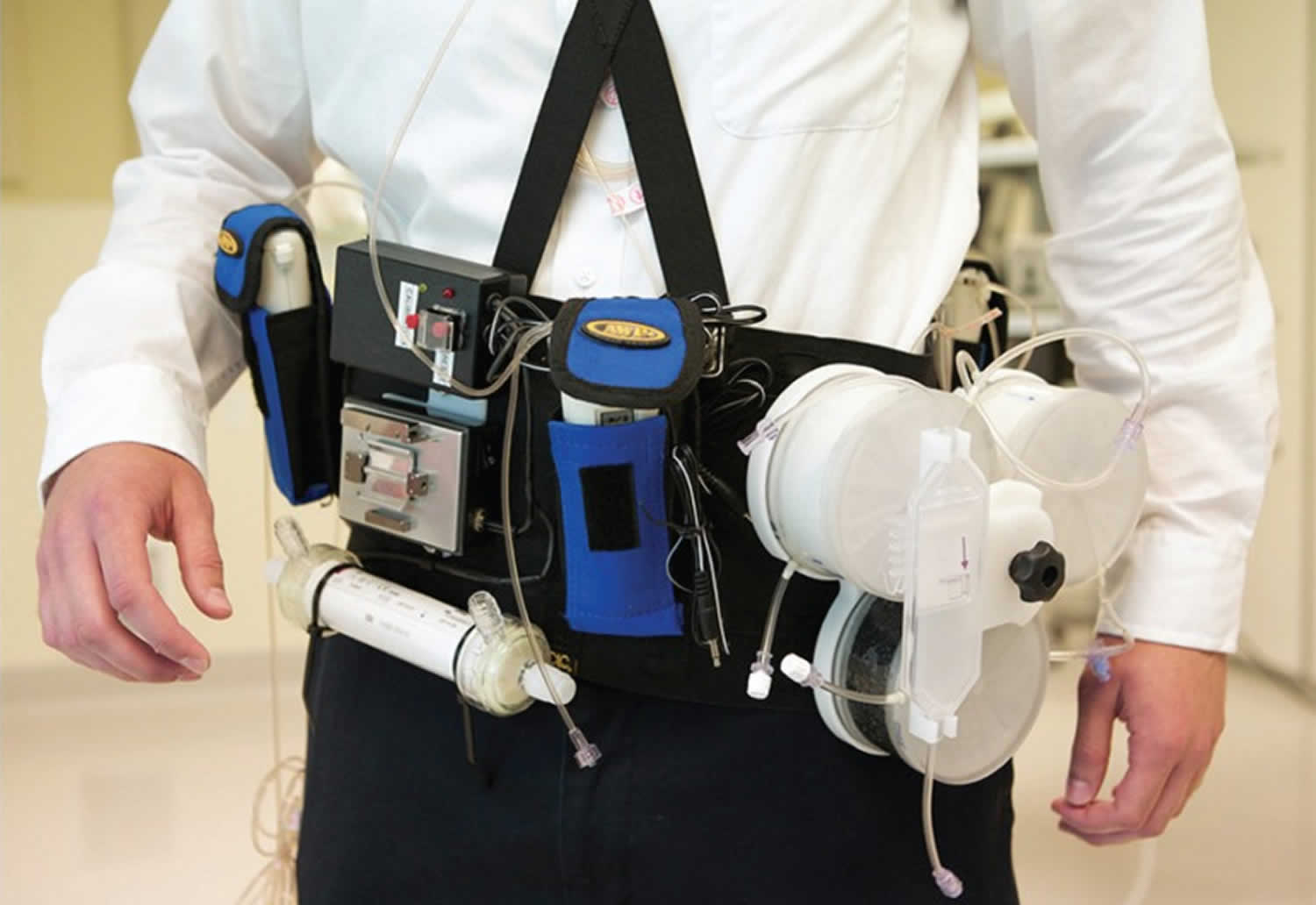
Artificial kidney
Researchers have attempted to develop a wearable artificial kidney since the early days of dialysis but were limited by the technologies available at the time 1. Since then, developments in various fields of research have made lightweight, wearable dialysis systems feasible. These advances include the miniaturization of sensors and pumps 2; small, long-lasting batteries 3; ultra-permeable membranes that reduce dialyzer size 4; and new filtration materials to cleanse and reuse dialysate solutions without the need for large quantities of purified water 5.
