What are oranges
The orange is the fruit of the citrus species Citrus sinensis in the family Rutaceae 1. It is also called sweet orange, to distinguish it from the bitter orange (also known as Citrus aurantium). The sweet orange reproduces asexually; varieties of sweet orange arise through mutations 2.
The orange is a hybrid between pomelo (Citrus maxima) and mandarin (Citrus reticulata). It has genes that are ~25% pomelo and ~75% mandarin 2; however, it is not a simple backcrossed BC1 hybrid, but hybridized over multiple generations. The chloroplast genes, and therefore the maternal line, seem to be pomelo 2. The sweet orange has had its full genome sequenced. Earlier estimates of the percentage of pomelo genes varying from ~50% to 6% have been reported 3.
The orange is unknown in the wild state; it is assumed to have originated in southern China, northeastern India, and perhaps southeastern Asia and that they were first cultivated in China around 2500 BC 2. As of 1987, orange trees were found to be the most cultivated fruit tree in the world 4. Orange trees are widely grown in tropical and subtropical climates for their sweet fruit. The fruit of the orange tree can be eaten fresh, or processed for its juice or fragrant peel 5. As of 2012, sweet oranges accounted for approximately 70% of citrus production with Brazil as the world’s leading orange producer, with an output of 17 million tonnes, followed by China, India, and the United States as the four major producers 6. Production of orange juice between the São Paulo (Brazil) and mid-south Florida areas makes up roughly 85% of the world market. Brazil exports 99% of its production, while 90% of Florida’s production is consumed in the United States 7.
Types of oranges
Common oranges (also called “white”, “round”, or “blond” oranges) constitute about two-thirds of all the orange production. The majority of this crop is used mostly for juice extraction.
Valencia orange
The Valencia orange is a sweet orange. It was first hybridized by pioneer American agronomist and land developer William Wolfskill in the mid-19th century on his farm in Santa Ana in southern California in the United States 1.
Primarily grown for processing and orange juice production, Valencia oranges have seeds, varying in number from zero to nine per fruit. Its excellent taste and internal color make it desirable for the fresh fruit markets, too. The fruit has an average diameter of 2.7 to 3 inches (70–76 mm), also a piece of this fruit which weighs 96 grams has 45 calories and 9 grams of sugar 8. After bloom, it usually carries two crops on the tree, the old and the new. The commercial harvest season in Florida runs from March to June. Worldwide, Valencia oranges are prized as the only variety of orange in season during summer. Furthermore, Valencia oranges bring benefits because of the vitamin C and flavonoids contained 9.
Figure 1. Valencia orange

Navel oranges are characterized by the growth of a second fruit at the apex, which protrudes slightly and resembles a human navel. They are primarily grown for human consumption for various reasons: their thicker skin makes them easy to peel, they are less juicy and their bitterness – a result of the high concentrations of limonin and other limonoids – renders them less suitable for juice 10. Their widespread distribution and long growing season have made navel oranges very popular. In the United States, they are available from November to April, with peak supplies in January, February, and March 11.
According to a 1917 study by Palemon Dorsett, Archibald Dixon Shamel and Wilson Popenoe of the United States Department of Agriculture (USDA), a single mutation in a Selecta orange tree planted on the grounds of a monastery near Bahia, Brazil, probably yielded the first navel orange between 1810 and 1820 12. Nevertheless, a researcher at the University of California, Riverside, has suggested that the parent variety was more likely the Portuguese navel orange (Umbigo). Today, navel oranges continue to be propagated through cutting and grafting. This does not allow for the usual selective breeding methodologies, and so all navel oranges can be considered fruits from that single, nearly two-hundred-year-old tree: they have exactly the same genetic make-up as the original tree and are, therefore, clones. On rare occasions, however, further mutations can lead to new varieties 12.
Figure 2. Navel oranges

Blood oranges
Blood oranges 4 are a natural mutation of Citrus sinensis, although today the majority of them are hybrids. High concentrations of anthocyanin a family of antioxidant pigments common to many flowers and fruit, but uncommon in citrus fruits 13 give the rind, flesh, and juice of the fruit their characteristic dark red color. Blood oranges were first discovered and cultivated in Sicily in the fifteenth century. Since then they have spread worldwide, but are grown especially in Spain and Italy under the names of sanguina and sanguinella, respectively.
Chrysanthemin (cyanidin 3-O-glucoside) is the main compound found in red oranges 14. The flesh develops its characteristic maroon color when the fruit develops with low temperatures during the night 15. Sometimes, dark coloring is seen on the exterior of the rind, as well, depending on the variety of blood orange. The skin can be tougher and harder to peel than that of other oranges. Blood oranges have a unique flavor profile compared to other oranges, being distinctly raspberry-like in addition to the usual citrus notes 15.
Figure 3. Blood oranges

Orange Nutrition Facts
The outermost layer of the orange rind can be thinly grated with a zester to produce orange zest. Orange zest is popular in cooking because it contains oils and has a strong flavor similar to that of the orange pulp. The white part of the rind, including the pith, is a source of pectin and has nearly the same amount of vitamin C as the flesh and other nutrients.
As with other citrus fruits, orange pulp is an excellent source of vitamin C, providing 64% of the Daily Value in a 100 g serving and high in fiber providing 3.1 gram per fruit ~ 10% of the Daily Value (Table 1). Most Americans are not getting enough fiber. According to the Dietary Guidelines for Americans 16, women should get 25–32 grams of fiber per day and men should get 30–35 grams of fiber per day. A diet that includes foods that are rich in fiber can help lower blood cholesterol and prevent diabetes and heart disease. When carbohydrates are combined with fiber, it slows the absorption of sugar and regulates insulin response. And food with fiber make us feel full, which discourages overeating. Also, fiber itself has no calories, and adequate amounts of fiber help move food through the digestive system, promoting healthy bowel function and protecting against constipation.
Oranges contain diverse phytochemicals, including carotenoids (beta-carotene, lutein and beta-cryptoxanthin), flavonoids (e.g. naringenin) and numerous volatile organic compounds producing orange aroma, including aldehydes, esters, terpenes, alcohols, and ketones.
Oranges, whose flavor may vary from sweet to sour, are commonly peeled and eaten fresh or squeezed for juice. The thick bitter rind is usually discarded, but the orange peel is edible and has significant contents of vitamin C, dietary fiber, total polyphenols, carotenoids, limonene and dietary minerals, such as potassium and magnesium 17.
