Esophageal stricture
Esophageal stricture is an abnormal narrowing of the esophagus (the tube from the mouth to the stomach). Esophageal stricture causes swallowing difficulties or dysphagia. People with esophageal strictures also have difficulty swallowing solid foods, but generally do not have problems with swallowing liquids. Swallowing problems may keep you from getting enough fluids and nutrients. Solid food, especially meat, can get stuck above the stricture. If this happens, endoscopy would be needed to remove the lodged food. There is also a higher risk of having food, fluid, or vomit enter the lungs with regurgitation. This can cause choking or aspiration pneumonia.
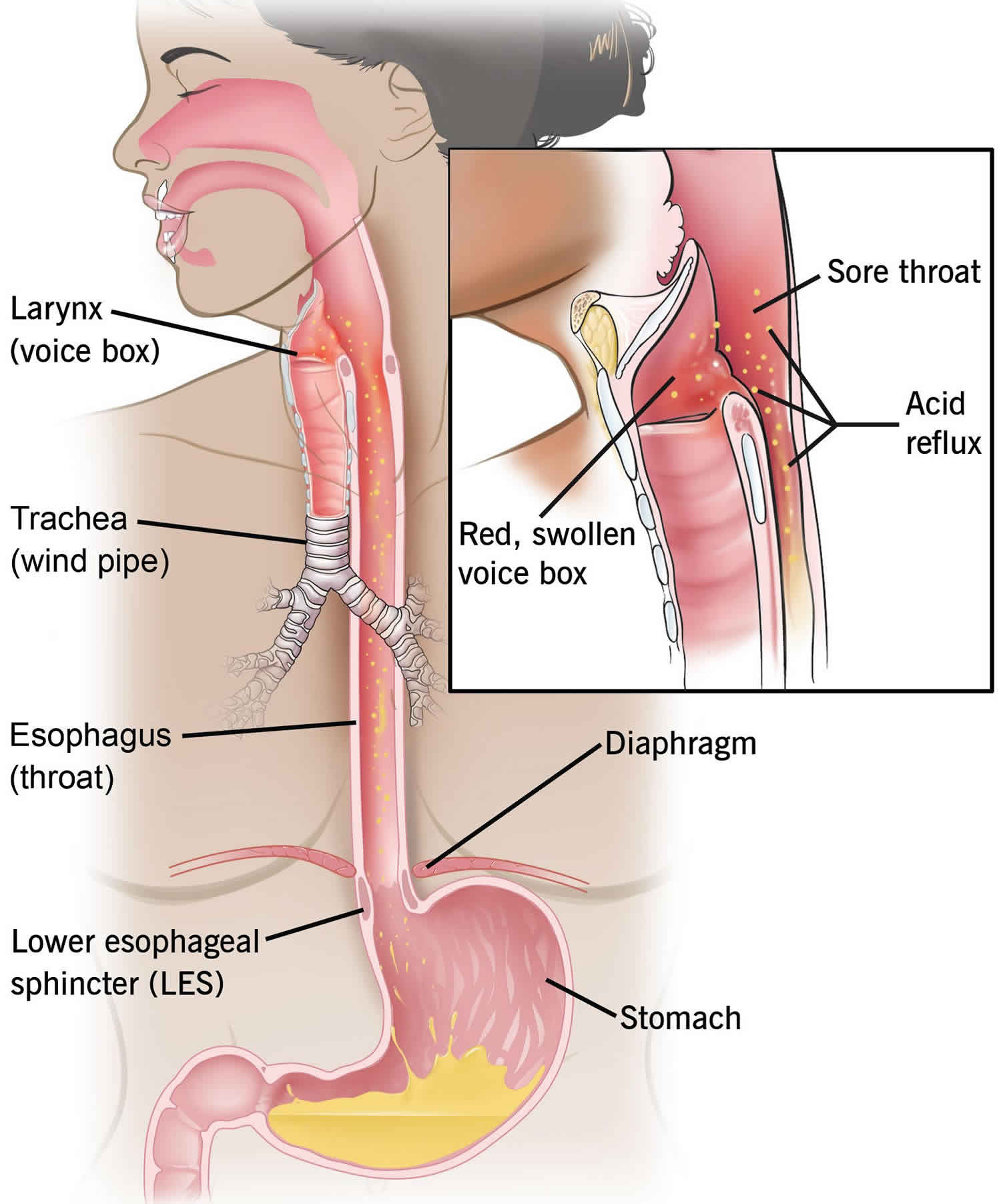
The esophagus is the narrow muscular organ that connects the mouth to the stomach and through which foods and liquids pass after being swallowed. After food is chewed and swallowed, the lump of food moves downward through the esophagus. If the esophagus is functioning normally, peristalsis, or a wave of coordinated contractions, takes place. Esophageal stricture formation is not common. One study reported an incidence rate of 1.1 per 10000 person-years, which also increases with age. A history of gastroesophageal reflux disease (GERD), hiatal hernia, prior dysphagia, peptic ulcer disease, and use of alcohol are known risk factors for esophageal stricture formation.
