Gray baby syndrome
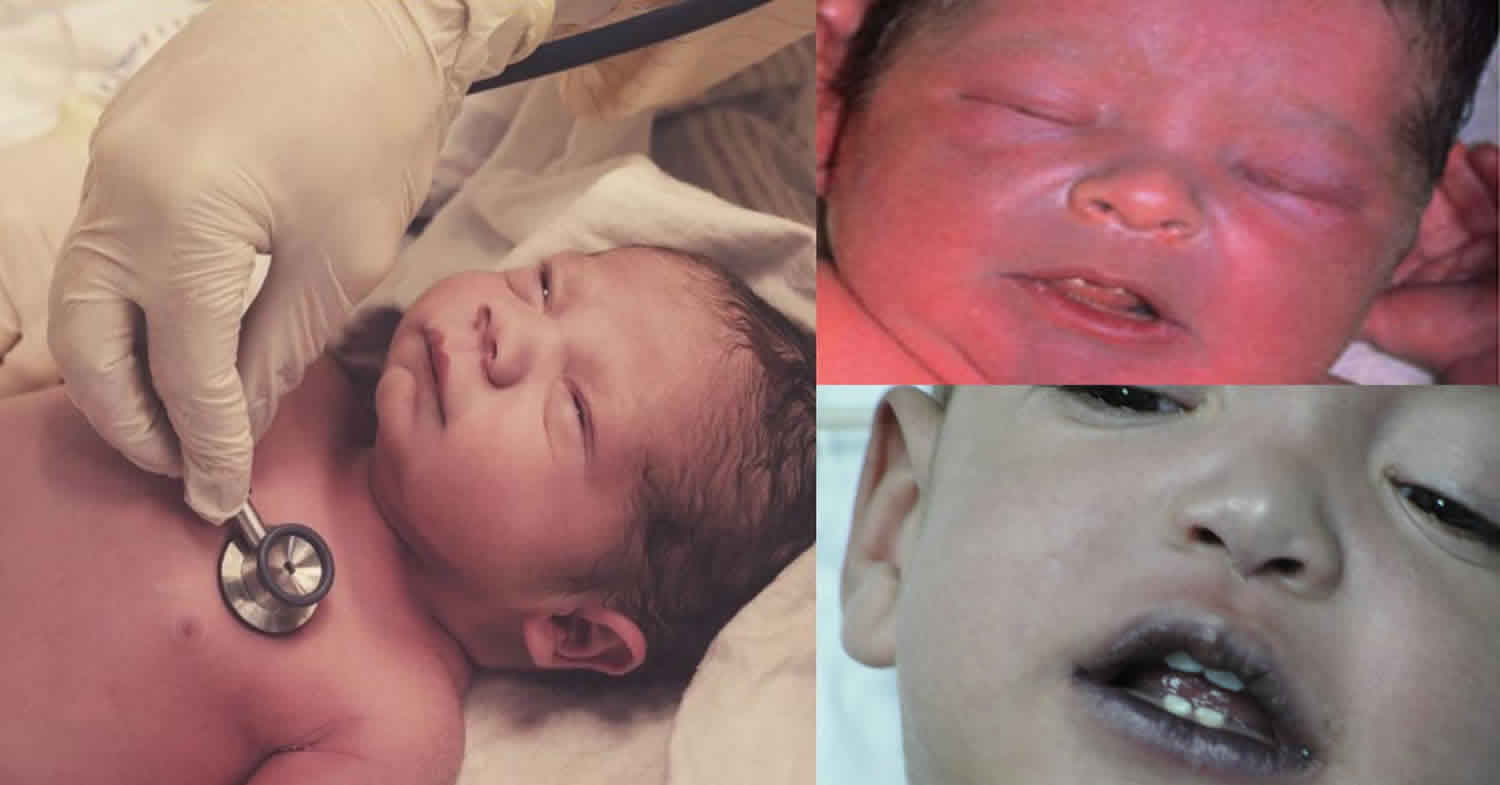
Gray baby syndrome also known as chloramphenicol grey baby syndrome, chloramphenicol-induced gray baby syndrome or grey baby syndrome, is a type of circulatory collapse that can occur in premature and newborn infants and is associated with excessively high serum levels of chloramphenicol. Similar syndromes have been encountered in adult patients with overdose and in patients with hepatic disease. Chloramphenicol is a broad spectrum antibiotic introduced into clinical practice in 1948 under the brand name Chloromycetin, but was subsequently shown to cause serious and fatal aplastic anemia and is now used rarely and reserved for severe, life-threatening infections for which other antibiotics are not available. Twelve years after its discovery, the first case report of a potentially fatal adverse reaction to chloramphenicol was discovered in neonates, with a predilection towards preterm infants. Neonates born at less than 37 weeks gestation were given chloramphenicol in an intravenous or oral formulation within two days of birth when they began to develop abdominal distention, vomiting, hypothermia, cyanosis, and cardiovascular instability. Vasomotor collapse resulting in mottling of skin and eventual ashen-gray skin discoloration led to the naming of this reaction as “gray-baby syndrome” 1. Currently, chloramphenicol is available only in parenteral forms, and its use is restricted to severe, life-threatening infections for which no other antibiotic is available because of antibiotic resistance or drug allergy.
