Eucalyptus oil
Eucalyptus oil also known as eucalyptus essential oil, is the generic name for distilled oil extracted from the leaves of various Eucalyptus species, a genus of the plant family Myrtaceae native to Australia and cultivated worldwide. Eucalyptus oil is a complex mixture of a variety of monoterpenes, sesquiterpenes, and aromatic phenols 1. The Eucalyptus oil has been known as antibacterial, antifungicidal, and antiseptic in nature 2. In recent years, the action of Eucalyptus oil has been scientifically proven, and there have been reports that Eucalyptus oil suppresses the production of chemokines, cytokines and lipid mediators in basophils, alveolar macrophages and monocytes 3. Eucalyptus oil is commonly used as an over-the-counter (OTC) medication, cough drops, lozenges, ointments, mouthwashes, and inhalants in most countries for treating common colds and sinusitis, in addition to its myriad use in pharmaceutical, flavoring, pesticide, perfumery, and industrial uses 4. However, its permissible limit is often unregulated. A product of encapsulated eucalyptus oil for intestinal release is available and anecdotal reports on its use for respiratory problems are highly favorable. A side effect can be constipation, which might be due to imbalance of gut flora or relaxation of smooth muscle contraction, similar to antispasmodic effects discussed below.
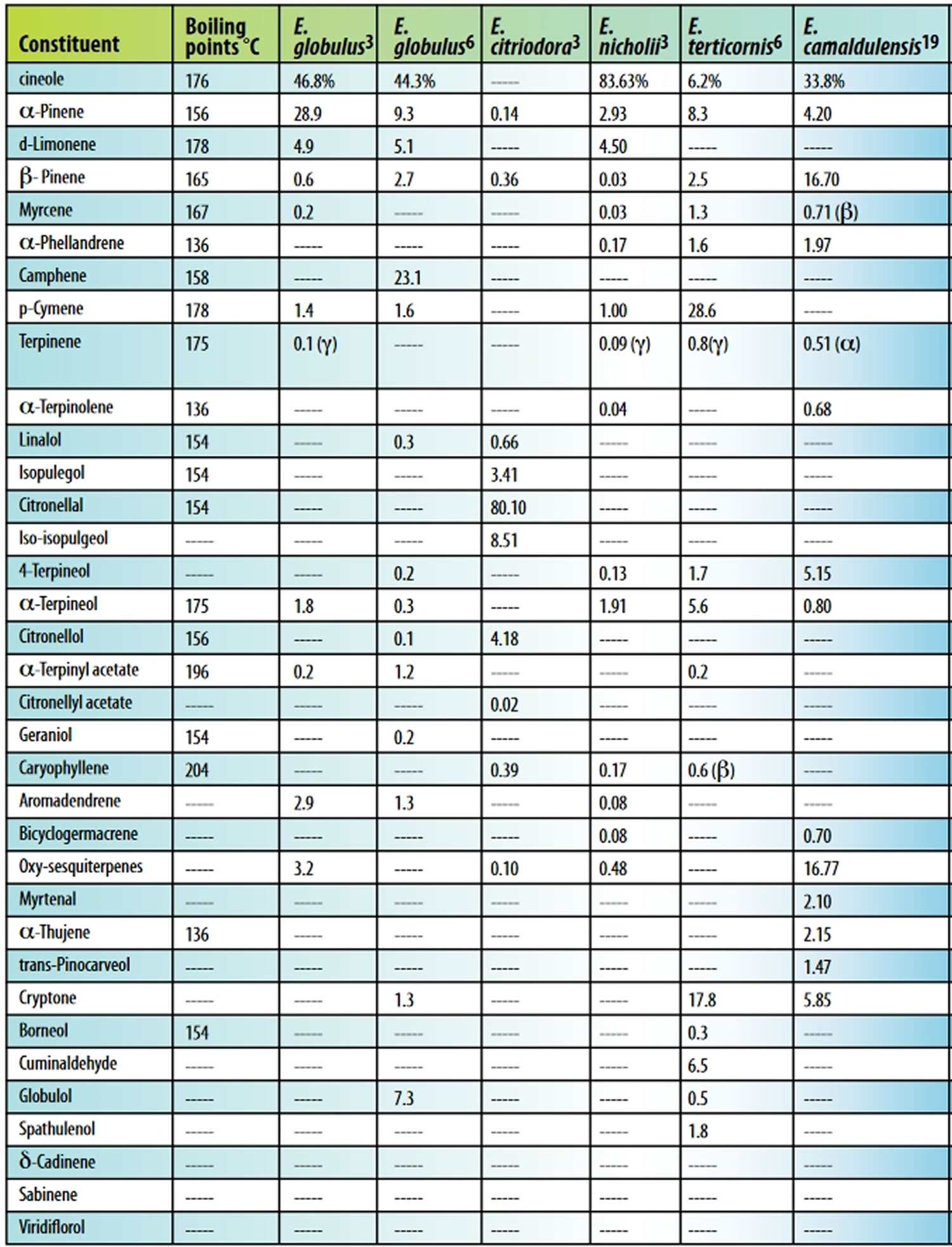
The main group of constituents of eucalyptus oil is monoterpenes such as α-pinene, myrcene, cineole (eucalyptol), fenchone, α-terpinolene, and β-terpinyl acetate), and the principal constituent of pharmaceutical‐grade eucalyptus oil is eucalyptol (1,8‐cineole), which must comprise at least 70% of the contents (see Table 1) 5. Eucalyptus kochii and Eucalyptus polybractea have the highest cineole content, ranging from 80% to 95% 6. Global production is dominated by Eucalyptus globulus. The composition of the extracted oil can change depending on the storage conditions of the raw material and the technique employed to extract the oil 7.
