What is artemisinin
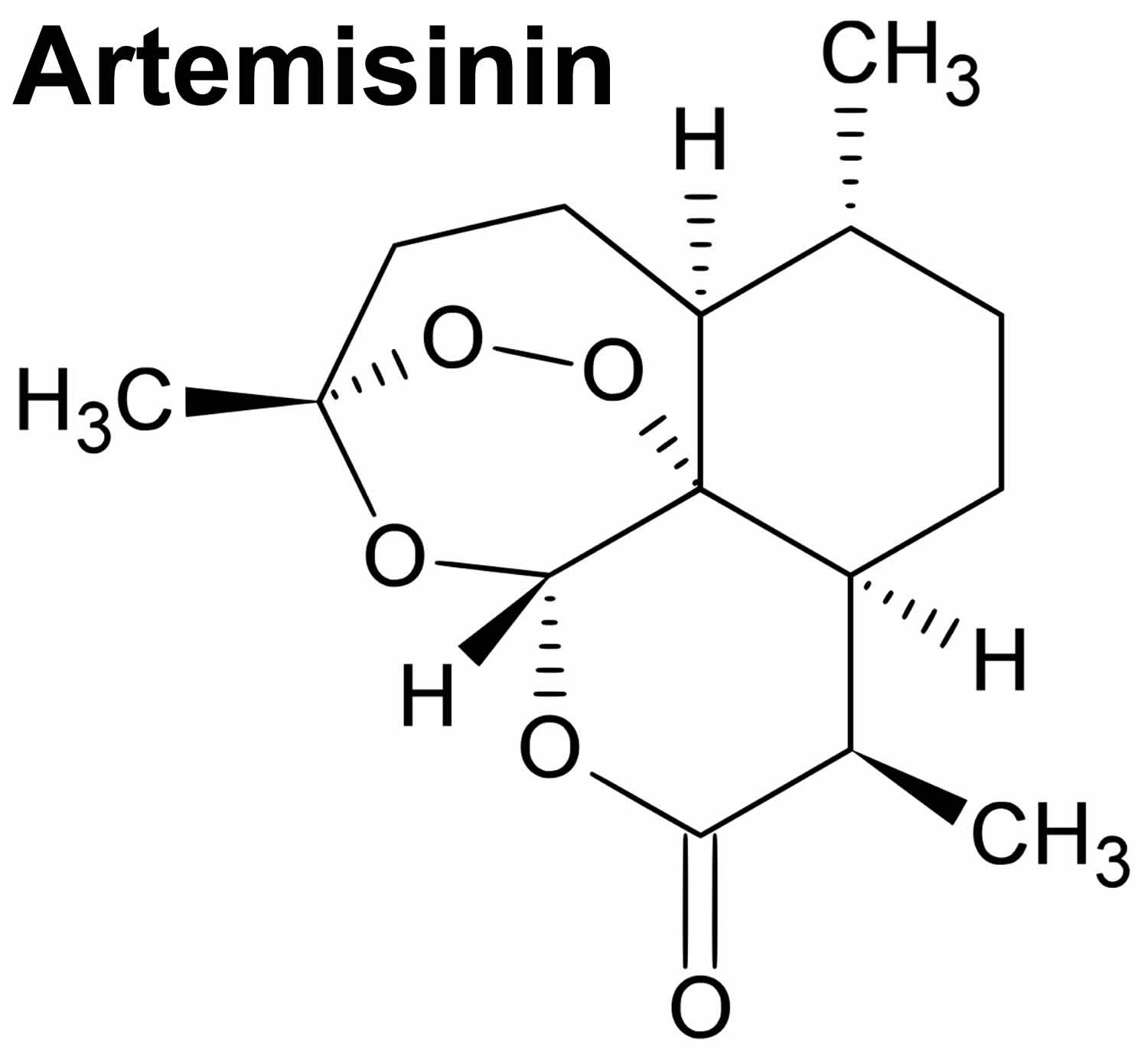
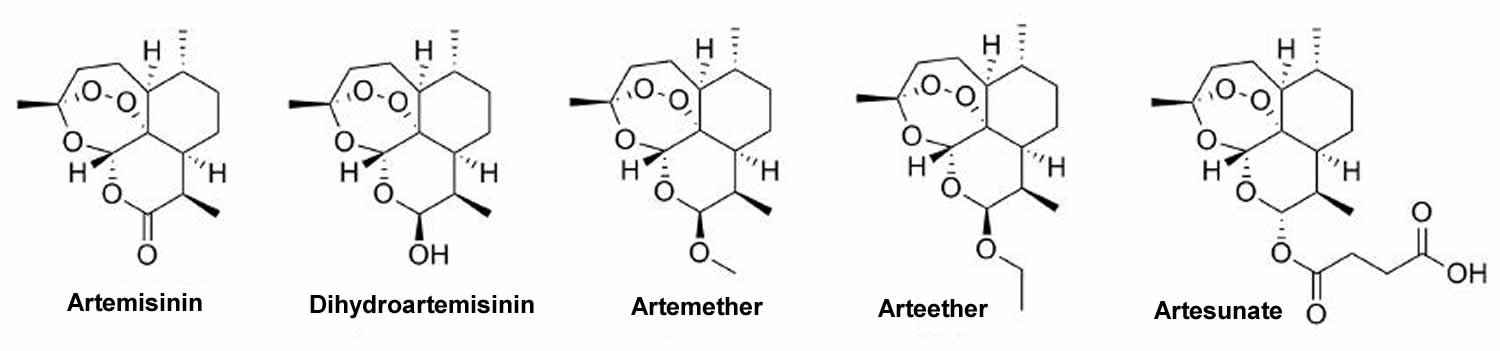
Artemisinin is the most effective antimalarial drug derived from Artemisia annua that was discovered by a Chinese researcher You-You Tu and her team in 1972 1 and in 2015 shared the Nobel Prize for Medicine for her discovery. Chemically, artemisinin is a sesquiterpene lactone with a unique endoperoxide structure, without the nitrogen containing heterocyclic ring like other antimalarial compounds 2. Artemisinin is a sensitive molecule for large-scale derivatization. The carbonyl group can be easily reduced to a hydroxyl group, preserving the crucial endoperoxide moiety, using sodium borohydride to obtain dihydroartemisinin. This led to the preparation of a series of semisynthetic first-generation analogues: artemether; arteether; dihydroartemisinin and artesunic acid, which is commercially known as artesunate (Figure 2). These analogues share the same basic structure of artemisinin with different substituents. The carbonyl exchange exerts influence on the solubility of each artemisinin derivative and on some of their pharmacokinetic properties. Artemisinin itself is poorly soluble in water but is soluble in many aprotic organic solvents. Artemisinin binds to heme, decomposing its endoperoxide bridge, leading to the release of toxic free radicals that damage the malaria (Plasmodium species) parasites, including chloroquine-resistant Plasmodium falciparum.
Artemisinins are derivatives of the Chinese herb known as “qinghao” or sweet wormwood plant (Artemisia annua) 3. The artemisinins have antimalarial activity in vitro and in vivo and are believed to act by release of free radicals into the parasite vacuoles. Artemisinin derivatives are currently the most active antimalarial drugs available and have been introduced around the world as an integral part of therapy of active malaria, always in combination with other antimalarials (also called Artemisinin-based combination therapies) to prevent resistance such as amodiaquine, lumefantrine and mefloquine. Several oral and parenteral formulations of artemisinin derivatives are available worldwide. In the United States, the combination of artemether (20 mg) and lumefantrine (120 mg) was approved for therapy of Plasmodium falciparum malaria in 2009 under the brand name Coartem. The recommended dose for adults is 4 tablets twice daily for 3 days (6 doses). Artesunate (Adamsunate) is also available on a named-patient basis from the Centers for Disease Control and Prevention (CDC) Malaria Hotline. Common side effects of artesunate include nausea, vomiting, anorexia, and dizziness. Potentially severe adverse events include prolongation of the QTc interval and cardiac arrhythmias. Artemisinin derivatives have been associated with a low rate of serum aminotransferase elevations (1% to 4%) that are generally asymptomatic, mild-to-moderate and self-limited, often resolving even with continuing therapy.
Figure 1. Artemisinin chemical structure
[Source 4 ]What is artemisinin used for?
Artemisinin and its derivatives, dihydroartemisinin, artemether, arteether and artesunate (Figure 2) are drugs that act by killing the malaria parasites at an early phase of their development, quickly decreasing their numbers. Other characteristics of artemisinin drugs are low bioavailability and short half-life, which, in addition to previous reports of resistance, make them inefficient as monotherapies for the treatment of malaria. Therefore, artemisinins are recommended in artemisinin-based combination therapy (ACT) 5.
Artemether has several proposed mechanisms of action, including interfering with plasmodial transport proteins and with mitochondrial electron transport and producing free radicals to reduce blood antioxidants and glutathione. When used as a monotherapy, artemether has a relatively high recrudescence rate and is rapidly absorbed after oral dosing, reaching a maximum concentration in adults after approximately 2 hours. Once in systemic circulation, artemether is hydrolyzed in the gut and liver to dihydroartemisinin. The bioavailability of artemether increases 2-fold when given in the presence of food 6.
Artesunate is the most important semisynthetic derivative due to its rapid antimalarial action, lack of considerable clinical resistance and significantly greater solubility in water than artemisinin, dihydroartemisinin or artemether, which is favorable for the preparation of formulations 7.
