.
One of the active research trials is the Dominantly Inherited Alzheimer Network (DIAN), which studies individuals with dominant Alzheimer’s mutations (PSEN1, PSEN2 or APP). This research network includes observational studies and clinical trials.
Family history and genetics
Your risk of developing Alzheimer’s is somewhat higher if a first-degree relative — your parent or sibling — has the disease. While a quarter of Alzheimer’s patients have a strong family history of the disease, only 1% directly inherit a gene mutation that causes early-onset Alzheimer’s disease, also known as familial Alzheimer’s disease 1. Most genetic mechanisms of Alzheimer’s disease among families remain largely unexplained, and the genetic factors are likely complex.
Genes control the function of every cell in your body. Some genes determine your basic characteristics, such as the color of your eyes and hair. Other genes can make you more likely to develop certain diseases, including Alzheimer’s disease. The most common gene associated with late-onset Alzheimer’s disease is a risk gene called apolipoprotein E (APOE). Apolipoprotein E (APOE) is a regulator of lipid metabolism that has an affinity for beta-amyloid protein and is another genetic marker that increases the risk of Alzheimer’s disease. There are three types of the APOE gene, called alleles: APOE e2, e3 and e4. Because you inherit one APOE gene from your mother and another from your father, you have two copies of the APOE gene. Everyone has two copies of the gene and the combination determines your APOE “genotype”—E2/E2, E2/E3, E2/E4, E3/E3, E3/E4, or E4/E4.
- APOE e2 — rarest form of APOE and carrying even one copy appears to reduce the risk of developing Alzheimer’s disease by up to 40%.
- APOE e3 — the most common allele and doesn’t seem to affect the risk of Alzheimer’s disease.
- APOE e4 — a little more common and is present in approximately 10-15% of the general population (located on chromosome 19) and it increases the risk of Alzheimer’s disease and is associated with getting the disease at an earlier age. Having one copy of E4 (E3/E4) can increase your risk by 2 to 3 times while two copies (E4/E4) can increase the risk by 12 times 2. APOE4 is just one of many risk factors for dementia and its influence can vary across age, gender, race, and nationality 3. For example, having one copy of the E4 allele may pose more risk to women while having two copies seems to affect men and women similarly 4.
Having at least one APOE e4 gene increases your risk of developing Alzheimer’s disease two to threefold. If you have two APOE e4 genes, your risk is even higher, approximately eight to twelvefold. But not everyone who has one or even two APOE e4 genes develops Alzheimer’s disease. And the disease occurs in many people who don’t even have an APOE e4 gene, suggesting that the APOE e4 gene affects risk but is not a cause. Other genetic and environmental factors likely are involved in the development of Alzheimer’s disease.
As research on the genetics of Alzheimer’s progresses, researchers are uncovering links between late-onset Alzheimer’s and a number of other genes. Several examples include:
- ABCA7. The exact role of ABCA7 isn’t clear, but the gene seems to be linked to a greater risk of Alzheimer’s disease. Researchers suspect that it may have something to do with the gene’s role in how the body uses cholesterol.
- CLU. This gene helps regulate the clearance of amyloid-beta from the brain. Research supports the theory that an imbalance in the production and clearance of amyloid-beta is central to the development of Alzheimer’s disease.
- CR1. A deficiency of the protein this gene produces may contribute to chronic inflammation in the brain. Inflammation is another possible factor in the development of Alzheimer’s disease.
- PICALM. This gene is linked to the process by which brain nerve cells (neurons) communicate with each other. Smooth communication between neurons is important for proper neuron function and memory formation.
- PLD3. Scientists don’t know much about the role of PLD3 in the brain. But it’s recently been linked to a significantly increased risk of Alzheimer’s disease.
- TREM2. This gene is involved in the regulation of the brain’s response to inflammation. Rare variants in this gene are associated with an increased risk of Alzheimer’s disease.
- SORL1. Some variations of SORL1 on chromosome 11 appear to be associated with Alzheimer’s disease.
Researchers are continuing to learn more about the basic mechanisms of Alzheimer’s disease, which may potentially lead to new ways to treat and prevent the disease.
As with APOE, these genes are risk factors, not direct causes. In other words, having a variation of one of these genes may increase your risk of Alzheimer’s. However, not everyone who has one will develop Alzheimer’s disease.
If several of your family members have developed dementia over the generations and particularly at a young age, you may want to seek genetic counseling for information and advice about your chances of developing Alzheimer’s disease when you’re older.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Down syndrome
Many people with Down syndrome develop Alzheimer’s disease. This is likely related to having three copies of chromosome 21 — and subsequently three copies of the gene for the protein that leads to the creation of beta-amyloid. Signs and symptoms of Alzheimer’s tend to appear 10 to 20 years earlier in people with Down syndrome than they do for the general population.
Sex
There appears to be little difference in risk between men and women, but, overall, there are more women with the disease because they generally live longer than men.
Mild cognitive impairment
Mild cognitive impairment is a decline in memory or other thinking skills that is greater than what would be expected for a person’s age, but the decline doesn’t prevent a person from functioning in social or work environments.
People who have mild cognitive impairment have a significant risk of developing dementia. When the primary mild cognitive impairment deficit is memory, the condition is more likely to progress to dementia due to Alzheimer’s disease. A diagnosis of mild cognitive impairment enables the person to focus on healthy lifestyle changes, develop strategies to compensate for memory loss and schedule regular doctor appointments to monitor symptoms.
Past head trauma
People who’ve had a severe head trauma have a greater risk of Alzheimer’s disease, but much research is still needed in this area.
Poor sleep patterns
Research has shown that poor sleep patterns, such as difficulty falling asleep or staying asleep, are associated with an increased risk of Alzheimer’s disease.
Lifestyle and heart health
Research has shown that the same risk factors associated with heart disease may also increase the risk of Alzheimer’s disease. These include:
- Lack of exercise
- Obesity
- Smoking or exposure to secondhand smoke
- High blood pressure
- High cholesterol
- Poorly controlled type 2 diabetes
These factors can all be modified. Therefore, changing lifestyle habits can to some degree alter your risk. For example, regular exercise and a healthy low-fat diet rich in fruits and vegetables are associated with a decreased risk of developing Alzheimer’s disease.
Studies have found an association between lifelong involvement in mentally and socially stimulating activities and a reduced risk of Alzheimer’s disease. Low education levels — less than a high school education — appear to be a risk factor for Alzheimer’s disease.
Aluminum
Aluminum is an element abundantly present in the earth. Aluminum occurs naturally in food and water and is widely used in products ranging from cans and cookware to medications and cosmetics. Some observational studies suggested a link between brain levels of aluminum and Alzheimer’s disease 5. Since the association was found, many studies have investigated whether aluminum increases the risk for Alzheimer’s disease. The findings are far from clear but to be safe, limiting excessive exposure may be a good idea.
Aluminum can come in many forms and its toxicity depends on the levels of trivalent aluminum (Al+3), which can react with water to produce aluminum superoxides, which in turn can deplete mitochondrial iron and promote generation of reactive oxygen species 6. Trivalent aluminum (Al+3)-induced formation of reactive oxygen species can lead to oxidative damage and apoptosis.
Drinking water
Several meta-analyses examined the association between aluminum levels in drinking water and dementia risk, and the evidence is mixed and inconclusive 7, 8, 9. The only high-quality study involved almost 4,000 older adults in southwest France, the Paquid study 10. It found that levels of aluminum consumption in drinking water in excess of 0.1 mg/day were associated with a doubling of dementia risk and a 3-fold increase in Alzheimer’s risk 11. For reference, the 2020 City of New York Drinking Water Quality Report states that the concentration of aluminum in NYC drinking water ranged from 0.008-0.040 mg/liter 12. Of the remaining 13 moderate quality studies, 6 found an association between higher aluminum levels in drinking water and increased dementia risk 13, 14, 15, 16, 17, 4 found no associations 18, 19, 20 and 1 found a protective effect of higher soil levels of aluminum 21. Other elements present in drinking water, such as fluoride, copper, zinc, or iron, could also affect cognitive function and the results of these studies 22.
Antacids
Some antacids contain high levels of aluminum, as aluminum hydroxide reduces stomach acidity. Among medications, antacids and anti-ulceratives contain the highest levels of aluminum (35-208 mg/dose for antacids and 35-1450 mg/dose for anti-ulceratives) 23, though aluminum-free options exist (e.g., Rolaids, Tums). A very large meta-analysis of 9 observational studies including more than 6,000 people reported that regular antacid use was not associated with Alzheimer’s disease 24. Even when the analysis was confined to people who used antacids regularly for over 6 months, there was no association with Alzheimer’s. Studies with longer follow-up may be needed to definitively exclude the association between antacid use and dementia risk.
Antiperspirants
Aluminum salts in antiperspirants dissolve into the skin’s surface and form a temporary barrier within sweat ducts, which stops the flow of sweat to the skin’s surface. No studies have directly examined the link between aluminum-containing antiperspirant use and Alzheimer’s disease risk. However, a few studies have evaluated the link between antiperspirant use and breast cancer. Two studies found no increase in breast cancer risk 25, 26, but one other study reported that patients who used antiperspirant products more frequently and longer on shaved underarms were diagnosed with breast cancer at an earlier age 27. Studies show that aluminum salts in antiperspirants are poorly absorbed by the body, and the little that is absorbed is flushed out by the kidneys 28. However, if you regularly shave with a razor, aluminum may be more readily absorbed via small nicks and abrasions. To limit potential risks, avoid application of antiperspirants shortly after shaving and limit excessive use.
Occupational aluminum dust exposure
A meta-analysis of 3 observational studies including more than 1,000 people reported that occupational aluminum dust exposure was not associated with Alzheimer’s disease 29. However, further studies that precisely ascertain aluminum exposure are needed. In a more recent 2016 meta-analysis including 4 studies, the relationship between aluminum exposure and dementia was mixed due to the studies being too small 7.
There is no consistent or compelling evidence to associate aluminum with Alzheimer’s disease. Although a few studies have found associations between aluminum levels and Alzheimer’s risk, many others found no such associations. Due to the inconclusive nature of the findings, it may be advisable to limit excessive exposure.
Alzheimer’s disease pathophysiology
Alzheimer’s disease is characterized by an accumulation of abnormal neuritic plaques and neurofibrillary tangles 30.
Plaques are spherical microscopic lesions that have a core of extracellular amyloid beta-peptide surrounded by enlarged axonal endings. Beta-amyloid peptide is derived from a transmembrane protein known as an amyloid precursor protein (APP). The beta-amyloid peptide is cleaved from APP by the action of proteases named alpha, beta, and gamma-secretase. Usually, APP is cleaved by either alpha or beta-secretase and the tiny fragments formed by them are not toxic to neurons. However, sequential cleavage by beta and then gamma-secretase results in 42 amino acid peptides (beta-amyloid 42). Elevation in levels of beta-amyloid 42 leads to aggregation of amyloid that causes neuronal toxicity. Beta-amyloid 42 favors the formation of aggregated fibrillary amyloid protein over normal APP degradation. APP gene is located on chromosome 21, one of the regions linked to familial Alzheimer’s disease. Amyloid deposition occurs around meningeal and cerebral vessels and gray matter in Alzheimer’s disease. Gray matter deposits are multifocal and coalesce to form milliary structures called plaques. However, brain scans have noted amyloid plaques in some persons without dementia and then other persons had dementia but brain scans did not find any plaques.
Neurofibrillary tangles are fibrillary intracytoplasmic structures in neurons formed by a protein called tau. The primary function of the tau protein is to stabilize axonal microtubules. Microtubules run along neuronal axons and are essential for intracellular transport. Microtubule assembly is held together by tau protein. In Alzheimer’s disease, due to aggregation of extracellular beta-amyloid, there is hyperphosphorylation of tau which then causes the formation of tau aggregates. Tau aggregates form twisted paired helical filaments known as neurofibrillary tangles. They occur first in the hippocampus and then may be seen throughout the cerebral cortex. Tau-aggregates are deposited within the neurons. There is a staging system developed by Braak and Braak based on the topographical staging of neurofibrillary tangles into 6 stages, and this Braak staging is an integral part of the National Institute on Aging and Reagan Institute neuropathological criteria for the diagnosis of Alzheimer disease. Tangles are more strongly correlated to Alzheimer’s than the plaques are.
Another feature of Alzheimer’s disease is granulovacuolar degeneration of hippocampal pyramidal cells by amyloid angiopathy. Some reports indicate that cognitive decline correlates more with a decrease in the density of presynaptic boutons from pyramidal neurons in laminae III and IV, rather than an increase in the number of plaques.
