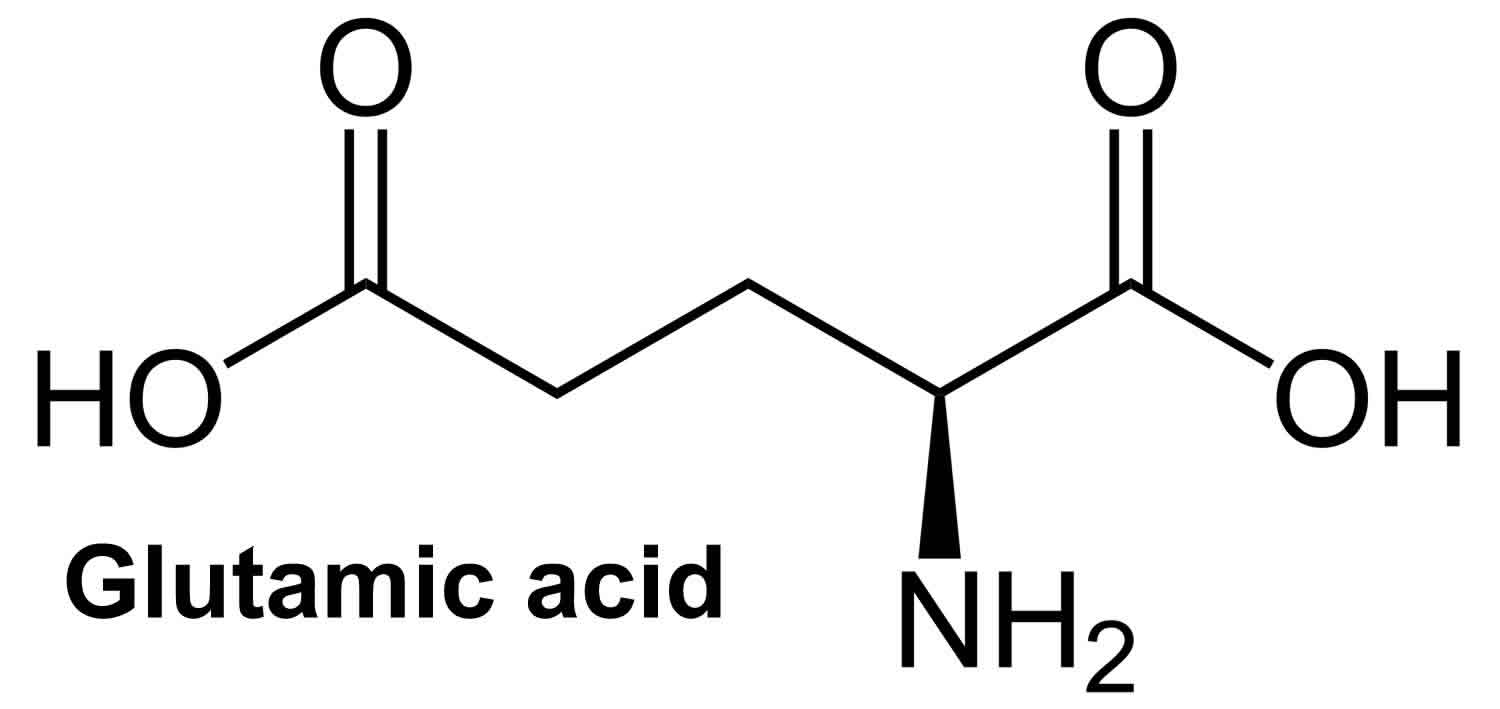
What is glutamic acid
Glutamic acid (C5H9O4N) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. Glutamic acid is non-essential amino acid in humans, meaning the body can synthesize it from oxoglutaric acid (formed in the metabolism of carbohydrates) and do not require dietary sources. In the body glutamic acid turns into glutamate. Glutamate is the most common excitatory (stimulating) neurotransmitter, in fact the most abundant one, in the vertebrate central nervous system. Glutamic acid serves as the precursor for other amino acids i.e., arginine and proline as well as for bioactive molecules such as gamma-aminobutyric acid (GABA) in GABA-ergic neurons and glutathione. GABA possesses several well-known physiological functions [i.e., anti-hypertension 1 and anti-diabetic 2] and glutathione plays a key role in the protection of the mucosa from peroxide damage and from dietary toxins 3. Furthermore, a number of studies have shown the possible usefulness of glutamic acid in enhancing nourishment in the elderly and in patients with poor nutrition 4.
