Chorioretinitis
Chorioretinitis also called retinochoroiditis, is an inflammatory process that involves the retina and the uveal tract of the eye (middle layer of eye), which consists of the iris, ciliary body, and choroid. Chorioretinitis is usually caused by congenital viral, bacterial, or protozoal infections in neonates. Congenital toxoplasma and cytomegalovirus (CMV) infection are the most common causes in this age group. Fungal infections are commonly identified, and emergent pathogens such as West Nile virus and lymphocytic choriomeningitis virus have been described 1. In rare instances, chorioretinitis is part of a systemic noninfectious process.
The extent of ocular involvement depends on the organism. Bilateral focal or extensive exudative chorioretinitis or panuveitis may be seen in patients with Toxoplasma gondii infection. A single large choroidal lesion with extensive inflammation or endophthalmitis is usually observed in patients with Toxocara canis, whereas interstitial keratitis or iritis is most common in patients with Treponema pallidum. Strabismus and optic atrophy may accompany chorioretinitis caused by cytomegalovirus (CMV). The central retinal lesions of CMV cannot be clinically distinguished from those of toxoplasmosis. However, unlike congenital toxoplasma infection, the retinitis caused by CMV does not progress 2.
Vessel trauma caused by other organisms, such as Toxocara or Baylisascaris larvae, may be associated with severe inflammatory responses.
Chorioretinitis associated with congenital viral infections like cytomegalovirus (CMV) tends to be stable or improve in infancy, whereas chorioretinitis associated with asymptomatic congenital toxoplasmosis progresses for years after birth and is more likely to be clinically significant at an older age 3.
If chorioretinitis is left untreated or if chorioretinitis does not respond to treatment, severe chorioretinitis can result in partial or total loss of vision in the affected eye. Morbidity is due to concurrent damage to major organ systems, especially damage to the brain (eg, developmental delays, seizures). Mortality due to chorioretinitis depends on the nature and progression of the underlying illness.
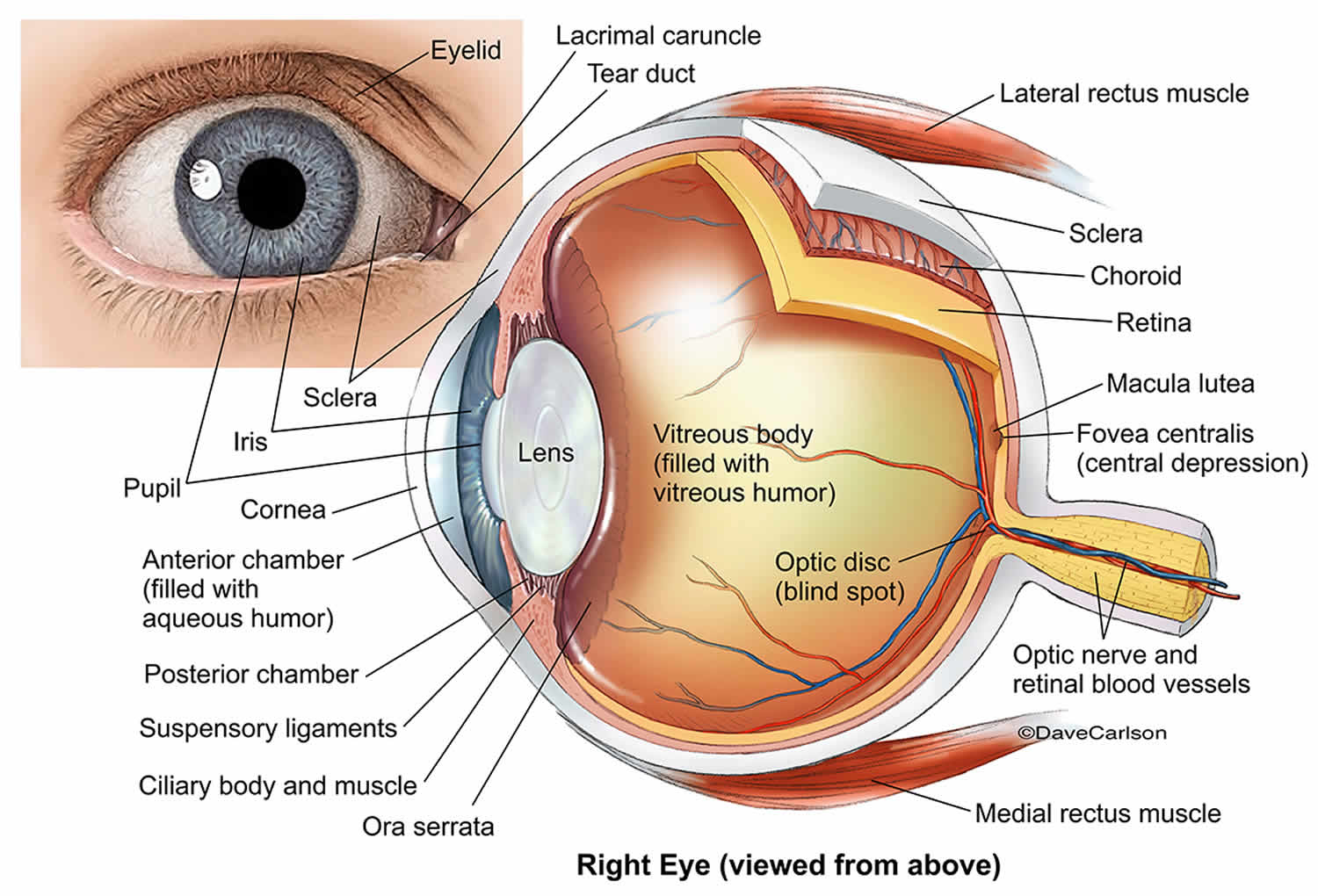
Eye anatomy
The inside of your eye contains a vitreous gel and is covered by an outer part consisting of three layers:
- The sclera – the white part of the eye
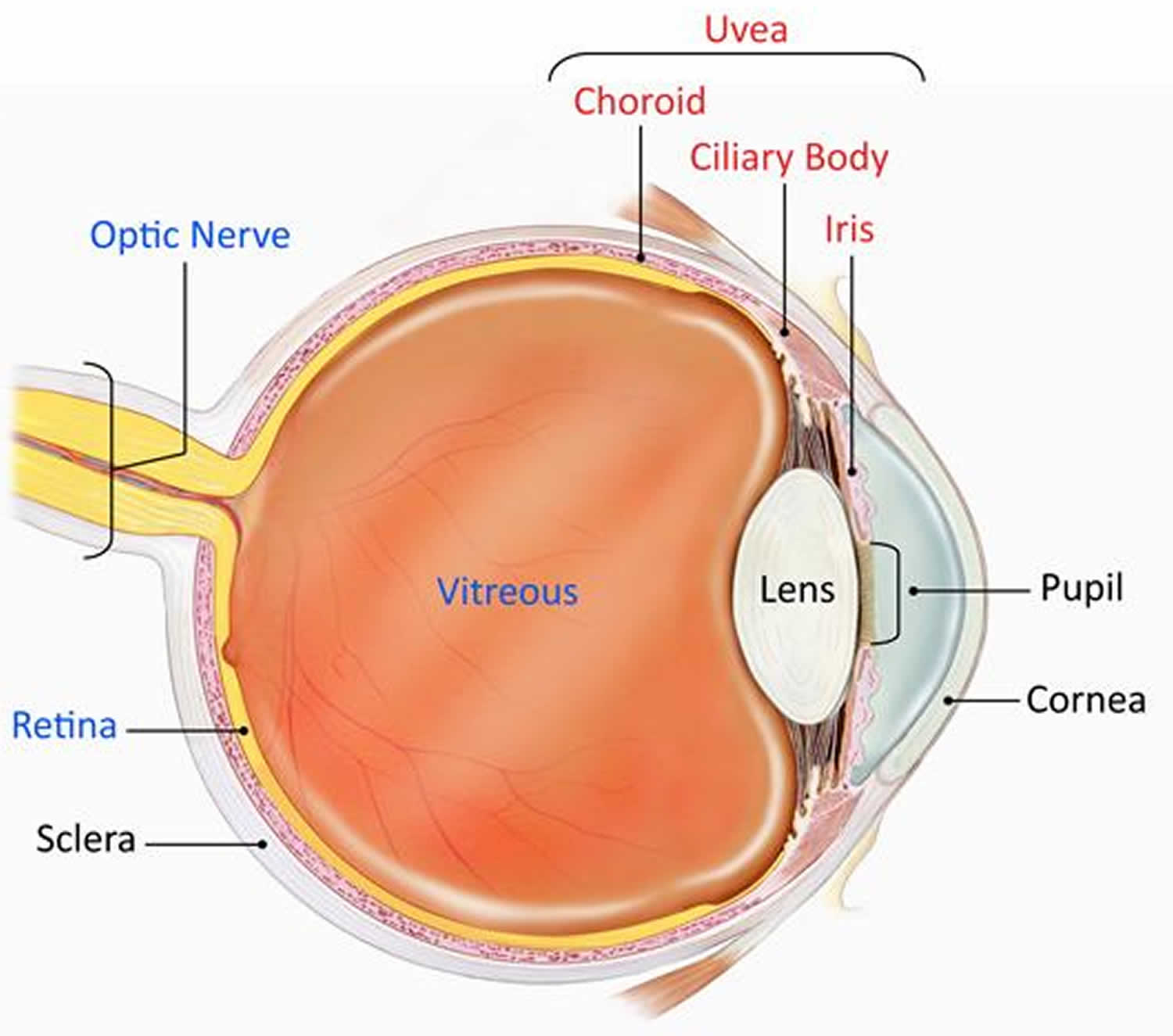
- The uvea (middle choroid layer) is the middle layer of the eye which contains much of the eye’s blood vessels to nourish the layers (see Figure 1)
- The innermost light sensitive layer – the retina. Retina is the layer of cells on the back, inside part of the eye that converts light into electrical signals sent to the brain.