Table 1. Orange (raw) – all commercial varieties
Nutrient | Unit | Value per 100 g | cup, sections 180 g | large (3-1/16″ dia) 184 g | small (2-3/8″ dia) 96 g | fruit (2-5/8″ dia) 131 g | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Water | g | 86.75 | 156.15 | 159.62 | 83.28 | 113.64 | |||||||||||||
| Energy | kcal | 47 | 85 | 86 | 45 | 62 | |||||||||||||
| Protein | g | 0.94 | 1.69 | 1.73 | 0.90 | 1.23 | |||||||||||||
| Total lipid (fat) | g | 0.12 | 0.22 | 0.22 | 0.12 | 0.16 | |||||||||||||
| Carbohydrate, by difference | g | 11.75 | 21.15 | 21.62 | 11.28 | 15.39 | |||||||||||||
| Fiber, total dietary | g | 2.4 | 4.3 | 4.4 | 2.3 | 3.1 | |||||||||||||
| Sugars, total | g | 9.35 | 16.83 | 17.20 | 8.98 | 12.25 | |||||||||||||
| Minerals | |||||||||||||||||||
| Calcium, Ca | mg | 40 | 72 | 74 | 38 | 52 | |||||||||||||
| Iron, Fe | mg | 0.10 | 0.18 | 0.18 | 0.10 | 0.13 | |||||||||||||
| Magnesium, Mg | mg | 10 | 18 | 18 | 10 | 13 | |||||||||||||
| Phosphorus, P | mg | 14 | 25 | 26 | 13 | 18 | |||||||||||||
| Potassium, K | mg | 181 | 326 | 333 | 174 | 237 | |||||||||||||
| Sodium, Na | mg | 0 | 0 | 0 | 0 | 0 | |||||||||||||
| Zinc, Zn | mg | 0.07 | 0.13 | 0.13 | 0.07 | 0.09 | |||||||||||||
| Vitamins | |||||||||||||||||||
| Vitamin C, total ascorbic acid | mg | 53.2 | 95.8 | 97.9 | 51.1 | 69.7 | |||||||||||||
| Thiamin | mg | 0.087 | 0.157 | 0.160 | 0.084 | 0.114 | |||||||||||||
| Riboflavin | mg | 0.040 | 0.072 | 0.074 | 0.038 | 0.052 | |||||||||||||
| Niacin | mg | 0.282 | 0.508 | 0.519 | 0.271 | 0.369 | |||||||||||||
| Vitamin B-6 | mg | 0.060 | 0.108 | 0.110 | 0.058 | 0.079 | |||||||||||||
| Folate, DFE | µg | 30 | 54 | 55 | 29 | 39 | |||||||||||||
| Vitamin B-12 | µg | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||||||
| Vitamin A, RAE | µg | 11 | 20 | 20 | 11 | 14 | |||||||||||||
| Vitamin A, IU | IU | 225 | 405 | 414 | 216 | 295 | |||||||||||||
| Vitamin E (alpha-tocopherol) | mg | 0.18 | 0.32 | 0.33 | 0.17 | 0.24 | |||||||||||||
| Vitamin D (D2 + D3) | µg | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||||||
| Vitamin D | IU | 0 | 0 | 0 | 0 | 0 | |||||||||||||
| Vitamin K (phylloquinone) | µg | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 0.015 | 0.027 | 0.028 | 0.014 | 0.020 | |||||||||||||
| Fatty acids, total monounsaturated | g | 0.023 | 0.041 | 0.042 | 0.022 | 0.030 | |||||||||||||
| Fatty acids, total polyunsaturated | g | 0.025 | 0.045 | 0.046 | 0.024 | 0.033 | |||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||||||||||||
| Cholesterol | mg | 0 | 0 | 0 | 0 | 0 | |||||||||||||
| Other | |||||||||||||||||||
| Caffeine | mg | 0 | 0 | 0 | 0 | 0 | |||||||||||||
Table 2. Valencia orange (raw) – California
Nutrient | Unit | Value per 100 g | cup sections, without membranes 180 g | fruit (2-5/8″ dia) 121 g | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Water | g | 86.34 | 155.41 | 104.47 | |||||||||||||||
| Energy | kcal | 49 | 88 | 59 | |||||||||||||||
| Protein | g | 1.04 | 1.87 | 1.26 | |||||||||||||||
| Total lipid (fat) | g | 0.30 | 0.54 | 0.36 | |||||||||||||||
| Carbohydrate, by difference | g | 11.89 | 21.40 | 14.39 | |||||||||||||||
| Fiber, total dietary | g | 2.5 | 4.5 | 3.0 | |||||||||||||||
| Minerals | |||||||||||||||||||
| Calcium, Ca | mg | 40 | 72 | 48 | |||||||||||||||
| Iron, Fe | mg | 0.09 | 0.16 | 0.11 | |||||||||||||||
| Magnesium, Mg | mg | 10 | 18 | 12 | |||||||||||||||
| Phosphorus, P | mg | 17 | 31 | 21 | |||||||||||||||
| Potassium, K | mg | 179 | 322 | 217 | |||||||||||||||
| Sodium, Na | mg | 0 | 0 | 0 | |||||||||||||||
| Zinc, Zn | mg | 0.06 | 0.11 | 0.07 | |||||||||||||||
| Vitamins | |||||||||||||||||||
| Vitamin C, total ascorbic acid | mg | 48.5 | 87.3 | 58.7 | |||||||||||||||
| Thiamin | mg | 0.087 | 0.157 | 0.105 | |||||||||||||||
| Riboflavin | mg | 0.040 | 0.072 | 0.048 | |||||||||||||||
| Niacin | mg | 0.274 | 0.493 | 0.332 | |||||||||||||||
| Vitamin B-6 | mg | 0.063 | 0.113 | 0.076 | |||||||||||||||
| Folate, DFE | µg | 39 | 70 | 47 | |||||||||||||||
| Vitamin B-12 | µg | 0.00 | 0.00 | 0.00 | |||||||||||||||
| Vitamin A, RAE | µg | 12 | 22 | 15 | |||||||||||||||
| Vitamin A, IU | IU | 230 | 414 | 278 | |||||||||||||||
| Vitamin D (D2 + D3) | µg | 0.0 | 0.0 | 0.0 | |||||||||||||||
| Vitamin D | IU | 0 | 0 | 0 | |||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 0.035 | 0.063 | 0.042 | |||||||||||||||
