What is colon cancer
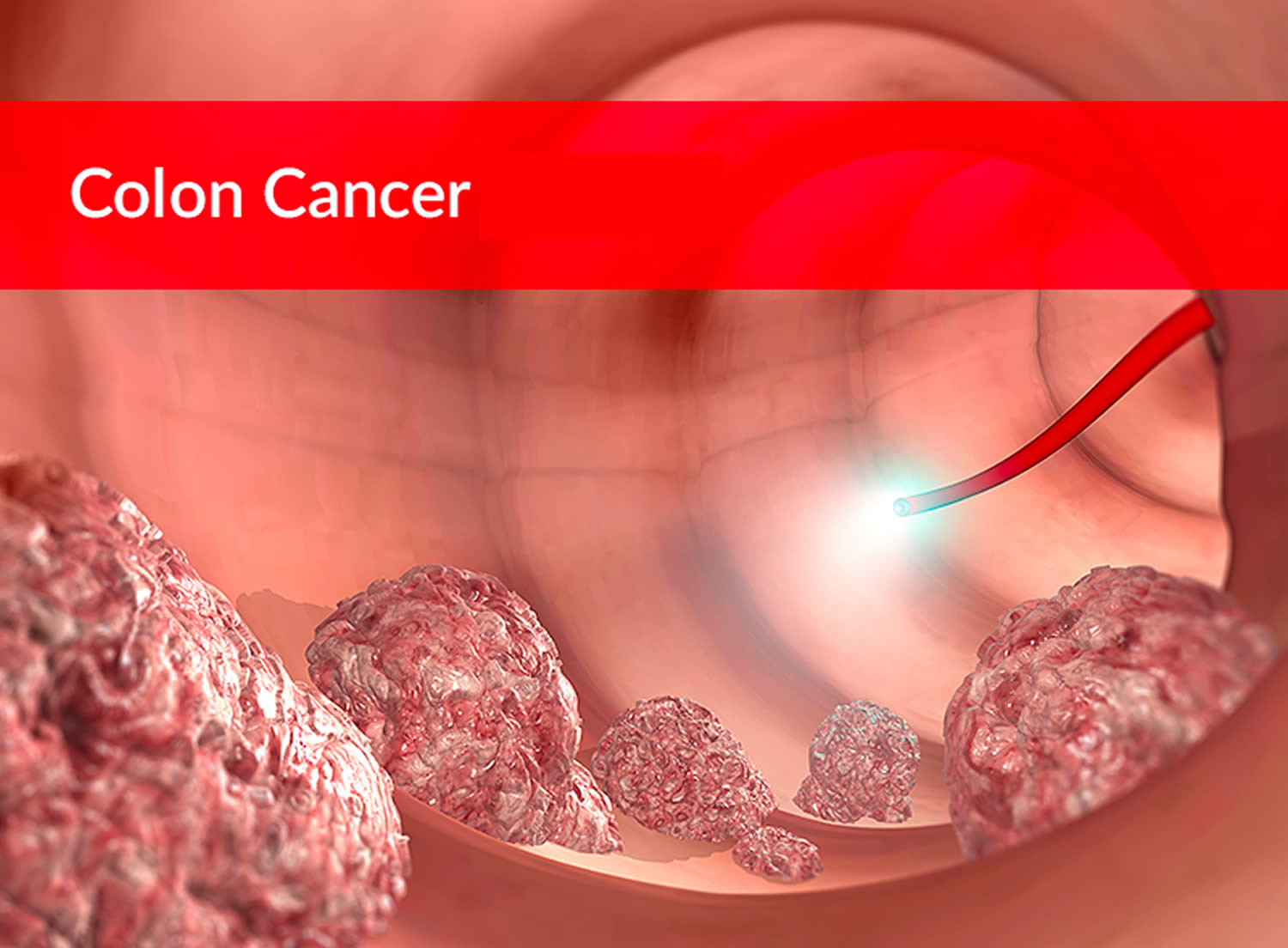
Colon cancer also known as bowel cancer or colorectal cancer (a term that combines colon cancer and rectal cancer which begins in the rectum), is cancer of the large intestine (colon or bowel), which is the final part of your digestive tract. Cancer that begins in the colon is called colon cancer, and cancer that begins in the rectum is called rectal cancer. Cancer that starts in either of these organs may also be called colorectal cancer. Depending on where the cancer starts, colon cancer is sometimes called colon or rectal cancer or colorectal cancer. Colon cancer and rectal cancer are often grouped together because they have many features in common. The large intestine (colon) extends from the distal end of the ileum to the anus, a distance of approximately 1.5 m in adults (5 ft) long and 6.5 cm (2.5 in.) in diameter. Together, the rectum and anal canal make up the last part of the large intestine and are about 6-8 inches long. The anal canal ends at the anus (the opening of the large intestine to the outside of the body). Most cases of colon cancer begin as small, noncancerous (benign) clumps of cells called adenomatous polyps. Over time some of these polyps can become colon cancers. Polyps may be small and produce few, if any, symptoms. For this reason, doctors recommend regular screening tests to help prevent colon cancer by identifying and removing polyps before they turn into cancer.
The chance of changing into a cancer depends on the kind of polyp. The 2 main types of polyps are:
- Adenomatous polyps (adenomas): These polyps sometimes change into cancer. Because of this, adenomas are called a pre-cancerous condition.
- Hyperplastic polyps and inflammatory polyps: These polyps are more common, but in general they are not pre-cancerous.
Other polyp characteristics that can increase the chances a polyp may contain cancer or increase someone’s risk of developing colorectal cancer besides the type include the size (larger than 1 cm), the number found (more than two), and if dysplasia is seen in the polyp after it is removed.
Dysplasia, another pre-cancerous condition, is an area in a polyp or in the lining of the colon or rectum where the cells look abnormal (but not like true cancer cells).
If cancer forms in a polyp, it can eventually begin to grow into the wall of the colon or rectum.
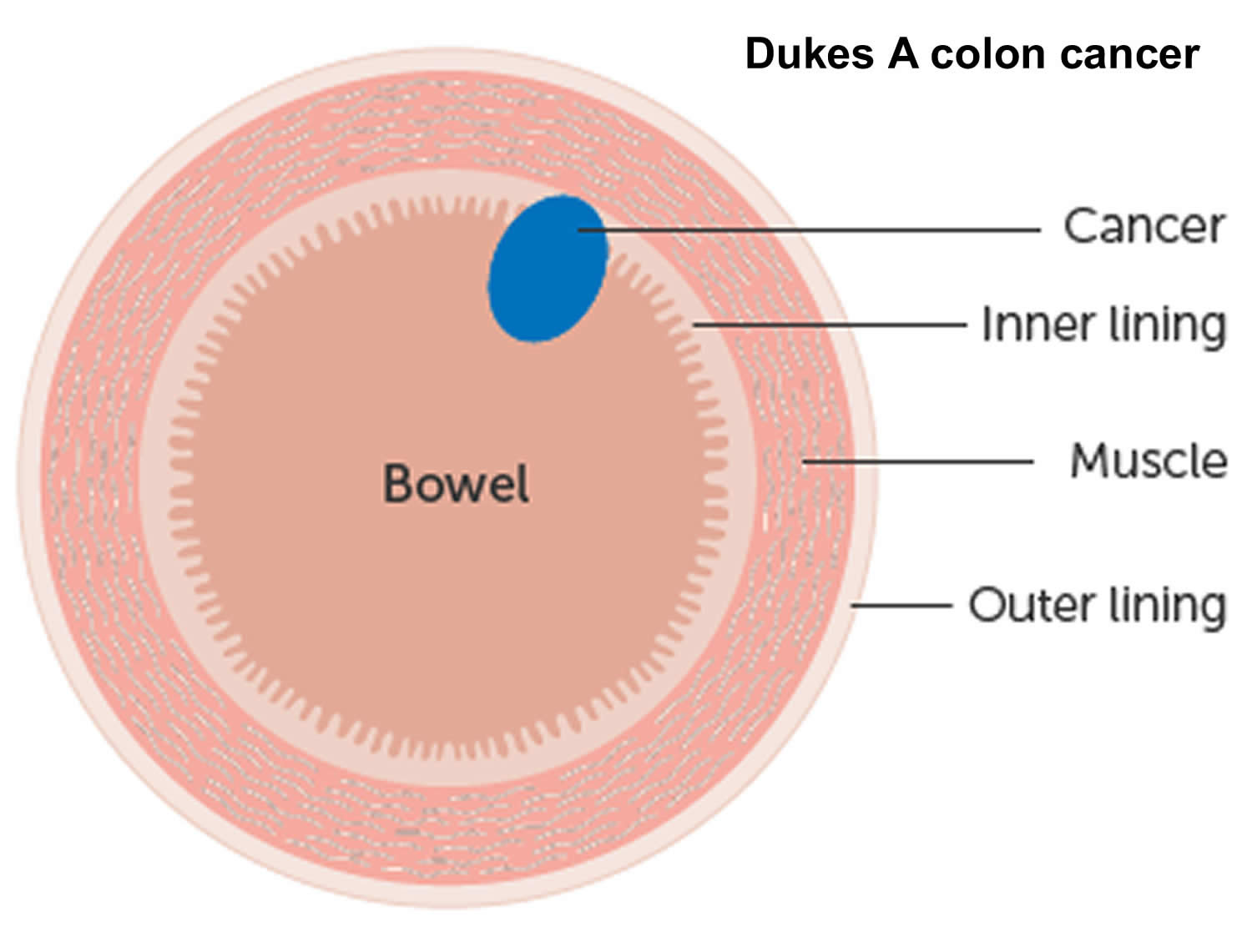
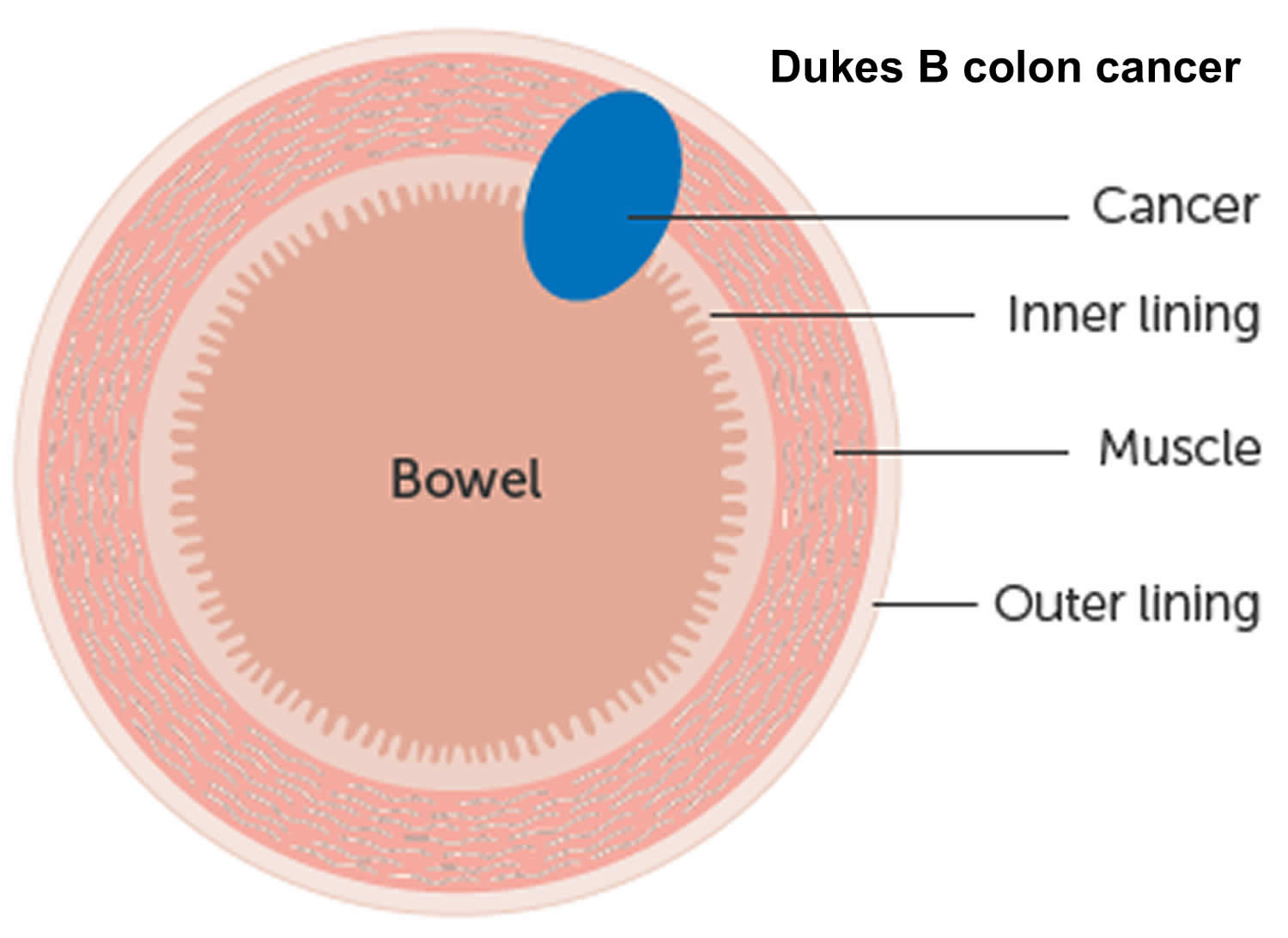
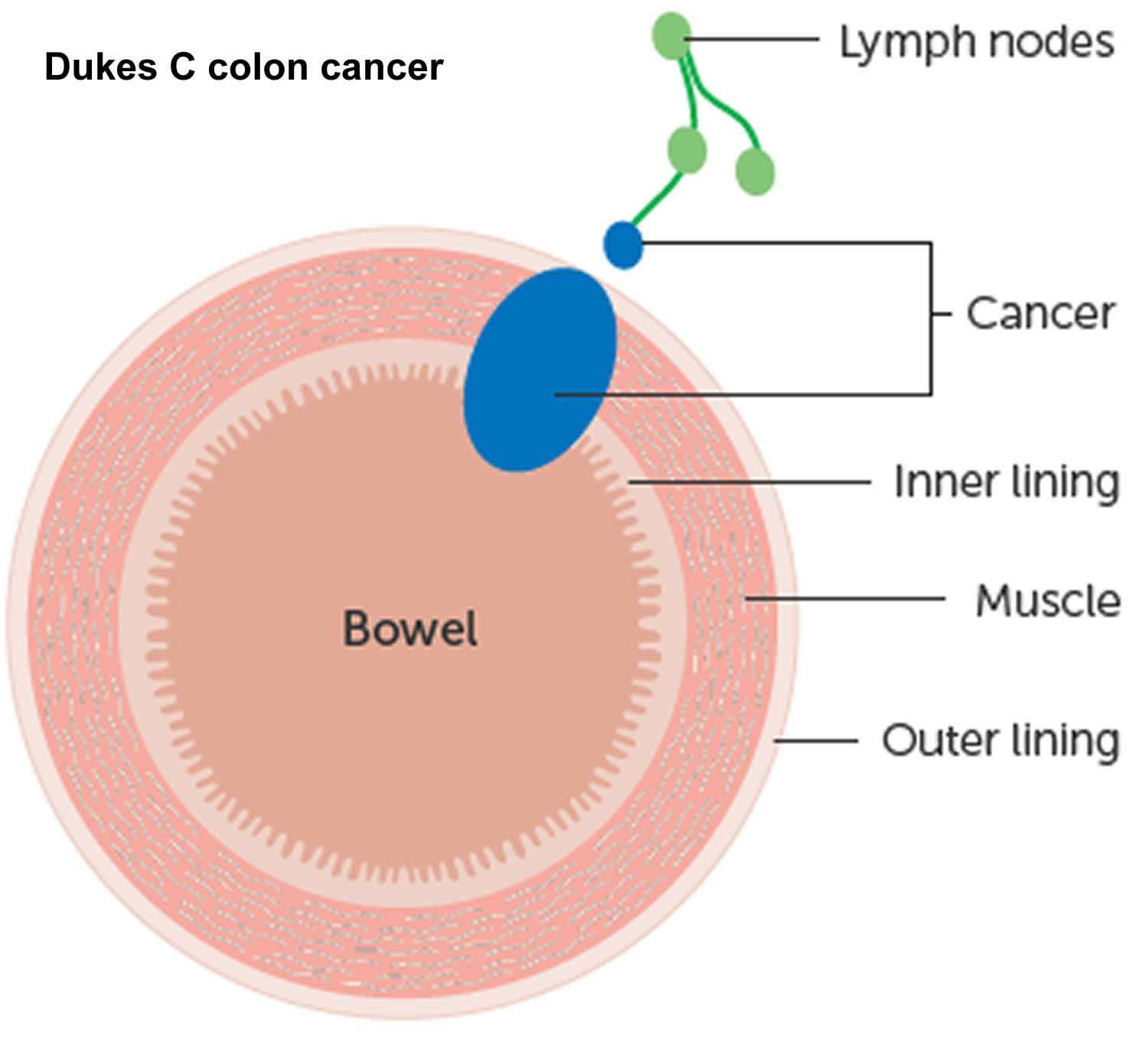
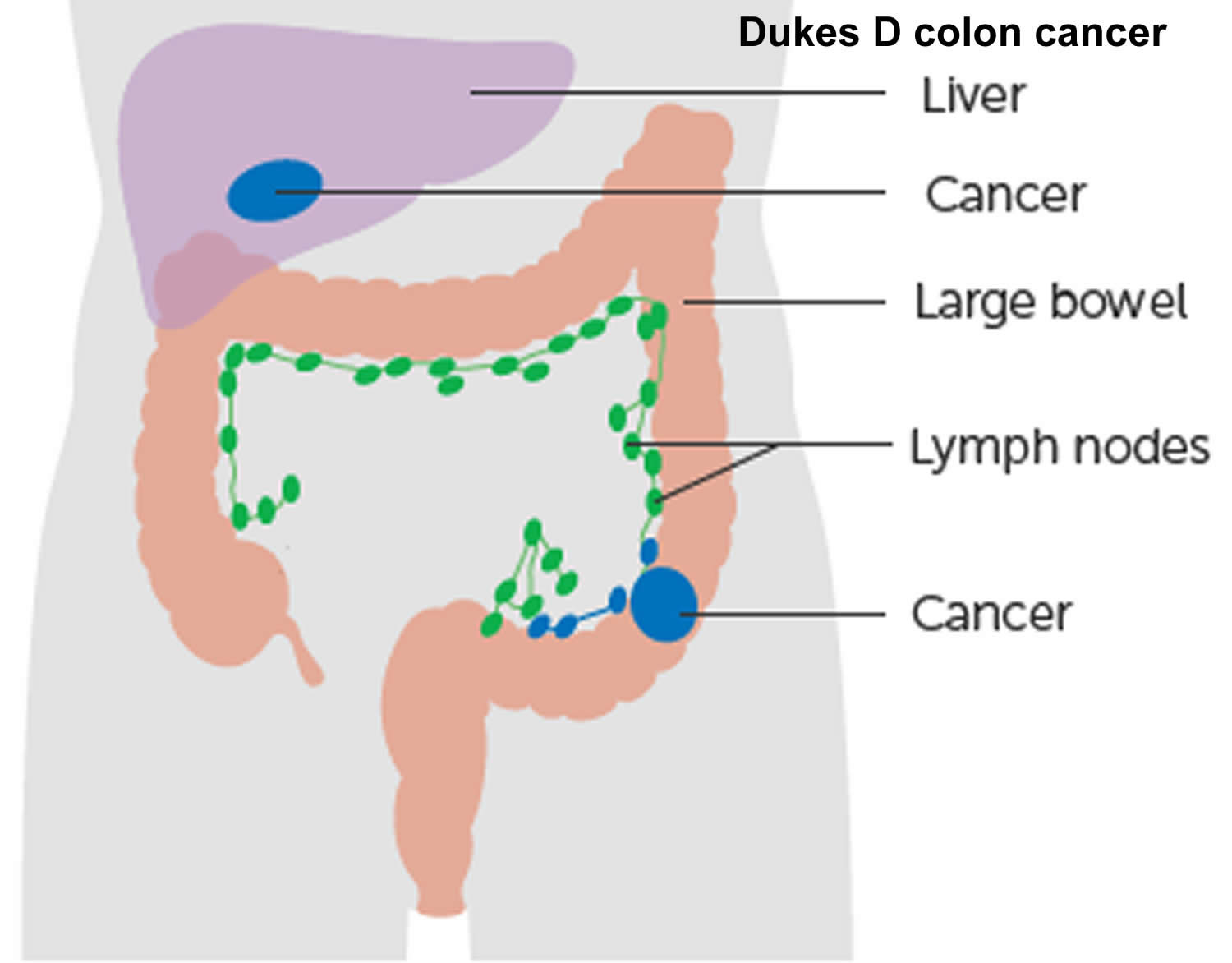
The wall of the colon and rectum is made up of several layers. Colorectal cancer starts in the innermost layer (the mucosa) and can grow outward through some or all of the other layers. When cancer cells are in the wall, they can then grow into blood vessels or lymph vessels (tiny channels that carry away waste and fluid). From there, they can travel to nearby lymph nodes or to distant parts of the body.
The stage (extent of spread) of a colorectal cancer depends on how deeply it grows into the wall and if it has spread outside the colon or rectum.
It can take as many as 10 to 15 years for a polyp to develop into colorectal cancer. Regular screening can often prevent colorectal cancer by finding and removing polyps before they have the chance to turn into cancer. Screening can also often find colorectal cancer early, when it might be easier to treat.