Cineole-based “oil of eucalyptus” is generally considered safe for adults if taken at a low dose, as in the case of flavoring agents or in pharmaceutical products. However, at higher-than-recommended doses, systemic toxicity can occur 8. Darben et al. 9 has reported a toxicity of topical Eucalyptus experienced by a 6-years old Caucasians girl, resulted with nausea, slurred speech, ataxia, and muscle weaknesses after application of 25 mL of Eucalyptus globulus on her skin. Eucalyptus oil taken from the eucalyptus tree (true eucalyptus oil) does not contain camphor 4. However, the cineole fraction of camphor laurel that is also used to manufacture eucalyptus oil (which is considered “fake eucalyptus oil”) may contain camphor 10. Like eucalyptus oil, camphor is also epileptogenic 11, with recent case reports of two patients with eucalyptus oil poisoning, both adults who unintentionally took eucalyptus oil and presented to the emergency room of our institution with seizures 4. Most people, even physicians, are not aware of the toxic potential of these seemingly innocuous substances including Eucalyptus oil induced seizure. Regulation regarding the permissible limits of these ingredients in substances that contain them should be strictly imposed. Also, the label of products that contain eucalyptus oil or camphor should have mandatory warnings of the potential toxic effects, including seizures 4.
Table 1. Eucalyptus oil components
Footnote: Table 1 lists the major components of eucalyptus oil from five species. The percentage of components varies with species, plant part, and batch. Oil yield from Eucalyptus leaves ranging from 3.57-10.6 mg/g volatile components was demonstrated when various species were steam-distilled. Eucalyptus globulus produced 5.25 mg oil/g of fresh leaf 12.
[Source 13 ]What is eucalyptus oil good for?
Eucalyptus oil is used for its aromatic properties and as an ingredient in pharmaceutical and industrial applications. Eucalyptus oil is used as a remedy for many ailments like cold, rhinitis, abdominal pain, and toothache 7. Eucalyptus oil has been used since ancient times for its bactericidal, anti-inflammatory, analgesic and sedative effects. Eucalyptus oil has been used by Australian natives as a remedy for wounds and inflammation 14. Essential oils obtained from Eucalyptus leaves are said to have anti-inflammatory, analgesic, sedative and bactericidal effects, and they are used in medicines and aromatherapy 15. The main chemical components of Eucalyptus oil, eucalyptol (1,8‐cineole) and alpha-terpineol, give the essential oil a soothing, cooling vapor. This makes Eucalyptus oil useful for massage. In the UK, oil extracted from Eucalyptus globulus is used to treat airway inflammation and various symptoms of rheumatism. In Brazil, Eucalyptus essential oil has been used in traditional medicine for numerous of medical diseases. In Japan, the essential oil obtained via steam distillation of the leaves of Eucalyptus globulus Labillardière or other closely related plants (Myrtaceae) is used as an anti-inflammatory and analgesic. According to Committee of Herbal Medicine Products, a committee of European Medicines Agency (European equivalent of the FDA) reported the traditionally application dosage of Eucalyptus globulus essential oil via transdermal administration at ranges between 1.7–4 g/100 L of water 16. This traditional application of Eucalyptus globulus essential oil was demonstrated for bath additive in the treatment of muscle aches that attributable to analgesic pharmacological activity of Eucalyptus essential oil. Besides, the main constituent of Eucalyptus globulus essential oil known as eucalyptol (1,8‐cineole) has been widely discussed for its natural analgesic properties 17.
Clinical studies confirmed the anti-inflammatory effects of Eucalyptus oil in patients with asthma, such as alleviating sinusitis symptoms and preventing the worsening of chronic obstructive pulmonary disease (COPD) 18. A comprehensive report on plant oil using basophils has reported the inhibitory effect of Eucalyptus oil on immunoglobulin E (IgE)-mediated basophil activation 19. It is suggested that Eucalyptus oil suppresses inflammation by directly acting on immune cells such as macrophages and monocytes 20, as supported by several studies. Specifically, Eucalyptus oil was demonstrated to inhibit lipopolysaccharide (LPS)-induced nitric oxide production in mouse macrophage cell lines10 improve the acute inflammatory response of the lungs in a mouse acute lung injury model 21 and inhibit active oxygen release by cultured alveolar macrophages from patients with chronic obstructive pulmonary disease (COPD) 22. Additionally, 1,8-cineole, the main component of Eucalyptus oil, blocked arachidonic acid metabolism in blood monocytes from patients with asthma 23 and inhibited LPS-induced IL-1β production by human monocytes 24. In recent years, it has also been reported that eucalyptol exerts anti-inflammatory effects in mice through effects on TRPM8 channels 25. Eucalyptus oil was shown to have an analgesic effect in the formalin and carrageenan paw edema test despite being applied topically 26. As a bath agent, Eucalyptus extract mixture was reported to improve the skin symptoms of patients with atopic dermatitis 27.
