What is a spleen
The spleen is the largest lymphoid organ and has a crucial function in the immune system. The spleen is responsible for the production and maturation of IgM, B lymphocytes, and opsonins 1. The spleen most importantly protects against infections from polysaccharide-encapsulated bacteria including Streptococcus pneumoniae, Haemophilus influenzae type B, Neisseria meningitidis, Escherichia coli, Salmonella, Klebsiella, and group B Streptococci (SHiNE SKiS) 1. The spleen also acts as the primary reservoir for platelets and as a filter for red blood cells (RBCs), removing damaged or malformed red blood cells from the circulation. In addition, the spleen performs extramedullary hematopoiesis.
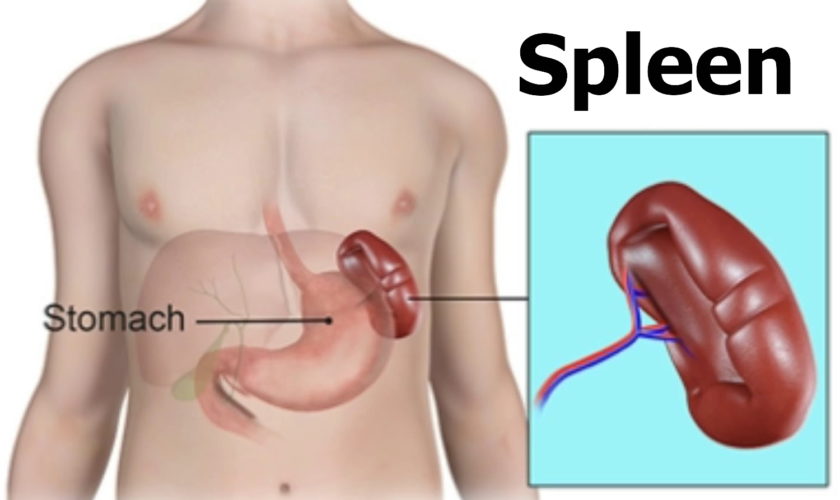
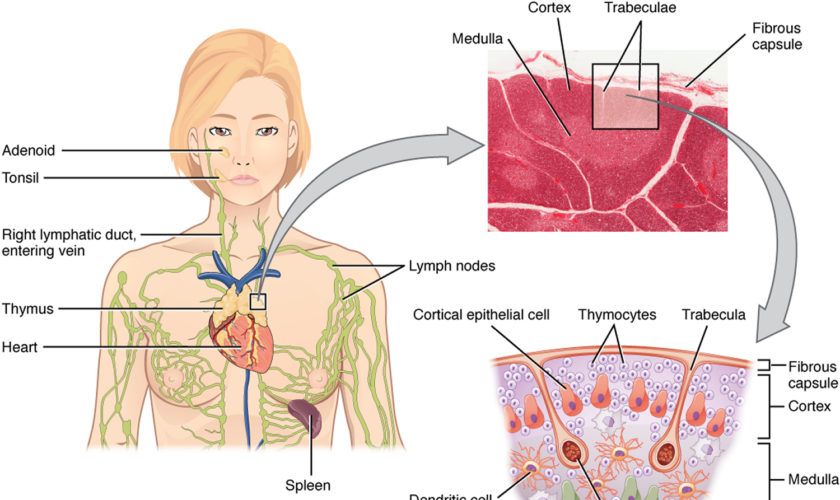
The normal position of the spleen is within the peritoneal cavity in the left upper quadrant of the abdominal cavity adjacent to ribs nine through 12, just beneath the left diaphragm. The spleen lies behind and to the left side of your stomach (see Figure 1). The normal sized spleen abuts the stomach, colon, and left kidney The spleen resembles a large lymph node and is subdivided into lobules (see Figure 3). However, unlike the lymphatic sinuses of a lymph node, the spaces in the spleen, called venous sinuses, are filled with blood instead of lymph.
