Cyclic neutropenia
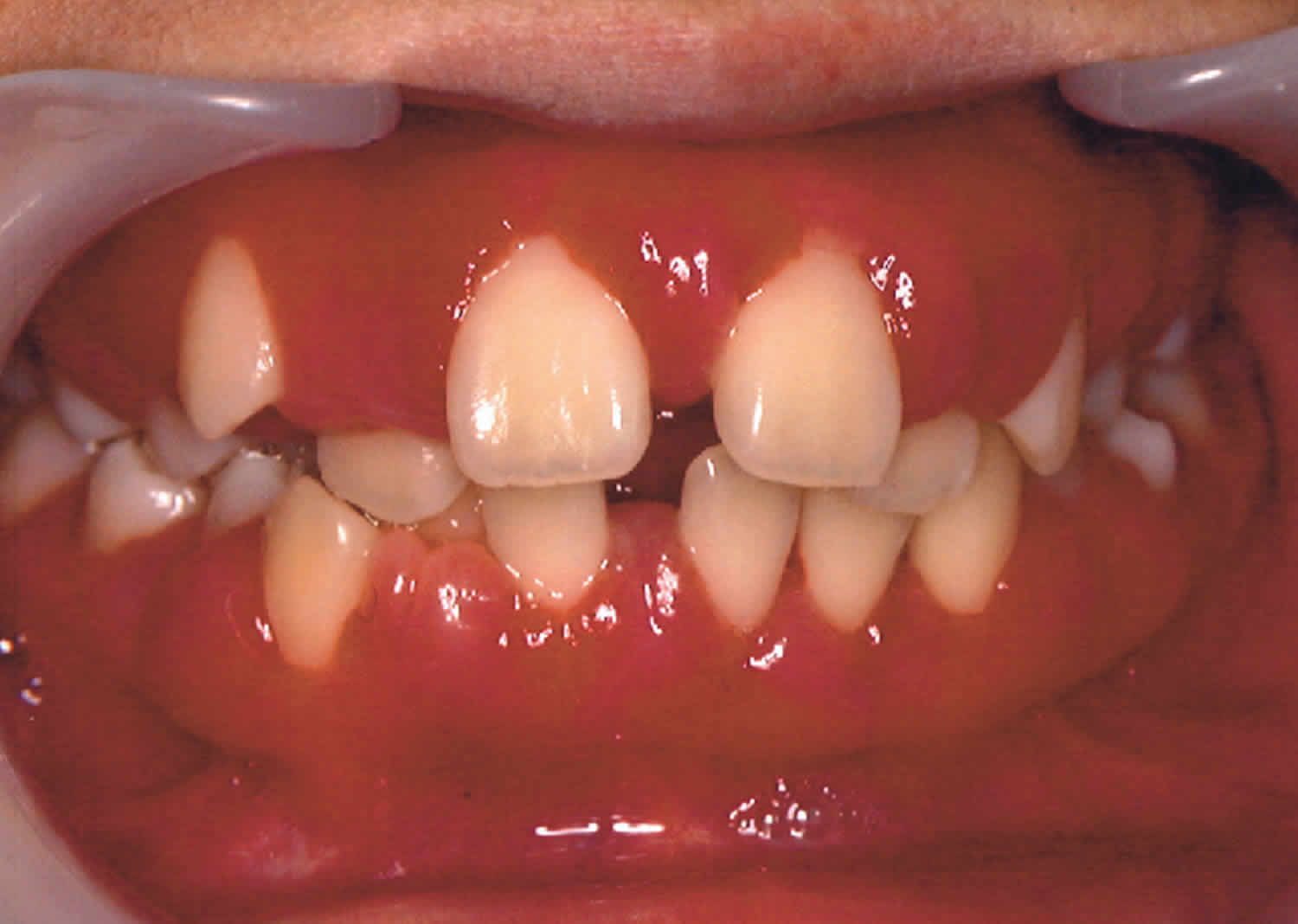
Cyclic neutropenia is a rare disorder that causes frequent infections and other health problems in affected individuals 1. People with cyclic neutropenia have recurrent episodes of neutropenia during which there is a shortage (deficiency) of neutrophils. The episodes of neutropenia are apparent at birth or soon afterward. For most affected individuals, neutropenia recurs every 21 days and lasts about 3 to 5 days 1. Patients with cyclic neutropenia often experience an early onset of severe periodontitis and are forced to undergo tooth extraction 2.
