What is beta glucan
Beta-glucan (β-glucan) or beta-(1,3)-d-glucan, is a naturally occurring soluble dietary fiber or glucose polymer (polysaccharide). Beta-glucan is frequently used in medicine, the cosmetics, and the food industry. Beta-glucans are produced by a variety of plants, such as oat, barley, and seaweed. In cereals, particularly, beta-glucans are presented as linear polysaccharides, in which glucose monomers are bound by β-(1,3) and β-(1,4) linkages and are mainly found in barley, oats, rye, and wheat, generally in amounts of about 7%, 5%, 2%, and less than 1% respectively 1. This structural organization confers water solubility to beta-glucans, which are therefore classified as soluble fibers 2. Recent studies suggest dietary fiber is linked with a lower risk of some types of cancer, especially colorectal cancer. But it is not clear whether it is the fiber or another component of high-fiber foods that is responsible for the link. These findings are one of the reasons that the health experts recommend eating high-fiber foods such as whole grains, vegetables, and fruits to help reduce cancer risk, but does not expressly recommend the use of fiber supplements. Beta-glucans are also recognized as having other important positive health benefits centered around their benefits in coronary heart disease, cholesterol lowering 3 and reducing the glycemic response 4. Such health benefits are linked to its high viscosity although it may be that some of these effects are due to appetite suppression. Beta-glucans might slow down the time it takes for food to travel through your digestive system, making you feel fuller. Beta-glucans seem to be safe (at up to 10 grams [g] a day for 12 weeks). Beta-glucans can cause flatulence.
Beta glucan is also a constituent of the cell wall of certain pathogenic bacteria (Pneumocystis carinii, Cryptococcus neoformans, Aspergillus fumigatus, Histoplasma capsulatum, Candida albicans) and fungi (Saccharomyces cerevisiae) 5. Beta-glucan has been purified from brewer’s and backer’s yeast 6, from oats and barley bran 7 or seaweeds 8. Beta-glucan has also been isolated from some mushrooms as shiitake (Lentinus edodes), maitake (Grifola frondosa) 9, schizophylan (Schizophillum commune), and SSG (Sclerotinia sclerotiorum) 10. Most beta-glucans are considered as non-digestible carbohydrates and are fermented to various degrees by the intestinal microbial flora 11. Beta-glucans aggregate in solution 12 to form thermoreversible infinite network gels. The primary use of beta-glucans is in texturizing as fat substitutes. Barley beta-glucan is highly viscous and pseudoplastic, both properties decreasing with increasing temperature 13. These properties cause difficulty in the brewing industry, negatively affecting fermentation and filtration.
Modern biomedical research has identified beta-glucans as biological response modifiers with anti-tumor properties that elicit potent immune responses through their recognition by a variety of pattern recognition receptors on dendritic cells, macrophages and neutrophils 14. Complement receptor-3 (CR3), lactosylceramides, scavenger receptors and dectin-1 are involved in beta-glucan recognition, triggering a series of signaling events that modulate innate and subsequently adaptive immune responses. Beta-glucan binding to specific receptors in dendritic cells and macrophages triggers their activation and maturation, increases their antigen-presentation ability and enhances the production of proinflammatory cytokines that stimulate the polarization of TH1 or TH17 responses, and induces the activation of antigen-specific CD8+ cytotoxic T lymphocytes. Moreover, large dendritic cellss can be degraded by macrophages into smaller moieties, when released, prime CR3 receptor on neutrophils and natural killer (NK) cells mediating CR3-dependent cellular cytotoxicity (CR3-DCC) of iC3b opsonized tumor cells. Elucidating the molecular mechanisms of beta-glucan-induced signaling in immune cells is essential for the design of new therapeutic strategies against cancer. Future studies should be done to translate beta-glucan research to the clinic.
