What is anovulation
Anovulation means absence of ovulation or when an egg is not released by your ovaries during a menstrual cycle. Anovulation is a not a disease but a sign, in much the same way that polycystic ovaries are the manifestation of a much larger disease process. Anovulation may manifest in a variety of clinical presentations, from luteal insufficiency to irregular periods (oligomenorrhea) or an absence of periods (amenorrhea). Ovulation is the result of a maturation process that occurs in the hypothalamic-pituitary-ovarian axis and is orchestrated by a neuroendocrine cascade terminating in the ovaries. Any alteration results in a failure to release a mature ovum, leading to anovulatory cycles.
Anovulation key points
- Absence of or inadequate ovulation is a common cause of infertility and in many cases can be treated effectively
- Amenorrhea and more commonly oligomenorrhea indicate that ovulation is not occurring, so a serum progesterone test is unhelpful
- Weight is important for the success of ovulation induction and outcome of pregnancy. The woman should achieve a body mass index of 20-29 before starting ovulation induction treatment
- Most couples in whom the only cause of subfertility is anovulation can overcome ovulation problems (60-98% cumulative conception rate at six months), but couples with concomitant male factor or tubal subfertility should be treated with appropriate assisted conception techniques
- Ovulation induction should be undertaken in a secondary or tertiary care setting. Couples must be warned of the risk of multiple pregnancy (5-10%) and ovarian hyperstimulation syndrome (<1%)
- The cumulative conception rate is lower for women with polycystic ovary syndrome (PCOS) than for those who have hypothalamic amenorrhea.
See your doctor about your period if:
- You have gone three months without a period and are not pregnant, breastfeeding, or in perimenopause or menopause.
- You get irregular periods (your period happens more often than every 24 days or less often than every 38 days, or lasts longer than 8 days).
- You feel dizzy, lightheaded, weak, or tired, or you have chest pain or trouble breathing during or after your period.
- You bleed through one or more pads or tampons every one to two hours.
- You suddenly get a fever and feel sick after using tampons.
- You have menstrual pain that doesn’t get better with over-the-counter pain medicine, such as ibuprofen or naproxen.
- You have period pain, cramps, or heavy bleeding that makes you miss work, school, or other daily activities.
- You get a migraine around your period or your regular migraine treatment stops working.
- You have blood clots in your menstrual flow that are larger than a quarter.
- You have bleeding after sex, more than once.
- You have spotting or bleeding any time in the menstrual cycle other than during your period.
- You have bleeding after menopause.
Menstruation cycle
The menstrual cycle is the monthly process in which female hormones stimulate an ovary to release an egg, thicken the lining of the uterus to support a pregnancy, and then cause the uterus to shed this lining (through menstruation) if there is no pregnancy. The average menstrual cycle is 28 days, but this varies between women and from month to month. In teens, the menstrual cycle can range from 21 to 45 days, but for most women, it is 21 to 35 days 1.
