What is benfotiamine
Benfotiamine (S-benzoylthiamine O-monophosphate) is a synthetic S-acyl derivative of thiamine or vitamin B1 (thiamin) 1. Benfotiamine is converted to thiamine, which serves as a key factor for three enzymes involved in generating energy from glucose 2. Oral administration of benfotiamine raises thiamine (vitamin B1) levels in blood and tissues to a much higher degree than the water-soluble thiamine salts. Benfotiamine is mainly dephosphorylated in the intestinal mucosal cells to generate a lipid-soluble compound, S-benzoythiamine 3. S-benzoythiamine can be promptly transformed to water-soluble thiamine (vitamin B1) and further transformed to several phosphorylated metabolites, such as thiamine monophosphate and thiamine diphosphate (TDP; also known as thiamine pyrophosphate) 4. Originally, benfotiamine was developed in Japan to treat alcoholic neuropathy and other painful neurological complications 5. Nowadays benfotiamine is largely used for treatment of type 2 diabetes and diabetic complications such as diabetic neuropathy 6, diabetic nephropathy, diabetic retinopathy 7 and cardiac angiopathy 8. Other reports suggest that benfotiamine can reverse cardiomyocyte contractile dysfunction 9 and reduce the neuropathic pain 10. During the last few years, there is considerable interest in the therapeutic potential of benfotiamine and its protective effect was elucidated in diabetic complications, such as diabetic neuropathy 8 and alcoholic neuropathy 11. Also, benfotiamine’s beneficial effect was shown in the animal model of Alzheimer’s disease 12, 13, 14. Although a pilot study of benfotiamine has found cognitive improvement in Alzheimer’s disease patients, no clinical studies have determined whether it can prevent age-related cognitive decline or dementia in healthy adults. Benfotiamine significantly reduced the formation of amyloid plaques in APP/PS1 mice 13 and was able to attenuate the glucose-induced increase in beta-amyloid protein synthesis in isolated HEK293 cells 15 and that it also could decrease the tau hyperphosphorylation and improve cognitive deficits in animal Alzheimer’s disease model 16. Further study has shown that benfotiamine has cognitive improvement in patients with mild to moderate Alzheimer’s disease 17. In spite of beneficial effects mentioned above, it also has been proposed that benfotiamine as a protective agent for Alzheimer’s disease treatment was based on the prevention of abnormal glucose metabolism 18. Benfotiamine appears to be safe when used at standard doses.
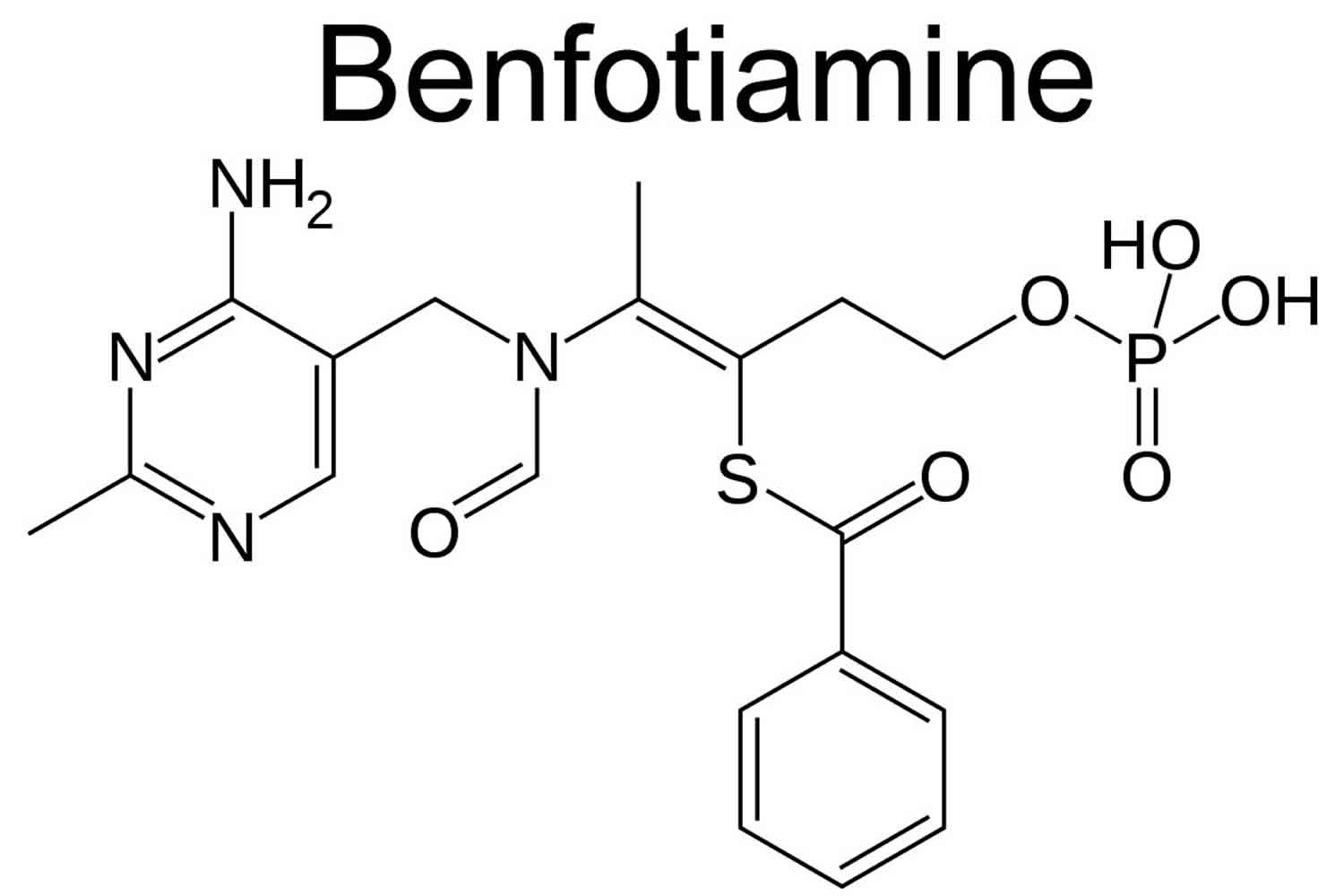
Unlike thiamine, benfotiamine’s structure contains an open thiazole ring that closes once it is absorbed, producing biologically active thiamine (see Figure 1). Several clinical trials in healthy adults have demonstrated the superior absorption of benfotiamine, a lipid-soluble thiamine analogues, compared to water-soluble thiamine salts 19. Higher plasma thiamine levels are achieved with oral benfotiamine administration and blood and tissue concentrations are maintained longer. Oral benfotiamine dosages in these studies ranged from 40-250 mg daily 20. Benfotiamine is absorbed via passive diffusion through the intestinal mucosa and is rapidly converted to biologically active thiamine. Peak plasma concentrations of thiamine after oral benfotiamine administration are at least five times greater than those observed after oral administration of water-soluble thiamine salts. Half-life of benfotiamine is similar to thiamine salts, but oral bioavailability of benfotiamine eight days after administration is roughly 25 percent of the original dose, about 3.6 times greater than after an oral dose of a thiamine salt 21.
Figure 1. Benfotiamine chemical structure
What is thiamine?
Thiamine also known as thiamin or vitamin B1, is naturally present in some foods, added to some food products, and available as a dietary supplement. Thiamine or vitamin B1 plays a critical role in energy metabolism and, therefore, in the growth, development, and function of cells 22. Thiamine is found naturally in many foods and is added to some fortified foods. Food sources of thiamine include whole grains, meat, and fish 23. Breads, cereals, and infant formulas in the United States and many other countries are fortified with thiamine 23. The most common sources of thiamine in the U.S. diet are cereals and bread 24. Pork is another major source of thiamine vitamin. Dairy products and most fruits contain little thiamine 25. About half of the thiamine in the U.S. diet comes from foods that naturally contain thiamine; the remainder comes from foods to which thiamine has been added 26.
You can get the recommended amounts of thiamine by eating a variety of foods, including the following:
- Whole grains and fortified bread, cereal, pasta, and rice
- Meat (especially pork) and fish
- Legumes (such as black beans and soybeans), seeds, and nuts
Heating foods containing thiamine can reduce their thiamine content. For example, bread has 20%–30% less thiamine than its raw ingredients, and pasteurization reduces thiamine content (which is very small to begin with) in milk by up to 20% 25. Because thiamine dissolves in water, a significant amount of the vitamin is lost when cooking water is thrown out 25. Processing also alters thiamine levels in foods; for example, unless white rice is enriched with thiamine, it has one tenth the amount of thiamine in unenriched brown rice 27.
Data on the oral bioavailability of thiamine from food are very limited 28. Some studies do show, however, that thiamine absorption increases when intakes are low 22. Several food sources of thiamine are listed in Table 2 below.
