What is guava
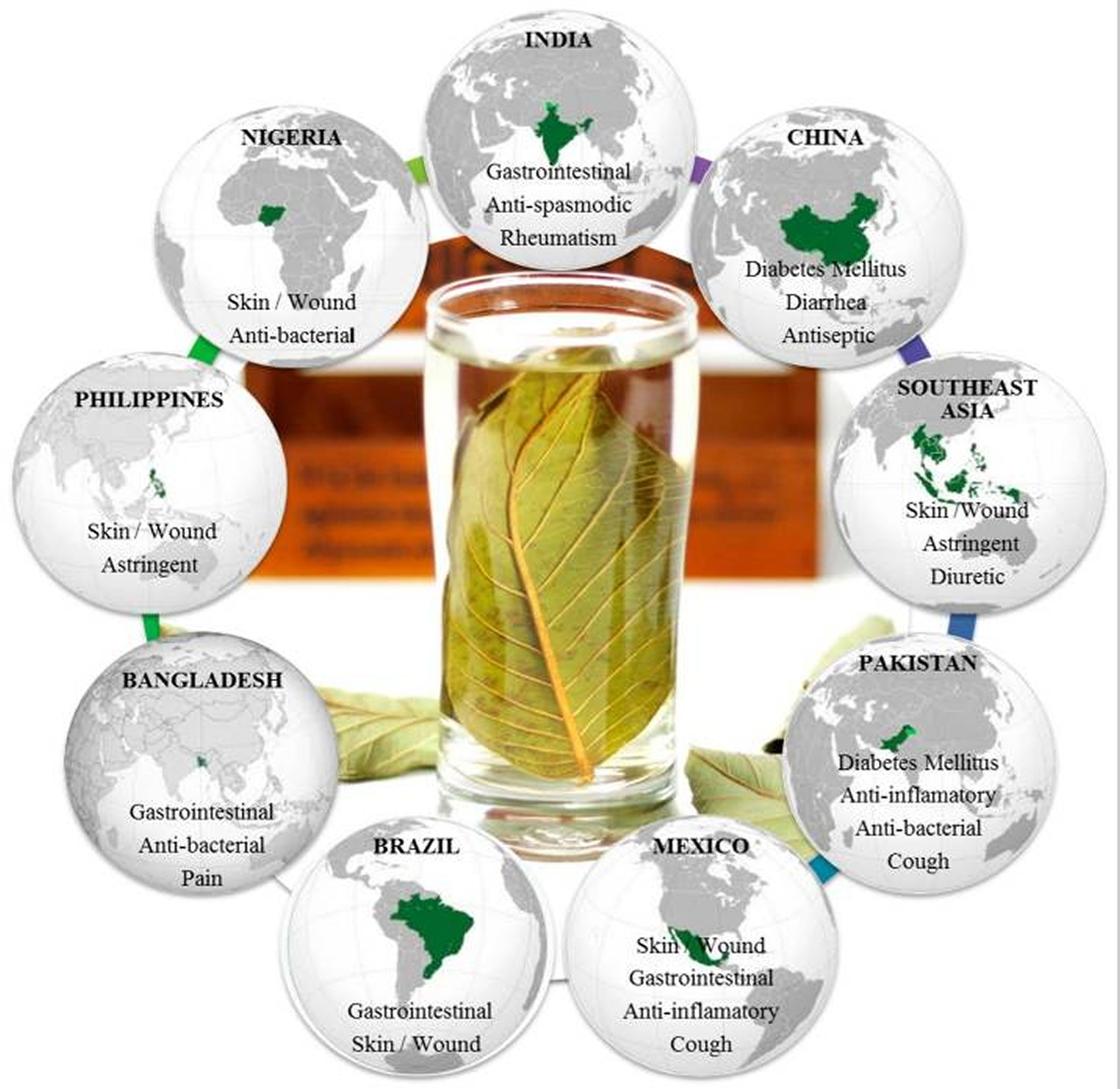
Guava (Psidium guajava Linn.) is a small tree belonging to the myrtle family (Myrtaceae) 1. There are about 100 species of tropical shrubs and small trees in the genus. Native to tropical areas from southern Mexico to northern South America, guava trees have been grown by many other countries having tropical and subtropical climates, thus allowing guava production around the world 2. Traditionally, preparations of guava leaves have been used in folk medicine in several countries, as anti-diarrheal remedy 3 to antimicrobial activity and anticancer property 3. Moreover, other several uses have been described elsewhere on all continents, with the exception of Europe 4, 5. Figure 3 summarizes the main traditional uses of guava leaves in the main guava producing countries. Depending upon the illness, the application of the remedy is either oral or topical. According to some researchers, the consumption of decoction, infusion, and boiled guava leaves preparations is the most common way to overcome several disorders, such as rheumatism, diarrhea, diabetes mellitus, and cough, in India, China, Pakistan, and Bangladesh 4, while in Southeast Asia the decoction is used as gargle for mouth ulcers 6 and as anti-bactericidal in Nigeria 7. For skin and wound applications, poultice is externally used in Mexico, Brazil, Philippines, and Nigeria 6. In addition, chewing stick is used for oral care in Nigeria 7. In recent years, guava leaves tea and some complementary guava products are available in several shops in Japan as well as on the Internet 8, because guava leaf phenolic compounds have been claimed to be food for specified health use, because they have beneficial health effects related to the modulation of blood–sugar level 9. A guava leaf tea is now commercially available in Japan which contains aqueous guava leaf extract and is approved as one of the Foods for Specified Health Uses 10.
In Mexico, the guava agua fresca beverage is popular. The entire fruit is a key ingredient in punch, and the juice is often used in culinary sauces (hot or cold), as well as artisan candies, dried snacks, fruit bars, desserts, or dipped in chamoy. Pulque de guava is a popular blend of the native alcoholic beverage.
In many countries, guava is eaten raw, typically cut into quarters or eaten like an apple, whereas in other countries it is eaten with a pinch of salt and pepper, cayenne powder or a mix of spices (masala). It is known as the winter national fruit of Pakistan. In the Philippines, ripe guava is used in cooking sinigang. Guava is a popular snack in Taiwan, sold on many street corners and night markets during hot weather, accompanied by packets of dried plum powder mixed with sugar and salt for dipping. In east Asia, guava is commonly eaten with sweet and sour dried plum powder mixtures. Guava juice is popular in many countries. The fruit is also often prepared in fruit salads.
Because of its high level of pectin, guavas are extensively used to make candies, preserves, jellies, jams, and marmalades (such as Brazilian goiabada and Colombian and Venezuelan bocadillo), and also for juices and aguas frescas or may be used in a marmalade jam on toast.
Red guavas can be used as the base of salted products such as sauces, substituting for tomatoes, especially to minimize acidity. A drink may be made from an infusion of guava fruits and leaves, which in Brazil is called chá-de-goiabeira, i.e., “tea” of guava tree leaves, considered medicinal.
Types of guava
The main difference between guavas lies within their fruit, which has flesh that ranges in color from red to white. There are even several varieties of fruit that share the common name guava that are not true guavas at all despite their similar appearance. The most frequently eaten species, and the one often simply referred to as “the guava”, is the apple guava (Psidium guajava).
Pink- and Red-Fleshed Guava
Sometimes referred to as dessert guavas, pink- and red-fleshed guava varieties produce sweet, intensely fragrant fruit. A common variety is the ‘Homestead’ guava (Psidium guajava ‘Homestead’), which produces fruit with sweet, flavorful flesh featuring a deep salmon-pink color.
Another pink-fleshed variety is ‘Hong Kong Pink’ (Psidium guajava ‘Hong Kong Pink,’ USDA zones 9b to 11), which produces large quantities of round, 6- to 8-ounce fruits. The pink-fleshed fruit ranges from sweet to mildly sub-acidic with a smooth texture and few seeds. ‘Red Indian’ (Psidium guajava ‘Red Indian,’ USDA zones 9b to 11) is an older pink-fleshed guava variety noted for its sweet, high quality fruit. Despite their seediness, the fruit is good for eating raw.
White- and Yellow-Fleshed Guava
White- or yellow-fleshed guavas typically produce mild, acidic fruit with little to no fragrance. The flesh ranges from creamy white to pale yellow and the skin typically stays green when ripe, although some varieties develop a pinkish blush. Common white-fleshed varieties include ‘Mexican Cream’ (Psidium guajava ‘Mexican Cream’) and ‘White Indian’ (Psidium guajava ‘White Indian’), both of which produce small- to medium-sized fruit with pale flesh. ‘Mexican Cream’ is noted for the sweet flavor of its fruit, while ‘White Indian’ produces somewhat tart and seedy fruit.
‘Detwiler’ (Psidium guajava ‘Detwiler’) stands out with its medium to large fruit, which is often 3 inches in diameter. The pale flesh ranges in color from yellowish to almost salmon pink and is noted for its sweetness and flavor. Another white- or yellow-fleshed variety that produces a heavy crop of large fruit is ‘Sweet White Indonesian’ (Psidium guajava ‘Sweet White Indonesian’). It is a fast-growing variety noted for its tasty, 4-inch-diameter fruit.
Guava Relatives
A handful of other fruit varieties bear the common name guava, including the strawberry guava (Psidium littoral or Psidium cattleianum) and pineapple guava (Feijoa sellowiana), which does not belong to the guava genus, Psidium.
The strawberry guava tree bears a close resemblance to the tropical guava, and it produces similarly sweet, almost spicy fruit. The fruit is typically 1 to 1 1/2 inches in diameter with wrinkled red skin and creamy white flesh. It is suitable for jellies and cooking, as well as fresh eating.
The fruit of pineapple guava trees share a flavor similar to tropical guavas, but they have rough, dark green skin even when ripe. Pineapple guava trees typically reach a mature height of 10 to 15 feet with an equal spread, making them suitable for use as a fruiting hedge or specimen shrub in USDA plant hardiness zones 8 to 10.