The menstrual cycle is characterized by regular, recurring changes in the endometrium, which culminate in menstrual bleeding (menses). Such cycles usually begin around age thirteen and continue into the early fifties, then cease.
A female’s first menstrual cycle, called menarche, occurs after the ovaries and other organs of the reproductive control system mature and begin responding
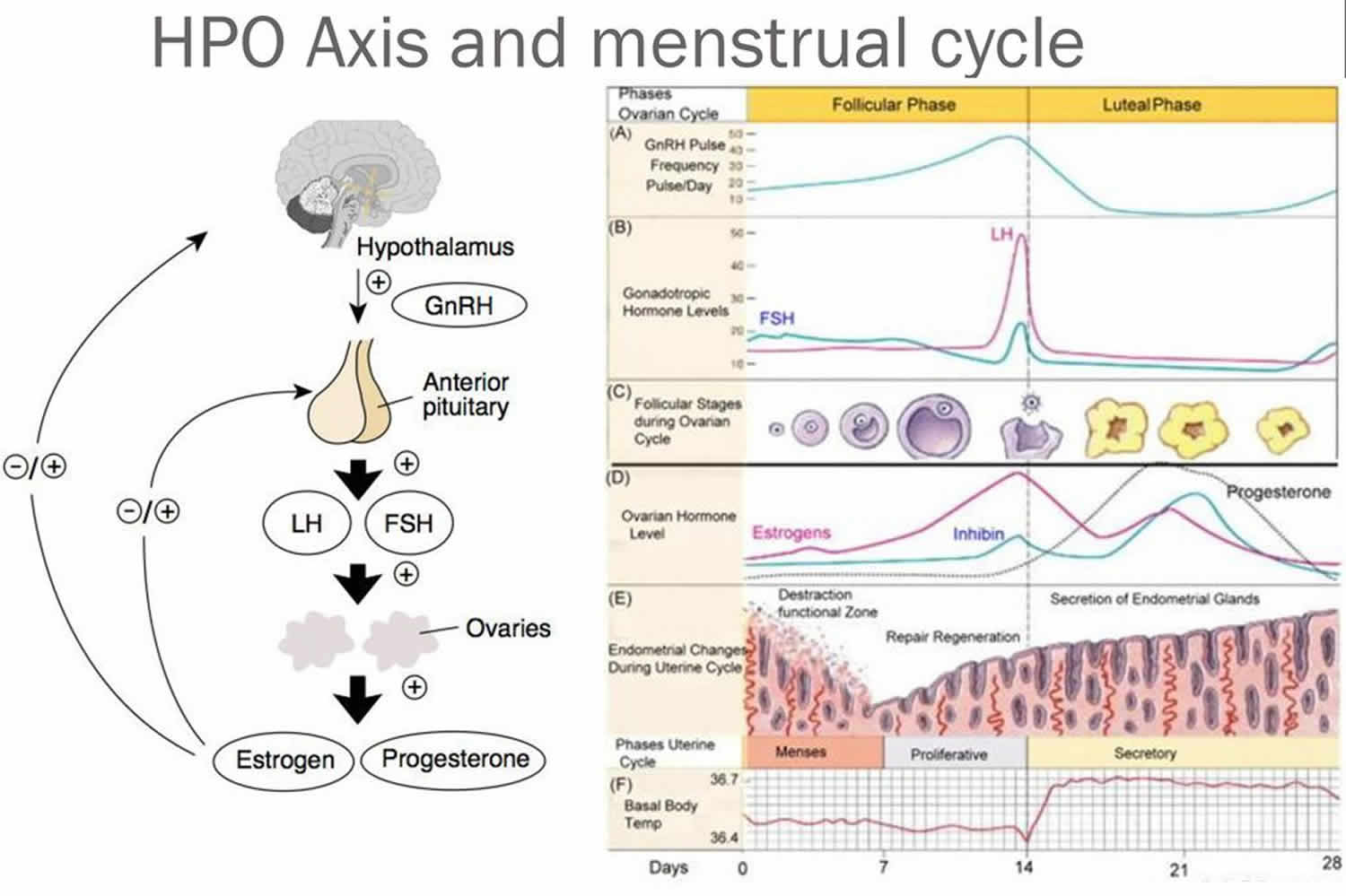
to certain hormones. Then, the hypothalamic secretion of gonadotropin-releasing hormone (GnRH) stimulates the anterior pituitary to release threshold levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Follicle-stimulating hormone (FSH) stimulates the final maturation of an ovarian follicle. The follicular cells produce increasing amounts of estrogens and some progesterone. Luteinizing hormone (LH) stimulates certain ovarian cells to secrete precursor molecules (such as testosterone), also used to produce estrogens.
In a young female, estrogens stimulate the development of secondary sex characteristics. Estrogens secreted during subsequent menstrual cycles continue the development and maintenance of these characteristics.
Figure 1. Pituitary gland hormones under the influence of the hypothalamus controlling the ovaries production of egg cell, ovulation and development of the female secondary sex characteristics
Figure 2. Ovarian Follicle Maturation
Figure 3. Ovarian activity during the Menstrual cycle
Note: Major events in the female menstrual cycle. (a) Plasma hormonal concentrations of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) affect follicle maturation in the ovaries. (b) Plasma hormonal concentrations of estrogen and progesterone influence changes in the uterine lining.
Increasing concentration of estrogens during the first week or so of a menstrual cycle changes the uterine lining, thickening the glandular endometrium (proliferative phase). Meanwhile, the follicle fully matures, and by around the fourteenth day of the cycle, the antral follicle appears on the ovary surface as a blisterlike bulge. Within the follicle, the follicular cells, which surround and connect the secondary oocyte to the inner wall, loosen. Follicular fluid accumulates.
While the follicle matures, it secretes estrogens that inhibit the release of luteinizing hormone (LH) from the anterior pituitary gland but allow luteinizing hormone (LH) to be stored in the gland. Estrogens also make anterior pituitary cells more sensitive to the action of gonadotropin-releasing hormone (GnRH), which is released from the hypothalamus in rhythmic pulses about ninety minutes apart.
Near the fourteenth day of follicular development, the anterior pituitary cells finally respond to the pulses of GnRH (gonadotropin-releasing hormone) and release the stored LH (luteinizing hormone). The resulting surge in LH concentration lasts about thirty-six hours. In response to the LH, the primary oocyte completes meiosis I. The LH (luteinizing hormone) also acts with FSH (follicle-stimulating hormone) inducing complex interactions with prostaglandins, progesterone, plasmin, and proteolytic enzymes, leading to the weakening and rupturing of the bulging follicular wall. This event sends the secondary oocyte and follicular fluid out of the ovary (ovulation).
Following ovulation, the space containing the follicular fluid fills with blood, which soon clots. Under the influence of LH (luteinizing hormone), the remnants of the follicle within the ovary form a temporary glandular structure in the ovary called a corpus luteum (“yellow body”).
