Hypohidrotic ectodermal dysplasia
Hypohidrotic ectodermal dysplasia is a rare inherited multisystem disorder that belongs to the group of diseases known as ectodermal dysplasias. Hypohidrotic ectodermal dysplasia is one of more than 100 types of ectodermal dysplasia. Starting before birth, ectodermal dysplasias result in the abnormal development of ectodermal tissues, particularly the skin, hair, nails, teeth, and sweat glands. Hypohidrotic ectodermal dysplasia is the most common form of ectodermal dysplasia. Hypohidrotic ectodermal dysplasia is estimated to occur in 1 in 20,000 newborns worldwide 1. Although some symptoms and findings associated with hypohidrotic ectodermal dysplasia are present shortly after birth such as heat intolerance, unexplained fever, and/or extensive peeling of the skin, the characteristic facial abnormalities may not be apparent in affected infants. Therefore, hypohidrotic ectodermal dysplasia often is not recognized in affected infants and children until associated dental and hair abnormalities become apparent.
Most people with hypohidrotic ectodermal dysplasia have a reduced ability to sweat (hypohidrosis) because they have fewer sweat glands than normal or their sweat glands do not function properly. Sweating is a major way that the body controls its temperature; as sweat evaporates from the skin, it cools the body. Reduced sweating can lead to a dangerously high body temperature (hyperthermia), particularly in hot weather. In some cases, hyperthermia can cause life-threatening health problems.
Affected individuals tend to have sparse scalp and body hair (hypotrichosis). The hair is often light-colored, brittle, and slow-growing. Hypohidrotic ectodermal dysplasia is also characterized by several missing teeth (hypodontia) or teeth that are malformed. The teeth that are present erupt from the gums later than usual and are frequently small and pointed.
Some people with hypohidrotic ectodermal dysplasia have distinctive facial features, including a prominent forehead, thick lips, and a flattened bridge of the nose. Additional features of this condition can include thin, wrinkled, and dark-colored skin around the eyes; chronic skin problems such as eczema; and a bad-smelling discharge from the nostrils (ozena).
Intellectual ability and growth are typically normal in people with hypohidrotic ectodermal dysplasia.
Hypohidrotic ectodermal dysplasia is caused by mutations in the EDA, EDAR, or EDARADD genes. It may be inherited in an X-linked recessive, autosomal recessive, or autosomal dominant manner depending on the genetic cause of the condition. The X-linked form is the most common form. X-linked hypohidrotic ectodermal dysplasia is a rare disorder that is fully expressed in males only. It is suspected to affect approximately 90 percent of affected individuals are male. However, females who carry a single copy of the disease gene (heterozygote carriers) may exhibit milder symptoms associated with the disorder. In those rare cases when hypohidrotic ectodermal dysplasia is inherited as an autosomal recessive genetic trait, males and females are affected in equal numbers. The forms have similar signs and symptoms, however the the autosomal dominant form tends to be the mildest.
Classic hypohidrotic ectodermal dysplasia can be diagnosed after infancy on the basis of physical features in most affected individuals. Identification of a hemizygous EDA pathogenic variant in an affected male or biallelic EDAR, EDARADD, or WNT10A pathogenic variants in an affected male or female confirms the diagnosis.
Treatment of hypohidrotic ectodermal dysplasia may include special hair care formulas or wigs, measures to prevent overheating, removal of ear and nose concretions, and dental evaluations and treatment (e.g., restorations, dental implants, or dentures) 2. Access to an adequate water supply and a cool environment during hot weather. Early dental treatment; bonding of conical shaped teeth; orthodontics as necessary; dental implants in the anterior portion of the mandibular arch in older children; replacement of dental prostheses as needed, often every 2.5 years; dental implants in adults; therapeutics to maintain oral lubrication and control caries; dietary counseling for individuals with chewing and swallowing difficulties. Nasal and aural concretions may be removed with suction devices or forceps as needed by an otolaryngologist. Prevention of nasal concretions through humidification of ambient air is helpful. Skin care products for eczema and exposures that exacerbate dry skin.
