CAMP test
CAMP test is used for the presumptive identification of Group B Streptococcus (group B strep) or Streptococcus agalactiae and Listeria monocytogenes 1. CAMP test is used to distinguish the species Streptococcus agalactiae from other species of beta-hemolytic Streptococcus 2. Streptococcus agalactiae also known as group B strep, is a bacterium that commonly cause of severe infections in newborns during the first week of life. More recently, experts recognized the increasing impact invasive group B Streptococcus disease has on adults. Streptococcus agalactiae is the only beta-hemolytic Streptococcus which yields a positive CAMP test.
CAMP test is an acronym for the authors of this test , Christie, Atkins, and Munch-Peterson, who described it in 1944.
Uses of CAMP test
- CAMP test is used to distinguish the species Streptococcus agalactiae from other species of beta-hemolytic Streptococcus.
- CAMP test is used to identify Listeria monocytogenes which also produces a positive CAMP reaction.
Procedure of CAMP test
- Streak a beta-lysin–producing strain of aureus down the center of a sheep blood agar plate.
- The streptococcal streak should be 3 to 4 cm long.
- Streak test organisms across the plate perpendicular to the aureus streak within 2 mm. (Multiple organisms can be tested on a single plate).
- Incubate at 35°-37°C in ambient air for 18-24 hours.
- Group B streptococci and a few other beta-streptococci produce an enhancement of the beta-lysin activity of the aureus strain.
CAMP test limitation
- A small percentage of group A streptococci may have a positive CAMP reaction.
- Some Group A Streptococcal will be CAMP test positive if the plate is incubated in a candle jar in an atmosphere or under anaerobic conditions. Therefore, ambient air incubation should be done.
- CAMP test should be limited to colonies with the characteristic group B streptococci morphology and narrow zone beta-hemolysis on sheep blood agar.
- Extended incubation times or elevated incubation temperatures may give false-positive results.
- Sheep blood agar plates are only used. Human, horse, rabbit, or guinea pig blood plates will not give a proper reaction.
- Colonies of Listeria monocytogenes have a narrow zone of beta-hemolysis on sheep blood agar and may be confused with group B beta-hemolytic streptococci, if catalase and gram stain are not performed.
CAMP test principle
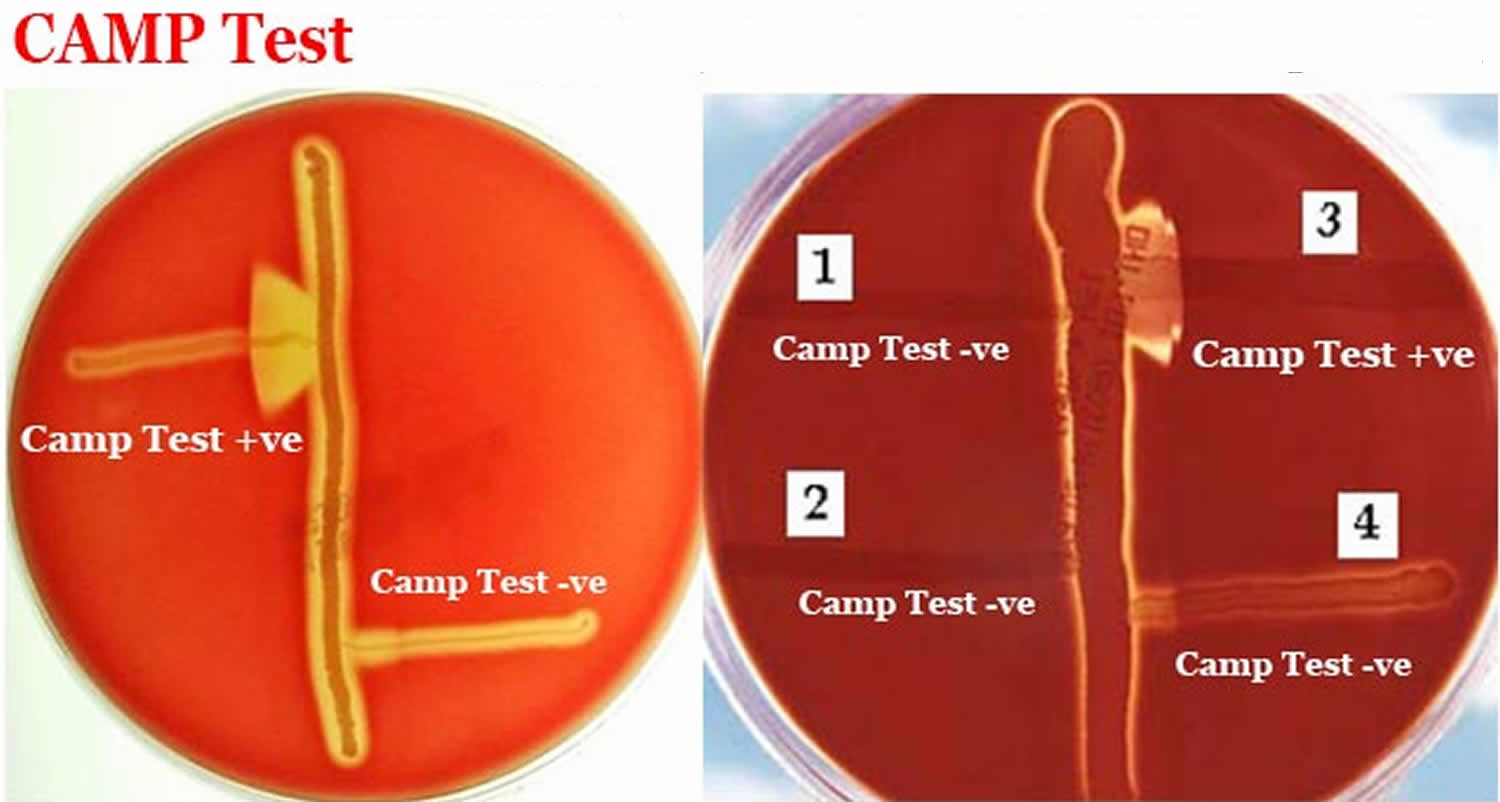