Physical and chemical properties of beta-glucan
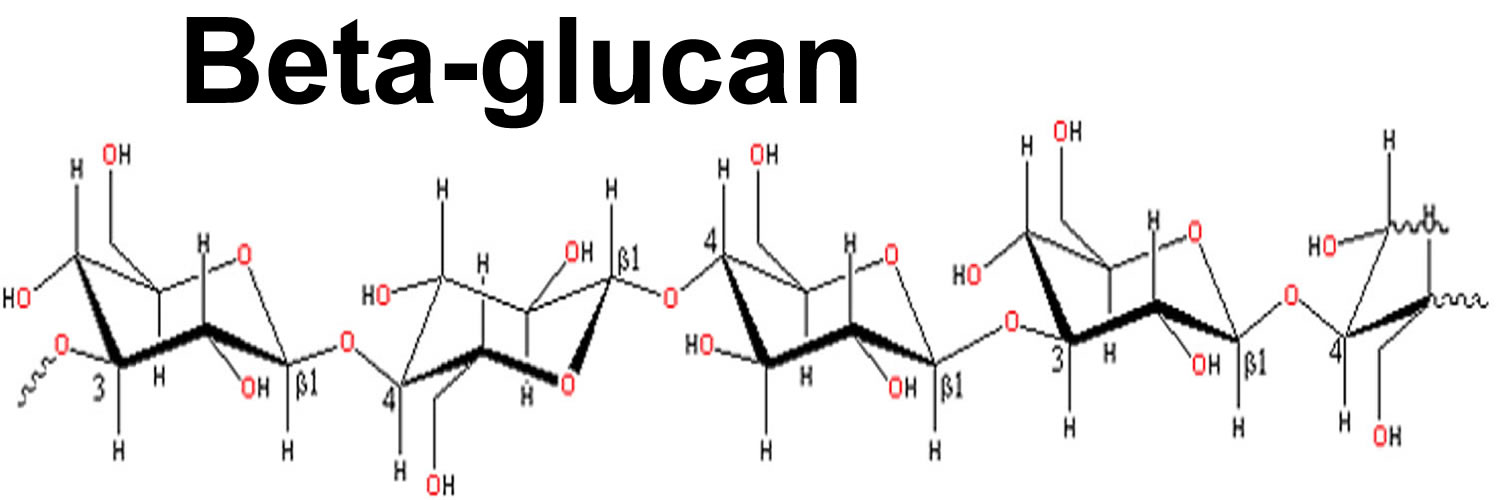
Beta-glucans are one of the most abundant forms of polysaccharides found inside the cell wall of bacteria and fungus. All beta-glucans share a common structure consisting of glucose polymers backbone of β(1,3)-linked β-d-glucopyranosyl units and they differ from each other by their length and branching structures (Figure 1) 15. Beta-glucans form ‘worm’-like cylindrical molecules containing up to about 250,000 glucose residues that may produce cross-links between regular areas containing consecutive cellotriose units 16. The branches derived from the glycosidic chain core are highly variable and the 2 main groups of branching are 1→4 or 1→6 glycosidic chains. These branching assignments appear to be species specific, for example, beta-glucans of fungus, that represent the most abundant polysaccharides found in the cell wall of fungi, have β(1,6)-linked branches coming off of the β(1,3) backbone, whereas those of bacteria have 1→4 side branches. The alignments of branching follow a particular ratio and branches can arise from branches (secondary branches). The structural diversity also depends on the fungal source 17. For example, beta-glucans of mushrooms have short β(1,6)-linked branches whereas those of yeast have β(1,6)-side branches with additional β(1,3) regions 18. Oat and barley beta glucans are primarily linear with large regions of b(1,4) linkages separating shorter stretches of b(1,3) structures 5. These structural differences can have large implications for the activity of the beta-glucan 5. For example, differences in the length of the polysaccharide chain, extent of branching, and the length of those branches can result in the difference between material extractable by hot water, as mushroom beta-glucans, and insoluble cell wall component, as yeast beta-glucan, and in different molecular weight. Of note, these structural differences may influence the immunogenic properties of beta-glucans and many studies have suggested that a higher degree of structural complexity is associated with enhanced beta-glucans-induced antimicrobial and anticancer activities 19. In general, test tube studies have suggested that large molecular weight or particular beta-glucans (such as zymosan) can directly activate leukocytes, stimulating their phagocytic, cytotoxic, and antimicrobial activities, including the production of reactive oxygen and nitrogen intermediates. Intermediate or low molecular weight beta-glucans (such as glucan phosphate) possess biological activity in animal study, but their cellular effects are less clear. Very short beta-glucans (<5000–10 000 molecular weight; such as laminarin) are generally considered inactive 20.