Glutamic acid is one of the most common natural amino acids and the most abundant amino acid in the diet. The flavor contributions made by glutamic acid and other amino acids were only scientifically identified early in the twentieth century. The substance was discovered and identified in the year 1866, by the German chemist Karl Heinrich Ritthausen who treated wheat gluten (for which it was named) with sulfuric acid 5. In 1908 Japanese researcher Kikunae Ikeda of the Tokyo Imperial University identified brown crystals left behind after the evaporation of a large amount of kombu broth as glutamic acid. These crystals, when tasted, reproduced the ineffable but undeniable flavor he detected in many foods, most especially in seaweed. Professor Ikeda termed this flavor “umami” 6. He then patented a method of mass-producing a crystalline salt of glutamic acid, monosodium glutamate (MSG). Monosodium glutamate (MSG), a salt of glutamic acid, is sometimes used as a condiment for flavoring foods and has been suggested to function as a sensory cue for protein ingestion 7. Monosodium glutamate (MSG) is a flavor enhancer that has been used effectively to bring out the best taste in foods, emphasizing natural flavors. Many researchers also believe that MSG imparts a fifth taste, independent of the four basic tastes of sweet, sour, salty and bitter.
The average American consumes about 11 grams of glutamate per day from natural protein sources and less than 1 gram of glutamate per day from MSG. This amount of added MSG is the same as adding 1 to 1.5 ounces of parmesan cheese. In contrast, the human body creates about 50 grams of glutamate daily for use as a vital component of metabolism.
Glutamic acid vs glutamine
In the body glutamic acid turns into glutamate. Glutamate is the anionic/salt form of glutamic acid and is considered the most abundant neurotransmitter in the brain 8. Glutamate is known to act on three families of ionotropic receptors: N-methyl-D-aspartate (NMDA), 2a‐amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and kainite 9. In addition, five glutamate transport enzymes, i.e. glial glutamate and aspartate transporter (GLAST), glial glutamate transporter (GLT), excitatory amino acid carrier 1 (EAAC1), excitatory amino acid transporter (EAAT) 4 and EAAT5 have been found in the mammalian nervous system 10. Although glutamate is an important neurotransmitter, it is also associated with neurotoxicity and chronic neurodegeneration 11. In addition, glutamate seems to mediate beta cell apoptosis through oxidative stress 12, whereas pre‐treating them with ceftriaxone, which is known to increase GLT1 expression, rescues these cells by enhancing the expression of glutamate transporter GLT1 13.
Glutamic acid function
Glutamic acid is a multifunctional amino acid involved in taste perception, excitatory neurotransmission 14 and intermediary metabolism 15. Glutamic acid (glutamine) is the most common neurotransmitter in the mammalian brain 14. Glutamic acid (glutamine) is a chemical that helps nerve cells in the brain send and receive information from other cells. Glutamate plays an important role in neuronal differentiation, migration and survival in the developing brain 14. This is largely through facilitating the entry of Ca++ 16. Blockade of N-methyl-D-aspartate (NMDA) receptors during the prenatal period [as by dizocilpine (MK-801), phencyclidine or ethanol] can induce apoptosis in vulnerable neurons (the selectivity of the vulnerability depending on developmental stage) 17.