A normal spleen ranges in length from 6 to 13 cm and in weight from 75 to 200 g 2. The spleen is not normally palpable except in slender young adults 2. When the spleen can be felt below the left costal (rib) margin, at rest or on inspiration, spleen enlargement should be assumed and the explanation sought. Although the normal-size or even the abnormally small, spleen can be involved in pathologic processes, with the exception of rubs associated with splenic infarcts, physical examination is generally not helpful in identifying the problem. Nevertheless, the enlarged and palpable spleen is an important clue to the presence of a variety of illnesses 2.
Figure 1. Spleen location
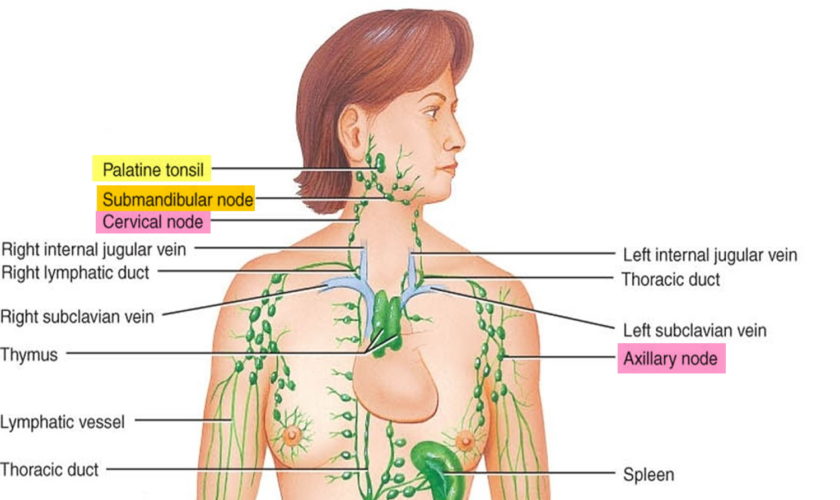
Figure 2. Spleen and the lymphatic system
What does the spleen do ?
The spleen plays a significant role in hematopoiesis and immunosurveillance. The major functions of the spleen include clearance of abnormal erythrocytes, removal of microorganisms and antigens as well as the synthesis of immunoglobulin G (IgG). The spleen also synthesizes the immune system peptides properdin and tuftsin 3. Also, approximately one-third of circulating platelets are stored in the spleen.
The tissues within splenic lobules are of two types (see Figure 3). The white pulp is distributed throughout the spleen in tiny islands. This tissue is composed of splenic nodules, which are similar to the lymphatic nodules in lymph nodes and are packed with lymphocytes (T lymphocyte cells and B lymphocyte cells). The red pulp, which fills the remaining spaces of the lobules, surrounds the venous sinuses. This pulp contains numerous red blood cells, which impart its color, plus many lymphocytes and macrophages.
Without an immune system, a human being would be just as exposed to the harmful influences of pathogens or other substances from the outside environment as to changes harmful to health happening inside of the body. The main tasks of the body’s immune system are:
- Neutralizing pathogens like bacteria, viruses, parasites or fungi that have entered the body, and removing them from the body
- Recognizing and neutralizing harmful substances from the environment
- Fighting against the body’s own cells that have changed due to an illness, for example cancerous cells.
The normal adult spleen contributes to the homeostasis of the body by removing from the blood useless or potentially injurious materials (e.g., abnormal or “wornout” red blood cells and microorganisms) and by synthesizing immunoglobulins and properdin 2.
Figure 3. Spleen anatomy
Blood capillaries in the red pulp are quite permeable. Red blood cells can squeeze through the pores in these capillary walls and enter the venous sinuses. The older, more fragile red blood cells may rupture during this passage, and the resulting cellular debris is removed by phagocytic macrophages in the venous sinuses. These macrophages also engulf and destroy foreign particles, such as bacteria, that may be carried in the blood as it flows through the venous sinuses. Thus, the spleen filters blood much as the lymph nodes filter lymph.