Chronic obstructive pulmonary disease (COPD)
Cineole (eucalyptol), a chemical found in eucalyptus oil, has mucolytic, bronchodilating and anti-inflammatory properties and reduces the exacerbation rate in patients suffering from chronic obstructive pulmonary disease (COPD), as well as ameliorates symptoms in patients suffering from asthma and rhinosinusitis. As part of a double-blind, placebo-controlled, multi-center-study, a total of 242 patients with confirmed acute bronchitis was randomly selected to participate 28. Over a period of 10 days, all patients were administered 3 x 200 mg of Cineole, or a respective placebo, per day. The primary outcome measure was a Bronchitis Sum Score, which summarizes the relevant symptoms of acute bronchitis. After 4 days of treatment it was notable, that the patient group treated with Cineole, showed significantly more improvements of the bronchitis-sum-score than those of the placebo group. The statistical significant difference of the individual outcome measures was especially underlined by the frequency of cough fits after 4 days. The effects of Cineole in the treatment of acute bronchitis were clearly measurable and could be proven after a treatment period of merely 4 days. This study corroborates the fact that Cineole actively and significantly reduces cough frequency after four days 28.
In another double-blind, placebo-controlled multi-center-study where 242 patients were randomly assigned with stable chronic obstructive pulmonary disease (COPD) to receive 200 mg of cineole or placebo 3 times daily as concomitant therapy for 6 months during winter-time 29. The frequency, duration and severity of exacerbations were combined as primary outcome measures for testing as multiple criteria. Secondary outcome measures included changes of lung function, respiratory symptoms and quality of life as well as the single parameters of the exacerbations. Baseline demographics, lung function and standard medication of both groups were comparable. During the treatment period of 6 months the multiple criteria frequency, severity and duration of exacerbations were significantly lower in the group treated with cineole in comparison to placebo. Secondary outcome measures validated these findings. Improvement of lung function, dyspnea and quality of life as multiple criteria were statistically significant relative to placebo. Adverse events were comparable in both groups. Concomitant therapy with cineole reduces exacerbations as well as dyspnea and improves lung function and health status. This study 29 further suggests cineole as an active controller of airway inflammation in COPD by intervening in the pathophysiology of airway inflammation of the mucus membrane.
Acute bronchitis
In other studies, research shows that taking a specific combination product containing eucalyptol, a chemical found in eucalyptus oil, and extracts of pine and lime by mouth for at least 2 weeks improves symptoms and reduces flare-ups in people with bronchitis 30, 31, 32.
Asthma
Leukotriene B4 (LTB4) and prostaglandin E2 (PGE2), both produced in the pathway of arachidonic acid metabolism, were measured in stimulated monocytes from 10 patients with bronchial asthma and 12 healthy controls after treatment with eucalyptol (1,8‐cineole) (200 mg three times daily) for three days 33. Forced expiratory volume in one second (FEV1) and airway resistance were measured the day before treatment, during treatment, and after discontinuing treatment. Significant inhibition of LTB4 and PGE2 was observed in both groups. The FEV1 increased by 23.7 percent and airway resistance decreased by 26.1 percent after three days of eucalyptol treatment 33. When lung function was checked four days after the end of treatment, FEV1 (28.7%) and airway resistance (-17.6%) were still significantly improved compared to before treatment 33.
The anti-inflammatory effect of oral euclyptol (1.8-cineole) (200 mg three times daily) was demonstrated in a double-blind, placebo-controlled, 12-week trial of asthma patients 34. The required oral glucocorticoid dosage was decreased by a mean of 3.75 mg in the euclyptol group compared to 0.91 mg in the control group. Prednisolone dose prior to treatment was 5-24 mg daily with average of 11 mg. Of note, the use of the rescue medication salbutamol (albuterol) was increased almost two-fold in the control group when prednisolone was lowered by 2.5 mg; whereas, there was no significant increase in rescue puffs in the euclyptol group, even with a decrease of 5 mg prednisolone 34. Some participants dropped out at that reduction (four in the euclyptol group, 11 in the placebo group). The euclyptol group maintained lung function capacity (peak expiratory flow rate, FEV1, and airway resistance) four times longer than placebo, even at a lower prednisolone dosage 34.
Rhinosinusitis
A study showed that euclyptol (1.8-cineole) is effective for the discomforts of non-purulent rhinosinusitis 35. Individuals (n=150) with subjective findings of headache with or without bending, tenderness to pressure points of the trigeminal nerve, impairment of general condition, nasal obstruction, and nasal secretions (rated by quantity and viscosity) were randomized in a double-blind, placebo-controlled trial 35. Subjects in the treatment group (n=75) showed over 80-percent improvement after seven days of oral 1,8-cineole (200 mg three times daily) compared to less than 50-percent improvement in the placebo group 35. Improvement was determined by symptoms-sum-score. Ultra-sonography at the end of the study showed that sinus shadowing remained in 37 patients in the placebo group and four patients in the euclyptol group. All patients also inhaled 100 mcg of the decongestant xylometazoline three times daily to relieve nasal congestion 35.