These drugs act on the Plasmodium gametocytes and blood schizonticides, preventing the transmission to other hosts and reducing the propagation of resistant forms. Literature reports have shown that, in the presence of the ferrous ions from the heme group of hemoglobin, the endoperoxide bridge of these substances can undergo a reductive cleavage, generating free radicals that can alkylate or modify the proteins of the parasite, causing its death. O’Neill and co-workers 8 described in detail the nature of the proposed radical and the mechanistic pathways bioactivation of artemisinin, the reductive scission model and the open peroxide model.
The combination of a fast-acting artemisinin derivative and a long-acting antimalarial with different mechanisms of action is called artemisinin-based combination therapy (ACT). The use of ACTs with at least two drugs is recommended by the World Health Organization (WHO) to prevent or reduce the development of antimalarial resistance. These combined therapies may act concomitantly through several mechanisms of action and in different biochemical targets of the parasite, achieving better results than monotherapy 5.
Replacement of monotherapy by artemisinin-based combination therapy (ACT) has started in all countries where P. falciparum is endemic. Artemisinin derivatives are considered the basis for the treatment of P. falciparum malaria and are used to combat uncomplicated malaria. Several new pharmaceutical fixed-dose combination formulations have been produced to treat this disease because of the high effectiveness and potency of artemisinin-based combination therapy (ACT) 5.
Mixed malaria infections are frequent in endemic areas. These infections are characterized by contamination by more than one species of Plasmodium. People infected with serious Plasmodium vivax can also have Plasmodium falciparum infections, for example. In addition, acute Plasmodium falciparum infections can be succeeded by a presumed relapse of Plasmodium vivax malaria. The treatment of choice for mixed malaria infections is ACTs because this therapy is efficient against all types of malaria 5.
The artemisinin-based combination therapies (ACTs) for the treatment of malaria are as follows:
- Dihydroartemisinin + piperaquine (Eurartesim®)
- Artemether + lumefantrine (Coartem®)
- Artesunate + mefloquine (ASMQ)
- Artesunate + amodiaquine (Winthrop® or Coarsucam™)
- Artesunate + sulfadoxine + pyrimethamine
- Pyronaridine + artesunate (Pyramax®)
- Artemisinin + naphthoquine (ARCO®)
The optimal regimen is the artemisinin-based combination therapy is artemether-lumefantrine (Coartem®). However, when it is not accessible, other artemisinin-based combinations may be used until artemether-lumefantrine can be obtained. Currently artesunate-amodiaquine (Winthrop® or Coarsucam™) is currently the preferred second line therapy. Dosing formulations for artesunate-amodiaquine are less standardized. When artemether-lumefantrine is unavailable and artesunate-amodiaquine will be used as a second option, Centers for Disease Control and Prevention (CDC) should be contacted for specific dosing instructions based on the formulation available in country.
Option 1
- Formulation of artemether-lumefantrine: tablets containing 20 mg of artemether plus 120 mg of lumefantrine.
- Dose: artemether-lumefantrine. Treat with the 6-dose schedule as described below in Table 1.
- Other instructions: Administer with food.
- Metabolism of drug: Maximum blood levels occur 6–12 hours after The half-life is 88 hours in healthy persons and twice as long in persons with malaria. The drug is excreted via the liver and feces.
- Side effects: dizziness, fatigue, anorexia, nausea, vomiting, abdominal pain, palpitations, myalgia, sleep disorders, arthralgia, headache, rash.
- Exceptions for the use of artemether-lumefantrine:
- Children weighing less than 5 kg
- Pregnant women in the first trimester
- Persons with known hypersensitivity to either component
- Alternatives approaches for persons who cannot receive artemether-lumefantrine:
- Children weighing less than 5 kg: Test with blood smear or rapid diagnostic test using a test kit agreed upon in consultation with CDC’s Division of Global Migration and Quarantine. Children who test positive for malaria should be treated according to national guidelines or consult CDC, if no national guidelines exist.
- Pregnant women: Test with blood smear or rapid diagnostic test using a test kit agreed upon in consultation with CDC’s Division of Global Migration and Quarantine. Pregnant or lactating women who test positive for malaria should be treated according to national guidelines or consult CDC, if no national guidelines exist.
- Persons with known hypersensitivity to either artemether or lumefantrine may receive alternative treatment. If allergic to both artemether component, discuss with CDC.
- Persons with symptomatic malaria should be treated according to national guidelines or consult CDC if no national guidelines exist.
Option 2
- Dosage and Formulation of Artesunate-amodiaquine combination therapy. Various formulations are available and CDC should be contacted prior to using for guidance based on available formulations in the country of departure.
- Metabolism
- Artesunate as in Option #1
- Amodiaquine is metabolized primarily in the liver, with plasma half-life of ~5 hours.