In aqueous solution, beta-glucans undergo conformational change into triple helix, single helix or random coils. The immune functions of beta-glucans are apparently dependent on their conformational complexity 21. It has been suggested that higher degree of structural complexity is associated with more potent immunmodulatory and anti-cancer effects 22.
Figure 1. Beta-glucan structure
 Footnote: Beta-glucan is one of the key components of the fungal cell wall. The basic subunit of the fungal beta-glucan is β-D-glucose linked to one another by 1→3 glycosidic chain (the b(1,3)-linked b-D-glucopyranosyl backbone) with 1→6 glycosidic side branches of varying distribution and length. The length and branches of the beta-glucan from various fungi are widely different.
[Source 22]
Footnote: Beta-glucan is one of the key components of the fungal cell wall. The basic subunit of the fungal beta-glucan is β-D-glucose linked to one another by 1→3 glycosidic chain (the b(1,3)-linked b-D-glucopyranosyl backbone) with 1→6 glycosidic side branches of varying distribution and length. The length and branches of the beta-glucan from various fungi are widely different.
[Source 22]Beta glucan benefits
It has been common knowledge in the scientific community that beta glucan is the most known powerful immune stimulant and a very powerful antagonist to both benign and malignant tumors; beta glucan lowers cholesterol and triglyceride level, normalizes blood sugar level, heals and rejuvenates the skin and has various other benefits 5.
Consumption of foods containing cereal beta-glucan have been shown to lower blood total and LDL “bad” cholesterol concentrations and this effect is related to a reduced risk of coronary heart disease 3. The evidence for these beneficial effects of beta-glucan in oats has been reviewed and accepted by the Food and Drug Administration (FDA) 23 and, later by the European Food Safety Authority (EFSA) 24, as a valid health claim. However, the mechanisms involved in the lowering of blood cholesterol remain unclear. The observed positive effects on blood cholesterol levels are thought to be mainly related to the physicochemical properties of the oat beta-glucan on a molecular scale, specifically its weight-average molecular weight (MW), solubility and the subsequent viscosity it generates 25.
The most documented mechanism seems to be the ability of beta-glucan to interfere with the recycling of bile salts and the impact on cholesterol metabolism 26. However, the physiology of cholesterol metabolism is complex and is likely to be also related to lipid metabolism 27. Since cholesterol and lipids are both hydrophobic, their digestion and absorption are interconnected. For instance, cholesterol and the products of lipolysis are incorporated together into bile salts mixed micelles before absorption by the enterocytes. The proportion of cholesterol present in the micelles is influenced by the lipid composition 28. However, this interconnectivity is not always observed in animal studies, certainly not when investigating the effect of water-soluble forms of dietary fiber, such as beta-glucan, on blood triacylglyceride and cholesterol concentrations 29. There is little detail available about the processes by which beta-glucan may directly affect lipid digestion 30. However, evidence suggests that water-soluble fibers may interfere with lipid emulsification 31 or lipase activity 32, and thus alter bile salt and cholesterol homeostasis.
While the effects of processing on the physicochemical properties of individual oat constituents, notably beta-glucan, have been investigated and shown to influence the extent of their bioactivity 33, the impact of processing of oat groats, including cooking, on lipid digestion and absorption has received little attention. Moreover, processing may also affect the structural characteristics of the cereal matrix on a macroscale, which can have an impact during digestion. The amount of beta-glucan released is dependent on the oat matrix, and a higher level of structural disintegration (flakes vs flour) resulted in an elevated beta-glucan solubilisation, whereas hydrothermal treatment decreased the polymer dissolution.