Esophageal stricture is either benign (non-cancerous) or malignant (cancerous). Benign esophageal stricture is a narrowing of the esophagus that is not caused by cancer. Benign esophageal strictures may occur due to buildup of fibrous tissue and collagen deposits due to ulcers or chronic inflammation of the esophagus.
Esophageal strictures can occur in any age group or population when one considers all the different possible etiologies. Strictures due to caustic esophagitis or eosinophilic esophagitis, however, are more common in children and young patients. Esophageal strictures related to acid reflux, iatrogenic or drug-induced esophagitis, on the other hand, are more common in adults. Malignant esophageal strictures are found in older people, as cancer prevalence is higher in older populations.
There are two major types of esophageal strictures: simple and complex.
- Simple esophageal stricture is symmetric with a diameter of more than 12 mm. Simple strictures are usually under 2 cm in size, straight and allow easy passage for the endoscope.
- Complex esophageal stricture is asymmetric and has a diameter of less than 12 mm. Complex esophageal strictures are typically longer than 2 cm, have an uneven surface, tortious margins, and a narrow diameter. Complex esophageal strictures are difficult to manage and require additional fluoroscopy or advanced thin-caliber endoscopes for further assessment 1.
The normal esophagus measures up to 30 mm in diameter. A esophageal stricture can narrow this down to 13 mm or less, causing dysphagia.
Esophageal stricture is a serious complication to many different disease processes and underlying causes. These include acid reflux, autoimmune, infectious, caustic, congenital, iatrogenic, medication-induced, radiation-induced, malignant, and idiopathic (unknown) disease processes. Esophageal stricture recognition and management should be prompt. Esophageal stricture formation can be due to inflammation, fibrosis or cancer involving the esophagus and often posing damage to the mucosa and/or submucosa.
Disease processes that can produce esophageal strictures can be grouped into three general categories:
- Intrinsic diseases that narrow the esophageal lumen through inflammation, fibrosis, or cancer;
- Extrinsic diseases that compromise the esophageal lumen by direct invasion or lymph node enlargement; and
- Diseases that disrupt esophageal peristalsis and/or lower esophageal sphincter (LES) function by their effects on esophageal smooth muscle and its innervation.
Generally, the term esophageal stricture is reserved for intraluminal esophageal disorders resulting in narrowing, although extrinsic esophageal compression and luminal compromise can sometimes occur, by direct invasion of cancer or lymph node enlargement for example, and therefore result in esophageal stricture as well 2. Regardless of cause, esophageal stricture is best managed promptly and aggressively to restore luminal patency; this is done for symptomatic improvement and/or palliative management in cases of cancer. New technological advancements in endoscopic therapy and different stent products have shown promising results with notable improvement in stricture management with low recurrence rates and fewer complications.
Appropriate management depends on identifying the correct cause for esophageal stricture.
Benign acid reflux related esophageal stricture is treated with dilation (stretching) of the esophagus using a thin cylinder or balloon that is inserted through an endoscope.You may need to have this treatment repeated after a period of time to prevent the esophageal stricture from narrowing again.
Proton pump inhibitors (acid-blocking medicines) can keep a peptic esophageal stricture from returning. Surgery is rarely needed.
If you have eosinophilic esophagitis, you may need to take medicines or make changes to your diet to reduce the inflammation. In some cases, dilation is done.
Esophageal stricture causes
Esophageal stricture is either benign or malignant.
Esophageal stricture can be caused by:
- Gastroesophageal reflux (GERD).
- Eosinophilic esophagitis.
- Injuries caused by an endoscope.