Figure 1. Guava fruit (apple guava)
Figure 2. Guava types
Figure 3. Main traditional uses of guava leaves in the principal producer countries
[Source 11]Guava nutrition
Guava fruit is rich in Vitamins A and C, folic acid, dietary fiber, as well as dietary minerals such as iron, manganese, potassium, and copper. It is known that a single guava fruit contains about four times the amount of Vitamin C as an orange (orange has ~ 53.2 mg vitamin C per 100 gram of orange) 12. In 100 g of guava are 228.3 mg of vitamin C. However, nutrient content varies across guava cultivars. Although the strawberry guava (P. littorale var. cattleianum) has only 25% of the amount found in more common varieties, its total vitamin C content in one 100 gram serving (37 mg) only provides 41% of the Recommended Dietary Allowances (RDAs) (RDA for men 90 mg and women 75 mg of vitamin C) (see Table 2).
Guava has 68 calories per 100 gram of fruit.
Table 1. Guava – common (raw) nutrition facts
| Nutrient | Unit | Value per 100 g | |||||
| Approximates | |||||||
| Water | g | 80.8 | |||||
| Energy | kcal | 68 | |||||
| Energy | kJ | 285 | |||||
| Protein | g | 2.55 | |||||
| Total lipid (fat) | g | 0.95 | |||||
| Ash | g | 1.39 | |||||
| Carbohydrate, by difference | g | 14.32 | |||||
| Fiber, total dietary | g | 5.4 | |||||
| Sugars, total | g | 8.92 | |||||
| Minerals | |||||||
| Calcium, Ca | mg | 18 | |||||
| Iron, Fe | mg | 0.26 | |||||
| Magnesium, Mg | mg | 22 | |||||
| Phosphorus, P | mg | 40 | |||||
| Potassium, K | mg | 417 | |||||
| Sodium, Na | mg | 2 | |||||
| Zinc, Zn | mg | 0.23 | |||||
| Copper, Cu | mg | 0.23 | |||||
| Manganese, Mn | mg | 0.15 | |||||
| Selenium, Se | µg | 0.6 | |||||
| Vitamins | |||||||
| Vitamin C, total ascorbic acid | mg | 228.3 | |||||
| Thiamin | mg | 0.067 | |||||
| Riboflavin | mg | 0.04 | |||||
| Niacin | mg | 1.084 | |||||
| Pantothenic acid | mg | 0.451 | |||||
| Vitamin B-6 | mg | 0.11 | |||||
| Folate, total | µg | 49 | |||||
| Folic acid | µg | 0 | |||||
| Folate, food | µg | 49 | |||||
| Folate, DFE | µg | 49 | |||||
| Choline, total | mg | 7.6 | |||||
| Vitamin B-12 | µg | 0 | |||||
| Vitamin B-12, added | µg | 0 | |||||
| Vitamin A, RAE | µg | 31 | |||||
| Retinol | µg | 0 | |||||
| Carotene, beta | µg | 374 | |||||
| Carotene, alpha | µg | 0 | |||||
| Cryptoxanthin, beta | µg | 0 | |||||
| Vitamin A, IU | IU | 624 | |||||
| Lycopene | µg | 5204 | |||||
| Lutein + zeaxanthin | µg | 0 | |||||
| Vitamin E (alpha-tocopherol) | mg | 0.73 | |||||
| Vitamin E, added | mg | 0 | |||||
| Vitamin D (D2 + D3) | µg | 0 | |||||
| Vitamin D | IU | 0 | |||||
| Vitamin K (phylloquinone) | µg | 2.6 | |||||
| Lipids | |||||||
| Fatty acids, total saturated | g | 0.272 | |||||
| 04:00:00 | g | 0 | |||||
| 06:00:00 | g | 0 | |||||
| 08:00:00 | g | 0 | |||||
| 10:00:00 | g | 0 | |||||
| 12:00:00 | g | 0 | |||||
| 14:00:00 | g | 0.019 | |||||
| 16:00:00 | g | 0.228 | |||||
| 18:00:00 | g | 0.025 | |||||
| Fatty acids, total monounsaturated | g | 0.087 | |||||
| 16:1 undifferentiated | g | 0.005 | |||||
| 18:1 undifferentiated | g | 0.082 | |||||
| 20:01:00 | g | 0 | |||||
| 22:1 undifferentiated | g | 0 | |||||
| Fatty acids, total polyunsaturated | g | 0.401 | |||||
| 18:2 undifferentiated | g | 0.288 | |||||
| 18:3 undifferentiated | g | 0.112 | |||||
| 18:04:00 | g | 0 | |||||
| 20:4 undifferentiated | g | 0 | |||||
| 20:5 n-3 (EPA) | g | 0 | |||||
| 22:5 n-3 (DPA) | g | 0 | |||||
| 22:6 n-3 (DHA) | g | 0 | |||||
| Fatty acids, total trans | g | 0 | |||||
| Cholesterol | mg | 0 | |||||
| Amino Acids | |||||||
| Tryptophan | g | 0.022 | |||||
| Threonine | g | 0.096 | |||||
| Isoleucine | g | 0.093 | |||||
| Leucine | g | 0.171 | |||||
| Lysine | g | 0.072 | |||||
| Methionine | g | 0.016 | |||||
| Phenylalanine | g | 0.006 | |||||
| Tyrosine | g | 0.031 | |||||
| Valine | g | 0.087 | |||||
| Arginine | g | 0.065 | |||||
| Histidine | g | 0.022 | |||||
| Alanine | g | 0.128 | |||||
| Aspartic acid | g | 0.162 | |||||
| Glutamic acid | g | 0.333 | |||||
| Glycine | g | 0.128 | |||||
| Proline | g | 0.078 | |||||
| Serine | g | 0.075 | |||||
| Other | |||||||
| Alcohol, ethyl | g | 0 | |||||
| Caffeine | mg | 0 | |||||
| Theobromine | mg | 0 | |||||
Table 2. Guava – strawberry (raw) nutrition facts
| Nutrient | Unit | Value per 100 g | |||||
| Approximates | |||||||
| Water | g | 80.66 | |||||
| Energy | kcal | 69 | |||||
| Energy | kJ | 289 | |||||
| Protein | g | 0.58 | |||||
| Total lipid (fat) | g | 0.6 | |||||
| Ash | g | 0.8 | |||||
| Carbohydrate, by difference | g | 17.36 | |||||
| Fiber, total dietary | g | 5.4 | |||||
| Minerals | |||||||
| Calcium, Ca | mg | 21 | |||||
| Iron, Fe | mg | 0.22 | |||||
| Magnesium, Mg | mg | 17 | |||||
| Phosphorus, P | mg | 27 | |||||
| Potassium, K | mg | 292 | |||||
| Sodium, Na | mg | 37 | |||||
| Vitamins | |||||||
| Vitamin C, total ascorbic acid | mg | 37 | |||||
| Thiamin | mg | 0.03 | |||||
| Riboflavin | mg | 0.03 | |||||
| Niacin | mg | 0.6 | |||||
| Vitamin B-12 | µg | 0 | |||||
| Vitamin A, RAE | µg | 5 | |||||
| Retinol | µg | 0 | |||||
| Vitamin A, IU | IU | 90 | |||||
| Lipids | |||||||
| Fatty acids, total saturated | g | 0.172 | |||||
| 14:00:00 | g | 0.012 | |||||
| 16:00:00 | g | 0.144 | |||||
| 18:00:00 | g | 0.016 | |||||
| Fatty acids, total monounsaturated | g | 0.055 | |||||
| 16:1 undifferentiated | g | 0.003 | |||||
| 18:1 undifferentiated | g | 0.052 | |||||
| Fatty acids, total polyunsaturated | g | 0.253 | |||||
| 18:2 undifferentiated | g | 0.182 | |||||
| 18:3 undifferentiated | g | 0.071 | |||||
| Fatty acids, total trans | g | 0 | |||||
| Cholesterol | mg | 0 | |||||
| Amino Acids | |||||||
| Tryptophan | g | 0.005 | |||||
| Threonine | g | 0.022 | |||||
| Isoleucine | g | 0.021 | |||||
| Leucine | g | 0.039 | |||||
| Lysine | g | 0.016 | |||||
| Methionine | g | 0.004 | |||||
| Phenylalanine | g | 0.001 | |||||
| Tyrosine | g | 0.007 | |||||
| Valine | g | 0.02 | |||||
| Arginine | g | 0.015 | |||||
| Histidine | g | 0.005 | |||||
| Alanine | g | 0.029 | |||||
| Aspartic acid | g | 0.037 | |||||
| Glutamic acid | g | 0.076 | |||||
| Glycine | g | 0.029 | |||||
| Proline | g | 0.018 | |||||
| Serine | g | 0.017 | |||||
Guava Pharmacological Properties
Anti-microbial and anti-parasitic activities
Aqueous and organic extracts of guava leaves have been demonstrated to have antibacterial activity due to an inhibitory effect against antibiotics-resistant clinical isolates of Staphylococcus aureus strains 13. Despite using the same diffusion method, differences are noticed in their inhibition zones, as shown in Table 3, probably due to extraction method or the dose assayed. A methanol extract exerted antibacterial effects, preventing the growth of different strains from several bacteria such as Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Proteus spp., and Shigella spp. 14. Furthermore, different extracts of the guava leaves such as aqueous, acetone–water, methanolic, spray-dried extracts, and the essential oil, showed potential inhibitory activity against Gram-positive and Gram-negative bacteria and fungi 15. In these studies, it is noticeable that Gram-positive bacteria exhibited greater inhibition zones and minimum inhibitory concentrations (MICs) than Gram-negative. Concerning the anti-fungal activity, less inhibition than bacteria is reported 16, except for Candida krusei and Candida glabrata which provided higher inhibition 17, and for Aspergillus spp. for which no activity was found 18 (Table 3). Moreover, Bezerra et al. 19 evaluated the effect of guava leaves on different bacterial strains, concluding that the synergistic action between the leaves and various antibiotics boosted its anti-bacterial activity. This effect was also observed by Betoni et al. 20 with target drugs for the protein synthesis, cell-wall synthesis, and folic acid. However, the latter did not find synergic effect with gentamicin, perhaps because the time of maceration was lower than the time used by Bezerra et al. 19, and also the solvent was different (Table 3).
