Fingernails
Nails are protective coverings on the ends of the fingers and toes. Fingernails and toenails are clear, hard derivatives of the stratum corneum. They are composed of very thin, dead, scaly cells, densely packed together and filled with parallel fibers of hard keratin. Each nail consists of a nail plate, which includes the free edge overhanging the tip of the finger or toe; that overlies a surface of skin called the nail bed. Specialized epithelial cells continuous with the epithelium of the skin produce the nail bed. The nail body, which is the visible attached part of the nail; and the nail root, which extends proximally under the overlying skin. The surrounding skin rises a bit above the nail as a nail fold, separated from the margin of the nail plate by a nail groove. The groove and the space under the free edge accumulate dirt and bacteria and require special attention when scrubbing for duty in an operating room or nursery.
The skin underlying the nail plate is the nail bed; its epidermis is called the hyponychium. At the proximal end of the nail, the stratum basale thickens into a growth zone called the nail matrix. Mitosis in the matrix accounts for the growth of the nail—about 1 mm per week in the fingernails and slightly slower in the toenails. The thickness of the matrix obscures the underlying dermal blood vessels and is the reason why an opaque white crescent, the lunule (lunula), often appears at the proximal end of a nail. A narrow zone of dead skin, the cuticle or eponychium, commonly overhangs this end of the nail.
The appearance of the fingertips and nails can be valuable in medical diagnosis. The fingertips become swollen or clubbed in response to long-term hypoxemia—a deficiency of oxygen in the blood stemming from conditions such as congenital heart defects and emphysema. Dietary deficiencies may be reflected in the appearance of the nails. An iron deficiency, for example, may cause them to become flat or concave (spoonlike) rather than convex. Contrary to popular belief, adding gelatin to the diet has no effect on the growth or hardness of the nails.
Figure 1. Fingernail
Fingernail problems
Fingernail fungus
Onychomycosis is a fungal infection of the fingernails or toenails that causes discoloration, thickening, and separation from the nail bed. Onychomycosis occurs in 10% of the general population but is more common in older adults; the prevalence is 20% in those older than 60 years and 50% in those older than 70 years 1. The increased prevalence in older adults is related to peripheral vascular disease, immunologic disorders, and diabetes mellitus. The risk of fungal infection of the fingernails or toenails is 1.9 to 2.8 times higher in persons with diabetes compared with the general population 2. In patients with human immunodeficiency virus infection, the prevalence ranges from 15% to 40% 3.
Fungal nail infection affects toenails more often than fingernails because of their slower growth, reduced blood supply, and frequent confinement in dark, moist environments. It may occur in patients with distorted nails, a history of nail trauma, genetic predisposition, hyperhidrosis, concurrent fungal infections, and psoriasis. It is also more common in smokers and in those who use occlusive footwear and shared bathing facilities 4.
Fungal fingernail infection is caused by various organisms, most often dermatophytes of the genus Trichophyton. Dermatophytes are fungi that require keratin for growth. These fungi can cause superficial infections of the skin, hair, and nails. Other organisms include Candida, which is more common in fingernail infections (Figure 2) and in patients with chronic mucocutaneous candidiasis 1. Nondermatophyte molds are a less common cause in the general population. Recent studies, however, have demonstrated that they are the predominant organisms in patients with fungal nail infection and human immunodeficiency virus infection 3.
Figure 2. Fingernail fungal infection (Candidal subungual onychomycosis)

