Best eye vitamins
Vitamins are essential for biochemical metabolic function. Deficiencies (or in some cases excess) of these essential vitamins can produce eye diseases. Patients at high risk for vitamin deficiency either from decreased intake (e.g., malnutrition or eating disorder) or poor absorption (bariatric surgery) or excess loss (e.g., vomiting) should be evaluated for vitamin deficiencies. Early recognition and prompt vitamin replacement may be vision or lifesaving.
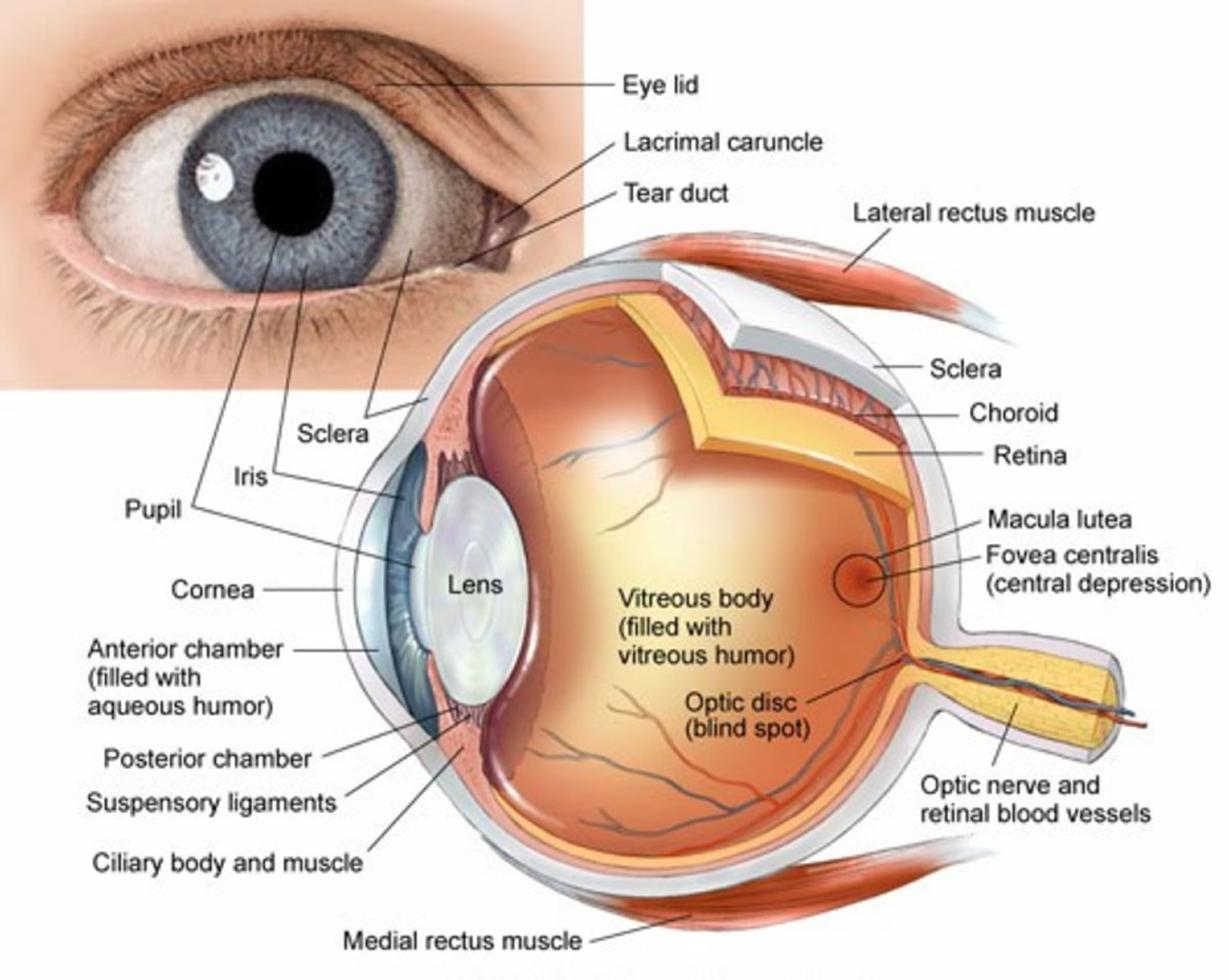
Many eye diseases can cause low vision. The main causes of vision loss in people older than 40 years of age are:
- Macular degeneration. This is caused by changes in the macula. The macula is the part of the eye that gives you clear, sharp vision.
- Glaucoma. This is usually caused by high pressure from the fluid inside the eye.
- Cataract. This is caused by a clouding of the lens inside the eye.
- Diabetic retinopathy. This affects people who have diabetes. It occurs when high blood sugar levels damage the blood vessels in the eyes.
Other common causes of vision loss include eye injury, eye infections, and vision changes associated with certain illnesses.
Eye diseases are more common in persons over 50 years of age. However, normal aging of the eye does not lead to low vision.
Because seeing involves both the eye and the brain, diseases that affect the brain, such as strokes, also can lead to low vision.
Low vision in a child can be caused by some of the same conditions as in adults. But there are other possible causes of childhood low vision 1.
Congenital Diseases (Present at Birth)
- Optic nerve hypoplasia (small optic nerves)
- Cataract
- Glaucoma
Inherited Diseases (Runs in a Family)
- Retinitis pigmentosa
- Optic atrophy
Acquired Diseases (Develop After Birth)
- Glaucoma
- Eye injury
- Retinopathy of prematurity (eye disease of premature infants)
- Cerebral/cortical visual impairment (from brain damage)
Vision loss may be prevented, depending on what is causing it. For example, you may prevent diabetic retinopathy by preventing type 2 diabetes. You may be able to prevent cataracts by wearing polarized sunglasses when you’re outside. However, you generally can’t prevent age-related vision loss.
Vitamin A
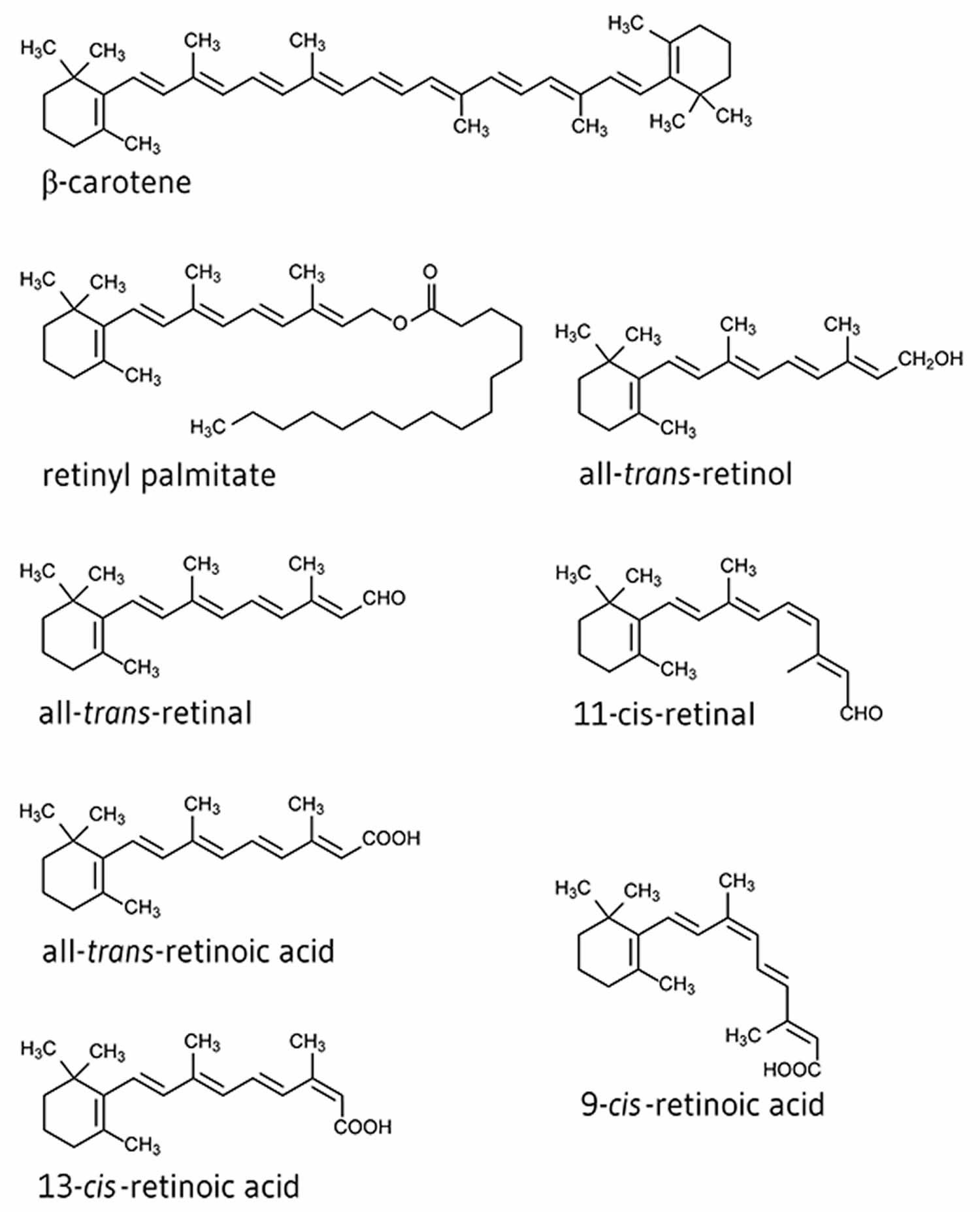
Vitamin A is a fat-soluble vitamin that is naturally present in many foods. Vitamin A is actually the name of a group of fat-soluble retinoids, including retinol, retinal, and retinyl esters 2. Vitamin A is important for normal vision, the immune system, reproduction and cellular communication 3. Vitamin A is critical for your vision as an essential component of rhodopsin, a protein that absorbs light in the retinal receptors, and because it supports the normal differentiation and functioning of the conjunctival membranes and cornea 4. Vitamin A also supports cell growth and differentiation, playing a critical role in the normal formation and maintenance of your heart, lungs, kidneys, and other organs 4.
There are two different types of vitamin A 5:
- The first type, preformed vitamin A (such as retinol and its esterified form, retinyl ester), is found in foods from animal sources, including dairy products, fish, and meat (especially liver). Concentrations of preformed vitamin A are highest in liver and fish oils 4. Other sources of preformed vitamin A are milk and eggs (egg yolks), which also include some provitamin A 4.
- The second type, provitamin A carotenoids (such as beta-carotene, alpha-carotene and beta-cryptoxanthin), is found in fruits, carrots, papaya, dark green leafy vegetables, and other plant-based products 6. The most common type of provitamin A in foods and dietary supplements is beta-carotene. Your body converts these plant pigments into vitamin A. Most dietary provitamin A comes from leafy green vegetables, orange and yellow vegetables, tomato products, fruits, and some vegetable oils 4.
The top food sources of vitamin A in the U.S. diet include dairy products, liver, fish, and fortified cereals; the top sources of provitamin A include carrots, broccoli, cantaloupe, and squash 3.
Both provitamin A and preformed vitamin A must be broken down inside a cell to retinal and retinoic acid, the active forms of vitamin A, to support the vitamin A’s important biological functions 4. Other carotenoids found in food, such as lycopene, lutein, and zeaxanthin, are not converted into vitamin A 5.
The various forms of vitamin A are solubilized into micelles in the intestinal lumen and absorbed by duodenal mucosal cells 6. Both retinyl esters and provitamin A carotenoids are converted to retinol, which is oxidized to retinal and then to retinoic acid 4. Most of your body’s vitamin A is stored in the liver in the form of retinyl esters.
Other forms of retinol are found in retinal pigment epithelium cells, such as 11-cis-retinal and all-trans-retinal, who both hold an essential role in the visual system 7. 11-cis-retinal binds opsin and holds the photoreceptor in its stable, inactive form. Photo isomerization of 11-cis-retinal to all-trans-retinal causes conformational alterations in the receptor, consequently producing meta-rhodopsin II. Meta-rhodopsin II production leads to a sequence of events resulting in a change in neurotransmitter release that is communicated to other retinal neurons and ultimately the brain 8.
The amount of vitamin A you need depends on your age and sex. Average daily recommended amounts are listed below in micrograms (mcg) of retinol activity equivalents (RAE). Recommended Dietary Allowances (RDAs) for vitamin A are given as retinol activity equivalents (RAE) to account for the different bioactivities of retinol and provitamin A carotenoids, all of which are converted by the body into retinol (see Table 1). 1 mcg RAE is equivalent to 1 mcg retinol, 2 mcg supplemental beta-carotene, 12 mcg dietary beta-carotene, or 24 mcg dietary alpha-carotene or beta-cryptoxanthin 6.
Vitamin A is now measured in micrograms (mcg) of retinol activity equivalents (RAE), but it was previously measured in International Units (IUs) 5. To convert International Units (IUs) to mcg RAE, use the following:
- 1 IU retinol = 0.3 mcg RAE
- 1 IU supplemental beta-carotene = 0.3 mcg RAE
- 1 IU dietary beta-carotene = 0.05 mcg RAE
- 1 IU dietary alpha-carotene or beta-cryptoxanthin = 0.025 mcg RAE
Retinol activity equivalents (RAE) can only be directly converted into IUs if the source or sources of vitamin A are known. For example, the RDA of 900 mcg RAE for adolescent and adult men is equivalent to 3,000 IU if the food or supplement source is preformed vitamin A (retinol) or if the supplement source is beta-carotene. This RDA is also equivalent to 18,000 IU beta-carotene from food or to 36,000 IU alpha-carotene or beta-cryptoxanthin from food. Therefore, a mixed diet containing 900 mcg RAE provides between 3,000 and 36,000 IU vitamin A, depending on the foods consumed.
Retinol and carotenoid levels are typically measured in plasma, and plasma retinol levels are useful for assessing vitamin A inadequacy 5. However, their value for assessing marginal vitamin A status is limited because they do not decline until vitamin A levels in the liver are almost depleted 9. Liver vitamin A reserves can be measured indirectly through the relative dose-response test, in which plasma retinol levels are measured before and after the administration of a small amount of vitamin A 6. A plasma retinol level increase of at least 20% indicates an inadequate vitamin A level 9. For clinical practice purposes, plasma retinol levels alone are sufficient for documenting significant deficiency.
A plasma retinol concentration lower than 0.70 micromoles/L (or 20 micrograms [mcg]/dL) reflects vitamin A inadequacy in a population, and concentrations of 0.70–1.05 micromoles/L could be marginal in some people6. In some studies, high plasma or serum concentrations of some provitamin A carotenoids have been associated with a lower risk of various health outcomes, but these studies have not definitively demonstrated that this relationship is causal.
Table 1. Recommended Dietary Allowances (RDAs) for Vitamin A
| Age | Male | Female | Pregnancy | Lactation |
| 0–6 months* | 400 mcg RAE | 400 mcg RAE | ||
| 7–12 months* | 500 mcg RAE | 500 mcg RAE | ||
| 1–3 years | 300 mcg RAE | 300 mcg RAE | ||
| 4–8 years | 400 mcg RAE | 400 mcg RAE | ||
| 9–13 years | 600 mcg RAE | 600 mcg RAE | ||
| 14–18 years | 900 mcg RAE | 700 mcg RAE | 750 mcg RAE | 1,200 mcg RAE |
| 19–50 years | 900 mcg RAE | 700 mcg RAE | 770 mcg RAE | 1,300 mcg RAE |
| 51+ years | 900 mcg RAE | 700 mcg RAE |
Footnotes:
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA).
*Adequate Intake (AI), equivalent to the mean intake of vitamin A in healthy, breastfed infants.
[Source 6 ]Figure 1. Vitamin A chemical structure
Food sources of vitamin A
Table 2 suggests many dietary sources of vitamin A. The foods from animal sources in Table 2 contain primarily preformed vitamin A, the plant-based foods have provitamin A, and the foods with a mixture of ingredients from animals and plants contain both preformed vitamin A and provitamin A.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin A in IUs arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminA-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminA-Food.pdf) and foods containing beta-carotene in mcg arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitA-betaCarotene-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitA-betaCarotene-Food.pdf).
Table 2. Vitamin A content of selected foods
| Food | Micrograms (mcg) RAE per serving | Percent DV* |
| Beef liver, pan fried, 3 ounces | 6582 | 731 |
| Sweet potato, baked in skin, 1 whole | 1403 | 156 |
| Spinach, frozen, boiled, ½ cup | 573 | 64 |
| Pumpkin pie, commercially prepared, 1 piece | 488 | 54 |
| Carrots, raw, ½ cup | 459 | 51 |
| Ice cream, French vanilla, soft serve, 1 cup | 278 | 31 |
| Cheese, ricotta, part skim, 1 cup | 263 | 29 |
| Herring, Atlantic, pickled, 3 ounces | 219 | 24 |
| Milk, fat free or skim, with added vitamin A and vitamin D, 1 cup | 149 | 17 |
| Cantaloupe, raw, ½ cup | 135 | 15 |
| Peppers, sweet, red, raw, ½ cup | 117 | 13 |
| Mangos, raw, 1 whole | 112 | 12 |
| Breakfast cereals, fortified with 10% of the DV for vitamin A, 1 serving | 90 | 10 |
| Egg, hard boiled, 1 large | 75 | 8 |
| Black-eyed peas (cowpeas), boiled, 1 cup | 66 | 7 |
| Apricots, dried, sulfured, 10 halves | 63 | 7 |
| Broccoli, boiled, ½ cup | 60 | 7 |
| Salmon, sockeye, cooked, 3 ounces | 59 | 7 |
| Tomato juice, canned, ¾ cup | 42 | 5 |
| Yogurt, plain, low fat, 1 cup | 32 | 4 |
| Tuna, light, canned in oil, drained solids, 3 ounces | 20 | 2 |
| Baked beans, canned, plain or vegetarian, 1 cup | 13 | 1 |
| Summer squash, all varieties, boiled, ½ cup | 10 | 1 |
| Chicken, breast meat and skin, roasted, ½ breast | 5 | 1 |
| Pistachio nuts, dry roasted, 1 ounce | 4 | 0 |
Footnote: *DV = Daily Value. FDA developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin A is 900 mcg RAE for adults and children age 4 years and older, where 1 mcg RAE = 1 mcg retinol, 2 mcg beta-carotene from supplements, 12 mcg beta-carotene from foods, 24 mcg alpha-carotene, or 24 mcg beta-cryptoxanthin. FDA does not require food labels to list vitamin A content unless vitamin A has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 10 ]Table 3. Common Food Sources of Retinol
| Food Sources of Retinol | Vitamin A (IU) |
|---|---|
| Liver, beef, cooked 3 oz. | 30,325 |
| Liver, chicken, cooked, 3 oz. | 13,920 |
| Egg substitute, fortified, ¼ cup | 1,355 |
| Fat-free milk, fortified with vitamin A, 1 cup | 500 |
| Cheese pizza, ⅛ of a 12-inch pie | 380 |
| Milk, whole, 3.25% fat, 1 cup | 305 |
| Cheddar cheese, 1 oz | 300 |
| Whole egg, 1 medium | 280 |
Vitamin A supplements
Vitamin A is available in multivitamins and as a stand-alone supplement, often in the form of retinyl acetate or retinyl palmitate 4. A portion of the vitamin A in some supplements is in the form of beta-carotene and the remainder is preformed vitamin A; others contain only preformed vitamin A or only beta-carotene. Supplement labels usually indicate the percentage of each form of the vitamin. The amounts of vitamin A in stand-alone supplements range widely 4. Multivitamin supplements typically contain 750–3,000 mcg RAE (2,500–10,000 IU) vitamin A, often in the form of both retinol and beta-carotene.
About 28%–37% of the general population uses supplements containing vitamin A 11. Adults aged 71 years or older and children younger than 9 are more likely than members of other age groups to take supplements containing vitamin A.
Vitamin A deficiency
Vitamin A deficiency can cause a spectrum of eye diseases including xerophthalmia (dryness of the conjunctiva and cornea). However, frank vitamin A deficiency is rare in the United States. Vitamin A deficiency is common in many developing countries, often because residents have limited access to foods containing preformed vitamin A from animal-based food sources and they do not commonly consume available foods containing beta-carotene due to poverty 4. In developing countries, vitamin A deficiency typically begins during infancy, when infants do not receive adequate supplies of colostrum or breast milk 12. Chronic diarrhea also leads to excessive loss of vitamin A in young children, and vitamin A deficiency increases the risk of diarrhea 13. The most common symptom of vitamin A deficiency in young children and pregnant women is xerophthalmia. One of the early signs of xerophthalmia is night blindness, or the inability to see in low light or darkness 14. In the early stages of vitamin A deficiency, nyctanopia also known as night blindness, may develop. In severe stages of vitamin A deficiency, keratomalacia may develop and this ulceration of the cornea can lead to blindness 7. Vitamin A deficiency also may cause squamous metaplasia of the conjunctiva and the formation of a Bitot’s spot (a well-demarcated area of keratinizing squamous metaplasia on the nasal bulbar or temporal conjunctiva) 7. Serum vitamin A concentrations are used to determine deficiency defined as serum retinol concentration of less than 0.70 micromoles/L in children, and less than 1.05 micromoles/L in adults 15. According to the World Health Organization (WHO), 190 million preschool-aged children and 19.1 million pregnant women around the world have a serum retinol concentration below 0.70 micromoles/L 12. In these countries, low vitamin A intake is most strongly associated with health consequences during periods of high nutritional demand, such as during infancy, childhood, pregnancy, and lactation.
People with vitamin A deficiency (and, often, xerophthalmia with its characteristic Bitot’s spots) tend to have low iron status, which can lead to anemia 9. Vitamin A deficiency also increases the severity and mortality risk of infections (particularly diarrhea and measles) even before the onset of xerophthalmia 16.
Worldwide, vitamin A deficiency is the leading cause of preventable blindness in children, mainly affecting countries in Southeast Asia and Africa 12. Over 250 million preschool children are deficient across the globe 17. In developing countries, vitamin A deficiency is because of malnutrition meanwhile in developed countries, although rare, is due to malabsorption following intestinal and bariatric surgery 18. Other causes of vitamin A deficiency include inadequate liver stores, liver disease, and the acute phase response 15.
Groups at risk of vitamin A inadequacy
The following groups are among those most likely to have inadequate intakes of vitamin A.
Premature Infants
In developed countries, clinical vitamin A deficiency is rare in infants and occurs only in those with malabsorption disorders 16. However, preterm infants do not have adequate liver stores of vitamin A at birth and their plasma concentrations of retinol often remain low throughout the first year of life 19. Preterm infants with vitamin A deficiency have an increased risk of eye, chronic lung, and gastrointestinal diseases 16.
Infants and Young Children in Developing Countries
In developed countries, the amounts of vitamin A in breast milk are sufficient to meet infants’ needs for the first 6 months of life. But in women with vitamin A deficiency, breast milk volume and vitamin A content are suboptimal and not sufficient to maintain adequate vitamin A stores in infants who are exclusively breastfed 20. The prevalence of vitamin A deficiency in developing countries begins to increase in young children just after they stop breastfeeding 9. The most common and readily recognized symptom of vitamin A deficiency in infants and children is xerophthalmia.
Pregnant and Lactating Women in Developing Countries
Pregnant women need extra vitamin A for fetal growth and tissue maintenance and for supporting their own metabolism 21. The World Health Organization estimates that 9.8 million pregnant women around the world have xerophthalmia as a result of vitamin A deficiency 12. Other effects of vitamin A deficiency in pregnant and lactating women include increased maternal and infant morbidity and mortality, increased anemia risk, and slower infant growth and development.
People with Cystic Fibrosis
Most people with cystic fibrosis have pancreatic insufficiency, increasing their risk of vitamin A deficiency due to difficulty absorbing fat 22. Several cross-sectional studies found that 15%–40% of patients with cystic fibrosis have vitamin A deficiency 23. However, improved pancreatic replacement treatments, better nutrition, and caloric supplements have helped most patients with cystic fibrosis become vitamin A sufficient 23. Several studies have shown that oral supplementation can correct low serum beta-carotene levels in people with cystic fibrosis, but no controlled studies have examined the effects of vitamin A supplementation on clinical outcomes in patients with cystic fibrosis 24.
Vitamin A Deficiency treatment
High-dose of oral vitamin A supplements are recommended for children to treat xerophthalmia and lower prophylactic dosing can be given for prevention of vitamin A deficiency. Women of reproductive age who are deficient may also need supplementation 15. If oral supplements are inefficient, then intravenous (IV) or intramuscular (IM) injections may be considered.
Vitamin A Palmitate
Dietary deficiency of vitamin A is traditionally treated with vitamin A palmitate in oil 60,000 IU orally once/day for 2 days, followed by 4500 IU orally once/day. If vomiting or malabsorption is present or xerophthalmia is probable, a dose of 50,000 IU for infants < 6 mo, 100,000 IU for infants 6 to 12 mo, or 200,000 IU for children > 12 mo and adults should be given for 2 days, with a third dose at least 2 wk later. The same doses are recommended for infants and children with complicated measles.
Vitamin A deficiency is a risk factor for severe measles; treatment with vitamin A can shorten the duration of the disorder and may reduce the severity of symptoms and risk of death. The WHO recommends that all children with measles in developing countries should receive 2 doses of vitamin A, (100,000 IU for children < 12 mo and 200,000 IU for those >12 months) given 24 hours apart.
Infants born of HIV-positive mothers should receive 50,000 IU (15,000 RAE) within 48 hours of birth. Prolonged daily administration of large doses, especially to infants, must be avoided because toxicity may result.
For pregnant or breastfeeding women, prophylactic or therapeutic doses should not exceed 10,000 IU (3000 RAE)/day to avoid possible damage to the fetus or infant.
Vitamin C
Vitamin C also known as ascorbic acid, L-ascorbic acid or ascorbate, is a water-soluble vitamin that is naturally present in some foods, added to others and available as a dietary supplement. Vitamin C is synthesized from D-glucose or D-galactose by many plants and animals. However, humans lack the enzyme L-gulonolactone oxidase required for ascorbic acid synthesis and must obtain vitamin C through food or supplements 25, 26. Vitamin C is found in many fruits and vegetables, including citrus fruits, tomatoes, potatoes, red and green peppers, kiwifruit, broccoli, strawberries, brussels sprouts, and cantaloupe. In the body, vitamin C acts as an antioxidant, helping to protect cells from the damage caused by free radicals. Free radicals are compounds formed when our bodies convert the food we eat into energy. People are also exposed to free radicals in the environment from cigarette smoke, air pollution, and ultraviolet light from the sun.
The Recommended Dietary Allowance (RDA; average daily level of intake sufficient to meet the nutrient requirement of 97–98% healthy individuals) for vitamin C ranges from 15 to 115 mg for infants and children (depending on age) and from 75 to 120 mg for nonsmoking adults; people who smoke need 35 mg more per day 27. The intestinal absorption of vitamin C is regulated by at least one specific dose-dependent, active transporter 28. Cells accumulate vitamin C via a second specific transport protein. In vitro studies have found that oxidized vitamin C, or dehydroascorbic acid, enters cells via some facilitated glucose transporters and is then reduced internally to ascorbic acid. The physiologic importance of dehydroascorbic acid uptake and its contribution to overall vitamin C economy is unknown.
Vitamin C plays a role in collagen, carnitine, hormone, and amino acid formation. It is essential for wound healing and facilitates recovery from burns. Vitamin C is also an antioxidant, supports immune function, and facilitates the absorption of iron 29. Vitamin C also plays an important role in both innate and adaptive immunity, probably because of its antioxidant effects, antimicrobial and antiviral actions, and effects on immune system modulators 30. Vitamin C helps maintain epithelial integrity, enhance the differentiation and proliferation of B cells and T cells, enhance phagocytosis, normalize cytokine production, and decrease histamine levels 31. Vitamin C might also inhibit viral replication 32.
Vitamin C deficiency impairs immune function and increases susceptibility to infections 31. Some research suggests that supplemental vitamin C enhances immune function 33, but its effects might vary depending on an individual’s vitamin C status 34.
