What is histamine intolerance
Histamine intolerance is the results of a disequilibrium of accumulated histamine and the capacity for histamine degradation 1. The main enzyme for the breakdown (metabolism) of ingested histamine is diamine oxidase (DAO) 2. An impaired histamine degradation based on a reduced diamine oxidase (DAO) activity and the resulting excess of histamine may cause numerous symptoms mimicking an allergic reaction. Ingestion of histamine-rich food 3, alcohol 4, or drugs (e.g., aminoguanidine, dihydralazine, chloroquine, pentamidine, cycloserine, clavulanic acid, dobutamine, pancuronium and others) 5 that release histamine or block diamine oxidase (DAO) enzyme may provoke diarrhea, headache 6, congestion of the nose, asthmatoid wheezing 7, hypotension, arrhythmia, urticaria 8, pruritus, flushing, and other conditions in these patients. Approximately 1% of the population has histamine intolerance, and 80% of those patients are middle-aged 9. Because of the multifaceted symptoms, the existence of histamine intolerance is frequently underestimated, or its symptoms are misinterpreted.
Histamine intolerance belongs to the group of non-IgE-mediated hypersensitivity-like reactions and is known as a pharmacological food intolerance 10. Currently, no valid in vitro tests can prove histamine intolerance; thus, a double-blind, placebo-controlled food challenge test remains the gold standard for diagnostic workup of non-IgE-mediated food intolerance 11.
In patients with typical symptoms of histamine intolerance that are triggered by histamine-rich food and alcohol, with intolerance of drugs that liberate histamine or block diamine oxidase (DAO), and with a negative diagnosis of allergy or internal disorders, histamine intolerance should be considered. A histamine-free diet, if necessary, supported by antihistamines or the substitution of diamine oxidase (DAO), leads to an improvement of symptoms. However, further studies investigating histamine intolerance due to double-blind, placebo-controlled provocations are indispensable.
Clinical symptoms and their provocation by certain foods and beverages appear similar in different diseases, such as food allergy and intolerance of sulfites, histamine, or other biogenic amines (e.g, tyramine). Therefore, the differentiation of the causal agent in adverse reactions to food, alcohol, and drugs is a difficult challenge. There is poor evidence of adverse reactions to these agents based on double-blind, placebo-controlled provocations 12. However, a better understanding of the pathophysiology, clinical picture, trigger factors, and diagnostic tools may help to clarify the confusing debate surrounding histamine intolerance.
Due to microbial contamination, food and beverages sometimes contain varying amounts of biogenic amines in relevant amounts. Therefore, spoiled or fermented foods can contain high levels of biogenic amines. In particular, food that undergoes microbial ripening, such as cheese, salami, sauerkraut, or red wine, can contain high levels of histamine (see Table 1 below) 1.
Histamine and drugs
The effect of drugs as specific diamine oxidase (DAO) inhibitors and their capacity to induce histamine intolerance have been shown in various studies with human placental diamine oxidase (DAO) and in animal experiments13. A clinically relevant activity via histamine release or inhibition of DAO has been observed for various drugs 14. Therefore, the intake of drugs, especially long-term medication, should be considered in interpretation of histamine intolerance symptoms and diamine oxidase (DAO) concentrations.
Drugs releasing histamine or inhibiting diamine oxidase (DAO)
| Substance class | Agent interfering with the histamine metabolism |
|---|---|
| Contrast media | |
| Muscle relaxants | Pancuronium, alcuronium, d-tubocurarine |
| Narcotics | Thiopental |
| Analgetics | Morphine, pethidine, nonsteroidal antiinflammatory drugs, acetylsalicylic acid, metamizole |
| Local anesthetics | Prilocaine |
| Antihypotonics | Dobutamine |
| Antihypertensive drugs | Verapamil, alprenolol, dihydralazine |
| Antiarrhythmics | Propafenone |
| Diuretics | Amiloride |
| Drugs influencing gut motility | Metoclopramide |
| Antibiotics | Cefuroxime, cefotiam, isoniazid, pentamidin, clavulanic acid, choroquine |
| Mucolytics | Acetylcysteine, ambroxol |
| Broncholytics | Aminophylline |
| H2-receptor antagonists | Cimetidine |
| Cytostatics | Cyclophosphamide |
| Antidepressants | Amitriptyline |
Histamine function
Histamine exerts its effects by binding to its 4 receptors [histamine 1 receptor (H1R), H2R, H3R, and and H4R] on target cells in various tissues (see Figure 1 and Table 1). Histamine causes smooth muscle cell contraction, vasodilatation (blood vessels to dilate), increased vascular permeability and mucus secretion, tachycardia (increase heart rate), alterations of blood pressure, and arrhythmias (abnormal heart rates or rhythms), and histamine stimulates gastric acid secretion and nociceptive nerve fibers. In addition, histamine has been known to play various roles in neurotransmission, immunomodulation, hematopoiesis (red blood cell formation), wound healing, day-night rhythm, and the regulation of histamine- and polyamine-induced cell proliferation and angiogenesis in tumor models 15 and intestinal ischemia 16.
Figure 1. Histamine effects
[Source 1]Table 1. Histamine effects according to plasma histamine concentration (ng/mL or nanogram per mililiter)
| Histamine | Clinical effect |
|---|---|
| 0–1 | Reference |
| 1–2 | ↑ Gastric acid secretion ↑ Heart rate |
| 3–5 | Tachycardia, headache, flush, urticaria, pruritus |
| 6–8 | ↓ Arterial pressure |
| 7–12 | Bronchospasm |
| ≈100 | Cardiac arrest |
Histamine metabolism
Histamine can be metabolized in 2 ways (Figure 2 and Table 2):
- By oxidative deamination by diamine oxidase (DAO) (former name: histaminase) or
- By ring methylation by histamine-N-methyltransferase 17.
