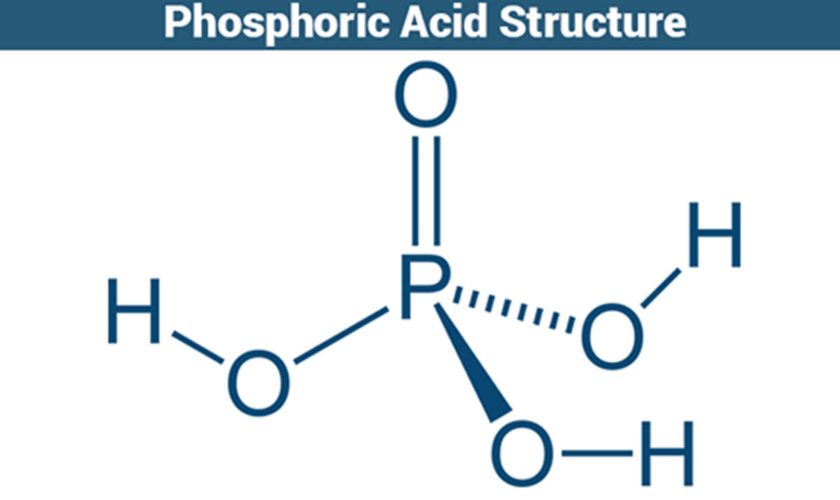
What is phosphoric acid
Phosphoric Acid (H3PO4) is a clear colorless liquid or transparent crystalline solid, an odorless phosphorus-containing strong inorganic acid. Phosphoric acid contains not less than 85% of H3PO4. Phosphoric acid is a sequestering agent which binds many divalent cations, including Fe++, Cu++, Ca++, and Mg++. Phosphoric acid is used in dentistry and orthodontics as an etching solution, to clean and roughen the surfaces of teeth where dental appliances or fillings will be placed. In addition, phosphoric acid is a constituent in bone and teeth, and plays a role in many metabolic processes. Phosphoric acid liquid is usually an 85% aqueous solution. Shipped as both a solid and liquid. Corrosive to metals and tissue. Phosphoric acid is also used in making fertilizers, detergents and in foods and beverages processing, water treatment, pickling and rust proofing metals, and for many other purposes. Phosphoric acid also used in photography (platinum printing) and as a wet etchant in semiconductor manufacturing at a standard concentration of 85%.
Phosphorus-containing substances occur very widely in natural foods usually as free phosphoric acid or as the potassium, sodium or calcium salts. Phosphate is found in highest concentrations (0.1-0.5% or more, in terms of phosphorus) in such foods as milk, cheese, nuts, fish, meat, poultry, eggs (yolk), and certain cereals 1.
Phosphoric acid in food is used as a sequestrant, an antioxidant and a “synergist” for other antioxidants; also as an acidulant and flavor in beverages and fruit
products 1.
Figure 1. Phosphoric acid
What does phosphoric acid do?
Phosphoric acid is an essential constituent of the human organism, not only in the bones and teeth, but also in many enzyme systems. Phosphorus plays an important role in carbohydrate, fat and protein metabolism.
The daily intake of phosphate necessary for human lies between 1 and 2 g 1. Insufficient supply of phosphate produces deficiency in the bones. Since the phosphate concentration of serum and tissues is maintained by physiological regulations, the intestinal absorption depends on requirements and is therefore limited. Doses of 2 to 4 g act as weak saline cathartics. Excretion takes place mainly in the feces as calcium phosphate, so that the continuous use of excessive amounts of sodium phosphate and phosphoric acid may cause a loss of calcium 1.
There have been a great many publications on phosphorus metabolism, on the interrelationships of calcium and phosphorus in foods and nutrition and on the impact thereon of the use of phosphate as a food additive.
Low-quality evidence from 1 study 2 showed that patients (any kidney stone type) with baseline soft drink consumption of more than 160 mL per day who were instructed to abstain from drinking soda had a reduced risk for symptomatic kidney stone recurrence compared with no treatment (33.7% vs. 40.6%). Subgroup analysis showed that the benefit was limited to patients who drank soda that was acidified by phosphoric acid (typically colas) rather than those acidified by citric acid (typically fruit-flavored sodas) (29.7% vs. 45.6%). Therefore decreasing soft drink intake in people with a high baseline intake of soft drinks acidified by phosphoric acid also decreased kidney stone recurrence 3.