| Fatty acids, total monounsaturated | g | 0.055 | 0.099 | 0.067 | |||||||||||||||
| Fatty acids, total polyunsaturated | g | 0.060 | 0.108 | 0.073 | |||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | 0.000 | |||||||||||||||
| Cholesterol | mg | 0 | 0 | 0 | |||||||||||||||
Table 3. Navel orange (raw)
Nutrient | Unit | Value per 100 g | cup sections, without membranes 165 g | fruit (2-7/8″ dia) 140 g | NLEA serving 154 g | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Water | g | 85.97 | 141.85 | 120.36 | 132.39 | ||||||||||||||
| Energy | kcal | 49 | 81 | 69 | 75 | ||||||||||||||
| Protein | g | 0.91 | 1.50 | 1.27 | 1.40 | ||||||||||||||
| Total lipid (fat) | g | 0.15 | 0.25 | 0.21 | 0.23 | ||||||||||||||
| Carbohydrate, by difference | g | 12.54 | 20.69 | 17.56 | 19.31 | ||||||||||||||
| Fiber, total dietary | g | 2.2 | 3.6 | 3.1 | 3.4 | ||||||||||||||
| Sugars, total | g | 8.50 | 14.03 | 11.90 | 13.09 | ||||||||||||||
| Minerals | |||||||||||||||||||
| Calcium, Ca | mg | 43 | 71 | 60 | 66 | ||||||||||||||
| Iron, Fe | mg | 0.13 | 0.21 | 0.18 | 0.20 | ||||||||||||||
| Magnesium, Mg | mg | 11 | 18 | 15 | 17 | ||||||||||||||
| Phosphorus, P | mg | 23 | 38 | 32 | 35 | ||||||||||||||
| Potassium, K | mg | 166 | 274 | 232 | 256 | ||||||||||||||
| Sodium, Na | mg | 1 | 2 | 1 | 2 | ||||||||||||||
| Zinc, Zn | mg | 0.08 | 0.13 | 0.11 | 0.12 | ||||||||||||||
| Vitamins | |||||||||||||||||||
| Vitamin C, total ascorbic acid | mg | 59.1 | 97.5 | 82.7 | 91.0 | ||||||||||||||
| Thiamin | mg | 0.068 | 0.112 | 0.095 | 0.105 | ||||||||||||||
| Riboflavin | mg | 0.051 | 0.084 | 0.071 | 0.079 | ||||||||||||||
| Niacin | mg | 0.425 | 0.701 | 0.595 | 0.655 | ||||||||||||||
| Vitamin B-6 | mg | 0.079 | 0.130 | 0.111 | 0.122 | ||||||||||||||
| Folate, DFE | µg | 34 | 56 | 48 | 52 | ||||||||||||||
| Vitamin B-12 | µg | 0.00 | 0.00 | 0.00 | 0.00 | ||||||||||||||
| Vitamin A, RAE | µg | 12 | 20 | 17 | 18 | ||||||||||||||
| Vitamin A, IU | IU | 247 | 408 | 346 | 380 | ||||||||||||||
| Vitamin E (alpha-tocopherol) | mg | 0.15 | 0.25 | 0.21 | 0.23 | ||||||||||||||
| Vitamin D (D2 + D3) | µg | 0.0 | 0.0 | 0.0 | 0.0 | ||||||||||||||
| Vitamin D | IU | 0 | 0 | 0 | 0 | ||||||||||||||
| Vitamin K (phylloquinone) | µg | 0.0 | 0.0 | 0.0 | 0.0 | ||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 0.017 | 0.028 | 0.024 | 0.026 | ||||||||||||||
| Fatty acids, total monounsaturated | g | 0.030 | 0.050 | 0.042 | 0.046 | ||||||||||||||
| Fatty acids, total polyunsaturated | g | 0.031 | 0.051 | 0.043 | 0.048 | ||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | 0.000 | 0.000 | ||||||||||||||
| Cholesterol | mg | 0 | 0 | 0 | 0 | ||||||||||||||

Health benefits of oranges
Anti-Cancer Effect of Citrus Fruits Flavonoids
Cancer and heart disease are two of the main pathologies worldwide, and the most common causes of death in old age. The two most important ways to reduce cancer risk are the avoidance of cancer-causing agents and finding preventive strategies to stop cancer onset. Citrus fruits like oranges, lemons, limes, bergamot, grapefruits, and tangerines are rich in vitamins and flavonoids, and have long been hypothesized to possess a protective effect against cancer.
Several compounds are responsible for citrus antitumoral effects; of these, vitamin C is considered an important micronutrient through which citrus fruits exert their antioxidant effects by trapping free radicals and reactive oxygen molecules, thus protecting against oxidative damage, inhibiting the formation of carcinogens and protecting DNA from damage 19. Flavonoids also exhibit antioxidant and free radical scavenging properties, interfering with the oxidative/anti-oxidative potential of the cell 20. Furthermore, there are numerous reports showing flavonoids to be able to act at various stages of carcinogenesis, and specifically to interact with proteins involved in cancer development.
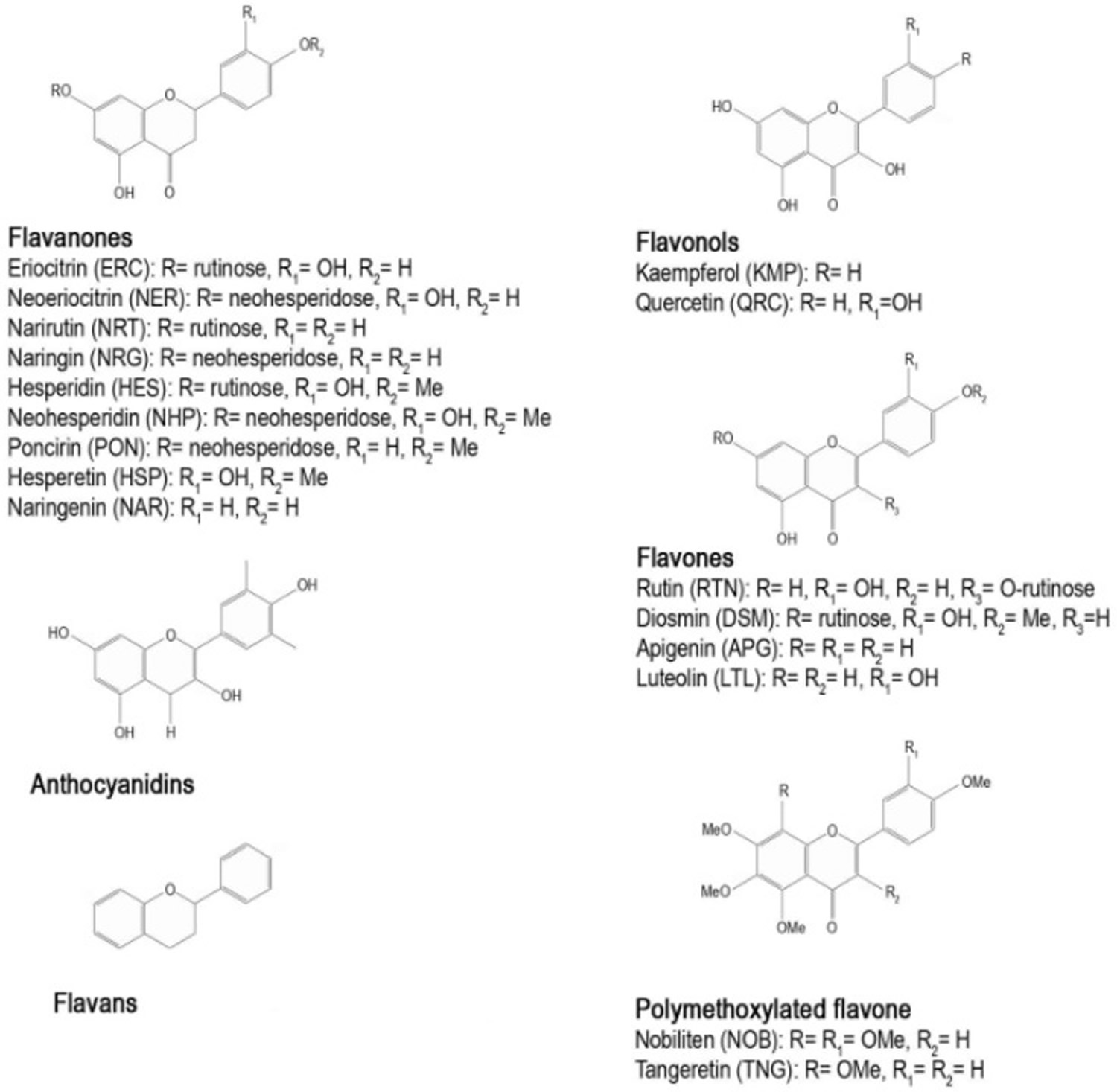
Flavonoids are pigments commonly present in the genus Citrus that are responsible for flower and fruit color. They are low molecular weight polyphenolic compounds, widely found in the plant kingdom as secondary metabolites. They are characterized by a common C6-C3-C6 structure consisting of two benzene rings (A and B) linked through a heterocyclic pyran ring (C) (see Figure 4).