Cyclic neutropenia is a rare condition and is estimated to occur in 1 in 1 million individuals worldwide.
Cyclic neutropenia appears to affect males and females in equal numbers. Most cases of cyclic neutropenia are thought to be present at birth (congenital); however, in some cases, the symptoms may not become obvious until childhood, adolescence, or early adulthood.
Cyclic neutropenia is a subdivision of severe chronic neutropenia. Severe chronic neutropenia is estimated to affect approximately 0.5 to 1 per million population in the United States 3.
Neutrophils are instrumental in fighting off infection by surrounding and destroying bacteria that enter the body. Neutropenia makes it more difficult for the body to fight off pathogens such as bacteria and viruses, so people with cyclic neutropenia typically develop recurrent infections of the sinuses, respiratory tract, and skin. Additionally, people with cyclic neutropenia often develop open sores (ulcers) in the mouth and colon, inflammation of the throat (pharyngitis) and gums (gingivitis), recurrent fever, or abdominal pain. People with cyclic neutropenia have these health problems only during episodes of neutropenia. At times when their neutrophil levels are normal, they are not at an increased risk of infection and inflammation.
Prompt, appropriate treatment of the infections associated with cyclic neutropenia is important. Such treatment may include antibiotic therapy. Careful oral and dental care is also required. In addition, individuals with cyclic neutropenia should avoid activities that may cause minor injuries.
A synthetic drug that stimulates the bone marrow’s production of neutrophils (recombinant human granulocyte-colony stimulating factor [rhG-CSF]) has been used to treat severe chronic neutropenia. One form, the orphan drug neupogen (Filgrastim), has been approved by the Food and Drug Administration (FDA) for use in the treatment of severe chronic neutropenia. Studies have shown that long-term therapy can elevate the numbers of neutrophils to normal range in most individuals, thereby reducing infections and other associated symptoms. Careful evaluation prior to initiation of such therapy and ongoing observation during therapy are essential to ensure the long-term safety and effectiveness of such treatment in individuals with severe chronic neutropenia. Neupogen is manufactured by Amgen Inc.
Genetic counseling may be of benefit for individuals with inherited forms of cyclic neutropenia and their families. Other treatment is symptomatic and supportive.
Cyclic neutropenia causes
Cyclic neutropenia may be inherited or acquired. Some cases are present at birth (congenital) and appear to occur randomly for no apparent reason (sporadically). There have been reports in the medical literature in which individuals within several multigenerational families (kindreds) have an increased incidence of cyclic neutropenia. In such familial cases, the disorder may be inherited as an autosomal dominant trait.
Investigators have determined that cases of sporadic and autosomal dominant cyclic neutropenia may be caused by disruption or changes (mutations) of the ELANE gene located on the short arm (p) of chromosome 19 (19p13.3) 3.
Mutations in the ELANE gene cause cyclic neutropenia. The ELANE gene provides instructions for making a protein called neutrophil elastase, which is found in neutrophils. When the body starts an immune response to fight an infection, neutrophils release neutrophil elastase. This protein then modifies the function of certain cells and proteins to help fight the infection.
ELANE gene mutations that cause cyclic neutropenia lead to an abnormal neutrophil elastase protein that seems to retain some of its function. However, neutrophils that produce abnormal neutrophil elastase protein appear to have a shorter lifespan than normal neutrophils. The shorter neutrophil lifespan is thought to be responsible for the cyclic nature of this condition. When the affected neutrophils die early, there is a period in which there is a shortage of neutrophils because it takes time for the body to replenish its supply.
