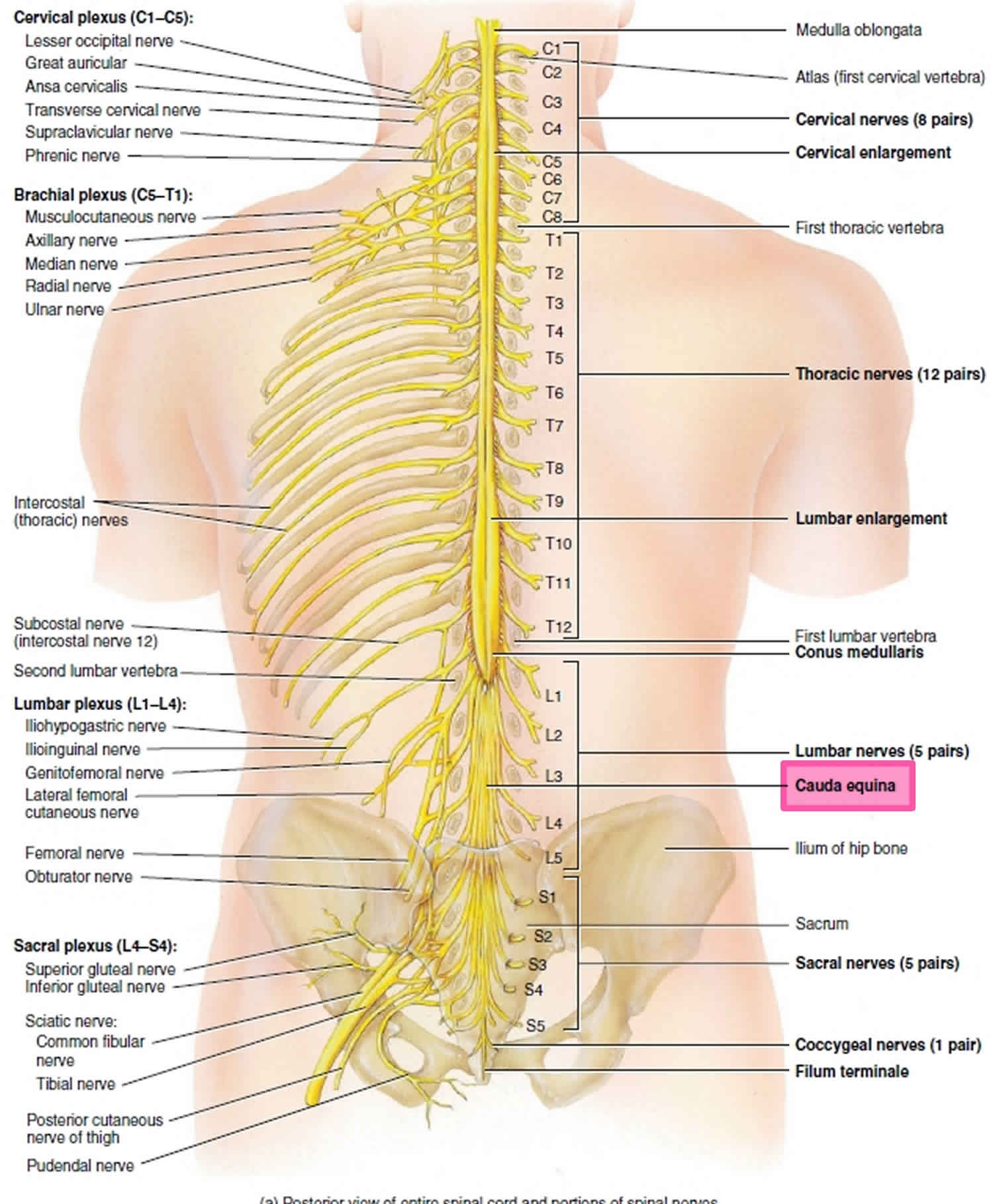
Cauda equina
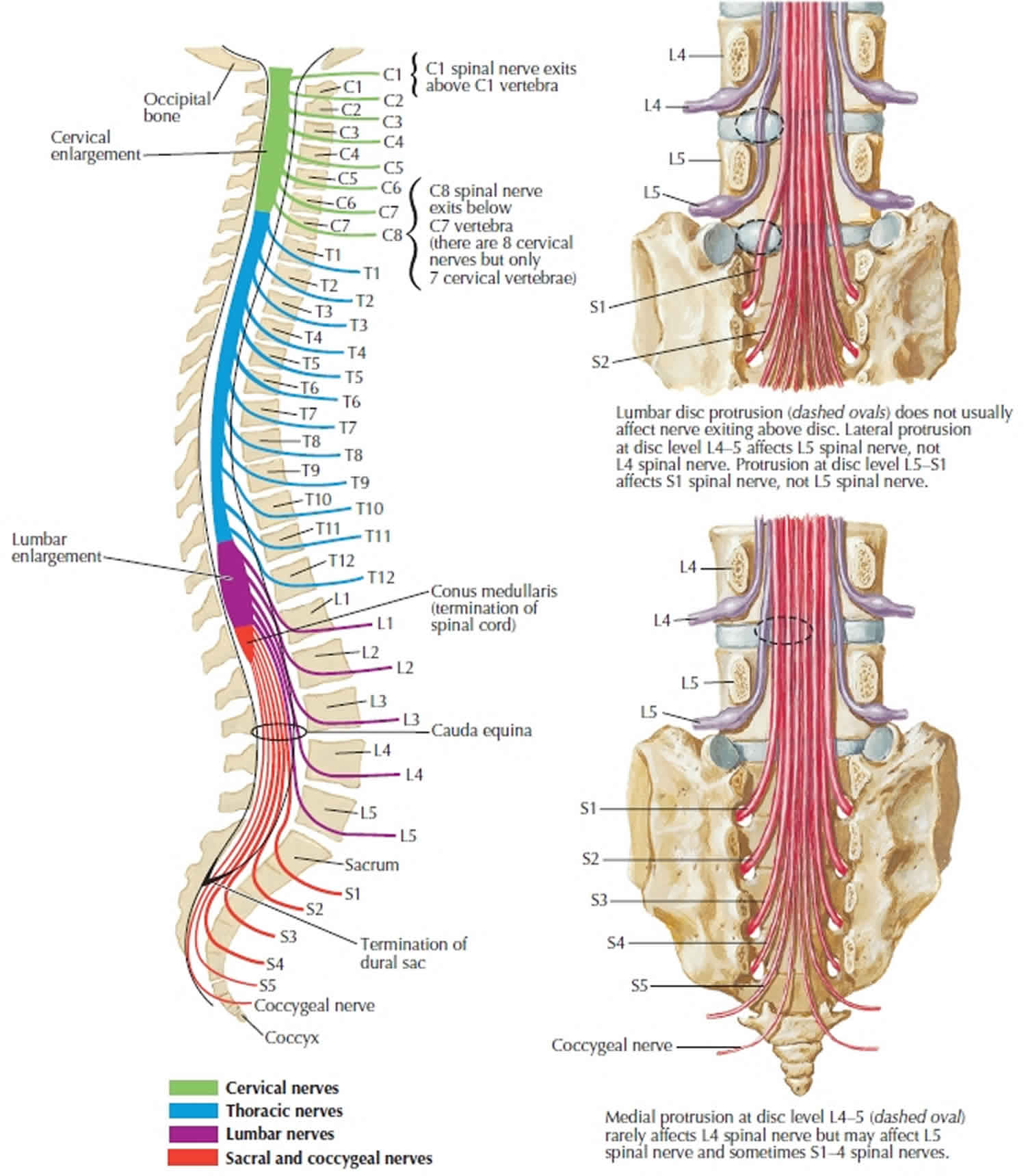
As spinal nerves branch from the spinal cord, they pass laterally to exit the vertebral canal through the intervertebral foramina between adjacent vertebrae. However, because the spinal cord is shorter than the vertebral column, nerves that arise from the lumbar, sacral, and coccygeal regions of the spinal cord do not leave the vertebral column at the same level they exit the spinal cord. The roots of these lower spinal nerves angle inferiorly alongside the filum terminale in the vertebral canal like wisps of hair. Accordingly, a rope-like tail of nerve fibers at the caudal end of the spinal cord are collectively named the cauda equina, from the Latin translation meaning “horse’s tail”. The cauda equina is a group of nerves and nerve roots stemming from the distal end of the spinal cord, typically levels L1-L5 and contains axons of nerves that give both motor and sensory innervation to the legs, bladder, anus, and perineum 1. Cauda equina syndrome is a medical emergency that results from compression and disruption of the function of these nerves and can be inclusive of the conus medullaris or distal to it, and most often occurs when damage occurs to the L3-L5 nerve roots 2. Cauda equina syndrome can present with back pain radiating to the legs, motor and sensory dysfunction of the lower extremities, bladder and/or bowel dysfunction, sexual dysfunction and saddle anesthesia 3. Cauda equina syndrome also carry a high risk of litigation as delays in diagnosis and management can lead to devastating life-long impairment 4.
The human spinal cord terminates at the L1-L2 vertebral level in a conical structure called the conus medullaris, which lies just caudad to the anatomical landmark of the 12th rib. The cauda equina contains a bundle of nerves which project distally within the enclosed cavity of the lumbar cistern from the spinal cord and conus medullaris toward the coccyx. Each nerve exits at its respective vertebral level toward targets which are supplied by the L2-S5 spinal cord level. Nerves contained within the cauda equina provide somatic efferent innervation to muscles of the lower extremity and somatic afferent sensations such as vibration, proprioception, pain, and temperature. Parasympathetic nerves provide visceral efferent signals to the urinary bladder from the S2 to S4 spinal cord levels and are responsible for micturition, accomplished by stimulating the detrusor muscle to contract while simultaneously relaxing the internal urethral sphincter. Sympathetic fibers from T11 through L2 exert urinary bladder filling by relaxing the detrusor muscle and contracting the internal urethral sphincter 5.