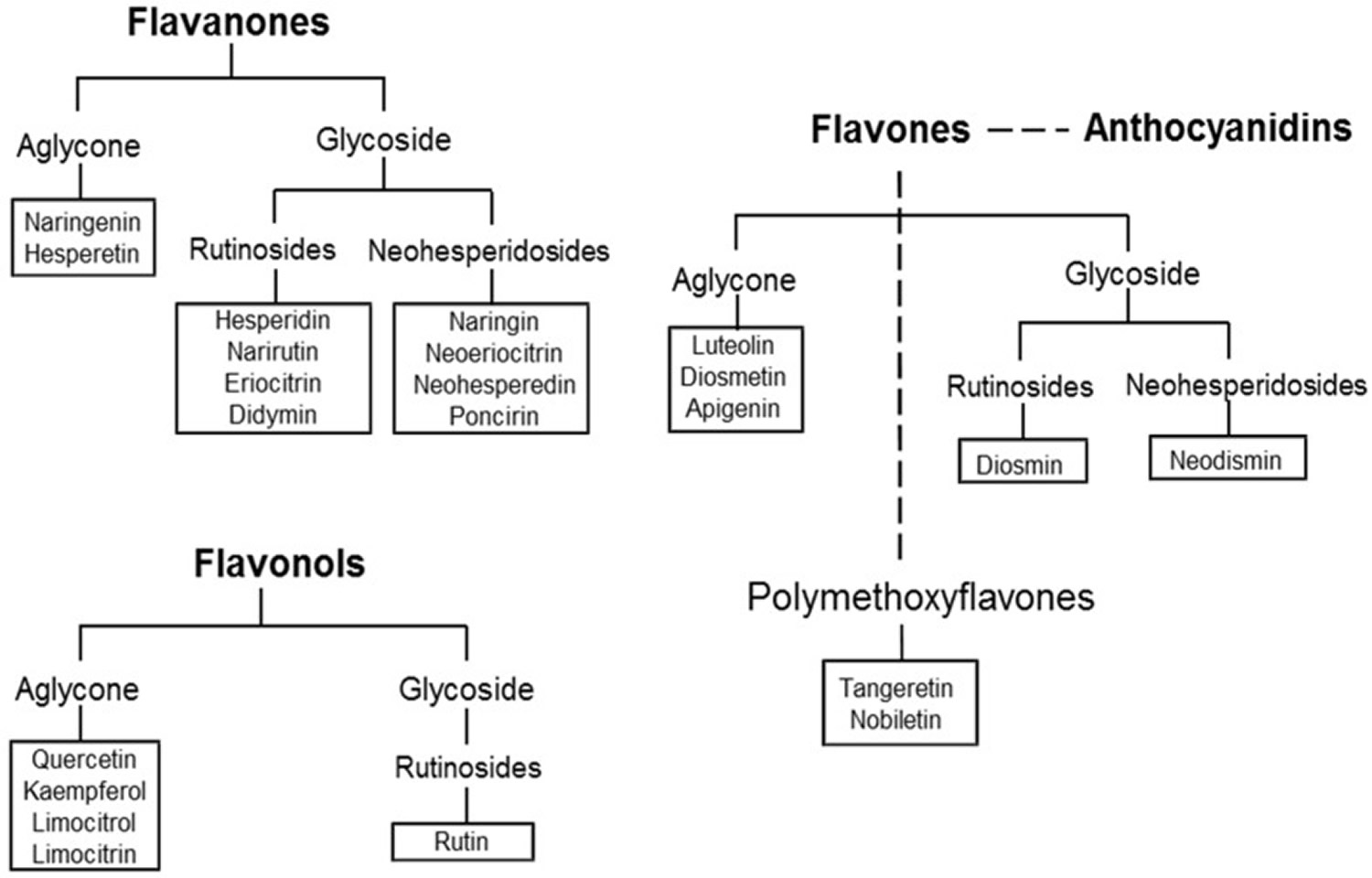
Flavonoids are divided into six classes on the basis of their chemical structures: flavones, flavanones, flavonols, isoflavones, anthocyanidins, and flavans. Flavonoids are mainly present in plants as glycosides, while aglycones (the forms lacking sugar moieties) occur less frequently.
The beneficial effects of flavonoids are mainly due to their anti-oxidant properties which can play a key role in fighting several degenerative diseases. However, there is recent increasing evidence linking the pharmacological activity of Citrus flavonoids to their ability to inhibit the activity of intracellular signaling molecules, such as phosphodiesterases, kinases, topoisomerases, and other regulatory enzymes 21.
Growing experimental evidence supports the view that Citrus flavonoids exert their anti-cancer effects through a number of different mechanisms. They may act as suppressing agents, preventing the formation of new cancers from pro-carcinogens or as blocking agents, disenabling carcinogens from achieving initiation, as well as preventing the onset of the tumor promotion stage. Moreover, Citrus flavonoids may function as transformation agents, facilitating the biotransformation of carcinogens into inactive metabolites. Finally, they behave as both anti-angiogenic and anti-metastatic agents, preventing the formation of new vessels and metastasis 21. Table 4 shows the principal cancer-related processes modulated by Citrus flavonoids.
More than sixty types of flavonoids have been identified in citrus fruits: flavanones are the flavonoids most widely present, followed by flavones, flavonols, and anthocyanins (the latter only in blood oranges). Some flavonoids, such as hesperidin, naringin, and polymethoxylated flavones are characteristic compounds contained in citrus while others like rutin and quercetin are common throughout the plant kingdom 22. Figure 4 shows the main structural formula of some flavonoids isolated from citrus fruits, and their chemical substituents.
The most abundant Citrus flavonoids are flavanones, e.g., hesperidin, naringin, or neohesperidin. However, there are flavones, e.g., diosmin, apigenin, or luteolin, that generally display higher biological activity, despite occuring in much lower concentrations. Of note are apigenin, which has shown particularly good anti-inflammatory activity, and diosmin and rutin that are important venotonic agents present in several pharmaceutical products.
