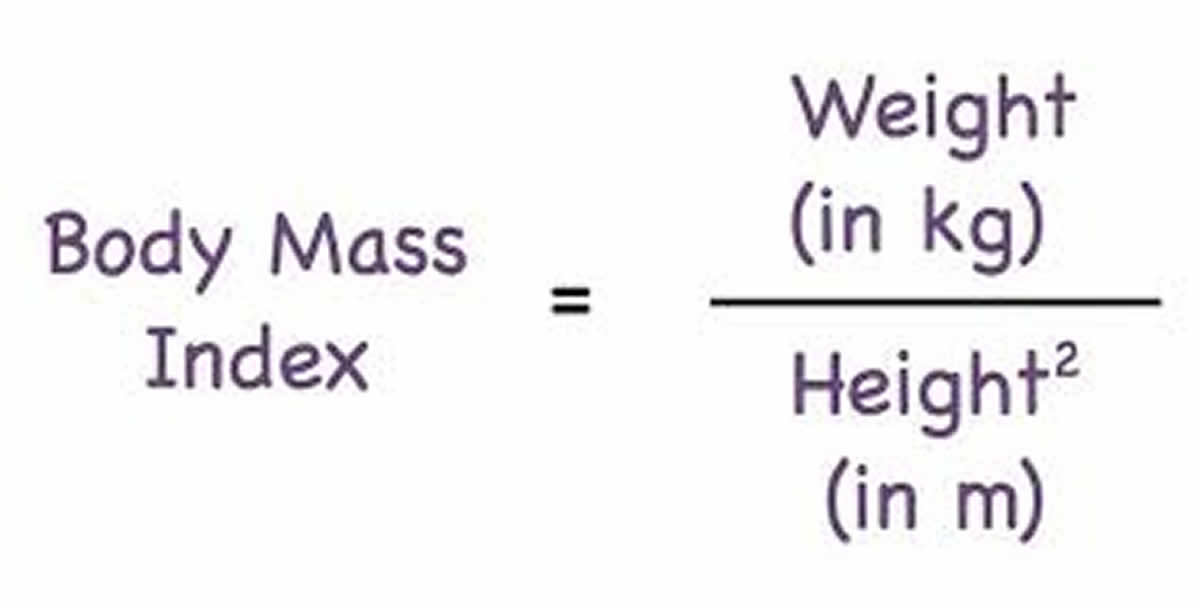Appetite suppressant
Traditional methods for weight loss include reducing calorie intake, increasing physical activity, and behavior therapy. Other weight-loss options include appetite suppressants approved for use in the United States as adjuncts in the treatment of obesity, the amphetamine congeners benzphetamine, desoxyephedrine (methamphetamine), phendimetrazine, diethylpropion, and phentermine, which were approved more than 50 years ago 1. These agents demonstrate a modest weight loss benefit when combined with dietary modifications and exercise. Few patients were exposed to these drugs for more than 12 weeks in subsequent efficacy assessments, and concerns existed about the potential for abuse and addiction. Consequently, the indication for weight loss was limited to short-term use only, specified in the labels as “a few weeks,” which is often interpreted as up to 12 weeks. Sibutramine (a serotonin and norepinephrine reuptake inhibitor) is an appetite suppressant that was withdrawn from the U.S. market in October 2010 for safety reasons. Sibutramine is known to increase blood pressure and/or pulse rate in some patients and may present a risk for patients with a history of coronary artery disease, congestive heart failure, arrhythmias, or stroke. Sibutramine may also interact, in life-threatening ways with other medications a consumer may be taking. Following the deaths of 2 young women taking sibutramine in Italy in 2002, that country temporarily suspended the drug’s marketing license 2. Moreover, numerous once-promising weight-loss drugs have been abandoned because of serious toxic effects 3: aminorex (which caused pulmonary hypertension), fenfluramine and dexfenfluramine (valvulopathy – association with valvular heart disorders), phenylpropanolamine (stroke), rimonabant (suicidal ideation and behavior), and most recently sibutramine (myocardial infarction and stroke).
Mild amphetamine analogs such as diethylpropion and phentermine are among the most commonly used appetite suppressants 4. Bupropion, an analog of diethylpropion, also produces weight loss 5. The mechanisms by which these pro-drugs produce their anorexic effect are complex, because they modulate the concentrations of serotonin, norepinephrine, and dopamine 6 and evoke responses in various cortical and subcortical areas involved in feeding 7.
In humans the above-named amphetamine congeners produce the sensation of fullness (serotonin) and increase agitation, insomnia, and energy expenditure (norepinephrine), as well as affecting motivation and reward pathways (dopamine) 8.
In this national study 9 estimating the prescription antiobesity drug use in the United States for the years 1991–2011 showed that prescription weight-loss drugs were predominantly used by women, users were mostly between 17 and 44 years old, and only a small proportion of users were not overweight or obese. Approximately 1 in 10 patients prescribed a weight-loss drug had a BMI of 26.9 kg/m2 or less. The duration of prescription antiobesity drug use was generally short, and most patients had only one episode of use during our 10-year study period. In about half of the patients, the longest treatment episode was 30 days or shorter, and only a quarter of the patients used antiobesity drugs for longer than 90 days.
To help obese and overweight Americans who have been unsuccessful in getting their weight under control with diet and exercise, the Food and Drug Administration (FDA) has recently in July 2012 approved two new medications—the first drugs for long-term weight management that FDA has approved in 13 years. Marketed as Belviq and Qsymia, these prescription medications would be taken for the rest of a person’s life 10. Belviq (lorcaserin) is a selective agonist of the serotonin (5-hydroxytryptamine or 5-HT) 2C (5-HT2C) receptor 11. Qsymia (phentermine plus extended-release topiramate, Vivus) is a fixed-dose combination of the sympathomimetic amine phentermine, which is an anorectic agent, and the antiepileptic drug topiramate 11. Both medications reduce appetite and, in some people, induce a negative energy balance.
You may be a candidate for taking Belviq or Qsymia if you are at least 18 and:
- your body mass index (BMI) is 30 or greater (obese); or
- your BMI is 27 or greater (overweight) and you have at least one other weight-related condition.
Women who are pregnant or thinking of becoming pregnant should not take either of these medications, Egan says, because weight loss offers no potential benefit to a pregnant woman and can cause fetal harm. Qsymia carries a risk for birth defects (cleft lip with or without cleft palate) in infants exposed during the first trimester of pregnancy.
Research suggests that safe weight loss involves combining a reduced-calorie diet with physical activity to lose 1/2 to 2 pounds a week (after the first few weeks of weight loss). Make healthy food choices. Eat small portions. Build exercise into your daily life. Combined, these habits may be a healthy way to lose weight and keep it off. These habits may also lower your chances of developing heart disease, high blood pressure, and type 2 diabetes.
To lose weight, reduce the number of calories you take in and increase the amount of physical activity you do each day. Create and follow a healthy eating plan that replaces less healthy options with a mix of fruits, veggies, whole grains, protein foods, and low-fat dairy:
- Eat a mix of fat-free or low-fat milk and milk products, fruits, veggies, and whole grains.
- Limit added sugars, cholesterol, salt (sodium), and saturated fat.
- Eat low-fat protein: beans, eggs, fish, lean meats, nuts, and poultry.
Health care providers use your Body Mass Index (BMI), to measure of your weight in relation to your height, to define overweight and obesity and is universally expressed in units of kg/m2, resulting from mass in kilograms and height in meters. People who have a BMI between 25 and 30 kg/m2 are considered overweight. Obesity is defined as having a BMI of 30 kg/m2 or greater. BMI is considered an important measure for understanding population trends. For individuals, it is one of many factors that should be considered in evaluating healthy weight, along with waist size, body fat composition, waist circumference, blood pressure, cholesterol level and blood sugar.
To calculate your body mass index, you divide your body weight in kilograms by your height in meter squared (commonly expressed as kg/m2), see the body mass index formula below.
To find out about your body mass index (BMI), you can use a FREE online BMI calculators from the Centers for Disease Control and Prevention (CDC) :
- Adults (BMI Calculator Adults. https://www.cdc.gov/healthyweight/assessing/bmi/adult_BMI/english_bmi_calculator/bmi_calculator.html)
For children and adolescents (younger than 20 years of age), overweight and obesity are based on the Centers for Disease Control and Prevention’s (CDC’s) BMI-for-age growth charts, which are available at Centers for Disease Control and Prevention (CDC Clinical Growth Charts https://www.cdc.gov/growthcharts/clinical_charts.htm).
The CDC has a BMI percentile calculator for children and teens at 12.