The cauda equina is supplied by arteries of the same name, which are small and may not be visualized radiographically. Each spinal nerve root has a corresponding medullary artery. A vasocorona surrounding the conus medullaris and the high degree of arterial anastomoses among the nerve roots predispose the vasculature patterns to significant diversity. Lymphatic capillaries occur nearly everywhere in the body except for a small number of sites, including the central nervous system (CNS); consequently, immune surveillance occurs within the layers of the meninges in a medium of cerebrospinal fluid (CSF). Cerebrospinal fluid bathes the brain, spinal cord, and cauda equina and is thought to serve as a protective layer of lubrication which provides nutrients and removes waste.
Table 1. Root and peripheral nerve innervation of the Lumbosacral Plexus
| Muscle | Nerve | Root |
|---|---|---|
| Iliopsoas | Femoral | L2, 3, 4 |
| Adductor longus | Obturator | L2, 3, 4 |
| Gracilis | Obturator | L2, 3, 4 |
| Quadriceps femoris | Femoral | L2, 3, 4 |
| Anterior tibial | Deep peroneal | L4, 5 |
| Extensor hallucis longus | Deep peroneal | L4, 5 |
| Extensor digitorum longus | Deep peroneal | L4,5 |
| Extensor digitorum brevis | Deep peroneal | L4, 5, S1 |
| Peroneus longus | Superficial peroneal | L5, S1 |
| Internal hamstrings | Sciatic | L4, 5, S1 |
| External hamstrings | Sciatic | L5, S1 |
| Gluteus medius | Superior gluteal | L4, 5, S1 |
| Gluteus maximus | Inferior gluteal | L5, S1, 2 |
| Posterior tibial | Tibial | L5, S1 |
| Flexor digitorum longus | Tibial | L5, S1 |
| Abductor hallucis brevis | Tibial (medial plantar) | L5, S1, 2 |
| Abductor digiti quinti pedis | Tibial (lateral plantar) | S1, 2 |
| Gastrocnemius lateral | Tibial | L5, S1, 2 |
| Gastrocnemius medial | Tibial | S1, 2 |
| Soleus | Tibial | S1, 2 |
Figure 1. Cauda equina
Cauda equina syndrome
Cauda equina syndrome refers to a collection of symptoms and signs that result from severe compression of the descending lumbar and sacral nerve roots at the lower end of the spinal cord called the cauda equina 7, 8, 9. The cauda equina, from the Latin word meaning “horse’s tail”, is a rope-like tail of nerve fibers at the caudal end of the spinal cord. The cauda equina is collection of nerves and nerve roots stemming from the distal end of the spinal cord, typically levels L1 to L5 and contains axons of nerves that give both motor and sensory innervation to your legs, bladder, anus, and perineum 1. Cauda equina syndrome disrupt the motor and sensory function to the lower extremities and bladder. Cauda equina syndrome symptoms include low back pain, sciatica (unilateral or, usually, bilateral), saddle sensory disturbances, bladder and bowel dysfunction, and variable lower extremity motor and sensory loss. Cauda equina syndrome is most commonly caused by an acutely extruded lumbar disc and is considered a diagnostic and surgical emergency 10, 7. Patients with cauda equina syndrome are often admitted to the hospital as a surgical emergency 11. Cauda equina syndrome can lead to incontinence and even permanent lower limbs paralysis.
If patients with cauda equina syndrome do not seek immediate treatment to relieve the pressure, it can result in permanent paralysis, impaired bladder and/or bowel control, loss of sexual sensation, and other problems 12. Even with immediate treatment, some patient may not recover complete function 7, 13. Patients with complete cauda equina syndrome have a poorer outcome 7. Approximately 20% of patients will have a poor outcome in terms of urological and/or sexual function, as well as lower limb paresthesia and weakness 14.
Although early treatment is required to prevent permanent problems, cauda equina syndrome may be difficult to diagnose.
Tandon and Sankaran described three variations of cauda equina syndrome 15:
- Rapid onset without a previous history of back problems.
- Acute bladder dysfunction with a history of low back pain and sciatica.
- Chronic backache and sciatica with gradually progressing cauda equina syndrome often with canal stenosis.
It is evident that the onset of cauda equina syndrome may be either acute within hours or gradual over weeks or months, and within these groups cauda equina syndrome may be complete with painless incontinence or incomplete with some sphincter function.
Additionally, cauda equina syndrome can be classified as incomplete (cauda equina syndrome is incomplete or CES-I) or complete (complete cauda equina syndrome) based on the presence of bowel and bladder symptoms 16, 8, 17, 14:
- In incomplete cauda equina syndrome, the patient has saddle anaesthesia but the bladder and bowel dysfunction has not progressed to full retention or incontinence. Patient has urinary difficulties of neurogenic origin including altered urinary sensation, loss of desire to void, poor urinary stream and the need to strain in order to urinate 17. Bladder or bowel symptoms that these patients may report include loss of urgency or altered urinary sensorium. Saddle and genital sensory deficit is often unilateral or partial and trigone sensation should be present 14.
- In complete cauda equina syndrome patients present with saddle anesthesia and painless urinary retention and overflow incontinence of bladder or bowel (cauda equina syndrome-retention or CES-R), when the bladder is no longer under executive control 17. There is usually extensive or complete saddle and genital sensory deficit with deficient trigone sensation 14. Patients with complete cauda equina syndrome have a poorer outcome 7. Approximately 20% of patients will have a poor outcome in terms of urological and/or sexual function, as well as lower limb paresthesia and weakness 14.
It is well established that the outcome for patients with cauda equina syndrome-incomplete (CES-I) at the time of surgery is generally favorable, whereas those who have deteriorated to cauda equina syndrome-retention (CES-R) when the compression is relieved have a poorer prognosis, although around 70% of cauda equina syndrome-retention (CES-R) patients have a socially acceptable long term outcome 17.
A useful test, the bulbocavernosus reflex (Figure 3), is the test for trigone sensitivity in which an inflated Foley catheter is gently pulled with the patient unaware 18, 19, 20. This should produce the urge to urinate. This will help to distinguish patients with a genuine neurological deficit from those who have purely pain-related retention which is not uncommon 21 as is retention as a result of constipation 22. Prompt MRI scanning will help to identify the likely cause of a cauda equina syndrome 23.
Cauda equina syndrome and conus medullaris syndrome are rare, with an estimated prevalence of 1 in 30,000 to 100,000 people per year 24. Estimates of annual incidence are between 1.5 to 3.4 per million people 24. The most common cause of compression in 45% of cauda equina syndrome is a herniated lumbar intervertebral disc at the L4-L5 and L5-S1 levels 3, 25. Cauda equina syndrome occurs in 3% of all disc herniations 17, 26, 27, 24. Cauda equina syndrome and conus medullaris syndrome are most common in young men, possibly due to this population group being more likely to experience compressive thoracolumbar trauma 24. One study estimated that in the U.S. we would expect to see 1016 new causes of cauda equina syndrome and 449 new cases of conus medullaris per year 24.