Figure 4. Citrus flavonoids (basic chemical structure)
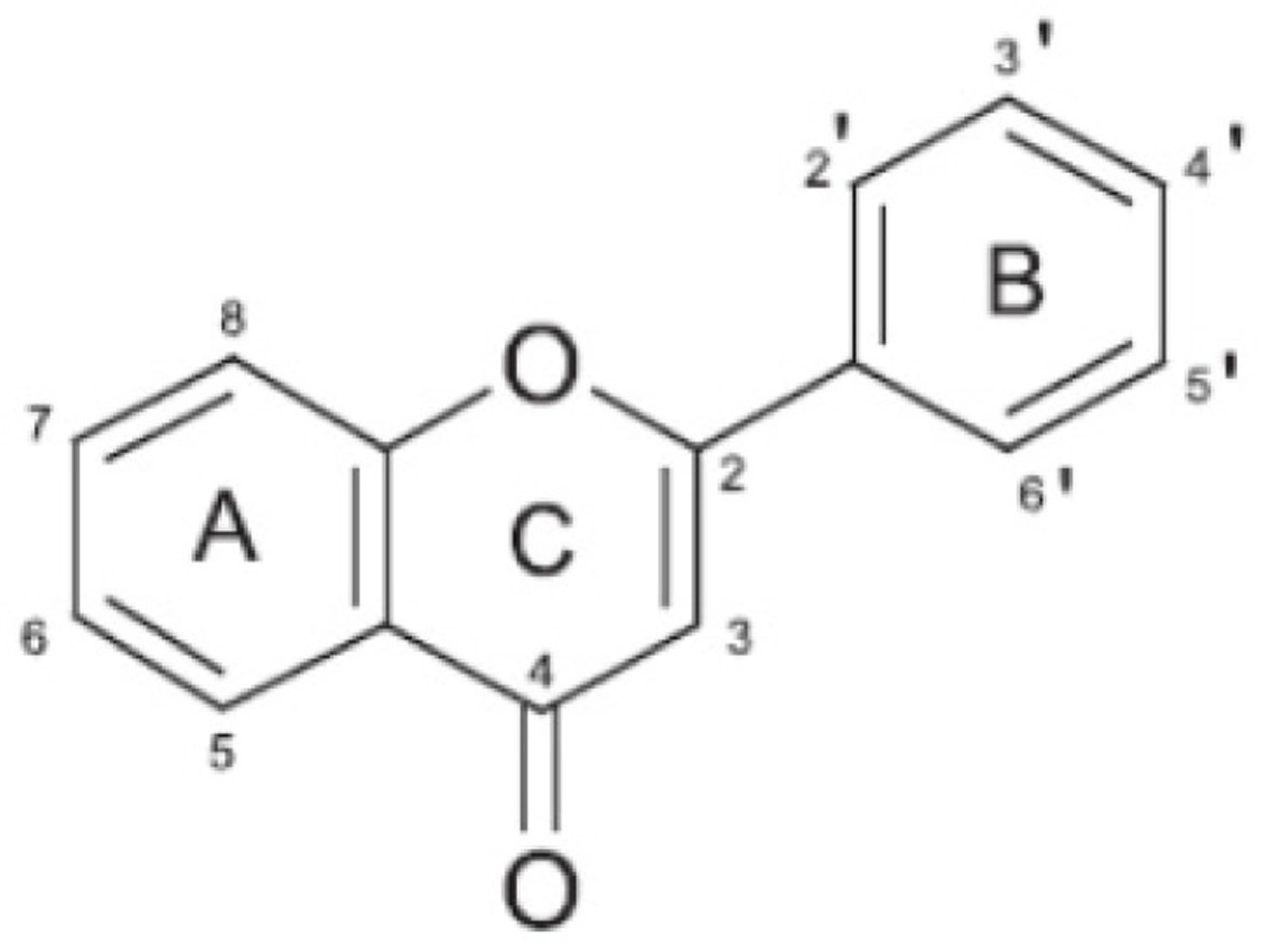
Figure 5. Classification of Citrus flavonoids
[Source 23]Table 5. Main mechanisms through which Citrus flavonoids may act as anti-cancer drugs
| Mechanism by Which Citrus Flavonoids May Fight against Cancer |
| Antioxidant activity, thus counteract oxidative stress |
| Anti-inflammatory effect |
| Phase II enzyme induction, hence enhancing detoxification |
| Phase I enzyme inhibition, thus stopping activation of carcinogens |
| Inhibition of cell proliferation |
| Inhibition of oncogene and/or induction of tumor suppressor gene |
| Induction of cell-cycle arrest |
| Induction of apoptosis |
| Inhibition of signal transduction pathways |
| Anti-angiogenic effect |
| Inhibition of cell adhesion, migration and invasion |
In line with this, some preclinical animal studies have indicated that Citrus juices and extracts may reduce cancer formation and progression. So et al. 24 were the first to show that concentrated Citrus sinensis (sweet orange) juice inhibits the development of mammary tumors induced by 5 mg of DMBA in rats, also suggesting the anti-cancer properties of naringin and quercetin. Two years later, the same authors 25 showed that a double-strength orange juice administration inhibited DMBA-induced mammary tumorigenesis in rats more effectively than double-strength grapefruit juice. Moreover, Miyagi and coworkers 26 showed that orange juice inhibits AOM-induced colon cancer in male rats, suggesting that flavonoids and limonoid glucosides might be responsible for this anti-cancer activity. Citrus reticulata (mandarin) juice has also long been investigated regarding its antitumoral activity. In particular, studies have demonstrated the capability of mandarin juice to suppress the chemically-induced carcinogenesis in colon, tongue, and lung cancers, especially when it is supplemented with added amounts of flavonoids, such as beta-cryptoxanthin and hesperidin 27. Recently, another study have investigated the effects of a flavonoid-rich extract from mandarin juice on three human anaplastic thyroid carcinoma cell lines (CAL-62, C-643, and 8505C cells), showing that mandarin juice reduced cell proliferation through a block of the cell cycle in the G2/M phase, accompanied by low cell death due to autophagy. Moreover, mandarin juice reduced activity of MMP-2, thus decreasing cell migration 28. In another study, Vanamala and coworkers 29 showed that grapefruit juice and limonin produce suppressive effects on AOM-induced colon carcinogenesis by lowering inducible nitric oxide synthases iNOS and cyclooxygenase-2 COX-2 levels and upregulating apoptosis, thereby reducing the formation of aberrant crypt foci. Furthermore, methanolic extract of lemon fruit triggered apoptosis of MCF-7 human breast cancer cells 30. An analogous effect was achieved on the same cell line using lemon seed extract 31.
One of the first population-based case-control studies evaluating whether Citrus intake is associated with a reduced cancer risk was carried out in Shanghai at the end of the 1990s. The aim of this study was to investigate the association between dietary factors and risk of nasopharyngeal carcinoma, Yuan et al. 32 found that high intake of oranges and tangerines was associated with a statistically significant reduction in the risk of nasopharyngeal carcinoma. The study included 935 nasopharyngeal carcinoma patients aged 15 to 74 years interviewed by a questionnaire. Authors concluded that oranges and tangerines are a rich source of vitamin C that can block nitrosamine formation, thereby offering a biological rationale for the anti-nasopharyngeal carcinoma effect. In the 1990s, Bosetti et al. 33 conducted a hospital-based case–control study in three areas of northern Italy on 304 patients affected by a squamous cell carcinoma of the esophagus and 743 controls who were asked to complete a questionnaire. The results of this observational study provide further evidence to support the theory that consumption of citrus fruit is inversely related to esophageal cancer risk. Steevens et al. 34 reached the same conclusions when studying a Netherlands cohort. High intake of citrus fruit has also been associated with reduced risk of cancer of the oral cavity and pharynx 35. Some years later, the same research group, performed a population-based case control study recruiting subjects in Northern Italy and Swiss Canton of Vaud in the 1990s showed that intake of citrus fruit may also reduce laryngeal cancer 36. In line with these findings, a prospective study on 42,311 US men in the Health Professionals Follow-up Study 37 reported that histologically-diagnosed oral premalignant lesions were suppressed by consumption of citrus fruit and citrus fruit juices (30% to 40% lower risk), thus upholding results previously obtained in Europe on smaller subject groups. Interestingly, a meta-analysis showed that the citrus fruit consumption exerts the strongest protective effect against oral cancer compared to all other kinds of fruits 38. Pourfarzi et al. 39 reported that regular intake of fruits could reduce the risk of gastric cancer by more than half. In particular, consumption of citrus fruit was more protective than all other fruits, and subjects eating them more than three times per week had about a 70% lower risk than those who never or infrequently ate citrus fruit. The beneficial effects of citrus fruit with respect to stomach cancer prevention were confirmed by a more recent cohort study performed in Netherlands 34. Epidemiological data from a network of case–control studies strengthen the hypothesis that increasing consumption of citrus fruit may reduce the risk of cancers of the digestive and upper respiratory tract 40. Gonzalez and co-workers 41 also observed a significant inverse correlation between total citrus fruit ingestion and gastric cancer risk.