- Side effects. Nausea, vomiting, abdominal pain, diarrhea, itching, bradycardia (less common). Prolonged QT (avoid with other medications that prolong QT). Can induce toxic hepatitis and fatal agranulocytosis (with prolonged use). Overdosage can cause syncope, spasticity, convulsions and involuntary movements.
- Exceptions of use for artesunate and amodiaquine
- Children weighing less than 5 kg (artesunate)
- First term of pregnancy (artesunate)
- Known hypersensitivity to either artesunate or amodiaquine
- Known abnormal white blood count, kidney disease or severe hepatic disorder/disease (amodiaquine)
- Caution should be exercised in patients on treatment drugs for HIV/AIDS (amodiaquine), cases should be discussed with CDC prior to presumptive treatment.
- Known prolonged QTc or another medication known to lengthen QTc
Artemisinin uses in cancer
The anticancer activity of artemisinin derivatives has been extensively studied since being first reported in 1993 9. Cancer cells are more prone to cytotoxic effect of artemisinins due to their high intracellular iron levels 10. Thus, introducing these derivatives into the anticancer armamentarium may address current critical challenges facing cancer chemotherapy, namely drug resistance and severe toxicity. In addition, artemisinin and its derivatives are active against human cytomegalovirus (CMV) infections and other viral infections, as well as Schistosoma sp., Fasciola hepatica, Babesia sp., etc., as discovered in the last few years 11.
However, at the present time there is no indication to use artemisinin to treat cancer. Laboratory test tube studies suggest strong co-relation of iron with various cancers 12 and by virtue of a higher rate of iron uptake, cancer cells would be selectively more vulnerable to the cytotoxicity of artemisinin 13. Various test tube (in-vitro) and animal (in-vivo) studies have been done to investigate the role of transferrin and its conjugates in iron-mediated effect of artemisinin in breast cancer 14, 15. Increased and decreased iron content in post and premenopausal women has been explored to be associated with increased breast cancer risk through pathways like oxidative stress and angiogenesis respectively. The endoperoxide moiety of artemisinin forms free radicals on reaction with iron that is essential for cell division and proliferation. Compared with non-cancerous cells, depending on the tumor aggressiveness, cancer cells have a higher number of cell surface transferrin receptors, which pick up iron via interaction with the plasma iron-carrying protein transferrin.
Various nano-formulations of artemisinin is tested for effective artemisinin targeting breast cancer both in-vitro and in-vivo 16. Also, combinational therapies have been done to study and compare the synergistic effect of artemisinin in breast cancer 17. In-vivo studies show the potential benefits of artemisinin in breast cancer treatment 18. Pharmacokinetics and toxicity of artemisinin have also been tested in breast cancer patients during phase-1 study 19. Mechanisms underlying artemisinin-mediated anti-proliferative and apoptosis inducing role in breast cancer have also been explored 20. Role of artemisinin in drug resistance has been studied as well 21. Artemisinin inhibited cell proliferation of estrogen receptor negative (ER-negative) breast cancer cells with fewer efficacies in comparison to estrogen receptor positive (ER-positive) ones. At the same time, cell viability and proliferation of normal breast epithelial MCF10A cells was un-affected. Artemisinin strongly inhibited cancer cell migration and invasion. Along with orphan nuclear receptors (ERRα, ERRβ and ERRγ), artemisinin altered the ERα/ERβ/PR/Her expression status of MCF-7 cells 22. The expression of genes involved in the signaling pathways associated with proliferation, migration, invasion and apoptosis was significantly altered which cooperatively resulted into reduced growth promoting activities of breast cancer cells, suggesting the possibility of artemisinin efficacy as an effective drug in breast cancer treatment 22. Interestingly, artemisinin exhibited inhibitory effect on histone deacetylases (HDACs).
Artemisinin dosage
Treatment of malaria depends on many factors including disease severity, the species of malaria parasite causing the infection, and the part of the world in which the infection was acquired. The latter two characteristics help determine the probability that the organism is resistant to certain antimalarial drugs. Additional factors such as age, weight, and pregnancy status may limit the available options for malaria treatment.
Ideally malaria treatment should not be initiated until the diagnosis has been established by laboratory testing. “Presumptive treatment” (that is without prior laboratory confirmation) should be reserved for extreme circumstances, such as strong clinical suspicion of severe disease in a setting where prompt laboratory diagnosis is not available.
