What is hypercapnia
Hypercapnia also known as hypercarbia, means high carbon dioxide (CO2) levels in arterial blood and tissues 1. The respiratory system serves a dual purpose: delivering oxygen to the pulmonary capillary bed from the environment and eliminating carbon dioxide from the bloodstream by removing it from the pulmonary capillary bed. Metabolic production of carbon dioxide occurs rapidly. Thus, a failure of ventilation promptly increases arterial blood CO2 tension [PaCO2]. Hypercapnia, or hypercarbia is often caused by hypoventilation or disordered breathing where not enough oxygen enters the lungs and not enough carbon dioxide is emitted. There are other causes of hypercapnia, as well, including some lung diseases.
Hypoventilation may be secondary to several mechanisms, including central respiratory drive depression, neuromuscular disorders, chest wall abnormalities, obesity hypoventilation, and chronic obstructive pulmonary disease (COPD). The specific causes can be summarized as follows:
- COPD
- Neuromuscular disorders 2 – Amyotrophic lateral sclerosis, muscular dystrophies (Duchenne and Becker dystrophies), diaphragm paralysis, Guillain-Barré syndrome, myasthenia gravis
- Chest wall deformities – Kyphoscoliosis, fibrothorax, thoracoplasty
- Central respiratory drive depression – Drugs (narcotics, benzodiazepines, barbiturates), neurologic disorders (encephalitis, brainstem disease, trauma, poliomyelitis, multiple sclerosis), primary alveolar hypoventilation
- Obesity-hypoventilation syndrome
- Carotid body resection and/or injury
- Myxedema (severe hypothyroidism)
The normal carbon dioxide (CO2) tension is considered to be within the range of 35–45 mmHg. Hypercapnia is clinically defined as an arterial blood partial pressure of CO2 of above 45 mmHg 3 and is a marker of poor prognosis in chronic obstructive pulmonary disease and other pulmonary disorders 1. Mild hypercapnia is noted to having carbon dioxide (CO2) partial pressure of up to 50 mmHg 4. Jung et. al 5, defined moderate hypercapnia as arterial blood carbon dioxide (CO2) levels between the range of 55 to 70 mmHg. Levels higher than 75 mmHg are generally regarded as severe hypercapnia 6. When considering these arbitrary levels of hypercapnia, it is important to emphasize that severity leveling of hypercapnia alone without a corresponding pH value, which is also affected by metabolic changes, may not represent the expected frame of clinical effects. Hypercapnia with and without acidosis has been observed in critically ill patients for many decades. This observation was either regarded as not beneficial or even harmful mostly due to its pH-related effects and complicated responses in the central nervous system 3.
Hypercapnia is a feature of many acute and chronic lung disease including obstructive sleep apnea syndrome, pneumonia, cystic fibrosis and chronic obstructive pulmonary disease (COPD) 7. Because O2 consumption is coupled to CO2 production, an intimate inverse relationship exists between the levels of these gases in cells and tissues. Furthermore, O2 and CO2 levels may become perturbed during certain pathophysiologic states. In acute lung injury hypoxia (low blood oxygen level) may arise, whereas permissive hypercapnia is often tolerated as a protective ventilatory strategy in patients presenting with this disorder 8. The intentional hypoventilation which allows, or specifically targets, high arterial blood CO2 tension [PaCO2] levels is known as permissive hypercapnia 9. Hypercapnia and hypoxia also influence inflammatory processes 10. During inflammation, oxygen consumption is significantly elevated, leading to tissue hypoxia. It is likely that this also has consequences for tissue CO2 levels 11.
Carbon dioxide (CO2) is a physiological gas found at low levels in the atmosphere and produced in cells during the process of aerobic respiration. Consequently, the levels of CO2 within tissues are usually significantly higher than those found externally. Carbon dioxide (CO2) plays a major role in the acid–base balance essential for intracellular and extracellular hemostasis 3. Carbon dioxide (CO2) has moderate water solubility. The reaction of dissolved CO2 and water will yield carbonic acid, with the enzyme carbonic anhydrase reversibly catalyzing the dissociation of carbonic acid. Carbon dioxide moves across the intracellular compartment via a concentration gradient. Carbon dioxide (CO2) is actively removed through exhalation by the respiratory system. Changes in partial pressure of CO2 will effect changes in hydrogen ion concentration. Shifts in tissue levels of CO2 (leading to either hypercapnia or hypocapnia [low CO2]) are associated with a number of pathophysiological conditions in humans. Clinical studies have indicated that such altered CO2 levels can impact upon disease progression. Recent advances in our understanding of the biology of CO2 has shown that like other physiological gases such as molecular oxygen (O2) and nitric oxide (NO), CO2 levels can be sensed by cells resulting in the initiation of physiological and pathophysiological responses 12. Acute CO2 sensing in neurons and peripheral and central chemoreceptors is important in rapidly activated responses including olfactory signalling, taste sensation and cardiorespiratory control 13. Furthermore, a role for CO2 in the regulation of gene transcription has recently been identified with exposure of cells and model organisms to high CO2 leading to suppression of genes involved in the regulation of innate immunity and inflammation 13. This latter, transcriptional regulatory role for CO2, has been largely attributed to altered activity of the NF-κB family of transcription factors.
Respiratory physiology
The respiratory control system tightly regulates ventilation. Alveolar ventilation is under the control of the central respiratory centers, which are located in the ventral aspects of the pons and medulla. The control of ventilation has metabolic and voluntary neural components. The metabolic component is spontaneous and receives chemical and neural stimuli from the chest wall and lung parenchyma and receives chemical stimuli from the blood levels of carbon dioxide and oxygen.
Metabolism rapidly generates a large quantity of volatile acid (carbon dioxide) and nonvolatile acid in the body. The metabolism of fats and carbohydrates leads to the formation of a large amount of carbon dioxide, which combines with water to form carbonic acid (H2CO3). The lungs excrete the volatile fraction via ventilation. Therefore, acid accumulation does not occur. PaCO2 is tightly maintained in a range of 39-41 mm Hg in normal states.
Ventilation is influenced and regulated by chemoreceptors for PaCO2, PaO2, and pH, located in the brainstem; by neural impulses from lung stretch receptors; and by impulses from the cerebral cortex. Failure of any of these mechanisms results in a state of hypoventilation and hypercapnia.
Hypoventilation and oxygen desaturation deteriorate during sleep secondary to a decrement in ventilatory response to hypoxia and increased PaCO2. In addition, diminished muscle tone develops during REM sleep, which further exacerbates hypoventilation secondary to insufficient respiratory effort.
Hypercapnia effects
Respiratory effects of hypercapnia
The main physiologic effect of increased CO2 in patients would result in a rightward shift of the oxygen dissociation curve which, in effect, improves unloading of oxygen at the tissue level. In a canine model, arterial hypercapnia produced a gradual and significant increase in oxygen carrying capacity 14. An increase in intracellular H+ led to decrease in pulmonary vascular resistance 15 abolishing hypoxic pulmonary vasoconstriction, reduction in intrapulmonary shunting and improvement of ventilation-perfusion mismatch 16. Hypercapnia also attenuates fluid dynamics in pulmonary vasculature, which would keep the diffusion distance between the pulmonary capillary and the alveoli short, thereby preserving optimal gas exchange and preventing edema formation 17. Although hypercapnia may notably increase pulmonary vascular resistance, due to its overall effects in decreasing pro-inflammatory response and fluid dynamics in the pulmonary vasculature, the overall effect may not always appear as increased pulmonary vascular resistance.
