Folate
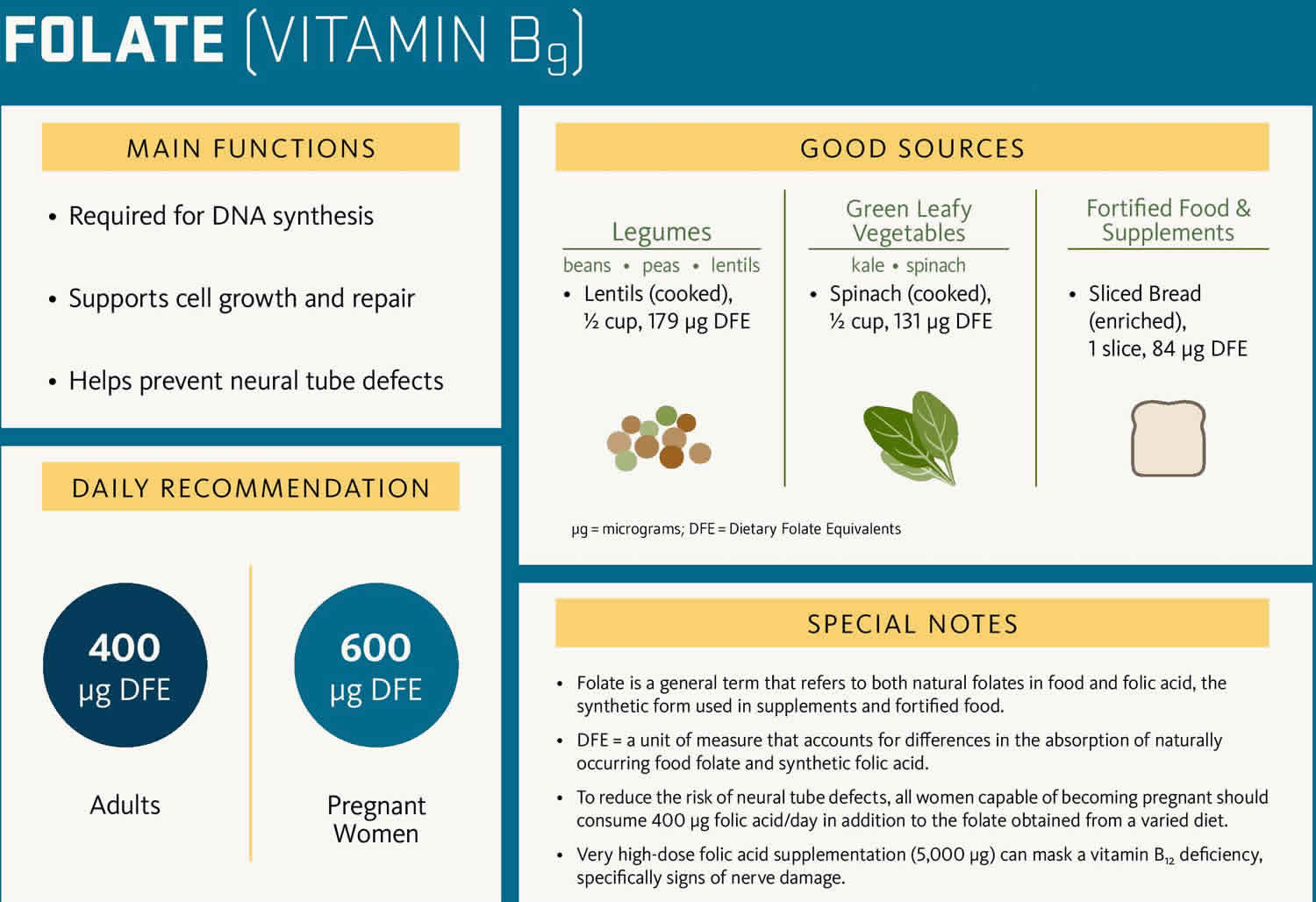
Folate also known as “folacin” or “vitamin B9,” is a water-soluble B vitamin that is naturally present in some foods is called “folates” and the synthetic or man-made folate is called “folic acid” that is used in dietary supplements and added to certain foods (fortified foods) 1. The potent form of folate is tetrahydrofolate (THF) 2. Your body needs folate to make DNA and other genetic material. Folic acid and folate also help your body make healthy new red blood cells. Red blood cells carry oxygen to all the parts of your body. If your body does not make enough red blood cells, you can develop anemia. Anemia happens when your blood cannot carry enough oxygen to your body, which makes you pale, tired, or weak. Also, if you do not get enough folic acid, you could develop a type of anemia called folate-deficiency anemia 3.
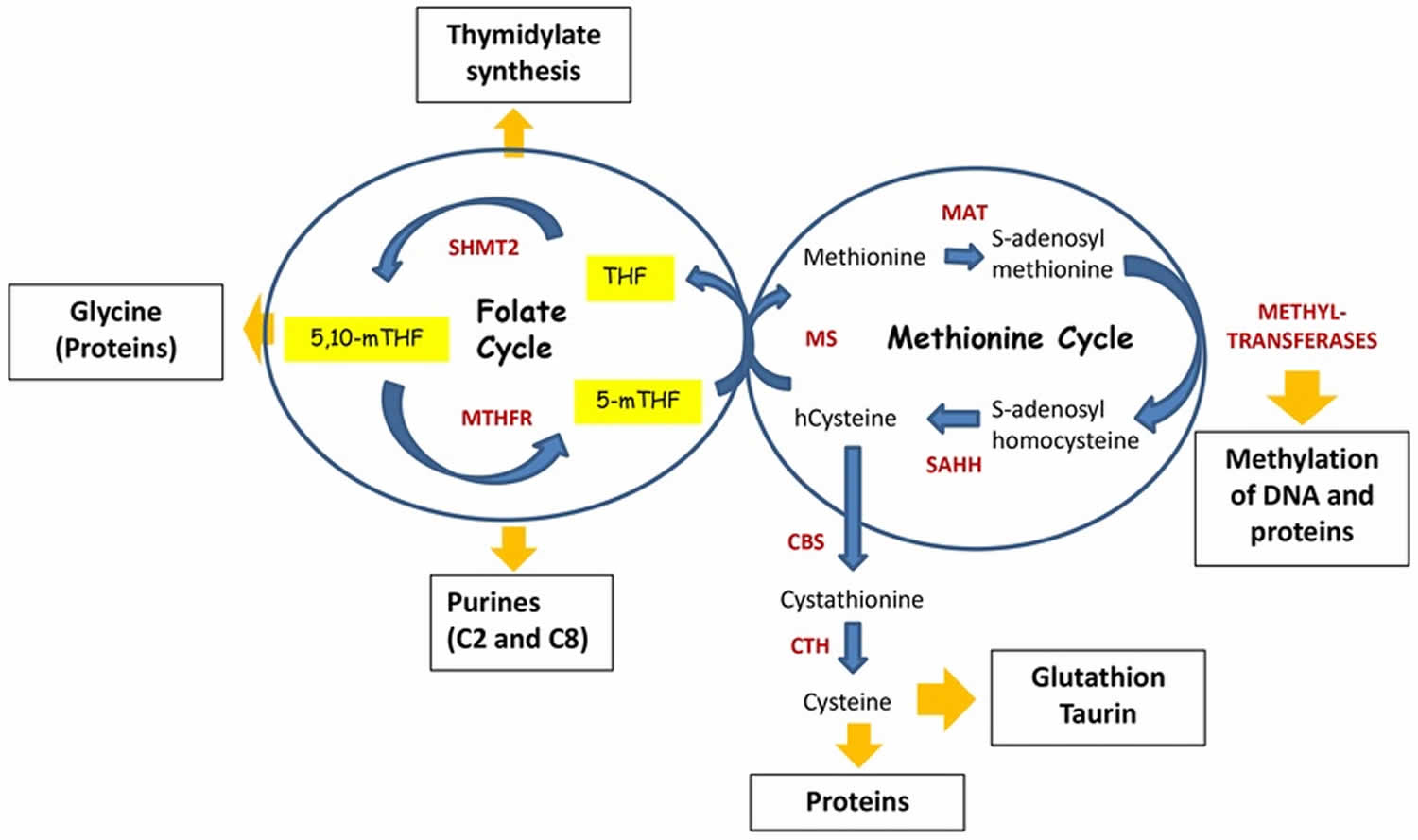
Folate functions as a coenzyme or cosubstrate in single-carbon transfers in the synthesis of nucleic acids (DNA and RNA) and metabolism of amino acids (Figures 1 and 2) 4, 5, 6. One of the most important folate-dependent reactions is the conversion of homocysteine to methionine in the synthesis of S-adenosyl-methionine (SAM), an important methyl donor 7. Another folate-dependent reaction, the methylation of deoxyuridylate to thymidylate in the formation of DNA, is required for proper cell division 1. An impairment of this reaction initiates a process that can lead to megaloblastic anemia, one of the hallmarks of folate deficiency 8.
When consumed, food folates are hydrolyzed to the monoglutamate form in the gut prior to absorption by active transport across the intestinal mucosa 9, 4. Passive diffusion also occurs when pharmacological doses of folic acid (the man made folate) are consumed 9. Before entering the bloodstream, the monoglutamate form is reduced to tetrahydrofolate (THF) and converted to either methyl or formyl forms. The main form of folate in plasma is 5-methyl-THF. Folic acid can also be found in the blood unaltered (known as unmetabolized folic acid), but whether this form has any biological activity or can be used as a biomarker of status is not known 10.
Some dietary supplements also contain folate in the monoglutamyl form, 5-methylenetetrahydrofolate or 5-methyl-folate (5-MTHF), also known as L-5-MTHF, L-methylfolate, and methylfolate 1. For some people with an methylenetetrahydrofolate reductase polymorphism (MTHFR polymorphism), supplementation with 5-methylenetetrahydrofolate (5-MTHF) might be more beneficial than with folic acid 11, 12. The bioavailability of 5-methylenetetrahydrofolate (5-MTHF) in supplements is the same as or greater than that of folic acid 13, 14, 15, 16. However, conversion factors between mcg and mcg dietary folate equivalent (DFE) for 5-MTHF (5-methyl-folate) have not been formally established. The U.S. Food and Drug Administration (FDA) allows manufacturers to use either a conversion factor of 1.7 to be comparable to folic acid, or their own established conversion factors not to exceed 1.7, where mcg DFE = mcg naturally occurring folate + (1.7 x mcg folic acid) 17. For example, a serving of food containing 60 microgram (mcg) of folate would provide 60 mcg of dietary folate equivalents (DFEs), while a serving of pasta fortified with 60 mcg of folic acid would provide 1.7 x 60 = 102 mcg of dietary folate equivalents (DFEs) due to the higher bioavailability of folic acid. A folic acid supplement of 400 mcg taken on an empty stomach would provide 800 mcg of dietary folate equivalents (DFEs). It should be noted that DFEs were determined in studies with adults and whether folic acid in infant formula is more bioavailable than folates in mother’s milk has not been studied. Use of dietary folate equivalents (DFEs) to determine a folate requirement for the infant would not be desirable.
Heating during cooking destroys folic acid 2. Folate is absorbed in the jejunum (the 2nd part of your small intestine) by active and passive transport mechanisms across the intestinal wall 2. Folate or folic acid is a water-soluble type of vitamin B. This means it is not stored in the fat tissues of the body. Leftover amounts of the vitamin leave the body through the urine. The body has about 1,000-20,000 mcg of folate stores, and adults need about 400 mcg/day to replenish the daily losses. Folate deficiency may take 8-16 weeks to become evident 2.
Folate is poorly stored, and folate deficiency can develop in weeks to months in persons with folate-deficient diets. Most of the serum folate is present in the inactive 5-methyltetrahydrofolate (5-methyl THF) form 2. Upon entering cells, 5-methyltetrahydrofolate (5-methyl THF) demethylates to tetrahydrofolate (THF), the biologically active form of folate that is involved in folate-dependent enzymatic reactions. Cobalamin (vitamin B12) serves as a co-factor for this demethylation to occur, and in the absence of vitamin B12, folate is “trapped” inside cells as 5-methyltetrahydrofolate (5-methyl THF). Tetrahydrofolate (THF) is involved in the formation of many coenzymes in metabolic systems, particularly for purine and pyrimidine synthesis, nucleoprotein synthesis, and maintenance in red blood cells formation 18. The deficiency of folate, as a result, leads to impairment of cell division, accumulation of toxic metabolites, and impartment of methylation reactions required for regulation of gene expression, resulting in megaloblastic anemia, which is characterized by large, abnormally nucleated red blood cells 8, 4.
The total body content of folate is estimated to be 10 to 30 mg; about half of this amount is stored in the liver and the remainder in blood and body tissues. A serum folate concentration is commonly used to assess folate status, with a value above 3 nanograms (ng)/mL indicating adequacy 19. This indicator, however, is sensitive to recent dietary intake, so it might not reflect long-term status. Erythrocyte folate concentration provides a longer-term measure of folate intakes, so when day-to-day folate intakes are variable—such as in people who are ill and whose folate intake has recently declined—it might be a better indicator of tissue folate stores than serum folate concentration 9. An erythrocyte folate concentration above 140 ng/mL indicates adequate folate status 9, although some researchers have suggested that higher values are optimal for preventing neural tube defects 20.
A combination of serum or red blood cell concentration and indicators of metabolic function can also be used to assess folate status. Plasma homocysteine concentration is a commonly used functional indicator of folate status because homocysteine levels rise when the body cannot convert homocysteine to methionine due to a 5-methyl-THF deficiency. Homocysteine levels, however, are not a highly specific indicator of folate status because they can be influenced by other factors, including kidney dysfunction and deficiencies of vitamin B12 and other micronutrients 21. The most commonly used cutoff value for elevated homocysteine is 16 micromoles/L, although slightly lower values of 12 to 14 micromoles/L have also been used 9.
Folate-deficiency anemia is most common during pregnancy. Other causes of folate-deficiency anemia include alcoholism and certain medicines to treat seizures, anxiety, or arthritis.
The symptoms of folate-deficiency anemia include:
- Fatigue
- Headache
- Pale skin
- Sore mouth and tongue
The latest research reveals the following about folic acid deficiency 2:
- There may be a link between elevated homocysteine (a marker for an increased risk for arteriosclerosis) and folate deficiency.
- A lowering of the risk of stroke but not adverse cardiac event when hyperhomocysteinemia is corrected with folic acid
- Reduction in the incidence of neural tube defects with folic acid supplementation during pregnancy.
- Lack of folic acid during pregnancy may increase the risk of diabetes-associated congenital disabilities and autism.
- Maternal folic acid during pregnancy may lower the risk of childhood leukemia.
- Folic acid supplementation may increase the risk of cancer.
If you have folate-deficiency anemia, your doctor may recommend taking folic acid vitamins and eating more foods with folate.
Folic acid is available in multivitamins and prenatal vitamins, supplements containing other B-complex vitamins, and supplements containing only folic acid. Common doses range from 680 to 1,360 mcg dietary folate equivalent (DFE) (400 to 800 mcg folic acid) in supplements for adults and 340 to 680 mcg DFE (200 to 400 mcg folic acid) in children’s multivitamins 22. About 85% of supplemental folic acid, when taken with food, is bioavailable 5, 8. When consumed without food, nearly 100% of supplemental folic acid is bioavailable 1.
Figure 1. Folate and methionine cycle
Footnote: Folate and methionine cycle. The Folate cycle begins with the conversion of dietary folate (vitamin B9) into dihydrofolate (DHF) then tetrahydrofolate (THF) by dihydrofolate reductase (DHFR). Next, tetrahydrofolate (THF) is converted to 5, 10-methylene-THF by serine hydroxymethyltransferase (SHMT) before being reduced into 5-methyl-THF (5-mTHF) by methylenetetrahydrofolate reductase (MTHFR). As part of the methionine cycle, 5-methyl-THF (5-mTHF) donates its carbon group to convert homocysteine (Hcy) to methionine (Met), which is catalyzed by methionine synthase (MS) and requires vitamin B12 as a cofactor, hence initiating Methionine cycle. In turn, methionine (Met) is used by methionine adenosyltransferase (MAT) to generate S-Adenosyl-Methionine (SAM) – the principal donor of methyl groups for DNA and proteins methylation. Thus, SAM is used by different methyltransferases, resulting in S-adenosylhomocysteine after its demethylation. Finally, S-adenosylhomocysteine hydrolase (SAHH) mediates deadenylation of S-adenosylhomocysteine to hcysteine, enclosing the methionine cycle. Homocysteine can be used by cystathionine synthase (CBS), which converts it to cystathionine. In turn, cystathionine is a substrate for cystathionine gamma-lyase (CTH), which uses it for synthesis of cysteine. Cysteine is required for the synthesis of proteins as well as for generation of taurine and glutathione, the latter is one of the critical molecules for redox homeostasis.
Abbreviations: Ado = adenosine; ATP = adeno-sine triphosphate; vitamin B6, vitamin B9, vitamin B12; Cbs = cystathionine beta synthase; DHF = dihydrofolate; DHFR = dihydrofolate reductase; Cys = cysteine; GSH = glutathione; Hcy = homocysteine; MAT = methionine adenosyltransferase; Met = methionine; 5–methylTHF = 5, 10–methyleneTHF; MTHFR = methylenetetrahydrofolate reductase or 5,10-methylene-trahydrofolate reductase; MS = methionine synthase, SAM = S-adenosyl-methionine; SAH = S-adenosylhomocysteine; SHMT = hydroxymethyltransferase; THF = tetrahydrofolate.
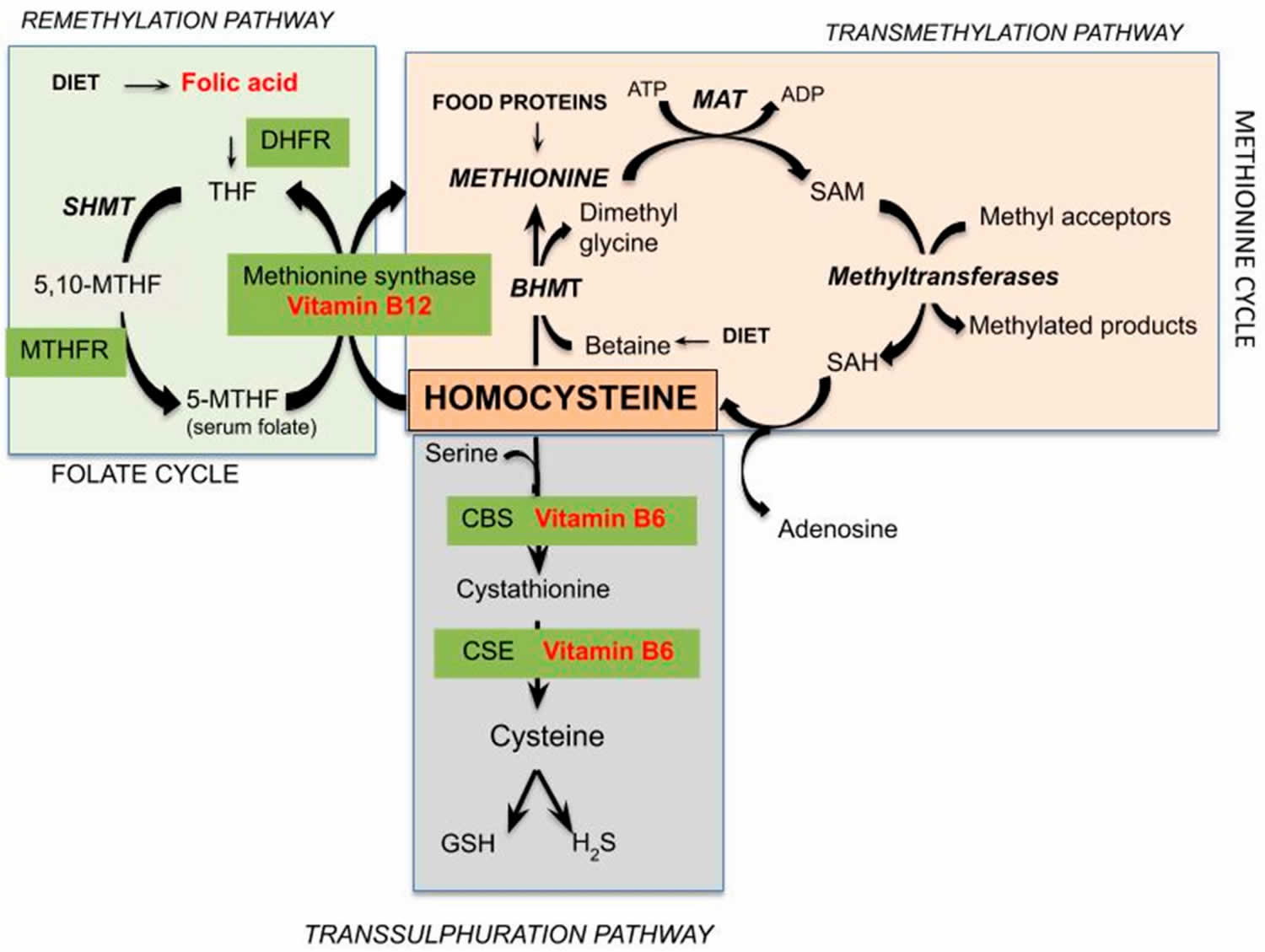
[Source 23 ]Figure 2. Homocysteine metabolism
Footnote: Schematic representation of pathways of homocysteine metabolism. The metabolism of homocysteine, an intermediate in the metabolism of sulfur-containing amino acids, provides an example of the interrelationships among nutrients necessary for optimal physiological function and health. Healthy individuals utilize two different pathways to metabolize homocysteine (Figure 3). One pathway (methionine synthase) synthesizes methionine from homocysteine and is dependent on both folate and vitamin B12 as cofactors. The other pathway converts homocysteine to another amino acid, cysteine, and requires two vitamin B6-dependent enzymes. Thus, the concentration of homocysteine in the blood is regulated by three B-vitamins: folate (vitamin B9), vitamin B12 (cobalamin), and vitamin B6 (pyridoxine) 24. In some individuals, riboflavin (vitamin B2) is also involved in the regulation of homocysteine concentrations.