- Long-term use of a nasogastric (NG) tube (tube through the nose into the stomach).
- Swallowing substances that harm the lining of the esophagus. These may include household cleaners, lye, disc batteries, or battery acid.
- Radiation treatment for cancer of the head, neck, or chest
- Surgery to treat esophageal cancer or Barrett’s esophagus
- Treatment of enlarged veins in the esophagus (esophageal varices)
- An allergic condition called eosinophilic esophagitis
- Cancer
- Recent advancement in the use of endoscopic procedures for diagnostic as well as therapeutic purposes has increased the occurrence of iatrogenic post-procedural esophageal stricture formation resulting from mucosal injury.
The most common type of esophageal stricture results from benign peptic stricture from long-standing gastroesophageal reflux disease (GERD), which accounts for 70 to 80% of adult cases 3. Unless it is treated, GERD can cause scarring and narrowing of the lower esophagus. However, now that more effective medications, such as proton pump inhibitors, have been developed to treat GERD, strictures in the lower esophagus resulting from acid reflux are less common.
In young children and adolescent populations, corrosive substance ingestion is the leading cause of stricture formation in the esophagus 2.
The following classification and list of common and uncommon causes for esophageal stricture formation in the esophagus can guide physicians in their approach to management:
Benign esophageal strictures
- Corrosive substance ingestion: Accidental ingestion of, or suicidal poisoning with, household cleaning products are not uncommon occurrences. Exposure to these corrosive substances is one of the top five most common causes of poisoning in adults and children below five years of age according to data from the American Association of Poison Control Centers 4. Substance ingestion could cause anything from mild injury to extensive full-thickness necrosis of the esophagus. Stricture development is a common consequence of ingesting such as toxic substances 5.
- Eosinophilic esophagitis: Eosinophilic esophagitis represents a distinct chronic, local immune-mediated esophageal disease clinically characterized by dysphagia and histologically by eosinophilic-predominant inflammation 6. Due to the chronicity of this condition and the recent rise in the prevalence of the disease, it contributes to a significant number of cases of esophageal strictures 7. The prevalence of esophageal stricture disease increases with diagnostic delay in eosinophilic esophagitis (From 17% at 0 to 2 years to 71% at over 20 years), given the time-dependent fibrotic features of eosinophilic esophagitis the correlate with the duration of untreated disease 8.
- Drug-induced esophagitis: Many medications can cause pill-induced esophagitis. Common culprits include nonsteroidal anti-inflammatory drugs (NSAIDs), potassium chloride tablets, and tetracycline antibiotics, among other medications. Frequently the initial symptoms are self-limiting, and therefore the patient continues with medication usage and is exposed persistently to the drug, causing esophagitis 9. This situation could lead to severe complication such as esophageal stricture in a small percentage of patients.
- Radiation injury: Radiation therapy, when offered alone or combined with surgery, can cause esophageal stricture as a side effect. Radiation is an integral part of head and neck cancer and lung cancer treatment. Radiation targeting the cervical or thoracic regions can cause damage to the surrounding normal soft tissue and result in one of the most common late complications (median duration of 6 months) called radiation-induced esophageal stricture 10. The risk of stricture increases substantially with a higher dose of radiation 11.
- Iatrogenic stricture post-endoscopic therapy: Upper gastrointestinal endoscopy is commonly an option for diagnostic and therapeutic interventions involving the esophagus. A routine biopsy may be performed in cases of suspected Barrett esophagus or malignancy. Therapeutically, the endoscopic mucosal and submucosal resections of superficial esophageal malignancy are other possibilities. A side effect of these interventions includes damage to the underlying regenerative cell layer, leading to fibrosis and stricture formation. The risk of stricture increases with extensive circumferential resection 12.
- Anastomotic stricture: Certain early-stage esophageal cancers and head & neck cancers are managed with an esophagectomy with a high end-esophagogastrostomy or bowel loop interposition. Such procedures have a postoperative risk of anastomotic stricture formation at the anastomosis; this can occur in 22 to 50% of cases and often require repeat endoscopic interventions to dilate the stricture due to high recurrence rates 13.
- Chemotherapy-induced esophageal stricture: Stricture occurrence related to chemotherapy is rare, and there are few case reports 14. In pediatric patients, it is reported as a rare event and a result of chemotherapy treatment, but it has been described to possibly have a multifactorial etiology, including infectious and inflammatory factors causing esophagitis 15.