Neuronal loss in Nucleus Basalis of Myenert, leading to low acetylcholine has also been noted.
Vascular contribution to the neurodegenerative process of Alzheimer’s disease is not fully determined. The risk of dementia is increased fourfold with subcortical infarcts. The cerebrovascular disease also exaggerates the degree of dementia and its rate of progression 31.
Alzheimer’s disease prevention
Alzheimer’s disease is not a preventable condition. However, a number of lifestyle risk factors for Alzheimer’s disease can be modified. Evidence suggests that changes in diet, exercise and habits — steps to reduce the risk of cardiovascular disease — may also lower your risk of developing Alzheimer’s disease and other disorders that cause dementia. Heart-healthy lifestyle choices that may reduce the risk of Alzheimer’s include the following:
- Exercise regularly. Exercise may protect against brain aging and improve mental function. Avoid long periods of physical inactivity and engage in moderate-intensity aerobic exercise for at least 30 minutes, 3 to 5 days per week.
- Eat a diet of fresh produce, healthy oils and foods low in saturated fat. Studies have shown that a brain-healthy diet consists of high levels of fruits, vegetables, fish, and legumes. With a recent observational study reported that people who consumed large amounts of processed meat had an elevated risk of dementia, including Alzheimer’s disease 32. There is also a growing evidence that specific diets— including the Mediterranean, Dietary Approaches to Stop Hypertension (DASH) and MIND (Mediterranean-DASH Intervention for Neurodegenerative Delay) diets—may promote brain health. These healthy, balanced options include whole foods such as fish, nuts, and vegetables rich in vitamins, nutrients, and omega-3 fatty acids. The mechanisms by which a healthy diet influences cognitive functions are not yet clear, but may be mediated by dietary components with antioxidant (e.g., fruits, vegetables) and anti-inflammatory (e.g., omega-3 fatty acids, polyphenols) properties 33. A healthy diet also decreases the risks for type-2 diabetes, high blood pressure, and high cholesterol, all of which have been associated with cognitive decline and dementia. Additional research is needed to determine which combination of foods and nutrients are best for optimal brain health. But based on this study and previous ones, a heart-healthy diet rich in fruits, vegetables, legumes, fish, and nuts, and low in full-fat dairy and red meat appear to be beneficial 34.
- Follow treatment guidelines to manage high blood pressure, diabetes and high cholesterol. Some chronic diseases can raise the risk of dementia and some medications can impair your brain’s function. Periodically review your medications and supplements with your physician and work with them to manage your brain health. Once you have the right treatment plan, make sure to take your medications as directed and periodically check with your pharmacist.
- Protect your head. Traumatic brain injury (TBI) is caused by a blow or jolt to the head – especially when the person is knocked out unconscious. Traumatic brain injuries can start a process in the brain where the substances that cause Alzheimer’s disease build up around the injured area. Wear a protective headgear in situations where there is a higher-than-normal risk of head injury – for example, riding a bike or scooter, working on a building site, horse-riding or playing football or cricket.
- If you smoke, ask your doctor for help to quit smoking. Smoking causes your arteries to become narrower, which can raise your blood pressure. It also increases your risk of cardiovascular disease, as well as several types of cancer.
- Keeping alcohol to a minimum. Drinking excessive amounts of alcohol increases your risk of stroke, heart disease and some cancers, as well as damaging your nervous system, including your brain.
- Maintaining a healthy weight. Being overweight or obese can increase your blood pressure and the risk of type 2 diabetes, both of which are linked to a higher risk of Alzheimer’s disease and vascular dementia. If you are overweight or obese, even losing 5% to 10% of the excess weight can help reduce your risk of dementia.
- Get Enough Sleep. Impaired sleep contributes to cognitive decline and may increase your risk of Alzheimer’s disease. To protect your brain, establish a bedtime routine, maintain a regular sleep schedule, and treat sleep-disordered breathing such as apnea. Don’t eat or exercise within 2–3 hours of bedtime and avoid over-use of sleeping pills and inducing sleep with alcohol, particularly if you have restless legs syndrome.
- Alleviate Stress. Prolonged stress can harm your brain, leading to fatigue, disturbed sleep, poor concentration, and memory lapses. Protect yourself by making changes to your lifestyle and learning ways to cope.
- Treat Depression. The relationship between dementia and depression is complex. It appears that having untreated depression increases your risk of developing dementia. However, depression can happen as part of the overall symptoms of dementia itself.
- Treat hearing loss. Hearing loss may increase your risk of getting dementia. However the reasons for this are still unclear. To avoid hearing loss increasing your risk of getting dementia, it’s important to get your hearing tested. Speak to your doctor about being referred to an audiologist (a health care professional for hearing). This will show up any hearing issues and provide ways of managing them, such as using a hearing aid. Often, managing hearing loss works best when you start doing it early on. This means protecting your hearing from a young age. For example, you can avoid listening to loud noises for long periods, and wear ear protection when necessary.
- Be Social. Loneliness and depression can impair cognitive health, causing memory loss and attention deficits. Maintain and build your social connections. And if you experience depression, get support.
- Keep Learning. Stimulate your brain throughout life by engaging intellectually. Education at any age may protect against cognitive decline. Studies have shown that preserved thinking skills later in life and a reduced risk of Alzheimer’s disease are associated with participating in social events, reading, dancing, playing board games, creating art, playing an instrument, and other activities that require mental and social engagement.
Mediterranean diet is low in saturated fat and high in fiber 35:
- Fruits, vegetables, grains, beans, nuts, and seeds are eaten daily and make up the majority of food consumed.
- Fat, much of it from olive oil, may account for up to 40% of daily calories.
- Small portions of cheese or yogurt are usually eaten each day, along with a serving of fish, poultry, or eggs.
- Red meat is consumed now and then.
- Small amounts of red wine are typically taken with meals.
- Main meals consumed daily should be a combination of three elements: cereals, vegetables and fruits, and a small quantity of legumes, beans or other (though not in every meal). Cereals in the form of bread, pasta, rice, couscous or bulgur (cracked wheat) should be consumed as one–two servings per meal, preferably using whole or partly refined grains. Vegetable consumption should amount to two or more servings per day, in raw form for at least one of the two main meals (lunch and dinner). Fruit should be considered as the primary form of dessert, with one–two servings per meal. Consuming a variety of colors of both vegetables and fruit is strongly recommended to help ensure intake of a broad range of micronutrients and phytochemicals. The less these foods are cooked, the higher the retention of vitamins and the lower use of fuel, thus minimizing environmental impact.
Dietary Approaches to Stop Hypertension (DASH) diet 36:
- Grains and grain products: 7–8 servings per day, more than half of which are whole-grain foods
- Fruits: 4–5 servings per day
- Vegetables: 4–5 servings per day
- Low-fat or non-fat dairy foods: 2–3 servings per day
- Lean meats, fish, poultry: 6 or less servings or fewer per day
- Nuts, seeds, and legumes: 4–5 servings per week
- Added fats and oils: 2–3 servings per day
- Sweets: 5 or less servings per week
- Salt (sodium): 1,500 milligrams (mg) sodium lowers blood pressure even further than 2,300 mg sodium daily.
MIND diet is short for Mediterranean-DASH Intervention for Neurodegenerative Delay diet 37. The MIND diet is similar to two other healthy meal plans: the DASH diet and the Mediterranean diet. Morris et al. 37 originally devised the MIND diet and found that the diet can slow cognitive decline over an average of 4.7 years in adults aged 58–98 years old. Interestingly, recent research found that the MIND diet and not the Mediterranean diet, protected against 12-year incidence of mild cognitive impairment and dementia in older adults 38. Also, a large observational study with older adults found that longer adherence to the MIND diet was associated with better verbal memory 39. The MIND diet promotes 10 healthy foods (Leafy greens, other veg, nuts, berries, fish, poultry, olive oil, beans, whole grains, red wine) and limits 5 other foods (red meat, butter, cheese, pastries and sweets, fried foods). While previous research shows that higher consumption of vegetables are associated with lower risk of cognitive decline 40, the strongest association was observed for higher intake of leafy greens 41. Previous research on cognitive function or dementia do not observe protective effects for overall fruit consumption 42. However, berries were shown to slow cognitive decline, particularly in global cognition and verbal memory in older adults 43.
- The MIND diet has 15 dietary components, including 10 “brain-healthy food groups” 44:
- Green leafy vegetables (like spinach and salad greens): At least six servings a week
- Other vegetables: At least one a day
- Nuts: Five servings a week
- Berries: Two or more servings a week
- Beans: At least three servings a week
- Whole grains: Three or more servings a day
- Fish: Once a week
- Poultry (like chicken or turkey): Two times a week
- Olive oil: Use it as your main cooking oil.
- Wine: One glass a day
- You AVOID:
- Red meat: Less than four servings a week
- Butter and margarine: Less than a tablespoon daily
- Cheese: Less than one serving a week
- Pastries and sweets: Less than five servings a week
- Fried or fast food: Less than one serving a week
- You AVOID:
The research found that high scores in all three diets were associated with a reduced risk of Alzheimer’s disease, but the MIND diet was the only diet in which even moderate adherence was beneficial. The MIND diet was also associated with a slower decline in global cognition, the equivalent of being 7.5 years younger in age cognitively. However, all of these studies are still observational, making it very difficult to confirm whether the benefits are caused by the diet or by other characteristics shared by the people who choose these foods. More research could help determine which of these diets has the most potential benefit for brain health. In the meantime, take note of the basic characteristics that they share: high levels of fruits, vegetables, fish, and legumes and low levels of processed foods, red meat, sweets, and sugars.
Dietary flavonols
Flavonols are a major class of the family of flavonoids, molecules that have interesting biological activity such as antioxidant, antimicrobial, hepatoprotective, anti-inflammatory, and vasodilatation effects, and they have been considered as potential anticancer agents 45. Flavonols are compounds found naturally in many fruits, vegetables, tea, and wine. A recent observational study reported that people with high intakes of flavonols had a significantly lower rate of developing Alzheimer’s disease compared to people with low intakes 46. Findings come from a study of 921 elderly participants (average age of 81.2 years old) who were free of dementia at the time of enrollment. Every year, participants received clinical neurologic examinations while also being interviewed on the frequency and amounts of different food items and beverages they consumed. Participants were followed for up to 12 years (average of 6.1 years), during which 220 participants developed Alzheimer’s disease. Researchers found that participants who were in the top 20% in total flavonol intake had a 48% lower rate of developing Alzheimer’s disease compared to those in the bottom 20%, after controlling for major lifestyle and other factors associated with dementia, such as age, education, genetic risk factor (ApoE), cognitive activity (e.g., reading, playing games, and writing letters), and physical activity (e.g., walking, biking, yard work) 46. Interestingly, the association between higher flavonol intake and reduced Alzheimer’s risk was stronger in men compared to women, such that men who were in the top 20% in total flavonol intake had a 76% lower rate of developing Alzheimer’s compared to men in the bottom 20%. The reasons for this sex difference are unknown 46.
Researchers further evaluated the different subclasses of flavonols, called kaempferol, myricetin, isorhamnetin, and quercetin.
- Kaempferol is abundant in kale, beans, tea, spinach, broccoli, capers, ginger, and dried goji berries 47. Participants who were in the top 20% in kaempferol intake had a 50% lower rate of developing Alzheimer’s disease compared to those in the bottom 20%.
- Myricetin is rich in tea, wine, kale, oranges, nuts, berries, and tomatoes 47. Participants who were in the top 20% in myricetin intake had a 38% lower rate of developing Alzheimer’s disease compared to those in the bottom 20%.
- Isorhamnetin is abundant in pears, olive oil, wine, and tomato sauce. Participants who were in the top 20% in isorhamnetin intake had a 38% lower rate of developing Alzheimer’s disease compared to those in the bottom 20%.
- Quercetin is rich in tomatoes, capers, red onions, kale, berries, apples, and tea 47. Dietary intake of quercetin was not associated with a lower rate of developing Alzheimer’s disease.
Because this study was an observational study and not a randomized clinical trial, it was not designed to prove that flavonols, or specific subclasses of flavonols like kaempferol, prevent Alzheimer’s disease. People who eat a diet rich in flavonols may be more likely to practice other healthy habits that are good for the brain. However, it is worth emphasizing that the associations between high flavonol intake and reduced Alzheimer’s disease risk remained significant even after controlling for some of these brain-healthy habits, such as cognitive and physical exercise.