Chloramphenicol-induced gray baby syndrome is an example of the potential dangers inherent in treating newborns based on dosing recommendations in adults. Grey baby syndrome is characterized by an ashen-gray color, abdominal distention, vomiting, flaccidity, cyanosis, circulatory collapse, and death. It usually starts 2 to 9 days after chloramphenicol treatment is started. Gray baby syndrome is a result of chloramphenicol impairing myocardial contractility by directly interfering with myocardial tissue respiration and oxidative phosphorylation. It is believed to occur more often in neonates due to their diminished ability to conjugate chloramphenicol and to excrete the active form in the urine. There have also been reports in small children and adults that have had accidental overdoses of the drug. Grey baby syndrome is generally associated with serum levels of chloramphenicol greater than 50 mg/L and may occur with unexplained metabolic acidosis. To accelerate drug removal, exchange transfusion and charcoal hemoperfusion have been used.
Premature infants and neonates are at the highest risk of the gray-baby syndrome from chloramphenicol exposure due to their decreased hepatic and renal function. Case reports of chloramphenicol toxicity have also been reported in children and adolescents. Various weight-based dosage adjustment has been suggested for newborns younger than 15 days, infants between 2 to 4 weeks days, and children older than one month 2.
Patients with gray baby syndrome should be admitted to a closely monitored telemetry setting, ideally in the intensive care unit. Changes in the dosing regimen (lower doses given at longer intervals) and careful monitoring of drug levels can help prevent this fatal complication of chloramphenicol administration in neonates and premature infants.
Gray baby syndrome causes
Gray baby syndrome is a type of circulatory collapse with cynosis that can occur in premature and newborn infants and is associated with excessively high serum levels of chloramphenicol (dose greater than 50 to 100 mg/kg) and in critically ill infants with hepatic disease. Grey baby syndrome has been seen in patients who were given doses greater than 200 mg daily 3. Exposure from maternal use has also been observed. Chloramphenicol has been assigned Pregnancy Category C (risk not ruled out) and is contraindicated during lactation (as it passes readily into breast milk). Chloramphenicol is detoxified in the liver primarily through conjugation with glucuronide. Neonates have a diminished ability to conjugate chloramphenicol and to excrete the active form in the urine.
Because of its broad spectrum of activity, chloramphenicol was widely used to treat a variety of infections in the 1950s, including nursery infections in neonates. Hospital-acquired bacterial infections in the newborn nursery were often due to less sensitive bacterial strains, compared to strains found outside the newborn nursery; consequently, premature and full-term infants were treated with adult doses scaled to the infant body weight or with doses exceeding those recommended in adults. In the late 1950s case reports of unexplained deaths in newborns who were receiving chloramphenicol appeared; these deaths and led to a controlled clinical trial of chloramphenicol therapy in premature newborns who were at high risk of infection because they were born more than 24 hours after spontaneous rupture of membranes. The standard of care for these newborns was empirical treatment with antibiotics, but newborns studied in this trial were assigned to one of four groups: (1) no antibiotics, (2) procaine penicillin and streptomycin, (3) chloramphenicol, and (4) all three antibiotics. Mortality was substantially higher in the newborns receiving chloramphenicol, and nearly two-thirds of the newborns receiving chloramphenicol died, compared to the <20% mortality rate in newborns who did not receive chloramphenicol. In infants weighing more than 2000 grams at birth, 45% receiving chloramphenicol died compared with 25% in the non-chloramphenicol-treated groups. This high mortality rate was ascribed to chloramphenicol toxicity.