Ingested thiamine from food and dietary supplements is absorbed by the small intestine through active transport at nutritional doses and by passive diffusion at pharmacologic doses 22. Most dietary thiamine is in phosphorylated forms and intestinal phosphatases hydrolyze them to free thiamine before the vitamin is absorbed 22. The remaining dietary thiamine is in free (absorbable) form 23. Humans store thiamine primarily in the liver, but in very small amounts 25. Thiamine (vitamin B1) has a short half-life, so people require a continuous supply of it from the diet.
About 80% of the approximately 25–30 mg of thiamine in the adult human body is in the form of thiamine diphosphate (TDP; also known as thiamine pyrophosphate), the main metabolically active form of thiamine. Bacteria in the large intestine also synthesize free thiamine and thiamine diphosphate (TDP), but their contribution, if any, to thiamine nutrition is currently unknown 29. Thiamine diphosphate (TDP) serves as an essential cofactor for five enzymes involved in glucose, amino acid and lipid metabolism 25.
Levels of thiamine in the blood are not reliable indicators of thiamine status. Thiamine status is often measured indirectly by assaying the activity of the transketolase enzyme, which depends on thiamine diphosphate (TDP), in erythrocyte hemolysates in the presence and absence of added thiamine diphosphate (TDP) 25. The result, known as the “TDP effect,” reflects the extent of unsaturation of transketolase with TDP. The result is typically 0%–15% in healthy people, 15%–25% in those with marginal deficiency, and higher than 25% in people with deficiency. Another commonly used measure of thiamine status is urinary thiamine excretion, which provides data on dietary intakes but not tissue stores 30. For adults, excretion of less than 100 microgram (mcg)/day thiamine in urine suggests insufficient thiamine intake, and less than 40 mcg/day indicates an extremely low intake 31.
How much thiamine do I need?
The amount of thiamine (vitamin B1) you need depends on your age and sex. Average daily recommended amounts are listed below in milligrams (mg). In the US the average dietary thiamine intake for young adult men is about 2 mg/day and 1.2 mg/day for young adult women. A survey of people over the age of 60 found an average dietary thiamine intake of 1.4 mg/day for men and 1.1 mg/day for women 32. However, institutionalization and poverty both increase the likelihood of inadequate thiamin intake in the elderly 33. Wholegrain cereals, legumes (e.g., beans and lentils), nuts, lean pork, and yeast are rich sources of thiamin 34. Because most of the thiamine is lost during the production of white flour and polished (milled) rice, white rice and foods made from white flour (e.g., bread and pasta) are fortified with thiamin in many Western countries. A number of thiamin-rich foods are listed in Table 2 below, along with their thiamine content in milligrams (mg). For more information on the nutrient content of foods, search USDA’s FoodData Central.
Table 1. Recommended Dietary Allowances (RDAs) for Thiamine
| Age | Male | Female | Pregnancy | Lactation |
| Birth to 6 months* | 0.2 mg | 0.2 mg | ||
| 7–12 months* | 0.3 mg | 0.3 mg | ||
| 1–3 years | 0.5 mg | 0.5 mg | ||
| 4–8 years | 0.6 mg | 0.6 mg | ||
| 9–13 years | 0.9 mg | 0.9 mg | ||
| 14–18 years | 1.2 mg | 1.0 mg | 1.4 mg | 1.4 mg |
| 19-50 years | 1.2 mg | 1.1 mg | 1.4 mg | 1.4 mg |
| 51+ years | 1.2 mg | 1.1 mg |
Footnote: * Adequate Intake (AI) = an intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA). Recommended Dietary Allowance (RDA) = average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
[Source 28 ]Food sources of thiamine
Food sources of thiamine include whole grains, meat, and fish 23. Breads, cereals, and infant formulas in the United States and many other countries are fortified with thiamine 23. The most common sources of thiamine in the U.S. diet are cereals and bread 24. Pork is another major source of thiamine vitamin. Dairy products and most fruits contain little thiamine 25. About half of the thiamine in the U.S. diet comes from foods that naturally contain thiamine; the remainder comes from foods to which thiamine has been added 26.
Table 2. Thiamine content of selected foods
| Food | Milligrams (mg) per serving | Percent Daily Value* |
| Rice, white, long grain, enriched, parboiled, ½ cup | 1.4 | 117 |
| Breakfast cereals, fortified with 100% of the daily value for thiamine, 1 serving | 1.2 | 100 |
| Egg noodles, enriched, cooked, 1 cup | 0.5 | 42 |
| Pork chop, bone-in, broiled, 3 ounces | 0.4 | 33 |
| Trout, cooked, dry heat, 3 ounces | 0.4 | 33 |
| Black beans, boiled, ½ cup | 0.4 | 33 |
| English muffin, plain, enriched, 1 muffin | 0.3 | 25 |
| Mussels, blue, cooked, moist heat, 3 ounces | 0.3 | 25 |
| Tuna, Bluefin, cooked, dry heat, 3 ounces | 0.2 | 17 |
| Macaroni, whole wheat, cooked, 1 cup | 0.2 | 17 |
| Acorn squash, cubed, baked, ½ cup | 0.2 | 17 |
| Rice, brown, long grain, not enriched, cooked, ½ cup | 0.1 | 8 |
| Bread, whole wheat, 1 slice | 0.1 | 8 |
| Orange juice, prepared from concentrate, 1 cup | 0.1 | 8 |
| Sunflower seeds, toasted, 1 ounce | 0.1 | 8 |
| Beef steak, bottom round, trimmed of fat, braised, 3 ounces | 0.1 | 8 |
| Yogurt, plain, low fat, 1 cup | 0.1 | 8 |
| Oatmeal, regular and quick, unenriched, cooked with water, ½ cup | 0.1 | 8 |
| Corn, yellow, boiled, 1 medium ear | 0.1 | 8 |
| Milk, 2%, 1 cup | 0.1 | 8 |
| Barley, pearled, cooked, 1 cup | 0.1 | 8 |
| Cheddar cheese, 1½ ounces | 0 | 0 |
| Chicken, meat and skin, roasted, 3 ounces | 0 | 0 |
| Apple, sliced, 1 cup | 0 | 0 |
Footnotes: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed daily values to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The Daily Value for thiamine is 1.2 mg for adults and children age 4 years and older 35. FDA does not require food labels to list thiamine content unless thiamine has been added to the food. Foods providing 20% or more of the Daily Value are considered to be high sources of a nutrient, but foods providing lower percentages of the Daily Value also contribute to a healthful diet.
The U.S. Department of Agriculture’s (USDA’s) Food Data Central website (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing thiamine arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Thiamin-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Thiamin-Food.pdf).
[Source 27 ]Who is at risk of thiamine deficiency?
The following groups are among those most likely to have inadequate thiamine (vitamin B1) status. Thiamine levels are significantly lower than normal in about 20 percent of alcoholics for several reasons: (1) the diet of an alcoholic tends to be heavy in carbohydrates, resulting in decreased thiamine intake; (2) absorption of thiamine and other nutrients is impaired due to the effects of chronic alcohol in-take on the gut’s absorptive mechanisms; (3) chronic alcohol consumption reduces the liver’s ability to store thiamine; and (4) acetaldehyde, an ethanol metabolite, interferes with thiamine utilization 36. These factors result in thiamine deficiency, which in many alcoholics does not respond to supplementation with oral water-soluble thiamine salts. Thiamine deficiency is also frequently observed in patients with diabetic neuropathy 37 and in patients who have undergone gastrectomy 38 or bariatric surgery 39 who subsequently develop neuropathies due to malabsorption.
People with alcohol dependence
In highly industrialized countries, chronic alcohol use disorders appear to be the most common cause of thiamine deficiency 22. Chronic alcohol abuse is associated with thiamine deficiency due to low dietary intake, impaired absorption and utilization, and increased excretion of the vitamin 34. Up to 80% of people with chronic alcoholism develop thiamine deficiency because ethanol reduces gastrointestinal absorption of thiamine, thiamine stores in the liver, and thiamine phosphorylation 40. Also, people with alcoholism tend to have inadequate intakes of essential nutrients, including thiamine.
Chronic alcohol feeding to rats showed a decrease in the active absorption of thiamine linked to the inhibition of thiamine membrane transporter thiamine transporter 1 (THTR-1) in the intestinal epithelium 41. Alcohol consumption in rats also decreases the levels of thiamine transporter 1 (THTR-1) and thiamine transporter 2 (THTR-2) in renal epithelial cells, thus limiting thiamine re-uptake by the kidneys 42.
Older adults
Up to 20%–30% of older adults have laboratory indicators that suggest some degree of thiamine deficiency 23. Possible reasons include low dietary intakes, a combination of chronic diseases, concomitant use of multiple medications, and low absorption of thiamine as a natural result of aging 43. Some small studies have found that the risk of thiamine deficiency is particularly high in elderly people who reside in an institution 44.