Vitamin C deficiency is uncommon in the United States, affecting only about 7% of individuals aged 6 years and older 35. People who smoke and those whose diets include a limited variety of foods (such as some older adults and people with alcohol or drug use disorders) are more likely than others to obtain insufficient amounts of vitamin C 33.
Vitamin C is required for the biosynthesis of collagen, L-carnitine, and certain neurotransmitters; vitamin C is also involved in protein metabolism 36. Collagen is an essential component of connective tissue, which plays a vital role in wound healing. Vitamin C is also an important physiological antioxidant 37 and has been shown to regenerate other antioxidants within the body, including vitamin E (alpha-tocopherol) 38. Ongoing research is examining whether vitamin C, by limiting the damaging effects of free radicals through its antioxidant activity, might help prevent or delay the development of certain cancers, cardiovascular disease, and other diseases in which oxidative stress plays a causal role. In addition to its biosynthetic and antioxidant functions, vitamin C plays an important role in immune function 38 and improves the absorption of nonheme iron 39, the form of iron present in plant-based foods. Insufficient vitamin C intake causes scurvy, which is characterized by fatigue or lassitude, widespread connective tissue weakness, and capillary fragility 40.
The intestinal absorption of vitamin C is regulated by at least one specific dose-dependent, active transporter 28. Cells accumulate vitamin C via a second specific transport protein. In vitro studies have found that oxidized vitamin C, or dehydroascorbic acid, enters cells via some facilitated glucose transporters and is then reduced internally to ascorbic acid. The physiologic importance of dehydroascorbic acid uptake and its contribution to overall vitamin C economy is unknown.
Oral vitamin C produces tissue and plasma concentrations that the body tightly controls. Approximately 70%–90% of vitamin C is absorbed at moderate intakes of 30–180 mg/day. However, at doses above 1 g/day, absorption falls to less than 50% and absorbed, unmetabolized ascorbic acid is excreted in the urine 38. Results from pharmacokinetic studies indicate that oral doses of 1.25 g/day ascorbic acid produce mean peak plasma vitamin C concentrations of 135 micromol/L, which are about two times higher than those produced by consuming 200–300 mg/day ascorbic acid from vitamin C-rich foods 41. Pharmacokinetic modeling predicts that even doses as high as 3 g ascorbic acid taken every 4 hours would produce peak plasma concentrations of only 220 micromol/L 41.
The total body content of vitamin C ranges from 300 mg (at near scurvy) to about 2 g 38. High levels of vitamin C (millimolar concentrations) are maintained in cells and tissues, and are highest in leukocytes (white blood cells), eyes, adrenal glands, pituitary gland, and brain. Relatively low levels of vitamin C (micromolar concentrations) are found in extracellular fluids, such as plasma, red blood cells, and saliva 38.
Even before the discovery of vitamin C in 1932, nutrition experts recognized that something in citrus fruits could prevent scurvy, a disease that killed as many as two million sailors between 1500 and 1800 42. Scurvy is a disease caused by a deficiency of vitamin C, characterized by swollen bleeding gums, malaise, lethargy, easy bruising, and spontaneous bleeding and the opening of previously healed wounds 43, which particularly affected poorly nourished sailors until the end of the 18th century 42. High-dose vitamin C has been studied as a treatment for patients with cancer since the 1970s. A Scottish surgeon named Ewan Cameron worked with Nobel Prize-winning chemist Linus Pauling to study the possible benefits of vitamin C therapy in clinical trials of cancer patients in the late 1970s and early 1980’s 44. In the 1970s, Linus Pauling promoted daily megadoses of vitamin C (the amount in 12 to 24 oranges) as a way to prevent colds and some chronic diseases 45. In the mid-20th century, a study hypothesized that cancer may be related to changes in connective tissue, which may be a consequence of vitamin C deficiency 46. A review of evidence published in 1974 suggested that high-dose ascorbic acid may increase host resistance and be a potential cancer therapy 47.
Table 4. Recommended Dietary Allowances (RDAs) for Vitamin C
| Age | Male | Female | Pregnancy | Lactation |
| 0–6 months | 40 mg* | 40 mg* | ||
| 7–12 months | 50 mg* | 50 mg* | ||
| 1–3 years | 15 mg | 15 mg | ||
| 4–8 years | 25 mg | 25 mg | ||
| 9–13 years | 45 mg | 45 mg | ||
| 14–18 years | 75 mg | 65 mg | 80 mg | 115 mg |
| 19+ years | 90 mg | 75 mg | 85 mg | 120 mg |
| Smokers | Individuals who smoke require 35 mg/day more vitamin C than nonsmokers. | |||
Footnote:
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
*Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA).
[Source 48 ]Food sources of vitamin C
Table 5 suggests many dietary sources of vitamin C. Fruits and vegetables are the best sources of vitamin C (see Table 5) 10. Citrus fruits, tomatoes and tomato juice, and potatoes are major contributors of vitamin C to the American diet 48. Other good food sources include red and green peppers, kiwifruit, broccoli, strawberries, Brussels sprouts, and cantaloupe (see Table 5) 48. Although vitamin C is not naturally present in grains, it is added to some fortified breakfast cereals. The vitamin C content of food may be reduced by prolonged storage and by cooking because ascorbic acid is water soluble and is destroyed by heat 40. Steaming or microwaving may lessen cooking losses. Fortunately, many of the best food sources of vitamin C, such as fruits and vegetables, are usually consumed raw. Consuming five varied servings of fruits and vegetables a day can provide more than 200 mg of vitamin C.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin C arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminC-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminC-Food.pdf).
Table 5. Vitamin C content of selected foods
| Food | Milligrams (mg) per serving | Percent (%) DV* |
| Red pepper, sweet, raw, ½ cup | 95 | 106 |
| Orange juice, ¾ cup | 93 | 103 |
| Orange, 1 medium | 70 | 78 |
| Grapefruit juice, ¾ cup | 70 | 78 |
| Kiwifruit, 1 medium | 64 | 71 |
| Green pepper, sweet, raw, ½ cup | 60 | 67 |
| Broccoli, cooked, ½ cup | 51 | 57 |
| Strawberries, fresh, sliced, ½ cup | 49 | 54 |
| Brussels sprouts, cooked, ½ cup | 48 | 53 |
| Grapefruit, ½ medium | 39 | 43 |
| Broccoli, raw, ½ cup | 39 | 43 |
| Tomato juice, ¾ cup | 33 | 37 |
| Cantaloupe, ½ cup | 29 | 32 |
| Cabbage, cooked, ½ cup | 28 | 31 |
| Cauliflower, raw, ½ cup | 26 | 29 |
| Potato, baked, 1 medium | 17 | 19 |
| Tomato, raw, 1 medium | 17 | 19 |
| Spinach, cooked, ½ cup | 9 | 10 |
| Green peas, frozen, cooked, ½ cup | 8 | 9 |
Footnote: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin C is 90 mg for adults and children age 4 years and older [13]. FDA does not require food labels to list vitamin C content unless vitamin C has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 10 ]Vitamin C and cataracts
Age-related macular degeneration (AMD) and cataracts are two of the leading causes of vision loss in older individuals. Oxidative stress might contribute to the cause of both conditions. Thus, researchers have hypothesized that vitamin C and other antioxidants play a role in the development and/or treatment of these diseases.
High dietary intakes of vitamin C and higher plasma ascorbate concentrations have been associated with a lower risk of cataract formation in some studies 49, 38. In a 5-year prospective cohort study conducted in Japan, higher dietary vitamin C intake was associated with a reduced risk of developing cataracts in a cohort of more than 30,000 adults aged 45–64 years 50. Results from two case-control studies indicate that vitamin C intakes greater than 300 mg/day reduce the risk of cataract formation by 70%–75% 49, 38. Use of vitamin C supplements, on the other hand, was associated with a 25% higher risk of age-related cataract extraction among a cohort of 24,593 Swedish women aged 49–83 years 51. These findings applied to study participants who took relatively high-dose vitamin C supplements (approximately 1,000 mg/day) and not to those who took multivitamins containing substantially less vitamin C (approximately 60 mg/day).
Data from clinical trials are limited. In one study, Chinese adults who took daily supplements of 120 mg vitamin C plus 30 mcg molybdenum for 5 years did not have a significantly lower cataract risk 52. However, adults aged 65–74 years who received 180 mg vitamin C plus 30 mcg molybdenum combined with other nutrients in a multivitamin/mineral supplement had a 43% significantly lower risk of developing nuclear cataracts than those who received a placebo 52. In the AREDS study 53, older individuals who received supplements of 500 mg vitamin C, 400 IU vitamin E, and 15 mg beta-carotene for an average of 6.3 years did not have a significantly lower risk of developing cataracts or of cataract progression than those who received a placebo. The AREDS2 study 54, which also tested formulations containing 500 mg vitamin C, confirmed these findings.
Vitamin C and macular degeneration
A population-based cohort study in the Netherlands found that adults aged 55 years or older who had high dietary intakes of vitamin C as well as beta-carotene, zinc, and vitamin E had a reduced risk of age-related macular degeneration 55. However, most prospective studies do not support these findings 56. The authors of a 2007 systematic review and meta-analysis of prospective cohort studies and randomized clinical trials concluded that the current evidence does not support a role for vitamin C and other antioxidants, including antioxidant supplements, in the primary prevention of early age-related macular degeneration 57.
Although research has not shown that antioxidants play a role in age-related macular degeneration development, some evidence suggests that they might help slow age-related macular degeneration progression 58. The Age-Related Eye Disease Study (AREDS), a large, randomized, placebo-controlled clinical trial, evaluated the effect of high doses of selected antioxidants (500 mg vitamin C, 400 IU vitamin E, 15 mg beta-carotene, 80 mg zinc, and 2 mg copper) on the development of advanced age-related macular degeneration in 3,597 older individuals with varying degrees of age-related macular degeneration 59. After an average follow-up period of 6.3 years, participants at high risk of developing advanced age-related macular degeneration (i.e., those with intermediate age-related macular degeneration or those with advanced age-related macular degeneration in one eye) who received the antioxidant supplements had a 28% lower risk of progression to advanced age-related macular degeneration than participants who received a placebo. A follow-up AREDS2 study confirmed the value of this and similar supplement formulations in reducing the progression of age-related macular degeneration over a median follow-up period of 5 years 60.
Overall, the currently available evidence does not indicate that vitamin C, taken alone or with other antioxidants, affects the risk of developing age-related macular degeneration, although some evidence indicates that the AREDS formulations might slow age-related macular degeneration progression in people at high risk of developing advanced age-related macular degeneration.
Vitamin C Deficiency
Acute vitamin C deficiency leads to scurvy 61. The timeline for the development of scurvy varies, depending on vitamin C body stores, but signs can appear within 1 month of little or no vitamin C intake (below 10 mg/day) 62. Initial symptoms can include fatigue (probably the result of impaired carnitine biosynthesis), malaise, and inflammation of the gums 63. As vitamin C deficiency progresses, collagen synthesis becomes impaired and connective tissues become weakened, causing petechiae, ecchymoses, purpura, joint pain, poor wound healing, hyperkeratosis, and corkscrew hairs 36. Additional signs of scurvy include depression as well as swollen, bleeding gums and loosening or loss of teeth due to tissue and capillary fragility 64. Iron deficiency anemia can also occur due to increased bleeding and decreased nonheme iron absorption secondary to low vitamin C intake 40. In children, bone disease can be present 40. Left untreated, scurvy is fatal 40.
Today, vitamin C deficiency and scurvy are rare in developed countries 48. Overt deficiency symptoms occur only if vitamin C intake falls below approximately 10 mg/day for many weeks 61. Vitamin C deficiency is uncommon in developed countries but can still occur in people with limited food variety.
Groups at risk of vitamin C inadequacy
Vitamin C inadequacy can occur with intakes that fall below the RDA but are above the amount required to prevent overt deficiency (approximately 10 mg/day). The following groups are more likely than others to be at risk of obtaining insufficient amounts of vitamin C.
Smokers and passive smokers
Studies consistently show that smokers have lower plasma and leukocyte vitamin C levels than nonsmokers, due in part to increased oxidative stress 48. For this reason, the IOM concluded that smokers need 35 mg more vitamin C per day than nonsmokers 48. Exposure to secondhand smoke also decreases vitamin C levels. Although the IOM was unable to establish a specific vitamin C requirement for nonsmokers who are regularly exposed to secondhand smoke, these individuals should ensure that they meet the RDA for vitamin C 38.
Infants fed evaporated or boiled milk
Most infants in developed countries are fed breastmilk and/or infant formula, both of which supply adequate amounts of vitamin C 65. For many reasons, feeding infants evaporated or boiled cow’s milk is not recommended. This practice can cause vitamin C deficiency because cow’s milk naturally has very little vitamin C and heat can destroy vitamin C 40.
Individuals with limited food variety
Although fruits and vegetables are the best sources of vitamin C, many other foods have small amounts of this nutrient. Thus, through a varied diet, most people should be able to meet the vitamin C recommended dietary allowance (RDA) or at least obtain enough to prevent scurvy. People who have limited food variety—including some elderly, indigent individuals who prepare their own food; people who abuse alcohol or drugs; food faddists; people with mental illness; and, occasionally, children—might not obtain sufficient vitamin C 63.
People with malabsorption and certain chronic diseases
Some medical conditions can reduce the absorption of vitamin C and/or increase the amount needed by the body. People with severe intestinal malabsorption or cachexia and some cancer patients might be at increased risk of vitamin C inadequacy 66. Low vitamin C concentrations can also occur in patients with end-stage renal disease on chronic hemodialysis 67.
Vitamin D
Vitamin D also known as calciferol, is a fat-soluble vitamin that is naturally present in a few foods, added to others, and available as a dietary supplement. Vitamin D is also produced by your body when ultraviolet (UV) rays from sunlight strike your skin and trigger vitamin D synthesis. The major source of vitamin D is sunlight. Vitamin D deficiency is typically due to limited sunlight exposure. Vitamin D obtained from sun exposure, foods, and supplements is biologically inert and must undergo two hydroxylations in the body for activation. Vitamin D enters the circulation from the skin or lymphatic system and goes to the liver for processing. The major circulating metabolite of vitamin D is 25-hydroxy vitamin D [25(OH)D] also known as “calcidiol”, is further hydroxylised to 1,25-dihydroxy vitamin D [1,25(OH)2D] also known as “calcitriol” by the kidney 68. Overall, vitamin D plays a role in the maintenance of serum calcium and phosphorus levels by increasing their absorption in the small intestine. Excessive amounts of vitamin D metabolites are catalyzed and excreted by the kidneys in the form of calcitroic acid 69. When your body is exposed to excessive sunlight, photolysis of the vitamin D skin metabolites occurs to prevent hypervitaminosis 70.
Vitamin D promotes calcium absorption in the gut and maintains adequate serum calcium and phosphate concentrations to enable normal bone mineralization and to prevent hypocalcemic tetany (involuntary contraction of muscles, leading to cramps and spasms). Vitamin D is also needed for bone growth and bone remodeling by osteoblasts and osteoclasts 71. Without sufficient vitamin D, bones can become thin, brittle, or misshapen. Vitamin D sufficiency prevents rickets in children and osteomalacia in adults 69. Together with calcium, vitamin D also helps protect older adults from osteoporosis 69.
Vitamin D has other roles in the body, including reduction of inflammation as well as modulation of such processes as cell growth, neuromuscular and immune function, and glucose metabolism 72. Many genes encoding proteins that regulate cell proliferation, differentiation, and apoptosis are modulated in part by vitamin D. Many tissues have vitamin D receptors, and some convert calcidiol [25(OH)D] to calcitriol [1,25(OH)2D].
In foods and dietary supplements, vitamin D has two main forms, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), that differ chemically only in their side-chain structures. Both forms are well absorbed in the small intestine. Absorption occurs by simple passive diffusion and by a mechanism that involves intestinal membrane carrier proteins 73. The concurrent presence of fat in the gut enhances vitamin D absorption, but some vitamin D is absorbed even without dietary fat. Neither aging nor obesity alters vitamin D absorption from the gut 73.
Serum concentration of 25-hydroxyvitamin D [25(OH)D] is currently the main indicator of vitamin D status. It reflects vitamin D produced endogenously and that obtained from foods and supplements 68. In serum, 25-hydroxyvitamin D [25(OH)D] has a fairly long circulating half-life of 15 days 68. Serum concentrations of 25(OH)D are reported in both nanomoles per liter (nmol/L) and nanograms per milliliter (ng/mL). One nmol/L is equal to 0.4 ng/mL, and 1 ng/mL is equal to 2.5 nmol/L.
Assessing vitamin D status by measuring serum 25-hydroxyvitamin D [25(OH)D] concentrations is complicated by the considerable variability of the available assays (the two most common ones involve antibodies or chromatography) used by laboratories that conduct the analyses 74. As a result, a finding can be falsely low or falsely high, depending on the assay used and the laboratory. The international Vitamin D Standardization Program has developed procedures for standardizing the laboratory measurement of 25-hydroxyvitamin D [25(OH)D] to improve clinical and public health practice 75.
In contrast to 25-hydroxyvitamin D [25(OH)D], circulating 1,25-dihydroxy vitamin D [1,25(OH)2D] is generally not a good indicator of vitamin D status because it has a short half-life measured in hours, and serum levels are tightly regulated by parathyroid hormone, calcium, and phosphate 68. Levels of 1,25-dihydroxy vitamin D [1,25(OH)2D] do not typically decrease until vitamin D deficiency is severe Norman AW, Henry HH. Vitamin D. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition, 10th ed. Washington DC: Wiley-Blackwell, 2012..
Although 25-hydroxyvitamin D [25(OH)D] functions as a biomarker of exposure, the extent to which 25(OH)D levels also serve as a biomarker of effect on the body (i.e., relating to health status or outcomes) is not clear 71.
Researchers have not definitively identified serum concentrations of 25-hydroxyvitamin D [25(OH)D] associated with deficiency (e.g., rickets), adequacy for bone health, and overall health. After reviewing data on vitamin D needs, an expert committee of the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine concluded that people are at risk of vitamin D deficiency at serum 25-hydroxyvitamin D [25(OH)D] concentrations less than 30 nmol/L (12 ng/mL; see Table 6 for definitions of “deficiency” and “inadequacy”) 68. Some people are potentially at risk of inadequacy at 30 to 50 nmol/L (12–20 ng/mL). Levels of 50 nmol/L (20 ng/mL) or more are sufficient for most people. In contrast, the Endocrine Society stated that, for clinical practice, a serum 25(OH)D concentration of more than 75 nmol/L (30 ng/mL) is necessary to maximize the effect of vitamin D on calcium, bone, and muscle metabolism 76. The Food and Nutrition Board committee also noted that serum concentrations greater than 125 nmol/L (50 ng/mL) can be associated with adverse effects (Table 6).
Optimal serum concentrations of 25-hydroxyvitamin D [25(OH)D] for bone and general health have not been established because they are likely to vary by stage of life, by race and ethnicity, and with each physiological measure used 77. In addition, although 25-hydroxyvitamin D [25(OH)D] levels rise in response to increased vitamin D intake, the relationship is nonlinear 78. The amount of increase varies, for example, by baseline serum levels and duration of supplementation.
Table 6. Serum 25-Hydroxyvitamin D [25(OH)D] Concentrations and Health
| nmol/L** | ng/mL* | Health status |
|---|---|---|
| <30 | <12 | Associated with vitamin D deficiency, leading to rickets in infants and children and osteomalacia in adults |
| 30 to <50 | 12 to <20 | Generally considered inadequate for bone and overall health in healthy individuals |
| ≥50 | ≥20 | Generally considered adequate for bone and overall health in healthy individuals |
| >125 | >50 | Emerging evidence links potential adverse effects to such high levels, particularly >150 nmol/L (>60 ng/mL) |
Footnotes:
* Serum concentrations of 25(OH)D are reported in both nanomoles per liter (nmol/L) and nanograms per milliliter (ng/mL).
** 1 nmol/L = 0.4 ng/mL and 1 ng/mL = 2.5 nmol/L.
Am I getting enough vitamin D?
Because you get vitamin D from food, sunshine, and dietary supplements, one way to know if you’re getting enough is a blood test that measures the amount of vitamin D in your blood. In the blood, a form of vitamin D known as 25-hydroxyvitamin D is measured in either nanomoles per liter (nmol/L) or nanograms per milliliter (ng/mL). One nmol/L is the same as 0.4 ng/mL.
- Levels of 50 nmol/L (20 ng/mL) or above are adequate for most people for bone and overall health.
- Levels below 30 nmol/L (12 ng/mL) are too low and might weaken your bones and affect your health.
- Levels above 125 nmol/L (50 ng/mL) are too high and might cause health problems.
In the United States, most people have adequate blood levels of vitamin D. However, almost one out of four people have vitamin D blood levels that are too low or inadequate for bone and overall health.
Some people are more likely than others to have trouble getting enough vitamin D:
- Breastfed infants. Breast milk alone does not provide infants with an adequate amount of vitamin D. Breastfed infants should be given a supplement of 10 mcg (400 IU) of vitamin D each day.
- Older adults. As you age, your skin’s ability to make vitamin D when exposed to sunlight declines.
- People who seldom expose their skin to sunshine because they do not go outside or because they keep their body and head covered. Sunscreen also limits the amount of vitamin D your skin produces.
- People with dark skin. The darker your skin, the less vitamin D you make from sunlight exposure.
- People with conditions that limit fat absorption, such as Crohn’s disease, celiac disease, or ulcerative colitis. This is because the vitamin D you consume is absorbed in the gut along with fat, so if your body has trouble absorbing fat, it will also have trouble absorbing vitamin D.
- People who are obese or have undergone gastric bypass surgery. They may need more vitamin D than other people.
How much vitamin D do I need?
The amount of vitamin D you need each day depends on your age. Average daily recommended amounts are listed below in micrograms (mcg) and International Units (IU). The Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine committee established Recommended Dietary Allowances (RDAs) for vitamin D to indicate daily intakes sufficient to maintain bone health and normal calcium metabolism in healthy people. Recommended Dietary Allowance (RDA) for vitamin D are listed in both micrograms (mcg) and international units (IU); 1 mcg vitamin D is equal to 40 IU (Table 7). Even though sunlight is a major source of vitamin D for some people, the Food and Nutrition Board based the vitamin D RDAs on the assumption that people receive minimal sun exposure 68. For infants, the Food and Nutrition Board committee developed Adequate Intake (AI) based on the amount of vitamin D that maintains serum 25(OH)D levels above 20 ng/mL (50 nmol/L) and supports bone development.
Table 7. Recommended Dietary Allowances (RDAs) for Vitamin D
| Age | Male | Female | Pregnancy | Lactation |
| 0-12 months* | 10 mcg (400 IU) | 10 mcg (400 IU) | ||
| 1–13 years | 15 mcg (600 IU) | 15 mcg (600 IU) | ||
| 14–18 years | 15 mcg (600 IU) | 15 mcg (600 IU) | 15 mcg (600 IU) | 15 mcg (600 IU) |
| 19–50 years | 15 mcg (600 IU) | 15 mcg (600 IU) | 15 mcg (600 IU) | 15 mcg (600 IU) |
| 51–70 years | 15 mcg (600 IU) | 15 mcg (600 IU) | ||
| >70 years | 20 mcg (800 IU) | 20 mcg (800 IU) |
Footnotes:
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
*Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
[Source 68 ]What foods provide vitamin D?
Very few foods naturally contain vitamin D. Fortified foods provide most of the vitamin D in the diets of people in the United States. Check the Nutrition Facts label for the amount of vitamin D in a food or beverage.
- Almost all of the U.S. milk supply is fortified with about 3 mcg (120 IU) vitamin D per cup. Many plant-based alternatives such as soy milk, almond milk, and oat milk are similarly fortified. But foods made from milk, like cheese and ice cream, are usually not fortified.
- Vitamin D is added to many breakfast cereals and to some brands of orange juice, yogurt, margarine, and other food products.
- Fatty fish (like trout, salmon, tuna, and mackerel) and fish liver oils are among the best natural sources of vitamin D.
- Beef liver, cheese, and egg yolks have small amounts of vitamin D.
- Mushrooms provide a little vitamin D. Some mushrooms have been exposed to ultraviolet light to increase their vitamin D content.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin D arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminD-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminD-Food.pdf). However, FoodData Central does not include the amounts of 25(OH)D in foods. A variety of foods and their vitamin D levels per serving are listed in Table 8.
The flesh of fatty fish (such as trout, salmon, tuna, and mackerel) and fish liver oils are among the best sources 79. An animal’s diet affects the amount of vitamin D in its tissues. Beef liver, cheese, and egg yolks have small amounts of vitamin D, primarily in the form of vitamin D3 and its metabolite 25(OH)D3. Mushrooms provide variable amounts of vitamin D2 79. Some mushrooms available on the market have been treated with UV light to increase their levels of vitamin D2. In addition, the Food and Drug Administration (FDA) has approved UV-treated mushroom powder as a food additive for use as a source of vitamin D2 in food products 80. Very limited evidence suggests no substantial differences in the bioavailability of vitamin D from various foods 81.
Animal-based foods typically provide some vitamin D in the form of 25(OH)D in addition to vitamin D3. The impact of this form on vitamin D status is an emerging area of research. Studies show that 25(OH)D appears to be approximately five times more potent than the parent vitamin for raising serum 25(OH)D concentrations 79. One study found that when the 25(OH)D content of beef, pork, chicken, turkey, and eggs is taken into account, the total amount of vitamin D in the food is 2 to 18 times higher than the amount in the parent vitamin alone, depending on the food 82. At the present time, the U.S. Department of Agriculture, Agricultural Research Service (USDA’s) Nutrient Database does not include 25(OH)D when reporting the vitamin D content of foods. Actual vitamin D intakes in the U.S. population may be underestimated for this reason.
Fortified foods provide most of the vitamin D in American diets 83. For example, almost all of the U.S. milk supply is voluntarily fortified with about 3 mcg/cup (120 IU), usually in the form of vitamin D3 84. In Canada, milk must be fortified with 0.88–1.0 mcg/100 mL (35–40 IU), and the required amount for margarine is at least 13.25 mcg/100 g (530 IU). Other dairy products made from milk, such as cheese and ice cream, are not usually fortified in the United States or Canada. Plant milk alternatives (such as beverages made from soy, almond, or oats) are often fortified with similar amounts of vitamin D to those in fortified cow’s milk (about 3 mcg [120 IU]/cup); the Nutrition Facts label lists the actual amount 85. Ready-to-eat breakfast cereals often contain added vitamin D, as do some brands of orange juice, yogurt, margarine, and other food products.
The United States mandates the fortification of infant formula with 1–2.5 mcg/100 kcal (40–100 IU) vitamin D; 1–2 mcg/100 kcal (40–80 IU) is the required amount in Canada 68.
Table 8. Vitamin D content of selected foods
| Food | Micrograms (mcg) per serving | International Units (IU) per serving | Percent DV* |
| Cod liver oil, 1 tablespoon | 34 | 1360 | 170 |
| Trout (rainbow), farmed, cooked, 3 ounces | 16.2 | 645 | 81 |
| Salmon (sockeye), cooked, 3 ounces | 14.2 | 570 | 71 |
| Mushrooms, white, raw, sliced, exposed to UV light, ½ cup | 9.2 | 366 | 46 |
| Milk, 2% milkfat, vitamin D fortified, 1 cup | 2.9 | 120 | 15 |
| Soy, almond, and oat milks, vitamin D fortified, various brands, 1 cup | 2.5-3.6 | 100-144 | 13-18 |
| Ready-to-eat cereal, fortified with 10% of the DV for vitamin D, 1 serving | 2 | 80 | 10 |
| Sardines (Atlantic), canned in oil, drained, 2 sardines | 1.2 | 46 | 6 |
| Egg, 1 large, scrambled** | 1.1 | 44 | 6 |
| Liver, beef, braised, 3 ounces | 1 | 42 | 5 |
| Tuna fish (light), canned in water, drained, 3 ounces | 1 | 40 | 5 |
| Cheese, cheddar, 1 ounce | 0.3 | 12 | 2 |
| Mushrooms, portabella, raw, diced, ½ cup | 0.1 | 4 | 1 |
| Chicken breast, roasted, 3 ounces | 0.1 | 4 | 1 |
| Beef, ground, 90% lean, broiled, 3 ounces | 0 | 1.7 | 0 |
| Broccoli, raw, chopped, ½ cup | 0 | 0 | 0 |
| Carrots, raw, chopped, ½ cup | 0 | 0 | 0 |
| Almonds, dry roasted, 1 ounce | 0 | 0 | 0 |
| Apple, large | 0 | 0 | 0 |
| Banana, large | 0 | 0 | 0 |
| Rice, brown, long-grain, cooked, 1 cup | 0 | 0 | 0 |
| Whole wheat bread, 1 slice | 0 | 0 | 0 |
| Lentils, boiled, ½ cup | 0 | 0 | 0 |
| Sunflower seeds, roasted, ½ cup | 0 | 0 | 0 |
| Edamame, shelled, cooked, ½ cup | 0 | 0 | 0 |
Footnotes:
* DV = Daily Value. The FDA developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin D is 20 mcg (800 IU) for adults and children aged 4 years and older. The labels must list vitamin D content in mcg per serving and have the option of also listing the amount in IUs in parentheses. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
** Vitamin D is in the yolk.
[Source 86 ]Can I get vitamin D from the sun?
