Hyperchloremic acidosis
Hyperchloremic acidosis also called normal anion gap acidosis and less precisely non-anion gap acidosis, is an acidosis (blood pH less than 7.35) that is caused by the loss of too much sodium bicarbonate from the body, which can happen with severe diarrhea, pancreatic fistula, nasojejunal tube suctioning from the duodenum, and chronic laxative use 1. Normal human physiological pH is 7.35 to 7.45. A decrease in pH below 7.35 this range is called acidosis, an increase in this range is alkalosis. Hyperchloremic acidosis is a metabolic disease state disease state where acidosis (pH less than 7.35) with an ionic chloride increase develops, because the kidneys reabsorb chloride (Cl–) instead of reabsorbing bicarbonate (HCO3–) and the anion gap equation ([Na+] − [Cl−] − [HCO3−]) will, therefore, remain the same, or “normal.” In other words, if bicarbonate (HCO3–) drops 10 mEq/L and chloride [Cl–] raises 10 mEq/L, the sum of the anions remains the same. The real question in normal anion gap metabolic acidosis is consequently: in what circumstances will there be an exchange of bicarbonate and chloride, or in other words, when will the decrease or loss of bicarbonate be replaced by chloride to maintain electroneutrality. This exchange of bicarbonate and chloride occurs in diseases of the gastrointestinal tract (severe diarrhea) and the kidneys. Understanding the physiological pH buffering process is important. The primary pH buffer system in the human body is the HCO3– (Bicarbonate)/CO2 (carbon dioxide) chemical equilibrium system 2.
The most common cause of hyperchloremic acidosis or “normal anion gap acidosis” is severe diarrhea with gastrointestinal loss of bicarbonate and a renal tubular acidosis being a distant second 3.
Bicarbonate (HCO3–) functions as an alkalotic substance. Carbon dioxide (CO2) functions as an acidic substance. Therefore, a decrease in serum bicarbonate (HCO3–) or an increase in CO2 (carbon dioxide) will make blood more acidic. Carbon dioxide (CO2) levels are physiologically regulated by the pulmonary system through respiration, whereas the serum bicarbonate (HCO3–) levels are regulated through your kidneys with reabsorption rates. Therefore, hyperchloremic metabolic acidosis is a decrease in bicarbonate (HCO3–) levels in the blood but with an increase in ionic chloride.
Anytime a metabolic acidosis is suspected, it is extremely useful to calculate the anion gap. The calculation of the serum anion gap:
- Serum anion gap = (Na+) – [(HCO3– + Cl–)]
Where Na+ is plasma sodium concentration, HCO3– is plasma bicarbonate concentration, and Cl– is plasma chloride concentration. The anions are negatively charged ions like chloride [Cl–] and bicarbonate [HCO3–]. The anion gap is the difference between measured cations (positively charged ions like sodium [Na+] and potassium [K+]) and measured anions (negatively charged ions like chloride [Cl–] and bicarbonate [HCO3–]) 4. The most common application of the anion gap is classifying cases of metabolic acidosis, states of lower than normal blood pH. Specifically, classifying into either those that do and those that do not have unmeasured anions in the plasma. The human body is electrically neutral; therefore, in reality, does not have a true anion gap 4. A normal serum anion gap is measured to be 5 to 16 mEq/L, with autoanalyzers using an ion-selective electrode. However, the anion gap value is dependent on the type of instrument used to measure its components 5. Therefore, you should know the reference range of the analyzer used and, if known, the patient’s baseline anion gap, too.
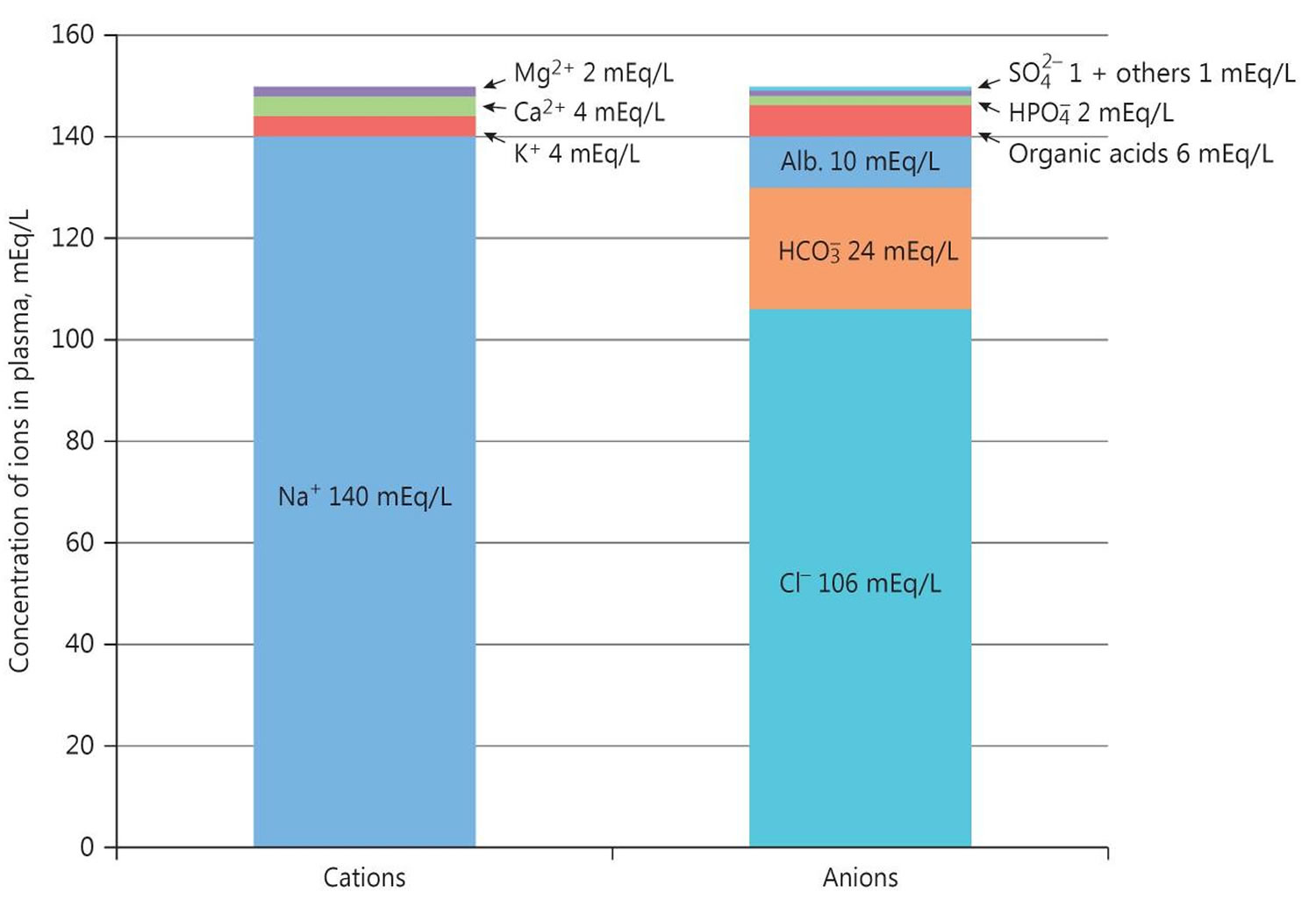
The anion gap is a calculation to determine the quantity of ionically active components within your blood that are not routinely measured. Serum anion gap is affected by the concentrations of all anions and cations which are not included in its calculations: i.e., albumin, globulin, potassium, calcium, magnesium, and organic and inorganic acids (see Figure 1). Because of the narrow extracellular concentration, most ions are omitted from the anion gap calculation. Since there are always components not directly measured, we expect this value to not equal 0. Most of this number is due to albumin (Alb); this anion is not accounted for in the anion gap formula, which is a large reason why the anion gap is not closer to zero. According to James Gamble 6, electrical neutrality in solution demands that the sum of the cations is equal to the sum of the anions (Figure 1). Sodium, chloride, bicarbonate, and albumin are quantitatively the major ions in the extracellular fluid compartment and are therefore used to calculate the anion gap 5. A true “ion gap,” however, does not exist in vivo which makes the anion gap a fundamental tool to evaluate acid-base disorders 7. Albumin is normally 4 mg/dL. Because of the large effect of albumin on anion gap, if a patient’s albumin level is abnormal, their expected anion gap will not be accurate 8. This can be corrected using simple math. The correction factor for albumin is 2.3–2.5 × [albumin], in g/dL 5. Therefore, each g/dL albumin decline will decrease the anion gap with about 2.5 mEq/L. To appreciate these facts, the anion gap formula should be: [Na+] − [Cl−] − [HCO3−] − 2.5 [albumin, in g/dL]. This equation is about zero in health, to stress the balance of ions, and also shows the relevance of albumin as a negative ion 5. As opposed to high anion gap acidosis which involves increased organic acid production, normal anion gap acidosis involves either increased production of chloride (hyperchloremic acidosis) or increased excretion of bicarbonate (HCO3–).