- Children (BMI Calculator Children. https://www.cdc.gov/healthyweight/bmi/calculator.html)
Body Mass Index for Men and Women Adults
The body mass index is an attempt to quantify the amount of tissue mass (muscle, fat, and bone) in an individual, and then categorize that person as underweight, normal weight, overweight, or obese based on that value. Commonly accepted body mass index ranges are:
A) Underweight: under 18.5 kg/m2,
B) Normal weight: 18.5 to 25 kg/m2,
C) Overweight: 25 to 30 kg/m2,
D) Obese: over 30 to 39.9 kg/m2.
E) Severely Obese: over 40 kg/m2.
FDA approved appetite suppressant
Weight-loss medicines, weight loss pills or prescription appetite suppressants approved by the Food and Drug Administration (FDA) might be an option for some people.
If you’re not successful at losing 1 pound a week after 6 months of using lifestyle changes, medicines may help. You should only use medicines as part of a program that includes diet, physical activity, and behavioral changes.
Prescription medications to treat overweight and obesity work in different ways. For example, some medications may help you feel less hungry or full sooner. Other medications may make it harder for your body to absorb fat from the foods you eat e.g. Orlistat – blocks your body from absorbing about a third of the fat you eat.
Weight-loss medicines might be suitable for adults who are obese (a BMI of 30 or greater). People who have BMIs of 27 or greater, and who are at risk for heart disease and other health conditions like type 2 diabetes or high blood pressure, also may benefit from weight-loss medicines. You’ll still need to focus on diet and exercise while taking these drugs, and they’re not for everyone.
FDA approved appetite suppressants:
- Amphetamine congeners:
- Benzphetamine,
- Desoxyephedrine (Methamphetamine),
- Diethylpropion,
- Phendimetrazine, and
- Phentermine
- Lorcaserin (Belviq)
- Phentermine-topiramate combination (Qsymia)
- Naltrexone-bupropion combination (Contrave)
- Liraglutide (Saxenda)
Only orlistat, lorcaserin (Belviq) and phentermine/topiramate (Qsymia) are FDA-approved for long-term use; the others are approved only for short-term use (usually considered ≤12 weeks).
Table 1. Drugs with an FDA-approved indication for obesity
| Generic Name | Trade Name(s) | Mechanism of action | Dosage | Whole-sale price per mo.* | Mean weight change relative to placebo at 1y, kg** | Interactions | Contraindications& | Common Adverse Events& | Cautions and Warnings& |
|---|---|---|---|---|---|---|---|---|---|
| Phenterminea | Adipex-P, Fastin, Oby-Cap, lonamin, Others | Noradrenergic causing appetite suppression | 15– 37.5mg/d | $6– $45 | Not Available | Guanethidine, CNS stimulants, alcohol, tricyclic antidepressants; requirements for insulin or oral hypoglycemic medications may be altered | Pregnancy or nursing, advanced cardiovascular disease, uncontrolled hypertension, hyperthyroidism, glaucoma, agitated states, history of drug abuse, MAOIs | Insomnia, elevation in heart rate, dry mouth, taste alterations, dizziness, tremors, headache, diarrhea, constipation, vomiting, gastro- intestinal distress, anxiety, and restlessness. | Do not increase beyond recommended dose if tolerance to the anorexiant effect develops. Caution prescribing to patients with even mild hypertension. Caution for patients using alcohol or other CNS active drugs or engaging in hazardous activity. |
| Diethylpropiona | Tenuate, Tenuate Dospan, Tepanil | Noradrenergic causing appetite suppression | 25mg 3 times/d or 75mg sustained- release/d | $47– $120 | Not Available | Same as phentermine | Same as phentermine | Same as phentermine | Same as phentermine |
| Phendimetrazineb | Bontril | Noradrenergic | 17.5–70mg 2–3 times/d or 105mg sustained- release/d | $6– $20 | Not Available | Same as phentermine | Same as phentermine | Same as phentermine | Same as phentermine |
| Benzphetamineb | Didrex | Noradrenergic causing appetite suppression | 25–50mg 1–3 times/d | $20– $50 | Not Available | Same as phentermine | Same as phentermine | Same as phentermine | Same as phentermine |
| Orlistatc | Xenical, Alli | Lipase inhibitor causing excretion of ~30% of ingested triglycerides in stool | 60 or 120mg 3 times/d within 1 hr of a fat- containing meals, plus a daily multi- vitamin | For 60mg TID: $45 For 120mg TID: $207 | For 60mg TID:−2.5 kg (−1.5 to −3.5) For 120mg TID: −3.4 kg (−3.2 to −3.6) | Decreased drug concentrations of cyclosporine and levothyroxine. Doses should be temporally separated from orlistat. Fat soluble vitamin absorption is decreased by orlistat | Pregnancy, chronic malabsorption syndromes, cholestasis | Oily Spotting, Flatus with Discharge, Fecal Urgency, Fatty/Oily Stool, Increased Defecation, Fecal Incontinence | Use with caution in those at risk for renal insufficiency, since treatment may increase urinary oxalate. Cholelithiasis and, rarely, severe liver injury including hepatocellular necrosis and acute hepatic failure leading to death, have been reported |
| Lorcaserina | Belviq | Highly selective serotonergic 5-HT2C receptor agonist causing appetite suppression | 10mg two times/d | $240 | −3.2 kg (−2.7 to −3.8) | Triptans, MAOIs including linezolid, SSRIs, SNRIs, dextro- methorphan, tricyclic antidepressants, bupropion, lithium, tramadol, tryptophan, and St. John’s Wort | Pregnancy | Headache, dizziness, fatigue, nausea, dry mouth, cough, and constipation, and back pain, cough and hypoglycemia in patients with type 2 diabetes. | Risk for Serotonin Syndrome or Neuroleptic Malignant Syndrome-like Reactions. Evaluate patients for signs or symptoms of valvular heart disease. Euphoria, hallucination, and dissociation have been seen with supra- therapeutic doses. Use with caution in men at risk for priapism |
| Phentermine / Topiramate-ERa | Qsymia | Noradrenergic + GABA- receptor activator, kainite/AMPA glutamate receptor inhibitor causing appetite suppression | 3.75/23mg /d for 2 weeks, then 7.5/46mg/ d, escalating to a maximum of 15/92mg/d | $140 – $195 | For 7.5/46mgd: −6.7 kg (−5.9 to −7.5) For 15/92mg/d −8.9 kg (−8.3 to −9.4) | Oral contraceptives, alcohol and other CNS depressants, non-potassium- sparing diuretics | Pregnancy, Glaucoma, Hyperthyroidism, MAOIs |
Abbreviations: MAOI = monoamine oxidase inhibitor, CNS = central nervous system, SSRI = selective serotonin-reuptake inhibitors, SNRI = selective serotonin-norepinephrine reuptake inhibitors.
*Reference prices found on March 8, 2013.
**Weight change relative to placebo (95 percentile confidence interval) using intent-to-treat analyses for each medication at 1 year. No studies for older noradrenergic agents (phentermine, diethylpropion, phendimetrazine, and benzphetamine) met inclusion criteria for length of treatment, sample size, and attrition.
a Medications listed on Drug Enforcement Administration Schedule IV are associated with a lower risk of abuse than
b Medications on Schedule III;
c Orlistat is a non-Drug Enforcement Administration scheduled drug.
& Common adverse events for noradrenergic agents include those listed as common in the NIDDK Weight-control Information Network Fact Sheet “Prescription Medications for the Treatment of Obesity”100 as adverse event frequency is not available in the drug package inserts for these agents. For orlistat, lorcaserin, and phentermine/topiramate ER, common adverse events are those listed in the drug package inserts that are reported to occur more frequently than placebo and with more than 5% prevalence.
Belviq—the trade name for the drug lorcaserin— is a 10 mg tablet taken twice a day that works by activating a part of the brain that controls hunger.
Belviq was tested in three clinical trials that lasted from 52 to 104 weeks and included nearly 8,000 obese and overweight patients.
- The average weight loss for patients taking Belviq ranged from 3 to 3.7 percent over those taking a placebo.
- In studies of patients without type 2 diabetes, about 47 percent of patients lost at least 5 percent of their weight compared with 23 percent of patients treated with placebo.
Belviq should be discontinued if you fail to lose 5 percent of your weight after 12 weeks of treatment, as it is unlikely that continued treatment will be successful.
Qsymia is a combination of two FDA-approved drugs: phentermine, an appetite suppressant, and topiramate, used to treat epilepsy and migraines. Qsymia is taken once a day, with patients starting at the lowest dose (3.75 mg phentermine/23 mg topiramate extended-release), then increasing to the recommended dose (7.5 mg/46 mg). In some circumstances, patients may have their dose increased to the highest dose (15 mg/92 mg).