Follicular cells secrete some progesterone during the first part of the menstrual cycle. During the second half of the cycle, cells of the corpus luteum secrete abundant progesterone and estrogens. Consequently, as a corpus luteum forms, the blood progesterone concentration sharply increases.
Progesterone causes the endometrium to become more vascular and glandular. It also stimulates the uterine glands to secrete more glycogen and lipids (secretory phase). The endometrial tissues fill with fluids containing nutrients and electrolytes, which provide a favorable environment for an embryo to develop.
High levels of estrogens and progesterone inhibit the anterior pituitary gland’s release of LH (luteinizing hormone) and FSH (follicle-stimulating hormone). Consequently, no other follicles are stimulated to complete development when the corpus luteum is active. However, if the secondary oocyte released at ovulation is not fertilized, the corpus luteum begins to degenerate (regress) on about the twenty-fourth day of the cycle. Eventually, connective tissue replaces it. The remnant of such a corpus luteum is called a corpus albicans, and is eventually absorbed.
When the corpus luteum ceases to function, concentrations of estrogens and progesterone rapidly decline, and in response, blood vessels in the endometrium constrict. This reduces the supply of oxygen and nutrients to the thickened endometrium (stratum functionalis and stratum basalis), and these lining tissues soon disintegrate and slough off. At the same time, blood leaves damaged capillaries, creating a flow of blood and cellular debris that passes through the vagina as the menstrual flow (menses). This flow usually begins about the twenty-eighth day of the cycle and continues for three to five days, while the concentrations of estrogens are relatively low. The beginning of the menstrual flow marks the end of a menstrual cycle and the beginning of the next cycle as a new developing antral follicle becomes available.
Low blood concentrations of estrogens and progesterone at the beginning of the menstrual cycle mean that the hypothalamus and anterior pituitary gland are no longer inhibited. Consequently, FSH (follicle-stimulating hormone) and LH (luteinizing hormone) concentrations soon increase, stimulating a new antral follicle to mature. As this follicle secretes estrogens, the uterine lining undergoes repair, and the endometrium begins to thicken again.
Day 1
The first day of bleeding is considered the first day of the menstrual cycle. After bleeding ends, usually around day 5, levels of the hormone estrogen begin to rise. The rise in estrogen causes the lining of the uterus to thicken as it prepares to hold a fertilized egg. At the same time, the changes in hormone levels cause follicles (the sacs in the ovary that contain eggs) to grow and mature, in preparation for one follicle to go through ovulation.
Ovulation
Around day 12 to 14 in an average 28-day cycle, the egg is released from a follicle on the ovary in a process called ovulation. Ovulation can occur anywhere between 10 and 21 days after the first day of a woman’s menstrual cycle. Ovulation is when the ovary releases an egg so it can be fertilized by a sperm to make a baby. A woman is most likely to get pregnant if she has sex without birth control in the three days before and up to the day of ovulation.
It may be difficult to know when you ovulate, but you can watch for signs. A few days before you ovulate, your vaginal mucus or discharge changes and becomes
more slippery and clear. A woman can also tell when she has begun ovulating using several methods, including at-home tests that measure levels of luteinizing hormone (LH) in the urine and keeping track of her body temperature, which typically rises slightly at ovulation. At mid-cycle, some women experience pain on one side of their pelvic area; this pain is called “Mittelschmerz” (meaning “middle pain,” because it occurs in the middle of the cycle) and may be a signal of ovulation 2.
More than 90 percent of women say they get symptoms of premenstrual syndrome (PMS) in the time after ovulation and before their period starts.
If a pregnancy does not occur, decreasing hormone levels signal for the lining of the uterus, called the endometrium, to be shed during menstruation.
The endometrium builds up and breaks down during the menstrual cycle. The endometrium is thickest halfway through the 28-day cycle. Then, if there is no pregnancy, it breaks down. This breakdown causes the bleeding of the menstrual phase. Figure 5 above illustrates an average 28-day cycle.
What causes anovulation
To understand anovulation, you must first understand what occurs during a normal ovulatory cycle. In normal physiology, ovulation is dependent on the presence of a functioning hypothalamic-pituitary-ovarian axis. The arcuate nucleus within the hypothalamus is composed of a collection of neurons and, when stimulated, releases gonadotropin-releasing hormone (GnRH) into the portal vessels of the pituitary stalk in a pulsatile fashion. GnRH stimulates receptors in the anterior pituitary gland to produce and secrete both luteinizing hormone (LH) and follicle stimulating hormone (FSH). In women, follicle stimulating hormone (FSH) induces maturation of ovarian follicles and eventual production of estrogen, while luteinizing hormone (LH) modulates the secretion of androgens from the ovarian theca cells 3. Estrogen, in turn, produces negative feedback on the pituitary gland.