Metwally et al. 21 associated the antimicrobial activity against some bacteria and fungi with five flavonoids isolated from guava leaves. This effect was also related to the concentration of tannins in the guava leaves 22 and to the content of gallic acid and catechin [19]. Additionally, the activity against bacterial and fungal pathogens was traced to betulinic acid and lupeol 23. In fact, these studies are focused on the activity of these compounds, rather than on the effect of the whole extract of guava leaves.
In addition, leaf acetone extract of guava has also exhibited moderate acaricidal and insecticidal activities causing the dead of Hippobosca maculata adult fly 24.
Furthermore, Adeyemi et al. 25 suggested that an ethanol extract from guava leaves function as a trypanocide agent, since its inhibition of Trypanosoma brucei brucei growth proved similar to that of the reference drugs. Kaushik et al. 26 proposed guava leaves as an anti-malaria agent, due to their inhibitory activity and the resistance indices. Furthermore, the effect of guava leaf essential oil against toxoplasmosis caused by the growth of Toxoplasma gondii were reported 27. Additionally, guava leaves were proposed for the treatment of diarrhea caused by enteric pathogens, since it showed significant inhibitory activity against Vibrio cholerae and Vibrio parahemolyticus, Aeromonas hydrophila, Escherichia coli, Shigella spp. and Salmonella spp. 28. In addition, a reduction was described for S. flexneri and V. cholera invasion and for their adherence to the human laryngeal epithelial cells, and for the production of E. coli heat labile toxin and cholera toxin, as well as their binding to ganglioside monosialic acid enzyme 29. Moreover, other studies also demonstrated the antimicrobial effect of some bacteria that cause gastrointestinal disorders by different methods. In contrast to previous results, no inhibition of the hydrodistillation and n-hexane extract was found against E. coli Salmonella spp. 28 (Table 3).
Furthermore, guava leaf tea helped control of the growth of influenza viruses, including oseltamivir-resistant strains, via the prevention of viral entry into host cells, probably due to the presence of flavonols 30.
Table 3. Guava leaves extracts in test tube studies against infectious and parasitic diseases
| Origin | Extraction Method | Major Constituent | Microorganisms | Assay | Main Results | Ref. |
|---|---|---|---|---|---|---|
| Saudi Arabia | Decoction (30 min) | - | Staphylococcus aureus strains | Agar well diffusion assay | At 200 µL: iz ≤ 30 mm. | 13 |
| India | Soxhlet with MeOH (12 h), maceration in H2O (4 h) | - | S. aureus strains | Agar well diffusion assay, time-kill of bacterial cell, SDS-PAGE analysis, and cellular toxicity to human erythrocytes assays | At 20 mg/L: iz ≤ 20 mm, MIC: 25 µg/mL (MeOH) and 7.5 mg/mL (H2O). MBC: 1.25 and 12.5 mg/mL, respectively, 10 h to kill bacteria, ↑ degradation of protein, no hemolysis. | 31 |
| Nigeria | Maceration in MeOH (48 h) | - | S. aureus, Escherichia coli, Pseudomonas aeruginosa, Proteus spp., and Shigella spp. | Agar well diffusion assay | At 20 mg/mL: iz ≤ 18 mm; 81.8% prevention growth. | 32 |
| India | Maceration with agitation in MeOH, Ac, and DMF (12 h) | - | G-p and G-n bacteria and fungi (91 clinically important strains) | Disc diffusion assay | At 25 mg/mL: against g-p 70% MeOH > 80% Ac > 50% DE, ↓ 76.36% g-n bacteria. Fungi 56% Ac > 38% ME > 31% DMF. No activity against Citrobacter spp., Alcaligenes fecalis, and Aspergillus spp. | 33 |
| India | Soxhlet with MeOH (4 h) | Phytochemical screening: mainly flavonoid-glycosides and tannins | Bacteria (Bacillus subtilis, S. aureus and E. coli), and fungi (Candida albicans and Aspergillus niger) | Paper disc diffusion assay | At 50 μg/mL: iz ≤ 12.6 mm and 10 mm for bacterial and fungi strains, respectively. E. coli: MIC 0.78 μg/mL, MBC 50 μg/mL, and MFC 12.5 μg/mL. | 16 |
| Brazil | Maceration with stirring in EtOH:H2O 70% (v/v) (50 °C, 1 h) | TPC: 25.93 (% m/m, dry base), TFC: 23.48 (mg/g, dry base) | Fungi (C. albicans, Candida krusei, and Candida glabrata), G-p (S. aureus) and G-n (E. coli and P. aeruginosa) | Microdilution assay | MIC = 80–100 µg/mL (C. krusei, C. glabrata and S. aureus) and MBC, MFC ≤ 250–1000 µg/mL (the others). | 34 |
| Brazil | Turbo-extraction with water or Ac:H2O 70% (v/v) (20 min) | Gallic acid: 0.065 µg/g, Catechin: 1.04 µg/g | G-p strains (S. aureus, Staphylococcus epidermidis, and Enterococcus faecalis) G-n (E. coli, Salmonella enteritidis, Shigella flexneri, and Klebsiella pneumoniae) | Agar-diffusion and microdilution assays | At 5 mg/mL: iz ≤ 20 mm, MIC = 39 μg/mL (S. epidermis), MIC | 35 |
| India | Soxhlet with n-hexane | Methyl 2,6,10-trimethyltridecanoate (28.86%) and Methyl octadecanoate (22.18%) | G-p: S. aureus, Streptoccocus faecalis, Bacillus subtillis, Lactobacillus spp., Enterococcus aerogenes, Acinetobacter spp. G-n: E. coli, Proteus vulgari, Enterobacter aerogenes, Salmonella typhimurium, P. aeruginosa, and K. pneumoniae | Agar well diffusion assay | At 80 µL: iz ≤ 27 mm, MIC = 3–10 µL. | 36 |
| Brazil | Maceration in EtOH:H2O 70% (v/v) (72 h) | - | E. coli, P. aeruginosa, and S. Aureus | Microdilution assay | Only S. aureus (MIC = 256 mg/mL). Synergic effect with ciprofloxacin and gentamicin at 1024 mg/mL. | 37 |
| Brazil | Maceration in MeOH:H2O 70% (v/v) (48h) | - | S. aureus strains | Disc diffusion assay | MIC 90% = 0.52 mg/mL, at 131.75 mg/mL synergic effect with tetracycline, chloramphenicol, erythromycin, vancomycin, oxacillin, cephalothin, ampicillin, cefoxitin, cotrimoxazole. | 38 |
| Egypt | Maceration in EtOH:H2O 50% (v/v) | Quercetin, avicularin, guajaverin, isoquercitrin, hyperin | S. aureus, E. coli, P. aeruginosa, and C. albicans | Agar well diffusion assay | S. aureus: ↑ iz quercetin (28 mm), MIC (μg/mL) guajaverin (0.09–0.19) | 39 |
| Indonesia | Maceration in EtOH:H2O 30% (v/v) (3 days) | Tannins (2.35 mg/g) | E. coli, P. aureginosa, S. aureus, A. niger and C. Albicans | Paper disc diffusion method | iz ≤ 15 mm. | 40 |
| India | Soxhlet with toluene (72 h) | Betulinic acid and lupeol | Fungi: Calletotricheme camellie, Fussarium equisitae, Alterneria alternate, Curvularia eragrostidies, and Colletrichum Gleosproides. Bacteria: E. Coli, B. Subtillis, S. aureus, and Enterobactor | Slide germination method | Bacteria: MIC | 41 |
| India | Soxhlet with Ac (8 h) | - | H. bispinosa Neumann (Acarina: Ixodidae) and H. maculata Leach (Diptera: Hippoboscidae) | Antiparasitic activity method of FAO (2004) | At 3 mg/mL: mortality 100% H. maculate, 78% H. bispinosa, parasite dead H. maculata (LC50 = 0.646 mg/mL). | 24 |
| Nigeria | Maceration with agitation in EtOH:H2O 20% and 80% (v/v) (24 h) | - | Trypanosoma brucei brucei and HEK293 | Alamar Blue assays | At 238.10 μg/mL: IC50 (T. b. brucei) = 6.3 μg/mL and 48.9 μg/mL for 80% and 20% extracts, respectively, IC50 (HEK293) 30.1 and 24.16%, respectively. | 42 |
| India | Soxhlet with ethyl acetate and MeOH (8 h) | - | Plasmodium falciparum strains | SYBR green assay | IC50 9–18 μg/mL, resistance indices = 0.6 and 1.4 in MeOH and ethyl acetate, respectively. | 43 |
| Malaysia | Hydrodistillation (3 h) | - | Toxoplasma gondii | MTT assay with Vero cells | At 200 µg/mL: No cytotoxic effect (EC50 = 37.54 µg/mL), anti-parasitic activity (EC50 of 3.94 µg/mL). | 44 |
| India | Soxhlet with EtOH, and maceration in H2O (6 days) | - | E. coli, Shigella spp., Salmonella spp., Aeromonas spp., S. aureus, and Candida spp. | Agar well diffusion assay | H2O: iz ≤ 30 mm (max C. albicans). EtOH: iz ≤ 31 mm (max Aeromonas hydrophila). | 45 |
| India | Soxhlet with EtOH:H2O 70% (v/v), MeOH, ethyl acetate, and H2O | Phytochemical analysis: tannins, saponins, flavonoids, terpenoids, sugars | E. coli, Salmonella spp., and Vibrio cholerae | Agar well diffusion assay | At 1000 µg/mL: iz ≤ 30 mm. MeOH: MIC (100%) > 250 µg/mL. EtOH:H2O:MICs (38–65%) > 500 µg/mL and > 750 µg/mL. Ethyl acetate and H2O: MICs > 750 µg/mL. | 28 |
| Bangladesh | Maceration in H2O and MeOH:H2O 75% (v/v) (48 h) | - | V. cholera | Agar well diffusion assay | MICs = 1250 µg/mL (H2O), 850 µg/mL for (MeOH:H2O). Antibacterial resistance to trimethoprim/sulfomethoxazole, furazolidone, tetracycline, and erythromycin. | 46 |