Hypercapnia effects on hemodynamics and oxygenation
Hypercapnia increases cardiac output, oxygen carrying capacity, mixed venous oxygen, and also peripheral tissue oxygenation 18. The increases in cerebral tissue oxygenation can be observed to some extent even when cardiac output was kept constant 19 which can possibly be explained by the relationship of oxygen supply and demand. Increase in cardiac output appears to be related to inotropic effect through ß-adrenergic receptors, and hypercapnia-induced sympathetic activation and catecholamines 20. Hypercapnia decreases systemic vascular resistance 21, which may further support tissue perfusion especially when intravascular volume status is maintained.
Cardiac index increases by about 10-15% for each 10-mmHg increments in arterial carbon dioxide tension within a range of 30 to 50 mmHg 22. Additionally, the rightward shift of the oxyhemoglobin dissociation curve and decreases in systemic vascular resistance increase oxygen availability to the peripheral tissue 22. Ratnaraj et al. 23 showed that increasing end-tidal carbon dioxide tension from 30 to 50 mmHg improved both subcutaneous tissue oxygen tension by ~ 23%, and intramural oxygenation in large and small intestine by 16-to-45% under general anesthesia in a pig model. Fleischmann et al. 24 showed that even under high inspired oxygen concentration of 0.80 in colorectal surgery patients who were assigned to mild hypercapnia (ET arterial blood CO2 tension [PaCO2] of 50 mmHg), subcutaneous tissue oxygenation increased by 38% and colon intramural oxygenation increased by about 100% compared to the patients who were assigned to normocapnia (ET arterial blood CO2 tension [PaCO2] of 35 mmHg). More recently, Schwartges et al. 25 elegantly showed that incremental levels of carbon dioxide increased cardiac output and systemic oxygen delivery in dogs, which was measured as dissolved oxygen and gastric mucosal oxygen saturation.
In spite of the positive oxygenation and perfusion effects by hypercapnia as described above, it should also be noted that myocardial depression due to overwhelming effects on inotropy and catecholamines may develop at CO2 concentrations greater than 10-15% (i.e., arterial blood CO2 tension [PaCO2] > 75 mmHg) 26 regardless of compensatory mechanisms to correct acidosis.
Hypercapnia and ventilator-associated lung injury (VALI)
Mechanical stress associated with overstretching alveoli can cause lung injury. This is clearly evident with the use of higher tidal volumes and increased transpulmonary pressure during mechanical ventilation resulting in ventilator associated lung injury (VALI). Experimental studies have shown that lung injury markers such as the presence of pulmonary edema, hyaline membranes, epithelial injury, filtration coefficient 27 and lymphatic flow are increased with higher tidal volumes 28. To minimize this insult, it has been advocated to utilize smaller tidal volumes i.e. 7 ml/kg or less 29 and to limit the plateau airway pressure to 35 cm H2O or lower 30. This in effect will cause a slow increase in PaCO2 hence implementation of permissive hypercapnia if there is no adjustment in respiratory rate 31. In a prospective randomized animal study with moderate to severe ventilation-induced lung injury, hypercapnic acidosis significantly reduced stretch-induced lung injury and resulted in higher arterial PO2 compared to normocapnia. Moreover, it also showed reduction in lung permeability as evidenced by bronchoalveolar protein concentrations and attenuated the decrease in static lung compliance in comparison with the normocapnia group 32. This study confirms the growing body of evidence that suggests hypercapnic acidosis involvement in the inhibition of nuclear factor kappa-B (NF-KB) that regulates genes central to lung injury, inflammation and repair.
In a large, randomized-controlled trial from the ARDS Network, Kregenow and colleagues demonstrated that permissive hypercapnia (pH < 7.35 and arterial blood CO2 tension [PaCO2] > 45 mmHg) reduced 28-day mortality in patients with acute lung injury who were ventilated with 12 mL/kg predicted body weight tidal volume 33. This is in part due the ability of hypercapnic acidosis to attenuate physiologic and histological indices of lung injury induced by very high levels of lung stretch 34, but not that due to the collapse and re-expansion of atelectatic lung 35. Laffey and Kavanagh 36 also stated that the combination of reduction of lung stretch and permissive hypercapnia is additive in preventing ventilator-associated lung injury (VALI).
Hypercapnia and ventilator-induced diaphragmatic injury
Ventilator-induced diaphragmatic dysfunction is another complication of prolonged controlled ventilation. It may progress to diaphragmatic muscle atrophy and result in long-term dependence on mechanical ventilation 37. Diaphragmatic muscle weakening can be explained by proteolysis, apoptosis and oxidative stress 38 and it appears to be directly proportional to duration of mechanical ventilation 39; anti-oxidant and anti-inflammatory agents such as steroids may attenuate the process 40.
Recently, Jung et al. 41 reported that after 72 hours of controlled mechanical ventilation, diaphragm contractions to maximal stimulus were preserved in hypercapnic animals compared to a 25% decrease in the normocapnic ventilated animals. Controlling lung injury, preventing diaphragmatic dysfunction, and altering exaggerated pro-inflammatory responses may suggest an important potential role for mild-to-moderate hypercapnia and hypercapnic acidosis in acutely developing systemic inflammatory processes 42.
Hypercapnia in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS)
The causes of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are varied, however, the mainstay in the management of both disease processes is mechanical ventilation, which can by itself further result in ventilator-associated lung injury (VALI) as described above. Hence, protective ventilator strategy with permissive hypercapnia has been studied both in human and animal models. Ischemia-reperfusion injury is highly associated with the development of acute lung injury (ALI). Shibata et al. 43 reported that hypercapnic acidosis inhibits xanthine oxidase, and therefore prevents an increase in capillary permeability, attenuates free-radical-induced lung injury, and possibly prevents apoptosis. Other mechanisms by which hypercapnic acidosis provide protection include attenuation of key etiologic factors that lead to acute lung injury (ALI), reduction of physical lung damage, inhibition of key aspects of the inflammatory response and direct protection of systemic organs 44. As mentioned above, the data published by the acute respiratory distress syndrome (ARDS) Network and others have shown that hypercapnic acidosis in this subset of patients had a reduced mortality 29.
Hypercapnia, pneumonia and sepsis
Pneumonia and sepsis are the leading causes of increased morbidity in critically ill patients 45. Hypercapnic acidosis has shown to depress immune function through various mechanisms via macrophage suppression, inhibition of phagocytic function of neutrophils and cytokine signaling 46. However, most of these data reported in animal studies and in vitro models. Curley et al. 32 stated that hypercapnic acidosis inhibits the activation of NF-KB, which is responsible for activation and regulation of pro-inflammatory and repair processes suggesting the reduction of pulmonary epithelial wound healing 47.
Such poor healing phenomenon was demonstrated in in vitro studies using human bronchial epithelial cells exposed to hypercapnia at neutral pH environment 48. Despite this mechanistic effect of hypercapnia on the immune system, hypercapnic acidosis has been shown to improve physiologic indices of injury in the setting of acute bacterial pneumonia 49. Ni Chonghaile et. al. 49 demonstrated that hypercapnic acidosis attenuated the increase in airway pressure and the decrease in both lung compliance and arterial oxygenation in their animal model via a neutrophil-independent mechanism. In a follow-up study, using their established pneumonia model, they showed that the protective effects of hypercapnic acidosis are further enhanced with the use of appropriate antibiotic therapy 50. On the contrary, in prolonged untreated pneumonia animal model, hypercapnic acidosis appeared to worsen lung indices in terms of static compliance and arterial oxygenation 51 and was associated with higher bacterial count and increased mortality 52.