For Plasmodium falciparum infections acquired in areas with chloroquine resistance, four treatment options are available. These include artemether-lumefantrine (Coartem™), which is the preferred option if readily available, and atovaquone-proguanil (Malarone™). These are fixed-dose combination therapies that can be used for pediatric patients ≥5 kg. Quinine sulfate plus doxycycline, tetracycline, or clindamycin is also a treatment option. For the quinine sulfate combination options, quinine sulfate plus either doxycycline or tetracycline is generally preferred to quinine sulfate plus clindamycin because there are more data on the efficacy of quinine sulfate plus doxycycline or tetracycline. Quinine should be given for 3 days, except for infections acquired in Southeast Asia where 7 days of treatment is required. The fourth option, mefloquine, is associated with rare but potentially severe neuropsychiatric reactions when used at treatment dose.We recommend this option only when the other options cannot be used. In addition, mefloquine is not recommended for infections acquired in certain parts of Southeast Asia due to drug resistance. Once a treatment regimen is started, if it is being tolerated, there is no need to switch regimens even if a preferred regimen becomes available. Options for treatment of pregnant women are presented in the “Alternatives for Pregnant Women” section below. Due to the risk of progression to severe disease, uncomplicated malaria treatment should be initiated as soon as possible with the regimen that is most readily available. In addition, clinicians should hospitalize patients with Plasmodium falciparum infection to monitor clinical response and check parasite density every 12–24 hours. Once clinical presentation improves and a decrease in parasite density becomes apparent, treating clinicians can consider outpatient completion of treatment.For pediatric patients, the treatment options are the same as for adults except the drug dose is adjusted by patient weight, and artemether-lumefantrine (Coartem™) and atovaquone-proguanil (Malarone™) can only be used in children ≥5 kg. The pediatric dose should never exceed the recommended adult dose. Pediatric dosing with quinine may be difficult due to unavailability of non-capsule forms of this antimalarial. If using a quinine-based regimen for children less than 8 years old, doxycycline and tetracycline are generally not recommended; therefore, quinine can be given in combination with clindamycin as recommended above. In rare instances, doxycycline or tetracycline can be used in combination with quinine in children less than 8 years old if other treatment options are not available or are not tolerated, and the benefit of adding doxycycline or tetracycline is judged to outweigh the risk. For infections attributed to “species not identified” in areas with chloroquine resistance that are subsequently diagnosed as being due to Plasmodium vivax or Plasmodium ovale, additional treatment with primaquine or tafenoquine should be administered.
For Plasmodium falciparum infections acquired in areas without chloroquine-resistant strains, which include Central America west of the Panama Canal, Haiti, and the Dominican Republic, patients can be treated with oral chloroquine, or, alternatively, hydroxychloroquine at recommended doses. In addition, any of the regimens listed for the treatment of chloroquine-resistant malaria may be used for the treatment of chloroquine-sensitive Plasmodium falciparummalaria. Prompt initiation of an effective regimen is vitally important,so using any one of the effective regimens that is readily available would be the preferred strategy.Due to the risk of progression to severe disease in patients with Plasmodium falciparum infection, patients should be hospitalized to monitor clinical response, and check parasite density every 12–24 hours until clinical presentation improves and a decrease in parasite density becomes apparent. Then, treating clinicians can consider outpatient completion of treatment for patients with improved clinical symptoms and decreasing parasite density. If infections initially attributed to “species not identified” are subsequently diagnosed as being due to Plasmodium vivax or Plasmodium ovale, additional treatment with primaquine or tafenoquine should be administered.
Table 1: Dosing of artemether-lumefantrine (Coartem™) for asymptomatic Plasmodium falciparum malaria
| Weight (kg) | Artemether-lumefantrine (Coartem™) |
|---|---|
| Number of tablets per dose | |
| Given at 0 hours, 8 hours, 24 hours, 36 hours, 48 hours, and 60 hours | |
| < 5 | Not recommended |
| 5–14 | 1 tablet |
| 15–24 | 2 tablets |
| 25–34 | 3 tablets |
| > 35 | 4 tablets |
Alternatives for Pregnant Women
Malaria in pregnant women is associated with high risks of both maternal and perinatal morbidity and mortality. While the mechanism is poorly understood, pregnant women have a reduced immune response and, therefore, less effectively clear malaria infections. In addition, malaria parasites sequester and replicate in the placenta. Pregnant women are three times more likely to develop severe disease than non-pregnant women who acquire malaria in the same geographic area. Malaria infection during pregnancy can lead to miscarriage, premature delivery, low birthweight, congenital infection, and/or perinatal death.Pregnant women diagnosed with uncomplicated malaria caused by chloroquine-resistant Plasmodium falciparuminfection in the second and third trimesters can be treated with artemether-lumefantrine. Artemether-lumefantrine may be used during the first trimester if other treatment options are not available, and if the potential benefit is judged to outweigh the potential risks. In addition, pregnant women of all gestational ages can be treated with mefloquine or a combination of quinine sulfate and clindamycin. Quinine treatment should continue for 7 days for Plasmodium falciparum infections acquired in Southeast Asia and for 3 days for infections acquired elsewhere; clindamycin treatment should continue for 7 days regardless of where the infection was acquired.
