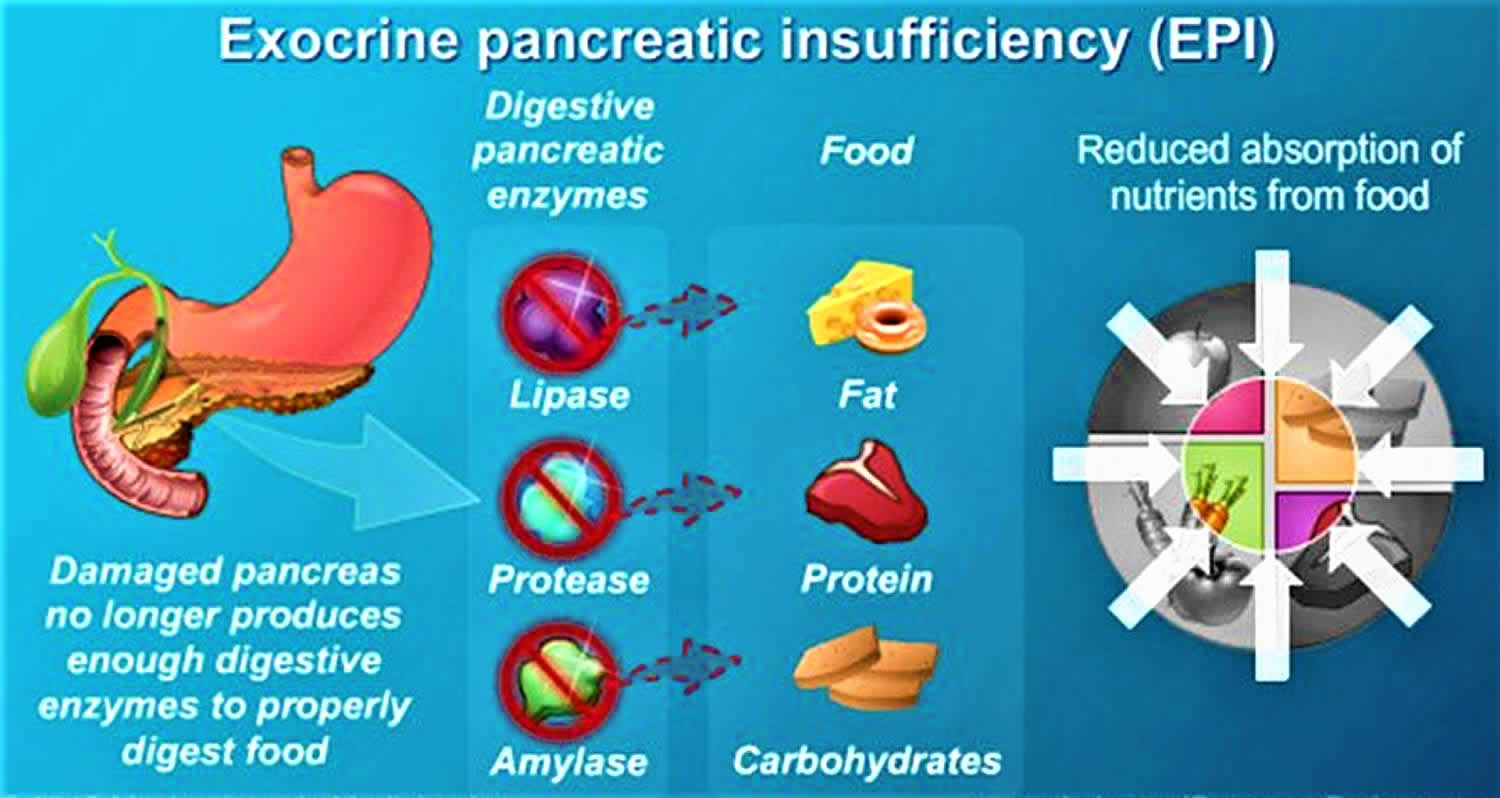
Exocrine pancreatic insufficiency
Exocrine pancreatic insufficiency (EPI) is a condition characterized by deficiency of the exocrine pancreatic enzymes, resulting in the inability to digest food properly, or maldigestion, resulting in malabsorption 1. This inadequate digestion with nutrient and, especially, fat malabsorption occurs when intraduodenal levels of lipase fall below 5–10% of normal enzyme output 2, leading to pancreatic steatorrhea, weight loss, and a potential decrease in quality of life 3. Furthermore, in exocrine pancreatic insufficiency, due to cystic fibrosis (CF) or chronic pancreatitis, there is decreased bicarbonate output causing a lower intestinal pH, which precipitates bile salt acids and impairs micelle formation of fats 4. Fat maldigestion is compounded by decreased pancreatic secretion of lipase and colipase, further dampening hydrolysis of intraluminal fat.
The cause of exocrine pancreatic insufficiency includes pancreatic and nonpancreatic causes 1. Numerous conditions account for the cause of exocrine pancreatic insufficiency, with the most common being diseases of the pancreatic tissue including chronic pancreatitis, cystic fibrosis, and a history of extensive necrotizing acute pancreatitis.
Many diagnostic tests are available to diagnose exocrine pancreatic insufficiency, however, the criteria of choice remain unclear and the causes for a false-positive test are not yet understood.
Treatment for exocrine pancreatic insufficiency includes dietary management, lifestyle changes (i.e., decrease in alcohol consumption and smoking cessation), and pancreatic enzyme replacement therapy 1.
Exocrine pancreatic insufficiency causes
The exocrine pancreas produces three main types of enzymes: amylase, protease, and lipase 5. Under normal physiologic conditions, the enzymes, specifically lipase, break undigested triglycerides into fatty acids and monoglycerides, which are then solubilized by bile salts. Because the exocrine pancreas retains a large reserve capacity for enzyme secretion, fat digestion is not clearly impaired until lipase output decreases to below 10% of the normal level 6.
A leading cause of exocrine pancreatic insufficiency is chronic pancreatitis 7. Other pancreatic causes include a history of extensive necrotizing acute pancreatitis, pancreatic cancer, pancreatic surgery, and cystic fibrosis. Non-pancreatic causes are celiac disease, diabetes mellitus, Crohn’s disease, gastric surgery, short bowel syndrome, and Zollinger–Ellison syndrome 8.
