What is egg white
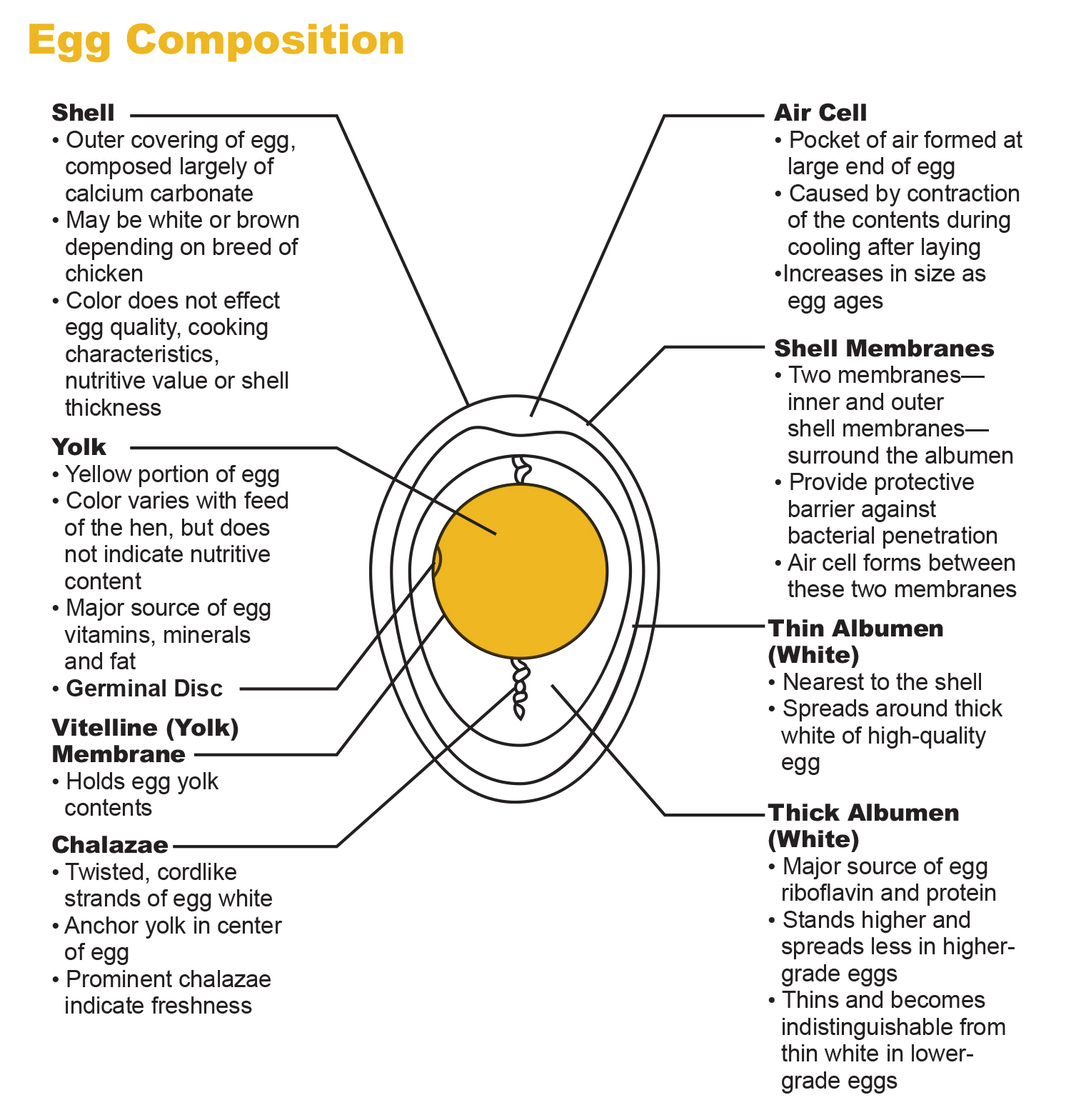
An egg consists of shell, membrane, albumen or egg white and egg yolk.
The shell. The shell of an egg has a rigid yet porous structure. The porous shell has great resistance to the entry of micro-organisms when kept dry and considerable resistance to the loss of moisture by evaporation. The color of the shell, which may be white or brown depending on the breed of the laying chicken, does not affect quality, flavor, cooking characteristics, nutritional value or shell thickness. For example, Leghorns produce white eggs, while Rhode Island Reds produce brown eggs.
Shell membrane. Inside the shell there are two membranes (as seen in Figure 1). The outer membrane is attached to the shell, the inner membrane is attached to the albumen or egg white. These two membranes provide a protective barrier against bacterial penetration.
Air space. An air space or air cell is a pocket of air usually found at the large end of the egg interior between the outer membrane and the inner membrane. This air cell is created by the contraction of the inner contents while the egg cools and by the evaporation of moisture after the egg has been laid. The air cell increases in size as time passes.