Studies of chloramphenicol pharmacokinetics were subsequently performed in newborns and children. High concentrations of chloramphenicol and its metabolites accumulated in newborns who developed toxicity, and presumably accounted for the severe toxicity. This accumulation of drug on a dosing regimen that is tolerable in adults resulted from the reduced capacity of newborns to metabolize chloramphenicol by glucuronide conjugation. When studied at lower doses in a group of children over a wider age range, the rate of chloramphenicol metabolism was found to be highly age dependent. The half-life was 26 hours in the newborns, 10 hours in the infants, and 4 hours in the older children.
Two pathophysiologic mechanisms are thought to play a role in the development of gray baby syndrome after exposure to the anti-microbial drug chloramphenicol. This condition is due to a lack of glucuronidation reactions occurring in the baby, thus leading to an accumulation of toxic chloramphenicol metabolites 4:
- The UDP-glucuronyl transferase enzyme system of infants, especially premature infants, is immature and incapable of metabolizing the excessive drug load.
- Insufficient renal excretion of the unconjugated drug.
A normally functioning liver will metabolize the chloramphenicol parent molecule (primarily by glucuronidation). The immature neonatal liver is unable to synthesize and recycle the UDP-glucuronyltransferase enzyme efficiently. Similarly, the neonatal kidneys are unable to excrete chloramphenicol and its metabolites efficiently. These two deficiencies result in elevated serum levels of chloramphenicol. The chloramphenicol molecule displaces unconjugated bilirubin from albumin, leading to kernicterus and eventually death if untreated 5.
The relative hepatic and renal dysfunction in neonates (especially in premature infants) result in elevated serum levels of chloramphenicol. This impairs electron transport within the mitochondrial and consequently cellular respiration, leading to direct cellular toxicity. The chloramphenicol parent molecule also displaces unconjugated bilirubin from albumin, giving way to kernicterus and eventually death or permanent neurological complications if left untreated 6.
Serum concentrations after a single oral or intravenous dose of chloramphenicol peak 1 to 2 hours after ingestion; chloramphenicol has excellent absorption in the gastrointestinal tract. Intramuscular chloramphenicol has variable absorption with serum concentrations reaching only 5% to 65% the concentration of the equivalent intravenous or oral dose. Roughly half of serum chloramphenicol is bound to albumin and other plasma proteins. Elimination happens primarily in the liver through O-glucuronidation, which puts neonates with immature hepatic metabolism at risk for the gray-baby syndrome. Urinary excretion of the parent chloramphenicol compound is approximately 20% in children and 10% to 12% in adults; the rest is excreted as the glucuronidated metabolite.
Gray baby syndrome prevention
Gray baby syndrome is a preventable problem. With the number of antibiotics available today, there should be no valid reason to use this agent for the management of infection in babies.
Gray baby syndrome symptoms
Gray baby syndrome may occur in neonates exposed to excessive doses of chloramphenicol. Gray baby syndrome is a state of cardiovascular collapse and usually begins 2–9 days after start of chloramphenicol treatment 7. The presentation of the gray-baby syndrome will vary depending on the level of toxicity from chloramphenicol. Gray baby syndrome primary symptoms include vomiting, refusal to suck, refusal to feed, respiratory distress (irregular and rapid respiration), abdominal distention, periods of cyanosis, passage of loose and green stools, flaccidity, ashen color, hypothermia and vascular collapse. After 24 hours, the children become flaccid, hypothermic, and severely ill while turning an ashen-gray color. Death occurs in about 40% of patients within 2 days of initial symptoms (usually by the fifth day of life).
Fifty-eight percent of the infants who received chloramphenicol and survived had similar signs that completely resolved 24–36 hours after discontinuing the drug.
Gray baby syndrome complications
Grey baby syndrome complications include:
- Bleeding
- Renal and liver failure
- Anemia
- Infection
- Confusion
- Marked Weakness
- Vision problems
- Shock
- Death
Gray baby syndrome diagnosis
Ideally, chloramphenicol exposure will be provided in the history from the caregiver. Poor feeding, fussiness, and vomiting are often elicited in the history.
A physical exam may reveal altered mental status from lethargy to obtundation, ashen-gray cyanosis, pallor, and abdominal distention/tenderness.