Cauda equina syndrome key points 7, 14:
- Cauda equina syndrome is usually characterized by these so-called ‘red flag’ symptoms:
- Severe low back pain
- Sciatica: often bilateral but sometimes absent, especially at L5/S1 with an inferior sequestration
- Saddle and/or genital sensory disturbance
- Bladder, bowel and sexual dysfunction
- Cauda equina syndrome clinical diagnosis is not easy and even in experienced hands is associated with a 43% false positive rate
- The investigation of choice is magnetic resonance imaging (MRI)
- Once urinary retention has occurred the prognosis is worse
- Good retrospective evidence supports urgent surgery especially in early cases 28, 17, 29, 30, 31, 32
Table 2. Symptoms and signs of cauda equina syndrome and conus medullaris syndrome
| Cauda Equina Syndrome | Conus Medullaris Syndrome | |
|---|---|---|
| Presentation | Gradual and unilateral | Sudden and bilateral |
| Reflexes | Both ankle and knee jerks affected | Knee jerks preserved but ankle jerks affected |
| Radicular pain | More severe | Less severe |
| Low back pain | Less | More |
| Sensory symptoms and signs | Numbness tends to be more localized to saddle area; asymmetrical, may be unilateral; no sensory dissociation; loss of sensation in specific dermatomes in lower extremities with numbness and paresthesia; possible numbness in pubic area, including glans penis or clitoris | Numbness tends to be more localized to perianal area; symmetrical and bilateral; sensory dissociation occurs |
| Motor strength | Asymmetric areflexic paraplegia that is more marked; fasciculations rare; atrophy more common | Typically symmetric, hyperreflexic distal paresis of lower limbs that is less marked; fasciculations may be present |
| Impotence | Less frequent; erectile dysfunction that includes inability to have erection, inability to maintain erection, lack of sensation in pubic area (including glans penis or clitoris), and inability to ejaculate | Frequent |
| Sphincter dysfunction | Urinary retention; tends to present late in course of disease | Urinary retention and atonic anal sphincter cause overflow urinary incontinence and fecal incontinence; tend to present early in course of disease |
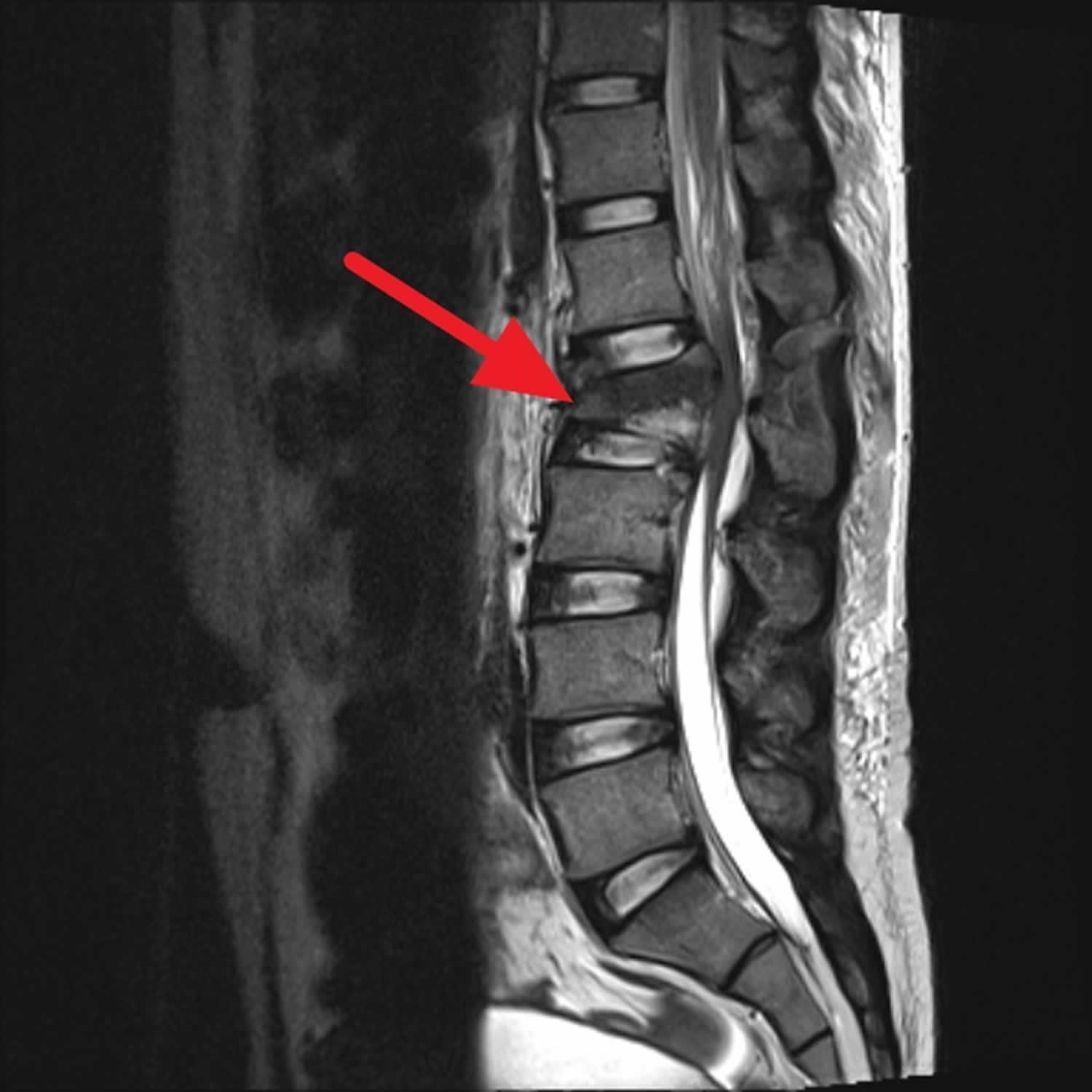
Figure 2. Cauda equina syndrome
Footnote: Young male worker falling from 20ft. Back pain and lower limb sensory deficit. Acute L2 vertebral body fracture (burst fracture), with significant retropulsion, severely narrowing the central canal (absolute stenosis) and impinging on the conus or the origin of the cauda equina. Small superior posterior L3 vertebral body fracture.
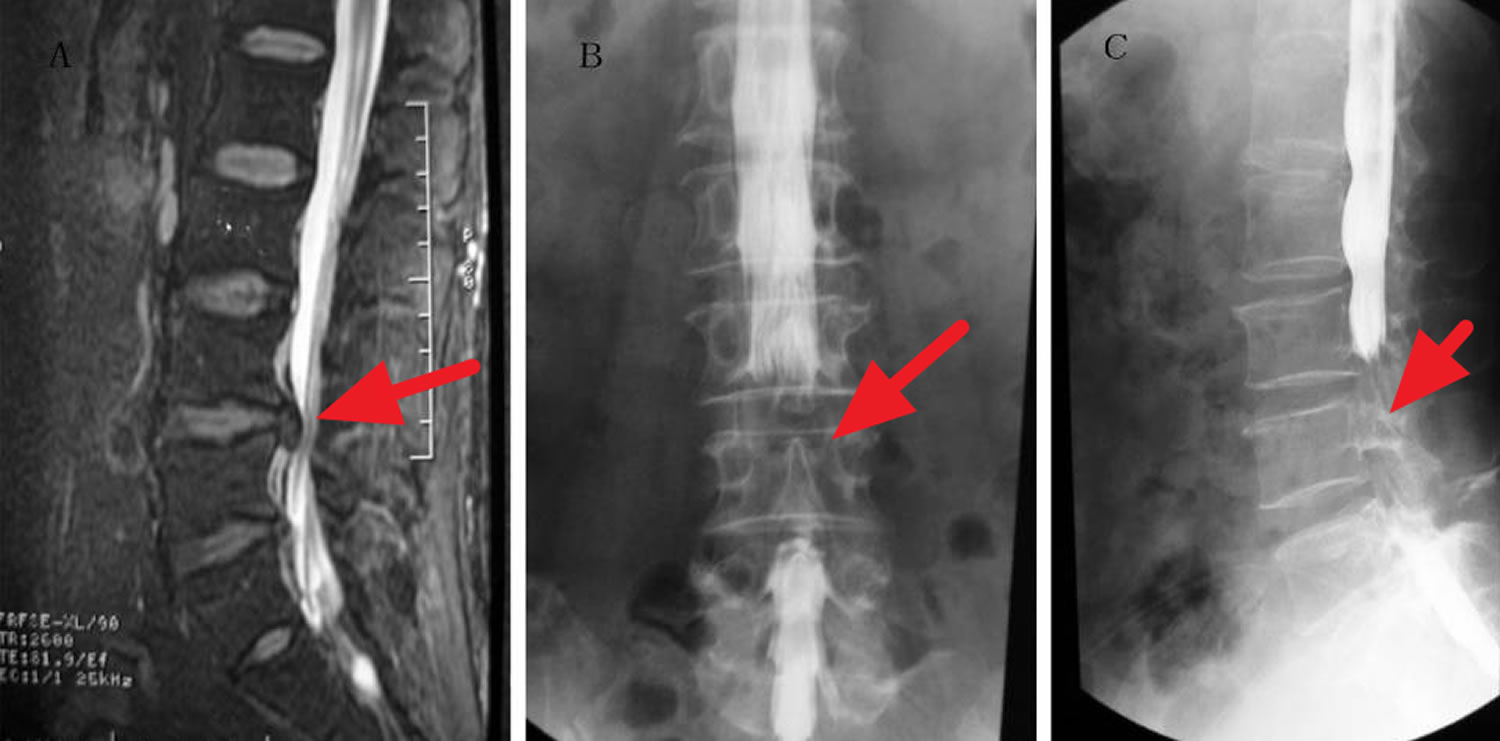
[Source 34 ]Figure 3. Cauda equina syndrome MRI
Footnote: A 38-year-old male experienced bowel incontinence and inability to achieve erection after chiropractic spinal manipulation. The dural sac and cauda equina were compressed at multiple levels, including L3-4, L4-5, and L5-S1. Myelography revealed severe dural sac compression with bilateral involvement. (A) Lumbar MRI sagittal radiograph showing the L4-5 herniated disk in the narrow area. (B) Frontal radiograph of the lumbar spinal canal showing a non-filling narrow area corresponding to the L4-5 herniated disk. (C) Lateral radiograph of the lumbar spinal canal showing a narrow non-filling area corresponding to the L4-5 herniated disk.
[Source 35 ]Cauda equina syndrome causes
Cauda equina syndrome and conus medullaris syndrome result from compression of the spinal cord and nerves/nerve roots arising from L1 to L5 levels. The most common cause of compression in 45% of cauda equina syndrome is a herniated lumbar intervertebral disc at the L4-L5 and L5-S1 levels 3, 25. Other causes include epidural abscess, spinal epidural hematoma, diskitis, tumor (either metastatic or a primary CNS cancer), trauma (particularly when there is retropulsion of bone fracture fragments), spinal stenosis and aortic obstruction 36. Rare reported cases exist in which cauda equina syndrome was associated with chiropractic manipulation, placement of interspinous devices, and thrombosis of the inferior vena cava 36.
Causes of cauda equina syndrome include (some of these are very rare) 37, 7, 8, 38:
- Degenerative
- lumbar disc herniation (most common, especially at L4/5 and L5/S1)
- lumbar spinal canal stenosis with a smaller disc prolapse 30
- spondylolisthesis
- hemorrhage into a Tarlov cyst (a sacral perineural cysts which are benign, cerebrospinal fluid containing lesions of the spinal nerve root sheath, between the endoneurium and perineurium) 39, 40, 41
- facet joint cysts
- after constipation 22
- Inflammatory
- both acute and chronic form may be seen in long-standing ankylosing spondylitis (2nd-5th decades; average 35 years) 42, 43, 44, 45.