Chronic pancreatitis
Chronic pancreatitis is an ongoing inflammatory process with irreversible morphological changes to the pancreas and a gradual loss in pancreatic parenchyma. There are three major groups of mutations that account for chronic pancreatitis (PRSS1, SPINK1, and CFTR). Theories of pathogenesis include oxidative stress, toxic-metabolic derangements, loss of ductal function or obstruction, and necrosis-fibrosis 9.
In chronic pancreatitis, approximately 20% of patients develop exocrine pancreatic insufficiency over time as a result of progressive loss of acinar cell function 10. Layer et al. 10 found that the median duration from the onset of symptomatic disease to exocrine pancreatic insufficiency was significantly longer in early-onset chronic pancreatitis (median age of onset being 19.2 years) than in late-onset idiopathic chronic pancreatitis (median age of 56.2 years) or alcoholic pancreatitis (median age of 13.1 years).
Cystic fibrosis
Cystic fibrosis is an autosomal recessive disorder caused by a mutation of the gene that encodes for a chloride channel called the cystic fibrosis transmembrane conductance regulator (CFTR). In ductal epithelial cells, CFTR is highly expressed and functions to transport fluid and anions into the lumen 11. Dysfunction of the CFTR gene leads to a decrease in luminal fluid volume and decreased pH, resulting in protein precipitation within the ductal lumen and loss of normal acinar cell function.
Exocrine pancreatic insufficiency is most commonly observed at birth or soon after due to in utero exocrine pancreatic damage. Waters et al. 12 showed that, during newborn screening, 63% of infants with cystic fibrosis are exocrine insufficient and almost 30% of the pancreas-sufficient group will become exocrine insufficient over the next 36 months. Individuals with class IV, V, or VI mutations (less severe CFTR mutations and hence some preserved CFTR function), tend to suffer from exocrine pancreatic insufficiency later in life 13. Corey et al. 14 compared 1000 patients from CF clinics in Boston and Toronto and demonstrated that prolonged untreated exocrine pancreatic insufficiency is associated with a worse long-term outcome and that patients maintained on a high fat diet (100 g per day) with higher doses of exogenous pancreatic enzymes did better than those on a low fat, lower pancreatic enzyme regimen. Overall, pancreatic insufficiency requiring lifelong pancreatic exocrine replacement therapy (PERT) is found in about 85% of CF patients 11.
A rare complication of PERT is seen only in patients with cystic fibrosis on very high doses of enzymes. Fitzsimmons et al. 15 found that, in children with cystic fibrosis, there was a strong correlation between high daily doses of PERT and the development of fibrosing colonopathy. However, this represents a small case series published in New England Journal of Medicine in 1997, which were not biopsy proven with very few cases reported since then. Factors related to cystic fibrosis, including thick intestinal secretions, dosing of PERT, and agents in the enteric coating of the pancrelipase preparations may be the precipitating factors causing this complication 15. Therefore, it has been recommended that, in children and adults with cystic fibrosis, the daily dose should remain below 2500 lipase units/kg of body weight per meal or 10000 lipase units/kg of body weight per day 16.
Post pancreatic surgery
Factors that contribute to exocrine pancreatic insufficiency following pancreatic surgery are a decrease in pancreatic tissue volume, extensive denervation following lymph node dissection, and surgically altered anatomy 4. Conditions such as pancreatic cancer, intraductal papillary mucinous neoplasms, premalignant mucinous cystic lesions, and benign tumors of the pancreas may all lead to exocrine pancreatic insufficiency via obstruction of the pancreatic duct. The degree of exocrine pancreatic insufficiency following pancreatic surgery is dependent on the extent of pancreatic resection combined with the degree of residual pancreatic parenchymal function with full manifestation of exocrine pancreatic insufficiency seen following a total pancreatectomy 17. The mechanism of exocrine pancreatic insufficiency in patients undergoing a Whipple procedure may be related to a mistiming of secreted endogenous pancreatic enzymes mixing with chyme.
Large systematic reviews report a 19–80% incidence of exocrine pancreatic insufficiency following a distal pancreatectomy 18; however, this wide variation may be in part a result of the different diagnostic methods employed 19. Post-operative incidence of exocrine pancreatic insufficiency after Whipple surgery is 56–98% 20. In addition, Halloran et al. 21 analyzed 40 patients following resection for pancreatic malignancy and found that exocrine pancreatic insufficiency was common and sustained after surgery, but was not associated with significant symptoms. These patients with newly developed exocrine pancreatic insufficiency, however, did have a tendency towards poorer quality of life.
Celiac disease
Celiac disease is a chronic inflammatory intestinal disorder that may occur in genetically predisposed people triggered by the ingestion of gluten. This disease has a United States and British prevalence of approximately 1% 22. In celiac disease, although exocrine pancreatic function is intrinsically normal, reduced levels of cholecystokinin release as a result of the duodenal villous atrophy, accounts for impaired gall bladder contraction and reduced exocrine pancreatic secretion 23.
Diabetes mellitus
Diabetes mellitus can predispose to exocrine pancreatic insufficiency and, conversely, longstanding exocrine pancreatic insufficiency can be associated with diabetes 24. In diabetes, there are several possible causes which can account for exocrine pancreatic insufficiency – the lack of the trophic action of insulin (and potentially of glucagon and somatostatin) on acinar cells, autoimmune damage of islet cells, causing destruction of both endocrine and exocrine tissue, and decreased exocrine pancreatic secretion as a complication of diabetic neuropathy 25. Therefore, the lack of insulin production and the autoimmunity in type I diabetes explain the higher observed prevalence of exocrine pancreatic insufficiency compared to those with type II diabetes (about 60% vs. 30%) 24. In addition, a recent article by Soave et al. 26 showed that the lower the immunoreactive trypsinogen levels at birth in newborns with cystic fibrosis, reflecting more severe exocrine pancreatic disease in utero, the earlier in life they developed cystic fibrosis-related endocrine disease (diabetes).
All infants
Based on the study by Lebenthal and Lee 27 indicating that the duodenal fluid of newborns and infants contained no amylase and negligible lipase at least for the first month of life, all healthy term infants are exocrine pancreatic insufficient. Normally, this is compensated for by amylase and lipase present in breastmilk. However, in formula-fed infants, exocrine pancreatic insufficiency would be expected. In fact, a recent study by Martin et al. 28 confirmed that formula-fed preterm infants had impaired fatty acid absorption evident through 6 or more weeks postnatal age compared to breastmilk-fed infants, and this is consistent with limited pancreatic lipase production by the pancreas 28. Thus, all infants, both term and preterm, represent the largest population of individuals with exocrine pancreatic insufficiency. The clinical implications of developmental pancreatic insufficiency in non-breast-fed infants is unknown, but may play a role in early nutrient deficits in critically ill newborns such as the preterm infant.
