Antimalarial drugs
Malaria is one of the world’s most common and important infectious diseases, affecting 200 to 300 million persons and accounting for half a million deaths yearly, mostly children. Malaria parasites of the Plasmodium genus are transmitted through the bite of infective mosquitoes. Female Anopheles species mosquitoes transmit four Plasmodium species that commonly cause illness in humans: Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae 1. Mixed infections with multiple species are possible and occur in areas where more than one species is in circulation 2. Rarely, humans can be infected with Plasmodium knowlesi, a predominantly simian malaria found in Southeast Asia. Malaria can be a severe, potentially fatal disease (especially when caused by Plasmodium falciparum) and treatment should be initiated as soon as possible. The majority of infections and deaths due to malaria occur in Africa and Asia. Between 1000 and 1500 cases are reported in the United States each year, virtually all being related to travel to endemic areas, and deaths being very rare and usually associated with misdiagnosis, inadequate therapy or lack of compliance.
Malaria is treated with prescription drugs to kill the parasite. The types of drugs and the length of treatment will vary, depending on:
- Which type of malaria parasite you have
- The area where the infection was acquired and its drug-resistance status
- The severity of your symptoms
- The clinical status of the patient
- Any accompanying illness or condition
- Your age
- Whether you’re pregnant
- Drug allergies, or other medications taken by the patient.
Patients who have severe malaria or who cannot take oral medications should be given the treatment by continuous intravenous infusion.
Most drugs used in treatment are active against the parasite forms in the blood (the form that causes disease) and include the following:
- Chloroquine: Chloroquine has been the standard antimalarial drug since its development during World War II and is used both for therapy and prophylaxis. Chloroquine resistance has become a growing problem, particularly for Plasmodium falciparum infections. Chloroquine rarely causes hepatic injury, although it can cause an acute exacerbation of porphyria cutanea tarda with hepatic involvement.
- Atovaquone-proguanil (Malarone®): The combination of atovaquone with proguanil is used for prevention and therapy of chloroquine-resistant Plasmodium falciparum infection.
- Artemether-lumefantrine (Coartem®)
- Mefloquine: Mefloquine is an aminoquinoline similar to quinine which is used both for prophylaxis and treatment of malaria, it being effective against many chloroquine-resistant Plasmodium falciparum infections. Mefloquine resistance has become a growing problem with it use as monotherapy. Mefloquine has only rarely been linked to hepatic injury, but it has frequent neuro-psychiatric side effects which has led to limitations on its use.
- Quinine: Quinine was the initial drug used for prophylaxis and therapy of malaria, being the active component of Cinchona bark that was used for centuries in South America for chills and fever, and that was introduced into Western medicine by Jesuit priests returning from Peru in the 17th Century. Quinine is active against malaria, but has been replaced by synthetic aminoquinolone derivatives such as chloroquine that are more potent and better tolerated. Quinine is still used rarely for therapy of chloroquine-resistant Plasmodium falciparum malaria. Quinine can cause an acute allergic response with fever, nausea, abdominal pain and liver injury, but it is rarely severe.
- Doxycycline (used in combination with quinine)
- Clindamycin (used in combination with quinine)
- Tetracycline (used in combination with quinine)
- Artesunate (not licensed for use in the United States, but available through the Centers for Disease Control and Prevention [CDC])
- Amodiaquine: Amodiaquine is an aminoquinolone structurally related to chloroquine which has some advantages, but which can cause severe hepatitis and agranulocytosis which led to its abandonment as prophylaxis. It is still used in combination with other agents for therapy of chloroquine-resistant Plasmodium falciparum.
- Mepacrine: Mepacrine is an acridine dye that was developed in the 1920s and found to have activity against Plasmodium vivax and falciparum infection. It was extensively used by the U.S. military in the South Pacific during World War II when it was known as atabrine. It was largely replaced by chloroquine thereafter, but found new uses in the treatment of other parasitic diseases and as an antiinflammatory agent in the therapy of lupus erythematosus. Mepacrine is commonly referred to as quinacrine in the United States, but it currently is not approved for any use, although available in some countries abroad or via the internet.
- Primaquine: Primaquine is an aminoquinolone that has been used for many decades as prophylaxis and therapy of malaria. It shares cross resistance with chloroquine.
- The combination of pyrimethamine and sulfadoxine (referred to as Fansidar) is also no longer used for prophylaxis against malaria because of hepatotoxicity and severe allergic reactions, but it is still used in combination with other agents in the treatment of Plasmodium falciparum malaria.
- Artemisinin: Artemisinin is an ancient Chinese herbal medication used for malarial fevers and recently shown to have excellent potency with a distinctive mode of action and little cross-resistance with the aminoquinolones. Artemisinin derivatives have been developed for oral and parenteral use and introduced throughout the world in combination with other agents for therapy of malaria. These derivatives include artesunate, artemisinin, dihydroartemisinin, artemether and arteether, many of which have been linked to instances of idiosyncratic liver injury. The combination of artemether with lumefantrine is available as therapy of Plasmodium falciparum malaria in the United States.
In addition, primaquine and tafenoquine are active against the dormant parasite liver forms (hypnozoites) and prevent relapses. Primaquine and tafenoquine should not be taken by pregnant women or by people who are deficient in G6PD (glucose-6-phosphate dehydrogenase). Patients should not take primaquine or tafenoquine until a quantitative test has excluded G6PD deficiency.
The three-page Malaria Treatment Guidelines (https://www.cdc.gov/malaria/resources/pdf/treatmenttable.pdf) table can be used as a guide for treatment of malaria in the United States. The drug or drug combinations recommended for treatment are listed in bold on the first line of each box in the adult and pediatric “drug and dose” columns. Each drug and its recommended dose are then listed individually on the lines below in the same box. It is important to note that the base/salt conversions for antimalarials are a continual source of confusion and can contribute to treatment errors. In this treatment table (where appropriate), the antimalarial dose is expressed in base with the salt equivalency noted in parentheses.
After initiation of treatment, the patient’s clinical and parasitologic status should be monitored. In infections with Plasmodium falciparum or suspected chloroquine-resistant Plasmodium vivax, blood smears should be made to confirm adequate parasitologic response to treatment (decrease in parasite density).
Because malaria cases are seen relatively rarely in North America, misdiagnosis by clinicians and laboratorians has been a commonly documented problem in published reports. However, malaria may be a common illness in areas where it is transmitted and therefore the diagnosis of malaria should routinely be considered for any febrile person who has traveled to an area with known malaria transmission in the past several months preceding symptom onset.
Symptoms of malaria are generally non-specific and most commonly consist of fever, malaise, weakness, gastrointestinal complaints (nausea, vomiting, diarrhea), neurologic complaints (dizziness, confusion, disorientation, coma), headache, back pain, myalgia, chills, and/or cough. The diagnosis of malaria should also be considered in any person with fever of unknown origin regardless of travel history.