With regard to systemic sepsis, animal models with hypercapnic acidosis demonstrated improvement in hemodynamic parameters, preserved central venous oxygen saturation, slowed the development of hypotension, and attenuated the associated increase in lactic acid concentration when compared to normocapnia 53. Additionally, hypercapnia may have some minimal effect in preventing other infection such as surgical site infections 54. The clinical implication of hypercapnia in effect is also dependent on the stage of the infection, i.e. early or late in the course of the disease process; and also the primary site of infection, may it be pulmonary or systemic source.
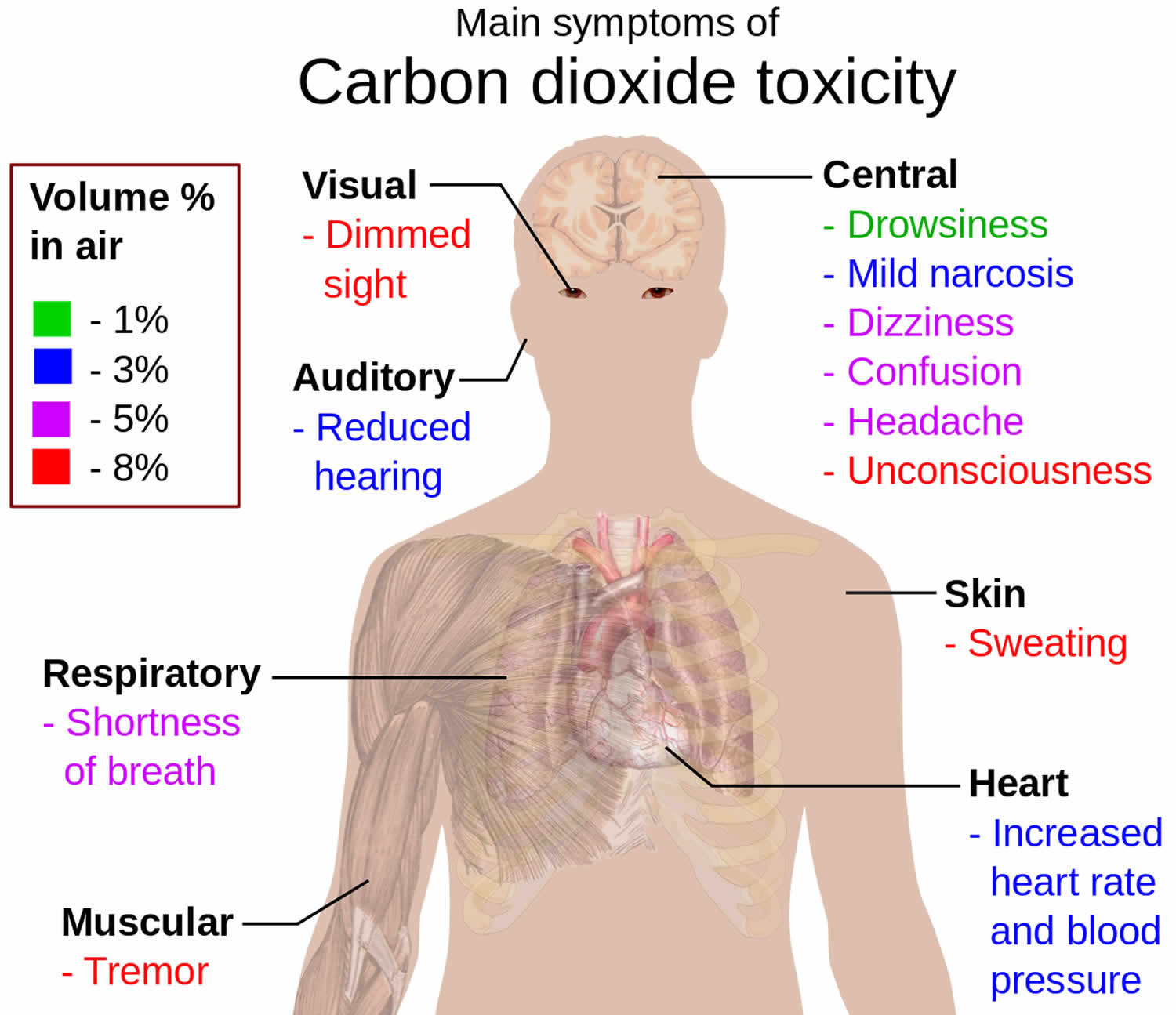
Hypercapnia signs and symptoms
Symptoms and signs of early hypercapnia include flushed skin, full pulse, tachypnea, dyspnea, extrasystoles, muscle twitches, hand flaps, reduced neural activity, and possibly raised blood pressure. Symptoms of mild hypercapnia might also include headache, confusion and lethargy. Hypercapnia can induce increased cardiac output, an elevation in arterial blood pressure, and a propensity toward cardiac arrhythmias 55. Hypercapnia may increase pulmonary capillary resistance. In severe hypercapnia (generally PaCO2 greater 75 mmHg), symptomatology progresses to disorientation, panic, hyperventilation, convulsions, unconsciousness, and eventually death 56.
In cases where symptoms are mild and develop slowly over time, people may not even realize they have hypercapnia. Therefore, it is important to be aware of both mild and severe symptoms.
Mild symptoms
The following are considered to be mild symptoms of hypercapnia:
- dizziness
- drowsiness
- excessive fatigue
- headaches
- feeling disoriented
- flushing of the skin
- shortness of breath
These symptoms of hypercapnia may arise from shorter periods of shallow or slow breathing, such as during deep sleep.
They may not always be a cause for concern, as the body is often able to correct the symptoms and balance carbon dioxide levels in the bloodstream without intervention.
However, if the above symptoms persist for several days, it is advisable to see a doctor.
Severe symptoms
The symptoms of severe hypercapnia require immediate medical attention, as they can cause long-term complications. Some cases may be fatal.
Alcohol and many illicit substances are known respiratory depressants. Their use in patients with hypoventilation syndromes may lead to coma and death 57
Severe hypercapnia symptoms include:
- confusion
- coma
- depressionor paranoia
- hyperventilation or excessive breathing
- irregular heartbeat or arrhythmia
- loss of consciousness
- muscle twitching
- panic attacks
- seizures
Hypercapnia causes
Hypercapnia is generally caused by hypoventilation, lung disease, or diminished consciousness. It may also be caused by exposure to environments containing abnormally high concentrations of carbon dioxide, such as from volcanic or geothermal activity, or by rebreathing exhaled carbon dioxide. It can also be an initial effect of administering supplemental oxygen on a patient with sleep apnea. In this situation the hypercapnia can also be accompanied by respiratory acidosis 58.
There are many causes of hypercapnia including the following:
Chronic obstructive pulmonary disease (COPD)
COPD is an umbrella term for several conditions that affect the breathing. Common forms of COPD include chronic bronchitis and emphysema.
Chronic bronchitis leads to inflammation and mucus in the airways, while emphysema involves damage to the air sacs or alveoli in the lungs.
Both conditions can cause increased levels of carbon dioxide in the bloodstream.
The main cause of COPD is long-term exposure to lung irritants. According to the National Heart, Lung, and Blood Institute, cigarette smoke is the most common lung irritant that causes COPD in the United States. Air pollution and exposure to chemicals or dust may also cause COPD.
Although not everyone with COPD will develop hypercapnia, a person’s risk increases as their COPD progresses.