Whether histamine is catabolized by diamine oxidase (DAO) or histamine-N-methyltransferase is supposed to depend on the localization of histamine. The diamine oxidase (DAO) protein is stored in plasma membrane–associated vesicular structures in epithelial cells and is secreted into the circulation on stimulation 18. Therefore, it has been proposed that diamine oxidase (DAO) may be responsible for scavenging extracellular histamine (e.g, after ingestion of histamine-rich food) after mediator release. Conversely, histamine-N-methyltransferase, the second most important enzyme inactivating histamine, is a cytosolic protein 19, which can convert histamine only in the intracellular space of cells 20. Thus, the enzymes do not seem to compete for the substrate, although they have a similar affinity for histamine and they are expressed in some overlapping tissues. Histamine-N-methyltransferase has a slightly higher affinity for histamine [Michaelis-Menten constant (kM): 6–13 μmol/L] than does diamine oxidase (DAO) (kM: 20 μmol/L). In mammals, diamine oxidase (DAO) expression is restricted to specific tissues; the highest activities are shown for small bowel and colon 21 and for placenta and kidney 20. Lower diamine oxidase (DAO) activity has been discussed as a potential indicator of intestinal mucosa damage in inflammatory and neoplastic diseases 22 and in persons undergoing chemotherapy 23. Histamine-N-methyltransferase is widely expressed in human tissues; the greatest expression is in kidney and liver, followed by spleen, colon, prostate, ovary, spinal cord cells, bronchi, and trachea24. Histamine-N-methyltransferase is regarded as the key enzyme for histamine degradation in the bronchial epithelium 25.
Figure 2. Histamine metabolism
Footnotes: Summary of the histamine metabolism. The biogenic amine histamine is synthesized by decarboxylation of the amino acid histidine catalyzed by l-histidine decarboxylase (HDC) (1). Histamine can be metabolized by extracellular oxidative deamination of the primary amino group by diamine oxidase (DAO) (2) or intracellular methylation of the imidazole ring by histamine-N-methyltransferase (HNMT) (3). Therefore, insufficient enzyme activity caused by enzyme deficiency or inhibition may lead to accumulation of histamine. Both enzymes can be inhibited by their respective reaction products in a negative feedbackloop (4). N-Methylhistamine is oxidatively deaminated to N-methyl-imidazole acetaldehyde by monoamine oxidase B (MAO B) (5) or by DAO (6). Because the methylation pathway takes place in the cytosolic compartment of cells, MAO B (5) has been suggested to catalyze this reaction in vivo 23.
[Source 1]Table 2. Characteristics of the histamine-degrading enzymes diamine oxidase (DAO) and histamine N-methyl-transferase (HNMT)
| DAO | HNMT | |
|---|---|---|
| Gene | ||
| Gene map locus | Chromosome 7q35 | Chromosome 2q22 |
| Gene | 10 kbp, 5 exons, 4 introns | 35 kbp, 6 exons |
| Associated with SNPs | Inflammatory and neoplastic gastrointestinal diseases such as food allergy, gluten-sensitive enteropathy, Crohn disease, ulcerative colitis, and colon adenoma | Asthma |
| Protein | Soluble homodimeric glycoprotein of MR 200 000 with subunits of 70–125 kDa; 750 amino acid residues | Soluble, cytosolic protein of MR 33 000 with subunits of 29–34 kDa; 292 amino acid residues |
| Enzyme | ||
| Group | Copper-containing amine oxidases | Methyltransferases |
| Active form | Homodimer with the active-site cofactor 2,4,5-trihydroxyphenylalanine quinone (Topa quinone) | Monomer with a 2-domain structure |
| Enzyme kinetics (km) | Histamine, 20 μmol/L | Histamine, 6–13 μmol/L |
| Putrescine, 350 μmol/L | S-adenosyl-l-methionine, 6–10 μmmol/L | |
| Spermidine, 3 mmol/L | ||
| Optimum pH | 7.2 | 7.5–9.0 |
| Inhibititors | Copper-chelating agents, eg cyanide Carbonylgroup reagents, eg, aminoguanidine, semibarbacide | Reaction products: N-methylhistamine, S-adenosyl-l-homocysteine |
| Sulphydryl groups: p-chloromercuriobenzoate | ||
| Major expression | Intestine, kidney, placenta | Highest: kidney and liver; considerable: spleen, colon, prostate, ovary, spinal cord cells, trachea, and bronchi; to a smaller amount, nearly ubiquitous expression |
| Storage | Plasma membrane–associated vesicular structures in epithelial cells, secretion into the circulation upon stimulation | Cytosolic compartment of the cells |
| Function | Extracellular scavenger of histamine and other diamines by oxidative deamination of the primary amino group of histamine | Intracellular histamine inactivation by methylation of the imidazole ring |
Abbreviations:
SNPs = single-nucleotide polymorphisms; kbp = kilobase pair; MR = molecular weight; kDa = kiloDalton; kM = Michaelis-Menten constant.
[Source 1]What causes histamine intolerance?
Different mechanisms have been proposed as causing histamine intolerance 26. Histamine intolerance can develop through both increased availability of histamine and impaired histamine degradation 1. Underlying conditions for increased availability may be an endogenous histamine overproduction caused by allergies, mastocytosis, bacterias, gastrointestinal bleeding, or increased exogenous ingestion of histidine or histamine by food or alcohol. Other biogenic amines, such as putrescine, may also be involved in displacing histamine from its mucosal mucine linkage, which results in an increase of free absorbable histamine in circulation. However, the main cause of histamine intolerance is an impaired enzymatic histamine degradation caused by genetic or acquired impairment of the enzymatic function of diamine oxidase (DAO) or histamine N-methyl-transferase (HNMT). Gastrointestinal diseases with altered enterocytes also may cause decreased production of diamine oxidase (DAO) 27. Yet another cause can be competitive inhibition of histamine degradation of diamine oxidase (DAO) by other biogenic amines, alcohol 4, or drugs 13. Acquired histamine intolerance may be transient and therefore reversible after the elimination of causes, such as by discontinuing diamine oxidase (DAO)-blocking drugs. Diamine oxidase (DAO) inhibits the transepithelial permeation of exogenous histamine 28 and impaired diamine oxidase (DAO) activity results in increased enteral histamine uptake with consequent increased plasma histamine concentrations 28 and corresponding symptoms. Increased amounts of histamine metabolites may also inhibit histamine N-methyl-transferase (HNMT), the second enzyme metabolizing histamine 29.
Reduced diamine oxidase (DAO) activity—or, rather, reduced diamine oxidase (DAO) release—after the application of heparin could be shown to be a marker of tissue damage in patients with chronic renal failure 30, viral hepatitis (101), or gut failure and of endotoxemia in patients with liver cirrhosis 31. Reduced diamine oxidase (DAO) activity has also been shown in patients with chronic urticaria as a typical histamine-mediated disease 32 combined with a reduced tolerance for infused histamine 8 and an improvement of urticaria by maintaining a histamine-free diet 33.
