Germinoma brain tumor
Germinoma is a type of germ cell tumor that shows good prognosis after treatment 1. Intracranial germinoma is the most common type of brain germ cell tumor, accounting for up to two-thirds of all intracranial germ cell tumor 2. Intracranial germinomas develop mostly in children, with a strong predilection for the young adult population 3. The most common germinoma tumor location was the pineal gland and suprasellar region, as solitary or multiple lesions 1. Intracranial germinomas present as bifocal germinoma in 6%-10% of patients, often synchronously in the pineal region and suprasellar area 4, and thus whether bifocal germinoma should be treated as a synchronous or disseminated disease is still debated 1. Some believe that bifocal germinoma is a synchronous local disease because of the excellent response to treatment, while others say it is a disseminated disease due to the subsequent leptomeningeal metastasis in some patients 5. Currently, bifocal germinoma is primarily considered a disseminated disease in the United States, while it is considered a disseminated disease in Europe 6.
At present, the preferred radiation treatment field for patients with disseminated germinoma is cranio-spinal irradiation 1. However, although cranio-spinal irradiation has shown excellent results for non-disseminated germinoma, the current recommended radiation therapy technique for non-disseminated germinoma is reduced field radiation treatment such as whole brain radiation therapy or whole ventricular radiation therapy due to the toxicity of cranio-spinal irradiation 7. Given that bifocal germinoma is yet to be established clearly as a synchronous or disseminated disease, the optimal radiation therapy field for bifocal germinoma also remains unclear 1.
Surgery followed by radiotherapy plus chemotherapy has achieved excellent survival outcomes for patients with intracranial germinomas 8. In about 10–20% of patients, however, the germinoma tumor recurs 10 years after first-line treatment 9. Furthermore, since the majority of patients with intracranial germinoma are children and adolescents, a large irradiated volume or high radiation dose results in late adverse effects such as growth disturbances or brain dysfunction 10. Thus, with this highly curable disease, alternative therapeutic strategies for treating refractory tumors, preventing avoidable morbidity and maintaining quality of life have become the main goals of current pediatric oncological efforts.
Figure 1. Intracranial germinoma
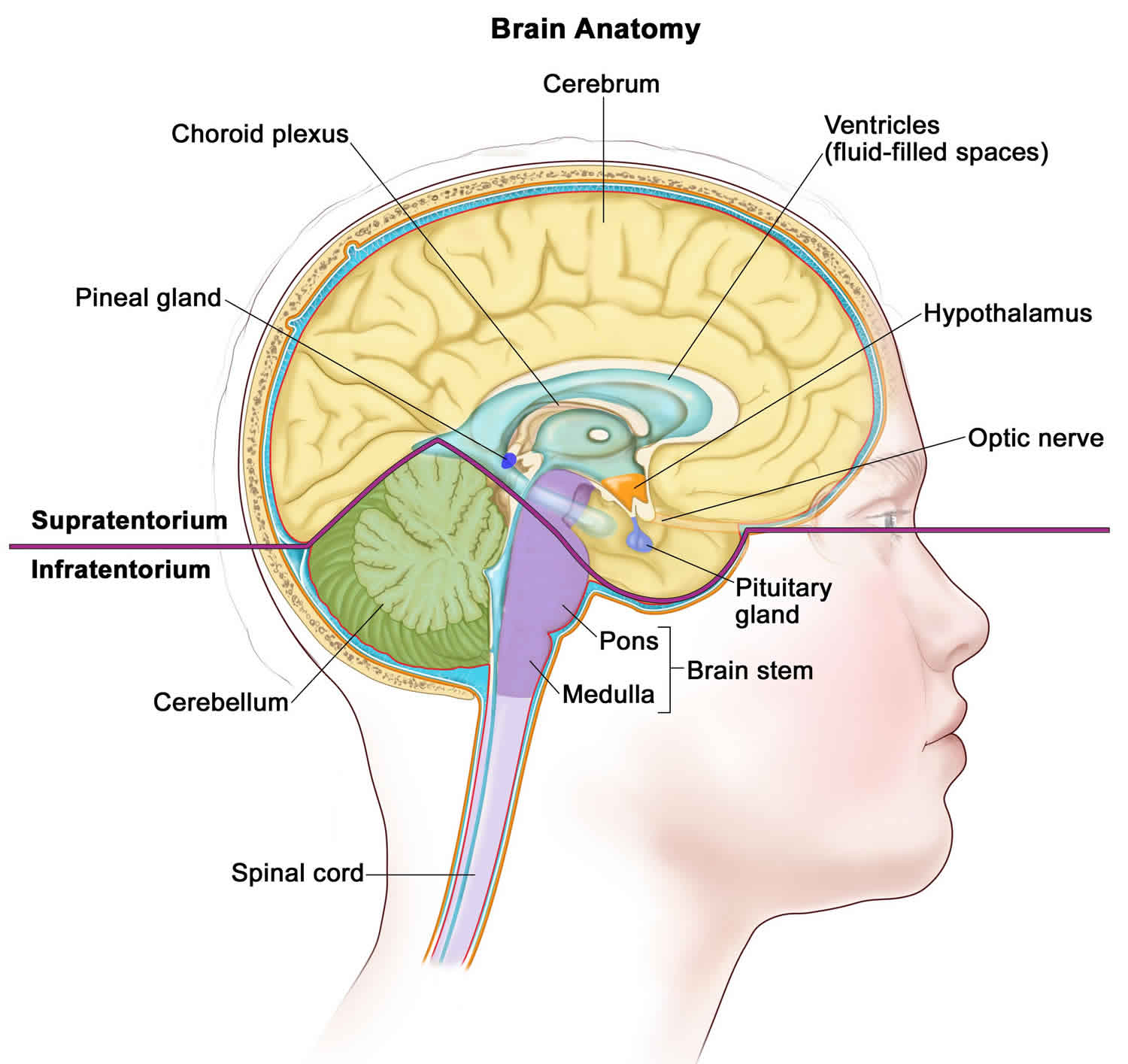
Footnote: Anatomy of the inside of the brain. The supratentorium contains the cerebrum, ventricles (with cerebrospinal fluid shown in blue), choroid plexus, hypothalamus, pineal gland, pituitary gland, and optic nerve. The infratentorium contains the cerebellum and brain stem.