Egg albumen or white. The albumen of the egg is composed of the outer thin albumen and the inner firm or thick albumen. The outer thin albumen spreads around the inner firm albumen. The inner firm albumen in high quality eggs stands higher and spreads less than the outer thin albumen.
White fibrous strips. These are twisted, cord-like strands of egg white, known as chalazae, which hold the yolk in position. Prominent thick chalazae indicate high quality and freshness.
Yolk. The yolk is almost spherical and is surrounded by a colorless membrane. The color of the yolk varies with the type of feed given to the laying hen. If the laying hen is fed yellow corn, alfalfa meal and fresh grass provide good pigment sources for a normal yellowish-orange yolk. The color of the yolk does not affect the nutritional content.
Egg weight. The weight of eggs varies widely depending on many factors such as the breed, the age of the layer and environmental temperature. In Africa, for example, the egg weight may range from 35 to 65 grams, while in Europe it may range from 45 to 70 grams. As a layer gets older the weight of the eggs increase.
The components of an egg weighing 60 grams are made up as follows:
- Yolk (29%) – 17.4 g
- White (61.5%) – 36.9 g
- Shell (9.5%) – 5.6 g
Figure 1. Egg composition
[Source 1]Egg nutritional value
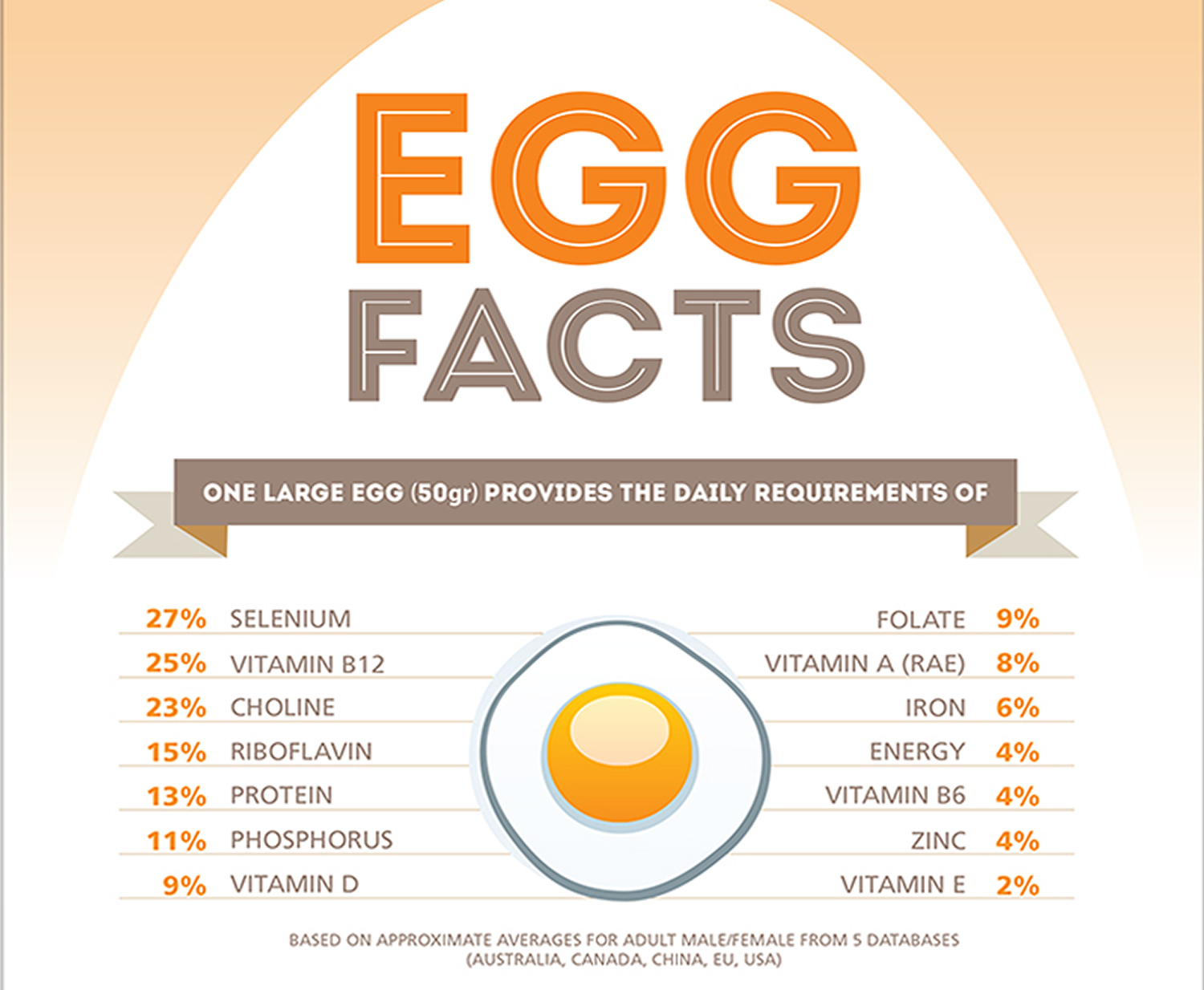
Eggs contain the highest quality protein and are often used as a standard to measure the quality of other protein sources. A single large egg provides 12 percent of the daily requirement of protein for 70 calories 1. Eggs also have the highest biological value of any protein, meaning that the essential amino acids they provide are used very efficiently by the body. Eggs also contain varying amounts of vitamins A, D, E, K, B6, B12, folate, and a variety of minerals (particularly riboflavin, phosphorus, and iron). Because eggs are very easy to digest, they are frequently included in therapeutic diets.
