Immune reconstitution syndrome
Immune Reconstitution Inflammatory Syndrome (IRIS) also known as Immune Reconstitution Syndrome or Immune Restoration Disease in HIV infection, is an exaggerated inflammatory reaction to a disease-causing microorganism that sometimes occurs when the immune system begins to recover following treatment with antiretroviral drugs 1, despite effective therapy and microbiologic cure and cannot be explained by a persistent primary infection, new secondary infection, or adverse drug events 2. IRIS can be mild or life-threatening.
Immune reconstitution inflammatory syndrome (IRIS) occurs in two forms:
- “Unmasking” IRIS refers to the flare-up of an underlying, previously undiagnosed infection soon after antiretroviral therapy is started;
- “Paradoxical” IRIS refers to the worsening of a previously treated infection after antiretroviral therapy is started.
IRIS has been classified as either “paradoxical,” in which a previously known opportunistic infection seems to worsen after initiation of antiretroviral therapy (ART) or “unmasking,” which is an inflammatory response to an opportunistic infection that was previously unrecognized. IRIS of either type usually occurs at the time of rapid viral load decrease or an increase in CD4 cell count. Most cases occur within the first 4 to 8 weeks after ART initiation in patients with initially low CD4 counts, but IRIS can occur at any CD4 count and many weeks to months after a patient starts or restarts ART 3.
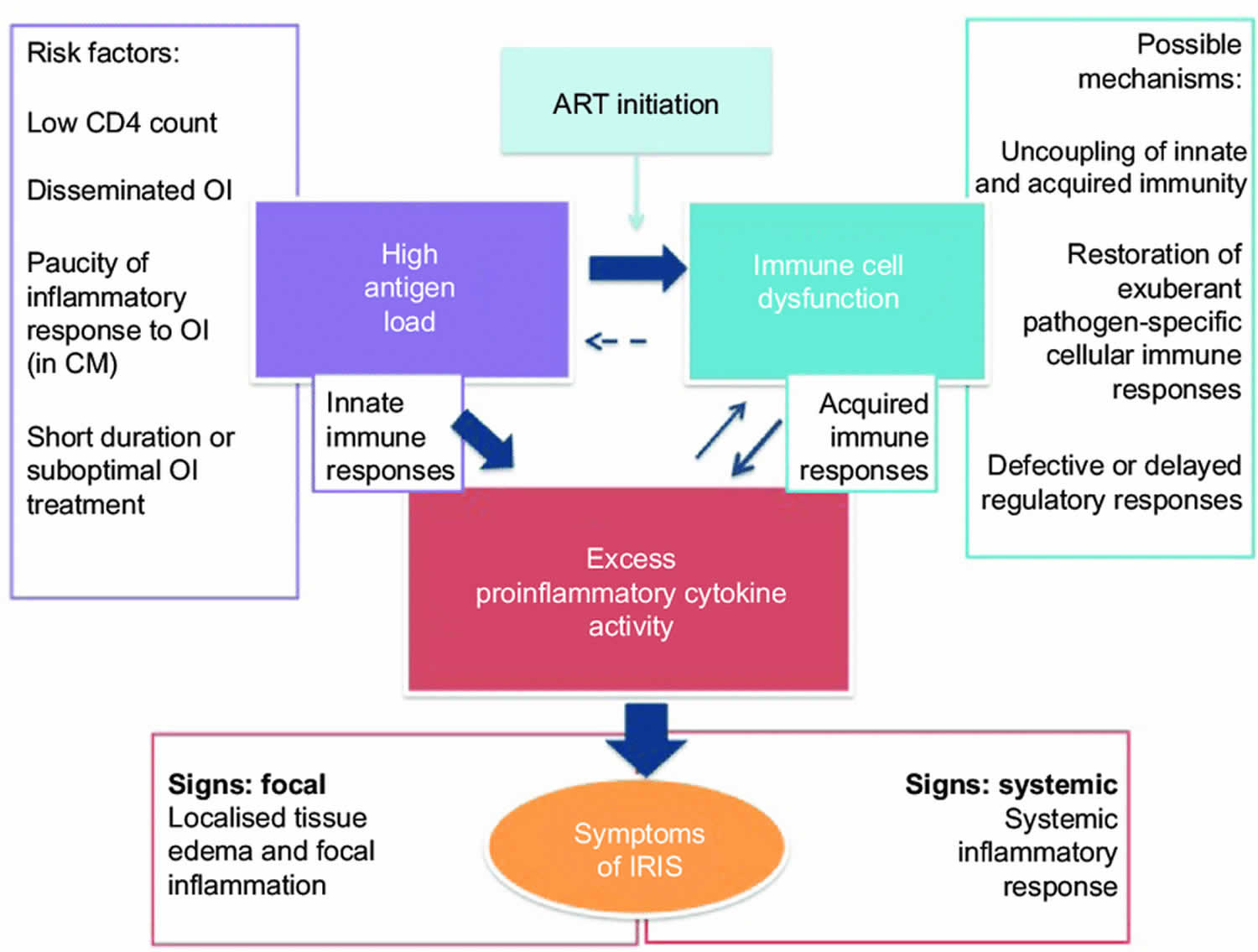
The immunopathogenesis of the immune reconstitution syndrome is unclear and appears to be result of unbalanced reconstitution of effector and regulatory T-cells, leading to exuberant inflammatory response in patients receiving antiretroviral therapy (ART) 4. Biomarkers, including interferon-γ (INF-γ), tumor necrosis factor-α (TNF-α), C-reactive protein (CRP) and interleukin (IL)-2, 6 and 7, are subject of intense investigation at present 4.
Potential mechanisms for immune reconstitution syndrome include a partial recovery of the immune system or exuberant host immunological responses to antigenic stimuli. The overall incidence of IRIS is unknown, but is dependent on the population studied and its underlying opportunistic infectious burden 5. IRIS has been reported in 10 to 32 per cent of patients starting ART 6. The variation in reported frequency reflect differences in case definitions, and more importantly, differences in study populations with differing risk profiles and underlying burden of opportunistic infections.