Figure 2. Germinoma brain cancer
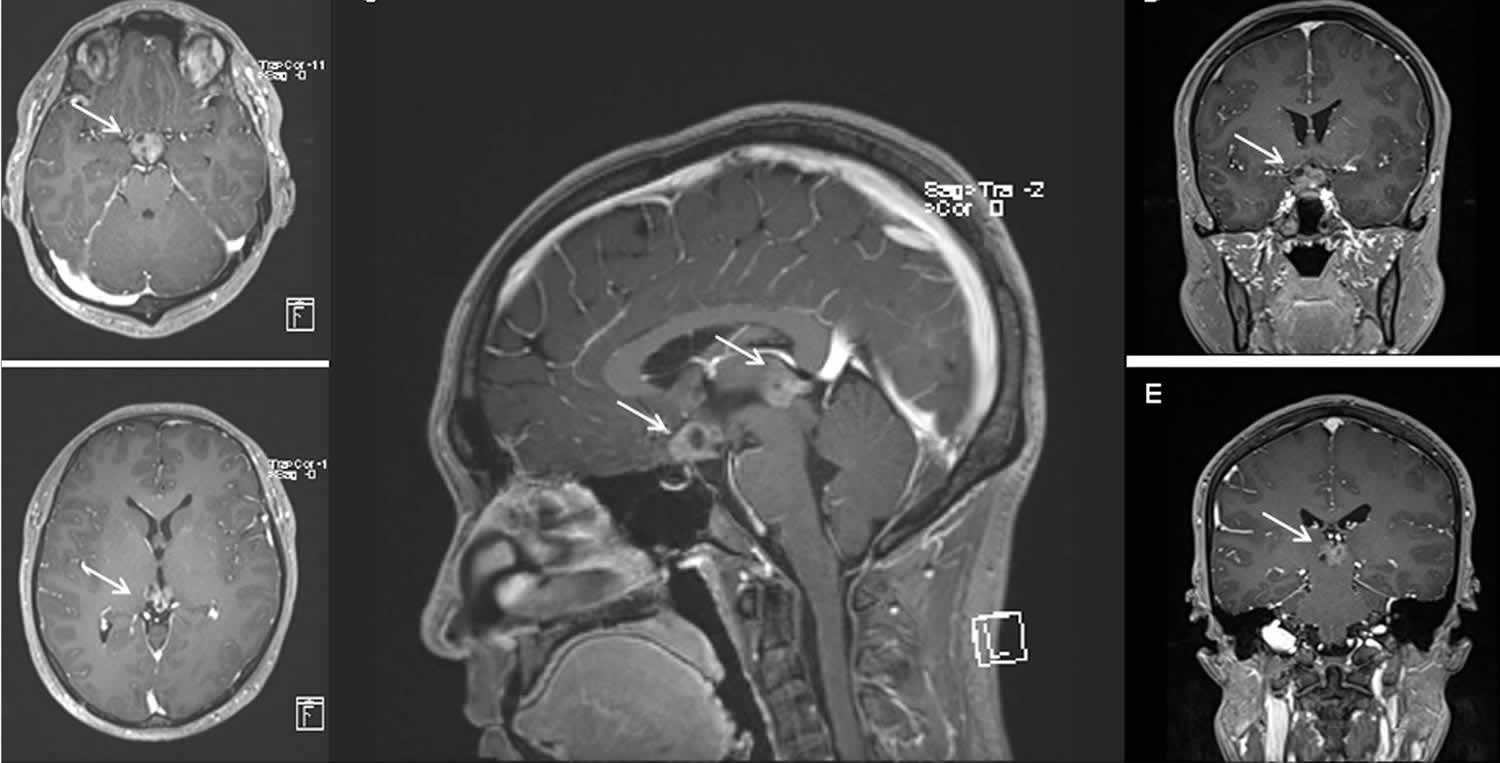
Footnote: A case of bifocal germinoma at diagnosis. (a) Suprasellar lesion (white arrow) on MRI T1 gadolinium axial image. (b) Pineal lesion (white arrow) on MRI T1 gadolinium axial image. (c) Bifocal lesions (white arrows) on MRI T1 gadolinium sagittal image. (d) Suprasellar lesion (white arrow) on MRI T1 gadolinium coronal image; E) Pineal lesion (white arrow) on MRI T1 gadolinium coronal image.