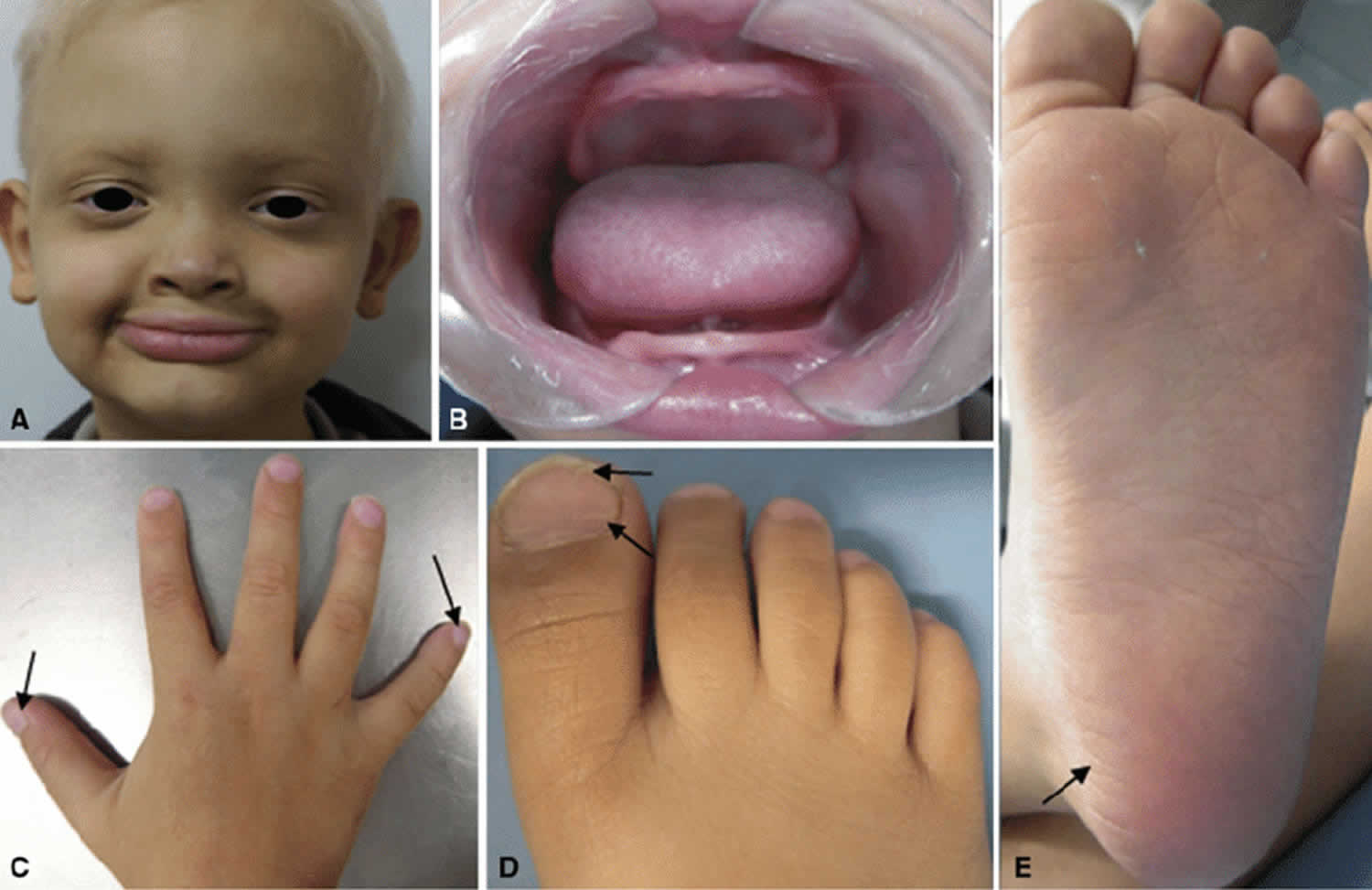
Figure 1. Hypohidrotic ectodermal dysplasia
Footnote: Proband case of X-linked hypohidrotic ectodermal dysplasia. The proband, (a) five-year-old male patient with the characteristic facial appearance, including a prominent forehead, saddle-shaped nose, prominent lips, and sparse, dry scalp hair, eyelashes, and eyebrows. (b) Apparent anodontia. (c, d, e) Arrows indicates changes in extremities. (c) Hand with convex nails. (d) Foot with nail dysplasia. (e) Dehydrated foot
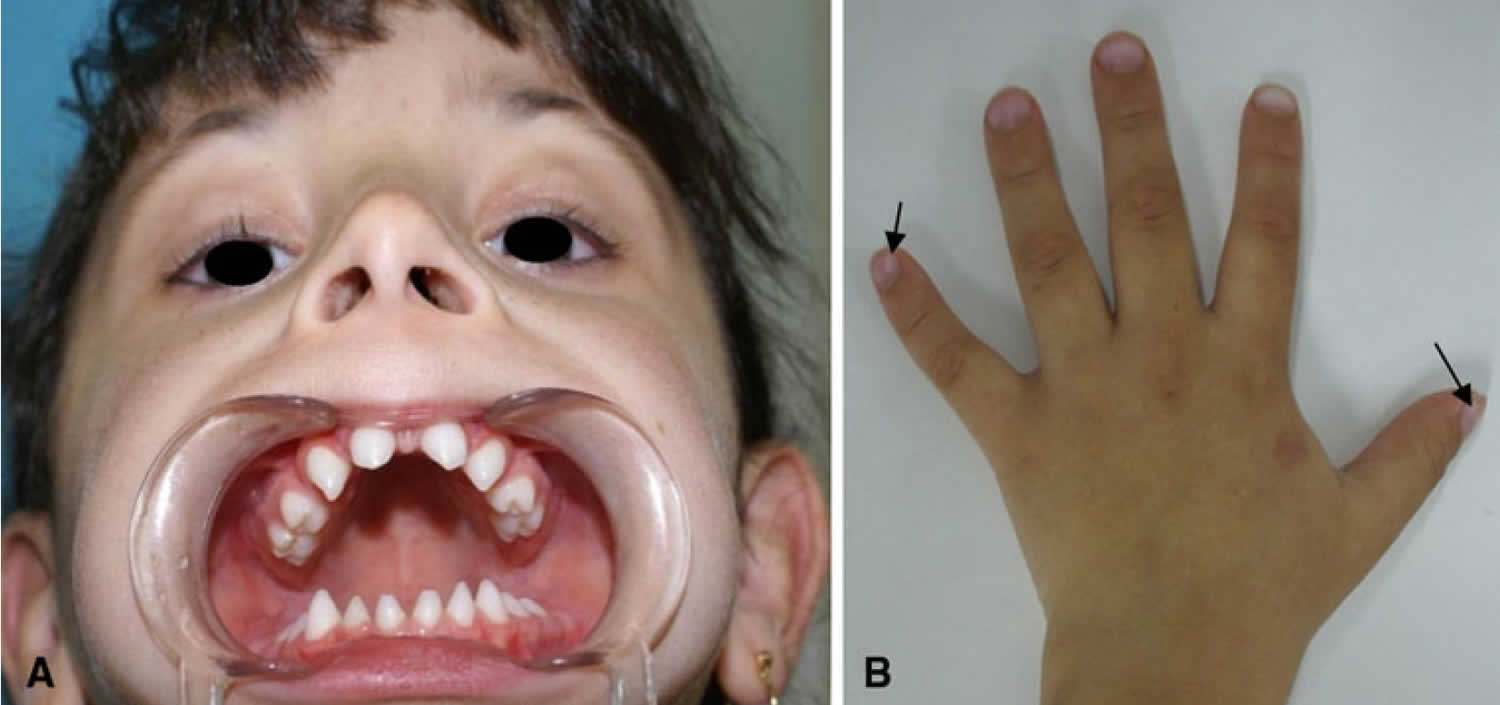
[Source 3 ]Figure 2. Female carrier of X-linked hypohidrotic ectodermal dysplasia
Footnote: Female carrier of X-linked hypohidrotic ectodermal dysplasia. Female carrier of X-linked hypohidrotic ectodermal dysplasia. (a) Five-year-old girl with the clinical features described for females in this family. She has a normal face and hair but agenesis of the lateral maxillary incisors and conical teeth. (b) Hand with convex nails (arrow)
[Source 3 ]Once someone has hypohidrotic ectodermal dysplasia, is there anything that can be done to correct the problems caused by the genetic mutation?