Most of the literature on epidemiology comes from the developed countries. In a series from southern India tuberculosis-associated IRIS (TB-IRIS) was reported in 7.6 per cent of patients 7. Sharma et al. 8 have reported incidence rates of 7.5 per cent for paradoxical TB-IRIS and 3 per cent for ART-associated TB in a retrospective study using consensus case-definitions. In a prospective study, using stringent case-definitions criteria 9, paradoxical TB-IRIS was seen in 4 per cent of patients and ART-associated TB in 7.5 per cent of patients. No cases of ART-associated TB fulfilling the criteria of unmasking TB-IRIS were identified in either of the studies. The higher incidence of TB-IRIS reported, particularly in the western literature, may be explained by leniency of clinical diagnostic criteria.
The infectious pathogens most frequently implicated in the immune reconstitution inflammatory syndrome are mycobacterial infections, fungi, varicella zoster, herpesviruses, and cytomegalovirus (CMV). Majority of patients with IRIS have a self-limiting disease course. No single treatment option exists and depends on the underlying infectious agent and its clinical presentation. Antiretroviral therapy (ART) is usually continued and treatment for the associated condition optimized. The overall mortality associated with IRIS is low; however, patients with central nervous system involvement with raised intracranial pressures in cryptococcal and tubercular meningitis, and respiratory failure due to acute respiratory distress syndrome (ARDS) have poor prognosis and require aggressive management including corticosteroids 4. Paradigm shifts in management of HIV with earlier initiation of ART is expected to decrease the burden of IRIS in developed countries; however, with enhanced rollout of ART in recent years and the enormous burden of opportunistic infections in developing countries like India, IRIS is likely to remain an area of major concern.
Immune reconstitution inflammatory syndrome causes
Despite numerous descriptions of the manifestations of IRIS, its pathogenesis remains largely speculative. Current theories concerning the pathogenesis of the syndrome involve a combination of underlying antigenic burden, the degree of immune restoration following highly active antiretroviral therapy (HAART), and host genetic susceptibility. These pathogenic mechanisms may interact and likely depend on the underlying burden of infectious or noninfectious agent.
Whether elicited by an infectious or noninfectious agent, the presence of an antigenic stimulus for development of the syndrome appears necessary. This antigenic stimulus can be intact, “clinically silent” organisms or dead or dying organisms and their residual antigens. IRIS that occurs as a result of “unmasking” of clinically silent infection is characterized by atypical exuberant inflammation and/or an accelerated clinical presentation suggesting a restoration of antigen-specific immunity. These characteristics differentiate IRIS from incident opportunistic infections that occur on ART as a result of delayed adequate immunity.
Table 1. Infectious and noninfectious causes of IRIS in HIV-infected patients
| Infectious causes | Noninfectious causes |
| Mycobacteria | Rheumatologic/Autoimmune |
| Mycobacterium tuberculosis 10 | Rheumatoid arthritis 11, Systemic lupus erythematosus (SLE) 12 |
| Graves disease 13, Autoimmune thyroid disease 14 | |
| Mycobacterium avium complex 15 | Sarcoidosis & granulomatous reactions 16 |
| Other mycobacteria 17 | Tattoo ink 18 |
| Cytomegalovirus 19 | AIDS-related lymphoma 20 |
| Herpes viruses | Guillain-Barre’ syndrome 21 |
| Herpes zoster virus 22 | Interstitial lymphoid pneumonitis 23 |
| Herpes simplex virus 24 | |
| Herpes virus-associated Kaposi’s sarcoma 24 | |
| Cryptococcus neoformans 15 | |
| Pneumocystis jirovecii pneumonia (PCP) 24 | |
| Histoplasmosis capsulatum 25 | |
| Toxoplasmosis 19 | |
| Hepatitis B virus 24 | |
| Hepatitis C virus 19 | |
| Progressive multifocal leukoencephalitis 26 | |
| Parvovirus B19 27 | |
| Strongyloides stercoralis infection 28 and other parasitic infections 29 | |
| Molluscum contagiosum & genital warts 24 | |
| Sinusitis 30 | |
| Folliculitis 31 |
Examples of IRIS in response to intact organisms include, but are not limited to, the unmasking of latent cryptococcal infection 32 and infection with Mycobacterium avium complex (MAC) 33. The most frequently reported IRIS symptoms in response to previously treated or partially treated infections include reports of clinical worsening and recurrence of clinical manifestations of Mycobacterium tuberculosis (TB) and cryptococcal meningitis following initiation of ART 34. In noninfectious causes of IRIS, autoimmunity to innate antigens plays a likely role in the syndrome. Examples include exacerbation of rheumatoid arthritis and other autoimmune diseases 11. Given the role of this antigenic stimulus, the frequency and manifestations of IRIS in a given population may be determined by the prevalence of opportunistic and non-opportunistic infections to initiation of ART.
The mechanism receiving the most attention involves the theory that the immune reconstitution inflammatory syndrome is precipitated by the degree of immune restoration following ART. In assessing this theory, investigators have examined the association between CD4 cell counts and viral loads and the risk of IRIS. Some studies suggest differences in the baseline CD4 profiles or quantitative viral load at ART initiation or their rate of change during HAART between IRIS and non-IRIS patients 35, while other studies demonstrate only trends or no significant difference between IRIS and non-IRIS patients 36. These immunological differences between groups have been difficult to verify due to small numbers of IRIS cases and lack of control groups. An alternative immunological mechanism may involve qualitative changes in lymphocyte function or lymphocyte phenotypic expression. For instance, following ART an increase in memory CD4 cell types is observed 37 possibly as a result of redistribution from peripheral lymphoid tissue 38. This CD4 phenotype is primed to recognize previous antigenic stimuli, and thus may be responsible for manifestations of IRIS seen soon after ART initiation. After this redistribution, naïve T cells increase and are thought to be responsible for the later quantitative increase in CD4 cell counts 39. These data suggest IRIS may be due to a combination of both quantitative restoration of immunity as well as qualitative function and phenotypic expression observed soon after the initiation of ART.
The third purported pathogenic mechanism for IRIS involves host genetic susceptibility to an exuberant immune response to the infectious or noninfectious antigenic stimulus upon immune restoration. Although evidence is limited, carriage of specific HLA alleles suggest associations with the development of IRIS and specific pathogens 40. Increased levels of interleukin-6 (IL-6) in IRIS patients may explain the exuberant Th1 response to mycobacterial antigens in subjects with clinical IRIS 41. Such genetic predispositions may partially explain why manifestations of IRIS differ in patients with similar antigenic burden and immunological responses to ART.