[Source 1 ]Germinoma causes
The exact cause of brain germ cell tumors is unknown. germ cell tumors appear to arise from primordial germ cells that migrate to the germinal ridges in the developing embryo 11. This process appears to be under the control of complex molecular events. Aberration in any of these molecular pathways may potentially give rise to germ cell tumors.
Important factors in cell migration include the extracellular matrix, which affects cell adherence and migration. Other factors, such as chemotropic factors, may also be involved in cell migration 12. In vitro studies have shown that tumor growth factor beta 1 may initiate the migration of primordial germ cells 13.
Some primordial germ cells that have left the yolk sac endoderm migrate aberrantly cranially towards the diencephalic midline structures rather than laterally to genital ridges.
Maturation of the fetal hypothalamus coincides with the migration of primordial germ cells. The fetal hypothalamus may secrete chemotrophic factors that attract primordial germ cells to the diencephalon 14.
The vacular theory may be an alternative event in which the primodial germ cells migrate into the mesenchyme of the mesentery and stimulate blood vessel formation and may reach intracranial locations via the circulation.
Once the primordial germ cells have reached their intracranial location through abnormal pathways, congenital or acquired aberrant molecular events occur in the primordial germ cell itself or in the surrounding microenvironment, leading to the formation of brain germ cell tumors.
The surge of the neuroendocrine functions of reproduction in the diencephalon may also be a cause or contributing factor to the development of brain germ cell tumors, as demonstrated by the location of these tumors and their predominance in the pubertal age group 15.
Germinoma symptoms
The signs and symptoms of brain germinoma tumors depend on the location of the tumor in the brain, as follows:
- Suprasellar germinoma. Patients with tumors arising in the suprasellar region often present with subtle or overt hormonal deficiencies and may experience a protracted prodrome lasting months to years. Diabetes insipidus caused by antidiuretic hormone deficiency occurs in 70% to 90% of patients and is the most common sentinel symptom; patients can usually compensate for this deficiency by drinking excessive amounts of fluid for months to years. Eventually, other hormonal symptoms and visual deficits may emerge as the tumor expands dorsally and compresses or invades the optic chiasm and/or fills the third ventricle to cause hydrocephalus 16.
- Patients with suprasellar germ cell tumors usually present with endocrine deficits. These include the following:
- Anterior hypopituitarism and diabetes insipidus
- Thyroid and/or cortisol deficiency
- Growth failure from growth hormone deficiency
- Delayed puberty from gonadotropin deficiency
- Regression of sexual development or sexual dysfunction
- Posterior pituitary dysfunction (vasopressin deficiency)
- Precocious puberty may develop in a pre-pubertal child (due to tumor-induced hypothalamic injury or secretion of human chorionic gonadotropin by the tumor).
- Visual disturbances may include diplopia, blurred vision, and diminished vision. Enuresis and psychiatric abnormalities may develop 17. In general, patients with symptoms of increased intracranial pressure and visual changes tend to present earlier in the disease course than patients with endocrine dysfunction.
- Patients with suprasellar germ cell tumors usually present with endocrine deficits. These include the following:
- Pineal germinoma. Patients with tumors in the pineal region usually have a shorter history of symptoms than do patients with tumors of the suprasellar or basal ganglionic region, with weeks to months of symptoms that include raised intracranial pressure and diplopia related to tectal and aqueductal compression. Symptoms and signs unique to masses in the pineal and posterior third ventricular region include Parinaud syndrome (vertical gaze impairment, convergence nystagmus, and light-near pupillary response dissociation), headache, and nausea and vomiting.
- Multifocal or bifocal germinoma tumors. Patients with multifocal or bifocal primary tumors may present with both suprasellar-region and pineal-region syndromes 18.
- Rare presentations of brain germ cell tumors include the following:
- Multiple lesions – germ cell tumors in the pineal, sellar region, corpus callosum, and ventricles was reported in an 18-year-old man who presented with psychosis 19.
- Wide skull base extension – This was reported in a 15-year-old girl with radiologic evidence of central skull base and suprasellar tumor extending into the cavernous sinus, intraorbital region, ethmoid sinus, sphenoid sinus, and pituitary fossa 20.
- Optic pathway – Intracranial germ cell tumors may occur primarily in the optic nerve and/or optic chiasma with progressive, painless visual loss 21; therefore, biopsy for definitive diagnosis may be required in patients with imaging studies suggestive of optic gliomas who have visual loss with hypothalamic-pituitary-adrenal dysfunction 22.
- Midbrain outflow tremor (Holmes tremor) – Holmes tremor is a hyperkinetic movement disorder that presents as mild to severe tremors, dystonia, and cerebellar deficits; it has been reported in patients with germinoma 23.
- Rare presentations of brain germ cell tumors include the following:
All patients had presenting symptoms at diagnosis such as headache and blurred vision, with a median symptom duration of 2 months (range, 1–36 months) 1. Nonspecific symptoms such as enuresis, anorexia, and psychiatric complaints 24 can lead to delays in a diagnosis, whereas signs of increased intracranial pressure or visual changes tend to result in an earlier diagnosis 25. A total of 17 patients (81%) showed endocrine dysfunction, and 9 patients (43%) had hydrocephalus 1.