Beta-glucan effect on cancer
Current data suggests that beta-glucans are potent immunomodulators with effects on both innate and adaptive immunity 22. The immunomodulatory and anti-cancer properties of beta-glucans result from their structure, molecular weight, degree of branching, conformation and its behavior in gastrointestinal tract 34. Moreover, these properties depend on an isolation procedure of beta-glucan.
In a solubilized form, beta-glucan binds to a lectin site within complement receptor 3 (CR3) on leukocytes, priming the receptor to trigger cytotoxic degranulation of leukocytes when leukocyte CR3 binds to complement 3 (iC3b)-coated tumors. Thus, the attachment of beta-glucan to CR3 of circulating leukocytes simulates leukocytes to kill iC3b-coated tumor cells in the same way as they kill iC3b-coated yeast.
The mechanisms by which beta-glucan can destroy cancer cells are very complex and still not fully understood. Some results suggest that immunomodulatory and anti-cancer features of beta-glucans is a consequence from their structure, molecular weight, degree of branching and conformation 35. At present most investigation has been focusing on cereals’ extract due to its good water-solubility. It is commonly known that beta-glucan is useful in adjuvant or supplementation therapy but not as a standard recurrent cancer treatment. Management of the standard cancer treatment protocol is still required 36.
Even though there is no complete evidence that beta-glucan can be effectively used as anti-cancer factors, there are many interesting investigations confirming its usefulness to affect cancer cells in vitro and in vivo 37. Recent data showed that the high molecular weight beta-glucan similar to low didn’t affect the normal human keratinocytes in concentration 50 to 200 μg/ml 35.
Figure 2. Beta-glucan in health and disease – beta-glucan effects on the Mononuclear Phagocyte System
Footnote: Model presenting the different consequences of beta-glucan recognition by mononuclear phagocytes in the context of anti-cancer activities, fungal infection recognitions, or trained immunity.
Abbreviations: DCs = dendritic cells; M2 = alternative macrophages; TAM = tumor-associated macrophages; M1 = classical macrophages.
[Source 38]In both tested lung cancer cell line scientists observed the proportional decrease of cell viability after incubation with high molecular weight beta-glucan 35. The low molecular weight beta-glucan causes only a slight cytotoxicity in these cells. The research of Hong et al. 39 is concentrated on the antitumor effect of glucan from microorganisms (Paenibacillus polymyxa JB115) on four different cancer lines (A549, Hela, Hep3B, Sarcoma 180). Significant cytotoxicity was observed in Hela and Sarcoma 180 cells. The cytotoxicity of beta-glucan was confirmed by Kim et al. 40. They studied colon cancer cells and postulated that viability of cancer cells is dependent on the applied dose of beta-glucan. They tested with MTT assay usage indicated that 200 μg/ml dose caused decreasing of viability of cancer cells about 50% 40. Other studies showed in contrast the inhibition rate of beta-(1–3) glucan isolated from Poria cocos mycelia on Sarcoma 180 as less than 10% 41. Moreover Zhang et al. 42 used water-soluble beta-glucan including mainly 1 → 3 and 1 → 4 linkages obtained from the mycelia of Poria cocos (PCM3-II). The dose effect of PCM3-II on MCF-7 cell line was studied by incubating these cells with 12.5–400 μg/ml of the glucan for 72 h. In this case the MTT examination showed that PCM3-II reduced proliferation and viability of the MCF-7 cells dose-dependently, so that the cancer-cell growth was reduced by 50% of the control level at 400 μg/ml of the beta-glucan 42.