The yolk makes up just over one third of an egg. It provides three-fourths of the calories, all of the fat-soluble vitamins (A, D, E, and K), and all of the choline, lutein, and zeaxanthin. The yolk also provides most of the phosphorus, iron, and folate and almost half of the protein and riboflavin. The white (albumen) provides more than half of the total protein and riboflavin. Hens given a high protein feed lay eggs with 1 to 2% more protein than in the other group 2. Choline, an essential nutrient, is shown to be important for proper brain development in the fetus and newborn and may play a role in memory function throughout life and into old age. Lutein and zeaxanthin may prevent macular degeneration, a leading cause of blindness in the elderly in the U.S. One large egg (50 grams) provides the daily requirements of 27% selenium, 25% vitamin B12, 23% choline, 15% riboflavin, 13% protein, 11% phosphorus, 9% vitamin D 3.
Though these nutrients are present only in small amounts in eggs, research shows that they may be more bioavailable, or absorbed and utilized by the body, when obtained from egg yolk than from richer sources.
Figure 2. Egg nutrition facts
[Source 3]When purchasing shell eggs, follow these guidelines:
- Accept only clean, sound and odor-free eggs.
- Purchase eggs according to grade and size desired and only in the quantity needed for one to two weeks.
- Accept only eggs delivered under refrigeration at a temperature of 45° F or below. Transfer to refrigerated storage promptly.
- Accept only eggs packed in snug-fitting fiberboard boxes to reduce breakage. Eggs are generally packed and purchased in 30-dozen cases or half cases of 15 dozen.
- Consider size and grade in relation to use and price. Also, compare prices for different sizes of eggs of the same grade.
- Check the grade of eggs delivered to you. Inspect the shells and then randomly break a few. These eggs should meet the guidelines for their given grades.
- A quick test for freshness is to check if the raw egg in the shell sinks in a basin of water. Fresh eggs stay at the bottom of the bowl while older eggs float because of the large air cell that forms in its base.
- If hard-boiling egg, it is best to use eggs a few days old. The fresher the egg, the more likely the white will stick to the shell.
How long do eggs last ?
The easiest way to maintain eggs at high quality is to store them in their original carton in the refrigerator as soon as possible after purchase. Cartons reduce water loss and protect flavours from other foods being absorbed into the eggs. Storing eggs loose, or in specially designed sections located on refrigerator doors is not recommended as this also exposes eggs to a greater risk of damage. The best conditions for egg storage are at a temperature of about – 1° C and relative humidity between 80 and 85 percent. At a temperature of 10° C, lower relative humidity is needed, between 75 and 80 percent. At all temperatures there is the risk of mould spoilage where the relative humidity is too high. Packaging materials that are too dry or are excessively moist and absorbent will also accentuate evaporation losses. Mould spores normally present on eggshells may, if sufficient time elapses, germinate and grow, penetrating the shell and causing spoilage. Generally this occurs only when eggs are in cold storage for several months or more under conditions of high humidity (above 85 percent). It can occur, however, at any temperature if the humidity is sufficiently high and the holding time long enough.
Fresh eggs can be kept refrigerated in their carton for 6 weeks from the date of pack. It is best to put eggs in the fridge as soon as you get them home, remember an egg ages 7 times quicker when left on the bench than when it is properly stored in the fridge. So to enjoy you fresh eggs for longer, store them in the fridge.
The main cause of spoilage by bacteria is the washing of dirty eggs before marketing. When the egg is washed, organisms from water – usually bacteria – can penetrate the shell. Once inside they multiply and eventually spoil the egg, causing green, black and red rots. Even when eggs become wet without any cleaning process, for example, by condensation after removal from refrigerated storage into a warm temperature, conditions may be favorable for the penetration of micro-organisms and rotting may follow. When eggs are kept dry, no such way is provided for bacteria to penetrate the shell.
