E-Cigarette
Electronic cigarettes are known by many different names, sometimes called “e-cigarettes,” “e-cigs,” “e-hookahs,” “mods,” “vape pens,” “vapes,” “tank systems,” and “electronic nicotine delivery systems (ENDS)” 1. Using an e-cigarette is sometimes called “vaping.” E-cigarettes are electronic devices that heat a liquid solution (an e‐liquid or “e‐juice”), often containing nicotine (the addictive drug in regular cigarettes, cigars, and other tobacco products), flavorings, and other chemicals and produce an aerosol, or mix of small particles in the air 2. Users inhale this aerosol into their lungs. Bystanders can also breathe in this aerosol when the user exhales into the air. E-cigarettes are sometimes also used to deliver marijuana (delta‐9‐tetrahydrocannabinoid or THC) or cannabis oil (cannabidiol or CBD oil) and other drugs. Tetrahydrocannabinol (THC) is the psychoactive mind-altering compound of marijuana that produces the “high.” E-cigarettes come in many shapes and sizes. Most have a battery, a heating element, a place to hold a liquid, an atomizer that transforms the liquid to an aerosol and a mouthpiece. Some e-cigarettes look like regular cigarettes, cigars, or pipes. Some look like USB flash drives, pens, and other everyday items. Larger devices such as tank systems, or “mods,” do not look like other tobacco products. Most adult users are current or former smokers who use e-cigarettes to reduce or quit cigarette smoking. E-cigarettes are not currently approved by the U.S. Food and Drug Administration (FDA) as a quit smoking devices. The U.S. Preventive Services Task Force, a group of health experts that makes recommendations about preventive health care, has concluded that evidence is insufficient to recommend e-cigarettes for smoking cessation in adults, including pregnant adults 3. e-Cigarettes are also popular among youth, with rates of e-cigarette use surpassing those of cigarette use in this population. Flavored products may be especially appealing to youth. Sweet flavors, such as “mango fruit medley” and “candy,” may be particularly appealing to youth, who can become addicted to nicotine and may have difficulty quitting e-cigarettes 4. Rates of secondhand exposure to e-cigarette vapor among youth are also high at 18% for middle schoolers and 29% for high schoolers 5. Secondhand e-cigarette exposure increases the risk of an asthma attack by 27% in youth with asthma 6. Youth e-cigarette use is also associated with increased risk of subsequent cigarette and marijuana use 7.
The e-cigarette aerosol that users breathe from the device and exhale can contain harmful and potentially harmful substances, including 8:
- Nicotine
- Ultrafine particles that can be inhaled deep into the lungs
- Flavoring such as diacetyl, a chemical linked to a serious lung disease
- Volatile organic compounds
- Cancer-causing chemicals
- Heavy metals such as nickel, tin, chromium, arsenic and lead 9
It is difficult for consumers to know what e-cigarette products contain. For example, some e-cigarettes marketed as containing zero percent nicotine have been found to contain nicotine 10. Although 99% of e-cigarettes contain nicotine, teens are more likely to report using e-cigarettes for substances other than nicotine, including flavors and marijuana 11.
e-Cigarettes have been marketed as healthier alternatives to traditional cigarettes because they do not combust tobacco 12. However, long-term health effects of e-cigarettes are unknown 13. e-Cigarettes pose several potential health risks, including exposure to heavy metals and toxicants, and nicotine poisoning. Although the impact of e-cigarette use in pregnancy is unknown, nicotine is a teratogen 14. A teratogen is a substance that interferes with the normal development of a fetus and can cause birth defects. Therefore, pregnant women should abstain from using all nicotine/tobacco products during pregnancy.
Are e-cigarettes less harmful than regular cigarettes?
Yes, but that doesn’t mean e-cigarettes are safe. E-cigarette aerosol generally contains fewer toxic chemicals than the deadly mix of 7,000 chemicals in smoke from regular cigarettes 15. However, e-cigarette aerosol is not harmless. It can contain harmful and potentially harmful substances, including nicotine, heavy metals like lead, volatile organic compounds, and cancer-causing agents 16.
In recent months the Centers for Disease Control and Prevention (CDC) has reported more than 2,500 cases of lung injury tied to vaping, mostly involving products that contain tetrahydrocannabinol (THC). The CDC and the Food and Drug Administration (FDA) recommend that people not use vaping products that contain THC, particularly from sources such as friends, family, or in-person or online dealers. The FDA is also warning people not to add THC, other oils or any other substances to vaping products. If you vape, watch for symptoms like coughing, shortness of breath and chest pain. Seek medical attention if you’re concerned about your health.
E-cigarettes containing nicotine aren’t considered safe for adolescents, young adults or pregnant women. Nicotine can harm brain development in children and young adults into their early 20s and is toxic to developing fetuses. Children and adults have also been poisoned by swallowing, breathing or absorbing e-cigarette liquid through their skin or eyes, according to the CDC.
In youth and adult nonsmokers, e-cigarette use also poses the risk of a nicotine addiction. This could lead to long-term use of e-cigarettes, the effects of which aren’t known, or to the use of traditional cigarettes. Research has shown that teen use of e-cigarettes is on the rise and associated with increased future use of traditional cigarettes.
Rarely, defective e-cigarette batteries have caused fires and explosions, mostly while the batteries are being charged.
Can e-cigarettes help adults quit smoking cigarettes?
E-cigarettes are not currently approved by the U.S. Food and Drug Administration (FDA) as a quit smoking devices. The U.S. Preventive Services Task Force, a group of health experts that makes recommendations about preventive health care, has concluded that evidence is insufficient to recommend e-cigarettes for smoking cessation in adults, including pregnant adults 3. Marketers claim it is easier to quit smoking if you switch to vaping first. But e-cigarettes contain nicotine and may even lead to a user becoming a traditional cigarette smoker. If you’re looking for help to stop smoking, there are several FDA-approved medications that have been shown to be safe and effective for this purpose. A combination of medication and counseling has been shown to work best. If you want to stop smoking, call 800-QUIT-NOW (800-784-8669) to connect to your state’s quit line.
E-cigarettes are not safe for youth, young adults, pregnant women, or adults who do not currently use tobacco products. However, e-cigarettes may help non-pregnant adults who smoke if used as a complete substitute for all cigarettes and other smoked tobacco products.