Phagocytosis removes foreign particles from the lymph as it moves from the interstitial spaces to the bloodstream. Phagocytes in the blood vessels and in the tissues of the spleen (and the liver and bone marrow) remove particles that reach the blood. Monocytes that leave the bloodstream by diapedesis become macrophages. These large cells may be free, or fixed in various tissues. The fixed macrophages can divide and produce new macrophages. Neutrophils, monocytes, and macrophages constitute the mononuclear phagocytic system (reticuloendothelial system).
Fever is body temperature elevated above an individual’s normal temperature due to an elevated setpoint. It is part of the innate defense because as a result of the fever the body becomes inhospitable to certain pathogens. Higher body temperature causes the spleen (and the liver) to sequester iron, which reduces the level of iron in the blood. Because bacteria and fungi require iron for normal metabolism, their growth and reproduction in a fever-ridden body slows and may cease. Also, phagocytic cells attack more vigorously when the temperature rises. For these reasons, low-grade fever of short duration may be a natural response to infection, not a treated symptom.
T Cells and the Cellular Immune Response
A lymphocyte must be activated before it can respond to an antigen. T cell activation requires that processed fragments of the antigen be attached to the surface of another type of cell, called an antigen-presenting cell (accessory cell). Macrophages, B cells, and several other cell types can be antigen-presenting cells.
T cell activation may occur when a macrophage phagocytizes a bacterium and digests it within a phagolysosome formed by the fusion of the vesicle containing the bacterium (phagosome) and a lysosome. Some of the resulting bacterial antigens are then displayed on the macrophage’s cell membrane near certain protein molecules that are part of a group of proteins called the major histocompatibility complex (MHC). MHC antigens help T cells recognize that a newly displayed antigen is foreign (nonself).
Activated T cells interact directly with antigen-bearing cells. Such cell-to-cell contact is called the cellular immune response, or cell-mediated immunity. T cells (and some macrophages) also synthesize and secrete polypeptides called cytokines that enhance certain cellular responses to antigens. For example, interleukin-1 and interleukin-2 stimulate the synthesis of several other cytokines from other T cells. Additionally, interleukin-1 helps activate T cells, whereas interleukin-2 causes T cells to proliferate. This proliferation increases the number of T cells in a clone, which is a group of genetically identical cells that descend from a single, original cell. Other cytokines, called colony stimulating factors (CSFs), stimulate leukocyte production in red bone marrow and activate macrophages. T cells may also secrete toxins that kill their antigen-bearing target cells, growth-inhibiting factors that prevent target cell growth, or interferon that inhibits the proliferation of viruses and tumor cells. Several types of T cells have distinct functions.
A specialized type of T cell, called a helper T cell, is activated when its antigen receptor combines with a displayed foreign antigen (Figure 9). Once activated, the
helper T cell proliferates and the resulting cells stimulate B cells to produce antibodies that are specific for the displayed antigen.
Another type of T cell is a cytotoxic T cell, which recognizes and combines with nonself antigens that cancerous cells or virally infected cells display on their surfaces near certain MHC proteins. Cytokines from helper T cells activate the cytotoxic T cell. Next, the cytotoxic T cell proliferates. Cytotoxic T cells then bind to the surfaces of antigen-bearing cells, where they release perforin protein that cuts pore like openings in the cell membrane, destroying these cells. In this way, cytotoxic T cells continually monitor the body’s cells, recognizing and eliminating tumor cells and cells infected with viruses. Cytotoxic T cells provide much of the body’s defense against HIV infection.
Some cytotoxic T cells do not respond to a nonself antigen on first exposure, but remain as memory T cells that provide for future immune protection. Upon subsequent exposure to the same antigen, these memory cells immediately divide to yield more cytotoxic T cells and helper T cells, often before symptoms arise.
Figure 4. T-cell (T lymphocyte) activation
Steps in Antibody Production
T Cell (T Lymphocyte) Activities
- Antigen-bearing agents enter tissues.
- An accessory cell, such as a macrophage, phagocytizes the antigen-bearing agent, and the macrophage’s lysosomes digest the agent.