Germinoma diagnosis
Radiographic characteristics of brain germinoma tumors cannot reliably differentiate germinomas from non-germ cell tumors or other brain tumors. The diagnosis of germ cell tumors is based on the following:
- Characteristic clinical signs and symptoms supported by neuroimaging.
- Histology, if available.
- Germ cell tumor marker analysis in the serum and lumbar cerebrospinal fluid (CSF).
The diagnosis of a suspected brain germ cell tumor and an assessment of the clinical deficits and extent of metastases can usually be confirmed with the following tests:
- Magnetic resonance imaging (MRI) of brain and spine with gadolinium.
- Alpha-fetoprotein (AFP) and beta subunit human chorionic gonadotropin (beta-HCG) in both serum and CSF. If preoperative lumbar CSF can be obtained safely and tumor markers are found to be elevated, histologic confirmation may not be needed. Before definitive therapy is initiated, a lumbar CSF assessment for cytology and tumor markers should be performed, if safe, to reconfirm the diagnosis and help monitor treatment response and control; the diagnostic utility of lumbar CSF is better validated and more reliable 26.
- Evaluation of pituitary/hypothalamic function.
- Visual-field and acuity examinations for suprasellar or hypothalamic tumors.
If possible, a baseline neuropsychologic examination should be performed after symptoms of endocrine deficiency and raised intracranial pressure are resolved.
A diagnosis of germ cell tumors often requires a tumor biopsy, except when characteristic increased tumor markers are found in the serum and/or CSF. When the tumor markers are negative or mildly elevated but below diagnostic criteria, or if there is any noncharacteristic finding, a tumor biopsy is performed.
It is crucial that appropriate staging is determined and that pure germinomas are distinguished from non-germinomatous germ cell tumors. Chemotherapy and radiation treatment plans differ significantly depending on germ cell tumor category and extent of disease.
The diagnosis and classification of brain germinoma tumors can be made on the basis of histology alone, tumor markers alone, or a combination of both 27. There is an effort to use tumor markers for prognostication on the basis of the presence and degree of elevation of alpha-fetoprotein (AFP) and beta-HCG. This is an evolving process, and cooperative groups in North America, Europe, and Japan have adopted slightly different criteria. For example, groups in the United States and Europe consider tumors to be secreting or mixed GCTs if serum and/or CSF alpha-fetoprotein (AFP) levels are 10 ng/mL or higher and/or serum and/or CSF beta-HCG levels are 50 IU/L or higher; however, several European and Asian groups designate tumors with serum and/or CSF alpha-fetoprotein (AFP) levels of 50 ng/mL or higher and/or beta-HCG levels of 100 IU/L or higher as secreting germinoma tumors. Patients with pure germinomas and teratomas usually present with negative markers, but low levels of beta-HCG can be detected in patients with germinomas 28.
Favorable-risk germinomas can secrete low levels of beta-HCG resulting from a syncytiotrophoblastic component. Non-germinomatous germ cell tumors can consist of one malignant non-germinomatous germ cell tumor type (e.g., embryonal carcinoma, yolk sac tumor, endodermal sinus tumor, or choriocarcinoma) or contain multiple elements of germ cell tumor components, including teratomatous or germinomatous constituents.
Stage information for childhood brain germ cell tumors
There is no universally accepted clinical staging system for germ cell tumors, but a modified Chang staging system has been traditionally used 29. Staging evaluation of central nervous system germ cell tumors includes the following:
- Magnetic resonance imaging (MRI). In addition to whole-brain MRI, MRI of the spine is required.
- Lumbar cerebrospinal fluid (CSF). When medically permissible, lumbar CSF should be obtained for the measurement of tumor markers (alpha-fetoprotein [AFP] and beta subunit human chorionic gonadotropin [beta-HCG]) and for cytopathologic review.
- Serum tumor markers are often obtained for alpha-fetoprotein (AFP) and beta-HCG; however, they do not serve as a substitute for CSF tumor markers, if lumbar CSF can be safely obtained 30.
Patients with localized disease and negative CSF cytology are considered to be M0 (metastatic negative); patients with positive CSF cytology or patients with drop metastasis (spinal or cranial subarachnoid metastases that arise from intracranial lesions) are considered to be M+ (metastatic positive). Appropriate staging is crucial because patients with metastatic disease may receive higher total doses of radiation and more extended radiation fields.
Germ cell tumors may be disseminated throughout the neuraxis at the time of diagnosis or at any disease stage. Several patterns of spread may occur in germinomas, such as subependymal dissemination in the lateral or third ventricles and parenchymal infiltration. Rarely, extracranial spread to lung and bone has also been reported 31.
Patients with bifocal intracranial germinomas limited to the suprasellar and pineal region are being treated in the same manner as are patients with synchronous, localized, nonmetastatic tumors in ongoing studies in North America (COG ACNS1123 [NCT01602666]) and Europe (SIOP CNS GCT II [NCT01424839]).
