What is epidural hematoma
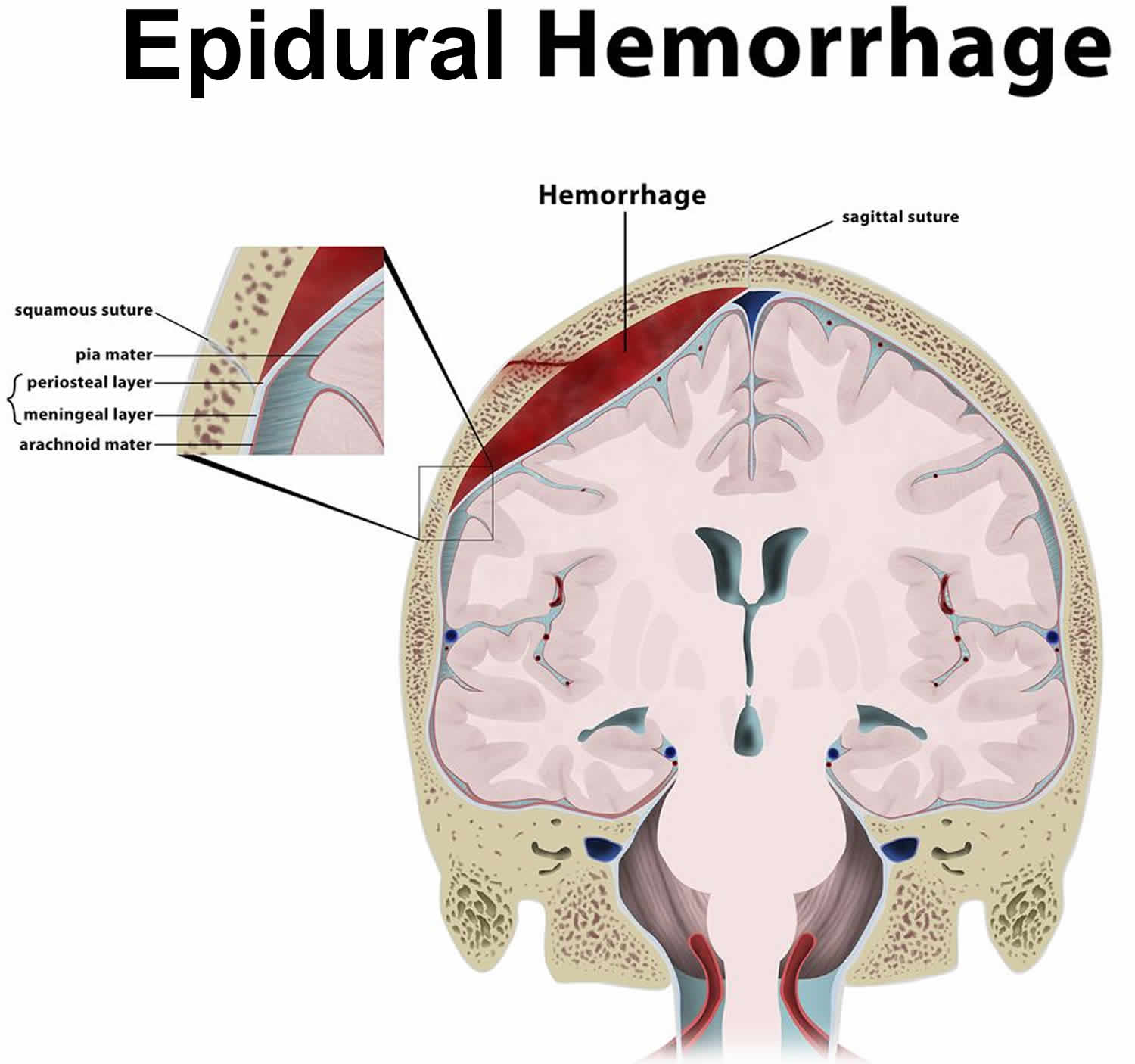
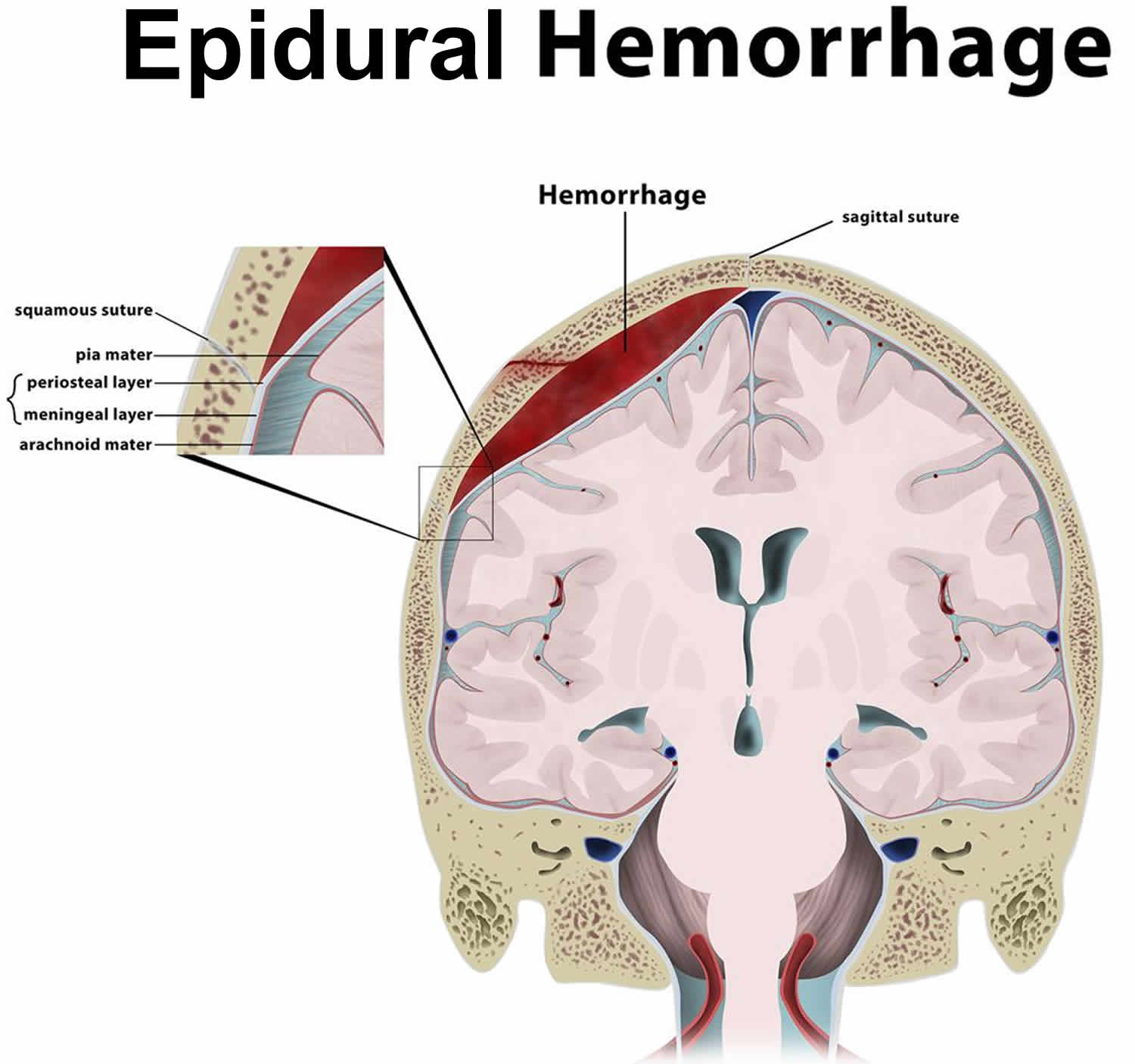
Epidural hematoma also known as an extradural hematoma, is a collection of blood that forms between the inner surface of the skull and outer layer of the dura mater, which is called the endosteal layer. Epidural hematoma is confined by the lateral sutures (especially the coronal sutures) where the dura mater inserts 1. Epidural hematoma is a life-threatening condition, which may require immediate intervention and can be associated with significant morbidity and mortality if left untreated 1. Epidural hematoma is usually associated with a history of head trauma and frequently associated skull fracture. The source of bleeding is usually arterial, most commonly from a torn middle meningeal artery 2. When the skull is fractured in high-energy injury, it can lead to tearing of the arteries sitting underneath the bone but above the dura mater. It is important to get these bleeds quickly recognized and controlled, usually under direct neurosurgical care. There can be a “lucid interval” as the brains in young patients can cope with the increased pressure from a bleed before deteriorating rapidly. There is also high risk of infection as these are usually open fractures. Rapid diagnosis and evacuation are important for a good outcome 3.