- Rheumatoid arthritis
- Traumatic 46, 26, 47
- spinal fracture or dislocation
- epidural hematoma (may also be spontaneous, post-operative, post-procedural or post-manipulation) 48, 49, 50
- gunshot wounds 51
- Infective 52
- arachnoiditis
- epidural abscess
- tuberculosis (Pott disease)
- infection in and around the vertebral bones (osteomyelitis)
- infection in the disc (diskitis)
- schistosomiasis
- Tumors 53
- Primary tumors
- myxopapillary ependymoma
- schwannoma
- spinal meningioma
- neurofibroma
- other tumors of the cauda equina
- Tumors in vertebral bodies
- lymphoma
- vertebral metastases
- Leptomeningeal carcinomatosis
- Primary tumors
- Endocrine and biochemical
- Vascular
- Congenital
- Spinal dysraphism
- Dwarfing syndromes,
- Congenital tumors:
- Numerous other rare space-occupying lesions e.g. sarcoid or after injections into the spine or spine surgery 54, 55
Risk factors for developing cauda equina syndrome
Risk factors for developing cauda equina syndrome include:
- A history of low back pain with sciatica, especially sciatica in both legs.
- Instability or deformity of the spine
- Trauma to the spine, often following a car accident
- Recent lumbar spine surgery
- A history of cancer
- Recent severe infection
- Cauda equina syndrome can develop suddenly even without these risk factors.
Cauda equina syndrome prevention
Prevention of cauda equina syndrome is focused on early diagnosis by identifying the symptoms described (below). While low back pain with leg pain and/or weakness is a common complaint that affects many people, cauda equina syndrome is a rare complication. Your doctors should be vigilant in identifying these cases. You should familiarize yourself with the signs and symptoms that could suggest possible cauda equina syndrome, including change in bowel or bladder function and loss of sensation in the groin.
Cauda equina syndrome symptoms
Cauda equina symptoms can vary in intensity and may evolve slowly over time.
See your doctor immediately if you have:
- Bladder and/or bowel dysfunction, causing you to retain urine or be unable to hold it.
- Severe or progressive problems in the lower extremities, including loss of or altered sensation between the legs, over the buttocks, the inner thighs and back of the legs (saddle area), and feet/heels.
The symptoms of cauda equina syndrome can vary, but the key signs doctors look for are 8, 37, 7:
- Severe low back pain and unilateral or bilateral sciatica
- Pain may be sudden and severe or gradually develop over weeks
- Motor weakness, sensory loss, or pain in one, or more commonly both legs
- Difficulty to pass urine or use the bowels. Prolonged inability to pass urine can result in urine leaking uncontrollably
- Numbness or tingling in the groin (“saddle region”)
- Sexual dysfunction
- Reduced or absent lower extremity reflexes
Low back pain can be divided into local and radicular pain. Local pain is generally a deep, aching pain resulting from soft-tissue and vertebral body irritation. Radicular pain is generally a sharp, stabbing pain resulting from compression of the dorsal nerve roots. Radicular pain projects in dermatomal distributions. Low back pain in cauda equina syndrome may have some characteristic that suggests something different from the far more common lumbar strain. Patients may report severity or a trigger, such as head turning, that seems unusual.
Severe pain is an early finding in 96% of patients with cauda equina syndrome secondary to spinal tumor. Later findings include lower extremity weakness due to involvement of the ventral roots. Patients generally develop hypotonia and hyporeflexia. Sensory loss and sphincter dysfunction are also common.
Urinary signs and symptoms of cauda equina syndrome include the following:
- Urin retention
- Difficulty initiating urination
- Decreased urethral sensation
Typically, urinary signs and symptoms begin with urinary retention and are later followed by an overflow urinary incontinence.
Bell et al demonstrated that the accuracy of urinary retention, urinary frequency, urinary incontinence, altered urinary sensation, and altered perineal sensation as indications of possible disk prolapse justified urgent MRI assessment 56, 57.
Bowel disturbances may include the following:
- Incontinence
- Constipation
- Loss of anal tone and sensation (loss of anal reflex)
The initial presentation of bladder or bowel dysfunction may be of difficulty starting or stopping a stream of urine. It may be followed by frank incontinence, first of urine then of stool. The urinary incontinence is on the basis of overflow. It is usually with associated saddle (perineal) anesthesia (the examiner can inquire if toilet paper feels different when the patient wipes).
Cauda equina syndrome complications
Cauda equina syndrome complications are increasingly likely if diagnosis and appropriate management is delayed, and include residual:
- Paralysis
- Sensory abnormalities
- Bladder, bowel, and sexual dysfunction
One study looked at 63-day outcomes on micturition, defecation, saddle anesthesia, sexual function, and sciatica in cauda equina syndrome. The data indicate that a large percentage of patients still experience residual symptoms irrespective of their time to surgical decompression 3. Micturition deficits such as retention requiring self-catheterization or presence of suprapubic or indwelling catheters and incontinence still presented in 47.7% of patients 3. Dysfunction with defecation decreased post-operatively significantly, but 41.8% of patients still had problems at 63 days post-operatively 3. Sexual dysfunction persisted in 53.3% of patients, and saddle anesthesia in 56.6% 3. Sciatica was present in 47.5% of patients 3. The best predictors of outcome are neurological status at presentation and degree of injury. Incomplete injuries tend to have better outcomes 3.
Cauda equina syndrome diagnosis
To diagnose cauda equina syndrome, your doctor will evaluate your medical history, give you a physical examination, and order multiple diagnostic imaging studies.
Medical history
A thorough history is necessary, with detailed questions regarding recent falls, trauma or injuries, use of anticoagulation, presented spinal instrumentation, intravenous drug use, history of malignancy, chiropractic manipulation, and constitutional symptoms like fevers/chills 3.
Patients can present with:
- Back pain and sciatica (seen in as many as 97% of patients)
- Weakness and changes in sensation in the lower extremities
- Bladder dysfunction (disruption of autonomic fibers results in either retention or incontinence in up to 92% of patients)
- Bowel dysfunction (retention or incontinence in up to 72% of patients)
- Saddle anesthesia or decreased sensation in the perineum (in up to 93% of patients)
- Sexual dysfunction (impotence in men) 3
Pain often is localized to the low back; local tenderness to palpation or percussion may be present 6. Pain in the legs or radiating to the legs is characteristic of cauda equina syndrome 6. Radicular pain is a common presentation in patients with cauda equina syndrome, usually in association with radicular sensory loss (saddle anesthesia), asymmetric paraplegia with loss of tendon reflexes, muscle atrophy, and bladder dysfunction 6. The presentation is somewhat similar to and is often confused with conus and epiconus lesions.
Reflex abnormalities may be present; they typically include loss or diminution of reflexes. Hyperactive reflexes may signal spinal cord involvement and exclude the diagnosis of cauda equina syndrome. Sensory abnormality may be present in the perineal area or lower extremities. Light touch in the perineal area should be tested. Anesthetic areas may show skin breakdown.
Muscle weakness may be present in muscles supplied by affected roots. Muscle wasting may occur in chronic cauda equina syndrome.
Poor anal sphincter tone is characteristic of cauda equina syndrome. Babinski sign or other signs of upper motor neuron involvement suggest a diagnosis other than cauda equina syndrome, possibly spinal cord compression.
In cauda equina syndrome, the peripheral nerve fibers from the sacral segments of the cord, as well as various lumbar dorsal and ventral nerve roots, may also be involved. This results in an asymmetric and higher distribution of motor and sensory symptoms and signs in the lower extremities. Incontinence of bowel and bladder is not severe and develops late for the same reason.
In conus and epiconus lesions, the sacral region neurons (S2-S4) are destroyed. The destruction of these neurons leads to an early and more severe involvement of bowel, urinary bladder, and sexual dysfunction than seen in those with cauda equina syndrome. In contrast, for the same reason, the motor and sensory symptoms in the lower extremities are often not very severe and only the distal parts of the limb musculature are involved.
The anatomical proximity of the conus medullaris, the epiconus, and the cauda equina can lead to 2 of these anatomical structures being involved via a single lesion, resulting in an overlap of symptomatology.
The symptoms above when presenting in isolation, are neither specific nor sensitive for cauda equina syndrome. However, several of the signs and symptoms mentioned above taken as a constellation should raise clinical suspicion 1. These symptoms also lack significant positive predictive value for the syndromes, especially early on 58. However, the onset of perineal anesthesia associated with bladder dysfunction is typical of the start of cauda equina syndrome and the time at which the clock starts on diagnosis and management 1. It is important also to note that painless urinary retention often has the greatest predictive value as a stand-alone symptom, but it is unfortunately indicative of late, often irreversible cauda equina syndrome 59.
Once clinical suspicion is established based on history, a thorough neurological examination is paramount. Findings to watch for include:
- Motor or sensory deficits in the legs – usually bilateral but can also be unilateral and asymmetrical (particularly in cases with an incomplete injury)
- Lower motor neuron signs in the legs – areflexia, hypotonia, atrophy (in cases of chronic cord compression resulting in cauda equina syndrome)
- Saddle anesthesia
- Absent or decreased rectal tone
- Absent or decreased bulbocavernosus reflex
- Palpable bladder indicating urinary retention 3
It is important to note that in the case of isolated conus medullaris syndrome, deficits of the lower extremities are more often bilateral and symmetric. Also, upper motor neuron signs can be present, such as spasticity and hyperreflexia 2.