Patients suspected of having malaria infection should be urgently evaluated. Treatment for malaria should not be initiated until the diagnosis has been confirmed by laboratory investigations. “Presumptive treatment” without the benefit of laboratory confirmation should be reserved for extreme circumstances (strong clinical suspicion orsevere disease in the setting of no availability of prompt laboratory confirmation, usually by microscopy).
Laboratory diagnosis of malaria can be made through microscopic examination of thick and thin blood smears. Thick blood smears are more sensitive in detecting malaria parasites because the blood is more concentrated allowing for a greater volume of blood to be examined; however, thick smears are more difficult to read. Thin smears aid in parasite species identification and quantification. Blood films need to be read immediately; off-hours, qualified personnel who can perform this function should be on-call. A negative blood smear makes the diagnosis of malaria unlikely. However, because non-immune individuals may be symptomatic at very low parasite densities that initially may be undetectable by blood smear, blood smears should be repeated every 12-24 hours for a total of 3 sets. If all 3 are negative, the diagnosis of malaria has been essentially ruled out.
After malaria parasites are detected on a blood smear, the parasite density should then be estimated. The parasite density can be estimated by looking at a monolayer of red blood cells (RBCs) on the thin smear using the oil immersion objective at 100x. The slide should be examined where the red blood cells are more or less touching (approximately 400 red blood cells per field). The parasite density can then be estimated from the percentage of infected red blood cells, after counting 500 to 2000 red blood cells.
In addition to microscopy, other laboratory diagnostic tests are available. Several antigen detection tests (rapid diagnostic tests or RDTs) using a “dipstick” or cassette format exist, but only one is approved for general diagnostic use in the United States. Rapid diagnostic tests can more rapidly determine that the patient is infected with malaria, but they cannot confirm the species or the parasitemia. Therefore, microscopy should also be done as soon as possible to confirm rapid diagnostic test results, species, and determine parasitemia. Laboratories that do not provide in-house on-the-spot microscopy services should maintain a stock of malaria rapid diagnostic tests so that they will be able to perform malaria diagnostic testing when urgently needed.
Parasite nucleic acid detection using polymerase chain reaction (PCR) is more sensitive and specific than microscopy but can be performed only in reference laboratories and so results are not often available quickly enough for routine diagnosis. However, PCR is a very useful tool for confirmation of species and detecting of drug resistance mutations. CDC offers malaria drug resistance testing for all malaria diagnosed in the United States free of charge. Serologic tests, also performed in reference laboratories, is not practical for routine diagnosis of acute malaria… Your state health department or the CDC can be contacted for more information on utilizing one of these tests.
Artemisinin derivatives
Artemisinin is an ancient Chinese herbal therapy for malarial fevers which has been recently found to have potent activity against many forms of malarial organisms, including chloroquine-resistant Plasmodium falciparum. Several artemisinin derivatives have been developed for clinical use in prevention and treatment of malaria.
The artemisinins including artesumate, arteeter, artemether, artemisinin, and dihydroartemisinin, are derivatives of the Chinese herb known as “qing hao” or sweet wormwood plant (Artemisia annua). The artemisinins have antimalarial activity in vitro and in vivo and are believed to act by release of free radicals into the parasite vacuoles. Artemisinin derivatives are currently the most active antimalarial drugs available and have been introduced around the world as an integral part of therapy of active malaria, always in combination with other antimalarials to prevent resistance such as amodiaquine, lumefantrine and mefloquine. Several oral and parenteral formulations of artemisinin derivatives are available worldwide. In the United States, the combination of artemether (20 mg) and lumefantrine (120 mg) was approved for therapy of Plasmodium falciparum malaria in 2009 under the brand name Coartem. The recommended dose for adults is 4 tablets twice daily for 3 days (6 doses). Artesunate (Adamsunate) is also available on a named-patient basis from the Centers for Disease Control and Prevention (CDC).
Artemisinin side effects
Common side effects of artesunate include nausea, vomiting, anorexia, and dizziness. Potentially severe adverse events include prolongation of the QTc interval and cardiac arrhythmias.
Amodiaquine
Amodiaquine is an aminoquinoline used for the therapy of malaria. Amodiaquine has been linked to severe cases of acute hepatitis which can be fatal, for which reason it is recommended for use only as treatment and not for prophylaxis against malaria.
Amodiaquine is a synthetic aminoquinoline that acts by binding to the protozoal or parasitic DNA and preventing DNA and RNA production and subsequent protein synthesis. It is active against the asexual erythrocytic forms of Plasmodium species. Amodiaquine is related in structure to chloroquine, and highly chloroquine-resistant Plasmodium falciparum is also resistant to amodiaquine. Amodiaquine remains a useful agent for treating falciparum malaria, but because of its potential for causing hepatotoxicity, it is no longer used for antimalarial prophylaxis. Amodiaquine is available in tablets of 150 to 600 mg in generic forms and under the brand names Camoquin and Flavoquine. The recommended dosage is 10 mg/kg of amodiaquine base once daily for 3 days usually in combination with other antimalarial agents.
Amodiaquine side effects
Common side effects of amodiaquine include nausea, diarrhea, skin rash and itching.
Atovaquone
Atovaquone is a naphthoquinone used for the prevention and treatment of Pneumocystis jevorici (formerly carinii) pneumonia and, in combination with proguanil, prevention and treatment of Plasmodium falciparum malaria.
Atovaquone is a synthetic naphthoquinone that acts by interfering with mitochondrial electron transport in susceptible organisms. Atovaquone is effective against chloroquine resistant Plasmodium falciparum, but is associated with a high rate of resistance, for which reason it is usually given in combination with other agents, most typically with proguanil. Atovaquone was approved for use in the United States in 1992 and the combination with proguanil in 2000. Current indications include treatment and prevention of Pneumocystis jiroveci (formerly carinii) pneumonia (atovaquone alone) and treatment and prevention of falciparum malaria (combined with proguanil). It is sometimes used off-label as a second line agent for Toxoplasma gondii. Atovaquone is available in tablets of 250 and 500 mg and as a suspension of 750 mg/5 mL under the brand name Mepron. A fixed combination of 250 mg of atovaquone and 100 mg of proguanil is available generically and under the brand name Malarone, which is used in a 3 day regimen (4 tablets daily) to treat drug resistant Plasmodium falciparum malaria and for the period of exposure (1 tablet daily) to prevent chloroquine resistant Plasmodium falciparium and vivax malaria. A pediatric formulation is also available. The recommended dosage of atovaquone varies by different indications. Specific recommendations on the use of atovaquone for management of opportunistic infections among persons with HIV infection are available at: https://aidsinfo.nih.gov/guidelines
Atovaquone side effects
Common side effects of atovaquone include headache, fever, anxiety, insomnia, vivid dreams, nausea, diarrhea, skin rash and itching.
Chloroquine
Chloroquine is an aminoquinoline used for the prevention and therapy of malaria. It is also effective in extraintestinal amebiasis and as an antiinflammatory agent for therapy of rheumatoid arthritis and lupus erythematosus.