Excluding skin cancers, colorectal cancer is the third most common cancer diagnosed in both men and women in the United States. The American Cancer Society’s estimates for the number of colorectal cancer cases in the United States for 2022 are 1, 2:
- New cases 106,180 (colon cancer only)
- New cases of rectal cancer: 44,850
- Deaths: 52,580 (colon and rectal cancers combined)
- About 4.2% of Americans are expected to develop colorectal cancer within their lifetime, and the lifetime risk of dying from colorectal cancer is 1.7% 3. Age-specific incidence and mortality rates show that most colorectal cancer cases are diagnosed after age 54 years and 78% of cases occur in patients aged 55 years and older; about 15% of colorectal cancer cases occur in patients aged 45 to 54 years 2, 4.
- Colorectal cancer is the second leading cause of cancer death in the United States. The death rate was 13.4 per 100,000 men and women per year based on 2015–2019 deaths, age-adjusted.
- Colorectal cancer represents 7.9% of all new cancer cases in the U.S.
- Colorectal cancer deaths represents 8.6% of all cancer deaths in the U.S.
- Rate of New Cases and Deaths per 100,000: The rate of new cases of colorectal cancer was 37.7 per 100,000 men and women per year. The death rate was 13.4 per 100,000 men and women per year. These rates are age-adjusted and based on 2015–2019 cases and deaths.
- Lifetime Risk of Developing colorectal cancer: Approximately 4.1 percent of men and women will be diagnosed with colorectal cancer at some point during their lifetime, based on 2017–2019 data.
- In 2019, there were an estimated 1,369,004 people living with colorectal cancer in the United States.
- 5-Year Relative Survival is 65.1%. Relative survival is an estimate of the percentage of patients who would be expected to survive the effects of their cancer. It excludes the risk of dying from other causes. Because survival statistics are based on large groups of people, they cannot be used to predict exactly what will happen to an individual patient. No two patients are entirely alike, and treatment and responses to treatment can vary greatly.
Overall, the lifetime risk of developing colorectal cancer is about 1 in 21 (4.7%) for men and 1 in 23 (4.4%) for women. This risk is slightly lower in women than in men. A number of other factors (described in Colorectal Cancer Risk Factors) can also affect your risk for developing colorectal cancer.
Colorectal cancer is the second leading cause of cancer death when numbers for both men and women are combined. The death rate (the number of deaths per 100,000 people per year) of colorectal cancer has been dropping for several decades. It is expected to cause about 50,260 deaths during 2017. One reason for this is that colorectal polyps are now more often found by screening and removed before they can develop into cancers.
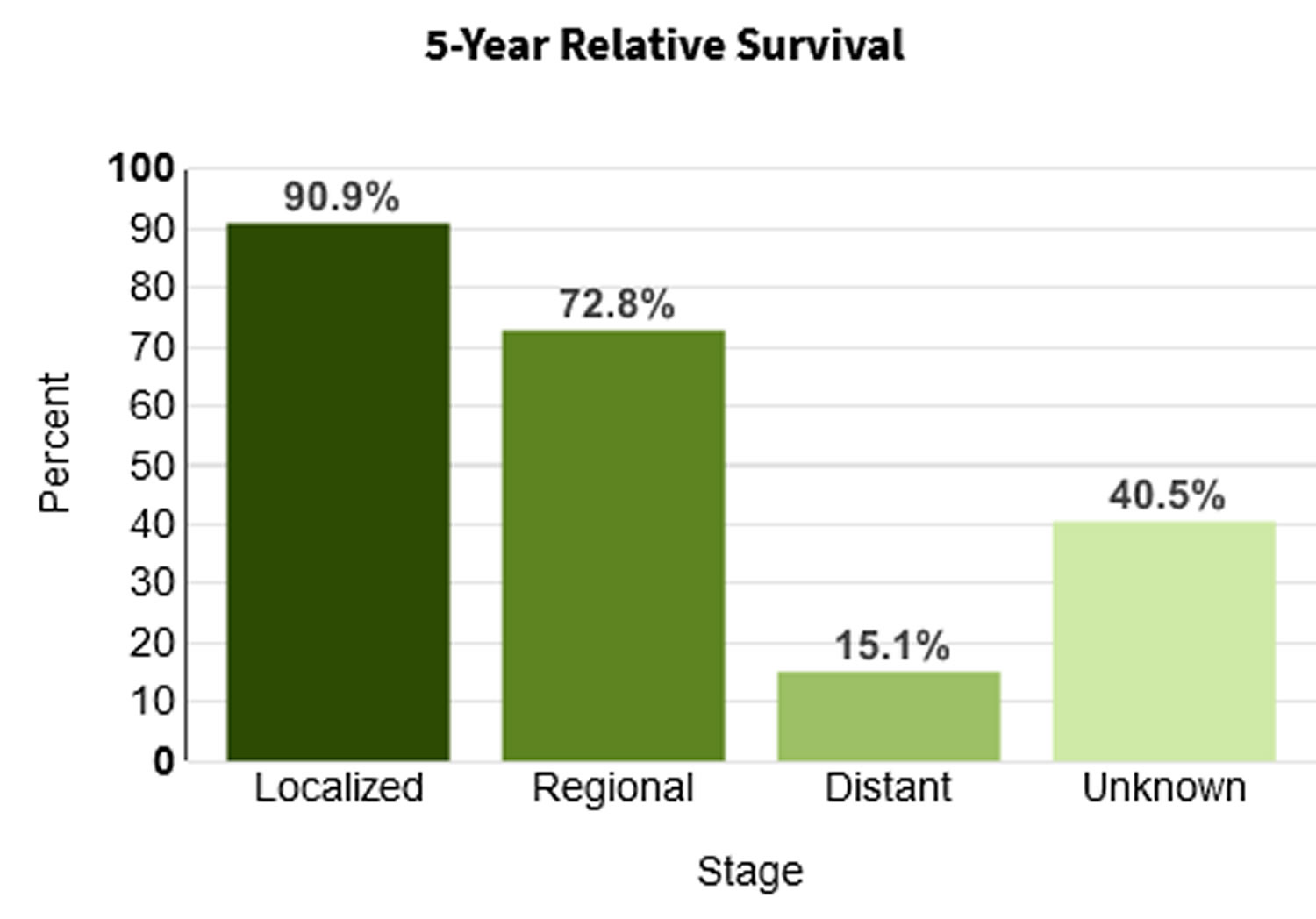
When colorectal cancer is found at an early stage before it has spread, the 5-year relative survival rate is about 90%. But only about 4 out of 10 colorectal cancers are found at this early stage. When cancer has spread outside the colon or rectum, survival rates are lower.
Unfortunately, only a little more than half of people who should get tested for colorectal cancer get the tests that they should. This may be due to things like lack of public and health care provider awareness of screening options, costs, and health insurance coverage issues.
Cancer of the colon is a highly treatable and often curable disease when localized to the bowel. Surgery is the primary form of treatment and results in cure in approximately 50% of the patients. Recurrence following surgery is a major problem and is often the ultimate cause of death.
The colon and rectum anatomy
To understand colorectal cancer, it helps to know about the normal structure and function of the colon and rectum.
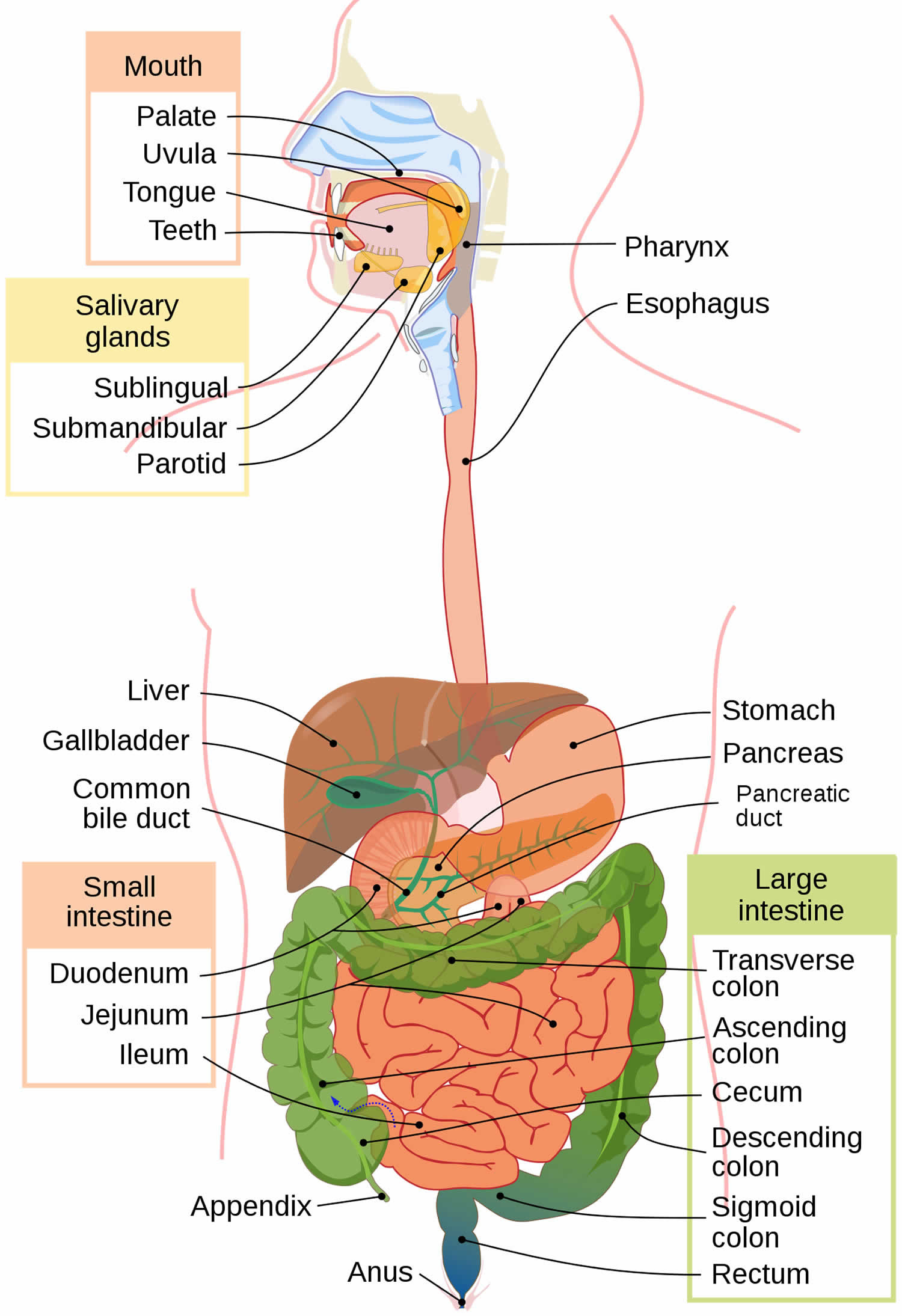
The colon and rectum make up the large intestine (or large bowel), which is part of the digestive system, also called the gastrointestinal (GI) system (see Figure 1 below). The digestive tract includes the mouth, esophagus, stomach, small intestine, large intestine, and rectum. The large intestine is approximately 5 feet (1.5 meters) long, making up one-fifth of the length of the gastrointestinal (GI) tract 5. The large intestine is responsible for processing indigestible food material (chyme) after most nutrients are absorbed in the small intestine. The large intestine performs an essential role by absorbing water, vitamins, and electrolytes from waste material 6, 7.
The large intestine is composed of 4 parts. It includes the cecum and ascending colon, transverse colon, descending colon, and sigmoid colon. The parts of the colon are named by which way the food is traveling through them.
- The first section is called the ascending colon. It starts with a pouch called the cecum, where undigested food is comes in from the small intestine. It continues upward on the right side of the abdomen (belly).
- The second section is called the transverse colon. It goes across the body from the right to the left side.
- The third section is called the descending colon because it descends (travels down) on the left side.
- The fourth section is called the sigmoid colon because of its “S” shape. The sigmoid colon joins the rectum, which then connects to the anus.
The ascending and transverse colon together are called the proximal colon. The descending and sigmoid colon are called the distal colon.
Figure 1. Gastrointestinal tract (human digestive system)
Figure 2. Large intestine (colon)
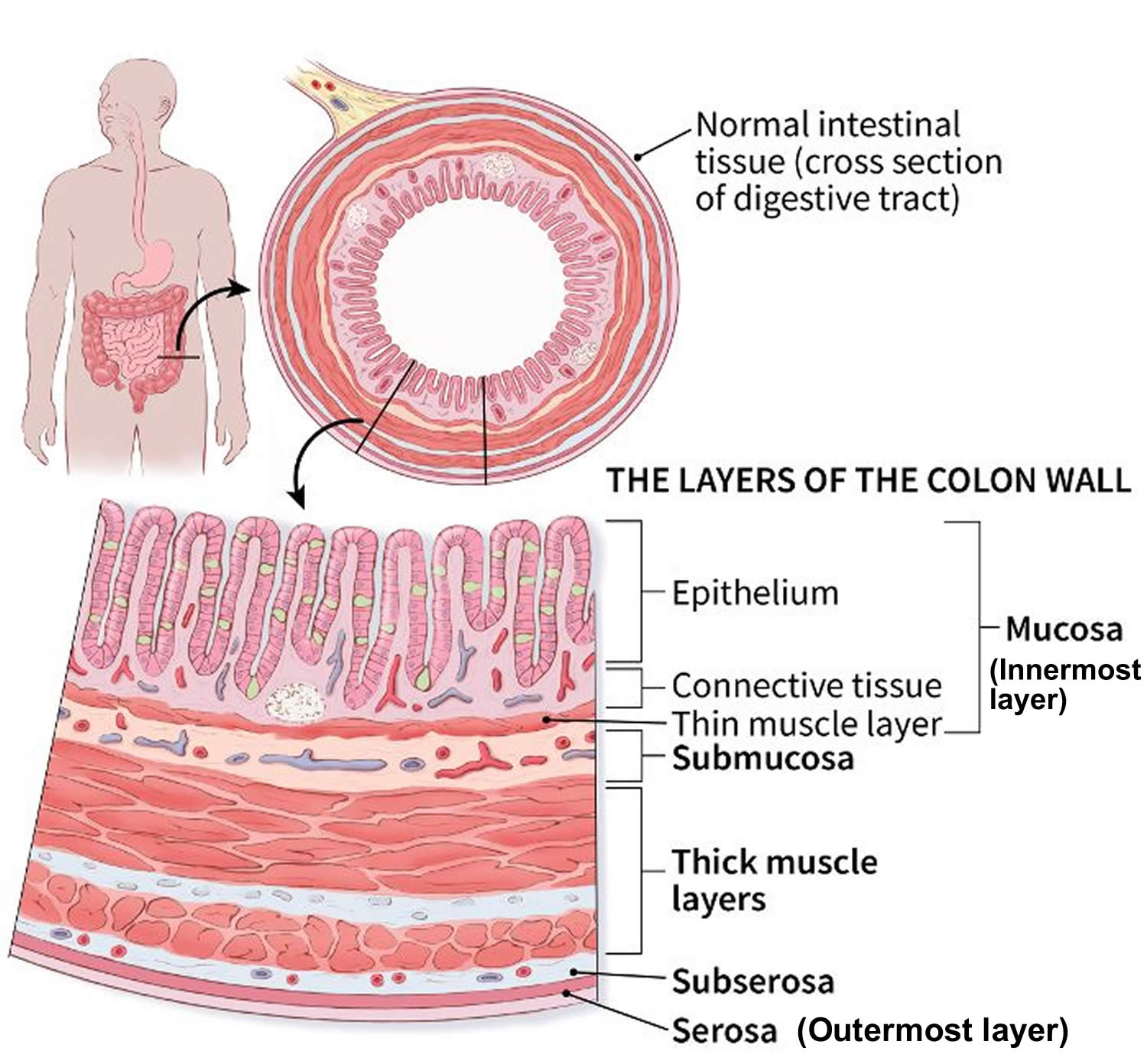
The intestinal wall
The intestinal wall is made up of multiple layers. The 4 layers of the large intestine from the lumen outward are the mucosa, submucosa, muscular layer, and serosa (Figure 2). The muscular layer is made up of 2 layers of smooth muscle, the inner, circular layer, and the outer, longitudinal layer.
Figure 3. Large intestine wall layers
Figure 4. Large intestine anatomy (normal)
What is a polyp in the colon?
A polyp is a projection (growth) of tissue from the inner lining of the colon into the lumen (hollow center) of the colon. Different types of polyps look different under the microscope. Polyps are benign (non-cancerous) growths, but cancer can start in some types of polyps. These polyps can be thought of as pre-cancers, which is why it is important to have them removed.
Polyps that tend to grow as slightly flattened, broad-based polyps are referred to as sessile.
Serrated polyps (serrated adenomas) have a saw-tooth appearance under the microscope. There are 2 types, which look a little different under the microscope:
- Sessile serrated adenomas (also called sessile serrated polyps)
- Traditional serrated adenomas
Both types need to be removed from your colon.
What is an adenoma (adenomatous polyp)?
An adenoma is a polyp made up of tissue that looks much like the normal lining of your colon, although it is different in several important ways when it is looked at under the microscope. In some cases, a cancer can start in the adenoma.
What are tubular adenomas, tubulovillous adenomas, and villous adenomas?
Adenomas can have several different growth patterns that can be seen under the microscope by the pathologist. There are 2 major growth patterns: tubular and villous. Many adenomas have a mixture of both growth patterns, and are called tubulovillous adenomas. Most adenomas that are small (less than ½ inch) have a tubular growth pattern. Larger adenomas may have a villous growth pattern. Larger adenomas more often have cancers developing in them. Adenomas with a villous growth pattern are also more likely to have cancers develop in them.
The most important thing is that your polyp has been completely removed and does not show cancer. The growth pattern is only important because it helps determine when you will need your next colonoscopy to make sure you don’t develop colon cancer in the future.
What does it mean if I have an adenoma (adenomatous polyp), such as a sessile serrated adenoma or traditional serrated adenoma?
These types of polyps are not cancer, but they are pre-cancerous (meaning that they can turn into cancers). Someone who has had one of these types of polyps has an increased risk of later developing cancer of the colon. Most patients with these polyps, however, never develop colon cancer.
What if my report mentions dysplasia?
Dysplasia is a term that describes how much your polyp looks like cancer under the microscope:
- Polyps that are only mildly abnormal (don’t look much like cancer) are said to have low-grade (mild or moderate) dysplasia.
- Polyps that are more abnormal and look more like cancer are said to have high-grade (severe) dysplasia.
The most important thing is that your polyp has been completely removed and does not show cancer. If high-grade dysplasia is found in your polyp, it might mean you need to have a repeat (follow-up) colonoscopy sooner than if high-grade dysplasia wasn’t found, but otherwise you do not need to worry about dysplasia in your polyp.
How does having an adenoma affect my future follow-up care?
Since you had an adenoma, you will need to have another colonoscopy to make sure that you don’t develop any more adenomas. When your next colonoscopy should be scheduled depends on a number of things, like how many adenomas were found, if any were villous, and if any had high-grade dysplasia. The timing of your next colonoscopy should be discussed with your treating doctor, as he or she knows the details of your specific case.
What if my adenoma was not completely removed?
If your adenoma was biopsied but not completely removed, you will need to talk to your doctor about what other treatment you’ll need. Most of the time, adenomas are removed during a colonoscopy. Sometimes, though, the adenoma may be too large to remove during colonoscopy. In such cases you may need surgery to have the adenoma removed.
What if my report also mentions hyperplastic polyps?
Hyperplastic polyps are typically benign (they aren’t pre-cancers or cancers) and are not a cause for concern.
Colon cancer signs and symptoms
Many people with colon cancer experience no symptoms in the early stages of the disease. When symptoms appear, they’ll likely vary, depending on the cancer’s size and location in your large intestine. Many of the symptoms of colon cancer can also be caused by something that isn’t cancer, such as infection, hemorrhoids, irritable bowel syndrome, or inflammatory bowel disease.
Colorectal cancer might not cause symptoms right away, but if it does, it may cause one or more of these symptoms:
- A change in bowel habits, such as diarrhea, constipation, or narrowing of the stool, that lasts for more than a few days
- A feeling that you need to have a bowel movement that’s not relieved by having one
- Rectal bleeding with bright red blood
- Blood in the stool, which might make the stool look dark brown or black
- Persistent abdominal discomfort, such as cramps, gas or pain
- A feeling that your bowel doesn’t empty completely
- Weakness or fatigue
- Unexplained weight loss
Signs of colon cancer include blood in the stool or a change in bowel habits.
These and other signs and symptoms may be caused by colon cancer or by other conditions. Check with your doctor if you have any of the following:
- A change in bowel habits.
- Blood (either bright red or very dark) in the stool.
- Diarrhea, constipation, or feeling that the bowel does not empty all the way.
- Stools that are narrower than usual.
- Frequent gas pains, bloating, fullness, or cramps.
- Weight loss for no known reason.
- Feeling very tired.
- Vomiting.
Colorectal cancers can often bleed into the digestive tract. Sometimes the blood can be seen in the stool or make it look darker, but often the stool looks normal. But over time, the blood loss can build up and can lead to low red blood cell counts (anemia). Sometimes the first sign of colorectal cancer is a blood test showing a low red blood cell count.
Some people may have signs that the cancer has spread to the liver with a large liver felt on exam, jaundice (yellowing of the skin or whites of the eyes), or trouble breathing from cancer spread to the lungs.