For pregnant women diagnosed with uncomplicated malaria caused by Plasmodium malariae, Plasmodium ovale,chloroquine-sensitivePlasmodium vivax, or chloroquine-sensitive Plasmodium falciparuminfection, prompt treatment with chloroquine or hydroxychloroquine (treatment schedule as for non-pregnant adult patients) is recommended. For chloroquine-resistant Plasmodium vivax infections, quinine plus clindamycin, or mefloquine should be given instead. For women in their second or third trimesters, artemether-lumefantrine is an additional option.
For Plasmodium vivaxor Plasmodium ovaleinfections, primaquine phosphate and tafenoquine for radical treatment of hypnozoites should not be given during pregnancy. Pregnant patients with Plasmodium vivaxor Plasmodium ovaleinfections should be maintained on chloroquine chemoprophylaxis for the duration of their pregnancy. The chemoprophylactic dose of chloroquine phosphate is 300 mg base (500 mg salt) orally once per week. After delivery, for pregnant patients with normal G6PD activity infected with Plasmodium vivax or Plasmodium ovale infections, subsequent treatment with primaquine phosphate or tafenoquine as described above is needed, but will depend on breastfeeding. If not breastfeeding, either drug can be used depending on the regimen used to treat the acute malaria episode. For women who are breastfeeding, infants should be tested for G6PD deficiency and if found to have normal activity, oral primaquine phosphate can be given to the mother. Tafenoquine is not recommended during breastfeeding. Women who after delivery cannot take primaquine or tafenoquine should be maintained on weekly chloroquine chemoprophylaxis for a total of 1 year after the acute malaria episode.
Pregnant women diagnosed with severe malaria should be treated aggressively with parenteral antimalarial therapy as described below. Doxycycline and tetracycline are generally not indicated for use in pregnant women. However, in rare instances, doxycycline or tetracycline can be used in combination with quinine if other treatment options are not available or are not being tolerated, and the benefit of adding doxycycline or tetracycline is judged to outweigh the risks.
Atovaquone-proguanil is not indicated for use in pregnant women because of the paucity of data on its safety in pregnant women. However, for pregnant women diagnosed with uncomplicated malaria caused by chloroquine-resistant Plasmodium falciparuminfection, atovaquone-proguanil may be used if other treatment options are not available or are not being tolerated, and if the potential benefit is judged to outweigh the potential risks.
Treatment of Severe Malaria
Patients with any manifestations of severe malaria, e.g. impaired consciousness/coma, hemoglobin <7 g/dL, acute kidney injury, acute respiratory distress syndrome, circulatory collapse/shock, acidosis, jaundice (with other signs of severe malaria), disseminated intravascular coagulation, and/or parasite density of ≥5% should be treated promptly and aggressively with parenteral antimalarial therapy regardless of the species of malaria seen on the blood smear. If severe malaria is strongly suspected but a laboratory diagnosis cannot be made at that time, blood should be collected for diagnostic testing to be done as soon as it becomes available and parenteral antimalarial drugs should be started.
All patients with severe malaria, regardless of infecting species, should be treated with intravenous (IV) artesunate. Clinicians caring for patients with severe malaria at a hospital where commercially-available IV artesunate cannot be obtained within 24 hours, should call the CDC to obtain IV artesunate, which is available through CDC as part of an expanded-use investigational new drug (IND) protocol.
Severe malaria can progress to a fatal outcome rapidly, so its treatment should be initiated as soon as possible. Cliniciansat hospitals where IV artesunate is not in stockshould consider interim treatment with an effective oral antimalarial while obtaining IV artesunate from a commercial source or CDC, whichever is fastest. If the patient is unable to tolerate oral medications, clinicians will need to consider alternative ways to administer oral medications while awaiting IV artesunate. For example, for patients with nausea and vomiting, an anti-emetic preceding the antimalarial may help, and, for comatose patients, a nasogastric tube can be considered.
The preferred antimalarial for interim oral treatment is artemether-lumefantrine (Coartem™) because of its fast onset of action. Other oral options include atovaquone-proguanil (Malarone™), quinine, and mefloquine. Intravenous or oral clindamycin and tetracyclines, such as doxycycline, are not adequate for interim treatment. These drugs are slow-acting antimalarials that would not take effect until well after 24 hours, and they are not effective antimalarials for treatment of severe malaria when used alone. As for any malaria treatment, the interim regimen should not include the medication used for chemoprophylaxis if possible.
When IV artesunate arrives, immediately discontinue the oral medication and start parenteral treatment. Each dose of IV artesunate is 2.4 mg/kg. A dose ofIV artesunate should be given at 0, 12, and 24 hours.
Note that the weight-based dosingapplies to both adults and children.Previously, weight-based dosing was differentiated between children <20kg and those ≥20kg. Current dosing in small children <20kg is based on an unpublished FDA analysis modeling pharmacokinetics in this population using available data. Patients on treatment for severe malaria should have one set of blood smears (thick and thin smear) performed every 12–24 hours until a negative result (no Plasmodium parasites are detected) is reported.