The genetic background of histamine intolerance
Recently, a potential genetic background of a reduced histamine metabolism has also been investigated. The human diamine oxidase (DAO) gene spans ≈10 kbp and is located on chromosome 7q35 17. Various single-nucleotide polymorphisms in the DAO gene have been shown to be associated with inflammatory and neoplastic gastrointestinal diseases, such as food allergy 34, gluten-sensitive enteropathy, Crohn disease, ulcerative colitis, and colon adenoma 35. No significant difference in the distribution of the investigated histamine N-methyl-transferase (HNMT) alleles could be shown between patients with gastrointestinal diseases and control subjects 35, but a functional relevant polymorphism of the HNMT gene (chromosome 2q22) has been described for white asthma patients 36. Conversely, this association could not be observed in Japanese 37, German pediatric 38, and East Indian 39 populations. Thus, histamine intolerance seems to be acquired mostly through the impairment of diamine oxidase (DAO) activity caused by gastrointestinal diseases or through the inhibition of diamine oxidase (DAO), but the high interindividual variations in the expression of diamine oxidase (DAO) in the gut and the association of single-nucleotide polymorphisms in the DAO gene with gastrointestinal diseases provide evidence for a genetic predisposition in a subgroup of patients with histamine intolerance 17.
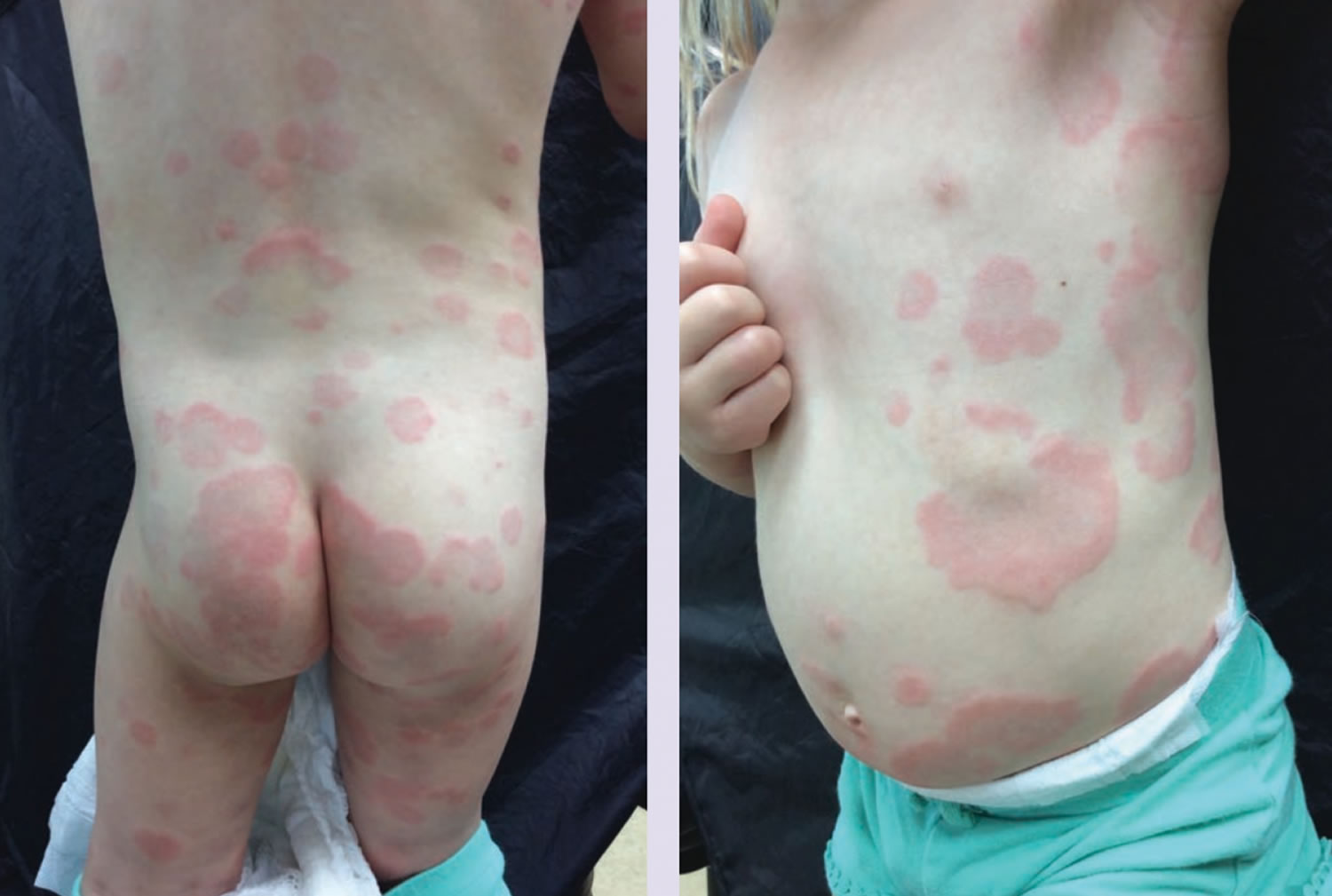
Histamine intolerance symptoms
Typical symptoms of histamine intolerance include gastrointestinal disorders, sneezing, rhinorrhea and congestion of the nose, headache 6, dysmenorrhea, hypotonia, arrhythmias 40, urticaria 32, pruritus, flushing, and asthma 41.
- Diarrhea,
- Headache 6,
- Congestion of the nose,
- Asthmatoid wheezing 7,
- Hypotension (low blood pressure),
- Arrhythmia (abnormal heart rate or rhythm),
- Urticaria 8,
- Pruritus,
- Flushing.
Histamine and headache
Headache can be induced dose-dependently by histamine in healthy persons as well as in patients with migraine 42. Histamine-induced headache is a vascular headache caused mainly by nitrate monoxide 43. Histamine releases endothelial nitrate monoxide upon stimulation of H1R (histamine 1 receptor), which is also expressed in the large intracranial arteries 44. In migraine patients, plasma histamine concentrations have been shown to be elevated both during headache attacks and during symptom-free periods. An increase in the number of brain mast cells is associated with pathologic conditions such as migraine, cluster headache, and multiple sclerosis 45. Many migraine patients have histamine intolerance evidenced by reduced diamine oxidase (DAO) activity, triggering of headache by food rich in histamine (e.g, long-ripened cheese or wine), and the alleviation of headache (i.e, disappearance of symptoms) under a histamine-free diet 46 and therapy with antihistamines 47.
Histamine and gastrointestinal tract
Besides headache, gastrointestinal ailments including diffuse stomach ache, colic, flatulence, and diarrhea are leading symptoms of histamine intolerance. Elevated histamine concentrations and diminished diamine oxidase (DAO) activities have been shown for various inflammatory and neoplastic diseases such as Crohn disease 22, ulcerative colitis 48, allergic enteropathy 27, food allergy 49, and colorectal cancers 50. In the colonic mucosa of patients with food allergy, a concomitant reduced histamine N-methyl-transferase (HNMT) 51 and an impaired total histamine degradation capacity 49 have been found 21, so that the enzymes cannot compensate each other. Therefore, an impaired histamine metabolism has been suggested to play a role in the pathogenesis of these diseases 1.