Findings from this current study is consistent with previous ones reporting that greater flavonoid intake is associated with higher cognitive scores and slower cognitive decline 43, 48, 49, 50. Thus, a diet rich in fruits, dark-colored vegetables, and legumes appears to be beneficial for brain health and dementia prevention.
Early-onset Alzheimer’s disease
Many people with early onset Alzheimer’s disease are in their 40s and 50s. They have families, careers or are even caregivers themselves when Alzheimer’s disease strikes. In the United States, it is estimated that approximately 200,000 people have early onset.
Since health care providers generally don’t look for Alzheimer’s disease in younger people, getting an accurate diagnosis of early-onset Alzheimer’s can be a long and frustrating process. Symptoms may be incorrectly attributed to stress or there may be conflicting diagnoses from different health care professionals. People who have early-onset Alzheimer’s may be in any stage of dementia — early stage, middle stage or late stage. The disease affects each person differently and symptoms will vary.
If you are experiencing memory problems:
- Have a comprehensive medical evaluation with a doctor who specializes in Alzheimer’s disease. Getting a diagnosis involves a medical exam and possibly cognitive tests, a neurological exam and/or brain imaging.
- Write down symptoms of memory loss or other cognitive difficulties to share with your health care professional.
- Keep in mind that there is no one test that confirms Alzheimer’s disease. A diagnosis is only made after a comprehensive medical evaluation.
What causes early onset Alzheimer’s disease?
Doctors do not understand why most cases of early-onset Alzheimer’s disease appear at such a young age. But in a few hundred families worldwide, scientists have pinpointed several rare genes that directly cause Alzheimer’s disease. People who inherit these rare genes tend to develop symptoms in their 30s, 40s and 50s. When Alzheimer’s disease is caused by deterministic genes, it is called “familial Alzheimer’s disease,” and many family members in multiple generations are affected.
Scientists have identified three genes in which mutations cause early-onset Alzheimer’s disease. If you inherit one of these mutated genes from either parent, you will probably have Alzheimer’s symptoms before age 65. The genes involved are:
- Amyloid precursor protein (APP)
- Presenilin 1 (PSEN1)
- Presenilin 2 (PSEN2)
Mutations of these genes cause the production of excessive amounts of a toxic protein fragment called amyloid-beta peptide. This peptide can build up in the brain to form clumps called amyloid plaques, which are characteristic of Alzheimer’s disease. A buildup of toxic amyloid-beta peptide and amyloid plaques may lead to the death of nerve cells and the progressive signs and symptoms of this disorder.
As amyloid plaques collect in the brain, tau proteins malfunction and stick together to form neurofibrillary tangles. These tangles are associated with the abnormal brain functions seen in Alzheimer’s disease.
However, some people who have early-onset Alzheimer’s don’t have mutations in these three genes. That suggests that some early-onset forms of Alzheimer’s disease are linked to other genetic mutations or other factors that haven’t been identified yet.
One of the active research trials is the Dominantly Inherited Alzheimer Network (DIAN), which studies individuals with dominant Alzheimer’s mutations (PSEN1, PSEN2 or APP). This research network includes observational studies and clinical trials.
Stages of Alzheimer’s disease
The symptoms of Alzheimer’s disease worsen over time, although the rate at which the disease progresses varies. On average, a person with Alzheimer’s lives four to eight years after diagnosis, but can live as long as 20 years, depending on other factors.
Changes in the brain related to Alzheimer’s begin years before any signs of the disease. This time period, which can last for years, is referred to as preclinical Alzheimer’s disease.
The stages below provide an overall idea of how abilities change once symptoms appear and should only be used as a general guide. (Dementia is a general term to describe the symptoms of mental decline that accompany Alzheimer’s and other brain diseases.)
Generally, the symptoms of Alzheimer’s disease are divided into 3 main stages: mild Alzheimer’s disease, moderate Alzheimer’s disease and severe Alzheimer’s disease. Be aware that it may be difficult to place a person with Alzheimer’s in a specific stage as stages may overlap.
Alzheimer’s disease typically progresses slowly in three general stages:
- Mild (early stage),
- Moderate (middle stage),
- Severe (late stage).
Since Alzheimer’s disease affects people in different ways, the timing and severity of dementia symptoms varies as each person progresses through the stages of Alzheimer’s disease differently.
Mild Alzheimer’s disease (early stage)
In the early stage of Alzheimer’s disease, a person may function independently. He or she may still drive, work and be part of social activities. Despite this, the person may feel as if he or she is having memory lapses, such as forgetting familiar words or the location of everyday objects.
Friends, family or others close to the individual begin to notice difficulties. During a detailed medical interview, doctors may be able to detect problems in memory or concentration. Common difficulties include:
- Problems coming up with the right word or name
- Trouble remembering names when introduced to new people
- Challenges performing tasks in social or work settings.
- Forgetting material that one has just read
- Losing or misplacing a valuable object
- Increasing trouble with planning or organizing
Moderate Alzheimer’s disease (middle stage)
Moderate Alzheimer’s disease is typically the longest stage and can last for many years. As the disease progresses, the person with Alzheimer’s will require a greater level of care.
During the moderate stage of Alzheimer’s, the dementia symptoms are more pronounced. A person may have greater difficulty performing tasks, such as paying bills, but they may still remember significant details about their life.
You may notice the person with Alzheimer’s confusing words, getting frustrated or angry, or acting in unexpected ways, such as refusing to bathe. Damage to nerve cells in the brain can make it difficult to express thoughts and perform routine tasks.
At this point, symptoms will be noticeable to others and may include:
- Forgetfulness of events or about one’s own personal history
- Feeling moody or withdrawn, especially in socially or mentally challenging situations
- Being unable to recall their own address or telephone number or the high school or college from which they graduated
- Confusion about where they are or what day it is
- The need for help choosing proper clothing for the season or the occasion
- Trouble controlling bladder and bowels in some individuals
- Changes in sleep patterns, such as sleeping during the day and becoming restless at night
- An increased risk of wandering and becoming lost
- Personality and behavioral changes, including suspiciousness and delusions or compulsive, repetitive behavior like hand-wringing or tissue shredding
Severe Alzheimer’s disease (late stage)
In the final stage of Alzheimer’s disease, dementia symptoms are severe. Individuals lose the ability to respond to their environment, to carry on a conversation and, eventually, to control movement. They may still say words or phrases, but communicating pain becomes difficult. As memory and cognitive skills continue to worsen, significant personality changes may take place and individuals need extensive help with daily activities.
At this stage, individuals may:
- Need round-the-clock assistance with daily activities and personal care
- Lose awareness of recent experiences as well as of their surroundings
- Experience changes in physical abilities, including the ability to walk, sit and, eventually, swallow
- Have increasing difficulty communicating
- Become vulnerable to infections, especially pneumonia
Alzheimer’s disease symptoms
Alzheimer’s disease is a progressive condition, which means the symptoms develop gradually over many years and eventually become more severe. It affects multiple brain functions.
Brain changes associated with Alzheimer’s disease lead to growing trouble with:
- Memory loss
- Thinking and reasoning problem
- Making judgments and decisions problem
- Planning and performing familiar tasks problem
- Changes in personality and behavior
The first sign of Alzheimer’s disease is usually minor memory loss. An early sign of Alzheimer’s disease is usually difficulty remembering recent events or conversations and forgetting the names of places and objects (short-term memory loss). As the disease progresses, memory impairments worsen and other symptoms develop, such as:
- confusion, disorientation and getting lost in familiar places
- difficulty planning or making decisions
- problems with speech and language
- problems moving around without assistance or performing self-care tasks
- personality changes, such as becoming aggressive, demanding and suspicious of others
- hallucinations (seeing or hearing things that are not there) and delusions (believing things that are untrue)
- low mood or anxiety.
At first, a person with Alzheimer’s disease may be aware of having difficulty with remembering things and organizing thoughts. A family member or friend may be more likely to notice how the symptoms worsen.
The rate at which the symptoms progress is different for each individual. And sometimes these symptoms are confused with other conditions and may initially be put down to old age.
In some cases, other conditions can be responsible for symptoms getting worse. These conditions include:
- infections
- stroke
- delirium
As well as these conditions, other things, such as certain medicines, can also worsen the symptoms of dementia.
Anyone with Alzheimer’s disease whose symptoms are rapidly getting worse should be seen by a doctor so these can be managed.
Early symptoms
In the early stages, the main symptom of Alzheimer’s disease is memory lapses.
For example, someone with early Alzheimer’s disease may:
- forget about recent conversations or events
- misplace items
- forget the names of places and objects
- have trouble thinking of the right word
- ask questions repetitively
- show poor judgement or find it harder to make decisions
- become less flexible and more hesitant to try new things
There are often signs of mood changes, such as increasing anxiety or agitation, or periods of confusion.
Middle-stage symptoms
As Alzheimer’s disease develops, memory problems will get worse. Someone with Alzheimer’s disease may find it increasingly difficult to remember the names of people they know and may struggle to recognize their family and friends.
Other symptoms may also develop, such as:
- increasing confusion and disorientation – for example, getting lost, or wandering and not knowing what time of day it is
- obsessive, repetitive or impulsive behavior
- delusions (believing things that are untrue) or feeling paranoid and suspicious about carers or family members
- problems with speech or language (aphasia)
- disturbed sleep
- changes in mood, such as frequent mood swings, depression and feeling increasingly anxious, frustrated or agitated
- difficulty performing spatial tasks, such as judging distances
- seeing or hearing things that other people do not (hallucinations)
Some people also have some symptoms of vascular dementia.
By this stage, someone with Alzheimer’s disease usually needs support to help them with everyday living. For example, they may need help eating, washing, getting dressed and using the toilet.
Later symptoms
In the later stages of Alzheimer’s disease, the symptoms become increasingly severe and can be distressing for the person with the condition, as well as their carers, friends and family.
Hallucinations and delusions may come and go over the course of the disease, but can get worse as the condition progresses.
Sometimes people with Alzheimer’s disease can be violent, demanding and suspicious of those around them.
A number of other symptoms may also develop as Alzheimer’s disease progresses, such as:
- difficulty eating and swallowing (dysphagia)
- difficulty changing position or moving around without assistance
- weight loss – sometimes severe
- unintentional passing of urine (urinary incontinence) or stools (bowel incontinence)
- gradual loss of speech
- significant problems with short- and long-term memory
In the severe stages of Alzheimer’s disease, people may need full-time care and assistance with eating, moving and personal care.
Memory loss
Everyone has occasional memory lapses. It’s normal to lose track of where you put your keys or forget the name of an acquaintance. But the memory loss associated with Alzheimer’s disease persists and worsens, affecting the ability to function at work or at home.
People with Alzheimer’s disease may:
- Repeat statements and questions over and over
- Forget conversations, appointments or events, and not remember them later
- Routinely misplace possessions, often putting them in illogical locations
- Get lost in familiar places
- Eventually forget the names of family members and everyday objects
- Have trouble finding the right words to identify objects, express thoughts or take part in conversations
According to the National Institute on Aging, in addition to memory problems, someone with Alzheimer’s disease may experience one or more of the following signs:
- Memory loss that disrupts daily life, such as getting lost in a familiar place or repeating questions.
- Trouble handling money and paying bills.
- Difficulty completing familiar tasks at home, at work or at leisure.
- Decreased or poor judgment.
- Misplaces things and being unable to retrace steps to find them.
- Changes in mood, personality, or behavioral.
If you or someone you know has several or even most of the signs listed above, it does not mean that you or they have Alzheimer’s disease. It is important to consult a health care provider when you or someone you know has concerns about memory loss, thinking skills, or behavioral changes.
- Some causes for symptoms, such as depression and drug interactions, are reversible. However, they can be serious and should be identified and treated by a health care provider as soon as possible.
- Early and accurate diagnosis provides opportunities for you and your family to consider or review financial planning, develop advance directives, enroll in clinical trials, and anticipate care needs.
Thinking and reasoning problem
Alzheimer’s disease causes difficulty concentrating and thinking, especially about abstract concepts such as numbers.
Multitasking is especially difficult, and it may be challenging to manage finances, balance checkbooks and pay bills on time. These difficulties may progress to an inability to recognize and deal with numbers.
Making judgments and decisions problem
The ability to make reasonable decisions and judgments in everyday situations will decline. For example, a person may make poor or uncharacteristic choices in social interactions or wear clothes that are inappropriate for the weather. It may be more difficult to respond effectively to everyday problems, such as food burning on the stove or unexpected driving situations.
Planning and performing familiar tasks problem
Once-routine activities that require sequential steps, such as planning and cooking a meal or playing a favorite game, become a struggle as the disease progresses. Eventually, people with advanced Alzheimer’s may forget how to perform basic tasks such as dressing and bathing.