Table 3. The salient features and findings of cauda equina syndrome versus conus medullaris syndrome
| Features | Cauda Equina syndrome | Conus Medullaris syndrome |
|---|---|---|
| Vertebral level | L2-sacrum | L1-L2 |
| Spinal level | Injury to the lumbosacral nerve roots | Injury of the sacral cord segment (conus and epiconus) and roots |
| Severity of symptoms and signs | Usually severe | Usually not severe |
| Symmetry of symptoms and signs | Usually asymmetric | Usually symmetric |
| Pain | Prominent, asymmetric, and radicular | Usually bilateral and in the perineal area |
| Motor | Weakness to flaccid paralysis | Normal motor function to mild or moderate weakness |
| Sensory | Saddle anesthesia, may be asymmetric | Symmetric saddle distribution, sensory loss of pin prick, and temperature sensations (Tactile sensation is spared.) |
| Reflexes | Areflexic lower extremities; bulbocavernosus reflex is absent in low cauda equina (sacral) lesions | Areflexic lower extremities (If the epiconus is involved, patellar reflex may be absent, whereas bulbocavernosus reflex may be spared.) |
| Sphincter and sexual function | Usually late and of lesser magnitude; lower sacral roots involvement can cause bladder, bowel, and sexual dysfunction | Early and severe bowel, bladder, and sexual dysfunction that results in a reflexic bowel and bladder with impaired erection in males |
| EMG | Multiple root level involvement; sphincters may also be involved | Mostly normal lower extremity with external anal sphincter involvement |
| Outcome | May be favorable compared with conus medullaris syndrome | The outcome may be less favorable than in patients with cauda equina syndrome |
Physical examination
Your doctor assesses stability, sensation, strength, reflexes, alignment and motion. He or she may ask you to stand, sit, walk on your heels and toes, bend forward, backward and to the sides, and lift your legs while lying down. Your doctor might check the tone and numbness of anal muscles. You may need blood tests.
Patients with an intact bulbocavernosus reflex and/or anal reflex would represent having an upper motor neuron (UMN) spinal cord lesion and if absent, most likely a lower motor neuron (LMN) spinal cord lesion 60. Bulbocavernosus reflexes may be absent or diminished 6. This should always be tested.
Anal sphincter tone is patulous and should always be tested since it can define the completeness of the injury (with bulbocavernosus reflex); it is also useful in monitoring recovery from the injury 6.
In cauda equina syndrome, muscle strength in the lower extremities is diminished 6. This may be specific to the involved nerve roots as listed below, with the lower lumbar and sacral roots more affected, leading to diminished strength in the glutei muscles, hamstring muscles (ie, semimembranosus, semitendinosus, biceps femoris), and the gastrocnemius and soleus muscles.
Sensation is decreased to pinprick and light touch in a dermatomal pattern corresponding to the affected nerve roots 6. This includes saddle anesthesia (sometimes including the glans penis or clitoris) and decreased sensation in the lower extremities in the distribution of lumbar and sacral nerves. Vibration sense may also be affected. Sensation of the glans penis or clitoris should be examined.
Muscle stretch reflexes may be absent or diminished in the corresponding nerve roots. Babinski reflex is diminished or absent.
Urinary incontinence could also occur secondary to loss of urinary sphincter tone; this may also present initially as urinary retention secondary to a flaccid bladder.
Muscle tone in the lower extremities is decreased, which is consistent with an lower motor neuron (LMN) lesion.
Table 4. Pain and deficits associated with specific nerve roots
| Nerve Root | Pain | Sensory Deficit | Motor Deficit | Reflex Deficit |
|---|---|---|---|---|
| L2 | Anterior medial thigh | Upper thigh | Slight quadriceps weakness; hip flexion; thigh adduction | Slightly diminished suprapatellar |
| L3 | Anterior lateral thigh | Lower thigh | Quadriceps weakness; knee extension; thigh adduction | Patellar or suprapatellar |
| L4 | Posterolateral thigh, anterior tibia | Medial leg | Knee and foot extension | Patellar |
| L5 | Dorsum of foot | Dorsum of foot | Dorsiflexion of foot and toes | Hamstrings |
| S1-2 | Lateral foot | Lateral foot | Plantar flexion of foot and toes | Achilles |
| S3-5 | Perineum | Saddle | Sphincters | Bulbocavernosus reflex; anal reflex |
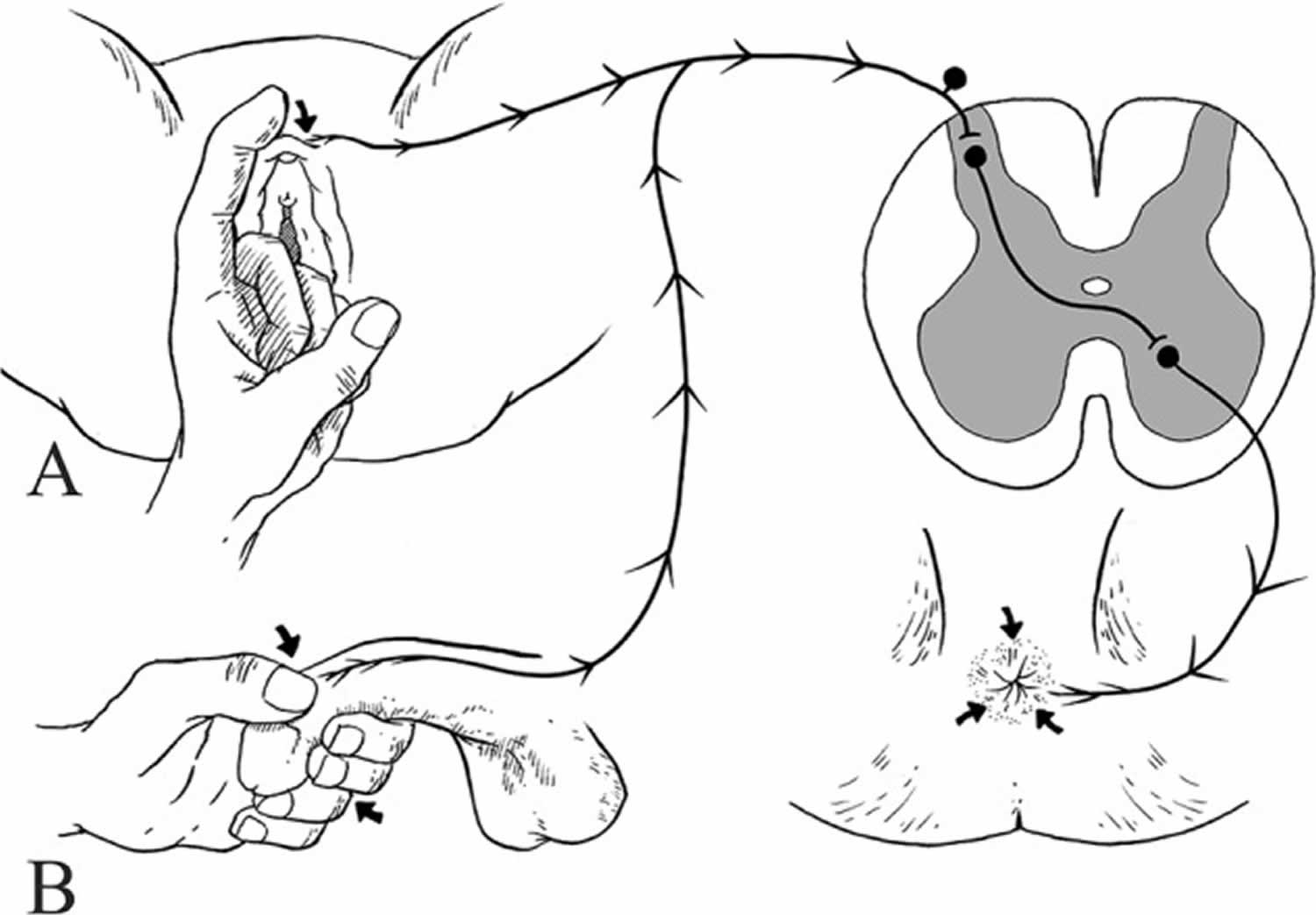
Bulbocavernosus reflex
Bulbocavernosus reflex also known as clitoroanal 61, penilo-cavernosus 62 and Osinski reflex 63 (see Figure 4) 64. Blaivas et al. 65 assessed the clinical utility of bulbocavernosus reflex and demonstrated a detectable reflex in 98% of able-bodied men and 81% of able-bodied females. However, in this study all patients with complete lower motor neuron (LMN) spinal cord lesions of the sacral spinal cord had absent bulbocavernosus reflex.
In women, different clinical methods have been described to elicit the bulbocavernosus reflex 66. Gently ‘squeezing the clitoris’ 67, ‘tapping and squeezing the clitoris’ 68, ‘gentle tapping of the clitoris’ 69 and ‘gently touching the labium minus lateral to the clitoris’ resulting in anal sphincter contraction, have been described as methods for eliciting the bulbocavernosus reflex in women 66. In men, the bulbocavernosus reflex is usually performed with the squeezing of the glans penis and observing anal sphincter contraction 64 or palpating the bulbocavernosus muscle contraction (see Figure 4) 70. Other effective stimuli to elicit this reflex have been described and include percussing the suprapubic area and observing anal sphincter contraction 71, or pulling the retention balloon of an indwelling Foley catheter against the bladder neck while a finger is inserted in the rectum 19.
The bulbocavernosus reflex is a multisynaptic spinal reflex mediated most commonly by root levels of S2 to S4 72, although occasionally the synapses may lie as high as L5 73 and the efferent innervation can include S5 74. Afferent impulses are conveyed via the dorsal nerve of the penis/clitoris or perineal nerve 75 and stimulate motor neurons of the external anal sphincter and bulbocavernosus muscles by the deep perineal 73 and inferior hemorrhoidal branches of the pudendal nerve 76.
Figure 4. Bulbocavernosus reflex
Footnote: Elicitation of bulbocavernosus reflex. (A) Bulbocavernosus reflex elicited by touching the labium minus lateral to the clitoris resulting in anal sphincter contraction in female. (B) Bulbocavernosus reflex elicited by squeezing of glans penis between the thumb and forefinger of the examiner resulting in anal sphincter contraction in male
[Source 60 ]Anal reflex
The anal reflex is reported being a constant appearance in normal subjects 77. The anal reflex is also known as the anal wink 78 , anocutaneous 79, and cutaneo-anal reflex 80 and is a multisynaptic spinal reflex mediated mainly by S2 to S4 81, 82, while others report S2 to S5 83, 84. The anal reflex has been also found to be a clinically feasible approach to assessment of sacral integrity 85 and a good predictor of recovery of the bladder and bowel functions in cauda equina syndrome 86. The anal reflex is usually elicited with pinprick stimulation at the mucocutaneous junction of the anus and observing for anal sphincter contraction 77, 82. Afferent pathways of the anal reflex lie in the pudendal nerve, which synapse in the spinal cord and travel via the inferior hemorrhoidal nerve to the external anal sphincter muscle 83, 84. Of key importance, the method to performing the anal reflex is the same examination technique recommended when performing the pinprick sensation at S4 to S5 as part of the International Standards for Neurological Classification of Spinal Cord Injury (see Figure 5) 87.