An epidural hematoma occurs in 2% of all head injuries and up to 15% of all fatal head traumas. Males are more often affected than are females 1. Furthermore, the incidence is higher among adolescents and young adults. The mean age of affected patients is 20 to 30 years, and it is rare after 50 to 60 years of age 1. As an individual’s age advances, the dura mater becomes more adherent to the overlying bone. This decreases the chance that a hematoma can develop in the space between the cranium and dura 4.
Epidural hematomas are typically biconvex in shape and can cause a mass effect with herniation 2. Epidural hematomas are usually limited by cranial sutures, but not by venous sinuses. Both CT and MRI are suitable to evaluate epidural hematomas. However, CT scan is the method for imaging in these events as it is quick, widely available and can show bleeds and bone injuries well 5. When the blood clot is evacuated promptly (or treated conservatively when small), the prognosis of epidural hematomas is generally good 2.
Typically epidural hematomas are seen in young patients who have sustained head trauma, usually with an associated skull fracture.
Unlike subdural hemorrhages, in which a history of head trauma is often difficult to clearly identify, epidural hemorrhages usually are precipitated by clearly defined head trauma.
A typical presentation is of a young patient involved in a head strike (either during sport or a result of a motor vehicle accident) who may or may not lose consciousness transiently. Following the injury, they regain a normal level of consciousness (lucid interval), but usually, have an ongoing and often severe headache. Over the next few hours, they gradually lose consciousness.
Due to the long cisternal course of the sixth cranial nerve (Abducens nerve, cranial nerve 6), it is often involved as downward herniation begins, usually on the side of the hemorrhage and can, in an emergency, guide exploratory burr holes.
Epidural hematoma may be intracranial (in the skull) or in the spine (spinal epidural hematoma).
- In intracranial epidural hematoma, a blood vessel outside the brain, usually in a groove on the inner side of the skull bursts. Because it is usually an artery that is involved, blood begins to rapidly accumulate between the inside of the skull and the strong outer covering of the brain (called dura mater). The pressure of the blood clot strips the dura mater away from its normal firm attachment to the inside of the skull. The blood clot then can press on the brain, causing injury, and if not diagnosed and treated promptly, may be fatal.
- In spinal epidural hematoma, the bleeding into the space between the spinal column and the outer lining of the spinal cord may be a result of trauma, bleeding disorders, underlying vascular abnormalities or may occur spontaneously.
Key facts
- Epidural hematoma is a neurosurgical emergency
- Suspect epidural hematoma if there is a history of head trauma leading to a period of loss of consciousness.
- Patients with small epidural hematoma may be asymptomatic
- Generally, occurs with a skull fracture, but may occur without bone fracture
- Epidural hematoma does not cross suture lines
- Although lucid interval is commonly described but not pathognomonic and may occur in patients with expanding mass lesions
- Patients with epidural hematoma may be unconscious, may regain consciousness after a brief loss of consciousness or may have no loss of consciousness;
- Patients with small epidural hematoma may be asymptomatic
- If epidural hematoma is rapidly detected and evacuated, the functional outcome is excellent
- If not diagnosed, epidural hematoma is associated with a high mortality, with mortality rates in excess of 15%
- The key prognostic feature is the level of consciousness at the time of presentation. Both bilateral epidural hematomas and posterior fossa epidural hematomas carry a very high mortality.
Epidural space (extradural space) anatomy
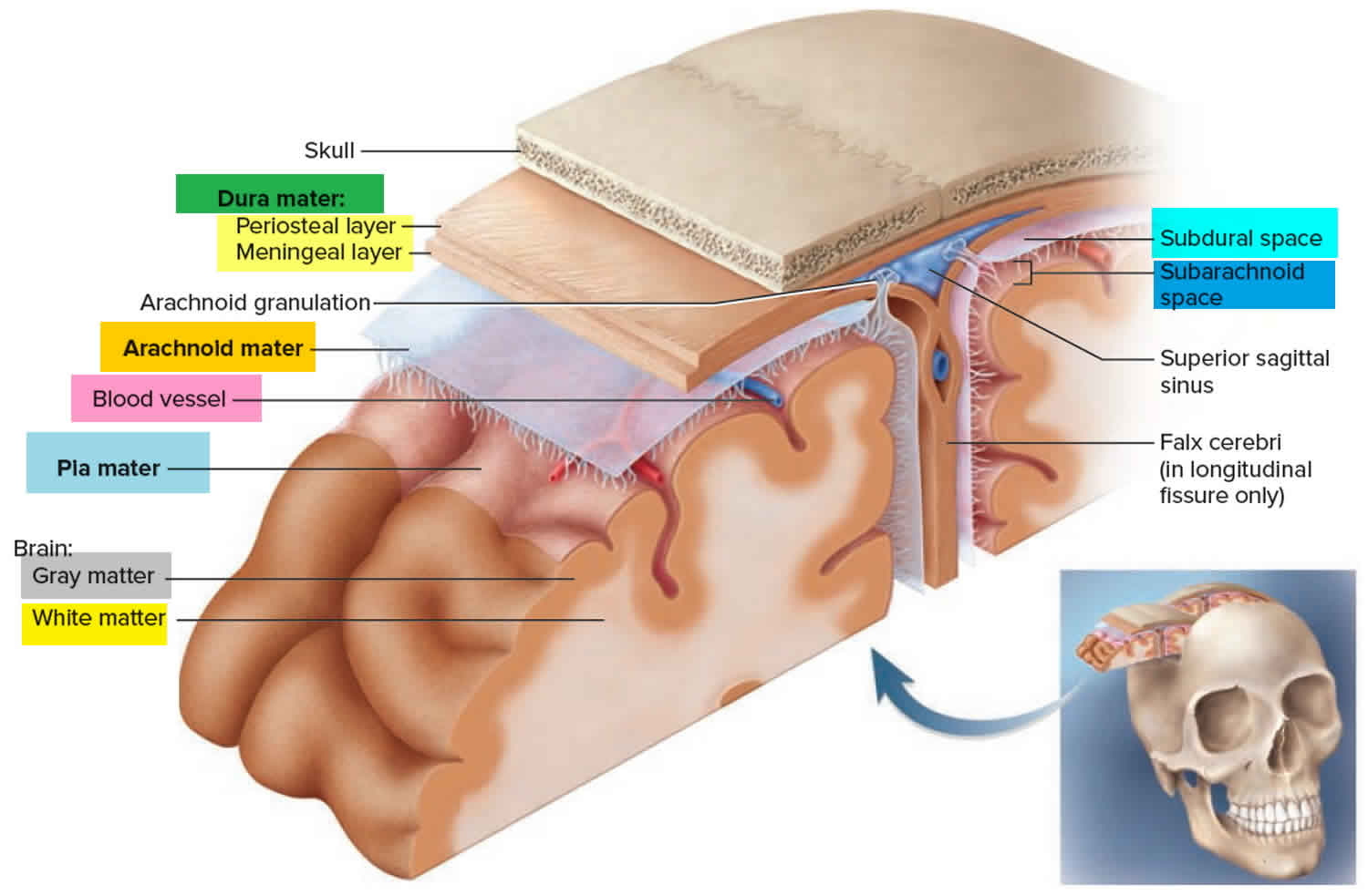
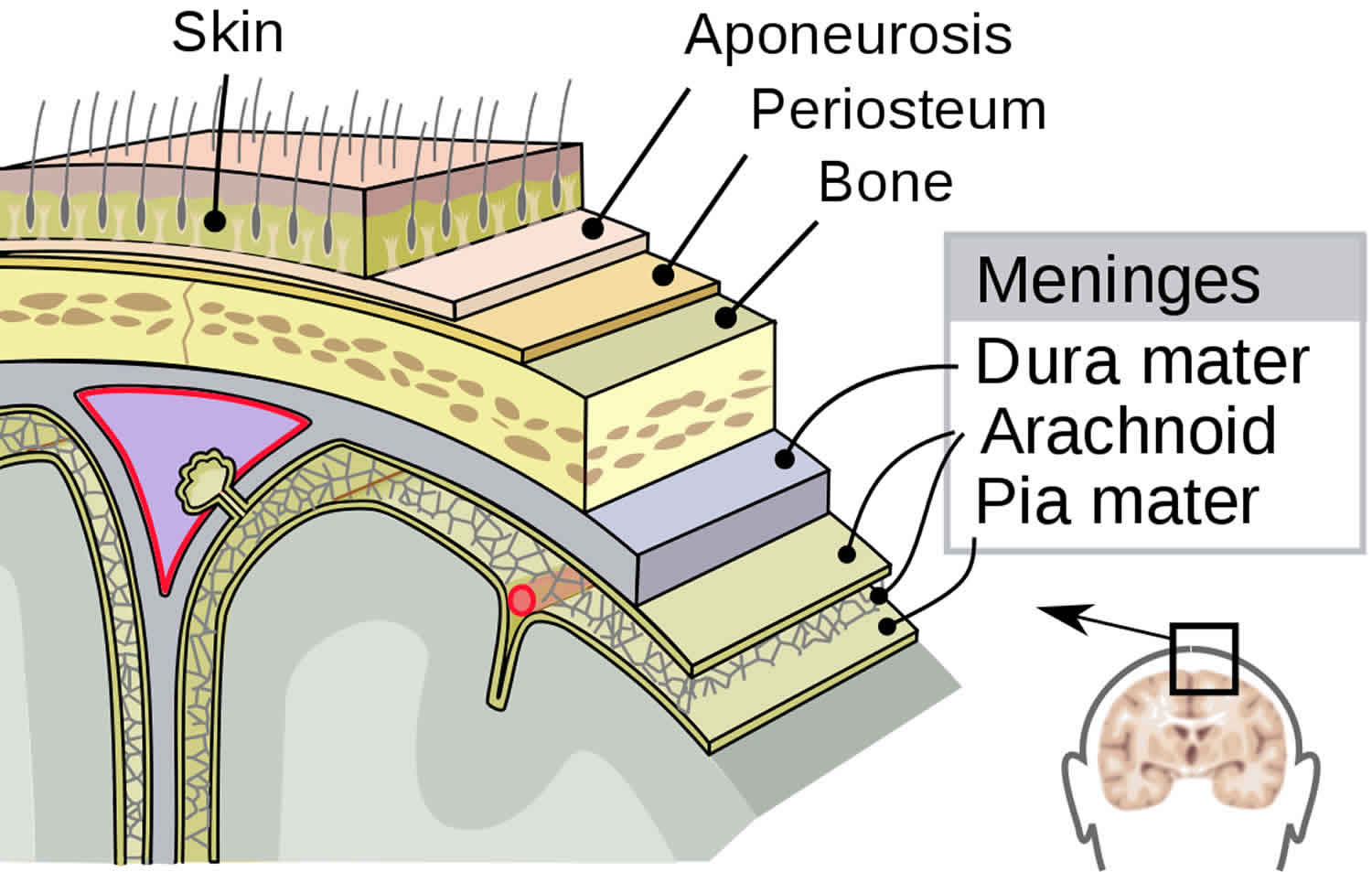
The epidural (extradural) space is a potential space between the cranial bones and the endosteal layer of the dura mater, which is otherwise adherent to the skull bone.