Figure 1. Normal anion gap levels
[Source 5 ]The management of hyperchloremic acidosis is with a multidisciplinary team that consists of a nephrologist, internist, endocrinologist, cardiologist, and a pulmonologist. Hyperchloremic metabolic acidosis can result in many symptoms and even lead to a cardiac arrest and respiratory failure. The key is to manage the primary condition causing the hyperchloremic acidosis. Patients with respiratory and cardiac symptoms may need close monitoring, and the acidosis may have to be reversed with bicarbonate. It is vital to rule out any medication causing the acidosis. For most patients, the prognosis is good as long as the primary condition is managed. Failure to manage the primary condition can lead to a high morbidity and mortality 9.
Acid-base disorders
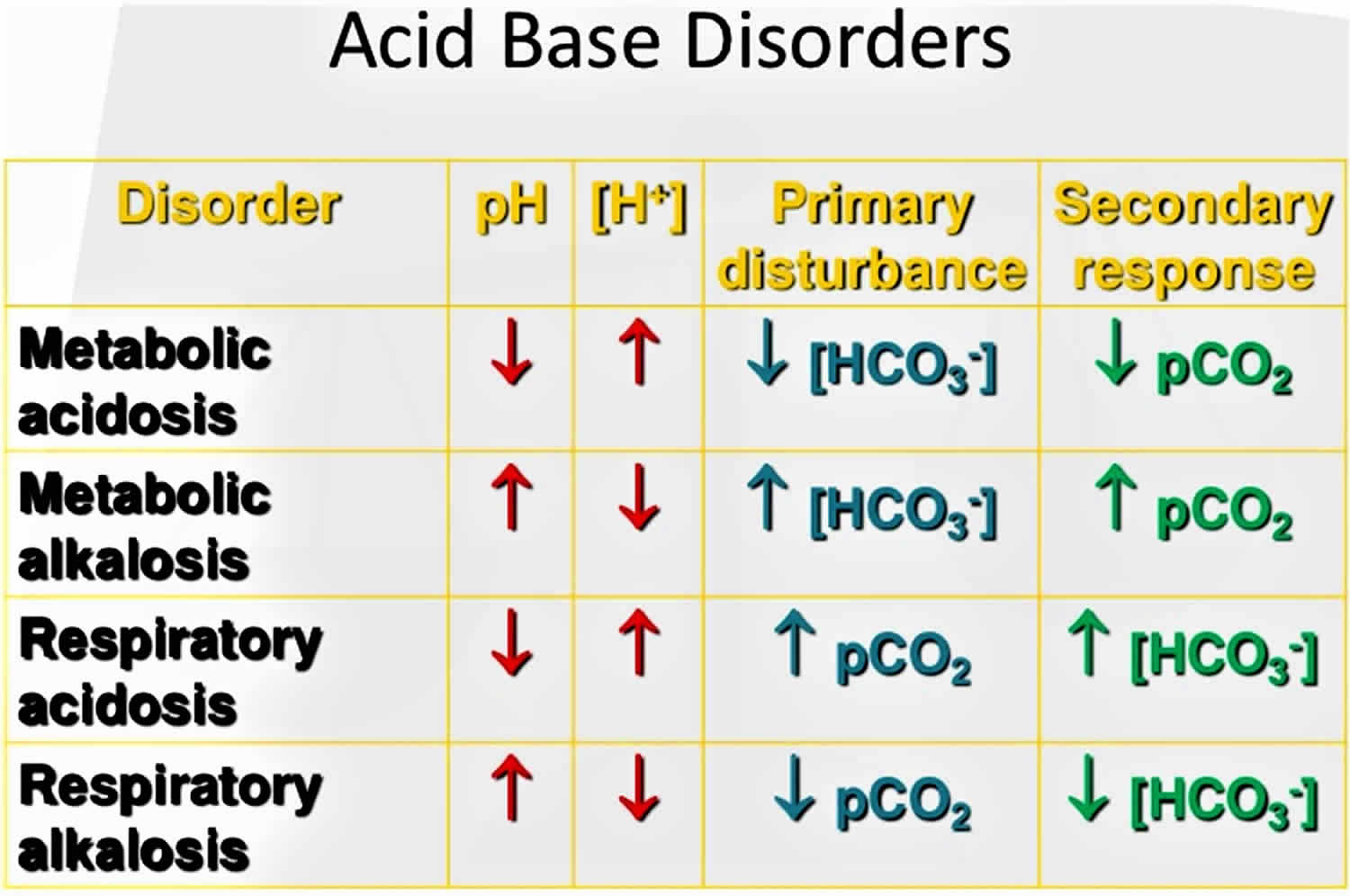
Acid-base disorders are divided into two broad categories:
- Those that affect respiration and cause changes in pH due to changes in CO2 concentration are called respiratory acidosis (low pH) and respiratory alkalosis (high pH). Respiratory acid-base disorders are commonly due to lung diseases or conditions that affect normal breathing.
- Disorders that affect metabolism and cause changes in pH due to either increased acid production or decreased base are called metabolic acidosis (low pH) and metabolic alkalosis (high pH). Metabolic acid-base disorders may be due to kidney disease, electrolyte disturbances, severe vomiting or diarrhea, ingestion of certain drugs and toxins, and diseases that affect normal metabolism (e.g., diabetes).
The human body experiences 2 main types of acidotic disorders: metabolic acidosis and respiratory acidosis. If one of these conditions occurs, the human body should induce a counterbalance in the form of an opposite condition. For example, if a person is experiencing a metabolic acidosis, their body will attempt to induce a respiratory alkalosis to compensate. It is rare for the compensation to make the pH completely normal at 7.4. When using the term acidosis (acidemia) or alkalosis (alkalemia), one is denoting that overall the pH is acidic or alkalotic, respectively. While not necessary, it can be useful to employ this terminology to distinguish between individual processes and the overall pH status of the patient since multiple imbalances can happen at the same time.
What is the difference between metabolic acidosis and respiratory acidosis?
Metabolic acidosis involves your digestive system and your urinary system. Your kidneys can’t properly filter acids from your bloodstream. Kidney disease, kidney failure, untreated diabetes, loss of bicarbonate and blood poisoning may cause a more acidic pH in your body.
Respiratory acidosis involves your respiratory system. Your lungs can’t remove enough carbon dioxide from your bloodstream. Asthma, brain injuries and excessive or disordered substance use may affect your lungs’ ability to remove carbon dioxide.
Acidosis compensation
The primary ways the body deals with excessive acidity are through renal adaptations, respiration, and buffering with calcium from bone.
It is vital for life that pH does not waiver too far from normal, and the body will always attempt to return an abnormal pH towards normal when acid-base balance is disturbed. Compensation is the name given to this life-preserving process. To understand compensation, it is important to recall that pH is governed by the ratio bicarbonate [HCO3–] (a base): arterial partial pressure of carbon dioxide (PaCO2) (an acid). So long as the ratio is normal, pH will be normal.