| India | Decoction | Major component: quercetin (2 mg/g) | E. coli (heat labile (HLT) and cholera toxin (CT)), V. cholerae, Shigella flexneri | Microtitre plate based assay. Assays for bacterial colonization (adherence and invasion) and enterotoxins | At 2.7 mg/mL: (EC50 = 0.98 (S. flexneri) and 2.88% (V. cholerae). ↓ adherence and invasion to epithelial cells (EC50 = 0.37–1.25% and 0.04–0.25%, respectively). The effect on adherence is not due to quercetin and the invasion is lower than with the extract. ↓ Production of HLT and CT (EC50 = 1.03 and 2.69%) and binding to glioside monosialic acid enzyme (EC50 = 0.06 and 2.51%). | 29 |
| Brazil | Soxhlet with n-hexane, ethyl acetate, MeOH, H2O (24 h) | - | S. aureus, Salmonella spp., and E. coli | Disc diffusion method | At 1938 µg/disc: iz = 7.00–11.25 mm (Soxhlet), and 11–18 mm (H2O). No inhibition to E. coli (H2O) and Salmonella spp. (hexane and ethyl acetate). | 47 |
| Nigeria | Soxhlet with EtOH:H2O 60% (v/v) (5 h), and H2O (3 h) | - | E. coli and S. aureus | Agar well diffusion assay | At 10 mg/mL: H2O: iz = 9–16 mm and 8–11 mm, MICs = 5 and 2.5 mg/mL (E. coli and S. aureus, respectively). EtOH:H2O: iz 12–21 and 11–14 mm, MICs = 1.25 and 0.625 mg/mL, respectively. | 48 |
| Japan | Infusion (8 min) | Tannin content: 1.11 mg/mL | H1N1 virus strains | 19-h Influenza growth inhibition assay | At 0.4 mg/mL: inhibition growth (IC50 = 0.05–0.42%). | 30 |
After checking the effect of guava leaf extract, in vitro, against Aeromonas hydrophila, in vivo experiments were carried out in tilapia (Oreochromis niloticus), indicating the potential use of guava as environmentally friendly antibiotic 49. Guava leaves also had anti-viral and anti-bacterial activity towards shrimp (Penaeus monodon) pathogens such as yellow-head virus, white spot syndrome virus, and Vibrio harvey. In addition, guava leaf extract improved the activities of prophenoloxidase and nitric oxide synthase in serum, and of superoxide dismutase, acid phosphatase, alkaline phosphatase, and lysozyme in serum and hepatopancreas 50.
Furthermore, guava leaves have been suggested for managing sleeping sickness, since they exhibited trypanocidal effect in albino rats 51; the extract ameliorate the tissue-lipid peroxidation associated to trypanosomosis, as well as raising the level of the glutathione concentration 52. The leaves also showed anti-malarial effect in BALB/c mice infected with Plasmodium berghei via parasitemia suppression 53. Moreover, guava leaves are also recommended for treating infectious diarrhea since they prevented intestinal colonization of Citrobacter rodentium in Swiss albino mice 54. In chicks, guava leaf extract enabled the control of diarrhea produced by E. coli and reduced the severity of its symptomatology 55. In mice, the improvement of cholera symptoms caused by V. cholerae, a human pathogen, was also confirmed by Shittu et al. 56.
In addition, anti-helminthic properties towards gastro-intestinal nematodes have been found, as a result of the presence of condensed tannins in the guava plant, which raised the levels of hemoglobin, packed cell volume, total protein, globulin, glucose, and calcium, and lowered the levels of blood urea 57.
All the results published regarding in vivo anti-bacterial properties have been summarized in Table 4.
Table 4. Guava leaves extracts animal studies against infectious and parasitic diseases
| Origin | Extraction Method | Subject | Treatment | Main Results | Ref. |
|---|---|---|---|---|---|
| Thailand | Maceration in H2O, EtOH, and ether (24 h) | Oreochromis niloticus | Aeromonas hydrophila | LD50 = 3.44 × 106 CFU/mL. ↓ Mortality of the subjects. | 49 |
| China | - | Penaeus monodon | Yellow-head virus, white spot syndrome virus, and Vibrio harveyi | Survival rate = 80–95% (↑ Weight (2 to 6 g)). In serum (↑ feed): ↓ PO (7.50 U/mL) and SOD (178.33 U/mL), ↑ NOS (64.80 U/mL). In hepato-pancreas: ↑ SOD (57.32 U/mg), ACP (23.28 U/mg), AKP (19.35 U/mg), and LSZ (3459.946 U/mg). | 50 |
| Nigeria | Maceration with agitation in EtOH:H2O 80% (v/v) (24 h) | Albino rats | T. b. brucei | At 300 mg/kg: ↓ parasitemia; ↑ survival in 24 days. | 58 |
| Nigeria | Maceration with agitation in EtOH:H2O 80% (v/v) (24 h) | Albino rats | T. b. brucei | Administration 1–7 days. ↑ GSH: liver (5.4 to 8.1), kidney (3.3 to 6.0), and serum (0.8 to 2.4), restored in kidney and serum. In the brain, no effect was found. ↓ MDA: serum (13.9 to 5.9), brain (42.8 to 18.1), kidney (27.3 to 17.6), and liver (38.2 to 19.2). | 59 |
| India | Decoction of the leaves (10 min) | BALB/c mice | Plasmodium berghei | At 350 and 1000 mg/kg ↓ parasitemia (73.7% and 85.8%); ↑ survival 15 and 18 days. | 53 |
| India | Extraction in EtOH:H2O 50% (v/v) | Swiss mice | Citrobacter rodentium | At 300 mg/kg: ↓ infection (day 4) of the treatment, and no infection at day 19 (control group at day 24). | 54 |
| Nigeria | Hidrodistillation and fractionation with ethyl acetate | ISA brown male chicks | E. coli | At 100 mg/kg: In 10 days ↓ signs of villous collapse (stunting, matting and fusion of villi), number of wet droppings (12-6); ↑ activity, weight gaining, and feed intake (from 27 to 45 g) in contrast to the infected ones (from 30 to 18 g); ↓ bacterial shedding load (from 60 to 45 CFU/mL). | 55 |
| Nigeria | Decoction of the leaves | Adult mice | V. cholera | At 250 mg/kg: Histopathological observations: mild degenerative, secretory, and inflammatory changes with goblet cells and with most of the exudate (neutrophils and lymphocytes). | 56 |
| India | - | Adult male goat | Haemonchus contortus | 90 Days feeding: ↑ Hb (7.2 to 8.6 g/dL), PCV (20.2 to 29.3%), total protein (4.8 to 6.3 g/dL), GLO (2.3 to 3.8 g/dL) (↑ control (2.8)), glucose (43.9 to 52.6 g/dL), and calcium (8.7 to 9.6 mg/dL); ↓ blood urea (47.9 to 29.8 mg/dL) (↓ control (41)). Phosphorus balance, serum albumin levels and serum enzyme activity did not show variation. | 57 |
Anti-cancer
Only one study is available on the anti-tumor effect that could be related to the phenolic composition of guava leaves. An ethanol extract of guava leaves was administrated to B6 mice after inoculation of melanoma cells. The results suggested that the extract had a vaccine effect, but not a therapeutic effect, against tumors through by depressing T regulatory cells 60.
Moreover, the meroterpene-enriched fraction of guava leaves, containing guajadial, psidial A, and psiguadial A and B, was evaluated in vivo in a solid Ehrlich murine breast-adenocarcinoma model. The results suggested that these compounds may act as phytoestrogens, presenting tissue-specific antagonistic and agonistic activity on estrogen receptors 61. These data partially confirmed the results in vitro obtained by Ryu et al. 62.
Diseases of the Blood and Immune System
Among blood diseases, anemia indicates a failure in the immune system. In this sense, guava extract presented an anti-anemic effect in trypanosomosis-infected Wistar rats, improving the values of hemoglobin, packed cell volume, red-blood cell counts, mean corpuscular volume, and mean concentration hemoglobin count while decreasing white-blood cell and neutrophil levels 63. Moreover, the same trend in the hematological analyses was also recorded in mice. After the administration of guava leaf extract, no alterations on the erythron were detected 64. Nevertheless, results differ because subjects under study are different, also the duration of the treatment, the extraction method and the dose assayed.
The anti-inflammatory response of guava leaves was dose-dependent in induced hyperalgesia in Sprague-Dawley rats, decreasing in paw-withdrawal latency, and significantly improving the survival rate of mice with lethal endotoxemia 65. Moreover, the anti-inflammatory activity of aqueous and acetone–water extracts of guava leaves was also confirmed in Swiss mice by reducing the amount of leukocyte migration. The acetone–water extract also exhibited peripheral analgesic activity, probably by blocking the effect or the release of endogenous substances that excite pain-nerve endings 15. The analgesic effect in albino rats was also reported. The ethanol extract reduced the writhing response [107], and a jumping response was found after the administration of a distilled extract (combination of methanol and aqueous extracts) 66. In this case, the writhing response for both Swiss mice and Wistar rats seems to be comparable, although the dose assayed is completely different.