- Thermal Injury: This is a rare cause of stricture formation in patients who accidentally drink hot edible foods and fluids, especially coffee or tea. The majority of these cases respond to conservative management successfully. A small number of published case reports, where esophageal strictures developed after accidental ingestion of hot substances, described the need for endoscopic dilation or surgical correction to manage the strictures 16.
- Infectious esophagitis: Viral infections with cytomegalovirus (CMV), herpes simplex (HSV), human immune deficiency virus (HIV) and fungal infections with Candida can cause esophageal mucosal inflammation and stricture formation. They tend to occur in immunocompromised patients, and odynophagia is usually present.
- Other Rare causes 17:
- Prolonged use of nasogastric tube
- Collagen vascular diseases such as scleroderma or systemic lupus erythematosus (SLE).
- Benign mucosal pemphigoid
- Graft versus host disease
- Esophageal web in Plummer-Vinson syndrome.
- Crohn disease
- Tuberculosis
Malignant esophageal stricture
- Esophageal adenocarcinoma
- Esophageal squamous cell carcinoma
- Metastatic esophageal neoplasm – usually from lung cancer
Esophageal stricture prevention
Use safety measures to avoid swallowing substances that can harm your esophagus. Keep dangerous chemicals out of the reach of children. See your doctor if you have GERD.
Esophageal stricture symptoms
Esophageal stricture symptoms may include:
- Trouble swallowing (dysphagia)
- Pain with swallowing
- Unintentional weight loss
- Regurgitation of food
Regardless of the nature of the esophageal stricture, patients typically present with one or all of the following symptoms: dysphagia (difficulty swallowing), food impaction, odynophagia (painful swallowing), chest pain, and weight loss 18. The most relevant symptom is progressive dysphagia to solid food, and this sometimes progresses to involve semisolid and liquid foods. The rate and type of symptom progression correlate with the underlying type of stricture. Physical examination findings are usually not significant in these patients.
Benign stricture follows a slow and insidious course, while malignant stricture develops rapidly. Sometimes dysphagia is combined with pain in the presence of acute esophagitis. Food impaction requires instant recognition and prompt management to avoid severe complications like aspiration or perforation. Patients presenting with such clinical symptoms have an underlying stricture in about 45% of cases diagnosed at endoscopy 19.
There is also a higher risk of having food, fluid, or vomit enter the lungs with regurgitation. This can cause choking or aspiration pneumonia.
Weight loss and anorexia along with long-standing weakness are more associated with malignant strictures or refractory strictures 20. The following historical information can also help understand the cause of stricture formation and guide management 21:
- A known medical history of GERD, Barrett esophagus, hiatal hernia, or medication use that could cause peptic ulcers and gastrointestinal irritations; any of these are risk factors for peptic stricture.
- The patient has a history of recent caustic product ingestion.
- A prior history of endoscopic treatment or esophageal surgery for any esophageal pathology. Such patients are at risk for developing anastomotic or iatrogenic stricture.
- There is a history of radiation therapy for any prior head & neck or chest malignancy.
- A history of medication use including alendronate, tetracycline or other antibiotics, NSAIDs and many others that predispose one to pill-induced esophagitis.
Esophageal stricture complications
Untreated esophageal stricture related complications:
- Food impaction
- Food particle aspiration
- Asthma from aspiration
- Severe chest pain
- Esophageal perforation from long-standing inflammation
- Fistula formation
Iatrogenic complication from stricture dilation and stent placement 22:
- Esophageal perforation
- Bleeding
- Hemorrhage
- Anesthesia-related complications (such as respiratory failure, sedation)
- Aspiration pneumonia
- Bacteremia – 22% of cases develop transient bacteremia from esophageal dilation
- It is usually associated with malignant stricture dilation and multiple dilations
- Periprocedural antibiotics are usually recommended to avoid this
- Re-stricture formation
- New stricture formation
- Traumatic stent removal
- Epithelization of the uncovered stent
- Displacement of stent proximally which could potentially cause choking sensation and difficulty in breathing
Esophageal stricture diagnosis
If symptoms such as difficulty swallowing or a burning sensation in the throat or chest are present, your doctor may perform various tests to determine the cause.