A large population-based case–control study was conducted on Chinese women in Shanghai by interview. Tangerines, oranges, and grapefruits were found to be inversely associated with breast cancer risk among pre-menopausal women, but the same data was not found to be statistically significant in post-menopausal women 42. However, a more recent study revealed a significant protective effect against breast cancer by oranges, orange juice, and other citrus fruit 43. Intake of either citrus fruit 44 or orange, grapefruit, and their juice 45 also reduced the risk of developing pancreatic cancer. Moreover, citrus fruit intake also seems to be inversely associated with prostate cancer risk 46, and high consumption of both tangerines and oranges was found to be protective against melanoma 47. Recently, a prospective study showed that Citrus consumption, especially if eaten daily, was correlated with reduced incidence of all cancers, although significant results were only obtained for prostate and pancreatic cancer 48. About 40,000 Japanese patients of Ohsaki were followed for up to 9 years to assess the Citrus consumption by a self-administered questionnaire. This study overcomes the bias of other studies described above due to their retrospective nature, confirming the ability of citrus fruit to reduce risk of first and second primary tumors 48. Interestingly, one prospective study indicated that high intake of citrus fruit may confer protection against the development of second primary cancers, particularly in the lung 49.
Furthermore, meta-analyses have confirmed the relationship between citrus fruit intake and decreased risk of cancers. In particular, Bae et al. 50 have provided evidence for the protective effects of high citrus fruit ingestion against stomach cancer risk. However, Bae and coworkers 51 found no association between citrus fruit intake and risk of prostate cancer. Another quantitative systematic review 51 has reported an inverse association between citrus fruit consumption and pancreatic cancer risk, although the effect was limited due to the weakness of study design. More recently, different meta-analyses have highlighted an inverse association between citrus fruit intake and the risk of various types of cancers, such as breast cancer 52 bladder cancers 53 and esophageal cancer 54. A very recent systematic literature review of prospective studies on citrus fruit intake and risk of esophageal and gastric cancers revealed only a marginally significant decreased risk of esophageal cancer and reported no significant inverse association for gastric cardia cancer, but data are still limited 55. On the contrary, some researchers have reported the ineffectiveness of citrus fruit in cancer prevention. For instance, the results from a large European prospective cohort suggested that higher consumption of fruits and vegetables is not associated with decreased risk of pancreatic cancer 56.
Essential Oils of Citrus sinensis (Sweet orange) with Antimutagenic and Antioxidant activity
Essential oils of natural origin are volatile liquid fractions, generally, steam-distillable containing substances responsible for the aroma of plants. Essential oils are important ingredients in the cosmetic industry, in food as flavorings or condiments and in the pharmaceutical production1. In particular, the Citrus genus is a source of vitamin C, flavonoids and terpenoids that have been the subject of study due to their demonstrated beneficial properties 57. The whole oils and its different components have been recognized as generally safe (GRAS) by the Food and Drug Administration (FDA) 58. Previous test tube study had demonstrated that the essential oils of citrus sinensis (sweet orange) and citrus latifolia are antimycotic against Candida albicans, Candida tropicalis, Candida guilliermondii, Candida glabrata and Candida lusitaniae strains isolated from the oral cavity of elder patients 57.
The main components of these essential oils were R-(+)-limonene and α-myrcene for citrus sinensis (sweet orange). Antonella et al. 2013 showed that the R-(+)-limonene, α-terpineol and its chemical derivative 1,8-cineol 59, were able to inhibit mutagenesis induced by 2-amino-anthracene, 2-amino-fluorene and alkylating agent Methyl-metano-sulfonate on S. typhimurium TA98, TA100 and E. coli uvrA strains 60. R-(+)-Limonene has been described as the bioactive component of several herbs and spices used in food preparation 61. The concentration and proportion of these components are variable, but several epidemiological reports indicate that their consumption may reduce gastric cancer risk 61. A study evaluating essential oils from sweet orange (Citrus sinensis) has shown that they act by several antimutagenic mechanisms; they are able to reduce alkylated DNA damages through a reduction in the expression of base-substitution mutations; also, both are antimutagenic by reducing the activation of pre-mutagens like 2AA, and, finally, against quinolones (NOR and 4NQO) as possible ROS (reactive oxygen species)-scavenging mixtures as proven by DPPH 62 (a popular assay used in natural product antioxidant studies), β-carotene bleaching and oxidative stress assays 57.
Citrus fruit peels, especially orange peel, have been used in traditional medicine due to their beneficial effects against digestive and respiratory problems 63. Extracts of orange peel contain up to 90% polymethoxyflavonoids, among which tangeretin, nobiletin and 3,3′;4′,5,6,7,8-heptamethoxyflavon are the most abundant. In a study in which the protective effects of orange peel against photodamage were investigated, Yoshizaki and colleagues 63 observed that treatment of HaCaT keratinocytes with extract of orange peel prior to UVB irradiation was able to modulate UVB-induced inflammatory response by suppression of cyclooxygenase (COX)-2 expression and prostaglandin (PG) E2 production via PPAR-γ activation 63. In another study on acne, sweet orange (Citrus sinensis) and sweet basil (Ocimum basilicum L.) essential oil gel formulations provided good to excellent results in the treatment of acne volunteers because of their antiseptic and keratolytic activity 64.
References- USDA Natural Resources Conservation Service. https://plants.usda.gov/core/profile?symbol=CISI3
- The draft genome of sweet orange (Citrus sinensis). Nat Genet. 2013 Jan;45(1):59-66. doi: 10.1038/ng.2472. Epub 2012 Nov 25. https://www.nature.com/ng/journal/v45/n1/full/ng.2472.html
- Andrés García Lor (2013). Organización de la diversidad genética de los cítricos. https://riunet.upv.es/bitstream/handle/10251/31518/Versi%C3%B3n3.Tesis%20Andr%C3%A9s%20Garc%C3%ADa-Lor.pdf
- Morton, J (1987). “Orange, Citrus sinensis. In: Fruits of Warm Climates”. NewCROP, New Crop Resource Online Program, Center for New Crops & Plant Products, Purdue University. pp. 134–142.
- www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?10782
- http://www.fao.org/faostat/en/#data/QC
- https://www.fas.usda.gov/data/citrus-world-markets-and-trade
- http://www.orangesonline.net/en/11-blog/16-orange-juice-calories?fc=module&module=psblog&controller=posts&post=16
- http://www.orangesonline.net/en/11-blog/30-the-orange-an-important-source-in-vitamin-c-that-helps-your-body?fc=module&module=psblog&controller=posts&post=30
- Kimball, Dan A. (June 30, 1999). “Citrus processing: a complete guide” (2d ed.). New York: Springer: 450. ISBN 0-8342-1258-7.
- https://www.sunkist.com/citrus/oranges/
- http://www.citrusvariety.ucr.edu/citrus/sweet_oranges.html
- Reliability of analytical methods for determining anthocyanins in blood orange juices. J Agric Food Chem. 2000 Jun;48(6):2249-52. http://pubs.acs.org/doi/abs/10.1021/jf991157h
- Influence of glucose on cyanidin 3-glucoside absorption in rats. Mol Nutr Food Res. 2008 Aug;52(8):959-64. doi: 10.1002/mnfr.200700377. http://onlinelibrary.wiley.com/doi/10.1002/mnfr.200700377/epdf
- McGee, Harold (2004). On food and cooking: the science and lore of the kitchen. New York: Scribner. pp. 376. ISBN 0-684-80001-2.