- Antigens from the digested antigen-bearing agents are displayed on the membrane of the accessory cell.
- Helper T cell becomes activated when it encounters a displayed antigen that fits its antigen receptors.
- Activated helper T cell releases cytokines when it encounters a B cell that has previously combined with an identical antigen-bearing agent.
- Cytokines stimulate the B cell to proliferate, enlarging its clone.
- Some of the newly formed B cells give rise to cells that differentiate into antibody-secreting plasma cells.
Figure 5. B (lymphocyte)-cell activation
B Cell (B Lymphocyte) Activities
- Antigen-bearing agents enter tissues.
- B cell encounters an antigen that fits its antigen receptors.
- Either alone or more often in conjunction with helper T cells, the B cell is activated. The B cell proliferates, enlarging its clone.
- Some of the newly formed B cells differentiate further to become plasma cells.
- Plasma cells synthesize and secrete antibodies whose molecular structure is similar to the activated B cell’s antigen receptors.
Antibody Actions
In general, antibodies react to antigens in three ways. Antibodies directly attack antigens, activate complement, or stimulate localized changes (inflammation) that help prevent the spread of pathogens or cells bearing foreign antigens.
In a direct attack, antibodies combine with antigens, causing them to clump (agglutination) or to form insoluble substances (precipitation). Such actions make it easier for phagocytic cells to recognize and engulf the antigen-bearing agents and eliminate them. In other instances, antibodies cover the toxic portions of antigen molecules and neutralize their effects (neutralization). However, under normal conditions, direct antibody attack is not as important as complement activation in protecting against infection.
When certain IgG or IgM antibodies combine with antigens, they expose reactive sites on antibody molecules. This triggers a series of reactions, leading to activation of the complement proteins, which in turn produce a variety of effects. These include:
- coating the antigen-antibody complexes (opsonization), making the complexes more susceptible to phagocytosis;
- attracting macrophages and neutrophils into the region (chemotaxis);
- rupturing membranes of foreign cells (lysis); agglutination of antigen-bearing cells; and
- neutralization of viruses by altering their molecular structure, making them harmless.
Other proteins promote inflammation, which helps prevent the spread of infectious agents.
Immune Responses
Activation of B cells or T cells after they first encounter the antigens for which they are specialized to react constitutes a primary immune response. During such a response, plasma cells release antibodies (IgM, followed by IgG) into the lymph. The antibodies are transported to the blood and then throughout the body, where they help destroy antigen bearing agents. Production and release of antibodies continues for several weeks.
Following a primary immune response, some of the B cells produced during proliferation of the clone remain dormant as memory cells. If the same antigen is encountered again, the clones of these memory cells enlarge, and they can respond rapidly by producing IgG to the antigen to which they were previously sensitized. These memory B cells, along with the memory cytotoxic T cells, produce a secondary immune response.
As a result of a primary immune response, detectable concentrations of antibodies usually appear in the blood plasma five to ten days after exposure to antigens. If the same type of antigen is encountered later, a secondary immune response may produce the same antibodies within a day or two. Although newly formed antibodies may persist in the body for only a few months or years, memory cells live much longer.
Naturally acquired active immunity occurs when a person exposed to a pathogen develops a disease. Resistance to that pathogen is the result of a primary immune response.
A vaccine is a preparation that produces artificially acquired active immunity. A vaccine might consist of bacteria or viruses that have been killed or weakened so that they cannot cause a serious infection, or only molecules unique to the pathogens. A vaccine might also be a toxoid, which is a toxin from an infectious organism that has been chemically altered to destroy its dangerous effects. Whatever its composition, a vaccine includes the antigens that stimulate a primary immune response, but does not produce symptoms of disease and the associated infections.
Specific vaccines stimulate active immunity against a variety of diseases, including typhoid fever, cholera, whooping cough, diphtheria, tetanus, polio, chickenpox, measles (rubeola), German measles (rubella), mumps, influenza, hepatitis A, hepatitis B, and bacterial pneumonia. A vaccine has eliminated naturally acquired smallpox from the world.