Exocrine pancreatic insufficiency symptoms
Symptoms of exocrine pancreatic insufficiency can include steatorrhea (clay-colored, loose, greasy, foul-smelling large stools), abdominal discomfort, bloating, and weight loss. Although floating stools are often thought of being indicative of steatorrhea, they are not; rather sticking to the toilet bowl is a more specific sign.
Exocrine pancreatic insufficiency diagnosis
A multitude of tests for exocrine pancreatic insufficiency have been developed over the past several decades and classified as direct versus indirect measures of exocrine pancreatic function. However, many of these have poor sensitivity or specificity (e.g. serum trypsin levels, qualitative stool fat) and/or are available at only limited centers such as with the 13C mixed triglyceride (13C-MTG) breath test.
72-hour fecal fat test
The gold standard has been the 72-hour stool collection while the patient consumes a diet containing 100 g of fat per day. Fat malabsorption is diagnosed at > 7 g of fat per 100 g of stool per day, with severe steatorrhea at ≥ 15 g per day 29. Unfortunately, this test is time-consuming and not easily tolerated due to bloating, abdominal discomfort, flatulence, and worsening steatorrhea. Additionally, errors can occur in stool collections and recording of fat intake 30. Diseases that impact mucosal fatty acid uptake, such as Crohn’s disease, bacterial overgrowth, and short bowel syndrome, can cause abnormal values despite normal exocrine pancreatic function. However, the 72-hour stool collection has served to measure the effectiveness of PERT in exocrine pancreatic insufficiency 31 for United States Food and Drug Administration (FDA) approval of PERTs.
Fecal elastase test
The pancreas produces pancreatic elastase 1, which is a highly stable enzyme during intestinal transit 32. This proteolytic enzyme can be measured in a fecal sample by an enzyme-linked immunosorbent assay 33. Because pancreatic elastase is highly stable during intestinal transit, the fecal concentration correlates well with exocrine pancreatic secretion 34. Diagnostic testing using fecal elastase has some advantages over other tests because it does not require a timed stool collection or special diet, has a high negative predictive value, and has a high sensitivity in moderate to severe exocrine pancreatic insufficiency when formed stools are analyzed 7. The reference range of less than 200 μg/g feces can be applied to both children and adults for the diagnosis of exocrine pancreatic insufficiency 35. Some consider values less than 100 μg/g feces as diagnostic of exocrine pancreatic insufficiency, with fecal elastase values between 100 and 200 μg/g to be indeterminate and difficult to interpret 36.
In mild to moderate exocrine pancreatic insufficiency, diagnostic testing using fecal elastase has a lower sensitivity (as low as 30%) and specificity, possibly resulting in an underestimation of exocrine pancreatic insufficiency 37. In childhood, however, fecal elastase is a useful noninvasive screening test for exocrine pancreatic insufficiency, demonstrating a negative predictive value of 99% for ruling out exocrine pancreatic insufficiency 30. Since fecal elastase is measured as a concentration in stool, watery stools will almost invariably result in low elastase values being measured and thus this non-invasive, pancreatic function test should be performed in a clinical setting where exocrine pancreatic insufficiency is suspected and a formed stool can be analyzed. This has replaced the more cumbersome 72-hour fecal fat test. In addition, PERTs do not have to be stopped for fecal elastase testing since the porcine enzymes do not cross react with the human fecal elastase antibody.
Laboratory studies
A complete laboratory evaluation is required not only to diagnose exocrine pancreatic insufficiency but also to determine the extent of the malabsorption and assess manifestations of the underlying disease, if present.
Blood tests
These can include the following:
- Complete blood count (CBC). A complete blood count (CBC) may reveal microcytic anemia due to iron deficiency or macrocytic anemia due to vitamin B-12 or folate malabsorption. Serum iron, vitamin B-12, and folate concentrations may help establish the diagnosis of exocrine pancreatic insufficiency. Prothrombin time (PT) may be prolonged because of malabsorption of vitamin K, a fat-soluble vitamin. A study by Lindkvist et al found that serum nutritional markers (eg, magnesium, albumin, prealbumin) can be used to determine the probability of exocrine pancreatic insufficiency in patients with chronic pancreatitis 38.
- Antigliadin and antiendomysial antibodies. Serum levels of antigliadin and antiendomysial antibodies can be used to help diagnose celiac sprue. The serum immunoglobulin A (IgA) level can be assessed to rule out IgA deficiency.
- Malabsorption can involve electrolyte imbalances such as hypokalemia, hypocalcemia, hypomagnesemia, and metabolic acidosis. Protein malabsorption may cause hypoproteinemia and hypoalbuminemia. Fat malabsorption can lead to low serum levels of triglycerides, cholesterol, and alpha- and beta-carotene. The Westergren erythrocyte sedimentation rate (ESR) may provide a clue to an underlying autoimmune disease.
Stool tests
Determination of fecal elastase and chymotrypsin (2 proteases produced by the pancreas) can be used to try to distinguish between pancreatic causes and intestinal causes of malabsorption.
Malabsorption tests
These can include the following:
- Fat absorption tests. See 72-hour fecal fat test above.
- D-xylose test. If the 72-hour fecal fat collection results demonstrate fat malabsorption, the D-xylose test is used to document the integrity of the intestinal mucosa. D-xylose is readily absorbed in the small intestine. Approximately half of the absorbed D-xylose is excreted in urine without being metabolized. If absorption of D-xylose is impaired by either a luminal factor (eg, bacterial overgrowth) or a reduced or damaged mucosal surface area (eg, from surgical resection or celiac disease), urinary excretion will be lower than normal. Cases of pancreatic insufficiency usually result in normal urinary excretion because absorption of D-xylose is still intact.