Intracranial germinoma treatment
For children older than 3 years and adults, radiation therapy has been an important component of therapy for germinomas and non-germinomatous germ cell tumors, although the optimal total dose and field size are debated.
- For children older than 3 years and adults, radiation therapy has been an important component of therapy for germinomas and non-germinomatous germ cell tumors, although the optimal total dose and field size are debated.
- Germ cell tumors arising in the central nervous system, similar to gonadal and extragonadal germ cell tumors, have demonstrated sensitivity to chemotherapy.
- Germinomas are highly chemosensitive and radiosensitive tumors. They are curable with craniospinal irradiation and local site–boost radiation therapy alone. However, the use of neoadjuvant or preirradiation chemotherapy allows reduced radiation therapy doses and volumes and, subsequently, reduced long-term radiation therapy–related effects. In North America, patients with localized germinomas are effectively treated with whole-ventricular irradiation supplemented with tumor site–boost radiation therapy. Focal irradiation to the tumor bed, regardless of response to chemotherapy, is considered inadequate treatment 32.
Table 1 outlines the treatment options for newly diagnosed and recurrent childhood CNS germ cell tumors. Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50% 33. Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment.
Table 1. Treatment options for childhood central nervous system germ cell tumors
| Treatment Group | Treatment Options |
|---|---|
| Newly diagnosed childhood germinomas | Radiation therapy |
| Neoadjuvant chemotherapy followed by response-based radiation therapy | |
| Newly diagnosed childhood teratomas | Surgery |
| Adjuvant therapy, for patients who had a subtotal resection (controversial): | |
| —Focal radiation therapy | |
| —Chemotherapy | |
| —Stereotactic radiosurgery | |
| Newly diagnosed childhood nongerminomatous germ cell tumors | Chemotherapy followed by radiation therapy |
| Surgery, if needed | |
| Recurrent childhood brain germ cell tumors | Chemotherapy followed by additional radiation therapy |
| High-dose chemotherapy with stem cell rescue with or without additional radiation therapy |
Treatment options for newly diagnosed childhood central nervous system (CNS) germinomas include the following:
- Radiation therapy.
- Neoadjuvant chemotherapy followed by response-based radiation therapy.
Radiation therapy
Germinomas are highly radiosensitive and have been traditionally treated successfully with radiation therapy alone. Historically, patients with nondisseminated disease have been treated with craniospinal irradiation plus a boost to the region of the primary tumor. The dose of craniospinal irradiation has ranged from 24 Gy to 36 Gy, although studies have used lower doses. The local tumor dose of radiation therapy has ranged between 40 Gy and 50 Gy. Studies of lower-dose craniospinal irradiation have shown excellent outcomes 34. This modification has resulted in 5-year overall survival rates of higher than 90% 35. These excellent survival rates have allowed investigators to focus on reducing radiation treatment volume and dose in an attempt to decrease late effects 32.
Patterns of relapse after craniospinal irradiation versus reduced-volume radiation therapy (whole-brain or whole-ventricular radiation therapy) have supported the omission of craniospinal irradiation for localized germinomas 36. On the basis of these results, the treatment for patients with localized germinomas has been modified to cover the whole ventricular system (24 Gy) followed by a boost to the primary site (40–45 Gy), rather than to deliver radiation therapy to the entire craniospinal axis or even to the whole brain. This change has not resulted in worse outcomes and is expected to minimize the acute and long-term toxicity of radiation therapy. Focal radiation therapy directed only to the tumor volume, even after neoadjuvant chemotherapy, results in inferior outcomes compared with whole-brain or whole-ventricle radiation therapy; therefore, focal radiation therapy is not recommended 32.
Neoadjuvant chemotherapy followed by response-based radiation therapy
Chemotherapy has been explored in an effort to reduce radiation therapy doses and associated neurodevelopmental morbidity. Several studies have confirmed the feasibility of this approach for maintaining excellent survival rates, but the number of treated patients is small 37.
Chemotherapy agents such as cyclophosphamide, ifosfamide, etoposide, cisplatin, and carboplatin are highly active in CNS germinomas. Patients receiving chemotherapy agents that require hyperhydration (e.g., cyclophosphamide, ifosfamide, and cisplatin) are often quite challenging to manage because of the possibility of diabetes insipidus in patients with primary tumors of the suprasellar region 38.
An international group of investigators has explored a chemotherapy-only approach primarily for younger children. The investigators were able to achieve a complete response in 84% of patients with germinomas treated with chemotherapy alone. Fifty percent of these patients suffered tumor relapse or progression; many recurrences were local, local plus ventricular, and ventricular alone and/or with leptomeningeal dissemination throughout the CNS, which required additional therapy, including radiation 39. Subsequent studies have continued to support the need for radiation therapy after chemotherapy and the likely requirement for whole-ventricular irradiation (24 Gy) with local tumor site–boost (total dose, 40 Gy) 40. Excellent results have also been reported for patients with metastatic germinomas who received craniospinal irradiation of 24 Gy with local tumor site–boost (total dose, 40 Gy) 29.
Optimal management of bifocal lesions is less clear, but most investigators consider this presentation a form of multifocal primary disease to be staged as M0. A meta-analysis of 60 patients demonstrated excellent progression-free survival after craniospinal irradiation alone. Chemotherapy plus localized radiation therapy, including whole-ventricular irradiation, also resulted in excellent disease control 41.