People with HIV or AIDS
People with HIV (human immunodeficiency virus) infection have an increased risk of thiamine deficiency and its complications, including beriberi and Wernicke-Korsakoff syndrome 45. Autopsies of 380 people with AIDS (acquired immunodeficiency syndrome) found that almost 10% had Wernicke’s encephalopathy 46 and some experts believe that thiamine deficiency is underdiagnosed in this population 47. The association between thiamine deficiency and HIV or AIDS is probably due to malnutrition as a result of the catabolic state associated with AIDS 48.
People with diabetes
Some small studies have found that thiamine levels in plasma are up to 76% lower in people with type 1 diabetes than in healthy volunteers and 50%–75% lower in people with type 2 diabetes 47. Other studies have shown a higher risk of thiamine deficiency in people with type 1 and/or type 2 diabetes based on tests of erythrocyte transketolase activity 49. These lower thiamine levels might be due to increases in clearance of thiamine by the kidneys. The relevance of these effects to clinical prognosis or outcomes is not known 48.
People who have undergone bariatric surgery
Bariatric surgery is surgery that affects the stomach and how food is digested. Bariatric surgery is designed to make the stomach much smaller, which causes the person to feel full after eating only a small amount of food. Bariatric surgery for weight loss is associated with some risks, including severe thiamine deficiency due to malabsorption that can lead to beriberi or Wernicke’s encephalopathy. A 2008 literature review identified 84 cases of Wernicke’s encephalopathy after bariatric surgery (primarily gastric bypass surgery) between 1991 and 2008 50. About half of these patients experienced long-lasting neurologic impairments. Micronutrient supplements that include thiamine are almost always recommended for patients following bariatric surgery to avoid deficiencies 51.
What are health risks from excessive thiamine?
Your body excretes excess amounts of thiamine in the urine 23. Because of the lack of reports of adverse effects from high thiamine intakes (50 mg/day or more) from food or supplements, the Food and Nutrition Board at the Institute of Medicine of the National Academies did not establish upper limits (ULs) for thiamine 28. They hypothesize that the apparent lack of toxicity may be explained by the rapid decline in absorption of thiamine at intakes above 5 mg. However, the Food and Nutrition Board at the Institute of Medicine of the National Academies noted that in spite of the lack of reported adverse events, excessive intakes of thiamine could have adverse effects.
What is benfotiamine used for?
Wernicke-Korsakoff syndrome
Wernicke-Korsakoff syndrome is a brain disorder, due to thiamine deficiency and one of the most severe neuropsychiatric complication of alcohol abuse that has been associated with both Wernicke’s encephalopathy and Korsakoff syndrome. The term Wernicke-Korsakoff syndrome refers to two different syndromes, each representing a different stage of the disease. Wernicke’s encephalopathy represents the “acute” phase and Korsakoff’s syndrome represents the “chronic” phase 52. However, they are used interchangeable in many sites. Wernicke’s encephalopathy is characterized by confusion, abnormal stance and gait (ataxia), and abnormal eye movements (nystagmus). Korsakoff’s syndrome is observed in a small number of patients. It is a type of dementia, characterized by memory loss and confabulation (filling in of memory gaps with data the patient can readily recall) and involvement of the heart, vascular, and nervous system. Wernicke-Korsakoff syndrome mainly results from chronic alcohol use, but also from dietary deficiencies, prolonged vomiting, eating disorders, systemic diseases (cancer, AIDS, infections), bariatric surgery, transplants, or the effects of chemotherapy 53. Studies indicate that there may be some genetic predisposition for the disease. Treatment involves supplementing the diet with thiamine. Wernicke encephalopathy is an acute syndrome and requires emergency treatment to prevent death and neurologic complications. In cases where the diagnosis is not confirmed, patients should still be treated while additional evaluations are completed 54.
The authors of a 2013 Cochrane review of thiamine to treat or prevent Wernicke-Korsakoff syndrome found only two studies that met their inclusion criteria, and one of these studies has not been published 55. These randomized, double-blind, placebo-controlled trials compared thiamine 5 mg/day by mouth for 2 weeks or daily intramuscular doses of 5 to 200 mg/day thiamine over 2 consecutive days in a total of 177 people with a history of chronic alcohol use. The Cochrane review authors concluded that the evidence from randomized clinical trials is insufficient to guide healthcare providers in selecting the appropriate dose, frequency, duration, or route of thiamine supplementation to treat or prevent Wernicke-Korsakoff syndrome in patients with alcohol abuse 55.
The authors of the European Federation of Neurological Societies guidelines for diagnosing, preventing, and treating Wernicke’s encephalopathy note that even high doses of oral thiamine supplements might not be effective in raising blood thiamine levels or curing Wernicke’s encephalopathy 56. They recommend 200 mg thiamine, preferably intravenously, three times daily (total of 600 mg/day) until the signs and symptoms stop, along with a balanced diet. In its guidelines for managing Wernicke’s encephalopathy in emergency departments, the Royal College of Physicians in London supports the administration of oral thiamine hydrochloride (100 mg three times a day) in patients with adequate dietary intakes of thiamine and no signs or symptoms of Wernicke’s encephalopathy 57. However, the authors recommend parenteral thiamine supplementation for patients at high risk, such as those with ataxia, confusion, and a history of chronic alcohol misuse, because oral supplementation is unlikely to produce adequate blood levels.
Diabetes
The proportion of people with type 1 or type 2 diabetes who have poor thiamine status based on erythrocyte transketolase activity ranges from 17% to 79% in studies conducted to date 58. In a study of 76 consecutive patients with type 1 or type 2 diabetes, for example, 8% had mild thiamine deficiency and 32% had moderate deficiency based on assays of the transketolase enzyme 49.
Some small studies have shown that oral supplementation with 150–300 mg/day thiamine can decrease glucose levels in patients with type 2 diabetes or impaired glucose tolerance 59. However, the authors of these studies did not assess the potential clinical significance of these findings.
A few small randomized studies have assessed the effects of benfotiamine supplements on diabetic neuropathy. Three studies found that, compared to placebo, 120–900 mg/day benfotiamine with or without other B-vitamins decreased the severity of neuropathy symptoms and lowered urinary albumin excretion (a marker of early-stage diabetic nephropathy) 60, 61. However, another study found no effect of 900 mg/day benfotiamine on urinary excretion of albumin or kidney injury molecule-1, a marker of kidney injury 62.
Well-designed studies with larger sample sizes and longer durations are required to determine whether thiamine supplements can reduce glucose levels in patients with diabetes or decrease diabetic compications.
Diabetic neuropathy
Benfotiamine is a transketolase activator that reduces tissue advanced glycation end-products (AGEs). Several independent pilot studies have demonstrated benfotiamine’s effectiveness in diabetic polyneuropathy 63. The Benfotiamine in the treatment of diabetic polyneuropathy (BEDIP) 3-week study used a 400 mg daily dose 64 and the Benfotiamine in Diabetic Polyneuropathy (BENDIP) 6-week study used 300 and 600 mg daily doses 65; both studies demonstrated subjective improvements in neuropathy scores in the groups receiving benfotiamine, with a pronounced decrease in reported pain levels 64. In a 12-week study, the use of benfotiamine plus vitamin B6 or vitamin B12 significantly improved nerve conduction velocity in the peroneal nerve along with appreciable improvements in vibratory perception. An alternate combination of benfotiamine (100 mg) and pyridoxine (100 mg) has been shown to improve diabetic polyneuropathy in a small number of diabetic patients 66. The use of benfotiamine in combination with other antioxidant therapies such as alpha-Lipoic acid are commercially available.
In a double-blind, randomized, placebo-controlled pilot study, 20 subjects with diabetic polyneuropathy were given two 50-mg tablets benfotiamine four times daily (400 mg total daily dose), and 20 subjects received placebo 64. Study duration was three weeks, and assessment was via neuropathy symptoms and vibration sensation scores from both physician and patient. In the treatment group a statistically significant improvement in the neuropathy score was reported compared to placebo. The most significant improvement reported was decrease in pain, whereas, there was no significant improvement in the tuning fork test – a measure of vibration perception 64.
Several studies have investigated the effect of benfotiamine in combination with other B vitamins in the treatment of diabetic neuropathy 66. One study included 45 patients with painful peripheral polyneuropathy. Thirty patients received Milgamma (50 mg benfotiamine and 250 μg vitamin B12 as cyanocobalamin per tablet) at a dose of two tablets four times daily for three weeks (total daily dose: 400 mg ben-fotiamine and 2,000 μg cyanocobalamin), followed by one tablet three times daily for nine weeks 67. The second group of 15 patients received a conventional B-vitamin supplement at a dose of two tablets three times daily for the entire 12-week period. Changes in pain severity and vibration perception thresholds were measured at baseline and at the end of three months 67. All Milgamma-treated patients experienced significant relief in neuropathic pain and a dramatic improvement in vibration perception thresholds. In patients receiving the conventional B-vitamin treatment, slight, non-statistically significant improvement was noted 67.