Your body makes vitamin D when your bare skin is exposed to the sun. Most people get at least some vitamin D this way. However, clouds, smog, old age, and having dark-colored skin reduce the amount of vitamin D your skin makes. Also, your skin does not make vitamin D from sunlight through a window.
Ultraviolet radiation from sunshine can cause skin cancer, so it’s important to limit how much time you spend in the sun. Although sunscreen limits vitamin D production, health experts recommend using sunscreen with a sun protection factor (SPF) of 15 or more when you’re out in the sun for more than a few minutes.
Vitamin D dietary supplements
Vitamin D is found in multivitamin/multimineral supplements. It is also available in dietary supplements containing only vitamin D or vitamin D combined with a few other nutrients. The two forms of vitamin D in supplements are D2 (ergocalciferol) and D3 (cholecalciferol). Both forms increase vitamin D in your blood, but D3 might raise it higher and for longer than D2. Because vitamin D is fat-soluble, it is best absorbed when taken with a meal or snack that includes some fat.
Vitamin D is found in supplements (and fortified foods) in two different forms: vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol). Both increase vitamin D in the blood.
In supplements and fortified foods, vitamin D is available in two forms, D2 (ergocalciferol) and D3 (cholecalciferol) that differ chemically only in their side-chain structure. Vitamin D2 is manufactured by the UV irradiation of ergosterol in yeast, and vitamin D3 is manufactured by the irradiation of 7-dehydrocholesterol from lanolin and the chemical conversion of cholesterol 87. The two forms have traditionally been regarded as equivalent based on their ability to cure rickets and, indeed, most steps involved in the metabolism and actions of vitamin D2 and vitamin D3 are identical. Both forms (as well as vitamin D in foods and from cutaneous synthesis) effectively raise serum Calcidiol [25-hydroxyvitamin D or 25(OH)D] levels 88. Firm conclusions about any different effects of these two forms of vitamin D cannot be drawn. However, it appears that at nutritional doses vitamins D2 and D3 are equivalent, but at high doses vitamin D2 is less potent. Some studies suggest that cholecalciferol (Vitamin D3) increases serum Calcidiol [25(OH) D] more efficiently than does ergocalciferol (Vitamin D2) 89.
- Vitamin D3 (cholecalciferol) is available in 400, 800, 1000, 2000, 5000, 10,000, and 60,000 IU capsules. It is available in some countries as an intramuscular injection (Arachital 600,000 IU, which maintains vitamin D levels for 1 year). However, it can be extremely painful 89.
- Vitamin D2 (ergocalciferol) is available for oral use in 400 and 50,000 unit capsules or in a liquid form (8000 IU/mL) 89.
The American Academy of Pediatrics (AAP) recommends that exclusively and partially breastfed infants receive supplements of 400 IU/day of vitamin D shortly after birth and continue to receive these supplements until they are weaned and consume ≥1,000 mL/day of vitamin D-fortified formula or whole milk 90. Similarly, all non-breastfed infants ingesting <1,000 mL/day of vitamin D-fortified formula or milk should receive a vitamin D supplement of 400 IU/day 90. The American Academy of Pediatrics also recommends that older children and adolescents who do not obtain 400 IU/day through vitamin D-fortified milk and foods should take a 400 IU vitamin D supplement daily. However, this latter recommendation (issued November 2008) needs to be reevaluated in light of the Food and Nutrition Board’s vitamin D RDA of 600 IU/day for children and adolescents (issued November 2010 and which previously was an AI of 200 IU/day).
What happens if you don’t get enough vitamin D?
People can become deficient in vitamin D because they don’t consume enough or absorb enough from food, their exposure to sunlight is limited, or their kidneys cannot convert vitamin D to its active form in the body. In children, vitamin D deficiency causes rickets, where the bones become soft and bend. It’s a rare disease but still occurs, especially among African American infants and children. In adults, vitamin D deficiency leads to osteomalacia, causing bone pain and muscle weakness.
A lack of vitamin D has been associated with:
- An impairment in memory and thinking skills in older adults
- Bone, back, or muscle pain
- Cancer (particularly colon cancer)
- Cardiovascular disease, and an increased risk of dying from a stroke or a heart attack
- Constant fatigue and tiredness
- Frequent infections (such as colds and flu)
- Hair loss
- Kidney disease
- Low mood or depression
- Osteomalacia
- Osteoporosis
- Poor dental health
- Rickets
- Severe asthma in children
- Skin wounds that take a long time to heal.
Research also suggests low vitamin D may be a factor in several other conditions such as type 2 diabetes, high blood pressure, and multiple sclerosis.
Multiple sclerosis
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system that damages the myelin sheath surrounding and protecting nerve cells in the brain and spinal cord. This damage hinders or blocks messages between the brain and body, leading to clinical features, such as vision loss, motor weakness, spasticity, ataxia, tremor, sensory loss, and cognitive impairment 91. Some people with multiple sclerosis eventually lose the ability to write, speak, or walk.
The geographical distribution of multiple sclerosis around the world is unequal. Few people near the equator develop the disease, whereas the prevalence is higher further north and south. This uneven distribution has led to speculation that lower vitamin D levels in people who have less sunlight exposure might predispose them to the disease 91.
Many epidemiological and genetic studies have shown an association between multiple sclerosis and low 25(OH)D levels before and after the disease begins 91. Observational studies suggest that adequate vitamin D levels might reduce the risk of contracting multiple sclerosis and, once multiple sclerosis is present, decrease the risk of relapse and slow the disease’s progression 92. One study, for example, tested 25(OH)D levels in 1,092 women in Finland an average of 9 years before their multiple sclerosis diagnosis and compared their outcomes with those of 2,123 similar women who did not develop multiple sclerosis 93. More than half the women who developed multiple sclerosis had deficient or insufficient vitamin D levels. Women with 25(OH)D levels of less than 30 nmol/L (12 ng/mL) had a 43% higher multiple sclerosis risk than women with levels of 50 nmol/L (20 ng/mL) or higher. Among the women with two or more serum 25(OH)D samples taken before diagnosis (which reduced random measurement variation), a 50 nmol/L increase in 25(OH)D was associated with a 41% reduced risk of multiple sclerosis, and 25(OH)D levels less than 30 nmol/L were associated with an multiple sclerosis risk that was twice as high as levels of 50 nmol/L or higher.
Two earlier prospective studies of similar design—one in the United States with 444 non-Hispanic White individuals 94 and the other with 576 individuals in northern Sweden 95—found that levels of 25(OH)D greater than 99.1 nmol/L (39.6 ng/mL) and at least 75 nmol/L (30 ng/mL), respectively, were associated with a 61–62% lower risk of multiple sclerosis.
No clinical trials have examined whether vitamin D supplementation can prevent the onset of multiple sclerosis, but several have investigated whether supplemental vitamin D can help manage the disease. A 2018 Cochrane review analyzed 12 such trials that had a total of 933 participants with multiple sclerosis; the reviewers judged all of these trials to be of low quality 91. Overall, vitamin D supplementation, when compared with placebo administration, had no effect on relevant clinical outcomes, such as recurrent relapse or worsened disability.
Experts have reached no firm consensus on whether vitamin D can help prevent multiple sclerosis given the lack of clinical trial evidence 96. In addition, studies have not consistently shown that vitamin D supplementation tempers the signs and symptoms of active multiple sclerosis or reduces rates of relapse.
Type 2 diabetes
Vitamin D plays a role in glucose metabolism. It stimulates insulin secretion via the vitamin D receptor on pancreatic beta cells and reduces peripheral insulin resistance through vitamin D receptors in the muscles and liver 97. Vitamin D might be involved in the pathophysiology of type 2 diabetes through its effects on glucose metabolism and insulin signaling as well as its ability to reduce inflammation and improve pancreatic beta-cell function 98.
Observational studies have linked lower serum 25(OH)D levels to an increased risk of diabetes, but their results might have been confounded by the fact that many participants were overweight or obese and were therefore more predisposed to developing diabetes and having lower 25(OH)D levels 68. A review of 71 observational studies in adults with and without type 2 diabetes from 16 countries found a significant inverse relationship between vitamin D status and blood sugar levels in participants who did and did not have diabetes 99.
In contrast to observational studies, clinical trials provide little support for the benefits of vitamin D supplementation for glucose homeostasis. One trial included 65 overweight or obese adult men and women (mean age 32 years) who were otherwise healthy, did not have diabetes, and had low serum vitamin D levels (at or below 50 nmol/L [20 ng/mL]) 100. The investigators randomly assigned participants to receive either a bolus oral dose of 2,500 mcg (100,000 IU) vitamin D3 followed by 100 mcg (4,000 IU)/day or a placebo for 16 weeks. In the 54 participants who completed the study, vitamin D supplementation did not improve insulin sensitivity or insulin secretion in comparison with placebo.
One systematic review and meta-analysis evaluated 35 clinical trials that included 43,407 adults with normal glucose tolerance, prediabetes, or type 2 diabetes who received a median of 83 mcg (3,332 IU)/day vitamin D supplements or placebo for a median of 16 weeks 101. Vitamin D had no significant effects on glucose homeostasis, insulin secretion or resistance, or hemoglobin A1c levels (HbA1c, a measure of average blood sugar levels over the previous 2–3 months), irrespective of the study population, vitamin D dose, or trial quality.
Several trials have investigated whether vitamin D supplementation can prevent the transition from prediabetes to diabetes in patients with adequate 25(OH)D levels, and all have had negative results. In a trial in Norway, 511 men and women aged 25–80 years (mean age 62 years) with prediabetes received 500 mcg (20,000 IU) vitamin D3 or a placebo each week for 5 years 102. The results showed no significant differences in rates of progression to type 2 diabetes; in serum glucose, insulin, or hemoglobin A1c levels; or in measures of insulin resistance. At baseline, participants had an adequate mean serum 25(OH)D level of 60 nmol/L (24 ng/mL).
The largest trial to date of vitamin D supplements for diabetes prevention randomized 2,423 men and women aged 25 years and older (mean age 60 years) with prediabetes who were overweight or obese (mean BMI of 32.1) to 100 mcg (4,000 IU)/day vitamin D3 or placebo for a median of 2.5 years 98. Most participants (78%) had adequate serum levels of vitamin D at baseline (at least 50 nmol/L [20 ng/mL]). Vitamin D did not significantly prevent the development of diabetes in comparison with placebo. However, a post hoc analysis showed a 62% lower incidence of diabetes among participants with low baseline serum 25(OH)D levels (less than 30 nmol/L [12 ng/mL]) who took the vitamin D supplement than among those who took the placebo 103.
Studies have also assessed the value of vitamin D supplementation for managing diabetes, and they have found that the vitamin offers limited benefits. One meta-analysis of 20 clinical trials compared the effects of 0.5 mcg (20 IU)/day to 1,250 mcg (50,000 IU)/week vitamin D supplementation for 2–6 months with those of placebo on glycemic control in 2,703 adults from around the world who had diabetes 97. The vitamin D reduced insulin resistance to a small but significant degree, especially in people taking more than 50 mcg (2,000 IU)/day who were vitamin D deficient at baseline, had good glycemic control, were not obese, and were of Middle Eastern ethnicity. However, the supplementation had no significant effects on fasting blood glucose, hemoglobin A1c (HbA1c) or fasting insulin levels.
Clinical trials to date provide little evidence that vitamin D supplementation helps maintain glucose homeostasis, reduces the risk of progression from prediabetes to type 2 diabetes, or helps manage the disease, particularly in vitamin D-replete individuals.
Vitamin D deficiency
People can develop vitamin D deficiency when usual intakes are lower over time than recommended levels, exposure to sunlight is limited, the kidneys cannot convert 25(OH)D to its active form, or absorption of vitamin D from the digestive tract is inadequate. Diets low in vitamin D are more common in people who have milk allergy or lactose intolerance and those who consume an ovo-vegetarian or vegan diet 68.
In children, vitamin D deficiency is manifested as rickets, a disease characterized by a failure of bone tissue to become properly mineralized, resulting in soft bones and skeletal deformities 104. In addition to bone deformities and pain, severe rickets can cause failure to thrive, developmental delay, hypocalcemic seizures, tetanic spasms, cardiomyopathy, and dental abnormalities 105.
Prolonged exclusive breastfeeding without vitamin D supplementation can cause rickets in infants, and, in the United States, rickets is most common among breastfed Black infants and children 106. In one Minnesota county, the incidence rate of rickets in children younger than 3 years in the decade beginning in 2000 was 24.1 per 100,000 107. Rickets occurred mainly in Black children who were breastfed longer, were born with low birthweight, weighed less, and were shorter than other children. The incidence rate of rickets in the infants and children (younger than 7) seen by 2,325 pediatricians throughout Canada was 2.9 per 100,000 in 2002–2004, and almost all patients with rickets had been breastfed 108.
The fortification of milk (a good source of calcium) and other staples, such as breakfast cereals and margarine, with vitamin D beginning in the 1930s along with the use of cod liver oil made rickets rare in the United States 109. However, the incidence of rickets is increasing globally, even in the United States and Europe, especially among immigrants from African, Middle-Eastern, and Asian countries 110. Possible explanations for this increase include genetic differences in vitamin D metabolism, dietary preferences, and behaviors that lead to less sun exposure 111.
In adults and adolescents, vitamin D deficiency can lead to osteomalacia, in which existing bone is incompletely or defectively mineralized during the remodeling process, resulting in weak bones 105. Signs and symptoms of osteomalacia are similar to those of rickets and include bone deformities and pain, hypocalcemic seizures, tetanic spasms, and dental abnormalities 111.
Screening for vitamin D status is becoming a more common part of the routine laboratory bloodwork ordered by primary-care physicians, irrespective of any indications for this practice 112. No studies have examined whether such screening for vitamin D deficiency results in improved health outcomes 113. The U.S. Preventive Services Task Force (USPSTF) found insufficient evidence to assess the benefits and harms of screening for vitamin D deficiency in asymptomatic adults 114. It added that no national professional organization recommends population screening for vitamin D deficiency.
Groups at risk of vitamin D inadequacy
Obtaining sufficient vitamin D from natural (nonfortified) food sources alone is difficult. For many people, consuming vitamin D-fortified foods and exposing themselves to some sunlight are essential for maintaining a healthy vitamin D status. However, some groups might need dietary supplements to meet their vitamin D requirements. The following groups are among those most likely to have inadequate vitamin D status.
Breastfed infants
Consumption of human milk alone does not ordinarily enable infants to meet vitamin D requirements, because it provides less than 0.6 to 2.0 mcg/L (25 to 78 IU/L) 115. The vitamin D content of human milk is related to the mother’s vitamin D status; studies suggest that the breastmilk of mothers who take daily supplements containing at least 50 mcg (2,000 IU) vitamin D3 have higher levels of the nutrient 116.
Although UVB exposure can produce vitamin D in infants, the American Academy of Pediatrics advises parents to keep infants younger than 6 months out of direct sunlight, dress them in protective clothing and hats, and apply sunscreen on small areas of exposed skin when sun exposure is unavoidable 117. The AAP recommends 10 mcg (400 IU)/day vitamin D supplements for exclusively and partially breastfed infants starting shortly after birth and lasting until they are weaned and consume at least 1,000 mL/day vitamin D-fortified formula or whole milk 115. The American Academy of Pediatrics also recommends 10 mcg (400 IU)/day supplemental vitamin D for all infants who are not breastfed and ingest less than 1,000 mL/day vitamin D-fortified formula or milk. An analysis of NHANES 2009–2016 data found that only 20.5% of breastfed infants and 31.1% of infants who were not breastfed ingested these recommended amounts of supplements 118.
Older adults
Older adults are at increased risk of developing vitamin D insufficiency in part because, as they age, skin cannot synthesize vitamin D as efficiently 119, they are likely to spend more time indoors, and they may have inadequate intakes of the vitamin 68. As many as half of older adults in the United States with hip fractures could have serum 25(OH)D levels <30 nmol/L (<12 ng/mL) 120.
People with limited sun exposure
Homebound individuals, women who wear long robes and head coverings for religious reasons, and people with occupations that limit sun exposure are unlikely to obtain adequate vitamin D from sunlight 121. The use of sunscreen also limits vitamin D synthesis from sunlight. However, because the extent and frequency of sunscreen use are unknown, the role that sunscreen may play in reducing vitamin D synthesis is unclear 68. Ingesting RDA levels of vitamin D from foods and/or supplements will provide these individuals with adequate amounts of this nutrient.
People with dark skin
Greater amounts of the pigment melanin in the epidermal layer result in darker skin and reduce the skin’s ability to produce vitamin D from sunlight 68. Various reports consistently show lower serum 25(OH)D levels in persons identified as black compared with those identified as white. However, it is not clear that lower levels of 25(OH)D for persons with dark skin have significant health consequences. Those of African American ancestry, for example, have reduced rates of fracture and osteoporosis compared with Caucasians 77. Ingesting RDA levels of vitamin D from foods and/or supplements will provide these individuals with adequate amounts of this nutrient.
People with conditions that limit fat absorption
Because vitamin D is a fat-soluble vitamin, its absorption depends on the gut’s ability to absorb dietary fat 73. Individuals who have a reduced ability to absorb dietary fat might require vitamin D supplementation 122. Fat malabsorption is associated with a variety of medical conditions, including some forms of liver disease, cystic fibrosis, celiac disease, and Crohn’s disease, as well as ulcerative colitis when the terminal ileum is inflamed 68, 123, 122. In addition, people with some of these conditions might have lower intakes of certain foods, such as dairy products (many of which are fortified with vitamin D), or eat only small amounts of these foods. Individuals who have difficulty absorbing dietary fat might therefore require vitamin D supplementation 122.
People who are obese or have undergone gastric bypass surgery
Individuals with a body mass index (BMI) of 30 or more have lower serum 25(OH)D levels than nonobese individuals. Obesity does not affect the skin’s capacity to synthesize vitamin D. However, greater amounts of subcutaneous fat sequester more of the vitamin 68. Obese people might need greater intakes of vitamin D to achieve 25(OH)D levels similar to those of people with normal weight 124.
Obese individuals who have undergone gastric bypass surgery can also become vitamin D deficient. In this procedure, part of the upper small intestine, where vitamin D is absorbed, is bypassed, and vitamin D that is mobilized into the bloodstream from fat stores might not raise 25(OH)D to adequate levels over time 125. Various expert groups—including the American Association of Metabolic and Bariatric Surgery, The Obesity Society, and the British Obesity and Metabolic Surgery Society—have developed guidelines on vitamin D screening, monitoring, and replacement before and after bariatric surgery 126.
Vitamin E
Vitamin E is a fat-soluble nutrient found in some foods, added to others, and available as a dietary supplement 127. “Vitamin E” is the collective name for a group of fat-soluble compounds with distinctive antioxidant activities 128. Naturally occurring vitamin E exists in eight chemical forms (alpha-, beta-, gamma-, and delta-tocopherol and alpha-, beta-, gamma-, and delta-tocotrienol) that have varying levels of biological activity 128. Alpha- (or α-) tocopherol is the only form that is recognized to meet human requirements. In the body, vitamin E acts as an antioxidant, helping to protect cells from the damage caused by free radicals. Free radicals are compounds formed when our bodies convert the food we eat into energy. People are also exposed to free radicals in the environment from cigarette smoke, air pollution, and ultraviolet light from the sun. Vitamin E is believed to serve as a chain-breaking antioxidant that stops the oxidative degradation of lipids, thus preventing free radical production and harm to the cell.
The body also needs vitamin E to boost its immune system so that it can fight off invading bacteria and viruses. It helps to widen blood vessels and keep blood from clotting within them. In addition, cells use vitamin E to interact with each other and to carry out many important functions.
Vitamin E is absorbed in the intestinal lumen, which is dependent upon various factors such as pancreatic secretions, micelle formation, and most importantly, chylomicron secretions. Chylomicron secretion is necessary for vitamin E absorption. Vitamin E is found in sunflower seeds, nuts, some oils, spinach, butternut squash, and many other food sources. Vitamin E deficiency has been linked to peripheral neuropathy in addition to spinocerebellar ataxia, skeletal myopathy and pigmented retinopathy. Interestingly, studies have reported vitamin E level in association to the development of cataracts 129.
Serum concentrations of vitamin E (alpha-tocopherol) depend on the liver, which takes up the nutrient after the various forms are absorbed from the small intestine. The liver preferentially resecretes only alpha-tocopherol via the hepatic alpha-tocopherol transfer protein 128; the liver metabolizes and excretes the other vitamin E forms 130. As a result, blood and cellular concentrations of other forms of vitamin E are lower than those of alpha-tocopherol and have been the subjects of less research 131.
Antioxidants protect cells from the damaging effects of free radicals, which are molecules that contain an unshared electron. Free radicals damage cells and might contribute to the development of cardiovascular disease and cancer 132. Unshared electrons are highly energetic and react rapidly with oxygen to form reactive oxygen species (ROS). The body forms ROS endogenously when it converts food to energy, and antioxidants might protect cells from the damaging effects of ROS. The body is also exposed to free radicals from environmental exposures, such as cigarette smoke, air pollution, and ultraviolet radiation from the sun. Reactive oxygen species (ROS) are part of signaling mechanisms among cells.
Vitamin E is a fat-soluble antioxidant that stops the production of reactive oxygen species (ROS) formed when fat undergoes oxidation. Scientists are investigating whether, by limiting free-radical production and possibly through other mechanisms, vitamin E might help prevent or delay the chronic diseases associated with free radicals.
In addition to its activities as an antioxidant, vitamin E is involved in immune function and, as shown primarily by in vitro studies of cells, cell signaling, regulation of gene expression, and other metabolic processes 128. Alpha-tocopherol inhibits the activity of protein kinase C, an enzyme involved in cell proliferation and differentiation in smooth muscle cells, platelets, and monocytes 127. Vitamin-E–replete endothelial cells lining the interior surface of blood vessels are better able to resist blood-cell components adhering to this surface. Vitamin E also increases the expression of two enzymes that suppress arachidonic acid metabolism, thereby increasing the release of prostacyclin from the endothelium, which, in turn, dilates blood vessels and inhibits platelet aggregation 127.
Vitamin E and macular degeneration
Age-related macular degeneration (AMD) and cataracts are among the most common causes of significant vision loss in older people. Their causes are usually unknown, but the cumulative effects of oxidative stress have been postulated to play a role. If so, nutrients with antioxidant functions, such as vitamin E, could be used to prevent or treat these conditions.
Prospective cohort studies have found that people with relatively high dietary intakes of vitamin E (e.g., 20 mg/day [30 IU]) have an approximately 20% lower risk of developing age-related macular degeneration than people with low intakes (e.g., <10 mg/day [<15 IU]) 133. However, two randomized controlled trials in which participants took supplements of vitamin E (500 IU/day [335 mg] d-alpha-tocopherol in one study 134 and 111 IU/day (50 mg) dl-alpha-tocopheryl acetate combined with 20 mg/day beta-carotene in the other study 135 or a placebo failed to show a protective effect for vitamin E on age-related macular degeneration. The Age-Related Eye Disease Study (AREDS) 59, a large randomized clinical trial, found that participants at high risk of developing advanced age-related macular degeneration (i.e., those with intermediate age-related macular degeneration or those with advanced age-related macular degeneration in one eye) reduced their risk of developing advanced age-related macular degeneration by 25% by taking a daily supplement containing vitamin E (400 IU [180 mg] dl-alpha-tocopheryl acetate), beta-carotene (15 mg), vitamin C (500 mg), zinc (80 mg), and copper (2 mg) compared to participants taking a placebo over 5 years. A follow-up AREDS2 study 60 confirmed the value of this and similar supplement formulations in reducing the progression of age-related macular degeneration over a median follow-up period of 5 years.
Overall, the available evidence is inconsistent with respect to whether vitamin E supplements, taken alone or in combination with other antioxidants, can reduce the risk of developing age-related macular degeneration or cataracts. However, the formulations of vitamin E, other antioxidants, zinc, and copper used in AREDS hold promise for slowing the progression of age-related macular degeneration in people at high risk of developing advanced age-related macular degeneration.
Vitamin E and cataracts
Several observational studies have revealed a potential relationship between vitamin E supplements and the risk of cataract formation. One prospective cohort study found that lens clarity was superior in participants who took vitamin E supplements and those with higher blood levels of the vitamin 136. In another study, long-term use of vitamin E supplements was associated with slower progression of age-related lens opacification 137. However, in the AREDS trial, the use of a vitamin E-containing (as dl-alpha-tocopheryl acetate) formulation had no apparent effect on the development or progression of cataracts over an average of 6.3 years 53. The AREDS2 study, which also tested formulations containing 400 IU (180 mg) vitamin E, confirmed these findings 54.
How much vitamin E do I need?
The amount of vitamin E you need each day depends on your age. Average daily recommended amounts are listed below in milligrams (mg). Naturally sourced vitamin E is called RRR-alpha-tocopherol (commonly labeled as d-alpha-tocopherol); the synthetically produced form is all rac-alpha-tocopherol (commonly labeled as dl-alpha-tocopherol).
Recommended Dietary Allowances (RDAs) for vitamin E are provided in milligrams (mg) and are listed in Table 9. One mg vitamin E (alpha-tocopherol) is equivalent to 1 mg RRR-alpha-tocopherol or 2 mg all rac-alpha-tocopherol. Because insufficient data are available to develop RDAs for infants, Adequate Intakes (AIs) were developed based on the amount of vitamin E consumed by healthy breastfed babies.
Vitamin E is listed on the new Nutrition Facts and Supplement Facts labels in milligram (mg). The U.S. Food and Drug Administration (FDA) required manufacturers to use these new labels starting in January 2020, but companies with annual sales of less than $10 million may continue to use the old labels that list vitamin E in international units (IUs) until January 2021 138. Conversion rules are as follows:
To convert from mg to IU:
- 1 mg of alpha-tocopherol is equivalent to 1.49 IU of the natural form or 2.22 IU of the synthetic form.
To convert from IU to mg:
- 1 IU of the natural form is equivalent to 0.67 mg of alpha-tocopherol.
- 1 IU of the synthetic form is equivalent to 0.45 mg of alpha-tocopherol.
For example, 15 mg of natural alpha-tocopherol would equal 22.4 IU (15 mg x 1.49 IU/mg = 22.4 IU). The corresponding value for synthetic alpha-tocopherol would be 33.3 IU (15 mg x 2.22 IU/mg).
Table 9. Recommended Dietary Allowances (RDAs) for vitamin E (alpha-tocopherol)
| Age | Males | Females | Pregnancy | Lactation |
| 0–6 months* | 4 mg | 4 mg | ||
| 7–12 months* | 5 mg | 5 mg | ||
| 1–3 years | 6 mg | 6 mg | ||
| 4–8 years | 7 mg | 7 mg | ||
| 9–13 years | 11 mg | 11 mg | ||
| 14+ years | 15 mg | 15 mg | 15 mg | 19 mg |
Footnotes:
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
*Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA).
[Source 139 ]What foods provide vitamin E?