Abbreviations: DHFR = dihydrofolate reductase; THF = tetrahydrofolate; SHMT = serinehydroxymethyltransferase; MTHF = methylenetetrahydrofolate; MTHFR = 5,10-methylene-THF reductase; ATP = adenosine triphosphate; MAT = methionine adenosyltransferase; ADP = adenosine diphosphate; SAM = S-adenosylmethionine; SAH = S-adenosylhomocysteine; BHMT = betaine-Hcy S-methyltransferase; CBS = cystathionine beta-synthase; CSE = cystathionase; GSH = glutathione; H2S = hydrogen sulphide
[Source 25 ]Why is Folic Acid Important?
Everyone needs folate (the naturally vitamin B9 present in some foods) or folic acid (the man made folate). Your body use folate to make new cells.
In women and pregnant mothers, folic acid is very important because it can help prevent some major birth defects of the baby’s brain and spine (anencephaly and spina bifida) 26. Centers for Disease Control and Prevention (CDC) urges women of childbearing age should obtain 400 mcg/day folic acid from dietary supplements and/or fortified foods in addition to the folate provided by a varied diet to help prevent major birth defects of the baby’s brain and spine 27. The American College of Obstetricians and Gynecologists recommends a prenatal folic acid of at least 400 micrograms starting at least 1 month before pregnancy and during the first 12 weeks of pregnancy for most pregnant women to ensure that they obtain adequate amounts of folic acid and other nutrients 28. Women who have had a child with an neural tube birth defect should take 4 milligrams (4000 micrograms) of folic acid each day as a separate supplement at least 3 months before pregnancy and for the first 3 months of pregnancy 28. You and your ob-gyn or other obstetric care provider can discuss whether you need to supplement with more than 400 micrograms daily 28.
Every woman needs folic acid every day, whether she’s planning to get pregnant or not, for the healthy new cells the body makes daily. Think about the skin, hair, and nails. These – and other parts of the body – make new cells each day.
Folate functions as a coenzyme or cosubstrate in single-carbon (1C-group) transfers in the synthesis of nucleic acids (DNA and RNA) and metabolism of amino acids (Figures 1 and 2) 4, 5, 6. One of the most important folate-dependent reactions is the conversion of homocysteine to methionine in the synthesis of S-adenosyl-methionine (SAM), an important methyl donor. Another folate-dependent reaction, the methylation of deoxyuridylate to thymidylate in the formation of DNA, is required for proper cell division. An impairment of this reaction initiates a process that can lead to megaloblastic anemia, one of the hallmarks of folate deficiency 8.
Once transported to the cell, folate undergoes covalent modification by polyglutamination 23. Folate is further substituted by the one-carbon moiety in the N5 and/or N10 position at different oxidation levels: formate (10-formyltetrahydrofolate), formaldehyde (5,10-methylenetetrahydrofolate), or methanol (5-methyltetrahydrofolate) 29. There are only two direct sources of one-carbon groups (1C-group) in one-carbon metabolism – serine and glycine 23. Therefore, the central reaction of the folate cycle is conversion of serine to glycine by serine hydroxymethyltransferase SHMT1 and SHMT2 enzymes. By transferring the one-carbon group (1C-group) from serine and tetrahydrofolate (THF), this reaction generates 5,10-methylenetetrahydrofolate – the first donor of one-carbon group (1C-group) in the folate cycle. Another source of 5,10-methylenetetrahydrofolate comes from the enzymatic cleavage of glycine by an enzyme called glycine decarboxylase (GLDC), which resides in mitochondria 23.
In turn, 5,10-methylenetetrahydrofolate can be used in three ways (Figures 1 and 2). First, it can serve as one-carbon-donor for the initial step of thymidylate biosynthesis, a reaction catalyzed by thymidylate synthase (TS). In this reaction 5,10-methylenetetrahydrofolate provides one-carbon group for the pyrimidine biosynthesis and is oxidized into dihydrofolate (DHF). In the next reaction dihydrofolate reductase (DHFR) reduces DHF to tetrahydrofolate (THF) enclosing this metabolic loop.
Second, 5,10-methylenetetrahydrofolate can be used by a cytosolic enzyme methylenetetrahydrofolate reductase 1 (MTHFD1), or mitochondrial tandem enzymes methylenetetrahydrofolate reductases MTHFD2L/MTHFD2, to generate 10-formyltetrahydrofolate 23. 10-formyltetrahydrofolate is a one-carbon-donor for the two reactions of purine biosynthesis catalyzed by Trifunctional enzyme Phosphoribosylglycinamide Formyltransferase/ Synthetase/ Phosphoribosylaminoimidazole Synthetase (GART) and Bifunctional 5-Aminoimidazole-4-Carboxamide Ribonucleotide Formyltransferase/IMP Cyclohydrolase (ATIC), both of which in turn generate tetrahydrofolate (THF).
Third, 5,10-methylenetetrahydrofolate is used by methylentetrahydrofolatereductase (MTHFR) to generate methyltetrahydrofolate. The latter donates a methyl group to homocycteine resulting in the formation of methionine and tetrahydrofolate (THF). By this way the Folate cycle is coupled with Methionine cycle (Shuvalov O, Petukhov A, Daks A, Fedorova O, Vasileva E, Barlev NA. One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget. 2017 Apr 4;8(14):23955-23977. doi: 10.18632/oncotarget.15053)). Finally, tetrahydrofolate (THF) is converted into 5,10-methylenetetrahydrofolate by serine hydroxymethyltransferase SHMT1 and SHMT2 enzymes thus enclosing the Folate cycle 23.
Another arm of the one-carbon group (1C-group)-metabolic process is the methionine cycle (Figures 1 and 2). It starts with methionine synthesis from homocysteine and methyltetrahydrofolate catalyzed by methionine synthase (MS). Subsequently, methionine adenyltransferase (MAT) synthesizes S-adenosyl-methionine (SAM), the main donor of methyl groups in the cell. After demethylation, S-adenosyl-methionine (SAM) is converted to S-adenosylhomocysteine (SAH). Finally, S-adenosyl homocysteine hydrolase (SAHH) mediates de-adenylation of SAHH resulting in homocysteine and full turn of the cycle 23.
One-carbon metabolism
The only function of folate coenzymes in the body appears to be in mediating the transfer of one-carbon units 30. Folate coenzymes act as acceptors and donors of one-carbon units in a variety of reactions critical to the metabolism of nucleic acids and amino acids (Figures 1 and 2) 31.
Nucleic acid metabolism
Folate coenzymes play a vital role in DNA metabolism through two different pathways. (1) The synthesis of DNA from its precursors (thymidine and purines) is dependent on folate coenzymes. (2) A folate coenzyme is required for the synthesis of methionine from homocysteine, and methionine is required for the synthesis of S-adenosylmethionine (SAM). SAM is a methyl group (one-carbon unit) donor used in most biological methylation reactions, including the methylation of a number of sites within DNA, RNA, proteins, and phospholipids. The methylation of DNA plays a role in controlling gene expression and is critical during cell differentiation. Aberrations in DNA methylation have been linked to the development of cancer.
Amino acid metabolism
Folate coenzymes are required for the metabolism of several important amino acids, namely methionine, cysteine, serine, glycine, and histidine. The synthesis of methionine from homocysteine is catalyzed by methionine synthase, an enzyme that requires not only folate (as 5-methyltetrahydrofolate) but also vitamin B12. Thus, folate and/or vitamin B12 deficiency can result in decreased synthesis of methionine and an accumulation of homocysteine. Elevated blood concentrations of homocysteine have been considered for many years to be a risk factor for some chronic diseases, including cardiovascular disease and dementia.
Why do women need folic acid?
Everyone needs folic acid to be healthy. But it is especially important for women:
- Before and during pregnancy. Folic acid protects unborn children against serious birth defects called neural tube defects. These birth defects happen in the first few weeks of pregnancy, often before a woman knows she is pregnant. Folic acid might also help prevent other types of birth defects and early pregnancy loss (miscarriage). Since about half of all pregnancies in the United States are unplanned 32, experts recommend all women get enough folic acid even if you are not trying to get pregnant.
- To keep the blood healthy by helping red blood cells form and grow. Not getting enough folic acid can lead to a type of anemia called folate-deficiency anemia. Folate-deficiency anemia is more common in women of childbearing age than in men.
Do Women need to take Folic Acid every day even if you’re not planning to get pregnant?
Yes. All women who can get pregnant need to take 400 to 800 micrograms of folic acid every day, even if you’re not planning to get pregnant 33. There are several reasons why:
- Your birth control may not work or you may not use birth control correctly every time you have sex. In a survey by the Centers for Disease Control and Prevention, almost 40% of women with unplanned pregnancies were using birth control 34.
- Birth defects of the brain and spine can happen in the first few weeks of pregnancy, often before you know you are pregnant. By the time you find out you are pregnant, it might be too late to prevent the birth defects.
- You need to take folic acid every day because it is a water soluble B-vitamin. Water soluble means that it does not stay in the body for a long time. Your body metabolizes (uses) folic acid quickly, so your body needs folic acid each day to work properly.
What can happen if women do not get enough folic acid during pregnancy?
If you do not get enough folic acid before and during pregnancy, your baby is at higher risk for neural tube defects.
Neural tube defects are serious birth defects that affect the spine, spinal cord, or brain and may cause death. These include:
- Spina bifida 35. This condition happens when an unborn baby’s spinal column does not fully close during development in the womb, leaving the spinal cord exposed. As a result, the nerves that control the legs and other organs do not work. Children with spina bifida often have lifelong disabilities. They may also need many surgeries.
- Anencephaly 36. This means that most or all of the brain and skull does not develop in the womb. Almost all babies with this condition die before or soon after birth.
How much folic acid do women need?
All women need 400 micrograms of folic acid every day. Women who can get pregnant should get 400 to 800 micrograms of folic acid from a vitamin or from food that has added folic acid, such as breakfast cereal 37. This is in addition to the folate you get naturally from food. Some women may need more folic acid each day.
Table 1. Recommended folic acid for women
| If you: | Amount of folic acid you may need daily 37 |
|---|---|
| Could get pregnant or are pregnant | 400–800 micrograms.37 Your doctor may prescribe a prenatal vitamin with more. |
| Had a baby with a neural tube defect (such as spina bifida) and want to get pregnant again | 4,000 micrograms. Your doctor may prescribe this amount. Research shows taking this amount may lower the risk of having another baby with spina bifida. 38 |
| Have a family member with spina bifida and could get pregnant | 4,000 micrograms. Your doctor may prescribe this amount. |
| Have spina bifida and want to get pregnant | 4,000 micrograms. Your doctor may prescribe this amount. Women with spina bifida have a higher risk of having children with the condition. |
| Take medicines to treat epilepsy, type 2 diabetes, rheumatoid arthritis, or lupus | Talk to your doctor or nurse. Folic acid supplements can interact with these medicines. |
| Are on dialysis for kidney disease | Talk to your doctor or nurse. |
| Have a health condition, such as inflammatory bowel disease or celiac disease, that affects how your body absorbs folic acid | Talk to your doctor or nurse. |
How can you be sure you get enough folic acid?
You can get enough folic acid from food alone. Many breakfast cereals have 100% of your recommended daily value (400 micrograms) of folic acid.
If you are at risk for not getting enough folic acid, your doctor may recommend that you take a vitamin with folic acid every day. Most U.S. multivitamins have at least 400 micrograms of folic acid. Check the label on the bottle to be sure. You can also take a pill that contains only folic acid.
If swallowing pills is hard for you, try a chewable or liquid product with folic acid.
Are some women at risk for not getting enough folic acid?
Yes, certain groups of women do not get enough folic acid each day 39.
- Women who can get pregnant need more folic acid (400 to 800 micrograms). 38
- Nearly one in three African-American women does not get enough folic acid each day.
- Spanish-speaking Mexican-American women often do not get enough folic acid. However, Mexican-Americans who speak English usually get enough folic acid. 40
Not getting enough folic acid can cause health problems, including folate-deficiency anemia, and problems during pregnancy for you and your unborn baby.
Do I need folic acid after menopause?
Yes. Women who have gone through menopause still need 400 micrograms of folic acid every day for good health. Talk to your doctor or nurse about how much folic acid you need.
How a Woman can get enough Folic Acid?
There are two easy ways to be sure to get enough folic acid each day:
- Take a vitamin that has folic acid in it every day. Most multivitamins sold in the United States have the amount of folic acid women need each day. Women can also choose to take a small pill (supplement) that has only folic acid in it each day.
- Multivitamins and folic acid pills can be found at most local pharmacy, grocery, or discount stores.
- Check the label to be sure it contains 100% of the daily value (DV) of folic acid, which is 400 micrograms (mcg).
- Eat a bowl of breakfast cereal that has 100% of the daily value of folic acid every day.
Not every cereal has this amount. Make sure you check the label on the side of the box, and look for one that has “100%” next to folic acid or 400 micrograms (mcg).
How much folate do I need?
The amount of folate you need depends on your age. Average daily recommended amounts are listed below in micrograms (mcg) of dietary folate equivalents (DFEs).
All women and teen girls who could become pregnant should consume 400 mcg of folic acid daily from supplements, fortified foods, or both in addition to the folate they get naturally from foods.
Table 2 lists the current Recommended Dietary Allowances (RDAs) for folate as mcg of dietary folate equivalents (DFEs). The Recommended Dietary Allowance (RDA) is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals. The Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine developed dietary folate equivalents (DFEs) to reflect the higher bioavailability of folic acid than that of food folate 5. At least 85% of folic acid is estimated to be bioavailable when taken with food, whereas only about 50% of folate naturally present in food is bioavailable 5, 4. Based on these values, the Food and Nutrition Board defined dietary folate equivalent (DFE) as follows:
- 1 microgram (mcg) of food folate provides 1 mcg DFE (1 mcg DFE = 1 mcg food folate)
- 1 mcg DFE = 0.6 mcg folic acid from fortified foods or dietary supplements consumed with foods (OR 1 mcg of folic acid taken with meals or as fortified food provides 1.7 mcg of DFEs)
- 1 mcg DFE = 0.5 mcg folic acid from dietary supplements taken on an empty stomach (OR 1 mcg of folic acid (supplement) taken on an empty stomach provides 2 mcg of DFEs)
For example, a serving of food containing 60 microgram (mcg) of folate would provide 60 mcg of DFEs, while a serving of pasta fortified with 60 mcg of folic acid would provide 1.7 x 60 = 102 mcg of DFEs due to the higher bioavailability of folic acid. A folic acid supplement of 400 mcg taken on an empty stomach would provide 800 mcg of DFEs. It should be noted that DFEs were determined in studies with adults and whether folic acid in infant formula is more bioavailable than folates in mother’s milk has not been studied. Use of DFEs to determine a folate requirement for the infant would not be desirable.
Factors for converting mcg dietary folate equivalent (DFE) to mcg for supplemental folate in the form of 5-methylenetetrahydrofolate (5-MTHF) have not been formally established 1.
For infants from birth to 12 months, the Food and Nutrition Board established an Adequate Intake (AI) for folate that is equivalent to the mean intake of folate in healthy, breastfed infants in the United States (see Table 2). Adequate Intake (AI) is the intake level that is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA) 1.
According to data from the 2013–2014 National Health and Nutrition Examination Survey (NHANES), most people in the United States consume adequate amounts of folate 1. Mean dietary intakes of folate from foods range from 417 to 547 mcg DFE per day for children aged 2–19 41. Average daily intakes of folate from food are 602 mcg DFE for males aged 20 and older and 455 mcg DFE for females 1.
Although most people consume adequate amounts of folate, certain groups, including women of childbearing age and non-Hispanic black women, are at risk of insufficient folate intakes 1. Even when intakes of folic acid from dietary supplements are included, 19% of female adolescents aged 14 to 18 years and 17% of women aged 19 to 30 years do not meet the Estimated Average Requirement (the average daily level of intake estimated to meet the requirements of 50% of healthy individuals; usually used to assess the nutrient intakes of groups of people and to plan nutritionally adequate diets for them; can also be used to assess the nutrient intakes of individuals) 42. Similarly, 23% of non-Hispanic black women have inadequate total intakes, compared with 13% of non-Hispanic white women.
About 35% of adults and 28% of children aged 1 to 13 years in the United States use supplements containing folic acid 42, 43. Adults aged 51 to 70 years are more likely than members of other age groups to take supplements containing folic acid. Use is also higher among non-Hispanic whites than non-Hispanic blacks or Mexican Americans. People aged 2 years and older who take supplements containing folic acid get a mean of 712 mcg DFE from those supplements 41.
Measurements of reb blood cell folate levels also suggest that most people in the United States have adequate folate status 1. According to an analysis of National Health and Nutrition Examination Survey (NHANES) 2003–2006 data, less than 0.5% of children aged 1 to 18 years have deficient erythrocyte folate concentrations 22. Mean concentrations in this age group range from 211 to 294 ng/mL depending on age, dietary habits, and supplement use. In adults, mean red blood cell folate concentrations range from 216 to 398 ng/mL, also indicating adequate folate status 44.
Some population groups are at risk of obtaining excessive folic acid. About 5% of men and women aged 51-70 and men aged 71 and older have folic acid intakes exceeding the Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) of 1,000 mcg per day, primarily because of the folic acid they obtain from dietary supplements 42. Furthermore, 30% to 66% of children aged 1 to 13 years who take folic acid-containing supplements have intakes of folic acid from both fortified food and dietary supplements exceeding the Tolerable Upper Intake Level (UL) of 300–600 mcg per day 43. Almost all children aged 1 to 8 years who consume at least 200 mcg/day folic acid from dietary supplements have total intakes that exceed the Tolerable Upper Intake Level (UL) 22. Little is known about the long-term effects of high folic acid doses in children 45.
Large amounts of folate can correct the megaloblastic anemia, but not the neurological damage, that can result from vitamin B12 deficiency 1. Excess intake of folate, especially in the elderly population high intakes of folate supplements might “mask” vitamin B12 deficiency until its neurological consequences become irreversible 1, 2. Questions about this possibility still remain, but the focus of concern has shifted to the potential for large amounts of folate to precipitate or exacerbate the anemia and cognitive symptoms associated with vitamin B12 deficiency 46, 47, 48, 49, 50.
Excess folate intake is also known to have a controversial and complex dual role in colorectal cancer 2. Concerns have also been raised that high folic acid intakes might accelerate the progression of preneoplastic lesions, increasing the risk of colorectal and possibly other cancers in certain individuals 51, 52, 53. In addition, folic acid from supplements intakes of 1,000 mcg per day or more during the periconception period have been associated with lower scores on several tests of cognitive development in children at ages 4–5 years than in children of mothers who took 400 mcg to 999 mcg 54.
Intakes of folic acid that exceed the body’s ability to reduce it to tetrahydrofolate (THF) lead to unmetabolized folic acid in the body, which has been linked to reduced numbers and activity of natural killer cells, suggesting that it could affect the immune system 55, 56. In addition, some scientists have hypothesized that unmetabolized folic acid might be related to cognitive impairment among older adults 57. These potential negative health consequences are not well understood and warrant further research 1, 4, 58.