In a second trial lasting six weeks, 36 subjects with painful diabetic neuropathy were divided into three groups of 12 each. The first group re-ceived Milgamma-N (40 mg benfotiamine, 90 mg pyridoxine, and 250 μg man-made vitamin B12 as cyanocobalamin per capsule) at a dose of two capsules four times daily (320 mg benfotiamine, 720 mg pyridoxine, and 2,000 μg cyanocobalamin daily) 68. The second group received Milgamma-N at a lower dose of one capsule three times daily (120 mg benfotiamine, 270 mg pyridoxine, and 750 μg cyanocobalamin daily), while the third group received one capsule three times daily of straight benfotiamine (150 mg benfotiamine daily). Neuropathy was assessed via pain and vibration sensation at baseline and after three and six weeks. Patients in all three groups reported beneficial therapeutic effects, even at three weeks, although the most significant improve-ment was reported by patients receiving the highest-dose benfotiamine 68.
A double-blind, randomized, placebo-controlled 12-week study examined the effectiveness of another benfotiamine combination containing both vitamins B6 and vitamin B12 in 24 diabetic patients with polyneuropathy. A statistically significant improvement in nerve conduction velocity in the peroneal nerve was observed in the treatment group compared to placebo. A trend toward improvement in vibration sensation was also reported in the treatment group; long-term observation of nine patients over a nine-month period supported the results 66.
Alcoholic neuropathy
Chronic alcoholics commonly develop polyneuropathy as a result of dietary deficiency and poor absorption of thiamine. Benfotiamine’s effect on neuropathy in alcoholics was investigated and compared to thiamine in a randomized, multicenter, placebo-controlled, double-blind study of 84 subjects over an eight-week period 69. Benfotiamine was given orally at 320 mg daily during weeks 1-4, followed by 120 mg daily during weeks 5-8. A second group received Milgamma-N (providing a total daily dose of 320 mg benfotiamine, 720 mg pyridoxine, and 2,000 μg man-made vitamin B12 cyanocobalamin) during weeks 1-4 and a total daily dose of 120 mg benfotiamine, 270 mg pyridoxine, and 750 μg cyanocobalamin during weeks 5-8; a third group received placebo 69.
Parameters measured included vibration perception in the great toe, ankle, and tibia; neural pain intensity; motor function and paralysis; sensory function; and overall neuropathy score and clinical assessment. Neuropathy was scored from 0 (maximum clinical expression of neuropathy symptoms) to 16 (free of symptoms) with scores of ≥10 representing a mild clinical picture. Although benfotiamine therapy was superior to Milgamma-N and placebo for all parameters, results reached statistical significance only for motor function and paralysis and overall neuropathy score. Patients taking benfotiamine had a significantly lower degree of paralysis (90%) than those in the placebo group (60.7%) or the Milgamma-N group (53.9%). Overall neuropathy scores in the benfotiamine group were ≥10 in 93.3 percent of patients, compared to 67.9 percent in the placebo group and 76.9 percent in the Milgamma-N group 69.
Why the benfotiamine-alone group had better results than the Milgamma-N group, despite the fact that the benfotiamine dosage was equivalent, is not completely understood. The authors hypothesize the vitamin B6 and vitamin B12 might compete with the effects of thiamine (vitamin B1) in the Milgamma-N group. On the other hand, in the case of diabetic neuropathy, the positive effects of the combination may be due to the fact that deficiencies of vitamins B1, B6, and B12 are implicated in its possible pathogenesis; whereas, alcoholic neuropathy is associated with primarily a thiamine (vitamin B1) deficiency.
Diabetic retinopathy
In diabetics, the development of microvascular disease is a leading cause of retinopathy and blindness 70. In a study with both animal and in vitro arms, researchers in Germany discovered benfotiamine administration prevented experimental diabetic retinopathy in rats 71.
Advanced glycation end-products (AGE) are the result of non-enzymatic addition of glucose or other saccharides to proteins, lipids, and nucleotides 63. In diabetes, excess glucose accelerates AGE generation that leads to intra- and extracellular protein cross-linking and protein aggregation. Activation of AGE receptors alters intracellular signaling and gene expression, releases pro-inflammatory molecules, and results in an increased production of reactive oxygen species (ROS) that contribute to diabetic microvascular complications 63.
Protein kinase C (PKC) activation is a critical step in the pathway to diabetic microvascular complications 63. It is activated by both hyperglycemia and disordered fatty-acid metabolism, resulting in increased production of vasoconstrictive, angiogenic, and chemotactic cytokines including transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), endothelin (ET-1), and intercellular adhesion molecules (ICAMs) 63.
Diabetic retinopathy is associated with increased advanced glycation end-products (AGE) (a sign of oxidative stress) and elevations in retinal protein kinase C (PKC) activity. In vitro, a 50 μM concentration of benfotiamine completely prevented increases in advanced glycation end-products (AGE) and protein kinase C (PKC) 71.
These same researchers also examined the in vivo effect of benfotiamine on retinas of diabetic rats; non-diabetic rats and untreated diabetic rats served as controls 71. Diabetic rats receiving benfotiamine for 36 weeks demonstrated a 2.5-fold increase in transketolase activity compared to untreated diabetic rats. Hexosamine pathway activity, protein kinase C (PKC) activity, advanced glycation end-products (AGE) formation, and nuclear factor-kappa B (NF-κB) activation in retinas of all three groups were analyzed. In diabetic rats, benfotiamine administration for 36 weeks reduced UDP-GlcNAc to levels lower than those observed in non-diabetic rats, normalized AGE levels and PKC activity, and inhibited activation of NF-κB in diabetic rat retinas. The results of this study indicate lipid-soluble benfotiamine may be of therapeutic benefit in patients with diabetic retinopathy by preventing or delaying the onset and progression of microvascular changes in the retina 71.
Heart failure
Severe thiamine deficiency (wet beriberi) can lead to impaired cardiac function and ultimately congestive heart failure (CHF). Although cardiac manifestations of beriberi are rarely encountered in industrialized countries, congestive heart failure due to other causes is common, especially in the elderly. The rates of poor thiamine status in patients with heart failure have ranged in studies from 21% to 98% 72. Explanations for this association include older age, comorbidities, insufficient dietary intake, treatment with diuretics, and frequent hospitalizations 73.
The authors of one study reported that 33% of 100 patients with chronic heart failure had thiamine deficiency compared to 12% of 50 healthy volunteers 74. Rates of thiamine deficiency were even higher when the investigators excluded those who used thiamine supplements. The different rates of thiamine deficiency in patients with heart failure in these and other studies are probably due to differences in nutrition status, comorbidities, medications and dietary supplements used, and techniques used to measure thiamine status 73.
Diuretics used in the treatment of congestive heart failure, notably furosemide, have been found to increase thiamine excretion, potentially leading to marginal thiamine deficiency 74. A number of studies have examined thiamine nutritional status in congestive heart failure patients and most found a fairly high incidence of thiamine deficiency, as measured by assays of transketolase activity. As in the general population, older congestive heart failure patients were found to be at higher risk of thiamine deficiency than younger ones 75. An important measure of cardiac function in congestive heart failure is the left ventricular ejection fraction (LVEF), which can be assessed by echocardiography. One study in 25 patients found that furosemide use, at doses of 80 mg/day or greater, was associated with a 98% prevalence of thiamine deficiency 76. In a randomized, double-blind study of 30 congestive heart failure patients, all of whom had been taking furosemide (80 mg/day) for at least three months, intravenous (IV) thiamine therapy (200 mg/day) for seven days resulted in an improved left ventricular ejection fraction (LVEF) compared to IV placebo 77. When all 30 of the congestive heart failure patients in that study subsequently received six weeks of oral thiamine therapy (200 mg/day), the average LVEF improved by 22%. This finding may be relevant because improvements in LVEF have been associated with improved survival in congestive heart failure patients 78. However, conclusions from studies published to date are limited due to the small sample sizes of the studies, lack of randomization in some studies, and a need for more precise assays of thiamine nutritional status. Presently, the need for thiamine supplementation in maintaining cardiac function in congestive heart failure patients remains controversial.
The authors of a systematic literature review and meta-analysis found two randomized, double-blind, placebo-controlled trials of thiamine supplementation in people with heart failure that met their eligibility criteria 79. In these trials, thiamine supplements significantly improved net change in left ventricular ejection fraction. The authors did not assess the clinical significance of this finding, however.
More research is needed to determine whether thiamine supplements might benefit people with heart failure, even if they have normal thiamine status.