Table 1. Common Pathogens in Onychomycosis (Fungal Nail Infections)
Dermatophytes (80% to 90%) |
Epidermophyton floccosum |
Microsporum species |
Trichophyton interdigitale |
Trichophyton mentagrophytes |
Trichophyton rubrum |
Trichophyton tonsurans |
Nondermatophyte molds (2% to 10%)* |
Acremonium species |
Alternaria species |
Aspergillus species |
Cladosporium carrionii |
Fusarium species |
Geotrichum candidum |
Lasiodiplodia theobromae |
Onychocola species |
Scopulariopsis species |
Scytalidium species |
Yeast (2% to 11%) |
Candida albicans |
Candida guilliermondii |
Candida parapsilosis |
*—Nondermatophyte molds are the predominant organism in patients with human immunodeficiency virus infection.
Classification of Fungal Nail Infection
Onychomycosis is divided into several classes based on morphologic patterns and mode of invasion of the nail (Table 2) 6. Classification provides a framework for diagnosis and expected response to treatment, and can help predict the prognosis. The classes include distal and lateral subungual onychomycosis (Figures 3 and 4), proximal subungual onychomycosis (Figure 5), superficial onychomycosis (Figure 6), and total dystrophic onychomycosis (Figure 7). A fifth class, endonyx subungual onychomycosis, is rare. Some nails have features from a combination of classes.
Table 2. Classification of Fungal Nail Infection
| Onychomycosis class | Clinical features | Causative organism* | Mode of infection | Comments |
|---|---|---|---|---|
Distal and lateral subungual | Begins distally at the hyponychium and spreads to the nail plate and bed; hyperkeratotic debris accumulates and results in onycholysis; nails thicken, chip, become dystrophic, and turn yellow-white or brown-black; infection can progress proximally, causing linear channels or “spikes” that can make treatment difficult; associated with paronychia | Epidermophyton floccosum | Fungal invasion through break in the skin at the lateral or distal undersurface of the nail | Most common form |
Trichophyton mentagrophytes | ||||
Trichophyton rubrum | ||||
Fusarium species | ||||
Scopulariopsis brevicaulis | ||||
Scytalidium species | ||||
Candida albicans | ||||
Endonyx subungual | Nail develops a milky white appearance, indentations, and lamellar splitting; no hyperkeratosis or onycholysis | Trichophyton soudanense | Fungus invades the full thickness of the nail from directly under the skin without infecting the nail bed | Rare; considered a subtype of distal and lateral subungual onychomycosis |
Trichophyton violaceum | ||||
Proximal subungual | Debris accumulates under the proximal portion of the nail, causing onycholysis and a white color that spreads distally | T. rubrum | Fungus invades the proximal nail fold and cuticle; may also develop secondary to paronychia | Suggests an immunosuppressive condition (e.g., human immunodeficiency virus infection) |
Aspergillus species | ||||
Fusarium species | ||||
C. albicans | ||||
Superficial | Nail appears to have powder-like patches of transverse striae on the surface | T. mentagrophytes | May appear on the superficial nail plate or emerge from under the nail fold; may be deep penetration of the superficial infection | Previously known as superficial white onychomycosis, but some organisms produce black debris |
T. rubrum | ||||
Acremonium species | ||||
Fusarium species | ||||
Scytalidium species | ||||
Total dystrophic | Complete destruction of the nail from long-standing infection; nail thickens, and nail structure is lost | — | — | Can result from any of the other classes, although it is most often from severe distal and lateral subungual onychomycosis |
NOTE: Candidal onychomycosis was previously considered a class of onychomycosis. This condition, which more commonly involves the fingernails, has recently been excluded as a separate type because it was inconsistent to base a class on the organism alone.
*—Dermatophytes are listed first, followed by nondermatophyte molds and yeast.
Figure 3. Distal and lateral subungual onychomycosis

Figure 4. Distal and lateral subungual onychomycosis with spike deformity

Figure 5. Proximal subungual onychomycosis

Figure 6. Superficial onychomycosis

Figure 7. Total dystrophic onychomycosis

Accurate diagnosis is crucial for successful treatment and requires identification of physical changes and positive laboratory analysis. Only 50% of nail problems are caused by onychomycosis 7, and clinical diagnosis by physical examination alone can be inaccurate. Psoriasis, chronic nail trauma, and other causes must also be considered. The differential diagnosis of onychomycosis is presented in Table 3.
Table 3. Common Conditions That Can Mimic Onychomycosis
| Condition | Features |
|---|---|
Infections | |
Chronic paronychia | Chronic inflammation of the proximal paronychium; cross-striations of the nail; Streptococcus, Staphylococcus, or Candida found on smear and culture; common in children |
Viral warts | Localized in nail folds and subungual tissue; longitudinal depressed grooves in the nail plate |
Skin disorders | |
Chronic dermatitis | Subungual dermatitis, hyperkeratosis, Beau lines, and pitting; thickened nail with corrugated surface |
Lichen planus | Longitudinal grooves and fissures; usually affects fingernails |
Psoriasis | Nail pitting, splinter hemorrhages, “oil staining,” yellow-gray or silvery white nails (eFigure B) |
Twenty-nail dystrophy | Dystrophy of all 20 nails; usually resolves in childhood; associated with the lesions of lichen planus (eFigure C) |
Trauma | |
Footwear | Oncholysis, ingrown toenails, subungual keratosis, nail plate discoloration and irregularities; caused by friction against the shoe |
Manipulation (e.g., manicures, pedicures, rubbing) | Horizontal parallel nail plate grooves, inflammation from Staphylococcus aureus or Pseudomonas infection (eFigure D) |
Tumors | |
Bowen disease | Squamous cell carcinoma; bleeding, pain, nail deformity, and nail discoloration |
Fibroma | Oval or spherical, white or yellow nodule; causes tunnel-like melanonychia; fibrous dermatofibroma or periungual fibroma |
Melanoma | Brown-yellow nail with dark pigment extending into the periungual skin folds; poor prognosis |
Figure 8. Nail pitting in a patient with psoriasis. The pits are enhanced by the presence of grease.