Normal body functions and metabolism generate large quantities of acids that must be neutralized and/or eliminated to maintain blood pH balance. Most of the acid is carbonic acid, which is created from carbon dioxide (CO2) and water (H2O). Carbon dioxide (CO2) is produced as the body uses glucose (sugar) or fat for energy. In its normal state, the body maintains carbon dioxide (arterial partial pressure of carbon dioxide [PaCO2]) in a well-controlled range from 35 to 45 mm Hg by balancing its production and elimination. Lesser quantities of lactic acid, ketoacids, and other organic acids are also produced.
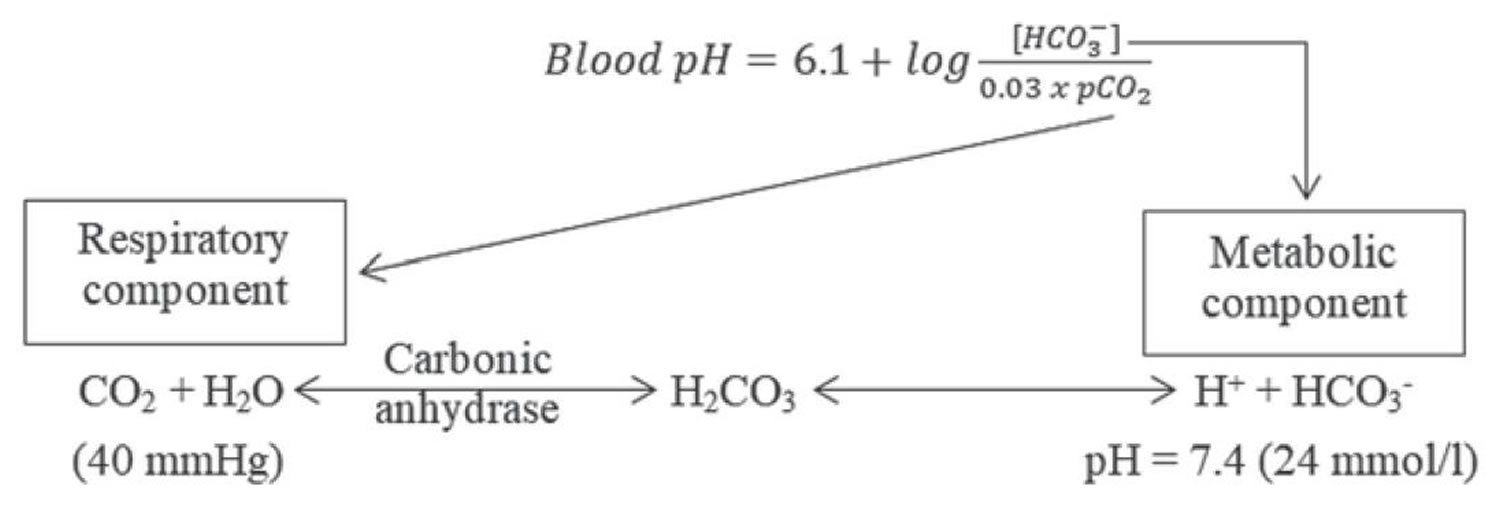
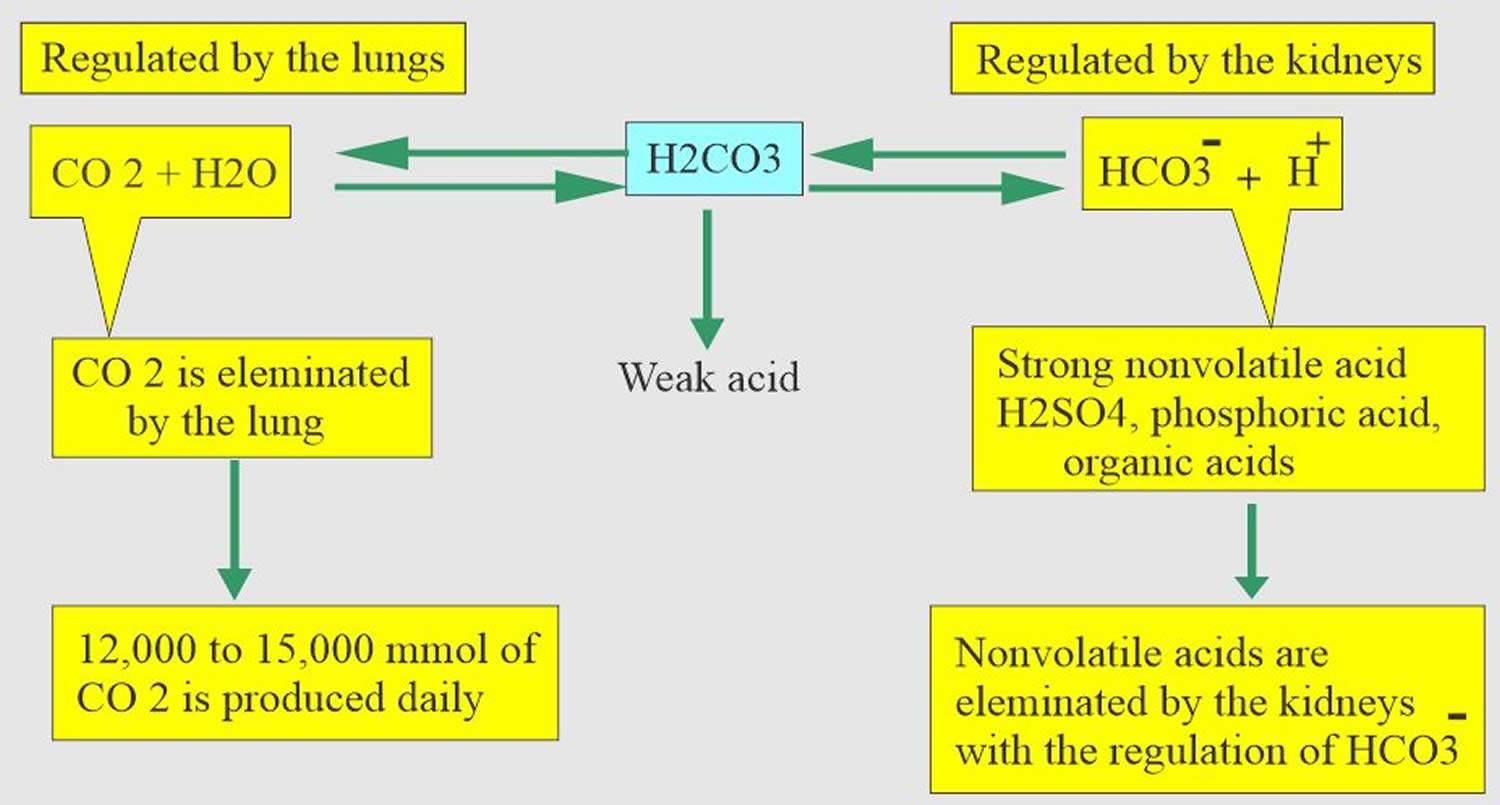
According to the Henderson-Hasselbalch equation (Figure 2), maintaining physiological pH depends on arterial partial pressure of carbon dioxide (PaCO2), which in turn depends on alveolar ventilation (hypoventilation causes acidosis and hyperventilation causes alkalosis). The kidneys participate in maintaining the stable pH by reabsorption of bicarbonate (3,600 mmol of bicarbonate is filtrated in glomeruli during 24 hour) and excretion of hydrogen ions from nonvolatile acids (including sulfur and phosphate) as titratable acidity (0.3 mmol hydrogen ions/kg/day) and in the form of ammonium ion (0.7 mmol hydrogen ions/kg/day) 10, 11.
The lungs and kidneys are the major organs involved in regulating blood pH. And to compensate for the metabolic acidosis, you increase your breathing rate (hyperventilation) to increase carbon dioxide (CO2) elimination 12, 13.
- The lungs flush acid out of your body by exhaling carbon dioxide (CO2). Raising and lowering the respiratory rate alters the amount of carbon dioxide (CO2) that is breathed out, and this can affect blood pH within minutes 14.