Alzheimer’s disease
Alzheimer’s disease is the most common type of dementia. Dementia is a syndrome (a group of related symptoms) associated with an ongoing decline of brain functioning. It can affect memory, thinking skills and other mental abilities. The exact cause of Alzheimer’s disease is not yet fully understood, although a number of things are thought to increase your risk of developing the condition. These include:
- increasing age
- a family history of the condition
- untreated depression, although depression can also be one of the symptoms of Alzheimer’s disease
- lifestyle factors and conditions associated with cardiovascular disease
According to animal model studies, thiamine deficiency might play a role in the development of Alzheimer’s disease 2. For example, thiamine deficiency produces oxidative stress in neurons, death of neurons, loss of memory, plaque formation, and changes in glucose metabolism—all markers of Alzheimer’s disease. Autopsy studies have shown that transketolase and other thiamine-dependent enzymes have decreased activity in the brains of people with Alzheimer’s disease 80.
Few studies have assessed the prevalence of thiamine deficiency in people with Alzheimer’s disease. One of these studies found that 13% of 150 patients with cognitive impairment and acute-onset behavioral disturbances were considered thiamine deficient based on plasma levels 81.
The authors of a 2001 Cochrane review assessed three double-blind, randomized trials (including two crossover trials) that compared the effects of 3 g/day oral thiamine to placebo on cognitive function in patients with Alzheimer’s type dementia 82. The three studies randomly assigned fewer than 20 patients each, and the two crossover studies did not include a washout period 83, 84, 85. The review authors stated that it was not possible to draw any conclusions from these three studies because they were small and the publications describing them did not provide enough detail to combine these data in a meta-analysis 82. Larger, well-designed studies are needed to determine whether thiamine supplements are beneficial for Alzheimer’s disease.
There is only one small open-label, uncontrolled study which showed that benfotiamine treatment for 18 months resulted in improved cognitive function in Alzheimer’s patients, though the treatment did not decrease levels of beta-amyloid, a protein common in people with Alzheimer’s disease 86. An ongoing phase II placebo-controlled clinical trial is testing whether benfotiamine can slow cognitive decline in patients with amnestic mild cognitive impairment and Alzheimer’s disease is now underway, however results have not yet been published 87.
End-stage renal disease
The thiamine pool in the body exists mainly as thiamine diphosphate (TDP), 80 percent of which is found in the erythrocytes (red blood cells). Many patients with chronic renal insufficiency demonstrate decreased erythrocyte transketolase activity (a sign of thiamine deficiency) compared to healthy subjects. Consequently, many of these patients also develop neuropathies secondary to kidney disease 88. In a clinical trial of 20 patients with end-stage renal disease, the effect of benfotiamine or thiamine nitrate on TDP blood levels and red blood cell transketolase activity was evaluated. Patients were given single oral doses of 100 mg thiamine nitrate or benfotiamine and blood levels analyzed via high-performance liquid chromatography over a 24-hour period. Compared to patients in the thiamine nitrate group, patients receiving benfotiamine experienced higher TDP concentrations in red blood cells as well as significantly improved erythrocyte transketolase activity. Benfotiamine may be of clinical benefit in thiamine-deficient patients with chronic renal insufficiency 89.
Peripheral vascular disease
In two separate studies, a group of Italian researchers from the University of Turin examined the effect of benfotiamine on endothelial defects and apoptosis in human umbilical vein endothelial cells cultured in the presence of high glucose 90, 91. Either thiamine or benfotiamine prevented AGE production 90 and apoptosis 91 in these cell cultures.
Aldose reductase activity and sorbitol levels are increased in human endothelial cells cultured in glucose, providing one mechanism for endothelial dysfunction in diabetes. Researchers found the addition of either thiamine or benfotiamine resulted in normalized intracellular glucose levels, decreased sorbitol concentrations, and reduction in aldose re-ductase activity 92.
Using an animal model of hind limb ischemia in diabetic mice, researchers in England investigated whether benfotiamine administration was of benefit in reparative neovascularization. Mice were randomly assigned to receive 80 mg/kg/day benfotiamine or placebo in drinking water. Two weeks after benfotiamine initiation, ischemia was surgically induced in the left hind limb, and limb recovery was determined at two weeks post-surgery. Parameters of limb recovery were analyzed via Doppler flowmetry and histological analysis of adductor muscles in the affected limb. It was demonstrated that benfotiamine improves healing and neovascularization in ischemic limbs of diabetic animals, probably via protein kinase B potentiation of angiogenesis, manifesting in increased perfusion and oxygenation of ischemic tissue and improved blood flow to the limb. Benfotiamine also stimulated capillarization and reduced apoptosis in ischemic muscle tissue 93.
Metabolic diseases
Thiamine supplementation is included in the clinical management of genetic diseases that affect the metabolism of carbohydrates and branched-chain amino acids (BCAAs).
Thiamine-responsive pyruvate dehydrogenase complex deficiency
Mutations in pyruvate dehydrogenase complex (PDHC) prevent the efficient oxidation of carbohydrates in affected individuals. Pyruvate dehydrogenase complex (PDHC) deficiency is commonly characterized by lactic acidosis, neurologic and neuromuscular degeneration, and death during childhood. The patients who respond to thiamine treatment (from few mg/day to doses above 1,000 mg/day) exhibit PDHC deficiency due to the decreased affinity of PDHC for thiamine pyrophosphate (TPP) 94. Although thiamine supplementation can reduce lactate accumulation and improve the clinical features in thiamine-responsive patients, it does not constitute a cure 95.
Maple syrup urine disease
Inborn errors of branched-chain amino acid (BCAA) metabolism lead to thiamine-responsive branched-chain ketoaciduria, also known as maple syrup urine disease. Alterations in the BCAA catabolic pathway result in neurologic dysfunction caused by the accumulation of branched-chain amino acids (BCAAs) and their derivatives, branched-chain ketoacids. The therapeutic approach includes a synthetic diet with reduced BCAA content, and thiamine (10-1,000 mg/day) is supplemented to patients with mutations in the E2 subunit of the BCKDH complex 96. In thiamine-responsive individuals, the supplementation has been proven effective to correct the phenotype without recourse to the BCAA restriction diet.
Thiamine-responsive megaloblastic anemia
Mutations in thiamine transporter 1 (THTR-1) that impair intestinal thiamine uptake and cause thiamine deficiency have been found in patients affected by thiamine-responsive megaloblastic anemia. This syndrome is characterized by megaloblastic anemia, diabetes mellitus, and deafness. A review of 30 cases reported additional neurologic, visual, and cardiac impairments 97. Oral doses of thiamine (up to 300 mg/day) maintain health and correct hyperglycemia in prepubescent children. However, after puberty, a decline in pancreatic function results in the requirement of insulin together with thiamine to control the hyperglycemia. One study also reported that the treatment of a four-month-old girl with 100 mg/day of thiamine did not prevent hearing loss at 20 months of age 98.
Biotin-responsive basal ganglia disease
Biotin-responsive basal ganglia disease, also called thiamine metabolism dysfunction syndrome-2, is caused by mutations in the gene coding for thiamine transporter 2 (THTR-2). The clinical features appear around three to four years of age and include sub-acute encephalopathy (confusion, drowsiness, altered level of consciousness), ataxia, and seizures. A retrospective study of 18 affected individuals from the same family or the same tribe in Saudi Arabia was recently conducted. The data showed that biotin monotherapy (5-10 mg/kg/day) efficiently abolished the clinical manifestations of the disease, although one-third of the patients suffered from recurrent acute crises. Often associated with poor outcomes, acute crises were not observed after thiamine supplementation started (300-400 mg/day) and for a five-year follow-up period. Early diagnostic and immediate treatment with biotin and thiamine led to positive outcomes 99.
Cancer
Thiamine deficiency has been observed in some cancer patients with rapidly growing tumors. Research in cell culture and animal models indicates that rapidly dividing cancer cells have a high requirement for thiamine 100. All rapidly dividing cells require nucleic acids at an increased rate, and some cancer cells appear to rely heavily on the TPP-dependent enzyme, transketolase, to provide the ribose-5-phosphate necessary for nucleic acid synthesis. A recent study found that the levels of THTR-1, transketolase, and TPP mitochondrial transporters were increased in samples of human breast cancer tissue compared to normal tissue, suggesting an adaptation in thiamine homeostasis in support of cancer metabolism 101. Thiamine supplementation in cancer patients is common to prevent thiamine deficiency, but Boros et al. 102 caution that too much thiamine may actually fuel the growth of some malignant tumors, suggesting that thiamine supplementation be reserved for those cancer patients who are actually deficient in thiamine. Presently, there is no evidence available from studies in humans to support or refute this theory. However, it would be prudent for individuals with cancer who are considering thiamine supplementation to discuss it with the clinician managing their cancer therapy.
Benfotiamine dosage
Benfotiamine supplements are available over-the-counter (OTC), often in capsules containing 150–300 mg. Based on clinical studies to date, daily doses of oral benfotiamine range from 300-450 mg daily in divided doses. In an open-label uncontrolled trial, a daily dose of 300 mg showed cognitive improvement in Alzheimer’s disease patients 86. Other studies in clinical populations have used Benfotiamine doses ranging from 200–600 mg/day 103, 104, 105.