Is phosphoric acid bad for you?
According to the Joint The Food and Agriculture Organization of the United Nations and the World Health Organization Expert Committee on Food Additives 4, “there is ample evidence to support the safety of the addition of small quantities of phosphoric acid to food. Thus, the use of 0.01-0.02% as a sequestrant, an antioxidant or “synergist” inantioxidant mixtures should present no health hazards whatsoever”.
The Joint The Food and Agriculture Organization of the United Nations and the World Health Organization Expert Committee on Food Additives 4, further added, “the use of phosphoric acid to compensate for deficiency of fruit acidity, as a flavor component and in other ways, essentially within the “normal” concentration of phosphates naturally occurring in foods, should present no problem”.
Estimate of acceptable daily intakes for man 4:
- Unconditional acceptance 0-5 mg/kg body-weight
- Conditional acceptance 5-15 mg/kg body-weight
The total dietary intake of phosphorus from both foods and food additives should not exceed 4:
- Unconditional acceptance up to 30 mg/kg body-weight
- Conditional acceptance 30-70 mg/kg body-weight
A report by the U.S. Food and Drug Administration [FDA] 5 found that high phosphate intakes can affect calcium distribution in the body and may in some cases produce soft tissue calcification and affect bone formation. Kidney damage, soft tissue calcification and bone effects were the main findings in laboratory animals fed phosphates 6. However, such effects were not observed in studies in humans, except in pa-tients with end stage renal disease. In ill individuals, hypophosphatemia is caused by vomiting and se-vere diarrhoea, and is associated with various liver diseases 7.
In the same FDA report 5, the FDA found no conclusive evidence of reproductive effects has been demonstrated in feeding studies with phosphate salts in animals. No carcinogenic potential was demonstrated in feeding studies in rats treated with phosphates; however several phosphates have been shown to
promote the effects of known carcinogens in rodents. Genotoxicity assays have yielded essentially negative results with phosphate salts 5.
Human Toxicity Studies
Studies on 15 students, who drank 2000-4000 mg of phosphoric acid in fruit juices every day for 10 days, and on 2 males who received 3900 mg of phosphoric acid every day for 14 days, revealed no observable change in urine composition indicative of a disturbed metabolism 8.
Consumption of soft drinks containing phosphoric acid has been linked to the recurrence of urinary stones in adult men 9. The mechanism is likely to involve increased calcium phosphate precipitation 9.
15 non-smoking adults aged 18-36 years were exposed to phosphoric acid aerosols. No airways irritation was reported at a concentration of 1.6 mg/m³. 18% of subjects reported airways irritation at 7.2 mg/cu m and 82% at 11.0 mg/m³ 10.
In a study in which the daily basal diet of 4 men contained 450 mg calcium and 1400 mg phosphorus, supplementation with 750 mg phosphorus as phosphoric acid for 1 week resulted in a slight decrease in urinary excretion of calcium. When the treatment was continued for 12 weeks, there was a further decrease in urinary calcium excretion 4.
A 42 year-old man presented with oropharyngeal burns and hematemesis one hour after ingesting 240 mL phosphoric acid. Endoscopy revealed moderate distal esophagitis and severe proximal gastritis. The duodenum was normal. The gastric injuries healed completely 11.
A 64 year-old man ingested 90-120 mL 20% hydrogen phosphate in attempted suicide. On admission one hour later he complained of a burning throat, hoarseness, mild abdominal pain and nausea. He had watery stools and vomited approximately 100 mL blood-stained liquid. The posterior pharynx was inflamed with discrete patches of mucosal pallor, the abdomen tender and the stool contained blood. Patient developed hyperphosphatemia, hypocalcaemia and a metabolic acidosis. Serum concentrations of calcium and phosphate were 2.05 mmol/L and 2.3 mmol/L respectively. Arterial blood gas analysis showed pH 7.19, HCO3- 6 mmol/L and an anion gap of 23 mmol/L. These abnormalities resolved within 36 hours following intravenous fluid and sodium bicarbonate plus oral aluminium hydroxide (as a phosphate binder). The patient recovered fully 11.