Chloroquine was developed in the 1940’s as a substitute for quinine in the prophylaxis and treatment of malaria, which had been a major problem among Allied troops in the Pacific. Chloroquine is a synthetic aminoquinoline that acts by binding to the protozoal or parasitic DNA and preventing DNA and RNA production and subsequent protein synthesis; it is active against the asexual erythrocytic forms of Plasmodium and Entamoeba species. Chloroquine is related in structure to quinine but more potent against Plasmodium falciparum, ovale, malariae and vivax, and better tolerated than quinine. Chloroquine remains the first choice of antimalarial prophylaxis as well as treatment. Chloroquine is available in tablets of 250 and 500 mg in generic forms and under the brand name Aralen. The recommended dosage for suppressive prophylaxis is 500 mg once weekly starting 1 to 2 weeks before and continuing for at 4 to 6 weeks after travel to an endemic area. Chloroquine has been replaced by hydroxychloroquine as an antiinflammatory agent in rheumatic diseases, and these are unapproved, off-label uses.
Chloroquine side effects
Common side effects of chloroquine include headache, blurred vision, anorexia, nausea, diarrhea, skin rash and itching.
Mefloquine
Mefloquine is a quinoline derivative used for the prevention and therapy of Plasmodium falciparum malaria.
Mefloquine is a quinoline methanol similar to quinine and is active against the asexual stages of malaria. Its exact mechanism of activity is unknown. Mefloquine is effective as prophylaxis against malaria and is widely used in therapy against chloroquine-resistant Plasmodium falciparum infection. Unfortunately, mefloquine resistance is becoming an enlarging problem. Mefloquine was approved for use in the United States in 1989 and is available in tablets of 250 mg in several generic forms and under the brand name Lariam. The recommended dosage for suppressive prophylaxis is 250 mg once weekly for 1 week before to 4 weeks after travel to an endemic area.
Mefloquine side effects
Common side effects of mefloquine include headache, fatigue, insomnia, vivid dreams, anorexia, nausea, diarrhea, abdominal discomfort, dizziness, rash and pruritus. Rare side effects include hallucinations, disorientation and seizures.
Mepacrine
Mepacrine also known as quinacrine or atabrine, is an acridine derivative initially used in the therapy and prevention of malaria and later as an antiprotozoal and immunomodulatory agent.
Mepacrine is an acridine derivative that was developed in the 1920s and extensively used as an antimalarial agent by the U.S. military in the South Pacific during World War II. It was recognized as causing a yellowing of the skin that resembled jaundice, but had few serious side effects, particularly at the low doses used for prophylaxis against malaria. Mepacrine was subsequently replaced by chloroquine and other more effective and better tolerated agents for malaria. Mepacrine also has activity against Giardia lamblia parasites as well as antiinflammatory and immunomodulatory activity, but is not approved for use in these conditions in the United States. Mepacrine continues to be used off-label, however, in lupus erythematosus, particularly for the dermatologic manifestations. While not commercially available or approved for use in the United States, mepacrine can be obtained overseas, via the internet and in some compounding pharmacies. The typical dose is 100 mg daily, and it is often combined with hydroxychloroquine, an accepted and widely used agent for rheumatologic disorders.
Mepacrine side effects
Side effects of mepacrine are not uncommon, but are generally mild including fatigue, abdominal discomfort, cramps, nausea, diarrhea, headache and dizziness. In high doses, mepacrine causes yellowing of the skin that can be mistaken for jaundice. It has occasionally been used to induce factitious illness. Serious side effects include lichen planus, exfoliative dermatitis and aplastic anemia.
Primaquine
Primaquine is an aminoquinoline that has been used for the prevention and therapy of malaria for more than 50 years. Primaquine is a synthetic aminoquinoline that acts by binding to the protozoal or parasitic DNA and preventing DNA and RNA production and subsequent protein synthesis; it is active against several of the stages in the development of the plasmodia including liver schizonts, hypnozoites and gametocytes and has most activity against Plasmodium ovale and vivax. Primaquine was approved for use in the United States in 1952. The current indications are for treatment of vivax malaria in combination with other antimalarial agents. It is also used as a second line agent in the prophylaxis against vivax malaria. Primaquine has been proposed as a major agent to prevent relapse after therapy of acute attacks of vivax malaria with more schizontocidal agents such as chloroquine or artemesinin. Primaquine is available in generic forms as tablets of 26.3 mg containing 15 mg of primaquine base. The recommended dosage for prophylaxis in adults is 30 mg of the base once daily starting before travel and continuing for at least 7 days after return from an endemic area. The dosage for therapy and prevention of relapse is usually 15 mg daily for 14 days or 45 mg once weekly for 8 weeks.
Primaquine side effects
Common side effects of primaquine include headache, anorexia, nausea, diarrhea, abdominal pain, skin rash and itching.
Proguanil
Proguanil is a biguanide derivative which is active against several protozoal species and is used in combination with atovaquone and chloroquine for the prevention and therapy of malaria. Proguanil has not been evaluated extensively as a single agent, but the combinations of proguanil with atovaquone or chloroquine have been used to treat malaria.
Proguanil is metabolized by the liver to cycloguanil which has potent antimalarial activity. Cycloguanil appears to act by inhibition of plasmodial dihydrofolate reductase-thymidine synthetase and thereby interfering with folate metabolism and DNA synthesis in a manner somewhat similar to pyrimethamine. In contrast, atovaquone is a naphthoquinone that acts by binding to the protozoal or parasitic DNA and preventing DNA and RNA production and subsequent protein synthesis. The two agents are synergistic in combination and demonstrate no cross resistance. The combination of atovaquone and proguanil has been extensively evaluated and was approved in the United States in 2000 for the prevention and treatment of malaria, particularly for drug resistant P. falciparum infection. This combination is available in tablets that contain 250 mg of atovaquone and 100 mg of proguanil in several generic forms and under the brand name Malarone. The recommended therapeutic regimen is four tablets daily for 3 days and one tablet daily for prophylaxis. Combinations of proguanil with chloroquine have also been used in both treatment and prevention of malaria, but not in the United States.
Proguanil side effects
Common side effects of atovaquone/proguanil include diarrhea, anorexia, nausea, vomiting, abdominal discomfort, headache, dizziness, vivid dreams, insomnia and oral ulcers.
Quinine
Quinine is a natural cinchona alkaloid that has been used for centuries in the prevention and therapy of malaria. Quinine is also used for idiopathic muscle cramps.