Studies have found unmetabolized folic acid in blood from children, adolescents, and adults 59, 60; breastmilk 61; and cord blood from newborns 62, 63. Limited research suggests that single doses of 300 mcg or 400 mcg folic acid (a common amount in folic acid-containing supplements or servings of fortified foods, such as breakfast cereals) result in detectable serum levels of unmetabolized folic acid, whereas doses of 100 mcg or 200 mcg do not 64, 65. In addition, a dose-frequency interaction appears to occur in which smaller amounts of folic acid consumed more frequently produce higher unmetabolized folic acid concentrations than the same total dose consumed in larger, less frequent amounts 66.
Based on the metabolic interactions between folate and vitamin B12, the Food and Nutrition Board established a Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) for the synthetic forms of folate available in dietary supplements and fortified foods (Table 3) 5. The Food and Nutrition Board did not establish a Tolerable Upper Intake Level (UL) for folate from food because high intakes of folate from food sources have not been reported to cause adverse effects 5. Thus, unlike the Recommended Dietary Allowances (RDAs), the Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) are in mcg, not mcg DFE. For folic acid, 1,000 mcg is equivalent to 1,667 mcg DFE because 0.6 mcg folic acid = 1 mcg DFE 66. The Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) do not apply to individuals taking high doses of supplemental folate under medical supervision 5.
Table 2. Recommended Dietary Allowances (RDAs) for Folate
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 65 mcg DFE |
| Infants 7–12 months | 80 mcg DFE |
| Children 1–3 years | 150 mcg DFE |
| Children 4–8 years | 200 mcg DFE |
| Children 9–13 years | 300 mcg DFE |
| Teens 14–18 years | 400 mcg DFE |
| Adults 19+ years | 400 mcg DFE |
| Pregnant teens and women | 600 mcg DFE |
| Breastfeeding teens and women | 500 mcg DFE |
Footnote: All women and teen girls who could become pregnant should consume 400 mcg of folic acid daily from supplements, fortified foods, or both in addition to the folate they get from following a healthy eating pattern.
[Source 67 ]Table 3. Tolerable Upper Intake Levels (ULs) for Folate from Supplements or Fortified Foods
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months | Not possible to establish* | Not possible to establish* | ||
| 7–12 months | Not possible to establish* | Not possible to establish* | ||
| 1–3 years | 300 mcg | 300 mcg | ||
| 4–8 years | 400 mcg | 400 mcg | ||
| 9–13 years | 600 mcg | 600 mcg | ||
| 14–18 years | 800 mcg | 800 mcg | 800 mcg | 800 mcg |
| 19+ years | 1,000 mcg | 1,000 mcg | 1,000 mcg | 1,000 mcg |
Footnote: * Breast milk, formula, and food should be the only sources of folate for infants.
[Source 67 ]What foods provide folate?
Folate is naturally present in a wide variety of foods including vegetables (especially dark green leafy vegetables), fruits and fruit juices, nuts, beans, peas, seafood, eggs, dairy products, meat, poultry, and grains (Table 4) 8, 68. Spinach, liver, asparagus, and brussels sprouts are among the foods with the highest folate levels 1.
Folate is naturally present in 67:
- Beef liver
- Vegetables (especially asparagus, brussels sprouts, and dark green leafy vegetables such as spinach and mustard greens)
- Fruits and fruit juices (especially oranges and orange juice)
- Nuts, beans, and peas (such as peanuts, black-eyed peas, and kidney beans)
Folic acid (man-made folate) is added to the following foods 67:
- Enriched bread, flour, cornmeal, pasta, and rice
- Fortified breakfast cereals
- Fortified corn masa flour (used to make corn tortillas and tamales, for example)
In January 1998, the U.S. Food and Drug Administration (FDA) began requiring manufacturers to add 140 mcg folic acid/100 g to enriched breads, cereals, flours, cornmeals, pastas, rice, and other grain products to reduce the risk of neural tube defects 69. Because cereals and grains are widely consumed in the United States, these products have become important contributors of folic acid to the American diet 1. The fortification program increased mean folic acid intakes in the United States by about 190 mcg/day 70. In April 2016, FDA approved the voluntary addition of up to 154 mcg folic acid/100 g to corn masa flour 71.
Since November 1, 1998, the Canadian government has also required the addition of 150 mcg folic acid/100 g to many grains, including enriched pasta, cornmeal, and white flour 72, 73. Many other countries, including Costa Rica, Chile, and South Africa, have also established mandatory folic acid fortification programs 73, 71.
To find out whether a food has added folic acid, look for “folic acid” on its Nutrition Facts label.
Table 4. Folate and Folic Acid content of selected foods
| Food | Micrograms (mcg) DFE per serving | Percent DV* |
|---|---|---|
| Beef liver, braised, 3 ounces | 215 | 54 |
| Spinach, boiled, ½ cup | 131 | 33 |
| Black-eyed peas (cowpeas), boiled, ½ cup | 105 | 26 |
| Breakfast cereals, fortified with 25% of the DV† | 100 | 25 |
| Rice, white, medium-grain, cooked, ½ cup† | 90 | 22 |
| Asparagus, boiled, 4 spears | 89 | 22 |
| Brussels sprouts, frozen, boiled, ½ cup | 78 | 20 |
| Spaghetti, cooked, enriched, ½ cup† | 74 | 19 |
| Lettuce, romaine, shredded, 1 cup | 64 | 16 |
| Avocado, raw, sliced, ½ cup | 59 | 15 |
| Spinach, raw, 1 cup | 58 | 15 |
| Broccoli, chopped, frozen, cooked, ½ cup | 52 | 13 |
| Mustard greens, chopped, frozen, boiled, ½ cup | 52 | 13 |
| Bread, white, 1 slice† | 50 | 13 |
| Green peas, frozen, boiled, ½ cup | 47 | 12 |
| Kidney beans, canned, ½ cup | 46 | 12 |
| Wheat germ, 2 tablespoons | 40 | 10 |
| Tomato juice, canned, ¾ cup | 36 | 9 |
| Crab, Dungeness, 3 ounces | 36 | 9 |
| Orange juice, ¾ cup | 35 | 9 |
| Turnip greens, frozen, boiled, ½ cup | 32 | 8 |
| Peanuts, dry roasted, 1 ounce | 27 | 7 |
| Orange, fresh, 1 small | 29 | 7 |
| Papaya, raw, cubed, ½ cup | 27 | 7 |
| Banana, 1 medium | 24 | 6 |
| Yeast, baker’s, ¼ teaspoon | 23 | 6 |
| Egg, whole, hard-boiled, 1 large | 22 | 6 |
| Cantaloupe, raw, cubed, ½ cup | 17 | 4 |
| Vegetarian baked beans, canned, ½ cup | 15 | 4 |
| Fish, halibut, cooked, 3 ounces | 12 | 3 |
| Milk, 1% fat, 1 cup | 12 | 3 |
| Ground beef, 85% lean, cooked, 3 ounces | 7 | 2 |
| Chicken breast, roasted, 3 ounces | 3 | 1 |
Footnotes: * DV = Daily Value. The U.S. Food and Drug Administration (FDA) developed Daily Value (DVs) to help consumers compare the nutrient contents of foods and dietary supplements within the context of a total diet. The DV (Daily Value) for folate is 400 mcg DFE for adults and children aged 4 years and older, where mcg DFE = mcg naturally occurring folate + (1.7 x mcg folic acid) 17. The labels must list folate content in mcg DFE per serving and if folic acid is added to the product, they must also list the amount of folic acid in mcg in parentheses. The FDA does not require food labels to list folate content unless folic acid has been added to the food. Foods providing 20% or more of the DV are considered to be high sources of a nutrient, but foods providing lower percentages of the DV also contribute to a healthful diet.
† Fortified with folic acid as part of the folate fortification program.
The U.S. Department of Agriculture’s FoodData Central lists the nutrient content of many foods and provides a comprehensive list of foods containing folate arranged by nutrient content and by food name.
[Source 1 ]Can food alone provide me with enough folate?
Yes, many people get enough folic acid from food alone. Some foods have high amounts of folic acid. For example, many breakfast cereals have 100% of the recommended daily value (400 micrograms) of folic acid in each serving. Check the label to be sure.
Some women, especially women who could get pregnant, may not get enough folic acid from food. African-American women and Mexican Americans are also at higher risk for not getting enough folic acid each day. Talk to your doctor or nurse about whether you should take a vitamin to get the 400 micrograms of folic acid you need each day. (source 3).
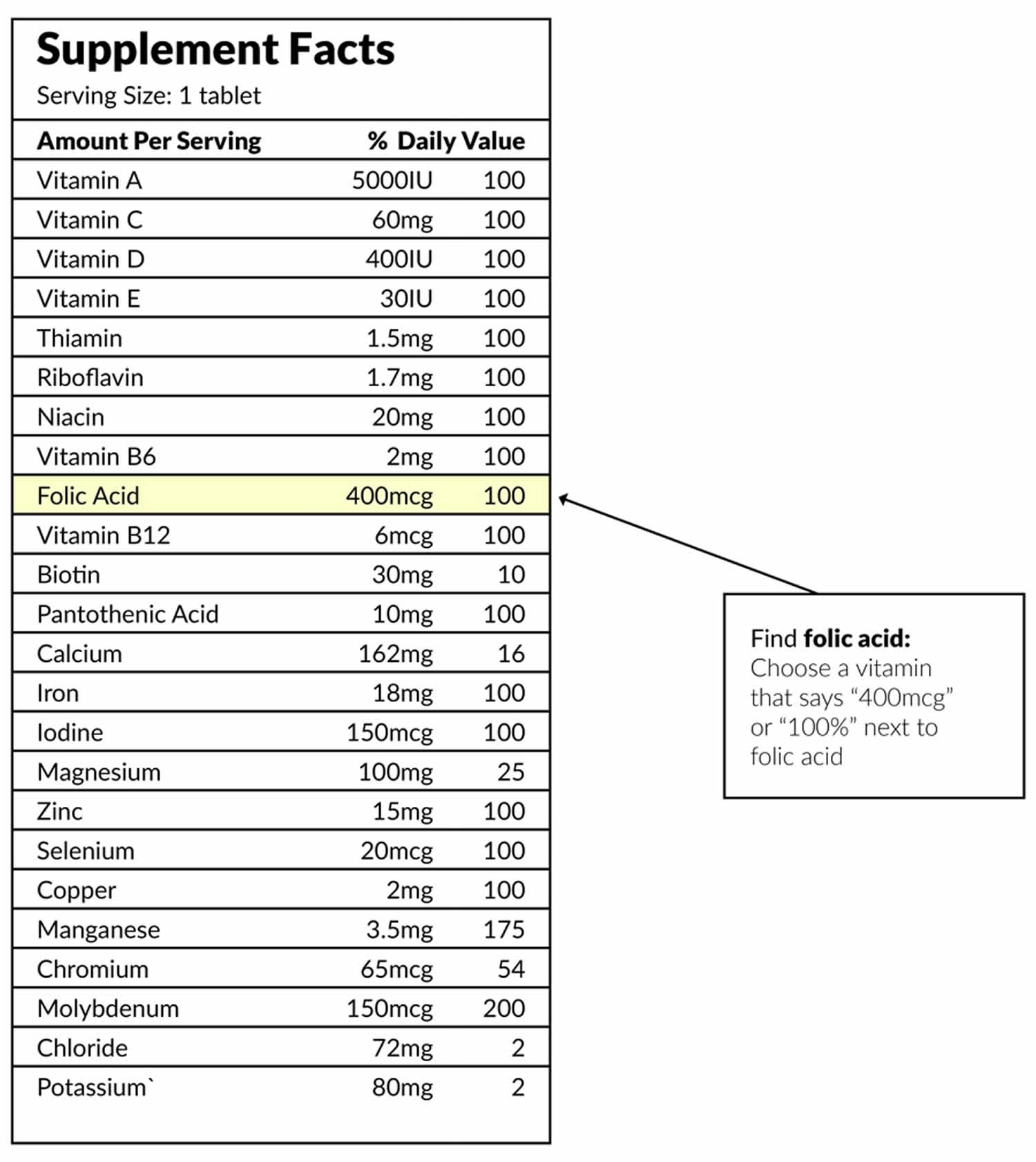
What should I look for when buying Vitamin Supplement with Folic Acid?
Look for “USP” or “NSF” on the label when choosing vitamins. These “seals of approval” mean the pills are made properly and have the amounts of vitamins it says on the label. Also, make sure the pills have not expired. If the bottle has no expiration date, do not buy it.
Ask your pharmacist for help with selecting a vitamin or folic acid-only pill. If you are pregnant and already take a daily prenatal vitamin, you probably get all the folic acid you need. Check the label to be sure.
Vitamin label
Check the “Supplement Facts” label to be sure you are getting 400 to 800 micrograms (mcg) of folic acid 37.
How do I know I’m getting enough folate?
Most people in the United States get enough folate. However, certain groups of people are more likely than others to have trouble getting enough folate:
- Teen girls and women aged 14–30 years (especially before and during pregnancy).
- Non-Hispanic black women.
- People with malabsorption disorders such as celiac disease, tropical sprue or inflammatory bowel disease
- People with alcohol use disorder
- Women of childbearing age
- Pregnant women
- People who eat overcooked fruits and vegetables. Folate can be easily destroyed by heat. Heating during cooking destroys folic acid 2.
- Certain medicines (such as phenytoin, sulfasalazine, or trimethoprim-sulfamethoxazole)
- Eating an unhealthy diet that does not include enough fruits and vegetables
- Kidney dialysis
What happens if you don’t get enough folate?
Folate deficiency is rare in the United States, but some people get barely enough. Getting too little folate can result in megaloblastic anemia, which causes weakness, fatigue, trouble concentrating, irritability, headache, heart palpitations, and shortness of breath. Folate deficiency can also cause open sores on the tongue and inside the mouth as well as changes in the color of the skin, hair, or fingernails.
Women who don’t get enough folate are at risk of having babies with neural tube defects, such as spina bifida. Folate deficiency can also increase the likelihood of having a premature or low-birth-weight baby.
Can folate be harmful?
Folate that is naturally present in food is not harmful. Folic acid in supplements and fortified foods, however, should not be consumed in amounts above the upper limit, unless recommended by a health care provider.
Taking large amounts of folic acid might hide a vitamin B12 deficiency. Folic acid can correct the anemia but not the nerve damage caused by vitamin B12 deficiency 1. This can lead to permanent damage of the brain, spinal cord, and nerves. Excess intake of folate, especially in the elderly population high intakes of folate supplements might “mask” vitamin B12 deficiency until its neurological consequences become irreversible 1, 2.
High doses of folic acid might also increase the risk of colorectal cancer and possibly other cancers in some people 2. Concerns have also been raised that high folic acid intakes might accelerate the progression of preneoplastic lesions, increasing the risk of colorectal and possibly other cancers in certain individuals 51, 52, 53. In addition, folic acid from supplements intakes of 1,000 mcg per day or more during the periconception period have been associated with lower scores on several tests of cognitive development in children at ages 4–5 years than in children of mothers who took 400 mcg to 999 mcg 54.
Some population groups are at risk of obtaining excessive folic acid. About 5% of men and women aged 51-70 and men aged 71 and older have folic acid intakes exceeding the Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) of 1,000 mcg per day, primarily because of the folic acid they obtain from dietary supplements 42. Furthermore, 30% to 66% of children aged 1 to 13 years who take folic acid-containing supplements have intakes of folic acid from both fortified food and dietary supplements exceeding the Tolerable Upper Intake Level (UL) of 300–600 mcg per day 43. Almost all children aged 1 to 8 years who consume at least 200 mcg/day folic acid from dietary supplements have total intakes that exceed the Tolerable Upper Intake Level (UL) 22. Little is known about the long-term effects of high folic acid doses in children 45.
Intakes of folic acid that exceed the body’s ability to reduce it to tetrahydrofolate (THF) lead to unmetabolized folic acid in the body, which has been linked to reduced numbers and activity of natural killer cells, suggesting that it could affect the immune system 55, 56. In addition, some scientists have hypothesized that unmetabolized folic acid might be related to cognitive impairment among older adults 57. These potential negative health consequences are not well understood and warrant further research 1, 4, 58.
Studies have found unmetabolized folic acid in blood from children, adolescents, and adults 59, 60; breastmilk 61; and cord blood from newborns 62, 63. Limited research suggests that single doses of 300 mcg or 400 mcg folic acid (a common amount in folic acid-containing supplements or servings of fortified foods, such as breakfast cereals) result in detectable serum levels of unmetabolized folic acid, whereas doses of 100 mcg or 200 mcg do not 64, 65. In addition, a dose-frequency interaction appears to occur in which smaller amounts of folic acid consumed more frequently produce higher unmetabolized folic acid concentrations than the same total dose consumed in larger, less frequent amounts 66.
Based on the metabolic interactions between folate and vitamin B12, the Food and Nutrition Board established a Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) for the synthetic forms of folate available in dietary supplements and fortified foods (Table 3) 5. The Food and Nutrition Board did not establish a Tolerable Upper Intake Level (UL) for folate from food because high intakes of folate from food sources have not been reported to cause adverse effects 5. Thus, unlike the Recommended Dietary Allowances (RDAs), the Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) are in mcg, not mcg DFE. For folic acid, 1,000 mcg is equivalent to 1,667 mcg DFE because 0.6 mcg folic acid = 1 mcg DFE 66. The Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) do not apply to individuals taking high doses of supplemental folate under medical supervision 5.
Can you get too much folic acid?
Yes, you can get too much folic acid, but only from man-made products such as multivitamins and fortified foods, such as breakfast cereals. You can’t get too much from foods that naturally contain folate.
You should not get more than 1,000 micrograms of folic acid a day, unless your doctor prescribes a higher amount. Too much folic acid can hide signs that you lack vitamin B12, which can cause nerve damage. (source 74).
Folate and Disease Prevention
Folate and folic acid are used for preventing and treating low blood levels of folate (folate deficiency) and high blood levels of homocysteine (homocysteinemia). Women who are pregnant or might become pregnant take folic acid to prevent serious birth defects such as spina bifida. Folic acid is also used for many other conditions including depression, stroke, decline in memory and thinking skills, and many others.
Neural tube defects
Neural tube defects result in malformations of the spine (spina bifida), skull, and brain (anencephaly). They are the most common major congenital malformations of the central nervous system and result from a failure of the neural tube to close at either the upper or lower end on days 21 to 28 after conception 75, 76. The prevalence rate of spina bifida and anencephaly (the two most common types of neural tube defects) in the United States is 5.5 to 6.5 per 10,000 births 77.
Because of folate’s role in the synthesis of DNA and other critical cell components, folate is especially important during phases of rapid cell growth 78. Although the mechanism has not been fully established, clinical trial evidence shows that adequate periconceptional folic acid consumption by women prevents a substantial proportion of neural tube defects 75, 76, 79, 80.
Since 1998, when mandatory folic acid fortification began in the United States, neural tube defect rates have declined by 28% 75. However, significant racial and ethnic disparities persist. Neural tube defect prevalence rates are highest among Hispanic women and lowest among non-Hispanic black women. Factors that might contribute to these disparities include differences in dietary and supplement-taking practices 81 as well as factors other than folate status—such as maternal diabetes, obesity, and intake of other nutrients (e.g., vitamin B12)—which are also believed to affect the risk of neural tube defects 80, 82, 83, 84. In addition, women with the 677C>T MTHFR polymorphism—which is more common in Hispanics than Caucasians, Asians, and African Americans—might have an increased risk of neural tube defects 4, 6, 85, 16. Another consideration is the fact that the data on neural tube defect prevalence rates were collected before 2016, when FDA approved the voluntary addition of folic acid to corn masa flour 71, an ingredient commonly consumed by Hispanic populations. Whether this policy change has affected the disparities in neural tube defect rates between Hispanic women and other populations is not yet known.
Because approximately 50% of pregnancies in the United States are unplanned, adequate folate status is especially important during the periconceptional period before a woman might be aware that she is pregnant. The Food and Nutrition Board advises women capable of becoming pregnant to “consume 400 mcg of folic acid daily from supplements, fortified foods, or both in addition to consuming food folate from a varied diet” 5. The U.S. Public Health Service and CDC have published similar recommendations 27. Consuming 400 mcg/day folic acid helps prevent neural tube defects, even in people with the 677C>T MTHFR polymorphism 86.