- To date, the few studies on the issue are mixed. A Cochrane Review found evidence from two randomized controlled trials that e-cigarettes with nicotine can help adults to stop smoking for at least six months or longer compared with placebo (non-nicotine) e-cigarettes 17. None of the included studies (short‐ to mid‐term, up to two years) detected serious adverse events considered possibly related to e-cigarettes use. The most commonly‐reported adverse effects were throat/mouth irritation, headache, cough, and nausea, which tended to dissipate with continued use. In some studies, reduced toxin concentrations and biomarkers of harm were observed in people who smoked and switched to vaping, consistent with reductions seen in smoking cessation. However, there are some limitations to the existing research, including the small number of trials, small sample sizes, and wide margins of error around the estimates.
- A recent Centers for Disease Control and Prevention (CDC) study found that many adults are using e-cigarettes in an attempt to quit smoking 18. However, most adult e-cigarette users do not stop smoking cigarettes and are instead continuing to use both products (known as “dual use”) 19. Dual use is not an effective way to safeguard your health, whether you’re using e-cigarettes, smokeless tobacco, or other tobacco products in addition to regular cigarettes. Because smoking even a few cigarettes a day can be dangerous, quitting smoking completely is very important to protect your health 20.
What are the dangers of e-cigarettes and vaping?
Experts have a number of concerns about the safety of e-cigarettes and vaping. E-cigarettes contain nicotine. In large doses, nicotine can be toxic.
- Makers claim that e-cigarettes don’t contain the harmful chemicals that cigarettes do. Of course, this is not true. Most devices contain nicotine. While there are some cartridges that don’t contain nicotine, most do. Any time a smoker inhales nicotine, they are inhaling an addicting and harmful chemical.
- Nicotine stimulates your central nervous system. This increases your blood pressure, breathing, and heart rate. Higher doses of nicotine can cause blood pressure and heart rate to go higher. This can lead to an abnormal heart rate (arrhythmia). In rare cases, this can cause heart failure or death. Over time, nicotine can lead to medical problems. These include heart disease, blood clots, and stomach ulcers.
- Nicotine increases the level of dopamine in your brain. This chemical messenger affects the part of the brain that controls feelings of pleasure. It can motivate you to use nicotine again and again to get that feeling of pleasure. You do this even though you know it is a risk to your health and well-being. That is what makes nicotine addictive.
- The ingredients in the liquid are not labeled. This means that we don’t know for sure what’s in the liquid or in what amounts.
- There are often chemicals in the liquid. Some of these are known to cause cancer. One study found a toxic chemical that is found in antifreeze.
- Tiny particles are released by the heating element and may be harmful. These particles can cause inflammation in the lungs, which can cause bacterial infections or pneumonia.
- The liquid in the cartridge can be poisonous if someone touches, sniffs, or drinks it. There has been an increase in poisoning cases of children under 5 who have had access to the liquid.
- “Secondhand smoke” is still a problem for e-cigarettes. Secondhand e-cigarette vapor contains chemicals that harm the lungs and hearts of people who aren’t vaping.
- They serve as an introductory product for preteens and teens. Many kids start with vaping and then move on to other tobacco products.
- Right now, there is little regulation when it comes to e-cigarettes.
Potential harms of e-Cigarettes
E-cigarettes are still fairly new, so scientists are still learning about their long-term health effects. Most e-cigarettes contain high concentrations of nicotine, which is highly addictive and can cause nicotine poisoning through ingestion, skin contact, or inhalation 21. e-Cigarette aerosol also delivers toxicants, including heavy metals (such as nickel, tin, chromium, arsenic and lead), although in lower concentrations than cigarette smoke 13. Little is known about the health impact of inhaling humectants (solvents), diluents (cutting agents), and flavorings from e-cigarettes. Harmful exposures related to vaping may occur from the high temperature‐induced degradation or reactions among the chemical constituents 22, metals released from the coil or e‐liquid itself (such as arsenic, cadmium, and nickel) 16 and/or fungal and bacterial contaminants on the coil or in the e‐liquid 23. Harm has also occurred from contact with the liquid nicotine solution, especially in children, and defective e-cigarette batteries may also explode, causing burns 24. Children exposed to e-cigarette liquid have more than five times the risk of hospital admission than children exposed to cigarettes 21. Small children are especially vulnerable to medical problems from e-cigarette exposure 21. Symptoms of nicotine poisoning include dizziness, tachycardia, vomiting, and seizures.
There are no data regarding the risks of e-cigarette use, including the relative risks vs. cigarette use, in pregnant women. However, nicotine itself is teratogenic, even in the absence of combustion byproducts. Therefore, pregnant women should abstain from using all nicotine/tobacco products during pregnancy. Cigarette use during pregnancy is associated with low birth weight, preterm birth, and sudden infant death syndrome (SIDS) 14. The risks of e-cigarette use, including relative risks versus cigarette use and risks of dual use (cigarettes and e-cigarettes used concurrently), for pregnant women and fetal development are unknown 25. However, although tobacco combustion byproducts are considered more harmful than nicotine for nonpregnant adults, nicotine itself is a developmental toxicant when used during pregnancy and is characterized by the FDA as teratogenic to human fetuses 14. In animal models, exposure to prenatal nicotine upregulates nicotine receptors, leading to changes in offspring brain structure and function 14.
Nicotine may have a detrimental impact on brain development in youth, and e-cigarettes may act as a gateway drug for cigarette or marijuana use. Youth are also susceptible to nicotine addiction. In animal models, nicotine lowers impulse control circuits, alters circuits related to mood and addiction, and affects brain regions dedicated to attention and learning 9. There is growing evidence that e-cigarette use increases risk of cigarette initiation 26. A meta-analysis is a statistical analysis that combines the results of multiple scientific studies. A recent meta-analysis indicated that in teens and young adults who have never smoked, the odds of smoking initiation are three to six times greater in those who have ever used e-cigarettes than in those who have never used e-cigarettes 27. One study also showed links between e-cigarette use and subsequent marijuana use in teens 7.
E-Cigarette or Vaping Product Use Associated Lung Injury (EVALI)
E-Cigarette or Vaping Product Use Associated Lung Injury also known as EVALI, encompasses a syndrome of respiratory, gastrointestinal, and constitutional symptoms 28. Symptoms of EVALI include nausea, vomiting, diarrhea, abdominal pain, chest pain, cough, and shortness of breath. Constitutional symptoms of fever, chills, fatigue, and recent weight loss are also frequently encountered. In a composite of 3 published EVALI case series, shortness of breath (dyspnea) (86%), cough (80%), and chest pain (49%) were the most commonly reported respiratory symptoms at presentation 29, 30, 31. In addition, subjective fever (79%), nausea (71%), and vomiting (70%) were the most commonly reported constitutional and gastrointestinal symptoms, respectively 28.