Quinine is the major alkaloid contained in the powered bark of the South American cinchona tree. The powder of the cinchona tree bark was used by native Quechna Indians in Peru to treat fever and was introduced into Western medicine when Jesuit priests took samples to Europe. The major effect of the cinchona tree bark was against malaria. Quinine acts against the asexual erythrocytic forms of malaria, including Plasmodium vivax, malariae and falciparum and is gametosidal to P. vivax and malariae. The use of quinine for malaria has been largely replaced by chloroquine, which is more potent and better tolerated. However, quinine is still used intravenously in some instances of drug resistant P. falciparum. In addition, quinine has been used frequently for nocturnal leg cramps, although the data in support of its efficacy is controversial, and quinine by prescription is no longer approved for this use. Quinine is available in multiple generic forms in low doses as an over-the-counter medication and as tablets of 324 mg for therapy of malaria. The use and dosage for malaria treatment and prevention requires higher doses than are used for leg cramps.
Quinine side effects
Common side effects of quinine include headache, dizziness, blurred vision, gastrointestinal upset, thrombocytopenia and hypersensitivity reactions.
Sulfadoxine and Pyrimethamine
The combination of sulfadoxine and pyrimethamine is used in the treatment and prophylaxis of chloroquine resistant strains of malaria and the treatment of toxoplasmosis.
Pyrimethamine is a diamino-pyrimidine and anti-folate, similar in structure and activity to proguanil, which has potent inibitory activity against malaria parasites as well as Toxoplasma gondii. Pyrimethamine has profound antimalarial synergy with sulfonamides and has been widely used in combination with sulfadoxine (sul” fa dox’ een) as prophylaxis and treatment of chloroquine-resistant malaria. In recent years, this combination has been replaced by other approaches, partially because of the frequency of hypersensitivity reactions including hepatotoxicity. However, it is still used for treatment of malaria (particularly in Africa) and for prophylaxis against chloroquine-resistant malaria in patients with contraindications to other agents. This combination is available under the brand name of Fansidar in tablets that combine 25 mg of pyrimethamine with 500 mg of sulfadoxine. Pyrimethamine is also available separately as 25 mg tables in generic forms and under the commerical name Daraprim for use in therapy of toxoplasmosis in combination with a sulfonamide. Pyrimethamine should not be used alone, either for treatment of toxoplasmosis or malaria.
Sulfadoxine and Pyrimethamine side effects
More common
- fever
- increased sensitivity of skin to sunlight
- irritation or soreness of tongue
- skin rash
Less common
- black, tarry stools
- bleeding or crusting sores on lips
- blood in urine or stools
- chest pain
- chills
- cough or hoarseness
- loss of appetite
- lower back or side pain
- muscle cramps or pain
- nausea
- painful or difficult urination
- pinpoint red spots on skin
- redness, blistering, peeling, or loosening of skin
- sore mouth
- sore throat
- sores, ulcers, and/or white spots in mouth
- sores on lips
- swelling in upper abdominal area
- unusual bleeding or bruising
- unusual tiredness or weakness
- vomiting
- yellow eyes or skin
Rare
- abdominal or stomach pain
- changes in facial skin color
- constipation
- fast or irregular breathing
- tenderness, itching, or burning of skin
- puffiness or swelling of the eyelids or around the eyes
- shortness of breath, troubled breathing, tightness in chest, and/or wheezing
- swelling of front part of neck
Symptoms of overdose
- bleeding or bruising (severe)
- clumsiness or unsteadiness
- convulsions (seizures)
- fever and sore throat
- irritation or soreness of tongue
- loss of appetite
- unusual tiredness or weakness
- trembling
- vomiting (severe)
Tafenoquine
Tafenoquine is an aminoquinoline that is used in combination with other antimalarials for the prevention of relapse of Plasmodium vivax malaria and by itself as prophylaxis against all species of malaria.
Tafenoquine is a synthetic 8-aminoquinoline that acts by binding to the protozoal or parasitic DNA and preventing DNA and RNA production and subsequent protein synthesis; it is active against several of the stages in the development of the plasmodia including liver schizonts, hypnozoites and gametocytes and has most activity against Plasmodium vivax. Tafenoquine was shown to decrease the risk of relapse in patients with Plasmodium vivax malaria who were treated with chloroquine but was less effective than current regimens when used as monotherapy for P. vivax malaria. Tafenoquine has also been shown to be effective as prophylaxis against malaria in travelers to regions with a high risk of malaria transmission. Tafenoquine was approved for use in the United States in 2018 for use in combination with other antimalarials such as chloroquine in prevention of relapse of P. vivax malaria, also referred to as “radical cure”. For this indication, tafenoquine is available as tablets of 150 mg under the brand name Krintafel and the recommended regimen is a single oral dose of 300 mg (2 tablets) given on day 1 or 2 of standard therapy of P. vivax malaria. Later in 2018, tafenoquine was approved for use as prophylaxis against malaria in travelers to high risk areas. For malarial prophylaxis, tafenoquine is available as 100 mg tablets under the brand name Arakoda, and the recommended regimen is a loading dose of 200 mg daily for three days before travel and maintenance dose of 200 mg once weekly to continue until one week after return from travel.
Tafenoquine side effects
Common side effects of tafenoquine include headache, dizziness, anorexia, nausea, pruritus and a decrease in hemoglobin. Testing for G6PD is recommended before using tafenoquine as it can induce hemolysis and methemoglobinemia in patients with G6PD deficiency. Other potentially severe but rare side effects include hypersensitivity reactions and severe psychiatric effects.
General approach to malaria treatment
It is preferable that treatment for malaria should not be initiated until the diagnosis has been established by laboratory investigations. “Presumptive treatment” without the benefit of laboratory confirmation should be reserved for extreme circumstances (strong clinical suspicion or severe disease in a setting where prompt laboratory diagnosis is not available).
Once the diagnosis of malaria has been made, appropriate antimalarial treatment must be initiated immediately. Treatment should be guided by three main factors:
- The infecting Plasmodium species
- The clinical status of the patient
- The drug susceptibility of the infecting parasites as determined by the geographic area where the infection was acquired and the previous use of antimalarial medicines
The infecting Plasmodium species: Determination of the infecting Plasmodium species for treatment purposes is important for three main reasons. Firstly, Plasmodium falciparum and Plasmodium knowlesi infections can cause rapidly progressive severe illness or death while the other species, Plasmodium vivax, Plasmodium ovale, or Plasmodium malariae, are less likely to cause severe manifestations. Secondly, Plasmodium vivax and Plasmodium ovale infections also require treatment for the hypnozoite forms that remain dormant in the liver and can cause a relapsing infection. Finally, Plasmodium falciparum and Plasmodium vivax species have different drug resistance patterns in differing geographic regions. For Plasmodium falciparum and Plasmodium knowlesi infections, the urgent initiation of appropriate therapy is especially critical.
The clinical status of the patient: Patients diagnosed with malaria are generally categorized as having either uncomplicated or severe malaria. Patients diagnosed with uncomplicated malaria can be effectively treated with oral antimalarials. However, patients who have one or more of the following clinical criteria (impaired consciousness/coma, severe normocytic anemia [hemoglobin < 7], acute kidney injury, acute respiratory distress syndrome, hypotension, disseminated intravascular coagulation, spontaneous bleeding, acidosis, hemoglobinuria, jaundice, repeated generalized convulsions, and/or parasitemia of ≥ 5%) are considered to have manifestations of more severe disease and should be treated aggressively with parenteral antimalarial therapy.