Benfotiamine side effects
Benfotiamine is generally considered safe for most people when taken at standard doses, though long-term safety has not been studied. Benfotiamine administration appears to be safe with no reports of toxicity in the scientific literature. In clinical trials, side effects were mild and included gastrointestinal issues and skin reactions 104. Because benfotiamine gets converted to thiamine (vitamin B1), and thiamine may cause low blood pressure or low blood glucose, people taking drugs or herbs to lower blood pressure or blood glucose should exercise caution 106.
Toxicity
The Food and Nutrition Board did not set a tolerable upper intake level (UL) for thiamine because there are no well-established toxic effects from consumption of excess thiamine in food or through long-term, oral supplementation (up to 200 mg/day). A small number of life-threatening anaphylactic reactions have been observed with large intravenous doses of thiamine 32.
Interactions with medications
Although benfotiamine is not known to interact with any medications, certain medications can have an adverse effect on thiamine levels. Some examples are provided below. Individuals taking these and other medications on a regular basis should discuss their thiamine status with their healthcare providers.
- Furosemide: Furosemide (Lasix®) is a loop diuretic used to treat edema and hypertension by increasing urinary output. Research has linked the use of furosemide to decreases in thiamine concentrations, possibly to deficient levels, as a result of urinary thiamine loss 76. Whether thiamine supplements are effective for preventing thiamine deficiency in patients taking loop diuretics needs to be determined in clinical studies.
- Chemotherapy with fluorouracil: Fluorouracil (also known as 5-fluorouracil; Adrucil®) is a chemotherapy drug that is commonly used to treat colorectal and other solid cancers. The published literature includes several cases of beriberi or Wernicke’s encephalopathy resulting from treatment with this drug, possibly because the drug might increase thiamine metabolism and block the formation of TDP, the active form of thiamine 107. Thiamine supplements might reverse some of these effects.
- Phenytoin: Reduced blood levels of thiamine have been reported in individuals with seizure disorders (epilepsy) taking the anticonvulsant medication, phenytoin, for long periods of time 108.
- Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A. The multifaceted therapeutic potential of benfotiamine. Pharmacol Res. 2010 Jun;61(6):482-8. doi: 10.1016/j.phrs.2010.02.008
- Gibson GE, Hirsch JA, Cirio RT, Jordan BD, Fonzetti P, Elder J. Abnormal thiamine-dependent processes in Alzheimer’s Disease. Lessons from diabetes. Mol Cell Neurosci. 2013 Jul;55:17-25. doi: 10.1016/j.mcn.2012.09.001
- Volvert ML, Seyen S, Piette M, et al. Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives. BMC Pharmacol. 2008;8(1):10. doi:10.1186/1471-2210-8-10
- Greb A, Bitsch R. Comparative bioavailability of various thiamine derivatives after oral administration. Int J Clin Pharmacol Ther. 1998 Apr;36(4):216-21.
- Fujiwara M (1954) Allithiamine: a newly found derivative of vitamin B. Journal of Biochemistry (Tokyo) 2, 273–285.
- Varkonyi T, Putz Z, Keresztes K, et al. Current options and perspectives in the treatment of diabetic neuropathy. Curr Pharm Des. 2013;19(27):4981–5007. doi:10.2174/13816128113199990310
- Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9(3):294–299. doi:10.1038/nm834
- Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A (2010) The multifaceted therapeutic potential of benfotiamine. Pharmacol Res 61: 482–488. 10.1016/j.phrs.2010.02.008
- Ceylan-Isik AF, Wu S, Li Q, Li SY, Ren J. High-dose benfotiamine rescues cardiomyocyte contractile dysfunction in streptozotocin-induced diabetes mellitus. J Appl Physiol (1985). 2006;100(1):150–156. doi:10.1152/japplphysiol.00988.2005
- Nacitarhan C, Minareci E, Sadan G. The effect of benfotiamine on mu-opioid receptor mediated antinociception in experimental diabetes. Exp Clin Endocrinol Diabetes. 2014;122(3):173–178. doi:10.1055/s-0033-1363977
- Manzardo AM, He J, Poje A, Penick EC, Campbell J, Butler MG (2013) Double-blind, randomized placebo-controlled clinical trial of benfotiamine for severe alcohol dependence. Drug Alcohol Depend. 133:562–70. 10.1016/j.drugalcdep.2013.07.035
- Markova N, Bazhenova N, Anthony DC, Vignisse J, Svistunov A, Lesch KP, Bettendorff L, Strekalova T. Thiamine and benfotiamine improve cognition and ameliorate GSK-3β-associated stress-induced behaviours in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2017 Apr 3;75:148-156. doi: 10.1016/j.pnpbp.2016.11.001
- Pan X, Gong N, Zhao J, Yu Z, Gu F, Chen J, Sun X, Zhao L, Yu M, Xu Z, Dong W, Qin Y, Fei G, Zhong C, Xu TL. Powerful beneficial effects of benfotiamine on cognitive impairment and beta-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain. 2010 May;133(Pt 5):1342-51. doi: 10.1093/brain/awq069
- Bozic, I., Savic, D., Laketa, D., Bjelobaba, I., Milenkovic, I., Pekovic, S., Nedeljkovic, N., & Lavrnja, I. (2015). Benfotiamine attenuates inflammatory response in LPS stimulated BV-2 microglia. PloS one, 10(2), e0118372. https://doi.org/10.1371/journal.pone.0118372
- Sun XJ, Zhao L, Zhao N, Pan XL, Fei GQ, Jin LR, et al. (2012) Benfotiamine prevents increased β-amyloid production in HEK cells induced by high glucose. Neurosci Bull. 28:561–6. 10.1007/s12264-012-1264-0
- Tapias V, Jainuddin S, Ahuja M, et al. Benfotiamine treatment activates the Nrf2/ARE pathway and is neuroprotective in a transgenic mouse model of tauopathy. Hum Mol Genet. 2018;27(16):2874–2892. doi:10.1093/hmg/ddy201
- Gibson GE, Luchsinger JA, Cirio R, et al. Benfotiamine and cognitive decline in Alzheimer’s disease: results of a randomized placebo-controlled Phase IIa clinical trial. J Alzheimers Dis. 2020;78(3):989–1010. doi:10.3233/JAD-200896
- Pan X, Gong N, Zhao J, et al. Powerful beneficial effects of benfotiamine on cognitive impairment and beta-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain. 2010;133(5):1342–1351. doi:10.1093/brain/awq069
- Benfotiamine. Alternative Medicine Review Volume 11, Number 3, September 2006. https://altmedrev.com/wp-content/uploads/2019/02/v11-3-238.pdf
- Ziems M, Netzel M, Bitsch I. Biokinetic parameters and metabolism of S-benzoylthiamine-O-monophosphate. BioFactors 2000;11:109-110.
- Loew D. Pharmacokinetics of thiamine derivatives especially of benfotiamine. Int J Clin Pharmcol Ther 1996;34:47-50.
- Said HM. Thiamin. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:748-53.
- Bettendorff L. Thiamin. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:261-79.
- Sharma S, Sheehy T, Kolonel LN. Ethnic differences in grains consumption and their contribution to intake of B-vitamins: results of the Multiethnic Cohort Study. Nutr J. 2013 May 20;12:65. doi: 10.1186/1475-2891-12-65
- Bemeur C, Butterworth RF. Thiamin. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:317-24.
- Fulgoni VL 3rd, Keast DR, Bailey RL, Dwyer J. Foods, fortificants, and supplements: Where do Americans get their nutrients? J Nutr. 2011 Oct;141(10):1847-54. doi: 10.3945/jn.111.142257
- U.S. Department of Agriculture, Agricultural Research Service. https://fdc.nal.usda.gov
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998.
- Nabokina SM, Said HM. A high-affinity and specific carrier-mediated mechanism for uptake of thiamine pyrophosphate by human colonic epithelial cells. Am J Physiol Gastrointest Liver Physiol 2012;303:G389-95.
- Allen L, de Benoist B, Dary O, Hurrell R, eds. Guidelines on Food Fortification with Micronutrients. Geneva: World Health Organization and Food and Agricultural Organization of the United Nations; 2006.
- Gibson GE, Blass JP. Thiamine-dependent processes and treatment strategies in neurodegeneration. Antioxid Redox Signal 2007;9:1605-19.
- Food and Nutrition Board, Institute of Medicine. Thiamin. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington D.C.: National Academy Press; 1998:58-86. https://www.nap.edu/read/6015/chapter/1
- Russell RM, Suter PM. Vitamin requirements of elderly people: an update. Am J Clin Nutr. 1993 Jul;58(1):4-14. doi: 10.1093/ajcn/58.1.4
- Tanphaichitr V. Thiamin. In: Shils M, Olson JA, Shike M, Ross AC, eds. Modern Nutrition in Health and Disease. 9th ed. Baltimore: Williams & Wilkins; 1999:381-389.