To diagnose esophageal stricture, you may need the following tests:
- Barium swallow to look for narrowing of the esophagus. Barium swallow requires you to swallow a solution containing barium. X-rays are taken while the barium moves down the esophagus. If a stricture is present, the barium may become stuck or slows down.
- Upper GI endoscopy to look for narrowing of the esophagus. In endoscopy a narrow tube called an endoscope is inserted into your esophagus. The endoscope has a light and tiny camera at one end so the doctor can observe the inside of the esophagus. After the presence of a stricture is confirmed, the most important step is to biopsy the stricture to rule out malignancy. Differentiation of benign stricture from malignant stricture is absolutely necessary to guide further management approaches.
- Esophageal manometry – if no structural abnormality is detected. It is performed to measure pressure waves inside the esophagus. The presence of unusually large numbers of simultaneous contractions in the lower esophagus is the major indicator of spasms.
- Esophageal motility test – measures muscular strength and coordination. The test is performed by inserting a small tube through the nose into the esophagus. The tube contains pressure-sensitive transducers, which remain in the esophagus after the tube is withdrawn. The patient swallows a small amount of water (about a teaspoon) at regular intervals to allow the transducers to measure the contractions during peristalsis. The test can also detect whether there is an abnormality in the valve at the lower end of the esophagus (lower esophageal sphincter).
The cause of esophageal stricture can usually be identified using radiologic and endoscopic modalities and can be confirmed by endoscopic visualization and tissue biopsy. Use of manometry can be diagnostic when dysmotility is suspected as the primary process. Computed tomography (CT) scanning and endoscopic ultrasonography are valuable aids in staging of malignant stricture. Fortunately, most benign esophageal strictures are amenable to pharmacologic, endoscopic, and/or surgical interventions.
Esophageal stricture treatment
There are a variety of methods available for treating simple benign (non-cancerous) esophageal strictures.
Dilation is used to widen the esophageal passageway to relieve dysphagia (difficulty swallowing). The technique uses dilators (long plastic or rubber cylinders of different sizes) to stretch the opening. Dilators with gradually increasing diameters are inserted through the mouth, sometimes over a guide wire.
Balloons may be passed through an endoscope and inflated to stretch the opening. Medication to reduce gastric acid production may be given before the procedure if the stricture is caused by GERD.
Complex strictures are often seen after radiation therapy to treat cancer of the head, neck or chest, or in cases where caustic substances were ingested. In cases for patients with malignant cancer, temporary metal stents (expandable tubes) may be used instead of balloon dilation to keep the esophagus open.
Esophageal stricture dilation
Dilator Selection:
There are currently two main types of dilators in use in clinical practice. Each has its own advantage and disadvantage 23.
- Mechanical (push type or bougie): They come with a variety of sizes and are made up of different types of materials such as rubber. Maloney bougie dilator can be freely passed without the use of the guidewire. While Savary-Gilliard has a guidewire to assist in the passage.
- Balloon: Expansion of a balloon produces radial force to dilate the lumen. Different sizes are available, and a balloon dilator gets passed through the scope 24. Newer balloon dilators from having an inbuilt guidewire and also allow for three different size expansions without changing the balloon.
Stricture dilation is an ambulatory outpatient procedure that requires certain levels of expertise from an endoscopist 25. Appropriate selection of dilator is determined usually based on the complexity, size, and site of the stricture. A lower esophageal stricture is commonly peptic in nature; due to their simple characteristic and small size, mechanical dilators are safe and effective in treating them. Complex strictures tend to undergo management with balloon dilators.
Technique:
First, the size of the dilator that is going to be used is estimated endoscopically by assessing the diameter of the stricture area. The first dilation performed should be the same size as the stricture. It is advanced in increments. No excessive force is used. The majority of surgeons or endoscopists follow the “rule of three,” performing up to three dilations per session while successively increasing the diameter of the dilator by 2 mm (6Fr) 26. Use of fluoroscopy is controversial, but can certainly be helpful in complex strictures. It can play a contributory role based on the endoscopist’s experience 27.
Use of Adjunctive Methods:
At present two main adjunctive treatment methods are employed based on preference: Intra-lesion injection of steroid, or oral steroid gel use and endoscopic stricturoplasty. Steroids help in decreasing the inflammation related to injury from dilation and, hence, reduce the chance of restenosis. However, long-term data are needed to establish its standard use 28. Other studies have shown better outcomes based on the lower recurrence of stenosis and the achievement of larger diameter patency 29. Four-quadrant stricturoplasty can be one option to consider in highly fibrotic strictures.