Risk factors for the development of IRIS
Risk factors identified for the development of IRIS in one cohort included male sex, a shorter interval between initiating treatment for opportunistic infections and starting ART, a rapid fall in HIV-1 RNA after ART, and being ART-naïve at the time of opportunistic infections diagnosis 15. Other significant predictors have also included younger age, a lower baseline CD4 cell percentage, a lower CD4 cell count at ART initiation, and a lower CD4 to CD8 cell ratio at baseline 24. It should be noted cohorts differ substantially in study populations and the type of IRIS (i.e. TB-IRIS only) examined, making conclusions regarding risk factors for IRIS difficult. Clinical factors associated with the development of IRIS are presented in Table 2.
Table 2. Clinical factors associated with the development of IRIS
| Risk factor | Reference |
| Male sex | [31] |
| Younger age | [32] |
| Lower CD4 cell count at ART initiation | [4] |
| Higher HIV RNA at ART initiation | [4] |
| Lower CD4 cell percentage at ART initiation | [32] |
| Lower CD4:CD8 ratio at ART initiation | [32] |
| More rapid initial fall in HIV RNA on ART | [31] |
| Antiretroviral naïve at time of OI diagnosis | [31] |
| Shorter interval between OI therapy initiation and ART initiation | [31] |
Footnote: Derived from cohorts where IRIS due to multiple pathogens were reported (i.e. cohorts which examined only TB-IRIS were excluded)
Immune reconstitution inflammatory syndrome symptoms
Presentations of IRIS may differ according to the triggering opportunistic infection. TB IRIS can present with worsening of the pulmonary or extrapulmonary symptoms of TB or with hepatotoxicity 42. Mycobacterium avium complex (MAC) IRIS may result in pulmonary or systemic inflammation that is indistinguishable from the original infection or with lymphadenitis, mass lesions, or osteomyelitis 43. Cryptococcal meningitis may worsen, resulting in rapid development of hearing or vision loss, ataxia, and elevated intracranial pressures, all of which have been reported 44. CMV retinitis IRIS can result in rapid and permanent vision loss with retinitis, vitritis, or uveitis 45. IRIS with hepatitis B or C can cause hepatitis flares and may be confused with medication toxicity 46. Brain lesions from progressive multifocal leukoencephalopathy may worsen or become unmasked with IRIS, as can Kaposi’s sarcoma, which can sometimes be fatal 47. Cerebral toxoplasmosis IRIS can present as cerebral abscess, encephalitis, or chorioretinitis 48. Exacerbation of autoimmune diseases such as sarcoid or Grave’s disease have been reported 49 and reactivations of herpes simplex virus (HSV), varicella zoster virus (VZV), folliculitis, or oral or genital warts can occur.
Mycobacterium tuberculosis IRIS
The commonest clinical manifestations of TB-IRIS are fever, lymphadenopathy and worsening respiratory symptoms 50. Pulmonary disorders, such as new pulmonary infiltrates, mediastinal lymphadenopathy, and pleural effusions are also common 51. Extrapulmonary presentations are also possible, including disseminated tuberculosis with associated acute renal failure 34, systemic inflammatory responses 52, and intracranial tuberculomas 53. Pulmonary TB-IRIS can be diagnosed by transient worsening of chest radiographs, especially if old radiographs are available for comparison. Other symptoms are nonspecific, and include persistent fever, weight loss, and worsening respiratory symptoms. Abdominal TB-IRIS can present with nonspecific abdominal pain and obstructive jaundice.
In most studies, TB-IRIS occurs within two months of ART initiation 34. Among 43 cases of Mycobacterium tuberculosis-associated IRIS, the median onset of IRIS was 12–15 days (range 2–114 days), with only four of these cases occurring more than four weeks after the initiation of antiretroviral therapy 35. These studies suggest the onset of mycobacterial-associated IRIS is relatively soon after initiation of ART, and clinicians should maintain a high level of vigilance during this period.
Paradoxical central nervous system TB reactions are well described in HIV-negative patients, and include expanding intracranial tuberculomas, tuberculous meningitis, and spinal cord lesions 54. TB-associated central nervous system IRIS has also been reported in HIV-positive patients 55. Compared to non-central nervous system TB-IRIS, symptoms tend to occur later, usually 5–10 months after ART initiation 54. Crump et al 56 described an HIV-seropositive patient in who developed cervical lymphadenopathy after five weeks of ART. Five months later, central nervous system symptoms associated with an expanding intracranial tuberculoma appeared after initiation of antituberculous therapy. The significant morbidity in this case illustrates the importance of maintaining a high clinical suspicion for the disease, particularly in endemic areas.
Treatment of tuberculosis-associated IRIS (TB-IRIS)
Treatment for mycobacterial-associated IRIS depends on the presentation and disease severity. Most patients present with non-life threatening presentations which respond to the institution of appropriate antituberculous therapy. However a range of life threatening presentations, such as acute renal failure 34 and acute respiratory distress syndrome (ARDS) 57, are described and have significant morbidity and mortality. Morbidity and mortality might also be greater in resource-limited settings where limited management options exist. Since the pathogenesis of the syndrome is an inflammatory one, systemic corticosteroids or nonsteroidal anti-inflammatory drugs (NSAIDS) may alleviate symptoms. In studies where therapy for IRIS was mentioned, the use of corticosteroids was variable 51 and anecdotally effective. Therapies ranged from intravenous methylprednisolone 40 mg every 12 hours to prednisone 20–70 mg/day for 5–12 weeks. These practices reflect the lack of evidence from controlled trials for the use of anti-inflammatory agents in IRIS. A randomized, placebo controlled trial examining doses of prednisone 1.5 mg/kg/day for two weeks followed by 0.75 mg/kg/day for two weeks in mild to moderate TB-IRIS is currently underway in South Africa. Until data become available, it is reasonable to administer corticosteroids for severe cases of IRIS such as tracheal compression due to lymphadenopathy, refractory or debilitating lymphadenitis, or severe respiratory symptoms, such as stridor and ARDS. Interruption of ART is rarely necessary but could be considered in life-threatening situations.