Cyclic neutropenia inheritance pattern
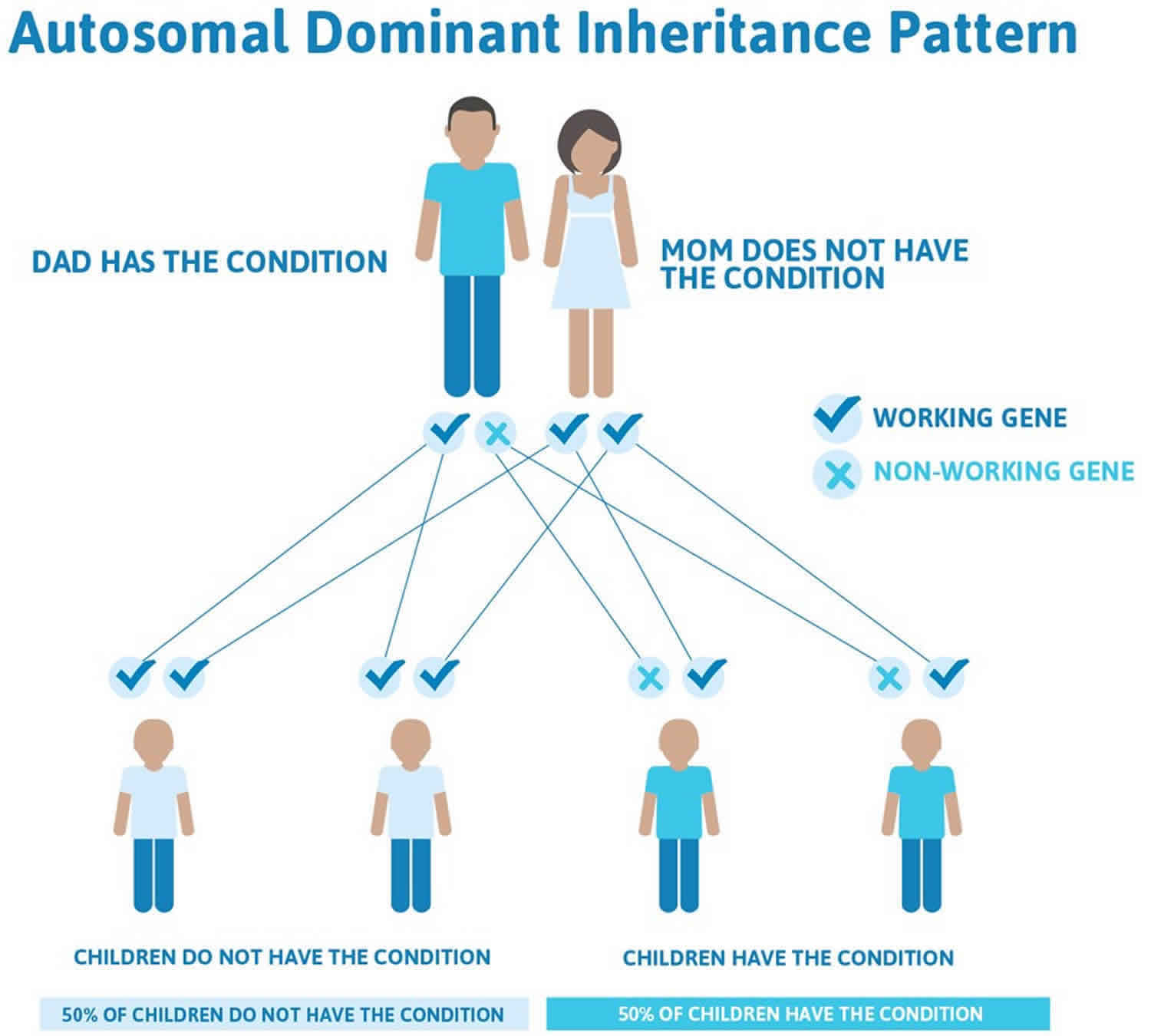
Cyclic neutropenia is inherited in an autosomal dominant pattern, which means one copy of the altered gene in each cell is sufficient to cause the disorder.