Enlarged spleen
Splenomegaly is defined as enlargement of the spleen measured by size or weight 3. The normal sized spleen measures up to 11 cm in length. A length of 11 cm-20 cm indicates splenomegaly and a length greater than 20cm is definitive of massive splenomegaly 3. The normal weight of the adult spleen is 70 g-200 g, spleen weight of 400 g-500 g indicates splenomegaly and spleen weight greater than 1000 g is definitive of massive splenomegaly 3. The normal sized spleen is usually not palpable in adults. However, it may be palpable due to variations in body habitus and chest wall anatomy. Splenomegaly may be diagnosed clinically or radiographically using ultrasound, CT imaging, or MRI. Splenomegaly may be a transient condition due to acute illness or may be due to serious underlying acute or chronic pathology.
Splenomegaly is a rare disease, with estimated prevalence approximately 2% of total US population 3. In adults, there has been no reported predominance in prevalence based on ethnicity, sex, or age.
Spleen enlargement can be associated with decreased or increased function, depending on the cause of the enlarged spleen.
Table 1. Causes of Splenomegaly (enlarged spleen)
| Vascular congestion |
| Cirrhosis |
| Splenic vein thrombosis |
| Portal vein thrombosis |
| Reticuloendothelial hyperplasia |
| Acute infections (e.g., typhoid fever, cytomegalovirus, Epstein-Barr virus infection) |
| Subacute or chronic infections (e.g., bacterial endocarditis, brucellosis, tuberculosis, histoplasmosis, malaria) |
| Collagen-vascular diseases and abnormal immune responses (e.g., systemic lupus erythematosus, serum sickness, sarcoidosis) |
| Work hypertrophy |
| Hemolytic anemias (e.g., spherocytosis) |
| Infiltrative or replacement processes |
| Nonmalignant hematologic disorders (e.g., polycythemia vera, myelofibrosis) |
| Leukemias |
| Lymphomas |
| Metastatic solid tumors |
| Storage diseases (e.g., Gaucher’s disease) |
| Amyloidosis |
| Benign tumor and cysts |
| Abscess |
| Subcapsular hemorrhage |
When spleen enlargement is associated with a change in splenic function, it is most frequently associated with splenic hyperfunction. This is reflected in the peripheral blood by thrombocytopenia, leukopenia, rapid red blood cell destruction, or a combination of these findings. This clinical syndrome of an enlarged spleen and peripheral cytopenias is often referred to as hypersplenism. When splenic enlargement is secondary to an infiltrative process (i.e., tumors or amyloidosis), splenic hypofunction can result. This is reflected in the peripheral blood by Howell–Jolly bodies and abnormal red blood cell forms. The presence of an enlarged spleen should lead to examination of the peripheral blood by the physician.
It is extremely important to correlate the presence of an enlarged spleen with the clinical history findings, other physical findings, laboratory results, and x-ray findings to identify the cause of splenic enlargement in a particular patient. For example, vascular spiders, red palms, and small testes in a patient with splenic enlargement would strongly suggest liver disease as the etiology. Roth spots and a new heart murmur would suggest endocarditis. Extensive lymphadenopathy, weight loss, night sweats, and an enlarged spleen would suggest a malignant lymphoproliferative disease. By making these correlations, it is possible to utilize the presence of an enlarged spleen to plan a patient’s subsequent evaluation and quickly and efficiently reach the correct diagnosis.
The mechanism underlying splenic enlargement varies based on the etiology. In the case of acute infectious illness, the spleen performs increased work in clearing antigens and producing antibodies and increases the number of reticuloendothelial cells contained within the spleen. These increased immune functions may result in splenic hypertrophy. In the case of liver disease and congestion, underlying illness causes increased venous pressure causing congestive splenomegaly. Extramedullary hematopoiesis exhibited in myeloproliferative disorders can lead to splenic hyperplasia.