Changes in personality and behavior
Brain changes that occur in Alzheimer’s disease can affect moods and behaviors. Problems may include the following:
- Depression
- Apathy
- Social withdrawal
- Mood swings
- Distrust in others
- Irritability and aggressiveness
- Changes in sleeping habits
- Wandering
- Loss of inhibitions
- Delusions, such as believing something has been stolen
Preserved skills
Many important skills are preserved for longer periods even while symptoms worsen. Preserved skills may include reading or listening to books, telling stories and reminiscing, singing, listening to music, dancing, drawing, or doing crafts.
These skills may be preserved longer because they are controlled by parts of the brain affected later in the course of the disease.
Alzheimer’s disease complications
Memory and language loss, impaired judgment, and other cognitive changes caused by Alzheimer’s can complicate treatment for other health conditions. A person with Alzheimer’s disease may not be able to:
- Communicate that he or she is experiencing pain — for example, from a dental problem
- Report symptoms of another illness
- Follow a prescribed treatment plan
- Notice or describe medication side effects
As Alzheimer’s disease progresses to its last stages, brain changes begin to affect physical functions, such as swallowing, balance, and bowel and bladder control. These effects can increase vulnerability to additional health problems such as:
- Inhaling food or liquid into the lungs (aspiration)
- Pneumonia and other infections
- Falls
- Fractures
- Bedsores
- Malnutrition or dehydration
Alzheimer’s disease diagnosis
There is no single diagnostic test that can determine if a person has Alzheimer’s disease. Physicians (often with the help of specialists such as neurologists, neuropsychologists, geriatricians and geriatric psychiatrists) use a variety of approaches and tools to help make a diagnosis. Although physicians can almost always determine if a person has dementia, it may be difficult to identify the exact cause.
Memory problems are not just caused by dementia, they can also be caused by:
- depression or anxiety
- stress
- medicines
- alcohol or drugs
- sleeping problems (insomnia)
- other health problems – such as hormonal disturbances or nutritional deficiencies
Medical history
During the medical workup, your health care provider will review your medical history, including psychiatric history and history of cognitive and behavioral changes. He or she will want to know about any current and past illnesses, as well as any medications you are taking. The doctor will also ask about key medical conditions affecting other family members, including whether they may have had Alzheimer’s disease or other dementias.
Physical exam and diagnostic tests
During a medical workup, you can expect the physician to:
- Ask about diet, nutrition and use of alcohol.
- Review all medications. (Bring a list or the containers of all medicines currently being taken, including over-the-counter drugs and supplements.)
- Check blood pressure, temperature and pulse.
- Listen to the heart and lungs.
- Perform other procedures to assess overall health.
- Collect blood or urine samples for laboratory testing. Blood tests may help your doctor rule out other potential causes of memory loss and confusion, such as a thyroid disorder or vitamin deficiencies.
Neurological exam
During a neurological exam, the physician will closely evaluate the person for problems that may signal brain disorders other than Alzheimer’s. The doctor will look for signs of small or large strokes, Parkinson’s disease, brain tumors, fluid accumulation on the brain, and other illnesses that may impair memory or thinking.
Your doctor will perform a physical exam and likely assess overall neurological health by testing the following:
- Reflexes
- Muscle tone and strength
- Ability to get up from a chair and walk across the room
- Sense of sight and hearing
- Coordination
- Balance
Information from a physical exam and laboratory tests can help identify health issues that can cause symptoms of dementia. Common causes of dementia-like symptoms are depression, untreated sleep apnea, delirium, side effects of medications, thyroid problems, certain vitamin deficiencies and excessive alcohol consumption. Unlike Alzheimer’s disease and other dementias, these conditions often may be reversed with treatment.
Mental status and neuropsychological testing
Your doctor may conduct a brief mental status test or a more extensive set of tests to assess memory and other thinking skills. Longer forms of neuropsychological testing may provide additional details about mental function compared with people of a similar age and education level. These tests are also important for establishing a starting point to track the progression of symptoms in the future.
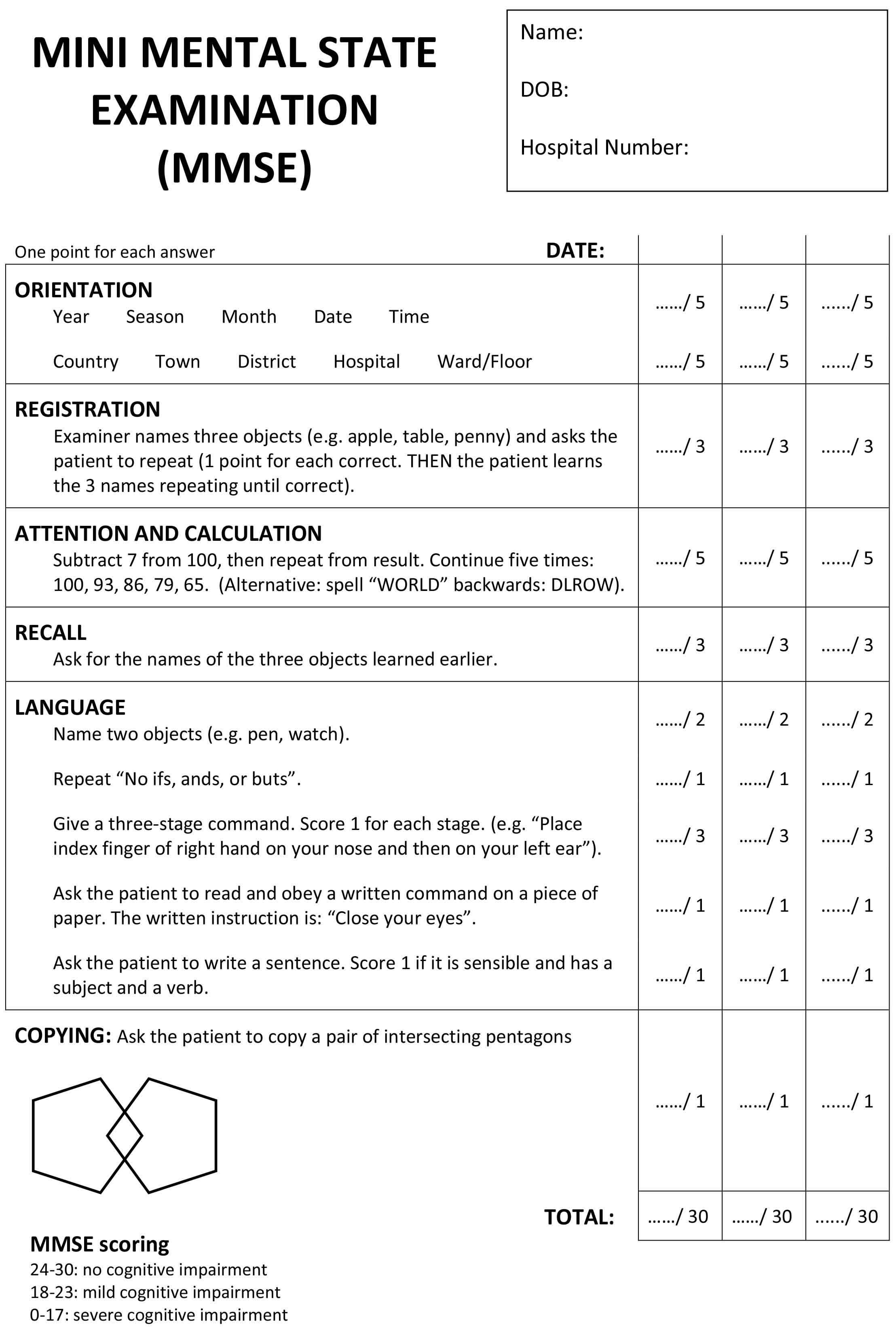
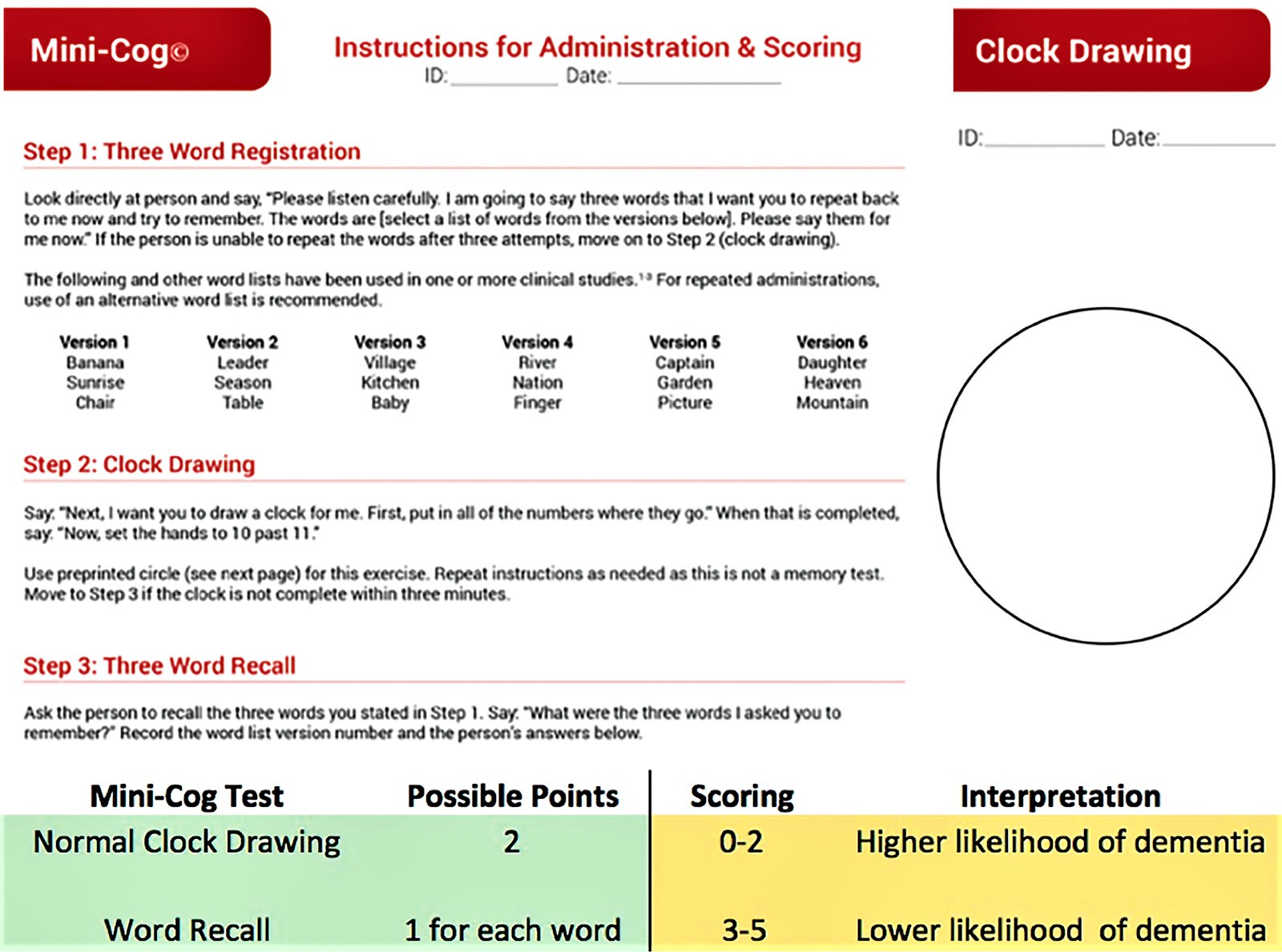
Mini-Mental State Exam (MMSE) and the Mini-Cog test
The Mini-Mental State Exam and Mini-Cog test are two commonly used assessments.
During the Mini-Mental State Exam, a health professional asks a patient a series of questions designed to test a range of everyday mental skills. The maximum MMSE score is 30 points. A score of 20 to 24 suggests mild dementia, 13 to 20 suggests moderate dementia, and less than 12 indicates severe dementia. On average, the MMSE score of a person with Alzheimer’s declines about two to four points each year.
During the Mini-Cog, a person is asked to complete two tasks:
- Remember and a few minutes later repeat the names of three common objects.
- Draw a face of a clock showing all 12 numbers in the right places and a time specified by the examiner.
The results of this brief test can help a physician determine if further evaluation is needed.