The authors of a 2017 systematic review concluded that folic acid supplementation protected users from neural tube defects in studies conducted before food fortification with folic acid began in the United States 87. Although studies conducted since that time do not demonstrate a clear protective association (possibly because of food fortification effects, study design flaws, or inadequate sample sizes) 87, the U.S. Preventive Services Task Force recommends that all women who are planning to become or capable of becoming pregnant take a daily supplement containing 400 to 800 mcg folic acid starting least 1 month before conception and continuing through the first 2 to 3 months of pregnancy 88.
The Food and Nutrition Board has not issued recommendations for women who have given birth to a child with an neural tube defect and plan to become pregnant again. However, other experts recommend that these women obtain 4,000 to 5,000 mcg supplemental folic acid daily starting at least 1 to 3 months before conception and continuing for 2½ to 3 months after conception 76, 89. These doses exceed the Tolerable Upper Intake Level (the maximum daily intake unlikely to cause adverse health effects) and should be taken only under medical supervision 89.
Preterm birth, congenital heart defects, and other congenital anomalies
According to observational studies, folic acid supplementation might increase mean gestational age and lower the risk of preterm birth 4, 90. In addition, folic acid in combination with a multivitamin supplement helps minimize the risk of congenital heart defects, possibly because cardiac tissue development depends on cells that require large amounts of folate 4, 75.
The authors of a large population-based cohort study of about 98% of all births in Canada from 1990 to 2011 concluded that folic acid fortification of foods was associated with an 11% reduction in the rate of nonchromosomal congenital heart defects 91. In a population-based case-control study in Atlanta involving 3,987 infants, congenital heart defects were 24% less common in the infants of women who took multivitamins containing folic acid during the periconceptional period than in the infants of women who did not 92. A case-control study in California in 866 infants had similar results 93. However, it is not possible to determine whether the findings from these studies could be attributed to components of multivitamins other than folic acid.
Studies have also found associations between the use of folic acid in combination with multivitamin supplements and reduced occurrence at birth of urinary tract anomalies, oral facial clefts, limb defects, and hydrocephalus, but the results of these studies have been inconsistent 5, 75.
Additional research is needed to fully understand the extent to which maternal consumption of folic acid might affect the risk of these adverse birth outcomes. However, folic acid’s established role in preventing neural tube defects—and possibly other birth defects—underscores its importance during the periconceptional period.
Autism spectrum disorder
Autism spectrum disorder (autism) is a neurodevelopmental disorder characterized by difficulty communicating and interacting with other people, limited interests, and repetitive behaviors 94. The classification and diagnosis of autism spectrum disorder was changed in 2013 to include conditions previously known as autistic disorder, Asperger’s syndrome, and pervasive developmental disorder not otherwise specified 94. The causes of autism spectrum disorder are not clear, but genetic and environmental factors (including infections) and prenatal exposure to certain drugs, pollutants, and pesticides are believed to play a role 95, 96, 97, 98.
Emerging evidence suggests that periconceptional folic acid supplementation might reduce the risk of autism spectrum disorder or mitigate the potentially increased risk of autism spectrum disorder from prenatal exposure to certain drugs and toxic chemicals 99, 100, 101. The mechanism of these potential benefits is unknown, but it might be related to folic acid’s role in DNA methylation, which, in turn, can affect neurodevelopment 99, 100, 101.
Some, but not all, observational studies have shown associations between maternal use of folic acid and/or multivitamin supplements before and/or during pregnancy and lower risk of autism spectrum disorder in the women’s offspring. For example, the prospective Norwegian Mother and Child Cohort Study that included 85,176 children aged 3.3 to 10.2 years found that children of mothers who took up to 400 mcg per day folic acid during all of part of the time from 4 weeks before to 8 weeks after the start of pregnancy were 39% less likely to have autistic disorder than those whose mothers did not take the supplements 102. The results showed no significant associations, however, between supplementation and Asperger’s syndrome or pervasive developmental disorder not otherwise specified. In a U.S. population-based, case-control study of 837 children, those born to mothers who consumed a mean of 600 mcg folic acid per day or more from supplements and fortified breakfast cereals during the first month of pregnancy had a 38% lower risk of autism spectrum disorder than those of mothers who consumed less than 600 mcg per day 103. This association was strongest for mothers and children with the 677C>T MTHFR polymorphism. Similarly, a 2018 case-control cohort study of 45,300 Israeli children demonstrated a significantly decreased risk of autism spectrum disorder in children of mothers who took folic acid and/or multivitamin supplements before and/or during pregnancy 104. Conversely, a longitudinal, population-based cohort of 35,059 pregnant Danish women and their children found no association between periconceptional folic acid or multivitamin use and autism spectrum disorder 105.
Periconceptional use of folic acid might mitigate the potentially increased risk of autism spectrum disorder in children exposed to certain drugs and neurotoxins in utero 96, 97, 98. An analysis of data from the Norwegian Mother and Child Cohort Study, which included 104,946 children, found that children exposed to antiepileptic drugs (known to reduce folate in vivo) in utero were 5.9 to 7.9 times more likely to have autistic traits at ages 18 and 36 months if their mothers did not take folic acid periconceptionally than if they did 96. In addition, the severity of autistic traits was inversely associated with both maternal plasma folate concentrations and folic acid doses. Similarly, in a U.S. study of 712 children, mothers exposed to any indoor pesticide during pregnancy who had folic acid intakes of 800 mcg or more per day during the first month of pregnancy were 1.7 times more likely to have a child with autism spectrum disorder than women with the same folic acid intakes who were not exposed to indoor pesticides 97. The risk of autism spectrum disorder was even higher (2.5 times) if the women were exposed to indoor pesticides and had daily folic acid intakes of less than 800 mcg, suggesting that folic acid might attenuate the potentially increased risk of autism spectrum disorder from pesticide exposure.
Overall, the evidence to date suggests a possible inverse association between mothers’ periconceptional folic acid intakes and risk of autism spectrum disorder in their offspring. However, most, if not all, of the currently available data are observational, and confounding weakens the ability to demonstrate causal inference. Additional research and validation in other studies are needed before firm conclusions can be drawn.
Cleft lip and cleft palate
Maternal folate status during pregnancy may influence the risk of congenital anomalies called orofacial clefts, namely cleft lip with or without cleft palate 106. A population-based case-control study in Norway investigated the impact of folic acid supplements in mothers of 377 newborns with cleft lip with or without cleft palate, 196 with cleft palate only (cleft palate only) and 763 controls 107. Although dietary folate intakes or supplements (during the first three months of pregnancy) on their own did not significantly modify the risk of cleft lip with or without cleft palate, the study reported a 64% lower risk among women taking multivitamin and folic acid (≥400 mcg daily) supplements in addition to dietary folates. In the same population, polymorphisms in the cystathionine β-synthase (CBS) gene (c.699C>T) or MTHFR gene (c.677C>T; when folate intake was below 400 mcg/day) appeared protective, while other gene variants in the folate/one-carbon metabolism could not be linked to cleft lip with or without cleft palate 108, 109. However, a recent meta-analysis of 18 studies showed an elevation of cleft lip with or without cleft palate risk with the maternal 677T/T homozygosity 110. Additional studies are needed to evaluate the risk of cleft lip with or without cleft palate while integrating both genetic polymorphism and folate intake parameters. Epidemiological evidence supporting a role for folate in the risk of cleft palate only is lacking.
Adverse pregnancy outcomes
Low birth weight has been associated with increased risk of mortality during the first year of life and may also influence health outcomes during adulthood 111. A recent systematic review and meta-analysis of eight randomized controlled trials found a positive association between folic acid supplementation and birth weight; no association with length of gestation was observed 112. Additionally, a prospective cohort study of 306 pregnant adolescents associated low folate intakes and maternal folate status during the third trimester of pregnancy with higher incidence of small for gestational age births (birth weight <10th percentile)113. Moreover, the maternal c.677C>T MTHFR genotype and increased homocysteine concentrations, considered an indicator of functional folate deficiency, have been linked to lower birth weights 114.
Elevated blood homocysteine concentrations have also been associated with increased incidence of miscarriage and other pregnancy complications, including preeclampsia and placental abruption 115. A large retrospective study showed that plasma homocysteine in Norwegian women was strongly related to adverse outcomes and complications, including preeclampsia, premature delivery, and very low birth weight, in previous pregnancies 116. A recent meta-analysis of 51 prospective cohort studies linked the c.677C>T MTHFR variant with increased risk of preeclampsia in Caucasian and East Asian populations, reinforcing the notion that folate metabolism may play a role in the condition 117. A large multicenter, randomized, controlled trial, the Folic Acid Clinical Trial (FACT), has been initiated to evaluate whether the daily supplementation of up to 5.1 mg of folic acid throughout pregnancy could prevent preeclampsia and other adverse outcomes (e.g., maternal death, placental abruption, preterm delivery) in high-risk women 118. Adequate folate intake during pregnancy protects against megaloblastic anemia 119. A recent case-control study found a reduction in risk of autism spectrum disorders with daily folic acid consumption of 600 μg or more before and during pregnancy when mother and child carried the c.677C>T MTHFR genotype 103.
Thus, it is reasonable to maintain folic acid supplementation throughout pregnancy, even after closure of the neural tube, in order to decrease the risk of other problems during pregnancy. Moreover, recent systematic reviews of observational studies found no evidence of an association between folate exposure during pregnancy and adverse health outcomes in offspring, in particular childhood asthma and allergies 120, 121.
Cancer
Several epidemiological studies have suggested an inverse association between folate intakes and status and the risk of colorectal, lung, pancreatic, esophageal, stomach, cervical, ovarian, breast, bladder, and other cancers 4, 122, 58, 123. Research has not established the precise nature of folate’s effect on carcinogenesis, but scientists hypothesize that folate might influence cancer development through its role in one-carbon metabolism and subsequent effects on DNA replication and cell division 123, 124. Evidence also indicates that folate might play a dual role in cancer initiation and progression 125. That is, folate might suppress some types of cancer during the early stages of development, whereas high doses of folic acid taken after preneoplastic lesions have been established might promote cancer development and progression 125.
Results from clinical trials involving folic acid supplementation have been mixed. In addition, most trials have included other B-vitamins (frequently at doses well above RDA levels) and sometimes other nutrients, making it difficult to disentangle the effects, if any, of folic acid alone. For example, in a trial in France, 2,501 people with a history of cardiovascular disease received daily supplements of 560 mcg folic acid, 3 mg vitamin B6, and 20 mcg vitamin B12 for 5 years 126. The researchers found no association between B-vitamin supplementation and cancer outcomes. In a combined analysis of two trials in Norway (where foods are not fortified with folic acid), supplementation with 800 mcg/day folic acid plus 400 mcg/day vitamin B12 for a median of 39 months in 3,411 people with ischemic heart disease increased cancer incidence rates by 21% and cancer mortality rates by 38% compared with no supplementation 127. Findings from these Norwegian trials have raised concerns about folic acid supplementation’s potential to raise cancer risk.
The most thorough research has focused on folate’s effect on the development of colorectal cancer and its precursor, adenoma 128. Several epidemiological studies have found inverse associations between high dietary folate intakes and the risk of colorectal adenoma and cancer 129, 130, 131. For example, in the NIH-AARP Diet and Health Study, a cohort study of more than 525,000 people aged 50 to 71 years in the United States, individuals with total folate intakes of 900 mcg/day or higher had a 30% lower risk of colorectal cancer than those with intakes lower than 200 mcg/day 130. Other studies, however, have found no significant associations between dietary folate intakes 132, 133 or circulating folate concentrations 134, 135 and colorectal cancer risk.
Several clinical trials have examined whether supplemental folic acid (sometimes in combination with other B-vitamins) reduces the risk of colorectal adenoma in individuals with or without a history of adenoma. In the Women’s Antioxidant and Folic Acid Cardiovascular Study, which included 1,470 older women at high risk of cardiovascular disease, daily supplementation with 2,500 mcg folic acid, 50 mg vitamin B6, and 1,000 mcg vitamin B12 did not affect rates of colorectal adenoma during 7.3 years of intervention and about 2 years of postintervention follow-up 136. A pooled analysis of three large clinical trials (one in Canada, one in both the United States and Canada, and one in both the United Kingdom and Denmark) found that folic acid supplementation for up to 3.5 years neither increased nor decreased adenoma recurrence rates in people with a history of adenoma 137. However, in one of the studies included in the analysis, folic acid supplementation (1,000 mcg/day) significantly increased the risks of having three or more adenomas and of noncolorectal cancers, although it had no effect on colorectal cancer risk 138.
Folic acid supplementation also had no effect on the risk of all cancer types combined in the pooled analysis of three clinical trials cited above 137. Similarly, a meta-analysis of 13 randomized trials showed no statistically significant effects of folic acid supplementation (median daily dose of 2,000 mcg) over an average treatment period of 5.2 years on overall cancer incidence or the incidence of colorectal, lung, breast, prostate, or other cancers 139.
Some research has found associations between folic acid supplementation and increased cancer risk. In a randomized clinical trial investigating osteoporotic fracture incidence in 2,919 participants aged 65 years or older with elevated homocysteine levels, those who received 400 mcg folic acid plus 500 mcg vitamin B12 and 600 IU vitamin D3 for 2 years reported a significantly higher cancer incidence, especially of colorectal and other gastrointestinal cancers, than those who received only 600 IU vitamin D3 140. In addition, a 2018 prospective study found that folic acid intake from fortified foods and supplements was positively associated with a risk of cancer recurrence among 619 patients with non–muscle-invasive bladder cancer, whereas natural folate intakes showed no significant association 141. Higher plasma folate concentrations have also been associated with an increased risk of breast cancer in women with a BRCA1 or BRCA2 mutation 142. A secondary analysis of the study by Cole and colleagues 138 found that folic acid supplementation significantly increased the risk of prostate cancer 142. Subsequent research has shown an association between increased cancer cell proliferation and higher serum folate concentrations in men with prostate cancer 143. A meta-analysis of six randomized controlled trials that included a total of 25,738 men found that the risk of prostate cancer was 24% higher in men receiving folic acid supplements than those taking a placebo 144.
The mixed findings from clinical trials, combined with evidence from laboratory and animal studies indicating that high folate status promotes tumor progression, suggest that folate might play dual roles in cancer risk, depending on the dosage and timing of the exposure. Modest doses of folic acid taken before preneoplastic lesions are established might suppress cancer development in healthy tissues, whereas high doses taken after the establishment of preneoplastic lesions might promote cancer development and progression 145, 53, 51. This hypothesis is supported by a 2011 prospective study that found an inverse association between folate intake and risk of colorectal cancer only during early pre-adenoma stages 146.
A 2015 expert panel convened by the National Toxicology Program and the National Institutes of Health Office of Dietary Supplements concluded that folic acid supplements do not reduce cancer risk in people with adequate baseline folate status 147. The panel also determined that the consistent findings from human studies that supplemental folic acid has an adverse effect on cancer growth justify additional research on the effects of folic acid supplementation on cancer risk 147. Several important questions about these effects remain, including the dose and timing of folic acid supplementation that might exert tumor-promoting effects and whether this effect is specific to synthetic folic acid or other forms of folate 125.
Overall, the evidence to date indicates that adequate dietary folate intake might reduce the risk of some forms of cancer. However, the effects of supplemental folic acid on cancer risk are unclear, especially among individuals with a history of colorectal adenomas or other forms of cancer. More research is needed to fully understand how dietary folate and supplemental folic acid affect cancer risk and whether their effects differ by timing of exposure.
Cardiovascular disease and stroke
An elevated homocysteine level has been associated with an increased risk of cardiovascular disease 4. Folate and other B vitamins are involved in homocysteine metabolism, and researchers have hypothesized that these micronutrients reduce cardiovascular disease risk by lowering homocysteine levels 4, 50.
Folic acid and vitamin B12 supplements lower homocysteine levels. However, these supplements do not actually decrease the risk of cardiovascular disease, although they appear to provide protection from stroke 148, 149, 150, 151. For example, in 5,442 U.S. women aged 42 or older who were at high risk of cardiovascular disease, daily supplements containing 2,500 mcg folic acid, 1 mg vitamin B12, and 50 mg vitamin B6 for 7.3 years did not reduce the risk of major cardiovascular events 152. In a substudy of 300 participants, the supplementation also had no significant effects on biomarkers of vascular inflammation 151, but it did lower homocysteine levels by a mean of 18.5% 152. Another clinical trial included 5,522 patients aged 55 years or older with vascular disease or diabetes from various countries (including the United States and Canada) that had a folic acid fortification program and some that did not 153. Patients received 2,500 mcg folic acid plus 50 mg vitamin B6 and 1 mg vitamin B12 or placebo for an average of 5 years. Compared with placebo, treatment with B vitamins significantly decreased homocysteine levels but did not reduce the risk of death from cardiovascular causes or myocardial infarction. Supplementation did, however, significantly reduce the risk of stroke by 25%.
In a large trial in regions of China without folic acid fortification among 20,702 adults with hypertension but no history of stroke or myocardial infarction, supplementation with 800 mcg folic acid plus 10 mg enalapril (used to treat high blood pressure) for a median of 4.5 years significantly reduced the risk of stroke by 21% compared with enalapril alone 149. The effect was more pronounced in participants with the lowest baseline levels of plasma folate. An analysis of 10,789 participants from this trial found that folic acid supplementation significantly reduced the risk of stroke by 73% among those who had a low platelet count and an elevated homocysteine level (increasing their risk of stroke) but had no significant effect on participants with a high platelet count and low homocysteine level 154. These findings suggest that folic acid supplementation might primarily benefit those with insufficient folate levels, which are less common in countries, such as the United States, with folic acid fortification 155.
The authors of a 2012 meta-analysis of 19 randomized controlled trials that included 47,921 participants concluded that B-vitamin supplementation has no effect on the risk of cardiovascular disease, myocardial infarction, coronary heart disease, or cardiovascular death, although it does reduce the risk of stroke by 12% 148. Likewise, the authors of the third update of a Cochrane review of the effects of homocysteine-lowering interventions on cardiovascular events concluded that folic acid supplementation alone or in combination with vitamin B6 and vitamin B12 does not affect the risk of myocardial infarction or death from any cause, but it does reduce the risk of stroke 156. Three other meta-analyses have also found that folic acid is effective for preventing stroke, especially in populations exposed to no or partial folic acid fortification 150, 157, 158.
Overall, the available evidence suggests that supplementation with folic acid alone or in combination with other B-vitamins reduces the risk of stroke, especially in populations with low folate status, but does not affect other cardiovascular endpoints.
Dementia, cognitive function, and Alzheimer’s disease
Most observational studies conducted to date have shown positive associations between elevated homocysteine levels and the incidence of both Alzheimer’s disease and dementia 159, 160, 161, 162, 163, 164, 164, 165. Scientists hypothesize that elevated homocysteine levels might have a negative effect on the brain via numerous mechanisms, including cerebrovascular ischemia leading to neuronal cell death, activation of tau kinases leading to tangle deposition, and inhibition of methylation reactions 164. Some, but not all, observational studies have also found correlations between low serum folate concentrations and both poor cognitive function and higher risk of dementia and Alzheimer’s disease 164, 161, 162, 166.
Despite this evidence, most clinical trial research has not shown that folic acid supplementation affects cognitive function or the development of dementia or Alzheimer’s disease, even though supplementation lowers homocysteine levels. In one randomized, double-blind, placebo-controlled trial in the Netherlands, 195 people aged 70 years or older with no or moderate cognitive impairment received 400 mcg folic acid plus 1 mg vitamin B12; 1 mg vitamin B12; or placebo for 24 weeks 167. Treatment with folic acid plus vitamin B12 reduced homocysteine concentrations by 36% but did not improve cognitive function. In another clinical trial in older adults (mean age 74.1 years) with elevated homocysteine levels, supplementation with 400 mcg folic acid plus 500 mcg vitamin B12 and 600 IU vitamin D3 for 2 years lowered homocysteine levels but did not affect cognitive performance compared with 600 IU vitamin D3 alone 168.
As part of the Women’s Antioxidant and Folic Acid Cardiovascular Study, 2,009 U.S. women aged 65 years or older at high risk of cardiovascular disease were randomly assigned to receive daily supplements containing 2,500 mcg folic acid plus 1 mg vitamin B12 and 50 mg vitamin B6 or placebo 169. After an average of 1.2 years, B-vitamin supplementation did not affect mean cognitive change from baseline compared with placebo. However, in a subset of women with a low baseline dietary intake of B vitamins, supplementation significantly slowed the rate of cognitive decline. In a trial that included 340 individuals in the United States with mild-to-moderate Alzheimer’s disease, daily supplements of 5,000 mcg folic acid plus 1 mg vitamin B12 and 25 mg vitamin B6 for 18 months did not slow cognitive decline compared with placebo 170.