The drug susceptibility of the infecting parasites: Finally, knowledge of the geographic area where the infection was acquired provides information on the likelihood of drug resistance of the infecting parasite and enables the treating clinician to choose an appropriate drug or drug combination and treatment course. In addition, if a malaria infection occurred despite use of a medicine for chemoprophylaxis, that medicine should not be a part of the treatment regimen. If the diagnosis of malaria is suspected and cannot be confirmed, or if the diagnosis of malaria is confirmed but species determination is not possible, antimalarial treatment effective against chloroquine-resistant Plasmodium falciparum must be initiated immediately.
Malaria is a nationally notifiable disease and all cases should be reported to your state health department, which are forwarded onto the CDC.
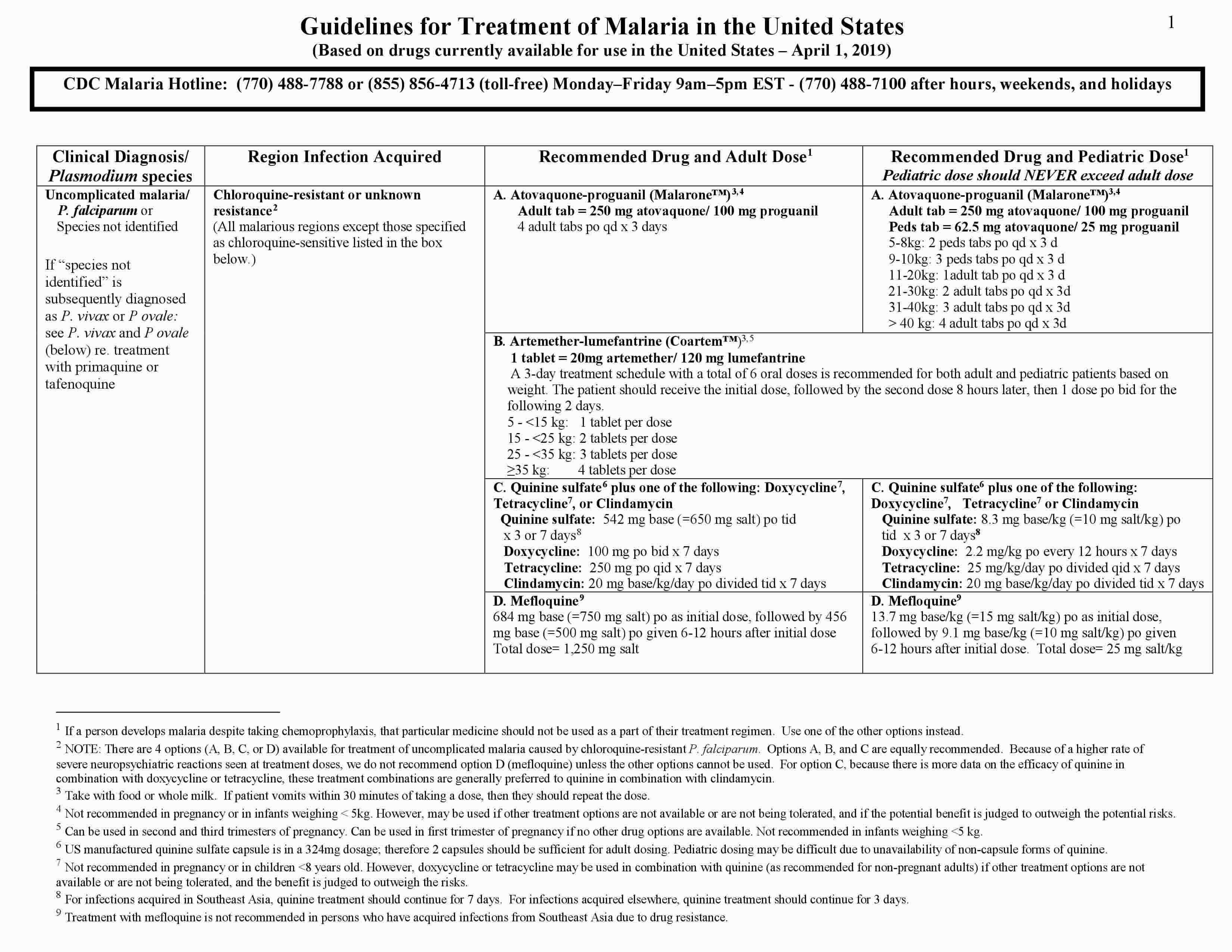
Figure 1. Antimalarial treatment guidelines
Treatment of Uncomplicated Malaria
Plasmodium falciparum or Species Not Identified – Acquired in Areas Without Chloroquine Resistance
For Plasmodium falciparum infections acquired in areas without chloroquine-resistant strains, which include Central America west of the Panama Canal, Haiti, and the Dominican Republic, patients can be treated with oral chloroquine. A chloroquine dose of 600 mg base (= 1,000 mg salt) should be given initially, followed by 300 mg base (= 500 mg salt) at 6, 24, and 48 hours after the initial dose for a total chloroquine dose of 1,500 mg base (=2,500 mg salt). Alternatively, hydroxychloroquine may be used at a dose of 620 mg base (=800 mg salt) po given initially, followed by 310 mg base (=400 mg salt) po at 6, 24, and 48 hours after the initial dose for a total hydroxychloroquine dose of 1,550 mg base (=2,000 mg salt).
In addition, any of the regimens listed below for the treatment of chloroquine-resistant malaria may be used for the treatment of chloroquine-sensitive malaria. Prompt initiation of an effective regimen is vitally important and so using any one of the effective regimens that is readily at hand would be the preferred strategy.
Plasmodium falciparum or Species Not Identified – Acquired in Areas With Chloroquine Resistance
For Plasmodium falciparum infections acquired in areas with chloroquine resistance, four treatment options are available. The first two treatment options are atovaquone-proguanil (Malarone) or artemether-lumefantrine (Coartem). These are fixed dose combination medicines that can be used for pediatric patients ≥5kg and for atoavaquone-proguanil, non-pregnant adults. Both of these options are very efficacious. Quinine sulfate plus doxycycline, tetracycline, or clindamycin is the next treatment option. For the quinine sulfate combination options, quinine sulfate plus either doxycycline or tetracycline is generally preferred to quinine sulfate plus clindamycin because there are more data on the efficacy of quinine plus doxycycline or tetracycline. Quinine treatment should continue for 7 days for infections acquired in Southeast Asia and for 3 days for infections acquired in Africa or South America. The fourth option, mefloquine, is associated with rare but potentially severe neuropsychiatric reactions when used at treatment doses. We recommend this fourth option only when the other options cannot be used.