Glutamic acid (glutamine) may be involved in learning and memory. At chemical synapses of the glutamatergic neurons, glutamate is stored in vesicles and is released from the pre-synaptic cell by nerve impulses. In the opposing post-synaptic cell, binding of glutamate lead to activation of specific glutamate receptors such as N-methyl-D-aspartate (NMDA) or α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA) 18. Glutamate plays an important role in synaptic plasticity in the brain and is involved in various cognitive functions, such as learning and memory 19. In fact, long-term potentiation (one of the plasticity forms) takes place at glutamatergic synapses in the neocortex, hippocampus and other parts of the brain 19. Another important role of glutamate is its ability to generate volume transmission, where extrasynaptic signaling is created via the summation of glutamate released from a neighboring synapse 20.
Glutamic acid plays various crucial roles in the intestinal tract, including (1) substrate for various metabolic pathways 21, (2) energy source for intestinal mucosa 22, (3) mediator for cell signaling 23, (4) regulator for oxidative reactions 24, as well as immune responses and barrier function 25.
Glutamic acid (glutamine) also play an important role in gastric phase digestion with multiplicity effects in the gastrointestinal tract when consumed with nutrients by enhancing gastric exocrine secretion 26. It may help people with hypochlorhydria (low stomach acid) or achlorhydria (no stomach acid). Furthermore, a number of studies have shown the possible usefulness of glutamic acid in enhancing nourishment in the elderly and in patients with poor nutrition 4.
Glutamic acid benefits
Glutamic acid benefits may include:
- Treatment for personality and childhood behavioral issues
- Help treat epilepsy and muscular dystrophy
- Treat intellectual disorders
- Treat low blood sugar (hypoglycemia) in people with diabetes
- Prevent nerve damage in people having chemotherapy
There may be other benefits that have not yet been proven through research.
Glutamic acid side effects
Glutamic acid has previously been shown to exert neurotoxic affects and is associated with neurodegenerative diseases 27. Glutamic acid has also been shown to be toxic to human pancreatic beta cells at physiological concentrations 12. In this study 8, Banday and Lejon reported an increased apoptosis among non‐obese diabetic mice beta cells after culture with glutamic acid. Banday and Lejon proposed that it is plausible that the increased glutamic acid levels leading to enhanced pancreatic beta cells apoptosis acts as one of the triggers in type 1 diabetes in non‐obese diabetic mice and that it is possible that a similar mechanism contributes to human type 1 diabetes. However, further studies are needed to support this hypothesis.
Glutamate and neurodegeneration
Glutamate is of particular interest to neurologists because of its possible involvement in acute or chronic neurodegenerative processes. It is useful to consider three distinct possible mechanisms.
One is the possibility that exogenous glutamate, or related compounds acting on glutamate receptors, can be consumed in the diet and damage the brain. There is one well-documented example of such a phenomenon in humans 14. In humans, the only decisively documented example of a dietary toxin producing pathology through action on a glutamate receptor is that of domoic acid 28. Domoic acid is synthesized by marine diatoms (Nitschia pungens) and enters the food chain when it is concentrated by blue mussels (Mytilus edulis) feeding on the algae. In an outbreak of such poisoning in eastern Canada in 1987, affected individuals developed acute symptoms within 1–4 hours of consuming 200–300 mg of domoate. An acute confusional state was the usual presenting feature, focal seizure signs were less commonly observed, but the picture was consistent with prolonged limbic seizure activity. A persistent anterograde amnesia was observed in some cases.
Neuropathologic studies in four elderly men who succumbed after days revealed extensive bilateral limbic system pathology with neuronal loss in cellular zones of hippocampus (CA1, CA3, dentate gyrus), amygdala, claustrum, septal area, thalamus and insular and subfrontal cortex. Similar patterns of damage can be induced by systemic injection of domoate or kainate in rodents, or by their focal injection into the hippocampus. The pathology is likely a consequence mainly of the limbic seizure activity rather than the effect of a direct excitotoxic action of domoate. This is shown by the observation that almost all of the pathology (commonly except for CA3 cell loss and sometimes some amygdala damage) is prevented by the administration of an NMDA receptor antagonist 29. It is likely that only the CA3 neurons are dying as a direct result of the excitotoxic action of domoate. A similar protective effect against remote damage after kainate-induced limbic seizure activity can be obtained with diazepam 30.