As the follicle grows through accumulation of follicular fluid, the cohort of granulosa cells acquire the necessary receptors to respond to luteinizing hormone (LH) with increased formation of cyclic adenosine monophosphate (cAMP). During the midcycle, the estrogen levels in the circulation reach a concentration that causes a positive feedback action on luteinizing hormone (LH) secretion. This is called the LH surge. Generally speaking, approximately 16-24 hours after the LH peak, ovulation occurs with the extrusion of a mature oocyte from the graafian follicle and the formation of the corpus luteum 4. These events are the culmination of a well-coordinated interplay between hormones and their appropriate receptors and proteolytic enzymes and prostaglandins acting in concert with one another, all directed by the hypothalamic-pituitary-ovarian axis.
The system is so sensitive that even the slightest alteration in any of these factors can disrupt its fluidity and lead to anovulation.
When problems arise at any of the many different levels involved in the normal menstrual cycle, it is sometimes helpful to separate the levels by organ system. The hypothalamus and the anterior pituitary can be considered the neuroendocrine components by virtue of their proximity to each another, while the ovaries are a separate compartment. The third aspect that can be defective is the signaling process that occurs between these 2 areas 4.
The initial stimulus must come from the hypothalamus in the form of gonadotropin-releasing hormone (GnRH); this decapeptide must be secreted in a pulsatile fashion within a critical range. For example, sexual maturity is not attained until the onset of regular ovulatory cycles, which may take months to years to occur. This maturation process is orchestrated by a neuroendocrine cascade and modified by autocrine and paracrine events in the ovaries, in which GnRH is the principal mediator 5.
Any alteration in the gonadotropin-releasing hormone (GnRH) pulse generator alters the hormonal milieu necessary for gonadotropin secretion and eventual response at the level of the ovary. Several entities (eg, hyperprolactinemia) are known to cause this type of dysregulation. Increasing levels of prolactin can cause a woman to progress from a deficient luteal phase to overt amenorrhea, usually associated with complete gonadotropin-releasing hormone (GnRH) suppression. More common causes of dysregulation include stress, anxiety, and eating disorders, which are also associated with an inhibition of normal GnRH pulsatility through excessive hypothalamic activity of corticotrophin-releasing hormone and stimulation of beta-endorphins 6.
How polycystic ovary syndrome (PCOS) is associated with anovulatory cycles has not been completely elucidated. Two associations with this disease entity are theorized to be at least somewhat responsible for its development. The first is the persistent elevation of LH levels in these patients; the second is the apparent arrest of antral follicle development at the 5- to 10-mm stage and consequent failure to enter the preovulatory phase of the cycle 7. This evidence indicates that the disturbance is mainly a central defect that initiates the cascade of events leading to its onset.
Similarly, any condition, whether primary or secondary, that results in either a persistent elevation or an insufficient attainment of estrogen levels can inhibit ovulation through a disruption of the mechanisms that induce the luteinizing hormone (LH) surge. To achieve the corresponding changes within the cycle, estradiol levels must rise and fall appropriately 4.
Causes of anovulation:
Hypothalamic
- Low concentration of gonadotrophin realeasing hormone (hypogonadotrophic hypogonadism)
- Weight or exercise related amenorrhoea
- Kallman’s syndrome
- Stress
- Idiopathic
Pituitary
- Hyperprolactinaemia
- Pituitary failure (hypogonadotrophic hypogonadism)
- Sheehan’s syndrome
- Craniopharyngioma or hypophysectomy
- Cerebral radiotherapy
Ovarian
- Polycystic ovaries
- Ovarian failure
- Idiopathic
- Radiotherapy or chemotherapy
- Surgical removal
- Genetic
- Autoimmune
- Chromosomal
- Turner’s syndrome (45,X)
- Androgen insensitivity syndrome (46,XY)
Other endocrine
- Hypothyroidism
- Congenital adrenal hyperplasia
Hypothalamic-pituitary causes
Hypogonadotrophic hypogonadism is characterized by a selective failure of the pituitary gland to produce luteinizing hormone (LH) and follicle stimulating hormone (FSH) 8. The commonest cause is excessive exercise, being underweight, or both. Women who have a low body mass index (for example, < 20) or who exercise excessively—for example, gymnasts, marathon runners, ballerinas—may develop amenorrhea because of a physiological reduction in the hypothalamic production of gonadotrophin releasing hormone. Women who are underweight for their height when they get pregnant are more likely to have “small for dates” babies; and children of women who have eating disorders are more likely to be admitted to hospital with failure to thrive.
Sheehan’s syndrome (panhypopituitarism), caused by infarction of the anterior pituitary venous complex (usually after massive postpartum haemorrhage or trauma), and Kallman’s syndrome (amenorrhea with anosmia caused by congenital lack of hypothalamic production of gonadotrophin releasing hormone) are rare. Children treated for a craniopharyngioma or some forms of leukemia may have hypogonadotrophic hypogonadism secondary to cerebral irradiation, which may affect the hypothalamus or the pituitary.