- Carbohydrate absorption test. A simple sensitive test for carbohydrate malabsorption is the hydrogen breath test, in which patients are given an oral solution of lactose 39. In cases of lactase deficiency, colonic organisms digest the unabsorbed lactose, which results in an elevated hydrogen content in the expired air. Bacterial overgrowth or rapid transit also can cause an early rise in breath hydrogen, in which case it is necessary to use glucose instead of lactose to make a diagnosis. However, 18% of patients are hydrogen nonexcretors, in whom the hydrogen breath test will yield false-negative test results.
- Bile salt absorption test. The bile salt breath test can determine the integrity of bile salt metabolism. The patient is given an oral conjugated bile salt, such as glycine cholic acid with the glycine radiolabeled in the carbon position. The bile salt is deconjugated and subsequently metabolized by bacteria. If interrupted enterohepatic circulation (eg, from bacterial overgrowth, ileal resection, or disease), a radioactively labeled elevated breath carbon dioxide level will be noted.
- Schilling test. Malabsorption of vitamin B-12 may occur as a consequence of an intrinsic factor deficiency (eg, from pernicious anemia or gastric resection), pancreatic insufficiency, bacterial overgrowth, ileal resection, or disease. The 3-stage Schilling test can often help differentiate these conditions.
- C13-D-xylose breath test. A study by Hope et al suggested that small intestinal malabsorption in chronic alcoholism may be identified by means of a C13-D-xylose breath test 40. The investigators evaluated this test in 14 alcoholics, compared the results with those obtained from untreated celiac disease patients and healthy control subjects, and correlated the breath test findings with the morphologic findings from the duodenal mucosa. In this study, absorption of C13-D-xylose was significantly less in the alcoholic patients than in healthy control subjects, whereas the time curve of C13-D-xylose absorption in the alcoholics was similar to that in the untreated celiac patients 41. In addition, although few changes were observed on light microscopy in the alcoholics, morphologic pathology (primarily reduced surface area of microvilli) was observed on electron microscopy in the majority of the patients.
Pancreatic function tests
These can include the following:
- Direct testing – Secretin test, cholecystokinin (CCK) test, secretin-CCK test
- Indirect testing – Qualitative fecal fat analysis, fecal elastase and fecal chymotrypsin level analysis
Pancreatic function can be measured directly by using endoscopy or the Dreiling tube method after stimulation with secretin or cholecystokinin (CCK). Direct pancreatic function testing is the most sensitive approach to assessment of exocrine pancreatic function and is usually performed at specialized centers 42. Various methods have been developed 43.
Direct testing
Whereas the cholecystokinin (CCK) test measures the ability of the acinar cells to secrete digestive enzymes, the secretin test measures the ability of the ductal cells to secrete bicarbonate. Although both tests yield abnormal results in advanced exocrine pancreatic insufficiency, it is not known which of the 2 secretagogues offers better sensitivity for early exocrine pancreatic insufficiency. In uncertain cases, both cholecystokinin (CCK) and secretin tests may be ordered.
Secretin test
In the secretin test, porcine or human synthetic secretin is given in doses ranging from 0.5 to 5 clinical units (CU)/kg. Duodenal fluid is continuously collected in 15-minute aliquots for 1 hour. The fluid is analyzed for bicarbonate concentration, volume, and total bicarbonate output.
A bicarbonate concentration lower than 80 mEq/L in all 4 aliquots represents exocrine insufficiency. A peak bicarbonate cutoff of 90 mEq/L has been advocated by some investigators. A peak bicarbonate concentration lower than 50 mEq/L is indicative of severe exocrine insufficiency. When the bicarbonate concentration is equivocal, volume and total bicarbonate output are used as secondary diagnostic parameters.
Cholecystokinin test
Use of a cholecystokinin (CCK) receptor agonist (eg, cerulein) as a hormonal stimulant provides information on pancreatic enzyme-secreting capacity. Two endoscopic tubes are placed: (1) a single-lumen gastric tube and (2) a double-lumen duodenal tube. The gastric tube continuously collects and discards gastric fluid to prevent acidification of the duodenum. One duodenal lumen continuously collects duodenal drainage fluid, whereas the other is used for administration of a mannitol-saline solution containing a nonabsorbable marker (polyethylene glycol [PEG]).
An accurate determination is made of enzyme concentration, enzyme output, and fluid volume on the basis of recovery of the PEG marker. Measurement of perfusion markers requires a specialized laboratory.
Secretin-cholecystokinin test
Many pancreatic research centers use the combined secretin-cholecystokinin (CCK) test, which allows simultaneous assessment of ductal and acinar secretory capacity. Many dosing regimens have been used for this test. The 2 hormones are administered, and the concentration and output of both bicarbonate and pancreatic enzymes are evaluated.
Indirect testing
Pancreatic function can also be measured indirectly. Qualitative fecal fat analysis via microscopic examination of random stool samples is used as a screening test only 44. In addition, measurement of fecal elastase and fecal chymotrypsin levels may serve as an indirect indicator of pancreatic function; however, sensitivity is limited to moderate or severe disease, and the result can be falsely positive as a result of dilution by watery stools 42. The typical findings in exocrine pancreatic insufficiency are increased fecal fat and decreased enzymes.
A prospective study by González-Sánchez et al suggested that with regard to sensitivity, specificity, and positive and negative predictive values, results from the fecal elastase-1 (FE-1) test are similar to those from the C13-mixed triglyceride breath test in the diagnosis of exocrine pancreatic insufficiency in chronic pancreatitis. However, the triglyceride breath test appeared to be more accurate than the FE-1 test in operated patients with chronic pancreatitis 45.
Abdominal imaging
Abdominal imaging can help in identifying features of chronic pancreatitis, which is the most common cause of exocrine pancreatic insufficiency.