Histamine and airways
During or immediately after the ingestion of histamine-rich food or alcohol, rhinorrhea or nasal obstruction may occur in patients with histamine intolerance; in extreme cases, asthma attacks also may occur. Reduced histamine N-methyl-transferase (HNMT) activity has been shown for patients with food allergy 51 and bronchial asthma 52.
Histamine intolerance diagnosis
Diagnosis of histamine intolerance has been based on low serum diamine oxidase (DAO) values, functional gastrointestinal disorders and improvement of symptoms with a histamine-reduced diet.
Basal plasma histamine concentrations of 0.3 to 1.0 ng/mL are considered normal 53. Exceeding the individual histamine tolerance gives rise to concentration-dependent histamine-mediated symptoms 54 (see Table 1). Even healthy persons may develop severe headache or flushing due to ingestion of massive amounts of histamine as is known from studies of scromboid poisoning 55. It has been shown that inhibition of diamine oxidase (DAO) followed by oral histamine administration may induce severe and even life-threatening reactions, such as hypotension, bronchospasm, or shock 13. Recurrent anaphylactic reactions have been reported in patients with hyperhistaminemia 56. In histamine-sensitive patients with reduced diamine oxidase (DAO) activity, symptoms occur even after the ingestion of the small amounts of histamine that are well tolerated by healthy persons. Symptoms can be manifest via the above mentioned actions of histamine in multiple organs, such as the gastrointestinal tract, lung, skin, cardiovascular system, and brain, according to the expression of histamine receptors.
Because of the multifaceted symptoms in multiple organs, a detailed history of the basal histamine-mediated symptoms, any triggering of symptoms after the intake of histamine-rich food or drugs interfering with the histamine metabolism, and concomitant gastrointestinal diseases or allergies is indispensable for diagnosis of histamine intolerance (Figure 3). Clinically, histamine-induced symptoms cannot always be assigned to the underlying pathomechanism. A massive intake of histamine from decomposed fish may result in the same symptoms as are seen in a person with an IgE-mediated fish allergy. Histamine actions may be possible causes of endogenous cell activation, increased exogenous uptake, decreased histamine degradation, or a combination of these mechanisms. An occult systemic mastocytosis should be excluded by measurement of the serum tryptase. Diagnosis of histamine intolerance is set by presentation of ≥2 typical symptoms of histamine intolerance 57 and improvement by histamine-free diet and antihistamines. The diagnosis of allergy using using the skin-prick test for food allergens or determination of specific IgE should be carried out to exclude food allergy. The diagnosis of allergy usually proves to be negative because histamine intolerance is a pseudoallergy. Keeping of a diet diary has proven useful in tracking significant improvement of symptoms with a histamine-free diet and relapses in histamine intolerance after dietary errors.
Figure 3. Diagnostic pathway for histamine intolerance
[Source 1]Histamine intolerance test
In a patient with clinical suspicion of histamine intolerance (i.e, ≥2 typical symptoms), improvement of symptoms by histamine-free diet or antihistamines, diamine oxidase (DAO) may be determined in serum 58 or tissue biopsy 59. Several radioextraction assays have been developed for the determination of the enzymatic activity of diamine oxidase (DAO) by using [3H]- or [14C]-labeled putrescinedihydrochloride as a substrate 60. Determination of the HNMT activity is based on transmethylation of histamine by S-adenosyl-l [methyl-14C] methionine 60. Furthermore, the total histamine degradation capacity can be measured 49. Plasma activity of DAO, which generally is relatively low, may be increased by the liberation of tissue-bound DAO through an injection of heparin 61, which was the main method used before the development of more sensitive assays. Serum diamine oxidase (DAO) concentrations showed no significant daily variations and no significant sex differences 14. In patients with a diamine oxidase (DAO) activity Histamine intolerance is presumably highly likely in patients with DAO activity <3 U/mL, likely (but less likely) in patients with diamine oxidase (DAO) activity <10 U/mL, and improbable in patients with diamine oxidase (DAO) activity ≥10 U/mL 9.
Conversely, in some patients with a clear clinical picture of histamine intolerance, normal diamine oxidase (DAO) activities have been observed, so that an additional determination of histamine concentrations and interpretation of laboratory data in view of the clinic seem advisable. Histamine can be measured in plasma or in urine, as can its degradation product N-methylhistamine 54. Deficiency of the DAO cofactors vitamin B-6, copper, and vitamin C, which are thought to supplement histamine degradation 62, has been discussed as being controversial 6. Elevated histamine concentrations, reduced diamine oxidase (DAO) activities, or both are classically found in histamine intolerance. A double-blind, placebo-controlled histamine provocation after a 4-week histamine-free diet is considered the gold standard in diagnosis. Because the amount of histamine in natural food varies tremendously according to storage and maturation, the provocation can be performed with alternate administration of capsules containing increasing doses of histamine-di-hydrochloride (0.75 and 1.5 mg/kg body weight, respectively) and placebo capsules 63. Blood pressure and heart rate should be continuously controlled, and positive reactions (e.g, hypotonia, tachycardia, urticaria, or other symptoms of an anaphylactoid reaction) should be immediately treated by a physician. Afterward, symptoms should be evaluated by using a standardized symptom-scoring system.
Histamine intolerance treatment
Histamine intolerance treatment is based on histamine-free diet. Alcohol and long-ripened or fermented (and therefore histamine-rich) food, such as aged cheese, cured meat, and yeast products; histamine-rich food, such as spinach or tomatoes; or histamine liberators, such as citrus fruit, should be avoided 46; the histamine-free diet can be complemented with adjuvant administration of H1 and H2 antagonists. Most antihistamines have no influence on diamine oxidase (DAO) activity, although inhibition of DAO by cimetidine and dihydralazine and increased activity by diphenhydramine have been observed 14. In patients consuming a strictly histamine-free diet, no additional benefit due to an intake of antihistamines could be observed 64. An increase in DAO activity with the histamine-free diet was shown in migraine patients 64. In addition, histamine degradation can be supported by the administration of vitamin C 62 and vitamin B-6, which leads to an increase in diamine oxidase (DAO) activity 6. Positive effects have been reported for mast cell stabilizers and pancreatic enzymes 65, especially with respect to gastrointestinal symptoms. Because of the frequent intolerant reactions toward drugs that interfere with the histamine metabolism, their intake should be avoided. Recently, capsules containing diamine oxidase (DAO) isolated from pig kidneys have been generated to supplement the lack of endogenous human diamine oxidase (DAO) in patients with histamine intolerance. These capsules contain only stabilizers—i.e, cellulose, sucrose, solanum tuberosum, polyacrylic acid, cellulose gum, triethyl citrate, and potato starch. Patients who are suspected of having histamine intolerance should be given a certificate noting that condition and stating that the administration of contrast and other drugs that release histamine should be avoided. If the administration of theses drugs is unavoidable, prior medication with antihistamines is recommended 66.