Analgesic effect
Three species of eucalyptus oil (Eucalyptus citriodora, Eucalyptus tereticornis and Eucalyptus globulus) showed dose-dependent and time-dependent peripheral and central acting analgesic properties in rodents compared to morphine, using standard experimental test models. Eucalyptus tereticornis had the greatest anti-inflammatory action in a model of rat paw edema 36. The anti-inflammatory action of eucalyptus oil compared to dexamethasone showed an average of 75-percent versus 97-percent inhibition, respectively, of neutrophil migration into the rat peritoneal cavity 36. A test for vascular permeability effect showed reduction, but widely varied by species and permeability agent 36.
When rats or mice were injected with proinflam-matory substances, 1,8-cineole (eucalyptol) was shown to prevent pain sensation. The opioid antagonist, naloxone, did not reverse the analgesic effect of eucalyptol (1,8-cineole), implying that eucalyptol does not work through the mu-opioid receptors in the body 37.
Using rats and mice in pain evaluation methods, eucalyptol (1,8-cineole) was found comparable to morphine in analgesic effect on the central and peripheral nervous system. A synergistic effect was observed between cineole and morphine, with naloxone failing to antagonize the effect of cineole. Eucalyptus oil with morphine can allow the same strength of analgesia with lower morphine dose. Beta-pinene had an anti-nociceptive supraspinal effect in rats; but in contrast to cineole, showed opioid antagonist activity on morphine comparable to naloxone 38.
Rat superior cervical ganglion was used to measure the nerve excitability (via intracellular recording) when exposed to eucalyptol at concentrations of 0.1, 1.0, 3.0, and 6.0 mM injected intracellularly. Inhibition of excitability was seen at 1.0, 3.0, and 6.0 mM. The 6.0 mM concentration showed a significant decrease in excitability, causing a complete action potential block in all the tested neurons. The authors state that one mechanism of action is indirectly due to depolarization of the neuronal cytoplasmic membrane 39.
Antispasmodic effect
Eucalyptus tereticornis oil and 1,8-cineole (eucalyptol) were evaluated on chemically- and electrically-induced muscle contraction of guinea pig tracheal smooth muscle 40. Eucalyptus oil inhibited potassium-induced smooth muscle contraction at 200-1,000 μg/mL, with 50-percent contraction inhibition at 248 μg/mL. Eucalyptus oil (200-400 μg/mL) enhanced the contrac-tions when induced by acetylcholine, but caused relaxation at 800-1,000 μg/mL. Eucalyptol (cineole) inhibited potassium-induced, smooth muscle contraction at 600-1,000 μg/mL with 50-percent contraction inhibition at 446 μg/mL. Eucalyptol (cineole) significantly enhanced the acetylcholine-induced contractions at all concentrations (10-1,000 μg/mL) 40.
Eucalyptol (cineole) applied to guinea pig tracheal smooth muscle resulted in a statistically significant decrease in contraction 41. Cineole also significantly relaxed smooth muscle when combined with ovalbumin stimulation (in previously sensitized animals), but no effect occurred with muscarinic-induced contractions, demonstrating the effect is from the sympathetic branch of the nervous system 41.
Another study demonstrated that eucalyptol (1,8-cineole) vapor had little to no effect on citric acid-induced cough in guinea pigs 42. However, the authors noted a possible dose-dependent, antitussive effect on the respiratory tract, but failed to increase the concentration to verify 42. It was noted that different species of eucalyptus oil might produce better or worse results depending on the cumulative effect of their respective constituents 42. Other studies using a- and b-pinene show spasmolytic effects on ileum preparations 43.
Antifungal activity
Two Eucalyptus species (Eucalyptus sideroxylon and Eucalyptus torquata) and their different plant parts (leaf, stem, and flower) were tested for antifungal activity. The eucalyptus oil showed (in order) strong antifungal activity against Candida albicans, Aspergillus flavus, and Aspergillus niger, compared to a fluconazole control (with no action from oil of Eucalyptus sideroxylon flower) 44.
Eucalyptus globulus oil and 29 other essential oils (including Tea Tree Oil) were evaluated against biofilm-forming Candida albicans strains, both sensitive and resistant to fluconazole. Eighteen oils showed anti-Candida activities with the strongest being eucalyptus oil, peppermint, ginger grass, and clove, with respective percentage inhibitions of 81-, 74-, 40-, and 29 percent. Fluconazole showed 78-percent inhibition, which was less than eucalyptus oil. Note that Tea Tree Oil was not among the highest inhibitors 45.
An herbal preparation with the active principal one-percent oil of Eucalyptus pauciflora was applied twice daily for three weeks to 50 patients with a skin infection by any of three species of Tinea 46. After two weeks, all patients were KOH-negative and after three weeks, 60 percent of patients completely recovered and 40 percent showed improvement; none of the recovered patients had relapsed at two months. A five-percent eucalyptus oil concentration produced no adverse skin effects during three weeks of application 46.
Antioxidant activity
Eucalyptus oil can react biologically as an antioxidant. The free radical scavenging capability of Eucalyptus tereticornis oil from fresh or decaying leaves and separate oil constituents was studied against superoxide anion and hydroxyl radical. Both essential oils showed strong antioxidant ability either comparable or exceeding the standard antioxidants ascorbic acid and t-butylhydroxytoluene, respectively. Interestingly, the sum of the major individual constituents of the essential oil did not match the exceptional results of the whole oil, suggesting a synergistic effect when in combination 47.