In HIV-negative patients, adjuvant corticosteroid use in tuberculous meningitis provides evidence of improved survival and decreased neurologic sequelae over standard therapy alone 58. Once other infectious etiologies, have been excluded, standard antituberculous therapy should be initiated or continued as the clinical situation dictates, and a course of corticosteroid therapy should be considered for central nervous system TB-IRIS. Continuation of ART is desirable, although its discontinuation may be necessary in unresponsive cases or in those presenting with advanced neurological symptoms.
Atypical mycobacterial IRIS
In general, Mycobacterium avium complex-associated IRIS typically presents with lymphadenitis, with or without abscess formation and suppuration 59. Other less common presentations include respiratory failure secondary to acute respiratory distress syndrome (ARDS) 17, leprosy 60, pyomyositis with cutaneous abscesses 33, intra-abdominal disease 61, and involvement of joints, skin, soft tissues, and spine 61.
Several studies have characterized the time of onset of Mycobacterium-associated IRIS. In one study of Mycobacterium avium complex lymphadenitis, the onset of a febrile illness was the first sign of IRIS and occurred between 6 and 20 days after initiation of antiretroviral therapy 59. In another study, the median time interval from the start of antiretroviral therapy to the development of mycobacterial lymphadenitis was 17 days (range 7–85 days) 62.
Treatment atypical mycobacterial IRIS
As with TB-IRIS, evidence for treatment of IRIS due to atypical mycobacteria are scarce. Occasionally, surgical excision of profoundly enlarged nodes or debridement of necrotic areas is anecdotally reported 33. However, healing is often poor leaving large, persistent sinuses. Needle aspiration is another option for enlarged, fluctuant and symptomatic nodes. Otherwise, treatment is similar to Mycobacterium tuberculosis IRIS (TB-IRIS).
Cytomegalovirus infection
In the pre-ART era, CMV retinitis, a vision-threatening disease, carried a high annual incidence and was one of the most significant AIDS-associated morbidities 63. After the introduction of HAART, Jacobson et al described five patients diagnosed with CMV retinitis 4–7 weeks after ART initiation. They speculated that an HAART-induced inflammatory response may be responsible for unmasking a subclinical infection 64. In addition to classical CMV retinitis, ART led to new clinical manifestations of the infection, termed immune recovery vitritis (IRV) or immune recovery uveitis (IRU), in patients previously diagnosed with inactive AIDS-related CMV retinitis 65. Distinct from the minimal intraocular inflammation of classic CMV retinitis, these manifestations exhibit significant posterior segment ocular inflammation thought to be due to the presence of residual CMV antigens or proteins which serve as the antigenic stimulus for the syndrome 66. Clinical manifestations include vision impairment and floaters.
In a retrospective cohort, CMV-related IRIS was common (6/33 of IRIS cases, or 18%) 10. In prospective cohorts, symptomatic vitritis occurred in 63% (incidence rate 83 per 100 p-yr) of ART responders who carried a previous diagnosis of CMV retinitis but had inactive disease at the onset of antiretroviral therapy. The median time from ART initiation to immune recovery vitritis was (43 weeks) 67. Another large prospective surveillance study 68 identified 374 patients with a history of CMV retinitis involving 539 eyes. Thirty-one of 176 ART responders (17.6%) were diagnosed with immune recovery uveitis. Male gender, use of ART, higher CD4 cell counts, and involvement of the posterior retinal pole as factors associated with a reduced risk of developing immune recovery uveitis, whereas prior use of intravitreous injections of cidofovir, large retinal lesions, and adequate immune recovery on ART were associated with increased risk.
The diagnosis of ocular manifestations of IRIS requires a high level of suspicion. In addition to signs of retinitis, inflammatory symptoms include vitritis, papillitis, and macular edema, resulting in symptoms of loss of visual acuity and floaters in affected eyes.
Treatment of IRIS associated CMV retinitis
Treatment of IRIS associated CMV retinitis and immune recovery vitritis may involve anti-CMV therapy with gancyclovir or valgancyclovir 69. However, the occurrence of immune recovery uveitis in patients receiving anti-CMV therapy draws its use into question [64,66,67]. The use of systemic corticosteroids has been successful, and immune recovery vitritis may require periocular corticosteroid injections 70. Due to its significant morbidity and varying temporal presentations, clinicians should maintain a high level of vigilance for ocular manifestations of CMV-associated IRIS.
Varicella zoster virus infection
Although complications such as encephalitis, myelitis, cranial and peripheral nerve palsies, and acute retinal necrosis can occur in immunocompromised HIV patients, the vast majority of patients exhibit typical or atypical dermatomal involvement without dissemination or systemic symptoms 71.
A randomized, controlled trial demonstrated oral acyclovir to be effective for dermatomal zoster in HIV-infected patients, facilitating healing and shortening the time of zoster-associated pain 72. Its use in cases of varicella zoster IRIS appears to be of clinical benefit 73. The benefit of corticosteroids in combination with acyclovir in acute varicella zoster has been demonstrated in two large randomized, controlled trials. The combination of corticosteroids and acyclovir decreased healing times, improved acute pain, and quality of life, but did not affect the incidence or duration of postherpetic neuralgia 74. The incidence of postherpetic neuralgia in immunocompetent individuals does not differ significantly from HIV-infected patients, but increases with increasing patient age 75. Successful symptomatic management involving opioids, tricyclic antidepressants, gabapentin, and topical lidocaine patches individually or in combination has been shown to be beneficial 76 and should be attempted in HIV patients with postherpetic neuralgia as a complication of herpes zoster IRIS.
Cryptococcus neoformans infection
Cryptococcus neoformans-induced IRIS meningitis symptoms range in onset from seven days to ten months after initiation of ART, with 20 (49%) occurring within four weeks of therapy 77. In one study 78, patients with Cryptococcus neoformans-related IRIS meningitis were compared to typical AIDS-related Cryptococcus neoformans meningitis. Patients with Cryptococcus neoformans-related IRIS meningitis exhibited no difference in clinical presentation. However, Cryptococcus neoformans-related IRIS patients exhibited had higher baseline plasma HIV RNA levels and higher CSF cryptococcal antigen titers, opening pressures, WBC counts, and glucose levels. Additionally, IRIS patients were more likely to have ART initiated within 30 days of previously diagnosed Cryptococcus neoformans meningitis. Most documented cases of Cryptococcus neoformans-induced IRIS meningitis have occurred in patients with CD4 counts <100 cells/mm³ 79.