Some patients with EVALI may appear ill and present with symptoms that closely mimic pneumonia with severe sepsis 28. Common presenting signs in the composite of the EVALI patients in the 3 studies are fever (79%), tachycardia (74%), tachypnea (55%), and/or hypoxia (42%) 29, 30, 31. Patients may be dehydrated from severe gastrointestinal fluid losses and insensible losses due to fever. Others may present with predominant gastrointestinal distress and little, if any, respiratory complaints.
On initial laboratory evaluation, patients with EVALI may have an elevated white blood cell count, mildly elevated liver transaminases (typically aspartate transaminase and alanine transaminase are <100 U/L), and an elevated erythrocyte sedimentation rate. Electrolyte derangement may also be present due to gastrointestinal losses 29. Creatinine elevation is infrequently seen but may occur due to prerenal causes, such as hypovolemia with dehydration.
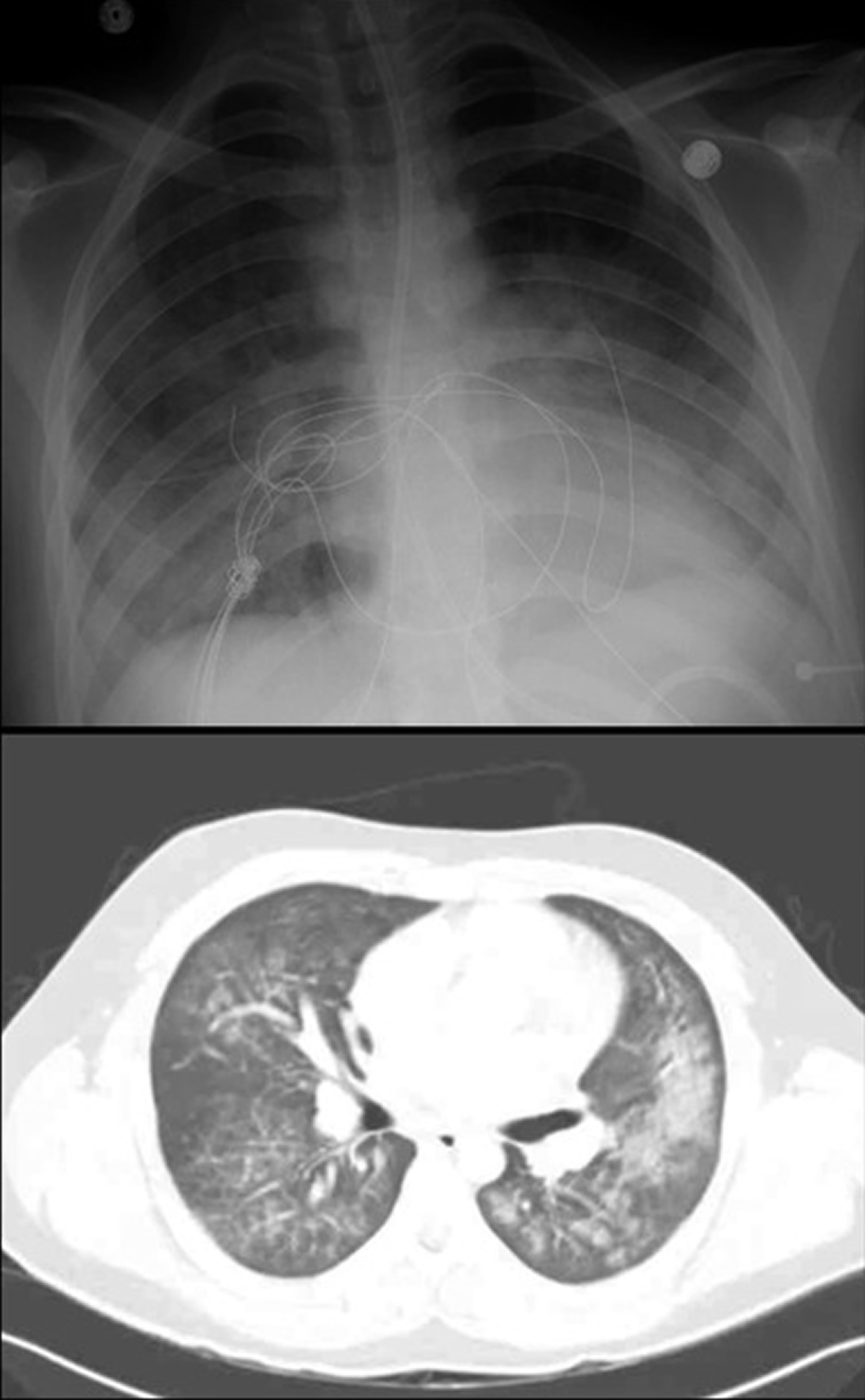
By the CDC inclusion criteria for EVALI, all patients have abnormal radiographs; either chest x‐ray or computed tomography (CT). Initial chest x‐ray was abnormal and 94% of patients in 3 case series, with findings including nonspecific bilateral opacities or infiltrates 29, 30, 31. The 6% with negative chest x‐rays have positive findings on chest CTs. The most frequent CT finding is bilateral diffuse and basilar ground glass opacities with subpleural sparing 32. This nonspecific pattern is also suggestive of atypical pneumonia or diffuse alveolar hemorrhage. Patients presenting with predominant gastrointestinal symptoms may have incidental pulmonary findings on CT of abdomen/pelvis, especially because pulmonary findings are typically seen in the lower dependent lung fields 33. In addition, CT is more sensitive for pneumothorax and/or pneumomediastinum, both of which have been reported in EVALI as illustrated by 2 recent case series where these were noted on CT in 18% and 10% of patients, respectively 29, 30.
As of February 18, 2020, the e‐cigarette or vaping product‐use associated lung injury (EVALI) epidemic has resulted in a total of 2,807 hospitalized EVALI cases with 64 deaths across 29 states and the District of Columbia 34. Most patients present with respiratory, gastrointestinal, and constitutional symptoms, such as fever 29.
Laboratory data show that vitamin E acetate, an additive in some tetrahydrocannabinol (THC)-containing e-cigarette, or vaping, products, is strongly linked to the EVALI outbreak 34.