Located between the sclera, the eye’s white outer coat, and the inner layer of the eye, called the retina, the uvea consists of the iris, ciliary body, and choroid. The middle choroid layer is made up of several components forming the uveal tract – the iris at the front, with the ciliary body near the front having muscles and ligaments to change the shape of the eye lens, and the choroid.
- Iris: The colored circle at the front of the eye. It defines eye color, secretes nutrients to keep the lens healthy, and controls the amount of light that enters the eye by adjusting the size of the pupil.
- Ciliary body: It is located between the iris and the choroid. It helps the eye focus by controlling the shape of the lens and it provides nutrients to keep the lens healthy.
- Choroid: A thin, spongy network of blood vessels, which primarily provides nutrients to the retina.
Uveitis disrupts vision by primarily causing problems with the lens, retina, optic nerve, and vitreous (see Figure 1).
- Lens: Transparent tissue that allows light into the eye.
- Optic Nerve: A bundle of nerve fibers that transmits electrical signals from the retina to the brain.
- Vitreous bdy: The fluid filled space inside the eye.
Inflammations that occur in the uveal layer are named with regard to where they are and they all end in “itis”, after the Greek word for inflammation:
- Iritis – Inflammation at the iris.
- Uveitis – Inflammation of the uveal tract
- Anterior uveitis – in the front area, from the Greek word for “before”
- Posterior uveitis – at the rear of the eye – also called choroiditis
- Retinitis – at the light sensitive layer
When an inflammation involves both the choroid and retinal layers then it is given the name “chorioretinitis” or sometimes “retinochoroiditis”.
Figure 1. Eye anatomy
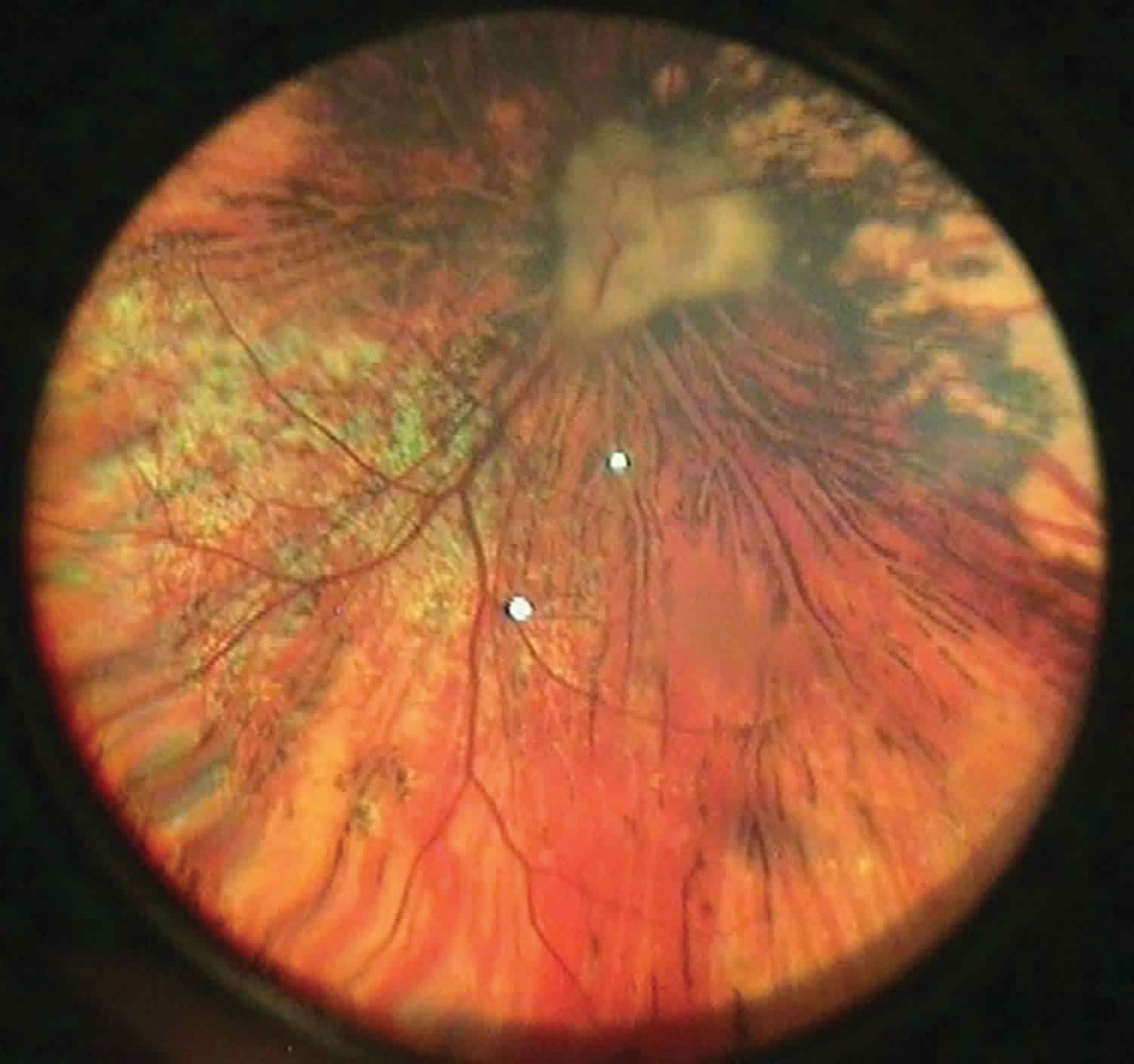
Figure 2. Chorioretinitis (fundoscopic image)
Chorioretinitis causes
In most individuals with chorioretinitis, the history may or may not aid in establishing causal agents. For example, in patients with chorioretinitis associated with congenital infections, eliciting the maternal history of primary viral or flulike illnesses during pregnancy is usually not easy. Dietary habits (preference of raw meat) and pet care (cleaning cat litter box) may imply toxoplasmosis or contact with kittens (catscratch disease). Lack of immunizations in a pregnant woman may also provide some clues to the diagnosis (eg, rubella).