In infant rats and mice (0–14 days old), the oral or intraperitoneal administration of high doses of glutamate or aspartate can be followed by acute neuronal degeneration in the retina (ganglion cells) and in various periventricular structures in the brain, including the arcuate nucleus of the hypothalamus 31. Whether this also occurs in primates is somewhat uncertain. Degeneration was reported by Olney et al. 32 but not seen by several other authors 33. The effect, in infant rodents, might possibly be related to the lesser capacity of their intestinal epithelium and liver to transaminate glutamate and aspartate, or to a lesser expression of the glial glutamate transporters GLT and GLAST in the hypothalamus at this developmental stage 34. Developmental changes in the expression of ionotropic glutamate receptors are known to influence excitotoxic phenomena 35 and may contribute to the pattern of vulnerability in the neonatal rodent.
Second, there is the possibility that endogenous glutamate released from neurons can contribute to acute neurodegeneration occurring in relation to cerebral ischemia or traumatic brain injury. In addition to glutamate receptors, neuronal and glial membranes contain glutamate transporters that are responsible for rapid removal of glutamate from extracellular space 36. Under stress conditions (such as brain injury or disease), glutamate transporters work in reverse leading to the accumulation of the excess glutamate in the extracellular space and promoting entrance of calcium to the cell via the NMDA receptor channels. This process is known as excitotoxicity, and it results in neuronal damage and eventual cell death. The excitotoxicity might occur as part of the ischemic cascade that is associated with stroke, autism, amyotrophic lateral sclerosis, lathyrism, some forms of mental retardation and Alzheimer’s disease 37. The decreased glutamate release is associated with phenylketonuria leading to the developmental disruption of glutamate receptor expression 38.
Third, there is the possibility that activation of glutamate receptors contributes to the process of cell death in chronic neurodegenerative disorders, such as motor neuron disease or amyotrophic lateral sclerosis (ALS), Huntington’s disease, Parkinson’s disease and Alzheimer’s disease 14.
Glutamate can be neurotoxic through an agonist effect on NMDA, AMPA, kainate or Group 1 metabotropic receptors and is thought to play an important role in cell death subsequent to status epilepticus, cerebral ischemia, perinatal asphyxia and traumatic brain injury 14. The relative contribution of these different classes of receptor varies according to the neurons involved and a variety of other circumstances. Selective neuronal death subsequent to status epilepticus appears to be highly dependent on NMDA receptor activation; thus NMDA receptor antagonists of all types (glutamate receptor competitive antagonists, glycine site competitive antagonists, open channel blockers and selective antagonists acting preferentially on a polyamine site or on the NR2B subunit of the NMDA receptor) protect against ischemic brain damage 39. NMDA receptors have different subunit composition according to their site of expression. Receptors with NR2B subunits are expressed particularly on GABAergic interneurons, so that antagonists acting selectively on these NMDA receptors may have effects differing from those of antagonists acting on NMDAI/NR2A receptors.
Acute neuronal degeneration after transient global or focal cerebral ischemia seems to be dependent on both NMDA and AMPA receptors.
It has been proposed that neurodegeneration in a variety of late onset neurological disorders is at least partially dependent on endogenous glutamate activating NMDA or AMPA receptors. These include motor neuron disease, Huntington’s disease, Parkinson’s disease and Alzheimer’s disease 14.