In the undifferentiated sick neonate presenting with cyanosis, a broad differential diagnosis must be considered, including but not limited to neonatal sepsis, non-accidental trauma, midgut volvulus, congenital heart disease, and inborn errors of metabolism. Blood work should include glucose, complete blood count with differential, complete metabolic panel, blood gas analysis, serum ammonia, serum lactic acid, serum ketones, and consideration for cardiac biomarkers including troponin and brain natriuretic peptide. In the setting of chloramphenicol toxicity, serum chloramphenicol levels may be drawn. Radiologic studies should include chest and abdominal films, and CT head or abdominal ultrasound (depending on the history). An electrocardiogram (ECG) should also be obtained 8.
Gray baby syndrome treatment
Management of chloramphenicol toxicity centers primarily around emergency measures and intensive supportive therapy in severe cases with cardiorespiratory impairment. The general approach to the ashen-gray hemodynamically unstable neonate starts with aggressive resuscitation and an early call to the pediatric intensive care unit or extracorporeal life support team, as some of these patients may be ideal candidates. These patients should be hemodynamically stabilized, appropriately oxygenated and ventilated, and intubated early. Checking a core temperature is critical as hypothermia is common in the gray neonate. Aggressive rewarming should be considered. A point-of-care glucose should also be checked, and hypoglycemia should be reversed if present. The differential diagnosis for an ashen-gray, cyanotic neonate should include chloramphenicol toxicity, congenital heart disease, adrenal insufficiency/hypothyroidism, inborn errors of metabolism, trauma, seizures, and of course, sepsis. Empiric administration of broad-spectrum antibiotics such as vancomycin, ampicillin (targeting Listeria), and a third-generation cephalosporin such as ceftriaxone or cefotaxime is recommended. Additional consideration should also be given to empiric prostaglandin administration in gray/cyanotic neonates, especially if a duct-dependent congenital cardiac lesion is present.
Modalities that have been used for the treatment of gray-baby syndrome are primarily aimed towards direct removal of the parent chloramphenicol molecule. This has been achieved through charcoal hemoperfusion and exchange transfusion. There have also been reports of phenobarbital being used for induction of the UDP-glucuronyltransferase enzyme. Consideration for cardiopulmonary bypass including extracorporeal membrane oxygenation may also be considered 9.
Exchange transfusion
This lifesaving procedure involves removing some of your baby’s blood and replacing the blood with freshly donated blood or plasma. The procedure is completed using a catheter.
Hemodialysis
This procedure uses a dialysis machine to cleanse toxins from your baby’s bloodstream. It also balances potassium and sodium levels and helps control your baby’s blood pressure.
In addition to the above treatments, your baby may be given oxygen therapy to improve breathing and oxygen delivery to the body. Your baby’s doctor may also recommend hemoperfusion. This treatment is similar to dialysis and helps remove toxins from the blood. Your baby’s blood will be monitored during treatment.
Gray baby syndrome prognosis
If the condition is diagnosed immediately and the drug is discontinued the prognosis is good but if the infant has developed organ dysfunction, the prognosis is guarded.
References- Chloramphenicol. In: Drugs and Lactation Database (LactMed). Bethesda (MD): National Library of Medicine (US); October 31, 2018.
- Knight M. Adverse drug reactions in neonates. J Clin Pharmacol. 1994 Feb;34(2):128-35.
- Cummings ED, Edens MA. Gray Baby Syndrome. [Updated 2019 Dec 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448133
- Brunton, Laurence L.; Lazo, John S.; Parker, Keith, eds. (2005). “Chapter 46. Protein Synthesis Inhibitors and Miscellaneous Antibacterial Agents”. Goodman & Gilman’s The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. ISBN 0-07-142280-3
- Beninger P. Pharmacovigilance: An Overview. Clin Ther. 2018 Dec;40(12):1991-2004.
- Tonni G, Leoncini S, Signorini C, Ciccoli L, De Felice C. Pathology of perinatal brain damage: background and oxidative stress markers. Arch. Gynecol. Obstet. 2014 Jul;290(1):13-20.
- Cummings ED, Edens MA. Gray Baby Syndrome. [Updated 2019 Dec 20]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK448133
- Long SS. 50 Years Ago in The Journal of Pediatrics: Visual Disturbances in Cystic Fibrosis following Chloramphenicol Administration. J. Pediatr. 2016 Jan;168:184.
- Ingebrigtsen SG, Didriksen A, Johannessen M, Škalko-Basnet N, Holsæter AM. Old drug, new wrapping – A possible comeback for chloramphenicol? Int J Pharm. 2017 Jun 30;526(1-2):538-546.