Cryptococcus neoformans infection treatment
A recent study 80 evaluated antifungal combination therapies in the treatment of Cryptococcus neoformans meningitis in HIV patients. Although significant log reductions in colony forming units were observed with all combinations, substantial numbers of patients remained culture positive 2 weeks after therapy. It may be important to delay ART until CSF sterility can be achieved with effective antifungal combinations such as amphotericin B and flucytosine. However, the exact timing of ART and whether attaining CSF culture sterility is important in avoiding IRIS is unknown. This is illustrated by cases of reactivation cryptococcal meningitis described in four patients who had received at least four weeks of antifungal therapy prior to ART 77. It is reasonable to administer systemic corticosteroids to alleviate unresponsive inflammatory effects, as anecdotal benefits have been observed in these patients 81. Furthermore, serial lumbar punctures may be required to manage persistent CSF pressure elevations in these patients 78. Although continuation of ART has been performed safely 81, interruption of antiviral therapy may be necessary in severe or unresponsive cases.
Immune reconstitution inflammatory syndrome management
To date, no prospective therapeutic trials concerning the management of IRIS have been conducted 5. All evidence regarding the management of IRIS in the literature relates to case reports and small case series reporting on management practice. This does not provide reliable evidence regarding either the safety or efficacy of these approaches, but merely guidance regarding the practice of others in managing this difficult condition.
The infectious pathogens most frequently implicated in the immune reconstitution inflammatory syndrome are mycobacterial infections, fungi, varicella zoster, herpesviruses, and cytomegalovirus (CMV). Majority of patients with IRIS have a self-limiting disease course. No single treatment option exists and depends on the underlying infectious agent and its clinical presentation.
In most cases, ART should not be interrupted. The Committee recommends symptomatic treatment of mild IRIS with nonsteroidal anti-inflammatory drugs (NSAIDs), drainage of abscesses, excision of inflamed or painful lymph nodes, and inhaled steroids for bronchospasm of cough 82.
In severe or life-threatening cases, the decision to interrupt ART is based on many factors individualized to the patient; consultation with experienced HIV care providers is recommended.
In cases of severe IRIS not caused by Kaposi’s sarcoma or Cryptococcus, corticosteroids are usually the treatment of choice. The recommended dose has not been standardized; this Committee recommends 1 to 2 mg/kg daily of prednisone or the equivalent for 1 to 2 weeks, followed by a taper. Corticosteroids can increase a patient’s risk of contracting other OIs and can also cause hyperglycemia, hypertension, mental status changes, or avascular necrosis, so careful monitoring is warranted.
One strategy to minimize the effect of IRIS is to coordinate initiation of antiretroviral therapy (ART) with the treatment of known opportunistic infections 82. In most cases, it is recommended that ART be started as soon as possible after HIV infection is diagnosed and within 2 weeks of initiating most opportunistic infection treatment 83. Exceptions to this include opportunistic infections that are known to carry a higher risk for severe or life-threatening IRIS when ART is initiated too soon, including tuberculous (TB) meningitis or other extrapulmonary TB 84, cytomegalovirus (CMV) retinitis 85 or Cryptococcus infections 86. Expert clinicians should be consulted in these situations.
References- Shelburne SA, 3rd, Hamill RJ, Rodriguez-Barradas MC, Greenberg SB, Atmar RL, Musher DW, Gathe JC, Jr., Visnegarwala F, Trautner BW. Immune reconstitution inflammatory syndrome: emergence of a unique syndrome during highly active antiretroviral therapy. Medicine (Baltimore) 2002;81:213–227. doi: 10.1097/00005792-200205000-00005
- Canfield GS, Henao-Martínez AF, Franco-Paredes C, et al. Corticosteroids for Posttransplant Immune Reconstitution Syndrome in Cryptococcus gattii Meningoencephalitis: Case Report and Literature Review [published correction appears in Open Forum Infect Dis. 2020 Jan 11;7(1):ofz542]. Open Forum Infect Dis. 2019;6(11):ofz460. Published 2019 Oct 23. doi:10.1093/ofid/ofz460 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6847472
- McComsey GA, Kitch D, Daar ES, et al. Inflammation markers after randomization to abacavir/lamivudine or tenofovir/emtricitabine with efavirenz or atazanavir/ritonavir. AIDS. 2012;26:1371-1385.
- Sharma SK, Soneja M. HIV & immune reconstitution inflammatory syndrome (IRIS). Indian J Med Res. 2011;134(6):866–877. doi:10.4103/0971-5916.92632 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3284095
- Murdoch DM, Venter WD, Van Rie A, Feldman C. Immune reconstitution inflammatory syndrome (IRIS): review of common infectious manifestations and treatment options. AIDS Res Ther. 2007;4:9. Published 2007 May 8. doi:10.1186/1742-6405-4-9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1871602
- Murdoch DM, Venter WDF, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–10.
- Kumarasamy N, Chaguturu S, Mayer KH, Solomon S, Yepthomi HT, Balakrishnan P, et al. Incidence of immune reconstitution syndrome in HIV/tuberculosis-coinfected patients after initiation of generic antiretroviral therapy in India. J Acquir Immune Defic Syndr. 2004;37:1574–6.
- Sharma SK, Dhooria S, Barwad P, Kadhiravan T, Ranjan S, Miglani S, et al. A study of TB-associated immune reconstitution inflammatory syndrome using the consensus case-definition. Indian J Med Res. 2010;131:804–8.
