What is plasmapheresis
Plasmapheresis is also called plasma exchange or apheresis, which involves being attached to a machine that removes blood from your vein to filter out the harmful antibodies such as monoclonal paraproteins and pathogenic autoantibodies, immune complexes, cryoglobulins, myeloma light chains, endotoxin, and cholesterol-containing lipoproteins 1, as well as replaces the deficient plasma components when plasma is used as the replacement fluid before returning the blood, primarily red blood cells, with various fluids to your body 2. The term plasmapheresis and plasma exchange is used interchangeably and synonymously. Therapeutic plasmapheresis has been successfully used in various antibody‐mediated diseases 3. Plasmapheresis was first used in 1952 in the setting of multiple myeloma to treat hyperviscosity 4 and since then has emerged as an important treatment modality for a number of neurological and other conditions 5.
More selective plasma separation methods can remove nonspecific immunoglobulins from the patient’s blood by new developed adsorbers 6. Since the pathogenetic relevance of auto‐antibodies could be defined in various diseases, disease‐specific adsorbers have been developed 7. These adsorbers also remove selective, immune complexes, immunoglobulins, and other substances from the patient’s blood.
The term autoimmune disease relates to diseases caused by antibodies acting against the body’s own tissue 2. They are also referred to as autoaggressional diseases. Autoimmune diseases, with exception of rheumatoid arthritis and autoimmune thyroiditis, are individually rare, but together they affect approximately 5% of the population in western countries 8. The cause of autoimmune reactions is still generally unknown.
Autoantibodies directly cause the destruction of the target cells in lysis. The cytotoxic antibodies react through complement activation with antigens of the cell surface and cause an intravascular lysis of erythrocytes through stages, (e.g., paroxysmal hemoglobulinuremia) particularly in the case of hematological diseases. In autoimmune hemolytic anemia for example, the affected erythrocytes can be opsonized by the antibodies. The process of binding of antibodies with complement participation changes the cells such that they are increasingly phagocytized, whereby the Fc‐parts of the bound antibodies are recognized by the Fc‐receptors of the phagocytizing cells and by the cells of the RES in the liver and the spleen. The so‐called immune clearance is the opsonization process, a physiologically effective way of removing intruding cells through immune bodies 6.
Inflammation is a complex set of events accompanied by the release of many different soluble antibodies that diffuse away from the side of their production. Autoantibodies can be detected in all tissues and can be directed against many non‐hematogenous tissues. Antibodies are transported via efferent lymphatics into the venous and circulate with the blood through the body. Antibodies of the IgG class can transverse blood vessel wall and enter extravascular tissue spaces 6.
Antibody occupation of cells or tissue structures does not necessarily mean that damage occurs. This only happens when mediators are involved. Autoantibodies can have a serious effect on an organ even without the activation of the complement system, especially when either functionally important receptors are blocked by antibodies or else important proteins are rendered inactive through the combination with antibodies, such as hormones or enzymes. Myasthenia gravis is a classic example of a receptor blockade.
The term immune complex disease refers to diseases caused by antigen‐antibody complexes. The antigen is sometimes of infectious origin. In most cases, however, neither the origin nor the structure of the antigen is clear. Immune complex formation is a physiological process for eliminating foreign material, such as bacteria, their components and viruses. Normally immune complexes are removed from the blood by adhesion of the Fc‐fragments of the antibodies to the corresponding phagocyte receptors in the liver and spleen. If the immune complexes activate the complement system (immune clearance) phagocytosis can even be enhanced. More than 80% of glomerulonephritis cases are caused by intra renal deposited circulating immune complexes. If the antigen adheres to the basal membrane and binds circulating antibodies, the immune complexes probably first form in situ 6.
Plasma is the largest single component of blood, and makes up about 55% of total blood volume (see Figure 2 below). It is a clear, straw-colored liquid, which carries platelets, red and white blood cells.
Plasma contains over 700 proteins and other substances, which can be extracted and which are key ingredients in medical products.
Once separated from blood cells, plasma can be:
- used in blood transfusions
- separated out into its many individual proteins which are used to make medical products
plasmapheresis vs plasma exchange,
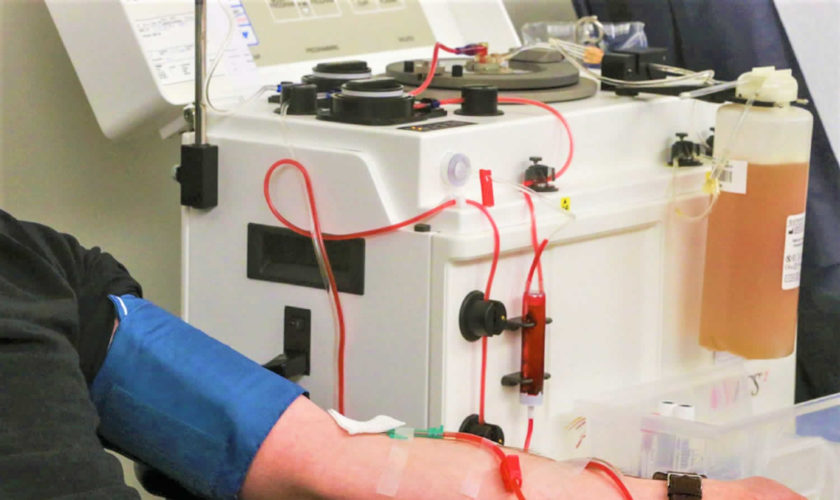
Figure 1. Plasmapheresis machine
What is blood plasma
How much blood you have depends mostly on your size and weight. A man who weighs about 70 kg (about 154 pounds) has about 5 to 6 liters of blood in his body. Blood is 55% blood plasma and about 45% different types of blood cells. Blood is composed of solid particles (red blood cells, white blood cells, and cell fragments called platelets) suspended in a fluid extracellular matrix called blood plasma. Over 99% of the solid particles present in blood are cells that are called red blood cells (erythrocytes) due to their red color. The rest are pale or colorless white blood cells (leukocytes) and platelets (thrombocytes).
The blood plasma is the clear, straw-colored, liquid portion of the blood in which the cells (red blood cells, white blood cells) and platelets are suspended. It is
approximately 92% water and less than 8% is dissolved substances, mostly proteins, a complex mixture of organic and inorganic biochemicals. Functions of plasma include transporting gases, vitamins, and other nutrients; helping to regulate fluid and electrolyte balance; and maintaining a favorable pH. Blood plasma also contain antibodies to fight infections (part of the immune system), glucose, amino acids and the proteins that form blood clots (part of the hemostatic system)..
Blood plasma contains electrolytes that are absorbed from the intestine or released as by-products of cellular metabolism. They include sodium, potassium, calcium, magnesium, chloride, bicarbonate, phosphate, and sulfate ions. Sodium and chloride ions are the most abundant. Bicarbonate ions are important in maintaining the pH of plasma. Like other plasma constituents, bicarbonate ions are regulated so that their blood concentrations remain relatively stable.
Blood transports a variety of materials between interior body cells and those that exchange substances with the external environment. In this way, blood helps maintain stable internal environmental conditions.