Vitamin E is found naturally in foods and is added to some fortified foods. Nuts, seeds, and vegetable oils are among the best sources of alpha-tocopherol, and significant amounts are available in green leafy vegetables and fortified cereals (see Table 10 for a more detailed list). Most vitamin E in American diets is in the form of gamma-tocopherol from soybean, canola, corn, and other vegetable oils and food products 131. You can get recommended amounts of vitamin E by eating a variety of foods including the following:
- Vegetable oils like wheat germ, sunflower, and safflower oils are among the best sources of vitamin E. Corn and soybean oils also provide some vitamin E.
- Nuts (such as peanuts, hazelnuts, and, especially, almonds) and seeds (like sunflower seeds) are also among the best sources of vitamin E.
- Green vegetables, such as spinach and broccoli, provide some vitamin E.
- Food companies add vitamin E to some breakfast cereals, fruit juices, margarines and spreads, and other foods. To find out which ones have vitamin E, check the product labels.
The U.S. Department of Agriculture’s (USDA’s) FoodData Central (https://fdc.nal.usda.gov) website lists the nutrient content of many foods, including, in some cases, the amounts of alpha-, beta-, gamma-, and delta-tocopherol. The USDA also provides a comprehensive list of foods containing vitamin E arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminE-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminE-Food.pdf).
Table 10. Vitamin E (Alpha-Tocopherol) content of selected foods
| Food | Milligrams (mg) per serving | Percent DV* |
| Wheat germ oil, 1 tablespoon | 20.3 | 135 |
| Sunflower seeds, dry roasted, 1 ounce | 7.4 | 49 |
| Almonds, dry roasted, 1 ounce | 6.8 | 45 |
| Sunflower oil, 1 tablespoon | 5.6 | 37 |
| Safflower oil, 1 tablespoon | 4.6 | 31 |
| Hazelnuts, dry roasted, 1 ounce | 4.3 | 29 |
| Peanut butter, 2 tablespoons | 2.9 | 19 |
| Peanuts, dry roasted, 1 ounce | 2.2 | 15 |
| Corn oil, 1 tablespoon | 1.9 | 13 |
| Spinach, boiled, ½ cup | 1.9 | 13 |
| Broccoli, chopped, boiled, ½ cup | 1.2 | 8 |
| Soybean oil, 1 tablespoon | 1.1 | 7 |
| Kiwifruit, 1 medium | 1.1 | 7 |
| Mango, sliced, ½ cup | 0.7 | 5 |
| Tomato, raw, 1 medium | 0.7 | 5 |
| Spinach, raw, 1 cup | 0.6 | 4 |
Footnote: *DV = Daily Value. FDA developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin E is 15 mg for adults and children age 4 years and older. 1 mg vitamin E = 1 mg RRR-alpha-tocopherol (commonly labeled as d-alpha-tocopherol) = 2 mg all rac-alpha-tocopherol (commonly labeled as dl-alpha-tocopherol). FDA does not require food labels to list vitamin E content unless vitamin E has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
Vitamin E supplements
Supplements of vitamin E typically provide only alpha-tocopherol, although “mixed” products containing other tocopherols and even tocotrienols are available. Naturally occurring alpha-tocopherol exists in one stereoisomeric form. In contrast, synthetically produced alpha-tocopherol contains equal amounts of its eight possible stereoisomers; serum and tissues maintain only four of these stereoisomers. A given amount of synthetic alpha-tocopherol (all rac-alpha-tocopherol; commonly labeled as “DL” or “dl”) is therefore only half as active as the same amount (by weight in mg) of the natural form (RRR-alpha-tocopherol; commonly labeled as “D” or “d”).
Two main things to consider when choosing a vitamin E supplement are:
- The amount of vitamin E: Most once-daily multivitamin-mineral supplements provide about 13.5 mg of vitamin E, whereas vitamin E-only supplements commonly contain 67 mg or more. The doses in most vitamin E-only supplements are much higher than the recommended amounts. Some people take large doses because they believe or hope that doing so will keep them healthy or lower their risk of certain diseases.
- The form of vitamin E: Although vitamin E sounds like a single substance, it is actually the name of eight related compounds in food, including alpha-tocopherol. Each form has a different potency, or level of activity in the body.
Vitamin E from natural sources is commonly listed as ”d-alpha-tocopherol” on food packaging and supplement labels. Synthetic (laboratory-made) vitamin E is commonly listed as ”dl-alpha-tocopherol.” The natural form is more potent; 1 mg vitamin E = 1 mg d-alpha-tocopherol (natural vitamin E) = 2 mg dl-alpha-tocopherol (synthetic vitamin E).
Some food and dietary supplement labels still list vitamin E in International Units (IUs) rather than mg. 1 IU of the natural form of vitamin E is equivalent to 0.67 mg. 1 IU of the synthetic form of vitamin E is equivalent to 0.45 mg.
Most vitamin-E-only supplements provide ≥67 mg (100 IU of natural vitamin E) of the nutrient. These amounts are substantially higher than the Recommended Dietary Allowances (RDAs).
Alpha-tocopherol in dietary supplements and fortified foods is often esterified to prolong its shelf life while protecting its antioxidant properties. The body hydrolyzes and absorbs these esters (alpha-tocopheryl acetate and succinate) as efficiently as alpha-tocopherol.
Some vitamin E supplements provide other forms of the vitamin, such as gamma-tocopherol, tocotrienols, and mixed tocopherols. Scientists do not know if any of these forms are superior to alpha-tocopherol in supplements.
Vitamin E deficiency
Frank vitamin E deficiency is rare and overt deficiency symptoms have not been found in healthy people who obtain little vitamin E from their diets 127. Premature babies of very low birth weight (<1,500 grams) might be deficient in vitamin E. Vitamin E supplementation in these infants might reduce the risk of some complications, such as those affecting the retina, but they can also increase the risk of infections 140.
Because the digestive tract requires fat to absorb vitamin E, people with fat-malabsorption disorders are more likely to become deficient than people without such disorders. Examples include Crohn’s disease, cystic fibrosis, and certain rare genetic diseases such as abetalipoproteinemia and ataxia with vitamin E deficiency (AVED). Vitamin E needs some fat for the digestive system to absorb it. Deficiency symptoms include peripheral neuropathy, ataxia, skeletal myopathy, retinopathy, and impairment of the immune response 141. People with Crohn’s disease, cystic fibrosis, or an inability to secrete bile from the liver into the digestive tract, for example, often pass greasy stools or have chronic diarrhea; as a result, they sometimes require water-soluble forms of vitamin E, such as tocopheryl polyethylene glycol-1000 succinate 128.
Some people with abetalipoproteinemia, a rare inherited disorder resulting in poor absorption of dietary fat, require enormous doses of supplemental vitamin E (approximately 100 mg/kg or 5–10 g/day) 128. Vitamin E deficiency secondary to abetalipoproteinemia causes such problems as poor transmission of nerve impulses, muscle weakness, and retinal degeneration that leads to blindness 142. Ataxia and vitamin E deficiency (AVED) is another rare, inherited disorder in which the liver’s alpha-tocopherol transfer protein is defective or absent. People with AVED have such severe vitamin E deficiency that they develop nerve damage and lose the ability to walk unless they take large doses of supplemental vitamin E 143.
Excessive vitamin E health risks
Research has not found any adverse effects from consuming vitamin E in food 139. However, high doses of alpha-tocopherol supplements can cause hemorrhage and interrupt blood coagulation in animals, and test tube studies data suggest that high doses inhibit platelet aggregation. Two clinical trials have found an increased risk of hemorrhagic stroke in participants taking alpha-tocopherol; one trial included Finnish male smokers who consumed 50 mg/day for an average of 6 years 144 and the other trial involved a large group of male physicians in the United States who consumed 400 IU (180 mg) of synthetic vitamin E every other day for 8 years 145. Because the majority of physicians in the latter study were also taking aspirin, this finding could indicate that vitamin E has a tendency to cause bleeding.
The Food and Nutrition Board at the Institute of Medicine of The National Academies has established Tolerable Upper Intake Levels (ULs – the maximum daily intake unlikely to cause adverse health effects) for vitamin E based on the potential for hemorrhagic effects (see Table 11). The Tolerable Upper Intake Levels (ULs) apply to all forms of supplemental alpha-tocopherol, including the eight stereoisomers present in synthetic vitamin E. Doses of up to 1,000 mg/day (1,500 IU/day of the natural form or 1,100 IU/day of the synthetic form) in adults appear to be safe, although the data are limited and based on small groups of people taking up to 3,200 mg/day of alpha-tocopherol for only a few weeks or months. Long-term intakes above the Tolerable Upper Intake Level (UL) increase the risk of adverse health effects 139. Vitamin E Tolerable Upper Intake Levels (ULs) for infants have not been established.
Two meta-analyses of randomized trials have also raised questions about the safety of large doses of vitamin E, including doses lower than the Tolerable Upper Intake Level (UL). These meta-analyses linked supplementation to small but statistically significant increases in all-cause mortality. One analysis found an increased risk of death at doses of 400 IU/day (form not specified), although the risk began to increase at 150 IU 146. In the other analysis of studies of antioxidant supplements for disease prevention, the highest quality trials revealed that vitamin E, administered singly (dose range 10 IU–5,000 IU/day; mean 569 IU [form not specified]) or combined with up to four other antioxidants, significantly increased mortality risk 147.
The implications of these analyses for the potential adverse effects of high-dose vitamin E supplements are unclear 127. Participants in the studies included in these analyses were typically middle-aged or older and had chronic diseases or related risk factors. These participants often consumed other supplements in addition to vitamin E. Some of the studies analyzed took place in developing countries in which nutritional deficiencies are common. A review of the subset of studies in which vitamin E supplements were given to healthy individuals for the primary prevention of chronic disease found no convincing evidence that the supplements increased mortality 148.
However, results from the recently published, large SELECT trial show that vitamin E supplements (400 IU/day [180 mg] as dl-alpha-tocopheryl acetate) may harm adult men in the general population by increasing their risk of prostate cancer 149. Follow-up studies are assessing whether the cancer risk was associated with baseline blood levels of vitamin E and selenium prior to supplementation as well as whether changes in one or more genes might increase a man’s risk of developing prostate cancer while taking vitamin E.
Table 11. Tolerable Upper Intake Levels (ULs) for Vitamin E
| Age | Male | Female | Pregnancy | Lactation |
| 1–3 years | 200 mg | 200 mg | ||
| 4–8 years | 300 mg | 300 mg | ||
| 9–13 years | 600 mg | 600 mg | ||
| 14–18 years | 800 mg | 800 mg | 800 mg | 800 mg |
| 19+ years | 1,000 mg | 1,000 mg | 1,000 mg | 1,000 mg |
Vitamin B9 and Vitamin B12
Vitamin B9 also known as folate acid, functions as a coenzyme or cosubstrate in single-carbon transfers in the synthesis of nucleic acids (DNA and RNA) and metabolism of amino acids 150. One of the most important folate-dependent reactions is the conversion of homocysteine to methionine in the synthesis of S-adenosyl-methionine, an important methyl donor 151. Another folate-dependent reaction, the methylation of deoxyuridylate to thymidylate in the formation of DNA, is required for proper cell division. An impairment of this reaction initiates a process that can lead to megaloblastic anemia, one of the hallmarks of folate deficiency 152.
Folate is found in green leafy vegetable, fruits, fortified cereals, and meats. The total body content of folate is estimated to be 15 to 30 mg; about half of this amount is stored in the liver and the remainder in blood and body tissues 153. Serum folate concentrations are commonly used to assess folate status; a value above 3 ng/mL indicates adequacy 154. This indicator, however, is sensitive to recent dietary intake, so it might not reflect long-term status. Erythrocyte folate concentrations provide a longer-term measure of folate intakes; a concentration above 140 ng/mL indicates adequate folate status 154. Folate deficiency can develop in the setting of malabsorptive diseases, excess alcohol, medications (e.g., antiepileptics), and pregnancy 153.
A combination of serum or erythrocyte folate concentration and indicators of metabolic function can also be used to assess folate status. Plasma homocysteine concentration is a commonly used functional indicator of folate status because homocysteine levels rise when the body cannot convert homocysteine to methionine due to a 5-MTHF deficiency 154. Homocysteine levels, however, are not a highly specific indicator of folate status because they can be influenced by other factors, including kidney dysfunction and deficiencies of vitamin B12 and other micronutrients 155. The most commonly used cutoff value for elevated homocysteine levels is 16 micromol/L, although slightly lower values of 12 to 14 micromol/L have also been used 156. A homocysteine cutoff of 10 micromol/L has been proposed for assessing folate status in populations 150.
Vitamin B12 also known as cobalamin, because vitamin B12 contains the mineral cobalt, compounds with vitamin B12 activity 157. Vitamin B12 is required for the development, myelination, and function of the central nervous system; healthy red blood cell formation; and DNA synthesis 158. Vitamin B12 functions as a cofactor for two enzymes, methionine synthase and L-methylmalonyl-CoA mutase 159. Methionine synthase catalyzes the conversion of homocysteine to the essential amino acid methionine 159. Methionine is required for the formation of S-adenosylmethionine, a universal methyl donor for almost 100 different substrates, including DNA, RNA, proteins, and lipids 160. L-methylmalonyl-CoA mutase converts L-methylmalonyl-CoA to succinyl-CoA in the metabolism of propionate, a short-chain fatty acid 159.
Vitamin B12 is absorbed with intrinsic factor, a glycoprotein of stomach cells, in the terminal ileum. Vitamin B12 status is typically assessed by measurements of serum or plasma vitamin B12 levels. The cutoff between normal vitamin B12 levels and deficiency varies by method and laboratory, but most laboratories define subnormal serum or plasma values as those lower than 200 or 250 pg/mL (148 or 185 pmol/L) 159. Levels of serum methylmalonic acid (MMA), a vitamin B12-associated metabolite, are the most sensitive markers of vitamin B12 status, and an methylmalonic acid level greater than 0.271 micromol/L suggests vitamin B12 deficiency 161. However, methylmalonic acid levels also rise with renal insufficiency and tend to be higher in older adults 162. Another marker is total plasma homocysteine levels, which rise quickly as vitamin B12 status declines; a serum homocysteine level higher than 15 micromol/L, for example, suggests vitamin B12 deficiency 163. However, this indicator has poor specificity because it is influenced by other factors, such as low folate levels and, especially, by declines in kidney function 164. Experts suggest that if a patient’s serum vitamin B12 level is less than 150 pg/ml (111 pmol/L), the patient’s serum methylmalonic acid levels should be checked to confirm a diagnosis of vitamin B12 deficiency 165.
Vitamin B12 deficiency most typically develops in patients following a vegetarian or vegan diet, with a history of bariatric surgery, and those with malabsorptive diseases. Elevated serum homocysteine and methylmalonic acid are an indicator of cobalamin deficiency 166.
Decreased levels of both vitamin B9 (folate) and vitamin B12 have been linked with age-related macular degeneration 167, a degeneration of the retina that results in central visual acuity and visual field loss (central scotoma) due to neovascular and non-neovascular derangements. Incorporating foods rich in folate and vitamin B12 is encouraged in patients with age-related macular degeneration due to its potential contribution to slowing its progression 167.
In addition to its role in age-related macular degeneration, patients with vitamin B12 deficiency have neurological manifestations. B12 deficiency particularly affects the dorsal spinal column clinically presenting as diminished vibratory and positional sense in the extremities, especially the lower limbs. Gait abnormalities and cognitive disturbances, such as decreased concentration and memory loss, are also noted 166.
How much vitamin B9 (folate) do I need?
The amount of folate you need depends on your age. Average daily recommended amounts are listed below in micrograms (mcg) of dietary folate equivalents (DFEs). The measure of mcg DFE is used because your body absorbs more folic acid from fortified foods and dietary supplements than folate found naturally in foods. Compared to folate found naturally in foods, you actually need less folic acid to get recommended amounts. For example, 240 mcg of folic acid and 400 mcg of folate are both equal to 400 mcg DFE.
All women and teen girls who could become pregnant should consume 400 mcg of folic acid daily from supplements, fortified foods, or both in addition to the folate they get from following a healthy eating pattern.
- 1 mcg DFE = 1 mcg food folate
- 1 mcg DFE = 0.6 mcg folic acid from fortified foods or dietary supplements consumed with foods
- 1 mcg DFE = 0.5 mcg folic acid from dietary supplements taken on an empty stomach
Table 12. Recommended Dietary Allowances (RDAs) for Folate
| Age | Male | Female | Pregnancy | Lactation |
| Birth to 6 months* | 65 mcg DFE* | 65 mcg DFE* | ||
| 7–12 months* | 80 mcg DFE* | 80 mcg DFE* | ||
| 1–3 years | 150 mcg DFE | 150 mcg DFE | ||
| 4–8 years | 200 mcg DFE | 200 mcg DFE | ||
| 9–13 years | 300 mcg DFE | 300 mcg DFE | ||
| 14–18 years | 400 mcg DFE | 400 mcg DFE | 600 mcg DFE | 500 mcg DFE |
| 19+ years | 400 mcg DFE | 400 mcg DFE | 600 mcg DFE | 500 mcg DFE |
Footnotes:
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
*Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an RDA.
[Source 156 ]What foods provide vitamin B9 (folate)?
Folate is naturally present in many foods, and folic acid is added to some foods. Folate is naturally present vegetables (especially dark green leafy vegetables), fruits and fruit juices, nuts, beans, peas, seafood, eggs, dairy products, meat, poultry, and grains (Table 13) 152. Spinach, liver, asparagus, and brussels sprouts are among the foods with the highest folate levels. You can get recommended amounts by eating a variety of foods, including the following.
Folate is naturally present in:
- Beef liver
- Vegetables (especially asparagus, brussels sprouts, and dark green leafy vegetables such as spinach and mustard greens)
- Fruits and fruit juices (especially oranges and orange juice)
- Nuts, beans, and peas (such as peanuts, black-eyed peas, and kidney beans)
Folic acid is added to the following foods:
- Enriched bread, flour, cornmeal, pasta, and rice
- Fortified breakfast cereals
- Fortified corn masa flour (used to make corn tortillas and tamales, for example)
In January 1998, the U.S. Food and Drug Administration (FDA) began requiring manufacturers to add 140 mcg folic acid/100 g to enriched breads, cereals, flours, cornmeals, pastas, rice, and other grain products 168 to reduce the risk of neural tube defects (NTDs). Because cereals and grains are widely consumed in the United States, these products have become important contributors of folic acid to the American diet. The fortification program increased mean folic acid intakes in the United States by about 190 mcg/day 169. In April 2016, FDA approved the voluntary addition of up to 154 mcg folic acid/100 g to corn masa flour 170.
Since November 1, 1998, the Canadian government has also required the addition of 150 mcg folic acid/100 g to many grains, including enriched pasta, cornmeal, and white flour 171. Many other countries, including Costa Rica, Chile, and South Africa, have also established mandatory folic acid fortification programs 171.
The U.S. Department of Agriculture’s FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing folate arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/Folate-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/Folate-Food.pdf).
Table 13. Folate and Folic Acid content of selected foods
| Food | Micrograms (mcg) DFE per serving | Percent DV* |
| Beef liver, braised, 3 ounces | 215 | 54 |
| Spinach, boiled, ½ cup | 131 | 33 |
| Black-eyed peas (cowpeas), boiled, ½ cup | 105 | 26 |
| Breakfast cereals, fortified with 25% of the DV† | 100 | 25 |
| Rice, white, medium-grain, cooked, ½ cup† | 90 | 22 |
| Asparagus, boiled, 4 spears | 89 | 22 |
| Brussels sprouts, frozen, boiled, ½ cup | 78 | 20 |
| Spaghetti, cooked, enriched, ½ cup† | 74 | 19 |
| Lettuce, romaine, shredded, 1 cup | 64 | 16 |
| Avocado, raw, sliced, ½ cup | 59 | 15 |
| Spinach, raw, 1 cup | 58 | 15 |
| Broccoli, chopped, frozen, cooked, ½ cup | 52 | 13 |
| Mustard greens, chopped, frozen, boiled, ½ cup | 52 | 13 |
| Bread, white, 1 slice† | 50 | 13 |
| Green peas, frozen, boiled, ½ cup | 47 | 12 |
| Kidney beans, canned, ½ cup | 46 | 12 |
| Wheat germ, 2 tablespoons | 40 | 10 |
| Tomato juice, canned, ¾ cup | 36 | 9 |
| Crab, Dungeness, 3 ounces | 36 | 9 |
| Orange juice, ¾ cup | 35 | 9 |
| Turnip greens, frozen, boiled, ½ cup | 32 | 8 |
| Peanuts, dry roasted, 1 ounce | 27 | 7 |
| Orange, fresh, 1 small | 29 | 7 |
| Papaya, raw, cubed, ½ cup | 27 | 7 |
| Banana, 1 medium | 24 | 6 |
| Yeast, baker’s, ¼ teaspoon | 23 | 6 |
| Egg, whole, hard-boiled, 1 large | 22 | 6 |
| Cantaloupe, raw, cubed, ½ cup | 17 | 4 |
| Vegetarian baked beans, canned, ½ cup | 15 | 4 |
| Fish, halibut, cooked, 3 ounces | 12 | 3 |
| Milk, 1% fat, 1 cup | 12 | 3 |
| Ground beef, 85% lean, cooked, 3 ounces | 7 | 2 |
| Chicken breast, roasted, 3 ounces | 3 | 1 |
Footnotes: * DV = Daily Value. The FDA developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for folate is 400 mcg DFE for adults and children aged 4 years and older, where mcg DFE = mcg naturally occurring folate + (1.7 x mcg folic acid). The labels must list folate content in mcg DFE per serving and if folic acid is added to the product, they must also list the amount of folic acid in mcg in parentheses. The FDA does not require food labels to list folate content unless folic acid has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
† Fortified with folic acid as part of the folate fortification program.
[Source 86 ]Folate supplements
Folic acid is available in multivitamins and prenatal vitamins, supplements containing other B-complex vitamins, and supplements containing only folic acid. Common doses range from 680 to 1,360 mcg DFE (400 to 800 mcg folic acid) in supplements for adults and 340 to 680 mcg DFE (200 to 400 mcg folic acid) in children’s multivitamins 172.
About 85% of supplemental folic acid, when taken with food, is bioavailable 152. When consumed without food, nearly 100% of supplemental folic acid is bioavailable.
Dietary supplements containing 5-methyl-tetrahydrofolate (also called methylfolate), a reduced form of folate, are also available. For some people, supplementation with 5-methyltetrahydrofolate might be more beneficial than with folic acid 173. The bioavailability of 5-methyltetrahydrofolate in supplements is the same as or greater than that of folic acid 174. However, conversion factors between mcg and mcg DFE for 5-methyltetrahydrofolate have not been formally established. The FDA allows manufacturers to use either a conversion factor of 1.7 to be comparable to folic acid, or their own established conversion factors not to exceed 1.7.
Folate deficiency
Isolated folate deficiency is uncommon; folate deficiency usually coexists with other nutrient deficiencies because of its strong association with poor diet, alcoholism, and malabsorptive disorders 152. Megaloblastic anemia, which is characterized by large, abnormally nucleated erythrocytes, is the primary clinical sign of folate or vitamin B12 deficiency 153. Its symptoms include weakness, fatigue, difficulty concentrating, irritability, headache, heart palpitations, and shortness of breath 156.
Folate deficiency can also produce soreness in and shallow ulcerations on the tongue and oral mucosa; changes in skin, hair, or fingernail pigmentation; gastrointestinal symptoms; and elevated blood concentrations of homocysteine 175.
Women with insufficient folate intakes are at increased risk of giving birth to infants with neural tube defects 156. Inadequate maternal folate status has also been associated with low infant birth weight, preterm delivery, and fetal growth retardation 176.
The following groups are among those most likely to be at risk of folate inadequacy.
People with alcohol use disorder
People with alcohol use disorder frequently have poor-quality diets that contain insufficient amounts of folate. Moreover, alcohol interferes with folate absorption and hepatic uptake, accelerates folate breakdown, and increases its renal excretion 154. An evaluation in Portugal, where the food supply is not fortified with folic acid, found low folate status in more than 60% of people with chronic alcoholism 177. Even moderate alcohol consumption of 240 ml (8 fluid ounces) red wine per day or 80 ml (2.7 fluid ounces) vodka per day for 2 weeks can significantly decrease serum folate concentrations in healthy men, although not to levels below the cutoff for folate adequacy of 3 ng/ml 178.
Women of childbearing age
All women capable of becoming pregnant should obtain adequate amounts of folate to reduce the risk of neural tube defects and other birth defects 179. However, some women of childbearing age get insufficient amounts of folate even if they take dietary supplements 180. Women of childbearing age should obtain 400 mcg/day folic acid from dietary supplements and/or fortified foods in addition to the folate provided by a varied diet 156.
Pregnant women
During pregnancy, demands for folate increase because of its role in nucleic acid synthesis 176. To meet this need, the FNB increased the folate RDA from 400 mcg DFE/day for nonpregnant women to 600 mcg DFE/day during pregnancy 156. This level of intake might be difficult for some women to achieve through diet alone. The American College of Obstetricians and Gynecologists recommends a prenatal vitamin supplement for most pregnant women to ensure that they obtain adequate amounts of folic acid and other nutrients 181.
People with malabsorptive disorders
Several medical conditions increase the risk of folate deficiency. People with malabsorptive disorders—including tropical sprue, celiac disease, and inflammatory bowel disease—might absorb less folate than people without these disorders 152; for example, about 20–60% of patients with inflammatory bowel disease have folate deficiency 182. Diminished gastric acid secretion associated with atrophic gastritis, gastric surgery, and other conditions can also reduce folate absorption 152.
People with the MTHFR polymorphism
People with a genetic polymorphism, 677C>T, in the methylenetetrahydrofolate reductase (MTHFR) gene have an impaired ability to convert folate to its active form, 5-methyltetrahydrofolate, because the methylenetetrahydrofolate reductase enzyme needed for this conversion is less active 183. About 25% of Hispanics, 10% of Caucasians and Asians, and 1% of African Americans are homozygous for the 677C>T MTHFR polymorphism 184. This polymorphism results in less biologically available 5-methyltetrahydrofolate and, thus, reduced methylation potential, leading to elevated homocysteine levels and an increased risk of neural tube defects 185. Although the research on the benefits of folate supplementation for people with this genetic polymorphism is inconclusive, these people might benefit from supplementation with 5-methyl-tetrahydrofolate (the “active” form of folic acid) 173.
How much vitamin B12 do I need?
The amount of vitamin B12 you need each day depends on your age. Average daily recommended amounts for different ages are listed below in micrograms (mcg). Table 14 lists the current Recommended Dietary Allowances (RDAs) for vitamin B12 157. For adults, the main criterion that the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine used to establish the RDAs was the amount needed to maintain a healthy hematological status and serum vitamin B12 levels. For infants aged 0 to 12 months, the Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine established an Adequate Intake (AI) that is equivalent to the mean intake of vitamin B12 in healthy, breastfed infants.
Table 14. Recommended Dietary Allowances (RDAs) for Vitamin B12
| Age | Male | Female | Pregnancy | Lactation |
| Birth to 6 months* | 0.4 mcg | 0.4 mcg | ||
| 7–12 months* | 0.5 mcg | 0.5 mcg | ||
| 1–3 years | 0.9 mcg | 0.9 mcg | ||
| 4–8 years | 1.2 mcg | 1.2 mcg | ||
| 9–13 years | 1.8 mcg | 1.8 mcg | ||
| 14–18 years | 2.4 mcg | 2.4 mcg | 2.6 mcg | 2.8 mcg |
| 19+ years | 2.4 mcg | 2.4 mcg | 2.6 mcg | 2.8 mcg |
Footnotes:
Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
*Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA).
[Source 157 ]What foods provide vitamin B12?
Vitamin B12 is found naturally in a wide variety of foods of animal origin (such as fish, meat, poultry, eggs, and dairy products) and manufacturers add it to some fortified foods (e.g., fortified breakfast cereals and fortified nutritional yeasts) 158. Plant foods have no vitamin B12 unless they are fortified 186. You can get recommended amounts of vitamin B12 by eating a variety of foods including the following:
- Fish, meat, poultry, eggs, milk, and other dairy products contain vitamin B12.