There is no specific treatment for hypohidrotic ectodermal dysplasia. Hypohidrotic ectodermal dysplasia is managed by treating the various symptoms. For patients with abnormal or no sweat glands, it is recommended that they live in places with air conditioning at home, school and work. In order to maintain normal body temperature, they should frequently drink cool liquids and wear cool clothing. Dental defects can be managed with dentures and implants. Artificial tears are used to prevent cornea damage for patients that do not produce enough tears. Surgery to repair a cleft palate is also helpful in improving speech and facial deformities 2.
Is hypohidrotic ectodermal dysplasia known to be associated with hypothyroidism?
An association between hypohidrotic ectodermal dysplasia and hypothyroidism has been described in three individuals in the medical literature – 2 male siblings and one unrelated female 4. The 2 brothers reportedly had hypohidrotic ectodermal dysplasia, primary hypothyroidism of gradual development in early childhood, and ciliary dyskinesia contributing to severe recurrent chest infections. In addition to sparse hair of the head and eyebrows and a shriveled appearance of fingernails and toenails, the brothers had urticaria pigmentosa-like skin and mucosal pigmentation. Their eyelashes and teeth were supposedly normal. The unrelated female described reportedly had similar findings 5. Due to these reports, the combination of findings was referred to as a separate condition called hypohidrotic ectodermal dysplasia with with hypothyroidism and ciliary dyskinesia.
Hypothyroidism in general has been reported to occur in about 3.7% of the population (approximately 1 in 27 individuals) 6. It is theoretically possible for an individual to have both hypohidrotic ectodermal dysplasia and hypothyroidism as distinct conditions without having the rare condition described above.
What is hypohidrotic ectodermal dysplasia with hypothyroidism and ciliary dyskinesia?
Hypohidrotic ectodermal dysplasia with hypothyroidism and ciliary dyskinesia is a rare condition characterized by alopecia (hair loss); nail dystrophy (abnormal development of the nails); ophthalmic (eye-related) complications; thyroid dysfunction (primary hypothyroidism); hypohidrosis; ephelides (freckles); enteropathy (disease of the intestine); and respiratory tract infections due to ciliary dyskinesia 4 These features have lead to the acronym ANOTHER syndrome as an alternative name for the condition. The gene that causes the condition is currently unknown but it is thought to be inherited in an autosomal recessive manner 4 Treatment is generally symptomatic and supportive.
Hypohidrotic ectodermal dysplasia cause
Hypohidrotic ectodermal dysplasia is a genetic condition that can result from mutations in one of several genes. These include EDA, EDAR, EDARADD, and WNT10A. EDA gene mutations are the most common cause of the disorder, accounting for more than half of all cases. EDAR, EDARADD, and WNT10A gene mutations each account for a smaller percentage of cases. In about 10 percent of people with hypohidrotic ectodermal dysplasia, the genetic cause is unknown.
The EDA, EDAR, and EDARADD genes provide instructions for making proteins that work together during embryonic development. These proteins form part of a signaling pathway that is critical for the interaction between two cell layers, the ectoderm and the mesoderm. In the early embryo, these cell layers form the basis for many of the body’s organs and tissues. Ectoderm-mesoderm interactions are essential for the formation of several structures that arise from the ectoderm, including the skin, hair, nails, teeth, and sweat glands.
Mutations in the EDA, EDAR, or EDARADD gene prevent normal interactions between the ectoderm and the mesoderm, which impairs the normal development of skin, hair, nails, teeth, and sweat glands. Mutations in any of these three genes lead to the major signs and symptoms of hypohidrotic ectodermal dysplasia described above.
The WNT10A gene provides instructions for making a protein that is part of a different signaling pathway known as Wnt signaling. Wnt signaling controls the activity of certain genes and regulates the interactions between cells during embryonic development. Signaling involving the WNT10A protein is critical for the development of ectodermal structures, particularly the teeth. The WNT10A gene mutations that cause hypohidrotic ectodermal dysplasia impair the protein’s function, which disrupts the development of teeth and other structures that arise from the ectodermal cell layer.