Figure 9. Twenty-nail dystrophy (also called sandpaper nails) is characterized by longitudinal ridges on all 20 nails. The nails may become discolored.

Figure 10. Median nail dystrophy caused by repetitive trauma to the nail from habitual rubbing.

Treatment of fungal nail infection
Onychomycosis is widely believed to be only a cosmetic problem, but it can be uncomfortable and can lead to cellulitis in older adults and foot ulcers in patients with diabetes. Eradication of the infection is key to improving appearance and avoiding these complications, but it is not easily accomplished because nails are made of keratin, which is nonvascular and impermeable to many agents 8. Because of poor drug delivery to nails, results of treatment may not be apparent for a year.
Treatment varies depending on the severity of nail changes, the organism involved, and concerns about adverse effects and drug interactions. Treatments also have varying effectiveness, based on cure parameters that are defined differently among studies. Mycotic cure denotes that no organism is identified on microscopy and culture. Clinical cure refers to improvement in the appearance of the nail, often defined as a normal appearance in 80% to 100% of the nail. It is a subjective measure that is difficult to compare across studies 9. Complete cure indicates that mycotic and clinical cure have been achieved.
Topical Agents
Several topical agents are used for the treatment of onychomycosis. These agents have few contraindications and no drug-drug interactions.
Ciclopirox 8% solution is the only topical prescription medication available in the United States for the treatment of onychomycosis. It is a synthetic hydroxypyridine antifungal formulated as a nail lacquer. Adverse effects include burning, itching, and stinging at the application site 10. It may be used in patients who cannot take oral antifungals and in those with less than 50% of the distal nail affected and no lunular involvement 11. It has been used in children, although it is not approved for use in patients younger than 12 years 12. When used alone, ciclopirox has a mycotic cure rate of 29% to 36%, and a clinical cure rate of 6% to 9% 11. A Cochrane review noted that the treatment failure rate was 61% to 64% after 48 weeks of use 13.
Ciclopirox has also been used in combination with oral agents to improve effectiveness. In one comparative study, a combination of ciclopirox and oral terbinafine had a mycotic cure rate of 88% and a complete cure rate of 68%, whereas terbinafine alone had a mycotic cure rate of 65% and a complete cure rate of 50% 14.
Nonprescription agents have also been used for treatment of onychomycosis. These therapies have been evaluated in only a small number of studies involving few patients. Topical mentholated ointment (Vicks Vaporub) was used in a small study involving 18 patients. After 48 weeks, 28% had mycotic and clinical cure, 56% had partial clearance, and 17% had no improvement 15. Tea tree oil (Melaleuca alternifolia) has been evaluated in two studies. Although one trial was favorable, combined data from both studies did not demonstrate significant benefit 12, 16. Snakeroot extract (Ageratina pichinchensis) is an antifungal derived from plants of the sunflower family. It was studied in a randomized trial involving 96 patients who applied the extract or ciclopirox for six months to nails with confirmed infections 17. Mycotic cure occurred in 59% of patients receiving the extract and in 64% of those receiving ciclopirox. Clinical cure occurred in 71% and 81% of patients, respectively. Differences between the two treatments were not statistically significant. A small study showed that a combination of cyanoacrylate, undecylenic acid, and hydroquinone (marketed as Renewed Nail) demonstrated mycotic cure in 78 of 154 participants (50%) 18.
Nail trimming and debridement are often performed concomitantly with other treatments and appear to offer benefit. Study groups that received nail debridement with oral terbinafine had higher clinical cure rates than those who received oral terbinafine alone 19. When debridement was performed with concurrent administration of ciclopirox, the mycotic cure rate was 77%, higher than that for ciclopirox alone 20. Improvement in nail appearance was reported, but clinical cure rates were not.
Laser and photodynamic therapies