Exocrine pancreatic insufficiency treatment
Management approaches to exocrine pancreatic insufficiency include the following 46:
- Lifestyle modifications (eg, avoidance of fatty foods, limitation of alcohol intake, cessation of smoking, and consumption of a well-balanced diet)
- Vitamin supplementation (primarily the fat-soluble vitamins A, D, E, and K)
- Pancreatic enzyme replacement therapy (PERT), which is the therapeutic mainstay
Long-term monitoring of patients with exocrine pancreatic insufficiency should focus on the following 2 issues:
- Correction of nutritional deficiencies
- Treatment of causative diseases (when possible); such treatment will vary according to the specific disease present
Dietary management and lifestyle changes
Fat malabsorption is the predominant cause of the symptoms of pancreatic steatorrhea resulting in weight loss as well as deficiencies of fat-soluble vitamins A, D, E, and K. In patients with chronic pancreatitis, a low fat diet has been the recommendation in order to minimize the pain of this disease and, in conjunction with pancreatic enzyme replacement therapy (PERT), to effectively treat steatorrhea. However, in patients with cystic fibrosis, a high fat diet in conjunction with increased amounts of pancreatic enzyme replacement therapies (PERTs) has been shown to improve the associated cystic fibrosis lung disease and thus low fat diets are no longer advocated in this disease. Fat soluble vitamins A, D, E, and K should be supplemented if indicated, and taken with pancreatic enzyme replacement therapy (PERT) 47. Consulting a dietitian is helpful to assess nutritional adequacy 48. In addition, smoking has been proven to be a risk factor in acute pancreatitis, chronic pancreatitis, pancreatic cancer 49, and to be associated with reduced exocrine pancreatic function 50. Therefore, smoking and alcohol cessation is recommended in exocrine pancreatic insufficiency due to chronic pancreatitis.
Pancreatic enzyme replacement therapy
The elimination of malabsorption, reduction of maldigestion-related symptoms, and the prevention of malnutrition-related morbidity and mortality is the goal for pancreatic enzyme replacement therapy (PERT) 48. This is most evident in cystic fibrosis, where prior to the availability of pancreatic enzyme replacement therapy (PERT), infants died within the first year of life. Prior to 2010, pancreatic enzymes were not FDA regulated and had variable consistency of activity. As a result, in 2010, the FDA mandated approval of all prescribed formulations of pancreatic enzyme replacement therapy (PERT). It should be noted that the clinical trials were relatively small (less than 40 subjects) and tested in subjects who were known to respond to PERTs. All pancreatic enzyme preparations are extracts from porcine pancreas (pancrelipase) and are available in preparations encapsulated in mini-microspheres or microtablets, which vary in particle size and pH-related release kinetics 51. Enteric-coated pancreatic microspheres are designed to be acid resistant and pH-sensitive to protect lipase from denaturation by gastric acid. Unfortunately, confusion has arisen due to the many different dosage strengths of PERTs (Table 1) 52.
Table 1. Current Food and Drug Administration (FDA) approved pancreatic enzyme replacement therapies (PERTs)
| Brand | Units of lipase |
|---|---|
| Creon | 3000; 6000; 12,000; 24,000; 36,000 |
| Zenpep | 3000; 5000; 10,000; 15,000; 20,000; 25,000 |
| Pancreaze | 4200; 10,500; 16,800; 21,000 |
| Ultresa | 13,800; 20,700; 23,000 |
| Viokase | 10,440; 20,880 (requires acid suppression) |
| Pertzye | 8000; 16,000 |
Enteric-coated pancreatic enzymes are most effective at a pH > 6. However, in patients with cystic fibrosis, the duodenal pH is < 6 53. The use of acid-suppression medications can increase gastric pH levels and theoretically improve the efficacy of pancreatic enzyme replacement therapy (PERT) and decrease exocrine pancreatic insufficiency symptoms 54. Current data may suggest a trial of acid blockers in patients with cystic fibrosis who have refractory steatorrhea 55. However, a recent retrospective study demonstrated no improvement of the coefficient of fat absorption (72-hour fecal fat test) when using a proton pump inhibitor in pediatric patients with cystic fibrosis 56.
Uncoated exogenous pancreatic enzymes, such as Viokase (Aptalis Pharma), are thought to mix well with intragastric nutrients and rapidly release high duodenal lipase amounts for fat digestion 51. The addition of acid-suppression medications is required to prevent degradation of non-enteric coated pancreatic enzymes 51. Only non-enteric pancreatic enzymes have been shown to improve the pain in a subset of patients with chronic pancreatitis. The use of unprotected exogenous enzymes in combination with enteric-coated enzymes has previously been recommended for the treatment of refractory exocrine pancreatic insufficiency 51; however, Kalnins et al. 57 showed no improvement in nutrient digestion (fecal fat, energy, and nitrogen output) when unprotected pancreatic enzymes were added to the conventional enteric-coated enzymes in 14 pediatric patients with cystic fibrosis.
Dosing and frequency of pancreatic enzyme replacement therapy administration
Dosing and frequency of administration are difficult aspects of pancreatic enzyme replacement therapy (PERT) treatment since different enteric-coated microspheres are not bioequivalent in vitro 58 and there are not enough clinical studies between preparations to define in vivo bioavailability. In these in vitro studies, the preparations varied in dissolution time (49–71 min half-life time) and in optimum pH (pH 5.0–5.8).
Several countries have recommended different doses of pancreatic enzyme replacement therapy (PERT). The Australasian Pancreatic Club 59, The Italian Association for the Study of the Pancreas 7 and The Spanish Pancreatic Club 60 recommend 25,000–50,000 lipase units per main meal in adults. Unfortunately, the evidence for these recommendations is relatively weak as emphasized by The Australasian Pancreatic Club in their recent study on the management of pancreatic exocrine insufficiency 59. In addition, a study from the Netherlands by Sikkens et al. 61 found that 70% (n = 161) of the patients with exocrine pancreatic insufficiency caused by chronic pancreatitis were under-treated and reported steatorrhea-related symptoms, despite pancreatic enzyme replacement therapy (PERT) (median enzyme intake of 6 capsules, 25,000 lipase units per day). These differences in recommendations demonstrate the significant confusion over dosing and administration amongst medical practitioners.
Likewise, there is no consensus over frequency of pancreatic enzyme replacement therapy (PERT) administration. In 1977, DiMagno et al. 62 demonstrated that administration of pancreatic enzyme replacement therapy (PERT) during a meal was as effective as hourly administration over the day to decrease steatorrhea. Other recommendations based on several reviews are to take 50% of the exogenous pancreatic enzymes at the beginning of the meal and 50% half-way through 63, pancreatic enzymes during or immediately following the meal 64, or lastly, 25% of the enzymes with the first bite, 50% during the meal, and 25% with the last bite 65. In addition, a recent randomized three-way crossover study of 24 patients using 40,000 lipase units per meal compared three different administration schedules with pancreatic enzyme replacement therapy (PERT) before meals, during meals, or after meals using the 13C-MTG breath test to measure fat absorption 66. The percentage of patients who normalized fat digestion was 50%, 54%, and 63%, respectively. Thus, no statistically significant differences were found between different administration schedules, however, they did recommend giving pancreatic enzyme replacement therapy (PERT) during or after meals.