Long-term success with dilation can often be challenging to achieve in all cases of the stricture. Unfavorable outcomes are more common in strictures from corrosive injury. Dilation is successful in only about 25% of such cases. Successful outcomes here refer to the ability to swallow solid food without intervention for 6 months after the first procedure 30.
The major problem one faces in stricture management is a recurrence. A stricture is recurrent when there is an inability to maintain a satisfactory luminal diameter for 4 weeks after achieving the target diameter of 14 mm. A stricture is refractory when there remains a persistent dysphagia score of 2 or more, as a result of an inability to successfully achieve a diameter of 14 mm over five sessions of dilation done at 2-week intervals 31.
Esophageal stents
Stents are often reserved for malignant stricture and refractory benign strictures. The goal of stent placement is to hold the stricture open for prolonged periods, causing the stricture, or the tissue around it, to remodel so that the stricture does not recur after stent removal. In malignant stricture, this could be either used for complete palliation in case of advanced cancer or temporary palliation in cases of ongoing neoadjuvant treatment 32.
Stents are the breakthrough inventions considering the extent of benefits they can provide in terms of symptom improvement and quality of life enhancement, especially in patients who are suffering from terminal cancer. Over the years, esophageal stents have evolved from rigid plastic conduits to self-expanding metal stents. Improvements continued to address the disadvantages of previous models. This trend is notable with self-expanding metal stents, where initially non-covered stents were the initial offering. Later, partially and then fully covered stents were developed to correct the issue of epithelialization and tumor growth in the previous older uncovered self-expanding metal stents, allowing feasibility of stent removal. Covered stents, however, show a higher displacement rate (20%) compared to there metal counterparts. To resolve this, biodegradable stents and suture fixation techniques are under clinical evaluation, and initial evidence shows promising outcomes 33. Biodegradable stents show superiority in outcomes and symptomatic improvements in patients with a corrosive esophageal stricture 34.
Benefits, safety, and feasibility of different stents have undergone comparison in various clinical and randomized controlled trials. Currently, no clear outcome benefit is apparent between the use of partial vs. full covered self-expanding metal stents for palliative management of malignant strictures in regards to recurrent obstruction and symptomatic success (the COPAC Study) 35. The FDA has approved the use of self-expanding plastic stents (SEPS) for the indication of benign esophageal stricture.
Surgical management
Surgical resection is reserved for malignant disease-causing esophageal stricture or benign conditions recalcitrant to less aggressive forms of medical and/or endoscopic therapy. When surgery is necessary for benign refractory peptic strictures, an antireflux procedure is selectively done to prevent further stenosis 36. Extensive surgery may be necessary in cases of malignant stricture, where concurrent removal of a mass also takes place if staging is favorable. In such cases, partial or complete esophagectomy, with gastric tube pull-up or bowel loop interposition and anastomosis is performed. Otherwise, palliative surgical approaches are considered to relieve symptoms or obstruction and to provide a route for enteral nutrition distal to a stricture, usually via gastrostomy tube placement.
Esophageal stricture prognosis
Esophageal stricture develops over time, and prognosis depends on the timing of evaluation and management as well as the underlying cause of stricture. While esophageal dilation is the first line management in cases of benign esophageal stricture, regardless of the underlying cause, it poses a 10 to 30% chance of re-stenosis. Stricture recurrence is the primary concern resulting in added risks and costs. Peptic stricture shows excellent prognosis when treated promptly with endoscopic dilation and long-term proton pump inhibitor therapy. Surgical correction of a hiatal hernia, if present, produces excellent results with minimal risk for re-stenosis and significant symptomatic improvement 37. To improve prognosis in terms of decreasing stricture recurrence, concurrent steroid injection therapy or steroid pill therapy have been used and are showing promising clinical outcomes 38. Stent placement is kept reserved for benign stricture cases where repeated dilation is not adequate and symptomatic control is poor. In the case of malignant stricture, the prognosis depends on the cancer type, tumor invasion, and disease stage. Surgical resection demonstrates better prognosis for cancer, which hasn’t yet invaded lymph nodes and surrounding tissue. When stricture management is with stent placement for palliation, the prognosis is almost always poor.