Figure 5. Anal reflex
Footnote: Elicitation of anal reflex. Anal reflex elicited by applying a pinprick to the mucocutaneous junction of the anus and evaluating for anal sphincter contraction
[Source 60 ]Muscle strength
Physical examination for cauda equina syndrome or conus medullaris syndrome would be incomplete without tests for sensation of the saddle and perineal areas, bulbocavernosus reflex, cremasteric reflex, and anal sphincter tone, findings for all of which are likely to be abnormal.
Muscle strength of the following muscles should be tested to determine the level of lesion:
- L2 – Hip flexors (iliopsoas)
- L3 – Knee extensors (quadriceps)
- L4 – Ankle dorsiflexors (tibialis anterior)
- L5 – Big toe extensors (extensor hallucis longus)
- S1 – Ankle plantar flexors (gastrocnemius/soleus)
American Spinal Cord Injury Association impairment scale
In defining impairments associated with a spinal cord lesion, the American Spinal Cord Injury Association (ASIA) impairment scale is used in determining the level and extent of injury.
The American Spinal Cord Injury Association (ASIA) scale should also be used in defining the extent of conus medullaris syndrome or cauda equina syndrome.
The American Spinal Cord Injury Association (ASIA) scale is as follows:
- A – Complete; no sensory or motor function preserved in sacral segments S4-S5
- B – Incomplete; sensory, but not motor, function preserved below the neurologic level and extends through sacral segments S4-S5
- C – Incomplete; motor function preserved below the neurologic level, and the majority of key muscles below the neurologic level have a muscle grade less than 3
- D – Incomplete; motor function preserved below the neurologic level, and the majority of key muscles below the neurologic level have a muscle grade greater than or equal to 3
- E – Normal; sensory and motor function normal
The injury should be described using this scale, for example, ASIA class A. Most patients with cauda equina/conus medullaris syndrome are in ASIA class A or B initially and gradually improve to class C, D, or E.
Diagnostic imaging
Your doctor may order x-rays, magnetic resonance imaging (MRI) scans, and computed tomography (CT) scans to help assess the problem. Plain x-rays is unlikely to be helpful in cauda equina syndrome but may be performed in cases of traumatic injury or in search of destructive changes, disk-space narrowing, or spondylolysis. For example, plain radiographs of the lumbosacral spine may depict early changes in vertebral erosions secondary to tumors and spina bifida.
The following tests may be helpful in diagnosing cauda equina syndrome:
- Magnetic resonance imaging (MRI): A diagnostic test that produces three-dimensional images of body structures using magnetic fields and computer technology. MRI produces images of the spinal cord, nerve roots, and surrounding areas.
- Myleogram: An x-ray of the spinal canal following injection of a contrast material into the surrounding cerebrospinal fluid spaces; can show displacement on the spinal cord or spinal nerves due to herniated discs, bone spurs, tumors, etc.
The gold standard method of evaluation for cauda equina syndrome is obtaining urgent MRI imaging with sagittal and axial T1 and T2 sequences 1. There has not been a specific timeframe established for “door to MRI time” in the ED, but early MRI and neurosurgical or orthopedic consultation is imperative. An ideal goal for MRI is one hour from the patient presentation 1. For patients with contraindications to MRI, such as those with metal implants, a CT myelogram is a viable option 1. This imaging modality has limited utility as it requires injecting contrast through a spinal tap to visualize the spinal cord and its associated structures. A bladder scan checking for a post-void residual volume should also be obtained to evaluate for urinary retention 1.
Blood studies
The following blood tests may help to define possible causes and any associated pathology, especially other causes of lesions in the lower spinal cord or cauda equina:
- CBC count, blood glucose, electrolytes, blood urea nitrogen (BUN), and creatinine – As part of the workup to rule out associated anemia, infection, and renal dysfunction, especially in associated retroperitoneal mass
- Erythrocyte sedimentation rate (ESR) – Elevation may point to an inflammatory pathology
- Syphilis serology to rule out meningovascular syphilis.
Other tests and procedures
Needle electromyography (EMG) may show evidence of acute denervation, especially in cauda equina lesions and multilevel lumbar spinal stenosis 88. EMG studies also could help in predicting prognosis and monitoring recovery. Performing needle EMG of the bilateral external anal sphincter muscles is recommended.
Nerve conduction studies especially of the pudendal nerve, may rule out more distal peripheral nerve lesions 89.
Somatosensory evoked potentials (SSEPs) could be done as part of the workup to rule out multiple sclerosis, which could present initially as a lower spinal cord syndrome 89.
Duplex ultrasound of peripheral vessels may rule out compromised vasculature as a possible cause of associated claudication.
Lumbar puncture should be performed to examine the CSF to rule out inflammatory disease of the meninges or spinal cord.
Cauda equina syndrome differential diagnosis
Cauda equina syndrome differential diagnoses include:
- Acute Inflammatory Demyelinating Polyradiculoneuropathy
- Amyotrophic Lateral Sclerosis
- Diabetic Neuropathy
- Guillain-Barré Syndrome
- Multiple Sclerosis
- Spinal cord neoplasms
- Neuromuscular and Myopathic Complications of HIV
- Neurosarcoidosis
- Spinal Cord Infections
- Traumatic Peripheral Nerve Lesions
Other problems to be considered include the following 90:
- Amyloidosis with deposits in the spinal cord
- Ankylosing spondylitis and other spondyloarthropathy
- Charcot-Marie-Tooth disease (types 1 and 3)
- Intravascular lymphomatosis
- Lipomas within the spine
- Lumbar stenosis (multilevel)
- Paget disease of the spine
- Spinal infection/abscess and meningitis
- Tethered cord syndrome or short filum terminale
- Vascular intermittent claudication
Cauda equina syndrome treatment
Cauda equina syndrome is considered a diagnostic and surgical emergency, although there is some debate about the timing of surgery, which is also dependent on whether the pathology is acute or chronic. Surgical decompression within 24 hours seems to have the best outcome 37, 7, 14.
If you have cauda equina syndrome, you may need urgent surgery to remove the material that is pressing on the nerves. The goal is to reverse the symptoms of neural dysfunction. The surgery may prevent pressure on the nerves from reaching the point at which damage is irreversible.
Treating patients within 48 hours after the onset of the cauda equina syndrome provides a significant advantage in improving sensory and motor deficits as well as urinary and rectal function 58. But even patients who undergo surgery after the 48-hour ideal timeframe may experience considerable improvement.
Methylprednisolone should be administered and must be started within 8 hours of injury 91. No evidence exists of any benefit if it is started more than 8 hours after injury; on the contrary, late treatment may have detrimental effects 91.
Administration of ganglioside GM1 sodium salt beginning within 72 hours of injury may be beneficial; the dose is 100 mg IV every day for 18-32 days 91.
Tirilazad mesylate (a nonglucocorticoid 21-aminosteroid) has been proven to be of benefit in animals and is currently under investigation 91. It inhibits lipid peroxidation and hydrolysis in the same manner as glucocorticoids.
Although short-term recovery of bladder function may lag behind reversal of lower extremity motor deficits, the function may continue to improve years after surgery. Following surgery, drug therapy coupled with intermittent self-catheterization can help lead to slow, but steady recovery of bladder and bowel function.
Other medical treatment options are useful in certain patients, depending on the underlying cause of the cauda equina syndrome 91. Anti-inflammatory agents and steroids can be effective in patients with inflammatory processes, including ankylosing spondylitis 91.
Patients with cauda equina syndrome secondary to infectious causes should receive appropriate antibiotic therapy 91. Patients with spinal neoplasms should be evaluated for the suitability of chemotherapy and radiation therapy.
Caution should be used in all forms of medical management for cauda equina syndrome. Any patient with true cauda equina syndrome with symptoms of saddle anesthesia and/or bilateral lower extremity weakness or loss of bowel or bladder control should undergo no more than 24 hours of initial medical management 91. If no relief of symptoms is achieved during this period, immediate surgical decompression is necessary to minimize the chances of permanent neurologic injury 91.
Cauda equina syndrome recovery
Surgery will not repair permanent nerve damage. If this occurs as a result of cauda equina syndrome, you can learn how to improve your quality of life.
Some suggestions:
- In addition to medical personnel, you may want to get help from an occupational therapist, social worker, continence advisor, or sex therapist.
- Involve your family in your care.
- To learn all you can about managing the condition, you may want to join a cauda equina syndrome support group.
Several studies have looked at prognosis and outcomes based on the timing of surgical decompression. Early intervention by surgical decompression in patients with conus medullaris and cauda equina syndromes is associated with a better prognosis, particularly when surgery occurs within 48 hours of initial presentation 58. The longer the compression continues, the worse the permanent structural and functional impairment, and the poorer the prognosis 1. It is important to note that the presence of bladder dysfunction prior to surgery has been linked to poorer outcomes regardless of the timing of decompression, although early decompression is still the recommendation for a better prognosis irrespective of clinical status at initial presentation 1.
Managing bladder and bowel function
Some bladder and bowel function is automatic, but the parts under voluntary control may be lost if you have cauda equina syndrome. This means you may not know when you need to urinate or move your bowels, and/or you may not be able to eliminate waste normally.