The epidural (extradural) space is a potential space inside the cranial vault and is not normally appreciable unless there is an underlying pathology 6.
This is in contrast to the epidural space of the spinal cord which contains epidural fat, lymphatics, blood vessels, and nerve roots 7. The two spaces are not continuous.
Meningeal arteries exist within the cranial extradural space 8. These include branches of the middle meningeal artery 6. These vessels may be damaged in trauma and can lead to a collection of blood known as an epidural hematoma.
Figure 1. Layers of the scalp and meninges
Figure 2. Skull anatomy
Figure 3. Epidural hematoma (intracranial epidural hemorrhage)
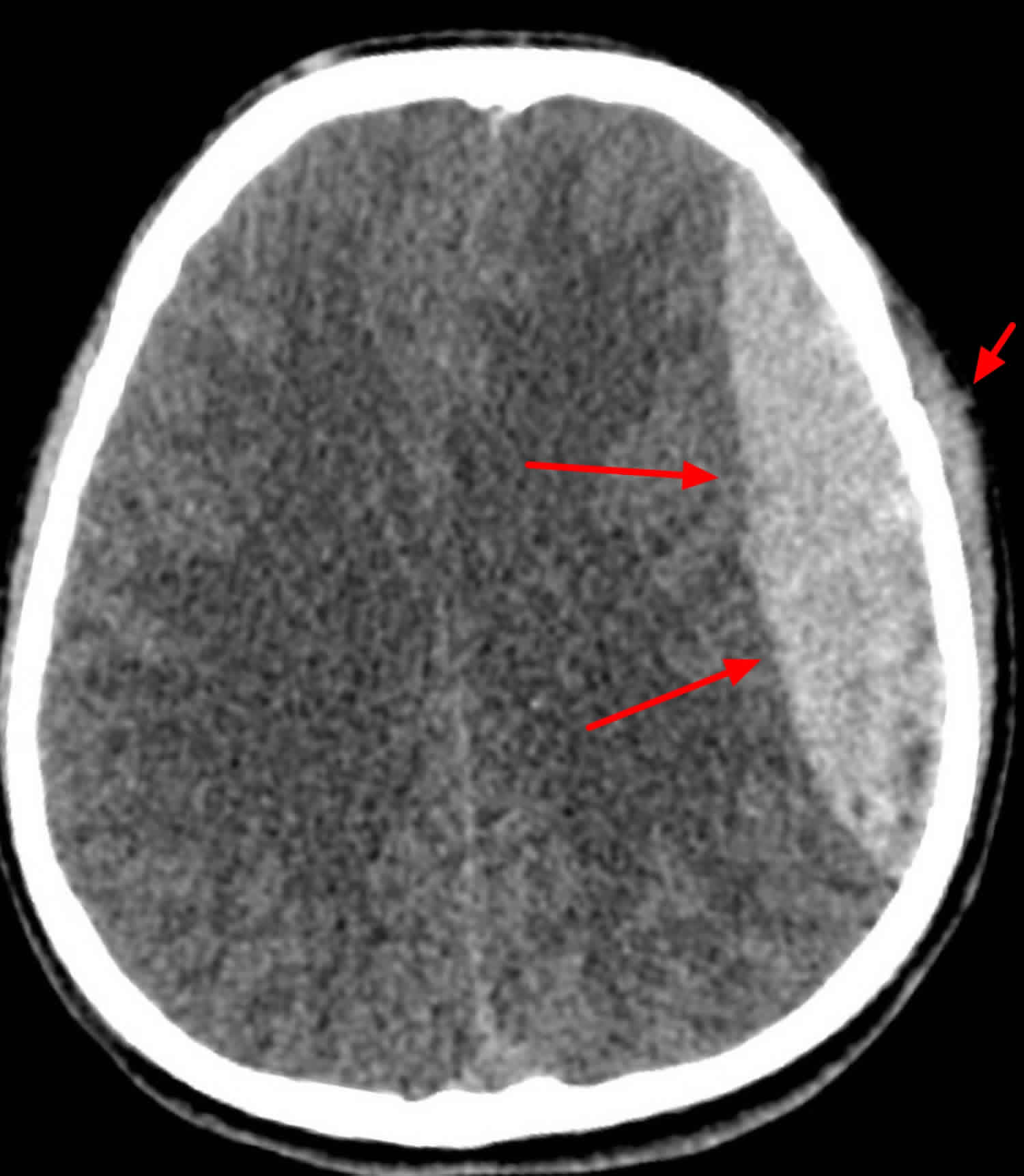
Figure 4. Epidural hematoma (CT scan head)
Footnote: 33 year old male with trauma to the left side of the head. Reduced Glasgow Coma Score. Boggy left temporal swelling. Large left sided bi-convex (or lenticular) collection under the skull. It is hyperdense in comparison to surround brain tissue/parenchyma. There is midline shift with compression of the left lateral ventricle. There is also marked soft tissue swelling over the skull on the left side. Underyling left temporoparietal fracture.