Many of these symptoms can be caused by conditions other than colorectal cancer, such as infection, hemorrhoids, or irritable bowel syndrome. Still, if you have any of these problems, it’s important to see your doctor right away so the cause can be found and treated, if needed.
If you notice any persistent symptoms that worry you, make an appointment with your doctor.
Talk with your doctor about when to begin colon cancer screening. Guidelines generally recommend that colon cancer screenings begin around 50. Your doctor may recommend more frequent or earlier screening if you have other risk factors, such as a family history of the disease.
Colon cancer causes
In most cases, it’s not clear what causes colon cancer. Doctors know that colon cancer occurs when healthy cells in the colon develop errors in their genetic blueprint, the DNA. A cell’s DNA contains a set of instructions that tell a cell what to do.
Healthy cells grow and divide in an orderly way to keep your body functioning normally. But when a cell’s DNA is damaged and becomes cancerous, cells continue to divide — even when new cells aren’t needed. As the cells accumulate, they form a tumor.
With time, the cancer cells can grow to invade and destroy normal tissue nearby. And cancerous cells can travel to other parts of the body to form deposits there (metastasis).
Inherited gene mutations that increase the risk of colon cancer
Inherited gene mutations that increase the risk of colon cancer can be passed through families, but these inherited genes are linked to only a small percentage of colon cancers. Inherited gene mutations don’t make cancer inevitable, but they can increase an individual’s risk of cancer significantly.
The most common forms of inherited colon cancer syndromes are:
- Hereditary nonpolyposis colorectal cancer (HNPCC) also called Lynch syndrome. Hereditary nonpolyposis colorectal cancer increases the risk of colon cancer and other cancers. People with hereditary nonpolyposis colorectal cancer tend to develop colon cancer before age 50. Lynch syndrome (hereditary non-polyposis colon cancer or HNPCC) is caused by changes in genes that normally help a cell repair damaged DNA. A mutation in one of the DNA repair genes like MLH1, MSH2, MSH6, PMS2, and EPCAM, can allow DNA errors to go unfixed. These errors will sometimes affect growth-regulating genes, which may lead to the development of cancer.
- Familial adenomatous polyposis (FAP). About 1% of all colorectal cancers are caused by familial adenomatous polyposis (FAP). Familial adenomatous polyposis (FAP) is caused by inherited changes in the APC gene. The APC gene is a tumor suppressor gene; it normally helps keep cell growth in check. In people with inherited changes in the APC gene, this “brake” on cell growth is turned off, causing hundreds of polyps to form in the colon. Over time, cancer will nearly always develop in one or more of these polyps. Familial adenomatous polyposis (FAP) is a rare disorder that causes you to develop thousands of polyps in the lining of your colon and rectum. In the most common type of FAP, hundreds or thousands of polyps develop in a person’s colon and rectum, often starting at ages 10 to 12 years. Cancer usually develops in 1 or more of these polyps as early as age 20. By age 40, almost all people with FAP will have colon cancer if their colon hasn’t been removed to prevent it. People with FAP also have an increased risk for cancers of the stomach, small intestines, pancreas, liver, and some other organs. There are 3 sub-types of familial adenomatous polyposis (FAP):
- In attenuated FAP (AFAP), patients have fewer polyps (less than 100), and colorectal cancer tends to occur at a later age (40s and 50s).
- Gardner syndrome is a type of FAP that also causes non-cancer tumors of the skin, soft tissue, and bones.
- Turcot syndrome is a rare inherited condition in which people have a higher risk of many adenomatous polyps and colorectal cancer. People with Turcot syndrome who have the APC gene are also at risk of a specific type of brain cancer called medulloblastoma.
- Peutz-Jeghers syndrome is caused by inherited changes in the STK11 (LKB1) gene, a tumor suppressor gene. People with Peutz-Jeghers syndrome tend to have freckles around the mouth (and sometimes on their hands and feet) and a special type of polyp called hamartomas in their digestive tracts. These people are at a much higher risk for colorectal cancer, as well as other cancers, such as breast, ovary, and pancreas. They usually are diagnosed at a younger than usual age.
- MUTYH-associated polyposis (MAP) is caused by mutations in the MUTYH gene, which is involved in how the cell “proofreads” or checks the DNA and fixes errors when cells divide. People with MUTYH-associated polyposis (MAP) develop many colon polyps. These will almost always become cancer if not watched closely with regular colonoscopies. These people also have an increased risk of other cancers of the GI (gastrointestinal) tract and thyroid.
Familial adenomatous polyposis, hereditary nonpolyposis colorectal cancer and other, rarer inherited colon cancer syndromes can be detected through genetic testing. If you’re concerned about your family’s history of colon cancer, talk to your doctor about whether your family history suggests you have a risk of these conditions. You may want to ask your doctor about genetic counseling and genetic testing.
Association between diet and increased colon cancer risk
Studies of large groups of people have shown an association between a typical Western diet and an increased risk of colon cancer. A typical Western diet is high in fat and low in fiber.
A diet that’s high in red meats (such as beef, pork, lamb, or liver) and processed meats (like hot dogs and some luncheon meats) raises your colorectal cancer risk.
Cooking meats at very high temperatures (frying, broiling, or grilling) creates chemicals that might raise your cancer risk. It’s not clear how much this might increase your colorectal cancer risk.
Having a low blood level of vitamin D may also increase your risk.
When people move from areas where the typical diet is low in fat and high in fiber to areas where the typical Western diet is most common, the risk of colon cancer in these people increases significantly. It’s not clear why this occurs, but researchers are studying whether a high-fat, low-fiber diet affects the microbes that live in the colon or causes underlying inflammation that may contribute to cancer risk. This is an area of active investigation and research is ongoing.
Following a healthy eating pattern that includes plenty of fruits, vegetables, and whole grains, and that limits or avoids red and processed meats and sugary drinks probably lowers risk.
Risk factors for colon cancer
Anything that increases your chance of getting a disease is called a risk factor. Having a risk factor does not mean that you will get cancer; not having risk factors doesn’t mean that you will not get cancer. Talk to your doctor if you think you may be at risk for colorectal cancer.
Factors that may increase your risk of colon cancer include:
- Older age. The great majority of people diagnosed with colon cancer are older than 50. Colon cancer can occur in younger people, but it occurs much less frequently.
- African-American race. African-Americans have a greater risk of colon cancer than do people of other races in the US.
- Jews of Eastern European descent (Ashkenazi Jews) have one of the highest colorectal cancer risks of any ethnic group in the world.
- Having a personal history of cancer of the colon, rectum, or ovary.
- Having a personal history of high-risk adenomas (colorectal polyps that are 1 centimeter or larger in size or that have cells that look abnormal under a microscope).
- Having a personal history of inflammatory intestinal conditions for 8 years or more. Chronic inflammatory diseases of the colon, such as ulcerative colitis and Crohn’s disease, can increase your risk of colon cancer.
- Having inherited syndromes that increase colon cancer risk. Genetic syndromes passed through generations of your family can increase your risk of colon cancer. These syndromes include familial adenomatous polyposis (FAP) and hereditary nonpolyposis colorectal cancer, which is also known as Lynch syndrome.
- Family history of colon or rectal cancer. You’re more likely to develop colon cancer if you have a parent, sibling or child with the disease (first-degree relative). If more than one family member has colon cancer or rectal cancer, your risk is even greater.
- Low-fiber, high-fat diet. Colon cancer and rectal cancer may be associated with a diet low in fiber and high in fat and calories. Research in this area has had mixed results. Some studies have found an increased risk of colon cancer in people who eat diets high in red meat and processed meat.
- A sedentary lifestyle. If you’re inactive, you’re more likely to develop colon cancer. Getting regular physical activity may reduce your risk of colon cancer.
- Diabetes. People with diabetes and insulin resistance have an increased risk of colon cancer. Both type 2 diabetes and colorectal cancer share some of the same risk factors (such as being overweight and physical inactivity). But even after taking these factors into account, people with type 2 diabetes still have an increased risk. They also tend to have a less favorable prognosis (outlook) after diagnosis.
- Obesity. People who are obese have an increased risk of colon cancer and an increased risk of dying of colon cancer when compared with people considered normal weight.
- Smoking. People who smoke may have an increased risk of colon cancer.
- Alcohol. Having three or more alcoholic drinks per day increases your risk of colon cancer.
- Radiation therapy for cancer. Radiation therapy directed at the abdomen to treat previous cancers increases the risk of colon and rectal cancer.
Colon cancer prevention
Get screened for colon cancer
People with an average risk of colon cancer can consider screening beginning at age 50. The American Cancer Society recommends that people at average risk of colorectal cancer start regular screening at age 45 8. But people with an increased risk, such as those with a family history of colon cancer, should consider screening sooner.
People who are in good health and with a life expectancy of more than 10 years should continue regular colorectal cancer screening through the age of 75.
For people ages 76 through 85, the decision to be screened should be based on a person’s preferences, life expectancy, overall health, and prior screening history.
People over 85 should no longer get colorectal cancer screening.
*For screening, people are considered to be at average risk if they do NOT have:
- A personal history of colorectal cancer or certain types of polyps
- A family history of colorectal cancer
- A personal history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease)
- A confirmed or suspected hereditary colorectal cancer syndrome, such as familial adenomatous polyposis (FAP) or Lynch syndrome (hereditary non-polyposis colon cancer or HNPCC)
- A personal history of getting radiation to the abdomen (belly) or pelvic area to treat a prior cancer
Several screening options exist — each with its own benefits and drawbacks. Talk about your options with your doctor, and together you can decide which tests are appropriate for you. The most important thing is to get screened, no matter which test you choose.
Several screening tests can be divided into 2 main groups:
- Stool-based tests: These tests check the stool (feces) for signs of cancer. These tests are less invasive and easier to have done, but they need to be done more often.
- Visual (structural) exams: These tests look at the structure of the colon and rectum for any abnormal areas. This is done either with a scope (a tube-like instrument with a light and tiny video camera on the end) put into the rectum, or with special imaging (x-ray) tests.
These tests each have different risks and benefits (see below), and some of them might be better options for you than others.
Make lifestyle changes to reduce your risk
You can take steps to reduce your risk of colon cancer by making changes in your everyday life. Take steps to:
- Eat a variety of fruits, vegetables and whole grains. Fruits, vegetables and whole grains contain vitamins, minerals, fiber and antioxidants, which may play a role in cancer prevention. Choose a variety of fruits and vegetables so that you get an array of vitamins and nutrients.
- Drink alcohol in moderation, if at all. If you choose to drink alcohol, limit the amount of alcohol you drink to no more than one drink a day for women and two for men.
- Stop smoking. Talk to your doctor about ways to quit that may work for you.
- Exercise most days of the week. Try to get at least 30 minutes of exercise on most days. If you’ve been inactive, start slowly and build up gradually to 30 minutes. Also, talk to your doctor before starting any exercise program.
- Maintain a healthy weight. If you are at a healthy weight, work to maintain your weight by combining a healthy diet with daily exercise. If you need to lose weight, ask your doctor about healthy ways to achieve your goal. Aim to lose weight slowly by increasing the amount of exercise you get and reducing the number of calories you eat.
Colon cancer prevention for people with a high risk
Some medications have been found to reduce the risk of precancerous polyps or colon cancer. However, not enough evidence exists to recommend these medications to people who have an average risk of colon cancer. These options are generally reserved for people with a high risk of colon cancer.
For instance, some evidence links a reduced risk of polyps and colon cancer to regular use of aspirin or aspirin-like drugs. But it’s not clear what dose and what length of time would be needed to reduce the risk of colon cancer. Taking aspirin daily has some risks, including gastrointestinal bleeding and ulcers, so doctors typically don’t recommend this as a prevention strategy unless you have an increased risk of colon cancer.
Screening Tests for Colorectal Cancer
Doctors recommend certain screening tests for healthy people with no signs or symptoms in order to look for early colon cancer. Finding colon cancer at its earliest stage provides the greatest chance for a cure. Screening (which is a process of looking for cancer in people who have no symptoms) has been shown to reduce your risk of dying of colon cancer.
People with an average risk of colon cancer can consider screening beginning at age 45. But people with an increased risk, such as those with a family history of colon cancer, should consider screening sooner. African-Americans and American Indians may consider beginning colon cancer screening at age 45.
Both men and women should have a colon cancer screening test starting at age 45 (if following the American Cancer Society Guideline). Some providers recommend that African Americans begin screening at age 45.
With a recent increase in colon cancer in people in their 40s, the American Cancer Society recommends that healthy men and women start screening at age 45. Talk to your doctor if you’re concerned.
Several screening options exist — each with its own benefits and drawbacks. Talk about your options with your doctor, and together you can decide which tests are appropriate for you. If a colonoscopy is used for screening, polyps can be removed during the procedure before they turn into cancer.
The American Cancer Society believes that preventing colorectal cancer (and not just finding it early) should be a major reason for getting tested. Having polyps found and removed keeps some people from getting colorectal cancer. You are encouraged to have tests that have the best chance of finding both polyps and cancer if these tests are available to you and you are willing to have them. But the most important thing is to get tested, no matter which test you choose.
*For screening, people are considered to be at AVERAGE risk if they DO NOT have:
- A personal history of colorectal cancer or certain types of polyps
- A family history of colorectal cancer
- A personal history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease)
- A confirmed or suspected hereditary colorectal cancer syndrome, such as familial adenomatous polyposis (FAP) or Lynch syndrome (hereditary non-polyposis colon cancer or HNPCC)
- A personal history of getting radiation to the abdomen (belly) or pelvic area to treat a prior cancer
People who are in good health and with a life expectancy of more than 10 years should continue regular colorectal cancer screening through the age of 75.
For people ages 76 through 85, the decision to be screened should be based on a person’s preferences, life expectancy, overall health, and prior screening history.
People over 85 should no longer get colorectal cancer screening.
When colon cancer is found early, before it has spread, the 5-year relative survival rate is 90%. This means 9 out of 10 people with early-stage cancer survive at least 5 years. But if the cancer has had a chance to spread outside the colon, survival rates are lower.
Starting at age 45, men and women at average risk for developing colorectal cancer should use one of the screening tests below:
Screening is the process of looking for cancer in people who have no symptoms. Several tests can be used to screen for colorectal cancers. These tests can be divided into 8:
Visual (structural) exams of the colon and rectum
- Colonoscopy every 10 years
- CT colonography (virtual colonoscopy) every 5 years*
- Flexible sigmoidoscopy every 5 years*
- Double-contrast barium enema every 5 years*
Stool-based tests
- Fecal immunochemical test (FIT) every year*,**
- Guaiac-based fecal occult blood test (gFOBT) every year*,**
- Stool DNA test every 3 years*
*Colonoscopy should be done if test results are positive.
** Highly sensitive versions of these tests should be used with the take-home multiple sample method. A guaiac-based fecal occult blood test (gFOBT) or fecal immunochemical test (FIT) done during a digital rectal exam in the doctor’s office is not enough for screening.
Tests that can find both colorectal polyps and cancer are encouraged if they are available and you are willing to have them. But the most important thing is to get tested, no matter which test you choose.
These tests, as well as others, can also be used when people have symptoms of colorectal cancer and other digestive diseases such as inflammatory bowel disease.
People at increased or high risk
If you are at an increased or high risk of colorectal cancer, you might need to start colorectal cancer screening before age 45 and/or be screened more often. The following conditions make your risk higher than average:
- A personal history of colorectal cancer or adenomatous polyps
- A personal history of inflammatory bowel disease (ulcerative colitis or Crohn’s disease)
- A personal history of radiation to the abdomen (belly) or pelvic area to treat a prior cancer
- A strong family history of colorectal cancer or polyps
- A known family history of a hereditary colorectal cancer syndrome such as familial adenomatous polyposis (FAP) or Lynch syndrome (hereditary non-polyposis colon cancer or HNPCC)
The tables below suggest screening guidelines for people with increased or high risk of colorectal cancer based on specific risk factors. Some people may have more than one risk factor. Refer to the tables below and discuss these recommendations with your health care provider. Your doctor can suggest the best screening option for you, as well as any changes in the schedule based on your individual risk.
People at increased risk for colorectal cancer
- People with one or more family members who have had colon or rectal cancer. Screening recommendations for these people depend on who in the family had cancer and how old they were when it was diagnosed. Some people with a family history will be able to follow the recommendations for average risk adults, but others might need to get a colonoscopy (and not any other type of test) more often, and possibly starting before age 45.
- People who have had certain types of polyps removed during a colonoscopy. Most of these people will need to get a colonoscopy again after 3 years, but some people might need to get one earlier (or later) than 3 years, depending on the type, size, and number of polyps.
- People who have had colon or rectal cancer. Most of these people will need to start having colonoscopies regularly about one year after surgery to remove the cancer. Other procedures like MRI or proctoscopy with ultrasound might also be recommended for some people with rectal cancer, depending on the type of surgery they had.
- People who have had radiation to the abdomen (belly) or pelvic area to treat a prior cancer. Most of these people will need to start having colorectal screening (colonoscopy or stool based testing) at an earlier age (depending on how old they were when they got the radiation). Screening often begins 5 years after the radiation was given or at age 30, whichever comes last. These people might also need to be screened more often than normal (such as at least every 3 to 5 years).
People at high risk for colorectal cancer
- People with inflammatory bowel disease (Crohn’s disease or ulcerative colitis). These people generally need to get colonoscopies (not any other type of test) starting at least 8 years after they are diagnosed with inflammatory bowel disease. Follow-up colonoscopies should be done every 1 to 3 years, depending on the person’s risk factors for colorectal cancer and the findings on the previous colonoscopy.
- People known or suspected to have certain genetic syndromes. These people generally need to have colonoscopy (not any of the other tests). Screening is often recommended to begin at a young age, possibly as early as the teenage years for some syndromes – and needs to be done much more frequently. Specifics depend on which genetic syndrome you have, and other factors. If you’re at increased or high risk of colorectal cancer (or think you might be), talk to your health care provider to learn more. Your provider can suggest the best screening option for you, as well as determine what type of screening schedule you should follow, based on your individual risk.
Note: As of 2022, the American Cancer Society no longer have screening guidelines specifically for people at increased or high risk of colorectal cancer. The tables below were from American Cancer Society prior to them removing their screening guidelines for people at increased or high risk of colorectal cancer. But we have decided to leave them here for your reference.