- A recent study analyzed samples from 51 EVALI cases from 16 states and a comparison group of samples from 99 comparison individuals without EVALI for vitamin E acetate, plant oils, medium chain triglyceride (MCT) oil, coconut oil, petroleum distillates, and diluent terpenes 35.
- Vitamin E acetate was identified in bronchoalveolar lavage fluid samples (fluid samples collected from the lungs) from 48 of the 51 EVALI patients, but not in the bronchoalveolar lavage fluid from the healthy comparison group.
- No other toxicants were found in bronchoalveolar lavage fluid from either group, except for coconut oil and limonene (1 EVALI patient each).
Figure 1. EVALI chest x‐ray and chest CT Imaging in an otherwise healthy adolescent
Footnote: Imaging findings of EVALI may be overlooked due to unfamiliarity of this diagnosis by radiologists. While pulmonary infiltrates are widely considered inclusion criteria 36, no formal diagnostic imaging criteria exist for EVALI 37. Complicating radiologic diagnosis, a number of poorly understood pulmonary complications and diseases, such as hypersensitivity pneumonitis, acute lung injury/diffuse alveolar hemorrhage, acute eosinophilic pneumonia, lipoid pneumonia, giant cell interstitial pneumonia, and respiratory-associated bronchiolitis-associated interstitial lung disease, have been attributed to, or may occur with, EVALI 38
[Source 28 ]What causes EVALI?
The exact pathophysiology of EVALI is not known and may represent a spectrum of disease rather than a distinct entity. Butt et al 39 reviewed lung biopsies from cases of confirmed or suspected EVALI, finding that all cases demonstrated histopathological findings of acute lung injury, including diffuse alveolar hemorrhage, organizing pneumonia, and/or acute fibrinous pneumonitis. While the researchers found no histological findings specific to EVALI, they identified foamy macrophages and pneumocyte vacuolization in all cases. The authors concluded that EVALI represents a form of chemical pneumonitis from a yet-to-be identified group of toxic substances 39.
The CDC and FDA, in coordination with state/local health departments and other professional societies, have performed a multi‐angle investigation of possible EVALI causes. No single brand and/or site of purchase have been linked throughout the investigation 28. Although both nicotine and THC vaping were initially linked to EVALI, >85% of patients with EVALI reported recent THC vaping 40. THC use may still be underreported due to the stigma and fear of ramifications of illicit drug use 41. At this time, the amount of exposure required to cause EVALI is unclear. Proposed factors that help pinpoint the patients who are at most risk for EVALI include (i) using THC containing products, (ii) using >5 times per day, and (iii) acquiring products from informal sources (eg, dealer or friend) 40.
Testing remnants from liquid cartridges has linked one component in particular, vitamin E acetate 42. Interestingly, most THC cartridges (52%) seized and analyzed during the EVALI outbreak in 2019 contained vitamin E acetate, whereas THC cartridges seized by law enforcement in 2018 did not contain any vitamin E acetate 43. Further linking vitamin E acetate to EVALI, a recent study found vitamin E acetate in 48 of 51 (94%) bronchoalveolar lavage fluid samples from 16 different states, whereas vitamin E acetate was not found in bronchoalveolar lavage samples from e‐cigarette users with no lung injury 44. The exact pathophysiologic mechanism linking vitamin E acetate to lung injury may stem from its inability to be absorbed by the pulmonary tissue leading to accumulation and interference with surfactant 44. Although vitamin E acetate in THC containing cartridges is the prime suspected cause for EVALI, 13% of EVALI patients endorsed exclusive vaping of nicotine‐containing cartridges or e‐liquids 41. At this point, the possibility of an alternative cause or multiple implicating agents cannot be excluded.
Key facts about vitamin E acetate:
- Vitamin E is a vitamin found in many foods, including vegetable oils, cereals, meat, fruits, and vegetables. It is also available as a dietary supplement and in many cosmetic products, like skin creams.
- Vitamin E acetate usually does not cause harm when ingested as a vitamin supplement or applied to the skin. However, previous research suggests that when vitamin E acetate is inhaled, it may interfere with normal lung functioning.
- Vitamin E acetate is used as an additive, most notably in tetrahydrocannabinol (THC)-containing e-cigarette, or vaping, products.
EVALI diagnosis
Because the symptoms of EVALI are easily misinterpreted for other pulmonary or gastrointestinal disease processes such as pneumonitis, gastroenteritis, or even appendicitis, EVALI is a diagnosis of exclusion, empiric infectious treatment and workup should begin immediately. The CDC has recommended minimum testing to include complete blood count, comprehensive metabolic panel, respiratory infectious panel (including influenza), urine legionella if indicated by history, urinalysis, blood cultures, and chest x‐ray (Table 1). Testing considerations should also include other infectious causes with similar clinical and radiological features, such as coronavirus disease 2019 (COVID‐19). Chest CT with or without contrast is also highly recommended if patients exhibit significant respiratory complaints and have risk factors for EVALI even if initial chest x‐ray is normal 45. Chest CT with intravenous contrast may also be helpful in ruling out other causes of hypoxia, such as pulmonary embolism.
The CDC has published guidelines for establishing probable or confirmed cases of EVALI (see Table 2) 45. The first and most important criterion is the use of a vaping device within 90 days of symptom onset. Due to stigma or fear of legal ramifications regarding illicit substance use (such as THC), patients may be reluctant to discuss this history. In adolescents or the seriously ill, gathering a collateral history from family or friends may reveal a history of use. Similarly, history should be obtained from the patient privately to address reluctance of discussing illicit use in front of family and friends. Like other sensitive history gathered in the emergency room, confidentiality and nonjudgmental questioning should be employed. In addition, repeat questioning may help as the patient‐physician relationship is being established 46.
Similar to eliciting medication history where over‐the‐counter medications are sometimes overlooked, clinicians should recognize that patients will not willingly volunteer e‐cigarette use even if asked about smoking habits. When gathering a history of e‐cigarette use, eliciting specifics may inform the plan of care: how often does the patient vape, when was the patient’s last use, what type of oil was used (nicotine, THC, etc), where did the patient get the oil (illicit or legitimate means), is the patient refilling the cartridge, and is the patient mixing his/her own e‐liquid 46.