Egg white nutrition facts
Egg white, also referred to as egg albumen, contains 56 percent of the whole egg’s total protein along with the majority of the egg’s niacin, riboflavin, choline, magnesium, potassium, sodium and sulfur 4. Fifteen grams of egg white protein contain 1341 mg of leucine, 837 mg of isoleucine, and 1096 mg of valine, and there is also an abundant source of branched amino acids and aromatic amino acids. It is well established that essential amino acids 5 stimulate skeletal muscle protein synthesis in animal and human models 6, 7, 8, 9. Recent data showed that leucine induces a maximal skeletal muscle protein anabolic response in young adults 10, which suggests that leucine-rich egg white protein intake might have an important effect on body mass increase.
Alone, egg whites are about 88 percent water, 10 percent protein and almost completely free of fat and cholesterol, making it a very attractive ingredient in today’s food formulating industry. In fact, egg whites are a high‐quality, nutrient dense food ingredient, as the protein in egg white has a very high biological value. It has also been shown to provide satiety and thus assist in weight loss diets 4.
Table 1. Raw egg white (fresh) nutrition facts
Nutrient | Unit | Value per 100 g | large 33 g | cup 243 g | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Water | g | 87.57 | 28.90 | 212.80 | |||||||||||||||
| Energy | kcal | 52 | 17 | 126 | |||||||||||||||
| Protein | g | 10.90 | 3.60 | 26.49 | |||||||||||||||
| Total lipid (fat) | g | 0.17 | 0.06 | 0.41 | |||||||||||||||
| Carbohydrate, by difference | g | 0.73 | 0.24 | 1.77 | |||||||||||||||
| Fiber, total dietary | g | 0.0 | 0.0 | 0.0 | |||||||||||||||
| Sugars, total | g | 0.71 | 0.23 | 1.73 | |||||||||||||||
| Minerals | |||||||||||||||||||
| Calcium, Ca | mg | 7 | 2 | 17 | |||||||||||||||
| Iron, Fe | mg | 0.08 | 0.03 | 0.19 | |||||||||||||||
| Magnesium, Mg | mg | 11 | 4 | 27 | |||||||||||||||
| Phosphorus, P | mg | 15 | 5 | 36 | |||||||||||||||
| Potassium, K | mg | 163 | 54 | 396 | |||||||||||||||
| Sodium, Na | mg | 166 | 55 | 403 | |||||||||||||||
| Zinc, Zn | mg | 0.03 | 0.01 | 0.07 | |||||||||||||||
| Vitamins | |||||||||||||||||||
| Vitamin C, total ascorbic acid | mg | 0.0 | 0.0 | 0.0 | |||||||||||||||
| Thiamin | mg | 0.004 | 0.001 | 0.010 | |||||||||||||||
| Riboflavin | mg | 0.439 | 0.145 | 1.067 | |||||||||||||||
| Niacin | mg | 0.105 | 0.035 | 0.255 | |||||||||||||||
| Vitamin B-6 | mg | 0.005 | 0.002 | 0.012 | |||||||||||||||
| Folate, DFE | µg | 4 | 1 | 10 | |||||||||||||||
| Vitamin B-12 | µg | 0.09 | 0.03 | 0.22 | |||||||||||||||
| Vitamin A, RAE | µg | 0 | 0 | 0 | |||||||||||||||
| Vitamin A, IU | IU | 0 | 0 | 0 | |||||||||||||||
| Vitamin E (alpha-tocopherol) | mg | 0.00 | 0.00 | 0.00 | |||||||||||||||
| Vitamin D (D2 + D3) | µg | 0.0 | 0.0 | 0.0 | |||||||||||||||
| Vitamin D | IU | 0 | 0 | 0 | |||||||||||||||
| Vitamin K (phylloquinone) | µg | 0.0 | 0.0 | 0.0 | |||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 0.000 | 0.000 | 0.000 | |||||||||||||||
| Fatty acids, total monounsaturated | g | 0.000 | 0.000 | 0.000 | |||||||||||||||
| Fatty acids, total polyunsaturated | g | 0.000 | 0.000 | 0.000 | |||||||||||||||
| Cholesterol | mg | 0 | 0 | 0 | |||||||||||||||
| Other | |||||||||||||||||||
| Caffeine | mg | 0 | 0 | 0 | |||||||||||||||
Refrigerated Liquid or Frozen Egg White
The proteins in egg whites are very functional, and assist food product developers with overcoming certain formulating challenges. An increasingly popular challenge in today’s food industry is to satisfy the restrictions set by natural foods stores on what a product may or may not contain. Egg whites have always been a good choice, as it is all‐natural and a nutrition powerhouse.
Egg whites help formulators with producing high‐volume foams and with leavening 4. When combined with other ingredients such as water or milk, it can be used to glaze pocket‐style sandwiches, rolls and breads, preventing the crusts from drying. Egg whites also act as an adhesive in both breading and coating processes, as well as with topical application of nuts and seeds.