Hemostasis refers to the process that stops bleeding, which is vitally important when blood vessels are damaged. Following an injury to the blood vessels, several actions may help to limit or prevent blood loss. These include vascular spasm, platelet plug formation, and blood coagulation.
Platelets adhere to any rough surface, particularly to the collagen in connective tissue. When a blood vessel breaks, platelets adhere to the collagen underlying the endothelium lining blood vessels. Platelets also adhere to each other, forming a platelet plug in the vascular break. A plug may control blood loss from a small break, but a larger break may require a blood clot to halt bleeding.
Coagulation, the most effective hemostatic mechanism, forms a blood clot in a series of reactions, each one activating the next. Blood coagulation is complex and utilizes many biochemicals called clotting factors. Some of these factors promote coagulation, and others inhibit it. Whether or not blood coagulates depends on the balance between these two groups of factors. Normally, anticoagulants prevail, and the blood does not clot. However, as a result of injury (trauma), biochemicals that favor coagulation may increase in concentration, and the blood may coagulate. The resulting mass is a blood clot, which may block a vascular break and prevent further blood loss. The clear, yellow liquid that remains after the clot forms is called serum. Serum is plasma minus the clotting factors.
Note: Blood is a complex mixture of formed elements in a liquid extracellular matrix, called blood plasma. Note that water and proteins account for 99% of the blood plasma.
Figure 2. Blood composition
Note: Blood consists of a liquid portion called plasma and a solid portion (the formed elements) that includes red blood cells, white blood cells, and platelets. When blood components are separated by centrifugation, the white blood cells and platelets form a thin layer, called the “buffy coat,” between the plasma and the red blood cells, which accounts for about 1% of the total blood volume. Blood cells and platelets can be seen under a light microscope when a blood sample is smeared onto a glass slide.
Blood Plasma Proteins
Blood plasma proteins are the most abundant of the dissolved substances (solutes) in plasma. These proteins remain in the blood and interstitial fluids, and ordinarily are not used as energy sources. The three main types of blood plasma proteins—albumins, globulins, and fibrinogen—differ in composition and function.
Albumins are the smallest plasma proteins, yet account for about 60% of them by weight. Albumins are synthesized in the liver.
Plasma proteins are too large to pass through the capillary walls, so they are impermeant. They create an osmotic pressure that holds water in the capillaries, despite blood pressure forcing water out of capillaries by filtration. The term colloid osmotic pressure is used to describe this osmotic effect due to the plasma proteins. Because albumins are so plentiful, they are an important determinant of the colloid osmotic pressure of the plasma.
By maintaining the colloid osmotic pressure of plasma, albumins and other plasma proteins help regulate water movement between the blood and the tissues. In doing so, the blood plasma proteins help control blood volume, which, in turn, directly affects blood pressure.
If the concentration of plasma proteins falls, tissues swell. This condition is called edema. As the concentration of plasma proteins drops, so does the colloid osmotic pressure. Water leaves the blood vessels and accumulates in the interstitial spaces, causing swelling. A low plasma protein concentration may result from starvation, a protein-deficient diet, or an impaired liver that cannot synthesize plasma proteins.
Globulins make up about 36% of the plasma proteins. They can be further subdivided into alpha, beta, and gamma globulins. The liver synthesizes alpha and
beta globulins, which have a variety of functions. The globulins transport lipids and fat-soluble vitamins. Lymphatic tissues produce the gamma globulins, which are a type of antibody.
Fibrinogen constitutes about 4% of the plasma proteins, and functions in blood coagulation (clotting). Fibrinogen is synthesized in the liver, fibrinogen is the largest of the plasma proteins.
Plasma products and their uses
Plasma products can be grouped into three main types:
- clotting or coagulation factors
- albumin solutions
- immunoglobulins
Coagulation or clotting factors
Coagulation is the name for the complex process of blood clotting. Clotting factors are proteins that work together with platelets to clot blood.
People need clotting factors for their blood to successfully clot. Missing one or more of these factors leads to blood clotting disorders such as haemophilia and Von Willebrand disease.
In the US, hemophilia is commonly treated with ‘recombinant factors’ that are manufactured in a laboratory and do not come from donated plasma.
Other blood clotting disorders that are treated with coagulation factors made from donated plasma.
Albumin
Albumin is the most common protein in blood plasma. It helps to:
- carry substances around the body
- maintain the right amount of fluid circulating in the body
If the circulation is working properly, vital hormones, cells and enzymes are transported to the right parts of the body to do their job.
If it’s not working properly, the circulatory system starts to break down, with serious consequences such as fluids being retained in the cells.
This can be treated by using human albumin solution which makes sure that the right amount of fluid is circulating in the blood stream.
Albumin can also be used to treat people with some types of liver or kidney disease and patients who have suffered burns.
Immunoglobulins
Immunoglobulins are protective antibodies which are produced by the body to fight against invading viruses or bacteria. There are two different types of immunoglobulins, specific and non-specific.
Specific immunoglobulins
Specific immunoglobulins contain high levels of antibody to a particular illness. These are given to people who have been exposed to a specific infection.
Antidotes to tetanus, rabies, chickenpox and hepatitis are all examples of specific immunoglobulins.
For example, a donor who has had chicken pox will have high levels of chicken pox antibodies. So their plasma would be ideal to treat a child with leukaemia who has been exposed to chicken pox.
Non-specific immunoglobulins
Non-specific immunoglobulins contain a wide variety of antibodies. These are given to people:
- who make faulty antibodies, or can’t make their own antibodies
- who are having treatments for diseases like cancer, where the treatment harms their ability to make antibodies
People with a faulty immune system need these products to live. Over 1,000 donations of plasma contribute to a single dose of an immunoglobulin product that contains all the necessary antibodies.
Table 1 summarizes the characteristics of the blood plasma proteins.
Table 1. Blood plasma proteins
Protein | Percentage of Total | Origin | Function |
Albumins | 60% | Liver | Help maintain colloid osmotic pressure |
Globulins | 36% | ||
Alpha globulins | Liver | Transport lipids and fat-soluble vitamins | |
Beta globulins | Liver | Transport lipids and fat-soluble vitamins | |
Gamma globulins | Lymphatic tissues | Constitute the antibodies of immunity | |
Fibrinogen | 4% | Liver | Plays a key role in blood coagulation |
Plasmapheresis indications
Plasmapheresis indication guidelines have been defined and revised in 2010 by the American Society for Apheresis and divided into four categories from 1 to 4 on the basis of available literature 9. Category 1 disorders are those for which plasmapheresis is accepted as first-line therapy either as primary standalone treatment or in conjunction with other models of treatment and include disorders such as Guillain–Barre syndrome (GBS), myasthenia gravis, chronic inflammatory demyelinating polyneuropathy, thrombotic thrombocytopenic purpura, Goodpasture’s syndrome, and atypical hemolytic uremic syndrome. The separation of plasma from blood can be achieved by centrifugation devices or with the use of hemodialysis machine and plasma filters. Although an Indian Society for Apheresis was created in 1985, there is a scarcity of data on PE from the Indian subcontinent. This is partly because the facility for PE is available in only large centers located mainly in the cities. With the aim of improving data collection about plasmapheresis procedures in the country, we undertook this retrospective study aiming to look at plasmapheresis procedures conducted in the nephrology department over a fixed time period.