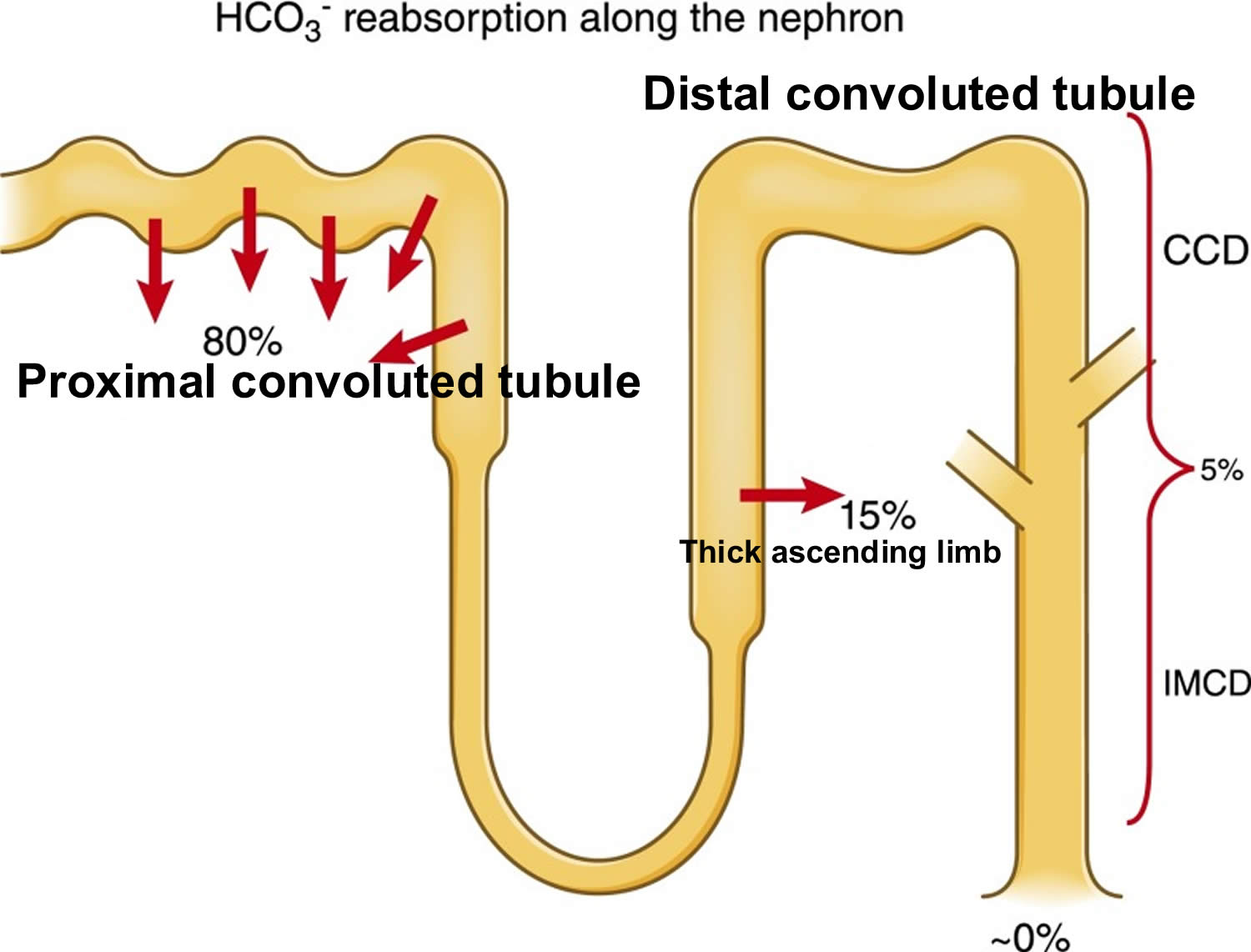
- The kidneys excrete acids in the urine, and they regulate the concentration of bicarbonate (HCO3–, a base) in blood. Acid-base changes due to increases or decreases in bicarbonate [HCO3–] concentration occur more slowly than changes in carbon dioxide (CO2), taking hours or days. Bicarbonate (HCO3–) reabsorption occurs in the kidneys in every part of the tubules. About 85–90% of the filtered bicarbonate is reabsorbed in the proximal tubules, 10% in the ascending arms of the Henle loop, 6% in the distal tubules, and 4% in the collecting tubules 10, 11.
Both of these processes are always at work, and they keep the blood pH in healthy people tightly controlled. The absolute quantities of acids or bases are less important than the balance between the two and its effect on blood pH.
Buffering systems that resist changes in pH also contribute to the regulation of acid and base concentrations. The main buffers in blood are hemoglobin (in red blood cells), plasma proteins, carbon dioxide (CO2), bicarbonate (HCO3–) and phosphates.
Carbon dioxide (CO2) plays a remarkable role in the human body mainly through pH regulation of the blood. The pH is the primary stimulus to initiate ventilation. In its normal state, the body maintains carbon dioxide (CO2) in a well-controlled range from 35 to 45 mm Hg by balancing its production and elimination. In a state of hypoventilation (breathing that is too shallow or too slow to meet the needs of the body), the body produces more carbon dioxide (CO2) than it can eliminate, causing a net retention of carbon dioxide (CO2). The increased carbon dioxide (CO2) is what leads to an increase in hydrogen ions (H+) and a slight increase in bicarbonate (HCO3–), as seen by a right shift in the following equilibrium reaction of carbon dioxide:
Carbon dioxide (CO2) + water (H2O) -> H2CO3 (carbonic acid) -> HCO3– + H+
The buffer system created by carbon dioxide consists of the following three molecules in equilibrium: carbon dioxide (CO2), H2CO3 (carbonic acid), and bicarbonate (HCO3–). When hydrogen ions (H+) is high, bicarbonate (HCO3–) buffers the low pH. When hydroxide (OH–) is high, H2CO3 (carbonic acid) buffers the high pH. In respiratory acidosis, the slight increase in bicarbonate (HCO3–) serves as a buffer for the increase in hydrogen ions (H+), which helps minimize the drop in pH. The increase in hydrogen ions inevitably causes the decrease in pH, which is the mechanism behind metabolic acidosis.
Figure 2. Henderson-Hasselbalch equation
[Source 15 ]Figure 3. Acid-base buffering system
Respiration
The pulmonary system adjusts pH using carbon dioxide (CO2); upon expiration, carbon dioxide (CO2) is projected into the environment. Due to carbon dioxide (CO2) forming carbon dioxide (CO2) in the body when combining with water (H2O), the amount of carbon dioxide (CO2) expired can cause pH to increase or decrease. When the respiratory system is utilized to compensate for metabolic pH disturbances, the effect occurs in minutes to hours 16.
Renal adaptation
The renal system affects pH by reabsorbing bicarbonate (HCO3–) and excreting fixed acids 16, 17. Whether due to pathology or necessary compensation, the kidney excretes or reabsorbs these substances which affect pH. The nephron is the functional unit of the kidney. Blood vessels called glomeruli transport substances found in the blood to the renal tubules so that some can be filtered out while others are reabsorbed into the blood and recycled. This is true for hydrogen ions and bicarbonate. If bicarbonate (HCO3–) is reabsorbed and/or acid is secreted into the urine, the pH becomes more alkaline (pH increases). When bicarbonate (HCO3–) is not reabsorbed or acid is not excreted into the urine, pH becomes more acidic (pH decreases). The metabolic compensation from the renal system takes longer to occur, days rather than minutes or hours.
The renal adaptations are extensive 18:
- Increased urinary excretion of sulfate, phosphate, urate, and chloride;
- Increased urinary excretion of calcium;
- Decreased urinary excretion of citrate;
- Increased urinary excretion of ammonium ions; and
- Kidney vasodilatation and increased glomerular filtration rate.
The kidneys mitigate but do not eliminate all the excess acidity. As the kidneys lose function with aging (when GFR is lower than 30 mL/min/1.73 m²), their ability to excrete acid becomes impaired, which may be another explanation for the loss of bone with aging 19. In fact, counteracting metabolic acidosis helps to preserve muscle mass and to improve bone metabolism 20, 21, 22.