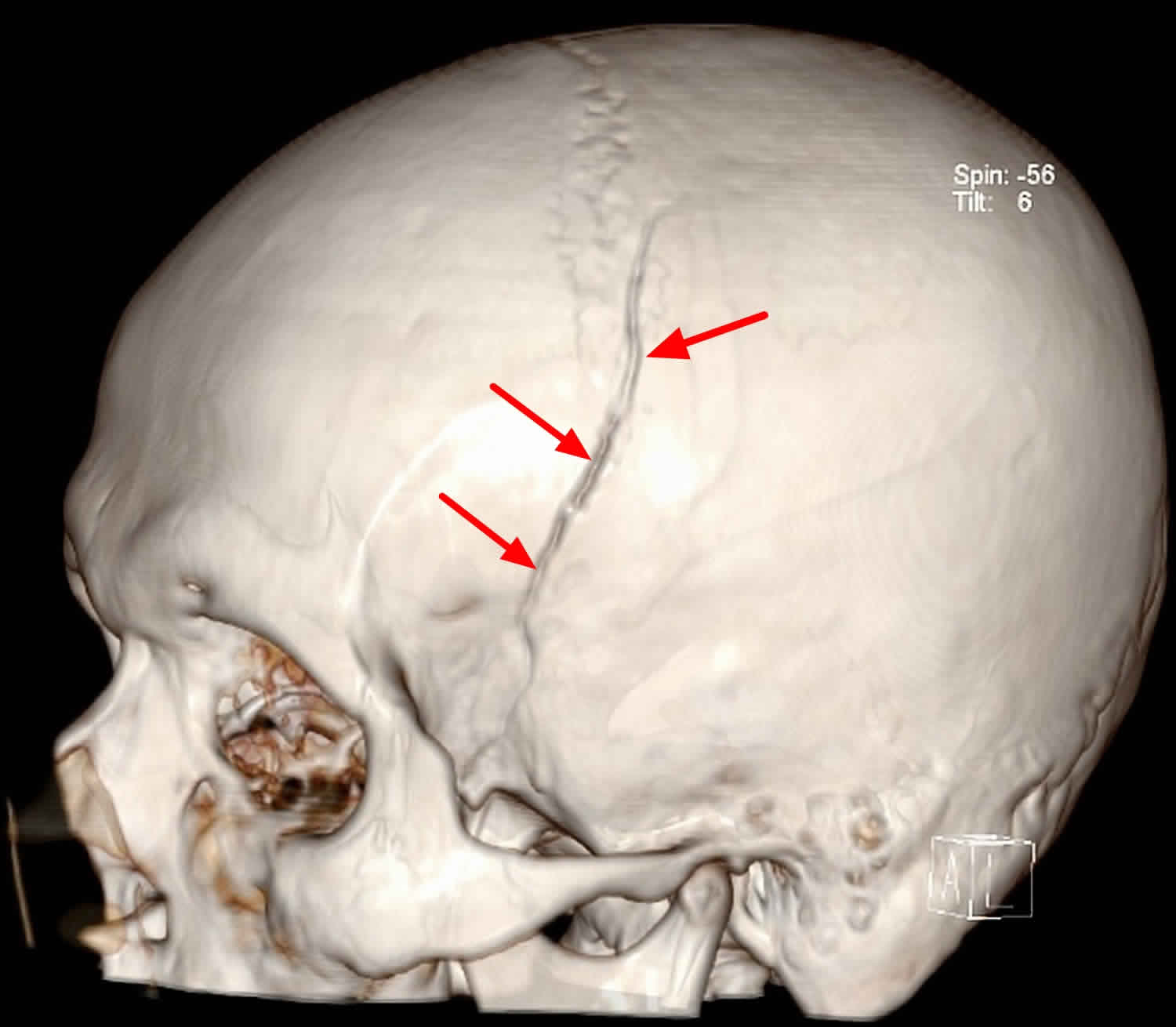
[Source 9 ]Figure 5. 3D image of the skull showing skull vault fractures (same patient as in Figure 4).
Venous epidural hematomas
Venous epidural hemorrhages are a relatively uncommon subtype of epidural hemorrhages, differing from arterial epidural hemorrhages not only in the cause, but also location and prognosis.
Venous epidural hemorrhages occur as a result of damage to the dural venous sinuses and often result in the displacement of the sinus away from the underlying bone.
Venous epidural hematoma location
Three locations are characteristic of venous epidural hemorrhage:
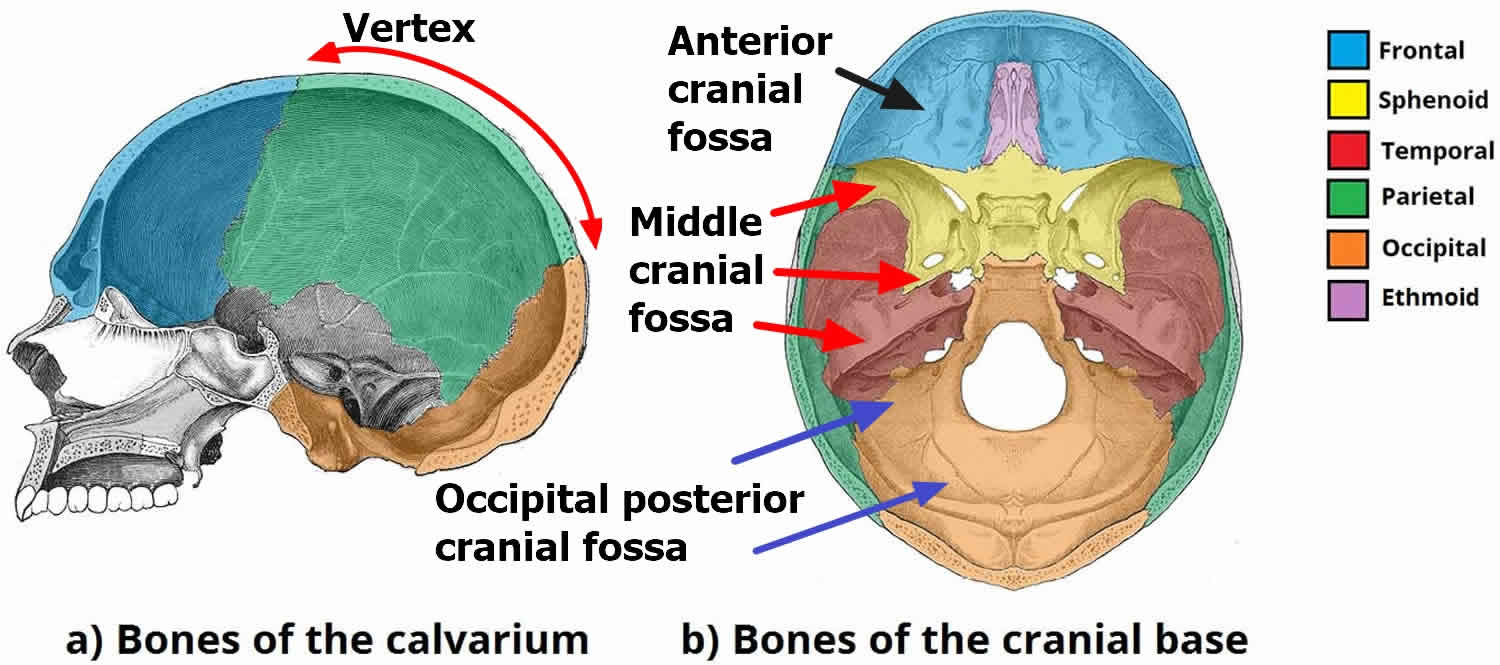
- Vertex
- Anterior middle cranial fossa
- Occipital posterior fossa
Vertex
Epidural hemorrhages located at the vertex are usually due to diastasis +/- fracture involving the superior sagittal sinus. Venous blood from the sinus or closely associated vein accumulates between the endosteal layer of the dura and the overlying inner table of the skull. Because the sinus runs in line with the disrupted sagittal suture, this is one of the occasions when an Epidural hematoma crosses the suture and in so doing displaces the superior sagittal sinus inferiorly.
Anterior middle cranial fossa
Anterior middle cranial fossa Epidural hemorrhages are thought to arise from the sphenoparietal sinus which runs along the superior margin of the greater wing of sphenoid 10. Due to their location, the anatomy of dural attachments and venous origin, they do not cause midline shift or herniation, rarely grow, and can generally be managed conservatively 10.
Occipital posterior fossa
Although most extradural hematomas in the posterior fossa are due to temporal bone fractures involving the middle meningeal artery, in the setting of occipital trauma, particularly fractures of the occipital bone, venous hemorrhage is more common 11. As is the case with vertex extradural hemorrhages, bleeding is from the adjacent sinus, in this case, the transverse sinus, which can be elevated away from the underlying bone.
Spinal epidural hematoma
Spinal epidural hematoma is a collection of blood in the potential space between the dura and the bone, along the spinal canal. Significant bleeding can lead to spinal cord damage, causing neurological injury and deficit. Spinal epidural hematomas are a rare spinal pathology can result in serious morbidity with delayed or non-treatment. Spinal epidural hematomas are typically considered a surgical emergency 12. The patient’s symptoms and signs will depend on the location of the epidural hematoma, and degree of spinal cord / cauda equina compression, but invariably there will be a combination of severe pain and neurological deficit.
Overall incidence approximately 1 per 1,000,000 people annually 13. The source of bleeding is usually venous 14.
Spinal epidural hematoma is most commonly spontaneous venous bleeds, often in the setting of coagulopathy or over-anticoagulation. They are anatomically located in what is known as the extradural neural axis compartment, located between the dura propria (visceral layer) and periosteum.
Spinal epidural hematoma causes
- Spontaneous spinal epidural hematoma (most common) 15, especially in the context of a bleeding disorder or over-anticoagulation
- Trauma, e.g. vertebral fracture
- Iatrogenic, e.g. lumbar puncture, epidural anesthesia. The risk of bleeding after procedures increases with anatomical factors such as vertebral abnormalities, multiple attempts at needle placement, and use of anticoagulation or antiplatelet therapy 16
- Spinal arteriovenous malformations or other vascular anomalies
- Spinal tumors
- Pregnancy
Spinal epidural hematoma signs and symptoms
Spinal epidural hematoma symptom:
- Patients typically complain of sudden onset of severe back pain, or radicular pain, depending on the location of the bleeding
- Pain may be exacerbated by percussion of the spine or by movements that increase intraspinal pressure, such as coughing or sneezing 13
- Neurological symptoms may not develop until hours to days after the onset of back pain, making this a difficult early diagnosis to make
Spinal epidural hematoma signs:
- There may be tenderness to palpation of the spine
- Motor and sensory deficits depend on the level and size of the hematoma
- Clinical presentation can range from focal weakness to paraplegia or quadraplegia, and from paresthesias to complete loss of sensation below a certain level 17
Spinal epidural hematoma location
Spinal epidural hematoma can occur throughout the spine but is most common in the cervicothoracic region, usually posterior to the thecal sac over 2-4 vertebral levels 18.