For purposes of Medicare coverage, plasmapheresis (apheresis) is defined as an autologous procedure, i.e., blood is taken from the patient, processed, and returned to the patient as part of a continuous procedure (as distinguished from the procedure in which a patient donates blood preoperatively and is transfused with the donated blood at a later date).
Medicare reimburseable indications for plasmapheresis – this may not be an exhaustive list of all applicable Medicare benefit categories for this item or service 10.
Apheresis is covered for the following indications:
- Plasma exchange for acquired myasthenia gravis;
- Leukapheresis in the treatment of leukemia;
- Plasmapheresis in the treatment of primary macroglobulinemia (Waldenstrom);
- Treatment of hyperglobulinemias, including (but not limited to) multiple myelomas, cryoglobulinemia and hyperviscosity syndromes;
- Plasmapheresis or plasma exchange as a last resort treatment of thromobotic thrombocytopenic purpura (TTP);
- Plasmapheresis or plasma exchange in the last resort treatment of life threatening rheumatoid vasculitis;
- Plasma perfusion of charcoal filters for treatment of pruritis of cholestatic liver disease;
- Plasma exchange in the treatment of Goodpasture’s Syndrome;
- Plasma exchange in the treatment of glomerulonephritis associated with antiglomerular basement membrane antibodies and advancing renal failure or pulmonary hemorrhage;
- Treatment of chronic relapsing polyneuropathy for patients with severe or life threatening symptoms who have failed to respond to conventional therapy;
- Treatment of life threatening scleroderma and polymyositis when the patient is unresponsive to conventional therapy;
- Treatment of Guillain-Barre Syndrome; and
- Treatment of last resort for life threatening systemic lupus erythematosus (SLE) when conventional therapy has failed to prevent clinical deterioration.
Apheresis is covered only when performed in a hospital setting (either inpatient or outpatient) or in a nonhospital setting, e.g., a physician directed clinic when the following conditions are met:
- A physician (or a number of physicians) is present to perform medical services and to respond to medical emergencies at all times during patient care hours;
- Each patient is under the care of a physician; and
- All nonphysician services are furnished under the direct, personal supervision of a physician.
Plasmapheresis for neurologic disorders
Plasmapheresis is established as effective and should be offered in severe acute inflammatory demyelinating polyneuropathy/Guillain-Barré syndrome and in the short-term management of chronic inflammatory demyelinating polyneuropathy (Class I studies, Level A) 11. Plasmapheresis is established as ineffective and should not be offered for chronic or secondary progressive multiple sclerosis (MS) (Class I studies, Level A) 11. Plasmapheresis is probably effective and should be considered for mild acute inflammatory demyelinating polyneuropathy/Guillain-Barré syndrome, as second-line treatment of steroid-resistant exacerbations in relapsing forms of MS, and for neuropathy associated with immunoglobulin A or immunoglobulin G gammopathy, based on at least one Class I or 2 Class II studies (Level B) 11. Plasmapheresis is probably not effective and should not be considered for neuropathy associated with immunoglobulin M gammopathy, based on one Class I study (Level B). Plasmapheresis is possibly effective and may be considered for acute fulminant demyelinating CNS disease (Level C) 11. There is insufficient evidence to support or refute the use of plasmapheresis for myasthenia gravis, pediatric autoimmune neuropsychiatric disorders associated with streptococcus infection, and Sydenham chorea (Class III evidence, Level U) 11.
Plasmapheresis for myasthenia gravis
Because of the lack of randomized controlled studies with masked outcomes, there is insufficient evidence to support or refute the efficacy of plasmapheresis in the treatment of myasthenic crisis or myasthenia gravis prethymectomy 11.
Plasmapheresis for MS
Plasmapheresis should not be offered for chronic progressive or secondary progressive MS (multiple sclerosis) 11. There is no studies on the efficacy of plasmapheresis compared to other treatment options in MS are available 11. There is good evidence plasmapheresis should be considered for the adjunctive treatment of exacerbations in relapsing forms of MS 11. There is weak evidence plasmapheresis may be considered in the treatment of fulminant CNS demyelinating diseases that fail to respond to high-dose corticosteroid treatment 11.
Plasmapheresis for acute inflammatory demyelinating polyneuropathy or Guillain-Barre Syndrome
There is strong evidence plasmapheresis should be offered in the treatment of acute inflammatory demyelinating polyneuropathy/Guillain-Barre Syndrome severe enough to impair independent walking or to require mechanical ventilation 11. There is good evidence plasmapheresis should be considered in the treatment of milder clinical presentations of acute inflammatory demyelinating polyneuropathy/Guillain-Barre Syndrome 12. IV immunoglobulin (IVIg) is an alternative treatment used in patients with acute inflammatory demyelinating polyneuropathy/Guillain–Barré syndrome. There is insufficient evidence to demonstrate the superiority of plasmapheresis over IV immunoglobulin (IVIg).
Plasmapheresis for chronic inflammatory demyelinating neuropathy
There is strong evidence plasmapheresis should be offered as a short-term treatment for patients with chronic inflammatory demyelinating neuropathy 12. Steroids, IV immunoglobulin (IVIg), and immunosuppressants also have been used in the treatment of chronic inflammatory demyelinating neuropathy 12.
Plasmapheresis for polyneuropathy associated with IgA and IgG monoclonal gammopathy of undetermined significance (MGUS)
There is good evidence plasmapheresis should be considered in polyneuropathy associated with IgA and IgG monoclonal gammopathy of undetermined significance (MGUS) 12. Plasmapheresis should not be considered in the treatment of polyneuropathy associated with IgM MGUS 12.
Plasmapheresis for hematological diseases
Therapeutic plasmapheresis is indicated in the management of various hematological diseases. For most of these diseases, clear pathogenetic mechanisms of the disease are understood, and there are well‐defined criteria with regard to the therapy 13. Most medical management of immunohematological disorders requires the use of plasmapheresis, serological immunomodulation, and classical pharmacological immunosuppression with steroids, cytotoxic agents, and antimetabolites, where overall therapy is individually tailored to the needs of the patient. Controlled trials are difficult if not impossible because of variables such as severity of disease, degree of organ system damage before intervention, age and the existence of co‐morbid conditions. In some rare hematological diseases, it is impossible to recruit a large number of cases to perform a controlled clinical trial. Therefore, for most of these diseases only small series of cases are available for analysis.
Therapeutic plasmapheresis, semi‐selective cascade filtration or IA aimed at the causative antibodies can be used in diseases caused by antibodies or immune complexes. Adjuvant drug therapies are different for different diseases and are typically individualized in type, dose and duration of use. The plasmapheresis method chosen depends on the pathophysiological origin of a given disease. The physician who has chosen the plasmapheresis method must be knowledgeable concerning the half‐life time, the compartmental distribution of pathogenic plasma proteins, and the elimination of other toxic substances and complement components. Table 2 shows a selection of hematological and hemostasiological diseases in which plasmapheresis has been implemented with the categories and the recommendation grade (RG) from the AAC.