Qsymia, was tested in two clinical trials which included nearly 3,700 obese and overweight patients treated for up to one year.
- The average weight loss of patients taking Qsymia ranged from 6.7 percent (lowest dose) to 8.9 percent (recommended dose) over those taking a placebo.
- Sixty-two percent of patients on the lowest dose and 70 percent on the recommended dose lost at least 5 percent of their weight compared with 20 percent treated with placebo.
If after 12 weeks, you have not lost 3 percent of your weight on the recommended dose of Qsymia, FDA recommends that treatment be discontinued or increased to the highest dose. If after an additional 12 weeks on the highest dose, you do not lose at least 5 percent of weight, Qsymia should be discontinued gradually.
Who might benefit from weight-loss medications?
Weight-loss medications are meant to help people who may have health problems related to overweight or obesity. Before prescribing a weight-loss medication, your doctor also will consider:
- the likely benefits of weight loss
- the medication’s possible side effects
- your current health issues and other medications
- your family’s medical history
- cost
Your doctor may prescribe a medication to treat your overweight or obesity if you are an adult with
- a BMI of 30 or more or
- a BMI of 27 or more and you have weight-related health problems, such as high blood pressure or type 2 diabetes.
Weight-loss medications aren’t for everyone with a high BMI. Some people who are overweight or obese may lose weight with a lifestyle program that helps them change their behaviors and improve their eating and physical activity habits. A lifestyle program may also address other factors that affect weight gain, such as eating triggers and not getting enough sleep.
Orlistat (Xenical)
How it works: Blocks your body from absorbing about a third of the fat you eat. Orlistat is a gastrointestinal lipase inhibitor which, when taken three times a day during or up to 1 hour after meals, leads to the excretion of approximately 30% of ingested fat.
Orlistat is available both in prescription (120mg) and over-the-counter (60mg) strength. Orlistat 120mg is FDA-approved for use in adults and adolescents age 12–16years.
When a doctor prescribes orlistat, it’s called Xenical (Orlistat 120mg). If you get it without a prescription, it’s called Alli, which has half of Xenical’s dose. Alli is the reduced-strength, 60-milligram version of orlistat (Xenical) a 120-milligram prescription drug.
The mean weight reduction attributable to orlistat 120mg three times daily at 12 months is modest: among adults participating in behavioral weight control programs and prescribed a lower fat diet (~30% of calories from fat), orlistat-treated patients lost on average 3.4 kg (~3.1% of initial weight) more than placebo-treated participants. The percentage of orlistat 120mg-treated participants who achieved clinically-meaningful (≥5%) weight loss at 1 year varied from 35–73% and the proportion losing ≥10% varied from 14–41%, with both ≥5% and ≥10% weight loss at 1 year significantly greater for orlistat-treated than for placebo-treated participants. At the end of a second year of treatment when a weight-maintenance diet was prescribed, orlistat 120mg-treated participants had lost approximately 3.3 kg (~3.3% of initial weight) more and orlistat 60mg-treated participants had lost approximately 2.5 kg (~2.5% of initial weight) more than those given placebo.
Because of its weight-loss related and weight-loss independent 14 actions, orlistat 120mg treatment is associated with significant improvements in cardiovascular risk factors including decreases in total- and LDL- cholesterol, fasting glucose, and systolic and diastolic blood pressures after 1 year of treatment 15.
Data from the XENDOS trial 16 of 3,305 patients treated for up to 4 years (attrition at 4 years: 48% for orlistat-treated and 66% for placebo-treated) found, in an intention-to-treat approach, that orlistat use decreased body weight over 4 years by 2.7 kg (approximately 2.4% of initial body weight) more than placebo and significantly decreased risk for developing type 2 diabetes from 9.0% with placebo to 6.2% with orlistat. Because orlistat leads to obligate increases in undigested stool triglycerides, it may cause considerable gastrointestinal adverse effects that may be decreased by co-administration of fiber-containing supplements 17.
Side effects include abdominal cramping, passing gas, leaking oily stool, having more bowel movements, and not being able to control bowel movements.
These side effects are generally mild and temporary. But they may get worse if you eat high-fat foods.
These adverse effects may cause patients who do not reduce their fat intake to discontinue therapy. Indeed, despite being FDA-approved in 1999 for indefinite treatment of obesity, among those prescribed orlistat 120mg clinically, fewer than 10% take it for at least 1y and <2% of patients use the medication for 2 years 4.
Rare cases of severe liver injury have been reported in people taking orlistat, but it’s not certain that the drug caused those problems.
What else you should know: You should be on a low-fat diet (less than 30% of your daily calories from fat) before taking orlistat.
Also, take a multivitamin at least 2 hours before or after taking orlistat, because the drug temporarily makes it harder for your body to absorb vitamins A, vitamin D, vitamin E, and vitamin K.
Orlistat is the only drug of its kind that’s approved in the U.S. All other prescription weight loss drugs curb your appetite, including the following.
Belviq (Lorcaserin Hydrochloride)
How it works: Curbs your appetite. Lorcaserin is a selective serotonin 2C (5HT2c) receptor agonist that was anticipated to recapitulate the weight loss effects of fenfluramine without its adverse cardiac effects 18. Lorcaserin 10mg twice daily was FDA-approved in 2012 on the basis of two large randomized, placebo-controlled trials in nondiabetic patients (BLOOM n=3182, 50% attrition) 19; BLOSSOM n=4004, 45% attrition) 20, along with a third, smaller trial in adults with type 2 diabetes (BLOOM-DM n=603, 34% attrition) 21. In these trials, participants received low-intensity nutritional and exercise counseling. Lorcaserin decreased body weight modestly, by about 3.2 kg (~3.2% of initial body weight) more than placebo 22. However, significantly more patients treated with lorcaserin 10mg twice daily than placebo lost ≥5% (BLOOM: 47 vs. 20%, BLOSSOM: 47 vs. 25%, BLOOM-DM: 37 vs. 16%) or ≥10% (BLOOM: 23 vs. 8%, BLOSSOM: 23 vs. 10%, BLOOM-DM: 16 vs. 4%) of their initial weight. Reduction in body weight below baseline in the one study 19 with data from participants who took lorcaserin for 2 years had average weight loss of 5.6 kg, versus 2.4 kg among placebo-treated participants. Blood pressure, total cholesterol, LDL”bad” cholesterol, and triglycerides also decreased significantly more in lorcaserin-treated participants 23. Among patients with diabetes, lorcaserin treatment led to lower body weight and improved glycated hemoglobin concentrations.30 Adverse effects (Table 1) include headache, nausea, fatigue, and dizziness 23. Although neither incidence of valvulopathy nor hypertension was statistically greater during lorcaserin than placebo treatment, both were numerically somewhat more prevalent and the FDA has requested that a post-approval trial to assess the long-term cardiovascular effects of lorcaserin be conducted 24.
Side effects: The most common side effects in people who don’t have diabetes are headache, dizziness, nausea, fatigue, dry mouth, and constipation.
The most common side effects in those who have diabetes are low blood sugar (hypoglycemia), headache, back pain, cough, and fatigue.
People taking some depression medications with Belviq need to be monitored very closely for a rare but serious reaction that includes fever and confusion.
Women who are pregnant or planning to get pregnant shouldn’t take Belviq.
What else you should know: If you don’t lose 5% of your weight after 12 weeks of taking Belviq, you should stop taking it, because it’s unlikely to work for you, the FDA says.
Contrave (naltrexone and bupropion)
How it works: Contrave is a combination of two FDA-approved drugs, naltrexone and bupropion, in an extended-release formula. Naltrexone is approved to treat alcohol and opioid dependence. Bupropion is approved to treat depression, seasonal affective disorder, and help people stop smoking.
Side effects: The most common side effects include nausea, constipation, headache, vomiting, dizziness, insomnia, dry mouth, and diarrhea. Contrave has a boxed warning about the increased risk of suicidal thoughts and behaviors associated with bupropion. The warning also notes that serious neuropsychiatric issues linked to bupropion have been reported. Contrave can cause seizures and must not be used in patients who have seizure disorders. The drug can also increase blood pressure and heart rate.
What else you should know: If you don’t lose 5% of your weight after 12 weeks of taking Contrave, you should stop taking it, because it’s unlikely to work for you, the FDA says.
Saxenda
How it works: Saxenda is a higher dose of the type 2 diabetes drug Victoza. It mimics an intestinal hormone that tells the brain your stomach is full.
Side effects: Nausea, vomiting, diarrhea, constipation, low blood pressure, and increased appetite. Serious side effects can include raised heart rate, pancreatitis, gallbladder disease, kidney problems, and suicidal thoughts.