Hyperprolactinaemia is usually caused by a pituitary microadenoma. This leads to a reduction in the production of pituitary luteinizing hormone (LH) and follicle stimulating hormone (FSH). Although the commonest presentation is secondary amenorrhea, some women may present with galactorrhea. A smaller number may have headaches or disturbed vision that may indicate a macroadenoma, which needs urgent investigation and treatment. A microadenoma is easily treated with drugs with a subsequent resumption of menses and fertility.
Ovarian causes
Polycystic ovary syndrome is the commonest cause (70%) of anovulatory subfertility. The primary abnormality seems to be an excess of androgen production within the ovary that leads to the recruitment of large numbers of small preovulatory follicles, which fail to respond to normal concentrations of follicle stimulating hormone. Thus, a dominant follicle is rarely produced. Women with polycystic ovary syndrome (PCOS) commonly present in their late teens or early 20s with hirsutism, acne, or irregular periods (cycle length > 35 days). Even if they ovulate, the chance of conception for these women is reduced because fewer ovulatory events occur in a given time frame. Only a third of women with polycystic ovary syndrome (PCOS) are obese, but obesity increases the likelihood of a woman with the syndrome developing anovulation.
Premature ovarian failure (premature menopause)
Unfortunately this is an irreversible condition. The only treatment option that can result in conception is the use of donated eggs with in vitro fertilization. Patients will need hormone replacement therapy to alleviate menopause symptoms and to reduce loss of bone density.
Genetic abnormalities
The commonest genetic abnormality is Turner’s syndrome (45,X), in which underdeveloped (streak) ovaries result in primary ovarian failure (premature menopause). With adequate estrogen replacement the uterus can grow large enough for the woman to conceive using donated eggs with in vitro fertilization. Some translocations and deletions of the X chromosome also cause ovarian failure.
Ten per cent of primary amenorrhea is caused by androgen insensitivity syndrome (formerly testicular feminization). These women have a 46,XY karyotype and intra-abdominal gonads that are testes but have developed as phenotypically female because of the absence of, or non-functionality of androgen receptors. The vagina usually ends blindly and, as there is no uterus, pregnancy is impossible. The gonads should be removed because of an increased risk of malignant change. Explaining the nature of the problem to the patient needs care and sensitivity, and longer term psychological support may be needed.
Anovulation signs and symptoms
Anovulation signs and symptoms depend on the underlying cause. Anovulation occurs only in women of reproductive age. Almost all women experience anovulatory cycles at some point in their reproductive lives. Estimates of chronic anovulation rates range from 6-15% of women during the reproductive years.
Anovulation is physiologic at the extremes of reproductive age. During menarche, absence of ovulation is due to immaturity of the hypothalamic-pituitary-ovarian axis, leading to an uncoordinated secretion of GnRH (pulsatility).
When anovulation occurs outside of the perimenarchal or perimenopausal years, extrinsic and intrinsic causes must be excluded.
Polycystic ovary syndrome (PCOS) happens when a woman’s ovaries or adrenal glands produce more male hormones than normal. PCOS causes cysts (fluid-filled sacs) to grow on the ovaries. Symptoms include:
- Irregular menstrual periods
- Infertility
- Pelvic pain
- Excess hair growth on the face, chest, stomach, or thighs
- Weight gain
- Acne or oily skin
- Patches of thickened skin
Women with PCOS are at higher risk of diabetes, metabolic syndrome, heart disease, and high blood pressure.
PCOS is more common in women who have obesity, or have a mother or sister with PCOS. To diagnose PCOS, your health care provider may do a physical exam, pelvic exam, blood tests, and an ultrasound.