- https://health.gov/dietaryguidelines/
- Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012 Oct 15;134(4):1892-8. doi: 10.1016/j.foodchem.2012.03.090. Epub 2012 Mar 30. http://www.sciencedirect.com/science/article/pii/S0308814612005730
- United States Department of Agriculture Agricultural Research Service. National Nutrient Database for Standard Reference Release 28. https://ndb.nal.usda.gov/ndb/search/list
- Vitamin C: update on physiology and pharmacology. Mandl J, Szarka A, Bánhegyi G. Br J Pharmacol. 2009 Aug; 157(7):1097-110. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2743829/
- Flavonoids: antioxidants or signalling molecules? Williams RJ, Spencer JP, Rice-Evans C. Free Radic Biol Med. 2004 Apr 1; 36(7):838-49. https://www.ncbi.nlm.nih.gov/pubmed/15019969/
- Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular, and anti-inflammatory activity. Benavente-García O, Castillo J. J Agric Food Chem. 2008 Aug 13; 56(15):6185-205. https://www.ncbi.nlm.nih.gov/pubmed/18593176/
- Flavonoid composition of fruit tissues of citrus species. Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Biosci Biotechnol Biochem. 2006 Jan; 70(1):178-92. https://www.jstage.jst.go.jp/article/bbb/70/1/70_1_178/_article
- Cirmi S, Ferlazzo N, Lombardo GE, et al. Chemopreventive Agents and Inhibitors of Cancer Hallmarks: May Citrus Offer New Perspectives? Nutrients. 2016;8(11):698. doi:10.3390/nu8110698. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5133085/
- Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. So FV, Guthrie N, Chambers AF, Moussa M, Carroll KK. Nutr Cancer. 1996; 26(2):167-81. https://www.ncbi.nlm.nih.gov/pubmed/8875554/
- Inhibition of mammary cancer by citrus flavonoids. Guthrie N, Carroll KK. Adv Exp Med Biol. 1998; 439():227-36. https://www.ncbi.nlm.nih.gov/pubmed/9781306/
- Inhibition of azoxymethane-induced colon cancer by orange juice. Miyagi Y, Om AS, Chee KM, Bennink MR. Nutr Cancer. 2000; 36(2):224-9. https://www.ncbi.nlm.nih.gov/pubmed/10890034/
- Kohno H., Maeda M., Honjo S., Murakami M., Shimada R., Masuda S., Sumida T., Azuma Y., Ogawa H., Tanaka T. Prevention of colonic preneoplastic lesions by the β-cryptoxanthin and hesperidin rich powder prepared from Citrus unshiu marc. Juice in male f344 rats. J. Toxicol. Pathol. 1999;12:209–215.
- Flavonoid Fraction of Citrus reticulata Juice Reduces Proliferation and Migration of Anaplastic Thyroid Carcinoma Cells. Celano M, Maggisano V, De Rose RF, Bulotta S, Maiuolo J, Navarra M, Russo D. Nutr Cancer. 2015; 67(7):1183-90. https://www.ncbi.nlm.nih.gov/pubmed/26365817/
- Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Vanamala J, Leonardi T, Patil BS, Taddeo SS, Murphy ME, Pike LM, Chapkin RS, Lupton JR, Turner ND. Carcinogenesis. 2006 Jun; 27(6):1257-65. https://www.ncbi.nlm.nih.gov/pubmed/16387741/
- Apoptosis-mediated inhibition of human breast cancer cell proliferation by lemon citrus extract. Alshatwi AA, Shafi G, Hasan TN, Al-Hazzani AA, Alsaif MA, Alfawaz MA, Lei KY, Munshi A. Asian Pac J Cancer Prev. 2011; 12(6):1555-9. https://www.ncbi.nlm.nih.gov/pubmed/22126498/
- Evaluation of chemopreventive and cytotoxic effect of lemon seed extracts on human breast cancer (MCF-7) cells. Kim J, Jayaprakasha GK, Uckoo RM, Patil BS. Food Chem Toxicol. 2012 Feb; 50(2):423-30. https://www.ncbi.nlm.nih.gov/pubmed/22056335/
- Preserved foods in relation to risk of nasopharyngeal carcinoma in Shanghai, China. Yuan JM, Wang XL, Xiang YB, Gao YT, Ross RK, Yu MC. Int J Cancer. 2000 Feb 1; 85(3):358-63. https://www.ncbi.nlm.nih.gov/pubmed/10652427/
- Food groups and risk of squamous cell esophageal cancer in northern Italy. Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, Trichopoulos D, Lagiou P, Bardini R, Franceschi S. Int J Cancer. 2000 Jul 15; 87(2):289-94. https://www.ncbi.nlm.nih.gov/pubmed/10861489/
- Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Int J Cancer. 2011 Dec 1; 129(11):2681-93. https://www.ncbi.nlm.nih.gov/pubmed/21960262/
- Food groups, oils and butter, and cancer of the oral cavity and pharynx. Franceschi S, Favero A, Conti E, Talamini R, Volpe R, Negri E, Barzan L, La Vecchia C. Br J Cancer. 1999 May; 80(3-4):614-20. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2362347/
- Food groups and laryngeal cancer risk: a case-control study from Italy and Switzerland. Bosetti C, La Vecchia C, Talamini R, Negri E, Levi F, Dal Maso L, Franceschi S. Int J Cancer. 2002 Jul 20; 100(3):355-60. https://www.ncbi.nlm.nih.gov/pubmed/12115553/
- Prospective study of fruits and vegetables and risk of oral premalignant lesions in men. Maserejian NN, Giovannucci E, Rosner B, Zavras A, Joshipura K. Am J Epidemiol. 2006 Sep 15; 164(6):556-66. https://www.ncbi.nlm.nih.gov/pubmed/16847039/
- Association between fruit and vegetable consumption and oral cancer: a meta-analysis of observational studies. Pavia M, Pileggi C, Nobile CG, Angelillo IF. Am J Clin Nutr. 2006 May; 83(5):1126-34. http://ajcn.nutrition.org/content/83/5/1126.long
- The role of diet and other environmental factors in the causation of gastric cancer in Iran–a population based study. Pourfarzi F, Whelan A, Kaldor J, Malekzadeh R. Int J Cancer. 2009 Oct 15; 125(8):1953-60. https://www.ncbi.nlm.nih.gov/pubmed/19569234/
- Citrus fruit and cancer risk in a network of case-control studies. Foschi R, Pelucchi C, Dal Maso L, Rossi M, Levi F, Talamini R, Bosetti C, Negri E, Serraino D, Giacosa A, Franceschi S, La Vecchia C. Cancer Causes Control. 2010 Feb; 21(2):237-42. https://www.ncbi.nlm.nih.gov/pubmed/19856118/
- Fruit and vegetable intake and the risk of gastric adenocarcinoma: a reanalysis of the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study after a longer follow-up. Gonzalez CA, Lujan-Barroso L, Bueno-de-Mesquita HB, Jenab M, Duell EJ, Agudo A, Tjønneland A, Boutron-Ruault MC, Clavel-Chapelon F, Touillaud M, Teucher B, Kaaks R, Boeing H, Steffen A, Trichopoulou A, Roukos D, Karapetyan T, Palli D, Tagliabue G, Mattiello A, Tumino R, Ricceri F, Siersema PD, Numans ME, Peeters PP, Parr CL, Skeie G, Lund E, Quirós JR, Sánchez-Cantalejo E, Navarro C, Barricarte A, Dorronsoro M, Ehrnström R, Regner S, Khaw KT, Wareham N, Key TJ, Crowe FL, Blaker H, Romieu I, Riboli E. Int J Cancer. 2012 Dec 15; 131(12):2910-9. https://www.ncbi.nlm.nih.gov/pubmed/22473701/