For ease of convenience, egg product manufacturers separate egg whites from egg yolks, and sell them as individual ingredients. Liquid egg whites are available refrigerated, ready‐to‐use, or frozen 4. The advantage to frozen egg whites is a lengthier shelf life. Food manufacturers can thaw and use on an as-needed basis.
Regardless if liquid egg whites are sold refrigerated or frozen, they are always pasteurized for safety. Egg whites can also be formulated to include other ingredients such as salt or sugar for added shelf life and enhanced functionality. At the industrial level, liquid white—refrigerated or thawed frozen—can be added with other wet ingredients. Liquid whites readily integrate into manufacturing systems, including pumping and extrusion.
Availability:
- Egg White
- Salted Egg White
- Extended Shelf Life Egg White
- High-Gel Egg White
- High-Whip Egg White
Table 2. 100% Liquid egg whites nutrition facts
Nutrient | Unit | 46 g | Value per 100 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Energy | kcal | 25 | 54 | ||||||||||||||||
| Protein | g | 5.00 | 10.87 | ||||||||||||||||
| Total lipid (fat) | g | 0.00 | 0.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 1.00 | 2.17 | ||||||||||||||||
| Fiber, total dietary | g | 0.0 | 0.0 | ||||||||||||||||
| Sugars, total | g | 0.00 | 0.00 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Calcium, Ca | mg | 0 | 0 | ||||||||||||||||
| Iron, Fe | mg | 0.00 | 0.00 | ||||||||||||||||
| Potassium, K | mg | 60 | 130 | ||||||||||||||||
| Sodium, Na | mg | 75 | 163 | ||||||||||||||||
| Vitamins | |||||||||||||||||||
| Vitamin C, total ascorbic acid | mg | 0.0 | 0.0 | ||||||||||||||||
| Folic acid | µg | 0 | 0 | ||||||||||||||||
| Vitamin A, IU | IU | 0 | 0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 0.000 | 0.000 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 0.000 | 0.000 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 0.000 | 0.000 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
Table 3. Egg white raw pasteurized and frozen nutrition facts
Nutrient | Unit | Value per 100 g | oz 28 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Water | g | 88.17 | 24.69 | ||||||||||||||||
| Energy | kcal | 48 | 13 | ||||||||||||||||
| Protein | g | 10.20 | 2.86 | ||||||||||||||||
| Total lipid (fat) | g | 0.00 | 0.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 1.04 | 0.29 | ||||||||||||||||
| Fiber, total dietary | g | 0.0 | 0.0 | ||||||||||||||||
| Sugars, total | g | 0.25 | 0.07 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Calcium, Ca | mg | 8 | 2 | ||||||||||||||||
| Iron, Fe | mg | 0.04 | 0.01 | ||||||||||||||||
| Magnesium, Mg | mg | 11 | 3 | ||||||||||||||||
| Phosphorus, P | mg | 13 | 4 | ||||||||||||||||
| Potassium, K | mg | 169 | 47 | ||||||||||||||||
| Sodium, Na | mg | 169 | 47 | ||||||||||||||||
| Zinc, Zn | mg | 0.07 | 0.02 | ||||||||||||||||
| Vitamins | |||||||||||||||||||
| Vitamin C, total ascorbic acid | mg | 0.0 | 0.0 | ||||||||||||||||
| Thiamin | mg | 0.023 | 0.006 | ||||||||||||||||
| Riboflavin | mg | 0.423 | 0.118 | ||||||||||||||||
| Niacin | mg | 0.093 | 0.026 | ||||||||||||||||
| Vitamin B-6 | mg | 0.005 | 0.001 | ||||||||||||||||
| Folate, DFE | µg | 10 | 3 | ||||||||||||||||
| Vitamin B-12 | µg | 0.03 | 0.01 | ||||||||||||||||
| Vitamin A, RAE | µg | 0 | 0 | ||||||||||||||||
| Vitamin A, IU | IU | 0 | 0 | ||||||||||||||||
| Vitamin E (alpha-tocopherol) | mg | 0.00 | 0.00 | ||||||||||||||||
| Vitamin D (D2 + D3) | µg | 0.0 | 0.0 | ||||||||||||||||
| Vitamin D | IU | 0 | 0 | ||||||||||||||||
| Vitamin K (phylloquinone) | µg | 0.0 | 0.0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 0.000 | 0.000 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 0.000 | 0.000 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 0.000 | 0.000 | ||||||||||||||||
| Fatty acids, total trans | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
| Other | |||||||||||||||||||
| Caffeine | mg | 0 | 0 | ||||||||||||||||
Dried Egg White
By having most of the moisture removed, dried egg whites are viewed as more convenient than fresh or liquid egg whites, as the dried version has a longer shelf life and is shelf stable 12. Dried egg white also readily reconstitutes and easily blends with other dry ingredients.