Treatment options under clinical evaluation for newly diagnosed childhood brain germinomas
Early-phase therapeutic trials may be available for selected patients. These trials may be available via the Children’s Oncology Group (COG), the Pediatric Brain Tumor Consortium, or other entities.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- COG-ACNS1123 (NCT01602666) (Chemotherapy Followed by Radiation Therapy in Treating Younger Patients With Newly Diagnosed Localized CNS Germ Cell Tumors [GCTs]): COG-ACNS1123 is a COG cooperative multi-institutional trial. This phase II trial of response-based radiation therapy for patients with localized germinoma (stratum 2) will compare the event-free survival and overall survival rates of a short course of chemotherapy followed by response-based, whole-ventricular radiation therapy, with a boost to the primary site. For patients who obtain a complete response after chemotherapy, the whole-ventricular radiation dose will be 25% lower than the standard whole-ventricular dose; for patients who have a less-than-complete response after chemotherapy, the standard whole-ventricular dose will be used, with or without second-look surgery.
- SIOP CNS GCT II (NCT01424839) (Prospective Trial for the Diagnosis and Treatment of Intracranial Germ Cell Tumors): This prospective, nonrandomized, multicenter study stratifies treatment according to risk groups.
Intracranial germinoma prognosis
Germinomas are generally associated with an excellent prognosis. Even in patients with syncytiotrophoblasts that secrete β-hCG, 5-year survival is 70-90% and 10-year survival is 70% 42. With mixed germ cell tumors, 5-year survival is 60-80%. With nongerminomatous germ cell tumors, 5-year survival is 30-50%.
Patients with pure germinomas have a 10-year survival rate of 90%. For nongerminomatous germ cell tumor, the 10-year overall survival rates were reported to be 30%-80% 43.
Diabetes insipidus, hypopituitarism, and visual field deficits are the most common presentation of CNS germ cell tumors and may persist despite therapy. Parinaud syndrome is common in patients with pineal tumors and often persists even after therapy.
Surgery of deep-seated structures within the brain may be associated with significant morbidity. However, modern neurosurgical navigation techniques have minimized this risk. Tissue sampling by stereotactic biopsy is a safe and rapid method of determining tumor histology. Pineal-region tumors have a surgical morbidity of 2-5%. Patients may suffer from transient movement abnormalities of eyes, ataxia, and cognitive dysfunction.
Late sequelae of radiation therapy to the CNS include growth effects, hearing loss, neuropsychological and cognitive impairments, and neuro-endocrine disorders 44. Risks of treatment-related secondary cancers are well described. Larger irradiation volume and dose both adversely affect intellectual functions, concept, executive function, memory, decline in neurocognitive function, and performance IQs, particularly in children 44.
Patients may have persistent neurological deficits, even after tumor control. Neurological deficits may be significant and are multifactorial in origin. Damage by the tumor itself, surgical intervention, radiation therapy, and chemotherapy all contribute to neurological impairment irrespective of age. Patients with tumors located in the basal ganglia perform poorly compared with those who have tumors in the pineal and suprasellar regions; they have lower full-scale IQs and short-term retention of verbal and visual stimuli 45.
Several long-term studies have demonstrated poor performance in adaptive skills, particularly in psychosocial domains, behavioral dysfunction, and financial difficulties, leading to poor quality of life 46. Patients who had undergone surgical biopsies did worse than patients who had surgical resection. Lower Karnofsky performance status scale scores following surgery have been associated with impaired neurocognitive function that may decline over time, particularly in children 47.
More than 50% of patients may continue to suffer from endocrine abnormalities, with growth hormone deficiency and growth retardation, hypopituitarism, and hypothyroidism. They may require lifelong hormonal replacement therapy 47.
Brain injury in the form of atrophy, multifocal encephalomalacia, leukoencephalopathy, and focal necrosis has been reported in patients with intracranial germ cell tumors 48. The occurrence of cerebrovascular occlusion may lead to the development of strokes, with an almost 59-fold increase risk of death in long-term survivors 46.
Patients with intracranial germ cell tumors have a cumulative incidence of secondary cancer of 6%, with a cumulative risk of death due to malignancy of 16%. Radiation therapy and chemotherapy may both promote the development of secondary cancers, including but not limited to acute myeloid leukemia and radiation-induced brain neoplasms 46.
References- Chung SY, Han JW, Kim DS, Yoon HI, Suh CO. Treatment outcomes based on radiation therapy fields for bifocal germinoma: Synchronous or disseminated disease?. PLoS One. 2019;14(10):e0223481. Published 2019 Oct 3. doi:10.1371/journal.pone.0223481 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6776334
- Liu B, Arakawa Y, Yokogawa R, et al. PD-1/PD-L1 expression in a series of intracranial germinoma and its association with Foxp3+ and CD8+ infiltrating lymphocytes. PLoS One. 2018;13(4):e0194594. Published 2018 Apr 4. doi:10.1371/journal.pone.0194594 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5884516
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellision DW, Figarella-Branger D, et al. WHO classification and grading of tumours of the central nervous system International Agency for Research on Cancer, France: IARC Press; 2016.