Although they are expensive, laser and photodynamic therapies have become popular based on the success of in-vitro studies. Several neodymium:yttrium-aluminum-garnet (Nd:YAG) laser therapies have been approved by the U.S. Food and Drug Administration for treatment of onychomycosis 21. The Pinpointe Foot-laser, Cutera GenesisPlus laser, and Cooltouch Varia laser are short-pulse laser systems, whereas the Light Age Q-Clear laser is a Q-switched laser. However, there are only limited data about the use of these therapies in patients. In one study, Nd:YAG laser light was used to treat 37 nails, with one to three treatments given four to eight weeks apart. At 16 weeks, 61% were completely cured, 19% had significant improvement in the nail appearance, and 11% had moderate improvement in the nail appearance 22.
Another laser treatment, the dual-wavelength near-infrared laser (Noveon), is approved for dermatologic use, but not specifically for treatment of onychomycosis 23. This treatment was used on 26 nails on days 1, 14, 42, and 120. After 180 days, 91% of nails with mild infection showed clinical improvement (3 to 4 mm of the nail free of clinical infection); however, only 30% had mycotic cure 24.
Photodynamic therapy using photosensitizing drugs and light to destroy fungal cells has shown some success in the treatment of onychomycosis, but further evaluation is needed 25.
Oral Anti-Fungal Medications
Antifungals from the azole and allylamine classes are the most widely used oral medications for the treatment of onychomycosis. The azole class includes itraconazole (Sporanox), fluconazole (Diflucan), and ketoconazole; however, ketoconazole is rarely prescribed because of drug interactions and hepatotoxicity. The allylamine class is represented by terbinafine (Lamisil).
A meta-analysis of treatments for toenail onychomycosis determined that mycotic cure rates were 76% for terbinafine, 63% for itraconazole with pulse dosing, 59% for itraconazole with continuous dosing, and 48% for fluconazole 26. Clinical cure rates were 66% for terbinafine, 70% for itraconazole with pulse dosing, 70% for itraconazole with continuous dosing, and 41% for fluconazole. Common adverse effects included headache, gastrointestinal problems, and rash; these drugs also have been associated with Stevens-Johnson syndrome, prolonged QT interval, and ventricular dysfunction. The use of these agents is discouraged in patients with liver, renal, or heart disease, and in those receiving medications with which there may be significant drug-drug interactions 27. Liver function studies are recommended before beginning treatment and after one month of therapy. A meta-analysis concluded that the risk of asymptomatic elevation of transaminase levels in immunocompetent patients receiving oral antifungal agents was 2%, and that the risk of elevations requiring termination of therapy was 1% 28. Although these medications are not approved for use in children, they have been used in children with positive results 12.
Dark line on fingernail
The assessment and management of pigmented nail bed lesions can be a significant challenge for clinicians. The difficulty arises from the wide spectrum of differential diagnoses, including potentially life-threatening subungual melanoma.
Figure 1 shows the anatomy of the nail apparatus and location of the nail matrix, an important site for the development of nail bed pathology.
Differential diagnoses of dark line on fingernail could include 29:
- melanocytic lesions
- naevi (moles)
- lentigo (increased single melanocytes)
- melanoma in situ
- melanoma
- subungal squamous cell carcinoma (SCC)
- subungual haemorrhage
- fungal infection
- systemic illness (including systemic lupus erythematosus, scleroderma)
- drug-induced pigmentation
- ethnic-type pigmentation (can often involve multiple digits).
Longitudinal melanonychia is a specific appearance of a linear, pigmented band on the nail plate. This appearance in itself is non-specific and can result from the same variety of diagnoses listed above for any subungual pigmentation. Early subungual melanoma often presents as longitudinal melanonychia.
The differential diagnoses for a patient presenting with a subungual lesion are broad. Lesions can be divided into melanocytic and non-melanocytic. They can also be categorised as neoplastic, traumatic, infective, systemic and drug-induced. Figure 11 illustrates the clinical appearance of some common differentials.
Figure 11. Benign pigmented subungual lesions