Anovulation diagnosis
Laboratory Studies
Laboratory evaluation in patients with anovulation includes the following:
- Pregnancy test – Quantitative beta-HCG in all women of reproductive age
- FSH – Important in assessing for premature ovarian failure
- LH – In combination with FSH, helps establish a diagnosis of PCOS (with LH/FSH ratio >2:1)
- Ovarian steroid hormones – Estradiol, progesterone (midluteal)
- TSH – Hypothyroidism
- Prolactin – Hyperprolactinemia
- Glucose – Using a 2-hour glucose tolerance test after 75-g glucose load
- Cortisol with or without ACTH stimulation test – Helps determine presence of adrenal insufficiency
- Total testosterone/free testosterone – In the presence of hirsutism or virilization, can help distinguish ovarian versus adrenal origin
- Dehydroepiandrosterone sulfate (DHEAS) – Hirsutism or virilization of adrenal origin
- 17-Hydroxyprogesterone – CAH
- Pregnenolone – 17-alpha-hydroxylase deficiency
Workup for autoimmune disorders may be considered when initial test results are uninformative and may include the following studies:
- Complete blood count (CBC)
- Complete metabolic profile – Electrolytes, albumin, renal function tests, liver function tests
- Antinuclear antibodies
- Rheumatoid factor
- Erythrocyte sedimentation rate
- C-reactive protein
- Thyroid antibodies
Other tests may include the following:
- Karyotype – Usually performed in patients younger than 30 years to rule out presence of Y chromosome (frequency of germ cell tumors in patients >30 y is negligible)
- Galactose-1-phosphate – Galactosemia
Table 1. Investigations for anovulation
| Investigation | When done | Interpretation |
|---|---|---|
| Progesterone | Mid-luteal phase of cycle (for example, day 21 of 28 day cycle or day 28 of 35 day cycle) | >30 nmol/l confirms ovulation; if 10-30 nmol/l check when sample taken in relation to cycle length |
| Follicle stimulating hormone | Early follicular phase | >10 IU/l indicates reduced ovarian reserve; >40 IU/l indicates ovarian failure; <5 IU/l may indicate pituitary or hypothalamic problem |
| Luteinising hormone | Early follicular phase | >10 IU/l indicates polycystic ovaries; <5 IU/l may indicate pituitary or hypothalamic problem |
| Testosterone | Any time in cycle | >2.4 nmol/l indicates polycystic ovaries >5 nmol/l suggests congenital adrenal hyperplasia; check DHEAS and 17-OHP |
| Prolactin | Any time in cycle (but not after exercise or stress) | >1000 IU/l indicates pituitary adenoma; needs repeating |
| Thyroid stimulating hormone (TSH) | Any time in cycle if woman has symptoms or signs of hypothyroidism or has hyperprolactinaemia | High thyroid stimulating hormone indicates hypothyroidism |
| Transvaginal ultrasound scan | Oligomenorrhoea or amenorrhoea; raised luteinising hormone or testosterone | Identifies polycystic ovaries |
| MRI/CT of pituitary | abcboxtIf two prolactin levels >1000 IU/l | Identifies macroadenomas |
| Karyotype | Primary amenorrhoea and premature menopause | Identifies karyotypic abnormalities—for example, Turner’s syndrome (45,X), translocations, and androgen insensitivity syndrome (46,XY) |
| Body mass index (BMI) | Oligomenorrhoea or amenorrhoea | Body mass index >30 suggests polycystic ovary syndrome; body mass index <20 suggests hypogonadotrophic hypogonadism |
Abbreviations: CT=computed tomogram; DHEAS=dihydroepiandrosterone sulphate; MRI=magnetic resonance imaging scan; 17-OHP=17-hydroxyprogesterone
[Source 8 ]Imaging Studies
Radiologic studies in the evaluation of patients with anovulation includes the following:
- Ultrasonography – Evaluation of ovaries and endometrium (transvaginal), adrenals (abdominal). Evaluate for the presence of 12 or more follicles in each ovary measuring 2-9 mm in diameter and/or increased ovarian volume greater than 10 mL 9. A thickened heterogeneous endometrium in the setting of chronic anovulation should prompt suspicion for endometrial hyperplasia regardless of the patient’s age.
- Computed tomography scanning (CT scan) – Adrenals (abdominal)
- Magnetic resonance imaging (MRI) – Pituitary glands, adrenals
- Bone density scanning (ie, dual-energy x-ray absorptiometry [DEXA scan]) – Vertebrae and femur (primarily in hypoestrogenic states)
- Nuclear thyroid scanning – Hot versus cold nodules in the presence of positive physical findings and symptoms
Procedures
Endometrial biopsy may be performed to exclude endometrial hyperplasia. An endometrial biopsy should be performed in all women older than 35 years who have irregular uterine bleeding, whether in the presence of anovulatory or ovulatory cycles. Biopsy is also indicated in women younger than 35 years who have a long-standing history of anovulation and concomitant risk factors for endometrial hyperplasia, such as obesity (unopposed estrogenic environment). The most important aspect is that age should not be a factor in deciding whether to perform an endometrial biopsy.
Anovulation treatment
The medical management of anovulation is complex because it involves treating the specific causes of anovulation.
Consider consultations with the following specialists:
- Neurosurgeons – In the presence of a macroadenoma unresponsive to medical management with bromocriptine
- Psychiatrists/psychologists – For patients with body dysmorphic disorder and concomitant anorexia nervosa and bulimia
- Nutritionists – For patients with anorexia nervosa and bulimia
- Endocrinologists – When anovulation is due to adrenal disorders such as Cushing syndrome, Addison disease, overt type 2 diabetes mellitus, panhypopituitarism (ie, Sheehan syndrome), refractory thyroid disease
- Gynecologic oncologists/general surgeons – In the case of either an adnexal mass or adrenal mass of benign or malignant origin
- Reproductive endocrinologists and infertility specialists – When fertility is desired in order to appropriately monitor ovulation induction with either clomiphene citrate or gonadotropins or in the management of PCOS. A systematic review and network meta-analysis by Wang et al 10 reported that letrozole and the combination of clomiphene and metformin had higher pregnancy rates and ovulation rates than clomiphene alone in women with WHO group II anovulation.