Table 1. Relevant assessment questions for patients with suspected EVALI
| Vaping history components for suspected EVALI | |
| Substance vaped | THC, nicotine, and/or other |
| The following should be asked of each substance vaped: | |
| Substance origin | Illicit or legitimate means |
| Diluents | Cutting oils |
| Flavoring | Flavored or unflavored |
| Cartridge cleanliness | Disposable or refilled |
| Vaping onset | Recent or long‐term |
| Last vaping use | ≤90 days (included in case definition) |
| Frequency | Daily, weekly, or monthly (multiple times daily poses risk) |
| Additional social history | Other substance use |
Table 2. CDC case definition of probable versus confirmed EVALI
| Confirmed case | Vaping or dabbing history within 90 days before symptom onset + Pulmonary infiltrate or ground‐glass opacities on chest x‐ray OR chest CT + Negative for pulmonary infection on initial workup after studies including a minimum of negative respiratory viral panel and influenza PCR; other studies if suspected include urine antigen testing for Streptococcus pneumoniae and Legionella, sputum culture if productive cough is reported, bronchoalveolar lavage culture if performed, and HIV‐related opportunistic respiratory infection testing a + No evidence of alternative diagnoses, such as autoimmune disease or malignant process |
| Probable case | Vaping or dabbing history within 90 days before symptom onset + Pulmonary infiltrate or ground‐glass opacities on chest x‐ray OR chest CT + Positive for pulmonary infection on initial workup, but the primary clinical team believes that the infection is not the single cause of the underlying pulmonary disease OR the minimum infectious testing was not performed + No evidence of alternative diagnoses, such as autoimmune disease or malignant process |
Footnotes: a Minimum criteria include negative respiratory viral panel, influenza polymerase chain reaction or rapid test if local epidemiology supports testing. All other clinically indicated respiratory infectious disease testing must be negative (such as blood culture, urine antigen for Streptococcus pneumoniae and Legionella, sputum culture if productive cough is present, bronchoalveolar lavage culture if performed, and human immunodeficiency virus‐related opportunistic respiratory infections are of concern).
[Source 28 ]Table 3. Suggested diagnostic criteria for EVALI
| Use of e-cigarettes |
| Pulmonary infiltrate on chest radiograph or ground glass opacities on computerized tomography (CT) scan |
| Elevated white blood cell count and inflammatory markers (c-reactive protein, erythrocyte sedimentation rate) |
| Absence of pulmonary infection—negative for respiratory viruses including influenza, negative HIV or HIV-related infections, negative blood, sputum and/or bronchial alveolar lavage (BAL) cultures |
| Foamy macrophages containing vitamin E acetate on bronchial alveolar lavage/lung pathology 30 |
| No evidence of alternative medical causes (e.g., heart failure, rheumatologic disease, cancer) |
EVALI complications
The main serious complications of e-cigarette or vaping product use associated lung injury (EVALI) are 47:
- Acute respiratory distress syndrome (ARDS)
- Respiratory failure
- Need for intubation and mechanical ventilation
- Death
EVALI treatment
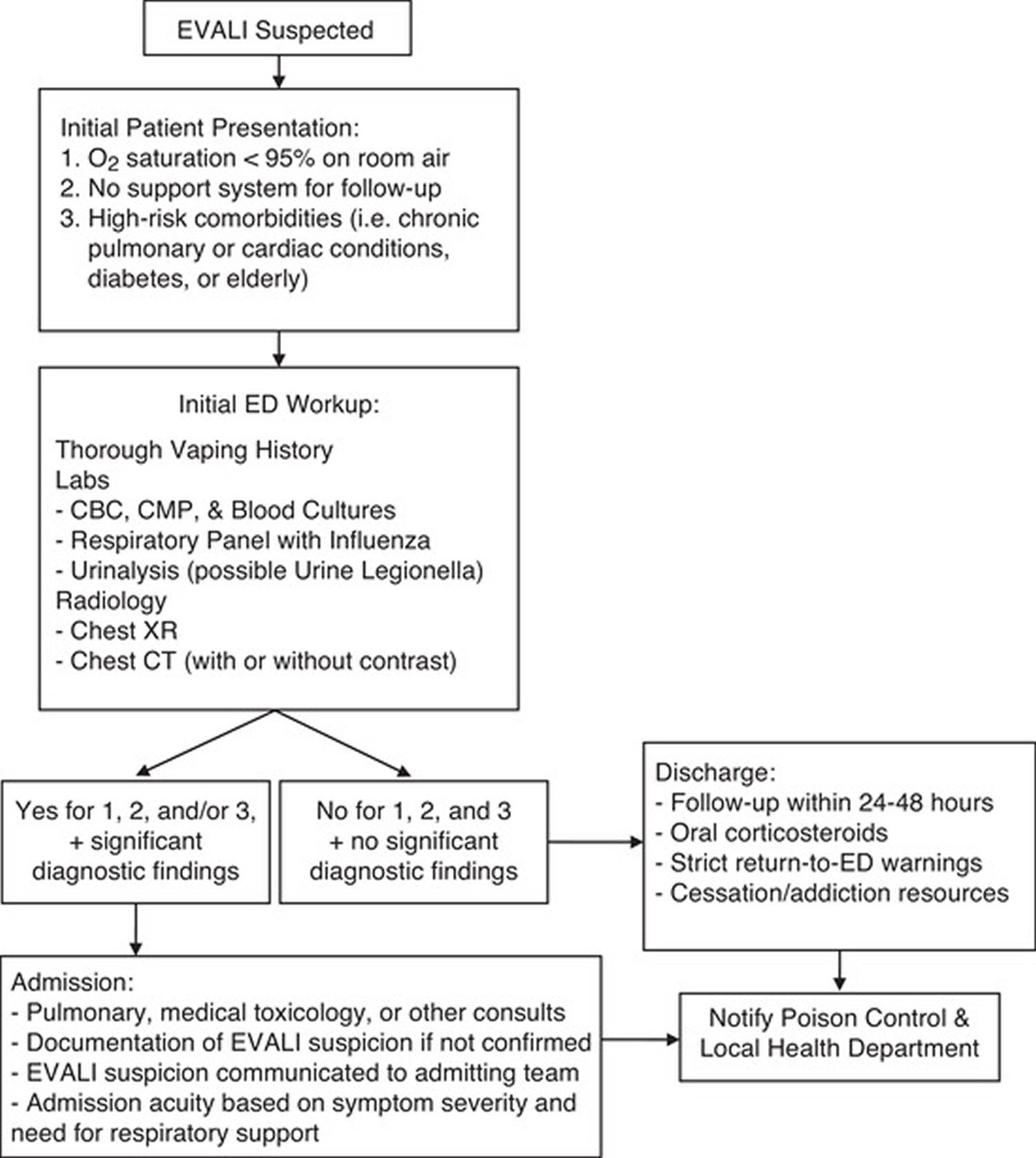
Determining need for hospital admission versus discharge requires careful clinical consideration by emergency physicians due to the high risk of rehospitalization and mortality among EVALI patients (see Figure 2). One out of every 7 EVALI deaths occurred in a median of 3 days after hospital discharge 48.