- Clams and beef liver are some of the best source of vitamin B12.
- Some breakfast cereals, nutritional yeasts, and other food products are fortified with vitamin B12.
The U.S. Department of Agriculture’s FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing vitamin B12 arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/VitaminB12-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/VitaminB12-Food.pdf).
The average vitamin B12 level in the breast milk of women with vitamin B12 intakes above the RDA is 0.44 mcg/L 187. The U.S. Food and Drug Administration (FDA) specifies that infant formulas sold in the United States must provide at least 0.15 mcg vitamin B12 per 100 kcal 188.
The estimated bioavailability of vitamin B12 from food varies by vitamin B12 dose because absorption decreases drastically when the capacity of intrinsic factor is exceeded (at 1–2 mcg of vitamin B12) 189. Bioavailability also varies by type of food source. For example, the bioavailability of vitamin B12 appears to be about three times higher in dairy products than in meat, fish, and poultry, and the bioavailability of vitamin B12 from dietary supplements is about 50% higher than that from food sources 190.
A variety of foods and their vitamin B12 levels per serving are listed in Table 15.
Table 15. Vitamin B12 content of selected foods
| Food | Micrograms per serving | Percent DV* |
| Beef liver, cooked, pan-fried, 3 ounces | 70.7 | 2944 |
| Clams (without shells), cooked, 3 ounces | 17 | 708 |
| Tuna, bluefin, cooked, dry heat, 3 ounces | 9.3 | 385 |
| Nutritional yeast, fortified, from several brands (check label), about ¼ cup | 8.3 to 24 | 346 to 1,000 |
| Salmon, Atlantic, cooked, 3 ounces | 2.6 | 108 |
| Beef, ground, 85% lean meat/15% fat, pan-browned, 3 ounces | 2.4 | 100 |
| Milk, 2% milkfat, 1 cup | 1.3 | 54 |
| Yogurt, plain, fat free, 6-ounce container | 1 | 43 |
| Breakfast cereals, fortified with 25% of the DV for vitamin B12, 1 serving | 0.6 | 25 |
| Cheese, cheddar, 1½ ounces | 0.5 | 19 |
| Egg, whole, cooked, 1 large | 0.5 | 19 |
| Turkey, breast meat, roasted, 3 ounces | 0.3 | 14 |
| Tempeh, 1/2 cup | 0.1 | 3 |
| Banana, 1 medium | 0 | 0 |
| Bread, whole-wheat, 1 slice | 0 | 0 |
| Strawberries, raw, halved, 1/2 cup | 0 | 0 |
| Beans, kidney, boiled, 1/2 cup | 0 | 0 |
| Spinach, boiled, drained, 1/2 cup | 0 | 0 |
Footnotes: *DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed DVs to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV for vitamin B12 is 2.4 mcg for adults and children aged 4 years and older. FDA does not require food labels to list vitamin B12 content unless vitamin B12 has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
[Source 191 ]Vitamin B12 supplements
Vitamin B12 is available in multivitamin/mineral supplements, in supplements containing other B-complex vitamins, and in supplements containing only vitamin B12. Multivitamin/mineral supplements typically contain vitamin B12 at doses ranging from 5 to 25 mcg. Vitamin B12 levels are higher, generally 50–500 mcg, in supplements containing vitamin B12 with other B-complex vitamins and even higher, typically 500–1,000 mcg, in supplements containing only vitamin B12.
The most common form of vitamin B12 in dietary supplements is cyanocobalamin 192. Other forms of vitamin B12 in supplements are adenosylcobalamin, methylcobalamin, and hydroxycobalamin.
No evidence indicates that absorption rates of vitamin B12 in supplements vary by form of the vitamin. These rates are about 50% at doses (less than 1–2 mcg) that do not exceed the cobalamin-binding capacity of intrinsic factor and are substantially lower at doses well above 1–2 mcg 192. For example, absorption is only about 2% at doses of 500 mcg and 1.3% at doses of 1,000 mcg 193.
In addition to oral dietary supplements, vitamin B12 is available in sublingual preparations as tablets or lozenges. Evidence suggests no difference in efficacy between oral and sublingual forms 194.
Vitamin B12 deficiency
Vitamin B-12 deficiency is characterized by megaloblastic anemia, fatigue, weakness, constipation, loss of appetite, and weight loss 195, 196, 197. Neurological changes, such as numbness and tingling in the hands and feet, can also occur 198, 199. Additional symptoms of Vitamin B-12 deficiency include difficulty maintaining balance, depression, confusion, dementia, poor memory, and soreness of the mouth or tongue 200. The neurological symptoms of Vitamin B-12 deficiency can occur without anemia, so early diagnosis and intervention is important to avoid irreversible damage 201. During infancy, signs of a Vitamin B-12 deficiency include failure to thrive, movement disorders, developmental delays, and megaloblastic anemia 202. Many of these symptoms are general and can result from a variety of medical conditions other than Vitamin B-12 deficiency.
Causes of vitamin B12 deficiency include difficulty absorbing vitamin B12 from food, lack of intrinsic factor (e.g., because of pernicious anemia), surgery in the gastrointestinal tract, prolonged use of certain medications (e.g., metformin or proton pump inhibitors, discussed in more detail below in the section on interactions with medications), and dietary deficiency 165. Because people who have difficulty absorbing vitamin B12 from food absorb free vitamin B12 normally, their vitamin B12 deficiency tends to be less severe than that of individuals with pernicious anemia, who cannot absorb either food-bound or free vitamin B12. Certain congenital conditions, such as hereditary intrinsic factor defects and congenital vitamin B12 malabsorption (Imerslund-Gräsbeck disease), can also cause severe vitamin B12 deficiency 158.
Because the body stores about 1 to 5 mg vitamin B12 (or about 1,000 to 2,000 times as much as the amount typically consumed in a day), the symptoms of vitamin B12 deficiency can take several years to appear 203.
Vitamin B12 deficiency with the classic hematologic and neurologic signs and symptoms is uncommon 163. However, low or marginal vitamin B12 status (200–300 pg/mL [148–221 pmol/L]) without these symptoms is much more common, at up to 40% in Western populations, especially in those with low intakes of vitamin B12-rich foods 204. The prevalence of vitamin B12 deficiency varies by cutoff level and biomarker used. For example, among adults aged 19 and older who participated in the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2004, the rate of low vitamin B12 levels in serum was 3% with a cutoff of less than 200 pg/mL (148 pmol/L) and 26% with a cutoff of less than 350 pg/mL (258 pmol/L) 205. Approximately 21% of adults older than 60 had abnormal levels of at least one vitamin B12 biomarker 205.
Typically, vitamin B12 deficiency is treated with vitamin B12 injections, because this method bypasses any barriers to absorption. However, high doses of oral vitamin B12 might also be effective. A 2018 Cochrane review included three randomized controlled trials that compared very high doses (1,000–2,000 mcg) of oral with intramuscular vitamin B12 for vitamin B12 deficiency in a total of 153 participants 206. The evidence from these studies, although of low quality, showed that the ability of high oral doses of vitamin B12 supplements to normalize serum vitamin B12 was similar to that of intramuscular vitamin B12. Overall, an individual patient’s ability to absorb Vitamin B-12 is the most important factor in determining whether Vitamin B-12 should be administered orally or via injection 207. In most countries, the practice of using intramuscular Vitamin B-12 to treat Vitamin B-12 deficiency has remained unchanged 208.
Omega 3 fatty acids (fish oil)
Omega-3 fatty acids are found in foods, such as fish and flaxseed, and in dietary supplements, such as fish oil 209. The three main omega-3 fatty acids are alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). Alpha-linolenic acid (ALA) is found mainly in plant oils such as flaxseed, soybean, and canola oils 210. DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid) are present in fish, fish oils, krill oils and other seafood, but they are originally synthesized by microalgae, not by the fish. When fish consume phytoplankton that consumed microalgae, they accumulate the omega-3s in their tissues 210.
Alpha-linolenic acid (ALA) is an essential fatty acid, meaning that your body can’t make it, so you must get it from the foods and beverages you consume. Your body can convert some alpha-linolenic acid (ALA) into EPA (eicosapentaenoic acid) and then to DHA (docosahexaenoic acid), but only in very small amounts 209. Therefore, getting DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid) from foods (and dietary supplements if you take them) is the only practical way to increase levels of these omega-3 fatty acids in your body.
Omega-3s are important components of the membranes that surround each cell in your body. DHA (docosahexaenoic acid) levels are especially high in retina (eye), brain, and sperm cells. Omega-3s also provide calories to give your body energy and have many functions in your heart, blood vessels, lungs, immune system, and endocrine system (the network of hormone-producing glands).
According to data from the 2011–2012 National Health and Nutrition Examination Survey (NHANES), most children and adults in the United States consume recommended amounts of omega-3s as ALA 211. Among children and teens aged 2–19 the average daily ALA intake from foods is 1.32 g for females and 1.55 g for males. In adults aged 20 and over, the average daily ALA intake from foods is 1.59 g in females and 2.06 g in males.
Consumption of DHA and EPA from foods contributes a very small amount to total daily omega-3 intakes (about 40 mg in children and teens and about 90 mg in adults) 211. Use of dietary supplements containing omega-3s also contributes to total omega-3 intakes. Fish oil is one of the most commonly used nonvitamin/nonmineral dietary supplements by U.S. adults and children 212, 213. Data from the 2012 National Health Interview Survey indicate that 7.8% of U.S. adults and 1.1% of U.S. children use supplements containing fish oil, omega-3s, and/or DHA or EPA 212, 213. According to an analysis of 2003–2008 NHANES data, use of these supplements adds about 100 mg to mean daily ALA intakes, 10 mg to mean DHA intakes, and 20 mg to mean EPA intakes in adults 214.
A deficiency of essential fatty acids—either omega-3s or omega-6s—can cause rough, scaly skin and dermatitis 215. Plasma and tissue concentrations of DHA decrease when an omega-3 fatty acid deficiency is present. However, there are no known cut-off concentrations of DHA or EPA below which functional endpoints, such as those for visual or neural function or for immune response, are impaired.
How much omega-3 do I need?
Experts have not established recommended amounts for omega-3 fatty acids, except for alpha-linolenic acid (ALA). Average daily recommended amounts for alpha-linolenic acid (ALA) are listed below in grams (g). The amount you need depends on your age and sex.
Table 16 lists the current Adequate Intakes (AIs) for omega-3s in grams per day. Human milk contains omega-3s as ALA, EPA and DHA, so the Food and Nutrition Board of the Institute of Medicine established an AI for infants from birth to 12 months that is equivalent to the mean intake of omega-3s in healthy, breastfed infants 215.
For infants, the Adequate Intakes (AIs) apply to total omega-3s. For ages 1 and older, the AIs apply only to ALA because ALA is the only omega-3 that is essential. The Food and Nutrition Board of the Institute of Medicine did not establish specific intake recommendations for EPA, DHA or other omega-3s 215.
- Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
- Adequate Intake (AI): Intake at this level is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA).
Table 16. Recommended daily intake for Omega-3s
| Age | Male | Female | Pregnancy | Lactation |
| Birth to 6 months* | 0.5 g | 0.5 g | ||
| 7–12 months* | 0.5 g | 0.5 g | ||
| 1–3 years** | 0.7 g | 0.7 g | ||
| 4–8 years** | 0.9 g | 0.9 g | ||
| 9–13 years** | 1.2 g | 1.0 g | ||
| 14–18 years** | 1.6 g | 1.1 g | 1.4 g | 1.3 g |
| 19-50 years** | 1.6 g | 1.1 g | 1.4 g | 1.3 g |
| 51+ years** | 1.6 g | 1.1 g |
Footnotes:
*As total omega-3s
**As alpha-linolenic acid (ALA)
[Source 215 ]Food sources of omega-3
Omega-3s are found naturally in some foods and are added to some fortified foods. You can get adequate amounts of omega-3s by eating a variety of foods, including the following:
- Fish and other seafood (especially cold-water fatty fish, such as salmon, mackerel, tuna, herring, and sardines)
- Nuts and seeds (such as flaxseed, chia seeds, and walnuts)
- Plant oils (such as flaxseed oil, soybean oil, and canola oil)
- Fortified foods (such as certain brands of eggs, yogurt, juices, milk, soy beverages, and infant formulas)
Plant oils that contain alpha-linolenic acid (ALA) include flaxseed (linseed), soybean, and canola oils 216. Chia seeds and walnuts also contain alpha-linolenic acid (ALA).
The omega-3 content of fish varies widely. Cold-water fatty fish, such as salmon, mackerel, tuna, herring, and sardines, contain high amounts of omega-3s, whereas fish with a lower fat content—such as bass, tilapia and cod—as well as shellfish contain lower levels 210. The omega-3 content of fish also depends on the composition of the food that the fish consumes 217. Farmed fish usually have higher levels of DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid) than wild-caught fish, but it depends on the food they are fed 218. An analysis of the fatty acid composition of farm-raised Atlantic salmon from Scotland showed that the EPA and DHA content significantly decreased between 2006 and 2015 due to the replacement of traditional marine ingredients in fish feed with other ingredients 219.
Beef is very low in omega-3s, but beef from grass-fed cows contains somewhat higher levels of omega-3s, mainly as ALA, than that from grain-fed cows 220.
Some foods, such as certain brands of eggs, yogurt, juices, milk, and soy beverages, are fortified with DHA and other omega-3s. Since 2002, manufacturers have added DHA and arachidonic acid (the two most prevalent polyunsaturated fatty acids (PUFAs) in the brain) to most infant formulas available in the United States 221.
Several food sources of ALA, DHA, and/or EPA are listed in Table 17. The U.S. Food and Drug Administration (FDA) has established a Daily Value (DV) of 65 g for total fat but not for omega-3s. Thus, Table 17 presents the amounts of omega-3 fatty acids in grams per serving only and not the percent of the Daily Value (DV).
Table 17. Omega-3 content of selected foods
| Food | Grams per serving | ||
| ALA (alpha-linolenic acid) | DHA (docosahexaenoic acid) | EPA (eicosapentaenoic acid) | |
| Flaxseed oil, 1 tbsp | 7.26 | ||
| Chia seeds, 1 ounce | 5.06 | ||
| English walnuts, 1 ounce | 2.57 | ||
| Flaxseed, whole, 1 tbsp | 2.35 | ||
| Salmon, Atlantic, farmed cooked, 3 ounces | 1.24 | 0.59 | |
| Salmon, Atlantic, wild, cooked, 3 ounces | 1.22 | 0.35 | |
| Herring, Atlantic, cooked, 3 ounces* | 0.94 | 0.77 | |
| Canola oil, 1 tbsp | 1.28 | ||
| Sardines, canned in tomato sauce, drained, 3 ounces* | 0.74 | 0.45 | |
| Mackerel, Atlantic, cooked, 3 ounces* | 0.59 | 0.43 | |
| Salmon, pink, canned, drained, 3 ounces* | 0.04 | 0.63 | 0.28 |
| Soybean oil, 1 tbsp | 0.92 | ||
| Trout, rainbow, wild, cooked, 3 ounces | 0.44 | 0.4 | |
| Black walnuts, 1 ounce | 0.76 | ||
| Mayonnaise, 1 tbsp | 0.74 | ||
| Oysters, eastern, wild, cooked, 3 ounces | 0.14 | 0.23 | 0.3 |
| Sea bass, cooked, 3 ounces* | 0.47 | 0.18 | |
| Edamame, frozen, prepared, ½ cup | 0.28 | ||
| Shrimp, cooked, 3 ounces* | 0.12 | 0.12 | |
| Refried beans, canned, vegetarian, ½ cup | 0.21 | ||
| Lobster, cooked, 3 ounces* | 0.04 | 0.07 | 0.1 |
| Tuna, light, canned in water, drained, 3 ounces* | 0.17 | 0.02 | |
| Tilapia, cooked, 3 ounces* | 0.04 | 0.11 | |
| Scallops, cooked, 3 ounces* | 0.09 | 0.06 | |
| Cod, Pacific, cooked, 3 ounces* | 0.1 | 0.04 | |
| Tuna, yellowfin, cooked 3 ounces* | 0.09 | 0.01 | |
| Kidney beans, canned ½ cup | 0.1 | ||
| Baked beans, canned, vegetarian, ½ cup | 0.07 | ||
| Ground beef, 85% lean, cooked, 3 ounces** | 0.04 | ||
| Bread, whole wheat, 1 slice | 0.04 | ||
| Egg, cooked, 1 egg | 0.03 | ||
| Chicken, breast, roasted, 3 ounces | 0.02 | 0.01 | |
| Milk, low-fat (1%), 1 cup | 0.01 | ||
Footnotes:
*Except as noted, the USDA database does not specify whether fish are farmed or wild caught.
**The USDA database does not specify whether beef is grass fed or grain fed.
The U.S. Department of Agriculture’s FoodData Central (https://fdc.nal.usda.gov) lists the nutrient content of many foods and provides a comprehensive list of foods containing alpha-linolenic acid (ALA) arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/ALA-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/ALA-Food.pdf), foods containing DHA (docosahexaenoic acid) arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/DHA-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/DHA-Food.pdf), and foods containing EPA (eicosapentaenoic acid) arranged by nutrient content (https://ods.od.nih.gov/pubs/usdandb/EPA-Content.pdf) and by food name (https://ods.od.nih.gov/pubs/usdandb/EPA-Food.pdf).
[Source 86 ]Omega 3 supplements
Omega-3s are present in several dietary supplement formulations, including fish oil, krill oil, cod liver oil, and vegetarian products that contain algal oil. A typical fish oil supplement provides about 1,000 mg fish oil, containing 180 mg EPA and 120 mg DHA, but doses vary widely 222. Cod liver oil supplements provide vitamin A and vitamin D in addition to omega-3s. Although seafood contains varying levels of methyl mercury (a toxic heavy metal) 223, omega-3 supplements have not been found to contain this contaminant because it is removed during processing and purification 224.
Dietary supplements can contain several different forms of omega-3s, including natural triglycerides, free fatty acids, ethyl esters, re-esterified triglycerides, and phospholipids 225. Natural triglycerides are the form that occur naturally in fish oil, whereas ethyl esters are synthesized from natural triglycerides by replacement of the glycerol molecule of the triglyceride with ethanol. Re-esterified triglycerides are formed by the conversion of ethyl esters back to triglycerides. Omega-3s as re-esterified triglycerides, natural triglycerides, and free fatty acids have somewhat higher bioavailability than ethyl esters, but consumption of all forms significantly increases plasma EPA and DHA levels 226.
Krill oil contains omega-3s primarily as phospholipids, and limited research suggests that these have somewhat higher bioavailability than the omega-3s in fish oil 227.
Plant-based sources of omega-3s from algal oil usually provide around 100–300 mg DHA; some contain EPA as well. These supplements typically contain omega-3s in the triglyceride form 224. According to a small study, the bioavailability of DHA from algal oil is equivalent to that from cooked salmon 228.
Formulations of omega-3 dietary supplements vary widely, so it is important to check product labels to determine the types and amounts of omega-3s in these products. The Dietary Supplement Label Database (http://www.dsld.nlm.nih.gov/dsld) from the National Institutes of Health contains label information from many dietary supplements on the market that contain omega-3s.
Age-Related Macular Degeneration (AMD) is a major cause of vision loss among older adults. In most cases, severe vision loss is associated with advanced macular degeneration, which consists of either central geographic atrophy (dry AMD, the most common form) or neovascular AMD (wet AMD) 229. Based on DHA’s presence as a structural lipid in retinal cellular membranes and the beneficial effects of EPA-derived eicosanoids on retinal inflammation, neovascularization, and cell survival, researchers have suggested that these omega-3s have cytoprotective effects in the retina that may help prevent the development or progression of AMD 230.
Results from observational studies suggest that people who consume higher amounts of fatty fish and/or dietary omega-3s have a lower risk of developing AMD. In the cross-sectional EUREYE study of 2,275 participants aged 65 years or older, those who ate fatty fish at least once per week had a 53% lower risk of neovascular AMD than those who consumed fatty fish less often 231. Results were similar in a study in 681 elderly male twins 232 and an analysis of 38,022 healthy female health professionals 229. In the latter study, women in the highest tertiles of dietary DHA plus EPA intake (median of 330 mg/day) had a 38% lower risk of developing AMD during an average of 10 years of follow-up than those in those in the lowest tertile (median intake of 80 mg/day). Higher serum and erythrocyte membrane levels of EPA (but not DHA) have also been associated with a lower risk of neovascular AMD 233.
In the AREDS study, a dietary supplement formulation containing 15 mg beta-carotene, 400 IU vitamin E, 500 mg vitamin C, 80 mg zinc, and 2 mg copper reduced the risk of advanced AMD in people with intermediate AMD or advanced AMD in one eye 59. Data from a nested cohort study within the AREDS population indicated that participants who reported the highest omega-3 intakes were about 30% less likely to develop central geographic atrophy and neovascular AMD than other participants 234.
These findings, combined with other epidemiological evidence, formed the basis for the AREDS2 clinical trial that examined whether adding 350 mg DHA and 650 mg EPA to the AREDS formulation further reduced the risk of progression to advanced AMD 235. The results showed that EPA and DHA did not provide any additional benefits after a median follow-up of 5 years. These findings are in line with those from a Cochrane review 236 that included the results from AREDS2 and the Nutritional AMD Treatment 2 study 237, a 3-year randomized clinical trial of omega-3 supplements (840 mg/day DHA and 270 mg/day EPA) in patients with early age-related maculopathy and neovascular AMD. The Cochrane review authors concluded that omega-3 supplementation for up to 5 years in people with AMD does not reduce the risk of progression to advanced AMD or of moderate to severe vision loss.
Omega-3 and dry eye disease
About 14% of adults in the United States have dry eye disease, a chronic condition in which decreased tear volume and quality leads to ocular surface inflammation and damage, causing discomfort and visual impairment 238. Older women, in particular, have a higher risk of dry eye disease than other groups, possibly because of hormonal changes that affect the tear-producing glands 239. Researchers hypothesize that omega 3s—particularly DHA (docosahexaenoic acid) and EPA (eicosapentaenoic acid)—might reduce the risk of dry eye disease and relieve its symptoms because of their anti-inflammatory activity, and many patients take them as adjunctive treatments to artificial tears and other medications.
Some, but not all, observational studies show inverse associations between self-reported dietary consumption of omega-3s and risk of dry eye disease. For example, in a cross-sectional study of 32,470 women aged 45–84 participating in the Women’s Health Study, those in the highest quintile of total dietary omega-3 intake (mean of 1,990 mg/day) had a 17% lower risk of dry eye disease than those in the lowest quintile (mean intake of 920 mg/day) 240. The study found a similar association for DHA—women in the highest versus the lowest quintiles of DHA intake had a 12% lower risk of dry eye disease; however, the results showed no significant associations for EPA. But in another cross-sectional study of 322 postmenopausal women, total dietary omega-3 intakes were not correlated with the prevalence of dry eye disease 239.
Results from clinical trials using omega-3 supplementation, primarily EPA and DHA, have had mixed results in reducing the symptoms and signs of dry eye disease. Furthermore, there is no consensus on the optimal dose, composition, or length of omega-3 treatment for this condition 241.
The studies that have found beneficial effects from omega-3 supplementation for symptoms and signs of dry eye disease include one showing that daily supplementation with 1,000 mg omega-3s (650 mg EPA plus 350 mg DHA) for 3 months in 518 men and women (mean age about 40 years) living in northern India reduced symptoms and some signs of dry eye disease compared with placebo 242. In another clinical trial of 105 men and women, daily treatment with supplements containing 2,240 mg omega-3s (1,680 mg EPA and 560 mg DHA as re-esterified triglycerides) for 12 weeks also reduced symptoms of dry eye disease compared with placebo 243. In addition, the supplements increased tear break-up time and decreased tear osmolarity (which would be likely to reduce ocular surface damage).
However, another large, randomized, double-blind clinical trial conducted in the United States found that EPA and DHA from fish oil supplements are no better than placebo at relieving symptoms or signs of dry eye disease 238. This 12-month trial included 535 participants (about 81% female) aged 18 years or older (mean age about 58 years) with at least a 6-month history of moderate to severe dry eye disease. Among them, 349 participants received daily supplements of 3,000 mg omega-3s (2,000 mg EPA plus 1,000 mg DHA), and 186 received a placebo containing 5,000 mg olive oil. Participants could continue taking medications for dry eyes, including artificial tears and prescription anti-inflammatory eye drops, as well as omega-3 supplements as long as the total dose of EPA plus DHA was less than 1,200 mg per day. At the end of the study, symptoms were less severe than at baseline in both groups, but the results showed no significant differences between groups. Groups also showed no significant differences compared with baseline in signs of dry eye disease, including conjunctive and cornea integrity as well as tear volume and quality.
Overall, the evidence to date shows no consistent relationship between omega-3s and dry eye disease. More research is warranted to fully understand whether increased intakes of dietary or supplemental omega-3s help reduce the risk of dry eye disease and whether they are beneficial as an adjunct treatment.
Best eye vitamins for macular degeneration
Macular degeneration also known as age-related macular degeneration (AMD) affects the macula, the part of the eye that allows you to see fine detail. Age-related macular degeneration causes no pain. An eye affected by macular degeneration will not be able to perceive detail or colors as well as a healthy eye. Progressive deterioration of the macula can lead to loss of central sight. However, it does not lead to complete blindness because the peripheral regions of the retina remain unaffected. Macular degeneration (age-related macular degeneration) is a major cause of significant severe, irreversible vision loss in Americans 60 and older 244. In 2004, examining a slightly expanded age group, it was estimated that approximately 1.75 million people aged 40 years or older in the United States were estimated to have either neovascular age-related macular degeneration or geographic atrophy in at least one eye, and 7.3 million were considered to have high-risk features, such as large drusen in one or both eyes 245. Overall, age-related macular degeneration is responsible for an estimated 46% of cases of severe visual loss (visual acuity 20/200 or worse) in persons over 40 years of age in the United States 246. While most consider the onset of age-related macular degeneration as occuring in individuals over the age of 50, there are variations in the epidemiologic literature. Cases of advanced age-related macular degeneration that occur between ages 40 and 50 is very low, yet detection of the earlier stages, which are precursors of more advanced age-related macular degeneration, may well occur during this decade. Therefore, the reader must keep in mind that age-related macular degeneration is a disease spectrum with early and later stages. Although an estimated 80% of age-related macular degeneration patients have non-neovascular or atrophic age-related macular degeneration 247, the neovascular form is responsible for nearly 90% of the severe visual acuity loss (20/200 or worse) from age-related macular degeneration 248.
There are two types of age related macular degeneration: wet and dry.
- Wet age-related macular degeneration happens when abnormal blood vessels grow under the macula. These new blood vessels often leak blood and fluid. This is the most serious kind. Wet age-related macular degeneration damages the macula quickly. Blurred vision is a common early symptom.
- Dry age-related macular degeneration happens when the light-sensitive cells in the macula slowly break down. It is the most common kind. Your gradually lose your central vision. A common early symptom is that straight lines appear crooked.
Risk Factors for macular degeneration
Macular degeneration tends to have a higher incidence of occurrence when the following risk factors are present:
- Age: Macular degeneration can occur at any age; however, the chance of its occurrence increases nearly five-fold after the age of sixty-five.
- Heredity: Macular degeneration tends to “run in families.” This means that there is a genetic factor that predisposes a person toward developing the condition. Anytime a “bloodline” relative has macular degeneration, you run an increased chance of developing the condition.
- Gender: Women have a slightly higher incidence of developing macular degeneration than men.
- Ethnic Background: Fair-skinned people of northern European ancestry (Scandinavian, English, or German descent) have a higher chance of developing macular degeneration.
- Eye Color: Blue-eyed individuals are more prone to develop the condition than brown-eyed persons.
- Cardiovascular History: A history of heart disease or stroke is associated with a higher incidence of macular degeneration.
- Smoking: Macular degeneration tends to occur more frequently in persons who smoke. Even after treatment, smokers are reported to have a greater chance of having macular problems recur.