The oils of three eucalyptus species (Eucalyptus polyanthemos, Eucalyptus globulus, and Eucalyptus perriniana) were assessed for antioxidant ability. Eucalyptus polyanthemos showed an antioxidant effect comparable to alpha-tocopherol, as it inhibited hexanal from oxidizing to hexanoic acid for at least 30 days. At 500 μg/mL, Eucalyptus polyanthemos, Eucalyptus globulus, and Eucalyptus perriniana inhibited hexanal oxidation by 99-, 55-, and 16 percent, respectively. In comparison, 50 μg/mL alpha-tocopherol inhibited hexanal oxidation by 98 percent 12.
Additional research demonstrates the antioxidant ability of eucalyptus oil by the same experimental method, although the species was not given 48. However, when Eucalyptus globulus was compared with 10 other essential oils for free radical scavenging ability, it performed poorly and the authors of the study attributed it to the high amount of monoterpenes. In regard to lipid oxidation, Eucalyptus globulus demonstrated moderate inhibition (48.6%) 49.
Eucalyptus oil dosage
The appropriate dose of eucalyptus oil depends on several factors such as the user’s age, health, and several other conditions. At this time there is not enough scientific information to determine an appropriate range of doses for eucalyptus oil. Keep in mind that natural products are not always necessarily safe and dosages can be important. Be sure to follow relevant directions on product labels and consult your pharmacist or physician or other healthcare professional before using.
Eucalyptus oil side effects and poisoning
Natural Medicines Comprehensive Database states that foods in the United States containing eucalyptus oil are safe. For pregnant or lactating women, small oral amounts of eucalyptus oil found as flavoring in food are considered safe. Use of eucalyptus oil by pregnant or lactating women for medicinal purposes should be avoided because of insufficient toxicity information 50. Ingesting 3.5 mL or more of the undiluted Eucalyptus oil at one time has been reported to be fatal, although other reports show ingestion of more than 3.5 mL to be non-fatal 50. Topically, the undiluted Eucalyptus oil potentially causes neurotoxicity when used for a prolonged period of time or administered in large amounts over the body 50. An example of toxicity was observed in a six-year-old Caucasian girl with full body pruritic urticaria, who had received some benefit from application of bandages soaked with a mixture of vinegar, olive oil, ethanol, and eucalyptus oil containing 80- to 85-percent cineole 9. The solution was applied over two days, culminating in a final one-time application of 50 mL of the eucalyptus oil in the form of the solution. The 6-years old Caucasians girl developed slurred speech and unsteady gait (ataxia), muscle weaknesses and lost consciousness within 30 minutes 9. Upon arousal, the patient had nausea, vomiting, inability to walk, decreased deep tendon reflexes, hypotonia, hypotension, and proteinuria. The patient made a full recovery after six hours following removal of eucalyptus oil from the skin 9. The initial body-wide pruritic urticaria resolved within 48 hours with no further treatment and no further recurrence 9.
The most inclusive review on eucalyptus oil poisoning in children (109 children, mean age two years) was conducted retrospectively over a 12-year period 51. Various extremes of eucalyptus oil poisoning were reported, with no notable effects occurring when 1.7 mL or less of pure eucalyptus oil was ingested. Minor poisoning occurred with doses around 2.0 mL, including ataxia, vomiting, abdominal pain, and/or miosis. Moderate poisoning occurred after a mean of 2.5 mL, which included a decrease in the level of consciousness or a Glasgow Coma Scale (GCS) of 8-14 51. Major poisoning occurred with ingestion of at least 7.5 mL and included unconsciousness, unresponsiveness to verbal command, absent eye opening, abnormal extensor/flexor responses to noxious stimuli, and/or a Glasgow Coma Scale score of 3-7 for any duration without hypoventilation 51. Life-threatening poisoning is considered unconsciousness with hypoventilation. Caution is to be exerted with a vomiting patient, since aspiration of pure eucalyptus oil can cause pneumonitis 51.
Eucalyptus oil poisoning is rare in adults but is not that uncommon in children 52 and it is usually unintentional 53. In the literature, eucalyptus oil poisoning in children presented with various clinical syndromes 54. The common Eucalyptus oil side effects in children include depression in the level of consciousness, ataxia, seizures, and vomiting 55. These manifestations can occur with therapeutic dosage or overdose. In children, the first episode of seizures or breakthrough seizures can occur with eucalyptus oil ingestion 56. Unlike in children, seizures are unusual in adult patients with eucalyptus oil poisoning 57. Most people, even physicians, are not aware of the toxic potential of these seemingly innocuous substances including Eucalyptus oil induced seizure. Regulation regarding the permissible limits of these ingredients in substances that contain them should be strictly imposed. Also, the label of products that contain eucalyptus oil or camphor should have mandatory warnings of the potential toxic effects, including seizures 4.