Table 1. Professional society recommendations on when to start and when to stop colorectal cancer screening
| Colorectal cancer screening start age | Colorectal cancer screening stop age | |
|---|---|---|
| Multi-Society Task Force, 2021 | “We suggest that clinicians offer colorectal cancer screening to all average-risk individuals age 45-49 (weak recommendation; low-quality evidence).” | “We suggest that individuals who are up to date with screening and have negative prior screening tests, particularly high-quality colonoscopy, consider stopping screening at age 75 years or when life expectancy is less than 10 years (weak recommendation, low-quality evidence).” |
| “For average-risk individuals who have not initiated screening before age 50, we recommend that clinicians offer colorectal cancer screening to all average-risk individuals beginning at age 50 (strong recommendation, high-quality evidence).” | “We suggest that persons without prior screening should be considered for screening up to age 85, depending on consideration of their age and comorbidities (weak recommendation, low-quality evidence).” | |
| National Comprehensive Cancer Network, 2021 9 | “Average risk: age ≥45. The panel has reviewed existing data for beginning screening of average-risk individuals at age <50 years. Based on their assessment, the panel agrees that the data are stronger to support beginning screening at 50 years but acknowledges that lower-level evidence supports a benefit for screening earlier. When initiating screening for all eligible individuals, the panel recommends a discussion of potential harms/risks and benefits, and the consideration of all recommended colorectal cancer screening options.” | Not provided |
| American College of Gastroenterology, 2021 10 | “We recommend colorectal cancer screening in average-risk individuals between ages 50 and 75 years to reduce incidence of advanced adenoma, colorectal cancer, and mortality from colorectal cancer.” Strong recommendation; moderate-quality evidence “We suggest colorectal cancer screening in average-risk individuals between ages 45 and 49 years to reduce incidence of advanced adenoma, colorectal cancer, and mortality from colorectal cancer.” Conditional recommendation; very low-quality evidence | “We suggest that a decision to continue screening beyond age 75 years be individualized (conditional recommendation strength, very low-Grading of Recommendations Assessment, Development and Evaluation quality of evidence).” |
| U.S. Preventative Services Task Force, 2021 11 | Grade A: “The U.S. Preventative Services Task Force recommends screening for colorectal cancer in all adults ages 50 to 75 years.” Grade B: “The U.S. Preventative Services Task Force recommends screening for colorectal cancer in adults aged 45 to 49 years.” | Grade C: “The U.S. Preventative Services Task Force recommends that clinicians selectively offer screening for colorectal cancer in adults aged 76 to 85 years. Evidence indicates that the net benefit of screening all persons in this age group is small. In determining whether this service is appropriate in individual cases, patients and clinicians should consider the patient’s overall health, prior screening history, and preferences.” |
| American College of Physicians, 2019 12 | “Clinicians should screen for colorectal cancer in average-risk adults between the ages of 50 and 75 years.” | “Clinicians should discontinue screening for colorectal cancer in average-risk adults older than 75 years or in adults with a life expectancy of 10 years or less.” |
| American Cancer Society, 2018 13 | “The American Cancer Society recommends that adults aged 45 and older with an average risk of colorectal cancer undergo regular screening with either a high-sensitivity stool-based test or a structural (visual) examination, depending on patient preference and test availability. As a part of the screening process, all positive results on non-colonoscopy screening tests should be followed up with timely colonoscopy.” | “Average-risk adults in good health with a life expectancy of greater than 10 years continue colorectal cancer screening through the age of 75 years (qualified recommendation).” |
| “The recommendation to begin screening at age 45 is a qualified recommendation.” | Clinicians should “individualize colorectal cancer screening decisions for individuals aged 76 through 85 years based on patient preferences, life expectancy, health status, and prior screening history (qualified recommendation).” | |
| “The recommendation for regular screening in adults aged 50 y and older is a strong recommendation.” | Clinicians should “discourage individuals over age 85 years from continuing colorectal cancer screening (qualified recommendation).” |
Table 2. American Cancer Society Guidelines on Screening and Surveillance for the Early Detection of Colorectal Adenomas and Cancer in People who have a history of polyps on prior colonoscopy
| INCREASED RISK – People who have a history of polyps on prior colonoscopy | |||
| Risk category | When to test | Recommended test(s) | Comment |
| People with small rectal hyperplastic polyps | Same age as those at average risk | Colonoscopy, or other screening options at same intervals as for those at average risk | Those with hyperplastic polyposis syndrome are at increased risk for adenomatous polyps and cancer and should have more intensive follow-up. |
| People with 1 or 2 small (no more than 1 cm) tubular adenomas with low-grade dysplasia | 5 to 10 years after the polyps are removed | Colonoscopy | Time between tests should be based on other factors such as prior colonoscopy findings, family history, and patient and doctor preferences. |
| People with 3 to 10 adenomas, or a large (at least 1 cm) adenoma, or any adenomas with high-grade dysplasia or villous features | 3 years after the polyps are removed | Colonoscopy | Adenomas must have been completely removed. If colonoscopy is normal or shows only 1 or 2 small tubular adenomas with low-grade dysplasia, future colonoscopies can be done every 5 years. |
| People with more than 10 adenomas on a single exam | Within 3 years after the polyps are removed | Colonoscopy | Doctor should consider possible genetic syndrome (such as FAP or Lynch syndrome). |
| People with sessile adenomas that are removed in pieces | 2 to 6 months after adenoma removal | Colonoscopy | If entire adenoma has been removed, further testing should be based on doctor’s judgment. |
Table 3. American Cancer Society Guidelines on Screening and Surveillance for the Early Detection of Colorectal Adenomas and Cancer in People who have had colorectal cancer
| INCREASED RISK – People who have had colorectal cancer | |||
| Risk category | When to test | Recommended test(s) | Comment |
| People diagnosed with colon or rectal cancer | At time of colorectal surgery, or can be 3 to 6 months later if person doesn’t have cancer spread that can’t be removed | Colonoscopy to look at the entire colon and remove all polyps | If the tumor presses on the colon/rectum and prevents colonoscopy, CT colonoscopy (with IV contrast) or double-contrast barium enema (DCBE) may be done to look at the rest of the colon. |
| People who have had colon or rectal cancer removed by surgery | Within 1 year after cancer resection (or 1 year after colonoscopy to make sure the rest of the colon/rectum was clear) | Colonoscopy | If normal, repeat in 3 years. If normal then, repeat test every 5 years. Time between tests may be shorter if polyps are found or there’s reason to suspect Lynch syndrome. After low anterior resection for rectal cancer, exams of the rectum may be done every 3 to 6 months for the first 2 to 3 years to look for signs of recurrence. |
Table 4. American Cancer Society Guidelines on Screening and Surveillance for the Early Detection of Colorectal Adenomas and Cancer in People with a family history of colorectal cancer or adenomatous polyps
| INCREASED RISK – People with a family history | |||
| Risk category | Age to start testing | Recommended test(s) | Comment |
| Colorectal cancer or adenomatous polyps in any first-degree relative before age 60, or in 2 or more first-degree relatives at any age (if not a hereditary syndrome). | Age 40, or 10 years before the youngest case in the immediate family, whichever is earlier | Colonoscopy | Every 5 years. |
| Colorectal cancer or adenomatous polyps in any first-degree relative aged 60 or older, or in at least 2 second-degree relatives at any age | Age 40 | Same test options as for those at average risk. | Same test intervals as for those at average risk. |
Table 5. American Cancer Society Guidelines on Screening and Surveillance for the Early Detection of Colorectal Adenomas and Cancer in People with familial adenomatous polyposis, Lynch syndrome & inflammatory bowel disease
| HIGH RISK | |||
| Risk category | Age to start testing | Recommended test(s) | Comment |
| Familial adenomatous polyposis (FAP) diagnosed by genetic testing, or suspected FAP without genetic testing | Age 10 to 12 | Yearly flexible sigmoidoscopy to look for signs of FAP; counseling to consider genetic testing if it hasn’t been done | If genetic test is positive, removal of colon (colectomy) should be considered. |
| Lynch syndrome (hereditary non-polyposis colon cancer or HNPCC), or at increased risk of Lynch syndrome based on family history without genetic testing | Age 20 to 25 years, or 10 years before the youngest case in the immediate family | Colonoscopy every 1 to 2 years; counseling to consider genetic testing if it hasn’t been done | Genetic testing should be offered to first-degree relatives of people found to have Lynch syndrome mutations by genetic tests. It should also be offered if 1 of the first 3 of the modified Bethesda criteria is met.* |
| Inflammatory bowel disease: -Chronic ulcerative colitis -Crohn’s disease | Cancer risk begins to be significant 8 years after the onset of pancolitis (involvement of entire large intestine), or 12-15 years after the onset of left-sided colitis | Colonoscopy every 1 to 2 years with biopsies for dysplasia | These people are best referred to a center with experience in the surveillance and management of inflammatory bowel disease. |
The US Preventive Services Task Force (USPSTF) colon cancer screening guidelines
The US Preventive Services Task Force (USPSTF) colon cancer screening guidelines is in the process of being updated in 2019.
The US Preventive Services Task Force (USPSTF) colon cancer screening guidelines – 2021 recommendations 15:
- Adults aged 45 to 75 years: The USPSTF recommends screening for colorectal cancer starting at age 50 years and continuing until age 75 years. The risks and benefits of different screening methods vary.
- Adults aged 76 to 85 years: The USPSTF recommends that clinicians selectively offer screening for colorectal cancer in adults aged 76 to 85 years. Evidence indicates that the net benefit of screening all persons in this age group is small. The decision to screen for colorectal cancer in adults aged 76 to 85 years should be an individual one, taking into account the patient’s overall health, prior screening history and preferences.
- Adults in this age group who have never been screened for colorectal cancer are more likely to benefit.
- Screening would be most appropriate among adults who 1) are healthy enough to undergo treatment if colorectal cancer is detected and 2) do not have comorbid conditions that would significantly limit their life expectancy.
The US Preventive Services Task Force (USPSTF) recommended screening strategies include 16:
- High-sensitivity guaiac fecal occult blood test (HSgFOBT) or fecal immunochemical test (FIT) every year
- Stool DNA-FIT every 1 to 3 years
- Computed tomography colonography every 5 years
- Flexible sigmoidoscopy every 5 years
- Flexible sigmoidoscopy every 10 years + annual FIT
- Colonoscopy screening every 10 years
Visual (structural) exams of the colon and rectum
Flexible sigmoidoscopy
During this test, the doctor looks at part of the colon and rectum with a sigmoidoscope (a flexible, lighted tube about the thickness of a finger with a small video camera on the end). It’s put in through the anus and into the rectum and moved into the lower part of the colon. Images from the scope are seen on a video screen.
Using the sigmoidoscope, your doctor can look at the inside of the rectum and part of the colon to detect (and possibly remove) any abnormality. The sigmoidoscope is only 60 centimeters (about 2 feet) long, so the doctor is able to see the entire rectum but less than half of the colon with this procedure.
This test is not widely used as a screening test for colorectal cancer in the United States.
Before the test: Be sure your doctor knows about any medicines you take. You might need to change how you take them before the test. Your insides must be empty and clean so your doctor can see the lining of the sigmoid colon and rectum. You will get specific instructions to follow to clean them out. You may be asked to follow a special diet (such as drinking only clear liquids) or to use enemas or strong laxatives the day before the test to clean out your colon.
During the test: A sigmoidoscopy usually takes about 10 to 20 minutes. Most people don’t need to be sedated for this test, but this might be an option you can discuss with your doctor. Sedation may make the test less uncomfortable, but you’ll need some time to recover from it and you’ll need someone with you to take you home after the test.
You’ll probably be asked to lie on a table on your left side with your knees pulled up near your chest. Before the test, your doctor may put a gloved, lubricated finger into your rectum to examine it. For the test itself, the sigmoidoscope is first lubricated to make it easier to insert into the rectum. The scope may feel cold as it’s put in. Air will be pumped into the colon through the sigmoidoscope so the doctor can see the walls of the colon better.
If you are not sedated during the procedure, you might feel pressure and slight cramping in your lower belly. To ease discomfort and the urge to have a bowel movement, it helps to breathe deeply and slowly through your mouth. You’ll feel better after the test once the air leaves your colon.
If a polyp is found during the test, the doctor may remove it with a small instrument passed through the scope. The polyp will be looked at in the lab. If a pre-cancerous polyp (an adenoma) or colorectal cancer is found, you’ll need to have a colonoscopy (see below) later to look for polyps or cancer in the rest of the colon.
Possible complications and side effects: This test may be uncomfortable because of the air put into the colon, but it should not be painful. Be sure to let your doctor know if you feel pain during the procedure. You might see a small amount of blood in your first bowel movement after the test. More serious bleeding and puncture of the colon are possible complications, but they are very uncommon.
Figure 5. Sigmoidoscopy. A thin, lighted tube is inserted through the anus and rectum and into the lower part of the colon to look for abnormal areas
Colonoscopy
For this test, the doctor looks at the entire length of the colon and rectum with a colonoscope, a thin, flexible, lighted tube with a small video camera on the end. It’s basically a longer version of a sigmoidoscope. It’s put in through the anus and into the rectum and colon. Special instruments can be passed through the colonoscope to biopsy (sample) or remove any suspicious-looking areas such as polyps, if needed.
Before the test: Be sure your doctor knows about any medicines you are taking. You might need to change how you take them before the test. The colon and rectum must be empty and clean so your doctor can see the lining of the entire colon and rectum during the test. This process of cleaning out the colon and rectum is sometimes unpleasant and can keep people from getting this important screening test done. However, newer kits are available to clean out the bowel and may be better tolerated than previous ones. Your doctor can discuss the options with you.
Your doctor will give you specific instructions. It’s important to read them carefully a few days ahead of time, since you may need to follow a special diet for at least a day before the test and to shop for supplies and laxatives. If you’re not sure about any of the instructions, call the doctor’s office and go over them with the nurse.
You will probably also be told not to eat or drink anything after midnight the night before your test. If you normally take prescription medicines in the mornings, talk with your doctor or nurse about how to manage them for that day.
Because a sedative is used during the test, you will need to arrange for someone you know to take you home after the test. You might need someone to help you get into your home if you are sleepy or dizzy, so many centers that do colonoscopies will not discharge people to go home in a cab or a ridesharing service. If transportation might be a problem, talk with your health care provider about the policy at your hospital or surgery center for using one of these services. There may be other resources available for getting home, depending on the situation.
During the test: The test itself usually takes about 30 minutes, but it may take longer if a polyp is found and removed. Before it starts, you’ll be given a sedating medicine (into a vein) to make you feel relaxed and sleepy during the procedure. For most people, this medicine makes them unaware of what’s going on and unable to remember the procedure afterward. You’ll wake up after the test is over, but might not be fully awake until later in the day.
During the test, you’ll be asked to lie on your side with your knees pulled up. A drape will cover you. Your blood pressure, heart rate, and breathing rate will be monitored during and after the test.
Your doctor might insert a gloved finger into the rectum to examine it before putting in the colonoscope. The colonoscope is lubricated so it can be inserted easily into the rectum. Once in the rectum, the colonoscope is passed all the way to the beginning of the colon, called the cecum.
If you’re awake, you may feel an urge to have a bowel movement when the colonoscope is inserted or pushed further up the colon. The doctor also puts air into the colon through the colonoscope to make it easier to see the lining of the colon and use the instruments to perform the test. To ease any discomfort, it may help to breathe deeply and slowly through your mouth.
The doctor will look at the inner walls of the colon as he or she slowly removes the colonoscope. If a small polyp is found, it may be removed and then sent to a lab to be checked if it has any areas that have changed into cancer. This is because some small polyps may become cancer over time.
If your doctor sees a larger polyp or tumor or anything else abnormal, a biopsy may be done. A small piece of tissue is taken out through the colonoscope. The tissue is checked in the lab to see if it’s cancer, a benign (non-cancerous) growth, or inflammation.
Possible side effects and complications: The bowel preparation before the test is unpleasant. The test itself might be uncomfortable, but the sedative usually helps with this, and most people feel normal once the effects of the sedative wear off. Because air is pumped into the colon during the test, people sometimes feel bloated, have gas pains, or have cramping for a while after the test until the air passes out.
Some people may have low blood pressure or changes in heart rhythm from the sedation during the test, but these are rarely serious.
If a polyp is removed or a biopsy is done during the colonoscopy, you might notice some blood in your stool for a day or 2 after the test. Serious bleeding is uncommon, but in rare cases, bleeding might need to be treated or can even be life-threatening.
Colonoscopy is a safe procedure, but in rare cases the colonoscope can puncture the wall of the colon or rectum. This is called a perforation. Symptoms can include severe abdominal (belly) pain, nausea, and vomiting. This can be a major (or even life-threatening) complication, because it can lead to a serious abdominal (belly) infection. The hole may need to be repaired with surgery. Ask your doctor about the risk of this complication.
Figure 6. Colonoscopy
Double-contrast barium enema (DCBE)
This test is also called an air-contrast barium enema or a barium enema with air contrast. It may also be called a lower GI series. It’s basically a type of x-ray test. Barium sulfate, which is a chalky liquid, and air are put into the colon and rectum through the anus to outline the inner lining. This can show abnormal areas on x-rays. If suspicious areas are seen on this test, a colonoscopy will need to be done to explore them further.
This test is not widely used as a screening test for colorectal cancer in the United States.
Before the test: It’s very important that the colon and rectum are empty and clean so they can be seen during the test. You’ll be given specific instructions on how to prepare for the test. For example, you may be asked to clean your bowel the night before with laxatives and/or take enemas the morning of the exam. You’ll probably be asked to follow a clear liquid diet for at least a day before the test. You may also be told to avoid eating or drinking dairy products the day before the test, and to not eat or drink anything after midnight the night before the test.
During the test: The test takes about 30 to 45 minutes, and sedation isn’t needed. You lie on a table on your side in an x-ray room. A small, flexible tube is put into your rectum, and barium sulfate is pumped in to partially fill and open up the colon and rectum. You are then turned on the x-ray table so the barium moves throughout the colon and rectum. Then air is pumped into the colon and rectum through the same tube to expand them. This might cause some cramping and discomfort, and you may feel the urge to have a bowel movement.
X-ray pictures of the lining of your colon and rectum are then taken to look for polyps or cancers. You may be asked to change positions to help move the barium and so that different views of the colon and rectum can be seen on the x-rays.
If polyps or other suspicious areas are seen on this test, you’ll probably need a colonoscopy to remove them or to study them fully.
Possible side effects and complications: You may have bloating or cramping after the test, and will probably feel the need to empty your bowels soon after the test is done. The barium can cause constipation for a few days, and your stool may look grey or white until all the barium is out. There’s a very small risk that inflating the colon with air could injure or puncture it, but this risk is thought to be much less than with colonoscopy. Like other x-ray tests, this test also exposes you to a small amount of radiation.
Figure 7. Barium enema

CT colonography (virtual colonoscopy)
This test is an advanced type of computed tomography (CT or CAT) scan of the colon and rectum. A CT scan uses x-rays, but instead of taking one picture, like a regular x-ray, a CT scanner takes many pictures as it rotates around you while you lie on a table. A computer then combines these pictures into detailed images of the part of your body being studied.
For CT colonography, special computer programs create both 2-dimensional x-ray pictures and a 3-dimensional view of the inside of the colon and rectum, which lets the doctor look for polyps or cancer.
This test may be especially useful for some people who can’t have or don’t want to have more invasive tests such as colonoscopy. It can be done fairly quickly, and sedation isn’t needed. But even though this test is not invasive like a colonoscopy, the same type of bowel prep is needed. Also, a small, flexible tube is put in the rectum to fill the colon with air. Another possible drawback is that if polyps or other suspicious areas are seen on this test, a colonoscopy will still probably be needed to remove them or to explore them fully.
Before the test: It’s important that the colon and rectum are emptied before this test to get the best images. You’ll probably be told to follow a clear liquid diet for at least a day before the test. There are a number of ways to clean out the colon before the test. Often, the evening before the procedure, you drink large amounts of a liquid laxative solution. This often results in spending a lot of time in the bathroom. The morning of the test, sometimes more laxatives or enemas may be needed to make sure the bowels are empty. Newer kits are available to clean out the bowel and may be better tolerated than previous ones. Your doctor can discuss the options with you.
During the test: This test is done in a special room with a CT scanner. It takes about 10 minutes. You may be asked to drink a contrast solution before the test to help “tag” any stool left in the colon or rectum, which helps the doctor when looking at the test images. You’ll be asked to lie on a narrow table that’s part of the CT scanner, and will have a small, flexible tube put into your rectum. Air is pumped through the tube into the colon and rectum to expand them to provide better images. The table then slides into the CT scanner, and you’ll be asked to hold your breath for about 15 seconds while the scan is done. You’ll likely have 2 scans: one while you’re lying on your back and one while you’re on your stomach or side.
Possible side effects and complications: There are usually few side effects after this test. You may feel bloated or have cramps because of the air in the colon and rectum, but this should go away once the air passes from the body. There’s a very small risk that inflating the colon with air could injure or puncture it, but this risk is thought to be much less than with colonoscopy. Like other types of CT scans, this test also exposes you to a small amount of radiation
Stool-based tests
These tests look at the stool (feces) for signs of cancer. Most people find these tests easier to have than tests like colonoscopy, and they can often be done at home. But these tests aren’t as good at finding polyps such as tests like colonoscopy. And if the result from one of these stool tests is positive (abnormal), you’ll probably still need a colonoscopy to see if you have cancer.
Guaiac-based fecal occult blood test (gFOBT)
One way to test for colorectal cancer is to look for occult (hidden) blood in stool. The idea behind this test is that blood vessels in larger colorectal polyps or cancers are often fragile and easily damaged by the passage of stool. The damaged vessels usually bleed into the colon, but only rarely is there enough bleeding for blood to be seen in the stool.
The guaiac-based fecal occult blood test (gFOBT) detects blood in the stool through a chemical reaction. This test can’t tell if the blood is from the colon or from other parts of the digestive tract (such as the stomach). If this test is positive, a colonoscopy will be needed to find the reason for the bleeding. Although blood in the stool can be from cancers or polyps, it can also have other causes, such as ulcers, hemorrhoids, diverticulosis (tiny pouches that form at weak spots in the colon wall), or inflammatory bowel disease (colitis).
Over time, this test has improved so that it’s now more likely to find colorectal cancer. The American Cancer Society recommends the more modern, highly sensitive versions of this test for screening.
This test must be done every year, unlike some other tests (like colonoscopy).
This test is done with a kit that you can use in the privacy of your own home that allows you to check more than one stool sample. A FOBT done during a digital rectal exam in the doctor’s office (which only checks one stool sample) is not enough for proper screening.
People having this test will get a kit with instructions from their doctor’s office or clinic. The kit will explain how to take stool samples at home (usually samples from 3 straight bowel movements are smeared onto small squares of paper). The kit is then returned to the doctor’s office or medical lab (usually within 2 weeks) for testing.
Before the test: Some foods or drugs can affect the results, so you may be instructed to avoid the following before this test:
- Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen (Advil), naproxen (Aleve), or aspirin (more than 1 adult aspirin per day), for 7 days before testing. (They can cause bleeding, which can lead to a false-positive result.) Note: People should try to avoid taking NSAIDs for minor aches. But if you take these medicines daily for heart problems or other conditions, don’t stop them for this test without talking to your doctor first.
- Vitamin C in excess of 250 mg daily from either supplements or citrus fruits and juices for 3 days before testing. (This can affect the chemicals in the test and make the result negative, even if blood is present.)
- Red meats (beef, lamb, or liver) for 3 days before testing. (Components of blood in the meat may cause a positive test result.)
Some people who are given the test never do it or don’t return it because they worry that something they ate may affect the test. Even if you are concerned that something you ate may alter the test, the most important thing is to get the test done.
Collecting the samples: Have all of your supplies ready and in one place. Supplies typically include a test kit, test cards, either a brush or wooden applicator, and a mailing envelope. The kit will give you detailed instructions on how to collect the stool samples. Be sure to follow the instructions that come with your kit, as different kits might have different instructions. If you have any questions about how to use your kit, contact your doctor’s office or clinic. Once you have collected the samples, return them as instructed in the kit.
If this test finds blood, you will need a colonoscopy to look for the source. It’s not enough to simply repeat the gFOBT or follow up with other types of tests.
Fecal immunochemical test (FIT)
The fecal immunochemical test (FIT) is also called an immunochemical fecal occult blood test (iFOBT). It tests for occult (hidden) blood in the stool in a different way than a guaiac-based FOBT. This test reacts to part of the human hemoglobin protein, which is found in red blood cells.
The FIT is done much like the gFOBT, in that small amounts of stool are collected on cards (or in tubes). Some people may find this test easier because there are no drug or dietary restrictions (vitamins and foods do not affect the FIT), and collecting the samples may be easier. This test is also less likely to react to bleeding from other parts of digestive tract, such as the stomach.