Figure 4. Kidneys control of plasma bicarbonate (HCO3–)
Abbreviations: CCD = cortical collecting duct; IMCD = inner medullary collecting duct
[Source 11 ]Bone for acid buffering
The major reservoir of base is the skeleton (in the form of alkaline salts of calcium), which provides the buffer needed to maintain blood pH and plasma bicarbonate concentrations when renal and respiratory adaptations are inadequate. Acid-promoting diets are associated with increased urinary excretion of both calcium and bone matrix protein and decreased bone density 23. Neutralizing acid intake with diet or alkalinizing supplements decreases urine Calcium and bone matrix protein excretion. Also, to a much smaller degree, skeletal muscle can act as a buffer.
Other buffer systems
Other buffer systems in the human body include the phosphate buffer system, proteins, and hemoglobin. All of these contain bases which accept hydrogen ions which keep the pH from plummeting. The phosphate buffer system, while present globally, is important for the regulation of urine pH. Proteins assist with intracellular pH regulation. Red blood cells use the reaction above to help hemoglobin buffer; carbon dioxide can diffuse across red blood cells and combine with water. This alone would cause an increase in hydrogen ions; however, hemoglobin can bind hydrogen ions. Hemoglobin also can bind carbon dioxide without this reaction. This depends on the amount of oxygen that is bound to hemoglobin. This is called the Haldane effect and the Bohr effect. When hemoglobin is saturated with oxygen, it has a lower affinity for carbon dioxide (CO2) and hydrogen ions and is able to release it.
Hyperchloremic metabolic acidosis causes
Hyperchloremic metabolic acidosis is a pathological state that results from bicarbonate loss, rather than acid production or retention. Bicarbonate (HCO3) loss leading to hyperchloremic metabolic acidosis occurs in a variety of ways: gastrointestinal (GI) causes, renal causes, and exogenous causes. Gastrointestinal loss of bicarbonate occurs through severe diarrhea, pancreatic fistula, nasojejunal tube suctioning from the duodenum, and chronic laxative use 24. Kidney sources of hyperchloremic acidosis include proximal renal tubular acidosis, distal renal tubular acidosis, and long-term use of carbonic anhydrase inhibitors 24. Exogenous causes include ingestion of acids such as ammonium chloride and hydrochloric acid and volume resuscitation with 0.9% normal saline 25.
Gastrointestinal causes
Normally, there is a degree of bicarbonate secreted into the intestinal lumen to allow for neutralization of the acidic environment of food from gastric emptying. Over the distance of the small intestines, this bicarbonate is reabsorbed as bile. However, in pathologies with profuse watery diarrhea, bicarbonate within the intestines is lost through the stool due to increased motility of the gut. This leads to further secretion of bicarbonate from the pancreas and intestinal mucosa leading to a net acidification of the blood from bicarbonate loss. Likewise, pancreatic fistula leads to excessive bicarbonate secretion from the pancreas into the intestines. This excess bicarbonate is ultimately lost in stools. Nasojejunal suctioning removes bicarbonate from the duodenal or jejunal space via direct suctioning of the luminal contents.The overarching theme with these pathologies is loss of bicarbonate from the gastrointestinal spaces which leads to an acidotic state in the blood via unopposed hydrogen in the buffering system as above.
Renal causes
Distal renal tubular acidosis (type 1 renal tubular acidosis) is a failure of the distal nephron to secrete hydrogen appropriately into urine. This results in alkalotic urine and acidosis of the blood. Failure to secrete hydrogen directly correlates with the ammonium (NH4) levels in urine and is able to be deduced via a positive urine anion gap as above. Proximal renal tubular acidosis (type 2 renal tubular acidosis) is a pathology where bicarbonate is failed to be reabsorbed appropriately. This leads to loss of bicarbonate into the urine. The net result is acidosis of blood and alkalotic urine. Both types of renal tubular acidosis are associated with hypokalemia. Carbonic anhydrase inhibitors such as acetazolamide create a medically induced type 2 proximal renal tubular acidosis scenario by inhibiting bicarbonate reabsorption in the proximal nephron.
Exogenous causes
Many of the exogenous causes of hyperchloremic acidosis as logical evaluations. When substances such as ammonium chloride and hydrochloric acid are supplemented into the body, they react with bicarbonate in an attempt to buffer the pH. However, this will deplete bicarbonate stores leading to an acidotic state. Large volume resuscitation with 0.9% normal saline leads to an overload of chloride ions into the blood. As stated previously, chloride and bicarbonate work together to maintain an ionic balance of the cellular space. Hyperchlorhydria forces bicarbonate to move intracellularly to maintain ionic equilibrium, thus reducing the available bicarbonate for the pH buffering system leading to net acidosis.
Hyperchloremic acidosis symptoms
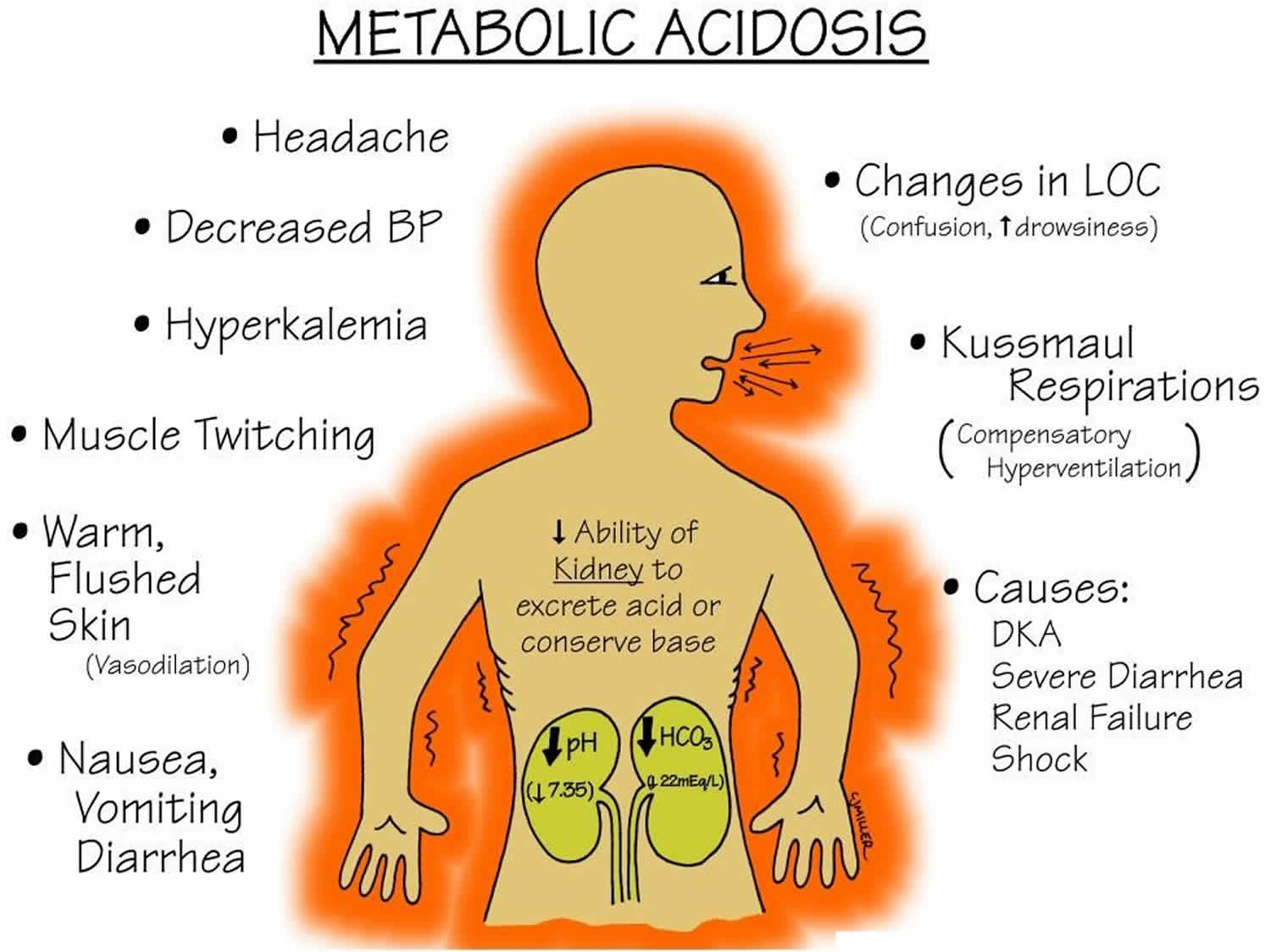
Patients with hyperchloremic acidosis have no effects due to the hyperchloremia necessarily. Hyperchloremic acidosis symptoms depend on the underlying disease or condition. Hyperchloremic metabolic acidosis itself causes rapid breathing. It is expected to see an increase in respiratory rate as the body attempts to decrease CO2 in compensation, however, in long-standing disease this may lead to muscle fatigue and respiratory failure. A headache, confusion, lack of energy, nausea, and vomiting are common complaints, however as acidosis worsens stupor, coma, myocardial instability or cardiac arrest may occur leading to shock or death.