Age-related macular degeneration’s cause is usually unknown, but the cumulative effect of oxidative stress is postulated to play a role. If so, supplements containing carotenoids with antioxidant functions, such as beta-carotene, lutein, and zeaxanthin, might be useful for preventing or treating this condition. Lutein and zeaxanthin, in particular, accumulate in the retina, the tissue in the eye that is damaged by age-related macular degeneration.
There’s currently no treatment for early macular degeneration, so your eye doctor (ophthalmologist) will probably just keep track of how your eyes are doing with regular eye exams. Eating healthy, getting regular exercise, and quitting smoking can also help.
- If you are diagnosed with intermediate or late macular degeneration, ask your eye doctor (ophthalmologist) about treatment options and how the condition may affect your vision in the future.
- Right now, there is no way to treat the dry form of macular degeneration. If you have intermediate or late macular degeneration, people with lots of drusen or serious vision loss might benefit from taking a certain combination of dietary (vitamins and minerals) supplements may be able to stop it from getting worse (see Age-Related Eye Diseases Studies (AREDS) below). Talk with your ophthalmologist about whether nutritional supplements are recommended for you. A large study (AREDS and the later AREDS 2 study) found those people may slow their dry macular degeneration by taking these vitamins and minerals daily:
- Vitamin C (500 mg)
- Vitamin E 180 mg (400 IU)
- Lutein (10 mg)
- Zeaxanthin (2 mg)
- Zinc (80 mg)
- Copper (2 mg)
- Your ophthalmologist can tell you if vitamins and minerals are recommended for your dry macular degeneration, as not all forms will benefit from the AREDS supplements. Beta carotene should not be used by smokers as it raised the risk of lung cancer.
- It is important to remember that nutritional supplements are not a cure for macular degeneration, but they may help to slow the disease in some people with early- to mid-stage macular degeneration.
For people with a type of late macular degeneration called “wet” or neovascular macular degeneration, there are other treatments that may be able to stop further vision loss:
- Medicines called anti-VEGF drugs (Vascular Endothelial Growth Factor Inhibitors) that the doctor injects in your eye. Anti-VEGF treatment helps reduce the number of abnormal blood vessels in your retina. It also slows any leaking from blood vessels. This medicine is delivered to your eye through a very slender needle. Injections are administered once monthly for four months or until vision stabilizes, then every three months thereafter. The safety and effectiveness of this intervention have not been evaluated past 24 months.
- Laser treatment, called photodynamic therapy (PDT). Laser surgery may also be used to treat some types of wet AMD. Your eye surgeon shines a laser light beam on the abnormal blood vessels. This reduces the number of vessels and slows their leaking.
The Age-Related Eye Disease Study (AREDS) 59, a large randomized clinical trial, found that participants at high risk of developing advanced age-related macular degeneration (i.e., those with intermediate AMD or those with advanced AMD in one eye) reduced their risk of developing advanced age-related macular degeneration by 25% by taking a daily supplement containing beta-carotene (15 mg), vitamin E (180 mg [400 IU] dl-alpha-tocopheryl acetate), vitamin C (500 mg), zinc (80 mg), and copper (2 mg) for 5 years compared to participants taking a placebo.
A follow-up AREDS2 study 60 confirmed the value of this supplement in reducing the progression of age-related macular degeneration over a median follow-up period of 5 years but found that adding lutein (10 mg) and zeaxanthin (2 mg) or omega-3 fatty acids to the formulation did not confer any additional benefits. Importantly, the study revealed that beta-carotene was not a required ingredient; the original AREDS formulation without beta-carotene provided the same protective effect against developing advanced age-related macular degeneration. In a more detailed analysis of results, supplementation with lutein and zeaxanthin reduced the risk of advanced age-related macular degeneration by 26% in participants with the lowest dietary intakes of these two carotenoids who took a supplement containing them compared to those who did not take a supplement with these carotenoids 60. The risk of advanced age-related macular degeneration was also 18% lower in participants who took the modified AREDS supplement containing lutein and zeaxanthin but not beta-carotene than in participants who took the formulation with beta-carotene but not lutein or zeaxanthin.
Individuals who have or are developing age-related macular degeneration should talk to their eye doctor (ophthalmologist) about taking one of the supplement formulations used in AREDS.
The formulation suggested by AREDS 1 is a daily dose of 59:
- Vitamin C (ascorbic acid) 500 mg
- Vitamin E 400 international units (IU)
- Beta carotene 15 mg
- Zinc oxide 80 mg
- Cupric oxide 2 mg (added to reduce the risk of copper-deficiency anemia).
This formulation was modified by AREDS 2 60. AREDS 2 (Age-Related Eye Disease Study 2) was a very large research study. It looked at taking vitamins and minerals daily for macular degeneration. This study found that certain nutritional supplements could help some people who have a lot of drusen. These supplements may also help people who have lost a lot of vision in at least one eye from macular degeneration. Taking the following nutritional supplements every day may help these people lower their risk of getting late-stage or wet macular degeneration:
- Vitamin C (ascorbic acid) 500 mg
- Vitamin E 400 international units (IU)
- Lutein 10 mg
- Zeaxanthin 2 mg
- Zinc (as zinc oxide) 80 mg
- Copper (as cupric oxide) 2 mg (added to reduce the risk of copper-deficiency anemia)
- Fish oil containing omega-3 fatty acids (docosahexaenoic acid [DHA] 350 mg + eicosapentaenoic acid [EPA] 650 mg)
- Beta carotene was removed as it increased the risk of lung cancer, especially in smokers. Macular pigments lutein and zeaxanthin provided an additional reduction of risk of progression of age-related macular degeneration (AMD).
So far, only people who have a considerably higher risk of developing late-stage macular degeneration have been shown to benefit from the AREDS supplements. People are at higher risk if they already have many deposits in their eyes, called drusen. Taking AREDS supplements regularly lowered the risk of late-stage macular degeneration associated with loss of vision in some of them. In order to be effective, the supplements had to be taken daily for several years.
It’s important to talk with your eye doctor (ophthalmologist) before taking these products because they aren’t suitable for everyone.
Figure 2. Human eye anatomy
Age is a major risk factor for age-related macular degeneration. The disease is most likely to occur after age 60, but it can occur earlier.
Other risk factors for age-related macular degeneration include:
- Smoking. Research shows that smoking doubles the risk of age-related macular degeneration.
- Race. age-related macular degeneration is more common among Caucasians than among African-Americans or Hispanics/Latinos.
- Family history and Genetics. People with a family history of age-related macular degeneration are at higher risk. At last count, researchers had identified nearly 20 genes that can affect the risk of developing age-related macular degeneration. Many more genetic risk factors are suspected. You may see offers for genetic testing for age-related macular degeneration. Because age-related macular degeneration is influenced by so many genes plus environmental factors such as smoking and nutrition, there are currently no genetic tests that can diagnose age-related macular degeneration, or predict with certainty who will develop it. The American Academy of Ophthalmology 249 currently recommends against routine genetic testing for age-related macular degeneration, and insurance generally does not cover such testing.
Researchers have found links between age-related macular degeneration and some lifestyle choices, such as smoking. You might be able to reduce your risk of age-related macular degeneration or slow its progression by making these healthy choices:
- Avoid smoking
- Exercise regularly
- Maintain normal blood pressure and cholesterol levels
- Eat a healthy diet rich in green, leafy vegetables and fish
Why are the AREDS and AREDS2 formulas different?
In the AREDS trial, taking the AREDS formula reduced the risk of advanced macular degeneration by about 25% over a five-year period. In the AREDS2 trial, adding omega-3 or lutein plus zeaxanthin to the AREDS formulation (containing beta-carotene) had no additional overall effect on the risk of advanced macular degeneration. However, trial participants who took AREDS containing lutein and zeaxanthin and no beta-carotene had a reduction in risk of advanced macular degeneration, compared with those who took AREDS with beta-carotene. Also, for participants with very low levels of lutein and zeaxanthin in their diet, adding these supplements to the AREDS formulation helped lower their risk of advanced macular degeneration. Finally, former smokers who took AREDS with beta-carotene had a higher incidence of lung cancer. The investigators found no significant changes in the effectiveness of the formulation when they lowered zinc.
How do lutein and zeaxanthin compare to beta-carotene?
During the AREDS trial, two large trials funded by the National Cancer Institute found that beta-carotene may increase lung cancer risk among people who smoke. Lutein and zeaxanthin are in the same family of nutrients as beta-carotene and are believed to have important functions in the retina. Lutein and zeaxanthin have not been associated with increased cancer risk.
Some studies prior to AREDS2 found that dietary intake of lutein, zeaxanthin and omega-3 fatty acids is associated with a lower risk of developing advanced macular degeneration. Analysis from the AREDS2 trial suggests that lutein plus zeaxanthin offers similar or better protective benefits against advanced macular degeneration compared with beta-carotene. In the trial, participants who took an AREDS formulation containing lutein + zeaxanthin lacking beta-carotene had an 18% lower risk of progressing to advanced macular degeneration compared with those who took AREDS containing beta-carotene (no lutein or zeaxanthin). Among participants who had the lowest dietary intake of lutein and zeaxanthin, those who took AREDS with lutein plus zeaxanthin had a 26% lower risk of progressing to advanced macular degeneration compared to participants taking the original AREDS formula.
Are the AREDS vitamins right for me?
In clinical trials, the AREDS and AREDS2 formulas benefited people with intermediate or late macular degeneration. There was no benefit for people with early macular degeneration or for people who do not have macular degeneration.
Your doctor or eye care provider is in the best position to advise you on how treat your macular degeneration. You may wish to discuss AREDS/AREDS2 supplements with your health care providers to decide which, if any, supplements are right for you.
Will taking the AREDS or AREDS2 supplements prevent macular degeneration?
Nutritional supplements cannot prevent macular degeneration. However, the AREDS/AREDS2 supplements may delay progression of intermediate to advanced macular degeneration and may help you keep your vision longer. The participants AREDS trial have now been followed for more than 10 years, and the benefits of the AREDS formulation have persisted over this time.
How effective is the AREDS formula?
The “AREDS study” (Age-Related Eye Disease Study), involving about 3,600 participants, suggest that the AREDS supplements have a positive effect. The study lasted 6 years. During this time, the progression of vision loss was slowed down a little in some people who used the AREDS formula, but most of them didn’t benefit from it. Expressed in numbers 250:
- Without treatment: Vision worsened considerably within the six years in about 43 out of 100 participants.
- With treatment: This happened in about 37 out of 100 participants who took the AREDS formula.
In other words, 6 out of 100 people benefited from taking these supplements every day for six years 250.
Can I take a daily multivitamin if I am taking one of the AREDS/AREDS2 formulas?
Yes. The AREDS and AREDS2 formulas do not substitute for multivitamins. In AREDS, two-thirds of the study participants took multivitamins along with the AREDS formulation. In AREDS2, almost nine of ten participants took multivitamins.
Can a daily multivitamin alone provide the same vision benefits as the AREDS or AREDS2 formulas?
No. The vitamins and minerals tested in AREDS and AREDS2 trials were provided in much higher doses than what is found in multivitamins. Also, it is important to remember that most of the trial participants took multivitamins. Taking an AREDS formulation clearly provided a benefit over and above multivitamins.
Can diet alone provide the same levels of antioxidants and zinc as the AREDS or AREDS2 formulas?
No. The high levels of vitamins and minerals are difficult to achieve from diet alone. However, previous studies have suggested that people who have diets rich in green, leafy vegetables—a good source of lutein and zeaxanthin—have a lower risk of developing AMD. In the AREDS2 trial, the participants who benefited most from taking lutein plus zeaxanthin were those who did not get much of these nutrients in their diet. Within this group, those who received lutein plus zeaxanthin supplements had a 26% reduced risk of developing advanced AMD compared with those who did not receive the supplements.
What is omega-3?
Omega-3 fatty acids are made by marine algae and enriched in fish oils. They are believed to be responsible for the health benefits associated with regularly eating fish, including lower rates of cardiovascular disease. The AREDS2 study focused on the omega-3 fatty acids docosahexanoic acid (DHA) and its precursor eicosapentanoic acid (EPA). DHA is needed for the integrity of retinal cells and has been shown to promote retinal development and repair in prior studies.
What is the function of copper in the AREDS and AREDS2 supplements?
In AREDS/AREDS2 trials, copper (as cupric oxide) was added to supplement formulas containing zinc. The goal was to reduce the risk of copper deficiency anemia, a condition associated with high levels of zinc intake. The studies showed clear benefits for patients who took an AREDS formula with zinc, with no evidence of anemia. There was no evidence that 2 mg copper was harmful, nor reason to suspect that it would be. So, in the AREDS investigators’ hands, the use of copper was safe and may have helped balance the effects of zinc.
What is the basis for the concentration of zinc in the AREDS supplements?
In the AREDS trial, the 80 mg zinc dose (alone or in combination with antioxidant vitamins) was found to be effective compared to a placebo. Although zinc was found to be an essential component of the AREDS formulation, some nutritional experts recommended a lower dose. In the AREDS2 trial, there was no placebo control. Instead, participants were given the option to take the original formula or to be randomly assigned to receive a modified version, such as a formula containing 25 mg zinc. The investigators did not find a difference in the effects of 80 mg vs. 25 mg zinc. Because AREDS2 did not include a placebo control, results from AREDS, placebo-controlled trial, are still considered the gold standard.
Zinc is found in vegetables, grains, and meat. Vegetables and grains contain other molecules that can prevent zinc absorption and thus reduce its bioavailability. Supplements contain purified zinc, without these competing molecules. Although the chemical form of zinc affects its rate of absorption in the stomach, it is not clear how this affects bioavailability (i.e., the amount of zinc that reaches the retina).
Best vitamins for dry eyes
Your eyes need tears to stay healthy and comfortable. If your eyes do not produce enough tears, it is called dry eye 251. Dry eye is also when your eyes do not make the right type of tears or tear film. A healthy tear film on the eye is necessary for good vision. When you blink, a film of tears spreads over the eye. This keeps the eye’s surface smooth and clear.
The tear film is made of three layers. Each layer of the tear film serves a purpose.
- An oily layer: The oily layer is the outside of the tear film. It makes the tear surface smooth and keeps tears from drying up too quickly. This layer is made in the eye’s meibomian glands.
- A watery layer: The watery layer is the middle of the tear film. It makes up most of what we see as tears. This layer cleans the eye, washing away particles that do not belong in the eye. This layer comes from the lacrimal glands in the eyelids.
- A mucus layer: The mucus layer is the inner layer of the tear film. This helps spread the watery layer over the eye’s surface, keeping it moist. Without mucus, tears would not stick to the eye. Mucus is made in the conjunctiva. This is the clear tissue covering the white of your eye and inside your eyelids.
Normally, your eyes constantly make tears to stay moist. If your eyes are irritated, or you cry, your eyes make a lot of tears. But, sometimes the eyes don’t make enough tears or something affects one or more layers of the tear film. In those cases, you end up with dry eyes.
Anyone can get dry eye, but you might be more likely to have dry eye if you:
- Are age 50 or older
- Are female
- Wear contact lenses
- Don’t get enough vitamin A (found in foods like carrots, broccoli, and liver) or omega-3 fatty acids (found in fish, walnuts, and vegetable oils)
- Have certain autoimmune conditions, like lupus or Sjögren syndrome
Dry eye can cause:
- A scratchy or gritty feeling, like there’s something in your eye
- Stinging or burning feelings in your eye
- Red eyes. This is especially true when you are in the wind or near cigarette smoke.
- Sensitivity to light
- Blurry vision, especially when reading
- Strings of mucus in or around your eyes
- It is painful to wear contact lenses.
- You have lots of tears in your eyes.
Having a lot of tears in your eyes with dry eye might sound odd. But your eyes make more tears when they are irritated by dry eye.
Figure 3. Eye tear gland and ducts
Dry eye causes
Normally, the lacrimal glands above your eyes make tears that keep your eyes wet. Dry eye happens when your tears don’t do their job. This could mean:
- Your glands don’t make enough tears to keep your eyes wet
- Your tears dry up too fast
- Your tears just don’t work well enough to keep your eyes wet
People tend to make fewer tears as they get older (age 50 or older) due to hormonal changes. Both men and women can get dry eye. However, it is more common in women—especially those who have gone through menopause.
Some other causes of dry eye:
- Certain diseases, such as rheumatoid arthritis, Sjögren’s syndrome, diabetes, thyroid disease, and lupus
- Blepharitis (when eyelids are swollen or red)
- Entropion (when eyelids turn in); ectropion (eyelids turn outward)
- Being in smoke, wind or a very dry environments. Spending time in these types of places can cause your tears to dry up faster and lead to dry eye.
- Looking at a computer screen for a long time, reading and other activities that reduce blinking. You may blink less when looking at computer or tablet screens, which can lead to dry eye.
- Using contact lenses for a long time
- Having refractive eye surgery, such as laser eye surgery LASIK (laser-assisted in situ keratomileusis). After some types of laser surgery, your eyes may produce fewer tears. Ask your eye doctor how long this side effect usually lasts, and let them know if you have any questions or concerns.
- Medicines. Dry eye can be a side effect of some medicines that treat conditions like colds and allergies, depression, and high blood pressure. Taking certain medicines, such as:
- Diuretics (water pills) for high blood pressure
- Beta-blockers, for heart problems or high blood pressure
- Allergy and cold medicines (antihistamines)
- Sleeping pills
- Anxiety and antidepressant medicines
- Heartburn medicines
Tell your eye doctor (ophthalmologist) about all the prescription and non-prescription medicines you take.
Dry eye diagnosis
Your ophthalmologist will begin with an eye exam. He or she will look at your eyelids and the surface of the eye. They will also check how you blink.
There are many different tests that help diagnose dry eyes. Your ophthalmologist may do a test that measures the quality or the thickness of your tears. He or she may also measure how quickly you produce tears.
Testing for dry eye
- Slit lamp test: In a slit lamp test, your eye doctor will use a microscope called a slit lamp to see if your eyes are making enough tears. First, they’ll put a drop in your eye that will make your tears easier to see. Then, they’ll shine a thin, bright light into your eye and look at your eye and eyelids with a microscope.
- Schirmer’s test: A Schirmer’s test also tells your eye doctor if your eyes are making enough tears. Your eye doctor will give you eye drops to numb your eye. Then they’ll put a small piece of paper on the edge of your eyelid and ask you to close your eyes for 5 minutes. After 5 minutes, your doctor will see how much moisture (wetness) is on the paper.
- Tear break up time (TBUT): A TBUT test checks how long your tear film (layer of tears on your eyes) lasts after you blink. Your eye doctor will place a small amount of dye in your eye, and you’ll blink to make the dye fully cover your eye. Then, you’ll look forward without moving your eyes or blinking. Your eye doctor will watch to see how long the dyed tear film covers your whole eye. If your tear film does not last long, you may have dry eye.
Dry eye treatment
The first step in dry eye treatment is artificial tears. These are eye drops that are like your own tears. These come as preserved (screw cap bottle) and unpreserved (twist open vial). Preserved tears are more convenient, but some people are sensitive to preservatives. There are many brands available without a prescription. Try a few until you find a brand that works best for you.
If you use artificial tears more than six times a day or are allergic to preservatives, you should use preservative-free tears. This is because if the tears with preservatives are used a lot, these chemicals may start to irritate your eyes.
Start using the artificial tear drops at least 2 to 4 times per day. If your symptoms are not better after a couple of weeks of regular use:
- Increase use (up to every 2 hours).
- Change to unpreserved drops if you have been using the preserved type.
- Try a different brand.
- Talk to your ophthalmologist if you cannot find a brand that works for you. Your ophthalmologist might have you use a prescription eyedrop medication. This helps your eyes make more of their own tears.
Other treatments may include:
- Fish oil (omega 3 fatty acids) 2 to 3 times per day
- Glasses, goggles or contact lenses that keep moisture in the eyes
- Prescription medicines. If your dry eye is more serious, your eye doctor may give you a prescription for medicines called cyclosporine (Restasis) or lifitegrast (Xiidra). These medicines are both types of eye drops that can help your eyes make more tears.
- Tiny plugs placed in the tear drainage ducts to help moisture stay on the surface of the eye longer. Your ophthalmologist may suggest blocking your tear ducts. This makes your natural tears stay in your eyes longer. Tiny silicone or gel plugs (called punctal plugs) may be inserted in your tear ducts. These plugs can be removed later as needed.
- Temporary/dissolving plugs: These are made of a material (such as collagen) that gradually breaks down and is absorbed by the body. These plugs can last in the eye from a few days to months. Temporary plugs are often used to keep the eye moist after having refractive surgery, such as LASIK. They are also used when you want to try out punctal plugs to see if they help relieve your dry eye.
- Semi-permanent plugs: These are made of a longer-lasting medical plastic (such as silicone or acrylic). These plugs are designed to stay in the eye for years. They can be removed by your ophthalmologist if needed. Another type of semi-permanent punctal plug is placed in a deeper part of the tear duct called the canaliculus. These plugs cannot be seen at all in the eye.
- Your ophthalmologist could also recommend surgery that permanently closes your tear ducts.
Other helpful steps include:
- DO NOT smoke.
- Avoid second-hand smoke, direct wind, and air conditioning.
- Use a humidifier, particularly in the winter.
- Limit allergy and cold medicines that may dry you out and worsen your symptoms.
- Purposefully blink more often. Rest your eyes once in a while.
- Clean eyelashes regularly and apply warm compresses.
Some dry eye symptoms are due to sleeping with the eyes slightly open. Lubricating ointments work best for this problem. You should use them only in small amounts since they can blur your vision. It is best to use them before sleep.
In some cases, dry eye can happen because your lower eyelids are too loose, causing tears to drain too quickly out of your eye. If this is the cause of your dry eye, your eye doctor may suggest surgery to fix your eyelids and help your tears stay on your eyes. This treatment is not very common.
Best vitamins for eye floaters
Eye floaters are small, squiggly lines or dark specks, dots, circles, clouds or cobwebs that move as if floating in your vision. While they seem to be in front of your eye, they are floating inside (see Figure 4). Eye floaters are tiny clumps of gel, microscopic protein fibers or cell debris inside the vitreous (a jelly-like substance) that fills the back of your eyes and helping them to maintain their round shape. What you see are the shadows these clumps cast on your retina. When light enters the eye, these clumps can cast a small shadow on your retina, and this shadow is what you see as floaters.
You usually notice floaters when looking at something plain, like a blank wall or a blue sky.
Eye floaters may be caused by the normal aging process or as a result from other diseases or conditions. As you age, your vitreous starts to thicken or shrink. Sometimes clumps or strands form in the vitreous. If the vitreous pulls away from the back of the eye, it is called posterior vitreous detachment. Floaters usually happen with posterior vitreous detachment. They are not serious, and they tend to fade or go away over time. Severe floaters can be removed by surgery, but this has risks and is seldom necessary.
You are more likely to get eye floaters if you:
- are nearsighted (you need glasses to see far away)
- have had surgery for cataracts
- have had inflammation (swelling) inside the eye
Other not as common causes for floaters are other types of eye surgery, eye disease, eye injury, or crystal-like deposits that form in the vitreous.
If you notice a sudden increase in eye floaters, you need to see your eye specialist immediately — especially if you also see light flashes or lose your peripheral vision. These can be symptoms of an eye emergency that requires prompt attention. These could be signs of a serious condition, such as, retinal tears, hemorrhaging due to diabetes, high blood pressure, or uveitis (a kind of eye inflammation). It is important that you see a doctor because retinal tears and hemorrhaging can cause vision loss.
Figure 4. Eye floaters
Contact an eye specialist immediately if you notice:
- Many more eye floaters than usual
- A sudden onset of new floaters
- Flashes of light in the same eye as the floaters
- Darkness on any side or sides of your vision (peripheral vision loss)
These painless symptoms could be caused by a retinal tear, with or without a retinal detachment — a sight-threatening condition that requires immediate attention.
What causes eye floaters?
Inside your eye, there is a clear, gel-like fluid called the vitreous. You may see floaters if some of the gel in your vitreous clumps together. Small flecks of protein or other material that were trapped in the vitreous when your eye was formed can also cause floaters. The floaters in your eye are seen as shadows by your retina. The retina is the light-sensitive inner layer of your eye.
Floaters are a natural part of the eye’s aging process. As you age, your vitreous gel shrinks and may detach from your retina. If this happens, it can cause a small amount of bleeding. This is a common cause for floaters in people who are very nearsighted or who have had cataract surgery. Floaters are not a definite sign of a retinal detachment.
Eye floaters may be a result from other diseases or conditions:
- Inflammation in the back of the eye. Posterior uveitis is inflammation in the layers of the uvea in the back of the eye. This condition can cause the release of inflammatory debris into the vitreous that are seen as floaters. Posterior uveitis may be caused by infection, inflammatory diseases or other causes.
- Bleeding in the eye. Bleeding into the vitreous can have many causes, including diabetes, hypertension, blocked blood vessels and injury. Blood cells are seen as floaters.
- Torn retina. Retinal tears can occur when a sagging vitreous tugs on the retina with enough force to tear it. Without treatment, a retinal tear may lead to retinal detachment — an accumulation of fluid behind the retina that causes it to separate from the back of your eye. Untreated retinal detachment can cause permanent vision loss.
- Eye surgeries and eye medications. Certain medications that are injected into the vitreous can cause air bubbles to form. These bubbles are seen as shadows until your eye absorbs them. Certain vitreoretinal surgeries add silicone oil bubbles into the vitreous that can also be seen as floaters.
Risk factors for eye floaters
Factors that can increase your risk of floaters include:
- Age over 50
- Nearsightedness
- Eye trauma
- Complications from cataract surgery
- Diabetic retinopathy
- Eye inflammation
Eye floaters diagnosis
Your doctor will conduct a complete eye exam including eye dilation to better see the back of your eyes and the vitreous to determine the cause of the floaters.
Eye floaters treatment
Surgery to remove floaters is rare and only suggested for very severe cases. However, most eye floaters don’t require treatment.
Eye floaters can be frustrating, and adjusting to them can take time. Generally, floaters are not treated and people who see floaters learn to ignore them. If a floater appears in your line of vision, you should move your eye around. This causes the fluid inside your eye to shift and allows the floater to move out of the way. Looking up and down may be more helpful for moving floaters than looking side to side.
Any underlying cause of the floaters, such as bleeding from diabetes or inflammation, will be treated.
If your eye floaters impair your vision, which happens rarely, you and your eye doctor may consider treatment. Options may include:
- Surgery to remove the vitreous. An ophthalmologist removes the vitreous through a small incision (vitrectomy) and replaces it with a solution to help your eye maintain its shape. Surgery may not remove all the floaters, and new floaters can develop after surgery. Risks of a vitrectomy include bleeding and retinal tears.
- Using a laser to disrupt the floaters. An ophthalmologist aims a special laser at the floaters in the vitreous, which may break them up and make them less noticeable. Some people who have this treatment report improved vision; others notice little or no difference. Risks of laser therapy include damage to your retina if the laser is aimed incorrectly. Laser surgery to treat floaters is used infrequently.
Best vitamin for dark circles
Dark circles under your eyes happen when the skin beneath both eyes appears darkened. It’s different from bruising around one eye from an injury or redness and swelling in one eye caused by an infection. Dark circles under your eyes usually are not a sign of a medical problem.
Dark circles under the eyes are usually caused by being tired or not getting enough sleep. Sometimes, what appear to be dark circles under your eyes may merely be shadows cast by puffy eyelids or hollows under your eyes that develop as a normal part of aging.
Some of the most common causes of true under-eye circles are:
- Increased pigmentation (melanin)
- Loss of fatty tissue in the eyelid or around the eye
- Bulging fat and muscle loss
- Puffy eyelids
- Thin, translucent skin
- Shadowing due to anatomic shape of the orbit
- Allergies
- Atopic dermatitis (eczema)
- Contact dermatitis
- Fatigue
- Hay fever (allergic rhinitis)
- Heredity
- Skin pigment irregularities
- Rubbing or scratching your eyes
- Sun exposure, which prompts your body to produce more melanin, the pigment that gives skin its color
- Skin changes that happen with aging
Those prone to dark circles under the eyes include:
- The elderly (but they are also a common complaint in adolescents)
- People of non-white ethnic background
- People with a genetic predisposition to dark circles under the eyes.
