What is guar gum
Guar gum also called guaran, guar flour or Gum cyamopsis, is mainly consisting of high molecular weight (50,000-8,000,000) polysaccharides composed of galactomannan with the mannose:galactose ratio about 2:1. Guar gum is extracted from the endosperm of the seed (guar beans) of the guar plant (Cyamopsis tetragonoloba L. Taub syn. Cyamopsis psoraloides) that has thickening and stabilizing properties useful in the food, feed and industrial applications. Guar gum is used as thickener, stabilizer and emulsifier, and approved in most areas of the world (e.g. EU, USA, Japan, and Australia). Guar gum food additive is E 412. The guar seeds (guar beans) are mechanically dehusked, hydrated, milled and screened according to application 1. Guar gum is typically produced as a free-flowing, off-white powder. Commercial food‐grade guar gum is reported to contain usually about 80% guaran, 5–6% crude protein, 8–15% moisture, 2.5% crude fiber, 0.5–0.8% ash, and small amounts of lipids composed mainly of free and esterified plant fatty acids.
Table 1. General composition of guar gum
| Constituent | Percentage |
|---|---|
| Galactomannan | 75–85 |
| Moisture | 8.0–14 |
| Protein (N x 6.25) | 5.0–6.0 |
| Fiber | 2.0–3.0 |
| Ash | 0.5–1.0 |
Possible impurities
The commercial samples of guar gum contain approximately 4-12% moisture, 2-5% acid-soluble ash, 0.4-1.2% ash, and 2-6% protein. The samples of clarified guar gum contain approximately 5-10% moisture, 0.2-0.8% acid-soluble matter, 0.1-0.5% ash, and 0.1-0.6% protein.
Apart from the gum content, the guar gum contains:
- husk residues represented by the acid-insoluble-matter criterion (not more than 7.0%)
- proteins from the germ represented by the protein criteria (not more than 10.0%)
- ethanol/isopropanol residues for washing or extraction solvent(not more than 1% singly or in combination)
- microbiological contamination
In the United States, guar gum is listed for use as an emulsifier, formulation aid and firming agent in the following foods:
| Food Category | Maximum Use Level (%) |
| Baked goods & baking mixes | 0.35 |
| Breakfast cereals | 1.2 |
| Cheeses | 0.8 |
| Daily products analogs | 1 |
| Fats and oils | 2 |
| Jams and jellies | 1 |
| Milk products | 0.6 |
| Processed vegetable and vegetable juices | 2 |
| Soup and soup mixes | 0.8 |
| Sweet sauces, toppings and syrups | 1 |
| All other foods | 0.5 |
Figure 1. Guar seeds (guar beens)
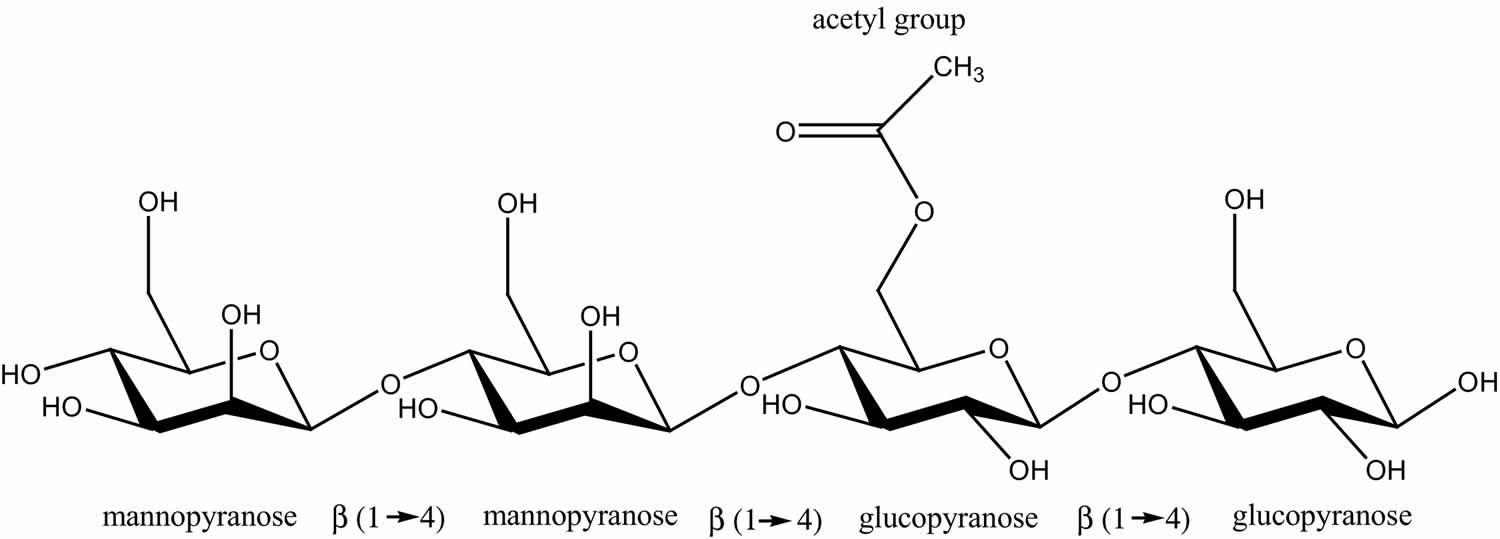
Figure 2. Galactomannan – the principal component of this gum is a galactomannan with a linear chain of (1 -> 4) linked ß-D-mannopyranose units with alpha-D-galactopyranose units attached by (1 -> 6) linkages to every alternate mannose.
The guar plant (Cyamopsis tetragonoloba L. Taub syn. Cyamopsis psoraloides) has been cultivated in India and Pakistan for centuries 1. It can also be cultivated in the southern hemisphere in semi-arid zones in Brazil, Australia and South Africa or in the Southern part of the USA, like Texas or Arizona. The guar kernel is composed of several layers, namely the husk (16-18%) on the outside, the germ (43-46%) and the endosperm (34-40%), which is composed of guar gum.
Guar splits are obtained after separation of the husk and the germ. After heat treatment, the hull is easy to separate by either attrition milling or various types of impact mills. The endosperm is recovered by sieving from the finer germ and hull fractions, and then milled to obtain powdered guar gum. The guar gum may be further purified clarified by dissolution in water, precipitation and recovery with ethanol or isopropanol. It is called as clarified (purified, extracted) guar gum. Clarified guar gum in the market is normally standardized with sugars.