For pediatric patients, the treatment options are the same as for adults except the drug dose is adjusted by patient weight. The pediatric dose should never exceed the recommended adult dose. Pediatric dosing may be difficult due to unavailability of non-capsule forms of quinine. If unable to provide pediatric doses of quinine, consider one of the other three options.
If using a quinine-based regimen for children less than eight years old, doxycycline and tetracycline are generally not indicated; therefore, quinine can be given in combination with clindamycin as recommended above. In rare instances, doxycycline or tetracycline can be used in combination with quinine in children less than 8 years old if other treatment options are not available or are not tolerated, and the benefit of adding doxycycline or tetracycline is judged to outweigh the risk.
If infections initially attributed to “species not identified” are subsequently diagnosed as being due to Plasmodium vivax or Plasmodium ovale, additional treatment with primaquine should be administered (see Plasmodium vivax and Plasmodium ovale, below).
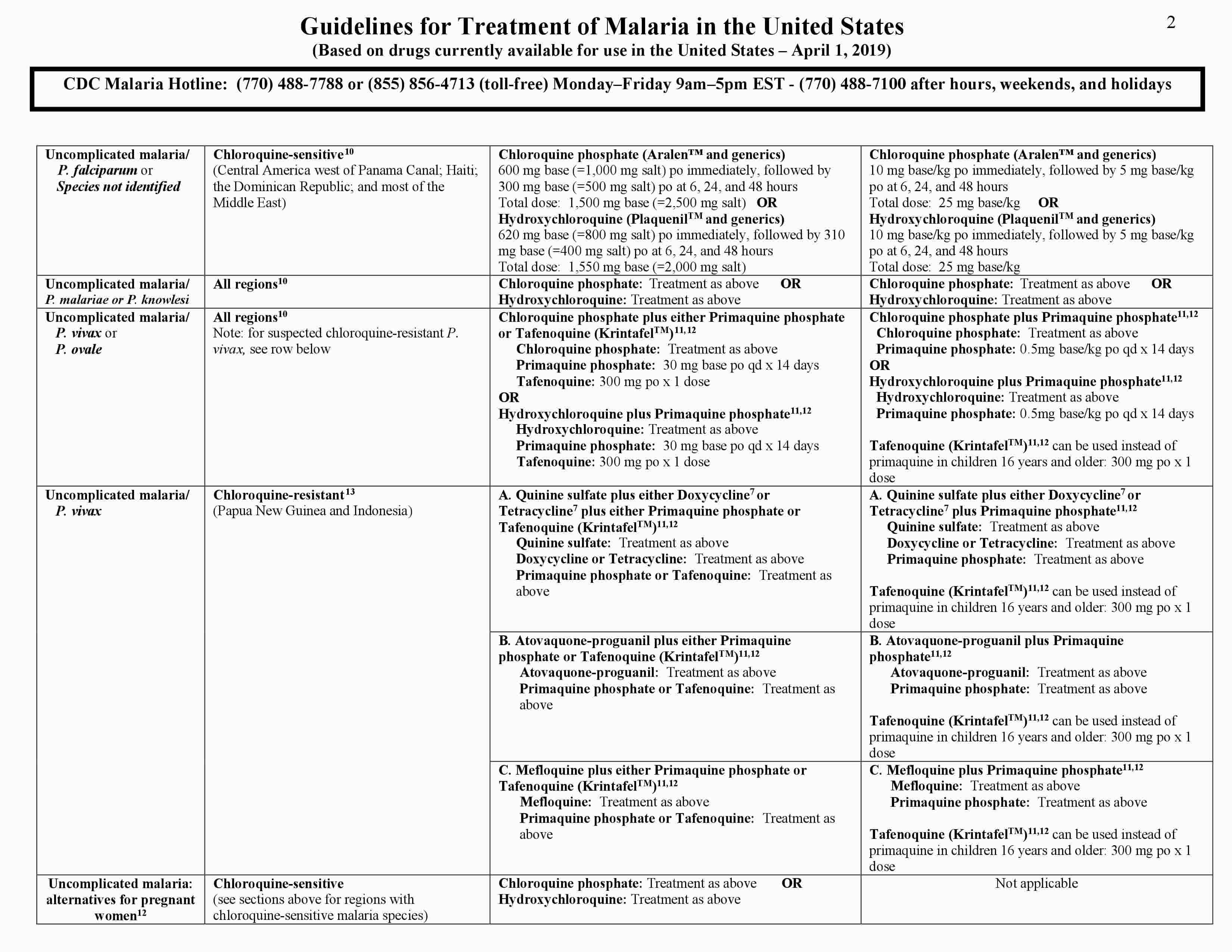
Plasmodium malariae and Plasmodium knowlesi
There has been no widespread evidence of chloroquine resistance in Plasmodium malariae and Plasmodium knowlesi species; therefore, chloroquine (or hydroxychloroquine) may still be used for both of these infections. In addition, any of the regimens listed above for the treatment of chloroquine-resistant malaria may be used for the treatment of Plasmodium malariae and Plasmodium knowlesi infections.
Plasmodium vivax and Plasmodium ovale
Chloroquine (or hydroxychloroquine) remains an effective choice for all Plasmodium vivax and Plasmodium ovale infections except for Plasmodium vivax infections acquired in Papua New Guinea or Indonesia. The regimens listed for the treatment of Plasmodium falciparum are also effective and may be used. Reports have confirmed a high prevalence of chloroquine-resistant Plasmodium vivax in these two specific areas. Rare cases of chloroquine-resistant Plasmodium vivax have also been documented in Burma (Myanmar), India, and Central and South America. Persons acquiring Plasmodium vivax infections from regions other than Papua New Guinea or Indonesia should initially be treated with chloroquine. If the patient does not respond to chloroquine, treatment should be changed to one of the two regimens recommended for chloroquine-resistant Plasmodium vivax infections, and your state health department and the CDC should be notified.
Persons acquiring Plasmodium vivax infections in Papua New Guinea or Indonesia should initially be treated with a regimen recommended for chloroquine-resistant Plasmodium vivax infections. The treatment regimens for chloroquine-resistant Plasmodium vivax infections are quinine sulfate plus doxycycline or tetracycline, or, atovaquone-proguanil, or artemether-lumefantrine, or mefloquine. These treatment options are equally recommended.
In addition to requiring blood stage treatment, infections with Plasmodium vivax and Plasmodium ovale can relapse due to hypnozoites that remain dormant in the liver. To eradicate the hypnozoites, patients should be treated with either tafenoquine (KrintafelTM) or primaquine phosphate. Tafenoquine can be used in those 16 years old and over, and is given as a single dose of 300 mg by mouth. If primaquoine phosphate is used, CDC recommends a dose of 30 mg (base) by mouth daily for 14 days. Because both tafenoquine and primaquine can cause hemolytic anemia in persons with glucose-6-phosphate-dehydrogenase (G6PD) deficiency, persons must be screened for G6PD deficiency prior to starting these drugs. For persons with borderline G6PD deficiency or as an alternate to the above regimen, primaquine may be given at the dose of 45 mg (base) orally one time per week for 8 weeks; consultation with an expert in infectious disease and/or tropical medicine is advised if this alternative regimen is considered in G6PD-deficient persons. Primaquine and tafenoquine must not be used during pregnancy. Tafenoquine must not be used in children less than 16 years old, or in those with a history of a psychotic disorder.