Patients potentially appropriate for outpatient management have normal oxygen saturations ≥95% with no respiratory distress on room air, lack high‐risk comorbidities such as chronic obstructive pulmonary disease or congestive heart failure, and have a support system for outpatient follow‐up 45. Due to the considerable degree of rehospitalization and death, ensuring follow‐up within 24–48 hours post‐discharge is imperative 28. Briefly discussing with the primary care provider can help ensure close follow up. If a patient lacks adequate outpatient support systems for follow‐up, the patient warrants admission due to the potential for rapid illness progression 45.
As expected, a higher incidence of chronic conditions (pulmonary, cardiac, advanced age, and diabetes) was present among patients who died post‐discharge 48. Discharge prescriptions may include a short course of oral corticosteroids with education on pharmacological tapering and antibiotic/antiviral prescriptions if warranted 45. The dosage and course may vary depending on clinical severity, and the decision may be in conjunction with pulmonary specialists. Glucocorticoids recommended by other institutions have been shown to improve disease severity and are a treatment modality consistent with standard management of steroid‐responsive pulmonary disease practiced by lung specialists 29, 30, 31. Education on strict return‐to‐emergency room warnings, annual influenza vaccination, and access to outpatient cessation and addiction resources should also be provided 46. If the patient intends to continue vaping, the patient should be instructed to avoid THC, illicit substances, and mixing or cutting substances 46. Patients who smoke nicotine e‐cigarettes should not switch to smoking traditional combustible cigarettes. In patients under 18 years of age, the CDC recommends that patients discontinue vaping altogether.
For EVALI patients presenting with abnormal vital signs that require admission, emergency physicians serve as the first line for gathering an adequate vaping history and should present this new differential diagnosis for consideration to the admitting team. EVALI patients benefit from consultation with pulmonology, medical toxicology (or local poison center), and/or psychiatry who may recommend further specific testing and evaluation once the patient is admitted 30. Intravenous or oral steroids are anecdotally beneficial in hospitalized EVALI patients. Recommended doses for admitted patients ranges from methylprednisone 120–500 mg daily 29 or 1 mg/kg for 1–2 days with a transition to oral prednisone 30.
Admission to the medical floor versus critical care area should be determined by the severity of presenting symptoms and need for noninvasive or invasive respiratory support. Pneumothorax and/or pneumomediastinum are reported in EVALI and may worsen with escalation of positive pressure ventilation 29. Extracorporeal membrane oxygenation (ECMO) has been successful in EVALI and is recommended for patients that are continually hypoxic after standard ventilation management 28.
Figure 2. EVALI diagnostic and treatment algorithm
[Source 28 ]EVALI prognosis
E-cigarette or vaping product use associated lung injury (EVALI) is a potentially fatal disease, with 68 deaths reported so far 34. The long-term effects and the risk for recurrence of EVALI are not known, as it is a newly described disease entity 49. A recent study of 98 patients showed that as many as 76% of the cases needed supplemental oxygen, 22% required non-invasive ventilation and 26% required intubation and mechanical ventilation 29. Poor prognostic indicators include age more than 35 years, comorbidities that compromise pulmonary reserve, and patients presenting with resting oxygen saturation less than 95% 50. These patients can rapidly deteriorate and end up developing acute respiratory distress syndrome (ARDS) 51.
Doctors are continuing to learn more about the long-term prognosis for patients with EVALI. Whereas many patients’ symptoms resolve, clinicians report that some patients have relapsed during corticosteroid tapers or with resumption of e-cigarette, or vaping, product use after hospitalization, underscoring the need for cessation and close follow-up. To prevent recurrence, the CDC advises avoiding the use of vaping devices with any nicotine or THC-containing juices, as both have been linked to EVALI 46.
References- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems; Eaton DL, Kwan LY, Stratton K, editors. Public Health Consequences of E-Cigarettes. Washington (DC): National Academies Press (US); 2018 Jan 23. 3, E-Cigarette Devices, Uses, and Exposures. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507187
- Brown, C. J., & Cheng, J. M. (2014). Electronic cigarettes: product characterisation and design considerations. Tobacco control, 23 Suppl 2(Suppl 2), ii4–ii10. https://doi.org/10.1136/tobaccocontrol-2013-051476
- Tobacco Smoking Cessation in Adults, Including Pregnant Persons: Interventions. https://www.uspreventiveservicestaskforce.org/uspstf/document/RecommendationStatementFinal/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
- Siqueira LM; Committee on Substance Use and Prevention. Nicotine and tobacco as substances of abuse in children and adolescents. Pediatrics. 2017;139(1):e20163436.
- Wang TW, Marynak KL, Agaku IT, et al. Secondhand exposure to electronic cigarette aerosol among US youths. JAMA Pediatr. 2017;171(5):490–492.
- Bayly JE, Bernat D, Porter L, et al. Secondhand exposure to aerosols from electronic nicotine delivery systems and asthma exacerbations among youth with asthma. Chest. 2019;155(1):88–93.
- Dai H, Catley D, Richter KP, et al. Electronic cigarettes and future marijuana use. Pediatrics. 2018;141(5):e20173787.
- About Electronic Cigarettes (E-Cigarettes). https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html
- US Department of Health and Human Services. E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC; 2016. https://e-cigarettes.surgeongeneral.gov/documents/2016_sgr_full_report_non-508.pdf
- Goniewicz ML, Gupta R, Lee YH, et al. Nicotine levels in electronic cigarette refill solutions: a comparative analysis of products from the U.S., Korea, and Poland. Int J Drug Policy. 2015;26(6):583–588.
- Marynak KL, Gammon DG, Rogers T, et al. Sales of nicotine-containing electronic cigarette products: United States, 2015. Am J Public Health. 2017;107(5):702–705.