Guar gum is mainly consisting of the high molecular weight polysaccharides composed of galactomannans which are consisting of a linear chain of (1→4)-linked β-D-mannopyranosyl units with (1→6)-linked α-D-galactopyranosyl residues as side chains. The mannose: galactose ratio is approximately 2:1. The molecular weight range is 50,000-8,000,000.
The clarified guar gum has higher galactomannans content and no longer contains the cell structure. The gum is a white to yellowish white, nearly odorless, free-flowing powder with a bland taste.
Guar gum is insoluble in organic solvents. The gum is soluble in cold water without heating to form a highly viscous so1ution. Guar gum solutions have buffering capacity and are very stable in the pH 4.0-10.5 range. Addition of a small amount of sodium borate to a water solution of guar gum will result in formation of a gel.
The caloric value was determined in groups of 10 rats fed for one week a 5 g basal diet supplemented with either 1 g or 3 g corn starch or 1 g and 3 g guar gum. At 1 g level guar gum was equivalent to corn starch but at the 3 g level there was a lower equivalence. In a further bioavailable calorie assay groups of 10 male weanling rats (Sprague-Dawley) were given 5 g basal diet or plus 0.5, 1, 2 g sucrose or 0.5, 1, 2 g guar gum for 10 days. Comparison of the carcass weight gain showed that guar gum was not a source of bioavailable calories 3. The rat can use guar flour as a precursor for liver glycogen but at a much reduced efficiency as shown by 15 controls receiving cocoa butter alone (<0.1% glycogen), or cocoa butter + 30% wheat flour (2.6% glycogen) and 18 test animals receiving cocoa butter + 30% guar flour (0.8% glycogen) for two days 4. The digestibility of guar gum in rats fed 0.4 g/day was estimated to be 76% 5. However another digestibility study in groups of five male and five female rats (Purdue strain) on a mannose-free diet showed that 83-100% of mannose fed as 1% guar gum in the diet for 18 hours was excreted in the feces over a total of 30 hours. Some decrease in chain length of galactomannans may have occurred probably through the action of the microflora as mammals are not known to possess mannosidase. Liberation of galactose was not determined 6. Incubation of solutions or suspensions with human gastric juice, duodenal juice + bile, pancreatic juice and succus entericus with or without added rabbit small gut membrane enzymes produced no evidence of hydrolysis (Semenza, 1975). Rat large gut
microflora partially hydrolyzed guar gum in vitro 7.
Feeding chicks for four weeks on a diet containing 3% cholesterol, 3% guar gum and 3% cholesterol + 3% guar gum reduced the serum cholesterol level especially if both cholesterol and guar gum were ingested. Liver cholesterol was only depressed if cholesterol and guar gum were fed 8. Groups each of eight male Holtzman rats were maintained on a purified synthetic diet, or the diet plus 1% cholesterol, or the diet plus 1% cholesterol and 10% guar gum for 28 days. The increased liver cholesterol and liver total lipid induced by cholesterol feeding was largely counteracted by concurrent feeding of guar gum. Ten per cent. but not 5% guar gum added concurrently to a casein/sucrose diet with 1% cholesterol and 10% corn oil significantly reduced serum and liver cholesterol. Five per cent. guar gum reduced only the liver cholesterol when only 5% corn oil was used with a commercial diet 9.
Is guar gum gluten free?
Yes.
Gluten-Containing Grains and Their Derivatives
- Wheat
- Varieties and derivatives of wheat such as:
- wheatberries
- durum
- emmer
- semolina
- spelt
- farina
- farro
- graham
- KAMUT® khorasan wheat
- einkorn wheat
- Rye
- Barley
- Triticale
- Malt in various forms including: malted barley flour, malted milk or milkshakes, malt extract, malt syrup, malt flavoring, malt vinegar
- Brewer’s Yeast
- Wheat Starch that has not been processed to remove the presence of gluten to below 20ppm and adhere to the FDA Labeling Law*
*According to the FDA, if a food contains wheat starch, it may only be labeled gluten-free if that product has been processed to remove gluten, and tests to below 20 parts per million of gluten. With the enactment of this law on August 5th, 2014, individuals with celiac disease or gluten intolerance can be assured that a food containing wheat starch and labeled gluten-free contains no more than 20ppm of gluten. If a product labeled gluten-free contains wheat starch in the ingredient list, it must be followed by an asterisk explaining that the wheat has been processed sufficiently to adhere to the FDA requirements for gluten-free labeling.
Guar gum allergy
A few case reports have been published on guar gum allergy, one in Finnish 10, another mentioned anaphylactic shock to guar gum (food additive E412) contained in a meal substitute 11, occupational allergic rhinitis from guar gum 12 and occupational asthma caused by guar gum 13.
Guar gum vs Xanthan gum
Xanthan gum is a high-molecular weight (of the order of 1000 kDa) polysaccharide gum comprising primarily of D-glucose and D-mannose as the dominant hexose units, along with D-glucuronic acidand pyruvic acid 14. Xanthan gum is produced by the fermentation of a carbohydrate source in a pure culture of Xanthomonas campestris, the naturally occurring bacteria. The fermentation medium comprises of a carbohydrate, a nitrogen source, and mineral salts. Once the fermentation process is complete, xanthan gum is recovered from the fermentation broth by alcohol precipitation in the form of a sodium, calcium, or potassium salt. The resulting coagulum is separated, rinsed, pressed, dried, and ground as part of down-stream processing and marketed as a cream-coloured powder.
Xanthan gum (E 415) is generally used as a thickener, stabiliser, emulsifier and foaming agent. The present assessment focuses in its proposed use as a thickener in infant formulae, follow-up formulae, and formulae for special medical purposes intended for infants.
An important property of xanthan gum solutions is the physicochemical interaction with plant galactomannans, such as locust bean gum and guar gum, or konjac glucomannan. The addition of any of these gums to a solution of xanthan gum at room temperature causes a synergistic increase in viscosity 15.
The European Food Safety Authority Panel noted that in cases, where xanthan gum (E 415) is added in combination with other gums, such as locust bean gum (E 410), guar gum (E 412) or konjac glucomannan (E 425 (ii)) to food, the synergistic increase in viscosity has to be taken into consideration. This may be relevant in particular for the above mentioned combined uses of xanthan gum and guar gum in infant food for special medical purposes.
Xanthan gum (E 415) is authorized as a food additive in the European Union (EU) according to Annex II and III to Regulation (EC) No 1333/2008 on food additives and it was previously evaluated by the European Union Scientific Committee for Food (SCF) and the Joint FAO/WHO Expert Committee on Food Additives (JECFA), who both allocated an acceptable daily intake (ADI) ‘not specified’ for this gum.