Although CMV is the most common congenital infection in the developed world, affecting approximately 1% of all infants born in the United States, only 10% of all infants born in the United States with congenital CMV infection have symptomatic disease at birth, including chorioretinitis 4.
Congenital disseminated infections such as CMV and toxoplasmosis may also manifest with extraocular findings such as intrauterine growth retardation, microcephaly, microphthalmia, cataract, uveitis, hearing defect, osteomyelitis, hepatosplenomegaly, lymphadenopathy, dermal erythropoiesis, carditis, and congenital heart disease.
Beyond the neonatal period, chorioretinitis can be diagnosed in diverse clinical conditions and can reflect newly acquired diseases or reactivation. Congenital toxoplasmosis is the most common cause of infectious chorioretinitis in immunocompetent children 5. Chorioretinitis can also result from a dissemination of parasitic infections like Toxocara or Baylisascaris (the raccoon roundworm) in immunocompetent patients 6. In severely immunodeficient patients, including those with acquired immunodeficiency syndrome (AIDS), chorioretinitis may be associated with Epstein-Barr virus (EBV), CMV, varicella-zoster virus, various fungi (eg, Candida, Aspergillus, Fusarium, dimorphic fungi), and Toxoplasma 7.
In addition, with increasing air travel and globalization, several emerging infectious diseases have been recognized as causing ocular disease, including retinitis, chorioretinitis, retinal vasculitis, and optic nerve involvement. These include rickettsiosis, Rift Valley fever, dengue fever, and Chikungunya virus 8.
Congenital infection
In immunocompetent children, chorioretinitis is usually associated with congenital infection; acquired infection is a less likely cause.
Toxoplasma gondii and CMV are the leading causes of congenital infections associated with chorioretinitis.
Viral causes include vertical or perinatal infections, including Herpes Simplex virus (HSV), rubella, varicella, Epstein-Barr virus (EBV), lymphocytic choriomeningitis virus, and, possibly flavivirus. With the recent increase in the incidence of congenital infection after being at a nadir since 1991, syphilis should be considered in an infant born with chorioretinitis whose mother has untreated or inadequately treated syphilis, particularly if she also has human immunodeficiency virus infection (HIV) 9.
Distinguishing these infections from perinatal transmission of other viral illnesses, including herpes simplex virus, hepatitis B, and HIV is important.
The risk of intrauterine infection is highest in infants of women with primary infection and is much less with recurrent infections.
Acquired chorioretinitis in immunocompetent children
Some children who ingest embryonated Toxoplasma canis or Baylisascaris procyonis eggs may develop visceral larva migrans or ocular larva migrans. Another acquired infection that may lead to chorioretinitis is Bartonella henselae 10. More than 90% of patients with catscratch disease have a history of recent contact with a cat, often a kitten, and 50-87% of these patients have been scratched.
Immunocompromised children
Chorioretinitis may be associated with systemic infection due to a vast array of pathogens. Any of the infections discussed above may be seen; however, the presentation in an immunocompromised individual may be atypical.
Other infections may include congenital or acquired Lyme disease, Yersinia enterocolitica, and Mycobacterium tuberculosis 11.
Invasive fungal infections may result from Candida,Cryptococcus species, and histoplasmosis 12.
A species of blackfly (Simulium species) can transmit onchocerciasis (in tropical Africa, Yemen, Saudi Arabia, and parts of Latin America) 13.
Noninfectious disease
Systemic noninfectious disease, such as sarcoidosis, collagen vascular disease, chronic granulomatous disease, Behcet disease, and juvenile rheumatoid arthritis may cause chorioretinitis 14.
Other possible noninfectious processes include chronic infantile, neurological, cutaneous, and articular syndrome (CINCA) syndrome, also known as neonatal onset multisystemic inflammatory disease 15.
Chorioretinitis symptoms
The signs and symptoms of chorioretinitis depend on the type of inflammation.