A physical exam may show altered mental status, tachycardia, tachypnea, accessory muscle use with respiration, neurological deficits, muscular weakness, cardiac arrhythmias, cardiac murmurs, respiratory wheezing, rales, or rhonchi.
Hyperchloremic acidosis complications
If hyperchloremic acidosis is not corrected, acidemia can cause arrhythmias which can prove fatal and increase mortality. pH less than 7.2 can impair myocardial contraction and increase the risk of ventricular fibrillation and heart failure. Tachypnea results in compensation of acidosis can cause respiratory muscle fatigue and if not corrected promptly can lead to respiratory arrest.
Hyperchloremic acidosis diagnosis
Your doctor will perform a physical examination and ask about your symptoms.
These tests can help diagnose acidosis. They can also determine whether the cause is a breathing problem (respiratory acidosis) or a metabolic problem (metabolic acidosis). Tests may include:
- Arterial blood gas (ABG)
- Basic metabolic panel, (a group of blood tests that measure your sodium, potassium, and chloride levels, kidney function, and other chemicals and functions)
- Urine pH
- Urine ketones or blood ketones
- Urinary anion gap is an essential measurement in hyperchloremic acidosis to establish the urine ammonium excretion status as discussed above. Distal renal tubular acidosis will have urinary pH greater than 5.3 and a positive urinary anion gap. In proximal renal tubular acidosis, urinary pH is usually less than 5.3, and urinary anion gap is variable 26.
- Lactic acid test
- A complete blood count (CBC) to evaluate for an infectious cause with elevated white blood count and fluid body status with hemoglobin and hematocrit values is useful.
Other tests that may be needed to determine the cause of the acidosis include:
- Pulmonary function test to measure breathing and how well the lungs are functioning
- Chest x-ray
- CT abdomen
The human body is very good at remaining balanced ionically under most scenarios. As a result, with loss of the negatively charged ion bicarbonate, negatively charged chloride ion is displaced to the extracellular space. This leads to a narrow anion gap, electrically neutral state without correcting the pathology that induced the acidosis. Likewise, increased Cl (chloride) may displace bicarbonate intracellularly. Determining exact cause of a narrow anion gap hyperchloremic acidosis requires another test, the urine anion gap. Urine anion gap is calculated:
The following is the equation for urine anion gap (UAG) where Na is sodium, K is potassium, and Cl is chloride:
- Urine anion gap = Urine Na + Urine K – Urine Cl
The urine anion gap provides an estimate of urinary ammonium (NH4+) excretion. The renal system attempts to ameliorate the effects of pathological metabolic acidosis by excreting ammonium (NH4+) into the urine. A urine anion gap between 20 to 90 mEq/L denotes low or normal ammonium (NH4+) secretion. One between minus 20 mEq/L (-20 mEq/L) and minus 50 mEq/L (-50 mEq/L) suggests the main cause of the metabolic acidosis is prolonged severe diarrhea. A urine anion gap approaching 0 is indeterminate 16.
Another important formula to use with metabolic acidosis is the Winter formula. This equation provides the clinician with the expected arterial partial pressure of carbon dioxide (PaCO2) value. This is important because there could be another acid-base disorder present.
The Winter formula is:
- Expected arterial partial pressure of carbon dioxide (PaCO2)= (1.5 X HCO3–) + 8 +/- 2
If the arterial partial pressure of carbon dioxide (PaCO2) value is within range of the expected arterial partial pressure of carbon dioxide (PaCO2), there is no mixed disorder, just respiratory compensation. When the value is lower or higher than expected, there is a mixed disorder; lower would mean a respiratory alkalosis and higher a respiratory acidosis. A shortcut for the Winter formula is that the last two digits of the pH +/- 2 is about equal to the expected arterial partial pressure of carbon dioxide (PaCO2) 5, 1.
Arterial blood gas (ABG) analysis
Arterial blood gas (ABG) sampling, is a test often performed in an inpatient setting to assess the acid-base status of a patient. A needle is used to draw blood from an artery, often the radial artery, and the blood is analyzed to determine parameters such as the pH, arterial partial pressure of carbon dioxide (PaCO2), arterial partial pressure of oxygen (PaO2), bicarbonate (HCO3–), oxygen saturation (O2 Sat) and more. This allows the physician to understand the status of the patient better. ABGs are especially important in the critically ill. They are the main tool utilized in adjusting to the needs of a patient on a ventilator.
- Arterial partial pressure of carbon dioxide (PaCO2) as carbon dioxide tension, this measures the level of carbon dioxide in your blood.
- Arterial partial pressure of oxygen (PaO2) also known as oxygen tension, this measures how well oxygen is being transferred into your blood.
- Oxygen saturation (O2 Sat) is an assessment of the amount of oxygen in your blood that is based on measuring levels of hemoglobin. Hemoglobin is a protein found inside red blood cells that is responsible for carrying oxygen throughout the body.
- Bicarbonate (HCO3–) concentration: Bicarbonate (HCO3–) is an electrolyte, which is a type of mineral involved in managing your body’s acid-base balance. Most of the carbon dioxide (CO2) in your blood is stored in the form of bicarbonate, so this measurement helps reflect carbon dioxide (CO2) levels.
- Although not universal, some arterial blood gases tests include measurements of hemoglobin as well as altered forms of the hemoglobin protein. Examples of these potential additional measurements include:
- Methemoglobin: Methemoglobin is a form of hemoglobin that has been oxidized, changing its heme iron configuration from the ferrous (Fe2+) to the ferric (Fe3+) state. Unlike normal hemoglobin, methemoglobin does not bind oxygen and as a result cannot deliver oxygen to the tissues.
- Carboxyhemoglobin: Carboxyhemoglobin is a stable complex of carbon monoxide and hemoglobin that forms in red blood cells upon contact with carbon monoxide. This abnormal form of hemoglobin attaches to carbon monoxide and can interfere with oxygen’s ability to travel in the blood.
- Oxyhemoglobin: Oxyhemoglobin represents the fraction of oxygenated hemoglobin in relation to the total hemoglobin present, including non-oxygen-binding hemoglobins. In healthy individuals, oxyhemoglobin and oxygen saturation are approximately equal.
- Deoxyhemoglobin: This is the form of hemoglobin without oxygen in the blood.
The following are the most important Normal Values on an ABG:
- pH = 7.35 to 7.45
- Arterial partial pressure of carbon dioxide (PaCO2) = 35 to 45 mmHg
- Arterial partial pressure of oxygen (PaO2) = 75 to 100 mmHg
- Bicarbonate (HCO3–) = 22 to 26 mEq/L
- O2 Sat = greater than 95%
The ability to quickly and efficiently read an ABG is paramount to quality patient care.
- Look at the pH. Decide whether it is acidotic, alkalotic, or within the physiological range
- Arterial partial pressure of carbon dioxide (PaCO2) level determines respiratory contribution; a high level means the respiratory system is lowering the pH and vice versa.
- Bicarbonate (HCO3–) level denotes metabolic/kidney effect. An elevated bicarbonate (HCO3–) is raising the pH and vice versa.