Central alveolar hypoventilation
The phrase “central alveolar hypoventilation” is used to describe patients with alveolar hypoventilation secondary to an underlying neurologic disease. Causes of central alveolar hypoventilation include drugs and central nervous system (CNS) diseases such as cerebrovascular accidents, trauma, and neoplasms.
- Primary and central alveolar hypoventilation: Patients with primary alveolar hypoventilation can voluntarily hyperventilate and normalize their PaCO2. These patients are unable to centrally integrate chemoreceptor signals, although the peripheral chemoreceptors appear to function normally.
- Congenital central hypoventilation syndrome: Hypoventilation may be caused by depression of the central respiratory drive. Congenital central hypoventilation syndrome, previously known as Ondine curse, is defined as the failure of automatic control of breathing 59. It generally presents in newborns and, in 90% of the cases, is caused by a polyalanine repeat expansion mutation in the PHOX2B gene. Patients heterozygous for PHOX2B may have milder forms of the disease and live into adulthood 60. Congenital central hypoventilation syndrome may occur in association with Hirschsprung disease. In addition, patients with CCHS are at increased risk for neuroblastoma and ganglioneuroma 60. These patients have absent or minimal ventilatory response to hypercapnia and hypoxemia during sleep and wakefulness. Since these individuals do not develop respiratory distress when challenged with hypercapnia or hypoxia, progressive hypercapnia and hypoxemia occurs during sleep. Ventilation in congenital central hypoventilation syndrome patients is more stable during rapid eye movement (REM) sleep than in non-REM sleep 61. The diagnosis is established after excluding another cause, either pulmonary, cardiac, metabolic, or neurologic, for central hypoventilation. Patients with congenital central hypoventilation syndrome require lifelong ventilatory support during sleep, and some may require 24-hour ventilatory support.
Obesity-hypoventilation syndrome
Patients with obesity-hypoventilation syndrome have a higher incidence of restrictive ventilatory defects when compared with patients who are obese but do not hypoventilate. Studies have shown that patients with obesity-hypoventilation syndrome have total lung capacities that are 20% lower and maximal voluntary ventilation that is 40% lower than patients who are obese who do not have hypoventilation 62.
Patients with obesity-hypoventilation syndrome demonstrate an excessive work of breathing and an increase in carbon dioxide production. Inspiratory muscle strength and resting tidal volumes also are reported to be decreased in patients with obesity hypoventilation. Pulmonary compliance is lower in patients with obesity-hypoventilation syndrome when compared with patients who are obese who do not have hypoventilation.
Obesity increases the work of breathing because of reductions in chest wall compliance and respiratory muscle strength. An excessive demand on the respiratory muscles leads to the perception of increased breathing effort and could unmask other associated respiratory and heart diseases.
Leptin deficiency or leptin resistance may also contribute to obesity-hypoventilation syndrome, by reducing ventilatory responsiveness and leading to carbon dioxide retention 63.
Despite the above-mentioned physiologic abnormalities, the most important factor in the development of hypoventilation in obesity-hypoventilation syndrome is likely a defect in the central respiratory control system. These patients have been shown to have a decreased responsiveness to carbon dioxide rebreathing, hypoxia, or both.
Chest wall deformities
In patients with chest wall deformities, hypoventilation develops secondary to decreased chest wall compliance, with a resultant decreased tidal volume 64. Alveolar dead space is unchanged, but the dead-space gas volume-to-tidal gas volume [VD/VT] ratio ratio is increased due to the reduced tidal volume.
The most common chest wall abnormality to cause hypoventilation is kyphoscoliosis. It is associated with a decrease in vital capacity and expiratory reserve volume, while the residual volume is only moderately reduced. These patients usually are asymptomatic until the late stages of disease, when the most severe deformity of the spine has occurred.
Sleep apnea
The National Sleep Foundation report that between 5 and 20 percent of adults have sleep apnea.
This common condition is characterized by shallow breathing, or pauses in breathing, during sleep. It can interfere with the level of oxygen in the bloodstream and throw off the body’s balance of carbon dioxide and oxygen.
Sleep apnea symptoms include daytime sleepiness, headaches upon waking, and difficulty concentrating.
Genetics
Rarely, a genetic condition where the liver fails to produce enough alpha-1-antitrypsin can cause hypercapnia. Alpha-1-antitrypsin is a protein that is necessary for lung health, so alpha-1-antitrypsin deficiency is a risk factor for COPD development.
Neuromuscular disorders
In some people, the nerves and muscles necessary for proper lung function may not work correctly. For example, muscular dystrophy can cause the muscles to weaken, eventually leading to breathing problems.
Neuromuscular diseases that can cause alveolar hypoventilation include myasthenia gravis, amyotrophic lateral sclerosis, Guillain-Barré syndrome, and muscular dystrophy 64. Patients with neuromuscular disorders have rapid, shallow breathing secondary to severe muscle weakness or abnormal motor neuron function.
Disorders of the nervous or muscular systems that can contribute to hypercapnia include:
- Amyotrophic lateral sclerosis(ALS), a progressive disease that affects nerve cells in the brain and spinal cord.
- Encephalitisor when a person has inflammation of the brain.
- Guillain-Barré syndromethat can be caused by an abnormal immune response.
- Myasthenia gravis, a chronic disease that can weaken the skeletal muscles responsible for breathing.
The central respiratory drive is maintained in patients with neuromuscular disorders. Thus, hypoventilation is secondary to respiratory muscle weakness. Patients with neuromuscular disorders have nocturnal desaturations that are most prevalent in the REM stage of sleep. The degree of nocturnal desaturation is correlated with the degree of diaphragm dysfunction. The nocturnal desaturations may precede the onset of daytime hypoventilation and gas exchange abnormalities.
Other causes
Other causes of high blood levels of carbon dioxide include:
- Activities that impact breathing, including diving or ventilator use.
- Brainstem stroke, which can affect breathing.
- Hypothermia, a medical emergency caused by rapid heat loss from the body.
- Obesityhypoventilation syndromes when overweight people cannot breathe deeply or quickly enough.
- An overdose of certain drugs, such as opioids or benzodiazepines.
Risk factors for developing hypercapnia
Some people are more at risk than others for the development of hypercapnia, especially if they:
- Smoke: People who smoke, especially heavy smokers, are at greater risk of COPD, hypercapnia, other breathing difficulties, and lung diseases.
- Have asthma: Because asthmacauses the airways to become inflamed and narrowed, it may impact breathing and the levels of carbon dioxide in the body when it is not well controlled.
- Work with lung irritants: Those who work with chemicals, dust, smoke, or other lung irritants are at greater risk of hypercapnia.
- Have COPD: Having COPD, especially if diagnosed at a later stage of disease progression, increases the likelihood of getting hypercapnia.
Hypercapnia prevention
Hypercapnia can be prevented by:
- treating existing lung conditions
- quitting smoking
- maintaining a healthy weight
- exercise regularly
- avoiding exposure to toxic fumes and chemicals.
Hypercapnia diagnosis
Some tests used to diagnose hypercapnia include:
- Arterial blood gas test: This checks for blood levels of carbon dioxide and oxygen.
- Spirometer test: This test involves blowing into a tube to assess how much air a person can move out of their lungs, and how fast they can do this.
- X-ray or CT scan: These imaging tests can check for the presence of lung damage and lung conditions.