Splenic sequestration crisis is a life-threatening illness common in pediatric patients with homozygous sickle cell disease and beta thalassemia 3. Up to 30% of these children may develop splenic sequestration crisis with a mortality rate of up to 15%. This crisis occurs when splenic vaso-occlusion causes a large percentage of total blood volume to become trapped within the spleen. Clinical signs include severe, rapid drop in hemoglobin leading to hypovolemic shock and death 3. Pediatric patients with sickle cell disease and beta thalassemia experience multiple splenic infarcts, resulting in splenic fibrosis and scarring. Over time, this leads to a small, auto infarcted spleen typically by the time patients reach adulthood. Splenic sequestration crisis can only occur in functioning spleens which may be why this crisis is rarely seen in adults. However, late adolescent or adult patients in this group who maintain splenic function may develop splenic sequestration crisis.
Symptoms of enlarged spleen
The most common physical symptom associated with splenomegaly is vague abdominal discomfort 3. Patients may complain of pain in the left upper abdomen or referred pain in the left shoulder.
- Abdominal bloating, distended abdomen, anorexia, and/or early satiety may also occur.
Commonly, patients will present with symptoms due to the underlying illness causing splenomegaly 3. Constitutional symptoms such as weakness, weight loss, and night sweats suggest malignant illness. Patients with splenomegaly due to acute infection may present with fever, rigors, generalized malaise, or focal infectious symptoms. Patients with underlying liver disease may present with symptoms related to alcohol abuse or hepatitis. Symptoms of anemia (lightheadedness, dyspnea, or exertion), easy bruising, bleeding, or petechiae may indicate splenomegaly due to underlying hemolytic process.
Diagnosis of enlarged spleen
A combination of serum testing and imaging studies may definitively diagnose splenomegaly and the underlying cause. Derangement in complete blood (cell) counts and morphology including white blood cell, red blood cell and platelets will vary based on underlying disease state. Abnormalities in liver function tests, lipase, rheumatologic panels, and disease-specific infectious testing aid in the diagnosis of causative disease.
Imaging may be used to diagnose splenomegaly and elucidate its underlying cause. The spleen has a similar attenuation as the liver when measured on CT imaging. In addition to diagnosing splenomegaly (splenic measurement of greater than 10 cm in craniocaudal length), abdominal CT may detect splenic abscess, mass lesions, vascular abnormalities, cysts, inflammatory changes, traumatic injury, intra-abdominal lymphadenopathy, or liver abnormalities.
Ultrasound is a useful imaging modality in measuring the spleen and spares the patient radiation from CT imaging. Normal spleen size measured via ultrasound is less than 13 cm superior to the inferior axis, 6 cm-7 cm in medial to lateral axis and 5 cm-6 cm in anterior to posterior plane.
MRI, PET scans, liver-spleen colloid scanning, and splenectomy and splenic biopsy may be indicated in certain cases.
Treatment and Management of enlarged spleen
Treatment of splenomegaly is targeted at treating the underlying disease and protecting the patient from complications of splenomegaly itself. Patients with splenomegaly from any cause are at increased risk of splenic rupture, and increased attention must be made to protect the patient from abdominal trauma. Treatment ranges from abdominal injury avoidance in the young healthy patient with splenomegaly due to infectious mononucleosis, to splenectomy of a massively enlarged spleen in a patient with Hairy cell leukemia. Likewise, the prognosis is largely dependent on underlying disease state.
Patients who undergo splenectomy are at increased risk of overwhelming infection due to encapsulated organisms such as Haemophilus Influenzae, Streptococcus pneumoniae, and Neisseria meningitidis should receive vaccinations against these organisms. Careful attention must be paid to post-splenectomy patients presenting with febrile illnesses as they may require more aggressive, empiric antibiotic therapy.
Ruptured spleen
Blunt splenic injury which is often times the first or second most commonly injured solid organ in the abdomen along with the liver 4. Rupture of the spleen is relatively common both immediately and in a delayed fashion following significant blunt abdominal injury 5. Massive hemorrhage commonly occurs from injuries to this friable vascular organ. The mortality rate from simple splenic rupture is 1% 6. Delayed diagnosis of a ruptured spleen increases the rate to 10% 6. While less common, cases of atraumatic rupture of diseased spleens 7, 8.