References- Shami VM. Endoscopic management of esophageal strictures. Gastroenterol Hepatol (N Y). 2014 Jun;10(6):389-91.
- Desai JP, Moustarah F. Esophageal Stricture. [Updated 2019 Jun 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK542209
- ASGE Standards of Practice Committee. Pasha SF, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD, Fisher DA, Foley KQ, Fonkalsrud L, Hwang JH, Jue TL, Khashab MA, Lightdale JR, Muthusamy VR, Sharaf R, Saltzman JR, Shergill AK, Cash B. The role of endoscopy in the evaluation and management of dysphagia. Gastrointest. Endosc. 2014 Feb;79(2):191-201.
- Mowry JB, Spyker DA, Cantilena LR, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila). 2014 Dec;52(10):1032-283.
- Chirica M, Bonavina L, Kelly MD, Sarfati E, Cattan P. Caustic ingestion. Lancet. 2017 May 20;389(10083):2041-2052.
- Lucendo AJ, Molina-Infante J, Arias Á, von Arnim U, Bredenoord AJ, Bussmann C, Amil Dias J, Bove M, González-Cervera J, Larsson H, Miehlke S, Papadopoulou A, Rodríguez-Sánchez J, Ravelli A, Ronkainen J, Santander C, Schoepfer AM, Storr MA, Terreehorst I, Straumann A, Attwood SE. Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 2017 Apr;5(3):335-358.
- Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003 Dec;125(6):1660-9.
- Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, Straumann A. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013 Dec;145(6):1230-6.e1-2.
- Kim SH, Jeong JB, Kim JW, Koh SJ, Kim BG, Lee KL, Chang MS, Im JP, Kang HW, Shin CM. Clinical and endoscopic characteristics of drug-induced esophagitis. World J. Gastroenterol. 2014 Aug 21;20(31):10994-9.
- Choi GB, Shin JH, Song HY, Lee YS, Cho YK, Bae JI, Kim JH, Jeong YH, Park MH. Fluoroscopically guided balloon dilation for patients with esophageal stricture after radiation treatment. J Vasc Interv Radiol. 2005 Dec;16(12):1705-10.
- Park JH, Kim KY, Song HY, Cho YC, Kim PH, Tsauo J, Kim MT, Jun EJ, Jung HY, Kim SB, Kim JH. Radiation-induced esophageal strictures treated with fluoroscopic balloon dilation: clinical outcomes and factors influencing recurrence in 62 patients. Acta Radiol. 2018 Mar;59(3):313-321.
- Martínek J, Juhas S, Dolezel R, Walterová B, Juhasova J, Klima J, Rabekova Z, Vacková Z. Prevention of esophageal strictures after circumferential endoscopic submucosal dissection. Minerva Chir. 2018 Aug;73(4):394-409.
- Park JY, Song HY, Kim JH, Park JH, Na HK, Kim YH, Park SI. Benign anastomotic strictures after esophagectomy: long-term effectiveness of balloon dilation and factors affecting recurrence in 155 patients. AJR Am J Roentgenol. 2012 May;198(5):1208-13.
- Sedhom D, Sedhom R, Mishra A, Razjouyan H, Rustgi V. Esophageal Stricture Resulting from Systemic Chemotherapy for Solid Malignancy. ACG Case Rep J. 2017;4:e99
- Kelly K, Storey L, O’ Sullivan M, Butler K, McDermott M, Corbally M, McMahon C, Smith OP, O’ Marcaigh A. Esophageal strictures during treatment for acute lymphoblastic leukemia. J. Pediatr. Hematol. Oncol. 2010 Mar;32(2):124-7
- Kitajima T, Momose K, Lee S, Haruta S, Shinohara H, Ueno M, Fujii T, Udagawa H. Benign esophageal stricture after thermal injury treated with esophagectomy and ileocolon interposition. World J. Gastroenterol. 2014 Jul 21;20(27):9205-9
- Mbiine R, Kabuye R, Lekuya HM, Manyillirah W. Tuberculosis as a primary cause of oesophageal stricture: a case report. J Cardiothorac Surg. 2018 Jun 05;13(1):58.