Spinal epidural hematoma diagnosis
Spinal epidural hematomas may extend over many segments. The total spine should always be imaged, even in patients with well localized clinical signs 19.
Magnetic resonance imaging (MRI)
- Gold standard imaging modality
- Superior to CT as it helps define the extent, volume, and precise location of the hematoma
- Should be performed with and without contrast
- Findings:
- In the first 24 hours, the hematoma is usually isointense or slightly hyperintense on T1-weighted imaged and hyperintense on T2-weighted images 20
- After 24 hours, the hematoma becomes mostly hyperintense on both T1- and T2-weighted images
Computed Tomography (CT)
- Non-contrast CT or CT myelography preferred imaging if MRI not available or if the patient cannot get an MRI 21
- Findings:
- The hematoma is usually visualized as a biconvex-shaped hyperdense lesion within the spinal canal, lying adjacent to the vertebral body (Post 1982)
- The lesion will be sharply demarcated and is separated from the less dense spinal canal
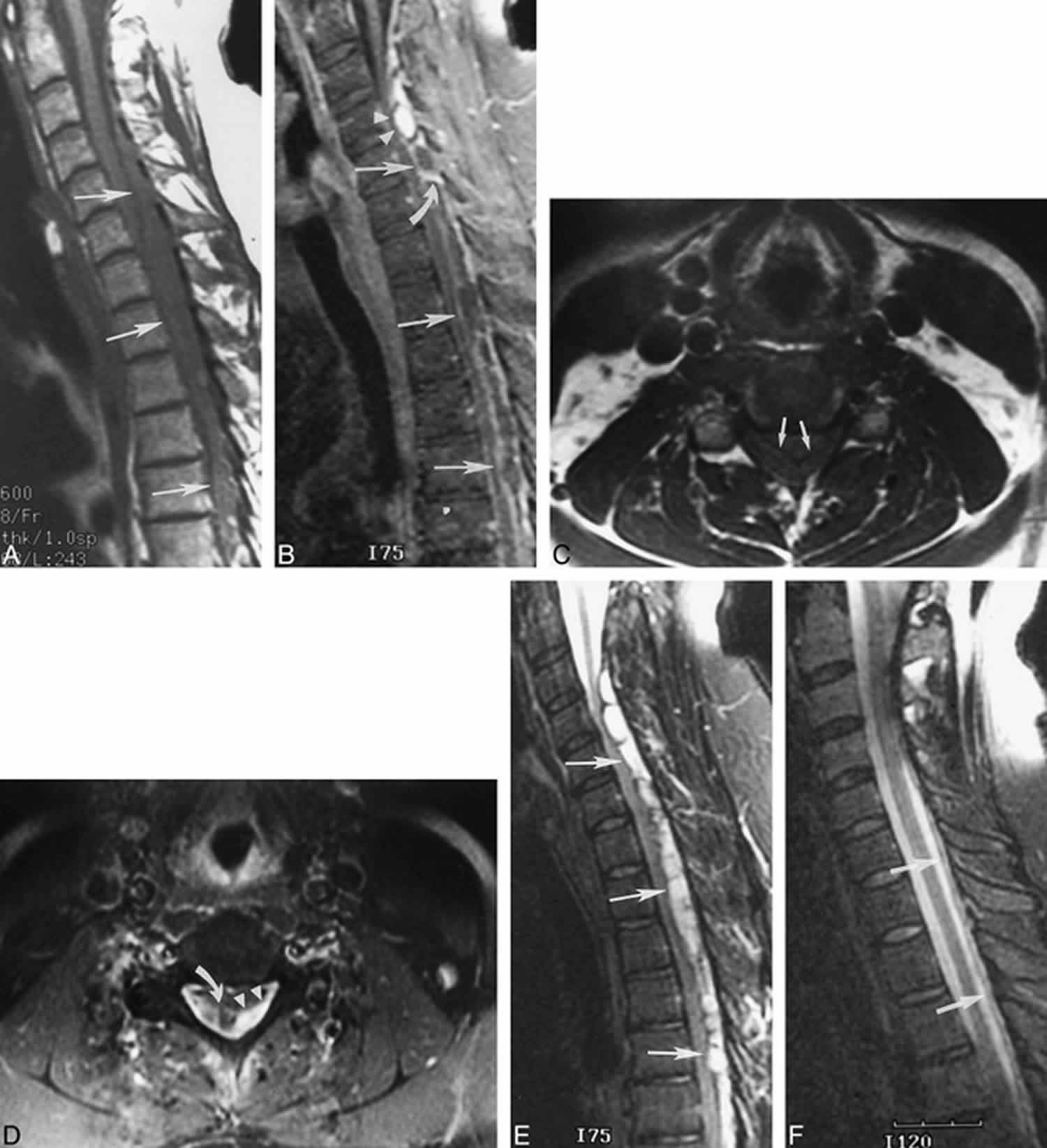
Figure 6. Spinal epidural hematoma (C2 to T6) – MRI images
Footnote: 42 year old man complaining of neck pain
(A) Initial sagittal T1-weighted image (600/8/2 [TR/TE/excitations]), obtained 120 hours after symptom onset, reveals a long-segment epidural hematoma that is isointense in signal to spinal cord and is causing severe cord compression (arrows).
(B) Sagittal T1-weighted image (400/8/2), obtained after the intravenous administration of contrast material, shows a mixed pattern of central (arrowheads) and peripheral (straight arrows) enhancement that likely reflects both enhancement of the hematoma itself and dural enhancement. Linear enhancement within the hematoma (curved arrow) may represent septa or vessels in lateral extensions of epidural fat.
(C) Axial T1-weighted image (500/9/2), obtained at the level of the central enhancement seen in B, shows the absence of hyperintensity within the hematoma before the administration of contrast material (arrows).
(D) Axial T1-weighted image (650/9/2), obtained after the intravenous administration of contrast material and at the level of the central enhancement seen in B, shows a mixed pattern of central (arrowheads) and peripheral (curved arrow) enhancement.
(E) Sagittal T2-weighted image (4000/98/2) shows that the hematoma is hyperintense to spinal cord with focal hypointensity that may represent deoxyhemoglobin and/or fibrous septa (arrows).
(F) Follow-up sagittal T2-weighted image, obtained 6 days after the initial imaging was performed, shows that nearly complete resolution of the epidural hematoma (arrows) was achieved with conservative management.
[Source 18 ]Spinal epidural hematoma diagnosis treatment
- Immediate consultation with neurosurgery
- Spinal epidural hematoma is a neurosurgical emergency that requires rapid surgical decompression 22
- Post-operative neurologic function is related to preoperative neurologic function and the time interval to decompression
- Delays in surgical management can lead to permanent neurological sequelae 16
- Full recovery after 72 hours of symptoms is rare 13
- Patients with mild symptoms may be managed conservatively after consultation with a neurosurgeon. Patients managed non-operatively may receive dexamethasone.
- There have been rare cases of spontaneous recovery of spinal epidural hematomas.
Subdural hematoma vs Epidural hematoma
Differentiating epidural hematoma from subdural hematoma in the head is usually straightforward, but occasionally it can be challenging. Subdural hematomas are more common and there are a few distinguishing features which are usually reliable.
Epidural hematomas occur in the context of head trauma with 95% (unlike subdural hematomas) having an underlying skull fracture.
The epidural hematoma collection is lentiform in appearance and when large cause considerable mass effect – this potentially life threatening injury is easily dealt with surgically provided prompt recognition and surgery is instigated.
History and mechanism of injury
Epidural hematoma
The typical presentation is of a young patient involved in a head strike (either during sport or a result of a motor vehicle accident) who may or may not lose consciousness transiently. Following the injury they regain a normal level of consciousness (lucid interval), but usually have an ongoing and often severe headache. Over the next few hours they gradually lose consciousness.
Subdural hematoma
In adults subdural hematomas are due to falls and there may not be a clear history of trauma. In young children, non accidental injury is a significant cause. The patients level of consciousness gradually decreases with increasing mass effect and confusion is often encountered in the elderly.