Phosphoric acid (orthophosphoric acid, metaphosphoric acid) topically may irritate and injure the eyes, owing to its acidity, but systemically phosphate has no poisonous action on the eye 12. Tested on human eyes, 0.16 M orthophosphoric acid buffered to pH 2.5 caused moderate brief stinging sensation but no injury when applied as a single drop. A drop of the same solution adjusted to pH 3.4 caused no discomfort.
A railroad accident in Somerville, Massachusetts led to a phosphorus trichloride liquid spillage 13. Attempted clean up with water led to the liberation of phosphorus trichloride, phosphorus acid, hydrogen chloride, and phosphorous oxides. Seventeen people exposed to this mixture were studied. Patients experienced eye irritation, lacrimation, nausea, vomiting, and dyspnea. Six patients had transient lactic dehydrogenase elevation. Although all patients had normal chest roentgenographic findings, pulmonary function tests showed statistically significant decreases in vital capacity, maximal breathing capacity, and forced expiratory volume vital capacity in those closest to the accident site. Patients exposed < 1.5 hour had significantly greater maximal expiratory flow rates at 25% of vital capacity when compared with patients who had been exposed longer. In seven patients, repeated pulmonary function tests one month later showed improvement, suggesting that the acute effects may have been due to phosphorus acid toxicity 13.
Phosphoric acid at high concentrations is corrosive to all tissues with which it comes in contact. It can cause severe skin burns at concentrations of 75% or greater. Inhalation of the vapor or mist can cause eye, nose, throat, and respiratory irritation or coughing. When ingested, it can produce nausea, vomiting, abdominal pain, bloody diarrhea, acidosis, shock, and irritation or burns of the oropharyngeal mucosa, esophagus, and stomach.
When used as an agent for metal cleaning, phosphoric acid may react with impurities in the metal and release phosphine gas.
Phosphoric acid toxicity
Ten to 25 per cent phosphoric acid solutions are irritant and more concentrated solutions corrosive.
Skin exposure, inhalation or ingestion of any quantity of a concentrated solution can be dangerous.
5 mL of a 1.0 per cent phosphoric acid solution was not caustic to the oral mucosa 14. A patient has survived ingestion of 90-120 mL of a metal cleaner containing 20 per cent “hydrogen phosphate” 15.
Skin
- Solutions greater than 10 per cent phosphoric acid are irritating to the skin and higher concentrations may cause burns.
Eyes
- Direct contact with phosphoric acid may irritate or burn the eye causing pain, blepharospasm, lacrimation and/or photophobia.
Inhalation
- Cough and retrosternal discomfort may be the only early features following phosphoric acid inhalation. Following significant exposure hoarseness, dyspnea (shortness of breath) and stridor (due to laryngeal edema) may develop. In the most severe cases the onset of non-cardiogenic pulmonary edema with increasing breathlessness, wheeze and cyanosis may be delayed for up to 36 hours.
- Bronchitis was reported in 46% and an obstructive lung function defect in 37% of 35 workers at a phosphoric acid production plant 11.
- A patient with no previous history of asthma developed wheeze associated with chemical pneumonitis after accidental phosphoric acid inhalation. Evidence of airways hyper-responsiveness persisted one year later 11.
Ingestion
- Ingestion of greater than 10 per cent phosphoric acid solutions will cause immediate burning of the mouth and throat possibly with retrosternal and abdominal pain, nausea and vomiting.
- Severe irritant or corrosive effects are likely following ingestion of greater than 20 per cent phosphoric acid solutions with hypersalivation, hematemesis (vomiting blood) and hypovolemic shock.
- There is a risk of gastric antrum ulceration, hemorrhage and perforation.
- The larynx may be burned, with edema causing airway obstruction.
- Obstructive symptoms due to esophageal or gastric stricture may develop weeks or months later.
- There is a single report of hyperphosphatemia, hypocalcemia and metabolic acidosis occurring after acute ingestion of 90-120 mL of 20 per cent phosphoric acid 15.