The main components of a wearable artificial kidney are, as follows 6:
- dialysis membrane
- dialysate regeneration
- vascular access
- patient monitoring
- power source
- pumping system 7
Three small safety-and-feasibility human trials of one device, the Wearable Artificial Kidney (WAK), have been published 6. One recent trial of the Wearable Artificial Kidney (WAK), published in 2016.12 This was a small, non-randomized pilot study of seven patients who wore the device in a US hospital 8. Five of the seven patients in the study used the WAK for the full 24-hour study period. All patients had conventional hemodialysis for four hours shortly before using the WAK and received anticoagulant therapy to prevent clotting. Participants were not required to follow any dietary restrictions during the study 8. However, several important technical issues with wearable artificial kidneys remain unresolved. Commercial availability of a wearable artificial kidney is still several years away 6.
The Wearable Artificial Kidney (WAK) is worn on a belt around the waist and weighs approximately 5 kg 8. The device includes a miniature, battery-powered pump to power the flow of both the blood and dialysate, and additional micro pumps to control ultrafiltration (fluid removal), the infusion of anticoagulants, and the delivery of other substances to the dialysate. Safety mechanisms include a bubble detector and wetness sensors at the arterial and venous access sites to detect blood leaks. Unlike hemodialysis systems intended for home use, which can require large volumes of purified water, the Wearable Artificial Kidney (WAK) requires only 400 mL of sterile water 8.
No wearable artificial kidney has received regulatory approval for marketing in Canada or elsewhere. In the US, the FDA accepted the Wearable Artificial Kidney (WAK) system for the Expedited Access Pathway (EAP) program 9. This program is intended to improve clinical data collection and reduce the time to regulatory approval for innovative technologies that serve important clinical needs 10.
Five wearable artificial kidneys appear to be in development (see Table 1). Only one of these devices — the Wearable Artificial Kidney or WAK (Blood Purification Technologies Inc., Los Angeles, California) — has published results from studies in humans 8.
Table 1. Wearable Artificial Kidneys in Development
| Device Name | Company/Developer (Country) | Type of Dialysis | Website |
|---|---|---|---|
| Wearable Artificial Kidney (WAK)8 | Blood Purification Technologies Inc. (formerly Xcorporeal Inc.) (US) | Hemodialysis | https://www |
| Automated Wearable Artificial Kidney Peritoneal Dialysis System (AWAK PD)11 | AWAK Technologies Pte. Ltd.(US/Singapore) | Peritoneal dialysis | http://www |
| Vicenza Wearable Artificial Kidney (ViWAK PD)12 | International Renal Research Institute of Vicenza/IRRIV, San Bortolo Hospital (Italy) | Peritoneal dialysis | http://www |
| Nanodialysis (the NaNo)13 | Nanodialysis BV (The Netherlands) | Hemodialysis and peritoneal dialysis combined | http://www |
| Carry Life Renal | Triomed AB (Sweden) | Peritoneal dialysis | http://triomed |
Who might benefit from artificial kidney?
The number of new cases of end-stage kidney disease in Canada, and worldwide, is increasing — mainly due to aging populations and increasing rates of diabetes and hypertension 14. The prevalence of end-stage kidney disease in Canada increased by approximately 3% each year from 2004 to 2009 15. The 2014 Canadian Organ Replacement Register reported that 35,281 Canadians (not including Quebecers) were living with end-stage kidney disease, and 20,690 Canadians were receiving dialysis 16. Each year, approximately 3,000 more Canadians begin long-term dialysis therapy 17.
Currently, 77% of Canadians receiving dialysis are treated with in-centre hemodialysis 16. The remainder receive some form of dialysis treatment at home. Peritoneal dialysis use varies across Canada, ranging from about 20% to 36% of dialysis patients (not including those in Quebec) 17. About half of these patients use automated nocturnal peritoneal dialysis 14.
In the US, the ECRI Institute 18 estimated that 40% to 60% of eligible American patients, particularly those already using home peritoneal dialysis and hemodialysis, may choose to use a wearable artificial kidney — if they are found to be as safe and effective as conventional forms of dialysis.