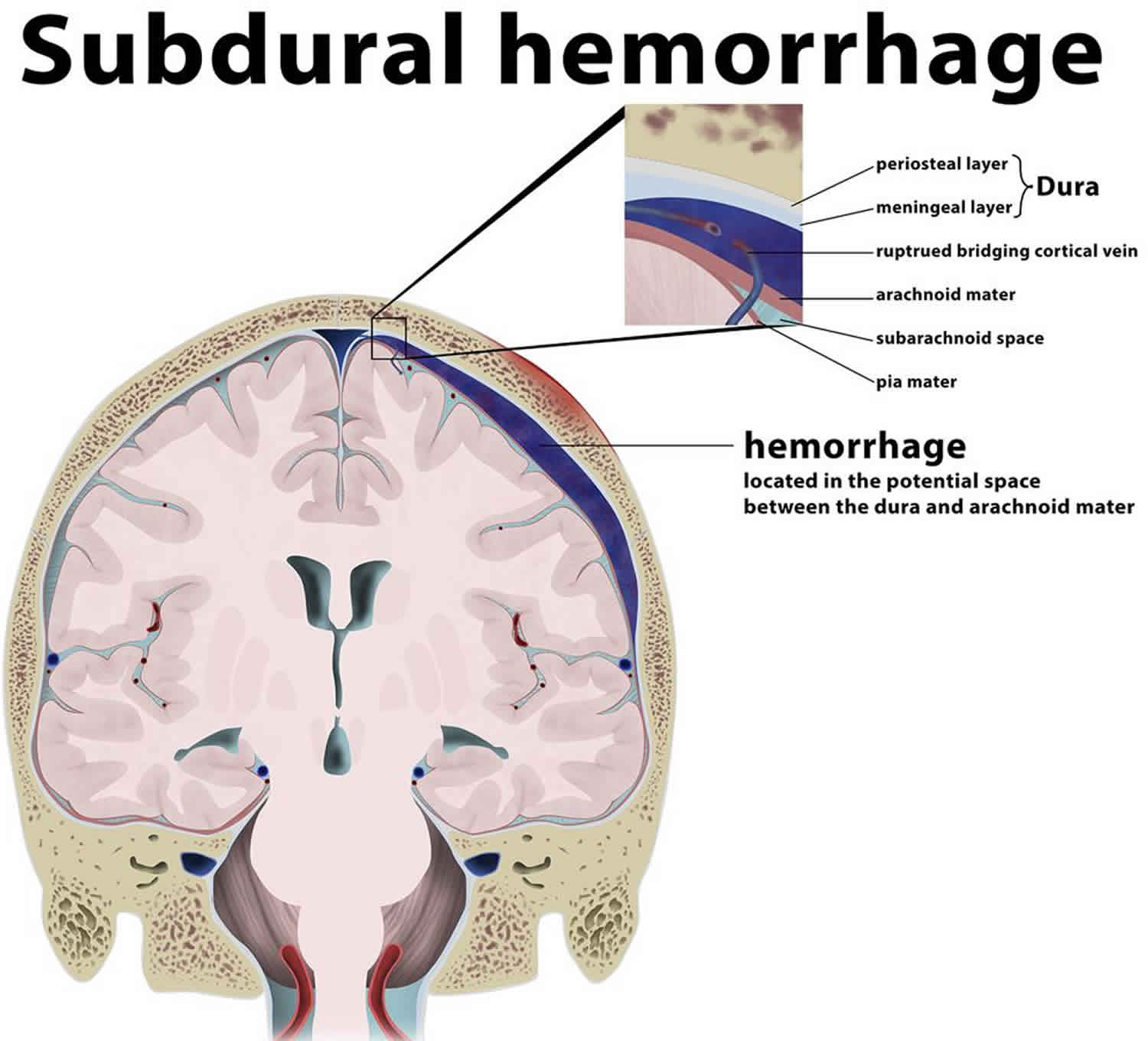
Subdural hemorrhage (subdural hematoma) is a collection of blood accumulating in the subdural space, the potential space between the dura and arachnoid mater of the meninges around the brain. subdural hematoma can happen in any age group, is mainly due to head trauma and CT scans are usually sufficient to make the diagnosis. Prognosis varies widely depending on the size and chronicity of the hemorrhage.
Subdural hematomas are seen in all age-groups although the cause will vary 23:
- Infants: non-accidental injury
- Young adults: motor vehicle accidents
- Elderly: falls (although a definite history of trauma may be lacking)
They are present in ~15% (range 10-20%) of all head trauma cases and occur in up to 30% of fatal injuries.
Clinical presentation
Acute subdural hemorrhages usually present in the setting of head trauma. This is especially the case in young patients, where they commonly co-exist with cerebral contusions.
Most patients (65-80%) present with a severely depressed conscious state, and pupillary abnormalities are seen in ~40% (range 30-50%) of cases (Jallo J, Loftus CM. Neurotrauma and Critical Care of the Brain. Thieme Medical Pub. (2009) ISBN:1604060328.)).
Occasionally spontaneous acute subdural hematomas are seen with an underlying bleeding disorder (e.g. anticoagulation medication, thrombocytopenia) or structural abnormality (e.g. dural arteriovenous fistula).
Clinical presentation of subacute/chronic subdural in the elderly is often vague and is one of the classic causes of a pseudodementia. A history of head trauma is often absent or very minor.
Pathology
Subdural hemorrhages are believed to be due to stretching and tearing of bridging cortical veins as they cross the subdural space to drain into an adjacent dural sinus. These veins rupture due to shearing forces when there is a sudden change in the velocity of the head. The arachnoid may also be torn, creating a mixture of blood and CSF (cerebrospinal fluid) in the subdural space.
10 to 30% of chronic subdural hematomas show evidence of repeated hemorrhage. Rebleeding usually occurs from the rupture of stretched cortical veins as they cross the enlarged fluid-filled subdural space or from the vascularized neomembrane on the outer (calvarial) side of the fluid collection.
Subdural hematomas are interposed between the dura and arachnoid. Typically crescent-shaped, they are usually more extensive than extradural hematomas. In contrast to extradural hemorrhage, subdural hematoma is not limited by sutures but are limited by dural reflections, such as the falx cerebri, tentorium, and falx cerebelli.
Some controversy, albeit of academic interest only, exists as to the exact location of a subdural hematoma. Classical teaching is that it is located in the potential space between the arachnoid layer and inner layer of the dura; however, no such space really exists. Rather the arachnoid-dura junction is composed of “avascular tissue with flake-like… cells stacked in several layers with narrow intercellular clefts” 24. Bleeding occurs within this multicellular layer, with these cells located on both sides of the hematoma 25. This possibly accounts for why some acute hematomas appear to have multiple compartments, usually ascribed to intermittent bleeding.
Figure 6. Subdural hematoma
Footnote: A parietal subdural hemorrhage is depicted, located between the arachnoid mater and meningeal (visceral) layer of the dura. Also depicted is a ruptured bridging cortical vein secondary to a non-fracturing cranial trauma, leading to the hemorrage in this location. Not that the hemorrhage is not limited by the sutural attachement of the dura, as it is subdural.
Source
Epidural hematoma
Almost always arterial explaining the progressive growth of the hematoma. Classically due to injury of the middle meningeal artery, a branch of the maxillary artery (from the external carotid artery).
Subdural hematoma
Almost always venous due to tearing of subdural cortical bridging veins which extend to the dural sinuses.
Radiographic features
Distribution and appearance
Epidural hematoma
Typically lentiform (lens-shaped, biconvex, lemon-shaped) and do not cross sutures as the periosteum crosses through the suture continuous with the outer periosteal layer.
Subdural hematoma
Typically crescentic (crescent moon-shaped, concave, banana-shaped) and more extensive than epidural hematoma, with the internal margin paralleling the cortical margin of the adjacent brain. As these occur in the subdural space, they cross sutures.
Treatment and prognosis
Epidural hematoma is treated with expedient evacuation via a craniotomy.
Subdural hematoma has various management strategies depending on the size, location and extent of mass effect and is either conservative (monitor with serial CT) or surgical (drainage with burr holes).
Epidural hematoma causes
An epidural hematoma is often caused by a skull fracture during childhood or adolescence. The majority of cases related to a traumatic mechanism are a result of head injury due to motor vehicle collisions, physical assaults, or accidental falls. This type of bleeding is more common in young people because the membrane covering the brain is not as closely attached to the skull as it is in older people and children younger than 2 years. Epidural hematoma occurs in approximately 10% of traumatic brain injuries (TBI) requiring hospitalization. Both by traumatic and non-traumatic mechanisms can cause an epidural hematoma 26.
Non-traumatic causes include the following:
- Infection/Abscess
- Coagulopathy
- Hemorrhagic Tumors
- Vascular Malformations
An epidural hematoma can also occur due to rupture of a blood vessel, usually an artery. The blood vessel then bleeds into the space between the dura and the skull.
The affected vessels are often torn by skull fractures. The fractures are most often the result of a severe head injury, such as those caused by motorcycle or automobile accidents.