According to the industry, during the fermentation process, the Xanthomonas campestris bacteria produce enzymes (i.e. amylases, cellulases or protease) which are reduced as much as possible or deactivated throughout the manufacturing process.
The European Food Safety Authority Panel noted that limits for possible residual bacterial enzymatic activities may be required in the EU specifications.
Xanthan gum (E 415) can be regarded as non‐toxic based on the results of acute oral toxicity studies.
From short‐term and subchronic toxicity studies, no toxicological relevant changes were reported apart from a decrease in red blood cell count and hemoglobin concentration in dogs receiving 2,000 mg/kg body weight per day for 12 weeks. This effect was marginal and it was not reproduced in a dog chronic toxicity study at 1,000 mg/kg body weight per day, the highest dose tested. The European Food Safety Authority Panel noted that decreased total serum cholesterol was frequently reported.
For genotoxicity, insufficient experimental data were available. However, taking into account the information on structure–activity relationships and considering that xanthan gum has a molecular weight far above the threshold for absorption, according to absorption, distribution, metabolism, and excretion data, it was not degraded in the intestine and is slightly fermented to non‐hazardous short‐chain fatty acids (SCFAs) by the gut microbiota, the European Food Safety Authority Panel concluded that xanthan gum (E 415) does not give rise to concerns for genotoxicity.
In chronic and long‐term studies, no adverse effects, including biochemical and hematological parameters, were reported in dogs and rats. The European Food Safety Authority Panel noted that decreased red blood cell counts reported in a subchronic toxicity study in dogs receiving 2,000 mg/kg body weight per day at 6 and 12 weeks, effect which was marginal and not reproduced in a dog chronic toxicity study at 1,000 mg/kg body weight per day for 107 weeks, the highest dose tested.
Dietary feeding of xanthan gum at levels of 0 (control), 250 and 500 mg/kg body weight per day to groups of albino rats of both sexes during a three‐generation reproduction study had no adverse effect on reproduction as judged by all the endpoints evaluated. No prenatal developmental toxicity studies were available to the European Food Safety Authority Panel.
In special studies in neonatal piglets, no test substance‐related effects in hematology or clinical chemistry parameters were observed at any dose. In the high‐dose group (3,750 mg/kg body weight per day) histopathological findings rated from minimal to moderate were observed in the large intestine (cecum, colon, rectum) and small intestine (duodenum). These effects were observed in fewer animals in the lower dose groups (375 and 750 mg/kg body weight per day) and the severity was considered minimal. The Panel considered the no‐observed‐adverse-effect‐level (NOAEL) for xanthan gum in neonatal piglets to be 375 mg/kg body weight per day, based on the changes of the feces (green, soft, watery, increased defecation) in the mid‐dose and high dose group, and the no‐observed‐adverse‐effect‐level (NOAEL) was 750 mg/kg body weight per day based on histopathological changes in the intestine in the high dose.
From a human study with repeated intake ranging from 10.4 to 12.9 g of xanthan gum per day (assuming a body weight of 70 kg corresponding to 149–184 mg/kg body weight per day), it was reported that xanthan gum acts as a bulk laxative causing no adverse dietary nor physiological effects. The only effects observed were moderate (10%) reduction in serum cholesterol and a significant increase in fecal bile acid concentrations 16.
A study investigating the effect of repeated intake of 15 g xanthan gum/day (assuming a body weight of 70 kg corresponding to 214 mg/kg body weight per day) on colonic function showed significant increases in stool output, frequency of defecation and flatulence due to the ingestion of the xanthan gum 17.
In clinical studies involving infants, the European Food Safety Authority Panel noted that consumption of xanthan gum in infant formula or formula for special medical purposes in infant was well tolerated, did not influence minerals (Ca, P, Mg), fat and nitrogen balance and did not affect growth characteristics up to concentration of 1,500 mg/L (232 mg/kg body weight per day) 18. These results were supported by the outcome of the post‐marketing surveillance with formulae containing xanthan gum at a concentration of approximately 750 mg/L of reconstituted formula 18.
The European Food Safety Authority Panel on Food Additives and Nutrient Sources added to Food provides a scientific opinion re‐evaluating the safety of xanthan gum (E 415) as food additive 18. Based on the reported use levels, a refined xanthan gum (E 415) exposure of up to 64 mg/kg body weight per day in children for the general population, 38 mg/kg body weight per day for children consumers only of food supplements at the high level exposure and 115 mg/kg body weight per day for infants consuming foods for special medical purposes and special formulae, were estimated. Xanthan gum (E 415) is unlikely to be absorbed intact and is expected to be fermented by intestinal microbiota. No adverse effects were reported at the highest doses tested in chronic and carcinogenicity studies and there is no concern with respect to the genotoxicity 18. Repeated oral intake by adults of xanthan gum up to 214 mg/kg body weight per day for ten days was well tolerated, but some individuals experienced abdominal discomfort, an undesirable but not adverse effect. The European Food Safety Authority Panel concluded that there is no need for a numerical Acceptable Daily Intake (ADI) for xanthan gum (E 415), and that there is no safety concern for the general population at the refined exposure assessment of xanthan gum (E 415) as food additive 18. Considering the outcome of clinical studies and post‐marketing surveillance, the European Food Safety Authority Panel concluded that there is no safety concern from the use of xanthan gum (E 415) in foods for special medical purposes and special formulae for infants and young children at concentrations reported by the food industry. The current re‐evaluation of xanthan gum (E 415) as a food additive is not considered to be applicable for infants under the age of 12 weeks.
Xantham Gum Safety Data for General population
Following the conceptual framework for the risk assessment of certain food additives re‐evaluated under European Commission Regulation No 257/2010 19:
- From all the data received, data were adequate for a refined exposure assessment for 25 out of 79 food categories;
- Based on the reported use levels, a refined exposure (non‐brand‐loyal scenario) of up to 64 mg/kg body weight per day in children (3–9 years) was estimated;
- Refined exposure assessments for consumers only of food supplements was also calculated and was up to 38 mg/kg body weight per day for children (3–9 years) considering high level exposure (95th percentile);
- Xanthan gum is unlikely to be absorbed intact and is expected to be partially fermented by intestinal microbiota;
- Adequate toxicity data were available;
- There was no concern with respect to genotoxicity;
- No adverse effects were reported in chronic studies in rats and dogs up to 1,000 mg/kg body weight per day, the highest dose tested. In rats, the compound was not carcinogenic;
- Repeated oral intake by adults of large amounts of xanthan gum up to 15,000 mg/person per day, corresponding to 214 mg/kg body weight per day for at least ten days was well tolerated, but some individuals experienced abdominal discomfort, which was considered by the European Food Safety Authority Panel as undesirable but not adverse;
The European Food Safety Authority Panel concluded that there is no need for a numerical acceptable daily intake (ADI) for xanthan gum (E 415), and that there is no safety concern at the refined exposure assessment for the reported uses and use levels of xanthan gum (E 415) as a food additive 18.