In cases where the autosomal dominant condition does run in the family, the chance for an affected person to have a child with the same condition is 50% regardless of whether it is a boy or a girl. These possible outcomes occur randomly. The chance remains the same in every pregnancy and is the same for boys and girls.
- When one parent has the abnormal gene, they will pass on either their normal gene or their abnormal gene to their child. Each of their children therefore has a 50% (1 in 2) chance of inheriting the changed gene and being affected by the condition.
- There is also a 50% (1 in 2) chance that a child will inherit the normal copy of the gene. If this happens the child will not be affected by the disorder and cannot pass it on to any of his or her children.
In most cases, an affected person inherits the mutation from one affected parent. Other cases result from new mutations in the gene and occur in people with no history of the disorder in their family. This is called a de novo mutation.
Figure 1 illustrates autosomal dominant inheritance. The example below shows what happens when dad has the condition, but the chances of having a child with the condition would be the same if mom had the condition.
Figure 1. Cyclic neutropenia autosomal dominant inheritance pattern
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Cyclic neutropenia symptoms
The primary finding associated with cyclic neutropenia is a severe chronic decrease in certain white blood cells (neutrophils). In most cases, episodes of neutropenia recur every 21 days (cyclic) and may last for three to six days. The cycling period usually remains constant and consistent among affected individuals. In addition, abnormal levels of red blood cells that assist in clotting (platelets), immature red blood cells (reticulocytes), and other types of white blood cells (monocytes) may occur. The monocyte count invariable increases during the periods of neutropenia.
During episodes of neutropenia, affected individuals may experience fever, a general feeling of ill health (malaise), inflammation and ulceration of the mucous membranes of the mouth (stomatitis), inflammation of the throat (pharyngitis), inflammation and degeneration of the tissues that surround and support the teeth (periodontal disease), and/or loss of appetite. Peridontal disease may result in loosening of teeth and early tooth loss in young children.
Individuals with cyclic neutropenia may be abnormally susceptible to various bacterial infections that often affect the skin, digestive (gastrointestinal) tract, and respiratory system. Such bacterial infections vary in severity and, in some cases, may result in life-threatening complications.
Cyclic neutropenia diagnosis
A diagnosis of cyclic neutropenia is made based upon a detailed patient history and thorough clinical evaluation. A diagnosis may be confirmed by monitoring an individual’s neutrophil count twice or three times per week for six weeks. Individuals with cyclic neutropenia should be genetically tested for mutations in the ELANE gene.
Cyclic neutropenia treatment
Prompt, appropriate treatment of the infections associated with cyclic neutropenia is important. Such treatment may include antibiotic therapy. Careful oral and dental care is also required. In addition, individuals with cyclic neutropenia should avoid activities that may cause minor injuries.
A synthetic drug that stimulates the bone marrow’s production of neutrophils (recombinant human granulocyte-colony stimulating factor [rhG-CSF]) has been used to treat severe chronic neutropenia. One form, the orphan drug neupogen (Filgrastim), has been approved by the Food and Drug Administration for use in the treatment of severe chronic neutropenia. Studies have shown that long-term therapy can elevate the numbers of neutrophils to normal range in most individuals, thereby reducing infections and other associated symptoms. Careful evaluation prior to initiation of such therapy and ongoing observation during therapy are essential to ensure the long-term safety and effectiveness of such treatment in individuals with severe chronic neutropenia. Neupogen is manufactured by Amgen Inc.
Genetic counseling may be of benefit for individuals with inherited forms of cyclic neutropenia and their families. Other treatment is symptomatic and supportive.