- If the pH is acidotic, look for the number that corresponds with a lower pH. If it is a respiratory acidosis, the carbon dioxide (CO2) should be high. If the patient is compensating metabolically, the bicarbonate (HCO3–) should be high as well. A metabolic acidosis will be depicted with an bicarbonate (HCO3–) that is low.
- If the pH is alkalotic, again, determine which value is causing this. A respiratory alkalosis will mean the carbon dioxide (CO2) is low; a metabolic alkalosis should lend an bicarbonate (HCO3–) that is high. Compensation with either system will be reflected oppositely; for a respiratory alkalosis the metabolic response should be a low bicarbonate (HCO3–) and for metabolic alkalosis, the respiratory response should be a high carbon dioxide (CO2).
- If the pH level is in the physiological range but the arterial partial pressure of carbon dioxide (PaCO2) and/or bicarbonate (HCO3–) are not within normal limits, there is likely a mixed disorder. Also, compensation does not always occur; this is when clinical information becomes paramount.
- Sometimes it is difficult to ascertain whether a patient has a mixed disorder.
Other tests that are important to perform when analyzing the acid-base status of a patient include those that measure electrolyte levels and renal function. This helps the clinician gather information that can be used to determine the exact mechanism of the acid-base imbalance as well as the factors contributing to the disorders 27, 28.
Urinalysis
Urine pH is normally acidic, at less than 5.0. In acidemia, the urine normally becomes more acidic. If the urine pH is above 5.5 in the face of acidemia, this finding is consistent with a type 1 renal tubular acidosis (RTA). Alkaline urine is typical in salicylate toxicity.
Patients with ethylene glycol toxicity may present with calcium oxalate crystals, which appear needle shaped, in the urine.
Urine Anion Gap
Calculating the urine anion gap is helpful in evaluating some cases of non-anion gap metabolic acidosis. The major measured urinary cations are Na+ and K+, and the major measured urinary anion is Cl-:
- Urine anion gap = Urine Na + Urine K – Urine Cl
In the face of metabolic acidosis, the kidneys increase the amount of NH3 synthesized to buffer the excess H+ and NH4 Cl excretion increases. The increased unmeasured ammonium (NH4+) thus increases the measured anion Cl- in the urine, and the net effect is a negative anion gap, representing a normal response to systemic acidification. The finding of a positive urine anion gap in the face of non-anion gap metabolic acidosis points toward a renal acidification defect (eg, renal tubular acidosis) 29.
Ketone level
Elevations of ketones indicate diabetic, alcoholic, and starvation ketoacidosis 30.
The nitroprusside test is used to detect the presence of ketoacids in the blood and the urine. This test measures only acetoacetate and acetone; therefore, it may underestimate the degree of ketonemia and ketonuria, because it will not detect the presence of beta-hydroxybutyrate. This limitation of the test can be especially problematic in patients with ketoacidosis who cannot convert beta-hydroxybutyrate to acetoacetate because of severe shock or liver failure.
An assay for beta-hydroxybutyrate is unavailable in some hospitals. An indirect method to circumvent this problem is to add a few drops of hydrogen peroxide to a urine specimen. This enzymatically will convert beta-hydroxybutyrate into acetoacetate, which will be detected by the nitroprusside test.
Serum Lactate level
The normal plasma lactate concentration is 0.5-1.5 mEq/L. Lactic acidosis is considered present if the plasma lactate level exceeds 4-5 mEq/L in an acidemic patient.
Most cases of lactic acidosis are due to tissue hypoxia (eg, from shock). Less commonly, underlying disease (eg, diabetic ketoacidosis), drugs, or toxins may be the cause 31.
Salicylate levels and Iron levels
Therapeutic salicylate levels range up to 20-35 mg/dL. Plasma levels exceeding 40-50 mg/dL are in the toxic range.
Plasma levels provide some information as to the severity of intoxication: 40-60 mg/dL is considered mild; 60-100 mg/dL is moderate; and greater than 100 mg/dL is considered severe.
Iron toxicity is associated with lactic acidosis. Iron levels greater than 300 mg/dL are considered toxic.
Special tests
Measuring the transtubular potassium gradient (TTKG) is useful in determining the cause of hyperkalemia or hypokalemia associated with metabolic acidosis.
- Transtubular Potassium Gradient (TTKG) = urine K+ × serum osmolality/serum K+ × urine osmolality
A transtubular potassium gradient (TTKG) of greater than 8 indicates that aldosterone is present and that the collecting duct is responsive to it. A transtubular potassium gradient (TTKG) of less than 5 in the presence of hyperkalemia indicates aldosterone deficiency or resistance. For the test to be interpretable, the urine Na+ level should be greater than 10 mEq/L and the urine osmolality should be greater than or equal to serum osmolality.
Plasma renin activity and plasma aldosterone levels are useful in determining the cause of the hyperkalemia and hypokalemia that accompany metabolic acidosis.
Calculation of fractional excretion of bicarbonate (FEHCO3–) is useful in the diagnosis of proximal renal tubular acidosis (RTA).
The ammonium chloride (NH4Cl) loading test is useful in patients with nephrocalcinosis and/or nephrolithiasis, who may have an incomplete form of distal renal tubular acidosis. These patients may not have a pH less than 7.35 or a drop in serum bicarbonate (HCO3–); metabolic acidosis can be induced by administration of NH4Cl (0.1 g/kg for 3 days). Under these circumstances of induced acidemia, a urine pH greater than 5.3 indicates distal renal tubular acidosis (RTA).
An alternative to the ammonium chloride (NH4Cl) loading test involves the simultaneous oral administration of furosemide to increase distal Na+ delivery and fludrocortisone to increase collecting duct Na+ absorption and proton secretion 32. Under these circumstances, a urine pH greater than 5.3 indicates distal renal tubular acidosis (RTA).
Measuring the urine-blood arterial partial pressure of carbon dioxide (PaCO2) gradient following an bicarbonate (HCO3–) load is useful in some patients with classic distal renal tubular acidosis to differentiate a permeability defect from other defects. This test is useful in patients with nephrocalcinosis in whom distal renal tubular acidosis (RTA) is suspected but urine is acidified appropriately in the face of metabolic acidosis. Some of these patients have a rate-dependent defect in proton secretion, revealed by a low urine-blood PaCO2 gradient following bicarbonate (HCO3–) loading.
Abdominal radiographs (eg, kidneys, ureters, bladder), CT scans, and/or renal ultrasound images may show renal stones or nephrocalcinosis in patients with distal renal tubular acidosis.
Hyperchloremic metabolic acidosis treatment
In every case of hyperchloremic acidosis, the primary treatment is aimed at identifying and treating the inciting event of pathology. If respiratory fatigue and failure occur, these patients will need to be intubated and placed on mechanical ventilation. Hyperventilation of the patient on ventilator control can help reduce the acid load. In gastrointestinal causes, it is essential to administer intravenous (IV) saline to maintain fluid load as patients will easily dehydrate from diarrhea or suctioning of the intestines. Additionally, electrolytes need to be monitored and replenished as applicable. Of specific importance is the potassium level. The acidosis is moderated by supplementing bicarbonate into the saline fluids until the underlying pathology is repaired. In renal tubular acidosis, large quantities of bicarbonate administration may be necessary. If fluid overload is a concern, diuretics with supplemental potassium may be administered for some effect. If the acidosis is resistant to therapy, it may be necessary to utilize dialysis therapy.
As always, a variety of medications are known to induce hyperchloremic acidosis and should be avoided or used with caution. Gastrointestinal bicarbonate loss is known to occur with calcium chloride, magnesium sulfate, and cholestyramine use. Proximal renal tubular acidosis is associated with streptozotocin, lead, mercury, arginine, valproic acid, gentamicin, ifosfamide, and outdated tetracycline usage. Distal renal tubular acidosis is associated with amphotericin B, toluene, nonsteroidal anti-inflammatory drugs, and lithium use.
Administration of an alkali is the mainstay of treatment for type 1 renal tubular acidosis. Adult patients should be given the amount required to buffer the daily acid load from the diet. This is usually approximately 1-3 mEq/kg/d and can be administered in any form, although the preferred form is as potassium citrate. Correction of acidosis usually corrects the hypokalemia, but K+ supplements may be necessary.