Some general recommendations for managing bladder and bowel dysfunction:
- Empty the bladder completely with a catheter 3 to 4 times each day. Drink plenty of fluids and practice regular personal hygiene to prevent urinary tract infection.
- Check for the presence of waste regularly and clear the bowels with gloved hands. You may want to use glycerin suppositories or enemas to help empty the bowels. Use protective pads and pants to prevent leaks.
- Quaile A. Cauda equina syndrome-the questions. Int Orthop. 2018 Oct 29
- Brouwers E, van de Meent H, Curt A, Starremans B, Hosman A, Bartels R. Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review. Spinal Cord. 2017 Oct;55(10):886-890
- Korse NS, Pijpers JA, van Zwet E, Elzevier HW, Vleggeert-Lankamp CLA. Cauda Equina Syndrome: presentation, outcome, and predictors with focus on micturition, defecation, and sexual dysfunction. Eur Spine J. 2017 Mar;26(3):894-904
- Rider IS, Marra EM. Cauda Equina And Conus Medullaris Syndromes. [Updated 2019 Jan 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537200
- Ridley LJ, Han J, Ridley WE, Xiang H. Cauda equina: Normal anatomy. J Med Imaging Radiat Oncol. 2018 Oct;62 Suppl 1:123
- Cauda Equina and Conus Medullaris Syndromes Clinical Presentation. https://emedicine.medscape.com/article/1148690-clinical#b3
- Lavy C, James A, Wilson-MacDonald J, Fairbank J. Cauda equina syndrome. BMJ. 2009 Mar 31;338:b936. doi: 10.1136/bmj.b936
- Gitelman A, Hishmeh S, Morelli BN, Joseph SA Jr, Casden A, Kuflik P, Neuwirth M, Stephen M. Cauda equina syndrome: a comprehensive review. Am J Orthop (Belle Mead NJ). 2008 Nov;37(11):556-62.
- Kobayashi S, Yoshizawa H, Yamada S. Pathology of lumbar nerve root compression. Part 1: intraradicular inflammatory changes induced by mechanical compression. J Orthop Res 2004;22:170–9. 10.1016/S0736-0266(03)00131-1
- Gautschi OP, Cadosch D, Hildebrandt G. Notfallszenario: Cauda-equina-Syndrom–Beurteilung und Management [Emergency scenario: cauda equina syndrome–assessment and management]. Praxis (Bern 1994). 2008 Mar 19;97(6):305-12. German. doi: 10.1024/1661-8157.97.6.305
- Mauffrey C, Randhawa K, Lewis C, Brewster M, Dabke H. Cauda equina syndrome: an anatomically driven review. Br J Hosp Med (Lond). 2008 Jun;69(6):344-7. doi: 10.12968/hmed.2008.69.6.29625
- Sato K, Kikuchi S. Clinical analysis of two-level compression of the cauda equina and the nerve roots in lumbar spinal canal stenosis. Spine (Phila Pa 1976). 1997 Aug 15;22(16):1898-903; discussion 1904. doi: 10.1097/00007632-199708150-00018
- Byrne TN. Disorders of the spinal cord and cauda equina. Curr Opin Neurol Neurosurg. 1993 Aug;6(4):545-8.
- Gardner A, Gardner E, Morley T. Cauda equina syndrome: a review of the current clinical and medico-legal position. Eur Spine J. 2011 May;20(5):690-7. doi: 10.1007/s00586-010-1668-3
- Tandon PN, Sankaran B. Cauda equina syndrome due to lumbar disc prolapse. Indian J Orthop. 1967;1:112–119.
- Kunam VK, Velayudhan V, Chaudhry ZA, Bobinski M, Smoker WRK, Reede DL. Incomplete Cord Syndromes: Clinical and Imaging Review. Radiographics. 2018 Jul-Aug;38(4):1201-1222. doi: 10.1148/rg.2018170178
- Gleave JRW, Macfarlane R. Cauda equina syndrome: what is the relationship between timing of surgery and outcome? Brit J Neurosurg. 2002;16(4):325–328. doi: 10.1080/0268869021000032887
- Previnaire JG. The importance of the bulbocavernosus reflex. Spinal Cord Ser Cases. 2018;4:2. doi: 10.1038/s41394-017-0012-0
- Alexander M, Aslam H, Marino RJ. Pulse article: how do you do the international standards for neurological classification of SCI anorectal exam? Spinal Cord Ser Cases. 2017;3:17078. doi: 10.1038/s41394-017-0015-x
- https://www.bmj.com/rapid-response/2011/11/02/clinical-assessment-cauda-equina-syndrome-and-bulbocavernosus-reflex
- Todd NV. Cauda equina syndrome. The timing of surgery probably does influence outcome. Br J Neurosurg. 2005;19:301–306. doi: 10.1080/02688690500305324
- Lawrentschuk N, Nguyen H. Cauda equina syndrome secondary to constipation: an uncommon occurrence. ANZ J Surg. 2005;75:498–500. doi: 10.1111/j.1445-2197.2005.03404.x
- Coscia M, Leipzig T, Cooper D. Acute cauda equina syndrome: diagnostic advantage of MRI. Spine. 1994;19:475–478. doi: 10.1097/00007632-199402001-00020
- Podnar S. Epidemiology of cauda equina and conus medullaris lesions. Muscle Nerve. 2007 Apr;35(4):529-31.
- Fraser S, Roberts L, Murphy E. Cauda equina syndrome: a literature review of its definition and clinical presentation. Arch Phys Med Rehabil. 2009 Nov;90(11):1964-8. doi: 10.1016/j.apmr.2009.03.021
- Harrop JS, Hunt GE Jr, Vaccaro AR. Conus medullaris and cauda equina syndrome as a result of traumatic injuries: management principles. Neurosurg Focus. 2004 Jun 15;16(6):e4. doi: 10.3171/foc.2004.16.6.4
- O’CONNELL JE. The indications for and results of the excision of lumbar intervertebral disc protrusions: a review of 500 cases. Ann R Coll Surg Engl. 1950 Jun;6(6):403-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2238544/pdf/annrcse00036-0039.pdf
- Heyes G, Jones M, Verzin Eet al.. Influence of timing of surgery on Cauda equina syndrome: outcomes at a national spinal centre. J Orthop 2018; 15: 210–215. 10.1016/j.jor.2018.01.020
- Hussain SA, Gullan RW, Chitnavis BP. Cauda equina syndrome: outcome and implications for management. Brit J Neurosurg. 2003;17(2):164–167. doi: 10.1080/0268869031000109098
- Kostuik JP. Medico-legal consequences of cauda equina syndrome: an overview. J Neurosurg Neurosurg Focus. 2004;16:39–41. doi: 10.3171/foc.2004.16.6.7
- O’Laoire SA, Crockard HA, Thomas DG. Prognosis for sphincter recovery after operation for cauda equina compression owing to lumbar disc prolapse. Brit Med J. 1981;282:1852–1854. doi: 10.1136/bmj.282.6279.1852
- Todd NV. Cauda equina syndrome: the timing of surgery probably does influence outcome. Br J Neurosurg 2009; 19: 301–306. 10.1080/02688690500305324
- Cauda Equina and Conus Medullaris Syndromes Clinical Presentation. https://emedicine.medscape.com/article/1148690-clinical
- Bickle I, Burst fracture with cauda equina syndrome. Case study, Radiopaedia.org https://doi.org/10.53347/rID-25701
- Shi J, Jia L, Yuan W, Shi G, Ma B, Wang B, Wu J. Clinical classification of cauda equina syndrome for proper treatment. Acta Orthop. 2010 Jun;81(3):391-5. doi: 10.3109/17453674.2010.483985
- Lavy C, James A, Wilson-MacDonald J, Fairbank J. Cauda equina syndrome. BMJ. 2009 Mar 31;338:b936.
- McNamee J, Flynn P, O’Leary S, Love M, Kelly B. Imaging in cauda equina syndrome–a pictorial review. Ulster Med J. 2013 May;82(2):100-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3756868
- Knipe H, Bell D, Bickle I, et al. Cauda equina syndrome. Reference article, Radiopaedia.org https://doi.org/10.53347/rID-28701
- Tarlov IM. Perineurial cysts of the spinal nerve roots. Arch Neurol Psychiatry 1938;40:1067–74. 10.1001/archneurpsyc.1938.0227012001700
- Tarlov IM. Spinal perineurial and meningeal cysts. J Neurol Neurosurg Psychiatry 1970;33:833–43. 10.1136/jnnp.33.6.833
- Yates JR, Jones CS, Stokes OM, Hutton M. Incomplete cauda equina syndrome secondary to haemorrhage within a Tarlov cyst. BMJ Case Rep. 2017 Aug 7;2017:bcr2017219890. doi: 10.1136/bcr-2017-219890
- Lan HH, Chen DY, Chen CC, Lan JL, Hsieh CW. Combination of transverse myelitis and arachnoiditis in cauda equina syndrome of long-standing ankylosing spondylitis: MRI features and its role in clinical management. Clin Rheumatol. 2007 Nov;26(11):1963-7. doi: 10.1007/s10067-007-0593-2
- Shaw PJ, Allcutt DA, Bates D, Crawford PJ. Cauda equina syndrome associated with multiple lumbar arachnoid cysts in ankylosing spondylitis: improvement following surgical therapy. J Neurol Neurosurg Psychiatry. 1990 Dec;53(12):1076-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC488319/pdf/jnnpsyc00522-0058.pdf
- Bowie EA, Glasgow GL. Cauda Equina Lesions Associated with Ankylosing Spondylitis. Br Med J. 1961 Jul 1;2(5243):24-7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1968962/pdf/brmedj03004-0032.pdf
- Rubinstein DJ, Alvarez O, Ghelman B, Marchisello P. Cauda equina syndrome complicating ankylosing spondylitis: MR features. J Comput Assist Tomogr. 1989;13:511–513. doi: 10.1097/00004728-198905000-00029
- Transfeldt E, White D, Bradford DS, Roche B. Delayed anterior decompression in patients with spinal cord and cauda equina injuries of the thoraco-lumbar spine. Spine. 1990;15:953–957. doi: 10.1097/00007632-199009000-00021
- Thongtrangan I, Le H, Park J, Kim DH. Cauda equina syndrome in patients with low lumbar fractures. Neurosurg Focus. 2004 Jun 15;16(6):e6.