Xantham Gum Safety Data for Infants and young children consuming foods for special medical purposes and special formulae
Concerning the use of xanthan gum (E 415) in ‘dietary foods for special medical purposes and special formulae for infants’ and in ‘dietary foods for babies and young children for special medical purposes:
- For populations consuming foods for special medical purposes and special formulae, the highest refined exposure estimates (p95) on the maximum reported data from food industry (750 mg/L for categories 13.1.5.1 and 250 mg/L for 13.1.5.2) were up to 115 mg/kg body weight per day for infants (12 weeks–11 months, brand loyal scenario);
- In a number of clinical studies, consumption of xanthan gum in infant formula or formula for special medical purposes in infant was well tolerated up to concentration of 1,500 mg/L (232 mg/kg body weight per day);
- No cases of adverse effects were reported from post‐marketing surveillance with formulae containing xanthan gum at a concentration of approximately of 750 mg/L of reconstituted formula which supported the results of the clinical studies;
The European Food Safety Authority Panel concluded, that there is no safety concern from the use of xanthan gum (E 415) in foods for special medical purposes consumed by infants and young children at concentrations reported by the food industry 18.
Xanthan gum possible impurities
Possible impurities of xantham gum include the nitrogen source from the fermentation medium, remaining alcohols used for the precipitation (isopropanol or ethanol) and heavy metals as contaminants. Microbiological contamination has also to be excluded.
An interested party has provided information on the content of lead (ND–2.0 mg/kg), arsenic (ND–2 mg/kg), cadmium (ND–0.1 mg/kg) and mercury (ND–1 mg/kg) in xanthan gum. According to the European Commission specifications, impurities of the toxic element lead are accepted up to concentration of 2 mg/kg.
Based on analytical data provided by the sponsor for three different batches of xanthan gum lead and arsenic concentrations are below the limit of detection about 4 parts per billion (ppb) or 4 µg/kg or 4 micrograms per kilogram which is 3 orders of magnitude lower than the limit in the specifications 2 parts per million (ppm) or 2 mg/kg or 2 milligrams per kilogram for lead. Isopropanol is found at concentrations 339-467 mg/Kg, close to the specification limit of 500 mg/Kg.
Is guar gum safe?
In the European Union, guar gum was evaluated by the Joint Food and Agriculture Organization (FAO)/World Health Organisation (WHO) Expert Committee on Food Additives (JECFA) in 1970, 1974 and 1975 20, who allocated an acceptable daily intake (ADI) ‘not specified’. Guar gum has been also evaluated by the Scientific Committee for Food in 1977 21 who endorsed the acceptable daily intake (ADI) ‘not specified’ allocated by JECFA. The European Food Safety Authority Panel considered that adequate exposure and toxicity data were available. Guar gum is practically undigested, not absorbed intact, but significantly fermented by enteric bacteria in humans. No adverse effects were reported in subchronic and carcinogenicity studies at the highest dose tested; no concern with respect to the genotoxicity. Oral intake of guar gum was well tolerated in adults. The European Food Safety Authority Panel concluded that there is no need for a numerical acceptable daily intake (ADI) for guar gum (E 412), and there is no safety concern for the general population at the refined exposure assessment of guar gum (E 412) as a food additive 22. The European Food Safety Authority Panel considered that for uses of guar gum in foods intended for infants and young children the occurrence of abdominal discomfort should be monitored and if this effect is observed doses should be identified as a basis for further risk assessment. Therefore, the European Food Safety Authority Panel concluded that the available data do not allow an adequate assessment of the safety of guar gum (E 412) in infants and young children consuming these foods for special medical purposes 22.
In 1998, the Scientific Committee for Food 23 accepted the use of guar gum in foods for special medical purposes for infants and young children at levels up to 10 g/L in ready‐to‐use liquid formulae containing extensively hydrolyzed protein and in ready‐to‐use liquid formulae containing partially hydrolyzed proteins for infants in good health at levels up to 1 g/L. In 2001, the Scientific Committee for Food accepted the use of guar gum in all weaning foods at levels up to 10 and up to 20 g/kg in gluten‐free cereal‐based foods, singly or in combination 24. In 2003, the Scientific Committee for Food re‐evaluated guar gum in the revision of the essential requirements of infant formulae and follow‐on formulae intended for the feeding of infants and young children 25.
The in vitro degradation and the in vivo digestibility of guar gum have been investigated in animals and humans which demonstrated that guar gum would not be absorbed unchanged and would not be metabolized by enzymes present in the gastrointestinal tract. However, it would be partially fermented to short‐chain fatty acids (SCFAs) during its passage through the large intestine by the action of the intestinal tract microflora 22. The rate of hydrolysis in the gastrointestinal tract in humans is unknown; however, it is expected that fermentation of guar gum would lead to the production of products such as short‐chain fatty acids (SCFAs) which were considered of no concern by the European Food Safety Authority Panel.
- Guar gum is regarded as not acutely toxic, based on the results of acute oral toxicity studies.
- In short‐term and subchronic studies in mice, rats, dogs and monkeys, no adverse effects were observed at the highest dose tested.
- The European Food Safety Authority Panel considered the available genotoxicity data on guar gum (E 412) to be sufficient to conclude that there is no concern with respect to genotoxicity.
- Overall, the European Food Safety Authority Panel considered guar gum as not carcinogenic 22.
Guar gum did not show reproductive effects (fertility) or developmental toxicity effects in the available studies. From a combined fertility/developmental study in rats 26, the European Food Safety Authority Panel could identify a no‐observed‐adverse‐effect‐level (NOAEL) of 5,200 mg/kg body weight per day for reproductive effects based on decreased number of corpora lutea and a NOAEL for developmental toxicity of 11,800 mg/kg body weight per day the highest dose tested.
Re‐evaluation of the use of guar gum (E 412) in foods for infants from 12 weeks of age and for young children. The European Food Safety Authority Panel acknowledged that consumption to the concerned food categories would be short and noted that it is prudent to keep the number of additives used in foods for infants and young children to the minimum necessary and that there should be strong evidence of need as well as safety before additives can be regarded as acceptable for use in infant formulae and foods for infants and young children 22. If guar gum is added in combination with locust bean gum and carrageenan to a follow‐on formula, the maximum level recommended by the Scientific Committee for Food for guar gum should not be exceeded by the total concentration of these three substances. The European Food Safety Authority Panel noted that it may be considered to establish specific purity criteria for the use of guar gum in food for infants and young children 22.