Note: A, C, narrow melanonychia with regular borders and parallel bands of pigment; B, D,corresponding dermatoscopic detail of narrow melanonychia with regular borders and parallel bands of pigment; E, F, subungual haematoma with homogenous reddish brown to black color.
Figure 12. Longitudinal melanonychia (vertical nail bands). These hyperpigmented bands occur as normal variants in 90 percent of black persons 30

The goal of initial assessment is to form an opinion of the likelihood of the lesion being either benign or malignant. This risk assessment guides whether patients are discharged with reassurance that a lesion is benign, enter a short, defined period of serial monitoring, or undergo a biopsy.
Serial monitoring of a lesion with the interval between assessments of 8–12 weeks can be a powerful aid in the management of lesions that are indeterminate on initial assessment. However, time intervals should be individualised and based on the likelihood of malignancy and the patient’s circumstances. The use of dermatoscopy and clinical photography can be particularly helpful and allows for direct comparisons of images obtained over a defined time interval. In cases of subungual haematoma, serial monitoring will often make the diagnosis readily apparent, as the lesion can be observed to migrate distally with normal nail plate growth proximal to the lesion.
Referral to a specialist centre with experience in managing subungual melanoma should be made in cases where the diagnosis remains unclear following a short period of monitoring or in cases where a malignant pathology is suspected from the initial assessment.
Biopsy
Biopsy of a nail bed lesion is a complex procedure. Improperly planned biopsies can risk obtaining an inadequate specimen or damaging the fragile sample of nail matrix so that histopathological examination is compromised. Furthermore, there is the hazard of causing permanent nail dystrophy from injury to the germinal matrix 31. As such, referral to a specialist group with experience in managing subungual pathology is often appropriate in cases where a biopsy is being considered.
Fingernail pain
Fingernail pain or paronychia, which can be acute or chronic, (Figure 13A and 13B) is an inflammatory reaction caused by bacterial invasion of the folds of tissue surrounding the nail 32. It is characterized by the rapid onset of erythema, edema, and tenderness at the proximal and lateral nail folds, usually following trauma 33. Most cases are caused by mixed flora. Staphylococcus aureus and Streptococcus pyogenes are the most common aerobic bacteria associated with paronychia, whereas anaerobic isolates include Bacteroides, Fusobacterium nucleatum, and grampositive cocci 34. Noninfectious causes of paronychia include excessive moisture, contact irritants, and trauma 33. Psoriasis, Reiter syndrome, and herpetic whitlow should be part of the differential diagnosis of recurrent cases.
Oral antibiotics may be used for severe infections in which an abscess has not developed. Coverage for methicillinresistant S. aureus should be considered in high-prevalence areas, and coverage for anaerobic bacteria should be considered when exposure to oral flora is suspected 35. If an abscess is present, surgical drainage is indicated 33.
The clinical manifestations of chronic paronychia are similar to those of acute paronychia; however, chronic cases last for more than six weeks. Because of constant infection, the cuticle may separate from the nail plate, forming a space for invasion of various microbes, including bacteria and fungi 35. Patients who are immunosuppressed or who have a systemic illness, such as diabetes or human immunodeficiency virus, are at increased risk of chronic paronychia 32. Treatment includes avoiding exposure to contact irritants and appropriate management of the underlying infection. A broad-spectrum topical antifungal, such as ketoconazole, can be used to treat the superinfection and prevent recurrence when a fungus, such as Candida albicans, is suspected 32.
Paronychia associated with a pseudomonal infection shares many of the same characteristics of acute and chronic paronychia; however, pseudomonal paronychia commonly leads to a green discoloration (Figure 13C). Pseudomonal superinfection is typically caused by repeated minor trauma to the nail apparatus in a chronically wet environment 32. Persons at risk of this condition include bartenders, dishwashers, and habitual nail-biters. Therapy for pseudomonal superinfection should include topical neomycin.
Figure 13. Fingernail pain or paronychia

Note: Paronychia – This condition is an inflammatory reaction caused by bacterial invasion of the folds of tissue surrounding the nail. (A) Acute paronychia is characterized by the rapid onset of erythema, edema, and tenderness at the proximal nail folds. (B) Chronic cases are similar in appearance to acute cases, but last more than six weeks and the cuticle may separate from the nail plate. (C) Paronychia associated with pseudomonal infection is typically caused by repeated minor trauma in a wet environment and often causes a green discoloration.
Fingernail Beau Lines
Beau lines (Figure 14) are horizontal grooves on the nail plate and generally involve most or all of the nails. They reflect an interruption of nail bed mitosis caused by severe illness, pemphigus, high fever, or chemotherapy 36. Beau lines also occur in patients with Raynaud disease. Treatment is aimed at the underlying etiology.
Figure 14. Fingernail Beau Lines

Fingernail Clubbing
Clubbing involves thickening of the nail bed’s soft tissue, particularly in the proximal end (Figure 15A). This condition usually affects all of the fingernails and rarely occurs in a single digit. Clubbing can be clinically diagnosed with an examination showing Schamroth sign1 (Figure 15B; absence of the diamond-shaped opening that normally appears when the digits are opposed). Lovibond angle (Figure 15C; the angle that forms between the nail plate and the soft tissue of the distal digit) is diagnostic of clubbing if it is greater than 180 degrees 37.
The pathogenesis of clubbing is thought to be secondary to altered vasculature. It is theorized that the thickening of the soft tissue develops from increased blood flow within the microvasculature, instead of within the larger capillaries 37. Clubbing can be a sign of numerous underlying diseases, such as cirrhosis, chronic obstructive pulmonary disease or celiac disease.
Figure 15. Fingernail clubbing

Spoon fingernail
Koilonychia (Figure 16) is a condition in which the nail becomes increasingly concave and therefore is often called spoon nail 36. It commonly occurs in association with iron deficiency anemia 37. Koilonychia can be a normal finding in infants, disappearing within the first few years of development 36.
Figure 16. Spoon fingernail (Koilonychia)