Symptoms of chorioretinitis include:
- Vision becoming blurred
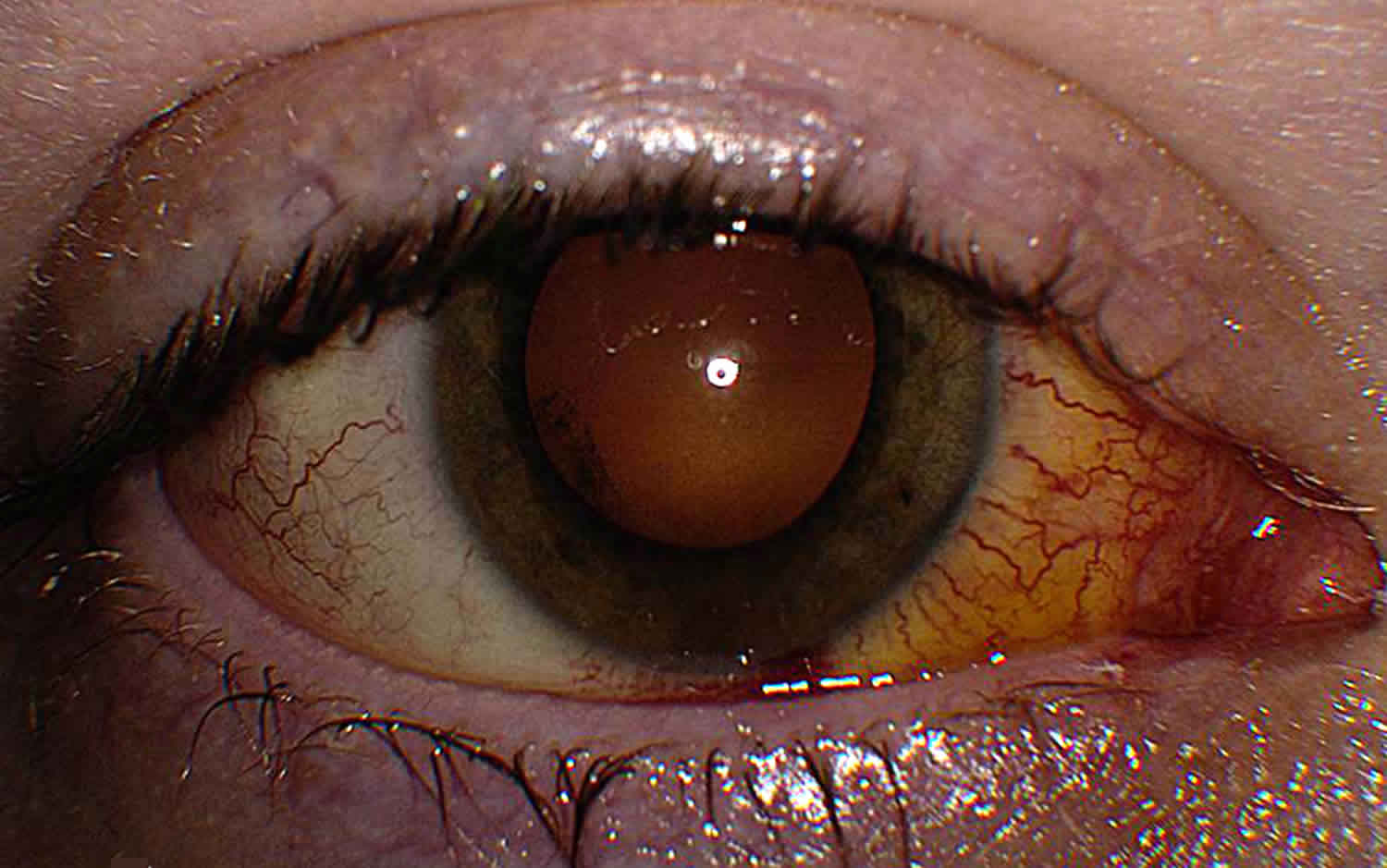
- “Pink” eye associated with pain
- Sensitivity to light sources (photophobia)
- Tears being continually produced
- Imagining to see “floaters” – cobweb-like structures that seem to float across the eyes which are induced because of material in the vitreous gel of the eye
An unusual symptom and one that can indicate the presence of other diseases is any difference seen in photographs taken with a flash and where the eyes do not give the same configuration of “camera red eye”.
Anyone suffering eye pain, severe light sensitivity, and any change in vision should immediately be examined by an ophthalmologist.
Chorioretinitis complications
A delay in the initiation of specific therapies for treatable causes of the patient with chorioretinitis results in further loss of vision especially in immunocompromised infants and children.
Chorioretinitis diagnosis
Diagnosis of chorioretinitis includes a thorough examination and the recording of the patient’s complete medical history. Laboratory tests may be done to rule out an infection or an autoimmune disorder.
The eye exams used include:
- An Eye Chart or Visual Acuity Test. This test measures whether a patient’s vision has decreased.
- A Funduscopic Exam. The pupil is widened (dilated) with eye drops and then a light is shown through with an instrument called an ophthalmoscope to noninvasively inspect the back, inside part of the eye.
- Ocular Pressure. An instrument, such a tonometer or a tonopen, measures the pressure inside the eye. Drops that numb the eye may be used for this test.