Like the gFOBT, the FIT may not detect a tumor that’s not bleeding, so multiple stool samples should be tested. This test must also be done every year. And if the results are positive for hidden blood, a colonoscopy will be needed to investigate further.
Collecting the samples: Have all of your supplies ready and in one place. Supplies typically include a test kit, test cards or tubes, long brushes or other collecting devices, waste bags, and a mailing envelope. The kit will give you detailed instructions on how to collect the samples. Be sure to follow the instructions that come with your kit, as different kits might have different instructions. If you have any questions about how to use your kit, contact your doctor’s office or clinic. Once you have collected the samples, return them as instructed in the kit.
Stool DNA test
A stool DNA test looks for certain abnormal sections of DNA from cancer or polyp cells. Colorectal cancer cells often have DNA mutations (changes) in certain genes. Cells from colorectal cancers or polyps with these mutations often get into the stool, where tests may be able to detect them. Cologuard®, the test currently available, also tests for blood in the stool.
Collecting the samples: You’ll get a kit in the mail to use to collect your entire stool sample. The kit will have a sample container, a bracket for holding the container in the toilet, a bottle of liquid preservative, a tube, labels, and a shipping box. The kit has detailed instructions on how to collect the sample. Be sure to follow the instructions that come with your kit. If you have any questions about how to use your kit, contact your doctor’s office or clinic. Once you have collected the sample, return it as instructed in the kit.
This test should be done every 3 years. If the test is positive (if it finds DNA changes or blood), a colonoscopy will be needed.
Table 6. Benefits and limitations of colorectal cancer screening tests
| Test | Pros | Cons |
|---|---|---|
| Flexible sigmoidoscopy | Fairly quick and safe Usually doesn’t require full bowel prep Sedation usually not used Does not require a specialist Done every 5 years | Looks at only about a third of the colon Can miss small polyps Can’t remove all polyps May be some discomfort Very small risk of bleeding, infection, or bowel tear Colonoscopy will be needed if abnormal |
| Colonoscopy | Can usually look at the entire colon Can biopsy and remove polyps Done every 10 years Can help find some other diseases | Can miss small polyps Full bowel prep needed Costs more on a one-time basis than other forms of testing Sedation is usually needed You will need someone to drive you home You may miss a day of work Small risk of bleeding, bowel tears, or infection |
| Double-contrast barium enema (DCBE) | Can usually see the entire colon Relatively safe Done every 5 years No sedation needed | Can miss small polyps Full bowel prep needed Some false positive test results Can’t remove polyps during testing Colonoscopy will be needed if abnormal |
| CT colonography (virtual colonoscopy) | Fairly quick and safe Can usually see the entire colon Done every 5 years No sedation needed | Can miss small polyps Full bowel prep needed Some false positive test results Can’t remove polyps during testing Colonoscopy will be needed if abnormal Still fairly new – may be insurance issues |
| Guaiac-based fecal occult blood test (gFOBT) | No direct risk to the colon No bowel prep Sampling done at home Inexpensive | Can miss many polyps and some cancers Can produce false-positive test results Pre-test diet changes are needed Needs to be done every year Colonoscopy will be needed if abnormal |
| Fecal immunochemical test (FIT) | No direct risk to the colon No bowel prep No pre-test diet changes Sampling done at home Fairly inexpensive | Can miss many polyps and some cancers Can produce false-positive test results Needs to be done every year Colonoscopy will be needed if abnormal |
| Stool DNA test | No direct risk to the colon No bowel prep No pre-test diet changes Sampling done at home | Can miss many polyps and some cancers Can produce false-positive test results Should be done every 3 years Colonoscopy will be needed if abnormal Still fairly new – may be insurance issues |
Colon cancer diagnosis
If you have symptoms that might be from colorectal cancer, or if a screening test shows something abnormal, your doctor will recommend one or more of the exams and tests below to find the cause.
Your doctor will also ask about your medical history to learn about possible risk factors, including your family history. You will also be asked if you’re having any symptoms and, if so, when they started and how long you’ve had them.
As part of a physical exam, your doctor will feel your abdomen for masses or enlarged organs, and also examine the rest of your body. You may also have a digital rectal exam (DRE). During this test, the doctor inserts a lubricated, gloved finger into your rectum to feel for any abnormal areas.
Diagnosing colon cancer
If your signs and symptoms indicate that you could have colon cancer, your doctor may recommend one or more tests and procedures, including:
Using a scope to examine the inside of your colon. Colonoscopy uses a long, flexible and slender tube attached to a video camera and monitor to view your entire colon and rectum. If any suspicious areas are found, your doctor can pass surgical tools through the tube to take tissue samples (biopsies) for analysis and remove polyps.
Virtual colonoscopy: A procedure that uses a series of x-rays called computed tomography to make a series of pictures of the colon. A computer puts the pictures together to create detailed images that may show polyps and anything else that seems unusual on the inside surface of the colon. This test is also called colonography or CT colonography.
Sigmoidoscopy: A procedure to look inside the rectum and sigmoid (lower) colon for polyps (small areas of bulging tissue), other abnormal areas, or cancer. A sigmoidoscope is inserted through the rectum into the sigmoid colon. A sigmoidoscope is a thin, tube-like instrument with a light and a lens for viewing. It may also have a tool to remove polyps or tissue samples, which are checked under a microscope for signs of cancer.
Biopsy: The removal of cells or tissues so they can be viewed under a microscope by a pathologist to check for signs of cancer.
Biopsy samples (from colonoscopy or surgery) are sent to the lab where they are looked at closely. Other tests may suggest that colorectal cancer is present, but the only way to be sure is to look at the biopsy samples under a microscope.
If cancer is found, other lab tests may also be done on the biopsy specimens to help better classify the cancer.
- Gene tests: Doctors may look for specific gene changes in the cancer cells that might affect how the cancer is best treated especially if the cancer has spread (metastasized). For example, doctors now typically test the cells for changes in the KRAS and NRAS and BRAF genes. Some doctors may also test for changes in the BRAF gene. Patients whose cancers have mutations in these genes typically do not benefit from treatment with certain targeted anti-cancer drugs.
- MSI and MMR testing: Colorectal cancer cells are typically tested to see if they show high levels of gene changes called microsatellite instability (MSI). Testing might also be done to see if the cancer cells have changes in any of the mismatch repair (MMR) genes (MLH1, MSH2, MSH6, and PMS2).
Changes in MSI or in MMR genes (or both) are often seen in people with Lynch syndrome (HNPCC). Most colorectal cancers do not have high levels of MSI or changes in MMR genes. But most colorectal cancers that are linked to Lynch syndrome do.
There are 2 possible reasons to test colorectal cancers for MSI or for MMR gene changes:
- To identify patients who should be tested for Lynch syndrome. A diagnosis of Lynch syndrome can help plan other cancer screenings for the patient (for example, women with Lynch syndrome may need to be screened for uterine cancer). Also, if a patient has Lynch syndrome, their relatives could also have it, and may want to be tested for it.
- To determine treatment options for colorectal cancer, where MSI or MMR results could change the way it is treated.
Blood tests. No blood test can tell you if you have colon cancer. But your doctor may test your blood for clues about your overall health, such as kidney and liver function tests.
Your doctor may also test your blood for a chemical sometimes produced by colon cancers called tumor markers that can be found in the blood. The most common tumor markers for colorectal cancer are carcinoembryonic antigen (CEA) and CA 19-9. Tracked over time, the level of CEA (carcinoembryonic antigen) in your blood may help your doctor understand your prognosis and whether your cancer is responding to treatment.
Blood tests for these tumor markers can sometimes suggest someone might have colorectal cancer, but they can’t be used alone to screen for or diagnose cancer. This is because tumor marker levels can sometimes be normal in someone who has cancer and can be abnormal for reasons other than cancer.
Tumor markers are used most often along with other tests to monitor patients who already have been diagnosed with colorectal cancer. They may help show how well treatment is working or provide an early warning that a cancer has returned.
If symptoms or the results of the physical exam or blood tests suggest that you might have colorectal cancer, your doctor could recommend more tests. This most often is colonoscopy, but sometimes other tests may be done first.
Barium enema: A series of x-rays of the lower gastrointestinal tract. A liquid that contains barium (a silver-white metallic compound) is put into the rectum. The barium coats the colon and x-rays are taken. This procedure is also called a lower GI series.
Colon cancer stages
Once you’ve been diagnosed with colon cancer, your doctor will order tests to determine the extent and how far the colon cancer has spread. This process is called staging. The stage of a cancer describes how much cancer is in the body. It helps determine how serious the cancer is and how best to treat it. Staging helps determine what treatments are most appropriate for you. Doctors also use a cancer’s stage when talking about survival statistics.
Staging tests may include imaging procedures such as abdominal, pelvic and chest CT scans. In many cases, the stage of your cancer may not be determined until after colon cancer surgery.
The earliest stage colorectal cancers are called stage 0 (a very early cancer or carcinoma in situ), and then range from stages I (1) through IV (4). As a rule, the lower the number, the less the cancer has spread. A higher number, such as stage IV, means cancer has spread more. And within a stage, an earlier letter means a lower stage. Although each person’s cancer experience is unique, cancers with similar stages tend to have a similar outlook and are often treated in much the same way.
The stages of colon cancer are:
- Stage O (a very early cancer). In stage O, abnormal cells are found in the mucosa (innermost layer) of the colon wall. These abnormal cells may become cancer and spread. Stage 0 is also called carcinoma in situ.
- Stage 1 colon cancer. In stage 1, cancer has formed in the mucosa (innermost layer) of the colon wall and has spread to the submucosa (layer of tissue under the mucosa). Cancer may have spread to the muscle layer of the colon wall.
- Stage 2 colon cancer. Stage 2 colon cancer is divided into stage 2A, stage 2B, and stage 2C.
- Stage 2A colon cancer: Cancer has spread through the muscle layer of the colon wall to the serosa (outermost layer) of the colon wall.
- Stage 2B colon cancer: Cancer has spread through the serosa (outermost layer) of the colon wall but has not spread to nearby organs.
- Stage 2C colon cancer: Cancer has spread through the serosa (outermost layer) of the colon wall to nearby organs.
- Stage 3 colon cancer. Stage 3 colon cancer is divided into stage 3A, stage 3B, and stage 3C.
- Stage 3A colon cancer:
- Cancer has spread through the mucosa (innermost layer) of the colon wall to the submucosa (layer of tissue under the mucosa) and may have spread to the muscle layer of the colon wall. Cancer has spread to at least one but not more than 3 nearby lymph nodes or cancer cells have formed in tissues near the lymph nodes; or
- Cancer has spread through the mucosa (innermost layer) of the colon wall to the submucosa (layer of tissue under the mucosa). Cancer has spread to at least 4 but not more than 6 nearby lymph nodes.
- Stage 3B colon cancer:
- Cancer has spread through the muscle layer of the colon wall to the serosa (outermost layer) of the colon wall or has spread through the serosa but not to nearby organs. Cancer has spread to at least one but not more than 3 nearby lymph nodes or cancer cells have formed in tissues near the lymph nodes; or
- Cancer has spread to the muscle layer of the colon wall or to the serosa (outermost layer) of the colon wall. Cancer has spread to at least 4 but not more than 6 nearby lymph nodes; or
- Cancer has spread through the mucosa (innermost layer) of the colon wall to the submucosa (layer of tissue under the mucosa) and may have spread to the muscle layer of the colon wall. Cancer has spread to 7 or more nearby lymph nodes.
- Stage 3C colon cancer:
- Cancer has spread through the serosa (outermost layer) of the colon wall but has not spread to nearby organs. Cancer has spread to at least 4 but not more than 6 nearby lymph nodes; or
- Cancer has spread through the muscle layer of the colon wall to the serosa (outermost layer) of the colon wall or has spread through the serosa but has not spread to nearby organs. Cancer has spread to 7 or more nearby lymph nodes; or
- Cancer has spread through the serosa (outermost layer) of the colon wall and has spread to nearby organs. Cancer has spread to one or more nearby lymph nodes or cancer cells have formed in tissues near the lymph nodes.
- Stage 3A colon cancer:
- Stage 4 colon cancer. The cancer has spread through the blood and lymph nodes to other parts of the body, such as the lung, liver, abdominal wall, or ovary.
- Stage 4 colon cancer is divided into stage 4A and stage 4B.
- Stage 4A colon cancer: Cancer may have spread through the colon wall and may have spread to nearby organs or lymph nodes. Cancer has spread to one organ that is not near the colon, such as the liver, lung, or ovary, or to a distant lymph node.
- Stage 4B colon cancer: Cancer may have spread through the colon wall and may have spread to nearby organs or lymph nodes. Cancer has spread to more than one organ that is not near the colon or into the lining of the abdominal wall.
- Stage 4 colon cancer is divided into stage 4A and stage 4B.
How is the colon cancer stage determined?
The staging system most often used for colorectal cancer is the American Joint Committee on Cancer (AJCC) TNM system, which is based on 3 key pieces of information:
- The extent (size) of the tumor (T): How far has the cancer grown into the wall of the colon or rectum? These layers, from the inner to the outer, include:
- The inner lining (mucosa), which is the layer in which nearly all colorectal cancers start. This includes a thin muscle layer (muscularis mucosa).
- The fibrous tissue beneath this muscle layer (submucosa)
- A thick muscle layer (muscularis propria)
- The thin, outermost layers of connective tissue (subserosa and serosa) that cover most of the colon but not the rectum
- The spread to nearby lymph nodes (N): Has the cancer spread to nearby lymph nodes?
- The spread (metastasis) to distant sites (M): Has the cancer spread to distant lymph nodes or distant organs such as the liver or lungs?
The system described below is the most recent American Joint Committee on Cancer system effective January 2018. It uses the pathologic stage (also called the surgical stage) which is determined by examining tissue removed during an operation. This is also known as surgical staging. This is likely to be more accurate than clinical staging, which takes into account the results of a physical exam, biopsies, and imaging tests, done before surgery.
Numbers or letters after T, N, and M provide more details about each of these factors. Higher numbers mean the cancer is more advanced. Once a person’s T, N, and M categories have been determined, this information is combined in a process called stage grouping to assign an overall stage.
Cancer staging can be complex, so ask your doctor to explain it to you in a way you understand.
Table 6. Colon cancer stages
| American Joint Committee on Cancer Stage | Stage grouping | Stage description* |
|---|---|---|
| 0 | Tis N0 M0 | The cancer is in its earliest stage. This stage is also known as carcinoma in situ or intramucosal carcinoma (Tis). It has not grown beyond the inner layer (mucosa) of the colon or rectum. Tis = Carcinoma in situ, intramucosal carcinoma (involvement of lamina propria with no extension through muscularis mucosae) N0 = No regional lymph node metastasis. M0 = No distant metastasis by imaging, etc.; no evidence of tumor in distant sites or organs. (This category is not assigned by pathologists.) |
| 1 | T1 or T2 N0 M0 | The cancer has grown through the muscularis mucosa into the submucosa (T1), and it may also have grown into the muscularis propria (T2). It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 2A | T3 N0 M0 | The cancer has grown into the outermost layers of the colon or rectum but has not gone through them (T3). It has not reached nearby organs. It has not spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 2B | T4a N0 M0 | The cancer has grown through the wall of the colon or rectum but has not grown into other nearby tissues or organs (T4a). It has not yet spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 2C | T4b N0 M0 | The cancer has grown through the wall of the colon or rectum and is attached to or has grown into other nearby tissues or organs (T4b). It has not yet spread to nearby lymph nodes (N0) or to distant sites (M0). |
| 3A | T1 or T2 N1/N1c M0 | The cancer has grown through the mucosa into the submucosa (T1), and it may also have grown into the muscularis propria (T2). It has spread to 1 to 3 nearby lymph nodes (N1) or into areas of fat near the lymph nodes but not the nodes themselves (N1c). It has not spread to distant sites (M0). |
| OR | ||
| T1 N2a M0 | The cancer has grown through the mucosa into the submucosa (T1). It has spread to 4 to 6 nearby lymph nodes (N2a). It has not spread to distant sites (M0). | |
| 3B | T3 or T4a, N1/N1c M0 | The cancer has grown into the outermost layers of the colon or rectum (T3) or through the visceral peritoneum (T4a) but has not reached nearby organs. It has spread to 1 to 3 nearby lymph nodes (N1a or N1b) or into areas of fat near the lymph nodes but not the nodes themselves (N1c). It has not spread to distant sites (M0). |
| OR | ||
| T2 or T3 N2a M0 | The cancer has grown into the muscularis propria (T2) or into the outermost layers of the colon or rectum (T3). It has spread to 4 to 6 nearby lymph nodes (N2a). It has not spread to distant sites (M0). | |
| OR | ||
| T1 or T2 N2b M0 | The cancer has grown through the mucosa into the submucosa (T1), and it may also have grown into the muscularis propria (T2). It has spread to 7 or more nearby lymph nodes (N2b). It has not spread to distant sites (M0). | |
| 3C | T4a N2a M0 | The cancer has grown through the wall of the colon or rectum (including the visceral peritoneum) but has not reached nearby organs (T4a). It has spread to 4 to 6 nearby lymph nodes (N2a). It has not spread to distant sites (M0). |
| OR | ||
| T3 or T4a N2b M0 | The cancer has grown into the outermost layers of the colon or rectum (T3) or through the visceral peritoneum (T4a) but has not reached nearby organs. It has spread to 7 or more nearby lymph nodes (N2b). It has not spread to distant sites (M0). | |
| OR | ||
| T4b N1 or N2 M0 | The cancer has grown through the wall of the colon or rectum and is attached to or has grown into other nearby tissues or organs (T4b). It has spread to at least one nearby lymph node or into areas of fat near the lymph nodes (N1 or N2). It has not spread to distant sites (M0). | |
| 4A | Any T Any N M1a | The cancer may or may not have grown through the wall of the colon or rectum (Any T). It might or might not have spread to nearby lymph nodes. (Any N). It has spread to 1 distant organ (such as the liver or lung) or distant set of lymph nodes, but not to distant parts of the peritoneum (the lining of the abdominal cavity) (M1a). |
| 4B | Any T Any N M1b | The cancer might or might not have grown through the wall of the colon or rectum (Any T). It might or might not have spread to nearby lymph nodes (Any N). It has spread to more than 1 distant organ (such as the liver or lung) or distant set of lymph nodes, but not to distant parts of the peritoneum (the lining of the abdominal cavity) (M1b). |
| 4C | Any T Any N M1c | The cancer might or might not have grown through the wall of the colon or rectum (Any T). It might or might not have spread to nearby lymph nodes (Any N). It has spread to distant parts of the peritoneum (the lining of the abdominal cavity), and may or may not have spread to distant organs or lymph nodes (M1c). |
Footnotes: * The following additional categories are not listed in the table above:
- TX: Main tumor cannot be assessed due to lack of information.
- T0: No evidence of a primary tumor.
- NX: Regional lymph nodes cannot be assessed due to lack of information.
Colon cancer stages
Footnote: At its earliest stage (stage 0), colon cancer is limited to the inner lining of your colon. As colon cancer progresses, it can grow through your colon and extend to nearby structures. The most advanced stage of colon cancer (stage IV or 4) indicates cancer has spread to other areas of the body, such as the liver or lungs.
Stage 0 colon cancer (Carcinoma in Situ)
Stage 1 colon cancer
Stage 1 colon cancer means that the cancer has grown through the inner lining of the bowel, or into the muscle wall, but no further. There is no cancer in the lymph nodes. In the TNM staging, stage 1 bowel cancer is the same as:
- T1, N0, M0
- T2, N0, M0
Stage 2 colon cancer
Stage 2 colorectal cancer has spread into the outer wall of the bowel or into tissue or organs next to the bowel. It has not spread to the lymph nodes or distant parts of the body. Stage 2 colon cancer is divided into 3 stages – 2A, 2B and 2C.
- Stage 2A colon cancer means that the cancer has grown into the outer lining of the bowel. In the TNM staging system, stage 2A bowel cancer is the same as T3, N0, M0.
- Stage 2B colon cancer means that the cancer has grown through the outer lining of the bowel into the tissue layer (peritoneum) covering the organs in the abdomen (belly). In the TNM staging system, stage 2B is the same as T4a, N0, M0.
- Stage 2C colon cancer means that the cancer has grown through the wall of the colon or rectum, into organs and tissues next to it. In the TNM staging system, stage 2C bowel cancer is the same as T4b, N0, M0.
Stage 3 colon cancer
Stage 3 bowel cancer has spread to nearby lymph nodes, but hasn’t spread to distant body parts. It’s divided into 3 groups – 3A, 3B and 3C.