When hypohidrotic ectodermal dysplasia results from WNT10A gene mutations, its features are more variable than when the condition is caused by mutations in the EDA, EDAR, or EDARADD gene. Signs and symptoms range from mild to severe, and mutations in the WNT10A gene are more likely to cause all of the permanent (adult) teeth to be missing.
Hypohidrotic ectodermal dysplasia inheritance pattern
Hypohidrotic ectodermal dysplasia has several different inheritance patterns. Most cases are inherited in an X-linked pattern and are caused by mutations in the EDA gene. A condition is considered X-linked if the mutated gene that causes the disorder is located on the X chromosome, one of the two sex chromosomes. In males (who have only one X chromosome), one altered copy of the gene in each cell is sufficient to cause the condition. In females, who have two copies of the X chromosome, one altered copy of the gene in each cell often leads to less severe features of the condition. Signs and symptoms can include a few missing or abnormal teeth, sparse hair, and mild problems with sweat gland function. However, some females with one copy of the mutated gene have more severe features of this disorder.
Less commonly, hypohidrotic ectodermal dysplasia has an autosomal dominant or autosomal recessive pattern of inheritance. Mutations in the EDAR, EDARADD, or WNT10A gene can cause either autosomal dominant or autosomal recessive hypohidrotic ectodermal dysplasia.
Autosomal dominant inheritance means one copy of the altered gene in each cell is sufficient to cause the disorder. Some affected individuals inherit the mutation from one affected parent. Other cases result from new mutations in the gene and occur in people with no history of the disorder in their family.
Autosomal recessive inheritance means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene. Some mutation carriers have mild signs and symptoms of hypohidrotic ectodermal dysplasia, including a somewhat reduced ability to sweat and less severe dental abnormalities.
Risk to family members with X-linked hypohidrotic ectodermal dysplasia (XLHED)
Parents of a male proband
- The father of an affected male will not have the disorder nor will he be hemizygous for the EDA pathogenic variant; therefore, he does not require further evaluation/testing.
- In a family with more than one affected individual, the mother of an affected male is an obligate heterozygote. Note: If a woman has more than one affected child and no other affected relatives and if the EDA pathogenic variant cannot be detected in her leukocyte DNA, she has germline mosaicism.
- Clinical examination may detect minimal manifestations of X-linked hypohidrotic ectodermal dysplasia in the mother (see mild hypohidrotic ectodermal dysplasia). Molecular genetic testing of the mother is indicated.
- If a male is the only affected family member (i.e., a simplex case), the mother may be a heterozygote or the affected male may have a de novo EDA pathogenic variant, in which case the mother is not a heterozygote.
Parents of a female proband
- A female proband may have inherited the EDA pathogenic variant from her father (who may be affected) or her mother (who may be mildly affected) or the pathogenic variant may be de novo.
- Clinical examination may clarify the status of the parents. Molecular genetic testing is indicated.
Siblings of a male proband. The risk to sibs depends on the genetic status of the mother:
- If the mother is heterozygous for an EDA pathogenic variant, the chance of transmitting it in each pregnancy is 50%. Male sibs who inherit the pathogenic variant will be affected; female sibs who inherit the pathogenic variant will be heterozygous and may show minimal manifestations (see Clinical Description, Mild hypohidrotic ectodermal dysplasia).
- If the proband represents a simplex case (i.e., a single occurrence in the family) and if the EDA pathogenic variant cannot be detected in the leukocyte DNA of the mother, the risk to sibs is presumed to be slightly greater than that of the general population (though still <1%) because of the theoretic possibility of maternal germline mosaicism.
Siblings of a female proband. The risk to sibs depends on the genetic status of the parents:
- If the mother of the proband has an EDA pathogenic variant, the chance of transmitting it in each pregnancy is 50%. Males who inherit the pathogenic variant will be affected; females who inherit the pathogenic variant will be heterozygotes and may show minimal manifestations (see mild hypohidrotic ectodermal dysplasia).
- If the father of the proband has an EDA pathogenic variant, he will transmit it to all of his daughters and none of his sons.
Offspring of a male proband. Affected males transmit the EDA pathogenic variant to:
- All of their daughters, who will be heterozygotes and may show minimal manifestations (see mild hypohidrotic ectodermal dysplasia);
- None of their sons.
Offspring of a female proband. Women with an EDA pathogenic variant have a 50% chance of transmitting the pathogenic variant to each child:
- Males who inherit the pathogenic variant will be affected.
- Females who inherit the pathogenic variant will be heterozygotes and may show minimal manifestations (see mild hypohidrotic ectodermal dysplasia).
Other family members. The risk to other family members depends on the status of the proband’s parents: if a parent is affected or has a pathogenic variant, family members are at risk.
People with specific questions about genetic risks or genetic testing for themselves or family members should speak with a genetics professional.
Resources for locating a genetics professional in your community are available online:
- The National Society of Genetic Counselors (https://www.findageneticcounselor.com/) offers a searchable directory of genetic counselors in the United States and Canada. You can search by location, name, area of practice/specialization, and/or ZIP Code.
- The American Board of Genetic Counseling (https://www.abgc.net/about-genetic-counseling/find-a-certified-counselor/) provides a searchable directory of certified genetic counselors worldwide. You can search by practice area, name, organization, or location.
- The Canadian Association of Genetic Counselors (https://www.cagc-accg.ca/index.php?page=225) has a searchable directory of genetic counselors in Canada. You can search by name, distance from an address, province, or services.
- The American College of Medical Genetics and Genomics (http://www.acmg.net/ACMG/Genetic_Services_Directory_Search.aspx) has a searchable database of medical genetics clinic services in the United States.