Fingernail Mees lines
Mees lines are transverse white lines that may extend the complete width of the nail plate. The lines can occur on a single digit or multiple digits. Mees lines migrate toward the distal end of the nail plate over time because the abnormality is in the nail plate and not the nail bed. The differential diagnosis for Mees lines is narrow, and it is thought to be caused primarily by arsenic poisoning 36. Other heavy metal poisonings and renal failure also may result in Mees lines 38. The timing of the poisoning can be estimated based on the rate of nail plate growth 36.
Figure 17. Fingernail Mees lines

Fingernail Muehrcke Lines
Muehrcke lines (Figure 18) are pairs of transverse white lines caused by localized pathology (e.g., edema from hypoalbuminemia) within the nail bed. Because they originate in the nail bed and not the nail plate, the lines do not migrate distally as the nail grows. The nail bed has abnormal vascular architecture that can be visualized microscopically 36. The lines disappear when pressure is applied to the nail plate because the abnormal blood supply is compressed. If the lines are caused by hypoalbuminemia, the nail findings improve as the underlying condition improves 39.
Figure 18. Fingernail Muehrcke Lines

Pincer fingernails
Pincer nail (Figure 19) is a transverse overcurvature of the nail plate and may be inherited or acquired. The exact etiology is unknown, but the condition has been associated with beta-blocker use, psoriasis, onychomycosis, tumors of the nail apparatus, systemic lupus erythematosus, Kawasaki disease, and malignancy 40. If pincer nail is associated with medication use, the nail plate returns to normal after cessation of the medication 41. Surgical treatment modalities include nail bed cutting with or without splinting 42.
Figure 19. Pincer fingernais

Fingernail Splinter hemorrhage
Splinter hemorrhages (Figure 20) are red-brown, longitudinal lines occurring in the nail bed (not the nail plate) that develop secondary to leaky capillaries 36. When pressure is applied to the nail, they do not disappear because the lines are caused by blood that has leaked out of the vasculature 37. Splinter hemorrhages historically have been associated with endocarditis, typically appearing in the midportion of the nail. However, only 15 percent of patients with endocarditis have them 36 and other causes of proximal nail bed splinter hemorrhages should be considered. Many causes of the condition have been identified. The most common cause is trauma, which often leads to distal nail bed splinter hemorrhages. Systemic causes include endocarditis; psoriasis; renal, pulmonary, and endocrine disease; and systemic skin conditions 43.
Figure 20. Fingernail Splinter hemorrhages