Table 2. Therapeutic plasmapheresis in hematological and hemostasiological diseases with immunologic origin
| Hematological and hemostasiological diseases | Apheresis Applications Committee of the ASFA, 2010, 2013 | |||||
|---|---|---|---|---|---|---|
| Therapeutic plasmapheresis modality | Category | Recommendation grade | Treated volume (TPV) | Replacement solution | Frequency | |
| Rhesus incompatibility | TPE | II | 2C | 1–1.5 | Human‐albumin‐electrolyte solution | Daily or every other day |
| Red cell alloimmunization in pregnancy | III | 2C | ||||
| Autoimmune hemolytic anemia | ||||||
| Warm autoimmune hemolytic disease | TPE | III | 2C | 1–1.5 | ||
| Cold agglutinin disease | II | 2C | ||||
| Aplastic anemia | TPE | III | 2C | 1–1.5 | ||
| Pure red cell aplasia | III | 1B | ||||
| ABO incompatible HPC Tx | TPE, RBC exchange ECP | II | 1B–2B | 1‐1.5 | Human‐albumin‐ electrolyte | Daily or every other day; series weekly |
| III | 2C | |||||
| Graft‐Versus‐Host Disease | ||||||
| Skin (acute) | II | 1C | 200–270 mL | |||
| Skin (chronic) | II | 1B | ||||
| Non skin (acute/chronic) | III | 2B | ||||
| Idiopathic thrombocytopenic purpura | TPE, IA‐Protein‐A | IV | 2C | 1–1.5 | Human‐albumin‐electrolyte, solution, plasma | Daily or every other day |
| III | 2C | |||||
| Post‐transfusion purpura | TPE | III | 2C | 1–1.5 | ||
| Coagulator factor inhibitors | ||||||
| Alloantibody | TPE, IA‐Protein‐A TPE, IA‐Protein‐A | IV | 2C | 1–1.5 | ||
| III | 2B | |||||
| Autoantibody | III | 2C | 1–1.5 | |||
| III | 1C | |||||
Footnotes:
- Category I: accepted for therapeutic plasmapheresis as first‐line therapy;
- Category II: accepted for therapeutic plasmapheresis as second‐line therapy;
- Category III: not accepted for therapeutic plasmapheresis, decision should be individualized;
- Category IV: not accepted for therapeutic plasmapheresis, Institutional Review Board (IRB) approval is desirable if therapeutic plasmapheresis is undertaken 14.
Abbreviations: ECP = extracorporeal photopheresis; IA = Protein‐A, Immunoadsorption on protein‐A; HPC = hematopoietic progenitor cell; Tx = transplantation.
Plasmapheresis for autoimmune diseases
The terms “systemic autoimmune disease” and “collagen vascular disease” describe a number of illnesses, the common characteristic of which is immune‐mediated destruction of intracellular structures in connective tissue, resulting in fibrinoid tissue damage 8. Based on an immune pathogenesis, the various organs form antigen components, which provoke formation of autoantibodies on the one hand, and circulating immune complexes causing inflammation in organ tissues on the other.
Antinuclear antibodies are to be found against most nuclear structures. The antibodies are typically directed against both cytoplasmic‐associated and cell membrane‐associated proteins, and also antibodies against cytoplasmic structures and cell membrane components. The different groups of antibodies observed in active and subclinical disease includes those against many extracellular antigens, such as collagen, myelin sheaths, immunoglobulins, basement membrane, intercellular bridges, hormones, and complement components 15.
Table 3. Therapeutic plasmapheresis in systemic lupus erythematosus (SLE), catastrophic antiphospholipid syndrome, and rheumatoid arthritis (RA)
| German Working Group of Clinical Nephrology | Apheresis Applications Committee of the ASFA, 2010, 2013 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Therapeutic plasmapheresis modality | Evidence class | Severity grade | Therapeutic plasmapheresis modality | Category | Recommendation grade | Treated Volume (TPV) | Replacement solution | Frequency | |
| Systemic lupus erythematosus (severe) | IA, Peptid‐GAM, Tryptophan, Dextran sulfate | III | B | TPE | II | 2C | 1‐1.5 | Human‐ albumin‐ electrolyte solution | Daily or every other day |
| Lupus nephritis | IV | 2B | |||||||
| Catastrophic antiphospholipid syndrome | III | C | TPE | III | 2C | ||||
| Rheumatoid arthritis | IA, Peptid‐GAM | Ib | A | IA | II | 2A | |||
Footnotes:
- Category I: accepted for therapeutic plasmapheresis as first‐line therapy;
- Category II: accepted for therapeutic plasmapheresis as second‐line therapy;
- Category III: not accepted for therapeutic plasmapheresis, decision should be individualized;
- Category IV: not accepted for therapeutic plasmapheresis, Institutional Review Board (IRB) approval is desirable if therapeutic plasmapheresis is undertaken 14.
Abbreviations: IA‐Protein‐A = Immunoadsorption on protein‐A; Peptid‐GAM = Immunoadsorption on synthetic Peptid‐GAM (Globaffin, Affina Immuntechnik, Germany); Tryptophan = immunoadsorption on tryptophan (Immunosorba, Asahi, Japan); Dextran sulfate, chemical adsorption on dextran sulfate (Liposorber, Kaneka, Japan).
Plasmapheresis for renal disorders
Many primary renal diseases are associated with autoantibodies, rendering them appealing indications for plasmapheresis. Some indications are well established by randomized controlled studies and are considered standard of care (Goodpasture and thrombotic thrombocytopenic purpura [TTP]). Others have less compelling or only anecdotal supporting evidence.
Anti–Glomerular Basement Membrane (anti-GBM) Antibody–Mediated Disease (Goodpasture syndrome)
A randomized controlled trial found plasmapheresis to provide a more rapid decrease in anti-glomerular basement membrane antibodies, lower posttreatment serum creatinine level, and decreased incidence of end-stage renal disease. Given these results and the integral role of the anti-glomerular basement membrane antibody, plasmapheresis as a means of rapidly decreasing anti-glomerular basement membrane titers has become the standard of care.
Treatment strategy:
- Early initiation of plasmapheresis is essential to avoid end-stage renal disease
- Initial prescription is 14 daily 4-L exchanges
- Continued plasmapheresis may be required if antibody titers remain increased
- Steroids, cyclophosphamide, or azathioprine are added to decrease production of anti-glomerular basement membrane antibody and minimize the inflammatory response
Crescentic Rapidly Progressive Glomerulonephritis (not associated with anti-GBM antibody)
Several controlled studies have failed to show a generalized benefit of plasmapheresis for all patients with rapidly progressive glomerulonephritis; however, subset analysis of all these studies showed plasmapheresis to be beneficial for patients presenting with severe disease or dialysis dependency. A more recent study (Jayne et al) limited to patients presenting with creatinine levels greater than 5.8 mg/dL (to convert creatinine in mg/dL to μmol/L, multiply by 88.4) appears to support this conclusion.
Patients with Wegener granulomatosis and microscopic polyarteritis who present with pulmonary hemorrhage appear to be more likely to present with IgM antineutrophil cytoplasmic antibodies (ANCAs). These patients may also respond to plasmapheresis.