While animal and test tube studies seem promising, evidences for a human clinical application of the beta-glucan are currently limited and does not fully support their recommendation 38. Most of the available clinical trials come from Eastern Countries and focused on the potential application of mushrooms in cancer therapy 38. A prospective clinical trial in patients with advanced breast cancer shows that administration of 10 mg capsules of soluble beta-glucan from Saccharomyces cerevisiae (brewer’s yeast) induces the proliferation and activation of peripheral blood monocytes with no clinical side effects 43. However, whether this can be clinically beneficial remains undisclosed. By contrast, the antimicrobial effect of beta-glucan has been poorly investigated by clinical trials and results remain controversial. A phase I and II trials show that treatment with PGG-glucan reduces infection rates in high-risk surgery patients. However, while PGG-glucan administration lead to a reduction of serious infections and death, an increased incidence of adverse events is observed in patients receiving beta-glucan treatment and phase III trial was terminated 44.
Summary
Several experimental evidences have demonstrated a crucial role for beta-glucan in the host–pathogen interaction during infections. Moreover, considerable efforts have been made to understand the cellular and molecular mechanisms of action of beta-glucan in fungal pathogenesis as well as how it promotes a phagocytic-mediated immune response. Similarly, administration of fungal beta-glucan is well known to stimulate the immune system and boost resistance to various infectious diseases and cancers, highlighting the multifaceted role of this molecule (Figure 2). However, although many in vivo studies have shown a beneficial effect of the beta-glucans isolated from different sources, a comprehensive investigation of the mechanism of action is still lacking. In addition, the absence of detailed methodology on experimentation, beta-glucan molecules source and purity reached render interpretation of the various results very complex 38. As such, discrepancies observed in the different studies are mainly related to the choice of purified components being used. In addition, unfortunately only few human studies are available and most of them have not been followed up with success 38. Hence, the possibility for clinical application of beta-glucan should be considered with caution and will require further investigation 38.
References- Izydorczyk M, Dexter J. Barley β-glucans and arabinoxylans: Molecular structure, physicochemical properties, and uses in food products–a Review. Food Res Int. 2008;41(9):850–68.
- Vasanthan T, Temelli F. Grain fractionation technologies for cereal beta-glucan concentration. Food Res Int. 2008;41(9):876–81
- Othman R.A., Moghadasian M.H., Jones P.J. Cholesterol-lowering effects of oat beta-glucan. Nutrition Reviews. 2011;69(6):299–309. https://www.ncbi.nlm.nih.gov/pubmed/21631511
- Grundy MML, Quint J, Rieder A, et al. The impact of oat structure and β-glucan on in vitro lipid digestion. Journal of Functional Foods. 2017;38(Pt A):378-388. doi:10.1016/j.jff.2017.09.011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5666125/
- Effects of beta-glucans on the immune system. Medicina (Kaunas). 2007;43(8):597-606. medicina.lsmuni.lt/med/0708/0708-01e.pdf
- Tokunaka K, Ohno N, Adachi Y, Tanaka S, Tamura H, Yadomae T. Immunopharmacological and immunotoxicological activities of a water-soluble (1–>3)-beta-D-glucan, CSBG from Candida spp. Int J Immunopharmacol 2000;22:383-94.
- Baur SK, Geisler G. Variability of beta-glucan content in oat caryopsis of 132 cultivated oat genotypes and 39 wild oat genotypes. J Agr Crop Sci 1996;176:151-7.
- Sawai M, Adachi Y, Kanai M, Matsui S, Yadomae T. Extraction of conformantionally stable (1-6)-branched (1-3)-β-D-glucans from premixed edible mushroom powders by cold alkaline solution. Int J Med Mushr. 2002;4:197–205. doi: 10.1615/IntJMedMushr.v4.i3.20
- Mizuno T, Saito H, Nishitoba T, Kawagishi H. Antitumoractive substances from mushrooms. Food Rev Int 1995;11:23-6.
- Brochers AT, Stern JS, Hackman RM, Keen LC, Gershwin ME. Mushrooms, tumors and immunity. Proc Soc Exp Biol Med 1999;221:281-93.