In a patient with suspected exocrine pancreatic insufficiency with a known history of pancreatic disease, empiric therapy with pancreatic enzyme replacement therapy (PERT)s may be indicated without formal testing. A clear response would be both diagnostic for exocrine pancreatic insufficiency as well as therapeutic. In addition, if there is a poor response to pancreatic enzyme replacement therapy (PERT), one should consider concurrent gastrointestinal comorbidities such as lactose intolerance, enteric bacterial infection, parasites (especially giardia), small intestinal bacterial overgrowth, biliary disease (cholestasis), colitis, celiac disease, short bowel syndrome, and Crohn’s disease 67. Other reasons could be insufficient dosing, lack of compliance, inadequate timing of pancreatic enzyme replacement therapy (PERT) administration, and poor diet (Table 2).
Table 2. Treatment strategies for lack of response to pancreatic enzyme replacement therapy (PERT)
| Treatment strategies |
|---|
| • Increase dosage |
| • Check compliance with the patient |
| • Add acid inhibitor |
| • Consider adding enzymes during and towards end of meal |
| • Consider microspheres, possibly adding a rapid release enzyme preparation |
| • Look for evidence of concurrent gastrointestinal disorder |
Pancreatic enzyme replacement therapy (PERT) should be taken with the first bite of a meal and consider adding extra enzymes during or towards the end of the meal. Thus, if consumption of a meal is less than 15 min, all enzymes can be taken at the beginning of the meal; for a 15- to 30-minute meal, we suggest taking half the enzyme capsules with the first bite and the other half in the middle of the meal; for more than 30 minutes, we recommend taking one third at the beginning, one third in the middle and one third at the end. The rationale for taking pancreatic enzymes throughout the meal is to mimic the action of our own endogenous pancreatic enzymes, where secretion from the gland occurs throughout a meal. Specifically, the more food that is ingested and/or the grater the amount of fat in the diet, the higher the amount of endogenous pancreatic enzyme secretion; thus, the number of pancreatic enzyme replacement therapy (PERT) capsules consumed should reflect this. Table 3 gives a suggested clinical overview of pancreatic enzyme replacement therapy (PERT) dosing for different age groups 67.
Table 3. Pancreatic enzyme replacement therapy (PERT) suggested dosing in different age groups
| Age group | Units of lipase |
|---|---|
| Infant | 2000–4000 units/120 mL formula or breastmilk |
| Child age < 4 years | 1000 units/kg/meal 500 units/kg/snack |
| Child age ≥ 4 years | 500 units/kg/meal 250 units/kg/snack |
| Adult starting dose | 50,000 units/meal 25,000 units/snack |
| Adult maximum dose | 150,000 units/meal 70,000 units/snack |
- Struyvenberg MR, Martin CR, Freedman SD. Practical guide to exocrine pancreatic insufficiency – Breaking the myths. BMC Med. 2017;15(1):29. Published 2017 Feb 10. doi:10.1186/s12916-017-0783-y https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5301368
- DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813–5. doi: 10.1056/NEJM197304192881603.
- Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: Present and future. Clin Exp Gastroenterol. 2011;4:55–73.
- Ghaneh P, Neoptolemos JP. Exocrine pancreatic function following pancreatectomy. Ann N Y Acad Sci. 1999;880:308–18. doi: 10.1111/j.1749-6632.1999.tb09534.x
- Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005 Jul. 54 Suppl 6:vi1-28.
- DiMagno EP, Go VL, Summerskill WH. Relations between pancreatic enzyme ouputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973 Apr 19. 288(16):813-5.
- Frulloni L, Falconi M, Gabbrielli A, Gaia E, Graziani R, Pezzilli R, Uomo G, Andriulli A, Balzano G, Benini L, Calculli L, Campra D, Capurso G, Cavestro GM, De Angelis C, Ghezzo L, Manfredi R, Malesci A, Mariani A, Mutignani M, Ventrucci M, Zamboni G, Amodio A, Vantini I. Italian consensus guidelines for chronic pancreatitis. Dig Liver Dis. 2010;42(Suppl 6):S381–406. doi: 10.1016/S1590-8658(10)60682-2
- Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54:1–28. doi: 10.1136/gut.2005.065946
- Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. Am J Gastroenterol. 2004;99:2256–70. doi: 10.1111/j.1572-0241.2004.40694.x
- Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481–7. doi: 10.1016/0016-5085(94)90553-3
- Olivier AK, Gibson-Corley KN, Meyerholz DK. Animal models of gastrointestinal and liver diseases. Animal models of cystic fibrosis: gastrointestinal, pancreatic, and hepatobiliary disease and pathophysiology. Am J Physiol Gastrointest Liver Physiol. 2015;308:G459–71. doi: 10.1152/ajpgi.00146.2014
- Waters DL, Dorney SF, Gaskin KJ, Gruca MA, O’Halloran M, Wilcken B. Pancreatic function in infants identified as having cystic fibrosis in a neonatal screening program. N Engl J Med. 1990;322:303–8. doi: 10.1056/NEJM199002013220505
- Bodewes FAJA, Verkade HJ, Taminiau JAJM, Borowitz D, Wilschanski M. Cystic fibrosis and the role of gastrointestinal outcome measures in the new era of therapeutic CFTR modulation. J Cyst Fibros. 2015;14:169–77. doi: 10.1016/j.jcf.2015.01.006
- Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol. 1988;41:583–91. doi: 10.1016/0895-4356(88)90063-7
- FitzSimmons SC, Burkhart GA, Borowitz D, Grand RJ, Hammerstrom T, Durie PR, Lloyd-Still JD, Lowenfels AB. High-dose pancreatic-enzyme supplements and fibrosing colonopathy in children with cystic fibrosis. N Engl J Med. 1997;336:1283–9. doi: 10.1056/NEJM199705013361803