Change of weight
Women with polycystic ovary syndrome who are overweight (body mass index > 30) should be advised to lose weight. Together with exercise, weight loss (even as little as 5% of body mass) reduces insulin and free testosterone levels, resulting in improved menstrual regularity, ovulation, and pregnancy rates. If a woman is obese when she is pregnant she is more likely to miscarry. Women who are underweight (body mass index < 20) should be encouraged to gain weight, and no infertility treatment should be offered until their body mass has returned to the lower limits of normal.
Hyperprolactinemia
Bromocriptine is safe and commonly used. Treatment should start with a dose of 1.25 mg (taken with food) at night for the first fortnight and then increased to 2.5 mg for another fortnight. The prolactin level should be checked, and if the level is below 1000 IU/l, the dose should be maintained. The side effects of bromocriptine (postural hypotension, nausea, vertigo, headache) can make it unacceptable to the patient. Cabergoline and quinagolide are newer long acting dopamine agonists with fewer side effects. Once prolactin levels have returned to below 1000 IU/l the woman’s periods should return and 70-80% of women will ovulate.
Hypothyroidism
In hypothyroidism thyrotropin releasing hormone may stimulate prolactin secretion in addition to thyrotropin releasing hormone from the anterior pituitary. Correction of the hypothyroidism with thyroxine replacement allows thyroid stimulating hormone and prolactin levels to return to normal, releasing the suppression to gonadotrophin secretion and ovulation.
Pulsatile gonadotrophin releasing hormone
Treatment with gonadotrophin releasing hormone that is started in a specialised hospital setting may be suitable for women who have a purely hypothalamic cause for their amenorrhoea, for example women with recovered weight related amenorrhoea but who are still not ovulating. The woman wears a small mechanical syringe pump that can deliver a pulse of gonadotrophin releasing hormone subcutaneously every 90 minutes, and this usually leads to unifollicular ovulation. Local reactions may occur at the injection site. Conception rates are similar to those in the normal population at around 20-30% per cycle and 80-90% after 12 months’ use.
Antiestrogen treatment
Clomifene acts by blocking oestrogen receptors in the pituitary leading to an increased production of follicle stimulating hormone, which then stimulates development of one or more dominant follicles. These drugs can be used only in conditions in which the hypothalamic-pituitary axis is functioning—for example, polycystic ovary syndrome. Ovulation induction with clomifene should be undertaken only in circumstances that allow access to ovarian ultrasound monitoring, because of the risk of multiple follicle development and the small but real risk of ovarian hyperstimulation syndrome. Seventy per cent of women with polycystic ovary syndrome will ovulate in response to clomifene, with a conception rate of 40-60% at six months. The incidence of twins is around 10%, and triplets 1%.
Metformin
Increasingly, studies report that metformin at doses of 1500 mg a day (in a similar way to weight loss) may improve menstrual regularity by reducing insulin and free testosterone concentrations in both lean and obese women with polycystic ovary syndrome who are not ovulating. However, caution is needed because metformin is not licensed for this indication, and the results of convincing trials are still awaited.
Follicle stimulating hormone injections
Treatment with follicle stimulating hormone is used in women with hypothalamic-pituitary causes of anovulation, and for women with polycystic ovary syndrome who have failed to respond to or conceive using clomifene. As the most serious complications of this therapy are ovarian hyperstimulation syndrome and high order multiple pregnancy, it is essential that this treatment is monitored by reproductive specialists with access to ultrasonography and tertiary care facilities.
Acute bleeding secondary to anovulation
Most causes of dysfunctional uterine bleeding respond to either oral or intravenous estrogen. Treatment using the parenteral route can be initiated with estrogen (Premarin) 25 mg IV q4h by accelerating the mitotic activity at the level of the endometrium. If no response is seen after 24 hours, suction dilation and curettage is warranted.
If the bleeding is not as vigorous, high-dose birth control pills (totalling 3 pills/d for 7 d), followed by continuation of oral contraceptives for a minimum of 3 months, has equal efficacy in reestablishing the endometrium.
Because of the menstrual irregularities associated with anovulation, anemia is a concern and must be treated with allogeneic blood transfusion if blood parameters fall below critical levels. Intravenous estrogen (Premarin) or high-dose combined oral contraceptives may be needed to ameliorate or terminate the acute bleeding episode. Dilation and curettage should never be the first-line treatment in this clinical setting; however, in the case of intractable bleeding, it may be the only alternative. Subtotal or total hysterectomy is rarely, if ever, necessary.
Anovulation and amenorrhea
Pregnancy test and hormonal studies measuring thyroid function, prolactin levels, and gonadotropin levels should be obtained. These hormonal assays should be followed by a progestational challenge to evaluate the endometrial lining and the presence of a hypoestrogenic state.