- Stage 3A colon cancer means one of the following:
- the cancer is still in the inner or muscle layer of the bowel wall, and it has spread to between 1 to 3 nearby lymph nodes or to areas of tissue close to the lymph nodes
- the cancer is in the inner layer of the bowel wall and has spread to between 4 and 6 nearby lymph nodes
- In TNM staging stage 3A is one of the following: T1 – T2, N1- N1c, M0 or T1, N2a, M0
- Stage 3B colon cancer means one of the following:
- the cancer has grown into the outer lining of the bowel wall or into the tissue layer covering the organs in the abdomen (belly) and it has spread to between 1 and 3 nearby lymph nodes or to areas of fat close to the lymph nodes
- the cancer has grown into the muscle or outer lining of the bowel wall and between 4 to 6 nearby lymph nodes contain cancer
- the cancer is still in the inner or muscle layer of the bowel wall and it has spread to 7 or more nearby lymph nodes
- In TNM staging, stage 3B is one of the following: T3 – T4a, N1-N1c, M0 or T2-T3, N2a, M0 or T1-T2, N2b, M0
- Stage 3C colon cancer means one of the following:
- the cancer has grown through the outer lining of the bowel wall into the tissue layer covering the organs in the abdomen (belly) and between 4 to 6 nearby lymph nodes contain cancer
- the cancer has grown into the outer lining of the bowel wall or into the tissue layer covering the organs in the abdomen and it has spread to 7 or more nearby lymph nodes
- the cancer has grown through the bowel wall into other nearby organs and it has spread to at least one nearby lymph node or to areas of fat close to the lymph nodes
- In the TNM staging, stage 3C the same as one of the following: T4a, N2a, M0 or T3-T4a, N2b, M0 or T4b, N1-N2, M0
Stage 3B colon cancer
Stage 3C colon cancer
Stage 4 colon cancer
Stage 4 colorectal cancer means the cancer has spread to other parts of the body, such as the liver or lungs. Stage 4 colon cancer is also called advanced bowel cancer. Stage 4 bowel cancer is divided into 3 stages – 4A, 4B and 4C.
- Stage 4A colon cancer means that the cancer has spread to 1 distant site or organ, for example the liver, but it hasn’t spread to the tissue lining your abdomen (peritoneum). The cancer spreads through the lymphatic system or the bloodstream. In the TNM staging, stage 4A is the same as any T, any N, M1a
- Stage 4B colon cancer means the cancer has spread to 2 or more distant organs, but it hasn’t spread to the tissue lining your abdomen (peritoneum). In the TNM staging, stage 4B is the same as any T, any N, M1b
- Stage 4C colon cancer means the cancer may have spread to distant organs and it has spread to the lining of your abdomen (peritoneum). In the TNM staging, stage 4C is the same as any T, any N, M1c
Dukes’ staging system
The Dukes staging system is a classification system for colorectal cancer. This system is now mainly of historical interest as it has largely been replaced by the American Joint Committee on Cancer (AJCC) TNM system staging system 19. But you might still hear your doctor talking about your bowel cancer as Dukes’ A,B,C or D.
- Dukes A colon cancer: The cancer is in the inner lining of the bowel. Or it is slightly growing into the muscle layer.
- Dukes B colon cancer: The cancer has grown through the muscle layer of the bowel.
- Dukes C colon cancer: The cancer has spread to at least 1 lymph node close to the bowel.
- Dukes D colon cancer: The cancer has spread to another part of the body, such as the liver, lungs or bones. In the number staging system, this is the same as stage 4 and it’s also called advanced bowel cancer.
Recurrent colon cancer
Recurrent colon cancer is cancer that has recurred (come back) after it has been treated. The cancer may come back in the colon or in other parts of the body, such as the liver, lungs, or both.
Colon cancer survival rate
Survival rates tell you what portion of people with the same type and stage of cancer are still alive a certain amount of time (usually 5 years) after they were diagnosed. They can’t tell you how long you will live, but they may help give you a better understanding about how likely it is that your treatment will be successful. Some people will want to know the survival rates for their cancer type and stage, and some people won’t. If you don’t want to know, you don’t have to.
What is a 5-year survival rate?
Statistics on the outlook for a certain type and stage of cancer are often given as 5-year survival rates, but many people live longer – often much longer – than 5 years. The 5-year survival rate is the percentage of people who live at least 5 years after being diagnosed with cancer. For example, a 5-year survival rate of 90% means that an estimated 90 out of 100 people who have that cancer are still alive 5 years after being diagnosed. Keep in mind, however, that many of these people live much longer than 5 years after diagnosis.
Relative survival rates are a more accurate way to estimate the effect of cancer on survival. These rates compare people with colorectal cancer to people in the overall population. For example, if the 5-year relative survival rate for a specific type and stage of cancer is 90%, it means that people who have that cancer are, on average, about 90% as likely as people who don’t have that cancer to live for at least 5 years after being diagnosed.
But remember, the 5-year relative survival rates are estimates – your outlook can vary based on a number of factors specific to you.
Cancer survival rates don’t tell the whole story
Survival rates are often based on previous outcomes of large numbers of people who had the disease, but they can’t predict what will happen in any particular person’s case. There are a number of limitations to remember:
- The numbers below are among the most current available. But to get 5-year survival rates, doctors have to look at people who were treated at least 5 years ago. As treatments are improving over time, people who are now being diagnosed with colorectal cancer may have a better outlook than these statistics show.
- These statistics are based on the stage of the cancer when it was first diagnosed. They do not apply to cancers that later come back or spread, for example.
- The outlook for people with colorectal cancer varies by the stage (extent) of the cancer – in general, the survival rates are better for people with earlier stage cancers. But many other factors can affect a person’s outlook, such as age and overall health, and how well the cancer responds to treatment. The outlook for each person is specific to his or her circumstances.
Your doctor can tell you how these numbers may apply to you, as he or she is familiar with your particular situation.
Colon cancer survival rates, by stage
The numbers below come from the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) database, looking at people diagnosed with colon cancer between 2012 and 2018. The National Cancer Institute’s SEER database does not group cancers according to the American Joint Committee on Cancer TNM stages (stage 1, stage 2, stage 3, etc.). Instead, it groups cancers into localized, regional, and distant stages 2:
- Localized: There is no sign that the cancer has spread outside of the colon or rectum.
- Regional: The cancer has spread outside the colon or rectum to nearby structures or lymph nodes.
- Distant: The cancer has spread to distant parts of the body such as the liver, lungs, or distant lymph nodes.
In general, if the cancer is found only in the part of the body where it started it is localized (sometimes referred to as stage 1). If it has spread to a different part of the body, the stage is regional or distant. The earlier colorectal cancer is caught, the better chance a person has of surviving five years after being diagnosed. For colorectal cancer, 37.2% are diagnosed at the local stage. The 5-year relative survival for localized colorectal cancer is 90.9%.
Remember, these survival rates are only estimates – they can’t predict what will happen to any individual person. We understand that these statistics can be confusing and may lead you to have more questions. Talk to your doctor to better understand your specific situation.
Table 7. 5-year relative survival rates for colon cancer
| National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) stage | 5-year relative survival rate |
|---|---|
| Localized | 90.9% |
| Regional | 72.8% |
| Distant | 15.1% |
| All SEER stages combined | 64.00% |
Footnote: SEER 2012–2018, All Races, Both Sexes by SEER Combined Summary Stage
Table 8. 5-year relative survival rates for rectal cancer
| National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) stage | 5-year relative survival rate |
|---|---|
| Localized | 90% |
| Regional | 73% |
| Distant | 17% |
| All SEER stages combined | 67% |
Footnotes:
- These numbers apply only to the stage of the cancer when it is first diagnosed. They do not apply later on if the cancer grows, spreads, or comes back after treatment.
- These numbers don’t take everything into account. Survival rates are grouped based on how far the cancer has spread, but your age, overall health, how well the cancer responds to treatment, whether the cancer started on the left or right side of the colon, and other factors can also affect your outlook.
- People now being diagnosed with colon or rectal cancer may have a better outlook than these numbers show. Treatments improve over time, and these numbers are based on people who were diagnosed and treated at least five years earlier.
These survival rates are only estimates – they can’t predict what will happen to any individual person. We understand that these statistics can be confusing and may lead you to have more questions. Talk to your doctor to better understand your specific situation.
[Source 20 ]Colon cancer treatment
The type of treatment your doctor recommends will depend largely on the location of your cancer, its stage and your other health concerns. Treatment for colon cancer usually involves surgery to remove the cancer. Other treatments, such as radiation therapy and chemotherapy, might also be recommended.
Colon cancer main treatments are:
- Surgery – the cancerous section of bowel is removed; it’s the most effective way of curing bowel cancer and in many cases is all you need
- Chemotherapy – where medicine is used to kill cancer cells
- Radiotherapy – where radiation is used to kill cancer cells
- Targeted therapies – a newer group of medicines that increases the effectiveness of chemotherapy and prevents the cancer spreading
- Immunotherapy. Immunotherapy is the use of medicines to help a person’s own immune system better recognize and destroy cancer cells. Immunotherapy can be used to treat some people with advanced colorectal cancer.
- Ablation and embolization therapy. When colon or rectal cancer has spread and there are a few small tumors in the liver or lungs, these metastases can sometimes be removed by surgery or destroyed by other techniques, such as ablation or embolization.
As with most types of cancer, the chance of a complete cure depends on how far your cancer spread by the time it’s diagnosed. If your cancer is confined to the bowel, surgery is usually able to completely remove it.
Keyhole or robotic surgery is being used more often, which allows surgery to be performed with less pain and a quicker recovery.
Surgery for early-stage colon cancer
If your colon cancer is very small, your doctor may recommend a minimally invasive approach to surgery, such as:
- Removing polyps (polypectomy) during a colonoscopy. If your cancer is small, localized and completely contained within a polyp and in a very early stage, your doctor may be able to remove it completely during a colonoscopy. This is usually done by passing a wire loop through the colonoscope to cut the polyp off the wall of the colon with an electric current.
- Endoscopic mucosal resection. Removing larger polyps may require also taking a small amount of the lining of the colon or rectum in a procedure called an endoscopic mucosal resection.
- Minimally invasive surgery. Polyps that can’t be removed during a colonoscopy may be removed using laparoscopic surgery. In this procedure, your surgeon performs the operation through several small incisions in your abdominal wall, inserting instruments with attached cameras that display your colon on a video monitor. The surgeon may also take samples from lymph nodes in the area where the cancer is located.
Surgery for invasive colon cancer
If the cancer has grown into or through your colon, your surgeon may recommend:
- Partial colectomy also called hemicolectomy or segmental resection. During this procedure, the surgeon removes the part of your colon that contains the cancer, along with a margin of normal tissue on either side of the cancer. Your surgeon is often able to reconnect the healthy portions of your colon or rectum. This procedure can commonly be done by a minimally invasive approach (laparoscopy).
- Surgery to create a way for waste to leave your body. When it’s not possible to reconnect the healthy portions of your colon or rectum, you may need an ostomy. This involves creating an opening in the wall of your abdomen from a portion of the remaining bowel for the elimination of stool into a bag that fits securely over the opening. Sometimes the ostomy is only temporary, allowing your colon or rectum time to heal after surgery. In some cases, however, the colostomy may be permanent. Sometimes the end of the small intestine (the ileum) instead of the colon is connected to a stoma in the skin. This is called an ileostomy.
- Lymph node removal. Nearby lymph nodes are usually also removed during colon cancer surgery and tested for cancer. At least 12 nearby lymph nodes are also removed so they can be checked for cancer.
- If all of the colon is removed, it’s called a total colectomy. Total colectomy isn’t often needed to remove colon cancer. It’s mostly used only if there’s another problem in the part of the colon without cancer, such as hundreds of polyps (in someone with familial adenomatous polyposis) or, sometimes, inflammatory bowel disease.
Side effects of colon surgery
Possible risks and side effects of surgery depend on several factors, including the extent of the operation and your general health before surgery. Problems during or shortly after the operation can include bleeding, infection, and blood clots in the legs.
When you wake up after surgery, you will have some pain and will need pain medicines for a few days. For the first couple of days, you may not be able to eat or you may be allowed limited liquids, as the colon needs some time to recover. Most people are able to eat solid food in a few days.
Sometimes after colon surgery, the bowel takes longer than normal to “wake up” and start working again after the surgery. This is called an ileus. It might be caused by the anesthesia or the actual handling of the bowel during the operation. Sometimes, too much pain medicine after the surgery can slow down the bowel function. If you develop an ileus, your doctor may want to delay eating solid food or even liquids, especially if you are having nausea and/or vomiting. More tests might also be done to make sure that the situation is not more serious.
Rarely, the new connections between the ends of the colon may not hold together and may leak. This can quickly cause severe pain, fever, and the belly to feel very hard. A smaller leak may cause you to not pass stool, have no desire to eat, and not do well or recover after surgery. A leak can lead to infection, and more surgery may be needed to fix it. It’s also possible that the incision (cut) in the abdomen (belly) might open up, becoming an open wound that may need special care as it heals.
After the surgery, you might develop scar tissue in your abdomen that can cause organs or tissues to stick together. These are called adhesions. Normally your intestines freely slide around inside your belly. In rare cases, adhesions can cause the bowels to twist up and can even block the bowel. This causes pain and swelling in the belly that’s often worse after eating. Further surgery may be needed to remove the scar tissue.
Surgery for advanced cancer
If your cancer is very advanced or your overall health very poor, your surgeon may recommend an operation to relieve a blockage of your colon or other conditions in order to improve your symptoms. This surgery isn’t done to cure cancer, but instead to relieve signs and symptoms, such as bleeding and pain.
Some patients have colon cancer that has spread to other parts of the body and also have tumors blocking the colon. In this case, surgery may be done to relieve the blockage without removing the part of the colon containing the cancer. Instead, the colon is cut above the tumor and attached to a stoma (an opening in the skin of the abdomen) to allow stool to come out. This is called a diverting colostomy. It can often help the patient recover enough to start other treatments (such as chemotherapy). It might also be done in cases where the cancer has not spread to distant areas.
If the cancer has spread to only one or a few spots (nodules) in the lungs or liver (and apparently nowhere else), surgery may be used to remove it. In most cases, this is only done if the cancer in the colon is also being removed (or was already removed). Depending on the extent of the cancer, this might help the patient live longer, or it could even cure the cancer. Deciding if surgery is an option to remove areas of cancer spread depends on their size, number, and location.
Chemotherapy
Chemotherapy uses drugs to destroy cancer cells. Chemotherapy for colon cancer is usually given after surgery (adjuvant chemo) if the cancer has spread to lymph nodes. In this way, chemotherapy may help reduce the risk of cancer recurrence and death from cancer. Sometimes chemotherapy may be used before surgery (neoadjuvant chemo) as well, with the goal of shrinking the cancer before an operation. Chemotherapy before surgery is more common in rectal cancer than in colon cancer.
Chemotherapy can also be given to relieve symptoms of colon cancer that has spread to other areas of the body.
Chemotherapy (chemo) may be used at different times during treatment for colorectal cancer:
- Adjuvant chemo is given after surgery. The goal is to kill cancer cells that might have been left behind at surgery because they were too small to see, as well as cancer cells that might have escaped from the main colon or rectal cancer to settle in other parts of the body but are too small to see on imaging tests. This helps lower the chance that the cancer will come back.
- Neoadjuvant chemo is given (sometimes with radiation) before surgery to try to shrink the cancer and make it easier to remove. This is often done for rectal cancer.
- For advanced cancers that have spread to other organs like the liver, chemo can be used to help shrink tumors and ease problems they’re causing. While it’s not likely to cure the cancer, this often helps people feel better and live longer.
Chemotherapy drugs used to treat colorectal cancer include:
- 5-Fluorouracil (5-FU)
- Capecitabine (Xeloda), a pill that is changed into 5-FU once it gets to the tumor.
- Irinotecan (Camptosar)
- Oxaliplatin (Eloxatin)
- Trifluridine and tipiracil (Lonsurf), a combination drug in pill form
Most often, combinations of 2 or 3 of these drugs are used. Sometimes, chemo drugs are given along with a targeted therapy drug.
Chemo drugs attack cells that are dividing quickly, which is why they work against cancer cells. But other cells in the body, such as those in hair follicles and in the lining of the mouth and intestines, are also dividing quickly. These cells can be affected by chemo too, which can lead to side effects.
The side effects of chemo depend on the type and dose of drugs given and how long you take them. Common side effects of chemo can include:
- Hair loss
- Mouth sores
- Loss of appetite or weight loss
- Nausea and vomiting
- Diarrhea
- Nail changes
- Skin changes
Chemo can also affect the blood-forming cells of the bone marrow, which can lead to:
- Increased chance of infections (from low white blood cell counts)
- Easy bruising or bleeding (from low blood platelet counts)
- Fatigue (from low red blood cell counts and other reasons)
Other side effects are specific to certain drugs. Ask your cancer care team about the possible side effects of the specific drugs you are getting. For example:
- Hand-foot syndrome can develop during treatment with capecitabine or 5-FU (when given as an infusion). It can start out as redness in the hands and feet, and then might progress to pain and sensitivity in the palms and soles. If it worsens, the skin may blister or peel, sometimes leading to painful sores. It’s important to tell your doctor right away about any early symptoms, such as redness or sensitivity, so that steps can be taken to keep things from getting worse.
- Neuropathy (nerve damage) is a common side effect of oxaliplatin. Symptoms include numbness, tingling, and even pain in the hands and feet. It can also cause intense sensitivity to cold in your throat, esophagus (the tube connecting the throat to the stomach), and the palms of your hands. This can cause pain when swallowing cold liquids or holding a cold glass. If you’ll be getting oxaliplatin, talk with your doctor about side effects beforehand, and let them know right away if you develop numbness and tingling or other side effects.
- Allergic or sensitivity reactions can happen in some people while getting the drug oxaliplatin. Symptoms can include skin rash; chest tightness and trouble breathing; back pain; or feeling dizzy, lightheaded, or weak. Be sure to tell your nurse right away if you notice any of these symptoms while you’re getting chemo.
- Diarrhea is a common side effect with many of these chemo drugs, but can be particularly bad with irinotecan. It needs to be treated right away — at the first loose stool — to prevent severe dehydration. This often means taking a drug like loperamide (Imodium). If you’re getting a chemo drug that will likely cause diarrhea, your doctor will give you instructions on what drugs to take and how often to take them to control this problem.
Most of these side effects tend to go away over time after treatment ends. Some, such as hand and foot numbness from oxaliplatin, may last for a long time. There are often ways to lessen these side effects. For example, you can be given drugs to help prevent or reduce nausea and vomiting or you may be told to keep ice chips in your mouth while chemo is being given to lower the chances of getting mouth sores.
Be sure to discuss any questions about side effects with your cancer care team. Also report any side effects or changes you notice while getting chemo so that they can be treated right away. In some cases, the doses of the chemo drugs may need to be reduced or treatment may need to be delayed or stopped to help keep the problem from getting worse.
Older people seem to be able to tolerate some types of chemo for colon or rectal cancer fairly well. Age is no reason to withhold treatment in otherwise healthy people.
Radiation therapy
Radiation therapy uses powerful energy sources, such as X-rays, to kill cancer cells, to shrink large tumors before an operation so that they can be removed more easily, or to relieve symptoms of colon cancer and rectal cancer. Radiation therapy either alone or combined with chemotherapy (chemoradiation) is one of the standard treatment options for the initial management of rectal cancer followed by surgery.
Different types of radiation therapy can be used to treat colon and rectal cancers.
- External-beam radiation therapy (EBRT). External-beam radiation therapy (EBRT) is the type of radiation therapy used most often for people with colon or rectal cancer. The radiation is focused on the cancer from a machine outside the body. It’s a lot like getting an x-ray, but the radiation is more intense. How often and how long a person gets radiation treatments depends on the reason the radiation is being given and other factors. Treatments might be given over the course of a few days or several weeks. Newer EBRT techniques, such as three-dimensional conformal radiation therapy (3D-CRT), intensity modulated radiation therapy (IMRT), and stereotactic body radiation therapy (SBRT), have been shown to help doctors treat colorectal cancers that have spread to the lungs or liver more accurately while lowering the radiation exposure to nearby healthy tissues. They are typically used if there is only a small number of tumors and if the tumor is causing symptoms and surgery is not an option.
- Internal radiation therapy (brachytherapy). Brachytherapy might be used to treat some rectal cancers, but more research is needed to understand how to best use and when to use brachytherapy. For this treatment, a radioactive source is put inside your rectum next to or into the tumor. This allows the radiation to reach the rectum without passing through the skin and other tissues of the belly (abdomen), so it’s less likely to damage nearby tissues.
- Endocavitary radiation therapy: For this treatment, a small balloon-like device is placed into the rectum to deliver high-intensity radiation for a few minutes. This is typically done in 4 treatments (or less), with about 2 weeks between each treatment. This can let some patients, particularly elderly patients, avoid major surgery and a colostomy. This type of treatment is used for some small rectal cancers or in cases where radiation was already given in the pelvic area and the rectal cancer has come back. Sometimes external-beam radiation therapy is also given.
- Interstitial brachytherapy: For this treatment, a tube is placed into the rectum and right into the tumor. Small pellets of radioactive material are then put into the tube for several minutes. The radiation travels only a short distance, limiting the harmful effects on nearby healthy tissues. It’s sometimes used to treat people with rectal cancer who are not healthy enough for surgery or have cancer that has come back in the rectum. This can be done a few times a week for a couple of weeks, but it can also be just a one-time procedure.
- Radioembolization. Radiation can also be given during an embolization procedure.
Possible side effects of radiation therapy
If you’re going to get radiation therapy, it’s important to ask your doctor about the possible short- and long-term side effects so that you know what to expect. Possible side effects of radiation therapy for colon and rectal cancer can include:
- Skin irritation at the site where radiation beams were aimed, which can range from redness to blistering and peeling
- Problems with wound healing if radiation was given before surgery
- Nausea
- Rectal irritation, which can cause diarrhea, painful bowel movements, or blood in the stool
- Bowel incontinence (stool leakage)
- Bladder irritation, which can cause problems like feeling like you have to go often (called frequency), burning or pain while urinating, or blood in the urine
- Fatigue/tiredness
- Sexual problems (erection issues in men and vaginal irritation in women)
- Scarring, fibrosis (stiffening), and adhesions that cause the tissues in the treated area to stick to each other
Most side effects should get better over time after treatment ends, but some problems may not go away completely. If you notice any side effects, talk to your doctor right away so steps can be taken to reduce or relieve them.