Rapid bleeding causes a collection of blood (hematoma) that presses on the brain. The pressure inside the head (intracranial pressure, ICP) increases quickly. This pressure may result in more brain injury.
Typically epidural hematomas are seen in young patients who have sustained head trauma, usually with an associated skull fracture. The source of bleeding is typically from a torn meningeal artery, usually the middle meningeal artery (75%) 27. An associated skull fracture is present in ~75% of cases 28. Pain (often severe headache) is caused by the stripping of dura from the bone by the expanding hemorrhage. The posterior fossa is a rare location for traumatic injury, in general, including epidural hematoma 29.
Occasionally, an epidural hematoma can form due to venous blood, typically a torn sinus with an associated fracture: see venous extradural hemorrhage above.
Young patients being affected is not only a result of the prevalent demographics of patients with a head injury but also relates to the changes that occur in the dura in older patients, as the dura is much more adherent to the inner surface of the skull.
It is important to realize that in the setting of sutural diastasis epidural hematomas can cross sutures, as the continuation of the parietal (periosteal) component of the dura through the suture – which usually limits spread – is likely also to be disrupted.
Pathophysiology
Arterial Injury
Most epidural hematomas result from arterial bleeding from a branch of the middle meningeal artery. The anterior meningeal artery or dural arteriovenous fistula at the vertex may be involved 30.
Venous Injury
Up to 10% of epidural hematomas are due to venous bleeding following the laceration of a dural venous sinus.
In adults, up to 75% of epidural hematomas occur in the temporal region. However, in children, they occur with similar frequency in the temporal, occipital, frontal, and posterior fossa regions.
A skull fracture is present in the majority of patients with epidural hematoma. These hematomas often present beneath a fracture of the squamous part of the temporal bone.
If this condition occurs within the spine, this entity is described as a spinal epidural hematoma.
Based on radiographic progression, it can be classified into one of the following:
- Type 1: Acute; occurs on day 1 and associated with a “swirl” of un-clotted blood
- Type 2: Subacute occurring between days 2 to 4 and usually solid
- Type 3: Chronic occurring between days 7 to 20; mixed or lucent appearance with contrast enhancement
Location
Epidural hematomas are generally unilateral in more than 95% of cases, however, bilateral or multiple epidural hematomas are reported.
95% are supratentorial
- temporoparietal: 60%
- frontal: 20%
- parieto-occipital: 20%
<5% are located infratentorially in posterior fossa 29
Special locations to consider, particularly those related to venous extradural bleeding, include:
- vertex epidural hematoma (which displaces the superior sagittal sinus) 31
- anterior middle cranial fossa 27
- likely venous bleeding (sphenoparietal sinus)
- do not cause midline shift or herniation
- rarely grow
- can be managed conservatively
Epidural hematoma signs and symptoms
The typical presentation is an initial loss of consciousness following trauma, a complete transient recovery (“often termed as a lucid interval”), culminating in a rapid progression of neurological deterioration 1. This occurs in 14% to 21% of patients with an epidural hematoma 1. However, these patients may be unconscious from the beginning or may regain consciousness after a brief coma or may have no loss of consciousness. Therefore, the presentations range from a temporary loss of consciousness to a coma.
- Contact your health care provider for any head injury that results in even a brief loss of consciousness, or if there are any other symptoms after a head injury (even without loss of consciousness). The typical pattern of symptoms that indicate an epidural hematoma is a loss of consciousness, followed by alertness, then loss of consciousness again. But this pattern may NOT appear in all people.
Beware that the lucid interval is not pathognomonic for an epidural hematoma and may occur in patients who sustain other expanding mass lesions. The classic lucid interval occurs in pure epidural hematomas that are very large and demonstrate a CT scan finding of active bleeding. The presentation of symptoms depends on how quickly the epidural hematoma is developing within the cranial vault. A patient with a small epidural hematoma may be asymptomatic, but this is rare. Also, an epidural hematoma may also develop in a delayed fashion.
A posterior fossa epidural hematoma is a rare event. This kind of epidural hematoma may account for approximately 5% of all posttraumatic intracranial mass lesions. Patients with posterior fossa epidural hematoma may remain conscious until late in the evolution of the hematoma, when they may suddenly lose consciousness, become apneic, and die. These lesions often extend into the supratentorial compartment by stripping the dura over the transverse sinus, resulting in a significant amount of intracranial bleeding.
This enlarging hematoma leads to eventual elevation of intracranial pressure which may be detected in a clinical setting by observing ipsilateral pupil dilation (secondary to uncal herniation and oculomotor nerve compression), the presence of elevated blood pressure, slowed heart rate, and irregular breathing. This triad is known as the “Cushing reflex.” These findings may indicate the need for immediate intracranial intervention to prevent central nervous system (CNS) depression and death.
The most important symptoms of an epidural hematoma are:
- Confusion
- Dizziness
- Drowsiness or altered level of alertness
- Enlarged pupil in one eye
- Headache (severe)
- Head injury or trauma followed by loss of consciousness, a period of alertness, then rapid deterioration back to unconsciousness
- Nausea or vomiting
- Weakness in part of the body, usually on the opposite side from the side with the enlarged pupil
The symptoms usually occur within minutes to hours after a head injury and indicate an emergency situation.
Sometimes, bleeding does not start for hours after a head injury. The symptoms of pressure on the brain also do not occur right away.
Epidural hematoma complications
- Mass effect: compression of the brain if bleeding is significant
- Herniation
- Seizures
Epidural hematoma diagnosis
The brain and nervous system (neurological) examination may show that a specific part of the brain is not working well (for instance, there may be arm weakness on one side). The exam may also indicate increased intracranial pressure (ICP).
If there is increased intracranial pressure (ICP), emergency surgery may be needed to relieve the pressure and prevent further brain injury.
Imaging studies such as a CT scan comprise the mainstay of diagnosis. Laboratory studies such as INR, partial thromboplastin time (PTT), thromboplastin time (PT), and liver function test (LFT) may be obtained to assess for increased bleeding risk or underlying coagulopathies 32.
CT Scan
CT scan is the most common imaging modality to assess for intracranial bleeding. Its popularity is related to its widespread availability in emergency departments. The majority of epidural hematomas are identifiable on a CT scan. The classic presentation is a biconvex or lens-shaped mass on brain CT scan, due to the limited ability of blood to expand within the fixed attachment of the dura to the cranial sutures. epidural hematomas does not cross suture lines.
Generally, radiologists use a standard formula for estimating the amount of blood present in an epidural hematoma. It is as follows:
ABC/2
- A: The maximum hemorrhage diameter on the CT slice with the largest area of hemorrhage
- B: The maximum diameter 90 degrees to A on the same CT slice
- C: The number of CT slices with hemorrhage multiplied by the slice thickness in centimeters
There are, however, other CT findings that may need to be taken into account when evaluating epidural hematoma. For example, continued bleeding may be indicated by areas of low density, or a “swirl-sign.” The latter may be used for prognosis, and often indicates the need for surgical intervention. If the epidural hematoma abuts brain tissue that is hemorrhagic or contused, it may appear shallow, and thus, may be overlooked if the CT scan is not carefully examined.
Several factors may lead to a non-diagnostic CT scan. These are as follows:
- A low-density blood collection may result from severe anemia (thus leading to misinterpretation).
- Arterial extravasation may be reduced secondary to severe hypotension.
- A positive finding on CT requires that enough blood accumulate for visualization. If the CT is obtained too soon after trauma, there may not be sufficient accumulation for appropriate interpretation.
- If the epidural hematoma is secondary to venous bleeding, blood accumulation may be slow. This could potentially result in difficulty with CT interpretation.
MRI
Brain MRI is more sensitive than a CT scan, particularly when assessing for epidural hematoma in the vertex. It should be obtained when there is high clinical suspicion for epidural hematoma, accompanying a negative initial head CT scan.
In the situation of a suspected spinal epidural hematoma, a spinal MRI is the preferred imaging modality, as it affords higher resolution versus a spinal CT.
Angiography
When evaluating epidural hematomas located in the vertex, the healthcare professional should evaluate for the presence of a dural arteriovenous (AV) fistula that may have arisen from the middle meningeal artery. Angiography may be required to evaluate the presence of such a lesion fully.
Epidural hematoma treatment
Epidural hematoma is a neurosurgical emergency. Epidural hematoma, therefore, requires urgent surgical evacuation to prevent irreversible neurological injury and death secondary to hematoma expansion and herniation. A neurosurgical consultation should be the urgently obtained as it is important to intervene within 1 to 2 hours of presentation 33.
The priority is to stabilize the patient, including the ABCs (airway, breathing, circulation), and these should be addressed urgently.