- Kebaish KM, Awad J. Spinal epidural haematoma causing acute cauda equina syndrome. J Neurosurg Neurosurg Focus. 2004;16:1–4. doi: 10.3171/foc.2004.16.6.1
- Shephard RH. Diagnosis and prognosis of cauda equina syndrome produced by protrusion of lumbar disc. Brit Med J. 1959;2:1434–1439. doi: 10.1136/bmj.2.5164.1434
- Ozgen S, Beyken N, Dogan IV, Deniz K, Pamir MN. Cauda equina syndrome after induction of spinal anaesthesia. J Neurosurg Neurosurg Focus. 2004;16:24–27.
- Flores LP, Nascimento Filho J deS S, Pereira Neto A, Suzuki K. Prognostic factors related to gunshot wounds to the spine in patients submitted to laminectomy. Arq De Neuro-Psyq. 1999;57:836–842. doi: 10.1590/S0004-282X1999000500016
- Cohen DB. Infectious origins of cauda equine syndrome. J Neurosurg Neurosurg Focus. 2004;16:5–10.
- Bagley CA, Gokaslan ZL. Cauda equina syndrome caused by primary and metastatic neoplasms. Neurosurg Focus. 2004 Jun 15;16(6):e3. doi: 10.3171/foc.2004.16.6.3
- Jensen RL. Cauda equina syndrome as a post operative complication of lumbar spine surgery. J Neurosurg Neurosurg Focus. 2004;16:34–38. doi: 10.3171/foc.2004.16.6.6
- Smith S, Leibrock LG, Gelber BR, Pierson EW. Acute herniated nucleus pulposus with cauda equina compression syndrome following chemonucleolysis. J Neurosurg. 1987;66:614–617. doi: 10.3171/jns.1987.66.4.0614
- Bell DA, Collie D, Statham PF. Cauda equina syndrome: what is the correlation between clinical assessment and MRI scanning? Br J Neurosurg. 2007 Apr;21(2):201-3. doi: 10.1080/02688690701317144
- Ma B, Wu H, Jia LS, Yuan W, Shi GD, Shi JG. Cauda equina syndrome: a review of clinical progress. Chin Med J (Engl). 2009 May 20;122(10):1214-22.
- Thakur JD, Storey C, Kalakoti P, Ahmed O, Dossani RH, Menger RP, Sharma K, Sun H, Nanda A. Early intervention in cauda equina syndrome associated with better outcomes: a myth or reality? Insights from the Nationwide Inpatient Sample database (2005-2011). Spine J. 2017 Oct;17(10):1435-1448
- Todd NV. Guidelines for cauda equina syndrome. Red flags and white flags. Systematic review and implications for triage. Br J Neurosurg. 2017 Jun;31(3):336-339.
- Kirshblum S, Eren F. Anal reflex versus bulbocavernosus reflex in evaluation of patients with spinal cord injury. Spinal Cord Ser Cases. 2020 Jan 7;6:2. doi: 10.1038/s41394-019-0251-3
- Campbell WW. The deep tendon or muscle stretch reflexes. In: Dejong’s The Neurologic Examination. 7th ed. Philadelphia: Kluwer Walters; 2013. p. 561–78.
- Podnar S. Saddle sensation is preserved in a few patients with cauda equina or conus medullaris lesions. Eur J Neurol. 2007;14:48–53. doi: 10.1111/j.1468-1331.2006.01542.x
- Vivek P. Acute spinal cord injury. In: Manipal manual of orthopaedics. 1st ed. London: Jaypee Brothers Medical; 2019. p. 110.
- Bors E, Blinn KA. Bulbocavernosus reflex. J Urol. 1959;82:128–30. doi: 10.1016/S0022-5347(17)65843-9
- Blaivas JG, Zayed AAH, Labib KB. The bulbocavernosus reflex in urology: a prospective study of 299 patients. J Urol. 1981;126:197–9. doi: 10.1016/S0022-5347(17)54445-6
- Wester C, FitzGerald MP, Brubaker L, Welgoss J, Benson JT. Validation of the clinical bulbocavernosus reflex. Neurourol Urodyn. 2003;22:589–91. doi: 10.1002/nau.10140
- Chancellor MB, Blaivas JG. Neuro-urologic examination. In: Chancellor MB, Blavias JG, editors. Practical neuro-urology: genitourinary complications in neurologic disease. Boston, Oxford: Butterworth-Heinemann; 1995. p. 55–62.
- Stanton SL. History and examination. In: Stanton SL, Monga AK, editors. Clinical urogynecology. 2nd ed. London: Churchill Livingstone; 2000. p. 77–84.
- Khullar V, Cardozo L. History and examination. In: Cardozo L, Staskin D, editors. Textbook of female urology and urogynecology. United Kingdom: Taylor and Francis; 2001. p. 153–65.
- Granata G, Padua L, Rossi F, De Franco P, Coraci D, Rossi V. Electrophysiological study of the bulbocavernosus reflex: normative data. Funct Neurol. 2013 Oct-Dec;28(4):293-5. doi: 10.11138/FNeur/2013.28.4.293
- Hargrove GK, Bors E. The suprapubic abdominal tap reflex: a useful method to assess the function of the sacral reflex arcs. J Urol. 1972;107:243–4. doi: 10.1016/S0022-5347(17)60992-3
- Vodušek DB. Neural control of pelvic floor muscles. In: Pelvic floor re-education. London: Springer London; 2008. p. 22–35.
- Lapides J, Bobbitt JM. Diagnostic value of bulbocavernous reflex. J Am Med Assoc. 1956;162:971–2. doi: 10.1001/jama.1956.72970270002010a
- Ko HY. Revisit spinal shock: pattern of reflex evolution during spinal Shock. Korean J Neurotrauma. 2018;14:47–54. doi: 10.13004/kjnt.2018.14.2.47
- Yang CC, Bradley WE. Reflex innervation of the bulbocavernosus muscle. BJU Int. 2000;85:857–63. doi: 10.1046/j.1464-410x.2000.00560.x
- Smith RP, Turek PJ. The Netter collection of medical iilustrations. Volume 1. In: Reproductive system. 2nd ed. Philadelphia, PA: Elsevier; 2011. p. 30
- Pedersen E, Harving H, Klemar B, Torring J. Human anal reflexes. J Neurol Neurosurg Psychiatry. 1978;41:813–8. doi: 10.1136/jnnp.41.9.813
- Alexander MS, Carr C, Chen Y, McLain A. The use of the neurologic exam to predict awareness and control of lower urinary tract function post SCI. Spinal Cord. 2017;55:840–3. doi: 10.1038/sc.2017.55
- Sanders C, Driver CP, Rickwood AMK. The anocutaneous reflex and urinary continence in children with myelomeningocele. BJU Int. 2002;89:720–1. doi: 10.1046/j.1464-410X.2002.02723.x
- Bartolo DCC, Jarratt JA, Read NW. The cutaneo-anal reflex: a useful index of neuropathy? Br J Surg. 1983;70:660–3. doi: 10.1002/bjs.1800701106
- Swash M. Early and late components in the human anal reflex. J Neurol Neurosurg Psychiatry. 1982;45:767–9. doi: 10.1136/jnnp.45.9.767
- Roberts MM. Neurophysiology in neurourology. Muscle Nerve. 2008;38:815–36. doi: 10.1002/mus.21001
- Campbell WW. The superficial (cutaneous) reflexes. In: Dejong’s the neurologic examination. 7th ed. Philadelphia: Wolters Kluwer; 2013. p. 581.
- Shiers S, Singal A, Korsten MA. Gastrointestinal system after spinal cord injury: assessment and intervention. In: Lin VW, Bono CM, editors. Spinal cord medicine, seconnd edition: Principles & Practice. 2nd ed. New York: Demos Medical; 2010. p. 395.
- King JC, Currie DM, Wright E. Bowel training in spina bifida: importance of education, patient compliance, age, and anal reflexes. Arch Phys Med Rehabil. 1994;75:243–7. doi: 10.1016/0003-9993(94)90022-1
- Dhatt S, Tahasildar N, Tripathy SK, Bahadur R, Dhillon M. Outcome of spinal decompression in cauda equina syndrome presenting late in developing countries: case series of 50 cases. Eur Spine J. 2011;20:2235–9. doi: 10.1007/s00586-011-1840-4
- American Spinal Injury Association/ International Medical Society of Paraplegia. International standards for neurological and functional classification of spinal cord injury patients. 7th ed. Chicago, Illinois: ASIA; 2015.
- Podnar, S. (2003), Electromyography of the anal sphincter: Which muscle to examine?. Muscle Nerve, 28: 377-379. https://doi.org/10.1002/mus.10417
- Rydevik B. Neurophysiology of cauda equina compression. Acta Orthop Scand Suppl. 1993;251:52-5. doi: 10.3109/17453679309160117
- Bardin, L.D., King, P. and Maher, C.G. (2017), Diagnostic triage for low back pain: a practical approach for primary care. Medical Journal of Australia, 206: 268-273. https://doi.org/10.5694/mja16.00828
- Cauda Equina and Conus Medullaris Syndromes Treatment & Management. https://emedicine.medscape.com/article/1148690-treatment