Renal Failure in Multiple Myeloma
After exclusion of other forms of renal failure associated with multiple myeloma (eg, hypercalcemia, volume depletion, hyperuricemia, infection, and amyloidosis), patients considered to have light-chain–related “cast nephropathy” may benefit from plasmapheresis. plasmapheresis can decrease serum levels of light chains more rapidly than chemotherapy alone. A randomized controlled study found plasmapheresis to provide a more likely return of renal function and better overall survival 16. However, despite a 50% decrease in need for dialysis, a recently reported study did not find a statistically significant benefit for plasmapheresis 17.
Treatment considerations:
- Demonstration of free light chains in serum is essential if plasmapheresis is to be considered a rational treatment option (by standard immunofixation or the new free light chain assay)
- Successful plasmapheresis prescription is 3 to 4 L of plasma exchanged on 5 consecutive days
- Well-established (chronic) renal failure considered to be caused by cast nephropathy may respond less dramatically
- Newly available highly permeable hemofilter membranes may allow for light chain removal without significant albumin loss 18
IgA Nephropathy and Henoch-Schönlein Purpura
Case reports and small clinical series suggest a possible beneficial effect of plasmapheresis in the treatment of IgA-associated rapidly progressive glomerulonephritis.
Cryoglobulinemia
Despite a lack of randomized controlled studies, most experts agree plasmapheresis can be a useful adjunct for severe active disease manifested by progressive renal failure, coalescing purpura, or advanced neuropathy. Plasmapheresis can rapidly decrease cryoglobulin levels without the use of immunosuppressive agents, which might be problematic in hepatitis C–associated disease.
Treatment strategy:
- A reasonable plasmapheresis prescription is to exchange 1 plasma volume 3 times weekly for 2 to 3 weeks
- An average of 13 treatments may be required to induce clinical improvement (range, 4 to 39)
- The replacement fluid can be 5% albumin, which must be warmed to prevent precipitation of circulating cryoglobulins
Thrombotic thrombocytopenic purpura
A large randomized controlled study found 78% survival with plasmapheresis and fresh frozen plasma (FFP) replacement compared with 50% survival with fresh frozen plasma infusions alone 19. Plasmapheresis with fresh frozen plasma replacement is the treatment of choice for thrombotic thrombocytopenic purpura and is considered standard of care.
Treatment considerations:
- Fresh frozen plasma (FFP) is required as replacement fluid to replace missing metalloprotease (ADAMTS13 [A Disintigrin-like And Metalloprotease with ThromboSpondin type 1 repeats])
- Plasma removal with plasmapheresis removes antibody to ADAMTS13
- Treatments are performed daily until the platelet count is normalized and hemolysis has largely ceased (normalization of lactate dehydrogenase)
- Exchanged volumes should be at least 1 plasma volume. Some experts recommend 1.5 plasma volume exchanges for the first week
- Previous recommendations suggest switching to cryoprecipitate-poor plasma in resistant cases because it may contain lower levels of von Willebrand factor. However, a recent review suggests that cryoprecipitate-poor plasma contains less ADAMTS13 and may be less effective than fresh frozen plasma 20
Hemolytic uremic syndrome in adults
Although renal failure tends to dominate the clinical presentation, unless a specific cause can be identified, hemolytic uremic syndrome is often difficult to distinguish from thrombotic thrombocytopenic purpura
Causes:
- Verotoxin induced by Escherichia coli 0157-H7: prodrome of bloody diarrhea
- Drugs: cyclosporine, tacrolimus, mitomycin, cisplatinum, quinine, oral contraceptives, antiplatelet agents, and so on
- Lupus
- Cancer
- Bone marrow transplant
- Post transplantation recurrence
Prognosis in adults is poor:
- Mortality between 25% and 50%
- End-stage renal disease in 40%
Although treatment success depends on the cause, hemolytic uremic syndrome in adults is often treated with plasmapheresis as with thrombotic thrombocytopenic purpura.
Hemolytic uremic syndrome in children
Prognosis is usually good in verotoxin-induced disease, with only a small percentage of patients experiencing strokes or sustained renal failure. Controlled trials with plasma infusion have shown only minimal benefit.
Plasmapheresis may be beneficial in children:
- Without a diarrheal prodrome
- Older than 5 years
- With significant central nervous system involvement
Lupus anticoagulant, Anticardiolipin Antibodies, and Antiphospholipid Antibody Syndrome
Lupus anticoagulant and anticardiolipin antibody are antiphospholid antibodies associated with thromboses, recurrent fetal loss, and renal disease. Plasmapheresis has been successful in removing antiphospholipid antibodies to avoid spontaneous abortion, treatment of lupus anticoagulant-associated renal failure, and in the management of catastrophic antiphospholipid syndrome (CAPS).
Scleroderma
Plasmapheresis may be useful in rare coexistence of scleroderma and ANCA-positive or antinuclear antibody (ANA)-positive renal disease.
Focal Segmental Glomerulosclerosis: Recurrence Posttransplantation
Fifteen percent to 55% of patients with end-stage renal disease secondary to focal segmental glomerulosclerosis have rapid recurrence of proteinuria after renal transplantation. Some patients with early recurrence of proteinuria have a circulating 30- to 50,000-d protein capable of increasing glomerular permeability to albumin. Standard plasmapheresis and immunoadsorption have been successful in decreasing the level of proteinuria. The addition of cyclophosphamide to plasmapheresis may lead to more prolonged remission. plasmapheresis may be effective in the treatment of recurrent focal segmental glomerulosclerosis if treatment is initiated promptly after the initiation of proteinuria.
Transplant Candidates With Cytotoxic Antibodies
Plasmapheresis and immunoadsorption have been successful in decreasing high levels of preformed cytotoxic antibodies (panel reactive antibody [PRA]), allowing for successful transplants for up to 34 months.
Often used with concomitant cyclophosphamide and prednisolone.
Renal Allograft Rejection
Plasmapheresis can provide a rapid decrease in anti-human leukocyte antigen (HLA) antibodies. However, 2 controlled trials of plasmapheresis for acute vascular rejection did not find this treatment to be useful.
Plasmapheresis together with cyclophosphamide and methylprednisolone has been reported to result in greater improvement in renal function and improved graft survival.
Renal Transplantation Across Blood Group Type ABO Groups
Plasmapheresis can be used to remove anti-A or anti-B antibodies before transplantation. Five-year graft survival has been as high as 78% when kidneys from donors in blood A2 or B subgroups are transplanted into group O recipients. Donor-specific skin grafting can be used to predict outcome.
Plasmapheresis for inflammatory eye disease
When conventional therapy with cortisone or immunosuppressive drugs fails or is inadequate in the treatment of immune‐mediated inflammatory eye disease with an auto immunologic pathogenesis, plasmapheresis may be indicated and is increasingly being implemented with success.