Artificial kidney safety
In the seven-patient US study, no serious adverse events were reported 8. Some patients reported mild hand or leg cramping, which resolved either when the ultrafiltration rate was decreased or without treatment.12 In five patients, temporary episodes of irregular heartbeat were noted 8. No signs of clinically significant hemolysis were apparent 8.
Vascular access is one of the most problematic areas for hemodialysis because of risks of blood loss, air embolism, infection, and clotting 19. In the UK pilot study, clotting occurred in two patients who were not receiving adequate doses of anticoagulant at the time 20. Vascular access for a wearable dialysis unit is also complicated by movement, which may dislodge the needle or the tubing, thus causing blood leakage, or kinking of the tubing thus causing blockage 19.
One patient in the UK study experienced needle dislodgement, but, as intended, the safety system of the device stopped the blood pump 20. Carbon dioxide bubbles were also reported in the dialysate but not in the blood compartment of the device. The investigators noted that this problem will need to be resolved in future iterations of the device 20.
In the Italian study 21, a clot formed in the catheter in one of the six patients and treatment was stopped after four hours. No other serious adverse events or technical problems were reported.
Dialysis patients who use a wearable artificial kidney will still need to be monitored by health professionals and attend regular clinic visits for the replacement of supplies and device maintenance. However, the frequency of these visits is not yet known 22.
As with other forms of home dialysis, patients, caregivers, and health care professionals will need training on the use of a wearable artificial kidney 21. No information specific to training requirements for wearable artificial kidneys was found, but it may be similar to the training provided for home dialysis.
Bioartificial kidneys
Still only in animal studies, researchers are trying to grow a kidney using embryonic kidney cells, adult stem cells, and by cloning kidney tissue 23. Several research groups are working on implantable, bioartificial kidney prototypes 24. The bioartificial kidney under development through The Kidney Project at the University of California in San Francisco is intended to be fully implantable, similar to a kidney transplant but without the need for immunosuppressant drugs; a wearable version may be developed in the interim 25.
Innovative BioTherapies, Inc. (Ann Arbor, Michigan) is developing a Bioartificial Renal Epithelial Cell System (BRECS) — a wearable bioartificial kidney grown from adult, progenitor kidney cells sourced from non-viable donor organs 26. According to the company website, preclinical trials followed by human trials are expected within three to five years 27.
Other innovations
Qidni Labs Inc. (Kitchener, Ontario) is investigating potential nanotechnologies for miniaturized dialysis components in implantable systems 28.
A peritoneal dialysis system that can recycle dialysate will require fewer connections and reconnections, possibly reducing the risk of peritonitis 1. Because the additional complications of vascular access are not involved, wearable peritoneal dialysis devices could reach the market before wearable hemodialysis devices 29.
References- Davenport A. Portable and wearable dialysis devices for the treatment of patients with end-stage kidney failure: Wishful thinking or just over the horizon? Pediatr Nephrol [Internet]. Dec, 2015. pp. 2053–60.
- Armignacco P, Lorenzin A, Neri M, Nalesso F, Garzotto F, Ronco C. Wearable devices for blood purification: principles, miniaturization, and technical challenges. Semin Dial. 2015 Mar;28(2):125–30
- Yee J. Rise of the small machines: salvation. Adv Chronic Kidney Dis. 2013 Nov;20(6):449–51.
- Burgin T, Johnson D, Chung H, Clark A, McGrath J. Analytical and Finite Element Modeling of Nanomembranes for Miniaturized, Continuous Hemodialysis. Membranes (Basel). 2015;6(1):6. Published 2015 Dec 31. doi:10.3390/membranes6010006 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4812412/
- Cheah WK, Ishikawa K, Othman R, Yeoh FY. Nanoporous biomaterials for uremic toxin adsorption in artificial kidney systems: A review. J Biomed Mater Res B Appl Biomater. 2016 Feb 23
- Topfer LA. Wearable Artificial Kidneys for End-Stage Kidney Disease. 2017 Jan 30. In: CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016-. 150. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425162
- Armignacco P, Lorenzin A, Neri M, Nalesso F, Garzotto F, Ronco C. Wearable devices for blood purification: principles, miniaturization, and technical challenges. Semin Dial. 2015 Mar;28(2):125–30.