Dried egg white manufacturing in the United States is defined in 21CFR160.145 13; however, the U.S. standard does not specify minimum or maximum moisture contents. Dried egg whites are usually produced by spraying atomized liquid egg white into a heated drier chamber. A continuous flow of accelerated heated air removes most of the moisture. The resulting ingredient is referred to as spray-dried egg white, spray-dried egg white solids or spray-dried egg albumen. Egg white can also be dried on trays or pans to create a flake or granular form.
Glucose, a reducing sugar, is removed from egg whites before drying to produce product with excellent storage stability. Whipping aids such as sodium lauryl sulfate may be added to dried egg white products at less than 0.1 percent by weight of the liquid prior to drying. Dried egg white with sodium lauryl sulfate is often referred to as high-whip dried egg white.
Food manufacturers use dried egg whites in a variety of applications including frozen desserts, bakery mixes, meringues, coatings and batters. For example, dried egg white, in combination with dried milk, flour and seasonings has been shown to make an excellent batter for deep-frying vegetables, meat and seafood. Egg white foams increase six to eight times in volume, and along with the gluten and starch in flour, establish the basic structure for angel food cakes. Dried egg white is also used in white cake formulas. Reconstituted dried egg white can be brushed on top of breads and other bakery products, acting like an adhesive for toppings such as seeds. The egg white also produces a light, shiny surface.
Availability:
- High-Whip Egg White Solids
- Instant Egg White Solids
- Pan Dried Albumen
- High-Gel Egg White Solids
Table 5. Egg white dried nutrition facts
Nutrient | Unit | Value per 100 g | oz 28 g | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approximates | |||||||||||||||||||
| Water | g | 5.80 | 1.62 | ||||||||||||||||
| Energy | kcal | 382 | 107 | ||||||||||||||||
| Protein | g | 81.10 | 22.71 | ||||||||||||||||
| Total lipid (fat) | g | 0.00 | 0.00 | ||||||||||||||||
| Carbohydrate, by difference | g | 7.80 | 2.18 | ||||||||||||||||
| Fiber, total dietary | g | 0.0 | 0.0 | ||||||||||||||||
| Sugars, total | g | 5.40 | 1.51 | ||||||||||||||||
| Minerals | |||||||||||||||||||
| Calcium, Ca | mg | 62 | 17 | ||||||||||||||||
| Iron, Fe | mg | 0.15 | 0.04 | ||||||||||||||||
| Magnesium, Mg | mg | 88 | 25 | ||||||||||||||||
| Phosphorus, P | mg | 111 | 31 | ||||||||||||||||
| Potassium, K | mg | 1125 | 315 | ||||||||||||||||
| Sodium, Na | mg | 1280 | 358 | ||||||||||||||||
| Zinc, Zn | mg | 0.10 | 0.03 | ||||||||||||||||
| Vitamins | |||||||||||||||||||
| Vitamin C, total ascorbic acid | mg | 0.0 | 0.0 | ||||||||||||||||
| Thiamin | mg | 0.005 | 0.001 | ||||||||||||||||
| Riboflavin | mg | 2.530 | 0.708 | ||||||||||||||||
| Niacin | mg | 0.865 | 0.242 | ||||||||||||||||
| Vitamin B-6 | mg | 0.036 | 0.010 | ||||||||||||||||
| Folate, DFE | µg | 18 | 5 | ||||||||||||||||
| Vitamin B-12 | µg | 0.18 | 0.05 | ||||||||||||||||
| Vitamin A, RAE | µg | 0 | 0 | ||||||||||||||||
| Vitamin A, IU | IU | 0 | 0 | ||||||||||||||||
| Vitamin E (alpha-tocopherol) | mg | 0.00 | 0.00 | ||||||||||||||||
| Vitamin D (D2 + D3) | µg | 0.0 | 0.0 | ||||||||||||||||
| Vitamin D | IU | 0 | 0 | ||||||||||||||||
| Vitamin K (phylloquinone) | µg | 0.0 | 0.0 | ||||||||||||||||
| Lipids | |||||||||||||||||||
| Fatty acids, total saturated | g | 0.000 | 0.000 | ||||||||||||||||
| Fatty acids, total monounsaturated | g | 0.000 | 0.000 | ||||||||||||||||
| Fatty acids, total polyunsaturated | g | 0.000 | 0.000 | ||||||||||||||||
| Cholesterol | mg | 0 | 0 | ||||||||||||||||
| Other | |||||||||||||||||||
| Caffeine | mg | 0 | 0 | ||||||||||||||||
Benefits of egg white
Protein intake is an important component of body building, and together with additional supplements (i.e., creatine and amino acids), is highly recommended for regular strength training. Long term, periodized resistance training (weights training) results in increases in skeletal muscle size and, ultimately, force generating capacity 14, 15. Sports nutrition scientists have attempted to increase training induced gains through a number of protocols, which generally attempt to augment and/or speed skeletal muscle regeneration. One such intervention has been to increase the provision of the branched chain amino acids, leucine, isoleucine, and valine, which make up more than one third of muscle protein 16. The branched chain amino acids are unique among the essential amino acids for their roles in protein metabolism 17, neural function 18, and blood glucose and insulin regulation 19. Moreover, Garlick and colleagues 20 have found that branched chain amino acids were able to stimulate skeletal muscle protein synthesis to the same degree as all 9 essential amino acids. Of the branched chain amino acids, only leucine was able to independently stimulate muscle protein synthesis 20. It is well known that vigorous exercise can induce a net negative protein balance in response to both endurance and resistance training 21. Norton and Layman proposed that consumption of branched chain amino acids, namely leucine, could turn individuals from a negative to a positive whole-body protein balance after intense exercise 17. In support, the consumption of a protein or essential amino acid complex that contains sufficient leucine has been shown to shift protein balance to a net positive state after intense exercise training 22. These findings led Norton and Wilson 23 to suggest that optimal protein intake per meal should be based on the leucine content of the protein consumed.