Severe uveitis is potentially associated with visual impairment or blindness in young patients 21. In posterior uveitis, progredient inflammatory processes can lead to morphologic changes in the chorioidea and retina, contributing to functional deterioration. In uveitis intermedia, inflammatory processes in the peripheral retina and in the area of the ciliary body require primary attention and aggressive treatment. In both cases, secondary destructive changes in the vessels can occur, causing reduced perfusion of the retina and chorioidea. Primary inflammatory vascular changes may lead to secondary morphologic chorioretinal changes, which may then further impair function. The inflammatory process and/or the reduced chorioretinal perfusion are important. Therefore, an anti‐inflammatory/immunomodulatory therapy, a hemorheologic therapy, or a combination of both treatments, should bring about improvement of the condition, insofar as no other specific therapy is indicated 6.
Detection of immune complexes or autoantibodies in uveitis is problematic. First, indications for the existence and possible pathomechanism of pathogenic substrates to retinal S antigen were found in patients with uveitis and in animal studies. Both improvement and deterioration in the condition can be regarded as an indication of elimination of a pathogenic substrate.
The improvement in hemorheologic parameters could contribute considerably to the therapeutic success in autoimmune eye diseases accompanied by primary or secondary vascular changes. With improved microcirculation, the damaged tissue can recover. In addition, other mechanisms, such as elimination of a pathogenic substrate or immunomodulatory effects of the exchange medium, probably contribute to the success of this therapy. The immunomodulating mechanism of plasmapheresis, which favors a prompter elimination of inflammation, increases ocular function, and reduces recurrence, has been clarified.
In recent years, the anti‐TNF‐α antibodies, infliximab and adalimumab, and others demonstrated significant efficacy in controlling uveitis associated with seronegative spondyloarthropathies and juvenile idiopathic arthritis 22. The majority of reports of biologic therapies in posterior uveitis have been uncontrolled or retrospective studies in patients with uveitis resistant to immunosuppression.
Biologic therapies have increased the treatment options for sight‐threatening uveitis. Despite an experimental rationale, the lack of evidence from randomized controlled studies limits our understanding of when to commence therapy, which agent to choose, and how long to continue treatment. Additionally, the high cost and potential side‐effects of the biologic agents have limited their current use to uveitis refractory to immunosuppression. Further controlled randomized multicenter studies of TPE and/or immunosuppression versus biologics are necessary to clarify efficacy, side‐effects, and costs.
Plasmapheresis for dermatological diseases
Dermatologic immune mediated diseases represent a heterogenous group of disorders associated with circulating autoantibodies against distinct adhesion molecules of the skin and/or mucosa. According to the level of split formation, the disorders can be divided in the intraepidermal blistering pemphigus, such as pemphigus vulgaris, pemphigus foliaceus, and paraneoplastic pemphigus, and the subepidermal blistering pemphigoid diseases, such as bullous pemphigoid, pemphigoid gestations, and dermatitis herpetiformis 23. The new developed sensitive and specific assays for circulating autoantibodies in these dermatological diseases now enable a serological diagnosis in about 90% of cases.
The incidences of autoimmune blistering skin diseases in Germany has doubled in the last 10 years, to about 25 new cases per million humans per year, because of improved diagnostic techniques as well as the aging of the population 23. There are an estimated 2000 new cases of autoimmune blistering skin diseases per year. The incidence of pemphigus in Europe is one to two cases per million humans per year, and 80% have pemphigus vulgaris. Bullous pemphigoid is the most common type of subepidermal autoimmune blistering skin disease in Europe, with an incidence of about 13 cases per million humans per year. The next common types are mucous membrane pemphigoid and pemphigoid gestationis 24.
The standard of diagnostic testing for autoimmune blistering skin diseases is the direct immunofluorescence (IF) microscopy to demonstrate tissue‐bound autoantibodies and/or C3 in the patients’ skin or mucous membranes. The direct IF microscopy of the patient’s serum can be used as a screening test for circulating antibodies. The diagnostic assessment of autoimmune blistering skin diseases can be expected to improve in the near future as new serological testing systems are developed that employ recombinant forms of the target antigens. But the treatments in use still need to be validated by prospective, controlled trials 66. An example for intradermal blistering pemphigus is pemphigus vulgaris.
Table 4. Therapeutic plasmapheresis in dermatological diseases with immunologic origin
| Dermatological diseases treated with therapeutic plasmapheresis | Apheresis Application Committee of the ASFA, 2010, 2013 | |||||||
|---|---|---|---|---|---|---|---|---|
| Therapeutic plasmapheresis modality | Treated with TA | Therapeutic plasmapheresismodality | Category | Recommendation grade | Treated Volume (TPV) | Replacement solution | Frequency | |
| Intraepidermal blistering pemphigus | 1‐1.5 | human‐albumin‐electrolyte solution | daily or every other day | |||||
| Pemphigus vulgaris | TPE, IA | + | TPE, IA, ECP | III | 2B, 2C | |||
| + | ||||||||
| Subepidermal blistering pemphigus | ||||||||
| Bullous pemphigoid | TPE, IA | + | – | – | – | |||
| + | ||||||||
| D‐penicillinamine induced pemphigus | TPE, IA | + | – | – | – | |||
| + | ||||||||
| Progressive scleroderma | TPE | + | TPE, ECP | III, III | 2C | |||
| 2B | ||||||||
| Pyoderma gangrenosum | TPE | + | – | – | – | |||
| Henoch‐Schönlein purpura | TPE | + | TPE | III | 2C | |||
Footnotes:
- Category I: accepted for therapeutic plasmapheresis as first‐line therapy;
- Category II: accepted for therapeutic plasmapheresis as second‐line therapy;
- Category III: not accepted for therapeutic plasmapheresis, decision should be individualized;
- Category IV: not accepted for therapeutic plasmapheresis, Institutional Review Board (IRB) approval is desirable if therapeutic plasmapheresis is undertaken 14.
Abbreviations: IA‐Protein‐A = Immunoadsorption on protein‐A; ECP = extracorporeal photopheresis
Plasmapheresis procedure
Plasmapheresis (also known as plasma exchange or apheresis) is similar to kidney dialysis (hemodialysis); however, plasmapheresis removes the plasma portion of the blood where the antibodies are located. Plasma is the almost clear part of the blood which carries red cells, white cells, platelets and other substances through your bloodstream. During plasmapheresis, you will need to have a working native fistula, graft or dialysis catheter. If you have a catheter, one line of the catheter is attached to tubing and takes blood to the plasmapheresis machine. A second line of the catheter is used to return the blood. If you have a fistula or graft, needles will be placed as they are for hemodialysis. You may feel some minor discomfort when the needles are placed in position. This is similar to what a blood donor experiences.
Plasmapheresis uses a machine that separates the plasma (the liquid part of the blood) that contains the abnormal antibodies such as monoclonal paraproteins and pathogenic autoantibodies, immune complexes, cryoglobulins, myeloma light chains, endotoxin, and cholesterol-containing lipoproteins from the blood cells. The plasma containing the abnormal protein is discarded, while the blood cells are mixed with salt solution and plasma from a donor and given back to the patient.
Plasmapheresis is done over a few hours while the person lies in a bed or sits in a reclining chair. The blood is removed through an IV line (usually in a vein in the arm), goes through the machine where the plasma is replaced, and then is returned to the body through another IV line. Sometimes, minor surgery is done before the procedure to put a single large catheter in a large vein just below the neck or under the collar bone instead of using IV lines in the arms. This type of catheter, called a central line or central venous catheter (CVC), has both IVs built in.