Four types of splenic injuries are recognised namely: intraparenchymal laceration, subcapsular haematoma, and splenic rupture and delayed rupture 9.
For many years it was accepted that splenic injury was best managed by splenectomy. However, it has more recently been realized that patients whose spleen has been removed are more liable to life-threatening infection 10. This is of particular consequence if splenectomy is performed in childhood, with a long life expectancy. The spleen has many functions including filtering or removing old poorly functioning red blood cells, catching bacteria, and producing antibodies. After removal of the spleen, these functions are lost and the patient could be susceptible to an overwhelming post-splenectomy infections from bacteria such as streptococcus pneumoniae, neisseria meningitidis, and hemophilus influenza 4. To reduce this risk, patients who undergo surgery to remove their spleen receive vaccines against these bacteria.With greater understanding of the splenic anatomy and function, and natural course of splenic injuries, the management has evolved into a more conservative approach though a splenectomy may still be required in some situations.
Symptoms of ruptured spleen
Most splenic injuries manifest at the moment of injury with symptoms of acute intraperitoneal hemorrhage and shock 11. At this point the patient falls into one of two categories:
- hemodynamically stable or
- hemodynamically unstable based on their vital signs (blood pressure and heart rate).
If the patient has a low blood pressure and/or a high heart rate (unstable), the trauma surgeon must identify the cause, which is often due to bleeding. Next the trauma surgeon determines the location of the bleeding. If it appears that the abdomen is the source, the patient, the patient may need to be taken to the operating room emergently for exploratory abdominal surgery. If a splenic injury with bleeding is found, removal of the spleen (splenectomy) may be required.
Treatment for splenic injury:
- Approximately 70% to 90% of children with splenic injury receive Non-operative management (NOM)
- Approximately 40% to 50% of adult patients with splenic injury receive Non-operative management
- 85% patient receive non-operative management in some centers
If instead the patient’s vital signs are normal (hemodynamically stable), a CT scan of the abdomen/pelvis may be done to evaluate potential trauma to the abdomen. At this point if the patient is found to have a splenic laceration it is graded according to the American Association for the Surgery of Trauma splenic injury scale (Table 1). Other factors such as intravenous contrast extravasation or “blush” can also be identified via the CT scan, which indicates that there may be active bleeding in progress in which case a hemodynamically stable patient may be sent to interventional radiology so that an angiogram can be performed and a potential active bleeding vessel can be embolized or coiled to stop any further bleeding. The patient then may be observed in the ICU/floor depending on severity and other trauma to the patient. Close monitoring of the patient’s condition, vital signs, blood tests and serial abdominal exams are required in order to assess the stability of the bleeding from the injury. The trauma surgeon must be prepared to operate 24/7 in case recurrent bleeding develops after a period of stability. Thus, these patients are best managed at a trauma center, which has the necessary resources to intervene quickly. If the patient remains stable the patient’s diet and activity can slowly be started after 24 hrs or depending on the individual institution’s protocols.
Whether the patient undergoes surgery or is managed non-operatively there are risks and complications associated with either strategy. After surgery there is always a small risk of infection and additionally bleeding from the procedure. If the spleen has been removed, the patient is at risk for certain bacterial infections, as discussed earlier and will require vaccinations. There is also a risk during the procedure of injuring the pancreas or other organs necessitating additional procedures. With the non-operative strategy there is a risk of delayed bleeding which may require an operation to remove the spleen. Also if the patient is selected for nonoperative management there is a chance of a missed associated injury in the abdomen such as a bowel injury.
As a result of understanding the function of the spleen, natural evolution of the splenic injury, improving technology and adjuncts such as angiogram the trauma surgeons are better able to manage blunt spleen injuries nonoperatively more successfully than before. Managing a hemodynamically unstable patient suspected of having an intra-abdominal injury often requires an immediate surgery for abdominal exploration. However, out of the patients managed nonoperatively there still is a set of patients that fail this type of management who will require surgical intervention and it is in those patients that the trauma surgeon must be vigilant. Blunt splenic trauma management has evolved significantly over the last few decades and as our understanding of the injury and its evolution improves so does our ability to manage the splenic injury whether it’s nonoperatively or surgically.