Histamine intolerance diet
Histamine and other biogenic amines are present to various degrees in many foods, and their presence increases with maturation 67. The formation of biogenic amines in food requires the availability of free amino acids, the presence of decarboxylase-positive microorganisms, and conditions allowing bacterial growth and decarboxylase activity. Free amino acids either occur as such in foods or may be liberated by proteolysis during processing or storage 68. Numerous bacteria and some yeasts display high L-histidine decarboxylase activity and thus have the capacity to form histamine. Histidine is generated from autolytic or bacterial processes 69. Therefore, high concentrations of histamine are found mainly in products of microbial fermentation, such as aged cheese 70, sauerkraut, wine 71, and processed meat 72 (see Table 3) or in microbially spoiled food. Thus, histamine, tyramine, putrescine, and cadaverine serve as indicators of hygienic food quality 68. Tyramine and putrescine also may lead to intolerance reactions in combination with histamine. Possible explanations may be the inhibition of diamine oxidase (DAO) by other amines 29 or the promotion of histamine liberation from the mucosa by putrescine 73.
Histamine foods
Alcohol, especially red wine, is rich in histamine and is a potent inhibitor of diamine oxidase (DAO) 74. The relation between the ingestion of wine, an increase in plasma histamine, and the occurrence of sneezing, flushing, headache, asthma attacks, and other anaphylactoid reactions and a reduction of symptoms by antihistamines has been shown in various studies 75. However, among the multitude of substances contained in wine, other biogenic amines such as tyramine 76 and sulfites 77 have been supposed to contribute to symptoms summarized as “wine intolerance” or “red wine asthma” 77. In double-blind, placebo-controlled wine tests with healthy persons 78 and in patients with chronic urticaria and wine intolerance 79, the histamine content did not influence wine tolerance. In the latter group, an increase in plasma histamine could be shown, paradoxically, after ingestion of the histamine-poor wine. In these patients, the ethanol metabolite acetaldehyde has been discussed as a histamine-releasing substance 79. However, the high percentage of responses to the placebo (87%) could be responsible for the absence of an effect in this study 12. Another randomized double-blind, placebo-controlled oral wine challenge in patients with a history of red wine–provoked asthma (n = 18) found no relation between wine tolerance and the wine’s content of histamine or other amines but did find a greater bronchoconstrictive response to wine with a high sulfite content 77. Sulfiting agents are widely used as antioxidants and preservatives in foods, beverages, and pharmaceuticals. Adverse reactions with a presumed relation to sulfites include anaphylactic shock, bronchospasm, urticaria, angioedema, nausea, abdominal pain, diarrhea, stroke, and death 80. Sulfite hypersensitivity has been reported mainly in patients with chronic asthma; the estimated prevalence is 5–10% in all patients 81. Asthmatic reactions have been attributed to reflex activation of the parasympathetic system by the irritating effect of sulfites, possibly enhanced by a deficiency of sulfite oxidase. Besides this pseudoallergic mechanism, in at least some cases of sulfite hypersensitity, an immunoglobulin E (IgE)–mediated immediate-type allergic reaction must be considered 82. Sulfites may be contained in wine, but they are also contained in foods that are poor in histamine, such as fruit juice, frozen vegetables, and lettuce. Thus, in patients reporting intolerance to wine, a careful history of reactions to other foods rich in histamine or sulfites should be taken. In patients who are suspected of having sulfite intolerance, skin testing and a double-blind, placebo-controlled challenge with capsules containing increasing doses of bisulfite or placebo should be performed.
In contrast to an IgE–mediated food allergy, in which the ingestion of even a small amount of the allergen elicits symptoms, in histamine intolerance, the cumulative amount of histamine is crucial. Besides variations in the amount of histamine in food according to storage and maturation, the quantity consumed, the presence of other biogenic amines, and the additional intake of alcohol or diamine oxidase (DAO)-blocking drugs are pivotal factors in the tolerance of the ingested food. Generally, an upper limit of 100 mg histamine/kg in foods and of 2 mg histamine/L in alcoholic beverages has been suggested 83. This threshold may be too high, considering the occurrence of histamine-mediated symptoms after oral ingestion of 75 mg histamine in 5 of 10 females without a history of histamine intolerance 7.
However, most of the positive studies for intolerant reactions to sulfite, histamine, and other biogenic amines do not fulfill the current scientific criteria for providing substantiated evidence of the clinical effect of these foods. Nevertheless, patients who have a conclusive history of adverse reactions to food, alcohol, drugs containing histamine, other biogenic amines, and sulfite but without proof of IgE exist. In such patients, a double-blind, placebo-controlled provocation of the suspected causal agents under close supervision by experienced specialists should be performed after exclusion of other causal diseases and informed consent of the patients—if the provocation is not unreasonably hazardous, considering the grade of the anaphylactoid reaction. Because of the great effort, time, and costs or because of patients’ fear of a repeated reaction, double-blind, placebo-controlled provocations often are not performed in clinical practice, even when they are indicated.