Plasmapheresis is not painful (aside from the IV lines being put in), but it can be hard to stay sitting or lying down in the same place for 2 or 3 hours. Calcium levels can drop in some people during treatment, causing numbness and tingling (especially in the hands and feet and around the mouth) and muscle spasms, which can sometimes be painful. This can be treated by giving the patient calcium.
Plasmapheresis works quickly to bring down the abnormal antibodies level. However, it does not treat the cause of the abnormal antibodies level, immune complexes, cryoglobulins, myeloma light chains, endotoxin, and cholesterol-containing lipoproteins from the blood cells, so it will go back up again without further treatment.
Figure 3. Plasmapheresis procedure
Plasmapheresis technique
Traditionally, plasmapheresis was performed with centrifugation devices used in blood-banking procedures. These devices offer the advantage of allowing for selective cell removal (cytapheresis). Plasmapheresis also can be performed using a highly permeable filter and standard dialysis equipment.
1) Centrifugation
Centrifugation separates the plasma by density gradients.Whole-blood constituents are layered into plasma (specific gravity [SG], 1.025 to 1.109), platelets (SG, 1.040), lymph (SG, 1.070), granulocytes (SG, 1.087 to 1.092), and red blood cells (SG, 1.093 to 1.096).
2) Filtration (membrane plasma separation)
Separation of plasma from the blood’s cellular components can also be accomplished by filtration though a highly permeable membrane. Blood is separated into its cellular and noncellular components by subjecting it to sieving through a membrane with pores that allow plasma proteins to pass, but that retain the larger cellular elements within the blood path.
3) Plasmapheresis with dialysis equipment
Plasmapheresis can be performed with a highly permeable filter connected to the blood pump and pressure monitoring system of the dialysis machine. The machine is used in its “isolated” ultrafiltration mode, bypassing the dialysate proportioning system.
4) Anticoagulation
For centrifugal techniques, anticoaglation is often provided by citrate. For membrane plasma separation with dialysis equipment, heparin can be used as during standard dialysis.
5) Replacement Fluids:
- Albumin
- Pros: no viral transmission, allergies are rare
- Cons: depletion coagulopathy, immunoglobulin depletion
- Electrolyte composition: Sodium, 145 ± 15 mEq/L; potassium, less than 2 mEq/L (sodium and potassium in mEq/L is equivalent to sodium and potassium in mmol/L)
- Anaphylactic reactions: Rare, antibodies to polymerized albumin
- “Depletion coagulopathy”: Replacement with albumin will lead to depletion of coagulation factors.
- After a single plasma exchange, prothrombin time (PT) increases 30%, partial thromboplastin time (PTT) doubles: these increases often reverse 1 day after treatment
- Multiple consecutive treatments result in prolonged increases in PT/PTT
- Fresh frozen plasma administered toward the end of the procedure can minimize hemorrhagic risks
- Immunoglobulin depletion
- A single 1-plasma volume exchange reduces serum immunoglobulin levels by 60%
- Multiple treatments can decrease immunoglobulin levels for several weeks
- A single infusion of immunoglobulin (IVIG) administered after a series of plasmapheresis treatments can reconstitute normal immunoglobulin levels
- Risk of viral transmission: Albumin is heat treated and considered to be devoid of transmissible virus.
- Fresh frozen plasma
- 3 L of fresh frozen plasma is obtained from 10 to 15 donors (15 separate units).
- Pros: Does not lead to postpheresis coagulopathy or immunoglobulin depletion. Fresh frozen plasma is essential for the treatment of thrombotic thrombocytopenic purpura.
- Cons: Anaphylactoid reactions, citrate toxicity, small risk of viral transmission.
- Anaphylactic reactions: Fever, rigors, urticaria, wheezing, hypotension, and laryngeal edema
- Angiotensin-converting enzyme (ACE) inhibitors should be avoided given their ability to inhibit kinin metabolism
- Consider pretreatment with diphenhydramine intravenously (IV): 0.3 to 0.5 mL of epinephrine (1:1,000 solution) should be available for subcutaneous administration for severe reactions
- Citrate toxicity: Fresh frozen plasma contains 14% citrate by volume; can lead to hypocalcemia and metabolic alkalosis
- Risk of viral transmission: 1/63,000 units for hepatitis B, 1/100,000 units for hepatitis C, 1/680,000 units for human immunodeficiency virus (HIV), and 1/641,000 units for human T-lymphotrophic virus
- Starch replacement for plasmapheresis
- Similar attributes with albumin, may be less expensive.
6) Vascular Access
- Antecubital veins:
- Ideal for low-flow treatments
- Increasingly difficult to use after multiple punctures
- Temporary vascular catheters: Catheter removal may be hazardous after an intensive run of plasmapheresis treatments, which can result in depletion coagulopathy and increased PT/PTT.
- Femoral vein cannulation
- Subclavian and internal jugular catheters
- Tunneled jugular venous catheters
- Permanent arteriovenous access: Preferred if treatments are to be repeated regularly (hyperlipidemia).
- Primary arteriovenous fistula
- Arteriovenous graft
7) Selective Plasmapheresis Techniques
- Designed to remove a particular pathogenic substance
- Decreases need for replacement fluid
- Minimizes risks of depletion coagulopathy and hypogammaglobulinemia
- Many systems available in Japan and Europe, few in United States
- Cascade filtration (“double filtration”)
- Separated plasma is refiltered through a secondary filter with smaller pore size
- Larger, unwanted molecules removed by secondary filter
- Indications: Waldenstrom macroglobulinemia, cryoglobulinemia, familial hypercholesterolemia, and immune complex–mediated disease
- Cryofiltration
- Removed plasma is cooled, causing certain substances to aggregate
- Increasing size allows for efficient secondary filtration
- Indications: cyroglobulins and immune complexes
- Immunoadsorbant techniques
- Systems for selective immunoadsorption
- Indications: nonselective immunoglobulin removal, low- density lipoprotein (LDL) cholesterol.
- Protein A columns: Protein A 42,000-d protein released from Staphylococcus aureus. Used for the ex vivo adsorption of 3 of the 4 classes of IgG (1, 2, and 4).
- Prosorba column (Cypress Biosciences Inc, San Diego, CA). Single-use nonregenerating system placed in series with a standard plasma exchange circuit. When the plasma is separated from the blood, it is slowly perfused over the column (at 20 mL/min). This column saturates rapidly with very limited IgG removal. Postulated mode of action is by “immunomodulation” of perfused plasma. Food and Drug Administration approved for idiopathic thrombocytopenic purpura (ITP) and rheumatoid arthritis. Secondary effects are common: fever, chills, musculoskeletal pains, hypotension. Contraindicated in patients using ACE inhibitors.
- Excorim (Lund, Sweden). Alternating columns repeatedly regenerated to allow for more efficient IgG removal. Renal indications: removal of anti-HLA antibodies in highly sensitized recipients, rapidly progressive glomerulonephritis.
- Protein A columns: Protein A 42,000-d protein released from Staphylococcus aureus. Used for the ex vivo adsorption of 3 of the 4 classes of IgG (1, 2, and 4).