- A curve fitting approach to estimate the extent of fermentation of indigestible carbohydrates. Wang H, Weening D, Jonkers E, Boer T, Stellaard F, Small AC, Preston T, Vonk RJ, Priebe MG. Eur J Clin Invest. 2008 Nov; 38(11):863-8.
- W. Li, S. W. Cui, Q. Wang and R. Y. Yada, Studies of aggregation behaviours of cereal β-glucans in dilute aqueous solutions by light scattering: Part I. Structure effects, Food Hydrocolloids, 25 (2011) 189-195.
- Z. Burkus and F. Temelli, Rheological properties of barley β-glucan, Carbohydrate Polymers, 59 (2005) 459-465.
- The Effects of β -Glucans on Dendritic Cells and Implications for Cancer Therapy. Anti-cancer agents VOLUME: 13; ISSUE: 5, 2013, page 689 – 698. http://www.eurekaselect.com/110381/article
- Stone BA, Clarke AE. Chemistry and biology of (1,3)-D-glucans. Victoria, Australia.: La Trobe University Press; 1992.
- β-Glucan. http://www1.lsbu.ac.uk/water/glucan.html
- Synytsya A, Novak M. Structural diversity of fungal glucans. Carbohydr Polym (2013) 92:792–809.10.1016/j.carbpol.2012.09.077
- Borchers AT, Keen CL, Gershwin ME. Mushrooms, tumors, and immunity: an update. Exp Biol Med (Maywood) (2004) 229:393–406.10.1177/153537020422900507
- Chen J, Seviour R. Medicinal importance of fungal beta-(1 – >3), (1 – >6)-glucans. Mycol Res (2007) 111:635–52.10.1016/j.mycres.2007.02.011
- Brown GD, Gordon S. Fungal b-glucans and mammalian immunity. Immunity 2003;19:311-15.
- Bohn J, BeMiller J. (1->3)-β-Glucans as biological response modifiers: a review of structure-functional activity relationships. Carbohydrate Polymers. 1995;28:3–14. doi: 10.1016/0144-8617(95)00076-3
- Chan GC-F, Chan WK, Sze DM-Y. The effects of β-glucan on human immune and cancer cells. Journal of Hematology & Oncology. 2009;2:25. doi:10.1186/1756-8722-2-25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2704234/
- US Food and Drug Administration FDA final rule for federal labeling: health claims; oats and coronary heart disease. Federal Register. 1997;62:3584–3681
- Efsa, NDA Panel Scientific opinion on the substantiation of health claims related to beta-glucans and maintenance of normal blood cholesterol concentrations (ID 754, 755, 757, 801, 1465, 2934) and maintenance or achievement of a normal body weight (ID 820, 823) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA Journal. 2009;7:1254–1271
- Wolever T.M.S., Tosh S.M., Gibbs A.L., Brand-Miller J., Duncan A.M., Hart V., …Wood P.J. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. American Journal of Clinical Nutrition. 2010;92(4):723–732
- Gunness P., Flanagan B.M., Mata J.P., Gilbert E.P., Gidley M.J. Molecular interactions of a model bile salt and porcine bile with (1,3:1,4)-beta-glucans and arabinoxylans probed by 13C NMR and SAXS. Food Chemistry. 2016;197(Pt A):676–685.
- Carey M.C., Hernell O. Digestion and absorption of fat. Seminars in Gastrointestinal Disease. 1992;3(4):189–208.
- Vinarov Z., Petrova L., Tcholakova S., Denkov N.D., Stoyanov S.D., Lips A. In vitro study of triglyceride lipolysis and phase distribution of the reaction products and cholesterol: effects of calcium and bicarbonate. Food and Function. 2012;3:1206–1220.