Cyclic neutropenia prognosis
The prognosis of a patient with neutropenia depends on the primary cause, duration, and severity of the neutropenia. Improved broad-spectrum antibiotic agents, combined with improved supportive care, have improved the prognosis for most patients with severe neutropenia. Ultimately, patient survival depends on the recovery of adequate neutrophil numbers.
Morbidity in those with neutropenia usually involves infections during severe, prolonged episodes of neutropenia. The infections may be superficial, involving mainly the oral mucosa, gums, skin, and sinuses, or they may be systemic, with life-threatening septicemia.
Serious medical complications occur in 21% of patients with cancer and neutropenic fever. Mortality correlates with the duration and severity of the neutropenia and the time elapsed until the first dose of antibiotics is administered for neutropenic fever 4. Neutropenic fever in cancer patients typically carries an overall mortality rate of 4-30%. A study of febrile neutropenia-related hospitalizations in patients with breast cancer reported an average inhospital mortality rate during 2009-2011 of 2.6%, but a rate of 4.4% in patients 65 years of age and older. Mean length of hospital stay was 5.7 days 5.
The three identified high-risk groups among cancer patients with neutropenic fever (many of whom have received aggressive chemotherapy) are as follows:
- Inpatients with fever while developing neutropenia
- Outpatients requiring acute hospital care for problems beyond neutropenia and fever
- Stable outpatients with uncontrolled cancer
However, a post-hoc analysis of the TROPIC trial in men with metastatic castration-resistant prostate cancer found that occurrence of grade ≥3 neutropenia during cabazitaxel therapy was associated with a prolonged overall survival (median 16.3 versus 14.0 months), a twice-longer progression-free survival (median 5.3 versus 2.6 months) and a higher confirmed prostate-specific antigen response ≥50% (49.8% versus 24.4%), as compared with patients who did not develop grade ≥3 neutropenia. These authors concluded that the inferior outcome in patients who failed to experience grade ≥3 neutropenia during therapy may suggest insufficient drug exposure or a limited impact on the tumor-associated immune response 6.
If agranulocytosis is untreated, the risk of dying is high. Death results from uncontrolled sepsis. If the condition can be reversed with treatment, the risk of dying is low. Antibiotic and antifungal medications can cure the infection if the absolute neutrophil count (ANC) rises. Agranulocytosis secondary to viral infections is usually self-limited, and patients with such conditions have a good prognosis.
Drug-induced agranulocytosis carries a mortality rate of 6-10%. If treated promptly and vigorously, patients with drug-induced agranulocytosis have a good prognosis.
References- Cyclic neutropenia. https://ghr.nlm.nih.gov/condition/cyclic-neutropenia
- Aota K, Kani K, Yamanoi T, et al. Management of tooth extraction in a patient with ELANE gene mutation-induced cyclic neutropenia: A case report. Medicine (Baltimore). 2019;98(39):e17372. doi:10.1097/MD.0000000000017372 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6775366
- Cyclic neutropenia. https://rarediseases.org/rare-diseases/cyclic-neutropenia/
- Krell D, Jones AL. Impact of effective prevention and management of febrile neutropenia. Br J Cancer. 2009 Sep. 101 Suppl 1:S23-6.
- Pathak R, Giri S, Aryal MR, Karmacharya P, Bhatt VR, Martin MG. Mortality, length of stay, and health care costs of febrile neutropenia-related hospitalizations among patients with breast cancer in the United States. Support Care Cancer. 2015 Jan 4.
- Meisel A, von Felten S, Vogt DR, Liewen H, de Wit R, de Bono J, et al. Severe neutropenia during cabazitaxel treatment is associated with survival benefit in men with metastatic castration-resistant prostate cancer (mCRPC): A post-hoc analysis of the TROPIC phase III trial. Eur J Cancer. 2016 Jan 29. 56:93-100.