From the refined brand‐loyal estimated exposure scenario taking into account the foods for special medical purposes, mean exposure to guar gum (E 412) from its use as a food additive ranged for infants between 325 and 609 mg/kg body weight per day and between 120 and 457 mg/kg body weight per day for toddlers. The 95th percentile of exposure ranged for infants between 912 and 1,555 mg/kg body weight per day and for toddlers between 310 and 743 mg/kg body weight per day.
The refined estimates are based on 51 out of 86 food categories in which guar gum (E 412) is authorized. The main food categories, in term of amount consumed, not taken into account were breakfast cereals, gluten‐free dietary foods for infants and young children, snacks and most of alcoholic beverages. However, based on the information in the Mintel Global New Products Database, in the EU market, no breakfast cereals are labelled with guar gum (E 412), and few alcoholic drinks are labelled with the additive. Therefore, the European Food Safety Authority Panel considered that the uncertainties identified would, in general, result in an overestimation of the exposure to guar gum (E 412) as a food additive in European countries for all scenarios.
The European Food Safety Authority Panel noted that use levels of guar gum (E 412) in food for infants under the age of 12 weeks would require a specific risk assessment in line with the recommendations given by JECFA 27 and the Scientific Committee for Food 23 and endorsed by the 2012 European Food Safety Authority Panel 28. Therefore, the current re‐evaluation of guar gum (E 412) as a food additive is not considered to be applicable for infants under the age of 12 weeks and will be performed separately.
General population
The European Food Safety Authority Panel concluded that there is no need for a numerical Acceptable Daily Intake (ADI) for guar gum (E 412), and that there is no safety concern for the general population at the refined exposure assessment for the reported uses of guar gum (E 412) as a food additive.
- Adequate exposure data were available; in the general population, the highest refined exposure assessments calculated based on the reported data from food industry were for infants (12 weeks–11 months) up to 812 mg/kg body weight per day (brand‐loyal scenario),
- Guar gum is practically undigested, not absorbed intact, but significantly fermented by enteric bacteria in humans,
- Adequate toxicity data were available,
- No adverse effects were reported in subchronic studies in rodents at the highest dose tested of 15,000 mg guar gum/kg body weight per day in mice and 18,000 mg guar gum/kg body weight per day in rats,
- There is no concern with respect to the genotoxicity of guar gum,
- No carcinogenic effects were reported at the highest dose tested of 7,500 mg guar gum/kg body weight per day in mice and 2,500 mg guar gum/kg body weight per day in rats,
- Oral intake of large amount of guar gum in (9,000–30,000 mg/person corresponding to 128–429 mg/kg body weight per day) was well tolerated in adults. In most studies after consumption of around 15,000 mg per day in adults corresponding to 214 mg/kg body weight per day, some individuals experienced abdominal discomfort which was considered by the European Food Safety Authority Panel as undesirable but not adverse,
- In one interventional study with diabetic children abdominal discomfort was reported in 5 out of 22 children given 13,500 mg guar gum per day corresponding to 314 mg/kg body weight per day,
- Using the refined exposure assessment (non brand‐loyal scenario), the European Food Safety Authority Panel noted that exposures for high level consumers (children and adults) would be below the level at which some abdominal discomfort was reported,
- No data on abdominal discomfort were available for infants and young children,
The European Food Safety Authority Panel considered that for uses of guar gum in foods intended for infants and young children the occurrence of abdominal discomfort should be monitored and if this effect is observed doses should be identified as a basis for further risk assessment.
Infants and young children consuming foods for special medical purposes and special formulae
Concerning the use of guar gum (E 412) in ‘dietary foods for special medical purposes and special formulae for infants’ and ‘in dietary foods for babies and young children for special medical purposes and given that:
- For populations consuming dietary foods for special medical purposes and special formulae, the highest refined exposure estimate (p95) calculated based on the reported data from food industry are for infants (12 weeks‐11 months) consuming dietary foods for special medical purposes and special formulae up to 1,555 mg/kg body weight per day (brand‐loyal scenario),
- Infants and young children consuming these foods may be exposed to a greater extent to guar gum (E 412) than their healthy counterparts because the permitted levels of guar gum (E 412) in products for special medical purposes are 10‐fold higher than in infant formulae and follow‐on formulae for healthy individuals,
- Infants and young children consuming foods belonging to these food categories may show a higher susceptibility to the gastrointestinal effects of guar gum than their healthy counterparts due to their underlying medical condition,
- No adequate specific studies addressing the safety of use of guar gum (E 412) in this population under certain medical conditions were available,
- It was not possible to assess at which exposure level of guar gum the gastrointestinal effects developed in this specific population,
The European Food Safety Authority Panel concluded that the available data do not allow an adequate assessment of the safety of guar gum (E 412) in infants and young children consuming these foods for special medical purposes 22.
The European Food Safety Authority Panel recommended that the maximum limits for the impurities of toxic elements (lead, mercury and arsenic) in the European Commission specification for guar gum (E 412) should be revised in order to ensure that guar gum (E 412) as a food additive will not be a significant source of exposure to those toxic elements in food in particular for infants and children. The European Food Safety Authority Panel noted that currently detected levels of these toxic elements were orders of magnitude below those defined in the European Union specifications, and therefore, the current limits could be lowered.
The European Food Safety Authority Panel recommended to harmonize the microbiological specifications in the European Union Regulation for polysaccharidic thickening agents, such as gums, and to include criteria for the absence of Salmonella spp. and Escherichia coli for total aerobic microbial count and for total combined yeasts and moulds count into the European Union specifications of guar gum (E 412).
The European Food Safety Authority Panel recommended to give separate specifications in the European Union regulation for guar gum and clarified guar gum differing significantly in the protein content.
The European Food Safety Authority Panel considered that no threshold dose can be established for allergic reactions. Therefore, it is advisable that exposure to eliciting allergens, such as proteinaceous compounds, is avoided as much as possible and therefore the European Food Safety Authority Panel recommended that their content should be reduced as much as possible, which can be achieved, for example, by clarification of guar gum.
The European Food Safety Authority Panel recommended that additional data should be generated to assess the potential health effects of guar gum (E 412) when used in ‘dietary foods for infants for special medical purposes and special formulae for infants’ and in ‘dietary foods for babies and young children for special medical purposes’ 22.