- Intake of fruits, vegetables and selected micronutrients in relation to the risk of breast cancer. Malin AS, Qi D, Shu XO, Gao YT, Friedmann JM, Jin F, Zheng W. Int J Cancer. 2003 Jun 20; 105(3):413-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1780272/
- Hormonal and metabolic modulation through nutrition: towards a primary prevention of breast cancer. Ronco AL, De Stéfani E, Stoll M. Breast. 2010 Oct; 19(5):322-32. https://www.ncbi.nlm.nih.gov/pubmed/20542695/
- Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Jansen RJ, Robinson DP, Stolzenberg-Solomon RZ, Bamlet WR, de Andrade M, Oberg AL, Hammer TJ, Rabe KG, Anderson KE, Olson JE, Sinha R, Petersen GM. Cancer Causes Control. 2011 Dec; 22(12):1613-25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3522747/
- Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area. Chan JM, Wang F, Holly EA. Cancer Epidemiol Biomarkers Prev. 2005 Sep; 14(9):2093-7. http://cebp.aacrjournals.org/content/14/9/2093.long
- Do dietary lycopene and other carotenoids protect against prostate cancer? Jian L, Du CJ, Lee AH, Binns CW. Int J Cancer. 2005 Mar 1; 113(6):1010-4. https://www.ncbi.nlm.nih.gov/pubmed/15514967/
- A protective effect of the Mediterranean diet for cutaneous melanoma. Fortes C, Mastroeni S, Melchi F, Pilla MA, Antonelli G, Camaioni D, Alotto M, Pasquini P. Int J Epidemiol. 2008 Oct; 37(5):1018-29. https://www.ncbi.nlm.nih.gov/pubmed/18621803/
- Citrus consumption and cancer incidence: the Ohsaki cohort study. Li WQ, Kuriyama S, Li Q, Nagai M, Hozawa A, Nishino Y, Tsuji I. Int J Cancer. 2010 Oct 15; 127(8):1913-22. https://www.ncbi.nlm.nih.gov/pubmed/20104526/
- Risk factors for the development of second primary tumors among men after laryngeal and hypopharyngeal carcinoma. Dikshit RP, Boffetta P, Bouchardy C, Merletti F, Crosignani P, Cuchi T, Ardanaz E, Brennan P. Cancer. 2005 Jun 1; 103(11):2326-33. https://www.ncbi.nlm.nih.gov/pubmed/15852357/
- Citrus fruit intake and stomach cancer risk: a quantitative systematic review. Bae JM, Lee EJ, Guyatt G. Gastric Cancer. 2008; 11(1):23-32. https://www.ncbi.nlm.nih.gov/pubmed/18373174/
- Citrus fruit intake and pancreatic cancer risk: a quantitative systematic review. Bae JM, Lee EJ, Guyatt G. Pancreas. 2009 Mar; 38(2):168-74. https://www.ncbi.nlm.nih.gov/pubmed/18824947/
- Citrus fruit intake and breast cancer risk: a quantitative systematic review. Song JK, Bae JM. J Breast Cancer. 2013 Mar; 16(1):72-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3625773/
- Intake of fruit and vegetables and risk of bladder cancer: a dose-response meta-analysis of observational studies. Yao B, Yan Y, Ye X, Fang H, Xu H, Liu Y, Li S, Zhao Y. Cancer Causes Control. 2014 Dec; 25(12):1645-58. https://www.ncbi.nlm.nih.gov/pubmed/25248495/
- Citrus Fruit Intake Substantially Reduces the Risk of Esophageal Cancer: A Meta-Analysis of Epidemiologic Studies. Wang A, Zhu C, Fu L, Wan X, Yang X, Zhang H, Miao R, He L, Sang X, Zhao H. Medicine (Baltimore). 2015 Sep; 94(39):e1390. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4616874/
- An update of the WCRF/AICR systematic literature review on esophageal and gastric cancers and citrus fruits intake. Vingeliene S, Chan DS, Aune D, Vieira AR, Polemiti E, Stevens C, Abar L, Rosenblatt DN, Greenwood DC, Norat T. Cancer Causes Control. 2016 Jul; 27(7):837-51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4923099
- Fruit and vegetable consumption and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition. Vrieling A, Verhage BA, van Duijnhoven FJ, Jenab M, Overvad K, Tjønneland A, Olsen A, Clavel-Chapelon F, Boutron-Ruault MC, Kaaks R, Rohrmann S, Boeing H, Nöthlings U, Trichopoulou A, John T, Dimosthenes Z, Palli D, Sieri S, Mattiello A, Tumino R, Vineis P, van Gils CH, Peeters PH, Engeset D, Lund E, Rodríguez Suárez L, Jakszyn P, Larrañaga N, Sánchez MJ, Chirlaque MD, Ardanaz E, Manjer J, Lindkvist B, Hallmans G, Ye W, Bingham S, Khaw KT, Roddam A, Key T, Boffetta P, Duell EJ, Michaud DS, Riboli E, Bueno-de-Mesquita HB. Int J Cancer. 2009 Apr 15; 124(8):1926-34. https://www.ncbi.nlm.nih.gov/pubmed/19107929/
- Toscano-Garibay JD, Arriaga-Alba M, Sánchez-Navarrete J, et al. Antimutagenic and antioxidant activity of the essential oils of Citrus sinensis and Citrus latifolia. Scientific Reports. 2017;7:11479. doi:10.1038/s41598-017-11818-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5597616/
- Espina L, et al. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food control. 2011;22:896–902. doi: 10.1016/j.foodcont.2010.11.021.
- Terpene compounds as possible precursors of 1,8-cineole in red grapes and wines. Fariña L, Boido E, Carrau F, Versini G, Dellacassa E. J Agric Food Chem. 2005 Mar 9; 53(5):1633-6. https://www.ncbi.nlm.nih.gov/pubmed/15740051/
- Antimutagenic and antioxidant activities of some bioflavours from wine. Antonella DS, Federico D, Grazia SM, Gabriela M. Food Chem Toxicol. 2013 Oct; 60():141-6. https://www.ncbi.nlm.nih.gov/pubmed/23891760/
- The role of herbs and spices in cancer prevention. Kaefer CM, Milner JA. J Nutr Biochem. 2008 Jun; 19(6):347-61. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2771684/
- Chemical Profile and Antioxidant Properties of Extracts and Essential Oils from Citrus × limon (L.) Burm. cv. Femminello Comune. Chem Biodivers. 2016 May;13(5):571-81. doi: 10.1002/cbdv.201500186. http://onlinelibrary.wiley.com/wol1/doi/10.1002/cbdv.201500186/full
- Orange peel extract, containing high levels of polymethoxyflavonoid, suppressed UVB-induced COX-2 expression and PGE2 production in HaCaT cells through PPAR-γ activation. Yoshizaki N, Fujii T, Masaki H, Okubo T, Shimada K, Hashizume R. Exp Dermatol. 2014 Oct; 23 Suppl 1():18-22. https://www.ncbi.nlm.nih.gov/pubmed/25234831/
- [Effectiveness of antimicrobial formulations for acne based on orange (Citrus sinensis) and sweet basil (Ocimum basilicum L) essential oils]. Biomedica. 2012 Jan-Mar;32(1):125-33. doi: 10.1590/S0120-41572012000100014. http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0120-41572012000100014&lng=en&nrm=iso&tlng=en