A secondary analysis of a study in Australia (which did not have mandatory folic acid fortification at the time of the study) found that daily supplementation with 400 mcg folic acid plus 100 mcg vitamin B12 for 2 years improved some measures of cognitive function, particularly memory, in 900 adults aged 60 to 74 years who had depressive symptoms 171. Another meta-analysis included 11 randomized controlled trials in over 20,000 older adults (mean age 60–82 years) that administered 400 to 2,500 mcg folic acid plus 20–1,000 mcg vitamin B12 in 10 trials and 3–50 mg vitamin B6 in 8 trials for 0.3 to 7.1 years. The supplementation significantly lowered homocysteine levels but did not affect cognitive aging, global cognitive function, or specific cognitive domains (including memory, speed, and executive function) 172.
Several large reviews have evaluated the effect of B vitamins on cognitive function. Most of the authors concluded that supplementation with folic acid alone or in combination with vitamins B12 or B6 does not appear to improve cognitive function in individuals with or without cognitive impairment 173, 174, 175, 176. Some noted, however, that when researchers took baseline homocysteine and B-vitamin status into account, B-vitamin supplementation slowed cognitive decline in individuals at high risk of cognitive decline 164, 165. For example, one trial in the Netherlands administered either 800 mcg folic acid or placebo daily for 3 years to 818 participants aged 50–70 years with elevated homocysteine levels (13 micromol/L or higher) and normal vitamin B12 levels 177. Folic acid supplementation reduced homocysteine concentrations by 26% and significantly improved global cognitive function, memory, and information processing speed compared with placebo, but it did not affect sensorimotor speed, complex speed, or word fluency.
Additional clinical trials are needed to better understand the effects of folic acid supplementation on cognitive function and cognitive decline.
Depression
Low folate status has been linked to depression and poor response to antidepressants in some, but not all, studies. The possible mechanisms are unclear but might be related to folate’s role in methylation reactions in the brain, neurotransmitter synthesis, and homocysteine metabolism 178, 179. However, secondary factors linked to depression, such as unhealthy eating patterns and alcohol use disorder, might also contribute to the observed association between low folate status and depression 180.
In an ethnically diverse population study of 2,948 people aged 15 to 39 years in the United States, serum and erythrocyte folate concentrations were significantly lower in individuals with major depression than in those who had never been depressed 180. An analysis of 2005-2006 NHANES data found that higher serum concentrations of folate were associated with a lower prevalence of depression in 2,791 adults aged 20 or older 179. The association was statistically significant in females, but not in males. However, another analysis showed no associations between folate intakes from both food and dietary supplements and depression among 1,368 healthy Canadians aged 67–84 years 178. Results from a study of 52 men and women with major depressive disorder showed that only 1 of 14 participants with low serum folate levels responded to antidepressant treatment compared with 17 of 38 with normal folate levels 181.
A few studies have examined whether folate status affects the risk of depression during pregnancy or after childbirth. A systematic review of these studies had mixed results 182. One study included in the review among 709 women in Singapore found that compared with women with higher plasma folate concentrations (mean 40.4 nmol/L [17.8 ng/mL]) at 26–28 weeks’ gestation, those with lower plasma folate concentrations (mean 27.3 nmol/L [12.0 ng/mL]) had a significantly higher risk of depression during pregnancy but not after giving birth 183. Another study of 2,856 women in the United Kingdom found no significant associations between red blood cell folate levels or folate intakes from food and dietary supplements before or during pregnancy and postpartum depressive symptoms 184. More recently, a cohort study of 1,592 Chinese women found a lower prevalence of postpartum depression in women who took folic acid supplements for more than 6 months during pregnancy than in those who took them for less time 185.
Studies have had mixed results on whether folic acid supplementation might be a helpful adjuvant treatment for depression when used with traditional antidepressant medications. In a clinical trial in the United Kingdom, 127 patients with major depression were randomly assigned to receive either 500 mcg folic acid or placebo in addition to 20 mg of fluoxetine daily for 10 weeks 186. Although the effects in men were not statistically significant, women who received fluoxetine plus folic acid had a significantly greater improvement in depressive symptoms than those who received fluoxetine plus placebo. Another clinical trial in the United Kingdom randomized 475 adults with moderate to severe depression who were taking antidepressant medications to either 5,000 mcg folic acid or placebo daily for 12 weeks in addition to their antidepressants 187. Measures of depression did not improve in participants taking folic acid compared with those taking placebo. The authors of a systematic review and meta-analysis of four trials of folic acid (<5,000 mcg/day in two trials; =5,000 mcg/day in two trials) in combination with fluoxetine or other antidepressants in patients with major depressive disorder concluded that less than 5,000 mcg/day folic acid might be beneficial as an adjunct to serotonin reuptake inhibitor (SSRI) therapy 188. The authors noted, however, that this conclusion was based on low-quality evidence. Another meta-analysis of four clinical trials found that 500–10,000 mcg folic acid per day for 6–12 weeks as an adjunctive treatment did not significantly affect measures of depression compared with placebo 189.
Other studies have examined the effects of 5-MTHF supplementation as an adjuvant treatment to antidepressants, and results suggest that it might have more promise than folic acid 190, 191, 188. In a clinical trial in 148 adults with major depressive disorder, supplementation with 7,500 mcg/day 5-MTHF for 30 days followed by 15,000 mcg/day for another 30 days, both in conjunction with SSRI treatment, did not improve measures of depression compared with SSRI treatment plus placebo 192. However, in a subsequent trial with the same study design in 75 adults, supplementation with 15,000 mcg/day 5-MTHF plus SSRI treatment for the full 60 days did significantly improve depression compared with SSRI treatment plus placebo 192.
The authors of a systematic review and meta-analysis of three trials of 5-MTHF (<15,000 mcg/day in one trial, and 15,000 mcg/day in two trials) in combination with fluoxetine or other antidepressants, concluded that 15,000 mcg/day 5-MTHF might be an effective adjunct to SSRI therapy in patients with major depressive disorder, although they noted that this conclusion was based on low-quality evidence 188. In addition, evidence-based guidelines from the British Association for Psychopharmacology 190 and the Canadian Network for Mood and Anxiety Treatments 191 state that 5-MTHF might be effective as an adjunct to SSRI treatment for depressive disorders.
Additional research is needed to fully understand the association between folate status and depression. Although limited evidence suggests that supplementation with certain forms and doses of folate might be a helpful adjuvant treatment for depressive disorders, more research is needed to confirm these findings. In addition, many of the doses of folate used in studies of depression exceed the Tolerable Upper Intake Level (UL) and should be taken only under medical supervision.
Folic acid deficiency
Folate deficiency usually coexists with other nutrient deficiencies because of its strong association with poor diet, alcoholism, and malabsorptive disorders 8. Megaloblastic anemia, which is characterized by large, abnormally nucleated red blood cells, is the primary clinical sign of folate or vitamin B12 deficiency 8, 4. Megaloblastic anemia symptoms include weakness, fatigue, difficulty concentrating, irritability, headache, heart palpitations, and shortness of breath 5. Folate deficiency can also produce soreness in and shallow ulcerations on your tongue and inside your mouth; changes in your skin, hair, or fingernail pigmentation; gastrointestinal symptoms; and elevated blood concentrations of homocysteine 4, 5, 8, 25, 160.
Women with inadequate folate intakes are at increased risk of giving birth to infants with birth defects of the brain and spine (neural tube birth defects) such as spina bifida and anencephaly 193, 5. Inadequate maternal folate status has also been associated with low infant birth weight, preterm delivery, and fetal growth retardation 4, 115. The Centers for Disease Control and Prevention (CDC) recommends ALL women of child bearing age should get 400 micrograms (mcg) of folic acid every day in addition to consuming food with folate from a varied diet 194, 193, 27. Whilst taking a higher dose of folic acid of more than 400 mcg each day is not necessarily better to prevent neural tube defects, unless a doctor recommends taking more due to other health conditions 193. The CDC further recommends that women who have already had a pregnancy affected by a neural tube defect consume 4,000 mcg of folic acid each day one month before becoming pregnant and through the first 3 months of pregnancy 193.
Folic acid deficiency causes
Isolated folate deficiency is rare in the United States and folic acid deficiency can arise from multiple causes, including inadequate dietary intake. The body has about 1,000-20,000 mcg of folate stores, and adults need about 400 mcg/day to replenish the daily losses. Folate deficiency may take 8-16 weeks to become evident 2.
The following groups are among those most likely to be at risk of folate deficiency 1:
- People with alcohol use disorder
- Women of childbearing age
- Pregnant women
- People with malabsorption disorders such as celiac disease, tropical sprue or inflammatory bowel disease
- People who eat overcooked fruits and vegetables. Folate can be easily destroyed by heat. Heating during cooking destroys folic acid 2.
- Certain medicines (such as phenytoin, sulfasalazine, or trimethoprim-sulfamethoxazole)
- Eating an unhealthy diet that does not include enough fruits and vegetables
- Kidney dialysis
Folate is absorbed in the jejunum (the 2nd part of your small intestine) by active and passive transport mechanisms across the intestinal wall 2. Hence, diseases such as celiac disease, tropical sprue, inflammatory bowel disease, short bowel syndrome, amyloidosis, gastric bypass surgery, or mesenteric vascular insufficiency can inhibit folate absorption resulting in a deficiency. Elevated pH, as occurs in achlorhydria, can also lead to poor folate absorption.
Drugs such as methotrexate, phenytoin, sulfasalazine, and trimethoprim can antagonize folate utilization, inhibit its absorption or conversation to its active form resulting in folate deficiency 2. Congenital deficiencies of enzymes required in folate metabolism can lead to folate deficiency. Folic acid deficiency can occur subsequent to vitamin B12 deficiency due to an impairment of methionine synthase resulting in the trapping of folate as methyltetrahydrofolate whereby methylene tetrahydrofolate accumulates in serum leading to folate trap phenomenon and increased urinary excretion of folate.
Alcoholism is a significant cause of folate deficiency. Pregnancy, hemolytic anemia, and dialysis can also result in folate deficiency.
The following groups are among those most likely to be at risk of folate deficiency 1.
People with alcohol use disorder
People with alcohol use disorder frequently have poor-quality diets that contain insufficient amounts of folate 1. Moreover, alcohol interferes with folate absorption and hepatic uptake, accelerates folate breakdown, and increases its renal excretion 4, 58. An evaluation in Portugal, where the food supply is not fortified with folic acid, found low folate status in more than 60% of people with chronic alcoholism 195. Even moderate alcohol consumption of 240 ml (8 fluid ounces) red wine per day or 80 ml (2.7 fluid ounces) vodka per day for 2 weeks can significantly decrease serum folate concentrations in healthy men, although not to levels below the cutoff for folate adequacy of 3 ng/ml 196.
Women of childbearing age
All women capable of becoming pregnant should obtain adequate amounts of folate to reduce the risk of neural tube birth defects such as spina bifida and anencephaly and other birth defects 193, 88. However, some women of childbearing age get insufficient amounts of folate even if they take dietary supplements 42. Women of childbearing age should obtain 400 mcg/day folic acid from dietary supplements and/or fortified foods in addition to the folate provided by a varied diet 27.
Pregnant women
During pregnancy, demands for folate increase because of its role in nucleic acid synthesis 115. To meet this need, the Food and Nutrition Board increased the folate Recommended Dietary Allowance (the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals) from 400 mcg DFE/day for nonpregnant women to 600 mcg DFE/day during pregnancy 5. This level of intake might be difficult for some women to achieve through diet alone. The American College of Obstetricians and Gynecologists recommends a prenatal folic acid of at least 400 micrograms starting at least 1 month before pregnancy and during the first 12 weeks of pregnancy for most pregnant women to ensure that they obtain adequate amounts of folic acid and other nutrients 28. Women who have had a child with an neural tube birth defect should take 4 milligrams (4000 micrograms) of folic acid each day as a separate supplement at least 3 months before pregnancy and for the first 3 months of pregnancy 28. You and your ob-gyn or other obstetric care provider can discuss whether you need to supplement with more than 400 micrograms daily 28.
People with malabsorptive disorders
Several medical conditions increase the risk of folate deficiency. People with malabsorptive disorders—including tropical sprue, celiac disease, and inflammatory bowel disease—might absorb less folate than people without these disorders 8; for example, about 20–60% of patients with inflammatory bowel disease have folate deficiency 197. Diminished gastric acid secretion associated with atrophic gastritis, gastric surgery, and other conditions can also reduce folate absorption 8.
People with an methylenetetrahydrofolate reductase (MTHFR) polymorphism
People with a genetic polymorphism, MTHFR C677T (MTHFR 677 C>T or MTHFR 677 C→T), in the methylenetetrahydrofolate reductase (MTHFR) gene have a reduced ability to convert folate to one of its active forms, 5-MTHF, because the methylenetetrahydrofolate reductase (MTHFR) enzyme needed for this conversion is less active 12. About 25% of Hispanics, 10% of Caucasians and Asians, and 1% of African Americans are homozygous for the 677C>T MTHFR polymorphism 16. A gene variant is a change in a DNA sequence that is different from the expected DNA sequence. This means in MTHFR C677T at the 677 position in the MTHFR gene, “C” is the expected DNA base and “T” is the gene variant 198.
The methylenetetrahydrofolate reductase (MTHFR) polymorphism results in less biologically available 5-MTHF and, thus, reduced methylation potential, leading to elevated homocysteine levels and an increased risk of neural tube defects 11, 85. Although the research on the benefits of folate supplementation for people with this genetic polymorphism is inconclusive, some of these people might benefit from supplementation with 5-MTHF 11, 12. However, the Centers for Disease Control and Prevention (CDC) recommends 400 mcg/day of folic acid, not 5-MTHF, for people who could become pregnant, even if they have a 677C>T MTHFR polymorphism 86.
Folate deficiency prevention
The best way to get folate your body needs is to eat a balanced diet. Most people in the United States eat enough folic acid because it is plentiful in the food supply.
Folate occurs naturally in the following foods:
- Beans and legumes
- Citrus fruits and juices
- Dark green leafy vegetables such as spinach, asparagus, and broccoli
- Liver
- Mushrooms
- Poultry, pork, and shellfish
- Wheat bran and other whole grains
The recommended daily amount of folic acid for adults is 400 mcg of folate daily. Specific recommendations depend on a person’s age, sex, and other factors (such as pregnancy and lactation) (see Table 2). Many foods, such as fortified breakfast cereals, now have extra folic acid added to help prevent birth defects.
The Centers for Disease Control and Prevention (CDC) recommends ALL women of child bearing age should get 400 micrograms (mcg) of folic acid every day in addition to consuming food with folate from a varied diet 194, 193, 27. Whilst taking a higher dose of folic acid of more than 400 mcg each day is not necessarily better to prevent neural tube defects, unless a doctor recommends taking more due to other health conditions 193. The CDC further recommends that women who have already had a pregnancy affected by a neural tube defect consume 4,000 mcg (4 mg) of folic acid each day one month before becoming pregnant and through the first 3 months of pregnancy 193.
Folate deficiency symptoms
Folate deficiency signs and symptoms include the following 199:
- Glossitis (inflammation of the tongue). Glossitis is a problem in which your tongue is swollen and inflamed making makes the surface of the tongue appear smooth. The tongue may appear swollen, beefy, red, or shiny, usually around the edges and tips initially. Some patients complain of a sore tongue or pain upon swallowing.
- Angular stomatitis also may be observed. These oral lesions typically occur at the time when folate depletion is severe enough to cause megaloblastic anemia, although, occasionally, lesions may occur before the anemia.
- Gastrointestinal signs and symptoms such as anorexia, nausea, vomiting, abdominal pain, diarrhea, especially after meals. Anorexia also is common and, in combination with nausea, vomiting, abdominal pain, diarrhea, may lead to marked weight loss. However, an underlying malabsorption disorder could be causing these symptoms, as well as folate deficiency. The lack of folate itself may not be the culprit.
- Manifestations of anemia. Folate deficiency causes a shortage of healthy red blood cells or anemia. In folate-deficiency anemia, the red blood cells are abnormally large (megaloblastic anemia). If you have anemia, your blood does not carry enough oxygen to the rest of your body. Anemia can produce other symptoms, such as:
- Paleness
- Shortness of breath
- Cold hands and feet
- Headaches
- Dizziness
- Fast, slow, or uneven heartbeat
- Brittle nails or hair loss
- Strange food cravings (known as pica)
- Neuropsychiatric symptoms include cognitive impairment, dementia, and depression. These manifestations overlap with those of vitamin B12 deficiency.{ref50)
- Patchy skin hyperpigmentation, especially particularly at the dorsal surfaces of the fingers, toes, and creases of palms and soles.
- Low-grade fever
- In severe folate deficiency you can have low levels of white blood cells and platelets
- Lack of folate causes a number of pregnancy-related complications, including placenta abruptio, spontaneous abortion, neural tube defects, and severe language deficits in children.
Folate deficiency in pregnant women can result in birth defects known as neural tube defects (anencephaly and spina bifida), which underlies the strong recommendation for folic acid supplementation in women of reproductive age 200, 193. Folate is important to the growth of the fetus’s spinal cord and brain. The Recommended Dietary Allowance (RDA) for folate during pregnancy is 600 micrograms (mcg)/day.
People with folate deficiency may also have darkening of the skin and mucous membranes, particularly at the upper side surfaces of the fingers, toes, and creases of palms and soles 201. Distribution typically is patchy. Fortunately, the skin hyperpigmentation gradually should resolve after weeks or months of folate treatment 201.
A modest temperature elevation (< 102°F) is common in patients who are folate deficient, despite the absence of any infection 201. Although the underlying mechanism is obscure, the temperature typically falls within 24-48 hours of vitamin treatment and returns to normal within a few days 201.
Folate deficiency complications
Failure to provide folic acid supplementation to pregnant females may lead to spontaneous abortion and fetal abnormalities, including neural tube defects and increased risk of severe language delay in the child 202.
Providing only folic acid supplementation to a patient who has cobalamin (vitamin B12) deficiency may lead to development of irreversible neuropathies 202.
Folate deficiency diagnosis
Folate deficiency can be diagnosed with a blood test. Pregnant women commonly have this blood test at prenatal checkups.
Medical history
In folate deficiency, the patient’s history is important because it may reveal the underlying reason for the folate deficiency. Very often, a patient presents with a history of excessive alcohol intake with concurrent poor diet intake. Other patients may be pregnant or lactating; may take certain drugs, such as phenytoin, sulfonamides, or methotrexate; may have chronic hemolytic anemia; or may have underlying malabsorption.
Blood test
Initial laboratory tests should include a complete blood count (CBC) and a peripheral blood smear. Laboratory tests in folic acid deficiency would reveal anemia, manifesting as a decrease in hemoglobin and hematocrit levels. The mean corpuscular volume (MCV) would be increased to a level greater than 100 consistent with a diagnosis of macrocytic anemia. In addition, a peripheral blood smear would show macrocytic red blood cells (RBCs)and/or megaloblasts and hypersegmented neutrophils. Ordering serum vitamin B12 (cobalamin) and folate levels can help differentiate between the two.
Serum folate and serum cobalamin testing
Ruling out vitamin B12 (cobalamin) deficiency is very important because deficiency of folic acid and vitamin B12 produce overlapping neurologic manifestations, and both cause megaloblastic anemia, but folate treatment will not improve neurologic abnormalities due to cobalamin deficiency 203. The reference range for serum vitamin B12 (cobalamin) is 200-900 pg/mL 204.
The usual reference range of serum folate is 2.5-20 ng/mL 204. By statistical definition, 2-5% of healthy individuals will have a serum folate level of less than 2.5 ng/mL; hence, the serum folate level cannot be used alone to establish the diagnosis of folate deficiency 204. Therefore, the serum folate test is definitive only when the level is greater than 5.0 ng/mL, which rules out folate deficiency. Otherwise, additional follow-up tests include serum homocysteine (reference range 5-16 mmol/L), which is elevated in vitamin B12 and folate deficiency, and serum methylmalonic acid (MMA) (reference range 70-270 mmol/L), which is elevated in vitamin B12 (cobalamin) deficiency only.
In most cases, serum folate testing alone is sufficient for assessment of folate status, but if there is strong clinical suspicion of folate deficiency and the serum folate level is normal and vitamin B12 (cobalamin) deficiency has been ruled out, the red blood cell (RBC) folate level may be measured 205. Red blood cell folate levels tend to reflect long-term folate status rather than acute changes in folate that are reflected in serum folate levels. However, many confounding factors, such as transfused red cells, can make this unreliable as a test for folate deficiency states 204.