Radiation for colon cancer
It’s not common to use radiation therapy to treat colon cancer, but it may be used in certain cases:
- Before surgery (along with chemo) to help shrink a tumor and make it easier to remove.
- After surgery, if the cancer has attached to an internal organ or the lining of the belly (abdomen). If this happens, the surgeon can’t be sure that all of the cancer has been removed. Radiation therapy may be used to try to kill any cancer cells that may have been left behind.
- During surgery, right to the area where the cancer was, to kill any cancer cells that may be left behind. This is called intraoperative radiation therapy or IORT.
- Along with chemo to help control cancer if a person is not healthy enough for surgery.
- To ease symptoms if advanced colon cancer is causing intestinal blockage, bleeding, or pain.
- To help treat colon cancer that has spread to other areas, such as the bones, lungs, or brain.
Radiation for rectal cancer
For rectal cancer, radiation therapy is a more common treatment and may be used:
- Either before and/or after surgery, often along with chemotherapy, to help keep the cancer from coming back. Many doctors now favor giving radiation therapy before surgery, as it may make it easier to remove the cancer, especially if the cancer’s size and/or location might make surgery difficult. This is called neoadjuvant treatment. Giving chemoradiation before surgery can also help lower the chances of damaging the sphincter muscles in the rectum when surgery is done. In either case, nearby lymph nodes are usually treated too.
- During surgery, right to the area where the tumor was, to kill any rectal cancer cells that may be left behind. This is called intraoperative radiation therapy or IORT.
- With or without chemo to help control rectal cancer if a person is not healthy enough for surgery or to ease symptoms if advanced rectal cancer is causing intestinal blockage, bleeding, or pain.
- To retreat rectal tumors that come back in the pelvis after radiation was given.
- To help treat rectal cancer that has spread to other areas, such as the bones, lungs, or brain.
Targeted drug therapy
Drugs that target specific malfunctions that allow cancer cells to grow are available to people with advanced colon cancer, including:
- Bevacizumab (Avastin)
- Cetuximab (Erbitux)
- Panitumumab (Vectibix)
- Ramucirumab (Cyramza)
- Regorafenib (Stivarga)
- Ziv-aflibercept (Zaltrap)
Targeted drugs can be given along with chemotherapy or alone. Targeted drugs are typically reserved for people with advanced colon cancer.
Some people are helped by targeted drugs, while others are not. Researchers have recently made progress in determining who is most likely to benefit from specific targeted drugs. Until more is known, doctors carefully weigh the possible benefit of targeted drugs against the risk of side effects and the cost when deciding whether to use these treatments.
Drugs that target blood vessel formation (VEGF)
Vascular endothelial growth factor (VEGF) is a protein that helps tumors form new blood vessels (a process known as angiogenesis) to get nutrients they need to grow. Drugs that stop VEGF from working can be used to treat some colon or rectal cancers. These include:
- Bevacizumab (Avastin)
- Ramucirumab (Cyramza)
- Ziv-aflibercept (Zaltrap)
These drugs are given as infusions into your vein (IV) every 2 or 3 weeks, in most cases along with chemotherapy. When combined with chemo, these drugs can often help people with advanced colon or rectal cancers live longer.
Possible side effects of drugs that target vascular endothelial growth factor (VEGF)
Common side effects of these drugs include:
- High blood pressure
- Extreme tiredness (fatigue)
- Bleeding
- Low white blood cell counts (with increased risk of infections)
- Headaches
- Mouth sores
- Loss of appetite
- Diarrhea
Rare but possibly serious side effects include blood clots, severe bleeding, holes forming in the colon (called perforations), heart problems, kidney problems, and slow wound healing. If a hole forms in the colon it can lead to severe infection and surgery may be needed to fix it.
Another rare but serious side effect of these drugs is an allergic reaction during the infusion, which could cause problems with breathing and low blood pressure.
Drugs that target cancer cells with EGFR changes
Epidermal growth factor receptor (EGFR) is a protein that helps cancer cells grow. Drugs that target EGFR can be used to treat some advanced colon or rectal cancers. These include:
- Cetuximab (Erbitux)
- Panitumumab (Vectibix)
Both of these drugs are given by IV infusion, either once a week or every other week.
These drugs typically don’t work by themselves in colorectal cancers that have mutations (defects) in the KRAS, NRAS or BRAF gene. Doctors commonly test the tumor for these gene changes before treatment, and only use these drugs in people who don’t have these mutations. One exception to this is when cetuximab is combined with the BRAF inhibitor encorafenib (see below). The combination of these two drugs appears to help people with advanced colorectal cancer live longer, even with one of these mutations.
Possible side effects of drugs that target epidermal growth factor receptor (EGFR)
The most common side effects of these drugs are skin problems such as an acne-like rash on the face and chest during treatment, which can sometimes lead to infections. An antibiotic cream or ointment may be needed to help limit the rash and related infections. Developing this rash often means the cancer is responding to treatment. People who develop this rash often live longer, and those who develop more severe rashes also seem to respond better than those with a milder rash. Other side effects can include:
- Headache
- Tiredness
- Fever
- Diarrhea
A rare but serious side effect of these drugs is an allergic reaction during the infusion, which could cause problems with breathing and low blood pressure. You may be given medicine before treatment to help prevent this.
Drugs that target cells with BRAF gene changes
Fewer than 10% of colorectal cancers have changes (mutations) in the BRAF gene. Colorectal cancer cells with these changes make an abnormal BRAF protein that helps them grow. Some drugs target this abnormal BRAF protein.
If you have colorectal cancer that has spread, your cancer will likely be tested to see if there is an abnormal BRAF gene. Drugs that target the abnormal BRAF protein (BRAF inhibitors) aren’t likely to work on colorectal cancers that have a normal BRAF gene.
BRAF inhibitors
Encorafenib (Braftovi) is a drug that attacks the abnormal BRAF protein directly.
This drug, when given with cetuximab (see above), can shrink or slow the growth of colorectal cancer in some people whose cancer has spread. The combination of these two drugs also appears to help people with advanced colorectal cancer live longer.
This drug is taken as pills or capsules, once a day.
Common side effects of encorafenib with cetuximab can include skin thickening, diarrhea, rash, loss of appetite, abdominal pain, joint pain, fatigue, and nausea.
Some people treated with a BRAF inhibitor might develop new squamous cell skin cancers. These cancers can often be treated by removing them. Still, your doctor will want to check your skin regularly during treatment and for several months afterward. You should also let your doctor know right away if you notice any new growths or abnormal areas on your skin.
Kinase inhibitor
Regorafenib (Stivarga) is a type of targeted therapy known as a kinase inhibitor. Kinases are proteins on or near the surface of a cell that carry important signals to the cell’s control center. Regorafenib blocks several kinase proteins that either help tumor cells grow or help form new blood vessels to feed the tumor. Blocking these proteins can help stop the growth of cancer cells.
This drug is used to treat advanced colorectal cancer, typically when other drugs are no longer helpful. It’s taken as a pill.
Common side effects include fatigue, rash, hand-foot syndrome (redness and irritation of the hands and feet), diarrhea, high blood pressure, weight loss, and abdominal pain.
Less common but more serious side effects can include severe bleeding or perforations (holes) in the stomach or intestines.
Immunotherapy
Some patients with advanced colon cancer have a chance to benefit from immunotherapy with antibodies such as pembrolizumab (Keytruda) and nivolumab (Opdivo). Whether a colon cancer has the chance to respond to these immunotherapies can be determined by a specific test of the tumor tissue.
An important part of the immune system is its ability to keep itself from attacking the body’s normal cells. To do this, it uses “checkpoints” – proteins on immune cells that need to be turned on (or off) to start an immune response. Colorectal cancer cells sometimes use these checkpoints to avoid being attacked by the immune system. Drugs that target these checkpoints (immune checkpoint inhibitors) help to restore the immune response against colorectal cancer cells.
Drugs called checkpoint inhibitors can be used for people whose colorectal cancer cells have tested positive for specific gene changes, such as a high level of microsatellite instability (MSI-H), or changes in one of the mismatch repair (MMR) genes. These drugs might be used to treat people whose cancer can’t be removed with surgery, has come back (recurred) after treatment, or has spread to other parts of the body (metastasized).
Pembrolizumab (Keytruda) and nivolumab (Opdivo)
- Pembrolizumab (Keytruda) and nivolumab (Opdivo) are drugs that target PD-1, a protein on immune system cells called T cells that normally help keep these cells from attacking other cells in the body. By blocking PD-1, these drugs boost the immune response against cancer cells.
- Pembrolizumab can be used as the first treatment for people with advanced or metastatic colorectal cancer. It is given as an intravenous (IV) infusion every 3 or 6 weeks.
- Nivolumab can be used alone or with ipilimumab (see below) for people with metastatic colorectal cancer that has grown after treatment with chemotherapy. It is typically given by itself as an IV infusion every 2 or 4 weeks. If it is used along with ipilimumab, then it is given every 3 weeks.
Ipilimumab (Yervoy)
- Ipilimumab (Yervoy) is another drug that boosts the immune response, but it has a different target. It blocks CTLA-4, another protein on T cells that normally helps keep them in check.
- This drug can be used along with nivolumab (Opdivo) to treat colorectal cancer, but it’s not used alone. It is given as an intravenous (IV) infusion, usually once every 3 weeks for 4 treatments.
Possible side effects of immunotherapy
Side effects of these drugs include fatigue, cough, nausea, diarrhea, skin rash, loss of appetite, constipation, joint pain, and itching.
Other, more serious side effects occur less often.
- Infusion reactions: Some people might have an infusion reaction while getting these drugs. This is like an allergic reaction, and can include fever, chills, flushing of the face, rash, itchy skin, feeling dizzy, wheezing, and trouble breathing. It’s important to tell your doctor or nurse right away if you have any of these symptoms while getting these drugs.
- Autoimmune reactions: These drugs work by basically removing one of the safeguards on the body’s immune system. Sometimes the immune system starts attacking other parts of the body, which can cause serious or even life-threatening problems in the lungs, intestines, liver, hormone-making glands, nerves, skin, kidney, or other organs.
It’s very important to report any new side effects during or after treatment with any of these drugs to your health care team promptly. If serious side effects do occur, you may need to stop treatment and take high doses of corticosteroids to suppress your immune system.
Ablation and embolization therapy
When colon or rectal cancer has spread and there are a few small tumors in the liver or lungs, these metastases can sometimes be removed by surgery or destroyed by other techniques, such as ablation or embolization.
When all of the primary cancer in the colon or rectum can be removed with surgery, ablation or embolization might be used to destroy small tumors in other places in the body
Ablation and embolization might also be good options for people whose metastatic tumors come back after surgery, whose cancer can’t be cured with surgery, or who can’t have surgery for other reasons. This might help a person live longer. It can also help treat problems the cancer is causing, like pain.
In most cases, patients don’t need to stay in the hospital for these treatments.
Ablation
Ablation techniques are used to destroy small (less than 4 cm across) tumors instead of removing them with surgery. There are many different types of ablation techniques. They can be used to treat tumors in other places, too.
- Radiofrequency ablation (RFA). Radiofrequency ablation (RFA) is one of the most common methods to treat cancer that has spread to the liver. It uses high-energy radio waves to kill cancer cells. A CT scan or ultrasound is used to guide a thin, needle-like probe through the skin and into the tumor. An electric current is then sent to the tip of the probe, releasing high-frequency radio waves that heat the tumor and destroy the cancer cells.
- Microwave ablation (MWA). Microwave ablation method is used to treat cancer that has spread to the liver. Imaging tests are used to guide a needle-like probe into the tumor. Electromagnetic microwaves are then sent through it to create high temperatures that kill the cancer quickly. This treatment has been used to treat larger cancers (up to 6 cm across).
- Ethanol (alcohol) ablation also known as percutaneous ethanol injection (PEI). In this technique, concentrated alcohol is injected right into the tumor to damage cancer cells. This is usually done through the skin using a needle, which is guided by ultrasound or CT scans. Sometimes multiple treatments of alcohol ablation may be needed to treat the whole tumor.
- Cryosurgery (cryotherapy or cryoablation). Cryosurgery destroys the tumor by freezing it with a thin metal probe. The probe is guided through the skin and into the tumor using ultrasound. Then very cold gas is passed through the end of the probe to freeze the tumor, killing the cancer cells. This method can treat larger tumors than the other ablation techniques, but sometimes general anesthesia (drugs used to put the patient into a deep sleep) is needed. Treatment can be repeated as needed to kill all the cancer cells.
Side effects of ablation therapy
Possible side effects after ablation therapy include:
- Abdominal (belly) pain
- Infection in the liver
- Fever
- Bleeding into the chest cavity or abdomen
- Abnormal liver tests.
Serious complications are rare, but they are possible.
Embolization
Embolization is used to treat tumors in the liver. In an embolization procedure, a substance is injected directly into an artery in the liver to block or reduce the blood flow to the tumor.
The liver is special in that it has 2 blood supplies. Most normal liver cells get blood from the portal vein, but cancer cells in the liver usually get their blood supply from the hepatic artery. Blocking the part of the hepatic artery that feeds the tumor helps kill the cancer cells, but it leaves most of the healthy liver cells unharmed because they get their blood supply from the portal vein.
Embolization can be used to treat tumors larger than 5cm (about 2 inches) across that are often too big to be treated with ablation. It can also be used along with ablation. Embolization does reduce some of the blood supply to the normal liver tissue, so it may not be a good option for patients with liver damage from diseases like hepatitis or cirrhosis.
There are 3 main types of embolization procedures used to treat colon or rectal cancer that has spread (metastasized) to the liver:
- Arterial embolization is also called trans-arterial embolization or TAE. In this procedure a catheter (a thin, flexible tube) is put into an artery through a small cut in the inner thigh and eased up into the hepatic artery in the liver. A dye is usually injected into the blood to help the doctor watch the path of the catheter using x-ray pictures. Once the catheter is in the right place, small particles are injected into the artery to plug it up, blocking oxygen and key nutrients from the cancer.
- Chemoembolization also called trans-arterial chemoembolization or TACE, combines arterial embolization with chemotherapy. TACE is done by giving chemotherapy through a catheter that’s put right into the artery that feeds the tumor, then plugging up the artery so the chemo can stay close to the tumor. Multiple treatments may be given over 4 to 6 weeks.
- Radioembolization combines embolization and radiation therapy. This is done by injecting tiny beads (called microspheres) coated with radioactive yttrium-90 (Y-90) into the hepatic artery. The beads lodge in the blood vessels near the tumor where they give off small amounts of radiation to the tumor site for several days. The radiation travels a very short distance, so its effects are limited mainly to the tumor.
Possible side effects of embolization
Possible side effects after embolization include:
- Belly (abdominal) pain
- Fever
- Nausea
- Infection in the liver
- Gallbladder inflammation
- Blood clots in the main blood vessels of the liver
- Abnormal liver tests
Because healthy liver tissue can be affected, there is a risk that liver function will get worse after embolization. This risk is higher if a large branch of the hepatic artery is embolized. Serious complications are not common, but they are possible.
Follow-up
Limited data and no level 1 evidence (evidence obtained from at least one properly designed randomized controlled trial) are available to guide patients and physicians about surveillance and management of patients after surgical resection and adjuvant therapy. The American Society of Clinical Oncology and the National Comprehensive Cancer Network recommend specific surveillance and follow-up strategies 21.
Following treatment of colon cancer, periodic evaluations may lead to the earlier identification and management of recurrent disease 22. The impact of such monitoring on overall mortality of patients with recurrent colon cancer, however, is limited by the relatively small proportion of patients in whom localized, potentially curable metastases are found. To date, no large-scale randomized trials have documented an overall survival benefit for standard, postoperative monitoring programs 23.
CEA is a serum glycoprotein frequently used in the management of patients with colon cancer. A review of the use of this tumor marker suggests the following 24:
- A CEA level is not a valuable screening test for colorectal cancer because of the large numbers of false-positive and false-negative reports.
- Postoperative CEA testing should be restricted to patients who would be candidates for resection of liver or lung metastases.
- Routine use of CEA levels alone for monitoring response to treatment should not be recommended.
The optimal regimen and frequency of follow-up examinations are not well defined because the impact on patient survival is not clear and the quality of data is poor 23.
Supportive (palliative) care
Palliative care is specialized medical care that focuses on providing relief from pain and other symptoms of a serious illness. Palliative care specialists work with you, your family and your other doctors to provide an extra layer of support that complements your ongoing care.
When palliative care is used along with all of the other appropriate treatments, people with cancer may feel better and live longer.
Palliative care is provided by a team of doctors, nurses and other specially trained professionals. Palliative care teams aim to improve the quality of life for people with cancer and their families. This form of care is offered alongside curative or other treatments you may be receiving.
Coping and support
A cancer diagnosis can be emotionally challenging. In time, people learn to cope in their own unique ways. Until you find what works for you, you might try to:
Know what to expect. Learn enough about your cancer to feel comfortable making treatment decisions.
Ask your doctor to tell you the type and stage of your cancer, as well as your treatment options and their side effects. The more you know, the more confident you’ll be when it comes to making decisions about your own care. Look for information in your local library and on reliable websites.
Keep friends and family close. Keeping your close relationships strong will help you deal with cancer. Friends and family can provide the practical support you’ll need, such as helping take care of your house if you’re in the hospital. And they can serve as emotional support when you feel overwhelmed by cancer.
Find someone to talk with. Find a good listener who is willing to listen to you talk about your hopes and fears. This may be a friend or family member. The concern and understanding of a counselor, medical social worker, clergy member or cancer support group also may be helpful.
Ask your doctor about support groups in your area. Or check your phone book, library or a cancer organization, such as the National Cancer Institute or the American Cancer Society.
Colon cancer treatment by Stage
Treatment for colon cancer is based largely on the stage (extent) of the cancer, but other factors can also be important.
People with colon cancers that have not spread to distant sites usually have surgery as the main or first treatment. Chemotherapy may also be used after surgery (called adjuvant treatment). Most adjuvant treatment is given for about 6 months.
Stage 0 colon cancer treatment
Since stage 0 colon cancers have not grown beyond the inner lining of the colon, surgery to take out the cancer is often the only treatment needed. In most cases this can be done by removing the polyp or taking out the area with cancer through a colonoscope (local excision). Removing part of the colon (partial colectomy) may be needed if a cancer is too big to be removed by local excision.
Stage 1 colon cancer treatment
Stage 1 colon cancers have grown deeper into the layers of the colon wall, but they have not spread outside the colon wall itself or into the nearby lymph nodes. Stage 1 bowel cancer includes cancers that were part of a polyp. If the polyp is removed completely during colonoscopy, with no cancer cells at the edges (margins) of the removed piece, no other treatment may be needed.
If the cancer in the polyp is high grade , or there are cancer cells at the edges of the polyp, more surgery might be recommended. You might also be advised to have more surgery if the polyp couldn’t be removed completely or if it had to be removed in many pieces, making it hard to see if cancer cells were at the edges.
For cancers not in a polyp, partial colectomy ─ surgery to remove the section of colon that has cancer and nearby lymph nodes ─ is the standard treatment. You typically won’t need any more treatment.
Stage 2 colon cancer treatment
Many stage 2 colon cancers have grown through the wall of the colon, and maybe into nearby tissue, but they have not spread to the lymph nodes.
Surgery to remove the section of the colon containing the cancer (partial colectomy) along with nearby lymph nodes may be the only treatment needed. But your doctor may recommend adjuvant chemotherapy (chemo after surgery) if your cancer has a higher risk of coming back (recurring) because of certain factors, such as:
- The cancer looks very abnormal (is high grade) when viewed closely in the lab.
- The cancer has grown into nearby blood or lymph vessels.
- The surgeon did not remove at least 12 lymph nodes.
- Cancer was found in or near the margin (edge) of the removed tissue, meaning that some cancer may have been left behind.
- The cancer had blocked (obstructed) the colon.
- The cancer caused a perforation (hole) in the wall of the colon.
The doctor might also test your tumor for specific gene changes, called MSI or MMR, to help decide if adjuvant chemotherapy would be helpful.
Not all doctors agree on when chemo should be used for stage 2 colon cancers. It’s important for you to discuss the risks and benefits of chemo with your doctor, including how much it might reduce your risk of recurrence and what the likely side effects will be.
If chemo is used, the main options include 5-FU and leucovorin, oxaliplatin, or capecitabine, but other combinations may also be used.
Stage 3 colon cancer treatment
Stage 3 colon cancers have spread to nearby lymph nodes, but they have not yet spread to other parts of the body.
Surgery to remove the section of the colon with the cancer (partial colectomy) along with nearby lymph nodes, followed by adjuvant chemo is the standard treatment for stage 3 colon cancers.
For chemo, either the FOLFOX (5-FU, leucovorin, and oxaliplatin) or CapeOx (capecitabine and oxaliplatin) regimens are used most often, but some patients may get 5-FU with leucovorin or capecitabine alone based on their age and health needs.
For some advanced colon cancers that cannot be removed completely by surgery, neoadjuvant chemotherapy given along with radiation (also called chemoradiation) might be recommended to shrink the cancer so it can be removed later with surgery. For some advanced cancers that have been removed by surgery, but were found to be attached to a nearby organ or have positive margins (some of the cancer may have been left behind), adjuvant radiation might be recommended. Radiation therapy and/or chemo may be options for people who aren’t healthy enough for surgery.