- Karmakar S, Sharma SK, Vashishtha R, Sharma A, Ranjan S, Gupta D, et al. Clinical characteristics of tuberculosis-associated immune reconstitution inflammatory syndrome in North Indian population of HIV/AIDS patients receiving HAART. Clin Dev Immunol. 2011;2011:239021
- French MA, Lenzo N, John M, Mallal SA, McKinnon EJ, James IR, Price P, Flexman JP, Tay-Kearney ML. Immune restoration disease after the treatment of immunodeficient HIV-infected patients with highly active antiretroviral therapy. HIV Med. 2000;1:107–115. doi: 10.1046/j.1468-1293.2000.00012.x
- Bell C, Nelson M, Kaye S. A case of immune reconstitution rheumatoid arthritis. Int J STD AIDS. 2002;13:580–581. doi: 10.1258/095646202760159747
- Behrens G, Knuth C, Schedel I, Mendila M, Schmidt RE. Highly active antiretroviral therapy. Lancet. 1998;351:1057–8; author reply 1058-9. doi: 10.1016/S0140-6736(05)79022-X
- Sereti I, Sarlis NJ, Arioglu E, Turner ML, Mican JM. Alopecia universalis and Graves’ disease in the setting of immune restoration after highly active antiretroviral therapy. Aids. 2001;15:138–140. doi: 10.1097/00002030-200101050-00026
- Calabrese LH, Kirchner E, Shrestha R. Rheumatic complications of human immunodeficiency virus infection in the era of highly active antiretroviral therapy: emergence of a new syndrome of immune reconstitution and changing patterns of disease. Semin Arthritis Rheum. 2005;35:166–174. doi: 10.1016/j.semarthrit.2005.03.007
- Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr., Hamill RJ. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. Aids. 2005;19:399–406. doi: 10.1097/01.aids.0000161769.06158.8a
- Mirmirani P, Maurer TA, Herndier B, McGrath M, Weinstein MD, Berger TG. Sarcoidosis in a patient with AIDS: a manifestation of immune restoration syndrome. J Am Acad Dermatol. 1999;41:285–286. doi: 10.1016/S0190-9622(99)70364-6
- Lawn SD. Acute respiratory failure due to Mycobacterium kansasii infection: immune reconstitution disease in a patient with AIDS. J Infect. 2005;51:339–340. doi: 10.1016/j.jinf.2005.02.004
- Silvestre JF, Albares MP, Ramon R, Botella R. Cutaneous intolerance to tattoos in a patient with human immunodeficiency virus: a manifestation of the immune restoration syndrome. Arch Dermatol. 2001;137:669–670.
- Jevtovic DJ, Salemovic D, Ranin J, Pesic I, Zerjav S, Djurkovic-Djakovic O. The prevalence and risk of immune restoration disease in HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 2005;6:140–143. doi: 10.1111/j.1468-1293.2005.00277.x
- Powles T, Thirlwell C, Nelson M, Bower M. Immune reconstitution inflammatory syndrome mimicking relapse of AIDS related lymphoma in patients with HIV 1 infection. Leuk Lymphoma. 2003;44:1417–1419. doi: 10.1080/1042819031000083280
- Piliero PJ, Fish DG, Preston S, Cunningham D, Kinchelow T, Salgo M, Qian J, Drusano GL. Guillain-Barre syndrome associated with immune reconstitution. Clin Infect Dis. 2003;36:e111–4. doi: 10.1086/368311
- Tangsinmankong N, Kamchaisatian W, Lujan-Zilbermann J, Brown CL, Sleasman JW, Emmanuel PJ. Varicella zoster as a manifestation of immune restoration disease in HIV-infected children. J Allergy Clin Immunol. 2004;113:742–746. doi: 10.1016/j.jaci.2004.01.768
- Ingiliz P, Appenrodt B, Gruenhage F, Vogel M, Tschampa H, Tasci S, Rockstroh JK. Lymphoid pneumonitis as an immune reconstitution inflammatory syndrome in a patient with CD4 cell recovery after HAART initiation. HIV Med. 2006;7:411–414. doi: 10.1111/j.1468-1293.2006.00389.x
- Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418–427. doi: 10.1086/499356
- Breton G, Adle-Biassette H, Therby A, Ramanoelina J, Choudat L, Bissuel F, Huerre M, Dromer F, Dupont B, Lortholary O. Immune reconstitution inflammatory syndrome in HIV-infected patients with disseminated histoplasmosis. Aids. 2006;20:119–121. doi: 10.1097/01.aids.0000199014.66139.39
- Safdar A, Rubocki RJ, Horvath JA, Narayan KK, Waldron RL. Fatal immune restoration disease in human immunodeficiency virus type 1-infected patients with progressive multifocal leukoencephalopathy: impact of antiretroviral therapy-associated immune reconstitution. Clin Infect Dis. 2002;35:1250–1257. doi: 10.1086/344056
- Intalapaporn P, Poovorawan Y, Suankratay C. Immune reconstitution syndrome associated with parvovirus B19-induced pure red cell aplasia during highly active antiretroviral therapy. J Infect. 2005
- Taylor CL, Subbarao V, Gayed S, Ustianowski AP. Immune reconstitution syndrome to Strongyloides stercoralis infection. Aids. 2007;21:649–650. doi: 10.1097/QAD.0b013e3280117f94
- Lawn SD, Wilkinson RJ. Immune reconstitution disease associated with parasitic infections following antiretroviral treatment. Parasite Immunol. 2006;28:625–633.
- Chan-Tack KM, Chengappa KS, Wolf JS, Kao GF, Reisler RB. Immune reconstitution inflammatory syndrome presenting as sinusitis with inflammatory pseudotumor in an HIV-infected patient: a case report and review of the literature. AIDS Patient Care STDS. 2006;20:823–828. doi: 10.1089/apc.2006.20.823
- Delfos NM, Collen AF, Kroon FP. Demodex folliculitis: a skin manifestation of immune reconstitution disease. Aids. 2004;18:701–702. doi: 10.1097/00002030-200403050-00019
- Woods ML, 2nd, MacGinley R, Eisen DP, Allworth AM. HIV combination therapy: partial immune restitution unmasking latent cryptococcal infection. Aids. 1998;12:1491–1494. doi: 10.1097/00002030-199812000-00011
- Lawn SD, Bicanic TA, Macallan DC. Pyomyositis and cutaneous abscesses due to Mycobacterium avium: an immune reconstitution manifestation in a patient with AIDS. Clin Infect Dis. 2004;38:461–463. doi: 10.1086/381033
- Jehle AW, Khanna N, Sigle JP, Glatz-Krieger K, Battegay M, Steiger J, Dickenmann M, Hirsch HH. Acute renal failure on immune reconstitution in an HIV-positive patient with miliary tuberculosis. Clin Infect Dis. 2004;38:e32–5. doi: 10.1086/381441
- Breton G, Duval X, Estellat C, Poaletti X, Bonnet D, Mvondo Mvondo D, Longuet P, Leport C, Vilde JL. Determinants of immune reconstitution inflammatory syndrome in HIV type 1-infected patients with tuberculosis after initiation of antiretroviral therapy. Clin Infect Dis. 2004;39:1709–1712. doi: 10.1086/425742
- Navas E, Martin-Davila P, Moreno L, Pintado V, Casado JL, Fortun J, Perez-Elias MJ, Gomez-Mampaso E, Moreno S. Paradoxical reactions of tuberculosis in patients with the acquired immunodeficiency syndrome who are treated with highly active antiretroviral therapy. Arch Intern Med. 2002;162:97–99. doi: 10.1001/archinte.162.1.97
- Autran B, Carcelain G, Li TS, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112
- Bucy RP, Hockett RD, Derdeyn CA, Saag MS, Squires K, Sillers M, Mitsuyasu RT, Kilby JM. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. J Clin Invest. 1999;103:1391–1398.