Hypohidrotic ectodermal dysplasia symptoms
Hypohidrotic ectodermal dysplasia is characterized by lack of or diminished sweating (anhidrosis or hypohidrosis), abnormally sparse hair (hypotrichosis), and/or absence (hypodontia) and/or malformation of certain teeth 7. In addition, affected individuals often have characteristic facial abnormalities, irregularities of the skin, abnormalities of the mucous membranes lining the respiratory and gastrointestinal (GI) tracts, an increased tendency to develop certain infections and allergic conditions, and/or other abnormalities. The range and severity of the symptoms and findings associated with hypohidrotic ectodermal dysplasia varies from case to case.
A primary feature of hypohidrotic ectodermal dysplasia is a lack of or diminished sweating (anhidrosis or hypohidrosis), resulting from underdevelopment of or partial or complete absence of certain sweat glands (eccrine glands). Because affected infants and children are unable to sweat appropriately when exposed to warm environments, they can experience repeated episodes of heat intolerance and “unexplained” high fevers that may remain unexplained until the disorder is diagnosed. In individuals with hypohidrotic ectodermal dysplasia, exertion can result in elevated body temperature (hyperpyrexia). Eating hot foods may also cause extreme discomfort. In some cases, without appropriate treatment, episodes of hyperpyrexia may result in life-threatening complications; particularly during the first two years of life.
Abnormal sparseness of hair (hypotrichosis) is also a primary characteristic of hypohidrotic ectodermal dysplasia, and is due to incomplete formation and reduced numbers of hair follicles. Scalp hair is usually blond or lightly pigmented; abnormally sparse, short, and fine; and, in some cases, stiff, dry, and unruly. Abnormal bald patches on the scalp (alopecia) may also be present. In addition, the eyebrows and eyelashes are often scanty or absent, although, in some cases, the eyelashes may appear normal. After puberty, male patterns of hair growth (e.g., moustache and beard) can be normal, while in other cases, facial and pubic hair growth may be sparse. In affected males and females, pubic and underarm (axillary) hair is typically scant. In some cases, hair may be absent from the arms, legs, and/or trunk.
The third primary characteristic typically associated with hypohidrotic ectodermal dysplasia is the absence (hypodontia) and/or malformation of teeth. In most cases, the majority of the primary (deciduous) and secondary (permanent) teeth are absent. The teeth most often present include front teeth (central incisors), teeth normally located next to the incisors (canines), and/or, in some cases, one or more molars. In most cases, the teeth that are present are widely spaced, with front teeth being pointed or cone shaped. In some rare cases, individuals with hypohidrotic ectodermal dysplasia may lack all upper and/or lower teeth (edentulous or anodontia). Some individuals can be missing all the teeth in one jaw and have some in the other jaw.
As a result of missing teeth the bony ridge of the jaws (alveolar process) that holds the teeth in place often fails to form properly. In addition, due to hypodontia, the lips may protrude outward (everted) and appear abnormally thick, the gums may be abnormally small or degenerated (atrophic), and the normally exposed red portion of the upper and lower lips (vermilion border) may not be noticeable.
Many individuals with hypohidrotic ectodermal dysplasia have additional, characteristic facial features, including a prominent forehead (frontal bossing); underdeveloped nostrils (hypoplastic alae nasi) and a low or sunken nasal bridge (so-called “saddle nose”); and underdeveloped, sunken cheeks (malar hypoplasia).
Distinctive skin changes may also be present. Many affected newborns have unusual scaling or peeling of the skin, while many children develop itchy (pruritic), scaling skin rashes (eczema). In the majority of individuals with hypohidrotic ectodermal dysplasia, the skin on most of the body is unusually thin and soft and can lack normal pigmentation (hypopigmentation). However, the skin around the eyes (periorbital) may be darkly pigmented (hyperpigmentation) and finely wrinkled, appearing prematurely aged. The skin may be extremely dry due to underdevelopment (hypoplasia) or absence (aplasia) of oil-secreting glands (sebaceous glands). Additionally there may be abnormalities in the skin ridge patterns (dermatoglyphic patterns) on the fingers, toes, palms of the hands, and/or soles of the feet. Some individuals with the disorder have unusually thin and brittle nails.
In many individuals with hypohidrotic ectodermal dysplasia, mucous glands within the membrane lining the respiratory and gastrointestinal (GI) tracts (e.g., in the lung, pharynx, larynx, trachea, upper esophagus, stomach, intestines) are underdeveloped (hypoplastic) or absent (aplastic). There are several rare hypohidrotic ectodermal dysplasia forms or subtypes that have abnormally decreased function of certain components of the immune system (e.g., depressed lymphocyte function, cellular immune hypofunction). The immune system works to protect the body against invading microorganisms, toxins, and other substances that are recognized as foreign to the body. In many infants and children with hypohidrotic ectodermal dysplasia, such mucous gland abnormalities and/or immune system irregularities cause an increased susceptibility to certain infections and/or allergic conditions.
Salivary glands can also be underdeveloped (hypoplastic), leading to abnormal dryness of the mouth and an altered sense of taste or smell. In addition, some individuals with hypohidrotic ectodermal dysplasia are unable to produce tears due to underdevelopment of the glands that secrete tears (hypoplastic lacrimal glands), hypoplasia of the ducts through which the tears pass (lacrimal ducts), and/or abnormal narrowing of the small openings in the inner corners of the eyelids where tears normally drain (stenotic lacrimal puncta). Eye (ocular) abnormalities may be present, including loss of transparency of the lens of the eyes (cataracts) and/or clouding of the portion of the eyes through which light passes (corneal opacities).