- Smith CD. Esophageal strictures and diverticula. Surg. Clin. North Am. 2015 Jun;95(3):669-81
- Gretarsdottir HM, Jonasson JG, Björnsson ES. Etiology and management of esophageal food impaction: a population based study. Scand. J. Gastroenterol. 2015 May;50(5):513-8.
- Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018 Jan;154(2):267-276.
- Kikendall JW. Pill-induced esophagitis. Gastroenterol Hepatol (N Y). 2007 Apr;3(4):275-6.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest. Endosc. 1998 Dec;48(6):563-7
- Cox JG, Winter RK, Maslin SC, Dakkak M, Jones R, Buckton GK, Hoare RC, Dyet JF, Bennett JR. Balloon or bougie for dilatation of benign esophageal stricture? Dig. Dis. Sci. 1994 Apr;39(4):776-81
- Taub S, Rodan BA, Bean WJ, Koerner RS, Mullin DM, Feng TS. Balloon dilatation of esophageal strictures. Am. J. Gastroenterol. 1986 Jan;81(1):14-8.
- Abele JE. The physics of esophageal dilatation. Hepatogastroenterology. 1992 Dec;39(6):486-9.
- Sami SS, Haboubi HN, Ang Y, Boger P, Bhandari P, de Caestecker J, Griffiths H, Haidry R, Laasch HU, Patel P, Paterson S, Ragunath K, Watson P, Siersema PD, Attwood SE. UK guidelines on oesophageal dilatation in clinical practice. Gut. 2018 Jun;67(6):1000-1023.
- Raymondi R, Pereira-Lima JC, Valves A, Morales GF, Marques D, Lopes CV, Marroni CA. Endoscopic dilation of benign esophageal strictures without fluoroscopy: experience of 2750 procedures. Hepatogastroenterology. 2008 Jul-Aug;55(85):1342-8.
- Yan X, Nie D, Zhang Y, Chang H, Huang Y. Effectiveness of an orally administered steroid gel at preventing restenosis after endoscopic balloon dilation of benign esophageal stricture. Medicine (Baltimore). 2019 Feb;98(8):e14565.
- Ramage JI, Rumalla A, Baron TH, Pochron NL, Zinsmeister AR, Murray JA, Norton ID, Diehl N, Romero Y. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. Am. J. Gastroenterol. 2005 Nov;100(11):2419-25.
- Tharavej C, Pungpapong SU, Chanswangphuvana P. Outcome of dilatation and predictors of failed dilatation in patients with acid-induced corrosive esophageal strictures. Surg Endosc. 2018 Feb;32(2):900-907
- Kochman ML, McClave SA, Boyce HW. The refractory and the recurrent esophageal stricture: a definition. Gastrointest. Endosc. 2005 Sep;62(3):474-5.
- Hindy P, Hong J, Lam-Tsai Y, Gress F. A comprehensive review of esophageal stents. Gastroenterol Hepatol (N Y). 2012 Aug;8(8):526-34.
- Li X, Zhang W, Zhang G. Endoscopic suturing device with Overstitch for esophageal stent fixation. Dig Endosc. 2019 Jan;31(1):e3-e4
- Karakan T, Utku OG, Dorukoz O, Sen I, Colak B, Erdal H, Karatay E, Tahtaci M, Cengiz M. Biodegradable stents for caustic esophageal strictures: a new therapeutic approach. Dis. Esophagus. 2013 Apr;26(3):319-22.
- Didden P, Reijm AN, Erler NS, Wolters LMM, Tang TJ, Ter Borg PCJ, Leeuwenburgh I, Bruno MJ, Spaander MCW. Fully vs. partially covered selfexpandable metal stent for palliation of malignant esophageal strictures: a randomized trial (the COPAC study). Endoscopy. 2018 Oct;50(10):961-971
- Pregun I, Hritz I, Tulassay Z, Herszényi L. Peptic esophageal stricture: medical treatment. Dig Dis. 2009;27(1):31-7.
- Pregun I, Hritz I, Tulassay Z, Herszényi L. Peptic esophageal stricture: medical treatment. Dig Dis. 2009;27(1):31-7
- Yan X, Nie D, Zhang Y, Chang H, Huang Y. Effectiveness of an orally administered steroid gel at preventing restenosis after endoscopic balloon dilation of benign esophageal stricture. Medicine (Baltimore). 2019 Feb;98(8):e14565