Table 2. Profile of patients with eucalyptus oil–induced seizures
| Time to seizure (minutes) | Duration of seizure (minutes) | Type of seizure | Postictal drowsiness (min) | History of febrile seizure | Past history of seizure | Family history of seizure | History of previous use of eucalyptus oil | MRI | Electroencephalogram (EEG) | Antiepileptic drug duration of treatment (weeks) | Follow‐up (months) | Recurrence |
| 5 | 15 | generalized tonic‐clonic seizures | 15 | No | No | No | No | Normal | Right frontal slowing | Levetiracetam (2 weeks) | 4 | No |
| 5 | 5 | generalized tonic‐clonic seizures | 20 | No | No | No | No | Normal | Normal | Levetiracetam | 3 | No |
| 2 | 5 | generalized tonic‐clonic seizures | 20 | No | No | No | No | Normal | Normal | Levetiracetam (4 weeks) | 3 | No |
| 2 | 3 | generalized tonic‐clonic seizures | 15 | No | No | No | No | Normal | Normal | No treatment | 3 | No |
| 5 | 3 | generalized tonic‐clonic seizures | 30 | No | No | No | No | Normal | Normal | No treatment | 12 | No |
| 3 | 3 | generalized tonic‐clonic seizures | 30 | No | No | No | No | Normal | Bifrontal and temporal slowing | Levetiracetam (4 weeks) | 4 | No |
| 10 | 8 | complex partial seizures (patient was in a state of altered sensorium for 10 minutes) | 10 | No | No | No | No | NA | NA | No treatment | 2 | No |
| 5 | 3 | generalized tonic‐clonic seizures | 20 | No | Yes | No | No | Normal | Normal | Previous topiramate continued | 12 | No |
| 2 | 1 | complex partial seizures (patient was in a state of altered sensorium for 10 minutes) | 10 | No | No | No | No | Normal | Normal | No treatment | 24 | No |
Eucalyptus oil overdose
Following the accidental exposure of human beings, death was reported in two cases after ingestion of 3.5-5 ml of essential eucalyptus oil, but a number of recoveries have also been described for much higher amounts of oil.
Eucalyptus oil overdose occurs when someone swallows a large amount of a product that contains this oil. This can be by accident or on purpose.
If you or someone you are with overdoses, call your local emergency number.
Do NOT induce vomiting. If vomiting occurs, lean patient forward or place on left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. For eye contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline during transport to the hospital.
Symptoms of Eucalyptus oil overdose
Below are symptoms of a eucalyptus oil overdose in different parts of the body.
AIRWAYS AND LUNGS
- Rapid breathing
- Shallow breathing
- Wheezing
EYES, EARS, NOSE, THROAT, AND MOUTH
- Difficulty swallowing
- Burning sensation in mouth
- Tiny pupils
HEART AND BLOOD
- Rapid, weak heartbeat
- Low blood pressure
MUSCLES AND JOINTS
- Muscle weakness
NERVOUS SYSTEM
- Drowsiness
- Headache
- Unconsciousness
- Dizziness
- Seizures
- Slurred speech
SKIN
- Redness and swelling (from the oil touching the skin)
STOMACH AND INTESTINES
- Abdominal pain
- Diarrhea
- Nausea and vomiting
Home Care
Seek medical help right away. DO NOT make the person throw up unless poison control or a health care provider tells you to.
If the oil is on the skin or in the eyes, flush with lots of water for at least 15 minutes.
Outlook (Prognosis) of Eucalyptus oil overdose
Survival past 48 hours is usually a good sign that recovery will occur. If any damage to the kidneys has occurred, it may take several months to heal. Drowsiness may persist for several days.
References- Batish D. R., Singh H. P., Kohli R. K., and Kaur S.. 2008. Eucalyptus essential oil as a natural pesticide. Forest Ecol. Manag. 256:2166–2174. doi:10.1016/j.foreco.2008.08.008
- Salari M. H., Amine G., Shirazi M. H., Hafezi R., and Mohammadypour M.. 2006. Antibacterial effects of Eucalyptus globulus leaf extract on pathogenic bacteria isolated from specimens of patients with respiratory tract disorders. Clin. Microbiol. Infect. 12:194–196. doi:10.1111/j.1469-0691.2005.01284.x
- Nakamura, T., Yoshida, N., Yamanoi, Y., Honryo, A., Tomita, H., Kuwabara, H., & Kojima, Y. (2020). Eucalyptus oil reduces allergic reactions and suppresses mast cell degranulation by downregulating IgE-FcεRI signalling. Scientific reports, 10(1), 20940. https://doi.org/10.1038/s41598-020-77039-5
- Ittyachen, A. M., George, G. R., Radhakrishnan, M., & Joy, Y. (2019). Eucalyptus oil poisoning: two case reports. Journal of medical case reports, 13(1), 326. https://doi.org/10.1186/s13256-019-2260-z
- Dhakad AK, Pandey VV, Beg S, et al. Biological, medicinal and toxicological significance of eucalyptus leaf essential oil: a review. J Sci Food Agric. 2018;98(3):833–848. doi: 10.1002/jsfa.8600
- Mathew, T., Kamath, V., Kumar, R. S., Srinivas, M., Hareesh, P., Jadav, R., & Swamy, S. (2017). Eucalyptus oil inhalation-induced seizure: A novel, underrecognized, preventable cause of acute symptomatic seizure. Epilepsia open, 2(3), 350–354. https://doi.org/10.1002/epi4.12065
- Vigan M. Essential oils: renewal of interest and toxicity. Eur J Dermatol. 2010 Nov-Dec;20(6):685-92. doi: 10.1684/ejd.2010.1066
- Darben T, Cominos B, Lee CT. Topical eucalyptus oil poisoning. Australas J Dermatol. 1998;39(4):265–267. doi: 10.1111/j.1440-0960.1998.tb01488.x
- Darben T, Cominos B, Lee CT. Topical Eucalyptus Oil Poisoning. Australas J. Dermatol. 1998;39:265–267. doi: 10.1111/j.1440-0960.1998.tb01488.x
- Ashurst PR, editor. Food flavorings. 3. Gaithersburg: Aspen; 1999.