Females who carry a single copy of the mutated EDA gene for X-linked hypohidrotic ectodermal dysplasia (heterozygote carriers) may have no symptoms or physical abnormalities or may have some of the characteristics associated with the disease. Approximately 70% of female carriers show symptoms that are typically milder than those associated with the fully expressed disorder. Female carriers of X-linked hypohidrotic ectodermal dysplasia may have dental abnormalities such as absence of certain teeth (hypodontia) and/or abnormally small, pointed, conical teeth; sparse hair (hypotrichosis); reduced sweating; and/or irregular dermatoglyphic patterns. In some cases, abnormalities of the breasts and nipples have been reported, and approximately 80 percent of carriers may experience difficulties nursing.
Hypohidrotic ectodermal dysplasia diagnosis
No guidelines regarding diagnostic criteria for hypohidrotic ectodermal dysplasia have been developed.
In most cases, hypohidrotic ectodermal dysplasia is diagnosed during early childhood when characteristic dental and hair abnormalities become apparent and prompt further testing. Such diagnosis is based upon a thorough clinical evaluation, identification of characteristic physical findings, a detailed patient and family history, and specialized laboratory testing. In some cases, during the newborn period, heat intolerance, unexplained fevers, and/or extensive skin peeling may lead to an earlier diagnosis.
Hypohidrotic ectodermal dysplasia should be suspected in an individual with:
- Hypotrichosis (sparseness of scalp and body hair). Scalp hair has thin shafts and is lightly pigmented. Note: Hair shafts can be brittle and twisted (pili torti) or have other anomalies on microscopic analysis; however, these findings are not sufficiently sensitive to be of diagnostic benefit 8. Secondary sexual hair (beard; axillary and pubic hair) can be normal.
- Hypohidrosis (reduced ability to sweat). Reduced ability to sweat in response to heat leads to hyperthermia:
- The function of sweat glands may be assessed by bringing the skin into contact with an iodine solution and raising ambient temperatures to induce sweating. The iodine solution turns color when exposed to sweat and can be used to determine the amount and location of sweating.
- The number and distribution of sweat pores can be determined by coating parts of the body (usually the hypothenar eminences of the palms) with impression materials commonly used by dentists.
- While skin biopsies have been used to determine the distribution and morphology of sweat glands, noninvasive techniques are equally effective. Live confocal microscope imaging is able to visualize the sweat ducts on the palms 9.
- Hypodontia (congenital absence of teeth):
- An average of nine permanent teeth – typically the canines and first molars –develop in individuals with classic hypohidrotic ectodermal dysplasia 10.
- Teeth are often smaller than average and have an altered morphology; the anterior teeth frequently have conical crowns.
- Dental radiographs are helpful for determining the extent of hypodontia and are useful in the diagnosis of mildly affected individuals. Taurodontism (elongation of the pulp chamber) is more common in molar teeth of individuals with hypohidrotic ectodermal dysplasia than in unaffected individuals.
Note: Anthropometric variations (measurements of facial form and tooth size) in hypohidrotic ectodermal dysplasia are subtle and have not proven clinically useful.
Specialized diagnostic testing
Specialized diagnostic testing may include microscopic examination of small samples of skin tissue removed from the palm, confirming partial or complete absence of eccrine sweat glands. In some cases, other types of sweat testing may be used to determine the reduction or absence of perspiration. One such test that is particularly helpful in detecting females who carry a single copy of the disease gene for X-linked hypohidrotic ectodermal dysplasia (heterozygotes) consists of the application of an iodine-in-alcohol solution over the entire back, followed by the application of a corn starch/castor oil suspension. During such testing, sweat glands become highlighted by a black dot. In heterozygous females, characteristic streaks will appear on the back in the shape of a “V”, demonstrating those areas that are devoid of sweat glands. Another method frequently used is the counting of sweat pores by direct observation. In cases of X-linked hypohidrotic ectodermal dysplasia, direct observation reveals no sweat pores in affected males and decreased numbers of sweat pores in female carriers. In males and females with the autosomal recessive form of hypohidrotic ectodermal dysplasia, such a count will also reveal decreased number of sweat pores.
Additional diagnostic tools are available and may include a test in which the sweat glands are stimulated by a drug called pilocarpine through the use of direct current (iontophoresis) and the resulting perspiration is measured and analyzed. In some cases, application of the substance o-phthalaldehyde may be applied directly to the skin (topically) of the palm. Such testing may reveal absence or reduction of sweating in affected individuals and female carriers.
In addition, dental x-rays to verify the absence of certain teeth and to further characterize associated dental abnormalities play an essential role in helping to confirm a diagnosis of hypohidrotic ectodermal dysplasia or identify carrier status.
Molecular testing for mutations in the EDA, EDAR, and EDARADD genes is available to confirm the diagnosis. Carrier testing is available if the disease-causing mutation(s) have been identified in an affected family member.
Carrier detection for X-linked hypohidrotic ectodermal dysplasia:
- Because females with X-linked hypohidrotic ectodermal dysplasia show mosaic patterns of sweat pore function and distribution, use of an iodine solution to assess sweat gland function or impression materials to assess number and distribution of sweat pores is particularly useful.
- Between 60% and 80% of females with X-linked hypohidrotic ectodermal dysplasia display some degree of hypodontia 11.
Establishing the diagnosis
Classic hypohidrotic ectodermal dysplasia is often diagnosed after infancy in affected individuals with the above characteristic features of hypotrichosis, hypohidrosis, and hypodontia.