The first possibility is normal levels with a positive withdrawal bleed. This indicates anovulation and unopposed estrogen stimulation. Treatment is focused on providing progesterone support and cyclicity in the form of oral contraceptives or progestin alone, which is paramount in the prevention of endometrial hyperplasia.
If TSH or prolactin levels are elevated, correcting the primary problem is usually enough to attain ovulatory cycles once again.
If gonadotropin levels are low or normal, a diagnosis of hypogonadotropic hypogonadism is assumed and a space-occupying lesion versus hypothalamic suppression due to exercise or weight fluctuations (eg, anorexia nervosa, bulimia) must be ruled out. Treatment again focuses on the cause of the suppression.
If FSH and LH levels are elevated (hypergonadotropic hypogonadism), the problem is usually related to an absence of inhibitory signals that originate from the ovary under normal conditions; therefore, ovarian failure is presumed. Generally, other signs and symptoms of hypoestrogenism, such as vaginal dryness, emotional lability, and hot flushes due to vasomotor spasm, help confirm the diagnosis. If this occurs before age 30 years, a karyotype is necessary to rule out the presence of a Y chromosome or fragile X premutation. Owing to the high rate of malignant germ cell tumors in this setting, a gonadectomy must be performed immediately. Other considerations are certain autoimmune and infectious processes that can destroy ovarian tissue through infiltration of autoimmune complexes.
Surgical Care
Surgical care is usually indicated to resolve the underlying cause for the anovulation, typically when medical therapy has failed.
Surgery is also indicated in rare cases, such as a macroadenoma of the pituitary with unrelenting growth eliciting severe symptoms (eg, headaches, bitemporal hemianopsia, diplopia). In the event of a benign or malignant neoplasm of ovarian or adrenal origin, exploratory laparotomy, resection, and staging are indicated.
Ovarian drilling and ovarian wedge resection are other surgical modalities used in the treatment of anovulation due to PCOS, with a spontaneous ovulation rate of more than 80% after the procedure.
While dilation and curettage is never first-line therapy for acute bleeding, practitioners are sometimes left with no other option. In even rarer cases, hysterectomy may be the only solution to the profound anemia stemming from acute blood loss.
Bariatric surgery has been advocated in the surgical treatment of severe obesity when accompanied by medical complications in which weight loss could be curative. Gastroplasty, vertical banded gastroplasty, gastric banding, and vertical stapling are commonly used but are less effective than the roux-en-Y gastric bypass. Typically patients with a BMI greater than 40 are candidates for surgery, assuming past attempts at medical treatment have failed, although patients with a BMI of 35-40 and underlying life-threatening medical problem may be considered as well 11.
Surgical induction
Laparoscopic ovarian diathermy or “drilling” has replaced wedge resection of the ovaries in women with polycystic ovary syndrome. At laparoscopy, five to six diathermy or laser punctures are made in the ovary. Success rates are comparable with follicle stimulating hormone administration, with lower risks of multiple pregnancy or ovarian hyperstimulation syndrome, but complications can arise from surgery and adhesion formation. If too much ovarian tissue is destroyed there is a potential risk of premature ovarian failure in the future, although this risk is still being evaluated.
References- McDowell, M. A., Brody, D. J., & Hughes, J.P. (2007). Has age at menarche changed? Results from the National Health and Nutrition Examination Survey (NHANES) 1999-2004. Journal of Adolescent Health, 40, 227–231.
- Krohn, P. L. (1949). Intermenstrual pain (the “Mittelschmerz”) and the time of ovulation. British Medical Journal, 1(4609), 803–805. Retrieved September 27, 2016
- Warren MP, Vu C. Central causes of hypogonadism–functional and organic. Endocrinol Metab Clin North Am. 2003 Sep. 32(3):593-612.
- Speroff L, Glass RH, Kase NG. Anovulation and the polycystic ovary syndrome. Clinical Gynecologic Endocrinology and Infertility. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. 487-513.
- Spence JE. Anovulation and monophasic cycles. Ann N Y Acad Sci. 1997 Jun 17. 816:173-6.
- Yen SC, Jaffe RB, Barbieri RL. Chronic anovulation due to CNS-hypothalamic-pituitary dysfunction. Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 4th ed. London, England: Elsevier Science; 1999.
- Franks S, Mason H, White D, Willis D. Etiology of anovulation in polycystic ovary syndrome. Steroids. 1998 May-Jun. 63(5-6):306-7.
- Hamilton-Fairley D, Taylor A. Anovulation. BMJ. 2003;327(7414):546–549. doi:10.1136/bmj.327.7414.546 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC192851
- The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004 Jan. 81(1):19-25.
- Wang R, Kim BV, van Wely M, Johnson NP, Costello MF, Zhang H, et al. Treatment strategies for women with WHO group II anovulation: systematic review and network meta-analysis. BMJ. 2017 Jan 31. 356:j138.
- Goldman L, Ausiello D. Obesity. Cecil Textbook of Medicine. 22nd ed. 2004. 1346.