- Collins L, Glasser AM, Abudayyeh H, et al. e-Cigarette marketing and communication: how e-cigarette companies market e-cigarettes and the public engages with e-cigarette information. Nicotine Tob Res. 2019;21(1):14–24.
- Electronic Cigarettes: Common Questions and Answers. Am Fam Physician. 2019 Aug 15;100(4):227-235. https://www.aafp.org/afp/2019/0815/p227.html
- England LJ, Aagaard K, Bloch M, et al. Developmental toxicity of nicotine: a transdisciplinary synthesis and implications for emerging tobacco products. Neurosci Biobehav Rev. 2017;72:176–189.
- Patnode CP, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral Counseling and Pharmacotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review of Reviews for the U.S. Preventive Services Task Force. Evidence Synthesis No. 134. AHRQ Publication No. 14-05200-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2015.
- Hess, C. A., Olmedo, P., Navas-Acien, A., Goessler, W., Cohen, J. E., & Rule, A. M. (2017). E-cigarettes as a source of toxic and potentially carcinogenic metals. Environmental research, 152, 221–225. https://doi.org/10.1016/j.envres.2016.09.026
- Hartmann-Boyce J, McRobbie H, Butler AR, Lindson N, Bullen C, Begh R, Theodoulou A, Notley C, Rigotti NA, Turner T, Fanshawe TR, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2021, Issue 9. Art. No.: CD010216. DOI: 10.1002/14651858.CD010216.pub6
- Quit Methods Used by US Adult Cigarette Smokers 2014-2016. https://www.cdc.gov/pcd/issues/2017/pdf/16_0600.pdf
- QuickStats: Cigarette Smoking Status Among Current Adult E-cigarette Users, by Age Group — National Health Interview Survey, United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:1177. https://www.cdc.gov/mmwr/volumes/65/wr/mm6542a7.htm
- Bjartveit K, Tverdal A. Health Consequences of Smoking 1-4 Cigarettes Per Day. Tobacco Control 2005;14(5):315–20.
- Govindarajan P, Spiller HA, Casavant MJ, et al. e-Cigarette and liquid nicotine exposures among young children. Pediatrics. 2018;141(5):e20173361.
- Flora JW, Meruva N, Huang CB, Wilkinson CT, Ballentine R, Smith DC, Werley MS, McKinney WJ. Characterization of potential impurities and degradation products in electronic cigarette formulations and aerosols. Regul Toxicol Pharmacol. 2016 Feb;74:1-11. doi: 10.1016/j.yrtph.2015.11.009
- Lee, M. S., Allen, J. G., & Christiani, D. C. (2019). Endotoxin and [Formula: see text] Contamination in Electronic Cigarette Products Sold in the United States. Environmental health perspectives, 127(4), 47008. https://doi.org/10.1289/EHP3469
- Patterson SB, Beckett AR, Lintner A, et al. A novel classification system for injuries after electronic cigarette explosions. J Burn Care Res. 2017;38(1):e95–e100.
- Stratton KR, Kwan LY, Eaton DL. National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of E-Cigarettes. National Academies Press; 2018.
- Jenssen BP, Walley SC; Section on Tobacco Control. e-Cigarettes and similar devices. Pediatrics. 2019;143(2):e20183652.
- Soneji S, Barrington-Trimis JL, Wills TA, et al. Association between initial use of e-cigarettes and subsequent cigarette smoking among adolescents and young adults. JAMA Pediatr. 2017;171(8):788–797.
- Aldy, K., Cao, D. J., Weaver, M. M., Rao, D., & Feng, S. Y. (2020). E-cigarette or vaping product use-associated lung injury (EVALI) features and recognition in the emergency department. Journal of the American College of Emergency Physicians open, 1(5), 1090–1096. https://doi.org/10.1002/emp2.12112
- Layden JE, Ghinai I, Pray I, Kimball A, Layer M, Tenforde MW, Navon L, Hoots B, Salvatore PP, Elderbrook M, Haupt T, Kanne J, Patel MT, Saathoff-Huber L, King BA, Schier JG, Mikosz CA, Meiman J. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin – Final Report. N Engl J Med. 2020 Mar 5;382(10):903-916. doi: 10.1056/NEJMoa1911614
- Blagev DP, Harris D, Dunn AC, Guidry DW, Grissom CK, Lanspa MJ. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet. 2019 Dec 7;394(10214):2073-2083. doi: 10.1016/S0140-6736(19)32679-0
- Kalininskiy A, Bach CT, Nacca NE, Ginsberg G, Marraffa J, Navarette KA, McGraw MD, Croft DP. E-cigarette, or vaping, product use associated lung injury (EVALI): case series and diagnostic approach. Lancet Respir Med. 2019 Dec;7(12):1017-1026. doi: 10.1016/S2213-2600(19)30415-1
- Henry TS, Kligerman SJ, Raptis CA, Mann H, Sechrist JW, Kanne JP. Imaging Findings of Vaping-Associated Lung Injury. AJR Am J Roentgenol. 2020 Mar;214(3):498-505. doi: 10.2214/AJR.19.22251
- Thakrar PD, Boyd KP, Swanson CP, Wideburg E, Kumbhar SS. E-cigarette, or vaping, product use-associated lung injury in adolescents: a review of imaging features. Pediatr Radiol. 2020 Mar;50(3):338-344. doi: 10.1007/s00247-019-04572-5
- CDC . Outbreak of Lung Injury Associated with the Use of E‐Cigarette, or Vaping, Products. https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
- Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N Engl J Med 2020; 382:697-705. https://www.nejm.org/doi/full/10.1056/NEJMoa1916433
- Layden J, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin — Preliminary Report. NEJM New Engl J Med. 2019. doi:10.1056/nejmoa1911614.
- Siegel D, Jatlaoui T, Koumans E, et al. Update: Interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping product use-associated lung injury — United States, October 2019. Center for Disease Control and Prevention. https://www.cdc.gov/ mmwr/volumes/68/wr/mm6841e3.htm
- Radiological Case: Electronic-Cigarette or Vaping Associated Lung Injury (EVALI). Appl Radiol. 2020;49(2):42-44.
- Butt Y, Smith M, Tazelaar H, et al. Pathology of vaping-associated lung injury. NEJM New Engl J Med. 2019. doi:10.1056/nejmc1913069.