Hypercapnia treatment
The treatment of hypoventilation primarily is directed at correcting the underlying disorder. Use caution when correcting chronic hypercapnia. Rapid correction of the hypercapnia can alkalinize the cerebrospinal fluid, which may cause seizures, and can induce a metabolic alkalosis, placing the patient at risk for cardiac dysrhythmias. Infusion of sodium HCO3 is not indicated for chronic hypoventilation syndromes.
Bronchodilators, such as beta agonists (eg, albuterol, salmeterol), anticholinergic agents (eg, ipratropium bromide), and methylxanthines (eg, theophylline), are helpful in treating patients with obstructive lung disease and severe bronchospasm. Additionally, theophylline may improve diaphragm muscle contractility and stimulate the respiratory center.
Drugs aimed at reversing the effects of certain sedative drugs may be helpful in the event of an overdose. Naloxone (Narcan) may be used to reverse the effects of narcotics, and flumazenil (Romazicon) may be used to reverse the effects of benzodiazepines.
Ventilation
There are two types of ventilation used for hypercapnia:
- Non-invasive ventilation: Breathing is assisted by a flow of air that comes through a mouthpiece or nasal mask. This is helpful for people with sleep apnea to keep the airways open at night and is also known as CPAP or continuous positive airway pressure.
- Mechanical ventilation: The person will have a tube inserted through their mouth into their airway. This is called intubation.
People with severe hypercapnia symptoms may be put on a ventilation device to assist with breathing.
Acute hypoventilation
Treatment of hypoventilation also is aimed at assisting ventilation. Therapies that may be beneficial are noninvasive ventilatory techniques, such as bilevel positive-pressure ventilation (PPV). Ventilatory assistance may be required in patients for the following indications:
- Symptoms of nocturnal hypoventilation, such as daytime hypersomnolence, morning headaches, fatigue, nightmares, and enuresis
- Dyspnea at rest
- Hypoventilation that causes pulmonary hypertension and cor pulmonale
- Nocturnal hypoxia (arterial oxygen saturation < 88%) despite supplemental oxygen
Patients with acute hypoventilation, diagnosed through symptoms and laboratory data, should be started on bilevel positive-pressure ventilation urgently. If this fails to improve symptoms and laboratory data within a short period (1-2 h), consideration for intubation and invasive mechanical ventilation should be undertaken. Acute hypercapnia may progress to cardiovascular instability, arrhythmia, cardiac or respiratory arrest, and death if untreated.
Chronic hypoventilation
Noninvasive ventilation using nocturnal bilevel positive-pressure ventilation using a mask interface is widely accepted as the ventilatory mode of choice in patients with chronic respiratory failure related to COPD, neuromuscular disease, thoracic deformities, and idiopathic hypoventilation. Nocturnal bilevel positive-pressure ventilation acts by reducing nocturnal hypoventilation and increasing carbon dioxide responsiveness 65. Nocturnal bilevel positive-pressure ventilation may obviate the need for tracheotomy and has improved many patient-oriented outcomes. Bilevel positive-pressure ventilation is the preferred method of noninvasive ventilation 66.
The indications for noninvasive bilevel positive-pressure ventilation for nocturnal hypoventilation syndromes have been formulated based on the available literature. Patients considered for this therapy should have at least 1 of the following:
- A disease known to cause hypoventilation
- Symptoms and signs of hypoventilation present
- Failure to respond to first-line therapies in mild cases of hypoventilation – ie, treatment of primary underlying disease with bronchodilators, respiratory stimulants, weight loss, supplemental oxygen, or continuous positive airway pressure (CPAP) 67
- Moderate to severe hypoventilation
Nocturnal bilevel positive-pressure ventilation is indicated for use in patients with neuromuscular disorders who exhibit morning headache, daytime hypersomnolence, sleep difficulties, or cognitive dysfunction.
In the absence of symptoms, nocturnal bilevel positive-pressure ventilation is recommended when PaCO2 is greater than 45 mm Hg or when PaO2 is less than 60mm Hg on a morning blood gas measurement 68.
Daytime ventilation should be used when these patients have PaCO2 greater than 50 mm Hg or less than 88% oxygen saturation 69.
Studies in patients with obesity-hypoventilation syndrome have demonstrated that one year of treatment with nocturnal bilevel positive-pressure ventilation improves blood gas values 70.
In patients unable to tolerate noninvasive ventilation or in patients in whom this is not effective, tracheostomy may be required.
Diet
Weight loss is an ideal treatment in obesity-hypoventilation syndrome. Weight loss improves the abnormal physiology and restores normal daytime gas exchange. In some individuals even a modest weight loss of 10 kg improves minute ventilation and normalizes daytime PaCO2. In concomitant obstructive sleep apnea, weight loss has been shown to decrease the number of sleep-disordered breathing events (apneas and hypopneas) and the severity of hypoxemia.
Weight loss
Weight loss should be encouraged in patients with obesity-hypoventilation syndrome. Diet regulation and exercise are prudent recommendations, and supervised weight loss programs should be offered to these patients. Unfortunately, many of these patients have numerous comorbidities that prevent them from performing an adequate level of exercise to facilitate significant weight loss.
Bariatric surgical procedures, such as gastric bypass procedures, should be offered to patients who are appropriate surgical candidates and who are willing to accept the risk of the surgical procedure. Obesity-hypoventilation syndrome is associated with a higher operative mortality 71.
Medication
Certain medications can assist breathing, such as:
- antibiotics to treat pneumonia or other respiratory infections
- bronchodilators to open the airways
- corticosteroids to reduce inflammation in the airway
Respiratory stimulants
Respiratory stimulants have been used in alveolar hypoventilation but have limited efficacy. These are generally a last resort and should only be considered with noninvasive pressure ventilation.
Medroxyprogesterone
Medroxyprogesterone increases the central respiratory drive and it has been shown to be effective in obesity-hypoventilation syndrome and central hypoventilation syndromes. Its effectiveness in COPD is not clear. Initial studies documenting a reduction in hypercapnia with treatment with medroxyprogesterone were performed in the 1960s 72.
More recent studies also have documented a decrease in hypercapnia in patients with obesity-hypoventilation syndrome with associated hypercapnia while receiving total daily doses of 60 mg of medroxyprogesterone in divided doses 2-3 times per day 73.
However, the drug does not improve apnea frequency or symptoms of sleepiness. In addition, the risk of venous thromboembolism is increased with progestational agents 74. Many experts do not currently recommend progesterone therapy.
Acetazolamide
Acetazolamide is a diuretic that inhibits carbonic anhydrase, increases HCO3 excretion, and causes metabolic acidosis. The metabolic acidosis subsequently stimulates ventilation. However, this medication must be used with caution. If the patient’s respiratory system cannot compensate for the metabolic acidosis it induces, the patient may suffer hyperkalemia and, potentially, a cardiac dysrhythmia.
Theophylline
Theophylline increases diaphragm muscle strength and stimulates the central ventilatory drive. In addition to being a stimulant, theophylline is also a bronchodilator. However, the effectiveness of this medication is limited.
Oxygen therapy
Because many patients with hypercapnia also are hypoxemic during the day, oxygen therapy may be indicated.
Oxygen therapy is indicated to prevent the sequelae of long-standing hypoxemia. Patients with COPD who meet the criteria for oxygen therapy have a decreased mortality when treated with continuous supplemental oxygen therapy 64. Oxygen therapy also has been shown to reduce pulmonary hypertension.
Use oxygen therapy with caution because it may worsen hypercapnia in some situations. In patients with COPD, the presence of worsening hypercapnia following oxygen therapy is a consequence of ventilation-perfusion mismatching rather than reduced ventilatory drive secondary to reduction in hypoxia 64.