Ingrown fingernail
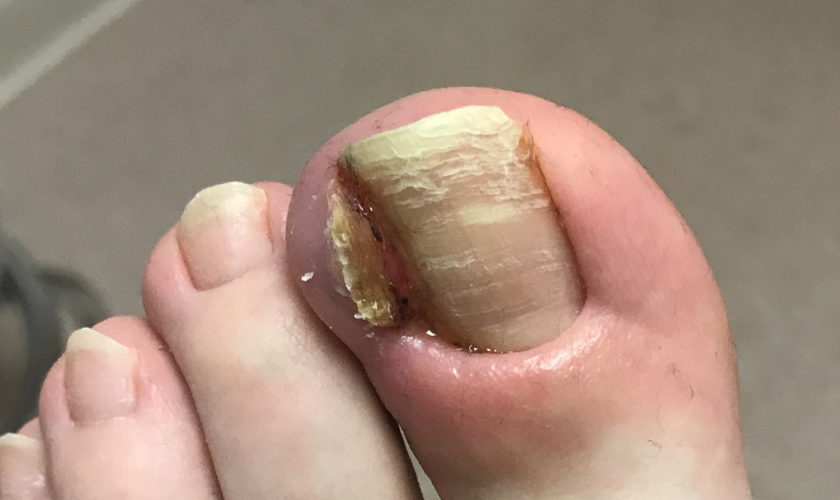
Ingrown nail or onychocryptosis, is a common condition, seen mostly in adolescents and young adults. The cause is multifactorial: repeated trauma, hyperhidrosis (excessive sweating), a broad nail plate, cutting the corners of the nail at an angle 44. The condition can be classified into 3 or 4 progressive stages. In stage 1, the lateral nail border is painful and slightly swollen; the later stages are characterized by marked hypertrophy of the lateral nail folds and the development of granulation tissue.
Home remedy for ingrown fingernail
Treating your fingernails gently can help prevent ingrown fingernail. Consider these simple tips:
- Keep your fingernails dry. Repeated or prolonged contact with water can contribute to split fingernails. Wear cotton-lined rubber gloves when washing dishes, cleaning or using harsh chemicals.
- Practice good nail hygiene. Keep your fingernails neatly trimmed, and round the tips in a gentle curve. When you use hand lotion, rub the lotion into your fingernails and cuticles, too. Don’t bite your fingernails or pick at your cuticles.
- Avoid harsh fingernail care products. Limit your use of nail polish remover. When using nail polish remover, opt for an acetone-free formula.
- Apply a protective layer. Applying a nail hardener might help strengthen fingernails.
If your best efforts to preventing ingrown fingernails don’t seem to help, see your doctor or dermatologist for additional suggestions. Some research suggests that the nutritional supplement biotin might help strengthen weak or brittle fingernails.
Treatment depends on the clinical stage: while conservative measures are sufficient in stage 1, surgery is indicated in stages 2 to 4.
In onychocryptosis, the aim of surgery is to eliminate granulation tissue and hypertrophic tissue and to perform a matricectomy. Various clinical studies have shown matricectomy with 88% phenol to be a simple technique that gives excellent results with minimum complications. Several surgical techniques have been described for removing excess soft tissue. The method most often used is the Howard-Dubois technique, which is usually effective in mild to moderate cases.
References- Thomas J, Jacobson GA, Narkowicz CK, Peterson GM, Burnet H, Sharpe C. Toenail onychomycosis: an important global disease burden. J Clin Pharm Ther. 2010;35(5):497–519.
- Mayser P, Freund V, Budihardja D. Toenail onychomycosis in diabetic patients: issues and management. Am J Clin Dermatol. 2009;10(4):211–220.
- Surjushe A, Kamath R, Oberai C, et al. A clinical and mycological study of onychomycosis in HIV infection. Indian J Dermatol Venereol Leprol. 2007;73(6):397–401.
- Gupta AK, Gupta MA, Summerbell RC, et al. The epidemiology of onychomycosis: possible role of smoking and peripheral arterial disease. J Eur Acad Dermatol Venereol. 2000;14(6):466–469.
- Onychomycosis: Current Trends in Diagnosis and Treatment. Am Fam Physician. 2013 Dec 1;88(11):762-770. http://www.aafp.org/afp/2013/1201/p762.html
- Hay RJ, Baran R. Onychomycosis: a proposed revision of the clinical classification. J Am Acad Dermatol. 2011;65(6):1219–1227.
- Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003;149(suppl 65):1–4.
- Baran R, Kaoukhov A. Topical antifungal drugs for the treatment of onychomycosis: an overview of current strategies for monotherapy and combination therapy. J Eur Acad Dermatol Venereol. 2005;19(1):21–29.
- Scher RK, Tavakkol A, Sigurgeirsson B, et al. Onychomycosis: diagnosis and definition of cure. J Am Acad Dermatol. 2007;56(6):939–944.
- Rotta I, Sanchez A, Gonçalves PR, Otuki MF, Correr CJ. Efficacy and safety of topical antifungals in the treatment of dermatomycosis: a systematic review. Br J Dermatol. 2012;166(5):927–933.
- Gupta AK, Fleckman P, Baran R. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J Am Acad Dermatol. 2000;43(4 suppl):S70–S80.
- Gupta AK, Skinner AR. Onychomycosis in children: a brief overview with treatment strategies. Pediatr Dermatol. 2004;21(1):74–79.
- Crawford F, Hollis S. Topical treatments for fungal infections of the skin and nails of the foot. Cochrane Database Syst Rev. 2007;(3):CD001434.
- Avner S, Nir N, Henri T. Combination of oral terbinafine and topical ciclopirox compared to oral terbinafine for the treatment of onychomycosis. J Dermatolog Treat. 2005;16(5–6):327–330.
- Derby R, Rohal P, Jackson C, Beutler A, Olsen C. Novel treatment of onychomycosis using over-the-counter mentholated ointment: a clinical case series. J Am Board Fam Med. 2011;24(1):69–74.