Endocrine and Metabolic Diseases
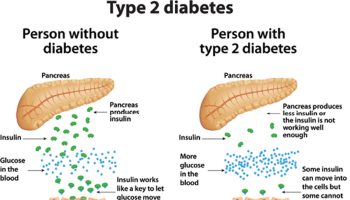
Guava leaves have shown their potential against one of the diseases with the highest incidence level worldwide, diabetes mellitus, and also towards biochemical changes caused by the disease. In spite of being leaves from different countries, treatments in different subjects or even different data, the same trend is followed in these works (Table 5).
The effect of aqueous guava leaf extract was investigated in rabbits, fed a high-cholesterol diet. Treatment with guava leaves reduced the plasma-cholesterol level, caused a remarkable spike in high-density lipoprotein, a dip in low-density lipoprotein levels, and significantly reduced the associated hyperglycemia. In addition, the extract showed hypolipidemic and hypoglycemic potentials in hypercholesterolemic rabbits 67. Furthermore, guava leaves reduced oxidative stress induced by hypercholesterolemia in rats 68.
In addition, the anti-diabetic effect was also evaluated in Leprdb/Leprdb mice and significant blood-glucose-lowering effects were observed. In addition, histological analysis revealed a significant reduction in the number of lipid droplets, which, furthermore, at least in part, could be mediated via the inhibition of protein tyrosine phosphatase 1B 69.
In streptozotocin-induced diabetic rats, the administration of oral doses of aqueous and ethanol extracts from guava leaves could alter the Ca:Mg ratio 70; however, in low-dose streptozotocin and nicotinamide-induced Sprague-Dawley diabetic rats, long-term administration of guava leaf extracts raised the plasma-insulin level, the glucose utilization, and the activity of hepatic enzymes 71. Moreover, the leaves also lowered blood glucose levels and decreased protein glycation 72.
In agreement with the above, a lower blood-glucose level was also reported in alloxan-induced diabetic rats. Additionally, no side effects were observed in certain liver enzymes (alkaline phosphatase and aspartate aminotransferase) whereas alanine aminotransferase activity declined 73. In alloxan-induced diabetic rats, a decrease was also found in blood glucose, total cholesterol, triglycerides, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, and a significant increase in high-density lipoprotein cholesterol after 21 days of treatment with guava leaf ethanolic extract 74.
Among the studies that evaluated only biochemical parameters, guava leaf extract promoted changes due to an alteration on the activity of alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, and acid phosphatase in the kidney, liver, and serum 75. In addition, Adeyemi and Akanji 76 evaluated the effect of daily administration of guava leaves, demonstrating the alteration of the serum homeostasis and the pathological variations in rat tissues.
Table 5. Guava leaves extract animal studies on endocrine and metabolic diseases
| Origin | Subject | Treatment | Main Results | Ref. |
|---|---|---|---|---|
| Nigeria | Rabbits | High-cholesterol diet | At 250 mg/kg: ↓ TC (15%); ↑ HDL (69%); ↓ LDL (74%); ↓ hyperglycemia 43%. | 67 |
| Brazil | Wistar rats | High-cholesterol diet | At 369.89 mg phenolic compound in the extract/g: ↓ TC (29–35%), TG (59–73%); ↑ HDL (46%); ↓ VLDL+LDL; ↓ enzyme activity (SOD (6.2 to 5.7 U/mg protein), GP (4.6 to 2.3 µmol/g protein). | 68 |
| Korea | Leprdb/Leprdb juvenile and adult mice | Diabetes spontaneous mutation | At 10 mg/kg: 87% inhibition PTP1B; ↓ glucose levels 31% and 42% respectively. | 69 |
| Iran | Wistar rat | Streptozotocin-induced diabetes | At 1mg/L: ↓ Ca/Mg ratio (18 to 12), glucose level, TG (100 to 65 mg/dL), TC (68 to 48 mg/dL), ↑ HDL (18 to 40 mg/dL), ↓ LDL, and VLDL to normal levels; ↓ alteration in vascular reactivity (110 to 50 mmHg). | 70 |
| Taiwan | Sprague-Dawley rats | Low-dose streptozotocin and nicotinamide-induced diabetes | At 400 mg/kg: ↓ blood glucose level (230 to 140 mg/dL); ↑ plasma insulin level and glucose utilization (normal levels); ↑ enzyme activity (hepatic hexokinase (8 to 11 U/mg protein), phosphofructokinase (18 to 25 U/mg protein) and glucose-6-phosphate dehydrogenase (11 to 25 U/mg protein). | 71 |
| India | Sprague-Dawley rats | Streptozotocin-induced diabetes | At 100 mg/kg: ↓ blood glucose level (4 to 1 mg/mL) and lipid peroxidation (2 to 1 mmol/100 g tissue); ↑ enzyme activity (CAT (6 to 10 × 103 U/mg protein), SOD (6 to 10 U/mg protein), GPx (0.4 to 0.6 U/mg protein), GRd (0.1 to 0.3 U/mg protein). | 72 |
| Nigeria | Albino rats | Alloxan-induced diabetes | At 200 mg/kg: ↑ average weight (99 to 209g); ↓ blood glucose level (15 to 8 mmol/L); ↓ alanine aminotransferase activity (32 to 24 U/L). | 73 |
| India | Albino rats | Alloxan-induced diabetes | At 500 mg/kg: ↓ blood glucose level, TC (231 to 163 mg/dL), TG (133 to 69 mg/dL), LDL (186 to 126 mg/dL), VLDL (26 to 13 mg/dL); ↑ HDL (18 to 23 mg/dL). | 74 |
| Nigeria | Wister rats | – | At 150 mg/kg: ↑ ALP (300, 175and 650 IU), AST (500, 400, 450 IU), ALT (1200, 1200, 1800 IU), ACP (750, 650, 900 IU) activity in the kidney, liver, and serum, respectively. | 75 |
| Nigeria | Mice | – | At 49.3 mg/mL: ↑ AST (93 to 126 iµ/L), ALT (30 to 35 iµ/L), ALP (57 to 66 iµ/L), conjugate bilirubin (0.2 to 0.3 mg/dL) and creatinine (0.9 to 1.2 mg/dL). | 64 |
| Nigeria | Albino rats | – | At 150 mg/kg: ↑ serum urea (2.9 to 6 mmol/L) and creatinine (2.7 to 4 mmol/L); ↓ concentration of serum Na+ (122 to 99 mmol/L). | 76 |
Notes: acid phosphatase (ACP); alanine aminotransferase (ALT); alkaline phosphatase (ALP); aspartate aminotransferase (AST); catalase (CAT); glutathione peroxidase (GPx); glutathione reductase (GRd); high-density lipoprotein (HDL) cholesterol; low-density lipoprotein (LDL) cholesterol; protein tyrosine phosphatase 1B (PTP1B); superoxide dismutase enzyme (SOD); total cholesterol (TC); triglycerides (TG); very low-density lipoprotein (VLDL) cholesterol; ↑ increases the affect; ↓ decreases the effect.
[Source 11]Cardiovascular diseases
Ademiluyi et al. 77 assessed the lipid peroxidation in rats after checking the antihypertensive effect, in vitro, of red and white guava leaves. The work concluded that the activity may be related to rosmarinic acid, eugenol, carvacrol, catechin, and caffeic acid since they were the major constituents of their extracts. In addition, this activity was supported by the biphasic and contractile effect on rat vascular smooth muscles 78.
In addition, atherosclerosis development was reduced in apoE-knockout mice by guava leaf extracts. In fact, the effect was connected to the presence of ethyl gallate and quercetin 79 In streptozotocin-induced diabetic rats, vascular reactivity to vasoconstrictor agents was reduced, as was vessel atherosclerosis [112]. Furthermore, Soman et al. 80 found that an ethyl acetate fraction of guava leaves reduced cardiac hypertrophy in streptozotocin-induced diabetic rats due to an anti-glycative effect.
Diseases of the Digestive System
In the digestive system, formed by the gastrointestinal tract plus the group organs necessary for the digestion, guava leaves have demonstrated activity towards different parts.
On the one hand, the leaves have shown the ability to protect the stomach against ulceration by inhibiting gastric lesions, reducing gastric secretory volume, and acid secretion, and raising the gastric pH 81. This anti-ulcer activity, resulting from the protection of the mucosa, was related to the flavonoids in guava leaves 82. The anti-diarrheal activity of guava leaf aqueous extract was evaluated on experimentally induced diarrhea in rodents. The extract performed in the same way as the control drugs, offering protection, inhibiting intestinal transit, and delaying gastric emptying 83. Another study attributed this activity to a dual action between the antimicrobial effect and the reduction in gastrointestinal motility ability of the extract 84. In rabbits, the anti-spasmodic effects were connected to a calcium channel blocking activity, which explains the inhibitory effect on gut motility. The anti-diarrheal protection was also tested in mice 85. The anti-diarrheal activity is dose-dependent, although the protection varied depending on the subjects.
Diseases of the Skin and Subcutaneous Tissue
Guava leaves have been suggested as a therapeutic agent to control pruritus in atopic dermatitis. The improvement of the skin lesions was due to a reduction in serum immunoglobulin E level and in the eczematous symptoms 86. Moreover, the epithelium was repaired with connective tissue and absence or moderate presence of inflammatory cells by guava leaves. As a result, guava leaves exhibited wound healing properties 87. Furthermore, guava leaf extract was tested on rat skin, and exhibited inhibitory activity towards an active cutaneous anaphylaxis reaction 88.