Table 3. High histamine foods
| Food categories | Histamine | Recommended upper limit for histamine | Tyramine | |||
|---|---|---|---|---|---|---|
| mg/kg | mg/L | mg/kg | mg/L | mg/kg | mg/L | |
| Fish (frozen/smoked or salted/canned) | 200 | ND | ||||
| Mackerel | 1–20/1–1788/ND–210 | |||||
| Herring | 1–4/5–121/1–479 | |||||
| Sardine | ND/14–150/3–2000 | |||||
| Tuna | ND/ND/1–402 | |||||
| Cheese | No recommendation | |||||
| Gouda | 10–900 | 10–900 | ||||
| Camembert | 0–1000 | 0–4000 | ||||
| Cheddar | 0–2100 | 0–1500 | ||||
| Emmental | 5–2500 | 0–700 | ||||
| Swiss | 4–2500 | 0–700 | ||||
| Parmesan | 10–581 | 0–840 | ||||
| Meat | No recommendation | |||||
| Fermented sausage | ND–650 | ND–1237 | ||||
| Salami | 1–654 | – | ||||
| Fermented ham | 38–271 | 123–618 | ||||
| Vegetables | ||||||
| Sauerkraut | 0–229 | 10 | 2–951 | |||
| Spinach | 30–60 | |||||
| Eggplant | 26 | |||||
| Tomato ketchup | 22 | |||||
| Red wine vinegar | 4 | |||||
| Alcohol | ||||||
| White wine | ND–10 | 2 | 1–8 | |||
| Red wine | ND–30 | 2 | ND–25 | |||
| Top-fermented beer | ND–14 | 1.1–36.4 | ||||
| Bottom-fermented beer | ND–17 | 0.5–46.8 | ||||
| Champagne | 670 | |||||
Abbreviation: ND = not detected
[Source 1]Table 4. Histamine food list
[Source 10]In addition to histamine-rich food, many foods such as citrus foods are considered to have the capacity to release histamine directly from tissue mast cells, even if they themselves contain only small amounts of histamine (Table 5). In vitro studies of persons with a history of pseudoallergic reactions to food have shown a fragility of duodenal mast cells with massive degranulation in the presence of histamine-releasing substances that is significantly greater than that shown by control subjects 84. However, clinical studies using oral challenge tests to support the hypothesis for the histamine-releasing capacity of foods are required 85.
Table 5. Foods with suggested histamine-releasing capacities
| Plant-derived | Animal-derived | Other |
|---|---|---|
| Citrus fruit | Fish | Additives |
| Papaya | Crustaceans | Liquorice |
| Strawberries | Pork | Spices |
| Pineapple | Egg white | |
| Nuts | ||
| Peanuts | ||
| Tomatoes | ||
| Spinach | ||
| Chocolate |
- Laura Maintz, Natalija Novak; Histamine and histamine intolerance, The American Journal of Clinical Nutrition, Volume 85, Issue 5, 1 May 2007, Pages 1185–1196, https://doi.org/10.1093/ajcn/85.5.1185
- Silla Santos MH. Biogenic amines: their importance in foods. Int J Food Microbiol 1996;29:213–31.
- Sattler J, Hafner D, Klotter HJ, Lorenz W, Wagner PK. Food-induced histaminosis as an epidemiological problem: plasma histamine elevation and haemodynamic alterations after oral histamine administration and blockade of diamine oxidase (DAO). Agents Actions 1988;23:361–5.
- Zimatkin SM, Anichtchik OV. Alcohol-histamine interactions. Alcohol Alcohol 1999;34:141–7.
- Wantke F, Hemmer W, Focke M, Stackl W, Gotz M, Jarisch R. Are adverse effects of sildenafil also caused by inhibition of diamine oxidase? Urol Int 2001;67:59–61. https://www.ncbi.nlm.nih.gov/pubmed/2128501
- Jarisch R, Wantke F. Wine and headache. Int Arch Allergy Immunol 1996;110:7–12.
- Wohrl S, Hemmer W, Focke M, Rappersberger K, Jarisch R. Histamine intolerance-like symptoms in healthy volunteers after oral provocation with liquid histamine. Allergy Asthma Proc 2004;25:305–11.
- Pollock I, Murdoch RD, Lessof MH. Plasma histamine and clinical tolerance to infused histamine in normal, atopic and urticarial subjects. Agents Actions 1991;32:359–65.
- Missbichler A. Diagnostischer Nachweis der Aktivität von Diaminooxidase in Serum oder Plasma. (Diagnostic proof of the DAO activity in serum and plasma.) In: Jarisch R. ed. Histamin-Intoleranz. Histamin und Seekrankheit. (Histamine intolerance. Histamine and motion sickness.) Stuttgart, Germany: Georg Thieme Verlag KG, 2004:8–17
- Chung BY, Cho SI, Ahn IS, et al. Treatment of Atopic Dermatitis with a Low-histamine Diet. Annals of Dermatology. 2011;23(Suppl 1):S91-S95. doi:10.5021/ad.2011.23.S1.S91. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3199434/
- Worm M, Fiedler EM, Dölle S, Schink T, Hemmer W, Jarisch R, et al. Exogenous histamine aggravates eczema in a subgroup of patients with atopic dermatitis. Acta Derm Venereol. 2009;89:52–56.
- Jansen SC, van DM, Bottema KC, Dubois AE. Intolerance to dietary biogenic amines: a review. Ann Allergy Asthma Immunol 2003;91:233–40.
- Sattler J, Lorenz W. Intestinal diamine oxidases and enteral-induced histaminosis: studies on three prognostic variables in an epidemiological model. J Neural Transm Suppl 1990;32:291–314.
- Wantke F, Proud D, Siekierski E, Kagey-Sobotka A. Daily variations of serum diamine oxidase and the influence of H1 and H2 blockers: a critical approach to routine diamine oxidase assessment. Inflamm Res 1998;47:396–400.
- Raithel M, Ulrich P, Hochberger J, Hahn EG. Measurement of gut diamine oxidase activity. Diamine oxidase as a new biologic marker of colorectal proliferation? Ann N Y Acad Sci 1998;859:262–6
- Kalchmair B, Klocker J, Perkmann R, et al. .Alterations in plasma amine oxidase activities in a compartment syndrome model. Inflamm Res 2003;52(suppl)1:S67–8.
- Schwelberger HG. Diamine oxidase (DAO) enzyme and gene. In: Falus A. ed. Histamine: biology and medical aspects. Budapest, Hungary: SpringMed Publishing, 2004:43–52.
- Schwelberger HG, Bodner E. Purification and characterization of diamine oxidase from porcine kidney and intestine. Biochim Biophys Acta 1997;1340:152–64.
- Brown DD, Tomchick R, Axelrod J. The distribution and properties of a histamine-methylating enzyme. J Biol Chem 1959;234:2948–50.
- Klocker J, Matzler SA, Huetz GN, Drasche A, Kolbitsch C, Schwelberger HG. Expression of histamine degrading enzymes in porcine tissues. Inflamm Res 2005;54(suppl):S54–7.
- Raithel M, Kufner M, Ulrich P, Hahn EG. The involvement of the histamine degradation pathway by diamine oxidase in manifest gastrointestinal allergies. Inflamm Res 1999;48(suppl):S75–6.
- Schmidt WU, Sattler J, Hesterberg R, et al. .Human intestinal diamine oxidase (DAO) activity in Crohn’s disease: a new marker for disease assessment? Agents Actions 1990;30:267–70.