Hypercapnia is best avoided by titration of oxygen delivery to maintain oxygen saturations in the range of 90-94% and PaO2 between 60 and 65mm Hg.
Approximately 50% of patients with obesity-hypoventilation syndrome require oxygen therapy in addition to nocturnal bilevel positive-pressure ventilation (PPV) 75. However, breathing 100% oxygen may cause worsening hypercapnia in stable patients with obesity-associated hypoventilation, due to a reduction in minute ventilation, resulting in alveolar hypoventilation and an associated increase in the volume of dead space-to-tidal volume ratio. Therefore, oxygen therapy should be administered with caution in patients who are morbidly obese 76. Oxygen use alone is often an inadequate therapy for obesity-hypoventilation syndrome.
Patients with neuromuscular disease should not usually be given oxygen therapy without ventilatory support.
Lifestyle changes
To reduce symptoms and avoid complications, a doctor may recommend changes to diet and physical activity. They will also encourage people with hypercapnia to avoid lung irritants by quitting smoking and limiting their exposure to chemicals, dust, and fumes.
Surgery
Surgery associated with hypoventilation includes bariatric procedures to promote weight loss and placement of an electrode on the phrenic nerve for diaphragm pacing. Some patients with thoracic deformities, such as kyphoscoliosis, may be candidates for corrective surgical procedures.
Refractory cases of hypoventilation due to advanced underlying disease, such as neuromuscular disease, chest wall deformities, or even obesity-hypoventilation syndrome, may require tracheostomy and assisted ventilation for optimal management.
If the lungs or airways are damaged, then surgery may be required. Options include lung volume reduction surgery to remove damaged tissue or a lung transplant where a damaged lung is replaced by a healthy lung from a donor.
Oxygen therapy for cystic fibrosis
Based on available data, oxygen therapy should be reserved for those individuals with objective evidence of hypoxemia, either at rest while awake or during either exercise or sleep 77. This 2013 Cochrane review has found an absence of data on which to dictate the prescription of long‐term oxygen therapy in people with cystic fibrosis with hypoxemia. In the subset of people with cystic fibrosis and documented nocturnal hypoxemia, oxygen therapy should be considered. In people with hypercapnia, assessment of the impact of oxygen supplementation on ventilation via capnometry may be indicated. In some of these individuals, nasal ventilation with oxygen supplementation during sleep may be a more optimal therapeutic choice. In people with cystic fibrosis with advanced lung disease and hypoxemia during exercise, the prescription of oxygen supplementation appears to prevent hypoxemia and has clear benefits regarding exercise capacity and duration. Although it is tempting to speculate that oxygen supplementation over time during exercise in people with cystic fibrosis with advanced lung disease might have cumulative benefits, there are no studies upon which to base this recommendation. Since there is some evidence that individuals with cystic fibrosis who have hypoxemia during exercise frequently have hypoxemia during sleep 78, overnight polysomnography should be considered in these individuals.
There are no published data to guide the prescription of chronic oxygen supplementation to people with advanced lung disease due to cystic fibrosis. Short‐term oxygen therapy during sleep and exercise improves oxygenation but is associated with modest and probably clinically inconsequential hypercapnia 77. There are improvements in exercise duration, time to fall asleep and regular attendance at school or work 77. There is a need for larger, well‐designed clinical trials to assess the benefits of long‐term oxygen therapy in people with cystic fibrosis administered continuously or during exercise or sleep or both.
Chronic obstructive pulmonary disease (COPD)
COPD (chronic obstructive pulmonary disease) makes it hard for you to breathe. The two main types of COPD are chronic bronchitis and emphysema. The main cause of COPD is long-term exposure to substances that irritate and damage the lungs. This is usually cigarette smoke. Air pollution, chemical fumes, or dust can also cause it.
At first, COPD may cause no symptoms or only mild symptoms. As the disease gets worse, symptoms usually become more severe. They include
- A cough that produces a lot of mucus
- Shortness of breath, especially with physical activity
- Wheezing
- Chest tightness
Many people with COPD experience increasing functional impairment and progressive loss of quality of life over many years 79. Acute exacerbations of COPD, defined as acute deterioration in respiratory health, contribute to functional impairment and risk of mortality in individual people with the disease 80.
Quitting smoking is the most important step you can take to treat COPD. COPD can also cause the oxygen level in the blood to fall; if this occurs, supplemental oxygen will be prescribed. Breathlessness, however, will happen with COPD even if you have good oxygen levels 81. Breathlessness is therefore not a good guide for oxygen use. To control symptoms of COPD, your breathing medications must be taken everyday, usually for life. Pulmonary rehabilitation, which is a personalized treatment program that teaches COPD management strategies to improve quality of life. Pulmonary rehabilitation programs may include plans that teach people how to breathe better and conserve their energy, as well as provide advice on food and exercise. Surgical procedures such as lung volume reduction surgery or lung transplantation may be helpful for some cases of COPD 81.
References- Gates KL, Howell HA, Nair A, et al. Hypercapnia Impairs Lung Neutrophil Function and Increases Mortality in Murine Pseudomonas Pneumonia. American Journal of Respiratory Cell and Molecular Biology. 2013;49(5):821-828. doi:10.1165/rcmb.2012-0487OC. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3931098/
- Paschoal IA, Villalba Wde O, Pereira MC. Chronic respiratory failure in patients with neuromuscular diseases: diagnosis and treatment. J Bras Pneumol. 2007 Feb. 33(1):81-92
- Bautista AF, Akca O. Hypercapnia: is it protective in lung injury? Medical Gas Research. 2013;3:23. doi:10.1186/2045-9912-3-23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3833649/
- Mild hypercapnia increases subcutaneous and colonic oxygen tension in patients given 80% inspired oxygen during abdominal surgery. Fleischmann E, Herbst F, Kugener A, Kabon B, Niedermayr M, Sessler DI, Kurz A. Anesthesiology. 2006 May; 104(5):944-9.
- Moderate and prolonged hypercapnic acidosis may protect against ventilator-induced diaphragmatic dysfunction in healthy piglet: an in vivo study. Jung B, Sebbane M, Le Goff C, Rossel N, Chanques G, Futier E, Constantin JM, Matecki S, Jaber S. Crit Care. 2013 Jan 24; 17(1):R15.
- Carbon dioxide tolerance: a review. Glatte HA Jr, Welch BE. Aeromed Rev. 1967; 5():1-28.
- Pulmonary hypertension, hypoxemia, and hypercapnia in obstructive sleep apnea patients. Krieger J, Sforza E, Apprill M, Lampert E, Weitzenblum E, Ratomaharo J. Chest. 1989 Oct; 96(4):729-37.
- Permissive hypercapnia: what to remember. Contreras M, Masterson C, Laffey JG. Curr Opin Anaesthesiol. 2015 Feb; 28(1):26-37. https://www.ncbi.nlm.nih.gov/pubmed/25500498/
- Dries DJ. Permissive hypercapnia. Journal of Trauma 1995;39:984‐9.
- Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Gates KL, Howell HA, Nair A, Vohwinkel CU, Welch LC, Beitel GJ, Hauser AR, Sznajder JI, Sporn PH. Am J Respir Cell Mol Biol. 2013 Nov; 49(5):821-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3931098/
- Regulation of gene expression by carbon dioxide. Taylor CT, Cummins EP. J Physiol. 2011 Feb 15; 589(Pt 4):797-803. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3060358/
- Turner MJ, Saint‐Criq V, Patel W, et al. Hypercapnia modulates cAMP signalling and cystic fibrosis transmembrane conductance regulator‐dependent anion and fluid secretion in airway epithelia. The Journal of Physiology. 2016;594(6):1643-1661. doi:10.1113/JP271309. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4799982/
- Taylor CT, Cummins EP. Regulation of gene expression by carbon dioxide. The Journal of Physiology. 2011;589(Pt 4):797-803. doi:10.1113/jphysiol.2010.201467. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3060358/
- Torbati D, Mangino MJ, Garcia E, Estrada M, Totapally BR, Wolfsdorf J. Acute hypercapnia increases the oxygen-carrying capacity of the blood in ventilated dogs. Crit Care Med. 1998;26(11):1863–1867. doi: 10.1097/00003246-199811000-00030
- Kantores C, McNamara PJ, Teixeira L, Engelberts D, Murthy P, Kavanagh BP, Jankov RP. Therapeutic hypercapnia prevents chronic hypoxia-induced pulmonary hypertension in the newborn rat. Am J Physiol Lung Cell Mol Physiol. 2006;291(5):L912–922. doi: 10.1152/ajplung.00480.2005
- Swenson ER, Robertson HT, Hlastala MP. Effects of inspired carbon dioxide on ventilation-perfusion matching in normoxia, hypoxia, and hyperoxia. Am J Respir Crit Care Med. 1994;149(6):1563–1569. doi: 10.1164/ajrccm.149.6.8004314
- Vengust M. Hypercapnic respiratory acidosis: a protective or harmful strategy for critically ill newborn foals? Can J Vet Res. 2012;76(4):275–280
- Gayan-Ramirez G, Testelmans D, Maes K, Racz GZ, Cadot P, Zador E, Wuytack F, Decramer M. Intermittent spontaneous breathing protects the rat diaphragm from mechanical ventilation effects. Crit Care Med. 2005;33(12):2804–2809. doi: 10.1097/01.CCM.0000191250.32988.A3
- Akca O, Sessler DI, Delong D, Keijner R, Ganzel B, Doufas AG. Tissue oxygenation response to mild hypercapnia during cardiopulmonary bypass with constant pump output. Br J Anaesth. 2006;96(6):708–714. doi: 10.1093/bja/ael093
- Blackburn JP, Conway CM, Leigh JM, Lindop MJ, Reitan JA. Pa CO2 and the pre-ejection period: the pa CO2 inotropy response curve. Anesthesiology. 1972;37(3):268–276. doi: 10.1097/00000542-197209000-00002
- Walley KR, Lewis TH, Wood LD. Acute respiratory acidosis decreases left ventricular contractility but increases cardiac output in dogs. Circ Res. 1990;67(3):628–635. doi: 10.1161/01.RES.67.3.628
- Mas A, Saura P, Joseph D, Blanch L, Baigorri F, Artigas A, Fernandez R. Effects of acute moderate changes in PaCO2 on global hemodynamics and gastric perfusion. Crit Care Med. 2000;28(2):360–365. doi: 10.1097/00003246-200002000-00012
- Ratnaraj J, Kabon B, Talcott MR, Sessler DI, Kurz A. Supplemental oxygen and carbon dioxide each increase subcutaneous and intestinal intramural oxygenation. Anesth Analg. 2004;99(1):207–211. doi: 10.1213/01.ANE.0000121308.26125.B0
- Fleischmann E, Herbst F, Kugener A, Kabon B, Niedermayr M, Sessler DI, Kurz A. Mild hypercapnia increases subcutaneous and colonic oxygen tension in patients given 80% inspired oxygen during abdominal surgery. Anesthesiology. 2006;104(5):944–949. doi: 10.1097/00000542-200605000-00009
- Schwartges I, Schwarte LA, Fournell A, Scheeren TW, Picker O. Hypercapnia induces a concentration-dependent increase in gastric mucosal oxygenation in dogs. Intensive Care Med. 2008;34(10):1898–1906. doi: 10.1007/s00134-008-1183-8
- Andersen M, Mouritzen C. Effect of acute respiratory and metabolic acidosis on cardiac output and peripheral resistance. Ann Surg. 1966;63(2):161–168
- Peevy KJ, Hernandez LA, Moise AA, Parker JC. Barotrauma and microvascular injury in lungs of nonadult rabbits: effect of ventilation pattern. Crit Care Med. 1990;18(6):634–637. doi: 10.1097/00003246-199006000-00012
- Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med. 1993;21(1):131–143. doi: 10.1097/00003246-199301000-00024
- Petrucci N, Iacovelli W. Lung protective ventilation strategy for the acute respiratory distress syndrome. Cochrane Database Syst Rev. 2007;3 CD003844
- Network TARDS. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308
- Rogovik A, Goldman R. Permissive hypercapnia. Emerg Med Clin North Am. 2008;26(4):941–952. doi: 10.1016/j.emc.2008.08.002. viii-ix
- Contreras M, Ansari B, Curley G, Higgins BD, Hassett P, O’Toole D, Laffey JG. Hypercapnic acidosis attenuates ventilation-induced lung injury by a nuclear factor-kappaB-dependent mechanism. Crit Care Med. 2012;40(9):2622–2630. doi: 10.1097/CCM.0b013e318258f8b4
- Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med. 2006;34(1):1–7. doi: 10.1097/01.CCM.0000194533.75481.03
- Sinclair SE, Kregenow DA, Lamm WJ, Starr IR, Chi EY, Hlastala MP. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2002;166(3):403–408. doi: 10.1164/rccm.200112-117OC
- O’Croinin D, Ni Chonghaile M, Higgins B, Laffey JG. Bench-to-bedside review: Permissive hypercapnia. Crit Care. 2005;9(1):51–59
- Laffey JG, Kavanagh BP. Biological effects of hypercapnia. Intensive Care Med. 2000;26(1):133–138. doi: 10.1007/s001340050027
- Jaber S, Jung B, Matecki S, Petrof BJ. Clinical review: ventilator-induced diaphragmatic dysfunction–human studies confirm animal model findings. Crit Care. 2011;15(2):206. doi: 10.1186/cc10023. doi: 10.1186/cc10023
- Petrof BJ, Jaber S, Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care. 2010;16(1):19–25. doi: 10.1097/MCC.0b013e328334b166. doi: 10.1097/MCC.0b013e328334b166
- Sassoon CS. Ventilator-associated diaphragmatic dysfunction. Am J Respir Crit Care Med. 2002;166(8):1017–1018. doi: 10.1164/rccm.2207008
- Maes K, Testelmans D, Cadot P, Deruisseau K, Powers SK, Decramer M, Gayan-Ramirez G. Effects of acute administration of corticosteroids during mechanical ventilation on rat diaphragm. Am J Respir Crit Care Med. 2008;178(12):1219–1226. doi: 10.1164/rccm.200702-296OC.