- Pakker NG, Notermans DW, de Boer RJ, Roos MT, de Wolf F, Hill A, Leonard JM, Danner SA, Miedema F, Schellekens PT. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nat Med. 1998;4:208–214. doi: 10.1038/nm0298-208
- Price P, Mathiot N, Krueger R, Stone S, Keane NM, French MA. Immune dysfunction and immune restoration disease in HIV patients given highly active antiretroviral therapy. J Clin Virol. 2001;22:279–287. doi: 10.1016/S1386-6532(01)00200-1
- Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, Vicaut E, Lagrange PH, Sereni D, Autran B. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. Aids. 2006;20:F1–7. doi: 10.1097/01.aids.0000202648.18526.bf
- Meintjes G, Rangaka MX, Maartens G, et al. Novel relationship between tuberculosis immune reconstitution inflammatory syndrome and antitubercular drug resistance. Clin Infect Dis. 2009;48:667-676.
- Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361-373.
- Gray F, Bazille C, Adle-Biassette H, et al. Central nervous system immune reconstitution disease in acquired immunodeficiency syndrome patients receiving highly active antiretroviral treatment. J Neurovirol. 2005;11 Suppl 3:16-22.
- Karavellas MP, Plummer DJ, Macdonald JC, et al. Incidence of immune recovery vitritis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J Infect Dis. 1999;179:697-700.
- Konopnicki D, Mocroft A, de Wit S, et al. Hepatitis B and HIV: prevalence, AIDS progression, response to highly active antiretroviral therapy and increased mortality in the EuroSIDA cohort. AIDS. 2005;19:593-601.
- Odongo FC. Fatal Disseminated Kaposi’s sarcoma due to immune reconstitution inflammatory syndrome following HAART initiation. Case Rep Infect Dis. 2013;2013:546578.
- Bowen LN, Smith B, Reich D, et al. HIV-associated opportunistic CNS infections: pathophysiology, diagnosis and treatment. Nat Rev Neurol. 2016;12:662-674.
- Rasul S, Delapenha R, Farhat F, et al. Graves’ disease as a manifestation of immune reconstitution in HIV-infected individuals after initiation of highly active antiretroviral therapy. AIDS Res Treat. 2011;2011:743597
- Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–373. doi: 10.1016/S1473-3099(05)70140-7
- Narita M, Ashkin D, Hollender ES, Pitchenik AE. Paradoxical worsening of tuberculosis following antiretroviral therapy in patients with AIDS. Am J Respir Crit Care Med. 1998;158:157–161.
- Furrer H, Malinverni R. Systemic inflammatory reaction after starting highly active antiretroviral therapy in AIDS patients treated for extrapulmonary tuberculosis. Am J Med. 1999;106:371–372. doi: 10.1016/S0002-9343(99)00015-7
- Crump JA, Tyrer MJ, Lloyd-Owen SJ, Han LY, Lipman MC, Johnson MA. Military tuberculosis with paradoxical expansion of intracranial tuberculomas complicating human immunodeficiency virus infection in a patient receiving highly active antiretroviral therapy. Clin Infect Dis. 1998;26:1008–1009
- Cheng VC, Ho PL, Lee RA, Chan KS, Chan KK, Woo PC, Lau SK, Yuen KY. Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients. Eur J Clin Microbiol Infect Dis. 2002;21:803–809. doi: 10.1007/s10096-002-0821-2
- Vidal JE, Cimerman S, Schiavon Nogueira R, Bonasser Filho F, Sztajnbok J, da Silva PR, Lins DL, Coelho JF. Paradoxical reaction during treatment of tuberculous brain abscess in a patient with AIDS. Rev Inst Med Trop Sao Paulo. 2003;45:177–178.
- Crump JA, Tyrer MJ, Lloyd-Owen SJ, Han LY, Lipman MC, Johnson MA. Military tuberculosis with paradoxical expansion of intracranial tuberculomas complicating human immunodeficiency virus infection in a patient receiving highly active antiretroviral therapy. Clin Infect Dis. 1998;26:1008–1009.
- Goldsack NR, Allen S, Lipman MC. Adult respiratory distress syndrome as a severe immune reconstitution disease following the commencement of highly active antiretroviral therapy. Sex Transm Infect. 2003;79:337–338. doi: 10.1136/sti.79.4.337
- Thwaites GE, Nguyen DB, Nguyen HD, Hoang TQ, Do TT, Nguyen TC, Nguyen QH, Nguyen TT, Nguyen NH, Nguyen TN, Nguyen NL, Nguyen HD, Vu NT, Cao HH, Tran TH, Pham PM, Nguyen TD, Stepniewska K, White NJ, Tran TH, Farrar JJ. Dexamethasone for the treatment of tuberculous meningitis in adolescents and adults. N Engl J Med. 2004;351:1741–1751. doi: 10.1056/NEJMoa040573
- Race EM, Adelson-Mitty J, Kriegel GR, Barlam TF, Reimann KA, Letvin NL, Japour AJ. Focal mycobacterial lymphadenitis following initiation of protease-inhibitor therapy in patients with advanced HIV-1 disease. Lancet. 1998;351:252–255. doi: 10.1016/S0140-6736(97)04352-3
- Lawn SD, Wood C, Lockwood DN. Borderline tuberculoid leprosy: an immune reconstitution phenomenon in a human immunodeficiency virus-infected person. Clin Infect Dis. 2003;36:e5–6. doi: 10.1086/344446
- Phillips P, Bonner S, Gataric N, Bai T, Wilcox P, Hogg R, O’Shaughnessy M, Montaner J. Nontuberculous mycobacterial immune reconstitution syndrome in HIV-infected patients: spectrum of disease and long-term follow-up. Clin Infect Dis. 2005;41:1483–1497. doi: 10.1086/497269
- Phillips P, Kwiatkowski MB, Copland M, Craib K, Montaner J. Mycobacterial lymphadenitis associated with the initiation of combination antiretroviral therapy. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:122–128.