Guava health benefits
To test the effect of guava leaf extract, several randomized clinical trials have been conducted during the last two decades, although only two studies are available in the last decade. One of the studies consisted of evaluating the effect of guava leaf extract pills on primary dysmenorrhea disorder. For this, 197 women were divided into four groups, and each received a different dosage: 3 and 6 mg extract/day, 300 mg placebo/day and 1200 mg ibuprofen/day. The administration took place over five days during three consecutive cycles. The results demonstrated that 6 mg extract/day alleviated menstrual pain and could replace the use of medicines like ibuprofen 89. However, a 2016 Cochrane review 90 on the efficacy and safety of dietary supplements for treating dysmenorrhoea involving 27 randomized controlled trials (3101 women) using 12 different herbal medicines (chamomile, cinnamon, Damask rose, dill, fennel, fenugreek, ginger, guava, rhubarb, uzara, valerian, and zataria), and five non-herbal supplements (fish oil, melatonin, vitamins B1 and E, and zinc sulphate) in a variety of formulations and doses. Supplements were compared with other supplements, placebo, no treatment, and non-steroidal anti-inflammatory drugs (NSAIDs). The review authors conclusion was there is no high quality evidence to support the effectiveness of any dietary supplement for dysmenorrhoea, and evidence of safety is lacking. However for several supplements there was some low quality evidence of effectiveness and more research is justified. Supplements for which there was some very limited evidence to suggest a potential benefit were fenugreek, ginger, valerian, zataria, zinc sulphate, fish oil, and vitamin B1 90.
Deguchi and Miyazaki 91 reviewed several studies regarding the effect of the intake of a commercial guava leaf tea (Bansoureicha®, Yakult Honsha, Tokyo, Japan) on different pathologies of diabetes mellitus illness such as the influence on postprandial blood glucose, on insulin resistance and on hypertriglyceridemia and hypercholesterolemia. The authors concluded that the ingestion of guava leaf tea can ameliorate the symptoms of diabetes mellitus and that it could be used as an alimentotherapy. In another clinical study with 40 patients, oral administration of capsules with 500 mg of guava fruit reduced the blood glucose level in weeks 3, 4, and 5 with a decrease of 12.3%, 24.79%, and 7.9%, respectively, as compared with the diabetic control group 92. The study also indicated that supplementation of 0.517 g/day of this extract could reduce fasting blood glucose.
Summary of guava benefits
It seems the health effects of guava come from its leaves which have been reported to contain bioactive compounds such as quercetin, catechin, vescalagin, gallic acid, peltatoside, hyperoside, isoquercitrin, and guaijaverin. However, based on the results of these traditional claims and the results of these animal and test tube studies, we cannot conclude whether or not guava or guava leaves extract will be of benefits for human health without good data from well conducted human clinical trials to establish their effectiveness.
References- Morton J.F. Fruits of Warm Climates. Creative Resource Systems, Inc.; Winterville, NC, USA: 1987.
- Salazar D.M., Melgarejo P., Martínez R., Martínez J.J., Hernández F., Burguera M. Phenological stages of the guava tree (Psidium guajava L.) Sci. Hortic. 2006;108:157–161. doi: 10.1016/j.scienta.2006.01.022.
- Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. Gutiérrez RM, Mitchell S, Solis RV. J Ethnopharmacol. 2008 Apr 17; 117(1):1-27. https://www.ncbi.nlm.nih.gov/pubmed/18353572/
- Shruthi S.D., Roshan A., Sharma S., Sunita S. A review on the medicinal plant Psidium guajava Linn. (Myrtaceae) J. Drug Deliv. Ther. 2013;3:162–168.
- Psidium guajava L., from ethnobiology to scientific evaluation: Elucidating bioactivity against pathogenic microorganisms. Morais-Braga MF, Carneiro JN, Machado AJ, Dos Santos AT, Sales DL, Lima LF, Figueredo FG, Coutinho HD. J Ethnopharmacol. 2016 Dec 24; 194():1140-1152. https://www.ncbi.nlm.nih.gov/pubmed/27845266/
- Morais-Braga M.F.B., Carneiro J.N.P., Machado A.J.T., dos Santos A.T.L., Sales D.L., Lima L.F., Figueredo F.G., Coutinho H.D.M. Psidium guajava L., from ethnobiology to scientific evaluation: Elucidating bioactivity against pathogenic microorganisms. J. Ethnopharmacol. 2016;194:1140–1152. doi: 10.1016/j.jep.2016.11.017. https://www.ncbi.nlm.nih.gov/pubmed/27845266
- Sanda K.A., Grema H.A., Geidam Y.A., Bukar-Kolo Y.M. Pharmacological aspects of P. guajava: An update. Int. J. Pharmacol. 2011;7:316–324.
- Bernal J., Mendiola J.A., Ibáñez E., Cifuentes A. Advanced analysis of nutraceuticals. J. Pharm. Biomed. Anal. 2011;55:758–774. doi: 10.1016/j.jpba.2010.11.033. https://www.ncbi.nlm.nih.gov/pubmed/21168989
- Arai S., Yasuoka A., Abe K. Functional food science and food for specified health use policy in Japan: State of the art. Curr. Opin. Lipidol. 2008;19:69–73. doi: 10.1097/MOL.0b013e3282f3f505. https://www.ncbi.nlm.nih.gov/pubmed/18196990
- Ishida Y. Food for specified health uses. In: Hosoya N, editor. FOSHU in Primary Care of Lifestyle-Related Disease (disorders) with Foods. Tokyo: Daiichi Shuppan; 2001. pp. 176–8.
- Díaz-de-Cerio E, Verardo V, Gómez-Caravaca AM, Fernández-Gutiérrez A, Segura-Carretero A. Health Effects of Psidium guajava L. Leaves: An Overview of the Last Decade. Battino M, ed. International Journal of Molecular Sciences. 2017;18(4):897. doi:10.3390/ijms18040897. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5412476/
- United States Department of Agriculture Agricultural Research Service. National Nutrient Database for Standard Reference Legacy Release. https://ndb.nal.usda.gov/ndb/search/list
- Milyani R. Inhibitory effect of some plant extracts on clinical isolates of Staphylococcus aureus. Afr. J. Microbiol. Res. 2012;6:6517–6524. doi: 10.5897/AJMR11.119
- Chah K.F., Eze C.A., Emuelosi C.E., Esimone C.O. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J. Ethnopharmacol. 2006;104:164–167. doi: 10.1016/j.jep.2005.08.070
- De Araújo A.A., Soares L.A.L., Assunção Ferreira M.R., de Souza Neto M.A., da Silva G.R., de Araújo R.F., Guerra G.C.B., de Melo M.C.N. Quantification of polyphenols and evaluation of antimicrobial, analgesic and anti-inflammatory activities of aqueous and acetone-water extracts of Libidibia ferrea, Parapiptadenia rigida and Psidium guajava. J. Ethnopharmacol. 2014;156:88–96. doi: 10.1016/j.jep.2014.07.031
- Dhiman A., Nanda A., Narasimhan B. In vitro antimicrobial activity of methanolic leaf extract of Psidium guajava L. J. Pharm. Bioallied Sci. 2011;3:226–229. doi: 10.4103/0975-7406.80776. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3103916/
- Fernandes M.R.V., Dias A.L.T., Carvalho R.R., Souza C.R.F., Oliveira W.P. Antioxidant and antimicrobial activities of Psidium guajava L. spray dried extracts. Ind. Crops Prod. 2014;60:39–44. doi: 10.1016/j.indcrop.2014.05.049
- Nair R., Chanda S. In Vitro antimicrobial activity of Psidium guajava L. leaf extracts against clinically important pathogenic microbial strains. Braz. J. Microbiol. 2007;38:452–458. doi: 10.1590/S1517-83822007000300013
- Bezerra Morais-Braga M.F., Lima Sales D., dos Santos Silva F., Pereira Chaves T., de Carvalho Nilo Bitu V., Torres Avilez W.M., Ribeiro-Filho J., Douglas Melo Coutinho H. Psidium guajava L. and Psidium brownianum Mart ex DC. potentiate the effect of antibiotics against Gram-positive and Gram-negative bacteria. Eur. J. Integr. Med. 2016;8:683–687. doi: 10.1016/j.eujim.2016.07.001
- Betoni J.E.C., Passarelli Mantovani R., Nunes Barbosa L., Di Stasi L.C., Fernandes Junior A. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem. Inst. Oswaldo Cruz. 2006;101:387–390. doi: 10.1590/S0074-02762006000400007
- Metwally A.M., Omar A.A., Harraz F.M., El Sohafy S.M. Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacogn. Mag. 2010;6:212–218. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2950385
- Mailoa M.N., Mahendradatta M., Laga A., Djide N. Antimicrobial activities of tannins extract from guava leaves (Psidium guajava L.) on pathogens microbial. Int. J. Sci. Technol. Res. 2014;3:236–241
- Ghosh P., Mandal A., Chakraborty P., Rasul M.G., Chakraborty M., Saha A. Triterpenoids from Psidium guajava with biocidal activity. Indian J. Pharm. Sci. 2010;72:504–507. doi: 10.4103/0250-474X.73936
- Zahir A.A., Rahuman A.A., Bagavan A., Santhoshkumar T., Mohamed R.R., Kamaraj C., Rajakumar G., Elango G., Jayaseelan C., Marimuthu S. Evaluation of botanical extracts against Haemaphysalis bispinosa Neumann and Hippobosca maculata Leach. Parasitol. Res. 2010;107:585–592. doi: 10.1007/s00436-010-1898-7
- Adeyemi O.S., Sykes M.L., Akanji M.A., Avery V.M. Anti-trypanosomal and cytotoxic activity of ethanolic extracts of Psidium guajava leaves in Alamar Blue based assays. Vet. Arh. 2011;81:623–633
- Kaushik N.K., Bagavan A., Rahuman A.A., Zahir A.A., Kamaraj C., Elango G., Jayaseelan C., Kirthi A.V., Santhoshkumar T., Marimuthu S., et al. Evaluation of antiplasmodial activity of medicinal plants from North Indian Buchpora and South Indian Eastern Ghats. Malar. J. 2015;14:65. doi: 10.1186/s12936-015-0564-z
- Lee W.C., Mahmud R., Noordin R., Pillai Piaru S., Perumal S., Ismail S. Free radicals scavenging activity, cytotoxicity and anti-parasitic activity of essential oil of Psidium guajava L. leaves against Toxoplasma gondii. J. Essent. Oil Bear. Plants. 2013;16:32–38. doi: 10.1080/0972060X.2013.764196
- Thiyagarajan S., Jamal A. Evaluation of Lethal Activity of Psidium guajava Linn. extracts on bacterial pathogens causing diarrheal infections. Int. J. Res. Ayurveda Pharm. 2015;6:111–117. doi: 10.7897/2277-4343.06124
- Birdi T., Daswani P., Brijesh S., Tetali P., Natu A., Antia N. Newer insights into the mechanism of action of Psidium guajava L. leaves in infectious diarrhoea. BMC Complement. Altern. Med. 2010;10:33 doi: 10.1186/1472-6882-10-33
- Sriwilaijaroen N., Fukumoto S., Kumagai K., Hiramatsu H., Odagiri T., Tashiro M., Suzuki Y. Antiviral effects of Psidium guajava Linn. (guava) tea on the growth of clinical isolated H1N1 viruses: Its role in viral hemagglutination and neuraminidase inhibition. Antivir. Res. 2012;94:139–146. doi: 10.1016/j.antiviral.2012.02.013
- Anas K., Jayasree P.R., Vijayakumar T., Manish Kumar P.R. In vitro antibacterial activity of Psidium guajava Linn. leaf extract on clinical isolates of multidrug resistant Staphylococcus aureus. Indian J. Exp. Biol. 2008;46:41–46. https://www.ncbi.nlm.nih.gov/pubmed/18697570
- Chah K.F., Eze C.A., Emuelosi C.E., Esimone C.O. Antibacterial and wound healing properties of methanolic extracts of some Nigerian medicinal plants. J. Ethnopharmacol. 2006;104:164–167. doi: 10.1016/j.jep.2005.08.070. https://www.ncbi.nlm.nih.gov/pubmed/16226414
- Nair R., Chanda S. In Vitro antimicrobial activity of Psidium guajava L. leaf extracts against clinically important pathogenic microbial strains. Braz. J. Microbiol. 2007;38:452–458. doi: 10.1590/S1517-83822007000300013.