Folate deficiency can be confirmed with a normal vitamin B12 and methylmalonic acid (MMA) level and elevated homocysteine levels, while vitamin B12 deficiency can be confirmed with elevated methylmalonic acid (MMA) and homocysteine levels and low vitamin B12 levels 18. Red blood cell folate levels are a very useful index of body stores and can help access the duration of folate deficiency 206, 203.
Other than folate or vitamin B12 (cobalamin) deficiency, the only other confounding causes for elevation of these compounds include kidney failure, intravascular volume depletion, and some rare inborn errors of metabolism involving folate or cobalamin-dependent enzymes.
Bone marrow biopsy
Bone marrow biopsy and aspirate is not required for evaluation of vitamin B12 or folate deficiency, but if done for other reasons in patients with folate deficiency may show hypercellular bone marrow with a megaloblastic maturation of cells 207. This cannot be differentiated from changes observed with vitamin B12 deficiency.
Folate deficiency treatment
All patients with folate deficiency should be offered supplemental folic acid for the correction of the deficiency. The dosage of folic acid needed to prevent or reverse folate deficiency varies with the clinical circumstances, as follows 205:
- Folate-deficient megaloblastic anemia due to dietary insufficiency or antiepileptic drugs: 5 mg/day folic acid for 4 months
- Malabsorption: Up to 15 mg/day folic acid for 4 months
- Chronic hemolytic states and kidney dialysis: for prophylaxis, 5 mg folic acid daily to weekly, depending on the patient’s diet and rate of hemolysis
Typically, oral folic acid (1 to 5 mg daily) suffices to treat folate deficiency 18. Intravenous, subcutaneous, or intramuscular formulations of folic acid can be used for patients unable to tolerate oral medications 2. Folinic acid also called leucovorin, a reduced form of folate, is primarily used to prevent the toxicities of methotrexate 2. The duration of therapy depends on whether the cause of the initial folate deficiency persists. Patients with malabsorption or short gut syndromes may typically require long-term treatment 2.
In patients who have a concomitant vitamin B12 deficiency, it is imperative to replenish vitamin B12 as well 2. Folate treatment alone does not improve neurological symptoms and signs due to B12 deficiency, which, if untreated, may likely progress and cause permanent neurological damage 206.
All patients should be encouraged to a diet rich in fruits and vegetables. Referral to a dietician may be indicated to ensure that the patient has appropriate dietary intake of folic acid. Fruits, vegetables, and fortified foods constitute the primary dietary source of folic acid 208.
Table 2 lists the current Recommended Dietary Allowances (RDAs) for folate as mcg of dietary folate equivalents (DFEs). The Recommended Dietary Allowance (RDA) is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals. The Food and Nutrition Board at the National Academies of Sciences, Engineering, and Medicine developed dietary folate equivalents (DFEs) to reflect the higher bioavailability of folic acid than that of food folate 5. At least 85% of folic acid is estimated to be bioavailable when taken with food, whereas only about 50% of folate naturally present in food is bioavailable 5, 4. Based on these values, the Food and Nutrition Board defined dietary folate equivalent (DFE) as follows:
- 1 mcg DFE = 1 mcg food folate
- 1 mcg DFE = 0.6 mcg folic acid from fortified foods or dietary supplements consumed with foods
- 1 mcg DFE = 0.5 mcg folic acid from dietary supplements taken on an empty stomach
Because of variable absorption rates, an approximation of total folate intake in a day can be calculated as follows:
Grams of DFEs provided = grams of food folate + 1.7 × (grams of folic acid supplementation)
Factors for converting mcg dietary folate equivalent (DFE) to mcg for supplemental folate in the form of 5-methylenetetrahydrofolate (5-MTHF) have not been formally established 1.
For infants from birth to 12 months, the Food and Nutrition Board established an Adequate Intake (AI) for folate that is equivalent to the mean intake of folate in healthy, breastfed infants in the United States (see Table 2). Adequate Intake (AI) is the intake level that is assumed to ensure nutritional adequacy; established when evidence is insufficient to develop an Recommended Dietary Allowance (RDA) 1.
Table 2. Recommended Dietary Allowances (RDAs) for Folate
| Life Stage | Recommended Amount |
|---|---|
| Birth to 6 months | 65 mcg DFE |
| Infants 7–12 months | 80 mcg DFE |
| Children 1–3 years | 150 mcg DFE |
| Children 4–8 years | 200 mcg DFE |
| Children 9–13 years | 300 mcg DFE |
| Teens 14–18 years | 400 mcg DFE |
| Adults 19+ years | 400 mcg DFE |
| Pregnant teens and women | 600 mcg DFE |
| Breastfeeding teens and women | 500 mcg DFE |
Footnote: All women and teen girls who could become pregnant should consume 400 mcg of folic acid daily from supplements, fortified foods, or both in addition to the folate they get from following a healthy eating pattern.
[Source 67 ]Folate deficiency prognosis
The prognosis is excellent for most patients with folic acid deficiency if it is corrected and generally reverses most clinical and biochemical abnormalities seen with folic acid deficiency 2. However, a lack of folate can lead to macrocytic anemia. In addition, lack of folate raises levels of homocysteine, which is associated with atherosclerotic disease 4, 5. Lack of folate causes a number of pregnancy-related complications, including placenta abruptio, spontaneous abortion, neural tube defects, and severe language deficits in children.
References- Folate. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional
- Khan KM, Jialal I. Folic Acid Deficiency. [Updated 2022 Jun 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK535377
- Office on Women’s Health, U.S. Department of Health and Human Services – Folic acid – https://www.womenshealth.gov/a-z-topics/folic-acid
- Bailey LB, Caudill MA. Folate. In: Erdman JW, Macdonald IA, Zeisel SH, eds. Present Knowledge in Nutrition. 10th ed. Washington, DC: Wiley-Blackwell; 2012:321-42.
- Institute of Medicine. Food and Nutrition Board. Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academy Press; 1998.
- Stover PJ. Folic acid. In: Ross AC, Caballero B, Cousins RJ, Tucker KL, Ziegler TR, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2012:358-68.
- Bailey LB, Gregory JFr (2006). Folate. Present Knowledge in Nutrition. B. Bowman and R. Russell. Washington, DC, International Life Sciences Institute. I: 278-301.
- Carmel R. Folic acid. In: Shils M, Shike M, Ross A, Caballero B, Cousins RJ, eds. Modern Nutrition in Health and Disease. 11th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005:470-81.
- Institute of Medicine. Food and Nutrition Board (1998). Dietary Reference Intakes: Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC, National Academy Press. https://www.nap.edu/catalog/6015/dietary-reference-intakes-for-thiamin-riboflavin-niacin-vitamin-b6-folate-vitamin-b12-pantothenic-acid-biotin-and-choline
- Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, et al. (2011). Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr 94(1): 303S-312S. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3127517/
- Scaglione F, Panzavolta G. Folate, folic acid and 5-methyltetrahydrofolate are not the same thing. Xenobiotica. 2014 May;44(5):480-8. doi: 10.3109/00498254.2013.845705
- Greenberg JA, Bell SJ, Guan Y, Yu YH. Folic Acid supplementation and pregnancy: more than just neural tube defect prevention. Rev Obstet Gynecol. 2011 Summer;4(2):52-9. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3218540
- Henderson AM, Aleliunas RE, Loh SP, Khor GL, Harvey-Leeson S, Glier MB, Kitts DD, Green TJ, Devlin AM. l-5-Methyltetrahydrofolate Supplementation Increases Blood Folate Concentrations to a Greater Extent than Folic Acid Supplementation in Malaysian Women. J Nutr. 2018 Jun 1;148(6):885-890. doi: 10.1093/jn/nxy057
- Green TJ, Liu Y, Dadgar S, Li W, Böhni R, Kitts DD. Wheat rolls fortified with microencapsulated L-5-methyltetrahydrofolic acid or equimolar folic acid increase blood folate concentrations to a similar extent in healthy men and women. J Nutr. 2013 Jun;143(6):867-71. doi: 10.3945/jn.113.174268
- Lamers Y, Prinz-Langenohl R, Brämswig S, Pietrzik K. Red blood cell folate concentrations increase more after supplementation with [6S]-5-methyltetrahydrofolate than with folic acid in women of childbearing age. Am J Clin Nutr. 2006 Jul;84(1):156-61. doi: 10.1093/ajcn/84.1.156
- Pietrzik K, Bailey L, Shane B. Folic acid and L-5-methyltetrahydrofolate: comparison of clinical pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2010 Aug;49(8):535-48. doi: 10.2165/11532990-000000000-00000
- Food Labeling: Revision of the Nutrition and Supplement Facts Labels. https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels
- Green R, Datta Mitra A. Megaloblastic Anemias: Nutritional and Other Causes. Med Clin North Am. 2017 Mar;101(2):297-317. doi: 10.1016/j.mcna.2016.09.013
- Carmel R (2005). Folic Acid. Modern Nutrition in Health and Disease. M. Shils, M. Shike, A. Ross, B. Caballero and R. Cousins. Baltimore, MD, Lippincott Williams & Wilkins: 470-481.
- Colapinto CK, O’Connor DL, Dubois L, Tremblay MS (2012). Folic acid supplement use is the most significant predictor of folate concentrations in Canadian women of childbearing age. Appl Physiol Nutr Metab 37(2): 284-292. http://www.nrcresearchpress.com/doi/full/10.1139/h11-161
- Green R (2011). Indicators for assessing folate and vitamin B-12 status and for monitoring the efficacy of intervention strategies. Am J Clin Nutr 94(2): 666S-672S. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3142735/
- Yeung LF, Cogswell ME, Carriquiry AL, Bailey LB, Pfeiffer CM, Berry RJ. Contributions of enriched cereal-grain products, ready-to-eat cereals, and supplements to folic acid and vitamin B-12 usual intake and folate and vitamin B-12 status in US children: National Health and Nutrition Examination Survey (NHANES), 2003-2006. Am J Clin Nutr. 2011 Jan;93(1):172-85. doi: 10.3945/ajcn.2010.30127
- Shuvalov O, Petukhov A, Daks A, Fedorova O, Vasileva E, Barlev NA. One-carbon metabolism and nucleotide biosynthesis as attractive targets for anticancer therapy. Oncotarget. 2017 Apr 4;8(14):23955-23977. doi: 10.18632/oncotarget.15053
- Gerhard GT, Duell PB. Homocysteine and atherosclerosis. Curr Opin Lipidol. 1999 Oct;10(5):417-28. doi: 10.1097/00041433-199910000-00006
- Azzini E, Ruggeri S, Polito A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int J Mol Sci. 2020 Feb 20;21(4):1421. doi: 10.3390/ijms21041421
- Centers for Disease Control and Prevention – Facts About Folic Acid – https://www.cdc.gov/ncbddd/folicacid/about.html
- Folic Acid Recommendations. https://www.cdc.gov/ncbddd/folicacid/recommendations.html
- Nutrition During Pregnancy. https://www.acog.org/womens-health/faqs/nutrition-during-pregnancy
- Stover PJ, Field MS. Trafficking of intracellular folates. Adv Nutr. 2011 Jul;2(4):325-31. doi: 10.3945/an.111.000596
- Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000 Feb;130(2):129-32. doi: 10.1093/jn/130.2.129
- Bailey LB, Gregory JF 3rd. Folate metabolism and requirements. J Nutr. 1999 Apr;129(4):779-82. doi: 10.1093/jn/129.4.779
- Finer, L.B., Zolna, M.R. (2016). Declines in unintended pregnancy in the United States, 2008-2011. The New England Journal of Medicine; 374(9):843–52.
- U.S. Preventive Services Task Force. (2016). Final Recommendation Statement: Folic Acid for the Prevention of Neural Tube Defects: Preventive Medication – https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/folic-acid-for-the-prevention-of-neural-tube-defects-preventive-medication
- Mosher, W.D., Jones, J., Abma, J.C. (2012). Intended and Unintended Births in the United States: 1982–2010 (PDF, 404 KB). National Health Statistics Reports; no. 55.
- CDC. (2016). Spina Bifida. – https://www.cdc.gov/ncbddd/spinabifida/facts.html
- CDC. (2015). Facts about Anencephaly. – https://www.cdc.gov/ncbddd/birthdefects/anencephaly.html
- U.S. Preventive Services Task Force. (2016). Final Recommendation Statement: Folic Acid for the Prevention of Neural Tube Defects: Preventive Medication
- CDC. (2016). Folic Acid Recommendations.- https://www.cdc.gov/ncbddd/folicacid/recommendations.html
- Bailey, R.L., Dodd, K.W., Gahche, J.J., Dwyer, J.T., McDowell, M.A., Yetley, E.A., et al. (2010). Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. American Journal of Clinical Nutrition; 91(1): 231–237.
- Hamner, H.C., Cogswell, M.E., Johnson, M.A. (2011). Acculturation factors are associated with folate intakes among Mexican American women. The Journal of Nutrition; 141(10): 1889–97.
- U.S. Department of Agriculture, Agricultural Research Service. What We Eat in America, 2013-2014. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweia-data-tables
- Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, McDowell MA, Yetley EA, Sempos CA, Burt VL, Radimer KL, Picciano MF. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003-2006. Am J Clin Nutr. 2010 Jan;91(1):231-7. doi: 10.3945/ajcn.2009.28427
- Bailey RL, McDowell MA, Dodd KW, Gahche JJ, Dwyer JT, Picciano MF. Total folate and folic acid intakes from foods and dietary supplements of US children aged 1-13 y. Am J Clin Nutr. 2010 Aug;92(2):353-8. doi: 10.3945/ajcn.2010.29652
- Yang Q, Cogswell ME, Hamner HC, Carriquiry A, Bailey LB, Pfeiffer CM, Berry RJ. Folic acid source, usual intake, and folate and vitamin B-12 status in US adults: National Health and Nutrition Examination Survey (NHANES) 2003-2006. Am J Clin Nutr. 2010 Jan;91(1):64-72. doi: 10.3945/ajcn.2009.28401. Epub 2009 Oct 14. Erratum in: Am J Clin Nutr. 2010 Oct;92(4):1001. Dosage error in article text.
- Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R, Curtin LR, Durazo-Arvizu RA, Eckfeldt JH, Green R, Gregory JF 3rd, Hoofnagle AN, Jacobsen DW, Jacques PF, Molloy AM, Massaro J, Mills JL, Nexo E, Rader JI, Selhub J, Sempos C, Shane B, Stabler S, Stover P, Tamura T, Tedstone A, Thorpe SJ, Coates PM, Johnson CL, Picciano MF. Biomarkers of folate status in NHANES: a roundtable summary. Am J Clin Nutr. 2011 Jul;94(1):303S-312S. doi: 10.3945/ajcn.111.013011
- Berry RJ, Carter HK, Yang Q. Cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007 Jul;86(1):265-7; author reply 267-9. doi: 10.1093/ajcn/86.1.265
- Carmel R. Does high folic acid intake affect unrecognized cobalamin deficiency, and how will we know it if we see it? Am J Clin Nutr. 2009 Dec;90(6):1449-50. doi: 10.3945/ajcn.2009.28835
- Mary Ann Johnson, PhD, If High Folic Acid Aggravates Vitamin B12 Deficiency What Should Be Done about It?, Nutrition Reviews, Volume 65, Issue 10, October 2007, Pages 451–458, https://doi.org/10.1111/j.1753-4887.2007.tb00270.x
- Selhub J, Morris MS, Jacques PF, Rosenberg IH. Folate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am J Clin Nutr. 2009 Feb;89(2):702S-6S. doi: 10.3945/ajcn.2008.26947C. Epub 2009 Jan 13. Erratum in: Am J Clin Nutr. 2009 Jun;89(6):1951
- Clarke R, Halsey J, Lewington S, Lonn E, Armitage J, Manson JE, Bønaa KH, Spence JD, Nygård O, Jamison R, Gaziano JM, Guarino P, Bennett D, Mir F, Peto R, Collins R; B-Vitamin Treatment Trialists’ Collaboration. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: Meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010 Oct 11;170(18):1622-31. doi: 10.1001/archinternmed.2010.348
- Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev. 2006 Feb;15(2):189-93. doi: 10.1158/1055-9965.EPI-152CO
- Edward Giovannucci, Meir J. Stampfer, Graham A. Colditz, Eric B. Rimm, Dimitrios Trichopoulos, Bernard A. Rosner, Frank E. Speizer, Walter C. Willett, Folate, Methionine, and Alcohol Intake and Risk of Colorectal Adenoma, JNCI: Journal of the National Cancer Institute, Volume 85, Issue 11, 2 June 1993, Pages 875–883, https://doi.org/10.1093/jnci/85.11.875
- Kim YI. Folate: a magic bullet or a double edged sword for colorectal cancer prevention? Gut. 2006 Oct;55(10):1387-9. doi: 10.1136/gut.2006.095463
- Valera-Gran D, Navarrete-Muñoz EM, Garcia de la Hera M, Fernández-Somoano A, Tardón A, Ibarluzea J, Balluerka N, Murcia M, González-Safont L, Romaguera D, Julvez J, Vioque J; INMA Project. Effect of maternal high dosages of folic acid supplements on neurocognitive development in children at 4-5 y of age: the prospective birth cohort Infancia y Medio Ambiente (INMA) study. Am J Clin Nutr. 2017 Sep;106(3):878-887. doi: 10.3945/ajcn.117.152769
- Paniz C, Bertinato JF, Lucena MR, De Carli E, Amorim PMDS, Gomes GW, Palchetti CZ, Figueiredo MS, Pfeiffer CM, Fazili Z, Green R, Guerra-Shinohara EM. A Daily Dose of 5 mg Folic Acid for 90 Days Is Associated with Increased Serum Unmetabolized Folic Acid and Reduced Natural Killer Cell Cytotoxicity in Healthy Brazilian Adults. J Nutr. 2017 Sep;147(9):1677-1685. doi: 10.3945/jn.117.247445
- Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, Ulrich CM. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006 Jan;136(1):189-94. doi: 10.1093/jn/136.1.189
- Morris MS, Jacques PF, Rosenberg IH, Selhub J. Circulating unmetabolized folic acid and 5-methyltetrahydrofolate in relation to anemia, macrocytosis, and cognitive test performance in American seniors. Am J Clin Nutr. 2010 Jun;91(6):1733-44. doi: 10.3945/ajcn.2009.28671. Epub 2010 Mar 31. Erratum in: Am J Clin Nutr. 2010 Oct;92(4):1002.
- Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF 3rd, Mills JL, Pfeiffer CM, Fazili Z, Zhang M, Ueland PM, Molloy AM, Caudill MA, Shane B, Berry RJ, Bailey RL, Hausman DB, Raghavan R, Raiten DJ. Biomarkers of Nutrition for Development-Folate Review. J Nutr. 2015 Jul;145(7):1636S-1680S. doi: 10.3945/jn.114.206599
- Stamm RA, March KM, Karakochuk CD, Gray AR, Brown RC, Green TJ, Houghton LA. Lactating Canadian Women Consuming 1000 µg Folic Acid Daily Have High Circulating Serum Folic Acid Above a Threshold Concentration of Serum Total Folate. J Nutr. 2018 Jul 1;148(7):1103-1108. doi: 10.1093/jn/nxy070. Erratum in: J Nutr. 2019 Apr 1;149(4):698.
- Pfeiffer CM, Sternberg MR, Fazili Z, Yetley EA, Lacher DA, Bailey RL, Johnson CL. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr. 2015 Mar;145(3):520-31. doi: 10.3945/jn.114.201210
- Page R, Robichaud A, Arbuckle TE, Fraser WD, MacFarlane AJ. Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am J Clin Nutr. 2017 May;105(5):1101-1109. doi: 10.3945/ajcn.116.137968
- Obeid R, Kasoha M, Kirsch SH, Munz W, Herrmann W. Concentrations of unmetabolized folic acid and primary folate forms in pregnant women at delivery and in umbilical cord blood. Am J Clin Nutr. 2010 Dec;92(6):1416-22. doi: 10.3945/ajcn.2010.29361
- Plumptre L, Masih SP, Ly A, Aufreiter S, Sohn KJ, Croxford R, Lausman AY, Berger H, O’Connor DL, Kim YI. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am J Clin Nutr. 2015 Oct;102(4):848-57. doi: 10.3945/ajcn.115.110783
- Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. 1997 Jun;65(6):1790-5. doi: 10.1093/ajcn/65.6.1790
- Sweeney MR, McPartlin J, Scott J. Folic acid fortification and public health: report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health. 2007 Mar 22;7:41. doi: 10.1186/1471-2458-7-41
- Sweeney MR, McPartlin J, Weir DG, Daly L, Scott JM. Postprandial serum folic acid response to multiple doses of folic acid in fortified bread. Br J Nutr. 2006 Jan;95(1):145-51. doi: 10.1079/bjn20051618
- Folate. https://ods.od.nih.gov/factsheets/Folate-Consumer
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. https://fdc.nal.usda.gov
- U.S. Food and Drug Administration. Food Standards: Amendment of Standards of Identity For Enriched Grain Products to Require Addition of Folic Acid. Federal Register 1996;61:8781-97. https://www.govinfo.gov/content/pkg/FR-1996-03-05/pdf/96-5014.pdf
- Choumenkovitch SF, Selhub J, Wilson PW, Rader JI, Rosenberg IH, Jacques PF. Folic acid intake from fortification in United States exceeds predictions. J Nutr. 2002 Sep;132(9):2792-8. doi: 10.1093/jn/132.9.2792
- Centers for Disease Control and Prevention (CDC). CDC Grand Rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep. 2010 Aug 13;59(31):980-4. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5931a2.htm
- Government of Canada. Regulations amending the food and drug regulations (1066). Canada Gazette 1998;132.