Surgical Intervention is recommended in patients with:
- Acute epidural hematoma
- Hematoma volume greater than 30 ml regardless of Glasgow coma score (GCS)
- Glasgow Coma Score less than 9 with pupillary abnormalities like anisocoria
Operative Management
In patients with acute and symptomatic epidural hematomas, the treatment is craniotomy and hematoma evacuation. Based on the available literature, “trephination” (or burr hole evacuation) is often a crucial form of intervention if more advanced surgical expertise is unavailable; it may even decrease mortality. However, the performance of a craniotomy, if feasible, can provide a more thorough evacuation of the hematoma.
Non-Operative Management
There is a scarcity of literature comparing conservative management with surgical intervention in patients with epidural hematoma. However, a non-surgical approach may be considered in a patient with acute epidural hematoma who has mild symptoms and meets all of the criteria listed below:
- Epidural hematoma volume of less than 30 ml
- Clot diameter of less than 15 mm
- Midline shift of less than 5 mm
- Glasgow coma score (GCS) greater than 8 and on physical examination, shows no focal neurological symptoms.
If the decision is made to manage acute epidural hematoma non-surgically, close observation with repeated neurological examinations and continuous surveillance with brain imaging is required, as the risk for hematoma expansion and clinical deterioration is present. The recommendation is to obtain a follow-up head CT scan within 6 to 8 hours following brain injury.
Epidural hematoma prognosis
In general, patients with pure epidural hematomas have an excellent prognosis of a functional outcome after surgical evacuation, when it is rapidly detected and evacuated. A delay in diagnosis and treatment increases the morbidity and the mortality.
Epidural hematomas caused by arterial bleeding develop rapidly and can be detected quickly. But those due to a dural sinus tear develop more slowly. Thus, clinical manifestations may be delayed, with a resultant delay in recognition and evacuation. Generally, an epidural hematoma volume greater than 50 cm prior to evacuation results in a worse neurological outcome and consequent mortality.
Factors that may influence outcome are as follows:
- Patient age
- Time lapsed between injury and treatment
- Immediate coma or lucid interval
- Presence of pupillary abnormalities
- Glasgow coma score (GCS)/motor score, on arrival
CT findings (hematoma volume, the degree of midline shift, the presence of signs of active hematoma bleeding, or associated intra-dural lesions)
Postoperative intracranial pressure (ICP)
Several markers that correlate with a poor prognosis of epidural hematoma include the following:
- A low Glasgow coma score (GCS) before surgery, or on arrival
- Abnormal pupil examination, in particular, un-reactive pupils (unilateral or bilateral)
- Advanced age
- The time between neurological symptoms and surgery
- Elevated intracranial pressure (ICP) in the post-operative period
Certain head CT findings can correlate with a poor prognosis:
- Hematoma volume of greater than 30 to 150 ml
- A midline shift greater than 10 to 12 mm
- “Swirl sign” indicating an active bleed
- Associated intracranial lesions (such as contusions, intracerebral hemorrhage, subarachnoid hemorrhage and diffuse brain swelling).
- Khairat A, Waseem M. Epidural Hematoma. [Updated 2018 Nov 15]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK518982
- Extradural hemorrhage. https://radiopaedia.org/articles/extradural-haemorrhage?lang=us
- Kanematsu R, Hanakita J, Takahashi T, Park S, Minami M. Radiologic Features and Clinical Course of Chronic Spinal Epidural Hematoma: Report of 4 Cases and Literature Review. World Neurosurg. 2018 Dec;120:82-89.
- Chicote Álvarez E, González Castro A, Ortiz Lasa M, Jiménez Alfonso A, Escudero Acha P, Rodríguez Borregán JC, Peñasco Martín Y, Dierssen Sotos T. Epidemiology of traumatic brain injury in the elderly over a 25 year period. Rev Esp Anestesiol Reanim. 2018 Dec;65(10):546-551.
- Extradural hemorrhage (teaching). https://radiopaedia.org/cases/extradural-haemorrhage-teaching
- Schulte E. Head and Neuroanatomy. Thieme Georg Verlag. ISBN:3131421010.
- Harold Ellis, The anatomy of the epidural space, Anaesthesia & Intensive Care Medicine, Volume 7, Issue 11, November 2006, Pages 402-404
- Extradural space. https://radiopaedia.org/articles/extradural-space?lang=us
- Extradural hematoma. https://radiopaedia.org/cases/extradural-haematoma-5
- Gean AD, Fischbein NJ, Purcell DD, Aiken AH, Manley GT, Stiver SI. Benign anterior temporal epidural hematoma: indolent lesion with a characteristic CT imaging appearance after blunt head trauma. Radiology. 257 (1): 212-8. doi:10.1148/radiol.10092075
- Khwaja HA, Hormbrey PJ. Posterior cranial fossa venous extradural haematoma: an uncommon form of intracranial injury. Emergency medicine journal : EMJ. 18 (6): 496-7.
- Spinal epidural hematoma. https://radiopaedia.org/articles/spinal-epidural-haematoma?lang=us
- Perron AD and Huff JS. Spinal Cord Disorders. In: Marx JA et al. Rosen’s Emergency Medicine: Concepts and Clinical Practice, 8th ed. Philadelphia: Saunders, 2013. 1419-1427.
- Aminoff, MJ. Lumbar Puncture: Technique, Indications, Contraindications, and Complications in Adults. Topic 4832, Version 14.0.
- Naidich TP, Castillo M, Cha S et-al. Imaging of the Spine. Saunders. ISBN:1437715516.
- Yi S et al. Postoperative Spinal Epidural Hematoma: Risk Factor and Clinical Outcome. Yonsei Med J. 2006:47(3):326-332.
- Myers M et al. Extensive Subarachnoid and Epidural Hematoma After Lumbar Puncture. Am J Emerg Med. 2015:33(4):603.e3-4
- Fukui MB, Swarnkar AS, Williams RL. Acute spontaneous spinal epidural hematomas. American Journal of Neuroradiology July 1999, 20 (7) 1365-1372 http://www.ajnr.org/content/20/7/1365.full
- Post MJ et al. CT Diagnosis of Spinal Epidural Hematoma. Am J Neuroradiol. 1982:3:190-192.
- Sklar EM et al. MRI of Acute Spinal Epidural Hematoma. J Comput Assist Tomogr. 1999:23(2):238-43.
- Zilkha A et al. Computed Tomography of Spinal Epidural Hematoma. Am J Neuroradiol. 1983:4:1073-76.
- Myers M et al. Extensive Subarachnoid and Epidural Hematoma After Lumbar Puncture. Am J Emerg Med. 2015:33(4):603.e3-4.
- Jallo J, Loftus CM. Neurotrauma and Critical Care of the Brain. Thieme Medical Pub. (2009) ISBN:1604060328.
- Orlin JR, Osen KK, Hovig T. Subdural compartment in pig: a morphologic study with blood and horseradish peroxidase infused subdurally. Anat. Rec. 1991;230 (1): 22-37. doi:10.1002/ar.1092300104
- Chung CK, Kim YM, Chi JG. Intralaminar dural haematoma developing in the contralateral convexity after temporal lobectomy. J. Neurol. Neurosurg. Psychiatr. 1999;66 (2): 248-9. doi:10.1136/jnnp.66.2.248
- Tamburrelli FC, Meluzio MC, Masci G, Perna A, Burrofato A, Proietti L. Etiopathogenesis of Traumatic Spinal Epidural Hematoma. Neurospine. 2018 Mar;15(1):101-107.
- Gean AD, Fischbein NJ, Purcell DD et-al. Benign anterior temporal epidural hematoma: indolent lesion with a characteristic CT imaging appearance after blunt head trauma. Radiology. 2010;257 (1): 212-8. doi:10.1148/radiol.10092075
- Irie F, Le Brocque R, Kenardy J et-al. Epidemiology of traumatic epidural hematoma in young age. J Trauma. 2011;71 (4): 847-53. doi:10.1097/TA.0b013e3182032c9a
- Takeuchi S, Wada K, Takasato Y et-al. Traumatic hematoma of the posterior fossa. Acta Neurochir. Suppl. 2013;118: 135-8. doi:10.1007/978-3-7091-1434-6_24
- Burjorjee JE, Rooney R, Jaeger M. Epidural Hematoma Following Cessation of a Direct Oral Anticoagulant: A Case Report. Reg Anesth Pain Med. 2018 Apr;43(3):313-316.
- Brant WE, Helms CA. Fundamentals of Diagnostic Radiology. Lippincott Williams & Wilkins. (2007) ISBN:0781761352
- Flaherty BF, Moore HE, Riva-Cambrin J, Bratton SL. Repeat Head CT for Expectant Management of Traumatic Epidural Hematoma. Pediatrics. 2018 Sep;142(3).
- Gutowski P, Meier U, Rohde V, Lemcke J, von der Brelie C. Clinical Outcome of Epidural Hematoma Treated Surgically in the Era of Modern Resuscitation and Trauma Care. World Neurosurg. 2018 Oct;118:e166-e174.