Figure 1. Mini-Mental State Exam (MMSE)
Figure 2. Mini-Cog test
Brain imaging
Images of the brain are now used chiefly to pinpoint visible abnormalities related to conditions other than Alzheimer’s disease — such as strokes, trauma or tumors — that may cause cognitive change. New imaging applications — currently used primarily in major medical centers or in clinical trials — may enable doctors to detect specific brain changes caused by Alzheimer’s disease.
Imaging of brain structures include the following:
- Magnetic resonance imaging (MRI). MRI uses radio waves and a strong magnetic field to produce detailed images of the brain. MRI scans are used primarily to rule out other conditions. While they may show brain shrinkage, the information doesn’t currently add significant value to making a diagnosis.
- Computerized tomography (CT). A CT scan, a specialized X-ray technology, produces cross-sectional images (slices) of your brain. It’s currently used chiefly to rule out tumors, strokes and head injuries.
Imaging of disease processes can be performed with positron emission tomography (PET). During a PET scan, a low-level radioactive tracer is injected into the blood to reveal a particular feature in the brain. PET imaging may include the following:
- Fluorodeoxyglucose PET scans show areas of the brain in which nutrients are poorly metabolized. Identifying patterns of degeneration — areas of low metabolism — can help distinguish between Alzheimer’s disease and other types of dementia.
- Amyloid PET imaging can measure the burden of amyloid deposits in the brain. This imaging is primarily used in research but may be used if a person has unusual or very early onset of dementia symptoms.
- Tau Pet imaging, which measures the burden of neurofibrillary tangles in the brain, is only used in research.
In special circumstances, such as rapidly progressive dementia or very early onset dementia, other tests may be used to measure abnormal beta-amyloid or tau in the cerebrospinal fluid.
Future diagnostic tests
Researchers are working on tests that can measure the biological evidence of disease processes in the brain. These tests may improve the accuracy of diagnoses and enable earlier diagnosis before the onset of symptoms.
Genetic testing generally isn’t recommended for a routine Alzheimer’s disease evaluation. The exception is people who have a family history of early-onset Alzheimer’s disease. Meeting with a genetic counselor to discuss the risks and benefits of genetic testing is recommended before undergoing any tests.
Genetic testing
Most experts don’t recommend genetic testing for late-onset Alzheimer’s disease. In some instances of early-onset Alzheimer’s disease, however, genetic testing may be appropriate.
Most clinicians discourage testing for the APOE genotype because the results are difficult to interpret. And doctors can generally diagnose Alzheimer’s disease without the use of genetic testing.
Testing for the mutant genes that have been linked to early-onset Alzheimer’s — APP, PSEN1 and PSEN2 — may provide more-certain results if you’re showing early symptoms or if you have a family history of early-onset disease. Genetic testing for early-onset Alzheimer’s disease may also have implications for current and future therapeutic drug trials as well as for family planning. Before being tested, it’s important to weigh the emotional consequences of having that information. The results may affect your eligibility for certain forms of insurance, such as disability, long-term care and life insurance.
Researchers are also studying genes that may protect against Alzheimer’s disease. One variant of the APOE gene, called APOE Christchurch, appears to be protective, with an effect similar to that of APOE e2. More research is needed to understand this variant’s effect on Alzheimer’s disease risk.
Alzheimer’s disease treatment
There is currently no known cure for Alzheimer’s disease.
Treatment addresses several different areas:
- Helping people maintain mental function.
- Managing behavioral symptoms.
- Slowing or delaying the symptoms of the disease.
Medications
The U.S. Food and Drug Administration (FDA) has approved medications that fall into two categories:
- Drugs that may delay clinical decline in people living with Alzheimer’s disease.
- Drugs that may temporarily mitigate some symptoms of Alzheimer’s disease.
When considering any treatment, it is important to have a conversation with your health care professional to determine whether it is appropriate. A physician who is experienced in using these types of medications should monitor people who are taking them and ensure that the recommended guidelines are strictly observed.
Alzheimer’s disease treatments overview:
- May delay clinical decline
- Aducanumab (Aduhelm™) approved for Alzheimer’s disease. Side effects include Amyloid Related Imaging Abnormalities (ARIA) which is swelling in areas of the brain, with or without small spots of bleeding in or on the surface of the brain; headache and fall.
- Treats cognitive symptoms (memory and thinking)
- Donepezil (Aricept®) approved for mild to severe dementia due to Alzheimer’s disease. Side effects include nausea, vomiting, loss of appetite, muscle cramps and increased frequency of bowel movements.
- Galantamine (Razadyne®) approved for mild to moderate dementia due to Alzheimer’s disease. Side effects include nausea, vomiting, loss of appetite and increased frequency of bowel movements.
- Rivastigmine (Exelon®) approved for mild to moderate dementia due to Alzheimer’s disease or Parkinson’s disease. Side effects include nausea, vomiting, loss of appetite and increased frequency of bowel movements.
- Memantine (Namenda®) approved for moderate to severe dementia due to Alzheimer’s disease. Side effects include headache, constipation, confusion and dizziness.
- Memantine + Donepezil (Namzaric®) approved for moderate to severe dementia due to Alzheimer’s disease. Side effects include nausea, vomiting, loss of appetite, increased frequency of bowel movements, headache, constipation, confusion and dizziness.
- Treats non-cognitive symptoms (behavioral and psychological)
- Suvorexant (Belsomra®) approved for insomnia in people living with mild to moderate Alzheimer’s disease. Side effects include impaired alertness and motor coordination, worsening of depression or suicidal thinking, complex sleep behaviors, sleep paralysis, compromised respiratory function.
Table 1. Alzheimer’s disease treatments summary
| Drug Name | Drug Type and Use | Delivery Method | How It Works | Manufacturer’s Recommended Dosage | Common Side Effects | Prescribing information |
|---|---|---|---|---|---|---|
| Aducanumab (Aduhelm®) | Disease-modifying immunotherapy prescribed to treat mild cognitive impairment or mild Alzheimer’s | Intravenous (IV): Dose is determined by a person’s weight; given over one hour every four weeks; most people will start with a lower dose and over a period of time increase the amount of medicine to reach the full prescription dose | Removes abnormal beta-amyloid to help reduce the number of plaques in the brain |
| Amyloid-related imaging abnormalities (ARIA), which can lead to fluid buildup or bleeding in the brain; also headache, dizziness, falls, diarrhea, confusion | https://www.biogencdn.com/us/aduhelm-pi.pdf |
| Donepezil (Aricept®) | Cholinesterase inhibitor prescribed to treat symptoms of mild, moderate, and severe Alzheimer’s | Tablet: Once a day; dosage may be increased over time if well tolerated Orally disintegrating tablet: Same dosing regimen as above | Prevents the breakdown of acetylcholine in the brain |
| Nausea, vomiting, diarrhea, muscle cramps, fatigue, weight loss | http://www.aricept.com |
| Rivastigmine (Exelon®) | Cholinesterase inhibitor prescribed to treat symptoms of mild, moderate, and severe Alzheimer’s | Capsule: Twice a day; dosage may be increased over time, at minimum two-week intervals, if well tolerated Patch: Once a day; dosage amount may be increased over time, at minimum four-week intervals, if well tolerated | Prevents the breakdown of acetylcholine and butyrylcholine (a brain chemical similar to acetylcholine) in the brain |
| Nausea, vomiting, diarrhea, weight loss, indigestion, muscle weakness | https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022083s008lbl.pdf |
| Memantine (Namenda®) | N-methyl D-aspartate (NMDA) antagonist prescribed to treat symptoms of moderate to severe Alzheimer’s | Tablet: Once a day; dosage may be increased in amount and frequency (up to twice a day) if well tolerated Oral solution: Same dosage as tablet Extended-release capsule: Once a day; dosage may increase in amount over time, at minimum one-week intervals, if well tolerated | Blocks the toxic effects associated with excess glutamate and regulates glutamate activation |
| Dizziness, headache, diarrhea, constipation, confusion | https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021487s010s012s014,021627s008lbl.pdf |
| Manufactured combination of memantine and donepezil (Namzaric®) | NMDA antagonist and cholinesterase inhibitor prescribed to treat symptoms of moderate to severe Alzheimer’s | Extended-release capsule: Once a day; initial dosage depends on whether the person is already on a stable dose of memantine and/or donepezil; dosage may increase over time, at minimum one-week intervals, if well tolerated | Blocks the toxic effects associated with excess glutamate and prevents the breakdown of acetylcholine in the brain |
| Headache, nausea, vomiting, diarrhea, dizziness, anorexia | https://www.rxabbvie.com/pdf/namzaric_pi.pdf |
| Galantamine (Razadyne®) | Cholinesterase inhibitor prescribed to treat symptoms of mild to moderate Alzheimer’s | Tablet: Twice a day; dosing may increase over time, at minimum four-week intervals, if well tolerated Extended-release capsule: Same dosage as tablet but taken once a day | Prevents the breakdown of acetylcholine and stimulates nicotinic receptors to release more acetylcholine in the brain |
| Nausea, vomiting, diarrhea, decreased appetite, dizziness, headache | https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021615s021lbl.pdf |
Footnote: * Available as a generic drug.
[Source: National Institutes of Health. National Institute on Aging 51 ]Drugs that may delay clinical decline
Drugs in this category may delay clinical decline with benefits to both cognition and function in people living with Alzheimer’s disease.
Aducanumab also known as aducanumab-avwa that is marketed as Aduhelm, is a human monoclonal antibody intravenous (IV) infusion therapy that is specific for beta-amyloid for the treatment of Alzheimer’s disease 52. Aducanumab (Aduhelm) is approved by the U.S. Food and Drug Administration (FDA) in June 2021 under the accelerated approval pathway, which provides patients with a serious disease earlier access to drugs when there is an expectation of clinical benefit despite some uncertainty about the clinical benefit 53. Continued approval for this indication may be contingent upon verification of clinical benefit in confirmatory trial(s). Aducanumab (Aduhelm) works by targeting beta-amyloid, a microscopic protein fragment that forms in the brain and accumulates into plaques. These plaques disrupt communication between nerve cells in the brain and may also activate immune system cells that trigger inflammation and devour disabled nerve cells. While scientists aren’t sure what causes cell death and tissue loss during the course of Alzheimer’s disease, amyloid plaques are one of the potential contributors. Aducanumab (Aduhelm) is the first therapy to demonstrate that removing beta-amyloid resulted in better clinical outcomes 54.
- Aduhelm was shown in clinical trials to delay the clinical decline of people living with early Alzheimer’s disease (mild cognitive impairment (MCI) due to Alzheimer’s or mild Alzheimer’s dementia).
- Some people who received Aduhelm experienced significant benefits on measures of cognition and function. Examples include abilities such as memory, orientation and language.
- Some also experienced benefits on activities of daily living, which refers to the everyday skills we need to take care of ourselves and live well independently. Conducting personal finances, performing household chores (such as cleaning, shopping and doing laundry) and independently traveling out of the home are all examples of activities of daily living.
In clinical trials, the most common side effects were ARIA-E (abnormal brain changes associated with anti-amyloid treatments — most often swelling in the brain — that are spotted with neuroimaging techniques like MRI), headache, ARIA-H (micro hemorrhage/superficial siderosis) and fall.
An FDA-approved diagnostic test is required before receiving aducanumab (Aduhelm); talk with your doctor about options.
Aducanumab (Aduhelm) can cause serious side effects including 55: Amyloid Related Imaging Abnormalities or “ARIA”. Amyloid Related Imaging Abnormalities (ARIA) is a common side effect that does not usually cause any symptoms but can be serious. Amyloid Related Imaging Abnormalities (ARIA) is most commonly seen as temporary swelling in areas of the brain that usually resolves over time. Some people may also have small spots of bleeding in or on the surface of the brain with the swelling. Although most people with swelling in areas of the brain do not have symptoms, some people may have symptoms such as:
- headache,
- confusion,
- dizziness,
- vision changes,
- nausea.
Enhanced clinical vigilance for ARIA is recommended during the first 8 doses of treatment with Aducanumab (Aduhelm), particularly during titration. If a patient experiences symptoms which could be suggestive of ARIA, clinical evaluation should be performed, including magnetic resonance imaging (MRI) scans if indicated.
The patient’s healthcare provider will do magnetic resonance imaging (MRI) scans before and during treatment with Aducanumab (Aduhelm) to check for amyloid related imaging abnormalities (ARIA). Patients should call their healthcare provider or go to the nearest hospital emergency room right away if they have any of the symptoms listed above.
The most common side effects of Aducanumab (Aduhelm) include 55:
- swelling in areas of the brain, with or without small spots of bleeding in or on the surface of the brain [Amyloid Related Imaging Abnormalities (ARIA)];
- headache and fall.