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels
- Thomson AD. Mechanisms of vitamin deficiency in chronic alcohol misusers and the development of the Wernicke-Korsakoff syndrome. Alcohol Alcohol Suppl 2000;35:2-7.
- Abbas ZG, Swai AB. Evaluation of the efficacy of thiamine and pyridoxine in the treatment of symptomatic diabetic peripheral neuropathy. East Afr Med J 1997;74:803-808.
- Koike H, Misu K, Hattori N, et al. Postgastrectomy polyneuropathy with thiamine deficiency. J Neurol Neurosurg Psychiatry 2001;71:357-362.
- Malinowski SS. Nutritional and metabolic complications of bariatric surgery. Am J Med Sci2006;331:219-225.
- Agabio R. Thiamine administration in alcohol-dependent patients. Alcohol Alcohol. 2005 Mar-Apr;40(2):155-6. doi: 10.1093/alcalc/agh106
- Subramanya SB, Subramanian VS, Said HM. Chronic alcohol consumption and intestinal thiamin absorption: effects on physiological and molecular parameters of the uptake process. Am J Physiol Gastrointest Liver Physiol. 2010 Jul;299(1):G23-31. doi: 10.1152/ajpgi.00132.2010
- Subramanian VS, Subramanya SB, Tsukamoto H, Said HM. Effect of chronic alcohol feeding on physiological and molecular parameters of renal thiamin transport. Am J Physiol Renal Physiol. 2010 Jul;299(1):F28-34. doi: 10.1152/ajprenal.00140.2010
- Vognar L, Stoukides J. The role of low plasma thiamin levels in cognitively impaired elderly patients presenting with acute behavioral disturbances. J Am Geriatr Soc. 2009 Nov;57(11):2166-8. doi: 10.1111/j.1532-5415.2009.02542.x
- Ito Y, Yamanaka K, Susaki H, Igata A. A cross-investigation between thiamin deficiency and the physical condition of elderly people who require nursing care. J Nutr Sci Vitaminol (Tokyo). 2012;58(3):210-6. doi: 10.3177/jnsv.58.210
- Lu’o’ng KV, Nguyen LT. The role of thiamine in cancer: possible genetic and cellular signaling mechanisms. Cancer Genomics Proteomics 2013;10:169-85.
- Boldorini R, Vago L, Lechi A, Tedeschi F, Trabattoni GR. Wernicke’s encephalopathy: occurrence and pathological aspects in a series of 400 AIDS patients. Acta Biomed Ateneo Parmense. 1992;63(1-2):43-9.
- Larsen TR, Dragu D, Williams M. Wernicke’s Encephalopathy: An Unusual Consequence of the Acquired Immune Deficiency Syndrome-Case Report and Literature Review. Case Rep Med. 2013;2013:709474. doi: 10.1155/2013/709474
- Thiamin. Fact Sheet for Health Professionals. https://ods.od.nih.gov/factsheets/Thiamin-HealthProfessional
- Jermendy G. Evaluating thiamine deficiency in patients with diabetes. Diab Vasc Dis Res. 2006 Sep;3(2):120-1. doi: 10.3132/dvdr.2006.014
- Aasheim ET. Wernicke encephalopathy after bariatric surgery: a systematic review. Ann Surg. 2008 Nov;248(5):714-20. doi: 10.1097/SLA.0b013e3181884308
- Xanthakos SA. Nutritional deficiencies in obesity and after bariatric surgery. Pediatr Clin North Am. 2009 Oct;56(5):1105-21. doi: 10.1016/j.pcl.2009.07.002
- Wernicke-Korsakoff Syndrome Information Page. https://www.ninds.nih.gov/Disorders/All-Disorders/Wernicke-Korsakoff-Syndrome-Information-Page
- Wernicke-Korsakoff Syndrome. https://emedicine.medscape.com/article/288379-overview#a7
- Wernicke encephalopathy. https://www.uptodate.com/contents/wernicke-encephalopathy
- Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff Syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013 Jul 1;2013(7):CD004033. doi: 10.1002/14651858.CD004033.pub3
- Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA; EFNS. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010 Dec;17(12):1408-18. doi: 10.1111/j.1468-1331.2010.03153.x
- Thomson AD, Cook CC, Touquet R, Henry JA; Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002 Nov-Dec;37(6):513-21. doi: 10.1093/alcalc/37.6.513. Erratum in: Alcohol Alcohol. 2003 May-Jun;38(3):291.
- Page GL, Laight D, Cummings MH. Thiamine deficiency in diabetes mellitus and the impact of thiamine replacement on glucose metabolism and vascular disease. Int J Clin Pract. 2011 Jun;65(6):684-90. doi: 10.1111/j.1742-1241.2011.02680.x
- Alaei Shahmiri F, Soares MJ, Zhao Y, Sherriff J. High-dose thiamine supplementation improves glucose tolerance in hyperglycemic individuals: a randomized, double-blind cross-over trial. Eur J Nutr. 2013 Oct;52(7):1821-4. doi: 10.1007/s00394-013-0534-6
- Carpenter KJ. The discovery of thiamin. Ann Nutr Metab. 2012;61(3):219-23. doi: 10.1159/000343109
- Rabbani N, Alam SS, Riaz S, Larkin JR, Akhtar MW, Shafi T, Thornalley PJ. High-dose thiamine therapy for patients with type 2 diabetes and microalbuminuria: a randomised, double-blind placebo-controlled pilot study. Diabetologia. 2009 Feb;52(2):208-12. doi: 10.1007/s00125-008-1224-4
- Alkhalaf A, Klooster A, van Oeveren W, Achenbach U, Kleefstra N, Slingerland RJ, Mijnhout GS, Bilo HJ, Gans RO, Navis GJ, Bakker SJ. A double-blind, randomized, placebo-controlled clinical trial on benfotiamine treatment in patients with diabetic nephropathy. Diabetes Care. 2010 Jul;33(7):1598-601. doi: 10.2337/dc09-2241
- Vinik A, Casellini C, Nevoret ML. Diabetic Neuropathies. [Updated 2018 Feb 5]. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK279175
- Haupt E, Ledermann H, Kopcke W: Benfotiamine in the treatment of diabetic polyneuropathy–a three-week randomized, controlled pilot study (BEDIP study). Int J Clin Pharmacol Ther 43:71-77, 2005.
- Stracke H, Gaus W, Achenbach U, Federlin K, Bretzel RG: Benfotiamine in Diabetic Polyneuropathy (BENDIP): Results of a Randomised, Double Blind, Placebo-controlled Clinical Study. Exp Clin Endocrinol Diabetes 2008.
- Stracke H, Lindemann A, Federlin K: A benfotiamine-vitamin B combination in treatment of diabetic polyneuropathy. Exp Clin Endocrinol Diabetes 104:311-316, 1996.
- Simeonov S, Pavlova M, Mitkov M, et al. Therapeutic efficacy of “Milgamma” in patients with painful diabetic neuropathy. Folia Med (Plovdiv) 1997;39:5-10.
- Winkler G, Pal B, Nagybeganyi E, et al. Effectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathy. Arzneimittelforschung 1999;49:220-224.
- Woelk H, Lehrl S, Bitsch R, Kopcke W. Benfotiamine in treatment of alcoholic polyneuropathy: an 8-week randomized controlled study (BAP I Study). Alcohol Alcohol 1998;33:631-638.
- No authors listed. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977-986.
- Hammes HP, Du X, Edelstein D, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med 2003;9:294-299.
- Wooley JA. Characteristics of thiamin and its relevance to the management of heart failure. Nutr Clin Pract. 2008 Oct-Nov;23(5):487-93. doi: 10.1177/0884533608323430
- DiNicolantonio JJ, Niazi AK, Lavie CJ, O’Keefe JH, Ventura HO. Thiamine supplementation for the treatment of heart failure: a review of the literature. Congest Heart Fail. 2013 Jul-Aug;19(4):214-22. doi: 10.1111/chf.12037
- Hanninen SA, Darling PB, Sole MJ, Barr A, Keith ME. The prevalence of thiamin deficiency in hospitalized patients with congestive heart failure. J Am Coll Cardiol. 2006 Jan 17;47(2):354-61. doi: 10.1016/j.jacc.2005.08.060
- Wilkinson TJ, Hanger HC, George PM, Sainsbury R. Is thiamine deficiency in elderly people related to age or co-morbidity? Age Ageing. 2000 Mar;29(2):111-6. doi: 10.1093/ageing/29.2.111
- Zenuk C, Healey J, Donnelly J, Vaillancourt R, Almalki Y, Smith S. Thiamine deficiency in congestive heart failure patients receiving long term furosemide therapy. Can J Clin Pharmacol. 2003 Winter;10(4):184-8.