Early research indicates that 2-3 g, or up to 0.05 g/kg bodyweight, of leucine are required to maximize muscle protein synthesis 23, 24, 25. However once this threshold has been reached, a protein’s beneficial effects on muscle protein synthesis effectively plateaus. For example, consuming 40 grams of egg protein (4 grams of leucine) did not enhance muscle protein synthesis over 20 grams of egg protein (2 grams of leucine) 26.
Moore et al. 27 conducted a dose response study of an egg protein supplement comparing 0 g, 5 g, 10 g, 20 g, and 40 g of egg protein delivered after a bout of exercise. After consumption of the supplement, muscle protein synthesis rates were monitored for four hours. Their results suggested that muscle protein synthesis was maximally stimulated with 20 g of egg protein, which contains 1.7 g of leucine. It was also observed that at double that dose (40 g, 3.4 g of leucine), no significant differences in muscle protein synthesis occurred 27. Male collegiate athletes consume more protein than what is recommended by the American Dietetic Association 28. Bianco et al. 29 reported that 30.1% of male athletes use dietary supplements during training as a “way to gain muscle and strength”, and also showed that whey protein shakes (50.0%) supplemented with creatine and amino acids (48.3%) were the most frequent choices amongst users.
Several reports describe how whey protein and amino acid supplementation increases muscle protein synthesis at rest 30, 31 and after weights training 32.
However this small study involving 30 young Japanese female college athletes showed that egg white protein supplementation (15 grams egg white protein; 75 kcal) or carbohydrate supplementation (17.5 g maltodextrin, 78 kcal). At baseline and 8 weeks after the regimen, participants performed 1RM strength tests by leg curl (LC), leg extension (LE), squat, and bench press exercises. The result was after 8 weeks ofegg white protein supplementation did not change body lean mass or muscle strength between those who consume egg white protein or carbohydrate 33. The findings showed that daily supplementation of either carbohydrate or egg white protein resulted in increase in the lean muscle mass and 1-RM (1-repetition maximum) muscle strength.
References- American Egg Board. Nutrition. http://www.aeb.org/food-manufacturers/why-eggs/55-production
- Food and Agriculture Organization of the United Nations. The Amino Acid Content of Hen’s Egg in Relation to Dietary Protein Intake, Breed and Environment. http://www.fao.org/docrep/meeting/009/ae906e/ae906e26.htm
- Food and Agriculture Organization of the United Nations. Egg facts. http://www.fao.org/resources/infographics/infographics-details/en/c/284410/
- American Egg Board. Refrigerated Liquid/Frozen Egg White. http://www.aeb.org/food-manufacturers/eggs-product-overview/egg-products-specifications/45-egg-white-types/138-frozen-liquid
- Mordier S, Deval C, Bechet D, Tassa A, Ferrara M. Leucine limitation induces autophagy and activation of lysosome-dependent proteolysis in C2C12 myotubes through a mammalian target of rapamycin-independent signaling pathway. J Biol Chem. 2000;275:29900–6. http://www.jbc.org/content/275/38/29900.long
- Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1–13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1–13C]valine. Am J Physiol. 1992;262:E372–6. https://www.ncbi.nlm.nih.gov/pubmed/1550230
- Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–8. http://ajpendo.physiology.org/content/275/1/E73.long
- Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3192452/
- Garlick PJ, Grant I. Amino-acid infusion increases the sensitivity of muscle protein-synthesis in vivo to insulin: effect of branched-chain amino-acids. Biochem J. 1988;254:579–84. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1135117/pdf/biochemj00224-0256.pdf
- Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. J Nutr. 2010 Nov; 140(11):1970-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2955876/
- United States Department of Agriculture Agricultural Research Service. USDA Branded Food Products Database. https://ndb.nal.usda.gov/ndb/search/list
- American Egg Board. Dried Egg White. http://www.aeb.org/food-manufacturers/eggs-product-overview/egg-products-specifications/45-egg-white-types/139-dried
- The U.S. Government Publishing Office. Electronic Code of Federal Regulations. https://www.ecfr.gov
- Nonlinear periodization maximizes strength gains in split resistance training routines. Monteiro AG, Aoki MS, Evangelista AL, Alveno DA, Monteiro GA, Piçarro Ida C, Ugrinowitsch C. J Strength Cond Res. 2009 Jul; 23(4):1321-6. https://www.ncbi.nlm.nih.gov/pubmed/19528843/
- Turner A. The science and practice of periodization: a brief review. Strength and Conditioning Journal. 2011;33:34–46.