- Jung B, Sebbane M, Goff CL, Rossel N, Chanques G, Futier E, Constantin JM, Matecki S, Jaber S. Moderate and prolonged hypercapnic acidosis may protect against ventilator-induced diaphragmatic dysfunction in healthy piglet: an in vivo study. Crit Care. 2013;17(1):R15. doi: 10.1186/cc12486. Epub ahead of print
- Akca O, Bautista A. Hypercapnia and ventilator-induced diaphragmatic dysfunction. Crit Care. 2013;17(2):129. doi: 10.1186/cc12563
- Shibata K, Cregg N, Engelberts D, Takeuchi A, Fedorko L, Kavanagh BP. Hypercapnic acidosis may attenuate acute lung injury by inhibition of endogenous xanthine oxidase. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1578–1584
- Laffey JG, O’Croinin D, McLoughlin P, Kavanagh BP. Permissive hypercapnia–role in protective lung ventilatory strategies. Intensive Care Med. 2004;30(3):347–356. doi: 10.1007/s00134-003-2051-1
- Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40(3):754–761. doi: 10.1097/CCM.0b013e318232db65. doi: 10.1097/CCM.0b013e318232db65
- Wang N, Gates KL, Trejo H, Favoreto S Jr, Schleimer RP, Sznajder JI, Beitel GJ, Sporn PH. Elevated CO2 selectively inhibits interleukin-6 and tumor necrosis factor expression and decreases phagocytosis in the macrophage. FASEB J. 2010;24(7):2178–2190. doi: 10.1096/fj.09-136895
- Curley G, Hayes M, Laffey JG. Can ‘permissive’ hypercapnia modulate the severity of sepsis-induced ALI/ARDS? Crit Care. 2011;15(2):212. doi: 10.1186/cc9994
- O’Toole D, Hassett P, Contreras M, Higgins BD, McKeown ST, McAuley DF, O’Brien T, Laffey JG. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-kappaB dependent mechanism. Thorax. 2009;64(11):976–982. doi: 10.1136/thx.2008.110304
- Ni Chonghaile M, Higgins BD, Costello JF, Laffey JG. Hypercapnic acidosis attenuates severe acute bacterial pneumonia-induced lung injury by a neutrophil-independent mechanism. Crit Care Med. 2008;36(12):3135–3144. doi: 10.1097/CCM.0b013e31818f0d13
- Chonghaile MN, Higgins BD, Costello J, Laffey JG. Hypercapnic acidosis attenuates lung injury induced by established bacterial pneumonia. Anesthesiology. 2008;109(5):837–848. doi: 10.1097/ALN.0b013e3181895fb7
- O’Croinin DF, Nichol AD, Hopkins N, Boylan J, O’Brien S, O’Connor C, Laffey JG, McLoughlin P. Sustained hypercapnic acidosis during pulmonary infection increases bacterial load and worsens lung injury. Crit Care Med. 2008;36(7):2128–2135. doi: 10.1097/CCM.0b013e31817d1b59
- Helenius IT, Krupinski T, Turnbull DW, Gruenbaum Y, Silverman N, Johnson EA, Sporn PH, Sznajder JI, Beitel GJ. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc Natl Acad Sci U S A. 2009;106(44):18710–18715. doi: 10.1073/pnas.0905925106
- Higgins BD, Costello J, Contreras M, Hassett P, D OT, Laffey JG. Differential effects of buffered hypercapnia versus hypercapnic acidosis on shock and lung injury induced by systemic sepsis. Anesthesiology. 2009;111(6):1317–1326. doi: 10.1097/ALN.0b013e3181ba3c11
- Akca O, Kurz A, Fleischmann E, Buggy D, Herbst F, Stocchi L, Galandiuk S, Iscoe S, Fisher J, Apfel CC. et al. Hypercapnia and surgical site infection: a randomized trial. Br J Anaesth. 2013;111(5):759–767. doi: 10.1093/bja/aet233
- Morgan GE, Jr., Mikhail MS, Murray MJ, “Chapter 3. Breathing Systems” (Chapter). Morgan GE, Jr., Mikhail MS, Murray MJ: Clinical Anesthesiology, 4th Edition
- Glatte Jr H. A.; Motsay G. J.; Welch B. E. (1967). “Carbon Dioxide Tolerance Studies”. Brooks AFB, TX School of Aerospace Medicine Technical Report. SAM-TR-67-77.
- Chen ML, Turkel SB, Jacobson JR, Keens TG. Alcohol use in congenital central hypoventilation syndrome. Pediatr Pulmonol. 2006 Mar. 41(3):283-5.
- Dement, Roth, Kryger, ‘Principles & Practices of Sleep Medicine’ 3rd edition, 2000, p. 88
- Hypoventilation Syndromes. https://emedicine.medscape.com/article/304381-overview
- Lesser DJ, Ward SL, Kun SS, Keens TG. Congenital hypoventilation syndromes. Semin Respir Crit Care Med. 2009 Jun. 30(3):339-47
- Eckert DJ, Jordan AS, Merchia P, Malhotra A. Central sleep apnea: Pathophysiology and treatment. Chest. 2007 Feb. 131(2):595-607.
- Balachandran JS, Masa JF, Mokhlesi B. Obesity Hypoventilation Syndrome Epidemiology and Diagnosis. Sleep Med Clin. 2014 Sep. 9(3):341-347.
- Piper AJ, Grunstein RR. Current perspectives on the obesity hypoventilation syndrome. Curr Opin Pulm Med. 2007 Nov. 13(6):490-6.
- Hypoventilation Syndromes https://emedicine.medscape.com/article/304381-overview
- Ozsancak A, D’Ambrosio C, Hill NS. Nocturnal noninvasive ventilation. Chest. 2008 May. 133(5):1275-86
- [Guideline] Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. National Guidelines Clearinghouse. 2008.
- Combs D, Shetty S, Parthasarathy S. Advances in Positive Airway Pressure Treatment Modalities for Hypoventilation Syndromes. Sleep Med Clin. 2014 Sep. 9(3):315-325.
- Ozsancak A, D’Ambrosio C, Hill NS. Nocturnal noninvasive ventilation. Chest. 2008 May. 133(5):1275-86.
- Paschoal IA, Villalba Wde O, Pereira MC. Chronic respiratory failure in patients with neuromuscular diseases: diagnosis and treatment. J Bras Pneumol. 2007 Feb. 33(1):81-92.
- Banerjee D, Yee BJ, Piper AJ, Zwillich CW, Grunstein RR. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest. 2007 Jun. 131(6):1678-84.
- Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest. 2007 Oct. 132(4):1322-36
- Lyons HA, Huang CT. Therapeutic use of progesterone in alveolar hypoventilation associated with obesity. Am J Med. 1968 Jun. 44(6):881-8
- Morrison DA, Goldman AL. Oral progesterone treatment in chronic obstructive lung disease: failure of voluntary hyperventilation to predict response. Thorax. 1986 Aug. 41(8):616-9.
- Powers MA. The obesity hypoventilation syndrome. Respir Care. 2008 Dec. 53(12):1723-30.
- Mokhlesi B, Tulaimat A. Recent advances in obesity hypoventilation syndrome. Chest. 2007 Oct. 132(4):1322-36.
- Wijesinghe M, Williams M, Perrin K, Weatherall M, Beasley R. The effect of supplemental oxygen on hypercapnia in subjects with obesity-associated hypoventilation: a randomized, crossover, clinical study. Chest. 2011 May. 139(5):1018-24
- Elphick HE, Mallory G. Oxygen therapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No.: CD003884. DOI: 10.1002/14651858.CD003884.pub4
- Bradley S, Solin P, Wilson J, et al. Hypoxemia and hypercapnia during exercise and sleep in patients with cystic fibrosis. Chest 1999;100(3):647‐54.
- Heyworth IT, Hazell ML, Linehan MF, Frank TL. How do common chronic conditions affect health‐related quality of life?. British Journal of General Practice 2009;59(568):e353‐8.
- Celli BR, Barnes PJ. Exacerbations of chronic obstructive pulmonary disease. European Respiratory Journal 2007;29(6):1224‐38.
- COPD. https://www.thoracic.org/statements/copd.php