- Whitehead A., Beck E.J., Tosh S., Wolever T.M. Cholesterol-lowering effects of oat beta-glucan: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition. 2014;100(6):1413–1421
- Lairon D., Play B., Jourdheuil-Rahmani D. Digestible and indigestible carbohydrates: interactions with postprandial lipid metabolism. Journal of Nutrition. 2007;18(4):217–227
- Zhai H., Gunness P., Gidley M.J. Effects of cereal soluble dietary fibres on hydrolysis of p-nitrophenyl laurate by pancreatin. Food & Function. 2016;7(8):3382–3389
- Wilcox M.D., Brownlee I.A., Richardson J.C., Dettmar P.W., Pearson J.P. The modulation of pancreatic lipase activity by alginates. Food Chemistry. 2014;146:479–484
- Tosh S.M., Chu Y. Systematic review of the effect of processing of whole-grain oat cereals on glycaemic response. British Journal of Nutrition. 2015;114(8):1256–1262.
- The effects of beta-glucan on human immune and cancer cells. Chan GC, Chan WK, Sze DM. J Hematol Oncol. 2009 Jun 10; 2():25.
- Choromanska A, Kulbacka J, Harasym J, Oledzki R, Szewczyk A, Saczko J. High- and low-Molecular Weight oat Beta-Glucan Reveals Antitumor Activity in Human Epithelial Lung Cancer. Pathology Oncology Research. 2018;24(3):583-592. doi:10.1007/s12253-017-0278-3. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5972159/
- Daou C, Zhang H. Oat Beta-glucan: its role in health promotion and prevention of diseases. Compr Rev Food Sci F. 2012;11:355–365. doi: 10.1111/j.1541-4337.2012.00189.x
- Kobayashi H, Yoshida R, Kanada Y, et al. Suppressing effects of daily oral supplementation of beta-glucan extracted from Agaricus blazei Murill on spontaneous and peritoneal disseminated metastasis in mouse model. J of Cancer Res and Clinical Oncol. 2005;131:527–538. doi: 10.1007/s00432-005-0672-1
- Camilli G, Tabouret G, Quintin J. The Complexity of Fungal β-Glucan in Health and Disease: Effects on the Mononuclear Phagocyte System. Frontiers in Immunology. 2018;9:673. doi:10.3389/fimmu.2018.00673. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5932370/
- Hong JH, Jung HK. Antioxidant and antitumor activities of β-glucanrich. Exopolysaccharides with different molecular weight from Paenibacillus polymyxa JB115. Korean Soc Appl Biol Chem. 2014;57:105–112. doi: 10.1007/s13765-013-4252-9.
- Kim MJ, Hong SY, Kim SK, Cheong CH, Park HJ, Chun HK, Jang KH, Yoon BD, Kim CH, Kang SA. Β-glucan enhanced apoptosis in human colon cancer cells SNU-C4. Nutrion Res an Practice. 2009;3:180–184. doi: 10.4162/nrp.2009.3.3.180
- Huang Q, Zhang L, Cheung PCK, Tan X. Evaluation of sulfated α-glucans from Poria Cocos mycelia as potential antitumor agent. Carbohydr Polym. 2006;64:337–344. doi: 10.1016/j.carbpol.2005.12.001.
- Zhang M, Chiu LC, Cheung PC, Ooi VE. Growth-inhibitory effects of a β-glukan from the mycelium Poria Cocos on human breast adenocarcinoma MCF-7-cells. Oncol Rep. 2006;15:637–643
- Demir G, Klein HO, Mandel-Molinas N, Tuzuner N. Beta glucan induces proliferation and activation of monocytes in peripheral blood of patients with advanced breast cancer. Int Immunopharmacol (2007) 7:113–6.10.1016/j.intimp.2006.08.011 https://www.ncbi.nlm.nih.gov/pubmed/17161824
- Dellinger EP, Babineau TJ, Bleicher P, Kaiser AB, Seibert GB, Postier RG, et al. Effect of PGG-glucan on the rate of serious postoperative infection or death observed after high-risk gastrointestinal operations. Betafectin Gastrointestinal Study Group. Arch Surg (1999) 134:977–83.10.1001/archsurg.134.9.977