CAMP test detects the CAMP factor, which is a diffusible, heat-stable, extracellular protein produced by Group B Streptococcus that acts synergistically with the beta-lysin of Staphylococcus aureus to cause enhanced lysis of sheep or bovine red blood cells but not human, rabbit or horse red blood cells. A known hemolytic strain of Staphylococcus aureus (ATCC 25923) is streaked in a straight line across the center of the sheep blood agar plate. Test inoculum is streaked in a straight line (2-3 cms in length) perpendicular to Staphylococcus aureus streak but without touching it. A known Group B Streptococcus may also be streaked similarly as a positive control. Four-five test organisms may be tested per plate. The plate is incubated at 37 °C for 18-24 hours. A positive test for CAMP factor appears as “arrowhead” hemolysis between the junction of growth of Staphylococcus aureus and Group B Streptococcus. There is no enhanced or “arrowhead” hemolysis if the test isolate is not Group B Streptococcus.
The hemolytic activity of the beta-hemolysin produced by most strains of Staphylococcus aureus is enhanced by extracellular protein (CAMP factor) produced by group B streptococci. Interaction of the beta-hemolysin with this factor causes “synergistic hemolysis,” which is easily observed on a blood agar plate. This phenomenon is seen with both hemolytic and non-hemolytic isolates of group B streptococci.
A similar test has been described for Listeria ivanovii, where an “arrowhead” hemolysis occurs appear between streaks of Listeria ivanovii and Rhodococcus equi.
CAMP test results
- CAMP test Positive: Enhanced hemolysis is indicated by an arrow head-shaped zone of beta-hemolysis at the junction of the two organisms.
- CAMP test Negative: No enhancement of hemolysis.
Quality control for CAMP test
- CAMP test positive: Streptococcus agalactiae (ATCC13813)—enhanced arrowhead hemolysis.
- CAMP test negative: Streptococcus pyogenes (ATCC19615)—beta-hemolysis without enhanced arrowhead formation.
Figure 1. CAMP test results
Reverse CAMP test
Reverse CAMP test can be used for differentiation of Clostridium perfringens from other Clostridium species. Here, a CAMP test positive Group B Streptococcus is streaked in the center of sheep blood agar, and Clostridium perfringens is streaked perpendicular to it. Following incubation at 37oC for 24-48 hours in anaerobic conditions, an “arrowhead” hemolysis is seen between growth of Clostridium perfringens and Group B Streptococcus. This is because of alpha toxin produced by Clostridium perfringens interacts with CAMP factor and produce synergistic hemolysis.
References