What causes dark circles under the eyes?
Pigmentation under the eyes is associated with dermal deposition of melanin. Dermal melanin deposition is often due to post-inflammatory pigmentation, which may follow:
- Sun exposure
- Atopic dermatitis
- Contact dermatitis
- Rubbing or scratching the eyes.
Loss of fatty tissue in the eyelid or around the eye (tear trough) is associated with:
- Ageing
- Genetic factors
- Smoking.
Bulging or puffy eyelids may be due to systemic conditions, particularly:
- Thyroid disease
- Dermatitis
- Hay fever (allergy).
Thin translucent skin is commonly observed with:
- Age
- Genetic factors.
Shadowing is more noticeable at times, due to:
- Fatigue or lack of sleep
- Periorbital oedema (puffy eyelids)
- Dehydration (sunken eyes).
Superficially located blood vessels and blood stasis may contribute to the darkened appearance.
Dark circles under the eyes diagnosis
Correct diagnosis of dark circles under the eyes can be difficult. It involves:
- Personal, medical and family history
- Physical examination
- Wood lamp evaluation, which allows the clinician to assess the depth of pigmentation.
Dark circles under the eyes treatment
Depending on what’s causing the circles under your eyes, your doctor may recommend prescription creams or a combination of treatments to erase or reduce discoloration. Laser therapy or chemical peels can be helpful in some cases. Hollows that cause shadows can be smoothed with injectable fillers, and surgery can eliminate puffy lids.
Home remedies for dark circles under your eyes may be all you need to help manage this condition. General measures include:
- Get adequate sleep. Although short nights don’t usually cause under-eye circles, a lack of sleep may make you paler, so shadows and circles you already have become more obvious.
- Smoking cessation
- Sleep with extra pillows to elevate the head and reduce eyelid swelling. Elevate your head with two or more pillows to prevent puffiness that develops when fluid pools in your lower eyelids.
- Massage temporary swelling while applying a cold compress
- Cold compresses also minimise the appearance of prominent blood vessels. Dilated under-eye blood vessels may contribute to dark circles under your eyes. Try holding a cold compress, a chilled teaspoon or a bag of frozen peas wrapped in a soft cloth against the area to make these vessels constrict.
- Cosmetic camouflage
- Light-reflecting concealers (these are often yellow or gold in colour) covered by translucent face powder. These should be applied in the shadows, not on the puffy skin.
Unfortunately, many of the remedies on the market lack evidence of efficacy.
Medical treatments to reduce pigmentation can include:
- Protection from sun exposure using sunglasses
- Topical agents; however dermal pigmentation responds poorly, and eyelids are sensitive so the stronger products may irritate (see melasma)
- Chemical peels to reduce fine lines and surface pigmentation
- Laser or intense pulsed light (IPL) treatments.
Loss of tissue (hollowing) and tear trough can be managed by aesthetic medical and surgical procedures:
- Fillers (dermal implants) eg hyaluronic acid injections or fat grafts
- Surgery to remove excess fat, muscle and skin (surgical blepharoplasty or laser eye-lifting procedure).
Considerable training and experience are required to optimize results. Improvement may be partial. An incorrect technique may make the dark circles look more prominent than before the procedure.
Best eye vitamins for blurry vision
Blurry vision is defined as the loss of sharpness of vision and the inability to see fine details. See your health care provider or an eye doctor if you have any problems with your eyesight.
Blurry vision and vision changes and problems can be caused by many different conditions. Some include:
- Presbyopia — Difficulty focusing on objects that are close. This problem often becomes noticeable in your early to mid-40s.
- Cataracts — Cloudiness over the eye lens, causing poor nighttime vision, halos around lights, and sensitivity to glare. Cataracts are common in older people.
- Glaucoma — Increased pressure in the eye, which is most often painless. Vision will be normal at first, but over time you can develop poor night vision, blind spots, and a loss of vision to either side. Some types of glaucoma can also happen suddenly, which is a medical emergency.
- Diabetic eye disease (diabetic retinopathy).
- Macular degeneration (age-related macular degeneration) — Loss of central vision, blurred vision (particularly while reading), distorted vision (straight lines will appear to be wavy), and colors that look faded. The most common cause of blindness in people over age 60 years.
- Eye infection, inflammation, or injury.
- Floaters — Tiny particles drifting inside the eye, which may be a sign of retinal detachment.
- Night blindness.
- Retinal detachment — Symptoms include floaters, sparks, or flashes of light in your vision, or a sensation of a shade or curtain hanging across part of your visual field.
- Optic neuritis — Inflammation of the optic nerve from infection or multiple sclerosis. You may have pain when you move your eye or touch it through the eyelid.
- Stroke or TIA.
- Brain tumor.
- Bleeding into the eye.
- Temporal arteritis — Inflammation of an artery in the brain that supplies blood to the optic nerve.
- Migraine headaches — Spots of light, halos, or zigzag patterns that appear before the start of the headache.
Medicines may also affect vision.
Seek emergency care from a medical professional who is experienced in dealing with eye emergencies if:
- You experience partial or complete blindness in one or both eyes, even if it is only temporary.
- You experience double vision, even if it is temporary.
- You have a sensation of a shade being pulled over your eyes or a curtain being drawn from the side, above, or below.
- Blind spots, halos around lights, or areas of distorted vision appear suddenly.
- You have sudden blurred vision with eye pain, particularly if the eye is also red. A red, painful eye with blurred vision is a medical emergency.
Get a complete eye exam if you have:
- Trouble seeing objects on either side.
- Difficulty seeing at night or when reading.
- Gradual loss of the sharpness of your vision.
- Difficulty telling colors apart.
- Blurred vision when trying to view objects near or far.
- Diabetes or a family history of diabetes.
- Eye itching or discharge.
- Vision changes that seem related to medicine. (DO NOT stop or change a medicine without talking to your doctor.)
- Causes of Low Vision. https://www.aao.org/eye-health/diseases/low-vision-cause
- Johnson EJ, Russell RM. Beta-Carotene. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:115-20.
- Solomons NW. Vitamin A. In: Bowman B, Russell R, eds. Present Knowledge in Nutrition. 9th ed. Washington, DC: International Life Sciences Institute; 2006:157-83.
- Ross CA. Vitamin A. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:778-91.
- Vitamin A. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press; 2001. https://www.nap.edu/read/10026/chapter/1
- Vitamins in Neuro-Ophthalmology. https://eyewiki.org/Vitamins_in_Neuro-Ophthalmology
- Blaner WS, Nau H, Agadir A. Retinoids: the Biochemical and Molecular Basis of Vitamin A and Retinoid Action. Berlin: Springer-Verlag; 1999. https://doi-org.ezproxy.med.cornell.edu/10.1007/978-3-642-58483-1
- Ross A. Vitamin A and Carotenoids. In: Shils M, Shike M, Ross A, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2006:351-75.
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central 2019. https://fdc.nal.usda.gov
- Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003-2006. J Nutr. 2011 Feb;141(2):261-6. doi: 10.3945/jn.110.133025
- World Health Organization. Global Prevalence of Vitamin A Deficiency in Populations at Risk 1995–2005: WHO Global Database on Vitamin A Deficiency. Geneva: World Health Organization; 2009. http://apps.who.int/iris/bitstream/handle/10665/44110/9789241598019_eng.pdf
- Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ. 2011 Aug 25;343:d5094. doi: 10.1136/bmj.d5094
- Sommer A. Vitamin a deficiency and clinical disease: an historical overview. J Nutr. 2008 Oct;138(10):1835-9. doi: 10.1093/jn/138.10.1835
- Bendich A, Deckelbaum RJ. Preventive Nutrition The Comprehensive Guide for Health Professionals. Cham: Springer International Publishing; 2018. DOI https://doi-org.ezproxy.med.cornell.edu/10.1007/978-1-59259-880-9_23
- Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don’t know, and what we need to know. Arch Dis Child Fetal Neonatal Ed. 2005 Mar;90(2):F103-8. doi: 10.1136/adc.2004.057547
- Micronutrient deficiencies. World Health Organization. https://www.who.int/health-topics/micronutrients
- Cheshire J, Kolli S. Vitamin A deficiency due to chronic malabsorption: an ophthalmic manifestation of a systemic condition. BMJ Case Reports. April 2017. doi:10.1136/bcr-2017-220024
- Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short and long-term morbidity in very low birthweight infants. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD000501. doi: 10.1002/14651858.CD000501.pub2. Update in: Cochrane Database Syst Rev. 2011;(10):CD000501
- Oliveira-Menegozzo JM, Bergamaschi DP, Middleton P, East CE. Vitamin A supplementation for postpartum women. Cochrane Database Syst Rev. 2010 Oct 6;(10):CD005944. doi: 10.1002/14651858.CD005944.pub2. Update in: Cochrane Database Syst Rev. 2016;3:CD005944
- van den Broek N, Dou L, Othman M, Neilson JP, Gates S, Gülmezoglu AM. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database Syst Rev. 2010 Nov 10;(11):CD008666. doi: 10.1002/14651858.CD008666.pub2. Update in: Cochrane Database Syst Rev. 2015;10:CD008666
- O’Neil C, Shevill E, Chang AB. Vitamin A supplementation for cystic fibrosis. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD006751. doi: 10.1002/14651858.CD006751.pub2. Update in: Cochrane Database Syst Rev. 2012;8:CD006751
- Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr 2002;35:246-59.
- Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002 Sep;35(3):246-59. doi: 10.1097/00005176-200209000-00004
- Naidu KA: Vitamin C in human health and disease is still a mystery? An overview. Nutr J 2: 7, 2003. https://www.ncbi.nlm.nih.gov/pubmed/14498993
- Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007;137:2171-84. https://www.ncbi.nlm.nih.gov/pubmed/17884994
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids Washington, DC: National Academy Press; 2000.
- Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care 2002;5:66-74. https://www.ncbi.nlm.nih.gov/pubmed/12134712
- Merck Sharp & Dohme Corp., Merck Manual. Vitamin C (Ascorbic Acid). https://www.merckmanuals.com/professional/nutritional-disorders/vitamin-deficiency,-dependency,-and-toxicity/vitamin-c
- Holford P, Carr AC, Jovic TH, Ali SR, Whitaker IS, Marik PE, Smith AD. Vitamin C-An Adjunctive Therapy for Respiratory Infection, Sepsis and COVID-19. Nutrients. 2020 Dec 7;12(12):3760. doi: 10.3390/nu12123760
- Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients. 2017 Nov 3;9(11):1211. doi: 10.3390/nu9111211
- Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev. 2013 Jan 31;2013(1):CD000980. doi: 10.1002/14651858.CD000980.pub4
- Johnston CS. Vitamin C. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition 11th ed. Cambridge, MA: Elsevier; 2020:155-69.
- Hemilä H. Vitamin C and Infections. Nutrients. 2017 Mar 29;9(4):339. doi: 10.3390/nu9040339
- Schleicher RL, Carroll MD, Ford ES, Lacher DA. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). Am J Clin Nutr. 2009 Nov;90(5):1252-63. doi: 10.3945/ajcn.2008.27016
- Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007 Oct;137(10):2171-84. doi: 10.1093/jn/137.10.2171
- Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6377-81. doi: 10.1073/pnas.86.16.6377
- Jacob RA, Sotoudeh G. Vitamin C function and status in chronic disease. Nutr Clin Care. 2002 Mar-Apr;5(2):66-74. doi: 10.1046/j.1523-5408.2002.00005.x
- Gershoff SN. Vitamin C (ascorbic acid): new roles, new requirements? Nutr Rev. 1993 Nov;51(11):313-26. doi: 10.1111/j.1753-4887.1993.tb03757.x
- Weinstein M, Babyn P, Zlotkin S. An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics. 2001 Sep;108(3):E55. doi: 10.1542/peds.108.3.e55
- Padayatty SJ, Sun H, Wang Y, Riordan HD, Hewitt SM, Katz A, Wesley RA, Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004;140:533-7. https://www.ncbi.nlm.nih.gov/pubmed/15068981
- Carpenter KJ. The history of scurvy and vitamin C. Cambridge: Cambridge University Press, 1986.
- Padayatty S, Espey MG, Levine M: Vitamin C. In: Coates PM, Betz JM, Blackman MR, et al., eds.: Encyclopedia of Dietary Supplements. 2nd ed. New York, NY: Informa Healthcare, 2010, pp 821-31.
- National Cancer Institute. High-Dose Vitamin C. https://www.cancer.gov/about-cancer/treatment/cam/patient/vitamin-c-pdq
- Pauling L. The significance of the evidence about ascorbic acid and the common cold. Proc Natl Acad Sci U S A 1971;68:2678-81. https://www.ncbi.nlm.nih.gov/pubmed/4941984
- McCormick WJ: Cancer: a collagen disease, secondary to a nutritional deficiency. Arch Pediatr 76 (4): 166-71, 1959. https://www.ncbi.nlm.nih.gov/pubmed/13638066?dopt=Abstract
- Cameron E, Pauling L: The orthomolecular treatment of cancer. I. The role of ascorbic acid in host resistance. Chem Biol Interact 9 (4): 273-83, 1974. https://www.ncbi.nlm.nih.gov/pubmed/4609626
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press, 2000. https://www.nap.edu/catalog/9810/dietary-reference-intakes-for-vitamin-c-vitamin-e-selenium-and-carotenoids
- Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999 Jun;69(6):1086-107. doi: 10.1093/ajcn/69.6.1086
- Yoshida M, Takashima Y, Inoue M, Iwasaki M, Otani T, Sasaki S, Tsugane S; JPHC Study Group. Prospective study showing that dietary vitamin C reduced the risk of age-related cataracts in a middle-aged Japanese population. Eur J Nutr. 2007 Mar;46(2):118-24. doi: 10.1007/s00394-006-0641-8
- Rautiainen S, Lindblad BE, Morgenstern R, Wolk A. Vitamin C supplements and the risk of age-related cataract: a population-based prospective cohort study in women. Am J Clin Nutr. 2010 Feb;91(2):487-93. doi: 10.3945/ajcn.2009.28528
- Sperduto RD, Hu TS, Milton RC, Zhao JL, Everett DF, Cheng QF, Blot WJ, Bing L, Taylor PR, Li JY, et al. The Linxian cataract studies. Two nutrition intervention trials. Arch Ophthalmol. 1993 Sep;111(9):1246-53. doi: 10.1001/archopht.1993.01090090098027
- Age-Related Eye Disease Study Research Group (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Archives of ophthalmology (Chicago, Ill. : 1960), 119(10), 1439–1452. https://doi.org/10.1001/archopht.119.10.1439
- Age-Related Eye Disease Study 2 (AREDS2) Research Group, Chew, E. Y., SanGiovanni, J. P., Ferris, F. L., Wong, W. T., Agron, E., Clemons, T. E., Sperduto, R., Danis, R., Chandra, S. R., Blodi, B. A., Domalpally, A., Elman, M. J., Antoszyk, A. N., Ruby, A. J., Orth, D., Bressler, S. B., Fish, G. E., Hubbard, G. B., Klein, M. L., … Bernstein, P. (2013). Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA ophthalmology, 131(7), 843–850. https://doi.org/10.1001/jamaophthalmol.2013.4412
- van Leeuwen R, Boekhoorn S, Vingerling JR, Witteman JC, Klaver CC, Hofman A, de Jong PT. Dietary intake of antioxidants and risk of age-related macular degeneration. JAMA. 2005 Dec 28;294(24):3101-7. doi: 10.1001/jama.294.24.3101
- Evans J. (2007). Primary prevention of age related macular degeneration. BMJ (Clinical research ed.), 335(7623), 729. https://doi.org/10.1136/bmj.39351.478924.BE
- Chong EW, Wong TY, Kreis AJ, Simpson JA, Guymer RH. Dietary antioxidants and primary prevention of age related macular degeneration: systematic review and meta-analysis. BMJ. 2007 Oct 13;335(7623):755. doi: 10.1136/bmj.39350.500428.47
- Evans JR. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst Rev. 2006 Apr 19;(2):CD000254. doi: 10.1002/14651858.CD000254.pub2. Update in: Cochrane Database Syst Rev. 2012;11:CD000254.
- Age-Related Eye Disease Study Research Group (2001). A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of ophthalmology (Chicago, Ill. : 1960), 119(10), 1417–1436. https://doi.org/10.1001/archopht.119.10.1417
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group*. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA. 2013;309(19):2005–2015. doi:10.1001/jama.2013.4997 https://jamanetwork.com/journals/jama/fullarticle/1684847
- Wang AH, Still C. Old world meets modern: a case report of scurvy. Nutr Clin Pract. 2007 Aug;22(4):445-8. doi: 10.1177/0115426507022004445
- Levine M, Rumsey SC, Daruwala R, Park JB, Wang Y. Criteria and recommendations for vitamin C intake. JAMA. 1999 Apr 21;281(15):1415-23. doi: 10.1001/jama.281.15.1415
- Francescone MA, Levitt J. Scurvy masquerading as leukocytoclastic vasculitis: a case report and review of the literature. Cutis. 2005 Oct;76(4):261-6.
- Stephen R, Utecht T. Scurvy identified in the emergency department: a case report. J Emerg Med. 2001 Oct;21(3):235-7. doi: 10.1016/s0736-4679(01)00377-8
- Bates CJ. Bioavailability of vitamin C. Eur J Clin Nutr. 1997 Jan;51 Suppl 1:S28-33.
- Hoffman FA. Micronutrient requirements of cancer patients. Cancer. 1985 Jan 1;55(1 Suppl):295-300. doi: 10.1002/1097-0142(19850101)55:1+<295::aid-cncr2820551315>3.0.co;2-x
- Deicher R, Hörl WH. Vitamin C in chronic kidney disease and hemodialysis patients. Kidney Blood Press Res. 2003;26(2):100-6. doi: 10.1159/000070991
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010.
- Vitamin D. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional
- Bartosik-Psujek H, Psujek M. Vitamin D as an immune modulator in multiple sclerosis. Neurologia i Neurochirurgia Polska. 2019;53(2):113-122. doi:10.5603/pjnns.a2019.0015
- Jones G. Vitamin D. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease, 11th ed. Philadelphia: Lippincott Williams & Wilkins, 2014.
- Norman AW, Henry HH. Vitamin D. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition, 10th ed. Washington DC: Wiley-Blackwell, 2012.
- Silva MC, Furlanetto TW. Intestinal absorption of vitamin D: a systematic review. Nutr Rev. 2018 Jan 1;76(1):60-76. doi: 10.1093/nutrit/nux034
- Sempos CT, Heijboer AC, Bikle DD, Bollerslev J, Bouillon R, Brannon PM, DeLuca HF, Jones G, Munns CF, Bilezikian JP, Giustina A, Binkley N. Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D. Br J Clin Pharmacol. 2018 Oct;84(10):2194-2207. doi: 10.1111/bcp.13652
- Sempos CT, Binkley N. 25-Hydroxyvitamin D assay standardisation and vitamin D guidelines paralysis. Public Health Nutr. 2020 May;23(7):1153-1164. doi: 10.1017/S1368980019005251
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911-30. doi: 10.1210/jc.2011-0385. Epub 2011 Jun 6. Erratum in: J Clin Endocrinol Metab. 2011 Dec;96(12):3908.
- Brown LL, Cohen B, Tabor D, Zappalà G, Maruvada P, Coates PM. The vitamin D paradox in Black Americans: a systems-based approach to investigating clinical practice, research, and public health – expert panel meeting report. BMC Proc. 2018 May 9;12(Suppl 6):6. doi: 10.1186/s12919-018-0102-4
- Institute of Medicine, Food and Nutrition Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press, 2010
- Roseland JM, Phillips KM, Patterson KY, Pehrsson PR, Taylor CL. Vitamin D in foods: An evolution of knowledge. Pages 41-78 in Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, eds. Vitamin D, Volume 2: Health, Disease and Therapeutics, Fourth Edition. Elsevier, 2018.
- U.S. Food and Drug Administration. Food additives permitted for direct addition to food for human consumption; vitamin D2 mushroom powder. Federal Register 2020;85:41916-20.
- Borel P, Caillaud D, Cano NJ. Vitamin D bioavailability: state of the art. Crit Rev Food Sci Nutr. 2015;55(9):1193-205. doi: 10.1080/10408398.2012.688897
- Taylor CL, Patterson KY, Roseland JM, Wise SA, Merkel JM, Pehrsson PR, Yetley EA. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr. 2014 May;144(5):654-9. doi: 10.3945/jn.113.189811
- Calvo MS, Whiting SJ, Barton CN. Vitamin D fortification in the United States and Canada: current status and data needs. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1710S-6S. doi: 10.1093/ajcn/80.6.1710S
- Yetley EA. Assessing the vitamin D status of the US population. Am J Clin Nutr. 2008 Aug;88(2):558S-564S. doi: 10.1093/ajcn/88.2.558S
- Vitamin D for Milk and Milk Alternatives. https://www.fda.gov/food/food-additives-petitions/vitamin-d-milk-and-milk-alternatives
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. https://fdc.nal.usda.gov/index.html
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. https://www.ncbi.nlm.nih.gov/pubmed/17634462?dopt=Abstract
- Cranney C, Horsely T, O’Donnell S, Weiler H, Ooi D, Atkinson S, et al. Effectiveness and safety of vitamin D. Evidence Report/Technology Assessment No. 158 prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-02.0021. AHRQ Publication No. 07-E013. Rockville, MD: Agency for Healthcare Research and Quality, 2007. https://www.ncbi.nlm.nih.gov/pubmed/18088161?dopt=Abstract
- Sahay M, Sahay R. Rickets–vitamin D deficiency and dependency. Indian Journal of Endocrinology and Metabolism. 2012;16(2):164-176. doi:10.4103/2230-8210.93732. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3313732/
- Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008;122:1142-1152. https://www.ncbi.nlm.nih.gov/pubmed/18977996?dopt=Abstract
- Jagannath VA, Filippini G, Di Pietrantonj C, Asokan GV, Robak EW, Whamond L, Robinson SA. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev. 2018 Sep 24;9(9):CD008422. doi: 10.1002/14651858.CD008422.pub3
- Sintzel MB, Rametta M, Reder AT. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol Ther. 2018 Jun;7(1):59-85. doi: 10.1007/s40120-017-0086-4
- Munger KL, Hongell K, Åivo J, Soilu-Hänninen M, Surcel HM, Ascherio A. 25-Hydroxyvitamin D deficiency and risk of MS among women in the Finnish Maternity Cohort. Neurology. 2017 Oct 10;89(15):1578-1583. doi: 10.1212/WNL.0000000000004489
- Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006 Dec 20;296(23):2832-8. doi: 10.1001/jama.296.23.2832
- Salzer J, Hallmans G, Nyström M, Stenlund H, Wadell G, Sundström P. Vitamin D as a protective factor in multiple sclerosis. Neurology. 2012 Nov 20;79(21):2140-5. doi: 10.1212/WNL.0b013e3182752ea8
- Marrie RA, Beck CA. Preventing multiple sclerosis: To (take) vitamin D or not to (take) vitamin D? Neurology. 2017 Oct 10;89(15):1538-1539. doi: 10.1212/WNL.0000000000004506
- Li X, Liu Y, Zheng Y, Wang P, Zhang Y. The Effect of Vitamin D Supplementation on Glycemic Control in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis. Nutrients. 2018 Mar 19;10(3):375. doi: 10.3390/nu10030375
- Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, Brodsky I, Ceglia L, Chadha C, Chatterjee R, Desouza C, Dolor R, Foreyt J, Fuss P, Ghazi A, Hsia DS, Johnson KC, Kashyap SR, Kim S, LeBlanc ES, Lewis MR, Liao E, Neff LM, Nelson J, O’Neil P, Park J, Peters A, Phillips LS, Pratley R, Raskin P, Rasouli N, Robbins D, Rosen C, Vickery EM, Staten M; D2d Research Group. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N Engl J Med. 2019 Aug 8;381(6):520-530. doi: 10.1056/NEJMoa1900906
- Rafiq S, Jeppesen PB. Is Hypovitaminosis D Related to Incidence of Type 2 Diabetes and High Fasting Glucose Level in Healthy Subjects: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients. 2018 Jan 10;10(1):59. doi: 10.3390/nu10010059
- Mousa A, Naderpoor N, de Courten MP, Teede H, Kellow N, Walker K, Scragg R, de Courten B. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D-deficient, overweight or obese adults: a randomized placebo-controlled trial. Am J Clin Nutr. 2017 Jun;105(6):1372-1381. doi: 10.3945/ajcn.117.152736
- Seida JC, Mitri J, Colmers IN, Majumdar SR, Davidson MB, Edwards AL, Hanley DA, Pittas AG, Tjosvold L, Johnson JA. Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014 Oct;99(10):3551-60. doi: 10.1210/jc.2014-2136. Epub 2014 Jul 25. Erratum in: J Clin Endocrinol Metab. 2015 Aug;100(8):3219.
- Jorde R, Sollid ST, Svartberg J, Schirmer H, Joakimsen RM, Njølstad I, Fuskevåg OM, Figenschau Y, Hutchinson MY. Vitamin D 20,000 IU per Week for Five Years Does Not Prevent Progression From Prediabetes to Diabetes. J Clin Endocrinol Metab. 2016 Apr;101(4):1647-55. doi: 10.1210/jc.2015-4013
- Pittas A, Dawson-Hughes B, Staten M. Vitamin D Supplementation and Prevention of Type 2 Diabetes. Reply. N Engl J Med. 2019 Oct 31;381(18):1785-1786. doi: 10.1056/NEJMc1912185
- Elder CJ, Bishop NJ. Rickets. Lancet. 2014 May 10;383(9929):1665-1676. doi: 10.1016/S0140-6736(13)61650-5
- Uday S, Högler W. Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Curr Osteoporos Rep. 2017 Aug;15(4):293-302. doi: 10.1007/s11914-017-0383-y. Erratum in: Curr Osteoporos Rep. 2017 Aug 14
- Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1697S-705S. doi: 10.1093/ajcn/80.6.1697S
- Thacher TD, Fischer PR, Tebben PJ, Singh RJ, Cha SS, Maxson JA, Yawn BP. Increasing incidence of nutritional rickets: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2013 Feb;88(2):176-83. doi: 10.1016/j.mayocp.2012.10.018
- Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ. 2007 Jul 17;177(2):161-6. doi: 10.1503/cmaj.061377
- Rajakumar K. Vitamin D, cod-liver oil, sunlight, and rickets: a historical perspective. Pediatrics. 2003 Aug;112(2):e132-5. doi: 10.1542/peds.112.2.e132
- Creo AL, Thacher TD, Pettifor JM, Strand MA, Fischer PR. Nutritional rickets around the world: an update. Paediatr Int Child Health. 2017 May;37(2):84-98. doi: 10.1080/20469047.2016.1248170
- Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Sävendahl L, Khadgawat R, Pludowski P, Maddock J, Hyppönen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Högler W. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab. 2016 Feb;101(2):394-415. doi: 10.1210/jc.2015-2175
- Taylor CL, Rosen CJ, Dwyer JT. Considerations in Dietetic Counseling for Vitamin D. J Acad Nutr Diet. 2019 Jun;119(6):901-909. doi: 10.1016/j.jand.2018.04.013
- Agency for Healthcare Research and Quality. Screening for vitamin D deficiency: Systematic review for the U.S. Preventive Services Task Force recommendation. Evidence Synthesis Number 118. AHRQ-Pub No. 13-05183-EF-1. June 2014.
- LeFevre ML; U.S. Preventive Services Task Force. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015 Jan 20;162(2):133-40. doi: 10.7326/M14-2450
- Wagner CL, Greer FR; American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics. 2008 Nov;122(5):1142-52. doi: 10.1542/peds.2008-1862. Erratum in: Pediatrics. 2009 Jan;123(1):197.