After the initial course of IV artesunate is completed, if parasite density is ≤1% (assessed on a blood smear collected 4 hours after the last dose of IV artesunate) and patient can tolerate oral treatment, a full treatment course with a follow-on regimen must be administered. Artemether-lumefantrine (Coartem™) is the preferred follow-on treatment but adequate alternatives are atovaquone-proguanil (Malarone™), quinine plus doxycycline or clindamycin, or mefloquine. Because of a risk of severe neuropsychiatric adverse events at treatment doses, mefloquine should only be used if other options are not available. If the patient received oral treatment prior to receiving IV artesunate, the same medication can be used as follow-on treatment, but a full regimen is required. As for any malaria treatment, the regimen selection should not include the medication used for chemoprophylaxis.
If, after the 3rd IV artesunate dose, the patient’s parasite density is> 1%, IV artesunate treatment should be continued with the recommended dose once a day for a maximum of 7 days until parasite density is ≤1%. Doses given at 0, 12, and 24 hours count as 1 day, which means up to 6 additional days. Clinicians should proceed with full course of oral follow-on treatment as above as soon as parasite density ≤1% and the patient is able to tolerate oral medications. Clinicians can consider placement of nasogastric tube or use of antiemetics to facilitate administration of oral treatment. Call CDC Malaria Hotline if additional artesunate is needed and/or advice on treatment.
For those patients with parasite density ≤1% but who still cannot tolerate oral medications after completing IV artesunate treatment, clinicians can continue IV artesunate, 1 dose daily not to exceed a total course of 7 days. Clinicians should call CDC Malaria Hotline if advice on treatment is needed.
Intravenous artesunate is safe in infants, children, and pregnant women in the second and third trimesters. There are limited clinical data on women taking IV artesunate in the first trimester of pregnancy, but no harmful effects have been observed. Given that severe malaria is especially life threatening for pregnant womenand their fetuses, and the lack of other treatment options for severe malaria in the United States, the benefits of treatment with IV artesunate outweigh the risks and IV artesunate should not be withheld. The only formal contraindication to IV artesunatetreatment is known allergy to IV artemisinins.
Artemisinin side effects
Common side effects of artesunate include nausea, vomiting, anorexia, and dizziness. Potentially severe adverse events include prolongation of the QTc interval and cardiac arrhythmias. Artemisinin derivatives have been associated with a low rate of serum aminotransferase elevations (1% to 4%) that are generally asymptomatic, mild-to-moderate and self-limited, often resolving even with continuing therapy.
IV artesunate is well tolerated. While rare, delayed post-artemisinin hemolytic anemia has been noted in published case reports following treatment of severe malaria with IV artesunate. Persons with higher parasite density seem to have a higher likelihood of delayed hemolytic anemia after treatment. All persons treated for severe malaria with IV artesunate should be monitored weekly for up to four weeks after treatment initiation for evidence of hemolytic anemia. Weekly laboratory evaluation should include hemoglobin measurement, reticulocyte count, haptoglobin, lactate dehydrogenase (LDH), and total bilirubin. Depending on the intensity of hemolysis and presence of anemia signs and symptoms, blood transfusion may be needed. Cases of delayed post-artemisinin hemolytic anemia in those receiving IV artesunate from CDC should be reported to CDC no longer than 24 hours after diagnosis. Cases of delayed post-artemisnin hemolytic anemia in patients who received Artesunate for Injection should be reported to MedWatch, FDA’s Safety Information and Adverse Event Reporting Program.
References- Tu Y. The discovery of artemisinin (qinghaosu) and gifts from Chinese medicine. Nat Med. 2011;17:1217–1220. doi: 10.1038/nm.2471
- Luo, X. D., and Shen, C. C. (1987). The chemistry, pharmacology, and clinical applications of qinghaosu (artemisinin) and its derivatives. Med. Res. Rev. 7, 29–52. doi: 10.1002/med.2610070103
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Artemisinin. [Updated 2017 Feb 8]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548419
- PINHEIRO, LUIZ C.S., FEITOSA, LÍVIA M., SILVEIRA, FLÁVIA F. DA, & BOECHAT, NUBIA. (2018). Current Antimalarial Therapies and Advances in the Development of Semi-Synthetic Artemisinin Derivatives. Anais da Academia Brasileira de Ciências, 90(1, Suppl. 2), 1251-1271. https://doi.org/10.1590/0001-3765201820170830
- Guidelines for the treatment of malaria. Third edition April 2015. https://www.who.int/malaria/publications/atoz/9789241549127/en/
- KARUNAJEEWA HA. 2012. Artemisinins: Artemisinin, Dihydroartemisinin, Artemether and Artesunate. In: Staines HM and Krishna S (Eds), Treatment and Prevention of Malaria. Antimalarial Drug Chemistry, Action and Use. London, United Kingdom. Springer Basel AG, p. 157-190.