- Manoguerra AS, Erdman AR, Wax PM. Camphor poisoning: an evidence-based practice guideline for out-of-hospital management. Clin Toxicol. 2006;44:357–370. doi: 10.1080/15563650600671696
- Lee KG, Shibamoto T. Antioxidant activities of volatile components isolated from Eucalyptus species. J Sci Food Agric 2001;81:1573-1579.
- Immune-Modifying and Antimicrobial Effects of Eucalyptus Oil and Simple Inhalation Devices. Altern Med Rev 2010;15(1):33-47. https://altmedrev.com/wp-content/uploads/2019/02/v15-1-33.pdf
- Hart PH, et al. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. 2000;49:619–626. doi: 10.1007/s000110050639
- Akhtar MA, Raju R, Beattie KD, Bodkin F, Munch G. Medicinal plants of the australian aboriginal Dharawal people exhibiting anti-inflammatory activity. Evid. Based Complement. Alternat. Med. 2016;2016:2935403. doi: 10.1155/2016/2935403
- Committee on Herbal Medicine Product. Assessment report on Eucalytus globulus Labill., Eucalyptus polybractea R.T. Baker and/or Eucalyptus smithii R.T. Baker, aetheroleum.
- Silva J, Abebe W, Sousa SM. Analgesic and Anti-Inflammatory Effects of Essential Oils Of Eucalyptus. J. Ethnopharmacol. 2003;89:277–283. doi: 10.1016/j.jep.2003.09.007
- Worth H, Schacher C, Dethlefsen U. Concomitant therapy with Cineole (Eucalyptole) reduces exacerbations in COPD: A placebo-controlled double-blind trial. Respir. Res. 2009;10:69. doi: 10.1186/1465-9921-10-69
- Ikawati Z, Wahyuono S, Maeyama K. Screening of several Indonesian medicinal plants for their inhibitory effect on histamine release from RBL-2H3 cells. J. Ethnopharmacol. 2001;75:249–256. doi: 10.1016/s0378-8741(01)00201-x
- Juergens UR. Anti-inflammatory properties of the monoterpene 1.8-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. (Stuttg) 2014;64:638–646. doi: 10.1055/s-0034-1372609
- Kim KY, Lee HS, Seol GH. Eucalyptol suppresses matrix metalloproteinase-9 expression through an extracellular signal-regulated kinase-dependent nuclear factor-kappa B pathway to exert anti-inflammatory effects in an acute lung inflammation model. J. Pharm. Pharmacol. 2015;67:1066–1074. doi: 10.1111/jphp.12407
- Rantzsch U, Vacca G, Duck R, Gillissen A. Anti-inflammatory effects of Myrtol standardized and other essential oils on alveolar macrophages from patients with chronic obstructive pulmonary disease. Eur. J. Med. Res. 2009;14(Suppl 4):205–209. doi: 10.1186/2047-783x-14-s4-205
- Juergens UR, Stober M, Schmidt-Schilling L, Kleuver T, Vetter H. Antiinflammatory effects of euclyptol (1.8-cineole) in bronchial asthma: Inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. Eur. J. Med. Res. 1998;3:407–412.
- Juergens UR, Stober M, Vetter H. Inhibition of cytokine production and arachidonic acid metabolism by eucalyptol (1.8-cineole) in human blood monocytes in vitro. Eur. J. Med. Res. 1998;3:508–510.
- Caceres AI, et al. Transient receptor potential cation channel subfamily M member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 2017;174:867–879. doi: 10.1111/bph.13760
- Gbenou JD, et al. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol. Biol. Rep. 2013;40:1127–1134. doi: 10.1007/s11033-012-2155-1
- Tukamoto K, et al. The examination of the usefulness for atopic dermatitis patient of Eucalyptus extract mixture bathing medicine. Nishinihon J. Dermatol. 1999;61:515–519. doi: 10.2336/nishinihonhifu.61.515
- Fischer J, Dethlefsen U. Efficacy of cineole in patients suffering from acute bronchitis: a placebo-controlled double-blind trial. Cough (London, England). 2013;9:25. doi:10.1186/1745-9974-9-25. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3842692/
- Worth H, Schacher C, Dethlefsen U. Concomitant therapy with Cineole (Eucalyptole) reduces exacerbations in COPD: A placebo-controlled double-blind trial. Respiratory Research. 2009;10(1):69. doi:10.1186/1465-9921-10-69. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2720945/
- Thom E and Wollan T. A controlled clinical study of Kanjang mixture in the treatment of uncomplicated upper respiratory tract infections. Phytother Res 1997;11:207-210.
- Sengespeik, H. C., Zimmermann, T., Peiske, C., and de Mey, C. [Myrtol standardized in the treatment of acute and chronic respiratory infections in children. A multicenter post-marketing surveillance study]. Arzneimittelforschung. 1998;48:990-994.