- Male proband. The diagnosis of classic hypohidrotic ectodermal dysplasia is established in a male proband with the above characteristic features. Identification of a hemizygous EDA pathogenic variant or biallelic EDAR, EDARADD, or WNT10A pathogenic variants confirms the diagnosis.
- Female proband. The diagnosis of classic hypohidrotic ectodermal dysplasia is established in a female proband with the above characteristic features. Identification of biallelic EDAR, EDARADD, or WNT10A pathogenic variants confirms the diagnosis.
Mild hypohidrotic ectodermal dysplasia
- Male proband. The diagnosis of mild hypohidrotic ectodermal dysplasia can be established in a male proband with the mild manifestations of the cardinal features. Identification of a heterozygous EDAR, EDARADD, or WNT10A pathogenic variant confirms the diagnosis.
- Female proband. The diagnosis of mild hypohidrotic ectodermal dysplasia due to an EDA pathogenic variant can be established in a female proband with a mosaic pattern of sweat pore function and distribution, hypodontia, and a family history suggestive of X-linked hypohidrotic ectodermal dysplasia. Identification of a heterozygous EDA pathogenic variant by molecular genetic testing confirms this diagnosis. The diagnosis of mild hypohidrotic ectodermal dysplasia can also be established in a female with mild manifestations by identification of a heterozygous pathogenic variant in EDAR, EDARADD, or WNT10A.
Molecular testing approaches can include serial single-gene testing and a multigene panel.
Serial single-gene testing can be considered if:
- The proband’s findings are classic and consistent with X-linked inheritance (i.e., males generally more severely affected than females, no male-to-male transmission). Sequence analysis of EDA is performed first, followed by gene-targeted deletion/duplication analysis of EDA if no pathogenic variant is found.
- The proband’s findings are classic and consistent with autosomal recessive inheritance, or mild and consistent with autosomal dominant inheritance. Sequence analysis of EDAR, EDARADD, and WNT10A should be performed, followed by deletion/duplication analysis if no pathogenic variant is found by sequence analysis.
If molecular genetic testing of EDA, EDAR, EDARADD, and WNT10A do not identify a pathogenic variant, other forms of ectodermal dysplasia should be considered.
A multigene panel that includes EDA, EDAR, EDARADD, WNT10A, and other genes of interest (see Differential Diagnosis) may also be considered. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview; thus, clinicians need to determine which multigene panel is most likely to identify the genetic cause of the condition at the most reasonable cost while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
Hypohidrotic ectodermal dysplasia treatment
The treatment of hypohidrotic ectodermal dysplasia is directed toward the specific symptoms that are apparent in each individual. Treatment may require the coordinated efforts of a team of specialists who need to systematically and comprehensively plan an affected individual’s treatment. Such specialists may include pediatricians or internists, physicians who treat disorders of the skin (dermatologists), dental specialists, physicians who diagnose and treat disorders of the ears, nose, and throat (otolaryngologists), allergists, and/or other health care professionals.
If possible, it is recommended that individuals with hypohidrotic ectodermal dysplasia live in a cool climate. Physicians may carefully monitor affected infants and young children and recommend supportive measures to help prevent episodes of severely elevated body temperature (hyperpyrexia). For children and adults with the disorder, preventive and protective measures should include avoidance of physical exertion, protection from high temperatures, and, during warm weather, large amounts of dietary fluids, cooling by water such as use of cool cloths and sponge baths, air conditioning, and/or other supportive measures.
Early dental intervention and restoration is also important. Artificial teeth and/or other devices (prosthetics) may be used to replace absent teeth. Braces, bridges, dental surgery, and/or other corrective measures may be used to help correct dental abnormalities and ensure appropriate nutrition. In addition, in affected individuals with alopecia, hairpieces or wigs may be helpful.
Physicians may recommend that impacted nasal secretions be carefully removed on a regular basis to help prevent or limit the severity of rhinitis. Physicians may also regularly monitor affected infants and children to help prevent respiratory infections and to ensure prompt, aggressive treatment should such infections occur.
In affected individuals with impaired tear secretion (alacrima), the use of artificial tears may help to prevent possible corneal damage.
Early intervention is important to ensure that children with hypohidrotic ectodermal dysplasia reach their potential. Special services that may be beneficial to affected children may include special education and special social support, and/or other medical, social, and/or vocational services.
Genetic counseling will be of benefit for affected children and their families. Other treatment is symptomatic and supportive.
Agents and circumstances to avoid
Individuals with severe hypohidrosis can have marked heat intolerance; care should be taken to prevent exposure to extreme heat and the potential for febrile seizures.
Treatment of manifestations
Management of affected individuals targets the three cardinal features and is directed at optimizing psychosocial development, establishing optimal oral function, and preventing hyperthermia.
Hypotrichosis
Wigs or special hair care formulas and techniques to manage sparse, dry hair may be useful. One report describes a child with hypohidrotic ectodermal dysplasia and alopecia who was treated with topical minoxidil to the scalp and had resultant hair growth 12.
Hypohidrosis
During hot weather, affected individuals must have access to an adequate supply of water and a cool environment, which may mean air conditioning, a wet T-shirt, and/or a spray bottle of water. Some individuals may benefit from “cooling vests.”
Affected individuals learn to control their exposure to heat and to minimize its consequences, but special situations may arise in which intervention by physicians and families is helpful. For example, a physician may have to prescribe an air conditioner before a school district complies, or parents may have to advocate for children who need to carry liquids into areas where they are prohibited.