Patients should call their healthcare provider for medical advice about side effects. Patients may report side effects to FDA at 1-800-FDA-1088.
Before receiving Aducanumab (Aduhelm), patients should tell their healthcare provider about all of their medical conditions, including if:
- they are pregnant or plan to become pregnant. It is not known if Aducanumab (Aduhelm) will harm your unborn baby. Tell your healthcare provider if you become pregnant during your treatment with Aducanumab (Aduhelm).
- they are breastfeeding or plan to breastfeed. It is not known if Aducanumab (Aduhelm) passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby while receiving Aducanumab (Aduhelm).
Serious allergic reactions such as swelling of the face, lips, mouth, or tongue and hives have happened during an Aducanumab (Aduhelm) infusion 55. Patients should tell their healthcare provider if they have any of the symptoms of a serious allergic reaction during or after an Aducanumab (Aduhelm) infusion.
Drugs that treat symptoms
Cognitive symptoms (memory and thinking)
As Alzheimer’s progresses, brain cells die and connections among cells are lost, causing cognitive symptoms to worsen. While these medications do not stop the damage Alzheimer’s causes to brain cells, they may help lessen or stabilize symptoms for a limited time by affecting certain chemicals involved in carrying messages among and between the brain’s nerve cells.
The following medications are prescribed to treat symptoms related to memory and thinking:
- Cholinesterase inhibitors (Aricept®, Exelon®, Razadyne®). Acetylcholinesterase (AChE) inhibitors increase levels of acetylcholine, a substance in the brain that helps nerve cells communicate with each other. Cholinesterase inhibitors are prescribed to treat symptoms related to memory, thinking, language, judgment and other thought processes. These medications (Aricept, Exelon, Razadyne) prevent the breakdown of acetylcholine, a chemical messenger important for memory and learning. These drugs support communication between nerve cells by preserving a chemical messenger that is depleted in the brain by Alzheimer’s disease. Additionally, cholinesterase inhibitors may delay or slow worsening of symptoms. Effectiveness varies from person to person.
- The cholinesterase inhibitors most commonly prescribed are:
- Donepezil (Aricept®): approved to treat all stages of Alzheimer’s disease.
- Rivastigmine (Exelon®): approved for mild-to-moderate Alzheimer’s as well as mild-to-moderate dementia associated with Parkinson’s disease.
- Galantamine (Razadyne®): approved for mild-to-moderate stages of Alzheimer’s disease.
- Though generally well-tolerated, if side effects occur, they commonly include nausea, vomiting, loss of appetite, increased frequency of bowel movements (diarrhea) and sleep disturbances. In people with cardiac conduction disorders, serious side effects may include cardiac arrhythmia.
- The cholinesterase inhibitors most commonly prescribed are:
- Glutamate regulators also known as N-Methyl D-aspartate (NMDA) antagonist (Namenda®). Glutamate regulators (N-Methyl D-aspartate [NMDA] antagonist) are prescribed to improve memory, attention, reason, language and the ability to perform simple tasks. This type of drug works by regulating the activity of glutamate, a different chemical messenger that helps the brain process information. Glutamate regulators drug is known as:
- Memantine (Namenda®): approved for moderate-to-severe Alzheimer’s disease. Memantine (Namenda®) slows the progression of symptoms with moderate to severe Alzheimer’s disease. Can cause side effects, including headache, constipation, confusion and dizziness.
- Memantine (Namenda®) can be used alone or with other Alzheimer’s disease treatments. There is some evidence that individuals with moderate to severe Alzheimer’s who are taking a cholinesterase inhibitor might benefit by also taking memantine.
As Alzheimer’s progresses, brain cells die and connections among cells are lost, causing cognitive symptoms to worsen. While current medications cannot stop the damage Alzheimer’s causes to brain cells, they may help lessen or stabilize symptoms for a limited time by affecting certain chemicals involved in carrying messages among the brain’s nerve cells. Doctors sometimes prescribe both types of medications together.
- Cholinesterase inhibitor + glutamate regulator (Namzeric®). This type of drug is a combination of a cholinesterase inhibitor and a glutamate regulator.
- Donepezil and memantine (Namzaric®): approved for moderate-to-severe Alzheimer’s disease. Possible side effects include nausea, vomiting, loss of appetite, increased frequency of bowel movements, headache, constipation, confusion and dizziness.
Sometimes other medications such as antidepressants may be prescribed to help control the behavioral symptoms associated with Alzheimer’s disease.
Behavioral and psychological symptoms
Alzheimer’s disease affects more than just memory and thinking. A person’s quality of life may be impacted by a variety of behavioral and psychological symptoms that accompany dementia, such as sleep disturbances, wandering, increased agitation, aggression, hallucinations and delusions. Some medications focus on treating these non-cognitive symptoms for a time, though it is important to try non-drug strategies to manage behaviors before adding medications. If coping strategies do not work, a consultant psychiatrist can prescribe risperidone or haloperidol, antipsychotic medicines, for those showing persistent aggression or extreme distress. These are the only medicines licensed for people with moderate to severe Alzheimer’s disease where there’s a risk of harm to themselves or others.
Risperidone should be used at the lowest dose and for the shortest time possible as it has serious side effects. Haloperidol should only be used if other treatments have not helped.
At this time, the FDA has approved one drug to address insomnia in people living with dementia, but trials into drugs that address other non-cognitive symptoms are underway.
- Orexin receptor antagonist (Suvorexant or Belsomra®). Prescribed to treat insomnia for individuals living with dementia, this drug is thought to inhibit the activity of orexin, a type of neurotransmitter involved in the sleep-wake cycle:
- Suvorexant (Belsomra®): approved for mild-to-moderate Alzheimer’s disease. Possible side effects include, but are not limited to: risk of impaired alertness and motor coordination (including impaired driving), worsening of depression or suicidal thinking, complex sleep behaviors (such as sleep-walking and sleep-driving), sleep paralysis and compromised respiratory function.
Antidepressants may sometimes be given if depression is suspected as an underlying cause of anxiety.
Sometimes other medications may be recommended to treat specific symptoms in behavioral and psychological symptoms of dementia, but these will be prescribed “off-label” (not specifically licensed for behavioral and psychological symptoms of dementia). It’s acceptable for a doctor to do this, but they must provide a reason for using these medications in these circumstances.
Alternative medicine
Various herbal remedies, vitamins and other supplements are widely promoted as preparations that may support cognitive health or prevent or delay Alzheimer’s. Clinical trials have produced mixed results with little evidence to support them as effective treatments.
Some of the treatments that have been studied recently include:
- Omega-3 fatty acids. Omega-3 fatty acids in fish or from supplements may lower the risk of developing dementia, but clinical studies have shown no benefit for treating Alzheimer’s disease symptoms.
- Curcumin. This herb comes from turmeric and has anti-inflammatory and antioxidant properties that might affect chemical processes in the brain. So far, clinical trials have found no benefit for treating Alzheimer’s disease.
- Ginkgo. Ginkgo is a plant extract containing several medicinal properties. A large study funded by the National Institutes of Health found no effect in preventing or delaying Alzheimer’s disease.
- Vitamin E. Although vitamin E isn’t effective for preventing Alzheimer’s disease, taking 2,000 international units daily may help delay the progression in people who already have the disease. However, study results have been mixed, with only some showing this benefit. Further research into the safety of 2,000 international units daily of Vitamin E in a dementia population will be needed before it can be routinely recommended.
Supplements promoted for cognitive health can interact with medications you’re taking for Alzheimer’s disease or other health conditions. Work closely with your health care team to create a safe treatment plan with any prescriptions, over-the-counter medications or dietary supplements.
Lifestyle and home remedies
Healthy lifestyle choices promote good overall health and may play a role in maintaining cognitive health.
Exercise
Regular exercise is an important part of a treatment plan. Activities such as a daily walk can help improve mood and maintain the health of joints, muscles and the heart. Exercise can also promote restful sleep and prevent constipation.
People with Alzheimer’s who develop trouble walking may still be able to use a stationary bike or participate in chair exercises. You may find exercise programs geared to older adults on TV or on DVDs.
Cognitive stimulation therapy
Cognitive stimulation therapy (CST) involves taking part in group activities and exercises designed to improve memory and problem-solving skills.
Cognitive rehabilitation
Cognitive rehabilitation works by getting you to use the parts of your brain that are working to help the parts that are not. Cognitive rehabilitation technique involves working with a trained professional, such as an occupational therapist, and a relative or friend to achieve a personal goal, such as learning to use a mobile phone or other everyday tasks.
Nutrition
People with Alzheimer’s disease may forget to eat, lose interest in preparing meals or not eat a healthy combination of foods. They may also forget to drink enough, leading to dehydration and constipation.
Offer the following:
- Healthy options. Buy healthy food options that the person with Alzheimer’s disease likes and can eat.
- Water and other healthy beverages. Try to ensure that a person with Alzheimer’s drinks several glasses of liquids every day. Avoid beverages with caffeine, which can increase restlessness, interfere with sleep and trigger a frequent need to urinate.
- High-calorie, healthy shakes and smoothies. You can supplement milkshakes with protein powders or make smoothies featuring favorite ingredients. This may be particularly important when eating becomes more difficult.
Social engagement and activities
Social interactions and activities can support the abilities and skills that are preserved. Doing things that are meaningful and enjoyable are important for the overall well-being of a person with Alzheimer’s disease. These might include:
- Listening to music or dancing
- Reading or listening to books
- Gardening or crafts
- Social events at senior or memory care centers
- Planned activities with children.
Reminiscence and life story work
Reminiscence work involves talking about things and events from your past. It usually involves using props such as photos, favorite possessions or music.
Life story work involves a compilation of photos, notes and keepsakes from your childhood to the present day. It can be either a physical book or a digital version.
These approaches are sometimes combined. Evidence shows they can improve mood and wellbeing.
Creating a safe and supportive environment
Adapting the living situation to the needs of a person with Alzheimer’s disease is an important part of any treatment plan. For someone with Alzheimer’s, establishing and strengthening routine habits and minimizing memory-demanding tasks can make life much easier.
You can take these steps to support a person’s sense of well-being and continued ability to function:
- Always keep keys, wallets, mobile phones and other valuables in the same place at home, so they don’t become lost.
- Keep medications in a secure location. Use a daily checklist to keep track of dosages.
- Arrange for finances to be on automatic payment and automatic deposit.
- Carry a mobile phone with location capability so that a caregiver can track its location. Program important phone numbers into the phone.
- Make sure regular appointments are on the same day at the same time as much as possible.
- Use a calendar or whiteboard in the home to track daily schedules. Build the habit of checking off completed items.
- Remove excess furniture, clutter and throw rugs.
- Install sturdy handrails on stairways and in bathrooms.
- Ensure that shoes and slippers are comfortable and provide good traction.
- Reduce the number of mirrors. People with Alzheimer’s may find images in mirrors confusing or frightening.
- Make sure that the person with Alzheimer’s carries identification or wears a medical alert bracelet.
- Keep photographs and other meaningful objects around the house.
Late stage Alzheimer’s disease care
A person with late-stage Alzheimer’s disease usually:
- Has difficulty eating and swallowing
- Needs assistance walking and eventually is unable to walk
- Needs full-time help with personal care
- Is vulnerable to infections, especially pneumonia
During the late stages, your role as a caregiver focuses on preserving quality of life and dignity. Although a person in the late stage of Alzheimer’s typically loses the ability to talk and express needs, research tells us that some core of the person’s self may remain. This means you may be able to continue to connect throughout the late stage of the disease.
At this point in the disease, the world is primarily experienced through the senses. You can express your caring through touch, sound, sight, taste and smell. For example, try:
- Playing his or her favorite music
- Reading portions of books that have meaning for the person
- Looking at old photos together
- Preparing a favorite food
- Rubbing lotion with a favorite scent into the skin
- Brushing the person’s hair
- Sitting outside together on a nice day
Late-stage care options
Since care needs are extensive during the late stage, they may exceed what you can provide at home, even with additional assistance. This may mean moving the person into a facility in order to get the care needed.
Deciding on late-stage care can be one of the most difficult decisions families face. Families that have been through the process tell us that it is best to gather information and move forward, rather than second guessing decisions after the fact. There are many good ways to provide quality care. Remember, regardless of where the care takes place, the decision is about making sure the person receives the care needed.
At the end of life, another option is hospice. The underlying philosophy of hospice focuses on quality and dignity by providing comfort, care and support services for people with terminal illnesses and their families. To qualify for hospice benefits under Medicare, a physician must diagnosis the person with Alzheimer’s disease as having less than six months to live.