- Tsujikawa T, Uda K, Ihara T, Andoh A, Fujiyama Y, Bamba T. Changes in serum diamine oxidase activity during chemotherapy in patients with hematological malignancies. Cancer Lett 1999;147:195–8.
- Schwelberger HG. Histamine N-methyltransferase (HNMT) enzyme and gene. In: Falus A. ed. Histamine: biology and medical aspects. Budapest, Hungary: SpringMed Publishing, 2004:53–9.
- Yamauchi K, Sekizawa K, Suzuki H, et al. .Structure and function of human histamine N-methyltransferase: critical enzyme in histamine metabolism in airway. Am J Physiol 1994;267:L342–9.
- Raithel M. Durchfälle und weicher Stuhl. In: Jarisch R. ed. Histamin-Intoleranz. Histamin und Seekrankheit. (Histamine intolerance. Histamine and motion sickness.) Stuttgart, Germany: Georg Thieme Verlag KG, 2004:77–110
- Raithel M, Matek M, Baenkler HW, Jorde W, Hahn EG. Mucosal histamine content and histamine secretion in Crohn’s disease, ulcerative colitis and allergic enteropathy. Int Arch Allergy Immunol 1995;108:127–33.
- Ahrens F, Gabel G, Garz B, Aschenbach JR. Release and permeation of histamine are affected by diamine oxidase in the pig large intestine. Inflamm Res 2002;51(suppl):S83–4.
- Sattler J, Lorenz W, Kubo K, Schmal A, Sauer S, Luben L. Food-induced histaminosis under diamine oxidase (DAO) blockade in pigs: further evidence of the key role of elevated plasma histamine levels as demonstrated by successful prophylaxis with antihistamines. Agents Actions 1989;27:212–4.
- Stein J, Scheuermann EH, Yazdi R, Lembcke B, Caspary WF. Reduced postheparin plasma diamine oxidase activity in patients with chronic renal failure. Z Gastroenterol 1994;32:236–9.
- Gang V, Stanjek J, Gaubitz W. [Diaminooxidase (histaminase) in liver diseases and experimental liver lesions]. Verh Dtsch Ges Inn Med 1976;82:434–6
- Lessof MH, Gant V, Hinuma K, Murphy GM, Dowling RH. Recurrent urticaria and reduced diamine oxidase activity. Clin Exp Allergy 1990;20:373–6.
- Guida B, De Martino CD, De Martino SD, et al. .Histamine plasma levels and elimination diet in chronic idiopathic urticaria. Eur J Clin Nutr 2000;54:155–8.
- Petersen J, Raithel M, Schwelberger HG. Characterisation of functional polymorphisms of the human diamine oxidase gene. Inflamm Res 2005;54(suppl):S58–9.
- Petersen J, Drasche A, Raithel M, Schwelberger HG. Analysis of genetic polymorphisms of enzymes involved in histamine metabolism. Inflamm Res 2003;52(suppl):S69–70.
- Yan L, Galinsky RE, Bernstein JA, Liggett SB, Weinshilboum RM. Histamine N-methyltransferase pharmacogenetics: association of a common functional polymorphism with asthma. Pharmacogenetics 2000;10:261–6.
- Sasaki Y, Ihara K, Ahmed S, et al. .Lack of association between atopic asthma and polymorphisms of the histamine H1 receptor, histamine H2 receptor, and histamine N-methyltransferase genes. Immunogenetics 2005;51:238–40.
- Deindl P, Peri-Jerkan S, Deichmann K, et al. .No association of histamine-N-methyltransferase polymorphism with asthma or bronchial hyperresponsiveness in two German pediatric populations. Pediatr Allergy Immunol 2005;16:40–2.
- Sharma S, Mann D, Singh TP, Ghosh B. Lack of association of histamine-N-methyltransferase (HNMT) polymorphisms with asthma in the Indian population. J Hum Genet 2005;50:611–7.
- Endou M, Levi R. Histamine in the heart. Eur J Clin Invest 1995;25(suppl):5–11.
- Wantke F, Hemmer W, Haglmuller T, Gotz M, Jarisch R. Histamine in wine. Bronchoconstriction after a double-blind placebo-controlled red wine provocation test. Int Arch Allergy Immunol 1996;110:397–400.
- Lassen LH, Heinig JH, Oestergaard S, Olesen J. Histamine inhalation is a specific but insensitive laboratory test for migraine. Cephalalgia 1996;16:550–3.
- Thomsen LL, Olesen J. Nitric oxide in primary headaches. Curr Opin Neurol 2001;14:315–21.
- Thomsen LL. Investigations into the role of nitrc oxide and the large intracanial arteries in migraine headache. Cephalalgia 1997;17:873–95.
- Huszti Z. Histamine in CNS-resident non-neuronal cells. In: Falus A, Grosman N, Darvas Z. eds. Histamine: biology and medical aspects. Budapest, Hungary: SpringMed Publishing, 2004:272–80.
- Wantke F, Gotz M, Jarisch R. Histamine-free diet: treatment of choice for histamine-induced food intolerance and supporting treatment for chronic headaches. Clin Exp Allergy 1993;23:982–5.
- Krabbe AA, Olesen J. Headache provocation by continuous intravenous infusion of histamine. Clinical results and receptor mechanisms. Pain 1980;8:253–9.
- Mennigen R, Kusche J, Streffer C, Krakamp B. Diamine oxidase activities in the large bowel mucosa of ulcerative colitis patients. Agents Actions 1990;30:264–6.
- Kuefner MA, Schwelberger HG, Ulrich P, Hahn EG, Raithel M. Total histamine degradation capacity (THDC) as an important biological marker of histamine metabolism in human colonic mucosa. Inflamm Res 2002;51(suppl):S87–8.
- Raithel M, Ulrich P, Hochberger J, Hahn EG. Measurement of gut diamine oxidase activity. Diamine oxidase as a new biologic marker of colorectal proliferation? Ann N Y Acad Sci 1998;859:262–6.
- Kuefner MA, Schwelberger HG, Weidenhiller M, Hahn EG, Raithel M. Both catabolic pathways of histamine via histamine-N-methyltransferase and diamine oxidase are diminished in the colonic mucosa of patients with food allergy. Inflamm Res 2004;53(suppl):S31–2.
- Preuss CV, Wood TC, Szumlanski CL, et al. .Human histamine N-methyltransferase pharmacogenetics: common genetic polymorphisms that alter activity. Mol Pharmacol 1998;53:708–17.
- Dyer J, Warren K, Merlin S, Metcalfe DD, Kaliner M. Measurement of plasma histamine: description of an improved method and normal values. J Allergy Clin Immunol 1982;70:82–7.