Guar gum uses
Guar gum is used as thickener, stabilizer and emulsifier, and as fiber source 29 and approved in most areas of the world (e.g. EU, USA, Japan, and Australia). Guar gum food additive is E 412. In the United States, guar gum is listed for use as an emulsifier, formulation aid and firming agent.
Beverages
Guar gum is used in beverages for thickening and viscosity control because of its several inherent properties. The important property of guar gum is its resistance to breakdown under low pH conditions present in beverages. Guar gum is soluble in cold water which makes it easy to use in beverage processing plants. It improves the shelf life of beverages.
Processed cheeses
In cheese product, syneresis or weeping is a problem of serious concern. Guar gum prevents syneresis or weeping by water phase management and thus also improves the texture and body of the product 30. In cheese products it is allowed up to 3% of the total weight. Guar gum in the soft cheeses enhances the yield of curd solids and gives a softer curve with separated whey. Low-fat cheese can be produced with addition of guar gum (at concentration 0.0025–0.01% w/v) without changing the rheology and texture compared with full-fat cheese.
Dairy products
Main purpose of using guar gum in frozen products is stabilization. Guar gum has important role in ice cream stabilization because of its water binding properties. Its use in high temperature short time processes is very favorable because such processes require hydrocolloids that can fully hydrate in a short processing time. According to McKiernan 31 locust bean gum has all the properties of an ideal gum but it hydrates slowly which is not favorable in high temperature short time process. Julien 32 obtained satisfactory results with guar as stabilizer in continuous ice cream processing. Guar gum should be used in ice cream mix at a concentration level of 0.3% 33. It was also used in combination with carrageenan in a mixed guar-carrageenan system developed for high temperature short time process. Like locust bean gum its performance can be improved when used in combination with other stabilizers 34. Guar gum in ice cream improves the body, texture, chewiness and heat shock resistance. Partially hydrolyzed guar gum (at 2–6% concentration level) decreases syneresis and improves the textural and rheological properties of low-fat yoghurt comparable with full-fat yoghurt 35.
Table 2. Food applications of guar gum
| Food | Dose level | Function |
| Chapati | 0.75% | Softness |
| Bread | 0.50% | Softness, loaf volume |
| Fried Products | 0.5–1.0% | Oil uptake reduction |
| Yoghurt | 2.00% | Texture improver |
| Cake | 0.15% | Fat replacer, Firmness |
| Sausage | 0.13–0.32% | Softness |
| Pasta | 1.50% | Texture improver |
| Ice cream | 0.50% | Smaller ice crystals |
| Baked goods | 1.00% | Dough improver |
| Tomato Ketchup | 0.5–1.0% | Consistency improver, |
| Tomato Ketchup | 0.5–1.0% | Serum loss reduction |
Processed meat products
Guar gum has strong water holding capacity in both hot and cold water. Hence, it is very effectively used as a binder and lubricant in the manufacturing of sausage products and stuffed meat products. It performs specific functions in processed meat products like syneresis control, prevention of fat migration during storage, viscosity control of liquid phase during processing and cooling and control of accumulation of the water in the can during storage. Guar gum also enhances the creaming stability and control rheology of emulsion prepared by egg yolk 37
Bakery products
Addition of guar gum in cake and biscuit dough improves the machinability of the dough that is easily removed from the mold and can be easily sliced without crumbling. At 1% addition of in batter of doughnuts, it gives desirable binding and film-forming properties that decreases the penetration of fats and oils. Guar gum in combination with starch is found to be effective in prevention of dehydration, shrinking and cracking of frozen-pie fillings 38. In wheat bread dough, addition of guar gum results in significant increase in loaf volume on baking 39. Guar gum along with xanthan gum retard staling in gluten-free rice cakes by decreasing the weight loss and retrogradation enthalpy 40. Similarly, guar gum also retards staling in chapati at room temperature as well as refrigerated temperature by controlling retrogradation of starch 41.
Salad dressings and sauces
Its cold water dispersibility and compatibility with high acidic emulsions enable it to use as thickener in salad dressing at about 0.2–0.8% of total weight. In salad dressings, it acts as an emulsion stabilizer by enhancing the viscosity of water phase and hence decreasing the separation rate of the water and oil phase 33. Guar gum has been found useful as a thickener in place of tragacanth in pickle and relish sauces 42. Guar gum enhances the consistency of tomato ketchup more prominently than other hydrocolloids like carboxy methyl cellulose, Sodium alginate, gum acacia and pectin. On addition of guar gum serum loss and flow values of tomato ketchup decreases which makes it a novel thickener for tomato ketchup 43.
Health benefits
Various studies have been conducted on animals to test for both harmful and beneficial effect of guar gum. Guar is completely degraded in the large intestine by Clostridium butyricum 44. Harmful effects are observed only when the guar gum is given to the animals at a high concentration of about 10–15% on weight basis. This high concentration will reduce growth of animal due to decreased feed intake and impaired digestion. It is considered that the high viscosity of the intestinal tract contents, resulting from intake of guar gum at higher concentration, is the major cause of the negative effects. Hence, guar gum can only be used for its beneficial effects at lower concentration of about 0.5–1.0%. Above this concentration it will show negative effects of higher viscosity, decreased protein efficacy and lipid utilization. High viscosity of guar gum when used at a higher concentration, above 1.0% will not only interfere with nutritional properties of the food but also with the physicochemical and sensory properties of the food product which is not accepted by the consumer. Partial hydrolysis of guar gum reduces the chain length and molecular weight of the polymer and ultimately the lower viscosity makes it a novel soluble fiber that resembles in basic chemical structure with native guar gum and has various applications in clinical nutrition associated with ingestion of dietary fiber. It solves all the problems of high viscosity of guar gum. With hydrolyzed guar gum it is possible to increase the dietary fiber content of various food products like beverages without disturbing the nutritional and sensory properties of the food products. Partial hydrolysis of guar gum supplementation to the diet also reduces the laxative requirement, incidence of diarrhea and symptoms of irritable bowel syndrome 45. For treatment of irritable bowel syndrome (IBS) water soluble non-gelling fibers are preferred. Due to its water solubility and non-gelling behavior, partially hydrolyzed guar gum decreased the symptoms in both forms of irritable bowel syndrome (IBS) i.e. constipation predominant and diarrhea predominant 46.
In vitro study shows that presence of guar gum significantly decreases the digestion of starch. It acts as a barrier between starch and starch hydrolyzing enzymes 47.