Ideally, discussions about end-of-life care wishes should take place while the person with the dementia still has the capacity to make decisions and share wishes about life-sustaining treatment.
Food and fluids
One of the most important daily caregiving tasks during late-stage Alzheimer’s is monitoring eating. As a person becomes less active, he or she will require less food. But, a person in this stage of the disease also may forget to eat or lose his or her appetite. Adding sugar to food and serving favorite foods may encourage eating; the doctor may even suggest supplements between meals to add calories if weight loss is a problem.
To help the person in late-stage Alzheimer’s stay nourished, allow plenty of time for eating and try these tips:
- Make sure the person is in a comfortable, upright position. To aid digestion, keep the person upright for 30 minutes after eating.
- Adapt foods if swallowing is a problem. Choose soft foods that can be chewed and swallowed easily. Thicken liquids such as water, juice, milk and soup by adding cornstarch or unflavored gelatin. You can also buy food thickeners at a pharmacy or health care supply store, try adding pudding or ice cream, or substitute milk with plain yogurt.
- Encourage self-feeding. Sometimes a person needs cues to get started. Begin by putting food on a spoon, gently putting his or her hand on the spoon, and guiding it to the person’s mouth. Serve finger foods if the person has difficulty using utensils.
- Assist the person with feeding, if needed. Alternate small bites with fluids. You may need to remind the person to chew or swallow. Make sure all food and fluid is swallowed before continuing on with the next bite.
- Encourage fluids. The person may not always realize that he or she is thirsty and may forget to drink, which could lead to dehydration. If the person has trouble swallowing water, try fruit juice, gelatin, sherbet or soup. Always check the temperature of warm or hot liquids before serving them.
- Monitor weight. While weight loss during the end of life is to be expected, it also may be a sign of inadequate nutrition, another illness or medication side effects. See the doctor to have weight loss evaluated.
Bowel and bladder function
Difficulty with toileting is very common at this stage in the disease. The person may need to be walked to the restroom and guided through the process. Incontinence is also common during late-stage Alzheimer’s.
To maintain bowel and bladder function:
- Set a toileting schedule. Keep a written record of when the person goes to the bathroom, and when and how much the person eats and drinks. This will help you track the person’s natural routine, and then you can plan a schedule. If the person is not able to get to the toilet, use a bedside commode.
- Limit liquids before bedtime. Limit — but do not eliminate — liquids at least two hours before bedtime. Be sure to provide adequate fluids for the person throughout the day to avoid dehydration.
- Use absorbent and protective products. Adult disposable briefs and bed pads can serve as a backup at night.
- Monitor bowel movements. It is not necessary for the person to have a bowel movement every day, but if there are three consecutive days without a bowel movement, he or she may be constipated. In such instances, it may help to add natural laxatives to the diet, such as prunes or fiber-rich foods (bran or whole-grain bread). Consult with the doctor if the constipation continues.
Skin and body health
A person with late-stage Alzheimer’s disease can become bedridden or chair-bound. This inability to move around can cause skin breakdown, pressure sores and “freezing” of joints.
To keep skin and body healthy:
- Relieve body pressure and improve circulation. Change the person’s position at least every two hours to relieve pressure and improve blood circulation.
- Make sure the person is comfortable and properly aligned. Use pillows to support arms and legs.
- Learn how to lift the person. A care provider, such as a nurse or physical therapist, can provide instructions on how to properly lift and turn the person without causing injury. Make sure not to ever lift by pulling on the person’s arms or shoulders.
- Keep skin clean and dry. Since skin can tear or bruise easily, use gentle motions and avoid friction when cleaning. Wash with mild soap and blot dry. Check daily for rashes, sores or breakdowns.
- Protect bony areas. Use pillows or pads to protect elbows, heels, hips and other bony areas. If you use skin moisturizer on these areas, apply it gently and do not massage it in.
- Prevent “freezing” of joints. Joint “freezing” (limb contractures) can occur when a person is confined to a chair or bed. It’s sometimes helpful to do range-of-motion exercises, such as carefully moving the arms and legs two to three times a day while the skin and muscles are warm, like right after bathing.
- Consult with the doctor before starting these exercises.
Infections and pneumonia
The inability to move around during late-stage Alzheimer’s disease can make a person more vulnerable to infections.
To help prevent infections:
- Keep the teeth and mouth clean. Good oral hygiene reduces the risk of bacteria in the mouth that can lead to pneumonia. Brush the person’s teeth after each meal. If the person wears dentures, remove them and clean them every night. Also, use a soft toothbrush or moistened gauze pad to clean the gums, tongue and other soft mouth tissues.
- Treat cuts and scrapes immediately. Clean cuts with warm soapy water and apply an antibiotic ointment. If the cut is deep, seek professional medical help.
- Protect against flu and pneumonia. The flu (influenza) can lead to pneumonia (infection in the lungs). It’s vital for the person with Alzheimer’s as well as his or her caregivers to get flu vaccines every year to help reduce the risk. A person can also receive a vaccine every five years to guard against pneumococcal pneumonia (a severe lung infection caused by bacteria).
Pain and illness
Communicating pain becomes difficult in the late stages. If you suspect pain or illness, see a doctor as soon as possible to find the cause. In some cases, pain medication may be prescribed.
To recognize pain and illness:
- Look for physical signs. Signs of pain and illness include pale skin tone; flushed skin tone; dry, pale gums; mouth sores; vomiting; feverish skin; or swelling of any part of the body.
- Pay attention to nonverbal signs. Gestures, spoken sounds and facial expressions (wincing, for example) may signal pain or discomfort.
- Be alert to changes in behavior. Anxiety, agitation, trembling, shouting and sleeping problems can all be signs of pain.
Living with Alzheimer’s disease
Alzheimer’s disease can affect all aspects of a person’s life, as well as those around them.
If you have been diagnosed with Alzheimer’s disease, it’s important to remember that:
- you’re still you, even though you have problems with memory, concentration and planning
- everyone experiences dementia differently
- focusing on the things you can still do and enjoy will help you to stay positive
With the right help and support when you need it, many people can, and do, live well with dementia for several years.
Tips to help cope with dementia
Coping with memory loss and problems with thinking speed can be distressing. But there are things that can help.
Try these tips:
- have a regular routine
- put a weekly timetable on the kitchen wall or fridge, and try to schedule activities for when you feel better (for example, in the mornings)
- put your keys in an obvious place, such as a large bowl in the hall
- keep a list of helpful numbers (including who to contact in an emergency) by the phone
- put regular bills on direct debits so you don’t forget to pay them
- use a pill organiser box (dosette box) to help you remember which medicines to take when (your pharmacist can help you get one)
- make sure your home is dementia-friendly and safe
There’s some evidence to suggest that rates of dementia are lower in people who remain mentally and socially active throughout their lives.
It may be possible to reduce your risk of Alzheimer’s disease and other types of dementia by:
- reading
- learning foreign languages
- playing musical instruments
- volunteering in your local community
- taking part in group sports, such as bowling
- trying new activities or hobbies
- maintaining an active social life
Interventions such as “brain training” computer games have been shown to improve cognition over a short period, but research has not yet demonstrated whether this can help prevent dementia.
Keeping in touch with people and engaging in social activities, such as going to the theatre or cinema, or being part of a walking group or choir, is good for your confidence and mental wellbeing.
If you have someone who helps care for you, an active social life is good for them, too. Many communities are now dementia-friendly. For example, cinemas put on dementia-friendly screenings of the latest films, and leisure centres run dementia-friendly swimming sessions as well as other activities.
It’s a good idea to join a local dementia-friendly group, perhaps at a memory café (a “dementia-friendly” café) or community center. You can share experiences and use tips from others who are living with dementia.
Look after your health
It’s important to look after your physical and mental health when you have dementia:
- Eat a healthy, balanced diet and drink plenty of fluids.
- Exercise regularly. This could be a daily walk or gardening, or you could try tai chi or dancing.
- Ask your doctor if you would benefit from flu vaccination and pneumonia vaccination.
- Get enough sleep. Try to avoid naps during the day and caffeine and alcohol at night.
- Depression is very common in dementia. Talk to your doctor, as there are talking treatments that can help.
- Have regular dental, eyesight and hearing check-ups.
If you have a long-term condition, such as diabetes or heart disease, try to attend regular check-ups with your doctor, which should include a review of the medicines you’re taking.
See your doctor if you feel unwell, as things like chest or urine infections can make you feel very confused if not treated promptly.
Telling people about your dementia
When you’re ready, it’s best to tell others about your diagnosis. It’s also good to tell them what you may have trouble with, such as following a conversation or remembering what was said.
You may find some people treat you differently than they did before. This may be because they don’t understand what dementia is or want to help but don’t know how. Try to explain what your diagnosis means and the ways in which they can help and support you. For example, if you’re no longer able to drive, they could take you to a weekly activity.
You may also find that you lose touch with some people. This may be because you no longer do the activities together that you used to do, or you find it harder to stay in touch.
This can be difficult to accept. But you can meet new people through activity and support groups. Focus on the people who are there for you.
When you need extra help and support
In the early stages of dementia you may be able to live at home, continuing to enjoy doing the things you have always done and having an active social life.
As the illness progresses, it’s likely that you’ll need extra help with daily activities, such as housework, shopping and cooking.
The first step is to apply for a needs assessment from the adult social services of your local council. This will help identify where you might benefit from help.
It’s advisable to do this soon after your diagnosis as a needs assessment can identify things you may not have thought of.
Alzheimer’s disease prognosis
Alzheimer’s disease is initially associated with memory impairment that progressively worsens. Over time, patients with Alzheimer’s disease can also display anxiety, depression, insomnia, agitation, and paranoia. As their disease progresses, patients with Alzheimer’s disease come to require assistance with basic activities of daily living, including dressing, bathing, and toileting. Eventually, difficulties with walking and swallowing may develop. Feeding may be possible only by gastrointestinal tube, and difficulty swallowing may lead to aspiration pneumonia.
On average, people with Alzheimer’s disease live between 3 and 11 years after diagnosis, but some survive 20 years or more. The degree of impairment at diagnosis can affect life expectancy. Patients with early-onset Alzheimer’s disease tend to have a more aggressive, rapid course than those with late-onset Alzheimer’s disease. The primary cause of death is intercurrent illness, such as pneumonia because impaired swallowing allows food or beverages to enter the lungs, where an infection can begin. Other common causes of death include dehydration, malnutrition, falls and other infections.
Alzheimer’s disease life expectancy
Alzheimer’s disease life expectancy from diagnosis to death varies as little as three or four years if the person is older than 80 when diagnosed, to as long as 10 or more years if the person is younger. Average life expectancy for a person age 65 years or older diagnosed with Alzheimer’s disease is about 4 to 8 years. Some individuals with Alzheimer’s disease may live up to 20 years after the first signs of disease. The most common cause of death in Alzheimer’s disease is pneumonia 56.
Alzheimer’s disease is currently ranked as the sixth leading cause of death in the United States, but recent estimates indicate that Alzheimer’s disease may rank third, just behind heart disease and cancer, as a cause of death for older people.
References- Bird TD. Alzheimer Disease Overview. 1998 Oct 23 [Updated 2018 Dec 20]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2021. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1161
- Michaelson DM. APOE ε4: the most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimers Dement. 2014 Nov;10(6):861-8. doi: 10.1016/j.jalz.2014.06.015
- Liu, C. C., Liu, C. C., Kanekiyo, T., Xu, H., & Bu, G. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature reviews. Neurology, 9(2), 106–118. https://doi.org/10.1038/nrneurol.2012.263 Erratum in: Nat Rev Neurol. 2013. doi: 10.1038/nmeurol.2013.32. Liu, Chia-Chan [corrected to Liu, Chia-Chen].