- Kaliner M, Shelhamer JH, Ottesen EA. Effects of infused histamine: correlation of plasma histamine levels and symptoms. J Allergy Clin Immunol 1982;69:283–9.
- Morrow JD, Margolies GR, Rowland J, Roberts LJ. Evidence that histamine is the causative toxin of scombroid-fish poisoning. N Engl J Med 1991;324:716–20.
- Hershko AY, Dranitzki Z, Ulmanski R, Levi-Schaffer F, Naparstek Y. Constitutive hyperhistaminaemia: a possible mechanism for recurrent anaphylaxis. Scand J Clin Lab Invest 2001;61:449–52.
- Jarisch R. Histamin-Intoleranz. (Histamine intolerance.) Aerztemagazin 2004;8:1–4
- Tufvesson G, Tryding N. Determination of diamine oxidase activity in normal human blood serum. Scand J Clin Lab Invest 1969;24:163–8.
- Kufner MA, Ulrich P, Raithel M, Schwelberger HG. Determination of histamine degradation capacity in extremely small human colon samples. Inflamm Res 2001;50(suppl):S96–7.
- Schwelberger HG, Klocker J, Sattler J, Bodner E. Determination of the activity of diamine oxidase in extremely small tissue samples. Inflamm Res 1995;44(suppl):S94–5.
- Mayer I, Missbichler A, Wantke F, et al. .Optimierter Radioextraktionsassay zur quantitativen Bestimmung der Aktivität von Diaminooxidase (DAO) in humanem Serum und Plasma. (Optimized radio-extraction assay for the quantitative measurement of the activity of diamine oxidase (DAO) in human serum and plasma.) Allergologie 2005;28:1–8
- Johnston CS. The antihistamine action of ascorbic acid. Subcell Biochem 1996;25:189–213.
- Fiedler EM, Pelchrzim R, Focke M, Zuberbier T, Worm M. Bedeutung von exogen zugeführtem Histamin bei Patienten mit atopischer Dermatitis. (Histamine intolerance. Effect of ingested histamine on the skin status of atopic patients.) Allergo J 2004;13:S49–50
- Steinbrecher I, Jarisch R. Histamin und Kopfschmerz. (Histamine and headache. ) Allergologie 2005;28:84–91
- Raithel M, Konturek PC, Wildner S, et al. .Evaluation der immunologischen Effekte von Bauchspeicheldrüsenenzymen bei gastrointestinal vermittelten Allergien (GMA) mittels doppel-blinder, placebokontrollierter Provokationstestung, ex vivo Mukosaosygenation und der in vitro Allergendegradation. (Evaluation of immunologic effects of pancreas enzymes in gastrointestinally mediated allergies (GMA) with the help of double-blind placebo-controlled provocation, ex vivo mucosa oxygenation, and in vitro allergen degradation.) Allergo J 2005;14:41
- Lorenz W, Ennis M, Doenicke A, Dick W. Perioperative uses of histamine antagonists. J Clin Anesth 1990;2:345–60.
- Bodmer S, Imark C, Kneubuhl M. Biogenic amines in foods: histamine and food processing. Inflamm Res 1999;48:296–300.
- Sarkadi L. Histamine in food. In: Falus A, Grosman N, Darvas Z. eds. Histamine: biology and medical aspects. Budapest, Hungary: SpringMed Publishing, 2004:176–85.
- Bover-Cid S, Holzapfel W. Biogenic amine production by bacteria. In: Morgan D, White A, Sánchez-Jiménez F, Bardócz S. eds. Biogenically active amines in food. Luxembourg City, Luxembourg: EC Publication, 2000:20–9.
- Beutling DM. Biogene Amine in der Ernährung. (Biogenic amines in nutrition.) Berlin, Germany: Springer, 1996
- Pechanek U, Pfannhauser W, Woidich H. [Content of biogenic amines in four food groups of the Austrian marketplace]. Z Lebensm Unters Forsch 1983;176:335–40
- Nordic Council of Ministers Present status of biogenic amines in foods in Nordic countries. Tema Nord 2002: 524 (ISBN: 92-893-0773-0). Cited by: Sarkadi L. Histamine in food. In: Falus A, Grosman N, Darvas Z. eds. Histamine: biology and medical aspects. Budapest, Hungary: SpringMed Publishing, 2004:176–85.
- Backhaus B, Raithel M, Hahn EG. Nicht-immunologisch induzierte Histaminfreisetzung an vitalen menschlichen Darmschleimhautbiopsien durch Stimulation mit Polyaminen. (Nonimmunologically induced histamine release of biopsies of vital human intestinal mucosa after stimulation with polyamines.) Allergo J 2005;14:41
- Izquierdo-Pulido, M. Biogenic amines in European beers. J Agric Food Chem 1996;44:33159–63.
- Wantke F, Hemmer W, Gotz M, Jarisch R. Adverse reactions to alcoholic beverages: a diagnostic guideline. Clin Exp Allergy 1997;27:343
- Littlewood JT, Gibb C, Glover V, Sandler M, Davies PT, Rose FC. Red wine as a cause of migraine. Lancet 1988;1:558–9.
- Dahl R, Henriksen JM, Harving H. Red wine asthma: a controlled challenge study. J Allergy Clin Immunol 1986;78:1126–9.
- Kanny G, Bauza T, Fremont S, et al. .Histamine content does not influence the tolerance of wine in normal subjects. Allerg Immunol (Paris) 1999;31:45–8.
- Kanny G, Gerbaux V, Olszewski A, et al. .No correlation between wine intolerance and histamine content of wine. J Allergy Clin Immunol 2001;107:375–8.
- Yang WH, Purchase EC. Adverse reactions to sulfites. CMAJ 1985;133:865–7, 880.
- Gunnison AF, Jacobsen DW. Sulfite hypersensitivity. A critical review. CRC Crit Rev Toxicol 1987;17:185–214.
- Przybilla B, Ring J. [Sulfite hypersensitivity]. Hautarzt 1987;38:445–8
- Brink B, Damink C., Joosten HM, Huisin’t Veld JH. Occurrence and formation of biologically active amines in foods Int J Food Microbiol 1990;11:73–84.
- Moneret-Vautrin DA, de Korwin JD, Tisserant J, Grignon M, Claudot N. Ultrastructural study of the mast cells of the human duodenal mucosa. Clin Allergy 1984;14:471–81.
- Vlieg-Boerstra BJ, van der HS, Oude Elberink JN, Kluin-Nelemans JC, Dubois AE. Mastocytosis and adverse reactions to biogenic amines and histamine-releasing foods: what is the evidence? Neth J Med 2005;63:244–9.