- Lloyd-Still JD. Cystic fibrosis and colonic strictures. A new “iatrogenic” disease. J Clin Gastroenterol. 1995;21(1):2–5. doi: 10.1097/00004836-199507000-00001
- Falconi M, Mantovani W, Crippa S, Mascetta G, Salvia R, Pederzoli P. Pancreatic insufficiency after different resections for benign tumours. Br J Surg. 2008;95:85–91. doi: 10.1002/bjs.5652
- Iacono C, Verlato G, Ruzzenente A, Campagnaro T, Bacchelli C, Valdegamberi A, Bortolasi L, Guglielmi A. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 2013;100:873–85. doi: 10.1002/bjs.9136
- Phillips ME. Pancreatic exocrine insufficiency following pancreatic resection. Pancreatology. 2015;15:449–55. doi: 10.1016/j.pan.2015.06.003
- Sikkens ECM, Cahen DL, de Wit J, Looman CWN, van Eijck C, Bruno MJ. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J Clin Gastroenterol. 2014;48:e43–6. doi: 10.1097/MCG.0b013e31828b2866
- Halloran CM, Cox TF, Chauhan S, Raraty MGT, Sutton R, Neoptolemos JP, Ghaneh P. Partial pancreatic resection for pancreatic malignancy is associated with sustained pancreatic exocrine failure and reduced quality of life: A prospective study. Pancreatology. 2012;11:535–45. doi: 10.1159/000333308
- West J, Logan RFA, Hill PG, Lloyd A, Lewis S, Hubbard R, Reader R, Holmes GKT, Khaw K-T. Seroprevalence, correlates, and characteristics of undetected coeliac disease in England. Gut. 2003;52:960–5. doi: 10.1136/gut.52.7.960
- Deprez P, Sempoux C, Van Beers BE, Jouret A, Robert A, Rahier J, Geubel A, Pauwels S, Mainguet P. Persistent decreased plasma cholecystokinin levels in celiac patients under gluten-free diet: Respective roles of histological changes and nutrient hydrolysis. Regul Pept. 2002;110:55–63. doi: 10.1016/S0167-0115(02)00162-3
- Piciucchi M, Capurso G, Archibugi L, Delle Fave MM, Capasso M, Delle FG. Exocrine pancreatic insufficiency in diabetic patients: Prevalence, mechanisms, and treatment. Int J Endocrinol. 2015;2015:595649. doi: 10.1155/2015/595649
- Ewald N, Kaufmann C, Raspe A, Kloer HU, Bretzel RG, Hardt PD. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c) Diabetes Metab Res Rev. 2012;28:338–42. doi: 10.1002/dmrr.2260
- Soave D, Miller MR, Keenan K, Li W, Gong J, Ip W, Accurso F, Sun L, Rommens JM, Sontag M, Durie PR, Strug LJ. Evidence for a causal relationship between early exocrine pancreatic disease and cystic fibrosis-related diabetes: a Mendelian randomization study. Diabetes. 2014;63:2114–9. doi: 10.2337/db13-1464
- Lebenthal E, Lee PC. Development of functional responses in human exocrine pancreas. Pediatrics. 1980;66:556–60
- Martin CR, Cheesman A, Brown J, Makda M, Kutner AJ, DaSilva D, Zaman M, Freedman SD. Factors determining optimal fatty acid absorption in preterm infants. J Pediatr Gastroenterol Nutr. 2016;62:130–6. doi: 10.1097/MPG.0000000000000934
- Nakajima K, Oshida H, Muneyuki T, Kakei M. Pancrelipase: An evidence-based review of its use for treating pancreatic exocrine insufficiency. Core Evid. 2012;7:77–91. doi: 10.2147/CE.S26705
- Beharry S, Ellis L, Corey M, Marcon M, Durie P. How useful is fecal pancreatic elastase 1 as a marker of exocrine pancreatic disease? J Pediatr. 2002;141:84–90. doi: 10.1067/mpd.2002.124829
- Berry AJ. Pancreatic enzyme replacement therapy during pancreatic insufficiency. Nutr Clin Pract. 2014;29:312–21. doi: 10.1177/0884533614527773
- Sziegoleit A, Krause E, Klör HU, Kanacher L, Linder D. Elastase 1 and chymotrypsin B in pancreatic juice and feces. Clin Biochem. 1989;22:85–9. doi: 10.1016/S0009-9120(89)80003-7
- Leeds JS, Hopper AD, Hurlstone DP, Edwards SJ, Mcalindon ME, Lobo AJ, Donnelly MT, Morley S, Sanders DS. Is exocrine pancreatic insufficiency in adult coeliac disease a cause of persisting symptoms? Aliment Pharmacol Ther. 2007;25:265–71. doi: 10.1111/j.1365-2036.2006.03206.x
- Löser C, Möllgaard A, Fölsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39:580–6. doi: 10.1136/gut.39.4.580
- Nissler K, Von Katte I, Huebner A, Henker J. Pancreatic elastase 1 in feces of preterm and term infants. J Pediatr Gastroenterol Nutr. 2001;33:28–31. doi: 10.1097/00005176-200107000-00005
- Lieb J-G. Pancreatic function testing: here to stay for the 21st century. World J Gastroenterol. 2008;14:3149. doi: 10.3748/wjg.14.3149
- Lankisch PG, Schmidt I, König H, Lehnick D, Knollmann R, Löhr M, Liebe S. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mild to moderate exocrine pancreatic insufficiency. Gut. 1998;42:551–4. doi: 10.1136/gut.42.4.551
- Lindkvist B, Dominguez-Munoz JE, Luaces-Regueira M, Castineiras-Alvarino M, Nieto-Garcia L, Iglesias-Garcia J. Serum nutritional markers for prediction of pancreatic exocrine insufficiency in chronic pancreatitis. Pancreatology. 2012 Jul-Aug. 12(4):305-10.
- Casterton PL, Verbeke KA, Brouns F, Dammann KW. Evaluation of sucromalt digestion in healthy children using breath hydrogen as a biomarker of carbohydrate malabsorption. Food Funct. 2012 Apr. 3(4):410-3.
- Hope HB, Tveito K, Aase S, Messelt E, Utzon P, Skar V. Small intestinal malabsorption in chronic alcoholism determined by 13C-D-xylose breath test and microscopic examination of the duodenal mucosa. Scand J Gastroenterol. 2010. 45(1):39-45.
- Hope HB, Tveito K, Aase S, Messelt E, Utzon P, Skar V. Small intestinal malabsorption in chronic alcoholism determined by C13-D-xylose breath test and microscopic examination of the duodenal mucosa. Scand J Gastroenterol. 2010. 45(1):39-45.
- Leeds JS, Oppong K, Sanders DS. The role of fecal elastase-1 in detecting exocrine pancreatic disease. Nat Rev Gastroenterol Hepatol. 2011 May 31. 8(7):405-15.