For pediatric patients greater than 8 years old, the treatment options, with the exception of tafenoquine, are the same as for adults except the drug dose is adjusted by patient weight. The pediatric dose should never exceed the adult recommended adult dose. For children less than 8 years old, doxycycline and tetracycline are generally not indicated therefore the other treatment options should be used.. For pediatric patients <5kg, mefloquine is the only option. If mefloquinemefloquine is not available or is not being tolerated and if the treatment benefits outweigh the risks, atovaquone-proguanil or artemether-lumefantrine should be used instead. Primaquine should be given to pediatric patients only after they have been screened for G6PD deficiency.
Alternatives for Pregnant Women
Malaria infection in pregnant women is associated with high risks of both maternal and perinatal morbidity and mortality. While the mechanism is poorly understood, pregnant women have a reduced immune response and therefore less effectively clear malaria infections. In addition, malaria parasites sequester and replicate in the placenta. Pregnant women are three times more likely to develop severe disease than non-pregnant women acquiring infections from the same area. Malaria infection during pregnancy can lead to miscarriage, premature delivery, low birth weight, congenital infection, and/or perinatal death.
For pregnant women diagnosed with uncomplicated malaria caused by Plasmodium malariae, Plasmodium vivax, Plasmodium ovale, or chloroquine-sensitive Plasmodium falciparum infection, prompt treatment with chloroquine (treatment schedule as with non-pregnant adult patients) is recommended. Alternatively, hydroxychloroquine, may be given instead. For women in their second or third trimesters, artemether-lumefantrine is an additional option. For pregnant women diagnosed with uncomplicated malaria caused by chloroquine-resistant Plasmodium falciparum infection, women in the second and third trimesters can be treated with artemether-lumfantrine, and for all trimesters, mefloquine or a combination of quinine sulfate and clindamycin is recommended. Quinine treatment should continue for 7 days for infections acquired in Southeast Asia and for 3 days for infections acquired elsewhere; clindamycin treatment should continue for 7 days regardless of where the infection was acquired. For pregnant women diagnosed with uncomplicated malaria caused by chloroquine-resistant Plasmodium vivax infection, prompt treatment with artemether-lumfantrine (second and third trimesters) or mefloquine (all trimesters) is recommended.
Doxycycline and tetracycline are generally not indicated for use in pregnant women. However, in rare instances, doxycycline or tetracycline can be used in combination with quinine if other treatment options are not available or are not being tolerated, and the benefit of adding doxycycline or tetracycline is judged to outweigh the risks.
According to its U.S. labels, atovaquone/proguanil is not indicated for use in pregnant women because there are no adequate, well-controlled studies in pregnant women. However, for pregnant women diagnosed with uncomplicated malaria caused by chloroquine-resistant Plasmodium falciparum infection, atovaquone-proguanil may be used if other treatment options are not available or are not being tolerated, and if the potential benefit is judged to outweigh the potential risks.
For Plasmodium vivax or Plasmodium ovale infections, primaquine phosphate and tafenoquine for radical treatment of hypnozoites should not be given during pregnancy. Pregnant patients with Plasmodium vivax or Plasmodium ovale infections should be maintained on chloroquine prophylaxis for the duration of their pregnancy. The chemoprophylactic dose of chloroquine phosphate is 300mg base (=500 mg salt) orally once per week. After delivery, for pregnant patients with Plasmodium vivax or Plasmodium ovale infections who do not have G6PD deficiency, subsequent treatment with primaquine phosphate or tafenoquine is needed, but will depend on breastfeeding. If not breastfeeding, either drug can be used. For women who are breastfeeding infants with normal G6PD activity, primaquine phosphate can be given. Tafenoquine is not recommended during breastfeeding. Pregnant women diagnosed with severe malaria should be treated aggressively with parenteral antimalarial therapy as described below.
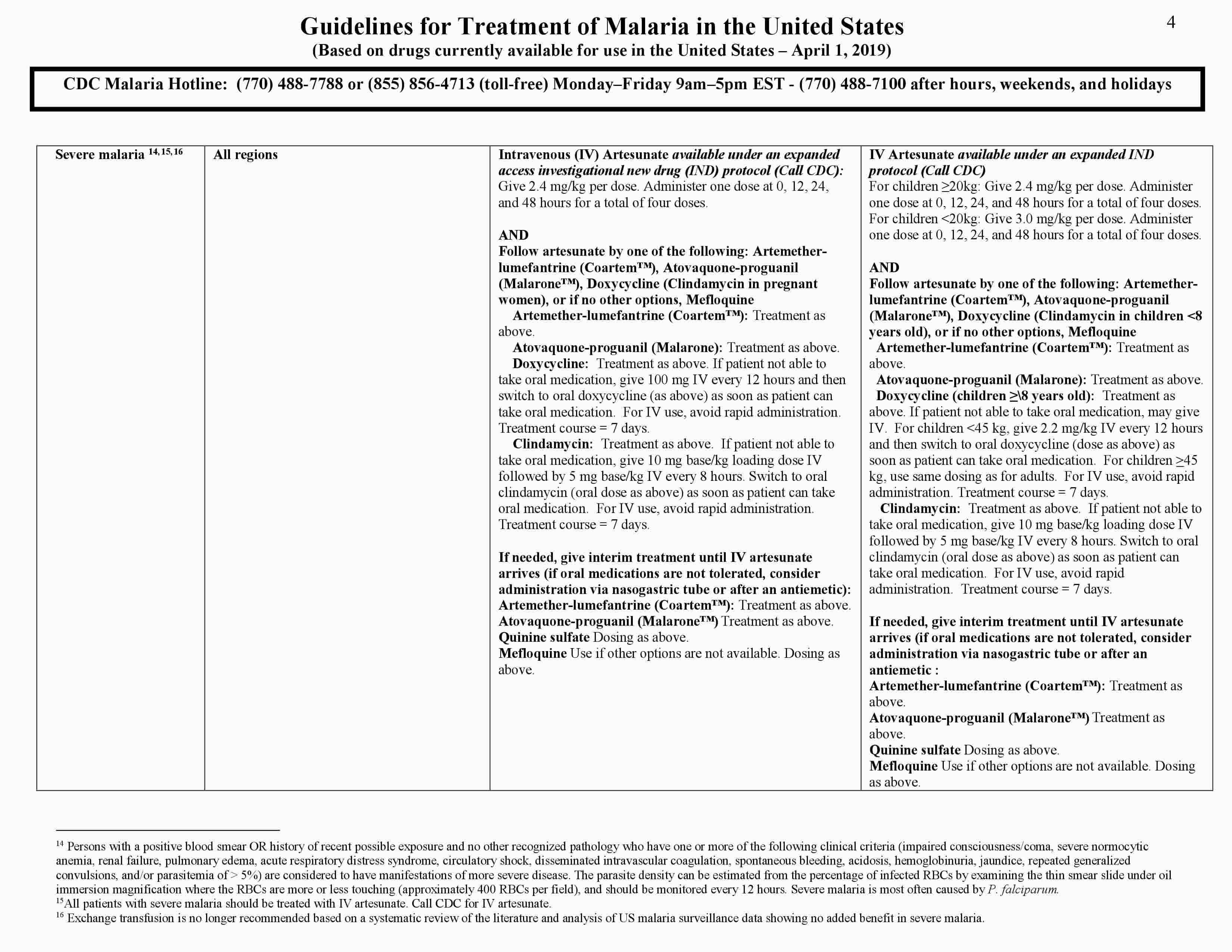
Treatment of Severe Malaria
Patients who are considered to have manifestations of more severe disease should be treated aggressively with parenteral antimalarial therapy regardless of the species of malaria seen on the blood smear. If severe malaria is strongly suspected but a laboratory diagnosis cannot be made at that time, blood should be collected for diagnostic testing as soon as it is available and parenteral antimalarial drugs should be started.