Guar gum shows cholesterol and glucose lowering effects because of its gel forming properties. It also helps in weight loss and obesity prevention. Due to gel forming capacity of guar gum soluble fiber, an increased satiation is achieved because of slow gastric emptying. Diet supplemented with guar gum decreased the appetite, hunger and desire for eating 48. Mechanism behind cholesterol lowering by guar gum is due to increase in excretion of bile acids in feces and decrease in enterohepatic bile acid which may enhances the production of bile acids from cholesterol and thus hepatic free cholesterol concentration is reduced 49. Hypotriacylglycerolemic effects are due to decrease in absorption of dietary lipids and reduced activity of fatty acid synthase in liver 50. Toxicity study on partially hydrolyzed guar gum has revealed that it is not mutagenic up to dose level of 2500 mg/day 51. Adequate intake of guar gum as dietary fiber helps in the maintenance of bowel regularity, significant reductions in total and LDL “bad” cholesterol, control of diabetes, enhancement of mineral absorption and prevention of digestive problems like constipation 52.
Other non-food uses
Other commercial importance of guar gum is because of its use in oil and gas well stimulation specifically hydraulic fracturing in which high pressure is used to crack rock 36. Guar gum makes the fracturing fluid thicker so that it can carry sand into fractured rock. This fracture remains open due to presence of sand which creates a path for gas or oil to flow to well bore. Guar derivatives for use in fracturing fluids are hydroxypropyl guar (HPG) and carboxymethyl hydroxypropyl guar (CMHPG). In textile and carpet printing, guar gum thickens the dye solutions which allow more sharply printed patterns to be produced. Guar gum has been used in explosives for over 25 years as an additive to dynamite for water blocking. In recent years, it has become the primary gelling agent in water based slurry explosives. Water blocking, swelling and gelling property of guar gum make it enable to use as an additive in explosive industry. Explosive property is maintained by mixing of ammonium nitrate, nitroglycerine and oil explosives with guar gum even in wet conditions. The production of paper is enhanced by an addition of small amounts of guar gum to the pulp. It serves as a fiber deflocculent and dry-strength additive. It provides denser surface to the paper used in printing. Research investigation shows that high viscosity guar gum derivatives can be obtained by treatment of guar gum with complexing agents like organic titanates, chromium salts and aluminum salts. These agents react with guar gum to form complexes with high viscosity gel.
Guar gum side effects
Guar gum can cause abdominal pain, flatulence, diarrhea, nausea, and cramps.
References- Guar Gum. Chemical and Technical Assessment. Prepared by Yoko Kawamura, Ph.D., for the 69th JECFA. http://www.fao.org/fileadmin/templates/agns/pdf/jecfa/cta/69/Guar_gum.pdf
- Chudzikowski RJ. Guar gum and its applications. J Soc Cosmet Chem. 1971;22:43–60.
- Robaislek, E. (1974) Bioavailable calorie assay of guar gum. Unpublished report from WARF Institute, Inc. submitted to the World Health Organization by Institut Européen des Industries de la Gomme de Caroube
- Krantz, J. C., jr (1948) The feeding of guar gum to rats (lifespan) and to monkeys. Unpublished report from the University of Maryland, School of Medicine submitted to the World Health Organization by General Mills Chemicals, Inc.
- Booth, A. N., Hendrickson, A. P. and De Eds, F. (1963) Physiologic effects of three microbial polysaccharides on rats, Toxicol. appl. Pharmacol., 5, 478-484
- Tsai, L. B. & Whistler, R. L. (1975) Digestibility of galactomannans. Unpublished report submitted to the World Health Organization by Professor H. Neukom, Chairman of the Technical Committee of Inst. Europ. des Industries de la Gomme de Caroube
- Semenza, G. (1975) Report on the possible digestion of locust bean gum in the stomach and/or in the small intestine in an in vitro study. Unpublished report from the Eidgenössische Technische Hochschule Zürich submitted to the World Health Organization by the Institut Européen des Industries de la Gomme de Caroube
- Couch, J. R., Bakshi, Y. K., Fergusson, T. M., Smith, E. B. & Creger, C. R. (1967) The effect of processing on the nutritional value of guar meal for broiler chicks, Brit. Poultry Sci., 8, 243-250
- Riccardi, B. A. & Fahrenback, H. J. (1967) Effect of guar gum and pectin N.F. on serum and liver lipids of cholesterol fed rats, Proc. Soc. Exp. Biol. Med., 124, (3), 749-752
- Duodecim. 1990;106(10):829-30. Finnish. No abstract available. Duodecim. 1990;106(10):829-30. https://www.ncbi.nlm.nih.gov/pubmed/1670299
- Anaphylactic shock to guar gum (food additive E412) contained in a meal substitute. Allergy. 2007 Jul;62(7):822. https://onlinelibrary.wiley.com/doi/full/10.1111/j.1398-9995.2007.01369.x
- Occupational allergic rhinitis from guar gum. Clin Allergy. 1988 May;18(3):245-52. https://www.ncbi.nlm.nih.gov/pubmed/3396193
- Occupational asthma caused by guar gum. J Allergy Clin Immunol. 1990 Apr;85(4):785-90. https://www.ncbi.nlm.nih.gov/pubmed/2324416
- XANTHAN GUM. 82nd JECFA – Chemical and Technical Assessment (CTA), 2016. Prepared by Eugenia Dessipri, Ph.D., and Reviewed by Madduri V. Rao, Ph.D. http://www.fao.org/3/a-br568e.pdf
- García‐Ochoa F, Santos VE, Casas JA and Gomez E, 2000. Xanthan gum: production, recovery, and properties. Biotechnology Advances, 18, 549–579.
- Eastwood MA, Brydon WG and Anderson DM, 1987. The dietary effects of xanthan gum in man. Food Additives and Contaminants, 4, 17–26.
- Daly J, Tomlin J and Read NW, 1993. The effect of feeding xanthan gum on colonic function in man: correlation with in vitro determinants of bacterial breakdown. British Journal of Nutrition, 69, 897–902.
- Re‐evaluation of xanthan gum (E 415) as a food additive. EFSA Journal 14 July 2017. https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4909
- EFSA ANS Panel (EFSA ANS Panel on Food Additives and Nutrient Sources added to Food), 2014. Statement on a conceptual framework for the risk assessment of certain food additives re‐evaluated under Commission Regulation (EU) No 257/2010. EFSA Journal 2014;12(6):3697, 11 pp. https://doi.org/10.2903/j.efsa.2014.3697
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1975a. In: Toxicological Evaluation of Certain Food Additives. 19th Report of the Joint FAO/WHO Expert Committee on Food Additives, World Health Organisation (WHO), tech. Rep. Ser. 1975, No 576; FAO Nutrition Meetings report Series, 1975, No 55. http://whqlibdoc.who.int/trs/WHO_TRS_576.pdf
- SCF (Scientific Committee for Food), 1978. Reports from the Scientific Committee for Food (7th series). Opinion expressed 1977. Food science and techniques, 1978.