- Siurin, S. A. [Effects of essential oil on lipid peroxidation and lipid metabolism in patients with chronic bronchitis]. Klin.Med (Mosk) 1997;75:43-45.
- Juergens UR, Stober M, Schmidt-Schilling L, et al. Antiinflammatory effects of euclyptol (1.8-cineole) in bronchial asthma: inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. Eur J Med Res1998;3:407-412.
- Juergens UR, Dethlefsen U, Steinkamp G, et al. Anti-inflammatory activity of 1,8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-con-trolled trial. Respir Med2003;97:250-256.
- Kehrl W, Sonnemann U, Dethlefsen U. Therapy for acute nonpurulent rhinosinusitis with cineole: results of a double-blind, randomized, placebo-controlled trial. Laryngoscope2004;114:738-742.
- Silva J, Abebe W, Sousa SM, et al. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J Ethnopharmacol 2003;89:277-283.
- Santos FA, Rao VS. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res 2000;14:240-244.
- Liapi C, Anifandis G, Chinou I, et al. Antinociceptive properties of 1,8-cineole and beta-pinene, from the essential oil of Eucalyptus camaldulensis leaves, in rodents. Planta Med 2007;73:1247-1254.
- Ferreira-da-Silva FW, Barbosa R, Moreira-Junior L, et al. Effects of 1,8-cineole on electrophysiological parameters of neurons of the rat superior cervical ganglion. Clin Exp Pharmacol Physiol 2009;36:1068-1073.
- Coelho-de-Souza LN, Leal-Cardoso JH, de Abreu Matos FJ, et al. Relaxant effects of the essential oil of Eucalyptus tereticornisand its main constituent 1,8-cineole on guinea-pig tracheal smooth muscle. Planta Med 2005;71:1173-1175.
- Bastos VP, Brito TS, Lima FJ, et al. Inhibitory effect of 1,8-cineole on guinea-pig airway challenged with ovalbumin involves a preferential action on electromechanical coupling. Clin Exp Pharmacol Physiol 2009;36:1120-1126.
- Laude EA, Morice AH, Grattan TJ. The antitussive effects of menthol, camphor and cineole in conscious guinea-pigs. Pulm Pharmacol 1994;7:179-184.
- Camara CC, Nascimento NR, Macedo-Filho CL, et al. Antispasmodic effect of the essential oil of Plectranthus barbatusand some major constituents on the guinea-pig ileum. Planta Med2003;69:1080-1085.
- Ashour HM. Antibacterial, antifungal, and anticancer activities of volatile oils and extracts from stems, leaves, and flowers of Eucalyptus sideroxylon and Eucalyptus torquata. Cancer Biol Ther2008;7:399-403.
- Agarwal V, Lal P, Pruthi V. Prevention of Candida albicans biofilm by plant oils. Mycopathologia 2008;165:13-19.
- Shahi SK, Shukla AC, Bajaj AK, et al. Broad spectrum herbal therapy against superficial fungal infections. Skin Pharmacol Appl Skin Physiol 2000;13:60-64.
- Singh HP, Mittal S, Kaur S, et al. Characterization and antioxidant activity of essential oils from fresh and decaying leaves of Eucalyptus tereticornis. J Agric Food Chem 2009;57:6962-6966.
- Grassmann J, Hippeli S, Dornisch K, et al. Antioxidant properties of essential oils. Possible explanations for their anti-inflammatory effects. Arzneimittelforschung 2000;50:135-139.
- Sacchetti G, Maietti S, Muzzoli M, et al. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimi-crobials in foods. Food Chem 2005;91:621-632.
- https://naturalmedicines.therapeuticresearch.com/databases/food,-herbs-supplements/professional.aspx?productid=700
- Tibballs J. Clinical effects and manage-ment of Eucalyptus oil ingestion in infants and young children. Med J Aust 1995;163:177-180.
- Webb NJ, Pitt WR. Eucalyptus oil poisoning in childhood: 41 cases in south-east Queensland. J Paediatr Child Health. 1993;29:368–371. doi: 10.1111/j.1440-1754.1993.tb00537.x
- Flaman Z, Pellechia-Clark S, Bailey B, et al. Unintentional exposure of young children to camphor and eucalyptus oils. Paediatr Child Health. 2001;6:80–83. doi: 10.1093/pch/6.2.80
- Webb NJ, Pitt WR. Eucalyptus oil poisoning in childhood: 41 cases in south-east Queensland. J Paediatr Child Health. 1993 Oct;29(5):368-71. doi: 10.1111/j.1440-1754.1993.tb00537.x
- Tibballs J. Clinical effects and management of eucalyptus oil ingestion in infants and young children. Med J Aust. 1995;163:177–180. doi: 10.5694/j.1326-5377.1995.tb124516.x
- Dudipala, S. C., Mandapuram, P., & Ch, L. K. (2021). Eucalyptus Oil-Induced Seizures in Children: Case Reports and Review of the Literature. Journal of neurosciences in rural practice, 12(1), 112–115. https://doi.org/10.1055/s-0040-1721199
- Kumar KJ, Sonnathi S, Anitha C, et al. Eucalyptus oil poisoning. Toxicol Int. 2015;22(1):170–171. doi: 10.4103/0971-6580.172259