What else you should know: If you don’t lose 4% of your weight after 16 weeks of taking Saxenda, you should stop taking it, because it’s unlikely to work for you, the FDA says.
Qsymia
How it works: Curbs your appetite. Qsymia combines phentermine with the seizure/migraine drug topiramate. Topiramate causes weight loss in several ways, including helping you feel full, making foods taste less appealing, and burning more calories.
Phentermine/topiramate-Extended Release (ER) is the first FDA-approved combination drug for obesity, combining low-dose phentermine with a non-standard dose of the antiepileptic medication topiramate-Extended Release (ER).
Phentermine/topiramate-ER, is administered as a once-daily capsule in 4 fixed-dose combinations: 3.75mg phentermine/23mg topiramate (starting dose); 7.5mg phentermine/46mg topiramate (recommended dose); 11.25mg phentermine/69mg topiramate (titration dose); and 15mg phentermine/92mg topiramate (top dose). Dosage is increased over 14 days to 7.5mg phentermine/46mg topiramate, with additional titration to the top dose if weight loss is inadequate 25.
Phentermine/topiramate-ER was recommended for approval based largely on 2 one-year Phase 3 clinical trials (EQUIP, n=1267) 26; CONQUER, n=2487) 27. All groups received a low-intensity lifestyle program. All underwent dose titration over 4 weeks to assigned dose followed by 52 weeks on drug or placebo. EQUIP 26; CONQUER, n=2487) 28 randomized a higher-risk sample of adults with BMI 27–45 kg/m² and ≥2 obesity-associated comorbid conditions, to placebo or phentermine/topiramate-ER. 31% of participants withdrew. One year weight loss was 8.1 kg (7.8%) with the recommended dose and 10.2 kg (9.8%) with the top dose, vs. 1.4 kg (1.2%) with placebo. In addition, 62% (recommended dose) and 70% (top dose) lost ≥5% of initial weight vs. 21% for placebo, with 37%, 48%, and 7% respectively losing ≥10% of initial weight. Many cardiovascular disease risk factors improved with active drug treatment at recommended- or top-dose 29. SEQUEL 30 an extension to CONQUER, followed 78% of CONQUER participants at sites selected for high enrollment and retention and who had completed the initial 56-week trial for a total of 108 weeks. 84% completed their second year of treatment with sustained weight loss of 9.3% and 10.5% at the recommended and top doses, respectively, vs. 1.8% for placebo, and continued differences in many cardiovascular disease risk factors. In addition, there was a significantly lower incidence of progression to type 2 diabetes in the top-dose group (0.9%) vs. placebo (3.7%).
An area of considerable concern, given that most users of obesity medications are women of reproductive age, is the potential for oral clefts in the offspring of women who become pregnant while taking topiramate 31. A risk evaluation and mitigation strategy was developed to minimize the likelihood of pregnancy in women with reproductive potential that includes provider training, dispensing only via certified pharmacies, and supplying patient information regarding risks and the necessity of using effective contraception 32. Women with childbearing potential should have a negative pregnancy test prior to starting phentermine/topiramate-ER and monthly thereafter 32. A small increase in resting heart rate has been observed in the clinical trials of phentermine/topiramate-ER at higher doses, with more patients on top-dose (56.1%) than placebo (42.1%) having increases of more than 10 beats per minute, leading to some concerns regarding its potential long-term effect on cardiovascular disease events. Phentermine/topiramate-ER was approved with a requirement for a post-marketing trial of to assess long-term cardiovascular safety 24. The labeling recommends against prescription in patients with recent or unstable cardiac or cerebrovascular disease, and suggests regular monitoring of resting heart rate 25.
Side effects: The most common side effects are tingling hands and feet, dizziness, altered sense of taste, insomnia, constipation, and dry mouth.
Serious side effects include certain birth defects (cleft lip and cleft palate), faster heart rate, suicidal thoughts or actions, and eye problems that could lead to permanent vision loss if not treated.
Women who might become pregnant should get a pregnancy test before taking Qsymia, and should use birth control and get monthly pregnancy tests while on the drug.
You also shouldn’t take Qsymia if you have glaucoma, hyperthyroidism, heart disease, or stroke. Get regular checks of your heart when starting the drug or increasing the dose.
What else you should know: If you don’t lose 3% of your weight after 12 weeks on Qsymia, the FDA recommends that you stop taking it or that your doctor increase your dose for the next 12 weeks — and if that doesn’t work, you should gradually stop taking it.
Phentermine
Your doctor may prescribe this under the names Adipex or Suprenza.
Phentermine is by far the most widely prescribed obesity medication in the US, with 25.3 million prescriptions dispensed to an estimated 6.2 million users between 2008–2011 4. Phentermine originally approved indication was obesity; and the drug was used on-label until 1977 when it, along with all other drugs approved for treating obesity, were approved a second time after an amendment to the Food Drug and Cosmetic Act required that the FDA approve new drugs based on efficacy as well as safety. There was protracted opposition to re-approval from those who maintained the sympathomimetic obesity drugs that had dangerous addiction potential. No evidence of addiction had appeared during 18 years of increasingly frequent use, but the FDA re-approved them all, having silenced the opposition by announcing the drugs would be approved for short-term use only 33. The FDA has jurisdiction over pharmaceutical companies but cannot regulate medical practice, jurisdiction over which resides in the individual US states. US physicians treating obesity, well aware of these statuary boundaries, continued to use phentermine and the other sympathomimetic amine anorectic drugs off-label long-term. Surveys of prescribing practices among physicians treating obesity have confirmed that a majority of these physicians continue to prescribe the sympathomimetics off-label in this manner 34.
A meta-analysis of 6 studies ranging from 2 to 24 weeks 35 found that patients using 15–30mg/day phentermine had a mean additional weight loss relative to placebo of 3.6 kg, with mean total weight loss of 6.3 kg. The longest published placebo-controlled trial of phentermine 36 lasted 36 weeks in 108 obese women treated with phentermine 30mg/day either continuously or intermittently (alternating months) and found similar weight loss in the continuous (12.2 kg) and intermittent (13.0 kg) arms vs. 4.8 kg with placebo. However, attrition was 41%, and data were presented only for completers, which is likely to overstate efficacy 36. Among completers, transient symptoms of central nervous system stimulation such as insomnia, irritability, and anxiety did not differ between those receiving continuous (24%) vs. intermittent (27%) therapy, compared with 8% for those taking placebo. Several short-term placebo-controlled studies of phentermine have shown elevations in pulse or smaller decreases in pulse and/or blood pressure than would be expected given the degree of weight loss 37.
Phentermine is also used off-label in several ways other than long-term. Phentermine is, and has long been, prescribed for patients whose excess adiposity is below the conventional BMI cutoffs. Maffetone et al. 38 have suggested the use of BMI cutoffs has seriously underestimated the extent of an “overfat pandemic comprised of people who exhibit metabolic health impairments associated with excess fat mass relative to lean body mass.” They point out that there are a very large number of individuals who have excess fat mass but with BMIs below the cutoffs, that such individuals have increased health risks, and imply that some deserve treatment. Viewed from Maffetone et al’s perspective, private obesity medicine practitioners have been treating these overfat patients with obesity drugs off-label despite not labeling them with the proposed terminology.
Prescribed phentermine doses higher than the limit suggested are common. The label lists 37.5 mg per day as the “usual dose” (30 mg of phentermine resin is the same dose). A separate paragraph warns, “When tolerance to the anorectic effect develops, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinued.” At the time the original phentermine label was written, tolerance was considered a prelude to addiction, but it is now well known that tolerance is not the same as dependence or addiction. The phenomenon referred to may be related both to intra-species variability of drug metabolism and dose–time induction of more rapid drug metabolism. Whatever the theoretical explanation, doses higher than 37.5 mg/day have been found effective and safe in a number of observational studies 39, 40.
Physicians treating attention-deficit/hyperactivity disorder (ADHD) use dose-to-effect titration with methamphetamine up to 1 mg/kg/day and methylphenidates up to 2 or 3 mg/kg/day 41. Attention-deficit/hyperactivity disorder (ADHD) medicines have considerably greater potentials for abuse than sympathomimetic anorectics. Current treatment recommendations for attention-deficit/hyperactivity disorder (ADHD) call for a starting dose of amphetamine of 0.3 mg/kg/day, then titrating up, if needed, to a maximum of 1.0 mg/kg/day. The variation in dose required for effective treatment is thought to be due to intra-individual variability in drug metabolism and plasma clearance. A study in baboons found that intravenous amphetamine and phentermine produced equivalent plasma levels of norepinephrine. This suggests that oral doses of these drugs would have equivalent effects in humans on plasma norepinephrine levels. Surveys indicate that the average phentermine dose employed in the USA is about 60 mg/day, and that some physicians have used up to 112 mg/day 42. These doses are comparable with the doses of amphetamine used to treat attention deficit with respect to norepinephrine plasma levels and are not excessive 42.