- Re‐evaluation of guar gum (E 412) as a food additive. EFSA Journal 24 February 2017. https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4669
- SCF (Scientific Committee for Food), 1998. Opinion of the Scientific Committee of Food on the applicability of the ADI (Acceptable Daily Intake) for food additives to infants. 17 September 1998.
- SCF (Scientific Committee on Food), 2001. Guidance on submissions for food additive evaluations by the scientific committee on food. Opinion expressed on 11 July 2001.
- SCF (Scientific Committee for Food), 2003. Report of the Scientific Committee on Food on the Revision of Essential Requirements of Infant Formulae and Follow‐on Formulae. Adopted on 4 April 2003. SCF/CS/NUT/IF/65 Final. 18 May 2003.
- Collins TF, Welsh JJ, Black TN, Graham SL and O’Donnell MW Jr, 1987. Study of the teratogenic potential of guar gum. Food and Chemical Toxicology, 25, 807–814.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1978. Evaluation of certain food additives. Twenty‐first report of the Joint FAO/WHO Expert Committee on Food Additives. No. 617. World Health Organization, Geneva.
- EFSA ANS Panel (European Food Safety Authority Panel on Food Additives and Nutrient Sources added to Food), 2012. Guidance for submission for food additive evaluations. EFSA Journal 2012;10(7):2760, 60 pp. doi:10.2903/j.efsa.2012.2760
- Morris JB. Morphological and reproductive characterization of guar (cyamopsis tetragonoloba) genetic resources regenerated in Georgia, USA. Genet Resour Crop Ev. 2010;57:985–993. doi: 10.1007/s10722-010-9538-8.
- Klis JB. Woody’s Chunk O’Gold cold-pack cheese food weeps no more. Food Processing Marketing. 1966;27:58–59.
- McKiernan BJ (1957) The role of gums in stabilizers. Paper presented at the Michigan Dairy Manufacturer’s Annual Conference, Michigan State University, East Lansing, Michigan
- Julien JP. A study of some stabilizers in relation to HTST pasteurization of Ice cream. Ice Cream Trade J. 1953;49:44.
- Goldstein AM, Alter EN (1959b) Guar Gum. In: Whistler (ed) Industrial gums, polysaccharides and their derivatives. Academic Press, New York, pp 321
- Weinstein B (1958) Stabilizers for ice cream type desserts. U.S. Patent 2856289
- Brennan CS, Tudorica CM. Carbohydrate-based fat replacers in the modification of the rheological, textural and sensory quality of yoghurt: comparative study of the utilisation of barley beta-glucan, guar gum and inulin. Int J Food Sci and Technol. 2008;43:824–833. doi: 10.1111/j.1365-2621.2007.01522.x
- Mudgil D, Barak S, Khatkar BS. Guar gum: processing, properties and food applications—A Review. Journal of Food Science and Technology. 2014;51(3):409-418. doi:10.1007/s13197-011-0522-x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3931889/
- Ercelebi EA, Ibanoglu E. Stability and rheological properties of egg yolk granule stabilized emulsions with pectin and guar gum. Int J Food Prop. 2010;13:618–630. doi: 10.1080/10942910902716984
- Werbin SJ (1950) Natural Gums in the Baking Industry, Paper presented at the American Association of Cereal Chemists, Dec. 5
- Cawley RW. The role of wheat flour pentosans in baking. II. Effect of added flour pentosans and other gums on gluten starch loaves. J Sci Food Agr. 1964;15:834–838. doi: 10.1002/jsfa.2740151204.
- Sumnu G, Koksel F, Sahin S, Basman A, Meda V. The effects of xanthan and guar gums on staling of gluten-free rice cakes baked in different ovens. Int J Food Sci Technol. 2010;45:87–93. doi: 10.1111/j.1365-2621.2009.02107.x
- Shaikh MI, Ghodke SK, Ananthanarayan L. Inhibition of staling in chapati (Indian unleavened flat bread) J Food Process Pres. 2008;32:378–403. doi: 10.1111/j.1745-4549.2008.00185.x
- Burrell JR. Pickles and sauces. Food Manuf. 1958;33:10–13.
- Gujral HS, Sharma A, Singh N. Effects of hydrocolloids, storage temperature and duration on the consistency of tomato ketchup. Int J Food Prop. 2002;5:179–191. doi: 10.1081/JFP-120015600
- Hartemink R, Schoustra SE, Rombouts FM. Degradation of guar gum by intestinal bacteria. Bioscience Microflora. 1999;18:17–25.
- Partially hydrolyzed guar gum: clinical nutrition uses. Slavin JL, Greenberg NA. Nutrition. 2003 Jun; 19(6):549-52.
- Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Giannini EG, Mansi C, Dulbecco P, Savarino V. Nutrition. 2006 Mar; 22(3):334-42. https://www.ncbi.nlm.nih.gov/pubmed/16413751/
- Dartois A, Singh J, Kaur L, Singh H. Influence of guar gum on the in vitro starch digestibility-rheological and microstructural characteristics. Food Biophysics. 2010;5:149–160. doi: 10.1007/s11483-010-9155-2
- Guar gum: a miracle therapy for hypercholesterolemia, hyperglycemia and obesity. Butt MS, Shahzadi N, Sharif MK, Nasir M. Crit Rev Food Sci Nutr. 2007; 47(4):389-96. https://www.ncbi.nlm.nih.gov/pubmed/17457723/
- Guar gum and similar soluble fibers in the regulation of cholesterol metabolism: current understandings and future research priorities. Rideout TC, Harding SV, Jones PJ, Fan MZ. Vasc Health Risk Manag. 2008; 4(5):1023-33. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2605338/
- Yamamoto Y. Hypolipidemic effects of a guar gum-xanthan gum mixture in rats fed high sucrose diet. Journal of Japanese Society of Nutrition and Food Science. 2001;54:139–145. doi: 10.4327/jsnfs.54.139
- Takahashi H, Yang SI, Fujiki M, Kim M, Yamamoto T, Greenberg NA. Toxicity studies of partially hydrolyzed guar gum. Int J Toxicol. 1994;13:273–278. doi: 10.3109/10915819409140599
- Chemical and physical properties, safety and application of partially hydrolized guar gum as dietary fiber. Yoon SJ, Chu DC, Raj Juneja L. J Clin Biochem Nutr. 2008 Jan; 42():1-7.