- Investigators described the death of one individual 19 days after ingestion of phosphoric acid as a result of recurrent internal hemorrhage. Necrosis of the and lower digestive tract and of the pancreas was evident at autopsy 16.
What is phosphoric acid used for?
The dominant use of phosphoric acid is for fertilizers, consuming approximately 90% of production 17.
Food additive
Food-grade phosphoric acid (additive E 338) is used to acidify foods and beverages such as various colas and jams 18. It provides a tangy or sour taste. Various salts of phosphoric acid, such as monocalcium phosphate, are used as leavening agents 17. Phosphoric acid in soft drinks has the potential to cause dental erosion 19. Phosphoric acid also has the potential to contribute to the formation of kidney stones, especially in those who have had kidney stones previously 20.
Rust removal
Phosphoric acid may be used to remove rust by direct application to rusted iron, steel tools, or other surfaces. The phosphoric acid changes the reddish-brown iron(III) oxide, Fe2O3 (rust), to ferric phosphate, FePO4.
Liquid phosphoric acid may be used for dipping, but phosphoric acid for rust removal is more often formulated as a gel. As a thick gel, it may be applied to sloping, vertical, or even overhead surfaces. Different phosphoric acid gel formulations are sold as “rust removers,” “rust killers” or “naval jelly.” Multiple applications of phosphoric acid may be required to convert all rust. Rust may also be removed by phosphate conversion coating. This process can leave a black phosphate coating that provides moderate corrosion resistance such protection is also provided by the superficially similar Parkerizing and blued electrochemical conversion coating processes.
In dentistry
Phosphoric acid is used in dentistry and orthodontics as an etching solution, to clean and roughen the surfaces of teeth where dental appliances or fillings will be placed. Phosphoric acid is also an ingredient in over-the-counter anti-nausea medications that also contain high levels of sugar (glucose and fructose). This acid is also used in many teeth whiteners to eliminate plaque that may be on the teeth before application.
Other applications
Among many applications, phosphoric acid is used:
- As a solution for anodizing.
- As an external standard for phosphorus-31 nuclear magnetic resonance (31P NMR).
- As a buffer agent in biology and chemistry. For example, a buffer for high-performance liquid chromatography.
- As a chemical oxidizing agent for activated carbon production, as used in the Wentworth process.[20]
- As the electrolyte in phosphoric acid fuel cells.
- With distilled water (2–3 drops per gallon) as an electrolyte in oxyhydrogen generators.
- As a catalyst in the hydration of alkenes to produce alcohols, predominantly ethanol.
- As an electrolyte in copper electropolishing for burr removal and circuit-board planarization.
- As a flux by metal workers and hobbyists (such as model railroaders) as an aid to soldering.
- In compound semiconductor processing, phosphoric acid is a common wet etching agent: for example, in combination with hydrogen peroxide and water it is used to etch InGaAs selective to InP 21.
- Heated in microfabrication to etch silicon nitride (Si3N4). It is highly selective in etching Si3N4 instead of SiO2, silicon dioxide 22.
- As a cleaner by construction trades to remove mineral deposits, cementitious smears, and hard-water stains.
- As a chelant in some household cleaners aimed at similar cleaning tasks.
- In hydroponics pH solutions to lower the pH of nutrient solutions. While other types of acids can be used, phosphorus is a nutrient used by plants, especially during flowering, making phosphoric acid particularly desirable.
- As a pH adjuster in cosmetics and skin-care products.
- As a dispersing agent in detergents and leather treatment.
- As an additive to stabilize acidic aqueous solutions within a wanted and specified pH range.
- As a sanitizing agent in the dairy, food, and brewing industries 23.
- As a main reactive agent in electrochemical weld cleaning.
- In soil organic carbon determination.