Table 1. Spleen injury scale
| Grade* | Injury type | Description of injury | ICD-9 | AIS-90 |
| I | Hematoma | Subcapsular, <10% surface area | 865-01 | 2 |
| 865.11 | ||||
| Laceration | Capsular tear, <1cm | 865.02 | 2 | |
| parenchymal depth | 865.12 | 2 | ||
| II | Hematoma | Subcapsular, 10%-50% surface area | 865.01 | |
| intraparenchymal, <5 cm in diameter | 865.11 | |||
| 2 | ||||
| Laceration | Capsular tear, 1-3cm parenchymal depth that does not | 865.02 | ||
| involve a trabecular vessel | 865.12 | |||
| 3 | ||||
| III | Hematoma | Subcapsular, >50% surface area or expanding; ruptured | ||
| subcapsular or parecymal hematoma; intraparenchymal | ||||
| hematoma > 5 cm or expanding | ||||
| Laceration | >3 cm parenchymal depth or involving trabecular vessels | 865.03 | 3 | |
| 865.13 | ||||
| IV | Laceration | Laceration involving segmental or hilar vessels producing | ||
| major devascularization (>25% of spleen) | 4 | |||
| V | Laceration | Completely shattered spleen | 865.04 | 5 |
| Vascular | Hilar vascular injury with devascularizes spleen | 865.14 | 5 | |
| *Advance one grade for multiple injuries up to grade III. | ||||
- Hijazi LS, Mead T. Functional Asplenism. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499949
- Armitage JO. Spleen. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston: Butterworths; 1990. Chapter 150. Available from: https://www.ncbi.nlm.nih.gov/books/NBK258/
- Chapman J, Bhimji SS. Splenomegaly. [Updated 2017 May 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2017 Jun-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430907/
- Blunt Splenic Trauma. American Association for the Surgery of Trauma. http://www.aast.org/GeneralInformation/BluntSplenicTrauma.aspx
- Olsen WR, Polley TZ Jr. A Second Look at Delayed Splenic Rupture. Arch Surg. 1977;112(4):422–425. doi: 10.1001/archsurg.1977.01370040074012. https://www.ncbi.nlm.nih.gov/pubmed/849149
- “Delayed rupture of the spleen” or delayed diagnosis of the splenic injury ? Isr J Med Sci. 1980 Sep-Oct;16(9-10):659-64. https://www.ncbi.nlm.nih.gov/pubmed/7429801
- Systematic review of atraumatic splenic rupture. Renzulli P, Hostettler A, Schoepfer AM, Gloor B, Candinas D. Br J Surg. 2009 Oct; 96(10):1114-21. https://www.ncbi.nlm.nih.gov/pubmed/19787754/
- Debnath D, Valerio D. Atraumatic rupture of the spleen in adults. J R Coll Surg Edinb. 2002;47:437–445. https://www.ncbi.nlm.nih.gov/pubmed/11874265
- Horgan GI. The paediatric Liver and Spleen. In: Rumack CR, Wilson SR, Charboneau J William, editors. Diagnostic ultrasound. Volume 2. Vol. 59. Mosby year Book; 1991. pp. 1175–1176.
- Conservative management of ruptured spleen. S Afr Med J. 1980 Apr 19;57(16):655-8. https://www.ncbi.nlm.nih.gov/pubmed/7376031
- Delayed splenic rupture: understanding the threat. J Trauma Nurs. 2002 Apr-Jun;9(2):34-40. https://www.ncbi.nlm.nih.gov/pubmed/15997614
- Spleen injury scale (1994 revision). American Association for the Surgery of Trauma. http://www.aast.org/Library/TraumaTools/InjuryScoringScales.aspx