The prevalence of overweight and obesity in patients with attention-deficit/hyperactivity disorder (ADHD) is high, perhaps as high as 30% 43 and patients with this combination frequently present at weight management clinics. Some of these patients are being treated for attention deficit, but many are not. It is common knowledge among obesity medicine practitioners that obese patients with attention deficit often experience clinical improvement when treated with phentermine, particularly if they are not currently being treated for their attention deficit. Effective dosages for weight loss or maintenance for these patients vary widely but most require or tolerate higher phentermine doses than patients without attention deficit.
The label suggests the drug not be used in pediatric patients aged <16 and be used with caution in older adults. Phentermine was used safely for treating overweight children by pediatricians until it became unpopular in the 1980s. There are no reports of harm from phentermine treatment in either very young or elderly patients 42. The surveys of obesity medicine physicians cited above indicate that a majority use phentermine in treating adolescents. The same surveys suggest that these physicians do not recognize an upper age limit for treatment. Populations selected for the long-term clinical trials for phentermine/topiramate did not include very young or very old subjects, so there are no modern clinical trial data on such populations. However, observational reports have included patients safely treated with phentermine as young as 3 years 44 and as old as 88 years 45.
How it works: Curbs your appetite.
Approved for long-term use ? No. It’s approved for short-term use (a few weeks) only.
Side effects can be serious, such as raising your blood pressure or causing heart palpitations, restlessness, dizziness, tremor, insomnia, shortness of breath, chest pain, and trouble doing activities you’ve been able to do.
Phentermine may make you drowsy, hampering your ability to drive or operate machinery. As with some other appetite suppressants, there’s a risk of becoming dependent upon the drug.
Less serious side effects include dry mouth, unpleasant taste, diarrhea, constipation, and vomiting.
Don’t take it late in the evening, as it may cause insomnia.
If you take insulin for diabetes, let your doctor know before you take phentermine, as you may need to adjust your insulin dose.
You should not take phentermine if you have a history of heart disease, stroke, congestive heart failure, or uncontrolled high blood pressure. You also shouldn’t take it if you have glaucoma, hyperthyroidism, or a history of drug abuse, or if you are pregnant or nursing.
What else you should know: Phentermine is an amphetamine. Because of the risk of addiction or abuse, such stimulant drugs are “controlled substances,” which means they need a special type of prescription.
Although there are no published data on the frequency of use, phentermine is occasionally prescribed for patients with label contraindications. Coronary artery disease, stroke, arrhythmias, congestive heart failure, and uncontrolled hypertension are listed specifically. In the absence of controlled data supporting these contraindications, there is no unambiguous evidence that suggests these conditions are absolute contraindications. However, US obesity medicine specialists, based on the known mechanism of action of phentermine, and the pathophysiology of the illness, would consider congestive heart failure, uncontrolled hypertension, untreated clinically significant arrhythmias, and severe advanced coronary artery disease to be absolute contraindications 42. However, in cases of less severe coronary artery disease, medically treated arrhythmias and patients who have past history of stroke, some US obesity medicine specialists would weigh the benefits of weight loss in obese, overweight and overfat subjects after a thorough assessment, that would likely include consultation with the patient’s cardiologist and/or medical specialist 42. This viewpoint is supported by reduced mortality observed in the Sibutramine Cardiovascular Outcomes (SCOUT) trial for patients with cardiovascular disease who had moderate weight loss 46. The expectation is that the same will be discovered with cardiovascular outcome trials that the FDA has mandated for the newer obesity drugs since weight loss in overweight and obese patients induces improvement in cardiac dysfunction common in such patients 47.
Other contraindications include hyperthyroidism, glaucoma and history of drug abuse 42. Most physicians would likely agree that phentermine not be used until hyperthyroidism has been treated but most would also agree a history of successful treatment of hyperthyroidism is not a contraindication. As with other medications that have anticholinergic side effects, phentermine is contraindicated in patients with narrow-angle glaucoma. However, it is not contraindicated in patients with open-angle glaucoma.
Phentermine use in clinical practice has not been associated with phentermine cravings, withdrawal, or excessive use leading to psychological or physical impairment. Although there is a widespread presumption that phentermine abuse is common, actual phentermine abuse is not common and appears limited to the use of the drug as a stimulant among people trying to stay awake and those trying to boost their energy level. Phentermine is longer acting than caffeine and does not have the adverse gastrointestinal effects of high doses of caffeine, so some students studying for exams and some long-haul truck drivers use it to stay awake and alert. Evidently, some patients with stimulant use disorder who use cocaine, methamphetamine or other strong stimulants add phentermine to a drug cocktail in an attempt to heighten the stimulant effects, but no data have been published on the frequency of this practice. A telephone survey of 50 addiction treatment centers in the USA found only 2 instances of patients using such cocktails among several thousand admissions, suggesting the addition of phentermine to such cocktails is uncommon 48. Stimulant use disorder due to phentermine alone as the favored drug has not been described, and no such entity is included in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) 49. Clinically significant psychiatric distress similar to that described in the DSM-5 in discussing stimulant use disorders has never been observed in overweight or obese patients treated with phentermine. A phentermine withdrawal syndrome has never been observed or described. Patients with either overt attention deficit or some of its symptoms typically “like” phentermine because they function better when taking it. Patients who have difficulty staying in control of eating also “like” phentermine because they have less cravings and enjoy better control of their eating behaviors. Patients with symptoms of attention deficit are quite common among the overweight and obese. For these reasons, “liking” phentermine should not be interpreted as an indication phentermine has high abuse potential. Phentermine treatment enhances control of impulsive behavior and control of other harmful eating behaviors; these are goals of behavioral treatment in obese patients.
It may be reasonable to prescribe phentermine for subjects who have a history of recovery from drug abuse. The author has found this safe in carefully selected patients provided their recovery is genuine, recovery has endured for at least several years and phentermine does not induce phentermine cravings or desire for their former drugs of abuse.
The label also lists contraindications that are absolute and seldom ignored, and these include recent monoamine oxidase inhibitor use, agitated states, pregnancy, nursing mothers and known hypersensitivity or idiosyncratic reactions to sympathetic amines.
Phentermine label warnings
Some consider prescribing phentermine despite specific label warnings to be an off-label use. It is recommended that the label warnings be included in an informed consent, and that any concern of the patient be thoroughly discussed and the discussion documented. The rationale for decisions to prescribe phentermine contrary to a warning would best be explicitly documented in the patient record.
Co-administration with other weight-loss drugs
US physicians view this warning as an anachronism since the FDA has approved the two combination drugs phentermine/topiramate and bupropion/naltrexone 42. The warning against combining weight-loss medicines appeared in the phentermine label in 2000 after 1997 reports of valvulopathy induced by the combination of phentermine and fenfluramine, and both dexfenfluramine and fenfluramine were taken off-market. Surveys of US physicians treating obesity have revealed these physicians frequently combined the various antiobesity drugs with other drugs approved for obesity and with a variety of other drugs that have an effect on weight loss. For example, these physicians combined phentermine and topiramate long before the FDA approved a fixed dose combination (Qsymia) in 2012 42. Other combinations in use are discussed.
Primary pulmonary hypertension
Primary pulmonary hypertension has not been associated with phentermine monotherapy. As discussed in a previous communication, isolated reports of primary pulmonary hypertension occurring in patients who have taken phentermine have relied on theoretical but unproven adverse effects and ignored the underlying incidence of idiopathic pulmonary hypertension 50.
Valvular heart disease
This warning first appeared in the label in 2000 after valvulopathy was discovered in patients taking phentermine and fenfluramine in 1997 and before publication of a report suggesting that fenfluramine, but not phentermine, activated cardiac 5HT2B serotonin receptors that then induced valvulopathy 51. Although the label states “… there have been rare cases of valvular heart disease in patients who reportedly have taken phentermine alone.”
Tolerance
Tolerance refers to a reduced response to a drug after repeated use. Tolerance is a normal physiologic process that occurs with substances of abuse but also with some medicines (e.g., diphenhydramine). Tachyphylaxis refers to very rapid development of tolerance.
Hazardous task ability
Labels for most drugs that work in the central nervous system include this warning. There is no specific evidence that phentermine degrades mental or physical performance. Generally, stimulants will enhance rather than degrade mental and physical performance until extremely high doses are used. The Federal Aviation Administration (FAA) has not accepted phentermine for pilots 50. The only antiobesity drug acceptable to the FAA is orlistat 50.