- Eighth Report of the Joint FAO/WHO Expert Committee on Food Additives, Wld Hlth Org. techn. Rep. Ser., 1965, 309; FAO Nutrition Meetings Report Series 1965, 38. http://www.inchem.org/documents/jecfa/jecmono/v38aje10.htm
- ShusterJJenkinsALoganCBarnettTRiehleRZacksonDet alSoft drink consumption and urinary stone recurrence: a randomized prevention trial., J Clin Epidemiol, 1992, vol. 45, pg. 911-6
- Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: A clinical practice guideline from the American College of Physicians”. Annals of Internal Medicine. 161 (9): 659–67. http://annals.org/aim/fullarticle/1920506/dietary-pharmacologic-management-prevent-recurrent-nephrolithiasis-adults-clinical-practice-guideline
- WHO/FAO; Joint Expert Committee on Food Additives (JECFA): Phosphoric acid and phosphate salts (WHO Food Additives Series 17 http://www.inchem.org/documents/jecfa/jecmono/v38aje10.htm
- GRAS Notice (GRN) No. 718 for Calcium acid pyrophosphate. https://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm593669.pdf
- Sanderson, P. H. (1959) Functional aspects of renal calcification in rats, Clin. Sei., 18,67-79
- Latner AL. 1975. Clinical biochemistry. Philadelphia: W.B. Saunders Co., 47-49, 279-315, 479, 842
- WHO/FAO: Expert Committee on food additives. FAO Nutrition Meetings Report Series 38a for Phosphoric acid (7664-38-2) (1964). http://www.inchem.org/documents/jecfa/jecmono/v38aje10.htm
- Shuster J, Jenkins A, Logan C, Barnett T, Riehle R, Zackson D, Wolfe. Soft drink consumption and urinary stone recurrence: A randomized prevention trial. J Clin Epidemiol 1992: 45: 911-6.
- National Poisons Information Service; United Kingdom Poison Information Documents (UKPID): Phosphoric Acid (7664-38-2) (January 28, 1998). http://www.inchem.org/documents/ukpids/ukpids/ukpid73.htm
- National Poisons Information Service; United Kingdom Poison Information Documents (UKPID): Phosphoric Acid (7664-38-2);January 28, 1998 http://www.inchem.org/documents/ukpids/ukpids/ukpid73.htm
- Grant, W.M. Toxicology of the Eye. 3rd ed. Springfield, IL: Charles C. Thomas Publisher, 1986., p. 733
- Phosphorus trichloride toxicity. Preliminary report. Am J Med. 1984 Dec;77(6):1039-42. https://www.amjmed.com/article/0002-9343(84)90185-2/pdf
- von Muhlendahl KE, Oberdisse U, Krienke EG. Local injuries by accidental ingestion of corrosive substances by children. Arch Toxicol 1978; 39: 299-314.
- Caravati EM. Metabolic abnormalities associated with phosphoric acid ingestion. Ann Emerg Med 1987; 16: 904-6.
- American Conference of Governmental Industrial Hygienists. Documentation of the TLV’s and BEI’s with Other World Wide Occupational Exposure Values. CD-ROM Cincinnati, OH 45240-4148 2010
- Klaus Schrödter, Gerhard Bettermann, Thomas Staffel, Friedrich Wahl, Thomas Klein, Thomas Hofmann “Phosphoric Acid and Phosphates” in Ullmann’s Encyclopedia of Industrial Chemistry 2008, Wiley-VCH, Weinheim. https://doi.org/10.1002/14356007.a19_465.pub3
- EU Approved additives and E Numbers. https://www.food.gov.uk/business-guidance/eu-approved-additives-and-e-numbers
- Dietary advice in dental practice. British Dental Journal volume 193, pages 563–568; 23 November 2002. https://www.nature.com/articles/4801628.pdf
- Dietary and pharmacologic management to prevent recurrent nephrolithiasis in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2014 Nov 4;161(9):659-67. doi: 10.7326/M13-2908. http://annals.org/aim/fullarticle/1920506/dietary-pharmacologic-management-prevent-recurrent-nephrolithiasis-adults-clinical-practice-guideline
- Wet Chemical Etching. http://terpconnect.umd.edu/~browns/wetetch.html
- Wolf, S.; R. N. Tauber (1986). Silicon processing for the VLSI era: Volume 1 – Process technology. p. 534. ISBN 0-9616721-6-1.
- http://www.fivestarchemicals.com/wp-content/uploads/StarSanTech-HB2.pdf