- Dawodu A, Tsang RC. Maternal vitamin D status: effect on milk vitamin D content and vitamin D status of breastfeeding infants. Adv Nutr. 2012 May 1;3(3):353-61. doi: 10.3945/an.111.000950
- Davis CD, Dwyer JT. The “sunshine vitamin”: benefits beyond bone? J Natl Cancer Inst. 2007 Nov 7;99(21):1563-5. doi: 10.1093/jnci/djm211
- Simon AE, Ahrens KA. Adherence to Vitamin D Intake Guidelines in the United States. Pediatrics. 2020 Jun;145(6):e20193574. doi: 10.1542/peds.2019-3574
- Chalcraft JR, Cardinal LM, Wechsler PJ, Hollis BW, Gerow KG, Alexander BM, Keith JF, Larson-Meyer DE. Vitamin D Synthesis Following a Single Bout of Sun Exposure in Older and Younger Men and Women. Nutrients. 2020 Jul 27;12(8):2237. doi: 10.3390/nu12082237
- Cranney C, Horsely T, O’Donnell S, Weiler H, Ooi D, Atkinson S, et al. Effectiveness and safety of vitamin D. Evidence Report/Technology Assessment No. 158 prepared by the University of Ottawa Evidence-based Practice Center under Contract No. 290-02.0021. AHRQ Publication No. 07-E013. Rockville, MD: Agency for Healthcare Research and Quality, 2007. https://www.ncbi.nlm.nih.gov/pubmed/18088161
- Sowah D, Fan X, Dennett L, Hagtvedt R, Straube S. Vitamin D levels and deficiency with different occupations: a systematic review. BMC Public Health. 2017 Jun 22;17(1):519. doi: 10.1186/s12889-017-4436-z
- Pappa HM, Bern E, Kamin D, Grand RJ. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008 Mar;24(2):176-83. doi: 10.1097/MOG.0b013e3282f4d2f3
- Holick MF. Vitamin D. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease, 10th ed. Philadelphia: Lippincott Williams & Wilkins, 2006.
- Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014 Nov 5;9(11):e111265. doi: 10.1371/journal.pone.0111265
- Chakhtoura M, Rahme M, El-Hajj Fuleihan G. Vitamin D Metabolism in Bariatric Surgery. Endocrinol Metab Clin North Am. 2017 Dec;46(4):947-982. doi: 10.1016/j.ecl.2017.07.006
- Chakhtoura MT, Nakhoul N, Akl EA, Mantzoros CS, El Hajj Fuleihan GA. Guidelines on vitamin D replacement in bariatric surgery: Identification and systematic appraisal. Metabolism. 2016 Apr;65(4):586-97. doi: 10.1016/j.metabol.2015.12.013
- Vitamin E. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional
- Traber MG. Vitamin E. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins R, eds. Modern Nutrition in Health and Disease. 10th ed. Baltimore, MD: Lippincott Williams & Wilkins, 2006;396-411.
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, D.C.: National Academy Press; 2000.
- Traber MG. Vitamin E regulatory mechanisms. Annu Rev Nutr. 2007;27:347-62. doi: 10.1146/annurev.nutr.27.061406.093819
- Dietrich M, Traber MG, Jacques PF, Cross CE, Hu Y, Block G. Does gamma-tocopherol play a role in the primary prevention of heart disease and cancer? A review. J Am Coll Nutr. 2006 Aug;25(4):292-9. doi: 10.1080/07315724.2006.10719538
- Verhagen H, Buijsse B, Jansen E, Bueno-de-Mesquita B. The state of antioxidant affairs. Nutr Today 2006;41:244-50.
- Evans J. Primary prevention of age related macular degeneration. BMJ. 2007 Oct 13;335(7623):729. doi: 10.1136/bmj.39351.478924.BE
- Taylor HR, Tikellis G, Robman LD, McCarty CA, McNeil JJ. Vitamin E supplementation and macular degeneration: randomised controlled trial. BMJ. 2002 Jul 6;325(7354):11. doi: 10.1136/bmj.325.7354.11
- Teikari JM, Virtamo J, Rautalahti M, Palmgren J, Liesto K, Heinonen OP. Long-term supplementation with alpha-tocopherol and beta-carotene and age-related cataract. Acta Ophthalmol Scand. 1997 Dec;75(6):634-40. doi: 10.1111/j.1600-0420.1997.tb00620.x
- Leske MC, Chylack LT Jr, He Q, Wu SY, Schoenfeld E, Friend J, Wolfe J. Antioxidant vitamins and nuclear opacities: the longitudinal study of cataract. Ophthalmology. 1998 May;105(5):831-6. doi: 10.1016/s0161-6420(98)95021-7
- Jacques PF, Taylor A, Moeller S, Hankinson SE, Rogers G, Tung W, Ludovico J, Willett WC, Chylack LT Jr. Long-term nutrient intake and 5-year change in nuclear lens opacities. Arch Ophthalmol. 2005 Apr;123(4):517-26. doi: 10.1001/archopht.123.4.517
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels and Serving Sizes of Foods That Can Reasonably Be Consumed at One Eating Occasion; Dual-Column Labeling; Updating, Modifying, and Establishing Certain Reference Amounts Customarily Consumed; Serving Size for Breath Mints; and Technical Amendments; Proposed Extension of Compliance Dates. https://www.federalregister.gov/documents/2017/10/02/2017-21019/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels-and-serving-sizes-of-foods-that
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academy Press, 2000. https://www.nap.edu/catalog/9810/dietary-reference-intakes-for-vitamin-c-vitamin-e-selenium-and-carotenoids
- Brion LP, Bell EF, Raghuveer TS. Vitamin E supplementation for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2003;(4):CD003665. doi: 10.1002/14651858.CD003665
- Kowdley KV, Mason JB, Meydani SN, Cornwall S, Grand RJ. Vitamin E deficiency and impaired cellular immunity related to intestinal fat malabsorption. Gastroenterology. 1992 Jun;102(6):2139-42. doi: 10.1016/0016-5085(92)90344-x
- Tanyel MC, Mancano LD. Neurologic findings in vitamin E deficiency. Am Fam Physician. 1997 Jan;55(1):197-201.
- Cavalier L, Ouahchi K, Kayden HJ, Di Donato S, Reutenauer L, Mandel JL, Koenig M. Ataxia with isolated vitamin E deficiency: heterogeneity of mutations and phenotypic variability in a large number of families. Am J Hum Genet. 1998 Feb;62(2):301-10. doi: 10.1086/301699
- Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994 Apr 14;330(15):1029-35. doi: 10.1056/NEJM199404143301501
- Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008 Nov 12;300(18):2123-33. doi: 10.1001/jama.2008.600
- Miller ER 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005 Jan 4;142(1):37-46. doi: 10.7326/0003-4819-142-1-200501040-00110
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007 Feb 28;297(8):842-57. doi: 10.1001/jama.297.8.842. Erratum in: JAMA. 2008 Feb 20;299(7):765-6.
- Huang HY, Caballero B, Chang S, Alberg A, Semba R, Schneyer C, et al. Multivitamin/Mineral Supplements and Prevention of Chronic Disease. Evidence Report/Technology Assessment No. 139. (Prepared by The Johns Hopkins University Evidence-based Practice Center under Contract No. 290-02-0018). AHRQ Publication No. 06-E012. Rockville, MD: Agency for Healthcare Research and Quality. May 2008.
- Klein EA, Thompson IM Jr, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD, Lieber MM, Walther PJ, Klotz L, Parsons JK, Chin JL, Darke AK, Lippman SM, Goodman GE, Meyskens FL Jr, Baker LH. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA. 2011 Oct 12;306(14):1549-56. doi: 10.1001/jama.2011.1437
- Stover PJ. Folic acid. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2012:358-68.
- Folate. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional
- Carmel R. Folic acid. In: Shils M, Shike M, Ross A, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005:470-81.
- Bailey LB, Caudill MA. Folate. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:321-42.
- Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF 3rd, Mills JL, Pfeiffer CM, Fazili Z, Zhang M, Ueland PM, Molloy AM, Caudill MA, Shane B, Berry RJ, Bailey RL, Hausman DB, Raghavan R, Raiten DJ. Biomarkers of Nutrition for Development-Folate Review. J Nutr. 2015 Jul;145(7):1636S-1680S. doi: 10.3945/jn.114.206599
- Green R. Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr. 2011 Aug;94(2):666S-72S. doi: 10.3945/ajcn.110.009613
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998. Available from: https://www.ncbi.nlm.nih.gov/books/NBK114310
- Stabler SP. Vitamin B12. In: Marriott BP, Birt DF, Stallings VA, Yates AA, eds. Present Knowledge in Nutrition. 11th ed. Washington, DC: Elsevier; 2020:257-71.
- Carmel R. Cobalamin (vitamin B12). In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2014:369-89.
- Allen LH. Vitamin B12. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:812-20.
- Maruvada P, Stover PJ, Mason JB, Bailey RL, Davis CD, Field MS, Finnell RH, Garza C, Green R, Gueant JL, Jacques PF, Klurfeld DM, Lamers Y, MacFarlane AJ, Miller JW, Molloy AM, O’Connor DL, Pfeiffer CM, Potischman NA, Rodricks JV, Rosenberg IH, Ross SA, Shane B, Selhub J, Stabler SP, Trasler J, Yamini S, Zappalà G. Knowledge gaps in understanding the metabolic and clinical effects of excess folates/folic acid: a summary, and perspectives, from an NIH workshop. Am J Clin Nutr. 2020 Nov 11;112(5):1390-1403. doi: 10.1093/ajcn/nqaa259
- Mineva EM, Sternberg MR, Zhang M, Aoki Y, Storandt R, Bailey RL, Pfeiffer CM. Age-specific reference ranges are needed to interpret serum methylmalonic acid concentrations in the US population. Am J Clin Nutr. 2019 Jul 1;110(1):158-168. doi: 10.1093/ajcn/nqz045
- Green R, Allen LH, Bjørke-Monsen AL, Brito A, Guéant JL, Miller JW, Molloy AM, Nexo E, Stabler S, Toh BH, Ueland PM, Yajnik C. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017 Jun 29;3:17040. doi: 10.1038/nrdp.2017.40. Erratum in: Nat Rev Dis Primers. 2017 Jul 20;3:17054.
- Allen LH, Miller JW, de Groot L, Rosenberg IH, Smith AD, Refsum H, Raiten DJ. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J Nutr. 2018 Dec 1;148(suppl_4):1995S-2027S. doi: 10.1093/jn/nxy201
- Langan RC, Goodbred AJ. Vitamin B12 Deficiency: Recognition and Management. Am Fam Physician. 2017 Sep 15;96(6):384-389.
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998. 4, Thiamin. Available from: https://www.ncbi.nlm.nih.gov/books/NBK114331
- Gopinath B, Flood VM, Rochtchina E, Wang JJ, Mitchell P. Homocysteine, folate, vitamin B-12, and 10-y incidence of age-related macular degeneration. The American Journal of Clinical Nutrition. 2013;98(1):129-135. doi:10.3945/ajcn.112.057091
- U.S. Food and Drug Administration. Food Standards: Amendment of Standards of Identity For Enriched Grain Products to Require Addition of Folic Acid. Federal Register 1996;61:8781-97. https://www.govinfo.gov/content/pkg/FR-1996-03-05/pdf/96-5014.pdf
- Choumenkovitch SF, Selhub J, Wilson PW, Rader JI, Rosenberg IH, Jacques PF. Folic acid intake from fortification in United States exceeds predictions. J Nutr. 2002 Sep;132(9):2792-8. doi: 10.1093/jn/132.9.2792
- Centers for Disease Control and Prevention (CDC). CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep. 2010 Aug 13;59(31):980-4.
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011 Mar;3(3):370-84. doi: 10.3390/nu3030370
- Yeung LF, Cogswell ME, Carriquiry AL, Bailey LB, Pfeiffer CM, Berry RJ. Contributions of enriched cereal-grain products, ready-to-eat cereals, and supplements to folic acid and vitamin B-12 usual intake and folate and vitamin B-12 status in US children: National Health and Nutrition Examination Survey (NHANES), 2003-2006. Am J Clin Nutr. 2011 Jan;93(1):172-85. doi: 10.3945/ajcn.2010.30127
- Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014 May;44(5):480-8. doi: 10.3109/00498254.2013.845705
- Henderson AM, Aleliunas RE, Loh SP, Khor GL, Harvey-Leeson S, Glier MB, Kitts DD, Green TJ, Devlin AM. l-5-Methyltetrahydrofolate Supplementation Increases Blood Folate Concentrations to a Greater Extent than Folic Acid Supplementation in Malaysian Women. J Nutr. 2018 Jun 1;148(6):885-890. doi: 10.1093/jn/nxy057
- Ho RC, Cheung MW, Fu E, Win HH, Zaw MH, Ng A, Mak A. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry. 2011 Jul;19(7):607-17. doi: 10.1097/JGP.0b013e3181f17eed
- Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr. 2000 May;71(5 Suppl):1295S-303S. doi: 10.1093/ajcn/71.5.1295s
- Glória L, Cravo M, Camilo ME, Resende M, Cardoso JN, Oliveira AG, Leitão CN, Mira FC. Nutritional deficiencies in chronic alcoholics: relation to dietary intake and alcohol consumption. Am J Gastroenterol. 1997 Mar;92(3):485-9.
- Gibson A, Woodside JV, Young IS, Sharpe PC, Mercer C, Patterson CC, McKinley MC, Kluijtmans LA, Whitehead AS, Evans A. Alcohol increases homocysteine and reduces B vitamin concentration in healthy male volunteers–a randomized, crossover intervention study. QJM. 2008 Nov;101(11):881-7. doi: 10.1093/qjmed/hcn112
- US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FA, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Phillips WR, Phipps MG, Pignone MP, Silverstein M, Tseng CW. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA. 2017 Jan 10;317(2):183-189. doi: 10.1001/jama.2016.19438
- Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, McDowell MA, Yetley EA, Sempos CA, Burt VL, Radimer KL, Picciano MF. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006. Am J Clin Nutr. 2010 Jan;91(1):231-7. doi: 10.3945/ajcn.2009.28427
- Nutrition During Pregnancy. https://www.acog.org/womens-health/faqs/nutrition-during-pregnancy
- Rossi RE, Whyand T, Murray CD, Hamilton MI, Conte D, Caplin ME. The role of dietary supplements in inflammatory bowel disease: a systematic review. Eur J Gastroenterol Hepatol. 2016 Dec;28(12):1357-1364. doi: 10.1097/MEG.0000000000000728
- Greenberg JA, Bell SJ, Guan Y, Yu YH. Folic Acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol. 2011 Summer;4(2):52-9.
- Pietrzik K, Bailey L, Shane B. Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2010 Aug;49(8):535-48. doi: 10.2165/11532990-000000000-00000
- Molloy AM, Pangilinan F, Brody LC. Genetic Risk Factors for Folate-Responsive Neural Tube Defects. Annu Rev Nutr. 2017 Aug 21;37:269-291. doi: 10.1146/annurev-nutr-071714-034235
- Damayanti D, Jaceldo-Siegl K, Beeson WL, Fraser G, Oda K, Haddad EH. Foods and Supplements Associated with Vitamin B12 Biomarkers among Vegetarian and Non-Vegetarian Participants of the Adventist Health Study-2 (AHS-2) Calibration Study. Nutrients. 2018 Jun 4;10(6):722. doi: 10.3390/nu10060722
- Henjum S, Manger M, Hampel D, Brantsæter AL, Shahab-Ferdows S, Bastani NE, Strand TA, Refsum H, Allen LH. Vitamin B12 concentrations in milk from Norwegian women during the six first months of lactation. Eur J Clin Nutr. 2020 May;74(5):749-756. doi: 10.1038/s41430-020-0567-x
- U.S. Food and Drug Adminstration. Code of Federal Regulations Title 21 § 107 – Infant Formula. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=107.100
- Doets EL, In ‘t Veld PH, Szczecińska A, Dhonukshe-Rutten RA, Cavelaars AE, van ‘t Veer P, Brzozowska A, de Groot LC. Systematic review on daily vitamin B12 losses and bioavailability for deriving recommendations on vitamin B12 intake with the factorial approach. Ann Nutr Metab. 2013;62(4):311-22. doi: 10.1159/000346968
- Allen LH. Bioavailability of vitamin B12. Int J Vitam Nutr Res. 2010 Oct;80(4-5):330-5. doi: 10.1024/0300-9831/a000041
- U.S. Department of Agriculture. FoodData Central. https://fdc.nal.usda.gov
- Paul C, Brady DM. Comparative Bioavailability and Utilization of Particular Forms of B12 Supplements With Potential to Mitigate B12-related Genetic Polymorphisms. Integr Med (Encinitas). 2017 Feb;16(1):42-49.
- Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood. 2008 Sep 15;112(6):2214-21. doi: 10.1182/blood-2008-03-040253
- Yazaki Y, Chow G, Mattie M. A single-center, double-blinded, randomized controlled study to evaluate the relative efficacy of sublingual and oral vitamin B-complex administration in reducing total serum homocysteine levels. J Altern Complement Med. 2006 Nov;12(9):881-5. doi: 10.1089/acm.2006.12.881
- Herbert V. Vitamin B-12 in Present Knowledge in Nutrition. 17th ed. Washington, DC: International Life Sciences Institute Press, 1996.
- Combs G. Vitamin B-12 in The Vitamins. New York: Academic Press, Inc., 1992.
- Bernard MA, Nakonezny PA, Kashner TM. The effect of Vitamin B-12 deficiency on older veterans and its relationship to health. J Am Geriatr Soc 1998;46:1199-206. https://www.ncbi.nlm.nih.gov/pubmed/9777900%20?dopt=Abstract
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B-12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press, 1998.
- Healton EB, Savage DG, Brust JC, Garrett TF, Lindenbaum J. Neurological aspects of cobalamin deficiency. Medicine 1991;70:229-44.
- Bottiglieri T. Folate, Vitamin B-12, and neuropsychiatric disorders. Nutr Rev 1996;54:382-90. https://www.ncbi.nlm.nih.gov/pubmed/9155210?dopt=Abstract
- Clarke R. B-vitamins and prevention of dementia. Proc Nutr Soc 2008;67:75-81. https://www.ncbi.nlm.nih.gov/pubmed/18234134?dopt=Abstract
- Monsen ALB, Ueland PM. Homocysteine and methylmalonic acid in diagnosis and risk assessment from infancy to adolescent. Am J Clin Nutr 2003;78:7-21. https://www.ncbi.nlm.nih.gov/pubmed/12816766?dopt=Abstract
- Hunt A, Harrington D, Robinson S. Vitamin B12 deficiency. BMJ. 2014 Sep 4;349:g5226. doi: 10.1136/bmj.g5226
- Hannibal L, Lysne V, Bjørke-Monsen AL, Behringer S, Grünert SC, Spiekerkoetter U, Jacobsen DW, Blom HJ. Biomarkers and Algorithms for the Diagnosis of Vitamin B12 Deficiency. Front Mol Biosci. 2016 Jun 27;3:27. doi: 10.3389/fmolb.2016.00027. Erratum in: Front Mol Biosci. 2017 Aug 08;4:53.
- Bailey RL, Carmel R, Green R, Pfeiffer CM, Cogswell ME, Osterloh JD, Sempos CT, Yetley EA. Monitoring of vitamin B-12 nutritional status in the United States by using plasma methylmalonic acid and serum vitamin B-12. Am J Clin Nutr. 2011 Aug;94(2):552-61. doi: 10.3945/ajcn.111.015222
- Wang H, Li L, Qin LL, Song Y, Vidal-Alaball J, Liu TH. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst Rev. 2018 Mar 15;3(3):CD004655. doi: 10.1002/14651858.CD004655.pub3
- Carmel R. How I treat cobalamin (Vitamin B-12) deficiency. Blood.2008;112:2214-21. https://www.ncbi.nlm.nih.gov/pubmed/18606874
- Vidal-Alaball J, Butler CC, Cannings-John R, Goringe A, Hood K, McCaddon A, et al. Oral Vitamin B-12 versus intramuscular Vitamin B-12 for Vitamin B-12 deficiency. Cochrane Database Syst Rev 2005;(3):CD004655. https://www.ncbi.nlm.nih.gov/pubmed/16034940
- Omega-3 Fatty Acids. https://ods.od.nih.gov/factsheets/Omega3FattyAcids-Consumer
- Harris WS. Omega-3 fatty acids. In: Coates PM, Betz JM, Blackman MR, et al., eds. Encyclopedia of Dietary Supplements. 2nd ed. London and New York: Informa Healthcare; 2010:577-86.
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America NHANES 2017-2018. https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/1718/wweia_2017_2018_data.pdf
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015 Feb 10;(79):1-16.
- Black LI, Clarke TC, Barnes PM, Stussman BJ, Nahin RL. Use of complementary health approaches among children aged 4-17 years in the United States: National Health Interview Survey, 2007-2012. Natl Health Stat Report. 2015 Feb 10;(78):1-19.
- Papanikolaou Y, Brooks J, Reider C, Fulgoni VL 3rd. U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003-2008. Nutr J. 2014 Apr 2;13:31. doi: 10.1186/1475-2891-13-31. Erratum in: Nutr J. 2014;13:64.
- Institute of Medicine, Food and Nutrition Board. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients). Washington, DC: National Academy Press; 2005.
- Jones PJH, Papamandjaris AA. Lipids: cellular metabolism. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:132-48.
- Miller MR, Nichols PD, Carter CG. n-3 Oil sources for use in aquaculture–alternatives to the unsustainable harvest of wild fish. Nutr Res Rev. 2008 Dec;21(2):85-96. doi: 10.1017/S0954422408102414
- Cladis DP, Kleiner AC, Freiser HH, Santerre CR. Fatty acid profiles of commercially available finfish fillets in the United States. Lipids. 2014 Oct;49(10):1005-18. doi: 10.1007/s11745-014-3932-5
- Sprague M, Dick JR, Tocher DR. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006-2015. Sci Rep. 2016 Feb 22;6:21892. doi: 10.1038/srep21892
- Van Elswyk ME, McNeill SH. Impact of grass/forage feeding versus grain finishing on beef nutrients and sensory quality: the U.S. experience. Meat Sci. 2014 Jan;96(1):535-40. doi: 10.1016/j.meatsci.2013.08.010
- Questions & Answers for Consumers Concerning Infant Formula. https://www.fda.gov/food/people-risk-foodborne-illness/questions-answers-consumers-concerning-infant-formula
- National Institutes of Health. Dietary Supplement Label Database. https://dsld.od.nih.gov/dsld/index.jsp
- Advice about Eating Fish. https://www.fda.gov/food/consumers/advice-about-eating-fish
- Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review. https://www.consumerlab.com/reviews/fish-oil-supplements-review/omega3
- Cunningham E. Are krill oil supplements a better source of n-3 fatty acids than fish oil supplements? J Acad Nutr Diet. 2012 Feb;112(2):344. doi: 10.1016/j.jand.2011.12.016
- Davidson MH, Kling D, Maki KC. Novel developments in omega-3 fatty acid-based strategies. Curr Opin Lipidol. 2011 Dec;22(6):437-44. doi: 10.1097/MOL.0b013e32834bd642
- Ulven SM, Holven KB. Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc Health Risk Manag. 2015 Aug 28;11:511-24. doi: 10.2147/VHRM.S85165
- Arterburn LM, Oken HA, Bailey Hall E, Hamersley J, Kuratko CN, Hoffman JP. Algal-oil capsules and cooked salmon: nutritionally equivalent sources of docosahexaenoic acid. J Am Diet Assoc. 2008 Jul;108(7):1204-9. doi: 10.1016/j.jada.2008.04.020
- Christen WG, Schaumberg DA, Glynn RJ, Buring JE. Dietary ω-3 fatty acid and fish intake and incident age-related macular degeneration in women. Arch Ophthalmol. 2011 Jul;129(7):921-9. doi: 10.1001/archophthalmol.2011.34
- SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005 Jan;24(1):87-138. doi: 10.1016/j.preteyeres.2004.06.002
- Augood C, Chakravarthy U, Young I, Vioque J, de Jong PT, Bentham G, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Vingerling JR, Fletcher AE. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr. 2008 Aug;88(2):398-406. doi: 10.1093/ajcn/88.2.398
- Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006 Jul;124(7):995-1001. doi: 10.1001/archopht.124.7.995
- Merle BM, Benlian P, Puche N, Bassols A, Delcourt C, Souied EH; Nutritional AMD Treatment 2 Study Group. Circulating omega-3 Fatty acids and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014 Mar 28;55(3):2010-9. doi: 10.1167/iovs.14-13916
- Sangiovanni, J. P., Agrón, E., Meleth, A. D., Reed, G. F., Sperduto, R. D., Clemons, T. E., Chew, E. Y., & Age-Related Eye Disease Study Research Group (2009). {omega}-3 Long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. The American journal of clinical nutrition, 90(6), 1601–1607. https://doi.org/10.3945/ajcn.2009.27594
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group*. Lutein + Zeaxanthin and Omega-3 Fatty Acids for Age-Related Macular Degeneration: The Age-Related Eye Disease Study 2 (AREDS2) Randomized Clinical Trial. JAMA. 2013;309(19):2005–2015. doi:10.1001/jama.2013.4997
- Lawrenson, J. G., & Evans, J. R. (2015). Omega 3 fatty acids for preventing or slowing the progression of age-related macular degeneration. The Cochrane database of systematic reviews, 2015(4), CD010015. https://doi.org/10.1002/14651858.CD010015.pub3
- Souied EH, Delcourt C, Querques G, Bassols A, Merle B, Zourdani A, Smith T, Benlian P; Nutritional AMD Treatment 2 Study Group. Oral docosahexaenoic acid in the prevention of exudative age-related macular degeneration: the Nutritional AMD Treatment 2 study. Ophthalmology. 2013 Aug;120(8):1619-31. doi: 10.1016/j.ophtha.2013.01.005
- Dry Eye Assessment and Management Study Research Group, Asbell, P. A., Maguire, M. G., Pistilli, M., Ying, G. S., Szczotka-Flynn, L. B., Hardten, D. R., Lin, M. C., & Shtein, R. M. (2018). n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. The New England journal of medicine, 378(18), 1681–1690. https://doi.org/10.1056/NEJMoa1709691
- Ziemanski, J. F., Wolters, L. R., Jones-Jordan, L., Nichols, J. J., & Nichols, K. K. (2018). Relation Between Dietary Essential Fatty Acid Intake and Dry Eye Disease and Meibomian Gland Dysfunction in Postmenopausal Women. American journal of ophthalmology, 189, 29–40. https://doi.org/10.1016/j.ajo.2018.01.004
- Miljanović, B., Trivedi, K. A., Dana, M. R., Gilbard, J. P., Buring, J. E., & Schaumberg, D. A. (2005). Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. The American journal of clinical nutrition, 82(4), 887–893. https://doi.org/10.1093/ajcn/82.4.887
- Hom MM, Asbell P, Barry B. Omegas and Dry Eye: More Knowledge, More Questions. Optom Vis Sci. 2015 Sep;92(9):948-56. doi: 10.1097/OPX.0000000000000655
- Bhargava, R., Kumar, P., Kumar, M., Mehra, N., & Mishra, A. (2013). A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. International journal of ophthalmology, 6(6), 811–816. https://doi.org/10.3980/j.issn.2222-3959.2013.06.13
- Epitropoulos, A. T., Donnenfeld, E. D., Shah, Z. A., Holland, E. J., Gross, M., Faulkner, W. J., Matossian, C., Lane, S. S., Toyos, M., Bucci, F. A., Jr, & Perry, H. D. (2016). Effect of Oral Re-esterified Omega-3 Nutritional Supplementation on Dry Eyes. Cornea, 35(9), 1185–1191. https://doi.org/10.1097/ICO.0000000000000940
- Vision Loss in Older Adults. Am Fam Physician. 2016 Aug 1;94(3):219-226. https://www.aafp.org/afp/2016/0801/p219.html
- Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564-72.
- Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004;122:477-85.
- Kahn HA, Leibowitz HM, Ganley JP, et al. The Framingham Eye Study. I. Outline and major prevalence findings. Am J Epidemiol 1977;106:17-32.
- Ferris FL III, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol 1984;102:1640-2.
- Age-Related Macular Degeneration PPP – Updated 2015. https://www.aao.org/preferred-practice-pattern/age-related-macular-degeneration-ppp-2015
- InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. Age-related macular degeneration (AMD): Do dietary supplements prevent AMD? 2015 Jul 29 [Updated 2018 May 3]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK315802
- What Is Dry Eye? https://www.aao.org/eye-health/diseases/what-is-dry-eye