Patients with type 2 renal tubular acidosis typically have hypokalemia and increased urinary K+ wasting. Administration of alkali in those patients leads to more bicarbonate (HCO3–) wasting and can worsen hypokalemia unless K+ is replaced simultaneously. Correcting this form of acidosis with alkali is difficult because a substantial proportion of the administered bicarbonate (HCO3–) is excreted in the urine, and large amounts are needed to correct the acidosis (10-30 mEq/kg/day). Potassium is also required when administering bicarbonate (HCO3–). Correction is essential in children for normal growth, while in adults aggressive correction to a normal level may not be required. Thiazide diuretics can be administered to induce diuresis and mild volume depletion, which, in turn, raises the proximal tubule threshold for bicarbonate (HCO3–) wasting.
Because hyperkalemia is central to the cause of type 4 renal tubular acidosis 33, major treatment goal is to lower the serum K+ level. This can be achieved by placing the patient on a low-potassium diet (1 mEq/kg K+/day) and by withdrawal of drugs that can cause hyperkalemia (eg, angiotensin-converting enzyme [ACE] inhibitors, nonsteroidal anti-inflammatory drugs). Loop diuretics can be helpful in reducing serum potassium levels as long as the patient is not hypovolemic. In resistant cases, fludrocortisone, a synthetic mineralocorticoid, can be used to increase K+ secretion, but this may increase Na+ retention. Alkali therapy is not usually required, because, in many patients, the mild degree of acidosis is corrected by achieving normokalemia. Hyperkalemia and acidosis worsen as kidney function declines further; eventually, the patient develops a high anion gap renal acidosis. Renal replacement therapy should be considered once the measures described fail to control hyperkalemia or acidosis.
Hyperchloremic metabolic acidosis prognosis
The prognosis of hypochloremic metabolic acidosis depends on the underlying cause of the condition. When the underlying cause is well managed, the prognosis is usually very good 1. Hyperchloremic metabolic acidosis can be detrimental in old age and those who already have a cardiopulmonary compromise.
References- Sharma S, Hashmi MF, Aggarwal S. Hyperchloremic Acidosis. [Updated 2022 Aug 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482340
- Farrokh S, Cho SM, Suarez JI. Fluids and hyperosmolar agents in neurocritical care: an update. Curr Opin Crit Care. 2019 Apr;25(2):105-109.
- Metabolic Acidosis. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/acid-base-regulation-and-disorders/metabolic-acidosis#sec12-ch157-ch157c-1035
- Pandey DG, Sharma S. Biochemistry, Anion Gap. [Updated 2019 Apr 6]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK539757
- Berend K. Review of the Diagnostic Evaluation of Normal Anion Gap Metabolic Acidosis. Kidney Dis (Basel). 2017 Dec;3(4):149-159. doi: 10.1159/000479279
- Gamble JL. Extracellular fluid and its maintenance. N Engl J Med. 1936;250:1150–1152.
- Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2015 Jan 8;372(2):195. doi: 10.1056/NEJMc1413880
- Hopkins E, Sanvictores T, Sharma S. Physiology, Acid Base Balance. [Updated 2022 Sep 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507807
- Huang L, Zhou X, Yu H. Balanced crystalloids vs 0.9% saline for adult patients undergoing non-renal surgery: A meta-analysis. Int J Surg. 2018 Mar;51:1-9.
- Koeppen BM. The kidney and acid-base regulation. Adv Physiol Educ. 2009 Dec;33(4):275-81. doi: 10.1152/advan.00054.2009
- Hamm LL, Nakhoul N, Hering-Smith KS. Acid-Base Homeostasis. Clin J Am Soc Nephrol. 2015 Dec 7;10(12):2232-42. doi: 10.2215/CJN.07400715
- Kisaka T, Cox TA, Dumitrescu D, Wasserman K. CO2 pulse and acid-base status during increasing work rate exercise in health and disease. Respir Physiol Neurobiol. 2015 Nov;218:46-56. doi: 10.1016/j.resp.2015.07.005
- Brinkman JE, Toro F, Sharma S. Physiology, Respiratory Drive. [Updated 2022 Jun 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482414
- Brinkman JE, Sharma S. Respiratory Alkalosis. [Updated 2022 Jul 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482117
- Adamczak M, Surma S. Metabolic Acidosis in Patients with CKD: Epidemiology, Pathogenesis, and Treatment. Kidney Dis (Basel). 2021 Jun 4;7(6):452-467. doi: 10.1159/000516371
- Hopkins E, Sharma S. Physiology, Acid Base Balance. [Updated 2018 Oct 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2018 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK507807
- Wesson DE, Nathan T, Rose T, Simoni J, Tran RM. Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int. 2007 Feb;71(3):210-7. doi: 10.1038/sj.ki.5002036
- Acidosis: An Old Idea Validated by New Research. Integr Med (Encinitas). 2015;14(1):8-12. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4566456/
- Frassetto LA, Morris RC, Jr, Sebastian A. Effect of age on blood acid-base composition in adult humans: role of age-related renal functional decline. Am J Physiol. 1996;271(6 Pt 2):F1114–F1122
- Kopple J.D., Kalantar-Zadeh K., Mehrotra R. Risks of chronic metabolic acidosis in patients with chronic kidney disease. Kidney Int. 2005;67:S21–S27. doi: 10.1111/j.1523-1755.2005.09503.x
- Dubey A.K., Sahoo J., Vairappan B., Haridasan S., Parameswaran S., Priyamvada P.S. Correction of metabolic acidosis improves muscle mass and renal function in chronic kidney disease stages 3 and 4: A randomized controlled trial. Nephrol. Dial. Transplant. 2020;35:121–129. doi: 10.1093/ndt/gfy214
- Noce A., Marrone G., Ottaviani E., Guerriero C., Di Daniele F., Pietroboni Zaitseva A., Di Daniele N. Uremic sarcopenia and its possible nutritional approach. Nutrients. 2021;13:147. doi: 10.3390/nu13010147
- Diet acids and alkalis influence calcium retention in bone. Buclin T, Cosma M, Appenzeller M, Jacquet AF, Décosterd LA, Biollaz J, Burckhardt P. Osteoporos Int. 2001; 12(6):493-9.
- Sharma S, Aggarwal S. Hyperchloremic Acidosis. [Updated 2019 Jun 22]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482340
- Alexander RT, Bitzan M. Renal Tubular Acidosis. Pediatr. Clin. North Am. 2019 Feb;66(1):135-157.
- Assimos DG. Re: Incomplete Distal Renal Tubular Acidosis and Kidney Stones. J. Urol. 2019 Mar;201(3):436-437.
- Patel S, Sharma S. Respiratory Acidosis. [Updated 2022 Jun 21]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482430
- Rajkumar P, Pluznick JL. Acid-base regulation in the renal proximal tubules: using novel pH sensors to maintain homeostasis. Am J Physiol Renal Physiol. 2018 Nov 1;315(5):F1187-F1190. doi: 10.1152/ajprenal.00185.2018
- Pereira PC, Miranda DM, Oliveira EA, Silva AC. Molecular pathophysiology of renal tubular acidosis. Curr Genomics. 2009 Mar;10(1):51-9. doi: 10.2174/138920209787581262
- Metabolic Acidosis Workup. https://emedicine.medscape.com/article/242975-workup#c12
- Seheult J, Fitzpatrick G, Boran G. Lactic acidosis: an update. Clin Chem Lab Med. 2017 Mar 1;55(3):322-333. doi: 10.1515/cclm-2016-0438
- Walsh SB, Shirley DG, Wrong OM, Unwin RJ. Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kidney Int. 2007 Jun;71(12):1310-6. doi: 10.1038/sj.ki.5002220
- Harris AN, Grimm PR, Lee HW, Delpire E, Fang L, Verlander JW, Welling PA, Weiner ID. Mechanism of Hyperkalemia-Induced Metabolic Acidosis. J Am Soc Nephrol. 2018 May;29(5):1411-1425. doi: 10.1681/ASN.2017111163