Hypodontia
- Dental treatment, ranging from simple restorations to dentures, must begin at an early age. Bonding of conical shaped teeth in young affected individuals improves aesthetics and chewing ability.
- Orthodontics may be necessary.
- Dental implants in the anterior portion of the mandibular arch have proven successful only in children age seven years and older 13.
- Children with hypohidrotic ectodermal dysplasia typically need to have their dental prostheses replaced every 2.5 years.
- Dental implants in adults can support aesthetic and functional dentition.
- Hyposalivation is present in some individuals, predisposing them to dental caries and the need for therapeutics directed at maintaining oral lubrication and caries control.
- Dietary counseling may be helpful for those individuals who have trouble chewing and swallowing despite adequate dental care.
Other
- Regular visits with an ENT physician may be necessary for management of the nasal and aural concretions. Commonly, nasal and aural concretions must be removed with suction devices or forceps and recommendations made about humidification of the ambient air to prevent their formation 14.
- Skin care products are useful for management of eczema and rashes and for dry skin associated with certain outdoor exposures like swimming.
- Lubrication eye drops can be helpful for dry eyes.
Prevention of secondary complications
Saliva substitutes and optimal fluoride exposure may be helpful in preventing dental caries in those individuals having a marked reduction in salivary flow. Other dental caries preventive approaches such as pit and fissure sealants can be beneficial as well.
Surveillance
The first dental visit should occur by age one year to monitor tooth and maxillary/mandibular development and for anticipatory guidance for parents. The developing dentition should be evaluated every six to 12 months to monitor existing treatments and to provide continued interventions as needed.
Pregnancy management
Optimal prenatal nutrition is recommended for mothers who are carriers of or affected with hypohidrotic ectodermal dysplasia. Affected women at risk for hyperthermia should take extra care not to become overheated during pregnancy. There are no other special recommendations for pregnancy management.
Some women may have difficulty breastfeeding their infants because of hypoplasia of the mammary glands.
Therapies under investigation
A Phase II clinical trial was conducted at several US and European medical centers to investigate the use of EDI200, developed by Edimer Pharmaceuticals, Inc 15. The results of the study were inconclusive. EDI200 is an ectodysplasin-A1 (EDA-A1) replacement protein that has been shown to bind specifically to the EDA-A1 receptor (EDAR) and activate the signaling pathway that leads to normal ectodermal development. EDI200 has demonstrated permanent correction of the disease manifestations in both mouse and dog models of X-linked hypohidrotic ectodermal dysplasia, with reduction in mortality and morbidity 15. For additional information on the clinical trial go here (https://clinicaltrials.gov/ct2/show/NCT01775462).
References- Hypohidrotic ectodermal dysplasia. https://ghr.nlm.nih.gov/condition/hypohidrotic-ectodermal-dysplasia
- Wright JT, Grange DK, Fete M. Hypohidrotic Ectodermal Dysplasia. 2003 Apr 28 [Updated 2017 Jun 1]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1112
- Aquino, Sibele & Paranaíba, Lívia & Swerts, Mário Sérgio & Martelli, Daniella & Barros, Letízia & Júnior, Hercílio. (2012). Orofacial Features of Hypohidrotic Ectodermal Dysplasia. Head and neck pathology. 6. 10.1007/s12105-012-0349-4.
- Hypohidrotic ectodermal dysplasia-hypothyroidism-ciliary dyskinesia syndrome. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=1882
- ECTODERMAL DYSPLASIA, HYPOHIDROTIC, WITH HYPOTHYROIDISM AND CILIARY DYSKINESIA. https://omim.org/entry/225050
- Hypothyroidism. https://emedicine.medscape.com/article/122393-overview
- Hypohidrotic ectodermal dysplasia. https://rarediseases.org/rare-diseases/hypohidrotic-ectodermal-dysplasia/
- Rouse C, Siegfried E, Breer W, Nahass G. Hair and sweat glands in families with hypohidrotic ectodermal dysplasia: further characterization. Arch Dermatol. 2004;140:850–5.
- Jones KB, Goodwin AF, Landan M, Seidel K, Tran DK, Hogue J, Chavez M, Fete M, Yu W, Hussein T, Johnson R, Huttner K, Jheon AH, Klein OD. Characterization of X-linked hypohidrotic ectodermal dysplasia (XL-HED) hair and sweat gland phenotypes using phototrichogram analysis and live confocal imaging. Am J Med Genet A. 2013;161A:1585–93.
- Lexner MO, Bardow A, Hertz JM, Nielsen LA, Kreiborg S. Anomalies of tooth formation in hypohidrotic ectodermal dysplasia. Int J Paediatr Dent. 2007;17:10–8.
- Cambiaghi S, Restano L, Paakkonen K, Caputo R, Kere J. Clinical findings in mosaic carriers of hypohidrotic ectodermal dysplasia. Arch Dermatol. 2000;136:217–24.
- Lee HE, Chang IK, Im M, Seo YJ, Lee JH, Lee Y. Topical minoxidil treatment for congenital alopecia in hypohidrotic ectodermal dysplasia. J Am Acad Dermatol. 2013;68:e139–40.
- Stanford CM, Guckes A, Fete M, Srun S, Richter MK. Perceptions of outcomes of implant therapy in patients with ectodermal dysplasia syndromes. Int J Prosthodont. 2008;21:195–200.
- Mehta U, Brunworth J, Lewis RA, Sindwani R. Rhinologic manifestations of ectodermal dysplasia. Am J Rhinol. 2007;21:55–8.
- Huttner K. Future developments in XLHED treatment approaches. Am J Med Genet. 2014;164A:2433–6.