- Buck DS, Nidorf DM, Addino JG. Comparison of two topical preparations for the treatment of onychomycosis: Melaleuca alternifolia (tea tree) oil and clotrimazole. J Fam Pract. 1994;38(6):601–605.
- Romero-Cerecero O, Zamilpa A, Jiménez-Ferrer JE, Rojas-Bribiesca G, Román-Ramos R, Tortoriello J. Double-blind clinical trial for evaluating the effectiveness and tolerability of Ageratina pichinchensis extract on patients with mild to moderate onychomycosis. A comparative study with ciclopirox [published correction appears in Planta Med. 2008;74(14):1767]. Planta Med. 2008;74(12):1430–1435.
- Rehder P, Nguyen TT. A new concept in the topical treatment of onychomycosis with cyanoacrylate, undecylenic acid, and hydroquinone. Foot Ankle Spec. 2008;1(2):93–96.
- Jennings MB, Pollak R, Harkless LB, Kianifard F, Tavakkol A. Treatment of toenail onychomycosis with oral terbinafine plus aggressive debridement: IRON-CLAD, a large, randomized, open-label, multicenter trial. J Am Podiatr Med Assoc. 2006;96(6):465–473.
- Malay DS, Yi S, Borowsky P, Downey MS, Mlodzienski AJ. Efficacy of debridement alone versus debridement combined with topical antifungal nail lacquer for the treatment of pedal onychomycosis: a randomized, controlled trial. J Foot Ankle Surg. 2009;48(3):294–308.
- Gupta AK, Simpson F. Newly approved laser systems for onychomycosis. J Am Podiatr Med Assoc. 2012;102(5):428–430.
- Kimura U, Takeuchi K, Kinoshita A, Takamori K, Hiruma M, Suga Y. Treating onychomycoses of the toenail: clinical efficacy of the sub-millisecond 1,064 nm Nd:YAG laser using a 5 mm spot diameter. J Drugs Dermatol. 2012;11(4):496–504.
- 510(k) summary: Noveon (Model LS1100-01-0968) dual wavelength leser instrument. http://www.accessdata.fda.gov/cdrh_docs/pdf7/K071815pdf
- Landsman AS, Robbins AH. Treatment of mild, moderate, and severe onychomycosis using 870- and 930-nm light exposure: some follow-up observations at 270 days. J Am Podiatr Med Assoc. 2012;102(2):169–171.
- Sotiriou E, Koussidou-Eremonti T, Chaidemenos G, Apalla Z, Ioannides D. Photodynamic therapy for distal and lateral subungual toenail onychomycosis caused by Trichophyton rubrum: preliminary results of a single-centre open trial. Acta Derm Venereol. 2010;90(2):216–217.
- Gupta AK, Ryder JE, Johnson AM. Cumulative meta-analysis of systemic antifungal agents for the treatment of onychomycosis. Br J Dermatol. 2004;150(3):537–544.
- Antifungal drugs. Treat Guidel Med Lett. 2009;7(88):95–102.
- Chang CH, Young-Xu Y, Kurth T, Orav JE, Chan AK. The safety of oral antifungal treatments for superficial dermatophytosis and onychomycosis: a meta-analysis. Am J Med. 2007;120(9):791–798.
- Pigmented lesions of the nail bed – Clinical assessment and biopsy. Australian Family Physician Volume 45, No.11, November 2016 Pages 810-813. https://www.racgp.org.au/afp/2016/november/pigmented-lesions-of-the-nail-bed-%E2%80%93-clinical-assessment-and-biopsy/
- Levit EK, Kagen MH, Scher RK, Grossman M, Altman E. The ABC rule for clinical detection of subungual melanoma. J Am Acad Dermatol. 2000;42(2 pt 1):269–274.
- Ruben BS. Pigmented lesions of the nail unit. Semin Cutan Med Surg 2015;34(2):101–08.
- Rigopoulos D, Larios G, Gregoriou S, Alevizos A. Acute and chronic paronychia. Am Fam Physician. 2008;77(3):339–346.
- Rockwell PG. Acute and chronic paronychia. Am Fam Physician. 2001;63(6):1113–1116.
- Wollina U. Acute paronychia: comparative treatment with topical antibiotic alone or in combination with corticosteroid. J Eur Acad Dermatol Venereol. 2001;15(1):82–84.
- Jebson PJ. Infections of the fingertip. Paronychias and felons. Hand Clin. 1998;14(4):547–555,viii.
- Fawcett RS, Linford S, Stulberg DL. Nail abnormalities: clues to systemic disease. Am Fam Physician. 2004;69(6):1417–1424.
- Gregoriou S, Argyriou G, Larios G, Rigopoulos D. Nail disorders and systemic disease: what the nails tell us. J Fam Pract. 2008;57(8):509–514.
- Chauhan S, D’Cruz S, Singh R, Sachdev A. Mees’ lines. Lancet. 2008;372(9647):1410.
- Zaiac MN, Daniel CR III. Nails in systemic disease. Dermatol Ther. 2002;15(2):99–106.
- Tosti A, Iorizzo M, Piraccini BM, Starace M. The nail in systemic diseases. Dermatol Clin. 2006;24(3):341–347.
- Bostanci S, Ekmekci P, Akyol A, Peksari Y, Gürgey E. Pincer nail deformity: inherited and caused by a beta-blocker. Int J Dermatol. 2004;43(4):316–318.
- Ghaffarpour G, Tabaie SM, Ghaffarpour G. A new surgical technique for the correction of pincernail deformity: combination of splint and nail bed cutting. Dermatol Surg. 2010;36(12):2037–2041.
- Saladi RN, Persaud AN, Rudikoff D, Cohen SR. Idiopathic splinter hemorrhages. J Am Acad Dermatol. 2004;50(2):289–292.
- Sánchez-Regaña M. Super U Technique for Ingrown Nails. Actas Dermosifiliogr. 2017;108:393