- Selective LDL cholesterol removal
- Limits the loss of plasma proteins and high-density lipoprotein (HDL) cholesterol
- Indicated in patients with familial hypercholesterolemia who cannot tolerate or whose condition is unresponsive to pharmacological treatment and who have either known cardiovascular disease and a plasma LDL cholesterol level greater than 200 mg/dL or no known cardiovascular disease and a plasma LDL cholesterol level greater than 300 mg/dL
- Four systems:
- Imunoadsorbant
- Dextran sulfate binding to apoprotein B. Contraindication for patients on ACE-inhibitor therapy
- Heparin-mediated extracorporal LDL precipitation (HELP)
- Direct adsorption of LDL (DALI). Does not require plasma separation, removes LDL directly from whole blood
- Endotoxin adsorption
- Fibers impregnated with polymyxin B. Can bind endotoxin fragments
- Japanese experience documents improvement in systemic hemodynamics of sepsis
- Not currently available in the United States
- Cascade filtration (“double filtration”)
Plasmapheresis complications
Plasmapheresis side effects include hypotension, allergic reactions, nausea, vomiting paresthesia, and cramps are the most common complications, and these may be seen in 3%–25% of procedures 25. Most of these events are mild and resolve without treatment. The reported incidence of paresthesia and cramps ranges from 1.5% to 9% 25. Reported incidence of hypotension ranges from 2.6% to 8.1% 26. However, the incidence of both of these was quite low in our study. The overall mortality in plasmapheresis is estimated to be 1–3/10,000 procedures 27.
References- Therapeutic Plasma Exchange: Core Curriculum 2008. American Journal of Kidney Diseases , Volume 52 , Issue 6 , 1180 – 1196. https://www.ajkd.org/article/S0272-6386(08)00707-5/fulltext
- Therapeutic Apheresis in Hematologic, Autoimmune and Dermatologic Diseases With Immunologic Origin. Bambauer R, Latza R, Burgard D, Schiel R. Ther Apher Dial. 2016 Oct; 20(5):433-452. https://onlinelibrary.wiley.com/doi/full/10.1111/1744-9987.12474
- Ahammed Nizar OT, Rai P, Rao SN, Shenoy MP. Plasmapheresis: A Retrospective Audit of Procedures from a Tertiary Care Center in Southern India. Indian Journal of Critical Care Medicine : Peer-reviewed, Official Publication of Indian Society of Critical Care Medicine. 2017;21(12):857-860. doi:10.4103/ijccm.IJCCM_177_17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5752796/
- Kaplan AA. Therapeutic plasma exchange: Core curriculum 2008. Am J Kidney Dis. 2008;52:1180–96. https://www.ncbi.nlm.nih.gov/pubmed/18562061
- Strauss RG, Ciavarella D, Gilcher RO, Kasprisin DO, Kiprov DD, Klein HG, et al. An overview of current management. J Clin Apher. 1993;8:189–94. https://www.ncbi.nlm.nih.gov/pubmed/8113206
- Bambauer R, Latza R, Schiel R. Therapeutic Plasma Exchange and Selective Plasma Separation Methods. In: Fundamental Technologies, Pathology and Clinical Results, 4th edn. D‐49525 Lengerich: Pabst Science Publishers, 2013.
- Kumilien G, Ullstrom L, Losvall A et al. Clinical experience with a new apheresis filter has specifically depletes ABO blood group antibodies. Transfusion 2006;46:1568–75. https://www.ncbi.nlm.nih.gov/pubmed/16965585
- Davidson A, Diamond B. Autoimmune Diseases. N Engl J Med 2001;345:340–50.
- Schwartz J, Padmanabhan A, Aqui N, Balogun RA, Connelly-Smith L, Delaney M, et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher. 2016;31:149–62. https://www.ncbi.nlm.nih.gov/pubmed/27322218
- National Coverage Determination (NCD) for Apheresis (Therapeutic Pheresis). https://www.cms.gov/medicare-coverage-database/details/ncd-details.aspx?ncdid=82
- Cortese I, Chaudhry V, So YT, Cantor F, Cornblath DR, Rae-Grant A. Evidence-based guideline update: Plasmapheresis in neurologic disorders: Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;76(3):294-300. doi:10.1212/WNL.0b013e318207b1f6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3034395/
- Update: Plasmapheresis in Neurologic Disorders. American Academy of Neurology guideline update. Neurology 2011;2011;76:294–300. https://www.aan.com/Guidelines/home/GetGuidelineContent/471
- Bambauer R, Latza R, Schiel R. Therapeutic Apheresis in Neurological and Hematological Diseases in Pediatrics. Clin Med Ins: Pediatr in press.
- Schwartz J, Winters JL, Padmanabhan A et al. Guidelines on the use of therapeutic apheresis in clinical practice‐evidence‐based approach from the Writing Committee of the American Society for Apheresis: the sixth special issue. J Clin Apher 2013;28:145–284.
- Berlit P. Lupus Erythematodes und Nervensystem. Dtsch Ärztebl 1989;86:A3176–82.
- Zucchelli P, Pasquali S, Cagnoli L, Ferrari G: Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int 33:1175-1189, 1988
- Clark WF, Stewart AK, Rock GA, et al: Plasma exchange when myeloma presents as acute renal failure: A randomized, controlled trial. Ann Intern Med 143:777-784, 2005
- Hutchison CA, Cockwell P, Reid S, et al: Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: In vitro and in vivo studies. J Am Soc Nephrol 18:886-895, 2007
- Rock GA, Shumak KH, Buskard NA, et al: Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med 325:393-397, 1991
- Raife TJ, Friedman KD, Dwyre DM: The pathogenicity of vWF factor in TTP: Reconsideration of treatment with cryopoor plasma. Transfusion 46:74-79, 2006
- Bodaghi B, Grendron G, Wechsler B et al. Efficacy of interferon alpha in the treatment of refractory and sight threatening uveitis: a retrospective monocentric study of 45 patients. Br Opthalmol 2007;91:335–9.
- Imrie FR, Dick D. Biologics in the treatment of uveitis. Curr Opin Ophthalmol 2007;18:481–6.
- Meyersburg D, Schmitt E, Kasperkiewicz M et al. Immunoadsorption in Dermatology. Ther Apher Dial 2012;16(4):311–20.
- Langan SM, Smeeth L, Hubbard R et al. Bullous pemphigoid and pemphigus vulgaris‐incidence and mortality in the UK: population based on cohort study. BMJ 2008;337:a180.
- Stegmayr B, Ptak J, Wikström B, Berlin G, Axelsson CG, Griskevicius A, et al. World apheresis registry 2003-2007 data. Transfus Apher Sci. 2008;39:247–54. https://www.ncbi.nlm.nih.gov/pubmed/18977177
- Shemin D, Briggs D, Greenan M. Complications of therapeutic plasma exchange: A prospective study of 1,727 procedures. J Clin Apher. 2007;22:270–6. https://www.ncbi.nlm.nih.gov/pubmed/17722046
- Ward DM. Conventional apheresis therapies: A review. J Clin Apher. 2011;26:230–8. https://www.ncbi.nlm.nih.gov/pubmed/21882233