The evidence that AMPA receptors on spinal motoneurons are involved in motor neuron disease (ALS or amyotrophic lateral sclerosis) is of several types 40. There appears to be a reduction in the expression of GLT-1, a glial glutamate transporter, in the spinal cord and brain regions showing loss of motoneurons 41. In organotypic cultures of spinal cord, glutamate transport inhibitors cause degeneration of motoneurons. This can be prevented by AMPA receptor antagonists such as GYKI 52466 42. AMPA receptor antagonists protect against the toxic effects of mutations in Cu/Zn superoxide dismutase in cultured mouse neurons 43.
Huntington’s disease may involve a primary metabolic or mitochondrial defect that causes striatal neurons to become vulnerable to excitotoxic effects of NMDA receptor activation.
Susceptibility to excitotoxic cell death is under genetic control in a variety of ways. Single-gene defects may enhance vulnerability, as in the case of superoxide dismutase. Some induced gene defects in mice confer protection against excitotoxic damage (e.g., neuronal nitric oxide synthase-knockout mice show reduced sensitivity to focal ischemia). Genetic background can be protective. Thus C57BL/6 and BALB/c mice are relatively insensitive to the excitotoxic effect of kainic acid in the hippocampus 44.
Glutamic acid foods
Glutamate occurs naturally in protein-containing foods such as cheese, milk, mushrooms, meat, fish, and many vegetables.
Monosodium glutamate or MSG, is the sodium salt of glutamate. When MSG is added to foods, it provides a similar flavoring function as the glutamate that occurs naturally in food. MSG is comprised of nothing more than water, sodium and glutamate. In the early 1900s, monosodium glutamate (MSG) was extracted from natural protein-rich foods such as seaweed. Today, MSG is made from starch, corn sugar or molasses from sugar cane or sugar beets. MSG is produced by a natural fermentation process that has been used for centuries to make such common foods as beer, vinegar and yogurt.
The human body treats glutamate that is added to foods in the form of monosodium glutamate (MSG) the same as the natural glutamate found in food. For instance, the body does not distinguish between free glutamate from tomatoes, cheese or mushrooms and the glutamate from MSG added to foods. Glutamate is glutamate, whether naturally present or from MSG.
Glutamate is also produced by the human body and is vital for metabolism and brain function.
Table 1. Glutamate contents of foods
| Food Size | Serving Glutamate (g/serving) | |
| Tomato juice | 1 cup | 0.827 |
| Tomato | 3 slices | 0.339 |
| Meat loaf dinner | 9 oz. | 0.189 |
| Human breast milk | 1 cup | 0.176 |
| Mushrooms | 1/4 cup | 0.094 |
| Parmesan cheese | 2 Tbsp | 0.047 |
| Corn | 1/2 cup | 0.031 |
| Peas | 1/2 cup | 0.024 |
| Cow’s milk | 1 cup | 0.016 |
| Canned tuna (in water) | 1/2 can | 0.008 |
- Inoue K., Shirai T., Ochiai H., Kasao M., Hayakawa K., Kimura M., Sansawa H. Blood pressure lowering effect of a novel fermented milk containing g amino butyric acid (GABA) in mild hypertensives. Eur. J. Clin. Nutr. 2003;27:490–495.
- Hagiwara H., Seki T., Ariga T. The effect of pre-germinated brown rice intake on blood glucose and PAI-1 levels in streptozotocin-induced diabetic rats. Biosci. Biotechnol. Biochem. 2004;68:444–447.
- Beyreuther K., Biesalski H., Fernstrom J., Grimm P., Hammes W., Heinemann U., Kempski O., Stehle P., Steinhart H., Walker R. Consensus meeting: Monosodium glutamate—An update. Eur. J. Clin. Nutr. 2006;61:304–313.
- Yamamoto S., Tomoe M., Toyama K., Kawai M., Uneyama H. Can dietary supplementation of monosodium glutamate improve the health of the elderly? Am. J. Clin. Nutr. 2009;90:844S–849S.
- R.H.A. Plimmer (1912) [1908]. R.H.A. Plimmer; F.G. Hopkins (eds.). The Chemical Constitution of the Protein. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co. p. 114.