- HAYNES RK. 2006. From artemisinin to new artemisinin antimalarials: biosynthesis, extraction, old and new derivatives, stereochemistry and medicinal chemistry requirements. Curr Top Med Chem 6: 509-537.
- O’NEILL PM, BARTON VE AND WARD SA. 2010. The Molecular Mechanism of Action of Artemisinin-The Debate Continues. Molecules 15: 1705-1721.
- Woerdenbag H.J., Moskal T.A., Pras N., Malingré T.M., El-Feraly F.S., Kampinga H.H., Konings A.W. Cytotoxicity of artemisinin-related endoperoxides to ehrlich ascites tumor cells. J. Nat. Prod. 1993;56(6):849–856. doi: 10.1021/np50096a007
- Lai H.C., Singh N.P., Sasaki T. Development of artemisinin compounds for cancer treatment. Invest. New Drugs. 2013;31(1):230–246. doi: 10.1007/s10637-012-9873-z.
- Liu K, Zuo H, Li G, Yu H, Hu Y. Global Research on Artemisinin and its Derivatives: Perspectives from Patents [published online ahead of print, 2020 Jun 23]. Pharmacol Res. 2020;159:105048. doi:10.1016/j.phrs.2020.105048 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7309871
- Paul I, Jones JM. Apoptosis block as a barrier to effective therapy in non small cell lung cancer. World J Clin Oncol. 2014;5(4):588–594. doi: 10.5306/wjco.v5.i4.588
- Mercer AE, Copple IM, Maggs JL, O’Neill PM, Park BK. The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J Biol Chem. 2011;286(2):987–996. doi: 10.1074/jbc.M110.144188
- Kim SH, Kang SH, Kang BS. Therapeutic effects of dihydroartemisinin and transferrin against glioblastoma. Nutr Res Prac. 2016;10(4):393–397. doi: 10.4162/nrp.2016.10.4.393
- Seo EJ, Wiench B, Hamm R, Paulsen M, Zu Y, Fu Y, Efferth T. Cytotoxicity of natural products and derivatives toward MCF-7 cell monolayers and cancer stem-like mammospheres. Phytomedicine Int J Phytotherapy Phytopharmacol. 2015;22(4):438–443. doi: 10.1016/j.phymed.2015.01.012
- Jabbarzadegan M, Rajayi H, Mofazzal Jahromi MA, Yeganeh H, Yousefi M, Muhammad Hassan Z, Majidi J. Application of arteether-loaded polyurethane nanomicelles to induce immune response in breast cancer model. Artificial Cells Nanomed Biotechnol. 2017;45(4):808–816. doi: 10.1080/21691401.2016.1178131
- Chen G, Gong R, Shi X, Yang D, Zhang G, Lu A, Yue J, Bian Z. Halofuginone and artemisinin synergistically arrest cancer cells at the G1/G0 phase by upregulating p21Cip1 and p27Kip1. Oncotarget. 2016;7(31):50302–50314. doi: 10.18632/oncotarget.10367
- Osaki T, Uto Y, Ishizuka M, Tanaka T, Yamanaka N, Kurahashi T, Azuma K, Murahata Y, Tsuka T, Itoh N, et al. Artesunate enhances the Cytotoxicity of 5-Aminolevulinic acid-based Sonodynamic therapy against mouse mammary tumor cells in vitro. Molecules. 2017;22(4).
- Konig M, von Hagens C, Hoth S, Baumann I, Walter-Sack I, Edler L, Sertel S. Investigation of ototoxicity of artesunate as add-on therapy in patients with metastatic or locally advanced breast cancer: new audiological results from a prospective, open, uncontrolled, monocentric phase I study. Cancer Chemother Pharmacol. 2016;77(2):413–427. doi: 10.1007/s00280-016-2960-7
- Hargraves KG, He L, Firestone GL. Phytochemical regulation of the tumor suppressive microRNA, miR-34a, by p53-dependent and independent responses in human breast cancer cells. Mol Carcinog. 2016;55(5):486–498. doi: 10.1002/mc.22296
- Chekhun VF, Lukianova NY, Borikun TV, Zadvorny TV, Mokhir A. Artemisinin modulating effect on human breast cancer cell lines with different sensitivity to cytostatics. Exp Oncol. 2017;39(1):25–29.
- Kumari K, Keshari S, Sengupta D, Sabat SC, Mishra SK. Transcriptome analysis of genes associated with breast cancer cell motility in response to Artemisinin treatment. BMC Cancer. 2017;17(1):858. Published 2017 Dec 15. doi:10.1186/s12885-017-3863-7 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5732364