- Thakkar JP, Chew L, Villano JL. Primary CNS germ cell tumors: current epidemiology and update on treatment. Med Oncol. 2013;30: 496 10.1007/s12032-013-0496-9
- Al-Mahfoudh R, Zakaria R, Irvine E, Pizer B, Mallucci CL. The management of bifocal intracranial germinoma in children. Childs Nerv Syst. 2014;30: 625–630. 10.1007/s00381-013-2287-1
- Rogers SJ, Mosleh-Shirazi MA, Saran FH. Radiotherapy of localised intracranial germinoma: time to sever historical ties? Lancet Oncol. 2005;6: 509–519. 10.1016/S1470-2045(05)70245-X
- Schoenfeld GO, Amdur RJ, Schmalfuss IM, Morris CG, Keole SR, Mendenhall WM, et al. Low-dose prophylactic craniospinal radiotherapy for intracranial germinoma. Int J Radiat Oncol Biol Phys. 2006;65: 481–485. 10.1016/j.ijrobp.2005.12.012
- Fu H, Guo X, Li R, Xing B. Radiotherapy and chemotherapy plus radiation in the treatment of patients with pure intracranial germinoma: A meta-analysis. Journal of clinical neuroscience: official journal of the Neurosurgical Society of Australasia. 2017. doi: 10.1016/j.jocn.2017.05.024
- Kawabata Y, Takahashi JA, Arakawa Y, Shirahata M, Hashimoto N. Long term outcomes in patients with intracranial germinomas: a single institution experience of irradiation with or without chemotherapy. J Neurooncol. 2008;88(2):161–7. doi: 10.1007/s11060-008-9542-4
- Kenjo M, Yamasaki F, Takayasu T, Nosaka R, Murakami Y, Kimura T, et al. Results of sequential chemoradiotherapy for intracranial germinoma. Jpn J Radiol. 2015;33(6):336–43. doi: 10.1007/s11604-015-0424-3
- Echevarría ME, Fangusaro J, Goldman S. Pediatric central nervous system germ cell tumors: a review. Oncologist. 2008 Jun. 13(6):690-9
- Pereda J, Motta PM. A unique fibrillar coat on the surface of migrating human primordial germ cells. Arch Histol Cytol. 1991 Oct. 54(4):419-25.
- Godin I, Wylie CC. TGF beta 1 inhibits proliferation and has a chemotropic effect on mouse primordial germ cells in culture. Development. 1991 Dec. 113(4):1451-7.
- Horowitz MB, Hall WA. Central nervous system germinomas. A review. Arch Neurol. 1991 Jun. 48(6):652-7.
- Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg. 1985 Aug. 63(2):155-67.
- Sethi RV, Marino R, Niemierko A, et al.: Delayed diagnosis in children with intracranial germ cell tumors. J Pediatr 163 (5): 1448-53, 2013.
- Malbari F, Gershon TR, Garvin JH, Allen JC, Khakoo Y, Levy AS, et al. Psychiatric manifestations as initial presentation for pediatric CNS germ cell tumors, a case series. Childs Nerv Syst. 2016 Aug. 32 (8):1359-62.
- Hoffman HJ, Otsubo H, Hendrick EB, et al.: Intracranial germ-cell tumors in children. J Neurosurg 74 (4): 545-51, 1991.
- Yang P, Li L, Kuang W, Li B, Zhou B, Yang J, et al. Intracranial multiple germ cell tumors: a case report and review of literature. Int J Clin Exp Pathol. 2014. 7(12):9002-7.
- Zhou ZH, Zhang HB, Rao J, Bian XW. Clinical diagnostic dilemma of intracranial germinoma manifesting as wide skull base extension. J Craniofac Surg. 2014 Sep. 25(5):e467-70.
- Chaudhry NS, Ahmad FU, Whittington E, Schatz N, Morcos JJ. Primary Intrinsic Chiasmal Germinoma. J Neuroophthalmol. 2015 Jan 22.
- Wilson JT, Wald SL, Aitken PA, Mastromateo J, Vieco PT. Primary diffuse chiasmatic germinomas: differentiation from optic chiasm gliomas. Pediatr Neurosurg. 1995. 23(1):1-5; discussion 6.
- Strowd RE, Burger P, Holdhoff M, Kleinberg L, Okun MS, Olivi A, et al. Steroid-responsive intracranial germinoma presenting as Holmes’ tremor: Importance of a tissue diagnosis. J Clin Neurosci. 2015 May. 22(5):911-3.
- Malbari F, Gershon TR, Garvin JH, et al.: Psychiatric manifestations as initial presentation for pediatric CNS germ cell tumors, a case series. Childs Nerv Syst 32 (8): 1359-62, 2016.
- Crawford JR, Santi MR, Vezina G, et al.: CNS germ cell tumor (CNSGCT) of childhood: presentation and delayed diagnosis. Neurology 68 (20): 1668-73, 2007.
- Allen J, Chacko J, Donahue B, et al.: Diagnostic sensitivity of serum and lumbar CSF bHCG in newly diagnosed CNS germinoma. Pediatr Blood Cancer 59 (7): 1180-2, 2012.