- Fernandes M.R.V., Dias A.L.T., Carvalho R.R., Souza C.R.F., Oliveira W.P. Antioxidant and antimicrobial activities of Psidium guajava L. spray dried extracts. Ind. Crops Prod. 2014;60:39–44. doi: 10.1016/j.indcrop.2014.05.049.
- De Araújo A.A., Soares L.A.L., Assunção Ferreira M.R., de Souza Neto M.A., da Silva G.R., de Araújo R.F., Guerra G.C.B., de Melo M.C.N. Quantification of polyphenols and evaluation of antimicrobial, analgesic and anti-inflammatory activities of aqueous and acetone-water extracts of Libidibia ferrea, Parapiptadenia rigida and Psidium guajava. J. Ethnopharmacol. 2014;156:88–96. doi: 10.1016/j.jep.2014.07.031.
- Nisha K., Darshana M., Madhu G., Bhupendra M.K. GC-MS Analysis and anti-microbial activity of Psidium guajava (leaves) grown in Malva region of India. Int. J. Drug Dev. Res. 2011;3:237–245.
- Bezerra Morais-Braga M.F., Lima Sales D., dos Santos Silva F., Pereira Chaves T., de Carvalho Nilo Bitu V., Torres Avilez W.M., Ribeiro-Filho J., Douglas Melo Coutinho H. Psidium guajava L. and Psidium brownianum Mart ex DC. potentiate the effect of antibiotics against Gram-positive and Gram-negative bacteria. Eur. J. Integr. Med. 2016;8:683–687. doi: 10.1016/j.eujim.2016.07.001.
- Betoni J.E.C., Passarelli Mantovani R., Nunes Barbosa L., Di Stasi L.C., Fernandes Junior A. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem. Inst. Oswaldo Cruz. 2006;101:387–390. doi: 10.1590/S0074-02762006000400007.
- Metwally A.M., Omar A.A., Harraz F.M., El Sohafy S.M. Phytochemical investigation and antimicrobial activity of Psidium guajava L. leaves. Pharmacogn. Mag. 2010;6:212–218.
- Mailoa M.N., Mahendradatta M., Laga A., Djide N. Antimicrobial activities of tannins extract from guava leaves (Psidium guajava L.) on pathogens microbial. Int. J. Sci. Technol. Res. 2014;3:236–241.
- Ghosh P., Mandal A., Chakraborty P., Rasul M.G., Chakraborty M., Saha A. Triterpenoids from Psidium guajava with biocidal activity. Indian J. Pharm. Sci. 2010;72:504–507. doi: 10.4103/0250-474X.73936.
- Adeyemi O.S., Sykes M.L., Akanji M.A., Avery V.M. Anti-trypanosomal and cytotoxic activity of ethanolic extracts of Psidium guajava leaves in Alamar Blue based assays. Vet. Arh. 2011;81:623–633.
- Kaushik N.K., Bagavan A., Rahuman A.A., Zahir A.A., Kamaraj C., Elango G., Jayaseelan C., Kirthi A.V., Santhoshkumar T., Marimuthu S., et al. Evaluation of antiplasmodial activity of medicinal plants from North Indian Buchpora and South Indian Eastern Ghats. Malar. J. 2015;14:65. doi: 10.1186/s12936-015-0564-z.
- Lee W.C., Mahmud R., Noordin R., Pillai Piaru S., Perumal S., Ismail S. Free radicals scavenging activity, cytotoxicity and anti-parasitic activity of essential oil of Psidium guajava L. leaves against Toxoplasma gondii. J. Essent. Oil Bear. Plants. 2013;16:32–38. doi: 10.1080/0972060X.2013.764196.
- Chanu T.R., Pai V., Chakraborty R., Raju B., Lobo R., Ballal M. Screening for antidiarrheal activity of Psidium guajava: A possible alternative in the treatment against diarrhea causing enteric pathogens. J. Chem. Pharm. Res. 2011;3:961–967.
- Rahim N., Gomes D.J., Watanabe H., Rahman S.R., Chomvarin C., Endtz H.P., Alam M. Antibacterial activity of Psidium guajava leaf and bark against multidrug-resistant Vibrio cholerae: Implication for cholera control. Jpn. J. Infect. Dis. 2010;63:271–274
- Gonçalves F.A., Andrade Neto M., Bezerra J.N.S., Macrae A., De Sousa O.V., Fonteles-Filho A.A., Vieira R.H.S.D.F. Antibacterial activity of guava, Psidium guajava Linnaeus, leaf extracts on diarrhea-causing enteric bacteria isolated from seabob shrimp, Xiphopenaeus kroyeri (Heller) Rev. Inst. Med. Trop. Sao Paulo. 2008;50:11–15. doi: 10.1590/S0036-46652008000100003
- Nwinyi O., Chinedu S.N., Ajani O.O. Evaluation of antibacterial activity of Pisidium guajava and Gongronema latifolium. J. Med. Plants Res. 2008;2:189–192.
- Pachanawan A., Phumkhachorn P., Rattanachaikunsopon P. Potential of Psidium guajava supplemented fish diets in controlling Aeromonas hydrophila infection in tilapia (Oreochromis niloticus) J. Biosci. Bioeng. 2008;106:419–424. doi: 10.1263/jbb.106.419
- Yin X.L., Li Z.J., Yang K., Lin H.Z., Guo Z.X. Effect of guava leaves on growth and the non-specific immune response of Penaeus monodon. Fish Shellfish Immunol. 2014;40:190–196. doi: 10.1016/j.fsi.2014.07.001
- Adeyemi S.O., Akanji M.A., Oguntoye S.A. Ethanolic leaf extract of Psidium guajava: Phyto-chemical and trypanocidal activity in rats infected with Trypanosoma brucei brucei. J. Med. Plants Res. 2009;3:420–423.
- Akanji M.A., Adeyemi O.S., Oguntoye S.O., Sulyman F. Psidium guajava extract reduces trypanosomosis associated lipid peroxidation and raises glutathione concentrations in infected animals. EXCLI J. 2009;8:148–154.