- Gura V, Rivara MB, Bieber S, et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight. 2016;1(8):e86397. doi:10.1172/jci.insight.86397 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4936831/
- ECRI Institute. U.S. clinical trial data announced on wearable artificial kidney prototype. Health Technol Trends. 2015 Nov;27(11):1–3. 8
- https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/ucm441467.htm
- Kim S, Roy S. Microelectromechanical systems and nephrology: the next frontier in renal replacement technology. Adv Chronic Kidney Dis. Nov, 2013. pp. 516–35
- Fissell WH, Roy S, Davenport A. Achieving more frequent and longer dialysis for the majority: wearable dialysis and implantable artificial kidney devices. Kidney Int. 2013 Aug;84(2):256–64.
- Wagemans ME, Simonis F, Hazenbrink DH, Gerritsen KG. A miniature artificial kidney for peritoneal dialysis [abstract] Nephrol Dial Transplant [Internet]. May, 2016. p. i235. Available from: http://ndt.oxfordjournals.org/content/31/suppl_1/i235.2.full.pdf+html
- Walker RC, Hanson CS, Palmer SC, Howard K, Morton RL, Marshall MR, et al. Patient and caregiver perspectives on home hemodialysis: a systematic review. Am J Kidney Dis. 2015 Mar;65(3):451–63
- Lance JM. Institut national d’excellence en santé et en services sociaux (INESSS). Budget impact analysis of increasing kidney transplantation in Québec. Montreal: INESSS; Dec 21, 2012.
- Treatment of end-stage organ failure in Canada, 2005 to 2014 [Internet]. Ottawa: Canadian Institute for Health Information; 2016. https://www.cihi.ca/sites/default/files/document/2016_corr_snapshot_enweb.pdf
- Sood MM, Tangri N, Hiebert B, Kappel J, Dart A, Levin A, et al. Geographic and facility-level variation in the use of peritoneal dialysis in Canada: a cohort study. CMAJ Open [Internet]. Jan, 2014. pp. E36–E44
- Wearable artificial kidney for kidney dialysis [Internet]. Plymouth Meeting (PA): ECRI Institute; 2014. https://www.ecri.org/
- Kooman JP, Joles JA, Gerritsen KG. Creating a wearable artificial kidney: where are we now? Expert Rev Med Devices. 2015 Jul;12(4):373–6
- Davenport A, Gura V, Ronco C, Beizai M, Ezon C, Rambod E. A wearable haemodialysis device for patients with end-stage renal failure: a pilot study. Lancet. 2007 Dec 15;370(9604):2005–10
- Gura V, Ronco C, Nalesso F, Brendolan A, Beizai M, Ezon C, et al. A wearable hemofilter for continuous ambulatory ultrafiltration. Kidney Int [Internet]. Feb, 2008. pp. 497–502. http://www.sciencedirect.com/science/article/pii/S0085253815530147
- Leonard EF, Cortell S, Jones J. The path to wearable ultrafiltration and dialysis devices [Internet] Blood Purif. 2011. pp. 92–5.
- Kim S, Fissell WH, Humes DH, Roy S. Current strategies and challenges in engineering a bioartificial kidney. Front Biosci (Elite Ed) [Internet]. 2015. pp. 215–28.
- Armignacco P, Garzotto F, Neri M, Lorenzin A, Ronco C. Wak engineering evolution. Blood Purif. 2015;39(1–3):110–4
- Schatel D. Home HD innovations at the HDU meeting [blog on the Internet]. Madison (WI): Home Dialysis Central; Oct 13, 2016 https://homedialysis.org/news-and-research/blog/173-home-hd-innovations-at-the-hdu-meeting?mc_cid=f77e3c7b36&mc_eid=64c36bc14a
- Buffington DA, Pino CJ, Chen L, Westover AJ, Hageman G, Humes HD. Bioartificial Renal Epithelial Cell System (BRECS): a compact, cryopreservable extracorporeal renal replacement device. Cell Med [Internet]. Jan, 2012. pp. 33–43.
- http://www.innbio.com/
- Strickland E. Three ways to build an artificial kidney. IEEE Spectrum [Internet]. Jun 24, 2016.
- Kooman JP, Joles JA, Gerritsen KG. Creating a wearable artificial kidney: where are we now? Expert Rev Med Devices. 2015 Jul;12(4):373–6.