- Shimon I, Almog S, Vered Z, Seligmann H, Shefi M, Peleg E, Rosenthal T, Motro M, Halkin H, Ezra D. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am J Med. 1995 May;98(5):485-90. doi: 10.1016/s0002-9343(99)80349-0
- Leslie D, Gheorghiade M. Is there a role for thiamine supplementation in the management of heart failure? Am Heart J. 1996;131(6):1248-1250.
- Dinicolantonio JJ, Lavie CJ, Niazi AK, O’Keefe JH, Hu T. Effects of thiamine on cardiac function in patients with systolic heart failure: systematic review and metaanalysis of randomized, double-blind, placebo-controlled trials. Ochsner J. 2013 Winter;13(4):495-9.
- Butterworth RF, Besnard AM. Thiamine-dependent enzyme changes in temporal cortex of patients with Alzheimer’s disease. Metab Brain Dis. 1990 Dec;5(4):179-84. doi: 10.1007/BF00997071
- O’Rourke NP, Bunker VW, Thomas AJ, Finglas PM, Bailey AL, Clayton BE. Thiamine status of healthy and institutionalized elderly subjects: analysis of dietary intake and biochemical indices. Age Ageing. 1990 Sep;19(5):325-9. doi: 10.1093/ageing/19.5.325
- Rodríguez-Martín JL, López-Arrieta JM, Qizilbash N. Thiamine for Alzheimer’s disease. Cochrane Database Syst Rev. 2000;(2):CD001498. doi: 10.1002/14651858.CD001498. Update in: Cochrane Database Syst Rev. 2001;(2):CD001498
- Blass JP, Gleason P, Brush D, DiPonte P, Thaler H. Thiamine and Alzheimer’s disease. A pilot study. Arch Neurol. 1988 Aug;45(8):833-5. doi: 10.1001/archneur.1988.00520320019008
- Nolan KA, Black RS, Sheu KF, Langberg J, Blass JP. A trial of thiamine in Alzheimer’s disease. Arch Neurol. 1991 Jan;48(1):81-3. doi: 10.1001/archneur.1991.00530130093025
- Meador K, Loring D, Nichols M, Zamrini E, Rivner M, Posas H, Thompson E, Moore E. Preliminary findings of high-dose thiamine in dementia of Alzheimer’s type. J Geriatr Psychiatry Neurol. 1993 Oct-Dec;6(4):222-9. doi: 10.1177/089198879300600408
- Pan X, Chen Z, Fei G, Pan S, Bao W, Ren S, Guan Y, Zhong C. Long-Term Cognitive Improvement After Benfotiamine Administration in Patients with Alzheimer’s Disease. Neurosci Bull. 2016 Dec;32(6):591-596. doi: 10.1007/s12264-016-0067-0
- Benfotiamine in Alzheimer’s Disease: A Pilot Study (Benfotiamine). https://clinicaltrials.gov/ct2/show/NCT02292238
- Mestyan I, Jobst K, Hazafi K, Green A. Erythrocyte transketolase activity in patients with chronic renal disease. Acta Med Hung 1986;43:315-319.
- Frank T, Bitsch R, Maiwald J, Stein G. High thiamine diphosphate concentrations in erythrocytes can be achieved in dialysis patients by oral administration of benfotiamine. Eur J Clin Pharmacol 2000;56:251-257.
- Pomero F, Molinar Min A, La Selva M, et al. Benfotiamine is similar to thiamine in correcting endothelial cell defects induced by high glucose. Acta Diabetol 2001;38:135-138.
- Beltramo E, Berrone E, Buttiglieri S, Porta M. Thiamine and benfotiamine prevent increased apoptosis in endothelial cells and pericytes cultured in high glucose. Diabetes Metab Res Rev2004;20:330-336.
- Berrone E, Beltramo E, Solimine C, et al. Regulation of intracellular glucose and polyol pathway by thiamine and benfotiamine in vascular cells cultured in high glucose. J Biol Chem2006;281:9307-9313.
- Gadau S, Emanueli C, Van Linthout S, et al. Benfotiamine accelerates the healing of ischaemic diabetic limbs in mice through protein kinase B/AKT mediated potentiation of angiogenesis and inhibition of apoptosis. Diabetologia 2006;49:405-420.
- Patel KP, O’Brien TW, Subramony SH, Shuster J, Stacpoole PW. The spectrum of pyruvate dehydrogenase complex deficiency: clinical, biochemical and genetic features in 371 patients. Mol Genet Metab. 2012 Jul;106(3):385-94. doi: 10.1016/j.ymgme.2012.03.017
- Lee, E. H., Ahn, M. S., Hwang, J. S., Ryu, K. H., Kim, S. J., & Kim, S. H. (2006). A Korean female patient with thiamine-responsive pyruvate dehydrogenase complex deficiency due to a novel point mutation (Y161C)in the PDHA1 gene. Journal of Korean medical science, 21(5), 800–804. https://doi.org/10.3346/jkms.2006.21.5.800
- Chuang DT, Chuang JL, Wynn RM. Lessons from genetic disorders of branched-chain amino acid metabolism. J Nutr. 2006 Jan;136(1 Suppl):243S-9S. doi: 10.1093/jn/136.1.243S
- Shaw-Smith C, Flanagan SE, Patch AM, Grulich-Henn J, Habeb AM, Hussain K, Pomahacova R, Matyka K, Abdullah M, Hattersley AT, Ellard S. Recessive SLC19A2 mutations are a cause of neonatal diabetes mellitus in thiamine-responsive megaloblastic anaemia. Pediatr Diabetes. 2012 Jun;13(4):314-21. doi: 10.1111/j.1399-5448.2012.00855.x
- Akın L, Kurtoğlu S, Kendirci M, Akın MA, Karakükçü M. Does early treatment prevent deafness in thiamine-responsive megaloblastic anaemia syndrome? J Clin Res Pediatr Endocrinol. 2011;3(1):36-9. doi: 10.4274/jcrpe.v3i1.08
- Alfadhel M, Almuntashri M, Jadah RH, Bashiri FA, Al Rifai MT, Al Shalaan H, Al Balwi M, Al Rumayan A, Eyaid W, Al-Twaijri W. Biotin-responsive basal ganglia disease should be renamed biotin-thiamine-responsive basal ganglia disease: a retrospective review of the clinical, radiological and molecular findings of 18 new cases. Orphanet J Rare Dis. 2013 Jun 6;8:83. doi: 10.1186/1750-1172-8-83
- Comín-Anduix B, Boren J, Martinez S, Moro C, Centelles JJ, Trebukhina R, Petushok N, Lee WN, Boros LG, Cascante M. The effect of thiamine supplementation on tumour proliferation. A metabolic control analysis study. Eur J Biochem. 2001 Aug;268(15):4177-82. doi: 10.1046/j.1432-1327.2001.02329.x
- Zastre JA, Hanberry BS, Sweet RL, McGinnis AC, Venuti KR, Bartlett MG, Govindarajan R. Up-regulation of vitamin B1 homeostasis genes in breast cancer. J Nutr Biochem. 2013 Sep;24(9):1616-24. doi: 10.1016/j.jnutbio.2013.02.002
- Boros LG, Brandes JL, Lee WN, Cascante M, Puigjaner J, Revesz E, Bray TM, Schirmer WJ, Melvin WS. Thiamine supplementation to cancer patients: a double edged sword. Anticancer Res. 1998 Jan-Feb;18(1B):595-602
- Haupt E, Ledermann H, Köpcke W. Benfotiamine in the treatment of diabetic polyneuropathy–a three-week randomized, controlled pilot study (BEDIP study). Int J Clin Pharmacol Ther. 2005 Feb;43(2):71-7. doi: 10.5414/cpp43071. Erratum in: Int J Clin Pharmacol Ther. 2005 Jun;43(6):304.
- Stracke H, Gaus W, Achenbach U, Federlin K, Bretzel RG. Benfotiamine in diabetic polyneuropathy (BENDIP): results of a randomised, double blind, placebo-controlled clinical study. Exp Clin Endocrinol Diabetes. 2008 Nov;116(10):600-5. doi: 10.1055/s-2008-1065351
- Fraser DA, Diep LM, Hovden IA, Nilsen KB, Sveen KA, Seljeflot I, Hanssen KF. The effects of long-term oral benfotiamine supplementation on peripheral nerve function and inflammatory markers in patients with type 1 diabetes: a 24-month, double-blind, randomized, placebo-controlled trial. Diabetes Care. 2012 May;35(5):1095-7. doi: 10.2337/dc11-1895
- Thiamin. https://www.mayoclinic.org/drugs-supplements-thiamin/art-20366430
- Rosen A, van Kuilenburg A, Assmann B, Kuhlen M, Borkhardt A. Severe encephalopathy, lactic acidosis, vegetative instability and neuropathy with 5-Fluorouracil treatment – pyrimidine degradation defect or beriberi? Case Rep Oncol. 2011 May;4(2):371-6. doi: 10.1159/000328803
- Flodin N. Pharmacology of micronutrients. New York: Alan R. Liss, Inc.; 1988.