- The effects of amino acid supplementation on hormonal responses to resistance training overreaching. Kraemer WJ, Ratamess NA, Volek JS, Häkkinen K, Rubin MR, French DN, Gómez AL, McGuigan MR, Scheett TP, Newton RU, Spiering BA, Izquierdo M, Dioguardi FS. Metabolism. 2006 Mar; 55(3):282-91. https://www.ncbi.nlm.nih.gov/pubmed/16483870/
- Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. Norton LE, Layman DK. J Nutr. 2006 Feb; 136(2):533S-537S. http://jn.nutrition.org/content/136/2/533S.long
- Carbohydrates, branched-chain amino acids, and endurance: the central fatigue hypothesis. Davis JM. Int J Sport Nutr. 1995 Jun; 5 Suppl():S29-38. https://www.ncbi.nlm.nih.gov/pubmed/7550256/
- Branched-chain amino acids: enzyme and substrate regulation. Brosnan JT, Brosnan ME. J Nutr. 2006 Jan; 136(1 Suppl):207S-11S. http://jn.nutrition.org/content/136/1/207S.long
- The role of leucine in the regulation of protein metabolism. Garlick PJ. J Nutr. 2005 Jun; 135(6 Suppl):1553S-6S. http://jn.nutrition.org/content/135/6/1553S.long
- Role of leucine in protein metabolism during exercise and recovery. Layman DK. Can J Appl Physiol. 2002 Dec; 27(6):646-63. https://www.ncbi.nlm.nih.gov/pubmed/12501002/
- An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Biolo G, Tipton KD, Klein S, Wolfe RR. Am J Physiol. 1997 Jul; 273(1 Pt 1):E122-9. https://www.ncbi.nlm.nih.gov/pubmed/9252488/
- Norton L, Wilson GJ. Optimal protein intake to maximize muscle protein synthesis. AgroFood industry hi-tech. 2009;20:54–57.
- Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. http://ajpendo.physiology.org/content/286/3/E321.long
- Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999;276:E628–E634. http://ajpendo.physiology.org/content/276/4/E628.long
- Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol. 2009;107:987–992. doi: 10.1152/japplphysiol.00076.2009. http://jap.physiology.org/content/107/3/987.long
- Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Am J Clin Nutr. 2009 Jan; 89(1):161-8. http://ajcn.nutrition.org/content/89/1/161.long
- Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. Rodriguez NR, DiMarco NM, Langley S, American Dietetic Association., Dietitians of Canada., American College of Sports Medicine: Nutrition and Athletic Performance. J Am Diet Assoc. 2009 Mar; 109(3):509-27. https://www.ncbi.nlm.nih.gov/pubmed/19278045/
- Bianco A., Mammina C., Paoli A., Bellafiore M., Battaglia G., Caramazza G., Palma A., Jemni M. Protein supplementation in strength and conditioning adepts: Knowledge, dietary behavior and practice in Palermo, Italy. J. Int. Soc. Sports Nutr. 2011;8:25. doi: 10.1186/1550-2783-8-25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3267647/
- Bohe J., Low J.F., Wolfe R.R., Rennie M.J. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J. Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2278544/
- Bohe J., Low A., Wolfe R.R., Rennie M.J. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose-response study. J. Physiol. 2003;552:315–324. doi: 10.1113/jphysiol.2003.050674. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2343318/
- Borsheim E., Tipton K.D., Wolf S.E., Wolfe R.R. Essential amino acid and muscle protein recovery from resistance exercise. Am. J. Physiol. Endocrinol. Metab. 2002;283:E648–E657. http://ajpendo.physiology.org/content/283/4/E648.long
- Hida A, Hasegawa Y, Mekata Y, et al. Effects of Egg White Protein Supplementation on Muscle Strength and Serum Free Amino Acid Concentrations. Nutrients. 2012;4(10):1504-1517. doi:10.3390/nu4101504. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3497008/