Risk of abuse and addiction
The Diagnostic and Statistical Manual of Mental Disorders: DSM-5 49 does not have criteria for “addiction” but instead sets forth criteria for diagnosing stimulant-related disorders and discusses “stimulant use disorder” and “stimulant withdrawal” with amphetamine and cocaine as prototypical stimulants. Phentermine is not specifically mentioned in the DSM-5. Stimulant use disorder is defined as a pattern of repeated use leading to clinically significant impairment or distress as manifested by two or more of a list of 11 symptoms, including intense cravings for the substance leading to self-destructive social and job or profession-related behaviors. In phentermine post-marketing studies, we found that long-term phentermine use, even at doses higher than 37.5 mg/day, did not induce phentermine cravings, and that abrupt cessation of long-term phentermine did not induce a stimulant withdrawal syndrome 52.
Assessment of drug “liking” is currently used as a measure of addiction potential 53. Phentermine has been used in studies as an example of a liked drug 54. However, patients taking phentermine often “like” the drug for a variety of valid reasons but do not have other signs or symptoms of addiction or physical dependence. Some like phentermine because they have lost weight taking it and enjoy a better quality of life. Many patients in medical weight management programs have adult attention deficit or at least some symptoms of attention deficit, and many are not taking specific attention medicines. These patients typically are less scattered, can focus better and are more productive when taking phentermine and, realizing this, “like” taking the drug. Discussions of drug liking of phentermine as a sign of addiction typically fail to consider the prevalence of attention deficit in the obese population as a reason for drug liking. Nor do such discussions differentiate between hedonic drug liking versus drug liking because of medical benefits.
Use with alcohol
There is an extensive literature on the effects of ethanol and a wide variety of stimulants in animals and humans. However, there are no specific reports on the effects of combining phentermine and ethanol in humans.
Hypertension
Phentermine-induced increases in blood pressure are often mentioned in both the medical and general literature, but few instances have been recorded 55. US obesity medicine practitioners typically do not prescribe phentermine with poorly controlled hypertension until it is under control, but they do prescribe it in the presence of controlled hypertension, then monitor the patient’s blood pressure closely, discontinuing phentermine if blood pressure rises 50.
Diethylpropion
Diethylpropion has a similar adverse-effect and weight loss profile to phentermine, but is much less frequently prescribed, with approximately 1 million prescriptions dispensed between 2008–2011 4. A meta-analysis of 9 small studies ranging from 6–52 weeks 56 found that patients using diethylpropion 75mg/day had a mean additional weight loss relative to placebo of 3.0 kg, with a mean total weight loss of 6.5 kg.
Phendimetrazine
Phendimetrazine, despite the paucity of randomized controlled trials 56 is prescribed three times more frequently than diethylpropion for obesity treatment, with more than 3 million phendimetrazine prescriptions estimated to have been filled between 2008–2011 4. In the completer’s analyses from two small 12-week trials 57, phendimetrazine appears to have similar weight loss to other noradrenergic drugs.
Benzphetamine
Benzphetamine is less commonly prescribed for obesity treatment than the other noradrenergic drugs 56 and there are few data from controlled trials evaluating its safety or efficacy 56.
Common adverse effects of noradrenergic drugs are shown in Table 1. Because these medications were approved prior to the requirements for long-term trials with adequate power to ascertain clinical endpoints, an adverse effect of noradrenergic obesity drugs on cardiovascular disease events cannot be excluded, and is of concern given their known effect on heart rate and blood pressure.
Drugs used off-label for weight management
Off-label drug use generally means that a drug is being used for an unapproved indication, population or at an unapproved dosage. In addition to these unapproved uses, antiobesity may be also employed for longer than recommended durations, when contraindicated, or in other ways contrary to the US FDA-approved label. Countries other than the USA typically have their own drug approval processes, but their labels commonly include language similar to that of the FDA label. Discussion of off-label drug use here is limited to US obesity medicine physicians and the US FDA label, and is intended to review some of the data on how US physicians are actually using drugs off-label. The uses of drugs that are approved for other diseases that have weight loss as a side effect and that are used in treating overweight or obese patients with the indicated diseases are not discussed.
While, in general, off-label drug use is neither illegal nor unethical, some jurisdictions may limit the use of specific medications for specific situations. For example, in the USA, the state of Ohio forbids proscribing any controlled substance for weight loss in any manner contrary to the FDA label (Ohio Administrative Code 4731-11-04). Penalties may include forfeiture of medical license. In addition, the state prosecutors may charge an offending doctor who dispenses medications with felony, drug trafficking and money laundering. Physicians should not presume off-label use of controlled substance antiobesity medicines is freely permitted in their own location but should investigate local regulations and laws carefully before prescribing these drugs off-label.
Drugs commonly used off-label for weight management are discussed in the following section.
Amphetamine
Methamphetamine (desoxyephedrine) was first approved in 1943 by the FDA for treating narcolepsy, mild depression, postencephalitic Parkinson syndrome, chronic alcoholism, cerebral arteriosclerosis, and hay fever. Later, in 1947, methamphetamine was also approved as the first drug for treating obesity. Both approvals were made at a time when when US law required the FDA to only consider whether new drugs were safe without consideration of their effectiveness. Later, in 1962, the US congress amended the Food Drug and Cosmetic Act requiring the FDA to approve new drugs based on their safety but only if the new drug demonstrated proven effectiveness for the stipulated indication. Today, methamphetamine remains FDA-approved for treating attention deficit (ADHD) and obesity and is still available as Desoxyn®. Methamphetamine is a category II controlled substance and although today this drug is apparently rarely used for treating obesity, such usage is not off-label. Physicians used 5 mg methamphetamine tablets up to 3 times daily before meals in the 1940s and 1950s for treating obesity, but then turned toward using sympathomimetic amines as these became available beginning with phenmetrazine in 1956; phentermine, diethylpropion and phendimetrazine in 1959; and benzphetamine in 1960.
Diabetic medicines
It is appropriate to monitor blood sugars during treatment for obesity since dose lowering or elimination of diabetic medicines is often a benefit and is to be expected with weight loss. Phentermine is not contraindicated in patients with either treated or untreated diabetes 50.
Metformin
Although the label for metformin specifies diabetes as the sole indication, the drug has been prescribed with increasing frequency for overweight and obese patients with impaired fasting glucose following a report that long-term metformin delayed or prevented diabetes and induced weight loss in such patients 58. Metformin is known to induce modest weight loss in overweight patients even without glucose abnormalities and is prescribed off-label as an adjunct to weight loss 59. Metformin, increasing used off-label in prediabetes and other insulin resistant states, produces small sustained weight losses of about 2% relative to placebo 60. Metformin improves insulin sensitivity, has a good safety profile, and long-term clinical experience. As weight loss attributable to metformin is small, its usefulness as monotherapy for obesity treatment is limited, but its salutary effects on body weight make it a good choice when other indications warrant its prescription. Metformin has also been used to prevent or ameliorate weight gain with atypical antipsychotic agents and mood stabilizers. A meta-analysis examining the effect of medications for attenuation of antipsychotic weight gain found an approximate 3 kg additional weight loss relative to placebo attributable to metformin 61.
Bupropion
Bupropion, a norepinephrine and dopamine reuptake inhibitor, was tested as monotherapy for up to one year as a weight loss medication. A pooled analysis of 3 studies ranging from 6 to 12 months showed additional weight loss relative to placebo of 2.8 kg in patients receiving 400mg/d bupropion, with total weight loss of 4.4 kg 62.
Topiramate
Topiramate was FDA-approved for treatment of refractory epilepsy in 1996. Weight loss was immediately noted as a side effect 63. Soon thereafter, reports began appearing that topiramate was effective in treating binge eating disorder 64 and obesity 65. A survey of US obesity medicine physicians performed in early 2008 and published in 2009 revealed that 50% were prescribing topiramate as monotherapy for obesity 66. In 2012, in a survey conducted before the FDA approved the combination phentermine and topiramate for obesity, 63% of those surveyed were already using topiramate for treating obesity and 61% were combining it with phentermine in some patients 67. Thus, topiramate has been used off-label, both as monotherapy and in combination.
Zonisamide
Zonisamide, an antiepileptic medication, also induces weight loss. A 12-month randomized controlled trial of 225 adults, with 97% follow-up found that a 400mg dose led to significantly greater weight loss than placebo (6.8% vs. 3.7%), as well as a greater proportion losing ≥5% and ≥10% of initial weight 68. However, adverse effects were limiting. Overall, fatigue was the only adverse effect that occurred at a significantly higher frequency for zonisamide treatment than for placebo. Although not observed frequently in this study, the following adverse effects occurred frequently with zonisamide therapy in epilepsy trials: dizziness, cognitive impairment, and somnolence 69. Because zonisamide is a sulfonamide, there is a potential for hypersensitivity reactions. Although rare, kidney stones and serious hematologic events have been reported with zonisamide therapy in patients with epilepsy. Consistent with data from epilepsy trials, an increase in serum creatinine concentration with zonisamide therapy, but not with placebo. Whereas the increase in the first 16 weeks (approximately 16%) was significant, there was no further increase in the extension phase; no value exceeded the upper limit of normal range, and there were no clinical events associated with the increase.