- Crider KS, Bailey LB, Berry RJ. Folic acid food fortification-its history, effect, concerns, and future directions. Nutrients. 2011 Mar;3(3):370-84. doi: 10.3390/nu3030370
- Morris, M.S., Jacques, P.F., Rosenberg, I.H., et al. (2007). Folate and vitamin B12 status in relation to anemia, macrocytosis and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr; 85(1):193–200.
- Wilson RD; GENETICS COMMITTEE; MOTHERISK. RETIRED: Pre-conceptional vitamin/folic acid supplementation 2007: the use of folic acid in combination with a multivitamin supplement for the prevention of neural tube defects and other congenital anomalies. J Obstet Gynaecol Can. 2007 Dec;29(12):1003-1013. English, French. doi: 10.1016/S1701-2163(16)32685-8. Erratum in: J Obstet Gynaecol Can. 2008 Mar;30(3):193. Goh, Ingrid [corrected to Goh, Y Ingrid].
- Pitkin RM. Folate and neural tube defects. Am J Clin Nutr. 2007 Jan;85(1):285S-288S. doi: 10.1093/ajcn/85.1.285S
- Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, Frohnert B, Kirby RS; Centers for Disease Control and Prevention. Updated estimates of neural tube defects prevented by mandatory folic Acid fortification – United States, 1995-2011. MMWR Morb Mortal Wkly Rep. 2015 Jan 16;64(1):1-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4584791
- Lamers Y. Folate recommendations for pregnancy, lactation, and infancy. Ann Nutr Metab. 2011;59(1):32-7. doi: 10.1159/000332073
- Scott JM. Evidence of folic acid and folate in the prevention of neural tube defects. Bibl Nutr Dieta. 2001;(55):192-5. doi: 10.1159/000059465
- Molloy AM, Kirke PN, Brody LC, Scott JM, Mills JL. Effects of folate and vitamin B12 deficiencies during pregnancy on fetal, infant, and child development. Food Nutr Bull. 2008 Jun;29(2 Suppl):S101-11; discussion S112-5. doi: 10.1177/15648265080292S114
- Williams LJ, Rasmussen SA, Flores A, Kirby RS, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995-2002. Pediatrics. 2005 Sep;116(3):580-6. doi: 10.1542/peds.2005-0592
- Rader JI, Schneeman BO. Prevalence of neural tube defects, folate status, and folate fortification of enriched cereal-grain products in the United States. Pediatrics. 2006 Apr;117(4):1394-9. doi: 10.1542/peds.2005-2745
- Shane B. Folate-responsive birth defects: of mice and women. Am J Clin Nutr. 2012 Jan;95(1):1-2. doi: 10.3945/ajcn.111.029595
- Omar Dary, Nutritional interpretation of folic acid interventions, Nutrition Reviews, Volume 67, Issue 4, 1 April 2009, Pages 235–244, https://doi.org/10.1111/j.1753-4887.2009.00193.x
- Molloy AM, Pangilinan F, Brody LC. Genetic Risk Factors for Folate-Responsive Neural Tube Defects. Annu Rev Nutr. 2017 Aug 21;37:269-291. doi: 10.1146/annurev-nutr-071714-034235
- MTHFR Gene, Folic Acid, and Preventing Neural Tube Defects. https://www.cdc.gov/ncbddd/folicacid/mthfr-gene-and-folic-acid.html
- Viswanathan M, Treiman KA, Kish-Doto J, Middleton JC, Coker-Schwimmer EJ, Nicholson WK. Folic Acid Supplementation for the Prevention of Neural Tube Defects: An Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2017 Jan 10;317(2):190-203. doi: 10.1001/jama.2016.19193
- US Preventive Services Task Force; Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, García FA, Kemper AR, Krist AH, Kurth AE, Landefeld CS, Mangione CM, Phillips WR, Phipps MG, Pignone MP, Silverstein M, Tseng CW. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA. 2017 Jan 10;317(2):183-189. doi: 10.1001/jama.2016.19438
- Centers for Disease Control (CDC). Use of folic acid for prevention of spina bifida and other neural tube defects–1983-1991. MMWR Morb Mortal Wkly Rep. 1991 Aug 2;40(30):513-6.
- Czeizel AE, Puhó EH, Langmar Z, Acs N, Bánhidy F. Possible association of folic acid supplementation during pregnancy with reduction of preterm birth: a population-based study. Eur J Obstet Gynecol Reprod Biol. 2010 Feb;148(2):135-40. doi: 10.1016/j.ejogrb.2009.10.016
- Liu S, Joseph KS, Luo W, León JA, Lisonkova S, Van den Hof M, Evans J, Lim K, Little J, Sauve R, Kramer MS; Canadian Perinatal Surveillance System (Public Health Agency of Canada). Effect of Folic Acid Food Fortification in Canada on Congenital Heart Disease Subtypes. Circulation. 2016 Aug 30;134(9):647-55. doi: 10.1161/CIRCULATIONAHA.116.022126
- Lorenzo D. Botto, Joseph Mulinare, J. David Erickson, Occurrence of Congenital Heart Defects in Relation to Maternal Multivitamin Use, American Journal of Epidemiology, Volume 151, Issue 9, 1 May 2000, Pages 878–884, https://doi.org/10.1093/oxfordjournals.aje.a010291
- Shaw GM, O’Malley CD, Wasserman CR, Tolarova MM, Lammer EJ. Maternal periconceptional use of multivitamins and reduced risk for conotruncal heart defects and limb deficiencies among offspring. Am J Med Genet. 1995 Dec 4;59(4):536-45. doi: 10.1002/ajmg.1320590428
- Autism Spectrum Disorder. https://www.nimh.nih.gov/health/topics/autism-spectrum-disorders-asd
- Berry RJ, Crider KS, Yeargin-Allsopp M. Periconceptional folic acid and risk of autism spectrum disorders. JAMA. 2013 Feb 13;309(6):611-3. doi: 10.1001/jama.2013.198
- Bjørk M, Riedel B, Spigset O, Veiby G, Kolstad E, Daltveit AK, Gilhus NE. Association of Folic Acid Supplementation During Pregnancy With the Risk of Autistic Traits in Children Exposed to Antiepileptic Drugs In Utero. JAMA Neurol. 2018 Feb 1;75(2):160-168. doi: 10.1001/jamaneurol.2017.3897. Erratum in: JAMA Neurol. 2018 Apr 1;75(4):518.
- Schmidt RJ, Kogan V, Shelton JF, Delwiche L, Hansen RL, Ozonoff S, Ma CC, McCanlies EC, Bennett DH, Hertz-Picciotto I, Tancredi DJ, Volk HE. Combined Prenatal Pesticide Exposure and Folic Acid Intake in Relation to Autism Spectrum Disorder. Environ Health Perspect. 2017 Sep 8;125(9):097007. doi: 10.1289/EHP604
- Goodrich AJ, Volk HE, Tancredi DJ, McConnell R, Lurmann FW, Hansen RL, Schmidt RJ. Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res. 2018 Jan;11(1):69-80. doi: 10.1002/aur.1885
- Caffrey A, Irwin RE, McNulty H, Strain JJ, Lees-Murdock DJ, McNulty BA, Ward M, Walsh CP, Pentieva K. Gene-specific DNA methylation in newborns in response to folic acid supplementation during the second and third trimesters of pregnancy: epigenetic analysis from a randomized controlled trial. Am J Clin Nutr. 2018 Apr 1;107(4):566-575. doi: 10.1093/ajcn/nqx069
- DeVilbiss EA, Gardner RM, Newschaffer CJ, Lee BK. Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. Br J Nutr. 2015 Sep 14;114(5):663-72. doi: 10.1017/S0007114515002470
- Roffman JL. Neuroprotective Effects of Prenatal Folic Acid Supplementation: Why Timing Matters. JAMA Psychiatry. 2018 Jul 1;75(7):747-748. doi: 10.1001/jamapsychiatry.2018.0378
- Surén P, Roth C, Bresnahan M, Haugen M, Hornig M, Hirtz D, Lie KK, Lipkin WI, Magnus P, Reichborn-Kjennerud T, Schjølberg S, Davey Smith G, Øyen AS, Susser E, Stoltenberg C. Association between maternal use of folic acid supplements and risk of autism spectrum disorders in children. JAMA. 2013 Feb 13;309(6):570-7. doi: 10.1001/jama.2012.155925
- Schmidt RJ, Tancredi DJ, Ozonoff S, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tassone F, Hertz-Picciotto I. Maternal periconceptional folic acid intake and risk of autism spectrum disorders and developmental delay in the CHARGE (CHildhood Autism Risks from Genetics and Environment) case-control study. Am J Clin Nutr. 2012 Jul;96(1):80-9. doi: 10.3945/ajcn.110.004416
- Levine SZ, Kodesh A, Viktorin A, Smith L, Uher R, Reichenberg A, Sandin S. Association of Maternal Use of Folic Acid and Multivitamin Supplements in the Periods Before and During Pregnancy With the Risk of Autism Spectrum Disorder in Offspring. JAMA Psychiatry. 2018 Feb 1;75(2):176-184. doi: 10.1001/jamapsychiatry.2017.4050
- Virk J, Liew Z, Olsen J, Nohr EA, Catov JM, Ritz B. Preconceptional and prenatal supplementary folic acid and multivitamin intake and autism spectrum disorders. Autism. 2016 Aug;20(6):710-8. doi: 10.1177/1362361315604076
- Badovinac, R.L., Werler, M.M., Williams, P.L., Kelsey, K.T. and Hayes, C. (2007), Folic acid–containing supplement consumption during pregnancy and risk for oral clefts: A meta-analysis. Birth Defects Research Part A: Clinical and Molecular Teratology, 79: 8-15. https://doi.org/10.1002/bdra.20315
- Wilcox AJ, Lie RT, Solvoll K, Taylor J, McConnaughey DR, Abyholm F, Vindenes H, Vollset SE, Drevon CA. Folic acid supplements and risk of facial clefts: national population based case-control study. BMJ. 2007 Mar 3;334(7591):464. doi: 10.1136/bmj.39079.618287.0B
- Boyles AL, Wilcox AJ, Taylor JA, Meyer K, Fredriksen A, Ueland PM, Drevon CA, Vollset SE, Lie RT. Folate and one-carbon metabolism gene polymorphisms and their associations with oral facial clefts. Am J Med Genet A. 2008 Feb 15;146A(4):440-9. doi: 10.1002/ajmg.a.32162
- Boyles AL, Wilcox AJ, Taylor JA, Shi M, Weinberg CR, Meyer K, Fredriksen A, Ueland PM, Johansen AM, Drevon CA, Jugessur A, Trung TN, Gjessing HK, Vollset SE, Murray JC, Christensen K, Lie RT. Oral facial clefts and gene polymorphisms in metabolism of folate/one-carbon and vitamin A: a pathway-wide association study. Genet Epidemiol. 2009 Apr;33(3):247-55. doi: 10.1002/gepi.20376
- Luo, Y.L., Cheng, Y.L., Ye, P., Wang, W., Gao, X.H. and Chen, Q. (2012), Association between MTHFR polymorphisms and orofacial clefts risk: A meta-analysis. Birth Defects Research Part A: Clinical and Molecular Teratology, 94: 237-244. https://doi.org/10.1002/bdra.23005
- Allen J Wilcox, On the importance—and the unimportance— of birthweight, International Journal of Epidemiology, Volume 30, Issue 6, December 2001, Pages 1233–1241, https://doi.org/10.1093/ije/30.6.1233
- Fekete K, Berti C, Trovato M, Lohner S, Dullemeijer C, Souverein OW, Cetin I, Decsi T. Effect of folate intake on health outcomes in pregnancy: a systematic review and meta-analysis on birth weight, placental weight and length of gestation. Nutr J. 2012 Sep 19;11:75. doi: 10.1186/1475-2891-11-75
- Baker PN, Wheeler SJ, Sanders TA, Thomas JE, Hutchinson CJ, Clarke K, Berry JL, Jones RL, Seed PT, Poston L. A prospective study of micronutrient status in adolescent pregnancy. Am J Clin Nutr. 2009 Apr;89(4):1114-24. doi: 10.3945/ajcn.2008.27097
- Lee HA, Park EA, Cho SJ, Kim HS, Kim YJ, Lee H, Gwak HS, Kim KN, Chang N, Ha EH, Park H. Mendelian randomization analysis of the effect of maternal homocysteine during pregnancy, as represented by maternal MTHFR C677T genotype, on birth weight. J Epidemiol. 2013 Sep 5;23(5):371-5. doi: 10.2188/jea.je20120219
- Scholl TO, Johnson WG. Folic acid: influence on the outcome of pregnancy. Am J Clin Nutr. 2000 May;71(5 Suppl):1295S-303S. doi: 10.1093/ajcn/71.5.1295s
- Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, Monsen AL, Ueland PM. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000 Apr;71(4):962-8. doi: 10.1093/ajcn/71.4.962
- Wang XM, Wu HY, Qiu XJ. Methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism and risk of preeclampsia: an updated meta-analysis based on 51 studies. Arch Med Res. 2013 Apr;44(3):159-68. doi: 10.1016/j.arcmed.2013.01.011
- Wen SW, Champagne J, Rennicks White R, Coyle D, Fraser W, Smith G, Fergusson D, Walker MC. Effect of folic acid supplementation in pregnancy on preeclampsia: the folic acid clinical trial study. J Pregnancy. 2013;2013:294312. doi: 10.1155/2013/294312
- Lassi ZS, Salam RA, Haider BA, Bhutta ZA. Folic acid supplementation during pregnancy for maternal health and pregnancy outcomes. Cochrane Database Syst Rev. 2013 Mar 28;(3):CD006896. doi: 10.1002/14651858.CD006896.pub2
- Crider KS, Cordero AM, Qi YP, Mulinare J, Dowling NF, Berry RJ. Prenatal folic acid and risk of asthma in children: a systematic review and meta-analysis. Am J Clin Nutr. 2013 Nov;98(5):1272-81. doi: 10.3945/ajcn.113.065623
- Susan B Brown, Katherine W Reeves, Elizabeth R Bertone-Johnson, Maternal folate exposure in pregnancy and childhood asthma and allergy: a systematic review, Nutrition Reviews, Volume 72, Issue 1, 1 January 2014, Pages 55–64, https://doi.org/10.1111/nure.12080
- He H, Shui B. Folate intake and risk of bladder cancer: a meta-analysis of epidemiological studies. Int J Food Sci Nutr. 2014 May;65(3):286-92. doi: 10.3109/09637486.2013.866641
- Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004 Nov;80(5):1123-8. doi: 10.1093/ajcn/80.5.1123
- Kim YI. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999 Feb;10(2):66-88. doi: 10.1016/s0955-2863(98)00074-6
- Kim YI. Folate and cancer: a tale of Dr. Jekyll and Mr. Hyde? Am J Clin Nutr. 2018 Feb 1;107(2):139-142. doi: 10.1093/ajcn/nqx076
- Andreeva VA, Touvier M, Kesse-Guyot E, Julia C, Galan P, Hercberg S. B vitamin and/or ω-3 fatty acid supplementation and cancer: ancillary findings from the supplementation with folate, vitamins B6 and B12, and/or omega-3 fatty acids (SU.FOL.OM3) randomized trial. Arch Intern Med. 2012 Apr 9;172(7):540-7. doi: 10.1001/archinternmed.2011.1450
- Ebbing M, Bønaa KH, Nygård O, Arnesen E, Ueland PM, Nordrehaug JE, Rasmussen K, Njølstad I, Refsum H, Nilsen DW, Tverdal A, Meyer K, Vollset SE. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009 Nov 18;302(19):2119-26. doi: 10.1001/jama.2009.1622
- Mason, J.B. (2011), Unraveling the complex relationship between folate and cancer risk. BioFactors, 37: 253-260. https://doi.org/10.1002/biof.174
- Kennedy DA, Stern SJ, Moretti M, Matok I, Sarkar M, Nickel C, Koren G. Folate intake and the risk of colorectal cancer: a systematic review and meta-analysis. Cancer Epidemiol. 2011 Feb;35(1):2-10. doi: 10.1016/j.canep.2010.11.004
- Gibson TM, Weinstein SJ, Pfeiffer RM, Hollenbeck AR, Subar AF, Schatzkin A, Mayne ST, Stolzenberg-Solomon R. Pre- and postfortification intake of folate and risk of colorectal cancer in a large prospective cohort study in the United States. Am J Clin Nutr. 2011 Oct;94(4):1053-62. doi: 10.3945/ajcn.110.002659
- Sanjoaquin, M.A., Allen, N., Couto, E., Roddam, A.W. and Key, T.J. (2005), Folate intake and colorectal cancer risk: A meta-analytical approach. Int. J. Cancer, 113: 825-828. https://doi.org/10.1002/ijc.20648
- Bassett JK, Severi G, Hodge AM, Baglietto L, Hopper JL, English DR, Giles GG. Dietary intake of B vitamins and methionine and colorectal cancer risk. Nutr Cancer. 2013;65(5):659-67. doi: 10.1080/01635581.2013.789114
- de Vogel S, Dindore V, van Engeland M, Goldbohm RA, van den Brandt PA, Weijenberg MP. Dietary folate, methionine, riboflavin, and vitamin B-6 and risk of sporadic colorectal cancer. J Nutr. 2008 Dec;138(12):2372-8. doi: 10.3945/jn.108.091157
- Neuhouser ML, Cheng TY, Beresford SA, Brown E, Song X, Miller JW, Zheng Y, Thomson CA, Shikany JM, Vitolins MZ, Rohan T, Green R, Ulrich CM. Red blood cell folate and plasma folate are not associated with risk of incident colorectal cancer in the Women’s Health Initiative observational study. Int J Cancer. 2015 Aug 15;137(4):930-9. doi: 10.1002/ijc.29453
- Shu-Chun Chuang, Matteo Rota, Marc J. Gunter, Anne Zeleniuch-Jacquotte, Simone J.P.M. Eussen, Stein Emil Vollset, Per Magne Ueland, Teresa Norat, Regina G. Ziegler, Paolo Vineis, Quantifying the Dose-Response Relationship Between Circulating Folate Concentrations and Colorectal Cancer in Cohort Studies: A Meta-Analysis Based on a Flexible Meta-Regression Model, American Journal of Epidemiology, Volume 178, Issue 7, 1 October 2013, Pages 1028–1037, https://doi.org/10.1093/aje/kwt083
- Song Y, Manson JE, Lee IM, Cook NR, Paul L, Selhub J, Giovannucci E, Zhang SM. Effect of combined folic acid, vitamin B(6), and vitamin B(12) on colorectal adenoma. J Natl Cancer Inst. 2012 Oct 17;104(20):1562-75. doi: 10.1093/jnci/djs370
- Figueiredo JC, Mott LA, Giovannucci E, Wu K, Cole B, Grainge MJ, Logan RF, Baron JA. Folic acid and prevention of colorectal adenomas: a combined analysis of randomized clinical trials. Int J Cancer. 2011 Jul 1;129(1):192-203. doi: 10.1002/ijc.25872
- Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, Allen JI, Robertson DJ, Beck GJ, Bond JH, Byers T, Mandel JS, Mott LA, Pearson LH, Barry EL, Rees JR, Marcon N, Saibil F, Ueland PM, Greenberg ER; Polyp Prevention Study Group. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007 Jun 6;297(21):2351-9. doi: 10.1001/jama.297.21.2351
- Vollset SE, Clarke R, Lewington S, Ebbing M, Halsey J, Lonn E, Armitage J, Manson JE, Hankey GJ, Spence JD, Galan P, Bønaa KH, Jamison R, Gaziano JM, Guarino P, Baron JA, Logan RF, Giovannucci EL, den Heijer M, Ueland PM, Bennett D, Collins R, Peto R; B-Vitamin Treatment Trialists’ Collaboration. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013 Mar 23;381(9871):1029-36. doi: 10.1016/S0140-6736(12)62001-7
- van Wijngaarden JP, Swart KM, Enneman AW, Dhonukshe-Rutten RA, van Dijk SC, Ham AC, Brouwer-Brolsma EM, van der Zwaluw NL, Sohl E, van Meurs JB, Zillikens MC, van Schoor NM, van der Velde N, Brug J, Uitterlinden AG, Lips P, de Groot LC. Effect of daily vitamin B-12 and folic acid supplementation on fracture incidence in elderly individuals with an elevated plasma homocysteine concentration: B-PROOF, a randomized controlled trial. Am J Clin Nutr. 2014 Dec;100(6):1578-86. doi: 10.3945/ajcn.114.090043
- Tu H, Dinney CP, Ye Y, Grossman HB, Lerner SP, Wu X. Is folic acid safe for non-muscle-invasive bladder cancer patients? An evidence-based cohort study. Am J Clin Nutr. 2018 Feb 1;107(2):208-216. doi: 10.1093/ajcn/nqx019
- Kim SJ, Zuchniak A, Sohn KJ, Lubinski J, Demsky R, Eisen A, Akbari MR, Kim YI, Narod SA, Kotsopoulos J. Plasma folate, vitamin B-6, and vitamin B-12 and breast cancer risk in BRCA1- and BRCA2-mutation carriers: a prospective study. Am J Clin Nutr. 2016 Sep;104(3):671-7. doi: 10.3945/ajcn.116.133470
- Tomaszewski JJ, Cummings JL, Parwani AV, Dhir R, Mason JB, Nelson JB, Bacich DJ, O’Keefe DS. Increased cancer cell proliferation in prostate cancer patients with high levels of serum folate. Prostate. 2011 Sep;71(12):1287-93. doi: 10.1002/pros.21346
- Wien TN, Pike E, Wisløff T, Staff A, Smeland S, Klemp M. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ Open. 2012 Jan 12;2(1):e000653. doi: 10.1136/bmjopen-2011-000653
- Mason JB, Tang SY. Folate status and colorectal cancer risk: A 2016 update. Mol Aspects Med. 2017 Feb;53:73-79. doi: 10.1016/j.mam.2016.11.010
- Lee JE, Willett WC, Fuchs CS, Smith-Warner SA, Wu K, Ma J, Giovannucci E. Folate intake and risk of colorectal cancer and adenoma: modification by time. Am J Clin Nutr. 2011 Apr;93(4):817-25. doi: 10.3945/ajcn.110.007781
- National Toxicology Program. NTP monograph: identifying research needs for assessing safe use of high intakes of folic acid. National Toxicology Program, 2015. https://ntp.niehs.nih.gov/ntp/ohat/folicacid/final_monograph_508.pdf
- Huang T, Chen Y, Yang B, Yang J, Wahlqvist ML, Li D. Meta-analysis of B vitamin supplementation on plasma homocysteine, cardiovascular and all-cause mortality. Clin Nutr. 2012 Aug;31(4):448-54. doi: 10.1016/j.clnu.2011.01.003
- Huo Y, Li J, Qin X, Huang Y, Wang X, Gottesman RF, Tang G, Wang B, Chen D, He M, Fu J, Cai Y, Shi X, Zhang Y, Cui Y, Sun N, Li X, Cheng X, Wang J, Yang X, Yang T, Xiao C, Zhao G, Dong Q, Zhu D, Wang X, Ge J, Zhao L, Hu D, Liu L, Hou FF; CSPPT Investigators. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: the CSPPT randomized clinical trial. JAMA. 2015 Apr 7;313(13):1325-35. doi: 10.1001/jama.2015.2274
- Huo Y, Qin X, Wang J, Sun N, Zeng Q, Xu X, Liu L, Xu X, Wang X. Efficacy of folic acid supplementation in stroke prevention: new insight from a meta-analysis. Int J Clin Pract. 2012 Jun;66(6):544-51. doi: 10.1111/j.1742-1241.2012.02929.x
- Christen WG, Cook NR, Van Denburgh M, Zaharris E, Albert CM, Manson JE. Effect of Combined Treatment With Folic Acid, Vitamin B6, and Vitamin B12 on Plasma Biomarkers of Inflammation and Endothelial Dysfunction in Women. J Am Heart Assoc. 2018 May 18;7(11):e008517. doi: 10.1161/JAHA.117.008517
- Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008 May 7;299(17):2027-36. doi: 10.1001/jama.299.17.2027
- Lonn E, Yusuf S, Arnold MJ, Sheridan P, Pogue J, Micks M, McQueen MJ, Probstfield J, Fodor G, Held C, Genest J Jr; Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006 Apr 13;354(15):1567-77. doi: 10.1056/NEJMoa060900. Epub 2006 Mar 12. Erratum in: N Engl J Med. 2006 Aug 17;355(7):746.