All patients with severe malaria, regardless of infecting species, should be treated with intravenous (IV) artesunate. Clinicians caring for patients with suspected severe malaria should call CDC to obtain IV artesunate.
Severe malaria can progress rapidly and must be treated as soon as possible. While timely delivery of IV artesunate is anticipated, health-care providers can consider treating the patient with an oral antimalarial while waiting for IV artesunate to arrive. Health-care providers will need to decide the most feasible route to administer the drug for patients unable to tolerate an oral antimalarial. For example, if this intolerance is due to nausea and vomiting, an anti-emetic preceding the antimalarial may help. For comatose patients, a nasogastric tube can be considered.
One of the antimalarials listed below can be administered. IV clindamycin and IV tetracyclines such as doxycycline are not recommended. These drugs are slow-acting antimalarials that would not take effect until well after 24 hours, and they are not effective antimalarials for treatment of severe malaria when used alone.
- Artemether/lumefantrine (Coartem®): 1 tablet=20 mg artemether and 120 mg lumefantrine. Give initial dose, then if still needed, follow with second dose 8 hours later.
- 5-14 kg: 1 tablet per dose
- 15-24 kg: 2 tablets per dose
- 25-34 kg: 3 tablets per dose
- ≥35 kg: 4 tablets per dose
- Atovaquone/proguanil (Malarone®): Adult (250 mg atovaquone/100 mg proguanil) and pediatric (62.5 mg atovaquone/25 mg proguanil) formulations are available.
- Adults: 4 adult tablets as one dose
- Children (≥5 kg only): Dosing based on weight
- 5–8 kg: 2 peds tabs
- 9–10 kg: 3 peds tabs
- 11–20 kg: 1 adult tab
- 21–30 kg: 2 adult tabs
- 31–40 kg: 3 adult tabs
- >40 kg: 4 adult tabs
- Quinine: Adults: 650 mg (salt) every 8 hours. Children: 10 mg (salt)/kg every 8 hours.
- Mefloquine (because of a risk of severe neuropsychiatric adverse events at treatment doses, mefloquine should only be used if atovaquone/proguanil or quinine is not available, and based on health-care provider judgement that treatment is needed prior to the arrival of IV artesunate): Adults: 750 mg salt, initially, then 500 mg salt 6–12 hours after initial dose. Children: 15 mg salt/kg, initially, then 10 mg salt/kg 6–12 hours after initial dose.
When IV artesunate arrives, discontinue the oral medication. The dosing of IV artesunate is as follows:
- Adults and children ≥20 kg: 2.4 mg/kg at 0 hour, 12 hours, and 24 hours; and 48 hours
- Children <20 kg: 3.0 mg/kg at 0 hour, 12 hours, and 24 hours; and 48 hours
After the course of IV artesunate is completed, a follow-on drug must be administered. Options include the following:
- Artemether/lumefantrine (Coartem®): 1 tablet=20 mg artemether and 120 mg lumefantrine. A 3-day treatment schedule with a total of 6 oral doses as follows: initial dose, second dose 8 hours later, then 1 dose twice a day for the following 2 days. Dosing as above.
- Atovaquone/proguanil (Malarone®): One dose daily for 3 days. Dosing as above.
- Doxycycline:
- Adults: 100 mg twice a day for 7 days.
- Children (8 years or older): 2 mg/kg twice a day for 7 days.
- Children under 8 years of age or pregnant women should instead receive clindamycin 20 mg base/kg/day divided three times a day for 7 days.
- Mefloquine (because of a risk of severe neuropsychiatric adverse events at treatment doses, mefloquine should only be used if other options are not available):
- Adults: 750 mg salt, initially, then 500 mg salt 6–12 hours after initial dose.
- Children: 15 mg salt/kg, initially, 10 mg salt/kg 6–12 hours after initial dose.
For those patients who still cannot tolerate oral medications after completing artesunate treatment, several treatment options are available, depending on the patient’s clinical and parasitological status. The most suitable course of treatment should be selected by the attending clinicians in consultation with CDC. Potential options include the following:
- Continue IV artesunate, 1 dose daily (see above for dosing) not to exceed a total course of 7 days.
- Switch to treatment with IV doxycycline (7 days) or IV clindamycin (7 days), dosing as above.
IV artesunate is safe in infants, children, and pregnant women in the second and third trimesters. There are limited clinical data on women taking IV artesunate in the first trimester of pregnancy; no harmful effects have been observed. Given that severe malaria is life threatening for pregnant women and their fetuses, and the lack of other treatment options for severe malaria in the United States, the benefits of treatment with IV artesunate outweigh the risks and IV artesunate should not be withheld. The only contraindication to IV artesunate is known allergy to IV artemisinins.
IV artesunate is well tolerated. While rare, delayed post-artemisinin hemolytic anemia has been noted in published case reports following treatment of severe malaria with IV artesunate in other non-endemic countries. Persons treated for severe malaria with IV artesunate should be monitored for up to 4 weeks after that treatment for evidence of hemolytic anemia. Persons with higher parasitemia seem to have a higher likelihood of delayed hemolytic anemia after treatment with IV artesunate. Depending on the amount of hemolysis, transfusion may be needed.
Previously, CDC recommended that exchange transfusion be considered for certain severely ill persons. However, exchange transfusion had not been proven beneficial in an adequately powered randomized controlled trial. In 2013 CDC conducted an analysis of cases of severe malaria treated with exchange transfusion and was unable to demonstrate a survival benefit of the procedure. CDC no longer recommends the use of exchange transfusion as an adjunct procedure for the treatment of severe malaria.
References