- Chowdhury RS, Forsmark CE. Review article: Pancreatic function testing. Aliment Pharmacol Ther. 2003 Mar 15. 17(6):733-50.
- Hammer HF. Pancreatic exocrine insufficiency: diagnostic evaluation and replacement therapy with pancreatic enzymes. Dig Dis. 2010. 28(2):339-43.
- Gonzalez-Sanchez V, Amrani R, Gonzalez V, Trigo C, Pico A, de-Madaria E. Diagnosis of exocrine pancreatic insufficiency in chronic pancreatitis: 13C-Mixed Triglyceride Breath Test versus Fecal Elastase. Pancreatology. 2017 Mar 6.
- Dominguez-Munoz JE. Pancreatic enzyme therapy for pancreatic exocrine insufficiency. Curr Gastroenterol Rep. 2007 Apr. 9(2):116-22.
- Sikkens ECM, Cahen DL, Koch AD, Braat H, Poley JW, Kuipers EJ, Bruno MJ. The prevalence of fat-soluble vitamin deficiencies and a decreased bone mass in patients with chronic pancreatitis. Pancreatology. 2013;13:238–42. doi: 10.1016/j.pan.2013.02.008
- Lindkvist B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J Gastroenterol. 2013;19:7258–66. doi: 10.3748/wjg.v19.i42.7258
- Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–61. doi: 10.1053/j.gastro.2013.01.068
- Law R, Parsi M, Lopez R, Zuccaro G, Stevens T. Cigarette smoking is independently associated with chronic pancreatitis. Pancreatology. 2010;10:54–9. doi: 10.1159/000225927
- Trang T, Chan J, Graham DY. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency in the 21(st) century. World J Gastroenterol. 2014;20:11467–85. doi: 10.3748/wjg.v20.i33.11467
- Updated Questions and Answers for Healthcare Professionals and the Public: Use an Approved Pancreatic Enzyme Product (PEP). https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/updated-questions-and-answers-healthcare-professionals-and-public-use-approved-pancreatic-enzyme
- Gelfond D, Ma C, Semler J, Borowitz D. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci. 2013;58:2275–81. doi: 10.1007/s10620-012-2209-1
- Proesmans M, De Boeck K. Omeprazole, a proton pump inhibitor, improves residual steatorrhoea in cystic fibrosis patients treated with high dose pancreatic enzymes. Eur J Pediatr. 2003;162:760–3. doi: 10.1007/s00431-003-1309-5
- Ng SM, Franchini AJ. Drug therapies for reducing gastric acidity in people with cystic fibrosis. Cochrane Database Syst Rev. 2014;8
- Woestenenk JW, van der Ent CK, Houwen RH. Pancreatic enzyme replacement therapy and coefficient of fat absorption in children and adolescents with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2015;61:355–60. doi: 10.1097/MPG.0000000000000784
- Kalnins D, Corey M, Ellis L, Durie PR, Pencharz PB. Combining unprotected pancreatic enzymes with pH-sensitive enteric-coated microspheres does not improve nutrient digestion in patients with cystic fibrosis. J Pediatr. 2005;146:489–93. doi: 10.1016/j.jpeds.2004.10.063
- Aloulou A, Puccinelli D, Sarles J, Laugier R, Leblond Y, Carrière F. In vitro comparative study of three pancreatic enzyme preparations: dissolution profiles, active enzyme release and acid stability. Aliment Pharmacol Ther. 2008;27:283–92. doi: 10.1111/j.1365-2036.2007.03563.x
- Working Party of the Australasian Pancreatic C. Smith RC, Smith SF, Wilson J, Pearce C, Wray N, Vo R, Chen J, Ooi CY, Oliver M, Katz T, Turner R, Nikfarjam M, Rayner C, Horowitz M, Holtmann G, Talley N, Windsor J, Pirola R, Neale R. Summary and recommendations from the Australasian guidelines for the management of pancreatic exocrine insufficiency. Pancreatology. 2016;16(2):164–80. doi: 10.1016/j.pan.2015.12.006
- Martínez J, Abad-González A, Aparicio JR, Aparisi L, Boadas J, Boix E, de las Heras G, Domínguez-Muñoz E, Farré A, Fernández-Cruz L, Gómez L, Iglesias-García J, García-Malpartida K, Guarner L, Lariño-Noia J, Lluís F, López A, Molero X, Moreno-Pérez O, Navarro S, Palazón JM, Pérez-Mateo M, Sabater L, Sastre Y, Vaquero E, de-Madaria E. The Spanish Pancreatic Club recommendations for the diagnosis and treatment of chronic pancreatitis: Part 1 (diagnosis) Pancreatology. 2012;13:18–28.
- Sikkens ECM, Cahen DL, Van Eijck C, Kuipers EJ, Bruno MJ. Patients with exocrine insufficiency due to chronic pancreatitis are undertreated: A Dutch national survey. Pancreatology. 2012;12:71–3. doi: 10.1016/j.pan.2011.12.010
- DiMagno EP, Malagelada JR, Go VL, Moertel CG. Fate of orally ingested enzymes in pancreatic insufficiency. Comparison of two dosage schedules. N Engl J Med. 1977;296:1318–22. doi: 10.1056/NEJM197706092962304
- Sikkens ECM, Cahen DL, Kuipers EJ, Bruno MJ. Pancreatic enzyme replacement therapy in chronic pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24:337–47. doi: 10.1016/j.bpg.2010.03.006
- Domínguez-Muñoz JE. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency: when is it indicated, what is the goal and how to do it? Adv Med Sci. 2011;56:1–5. doi: 10.2478/v10039-011-0005-3
- DiMagno MJ, DiMagno EP. Chronic pancreatitis. Curr Opin Gastroenterol. 2005;21:544–54. doi: 10.1097/01.mog.0000175543.42582.55
- Domínguez-Muñoz JE, Iglesias-García J, Iglesias-Rey M, Figueiras A, Vilariño-Insua M. Effect of the administration schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: A randomized, three-way crossover study. Aliment Pharmacol Ther. 2005;21:993–1000. doi: 10.1111/j.1365-2036.2005.02390.x
- Borowitz DS, Grand RJ, Durie PR. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. J Pediatr. 1995;127:681–4. doi: 10.1016/S0022-3476(95)70153-2