Stage 4 colon cancer treatment
Stage 4 colon cancers have spread from the colon to distant organs and tissues. Colon cancer most often spreads to the liver, but it can also spread to other places like the lungs, brain, peritoneum (the lining of the abdominal cavity), or to distant lymph nodes.
In most cases surgery is unlikely to cure these cancers. But if there are only a few small areas of cancer spread (metastases) in the liver or lungs and they can be removed along with the colon cancer, surgery may help you live longer. This would mean having surgery to remove the section of the colon containing the cancer along with nearby lymph nodes, plus surgery to remove the areas of cancer spread. Chemo is typically given after surgery, as well. In some cases, hepatic artery infusion may be used if the cancer has spread to the liver.
If the metastases cannot be removed because they’re too big or there are too many of them, chemo may be given before surgery (neoadjuvant chemo). Then, if the tumors shrink, surgery may be tried to remove them . Chemo might be given again after surgery. For tumors in the liver, another option may be to destroy them with ablation or embolization.
If the cancer has spread too much to try to cure it with surgery, chemo is the main treatment. Surgery might still be needed if the cancer is blocking the colon or is likely to do so. Sometimes, such surgery can be avoided by putting a stent (a hollow metal tube) into the colon during a colonoscopy to keep it open. Otherwise, operations such as a colectomy or diverting colostomy (cutting the colon above the level of the cancer and attaching the end to an opening in the skin on the belly to allow waste out) may be used.
If you have stage 4 cancer and your doctor recommends surgery, it’s very important to understand the goal of the surgery ─ whether it’s to try to cure the cancer or to prevent or relieve symptoms of the cancer.
Most people with stage 4 cancer will get chemo and/or targeted therapies to control the cancer. Some of the most commonly used regimens include:
- FOLFOX: leucovorin, 5-FU, and oxaliplatin (Eloxatin)
- FOLFIRI: leucovorin, 5-FU, and irinotecan (Camptosar)
- CAPEOX or CAPOX: capecitabine (Xeloda) and oxaliplatin
- FOLFOXIRI: leucovorin, 5-FU, oxaliplatin, and irinotecan
- One of the above combinations plus either a drug that targets VEGF, (bevacizumab [Avastin], ziv-aflibercept [Zaltrap], or ramucirumab [Cyramza]), or a drug that targets EGFR (cetuximab [Erbitux] or panitumumab [Vectibix])
- 5-FU and leucovorin, with or without a targeted drug
- Capecitabine, with or without a targeted drug
- Irinotecan, with or without a targeted drug
- Cetuximab alone
- Panitumumab alone
- Regorafenib (Stivarga) alone
- Trifluridine and tipiracil (Lonsurf)
The choice of regimens depends on several factors, including any previous treatments you’ve had and your overall health.
If one of these regimens is no longer working, another may be tried. For people with certain tumor changes in the MMR genes, another option after initial chemotherapy might be treatment with an immunotherapy drug such as pembrolizumab (Keytruda) or nivolumab (Opdivo).
For advanced cancers, radiation therapy can also be used to help prevent or relieve symptoms in the colon from the cancer such as pain. It might also be used to treat areas of spread such as in the lungs or bone. It may shrink tumors for a time, but it’s not likely to cure the cancer. If your doctor recommends radiation therapy, it’s important that you understand the goal of treatment.
Recurrent colon cancer treatment
Recurrent cancer means that the cancer has come back after treatment. The recurrence may be local (near the area of the initial tumor), or it may be in distant organs.
Local recurrence
If the cancer comes back locally, surgery (often followed by chemo) can sometimes help you live longer and may even cure you. If the cancer can’t be removed surgically, chemo might be tried first. If it shrinks the tumor enough, surgery might be an option. This might be followed by more chemo.
Distant recurrence
If the cancer comes back in a distant site, it’s most likely to appear in the liver first. Surgery might be an option for some people. If not, chemo may be tried to shrink the tumor(s), which may then be followed by surgery to remove them. Ablation or embolization techniques might also be an option to treat some liver tumors.
If the cancer has spread too much to be treated with surgery, chemo and/or targeted therapies may be used. Possible treatment schedules are the same as for stage 4 disease.
For people whose cancers are found to have certain gene changes, another option might be treatment with immunotherapy.
Your options depend on which, if any, drugs you had before the cancer came back and how long ago you got them, as well as your overall health. You may still need surgery at some point to relieve or prevent blockage of the colon or other local problems. Radiation therapy may be an option to relieve symptoms as well.
Recurrent cancers can often be hard to treat, so you might also want to ask your doctor if clinical trials of newer treatments are available.
Rectal cancer treatment by Stage
Treatment for rectal cancer is based mainly on the stage (extent) of the cancer, but other factors can also be important. People with rectal cancers that have not spread to distant sites are usually treated with surgery. Treatment with radiation and chemotherapy (chemo) may also be given before or after surgery.
Stage 0 rectal cancer treatment
Stage 0 rectal cancers have not grown beyond the inner lining of the rectum. Removing or destroying the cancer is typically all that’s needed. You can usually be treated with surgery such as a polypectomy (removing the polyp), local excision, or transanal resection. In rare cases, a more extensive surgery might be needed.
Stage 1 rectal cancer treatment
Stage 1 rectal cancers have grown into deeper layers of the rectal wall but have not spread outside the rectum itself. This stage includes cancers that were part of a polyp. If the polyp is removed completely during colonoscopy, with no cancer in the edges, no other treatment may be needed. If the cancer in the polyp was high grade or if there were cancer cells at the edges of the polyp, you might be advised to have more surgery. More surgery may also be advised if the polyp couldn’t be removed completely or if it had to be removed in many pieces, making it hard to see if there were cancer cells at the edges (margins).
For other stage 1 cancers, surgery is usually the main treatment. Some small stage 1 cancers can be removed through the anus without cutting the abdomen (belly), using transanal resection or transanal endoscopic microsurgery (TEM). For other cancers, a low anterior resection (LAR), proctectomy with colo-anal anastomosis, or an abdominoperineal resection (APR) may be done, depending on exactly where the cancer is located within the rectum.
Additional treatment typically isn’t needed after these operations, unless the surgeon finds the cancer is more advanced than was thought before surgery. If it is more advanced, a combination of chemo and radiation therapy is usually given. 5-FU and capecitabine are the chemo drugs most often used.
If you’re not healthy enough to have surgery, you may be treated with chemotherapy given with radiation therapy.
Stage 2 rectal cancer treatment
Many stage 2 rectal cancers have grown through the wall of the rectum and might extend into nearby tissues. They have not spread to the lymph nodes.
Most people with stage 2 rectal cancer will be treated with chemotherapy, radiation therapy, and surgery, although the order of these treatments might be different for some people. For example, here’s a common approach to treating these cancers:
- Many people get both chemo and radiation therapy (called chemoradiation) as their first treatment. The chemo given with radiation is usually either 5-FU or capecitabine (Xeloda).
- This is usually followed by surgery, such as a low anterior resection (LAR), proctectomy with colo-anal anastomosis, or abdominoperineal resection (APR), depending on where the cancer is in the rectum. If the chemo and radiation therapy shrink the tumor enough, sometimes a transanal resection can be done instead of a more invasive LAR or APR. This might help you avoid having a colostomy. But not all doctors agree with this method, because it doesn’t let the surgeon check the nearby lymph nodes for cancer.
- Additional chemo is then given after surgery, usually for a total of about 6 months. The chemo may be the FOLFOX regimen (oxaliplatin, 5-FU, and leucovorin), 5-FU and leucovorin, CAPEOX (capecitabine plus oxaliplatin) or capecitabine alone, based on what’s best suited to your health needs.
Another option might be to get chemotherapy alone first, followed by chemo plus radiation therapy, then followed by surgery.
For people who can’t have chemo plus radiation, surgery (such as an LAR, proctectomy with colo-anal anastomosis, or APR) might be done first. This might be followed by chemo, and sometimes radiation therapy.
Stage 3 rectal cancer treatment
Stage 3 rectal cancers have spread to nearby lymph nodes but not to other parts of the body.
Most people with stage 3 rectal cancer will be treated with chemotherapy, radiation therapy, and surgery, although the order of these treatments might differ.
Most often, chemo is given along with radiation therapy (called chemoradiation) first. This may shrink the cancer, often making it easier to take out larger tumors. It also lowers the chance that the cancer will come back in the pelvis. Giving radiation before surgery also tends to lead to fewer problems than giving it after surgery.
Chemoradiation is followed by surgery to remove the rectal cancer and nearby lymph nodes, usually by low anterior resection (LAR), proctectomy with colo-anal anastomosis, or abdominoperineal resection (APR), depending on where the cancer is in the rectum. If the cancer has reached nearby organs, a more extensive operation known as pelvic exenteration may be needed.
After surgery, chemo is given, usually for about 6 months. The most common regimens include FOLFOX (oxaliplatin, 5-FU, and leucovorin), 5-FU and leucovorin, CAPEOX (capecitabine plus oxaliplatin), or capecitabine alone. Your doctor will recommend the one best suited to your health needs.
Another option might be to get chemotherapy alone first, followed by chemo plus radiation therapy, then followed by surgery.
For people who can’t have chemo plus radiation for some reason, surgery (such as an LAR, proctectomy with colo-anal anastomosis, or APR) might be the first treatment. This might be followed by chemotherapy, sometimes along with radiation therapy.
Stage 4 rectal cancer treatment
Stage 4 rectal cancers have spread to distant organs and tissues such as the liver or lungs. Treatment options for stage 4 cancer depend to some extent on how widespread the cancer is.
If there’s a chance that all of the cancer can be removed (for example, there are only a few tumors in the liver or lungs), the most common treatment options include:
- Surgery to remove the rectal cancer and distant cancer, followed by chemo (and/or radiation therapy in some cases)
- Chemo, followed by surgery to remove the rectal cancer and distant cancer, usually followed by chemo and radiation therapy (chemoradiation)
- Chemo, followed by chemoradiation, followed by surgery to remove the rectal cancer and distant cancer. This might be followed by more chemotherapy.
- Chemoradiation, followed by surgery to remove the rectal cancer and distant cancer. This might be followed by chemotherapy.
These approaches may help you live longer. Surgery to remove the rectal cancer would usually be a low anterior resection (LAR), proctectomy with colo-anal anastomosis, or abdominoperineal resection (APR), depending on where it’s located.
If the only site of cancer spread is the liver, you might be treated with chemo that’s put right into the artery leading to the liver (hepatic artery infusion). This may shrink the cancers in the liver better than if the chemo is given into a vein (4) or by mouth.
If the cancer is more widespread and can’t be removed completely by surgery, treatment options depend on whether the cancer is causing a blockage of the intestine. If it is, surgery might be needed right away. If not, the cancer will likely be treated with chemo and/or targeted therapy drugs (without surgery). Some of the options include:
- FOLFOX: leucovorin, 5-FU, and oxaliplatin (Eloxatin)
- FOLFIRI: leucovorin, 5-FU, and irinotecan (Camptosar)
- CAPEOX or CAPOX: capecitabine (Xeloda) and oxaliplatin
- FOLFOXIRI: leucovorin, 5-FU, oxaliplatin, and irinotecan
- One of the above combinations, plus either a drug that targets VEGF (bevacizumab [Avastin], ziv-aflibercept [Zaltrap], or ramucirumab [Cyramza]), or a drug that targets EGFR (cetuximab [Erbitux] or panitumumab [Vectibix])
- 5-FU and leucovorin, with or without a targeted drug
- Capecitabine, with or without a targeted drug
- Irinotecan, with or without a targeted drug
- Cetuximab alone
- Panitumumab alone
- Regorafenib (Stivarga) alone
- Trifluridine and tipiracil (Lonsurf)
The choice of drugs or drug combinations depends on several factors, including any previous treatments, your overall health, and how well you can tolerate treatment.
If chemo shrinks the cancer, in some cases it may be possible to consider surgery to try to remove all of the cancer at this point. Chemo may then be given again after surgery.
If the cancer doesn’t shrink, a different drug combination may be tried. For people with certain gene changes in their cancer cells, another option after initial chemotherapy might be treatment with an immunotherapy drug such as pembrolizumab (Keytruda) or nivolumab (Opdivo).
For cancers that don’t shrink with chemo and widespread cancers that are causing symptoms, treatment is done to relieve symptoms and avoid long-term problems such as bleeding or blockage of the intestines. Treatments may include one or more of these:
- Removing the rectal cancer with surgery
- Surgery to create a colostomy and bypass the rectal cancer (a diverting colostomy)
- Using a special laser to destroy the cancer within the rectum
- Placing a stent (hollow metal tube) within the rectum to keep it open; this does not require surgery
- Chemoradiation therapy
- Chemo alone
If the cancer in the liver can’t be removed by surgery because they are too big or there are too many of them, it may be possible to destroy them (partially or completely) with ablation or embolization.
Recurrent rectal cancer treatment
Recurrent cancer means that the cancer has come back after treatment. It may come back near the area of the initial rectal cancer (locally) or in distant organs, like the lungs or liver. If the cancer does recur, it’s usually in the first 2 to 3 years after surgery, but it can also recur much later.
Local recurrence
If the cancer comes back in the pelvis (locally), it’s treated with surgery to remove the cancer, if possible. This surgery is often more extensive than the initial surgery. In some cases radiation therapy may be given during the surgery (this is called intraoperative radiotherapy) or afterward. Chemo may also be given after surgery. Radiation therapy might be used as well, if it was not used before.
Distant recurrence
If the cancer comes back in a distant part of the body, the treatment will depend on whether it can be removed by surgery.
If the cancer can be removed, surgery is done. Chemo may be given before surgery (see Treating stage 4 rectal cancer above for a list of possible drug options). Chemo can be given after surgery, too. When the cancer has spread to the liver, chemo may be given through the hepatic artery leading to the liver.
If the cancer can’t be removed by surgery, chemo and/or targeted therapy drugs may be used. For people with certain gene changes in their cancer cells, another option might be treatment with immunotherapy. The drugs used will depend on what drugs a person has received previously and on their overall health. If the cancer doesn’t shrink, a different drug combination may be tried.
As with stage 4 rectal cancer, surgery, radiation therapy, or other approaches may be used at some point to relieve symptoms and avoid long-term problems such as bleeding or blockage of the intestines.
These cancers can often be hard to treat, so you might also want to ask your doctor if there are any clinical trials of newer treatments that might be right for you.
Colon cancer prognosis
Colon cancer prognosis is clearly to the following 25:
- The degree of penetration of the tumor through the bowel wall.
- The presence or absence of lymph node involvement.
- The presence or absence of distant metastases.
These three characteristics form the basis for all staging systems developed for colon cancer.
Other prognostic factors include the following 25:
- Bowel obstruction and bowel perforation are indicators of poor prognosis 26.
- Elevated pretreatment serum levels of carcinoembryonic antigen (CEA) have a negative prognostic significance 27.
Many other prognostic markers have been evaluated retrospectively for patients with colon cancer, though most, including allelic loss of chromosome 18q or thymidylate synthase expression, have not been prospectively validated 28, 29. Microsatellite instability, also associated with HNPCC, has been associated with improved survival independent of tumor stage in a population-based series of 607 patients younger than 50 years with colorectal cancer 30. Patients with Lynch syndrome (hereditary nonpolyposis colorectal cancer [HNPCC]) reportedly have better prognoses in stage-stratified survival analysis than patients with sporadic colorectal cancer, but the retrospective nature of the studies and possibility of selection factors make this observation difficult to interpret 31.
Treatment decisions depend on factors such as physician and patient preferences and the stage of the disease, rather than the age of the patient 32.
Racial differences in overall survival after adjuvant therapy have been observed, without differences in disease-free survival, suggesting that comorbid conditions play a role in survival outcome in different patient populations 33.
References- American Cancer Society: Cancer Facts and Figures 2022. American Cancer Society, 2022. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf
- Cancer Stat Facts: Colorectal Cancer. https://seer.cancer.gov/statfacts/html/colorect.html
- SEER Cancer Statistics Review (CSR) 1975-2017. https://seer.cancer.gov/archive/csr/1975_2017
- Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med. 2002 Jun 6;346(23):1781-5. doi: 10.1056/NEJM200206063462304
- Azzouz LL, Sharma S. Physiology, Large Intestine. [Updated 2021 Aug 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507857
- Precup G, Vodnar DC. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: a comprehensive literature review. Br J Nutr. 2019 Jul 28;122(2):131-140. doi: 10.1017/S0007114519000680
- Sulaiman, S., & Marciani, L. (2019). MRI of the Colon in the Pharmaceutical Field: The Future before us. Pharmaceutics, 11(4), 146. https://doi.org/10.3390/pharmaceutics11040146
- American Cancer Society Guideline for Colorectal Cancer Screening. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html
- NCCN. Colorectal cancer screening guidelines. https://www.nccn.org/professionals/physician_gls/pdf/colorectal_screening.pdf
- Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021 Mar 1;116(3):458-479. doi: 10.14309/ajg.0000000000001122
- US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(19):1965–1977. doi:10.1001/jama.2021.6238
- Qaseem, A., Crandall, C. J., Mustafa, R. A., Hicks, L. A., Wilt, T. J., Clinical Guidelines Committee of the American College of Physicians, Forciea, M. A., Fitterman, N., Horwitch, C. A., Kansagara, D., Maroto, M., McLean, R. M., Roa, J., & Tufte, J. (2019). Screening for Colorectal Cancer in Asymptomatic Average-Risk Adults: A Guidance Statement From the American College of Physicians. Annals of internal medicine, 171(9), 643–654. https://doi.org/10.7326/M19-0642
- Wolf, A.M., et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA: A Cancer Journal for Clinicians, 68: 250-281. https://doi.org/10.3322/caac.21457
- Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Patel, Swati G. et al. Gastrointestinal Endoscopy, Volume 95, Issue 1, 1-15, November 15, 2021. https://doi.org/10.1016/j.gie.2021.06.012
- Colorectal Cancer: Screening Final Recommendation Statement https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/colorectal-cancer-screening
- Clinician Summary of USPSTF Recommendation. Screening for Colorectal Cancer May 2021. https://www.uspreventiveservicestaskforce.org/home/getfilebytoken/xL4XwcPKXBfTWgY5ouzLzy
- Colorectal Cancer Screening Tests. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/screening-tests-used.html
- Colorectal Cancer Stages. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/staged.html
- ASTLER, V. B., & COLLER, F. A. (1954). The prognostic significance of direct extension of carcinoma of the colon and rectum. Annals of surgery, 139(6), 846–852. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1609522/pdf/annsurg01327-0129.pdf
- Survival Rates for Colorectal Cancer. https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html
- Meyerhardt JA, Mangu PB, Flynn PJ, Korde L, Loprinzi CL, Minsky BD, Petrelli NJ, Ryan K, Schrag DH, Wong SL, Benson AB 3rd; American Society of Clinical Oncology. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2013 Dec 10;31(35):4465-70. doi: 10.1200/JCO.2013.50.7442
- Khoury DA, Opelka FG, Beck DE, Hicks TC, Timmcke AE, Gathright JB Jr. Colon surveillance after colorectal cancer surgery. Dis Colon Rectum. 1996 Mar;39(3):252-6. doi: 10.1007/BF02049461
- Benson AB 3rd, Desch CE, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Petrelli NJ, Pfister DG, Smith TJ, Somerfield MR; American Society of Clinical Oncology. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000 Oct 15;18(20):3586-8. doi: 10.1200/JCO.2000.18.20.3586
- Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol. 1996 Oct;14(10):2843-77. doi: 10.1200/JCO.1996.14.10.2843
- PDQ Adult Treatment Editorial Board. Colon Cancer Treatment (PDQ®): Health Professional Version. 2022 Jan 21. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK65858
- Steinberg SM, Barkin JS, Kaplan RS, Stablein DM. Prognostic indicators of colon tumors. The Gastrointestinal Tumor Study Group experience. Cancer. 1986 May 1;57(9):1866-70. doi: 10.1002/1097-0142(19860501)57:9<1866::aid-cncr2820570928>3.0.co;2-t
- Filella, X., Molina, R., Grau, J. J., Piqué, J. M., Garcia-Valdecasas, J. C., Astudillo, E., Biete, A., Bordas, J. M., Novell, A., & Campo, E. (1992). Prognostic value of CA 19.9 levels in colorectal cancer. Annals of surgery, 216(1), 55–59. https://doi.org/10.1097/00000658-199207000-00008
- Roth JA. p53 prognostication: paradigm or paradox? Clin Cancer Res. 1999 Nov;5(11):3345.
- McLeod, H. L., & Murray, G. I. (1999). Tumour markers of prognosis in colorectal cancer. British journal of cancer, 79(2), 191–203. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2362193/pdf/79-6690033a.pdf
- Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, Redston M, Gallinger S. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000 Jan 13;342(2):69-77. doi: 10.1056/NEJM200001133420201
- Watson P, Lin KM, Rodriguez-Bigas MA, Smyrk T, Lemon S, Shashidharan M, Franklin B, Karr B, Thorson A, Lynch HT. Colorectal carcinoma survival among hereditary nonpolyposis colorectal carcinoma family members. Cancer. 1998 Jul 15;83(2):259-66.
- Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol. 2002 Oct 1;20(19):3992-8. doi: 10.1200/JCO.2002.03.083
- Dignam JJ, Colangelo L, Tian W, Jones J, Smith R, Wickerham DL, Wolmark N. Outcomes among African-Americans and Caucasians in colon cancer adjuvant therapy trials: findings from the National Surgical Adjuvant Breast and Bowel Project. J Natl Cancer Inst. 1999 Nov 17;91(22):1933-40. doi: 10.1093/jnci/91.22.1933