- A Slit Lamp Exam. A slit lamp noninvasively inspects much of the eye. It can inspect the front and back parts of the eye and some lamps may be equipped with a tonometer to measure eye pressure. A dye called fluorescein, which makes blood vessels easier to see, may be added to the eye during the examination. The dye only temporarily stains the eye.
Laboratory Studies
- Herpes simplex virus (HSV) types I and II, Epstein-Barr virus (EBV), varicella, and human immunodeficiency virus (HIV) are elicited by cultures or polymerase chain reaction (PCR) of blood, cerebrospinal fluid (CSF), or tissues. Immunoglobulin titers (immunoglobulin [Ig]G) from CNS fluid, serum, and other body fluids (particularly in a newborn) are of uncertain value, except for screening purpose in HIV vertical infection, toxoplasmosis, and West Nile virus infection. Positive values in a newborn may represent passive transfer of maternal antibody.
- Congenital rubella can infect infants of nonimmune individuals and is diagnosed by interpreting results of viral culture and IgM and IgG titers in both infants and mothers.
- Cytomegalovirus (CMV) is shed in the urine of infants who are congenitally infected, and diagnosis is indicated by positive urine culture results in infants younger than 3 weeks. In immunodeficient hosts, a CMV antigenemia test using a peripheral blood sample is helpful in establishing recurrence or infection. The CMV antigenemia uses immunofluorescent assay on buffy coat polymorphonuclear cells to detect CMV-infected cells. CMV-PCR is also being increasingly used, although at the present time its interpretation in congenital infection remains unclear.
- Toxoplasma species are detected by specific IgA, IgM, IgG, Sabin-Feldman dye test, and polymerase chain reaction (PCR). Document Toxoplasma status of pregnant women and individuals who are immunocompromised.
- Lyme disease is detected by using polymerase chain reaction (PCR) assay of CSF and serum. The presence of specific IgM and IgG on Western blot result of serum may be significant for diagnosis and staging of infection.
- Yersinia species are detected by special stool culture and acute and convalescent IgG titers.
- Syphilis is detected by using serology tests (eg, nontreponemal rapid plasma reagent, Venereal Disease Research Laboratory test in CSF) and specific treponemal tests (eg, fluorescein treponemal antibody absorption, microhemagglutination-Treponema pallidum).
- Mantoux skin test, acid-fast stain, and cultures of bronchial washings or biopsy samples of tissues detect Mycobacterium tuberculosis. Mycobacterium tuberculosis-PCR is available in many reference laboratories to detect the presence of Mycobacterium tuberculosis from biopsy and tissue samples, blood, and CSF. The QuantiFERON tuberculosis (TB) test, which measures lymphocyte interferon response to tuberculosis antigen, may aid in the diagnosis of tuberculosis.
- Toxocariasis serology can be tested by using an enzyme-linked immunoabsorbent assay (ELISA) and confirmed by using a specific Western blot (available from the Centers for Disease Control and Prevention [CDC]).
- Several serology tests for catscratch disease have been developed, including indirect immunofluorescent antibody (IFA) testing, which shows good correlation with strict clinical criteria. Demonstration of rising IgG titer provides the best evidence of infection.
- Baylisascaris tests can be performed using serum and CSF. ELISA and Western blot are available from the Department of Veterinary Pathobiology at Purdue University.
Other studies
- Nitroblue tetrazolium test (or flow cytometry with dihydrorhodamine) is used to detect chronic granulomatous disease. In a healthy host, 95% or more of neutrophils produce superoxide radicals. In a host with a typical X-linked chronic granulomatous disease, fewer than 5% of neutrophils have such ability.
- Vertical HIV infection is suggested by hypergammaglobulinemia and a depressed CD4+ T-cell count. An HIV DNA PCR of the newborn can be used to confirm diagnosis of HIV infection in the presence of maternal antibodies. Testing with DNA PCR should be done when the infant is aged 2 weeks, 1 month, and 4 months in all infants at risk for vertical HIV infection.
- Some experts also recommend obtaining an HIV DNA polymerase chain reaction test at birth. For children aged 18 months and older, an HIV antibody test should be used.
- Perform a skin biopsy for rare cases of dermal hematopoiesis (blueberry muffin infant syndrome) in patients in whom congenital CMV or congenital toxoplasmosis is suspected.
Imaging Studies
- A pediatric ocular imaging system (RetCam3) may be used in newborns, infants, and uncooperative children.
- Perform sonography, CT scanning, and/or MRI of the CNS or specific organs in patients in whom congenital infections are suspected or in an immunocompromised host.
- Perform chest radiography, CT scanning, or both in patients in whom TB or sarcoidosis is suspected. CT scanning is helpful in identifying sites on which to perform biopsy for diagnostic purposes.
- Perform CT scanning of the abdomen for hepatic or splenic dissemination of disease.
- Perform radiography or bone scanning of the skeleton in patients in whom syphilis is suspected.
- Perform echocardiography in patients with congenital rubella infection.
Chorioretinitis treatment
Care for individuals with chorioretinitis is complex and requires thorough consideration of short-term and long-term care and goals to maintain quality of life 16.