Pramlintide
Pramlintide is a synthetic analogue of human amylin, which is administered subcutaneously at meal times as an adjunct to insulin for patients with type 1 and type 2 diabetes. A meta-analysis 70 of 8 studies in patients with type 2 diabetes and obese non-diabetic populations found additional weight loss relative to placebo of about 2.2 kg for both groups. One study 71 evaluating pramlintide in combination with phentermine vs. pramlintide alone found significantly greater weight loss with combination therapy, although diastolic blood pressure and heart rate increased despite greater weight loss with the combination.
Sympathomimetics other than phentermine
Other sympathomimetics include diethylpropion (1959), and phendimetrazine (1959), both of which have FDA-approved labels with indications, contraindications and warnings identical or similar to those in the phentermine label. Diethylpropion is a category IV controlled substance, and phendimetrazine is a category III. Both drugs are frequently used off-label in the US in manners similar to phentermine. In the 2012 survey, 63% of surveyed physicians used diethylpropion in an average of 18% of their patients and 60% used phendimetrazine in an average of 20% of their patients. Benzphetamine, a category III drug, is another older sympathomimetic drug that is still in use but apparently less often used than diethylpropion or phendimetrazine. The surveys did not include questions regarding benzphetamine, so we have no data but answers to queries to US pharmaceutical wholesalers reveal that their benzphetamine sales are much lower than the other sympathomimetics.
Drugs approved for uses other than obesity
Certain drugs approved for indications other than obesity but inducing weight loss when given on-label to overweight patients could potentially be used off-label to treat overweight and obese patients who do not have the indicated diagnosis. There is little or no data on whether any of these are actually being used off-label for obesity, but given the proclivity of obesity practitioners to adopt drugs that induce weight loss without regard to approved indications, one can expect some to use these off-label drugs for weight loss. Some of these drug candidates for off-label use are listed in the following section.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors
SGLT2 inhibitors, including canagliflozin, dapagliflozin, and empagliflozin, are FDA-approved for use with diet and exercise to lower blood sugar in adults with type 2 diabetes. In diabetics, these also induce modest weight loss. A recent report of a trial combining canagliflozen and phentermine for overweight patients without diabetes suggests this off-label combination can induce weight loss superior to diet and lifestyle modification without these medications 72.
Exenatide
Approved for treating diabetes, exenatide also induces weight loss in overweight diabetics 73. This drug has been used off-label for weight loss, but apparently infrequently.
Metreleptin
Approved for congenital or acquired generalized lipodystrophy, metreleptin is a synthetic leptin analog. Obese patients typically have high circulating leptin levels, and clinical trials have shown that leptin administration does not induce weight loss. However, leptin levels fall during weight loss and remain low afterward 74 and leptin administration after weight loss in obese patients reverses some of the neuroendrocrine adaptations involved in weight regain 74. These data suggest that daily leptin injections could possibly be useful in preventing weight regain after a significant weight loss. Resolution of the issue whether or not leptin analogs will be a solution to the problem of weight regain will require further research.
OTC appetite suppressant
There are very few proven choices in over-the-counter (OTC) or nonprescription medications for effective weight loss. One agent that is available without a prescription is Orlistat (a lower-dose version of the prescription drug Xenical) which blocks your body from absorbing about a third of the fat you eat and is not an appetite suppressant.
Do not take Orlistat if you are pregnant. Weight loss is not recommended during pregnancy.
You should not use Orlistat if you have a digestive disorder (problems absorbing food). You should not use Xenical if you have gallbladder problems, or if you are pregnant. Do not use Orlistat if you have had an organ transplant, if you use cyclosporine, or if you are not overweight.
Orlistat is only part of a complete program of treatment that also includes diet, exercise, and weight control. Your daily intake of fat, protein, and carbohydrates should be evenly divided over all of your daily meals. Follow your diet, medication, and exercise routines very closely.
Avoid a diet that is high in fat. High-fat meals taken in combination with orlistat can increase your risk of unpleasant side effects on your stomach or intestines.
Many people who are trying to lose weight may attempt to use dietary supplements or herbal medications, but most of these products have not been adequately studied for effectiveness or safety and none are approved by the U.S. Food and Drug Administration (FDA) for weight loss. Check with a healthcare provider for advice before using herbal or dietary supplements for weight loss.
References- Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Colman E. Ann Intern Med. 2005 Sep 6; 143(5):380-5. http://annals.org/aim/fullarticle/718716/anorectics-trial-half-century-federal-regulation-prescription-appetite-suppressants
- Willam P. Italian ban places question over anti-obesity drug. The Guardian. 8 March 2002.
- The FDA’s Assessment of Two Drugs for Chronic Weight Management. N Engl J Med 2012; 367:1577-1579 DOI: 10.1056/NEJMp1211277
- Use of prescription antiobesity drugs in the United States. Hampp C, Kang EM, Borders-Hemphill V. Pharmacotherapy. 2013 Dec; 33(12):1299-307. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4740913/
- Gadde KM, Xiong GL. Bupropion for weight reduction. Expert Rev Neurother 7: 17–24, 2007.
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse 36: 102–113, 2000.
- Safta L, Cuparencu B, Sirbu A, Secareanu A. Experimental observations on the effect of amphepramone on the behavior, locomotion, pentretrazol seizures and electroencephalogram. Psychopharmacology (Berl) 50: 165–169, 1976.
- Moyers SB. Medications as adjunct therapy for weight loss: approved and off-label agents in use. J Am Diet Assoc 105: 948–959, 2005
- Hampp C, Kang EM, Borders-Hemphill V. Use of Prescription Antiobesity Drugs in the United States. Pharmacotherapy. 2013;33(12):1299-1307. doi:10.1002/phar.1342. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4740913/
- Medications Target Long-Term Weight Control. https://www.fda.gov/forconsumers/consumerupdates/ucm312380.htm
- Drugs@FDA: FDA Approved Drug Products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
- Centers for Disease Control and Prevention. BMI Percentile Calculator for Child and Teen. https://www.cdc.gov/healthyweight/bmi/calculator.html
- Yanovski SZ, Yanovski JA. Long-term Drug Treatment for Obesity: A Systematic and Clinical Review. JAMA : the journal of the American Medical Association. 2014;311(1):74-86. doi:10.1001/jama.2013.281361.
- Cholesterol lowering effect of dietary weight loss and orlistat treatment–efficacy and limitations. Erdmann J, Lippl F, Klose G, Schusdziarra V. Aliment Pharmacol Ther. 2004 Jun 1; 19(11):1173-9. https://www.ncbi.nlm.nih.gov/pubmed/15153170/
- Long-term changes in blood pressure following orlistat and sibutramine treatment: a meta-analysis. Johansson K, Sundström J, Neovius K, Rössner S, Neovius M. Obes Rev. 2010 Nov; 11(11):777-91. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0030322/
- Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27(1):155–161 http://care.diabetesjournals.org/content/27/1/155.long
- Cavaliere H, Floriano I, Medeiros-Neto G. Gastrointestinal side effects of orlistat may be prevented by concomitant prescription of natural fibers (psyllium mucilloid) Int J Obes Relat Metab Disord. 2001;25(7):1095–1099.
- Connolly HM, Crary JL, McGoon MD, et al. Valvular heart disease associated with fenfluramine-phentermine. N Engl J Med. 1997;337(9):581–588.
- Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363(3):245–256.
- Fidler MC, Sanchez M, Raether B, et al. A One-Year Randomized Trial of Lorcaserin for Weight Loss in Obese and Overweight Adults: The BLOSSOM Trial. J Clin Endocrinol Metab. 2011
- O’Neil PM, Smith SR, Weissman NJ, et al. Randomized Placebo-Controlled Clinical Trial of Lorcaserin for Weight Loss in Type 2 Diabetes Mellitus: The BLOOM-DM Study. Obesity (Silver Spring) 2012;20(7):1426–1436.
- Chan EW, He Y, Chui CS, Wong AY, Lau WC, Wong IC. Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2013;14(5):383–392.
- BELVIQ (lorcaserin hydrochloride) tablets, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf
- Colman E, Golden J, Roberts M, Egan A, Weaver J, Rosebraugh C. The FDA’s assessment of two drugs for chronic weight management. The New England journal of medicine. 2012;367(17):1577–1579.