- Yamaguchi S, Ninomiya K. Umami and food palatability. J Nutr 2000;130:921S–6S.
- Torii K, Mimura T, Yugari Y. Biochemical mechanism of umami taste perception and effect of dietary protein on the taste preference for amino acids and sodium chloride in rats. In: Kawamura Y, Kare MR, eds. Umami: a basic taste. New York, NY: Marcel Dekker, 1987:513–63.
- Banday VS, Lejon K. Elevated systemic glutamic acid level in the non-obese diabetic mouse is Idd linked and induces beta cell apoptosis. Immunology. 2017;150(2):162-171. doi:10.1111/imm.12674 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5214223
- Meldrum BS. Glutamate as a neurotransmitter in the brain: review of physiology and pathology. J Nutr 2000; 130:1007s–15s.
- Seal RP, Amara SG. Excitatory amino acid transporters: a family in flux. Annu Rev Pharmacol Toxicol 1999; 39:431–56.
- Mitani A, Watanabe M, Kataoka K. Functional change of NMDA receptors related to enhancement of susceptibility to neurotoxicity in the developing pontine nucleus. J Neurosci 1998; 18:7941–52.
- Di Cairano ES, Davalli AM, Perego L, Sala S, Sacchi VF, La Rosa S, et al The glial glutamate transporter 1 (GLT1) is expressed by pancreatic beta‐cells and prevents glutamate‐induced beta‐cell death. J Biol Chem 2011; 286:14007–18.
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al Beta‐lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 2005; 433:73–7.
- Brian S. Meldrum, Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology, The Journal of Nutrition, Volume 130, Issue 4, April 2000, Pages 1007S–1015S, https://doi.org/10.1093/jn/130.4.1007S
- Kondoh T., Mallick H.N., Torii K. Activation of the gut-brain axis by dietary glutamate and physiologic significance in energy homeostasis. Am. J. Clin. Nutr. 2009;90:832S–837S.
- Yano, S., Tokumitsu, H. & Soderling, T. R. (1998) Calcium promotes cell survival through CaM-k kinase activation of the protein-kinase-B pathway. Nature (Lond.)396:584–587.
- Ikonomidou, C., Bosch, F., Miksa, M., Bittigau, P., Vöckler, J., Dikranian, K., Tenkova, T. I., Stefovska, V., Turski, L. & Olney, J. W. (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science (Washington DC)283:70–74.
- Uversky VN. The alphabet of intrinsic disorder: II. Various roles of glutamic acid in ordered and intrinsically disordered proteins. Intrinsically Disord Proteins. 2013;1(1):e24684. Published 2013 Apr 1. doi:10.4161/idp.24684 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5424795
- McEntee WJ, Crook TH. . Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology (Berl) 1993; 111:391 – 401; http://dx.doi.org/10.1007/BF02253527
- Okubo Y, Sekiya H, Namiki S, Sakamoto H, Iinuma S, Yamasaki M, et al. . . Imaging extrasynaptic glutamate dynamics in the brain. Proc Natl Acad Sci U S A 2010; 107:6526 – 31; http://dx.doi.org/10.1073/pnas.0913154107
- Feng ZM, Zhou XL, Wu F, Yao K, Kong XF, et al. (2014) Both Dietary Supplementation with Monosodium L-Glutamate and Fat Modify Circulating and Tissue Amino Acid Pools in Growing Pigs, but with Little Interactive Effect. PLOS ONE 9: e84533
- Watford M (2008) Glutamine metabolism and function in relation to proline synthesis and the safety of glutamine and proline supplementation. J Nutr 138: 2003s–2007s
- Zhang J, Yin YL, Shu X, Li TJ, Li FN, et al. (2013) Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids 45: 1169–1177.
- Wu GY, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134: 489–492.