- Riedel BC, Thompson PM, Brinton RD. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2016 Jun;160:134-47. doi: 10.1016/j.jsbmb.2016.03.012
- Virk SA, Eslick GD. Aluminum Levels in Brain, Serum, and Cerebrospinal Fluid are Higher in Alzheimer’s Disease Cases than in Controls: A Series of Meta-Analyses. J Alzheimers Dis. 2015;47(3):629-38. doi: 10.3233/JAD-150193
- Willhite, C. C., Karyakina, N. A., Yokel, R. A., Yenugadhati, N., Wisniewski, T. M., Arnold, I. M., Momoli, F., & Krewski, D. (2014). Systematic review of potential health risks posed by pharmaceutical, occupational and consumer exposures to metallic and nanoscale aluminum, aluminum oxides, aluminum hydroxide and its soluble salts. Critical reviews in toxicology, 44 Suppl 4(Suppl 4), 1–80. https://doi.org/10.3109/10408444.2014.934439
- Killin LO, Starr JM, Shiue IJ, Russ TC. Environmental risk factors for dementia: a systematic review. BMC Geriatr. 2016 Oct 12;16(1):175. doi: 10.1186/s12877-016-0342-y
- Wang Z, Wei X, Yang J, Suo J, Chen J, Liu X, Zhao X. Chronic exposure to aluminum and risk of Alzheimer’s disease: A meta-analysis. Neurosci Lett. 2016 Jan 1;610:200-6. doi: 10.1016/j.neulet.2015.11.014
- Cao L, Tan L, Wang HF, Jiang T, Zhu XC, Lu H, Tan MS, Yu JT. Dietary Patterns and Risk of Dementia: a Systematic Review and Meta-Analysis of Cohort Studies. Mol Neurobiol. 2016 Nov;53(9):6144-6154. doi: 10.1007/s12035-015-9516-4
- Dartigues JF, Gagnon M, Michel P, Letenneur L, Commenges D, Barberger-Gateau P, Auriacombe S, Rigal B, Bedry R, Alpérovitch A, et al. Le programme de recherche Paquid sur l’épidémiologie de la démence. Méthodes et résultats initiaux [The Paquid research program on the epidemiology of dementia. Methods and initial results]. Rev Neurol (Paris). 1991;147(3):225-30. French.
- Rondeau V, Jacqmin-Gadda H, Commenges D, Helmer C, Dartigues JF. Aluminum and silica in drinking water and the risk of Alzheimer’s disease or cognitive decline: findings from 15-year follow-up of the PAQUID cohort. Am J Epidemiol. 2009 Feb 15;169(4):489-96. doi: 10.1093/aje/kwn348
- https://www1.nyc.gov/assets/dep/downloads/pdf/water/drinking-water/drinking-water-supply-quality-report/2020-drinking-water-supply-quality-report.pdf
- Flaten TP. Geographical associations between aluminium in drinking water and death rates with dementia (including Alzheimer’s disease), Parkinson’s disease and amyotrophic lateral sclerosis in Norway. Environ Geochem Health. 1990 Mar;12(1-2):152-67. doi: 10.1007/BF01734064
- Forbes WF, Gentleman JF, Maxwell CJ. Concerning the role of aluminum in causing dementia. Exp Gerontol. 1995 Jan-Feb;30(1):23-32. doi: 10.1016/0531-5565(94)00050-d
- Frecker MF. Dementia in Newfoundland: identification of a geographical isolate? J Epidemiol Community Health. 1991 Dec;45(4):307-11. doi: 10.1136/jech.45.4.307
- McLachlan DR, Bergeron C, Smith JE, Boomer D, Rifat SL. Risk for neuropathologically confirmed Alzheimer’s disease and residual aluminum in municipal drinking water employing weighted residential histories. Neurology. 1996 Feb;46(2):401-5. doi: 10.1212/wnl.46.2.401
- Neri LC, Hewitt D. Aluminium, Alzheimer’s disease, and drinking water. Lancet. 1991 Aug 10;338(8763):390. doi: 10.1016/0140-6736(91)90531-s
- Forster DP, Newens AJ, Kay DW, Edwardson JA. Risk factors in clinically diagnosed presenile dementia of the Alzheimer type: a case-control study in northern England. J Epidemiol Community Health. 1995 Jun;49(3):253-8. doi: 10.1136/jech.49.3.253
- Martyn CN, Coggon DN, Inskip H, Lacey RF, Young WF. Aluminum concentrations in drinking water and risk of Alzheimer’s disease. Epidemiology. 1997 May;8(3):281-6. doi: 10.1097/00001648-199705000-00009
- Taylor GA, Newens AJ, Edwardson JA, Kay DW, Forster DP. Alzheimer’s disease and the relationship between silicon and aluminium in water supplies in northern England. J Epidemiol Community Health. 1995 Jun;49(3):323-4. doi: 10.1136/jech.49.3.323
- Shen XL, Yu JH, Zhang DF, Xie JX, Jiang H. Positive relationship between mortality from Alzheimer’s disease and soil metal concentration in mainland China. J Alzheimers Dis. 2014;42(3):893-900. doi: 10.3233/JAD-140153
- Frisardi V, Solfrizzi V, Capurso C, Kehoe PG, Imbimbo BP, Santamato A, Dellegrazie F, Seripa D, Pilotto A, Capurso A, Panza F. Aluminum in the diet and Alzheimer’s disease: from current epidemiology to possible disease-modifying treatment. J Alzheimers Dis. 2010;20(1):17-30. doi: 10.3233/JAD-2009-1340
- Tomljenovic L. Aluminum and Alzheimer’s disease: after a century of controversy, is there a plausible link? J Alzheimers Dis. 2011;23(4):567-98. doi: 10.3233/JAD-2010-101494
- Virk SA, Eslick GD. Brief Report: Meta-analysis of Antacid Use and Alzheimer’s Disease: Implications for the Aluminum Hypothesis. Epidemiology. 2015 Sep;26(5):769-73. doi: 10.1097/EDE.0000000000000326
- Fakri S, Al-Azzawi A, Al-Tawil N. Antiperspirant use as a risk factor for breast cancer in Iraq. East Mediterr Health J. 2006 May-Jul;12(3-4):478-82.
- Mirick DK, Davis S, Thomas DB. Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst. 2002 Oct 16;94(20):1578-80. doi: 10.1093/jnci/94.20.1578
- McGrath KG. An earlier age of breast cancer diagnosis related to more frequent use of antiperspirants/deodorants and underarm shaving. Eur J Cancer Prev. 2003 Dec;12(6):479-85. doi: 10.1097/00008469-200312000-00006
- Minshall C, Nadal J, Exley C. Aluminium in human sweat. J Trace Elem Med Biol. 2014 Jan;28(1):87-8. doi: 10.1016/j.jtemb.2013.10.002
- Virk SA, Eslick GD. Occupational Exposure to Aluminum and Alzheimer Disease: A Meta-Analysis. J Occup Environ Med. 2015 Aug;57(8):893-6. doi: 10.1097/JOM.0000000000000487
- Kumar A, Sidhu J, Goyal A, et al. Alzheimer Disease. [Updated 2021 Aug 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499922
- Vik-Mo AO, Bencze J, Ballard C, Hortobágyi T, Aarsland D. Advanced cerebral amyloid angiopathy and small vessel disease are associated with psychosis in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2019 Jun;90(6):728-730. doi: 10.1136/jnnp-2018-318445
- Zhang H, Greenwood DC, Risch HA, Bunce D, Hardie LJ, Cade JE. Meat consumption and risk of incident dementia: cohort study of 493,888 UK Biobank participants. Am J Clin Nutr. 2021 Mar 22:nqab028. doi: 10.1093/ajcn/nqab028
- McEvoy CT, Hoang T, Sidney S, Steffen LM, Jacobs DR Jr, Shikany JM, Wilkins JT, Yaffe K. Dietary patterns during adulthood and cognitive performance in midlife: The CARDIA study. Neurology. 2019 Apr 2;92(14):e1589-e1599. doi: 10.1212/WNL.0000000000007243
- Blumenthal JA, Smith PJ, Mabe S, Hinderliter A, Lin PH, Liao L, Welsh-Bohmer KA, Browndyke JN, Kraus WE, Doraiswamy PM, Burke JR, Sherwood A. Lifestyle and neurocognition in older adults with cognitive impairments: A randomized trial. Neurology. 2019 Jan 15;92(3):e212-e223. doi: 10.1212/WNL.0000000000006784
- Serra-Majem, L., Tomaino, L., Dernini, S., Berry, E. M., Lairon, D., Ngo de la Cruz, J., Bach-Faig, A., Donini, L. M., Medina, F. X., Belahsen, R., Piscopo, S., Capone, R., Aranceta-Bartrina, J., La Vecchia, C., & Trichopoulou, A. (2020). Updating the Mediterranean Diet Pyramid towards Sustainability: Focus on Environmental Concerns. International journal of environmental research and public health, 17(23), 8758. https://doi.org/10.3390/ijerph17238758
- Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG, Karanja N, Lin PH; DASH-Sodium Collaborative Research Group. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001 Jan 4;344(1):3-10. doi: 10.1056/NEJM200101043440101
- Morris, M. C., Tangney, C. C., Wang, Y., Sacks, F. M., Bennett, D. A., & Aggarwal, N. T. (2015). MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association, 11(9), 1007–1014. https://doi.org/10.1016/j.jalz.2014.11.009
- Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019 Apr;15(4):581-589. doi: 10.1016/j.jalz.2018.12.011
- Berendsen AM, Kang JH, Feskens EJM, de Groot CPGM, Grodstein F, van de Rest O. Association of Long-Term Adherence to the MIND Diet with Cognitive Function and Cognitive Decline in American Women. J Nutr Health Aging. 2018;22(2):222-229. doi: 10.1007/s12603-017-0909-0
- Chen X, Huang Y, Cheng HG. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: a 3-year cohort study. J Nutri Health Aging. 2012;16(6):549–552.
- Morris MC, Wang Y, Barnes LL, Bennett DA, Dawson-Hughes B, Booth SL. Nutrients and bioactives in green leafy vegetables and cognitive decline: Prospective study. Neurology. 2018 Jan 16;90(3):e214-e222. doi: 10.1212/WNL.0000000000004815
- Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006 Oct 24;67(8):1370-6. doi: 10.1212/01.wnl.0000240224.38978.d8
- Devore EE, Kang JH, Breteler MM, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012 Jul;72(1):135-43. doi: 10.1002/ana.23594
- Diet May Help Prevent Alzheimer’s. https://www.rush.edu/news/diet-may-help-prevent-alzheimers
- Small Molecule Models for Nonporphyrinic Iron and Manganese Oxygenases. Editor(s): Jan Reedijk, Kenneth Poeppelmeier. Comprehensive Inorganic Chemistry II (Second Edition), Elsevier, 2013, Pages 487-564, ISBN 9780080965291. https://doi.org/10.1016/B978-0-08-097774-4.00323-5
- Holland, T. M., Agarwal, P., Wang, Y., Leurgans, S. E., Bennett, D. A., Booth, S. L., & Morris, M. C. (2020). Dietary flavonols and risk of Alzheimer dementia. Neurology, 94(16), e1749–e1756. https://doi.org/10.1212/WNL.0000000000008981
- Bhagwat S, Haytowitz DB, Holden JM (2011) USDA Database for the Flavonoid Content of Selected Foods Release 3 US Department of Agriculture. https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav_R03.pdf
- Letenneur L, Proust-Lima C, Le Gouge A, Dartigues JF, Barberger-Gateau P. Flavonoid intake and cognitive decline over a 10-year period. Am J Epidemiol. 2007 Jun 15;165(12):1364-71. doi: 10.1093/aje/kwm036
- Engelhart MJ, Geerlings MI, Ruitenberg A, van Swieten JC, Hofman A, Witteman JC, Breteler MM. Dietary intake of antioxidants and risk of Alzheimer disease. JAMA. 2002 Jun 26;287(24):3223-9. doi: 10.1001/jama.287.24.3223
- Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, Dartigues JF. Intake of flavonoids and risk of dementia. Eur J Epidemiol. 2000 Apr;16(4):357-63. doi: 10.1023/a:1007614613771
- National Institutes of Health. National Institute on Aging. How Is Alzheimer’s Disease Treated ? https://www.nia.nih.gov/health/how-alzheimers-disease-treated
- Alexander GC, Emerson S, Kesselheim AS. Evaluation of Aducanumab for Alzheimer Disease: Scientific Evidence and Regulatory Review Involving Efficacy, Safety, and Futility. JAMA. 2021;325(17):1717–1718. doi:10.1001/jama.2021.3854
- Aducanumab (marketed as Aduhelm) Information. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/aducanumab-marketed-aduhelm-information
- Medications for Memory, Cognition and Dementia-Related Behaviors. https://www.alz.org/alzheimers-dementia/treatments/medications-for-memory
- ADUHELM (aducanumab-avwa) MEDICATION GUIDE. https://www.biogencdn.com/us/aduhelm-medication-guide.pdf
- Barnes, J., Bartlett, J. W., Wolk, D. A., van der Flier, W. M., & Frost, C. (2018). Disease Course Varies According to Age and Symptom Length in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD, 64(2), 631–642. https://doi.org/10.3233/JAD-170841