- Navon, L., Jones, C. M., Ghinai, I., King, B. A., Briss, P. A., Hacker, K. A., & Layden, J. E. (2019). Risk Factors for E-Cigarette, or Vaping, Product Use-Associated Lung Injury (EVALI) Among Adults Who Use E-Cigarette, or Vaping, Products – Illinois, July-October 2019. MMWR. Morbidity and mortality weekly report, 68(45), 1034–1039. https://doi.org/10.15585/mmwr.mm6845e1
- Lozier, M. J., Wallace, B., Anderson, K., Ellington, S., Jones, C. M., Rose, D., Baldwin, G., King, B. A., Briss, P., Mikosz, C. A., & Lung Injury Response Epidemiology/Surveillance Task Force (2019). Update: Demographic, Product, and Substance-Use Characteristics of Hospitalized Patients in a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injuries – United States, December 2019. MMWR. Morbidity and mortality weekly report, 68(49), 1142–1148. https://doi.org/10.15585/mmwr.mm6849e1
- Duffy, B., Li, L., Lu, S., Durocher, L., Dittmar, M., Delaney-Baldwin, E., Panawennage, D., LeMaster, D., Navarette, K., & Spink, D. (2020). Analysis of Cannabinoid-Containing Fluids in Illicit Vaping Cartridges Recovered from Pulmonary Injury Patients: Identification of Vitamin E Acetate as a Major Diluent. Toxics, 8(1), 8. https://doi.org/10.3390/toxics8010008
- Taylor, J., Wiens, T., Peterson, J., Saravia, S., Lunda, M., Hanson, K., Wogen, M., D’Heilly, P., Margetta, J., Bye, M., Cole, C., Mumm, E., Schwerzler, L., Makhtal, R., Danila, R., Lynfield, R., Holzbauer, S., & Lung Injury Response Task Force (2019). Characteristics of E-cigarette, or Vaping, Products Used by Patients with Associated Lung Injury and Products Seized by Law Enforcement – Minnesota, 2018 and 2019. MMWR. Morbidity and mortality weekly report, 68(47), 1096–1100. https://doi.org/10.15585/mmwr.mm6847e1
- Blount, B. C., Karwowski, M. P., Shields, P. G., Morel-Espinosa, M., Valentin-Blasini, L., Gardner, M., Braselton, M., Brosius, C. R., Caron, K. T., Chambers, D., Corstvet, J., Cowan, E., De Jesús, V. R., Espinosa, P., Fernandez, C., Holder, C., Kuklenyik, Z., Kusovschi, J. D., Newman, C., Reis, G. B., … Lung Injury Response Laboratory Working Group (2020). Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. The New England journal of medicine, 382(8), 697–705. https://doi.org/10.1056/NEJMoa1916433
- Evans, M. E., Twentyman, E., Click, E. S., Goodman, A. B., Weissman, D. N., Kiernan, E., Hocevar, S. A., Mikosz, C. A., Danielson, M., Anderson, K. N., Ellington, S., Lozier, M. J., Pollack, L. A., Rose, D. A., Krishnasamy, V., Jones, C. M., Briss, P., King, B. A., Wiltz, J. L., Lung Injury Response Clinical Task Force, … Lung Injury Response Clinical Working Group (2020). Update: Interim Guidance for Health Care Professionals Evaluating and Caring for Patients with Suspected E-cigarette, or Vaping, Product Use-Associated Lung Injury and for Reducing the Risk for Rehospitalization and Death Following Hospital Discharge – United States, December 2019. MMWR. Morbidity and mortality weekly report, 68(5152), 1189–1194. https://doi.org/10.15585/mmwr.mm685152e2
- Jatlaoui, T. C., Wiltz, J. L., Kabbani, S., Siegel, D. A., Koppaka, R., Montandon, M., Adkins, S. H., Weissman, D. N., Koumans, E. H., O’Hegarty, M., O’Sullivan, M. C., Ritchey, M. D., Chatham-Stephens, K., Kiernan, E. A., Layer, M., Reagan-Steiner, S., Legha, J. K., Shealy, K., King, B. A., Jones, C. M., … Lung Injury Response Clinical Working Group (2019). Update: Interim Guidance for Health Care Providers for Managing Patients with Suspected E-cigarette, or Vaping, Product Use-Associated Lung Injury – United States, November 2019. MMWR. Morbidity and mortality weekly report, 68(46), 1081–1086. https://doi.org/10.15585/mmwr.mm6846e2
- Zulfiqar H, Rahman O. Vaping Associated Pulmonary Injury. [Updated 2021 Jul 19]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560656
- Mikosz, C. A., Danielson, M., Anderson, K. N., Pollack, L. A., Currie, D. W., Njai, R., Evans, M. E., Goodman, A. B., Twentyman, E., Wiltz, J. L., Rose, D. A., Krishnasamy, V., King, B. A., Jones, C. M., Briss, P., Lozier, M., Ellington, S., & Lung Injury Response Epidemiology/Surveillance Task Force (2020). Characteristics of Patients Experiencing Rehospitalization or Death After Hospital Discharge in a Nationwide Outbreak of E-cigarette, or Vaping, Product Use-Associated Lung Injury – United States, 2019. MMWR. Morbidity and mortality weekly report, 68(5152), 1183–1188. https://doi.org/10.15585/mmwr.mm685152e1
- Crotty Alexander, L. E., Ware, L. B., Calfee, C. S., Callahan, S. J., Eissenberg, T., Farver, C., Goniewicz, M. L., Jaspers, I., Kheradmand, F., King, T. E., Jr, Meyer, N. J., Mikheev, V. B., Shields, P. G., Shihadeh, A., Strongin, R., & Tarran, R. (2020). E-Cigarette or Vaping Product Use-associated Lung Injury: Developing a Research Agenda. An NIH Workshop Report. American journal of respiratory and critical care medicine, 202(6), 795–802. https://doi.org/10.1164/rccm.201912-2332WS
- Lilly, C. M., Khan, S., Waksmundzki-Silva, K., & Irwin, R. S. (2020). Vaping-Associated Respiratory Distress Syndrome: Case Classification and Clinical Guidance. Critical care explorations, 2(2), e0081. https://doi.org/10.1097/CCE.0000000000000081
- Butt YM, Smith ML, Tazelaar HD, Vaszar LT, Swanson KL, Cecchini MJ, Boland JM, Bois MC, Boyum JH, Froemming AT, Khoor A, Mira-Avendano I, Patel A, Larsen BT. Pathology of Vaping-Associated Lung Injury. N Engl J Med. 2019 Oct 31;381(18):1780-1781. doi: 10.1056/NEJMc1913069