- Qsymia (phentermine and topiramate extended-release) capsules, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022580s004lbl.pdf
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012;20(2):330–342. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3270297/
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. https://www.ncbi.nlm.nih.gov/pubmed/21481449
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight a randomized adults without diabetes and with BMI ≥35 kg/m² to placebo, phentermine/topiramate-ER 3.75/23mg (starting dose), or 15/92mg (top dose). 40% of participants withdrew. At the top dose, mean 1 year weight loss was 10.9% vs. 1.6% of initial weight for placebo. 67% of patients given the top dose lost ≥5% of initial weight and 47% lost ≥10% of initial weight, compared with 17% and 7%, respectively for placebo. CONQUER ((Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–1352. https://www.ncbi.nlm.nih.gov/pubmed/21481449
- Davidson MH, Tonstad S, Oparil S, Schwiers M, Day WW, Bowden CH. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index >/=27 kg/m(2) The American journal of cardiology. 2013;111(8):1131–1138.
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297–308. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3260065/
- Margulis AV, Mitchell AA, Gilboa SM, et al. Use of topiramate in pregnancy and risk of oral clefts. American journal of obstetrics and gynecology. 2012;207(5):405, e401–407. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3484193/
- QSYMIA (phentermine and topiramate extended-release) Capsules. Risk evaluation and mitigation strategy (REMS). https://www.fda.gov/downloads/forindustry/userfees/prescriptiondruguserfee/ucm361075.pdf
- Colman E. Anorectics on trial: a half century of federal regulation of prescription appetite suppressants. Ann Intern Med. 2005;143(5):380–385
- Schmidt SL, Bryman D, Greenway FL, Hendricks EJ. How physician obesity medicine specialists treated obesity before 2012 new drug approvals. Obes Surg. 2015;25(1):186–190. https://www.ncbi.nlm.nih.gov/pubmed/25344465
- Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J Obes Relat Metab Disord. 2002;26(2):262–273
- Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J. 1968;1(5588):352–354. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1984840/pdf/brmedj02070-0040.pdf
- Ryan DH, Bray GA. Pharmacologic treatment options for obesity: what is old is new again. Curr Hypertens Rep. 2013;15(3):182–189.
- Maffetone PB, Rivera-Dominguez I, Laursen PB. Overfat and underfat: new terms and definitions long overdue. Front Public Health. 2016;4:279. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5206235/
- Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity (Silver Spring) 2011;19(12):2351–2360 https://www.ncbi.nlm.nih.gov/pubmed/21527891
- Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 2009;17(9):1730–1735 https://www.ncbi.nlm.nih.gov/pubmed/19300434
- Stevens JR, Wilens TE, Stern TA. Using stimulants for attention-deficit/hyperactivity disorder: clinical approaches and challenges. Prim Care Companion CNS Disord. 2013;15(2) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3733520/
- Hendricks EJ. Off-label drugs for weight management. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2017;10:223-234. doi:10.2147/DMSO.S95299. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5473499/
- Pagoto SL, Curtin C, Lemon SC, et al. Association between adult attention deficit/hyperactivity disorder and obesity in the US population. Obesity (Silver Spring) 2009;17(3):539–544. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3221303/
- Lorber J. Obesity in childhood. A controlled trial of anorectic drugs. Arch Dis Child. 1966;41(217):309–312. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2019558/pdf/archdisch01570-0083.pdf
- Hendricks EJ, Srisurapanont M, Schmidt SL, et al. Addiction potential of phentermine prescribed during long-term treatment of obesity. Int J Obes (Lond) 2014;38(2):292–298 https://www.ncbi.nlm.nih.gov/pubmed/23736363
- Caterson ID, Finer N, Coutinho W, et al. Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes Obes Metab. 2012;14(6):523–530.
- de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54(25):2376–2381.
- Bryman D, Hendricks EJ, Schmidt SL. Incidence of addiction and abuse due to phentermine, diethylpropion, and phendimetrazine in the United States. Telephone Survey of Emergency Rooms and Addiction Treatment Centers ed. Unpublished manuscript. 2013
- American Psychiatric Association, DSM-5 Task Force . Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. American Psychiatric Association: Arlington, VA; 2013.
- Hendricks EJ, Rothman RB. RE: pulmonary hypertension associated with use of phentermine? Yonsei Med J. 2011;52(5):869–870 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3159930/
- Rothman RB, Baumann MH, Savage JE, et al. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102(23):2836–2841.
- Hendricks EJ, Greenway FL. A study of abrupt phentermine cessation in patients in a weight management program. Am J Ther. 2011;18(4):292–299.
- Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016;71(8):670–679.
- Schoedel KA, Addy C, Chakraborty B, et al. Human abuse potential and cognitive effects of taranabant, a cannabinoid 1 receptor inverse agonist: a randomized, double-blind, placebo- and active-controlled, crossover study in recreational polydrug users. J Clin Psychopharmacol. 2012;32(4):492–502.
- Hendricks EJ, Rothman RB. Phentermine therapy for obesity does not elevate blood pressure. Diabetes Obes Metab. 2011;13(10):963–964. https://www.ncbi.nlm.nih.gov/pubmed/21896124
- Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Int J Obes Relat Metab Disord. 2002 Feb; 26(2):262-73. https://www.ncbi.nlm.nih.gov/pubmed/11850760/
- Sustained-action phendimetrazine in obesity. Hadler AJ. J Clin Pharmacol J New Drugs. 1968 Mar-Apr; 8(2):113-7. https://www.ncbi.nlm.nih.gov/pubmed/4871210/
- Diabetes Prevention Program Research Group Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35(4):731–737.
- Igel LI, Sinha A, Saunders KH, Apovian CM, Vojta D, Aronne LJ. Metformin: an old therapy that deserves a new indication for the treatment of obesity. Curr Atheroscler Rep. 2016;18(4):16.
- LeBlanc E, O’Connor E, Whitlock EP, Patnode C, Kapka T. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Agency for Healthcare Research and Quality; Rockville (MD): 2011. Screening for and Management of Obesity and Overweight in Adults. Report No.: 11-05159-EF-1.
- Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Maayan L, Vakhrusheva J, Correll CU. Neuropsychopharmacology. 2010 Jun; 35(7):1520-30. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3055458/
- Meta-analysis: pharmacologic treatment of obesity. Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, Hilton L, Suttorp M, Solomon V, Shekelle PG, Morton SC. Ann Intern Med. 2005 Apr 5; 142(7):532-46. https://www.ncbi.nlm.nih.gov/pubmed/15809465/
- Shorvon SD. Safety of topiramate: adverse events and relationships to dosing. Epilepsia. 1996;37(Suppl 2):S18–S22. https://www.ncbi.nlm.nih.gov/pubmed/8641242
- Shapira NA, Goldsmith TD, McElroy SL. Treatment of binge-eating disorder with topiramate: a clinical case series. J Clin Psychiatry. 2000;61(5):368–372. https://www.ncbi.nlm.nih.gov/pubmed/10847312
- Bray GA, Hollander P, Klein S, et al. A 6-month randomized, placebo-controlled, dose-ranging trial of topiramate for weight loss in obesity. Obes Res. 2003;11(6):722–733 https://www.ncbi.nlm.nih.gov/pubmed/12805393
- Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 2009;17(9):1730–1735
- Schmidt SL, Bryman D, Greenway FL, Hendricks EJ. How physician obesity medicine specialists treated obesity before 2012 new drug approvals. Obes Surg. 2015;25(1):186–190.
- Gadde KM, Franciscy DM, Wagner HR, 2nd, Krishnan KR. Zonisamide for weight loss in obese adults: a randomized controlled trial. JAMA. 2003;289(14):1820–1825. https://jamanetwork.com/journals/jama/fullarticle/196347
- Oommen KJ, Mathews S. Zonisamide: a new antiepileptic drug. Clin Neuropharmacol.1999;22:192-200.
- Singh-Franco D, Perez A, Harrington C. The effect of pramlintide acetate on glycemic control and weight in patients with type 2 diabetes mellitus and in obese patients without diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2011;13(2):169–180. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0031948/
- Aronne LJ, Halseth AE, Burns CM, Miller S, Shen LZ. Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity (Silver Spring) 2010;18(9):1739–1746.
- Hollander P, Bays HE, Rosenstock J, et al. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care. 2017;40(5):632–639.
- McAdam-Marx C, Nguyen H, Schauerhamer MB, et al. Glycemic control and weight outcomes for exenatide once weekly versus liraglutide in patients with type 2 diabetes: a 1-year retrospective cohort analysis. Clin Ther. 2016;38(12):2642–2651
- Sumithran P, Prendergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–1604.