Available treatment options for specific causes of chorioretinitis are as follows:
- Antivirals: Four drugs have been licensed for the systemic treatment of cytomegalovirus (CMV) infection. These include ganciclovir, valganciclovir (oral prodrug of ganciclovir), foscarnet, and cidofovir. Fomivirsen is licensed for intravitreal administration to treat CMV retinitis in patients with acquired immunodeficiency syndrome (AIDS). Newer drugs such as maribavir, which has the potential to be useful in ganciclovir-resistant strains of CMV, are under clinical investigation 2.
- For children with human immunodeficiency virus (HIV) infection, the drug of choice for initial treatment for CMV retinitis is intravenous ganciclovir. Oral valganciclovir is an option primarily for older children who are able to receive the adult dose and tablet formulation of valganciclovir. An alternative to treat CMV disease or for use in ganciclovir-resistant CMV infections in children with HIV is foscarnet. Combination therapy with ganciclovir and foscarnet delays progression of retinitis in certain patients failing monotherapy and can be used as initial therapy among children with sight-threatening disease. Intravenous ganciclovir and foscarnet may also be considered in initial therapy of CMV CNS disease. However, combination therapy is associated with substantial rates of adverse effects.
- Several agents are used to treat toxoplasmosis. Treatment with antiparasitic drugs is effective for active infections but not for the encysted form. The classic treatment includes triple drug therapy with pyrimethamine (0.5-1 mg/kg/day), sulfadiazine (120-150 mg/kg/day), and prednisone. Concurrent folinic acid helps to minimize bone marrow toxicity produced by the pyrimethamine. High-performance liquid chromatography with ultraviolet and mass spectrometric detection has been developed for monitoring the plasma levels of pyrimethamine and sulfadiazine during treatment using a small amount of plasma (25 mcL). This may be helpful in determining the relationship between plasma concentrations and treatment efficacy 17. Alternative antibiotic treatments include atovaquone (40 mg/kg/d has been used in adults, no dosage for children), azithromycin (5 mg/kg/day) and trimethoprim-sulfamethoxazole (40 mg/kg/day sulfamethoxazole, 8 mg/kg/day trimethoprim). Adjunctive clindamycin (20 mg/kg/day) is used for coverage against the encysted form. Treatment duration for congenital infection is typically 1 year. Prevention of fetal infection after maternal Toxoplasma seroconversion during pregnancy is attempted with spiramycin administration. A 60% decrease has been reported in the congenital infection rate in patients who received this treatment; however, it does not ameliorate the fate of infants who are infected.
- Catscratch disease is usually a self-limited disease in immunocompetent patients. Bartonella henselae is sensitive to many antibiotics in vitro, but only aminoglycosides have bactericidal activity. In immunocompetent patients, doxycycline at 200 mg/day is usually administered because of its property to cross the blood-brain and blood-ocular barrier. Caution should be used if administered to children because it may cause dental changes. Ciprofloxacin (1.5 g/day), gentamicin (3-5 mg/kg/day), erythromycin (20-50 mg divided into 3 doses; adults, 2 g/day), trimethoprim-sulfamethoxazole (40 mg/kg/day sulfamethoxazole, 8 mg/kg/day trimethoprim) are good alternatives and, like doxycycline, are usually given for 14-28 days. Immunodeficient patients need a more prolonged course of treatment, usually as long as 4 months. Steroids are also indicated for ocular disease 18.
- Treatment of chorioretinitis due to fungal infections can be difficult and prolonged. Intravitreous amphotericin B (5-10 mcg) has been used to treat serious fungal chorioretinitis.
- Candida species infection: Fluconazole (6-12 mg/kg/d) and amphotericin B (0.75-1 mg/kg/d) has been recommended as preferred antifungals for treatment of Candida endophthalmitis. New-generation triazoles (eg, voriconazole, posaconazole, ravuconazole) are changing the conventional approach to fluconazole-resistant Candida strains, as well as the approach to fungal endophthalmitis. Caspofungin is the first echinocandin approved to treat fungal endophthalmitis. Concomitant systemic caspofungin and voriconazole therapy has successfully treated endophthalmitis due to Candida albicans 19. Experimental intraocular voriconazole (≤ 25 mcg/mL) has been used for azole-resistant Candida infection with some success.
- Ocular histoplasmosis: Treatment is limited to photocoagulation of neovascular membranes, particularly when the macula is threatened. Antifungal therapy has no role in treatment of this disease because no actively replicating organisms are present. Amphotericin B is used to treat systemic disease (0.75-1 mg/kg/day) 20.
- Cryptococcus species infection: Amphotericin B (0.75-1 mg/kg/day) is used.
- Antituberculosis drugs are administered in patients with Mycobacterium tuberculosis and include isoniazid (10-30 mg/kg/day), rifampicin (10-20 mg/kg/day), pyrazinamide (30 mg/kg/day), and ethambutol (15 mg/kg/day). Other drugs, such as aminoglycosides and quinolones, may be used for treatment of drug-resistant organisms. Duration of therapy depends on the extent of the disease and on the immune status of the host.