- Kong X, Huang X, Zhao M, Xu B, Xu R, Song Y, Yu Y, Yang W, Zhang J, Liu L, Zhang Y, Tang G, Wang B, Hou FF, Li P, Cheng X, Zhao S, Wang X, Qin X, Li J, Huo Y. Platelet Count Affects Efficacy of Folic Acid in Preventing First Stroke. J Am Coll Cardiol. 2018 May 15;71(19):2136-2146. doi: 10.1016/j.jacc.2018.02.072
- Stampfer M, Willett W. Folate supplements for stroke prevention: targeted trial trumps the rest. JAMA. 2015 Apr 7;313(13):1321-2. doi: 10.1001/jama.2015.1961
- Martí-Carvajal AJ, Solà I, Lathyris D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2015 Jan 15;1:CD006612. doi: 10.1002/14651858.CD006612.pub4. Update in: Cochrane Database Syst Rev. 2017 Aug 17;8:CD006612
- Jenkins DJA, Spence JD, Giovannucci EL, Kim YI, Josse R, Vieth R, Blanco Mejia S, Viguiliouk E, Nishi S, Sahye-Pudaruth S, Paquette M, Patel D, Mitchell S, Kavanagh M, Tsirakis T, Bachiri L, Maran A, Umatheva N, McKay T, Trinidad G, Bernstein D, Chowdhury A, Correa-Betanzo J, Del Principe G, Hajizadeh A, Jayaraman R, Jenkins A, Jenkins W, Kalaichandran R, Kirupaharan G, Manisekaran P, Qutta T, Shahid R, Silver A, Villegas C, White J, Kendall CWC, Pichika SC, Sievenpiper JL. Supplemental Vitamins and Minerals for CVD Prevention and Treatment. J Am Coll Cardiol. 2018 Jun 5;71(22):2570-2584. doi: 10.1016/j.jacc.2018.04.020
- Tian T, Yang KQ, Cui JG, Zhou LL, Zhou XL. Folic Acid Supplementation for Stroke Prevention in Patients With Cardiovascular Disease. Am J Med Sci. 2017 Oct;354(4):379-387. doi: 10.1016/j.amjms.2017.05.020
- Kim S, Choi BY, Nam JH, Kim MK, Oh DH, Yang YJ. Cognitive impairment is associated with elevated serum homocysteine levels among older adults. Eur J Nutr. 2019 Feb;58(1):399-408. doi: 10.1007/s00394-017-1604-y
- Ho RC, Cheung MW, Fu E, Win HH, Zaw MH, Ng A, Mak A. Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry. 2011 Jul;19(7):607-17. doi: 10.1097/JGP.0b013e3181f17eed
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002 Feb 14;346(7):476-83. doi: 10.1056/NEJMoa011613
- Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am J Clin Nutr. 2005 Sep;82(3):636-43. doi: 10.1093/ajcn.82.3.636
- Clarke R. B-vitamins and prevention of dementia. Proc Nutr Soc. 2008 Feb;67(1):75-81. doi: 10.1017/S0029665108006046
- Smith AD, Refsum H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu Rev Nutr. 2016 Jul 17;36:211-39. doi: 10.1146/annurev-nutr-071715-050947
- Smith AD, Refsum H, Bottiglieri T, Fenech M, Hooshmand B, McCaddon A, Miller JW, Rosenberg IH, Obeid R. Homocysteine and Dementia: An International Consensus Statement. J Alzheimers Dis. 2018;62(2):561-570. doi: 10.3233/JAD-171042
- Hooshmand, B., Solomon, A., Kåreholt, I., Rusanen, M., Hänninen, T., Leiviskä, J., Winblad, B., Laatikainen, T., Soininen, H. and Kivipelto, M. (2012), Associations between serum homocysteine, holotranscobalamin, folate and cognition in the elderly: a longitudinal study. Journal of Internal Medicine, 271: 204-212. https://doi.org/10.1111/j.1365-2796.2011.02484.x
- Eussen SJ, de Groot LC, Joosten LW, Bloo RJ, Clarke R, Ueland PM, Schneede J, Blom HJ, Hoefnagels WH, van Staveren WA. Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: a randomized, placebo-controlled trial. Am J Clin Nutr. 2006 Aug;84(2):361-70. doi: 10.1093/ajcn/84.1.361
- van der Zwaluw NL, Dhonukshe-Rutten RA, van Wijngaarden JP, Brouwer-Brolsma EM, van de Rest O, In ‘t Veld PH, Enneman AW, van Dijk SC, Ham AC, Swart KM, van der Velde N, van Schoor NM, van der Cammen TJ, Uitterlinden AG, Lips P, Kessels RP, de Groot LC. Results of 2-year vitamin B treatment on cognitive performance: secondary data from an RCT. Neurology. 2014 Dec 2;83(23):2158-66. doi: 10.1212/WNL.0000000000001050
- Kang JH, Cook N, Manson J, Buring JE, Albert CM, Grodstein F. A trial of B vitamins and cognitive function among women at high risk of cardiovascular disease. Am J Clin Nutr. 2008 Dec;88(6):1602-10. doi: 10.3945/ajcn.2008.26404
- Aisen PS, Schneider LS, Sano M, Diaz-Arrastia R, van Dyck CH, Weiner MF, Bottiglieri T, Jin S, Stokes KT, Thomas RG, Thal LJ; Alzheimer Disease Cooperative Study. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008 Oct 15;300(15):1774-83. doi: 10.1001/jama.300.15.1774
- Walker JG, Batterham PJ, Mackinnon AJ, Jorm AF, Hickie I, Fenech M, Kljakovic M, Crisp D, Christensen H. Oral folic acid and vitamin B-12 supplementation to prevent cognitive decline in community-dwelling older adults with depressive symptoms–the Beyond Ageing Project: a randomized controlled trial. Am J Clin Nutr. 2012 Jan;95(1):194-203. doi: 10.3945/ajcn.110.007799. Epub 2011 Dec 14. Erratum in: Am J Clin Nutr. 2012 Aug;96(2):448. Dosage error in article text.
- Clarke R, Bennett D, Parish S, Lewington S, Skeaff M, Eussen SJ, Lewerin C, Stott DJ, Armitage J, Hankey GJ, Lonn E, Spence JD, Galan P, de Groot LC, Halsey J, Dangour AD, Collins R, Grodstein F; B-Vitamin Treatment Trialists’ Collaboration. Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am J Clin Nutr. 2014 Aug;100(2):657-66. doi: 10.3945/ajcn.113.076349
- Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007 Jan 8;167(1):21-30. doi: 10.1001/archinte.167.1.21
- Malouf R, Grimley Evans J. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane Database Syst Rev. 2008 Oct 8;(4):CD004514. doi: 10.1002/14651858.CD004514.pub2
- Dangour AD, Whitehouse PJ, Rafferty K, Mitchell SA, Smith L, Hawkesworth S, Vellas B. B-vitamins and fatty acids in the prevention and treatment of Alzheimer’s disease and dementia: a systematic review. J Alzheimers Dis. 2010;22(1):205-24. doi: 10.3233/JAD-2010-090940
- Ford AH, Almeida OP. Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials. J Alzheimers Dis. 2012;29(1):133-49. doi: 10.3233/JAD-2012-111739
- Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007 Jan 20;369(9557):208-16. doi: 10.1016/S0140-6736(07)60109-3
- Gougeon L, Payette H, Morais JA, Gaudreau P, Shatenstein B, Gray-Donald K. Intakes of folate, vitamin B6 and B12 and risk of depression in community-dwelling older adults: the Quebec Longitudinal Study on Nutrition and Aging. Eur J Clin Nutr. 2016 Mar;70(3):380-5. doi: 10.1038/ejcn.2015.202
- Huang X, Fan Y, Han X, Huang Z, Yu M, Zhang Y, Xu Q, Li X, Wang X, Lu C, Xia Y. Association between Serum Vitamin Levels and Depression in U.S. Adults 20 Years or Older Based on National Health and Nutrition Examination Survey 2005⁻2006. Int J Environ Res Public Health. 2018 Jun 9;15(6):1215. doi: 10.3390/ijerph15061215
- Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and folate status in the US Population. Psychother Psychosom. 2003 Mar-Apr;72(2):80-7. doi: 10.1159/000068692
- Papakostas GI, Petersen T, Mischoulon D, Ryan JL, Nierenberg AA, Bottiglieri T, Rosenbaum JF, Alpert JE, Fava M. Serum folate, vitamin B12, and homocysteine in major depressive disorder, Part 1: predictors of clinical response in fluoxetine-resistant depression. J Clin Psychiatry. 2004 Aug;65(8):1090-5. doi: 10.4088/jcp.v65n0810
- Trujillo J, Vieira MC, Lepsch J, Rebelo F, Poston L, Pasupathy D, Kac G. A systematic review of the associations between maternal nutritional biomarkers and depression and/or anxiety during pregnancy and postpartum. J Affect Disord. 2018 May;232:185-203. doi: 10.1016/j.jad.2018.02.004
- Chong MF, Wong JX, Colega M, Chen LW, van Dam RM, Tan CS, Lim AL, Cai S, Broekman BF, Lee YS, Saw SM, Kwek K, Godfrey KM, Chong YS, Gluckman P, Meaney MJ, Chen H; GUSTO study group. Relationships of maternal folate and vitamin B12 status during pregnancy with perinatal depression: The GUSTO study. J Psychiatr Res. 2014 Aug;55:110-6. doi: 10.1016/j.jpsychires.2014.04.006
- Blunden CH, Inskip HM, Robinson SM, Cooper C, Godfrey KM, Kendrick TR. Postpartum depressive symptoms: the B-vitamin link. Ment Health Fam Med. 2012 Jan;9(1):5-13. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3487611
- Yan J, Liu Y, Cao L, Zheng Y, Li W, Huang G. Association between Duration of Folic Acid Supplementation during Pregnancy and Risk of Postpartum Depression. Nutrients. 2017 Nov 2;9(11):1206. doi: 10.3390/nu9111206
- Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000 Nov;60(2):121-30. doi: 10.1016/s0165-0327(00)00153-1
- Bedson E, Bell D, Carr D, Carter B, Hughes D, Jorgensen A, Lewis H, Lloyd K, McCaddon A, Moat S, Pink J, Pirmohamed M, Roberts S, Russell I, Sylvestre Y, Tranter R, Whitaker R, Wilkinson C, Williams N. Folate Augmentation of Treatment–Evaluation for Depression (FolATED): randomised trial and economic evaluation. Health Technol Assess. 2014 Jul;18(48):vii-viii, 1-159. doi: 10.3310/hta18480
- Roberts E, Carter B, Young AH. Caveat emptor: Folate in unipolar depressive illness, a systematic review and meta-analysis. J Psychopharmacol. 2018 Apr;32(4):377-384. doi: 10.1177/0269881118756060
- Sarris J, Murphy J, Mischoulon D, Papakostas GI, Fava M, Berk M, Ng CH. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am J Psychiatry. 2016 Jun 1;173(6):575-87. doi: 10.1176/appi.ajp.2016.15091228
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier IN, Geddes J, Gilbody S, Haddad PM, Katona C, Lewis G, Malizia A, McAllister-Williams RH, Ramchandani P, Scott J, Taylor D, Uher R; Members of the Consensus Meeting. Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015 May;29(5):459-525. doi: 10.1177/0269881115581093
- Ravindran AV, Balneaves LG, Faulkner G, Ortiz A, McIntosh D, Morehouse RL, Ravindran L, Yatham LN, Kennedy SH, Lam RW, MacQueen GM, Milev RV, Parikh SV; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 5. Complementary and Alternative Medicine Treatments. Can J Psychiatry. 2016 Sep;61(9):576-87. doi: 10.1177/0706743716660290
- Papakostas GI, Shelton RC, Zajecka JM, Etemad B, Rickels K, Clain A, Baer L, Dalton ED, Sacco GR, Schoenfeld D, Pencina M, Meisner A, Bottiglieri T, Nelson E, Mischoulon D, Alpert JE, Barbee JG, Zisook S, Fava M. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012 Dec;169(12):1267-74. doi: 10.1176/appi.ajp.2012.11071114
- Folic Acid. https://www.cdc.gov/ncbddd/folicacid/about.html
- Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep. 1992 Sep 11;41(RR-14):1-7. https://www.cdc.gov/mmwr/preview/mmwrhtml/00019479.htm
- Glória L, Cravo M, Camilo ME, Resende M, Cardoso JN, Oliveira AG, Leitão CN, Mira FC. Nutritional deficiencies in chronic alcoholics: relation to dietary intake and alcohol consumption. Am J Gastroenterol. 1997 Mar;92(3):485-9.
- Gibson A, Woodside JV, Young IS, Sharpe PC, Mercer C, Patterson CC, McKinley MC, Kluijtmans LA, Whitehead AS, Evans A. Alcohol increases homocysteine and reduces B vitamin concentration in healthy male volunteers–a randomized, crossover intervention study. QJM. 2008 Nov;101(11):881-7. doi: 10.1093/qjmed/hcn112
- Rossi RE, Whyand T, Murray CD, Hamilton MI, Conte D, Caplin ME. The role of dietary supplements in inflammatory bowel disease: a systematic review. Eur J Gastroenterol Hepatol. 2016 Dec;28(12):1357-1364. doi: 10.1097/MEG.0000000000000728
- Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Renlund M, Stoll C, Alembik Y, Dott B, Czeizel AE, Gelman-Kohan Z, Scarano G, Bianca S, Ettore G, Tenconi R, Bellato S, Scala I, Mutchinick OM, López MA, de Walle H, Hofstra R, Joutchenko L, Kavteladze L, Bermejo E, Martínez-Frías ML, Gallagher M, Erickson JD, Vollset SE, Mastroiacovo P, Andria G, Botto LD. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J Med Genet. 2003 Aug;40(8):619-25. doi: 10.1136/jmg.40.8.619. Erratum in: J Med Genet. 2004 May;41(5):400. Redlund, M [corrected to Renlund, M].
- Folate Deficiency Clinical Presentation. https://emedicine.medscape.com/article/200184-clinical
- Li J, Xie Q, Gao J, Wang F, Bao Y, Wu L, Yang L, Liu Z, Guo R, Khan A, Dan Liu, Li C, Wu J, Xie J. Aberrant Gcm1 expression mediates Wnt/β-catenin pathway activation in folate deficiency involved in neural tube defects. Cell Death Dis. 2021 Mar 4;12(3):234. doi: 10.1038/s41419-020-03313-z
- Folate Deficiency Clinical Presentation. https://emedicine.medscape.com/article/200184-clinical#b2
- Folate Deficiency Clinical Presentation. https://emedicine.medscape.com/article/200184-clinical#b3
- Reynolds EH. The neurology of folic acid deficiency. Handb Clin Neurol. 2014;120:927-43. doi: 10.1016/B978-0-7020-4087-0.00061-9
- Folate Deficiency Workup. https://emedicine.medscape.com/article/200184-workup
- Devalia, V., Hamilton, M.S., Molloy, A.M. and (2014), Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br J Haematol, 166: 496-513. https://doi.org/10.1111/bjh.12959
- Okada A, Koike H, Nakamura T, Watanabe H, Sobue G. Slowly progressive folate-deficiency myelopathy: report of a case. J Neurol Sci. 2014 Jan 15;336(1-2):273-5. doi: 10.1016/j.jns.2013.10.032
- Folate Deficiency Workup. https://emedicine.medscape.com/article/200184-workup#c7
- Folate Deficiency Treatment & Management. https://emedicine.medscape.com/article/200184-treatment