- Jacobson MA, Mills J. Serious cytomegalovirus disease in the acquired immunodeficiency syndrome (AIDS). Clinical findings, diagnosis, and treatment. Ann Intern Med. 1988;108:585–594.
- Jacobson MA, Zegans M, Pavan PR, O’Donnell JJ, Sattler F, Rao N, Owens S, Pollard R. Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet. 1997;349:1443–1445. doi: 10.1016/S0140-6736(96)11431-8
- Karavellas MP, Lowder CY, Macdonald C, Avila CP, Jr., Freeman WR. Immune recovery vitritis associated with inactive cytomegalovirus retinitis: a new syndrome. Arch Ophthalmol. 1998;116:169–175.
- Schrier RD, Song MK, Smith IL, Karavellas MP, Bartsch DU, Torriani FJ, Garcia CR, Freeman WR. Intraocular viral and immune pathogenesis of immune recovery uveitis in patients with healed cytomegalovirus retinitis. Retina. 2006;26:165–169. doi: 10.1097/00006982-200602000-00007
- Karavellas MP, Plummer DJ, Macdonald JC, Torriani FJ, Shufelt CL, Azen SP, Freeman WR. Incidence of immune recovery vitritis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J Infect Dis. 1999;179:697–700. doi: 10.1086/314639
- Kempen JH, Min YI, Freeman WR, Holland GN, Friedberg DN, Dieterich DT, Jabs DA. Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology. 2006;113:684–694. doi: 10.1016/j.ophtha.2005.10.067
- Kosobucki BR, Goldberg DE, Bessho K, Koh HJ, Rodanant N, Labree L, Cheng L, Schrier RD, Azen SP, Freeman WR. Valganciclovir therapy for immune recovery uveitis complicated by macular edema. Am J Ophthalmol. 2004;137:636–638.
- Arevalo JF, Mendoza AJ, Ferretti Y. Immune recovery uveitis in AIDS patients with cytomegalovirus retinitis treated with highly active antiretroviral therapy in Venezuela. Retina. 2003;23:495–502. doi: 10.1097/00006982-200308000-00009
- Domingo P, Torres OH, Ris J, Vazquez G. Herpes zoster as an immune reconstitution disease after initiation of combination antiretroviral therapy in patients with human immunodeficiency virus type-1 infection. Am J Med. 2001;110:605–609. doi: 10.1016/S0002-9343(01)00703-3
- Gnann JW, Jr., Crumpacker CS, Lalezari JP, Smith JA, Tyring SK, Baum KF, Borucki MJ, Joseph WP, Mertz GJ, Steigbigel RT, Cloud GA, Soong SJ, Sherrill LC, DeHertogh DA, Whitley RJ. Sorivudine versus acyclovir for treatment of dermatomal herpes zoster in human immunodeficiency virus-infected patients: results from a randomized, controlled clinical trial. Collaborative Antiviral Study Group/AIDS Clinical Trials Group, Herpes Zoster Study Group. Antimicrob Agents Chemother. 1998;42:1139–1145.
- Martinez E, Gatell J, Moran Y, Aznar E, Buira E, Guelar A, Mallolas J, Soriano E. High incidence of herpes zoster in patients with AIDS soon after therapy with protease inhibitors. Clin Infect Dis. 1998;27:1510–1513.
- Whitley RJ, Weiss H, Gnann JW, Jr., Tyring S, Mertz GJ, Pappas PG, Schleupner CJ, Hayden F, Wolf J, Soong SJ. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376–383.
- Choo PW, Galil K, Donahue JG, Walker AM, Spiegelman D, Platt R. Risk factors for postherpetic neuralgia. Arch Intern Med. 1997;157:1217–1224. doi: 10.1001/archinte.157.11.1217
- Kanazi GE, Johnson RW, Dworkin RH. Treatment of postherpetic neuralgia: an update. Drugs. 2000;59:1113–1126. doi: 10.2165/00003495-200059050-00007
- Boelaert JR, Goddeeris KH, Vanopdenbosch LJ, Casselman JW. Relapsing meningitis caused by persistent cryptococcal antigens and immune reconstitution after the initiation of highly active antiretroviral therapy. Aids. 2004;18:1223–1224. doi: 10.1097/00002030-200405210-00023
- Shelburne SA, 3rd, Darcourt J, White AC, Jr., Greenberg SB, Hamill RJ, Atmar RL, Visnegarwala F. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:1049–1052. doi: 10.1086/428618
- York J, Bodi I, Reeves I, Riordan-Eva P, Easterbrook PJ. Raised intracranial pressure complicating cryptococcal meningitis: immune reconstitution inflammatory syndrome or recurrent cryptococcal disease? J Infect. 2005;51:165–171. doi: 10.1016/j.jinf.2005.04.022
- Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, Harrison TS. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363:1764–1767. doi: 10.1016/S0140-6736(04)16301-0
- King MD, Perlino CA, Cinnamon J, Jernigan JA. Paradoxical recurrent meningitis following therapy of cryptococcal meningitis: an immune reconstitution syndrome after initiation of highly active antiretroviral therapy. Int J STD AIDS. 2002;13:724–726. doi: 10.1258/095646202760326516
- Expert Commentary: NYSDOH AIDS Institute Updated Guideline on Management of Immune Reconstitution Inflammatory Syndrome (IRIS). https://www.medscape.com/viewarticle/889696
- Opportunistic infections and AIDS malignancies early after initiating combination antiretroviral therapy in high-income countries. AIDS. 2014;28:2461-2473.
- Namale PE, Abdullahi LH, Fine S, et al. Paradoxical TB-IRIS in HIV-infected adults: a systematic review and meta-analysis. Future Microbiol. 2015;10:1077-1099.
- Jabs DA, Ahuja A, Van Natta M, et al. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology. 2010;117:2152-61.e1-2.
- Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370:2487-2498.