- Ren W, Li Y, Yu X, Luo W, Liu G, et al. (2013) Glutamine modifies immune responses of mice infected with porcine circovirus type 2. Bri J Nutr 110: 1053–1060.
- Zolotarev V., Khropycheva R., Uneyama H., Torii K. Effect of free dietary glutamate on gastric secretion in dogs. Ann. N. Y. Acad. Sci. 2009;1170:87–90.
- Meldrum B, Garthwaite J. Excitatory amino‐acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci 1990; 11:379–87.
- Teitelbaum, J. S., Zatorre, R. J., Carpenter, S., Gendron, D., Evans, A. C, Gjedde, A. & Cashman, N. R. (1990) Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N. Engl. J. Med.322:1781–1787.
- Jarrard, L. & E & Meldrum, B. S. (1993) Selective excitotoxic pathology in the rat hippocampus. Neuropathol. Appl. Neurobiol.19:381–389.
- Ben-Ari, Y., Tremblay, E., Ottersen, O.-P. & Meldrum, B. S. (1980) The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res192:399–412.
- Olney, J. W. (1983) Excitotoxins: an overview. Fuxe, K. Roberts, P. Schwarcz, R. eds. Excitotoxins :82–96 Macmillan Press London, UK.
- Olney, J. W., Sharpe, L. G. & Feigin, R D. (1972) Glutamate-induced brain damage in infant primates. J. Neuropathol. Exp. Neurol.16:464–488.
- Meldrum, B. (1993) Amino acids as dietary excitotoxins: a contribution to understanding neurodegenerative disorders. Brain Res. Rev.18:293–314.
- Ullensvang, K., Lehre, K. P., Storm-Mathisen, J. & Danbolt, N. C. (1997) Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. Eur. J. Neurosci.9:1646–1655.
- Mitani, A., Watanabe, M. & Kataoka, K. (1998) Functional change of NMDA receptors related to enhancement of susceptibility to neurotoxicity in the developing pontine nucleus. J. Neurosci.18:7941–7952.
- Shigeri Y, Seal RP, Shimamoto K. . Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev 2004; 45:250 – 65; http://dx.doi.org/10.1016/j.brainresrev.2004.04.004
- Hynd MR, Scott HL, Dodd PR. . Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int 2004; 45:583 – 95; http://dx.doi.org/10.1016/j.neuint.2004.03.007
- Glushakov AV, Glushakova O, Varshney M, Bajpai LK, Sumners C, Laipis PJ, et al. . . Long-term changes in glutamatergic synaptic transmission in phenylketonuria. Brain 2005; 128:300 – 7; http://dx.doi.org/10.1093/brain/awh354
- Meldrum, B. S. (1990) Protection against ischaemic neuronal damage by drugs acting on excitatory neurotransmission. Cerebrovasc. Brain Metab. Rev.2:27–57.
- Ludolph, A. C., Meyer, T., Riepe, M. W. & Völkel, H. (1998) Amyotrophic lateral sclerosis and glutamate. Restor. Neurol. Neurosci.13:59–67.
- Rothstein, J. D., Van Kammen, M., Levey, A. I., Martin, L J. & Kuncl, R. W. (1995) Selective loss of glial glutamate transporter GLT-1 in amyotrophic lateral sclerosis. Ann. Neurol.38:73–84.
- Hirata, A., Nakamura, R., Kwak, S., Nagata, N. & Kamakura, K. (1997) AMPA receptor-mediated slow neuronal death in the rat spinal cord induced by long-term blockade of glutamate transporters with THA. Brain Res771:37–44.
- Roy, J., Minotti, S., Dong, L., Figlewicz, D. A. & Durham, H. D. (1998) Glutamate potentiates the toxicity of mutant Cu/Zn-superoxide dismutase in motor neurons by postsynaptic calcium-dependent mechanisms. J. Neurosci.18:9673–9684.
- Schauwecker, P. E. & Steward, O. (1997) Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc. Natl. Acad. Sci. U.S.A.94:4103–4108.