- Anthelmintics, including diethylcarbamazine (6 mg/kg/day), albendazole (400 mg oral twice daily), and mebendazole (100-200 mg oral twice daily), are usually administered with corticosteroids in patients with toxocariasis or baylisascariasis.
Etiologic treatments may not alter the clinical course of chorioretinitis because the pathologic changes may be due to an inflammatory and/or immunologic response instead of infection.
Treatment of other infectious causes, such as syphilis, yersiniosis, neuroborreliosis, depends on extent of disease but is likely to be successful in most patients.
Eye symptoms can be treated as follows:
- Steroids may have a role in the acute management of many vasculitides, collagen vascular diseases, or sarcoidosis; in some infectious processes (eg, Mycobacterium tuberculosis); or in some cases infections caused by Toxoplasma species.
- Laser treatment of retinal lesions is used in certain conditions with good results.
Surgical care
Vitrectomy is usually not needed and is reserved for severe cases that are resistant to conservative medical treatment.
References- Zinkernagel MS, Bolinger B, Krebs P, Onder L, Miller S, Ludewig B. Immunopathological basis of lymphocytic choriomeningitis virus-induced chorioretinitis and keratitis. J Virol. 2009 Jan. 83(1):159-66.
- Nassetta L, Kimberlin D, Whitley R. Treatment of congenital cytomegalovirus infection: implications for future therapeutic strategies. J Antimicrob Chemother. 2009 May. 63(5):862-7.
- Chorioretinitis. https://emedicine.medscape.com/article/962761-overview
- Stagno S, Britt W, et al. Cytomegalovirus Infections. Remington J, Klein J, Wilson C, eds. Infectious Diseases of the Fetus and Newborn Infant. 6th ed. Philadelphia, PA: Elsevier; 2006. 739-81.
- Hall BR, Oliver GE, Wilkinson M. A presentation of longstanding toxoplasmosis chorioretinitis. Optometry. 2009 Jan. 80(1):23-8.
- Wise ME, Sorvillo FJ, Shafir SC, Ash LR, Berlin OG. Severe and fatal central nervous system disease in humans caused by Baylisascaris procyonis, the common roundworm of raccoons: a review of current literature. Microbes Infect. 2005 Feb. 7(2):317-23.
- Egli A, Bergamin O, Mullhaupt B, et al. Cytomegalovirus-associated chorioretinitis after liver transplantation: case report and review of the literature. Transpl Infect Dis. 2008 Feb. 10(1):27-43.
- Khairallah M, Chee SP, Rathinam SR, Attia S, Nadella V. Novel infectious agents causing uveitis. Int Ophthalmol. 2010 Oct. 30(5):465-83.
- Woods CR. Congenital syphilis-persisting pestilence. Pediatr Infect Dis J. 2009 Jun. 28(6):536-7.
- Reed JB, Scales DK, Wong MT, Lattuada CP Jr, Dolan MJ, Schwab IR. Bartonella henselae neuroretinitis in cat scratch disease. Diagnosis, management, and sequelae. Ophthalmology. 1998 Mar. 105(3):459-66.
- Babu RB, Sudharshan S, Kumarasamy N, Therese L, Biswas J. Ocular tuberculosis in acquired immunodeficiency syndrome. Am J Ophthalmol. 2006 Sep. 142(3):413-8.
- Andreola C, Ribeiro MP, de Carli CR, Gouvea AL, Curi AL. Multifocal choroiditis in disseminated Cryptococcus neoformans infection. Am J Ophthalmol. 2006 Aug. 142(2):346-8.
- Ament CS, Young LH. Ocular manifestations of helminthic infections: onchocersiasis, cysticercosis, toxocariasis, and diffuse unilateral subacute neuroretinitis. Int Ophthalmol Clin. 2006 Spring. 46(2):1-10.
- Chalumeau M, Monnet D, Brezin AP, et al. Chorioretinal lesions as the unique feature of complete chronic granulomatous disease in an 8-year-old girl. Eur J Pediatr. 2007 Oct. 166(10):1069-70.
- Rigante D, Stabile A, Minnella A, et al. Post-inflammatory retinal dystrophy in CINCA syndrome. Rheumatol Int. 2009 May 8.
- Chorioretinitis Treatment & Management. https://emedicine.medscape.com/article/962761-treatment
- Johannessen JK, Christiansen I, Schmidt DR, Petersen E, Hansen SH. Simultaneous determination of pyrimethamine, sulfadiazine and N-acetyl-sulfadiazine in plasma for monitoring infants in treatment of congenital toxoplasmosis. J Pharm Biomed Anal. 2005 Jan 4. 36(5):1093-8.
- Accorinti M. Ocular bartonellosis. Int J Med Sci. 2009. 6(3):131-2.
- Shah CP, McKey J, Spirn MJ, Maguire J. Ocular candidiasis: a review. Br J Ophthalmol. 2008 Apr. 92(4):466-8.
- Prasad AG, Van Gelder RN. Presumed ocular histoplasmosis syndrome. Curr Opin Ophthalmol. 2005 Dec. 16(6):364-8.