- Rosenblum MK, Nakazato Y, Matsutani M: Germ cell tumours. In: Louis DN, Ohgaki H, Wiestler OD: WHO Classification of Tumours of the Central Nervous System. 4th rev.ed. Lyon, France: IARC Press, 2016, pp 286-91
- Calaminus G, Bamberg M, Harms D, et al.: AFP/beta-HCG secreting CNS germ cell tumors: long-term outcome with respect to initial symptoms and primary tumor resection. Results of the cooperative trial MAKEI 89. Neuropediatrics 36 (2): 71-7, 2005.
- Calaminus G, Kortmann R, Worch J, et al.: SIOP CNS GCT 96: final report of outcome of a prospective, multinational nonrandomized trial for children and adults with intracranial germinoma, comparing craniospinal irradiation alone with chemotherapy followed by focal primary site irradiation for patients with localized disease. Neuro Oncol 15 (6): 788-96, 2013.
- Fujimaki T, Mishima K, Asai A, et al.: Levels of beta-human chorionic gonadotropin in cerebrospinal fluid of patients with malignant germ cell tumor can be used to detect early recurrence and monitor the response to treatment. Jpn J Clin Oncol 30 (7): 291-4, 2000.
- Jennings MT, Gelman R, Hochberg F: Intracranial germ-cell tumors: natural history and pathogenesis. J Neurosurg 63 (2): 155-67, 1985.
- Joo JH, Park JH, Ra YS, et al.: Treatment outcome of radiation therapy for intracranial germinoma: adaptive radiation field in relation to response to chemotherapy. Anticancer Res 34 (10): 5715-21, 2014.
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.
- Bamberg M, Kortmann RD, Calaminus G, et al.: Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol 17 (8): 2585-92, 1999.
- Cho J, Choi JU, Kim DS, et al.: Low-dose craniospinal irradiation as a definitive treatment for intracranial germinoma. Radiother Oncol 91 (1): 75-9, 2009.
- Rogers SJ, Mosleh-Shirazi MA, Saran FH: Radiotherapy of localised intracranial germinoma: time to sever historical ties? Lancet Oncol 6 (7): 509-19, 2005.
- Kretschmar C, Kleinberg L, Greenberg M, et al.: Pre-radiation chemotherapy with response-based radiation therapy in children with central nervous system germ cell tumors: a report from the Children’s Oncology Group. Pediatr Blood Cancer 48 (3): 285-91, 2007.
- Afzal S, Wherrett D, Bartels U, et al.: Challenges in management of patients with intracranial germ cell tumor and diabetes insipidus treated with cisplatin and/or ifosfamide based chemotherapy. J Neurooncol 97 (3): 393-9, 2010.
- Balmaceda C, Heller G, Rosenblum M, et al.: Chemotherapy without irradiation–a novel approach for newly diagnosed CNS germ cell tumors: results of an international cooperative trial. The First International Central Nervous System Germ Cell Tumor Study. J Clin Oncol 14 (11): 2908-15, 1996.
- da Silva NS, Cappellano AM, Diez B, et al.: Primary chemotherapy for intracranial germ cell tumors: results of the third international CNS germ cell tumor study. Pediatr Blood Cancer 54 (3): 377-83, 2010.
- Weksberg DC, Shibamoto Y, Paulino AC: Bifocal intracranial germinoma: a retrospective analysis of treatment outcomes in 20 patients and review of the literature. Int J Radiat Oncol Biol Phys 82 (4): 1341-51, 2012.
- Villano JL, Propp JM, Porter KR, Stewart AK, Valyi-Nagy T, Li X. Malignant pineal germ-cell tumors: an analysis of cases from three tumor registries. Neuro Oncol. 2008 Apr. 10(2):121-30.
- Lee SH, Jung KW, Ha J, Oh CM, Kim H, Park HJ, et al. Nationwide Population-Based Incidence and Survival Rates of Malignant Central Nervous System Germ Cell Tumors in Korea, 2005-2012. Cancer Res Treat. 2017 Apr. 49 (2):494-501.
- Sugiyama K, Yamasaki F, Kurisu K, Kenjo M. Quality of life of extremely long-time germinoma survivors mainly treated with radiotherapy. Prog Neurol Surg. 2009. 23:130-9.
- Liang SY, Yang TF, Chen YW, Liang ML, Chen HH, Chang KP, et al. Neuropsychological functions and quality of life in survived patients with intracranial germ cell tumors after treatment. Neuro Oncol. 2013 Nov. 15(11):1543-51.
- Acharya S, DeWees T, Shinohara ET, Perkins SM. Long-term outcomes and late effects for childhood and young adulthood intracranial germinomas. Neuro Oncol. 2015 May. 17(5):741-6.
- Jinguji S, Yoshimura J, Nishiyama K, Aoki H, Nagasaki K, Natsumeda M, et al. Factors affecting functional outcomes in long-term survivors of intracranial germinomas: a 20-year experience in a single institution. J Neurosurg Pediatr. 2013 Apr. 11(4):454-63.
- Sutton LN, Radcliffe J, Goldwein JW, Phillips P, Janss AJ, Packer RJ, et al. Quality of life of adult survivors of germinomas treated with craniospinal irradiation. Neurosurgery. 1999 Dec. 45(6):1292-7; discussion 1297-8.