- Rajendran C., Begam M., Kumar D., Baruah I., Gogoi H.K., Srivastava R.B., Veer V. Antiplasmodial activity of certain medicinal plants against chloroquine resistant Plasmodium berghei infected white albino BALB/c mice. J. Parasit. Dis. 2014;38:148–152. doi: 10.1007/s12639-013-0252-2
- Gupta P., Birdi T. Psidium guajava leaf extract prevents intestinal colonization of Citrobacter rodentium in the mouse model. J. Ayurveda Integr. Med. 2015;6:50
- Geidam Y.A., Ambali A.G., Onyeyili P.A., Tijjani M.B., Gambo H.I., Gulani I.A. Antibacterial efficacy of ethyl acetate fraction of Psidium guajava leaf aqueous extract on experimental Escherichia coli (O78) infection in chickens. Vet. World. 2015;8:358–362. doi: 10.14202/vetworld.2015.358-362
- Shittu O.B., Ajayi O.L., Bankole S.O., Popoola T.O.S. Intestinal ameliorative effects of traditional Ogi-tutu, Vernonia amygdalina and Psidium guajava in mice infected with Vibrio cholera. Afr. Health Sci. 2016;16:620–628. doi: 10.4314/ahs.v16i2.33
- Jan O.Q., Kamili N., Ashraf A., Iqbal A., Sharma R.K., Rastogi A. Haematobiochemical parameters of goats fed tannin rich Psidium guajava and Carissa spinarum against Haemonchus contortus infection in India. J. Parasit. Dis. 2013;39:1–8. doi: 10.1007/s12639-013-0278-5
- Adeyemi S.O., Akanji M.A., Oguntoye S.A. Ethanolic leaf extract of Psidium guajava: Phyto-chemical and trypanocidal activity in rats infected with Trypanosoma brucei brucei. J. Med. Plants Res. 2009;3:420–423
- Akanji M.A., Adeyemi O.S., Oguntoye S.O., Sulyman F. Psidium guajava extract reduces trypanosomosis associated lipid peroxidation and raises glutathione concentrations in infected animals. EXCLI J. 2009;8:148–154
- Seo N., Ito T., Wang N., Yao X., Tokura Y., Furukawa F., Takigawa M., Kitanaka S. Anti-allergic Psidium guajava extracts exert an antitumor effect by inhibition of T regulatory cells and resultant augmentation of Th1 cells. Anticancer Res. 2005;25:3763–3770 http://ar.iiarjournals.org/content/25/6A/3763.long
- Rizzo L.Y., Longato G.B., Ruiz A.L.T.G., Tinti S.V., Possenti A., Vendramini-costa D.B. In vitro, in vivo and in silico analysis of the anticancer and estrogen-like activity of guava leaf extracts. Curr. Med. Chem. 2014;21:2322–2330. doi: 10.2174/0929867321666140120120031 https://www.ncbi.nlm.nih.gov/pubmed/24438525
- Ryu N.H., Park K.R., Kim S.M., Yun H.M., Nam D., Lee S.G., Jang H.J., Ahn K.S., Kim S.-H., Shim B.S., et al. A hexane fraction of guava leaves (Psidium guajava L.) induces anticancer activity by suppressing AKT/mammalian target of rapamycin/ribosomal p70 S6 kinase in human prostate cancer cells. J. Med. Food. 2011;15:231–241. doi: 10.1089/jmf.2011.1701 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3282482/
- Adeyemi O.S., Akanji M.A., Ekanem J.T. Anti-anaemic properties of the ethanolic extracts of Psidium guajava in Trypanosoma brucei brucei Infected Rats. Res. J. Pharmacol. 2010;4:74–77. doi: 10.3923/rjpharm.2010.74.77
- Udem S.C., Anyanwu M.U., Obidike R.I., Udem N.D. The effects of Psidium guajava Linn. (Myrtaceae) leaf chloroform extract on some hematological and biochemical parameters in mice. Comp. Clin. Pathol. 2011;20:47–51. doi: 10.1007/s00580-009-0950-4
- Jang M., Jeong S.-W., Cho S.K., Ahn K.S., Lee J.H., Yang D.C., Kim J.-C. Anti-Inflammatory Effects of an Ethanolic Extract of Guava (Psidium guajava L.) Leaves In Vitro and In Vivo. J. Med. Food. 2014;17:678–685. doi: 10.1089/jmf.2013.2936
- Stalin D. J. A study on the analgesic property of methanolic and aqueous extracts of dried leaf of Psidium guajava Linn. Int. J. Adv. Res. Pharm. Bio Sci. 2013;3:1–6
- Akinloye O., Akinmoladun A.C., Farombi E.O. Modulatory effect of Psidium guajava linn and ocimum gratissimum Linn on lipid profile and selected biochemical indices in rabbits fed high cholesterol diet. J. Complement. Integr. Med. 2010;7 doi: 10.2202/1553-3840.1336
- Mesquita Freire J., Patto de Abreu C.M., da Silveira Duarte S.M., Borges Araújo de Paula F., Ribeiro Lima A. Evaluation of the protective effect of guava fruits and leaves on oxidative stress. Acta Sci. Biol. Sci. 2014;36:35–40
- Oh W.K., Lee C.H., Lee M.S., Bae E.Y., Sohn C.B., Oh H., Kim B.Y., Ahn J.S. Antidiabetic effects of extracts from. Psidium Guajava. 2005;96:411–415
- Mansoori Bahrani A.H., Zaheri H., Soltani N., Kharazmi F. Effect of the administration of Psidium guava leaves on blood glucose, lipid profiles and sensitivity of the vascular mesenteric bed to Phenylephrine in streptozotocin-induced diabetic rats. J. Diabetes Mellit. 2012;2:138–145. doi: 10.4236/jdm.2012.21023
- Shen S.-C., Cheng F.-C., Wu N.-J. Effect of guava (Psidium guajava Linn.) leaf soluble solids on glucose metabolism in type 2 diabetic rats. Phytother. Res. 2008;22:1458–1464. doi: 10.1002/ptr.2476
- Soman S., Rauf A.A., Indira M., Rajamanickam C. Antioxidant and antiglycative potential of ethyl acetate fraction of Psidium guajava leaf extract in streptozotocin-induced diabetic rats. Plant Foods Hum. Nutr. 2010;65:386–391. doi: 10.1007/s11130-010-0198-9
- Ogueri C.C., Elekwa I., Ude V.C., Ugbogu A.E. Effect of aqueous extract of guava (Psidium guajava) leaf on blood glucose and liver enzymes in alloxan induced diabetic rats. Br. J. Pharm. Res. 2014;4:1079–1087. doi: 10.9734/BJPR/2014/7244
- Shakeera Banu M., Sujatha K., Sridharan G., Manikandan R. Antihyperglycemic and antihyperlipidemic potentials of Psidium guajava in alloxan-induced diabetic rats. Asian J. Pharm. Clin. Res. 2013;6:88–89.
- Adeyemi O.S., Akanji M.A. Biochemical changes in the kidney and liver of rats following administration of ethanolic extract of Psidium guajava leaves. Hum. Exp. Toxicol. 2011;30:1266–1274. doi: 10.1177/0960327110388534
- Adeyemi O.S., Akanji M.A. Psidium guajava leaf extract: Effects on rat serum homeostasis and tissue morphology. Comp. Clin. Pathol. 2012;21:401–407. doi: 10.1007/s00580-010-1106-2
- Ademiluyi A.O., Oboh G., Ogunsuyi O.B., Oloruntoba F.M. A comparative study on antihypertensive and antioxidant properties of phenolic extracts from fruit and leaf of some guava (Psidium guajava L.) varieties. Comp. Clin. Pathol. 2015;25:363–374. doi: 10.1007/s00580-015-2192-y
- Chiwororo W.D.H., Ojewole J.A.O. Biphasic effect of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract on rat isolated vascular smooth muscles. J. Smooth Muscle Res. 2008;44:217–229. doi: 10.1540/jsmr.44.217
- Takahashi Y., Otsuki A., Mori Y., Kawakami Y., Ito H. Inhibition of leukocyte-type 12-lipoxygenase by guava tea leaves prevents development of atherosclerosis. Food Chem. 2015;186:2–5. doi: 10.1016/j.foodchem.2015.03.105
- Soman S., Rajamanickam C., Rauf A.A., Indira M. Beneficial effects of Psidium guajava leaf extract on diabetic myocardium. Exp. Toxicol. Pathol. 2013;65:91–95. doi: 10.1016/j.etp.2011.06.005
- Umana Uduak E., Timbuak J.A., Musa S.A., Ikyembe D.T., Abdurrashid S., Hamman W.O. Ulceroprotective effect of methanol extract of Psidium guajava leaves on ethanol induced gastric ulcer in adult wistar rats. Asian J. Med. Sci. 2012;4:75–78
- Jayakumari S., Anbu J., Ravichandiran V., Anjana A., Siva Kumar G.M., Maharaj S. Antiulcerogenic and free radical scavenging activity of flavonoid fraction of Psidium guajava Linn leaves. Int. J. Pharm. Pharm. Sci. 2012;4:170–174
- Ojewole J.A.O., Awe E.O., Chiwororo W.D.H. Antidiarrhoeal activity of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract in rodents. J. Smooth Muscle Res. 2008;44:195–207. doi: 10.1540/jsmr.44.195
- Ezekwesili J.O., Nkemdilim U.U., Okeke C.U. Mechanism of antidiarrhoeal effect of ethanolic extract of Psidium guajava leaves. Biokemistri. 2010;22:85–90
- Shah A.J., Begum S., Hassan S.I., Ali S.N., Siddiqui B.S., Gilani A.-H. Pharmacological basis for the medicinal use of Psidium guajava leave in hyperactive gut disorders. Bangladesh J. Pharmacol. 2011;6:100–105. doi: 10.3329/bjp.v6i2.8692
- Choi J.H., Park B.H., Kim H.G., Hwang Y.P., Han E.H., Jin S.W., Seo J.K., Chung Y.C., Jeong H.G. Inhibitory effect of Psidium guajava water extract in the development of 2,4-dinitrochlorobenzene-induced atopic dermatitis in NC/Nga mice. Food Chem. Toxicol. 2012;50:2923–2929. doi: 10.1016/j.fct.2012.04.044
- Porta Santos Fernandes K., Kalil Bussadori S., Martins Marques M., Sumie Wadt Yamashita N., Bach E., Domingues Martins M. Healing and cytotoxic effects of Psidium guajava (Myrtaceae) leaf extracts. Braz. J. Oral Sci. 2010;9:9–14
- Baroroh H.N., Utami E.D. Harwoko inhibitory effect of ethanolic extract of Psidium guajava leaves in rat active cutaneus anaphylaxis reaction. Int. J. Pharm. Clin. Res. 2016;8:1–5.
- Vladislavovna Doubova S., Reyes Morales H., Flores Hernández S., Martínez-García M.D.C., González de Cossío Ortiz M., Chávez Soto M.A., Rivera Arce E., Lozoya X. Effect of a Psidii guajavae folium extract in the treatment of primary dysmenorrhea: A randomized clinical trial. J. Ethnopharmacol. 2007;110:305–310. doi: 10.1016/j.jep.2006.09.033 https://www.ncbi.nlm.nih.gov/pubmed/17112693
- Pattanittum P, Kunyanone N, Brown J, Sangkomkamhang US, Barnes J, Seyfoddin V, Marjoribanks J. Dietary supplements for dysmenorrhoea. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No.: CD002124. DOI: 10.1002/14651858.CD002124.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD002124.pub2/full
- Deguchi Y., Miyazaki K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr. Metab. Lond. 2010;7:1–10. doi: 10.1186/1743-7075-7-9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2831039/
- Yusof RM, Said M. Effect of high fibre fruit (Guava-Psidium guajava L.) on the serum glucose level in induced diabetic mice. Asia Pac J Clin Nutr. 2004;13(Suppl):S135