Inflammatory bowel disease
Inflammatory bowel disease (IBD) is a medical term that describes conditions characterized by long-standing (chronic) inflammation of the gastrointestinal tract. The inflammation lasts a long time before subsiding, but it usually comes back over and over again. The two most common types of inflammatory bowel diseases are Crohn’s disease and ulcerative colitis 1. Microscopic colitis are other common types of inflammatory bowel disease (IBD). In 2015, an estimated 1.3% of US adults (3 million) reported being diagnosed with some kind of IBD (either Crohn’s disease or ulcerative colitis) 2. Prevalence differed by several sociodemographic characteristics, including age, race/ethnicity, education, and poverty.
There are 2 main types of inflammatory bowel disease (IBD):
- Crohn’s disease
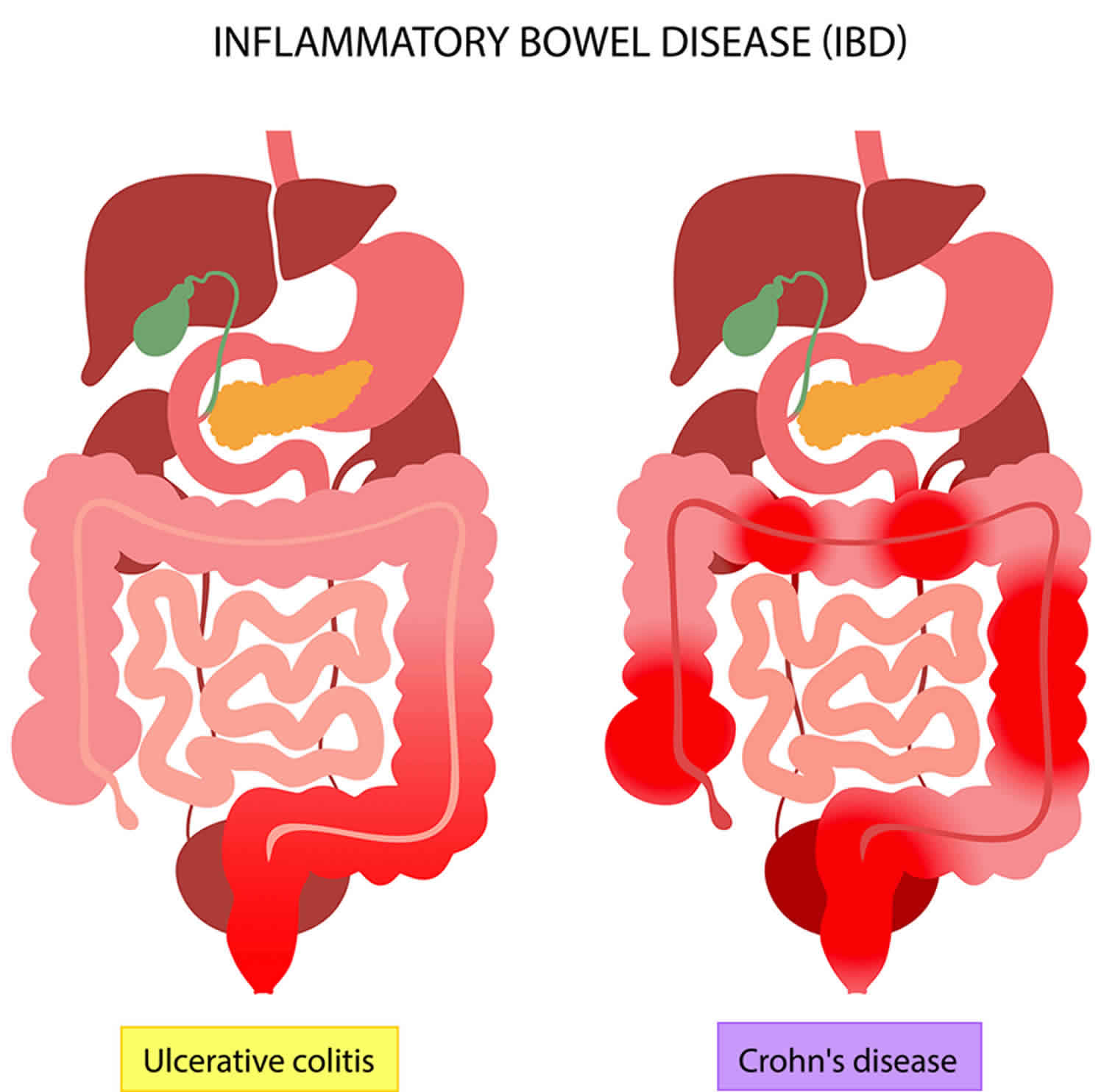
- Crohn’s disease is an IBD that causes ulcers to form in the gastrointestinal (GI) tract anywhere from the mouth to the anus. Most often it affects the portion of the small intestine before the large intestine or colon.
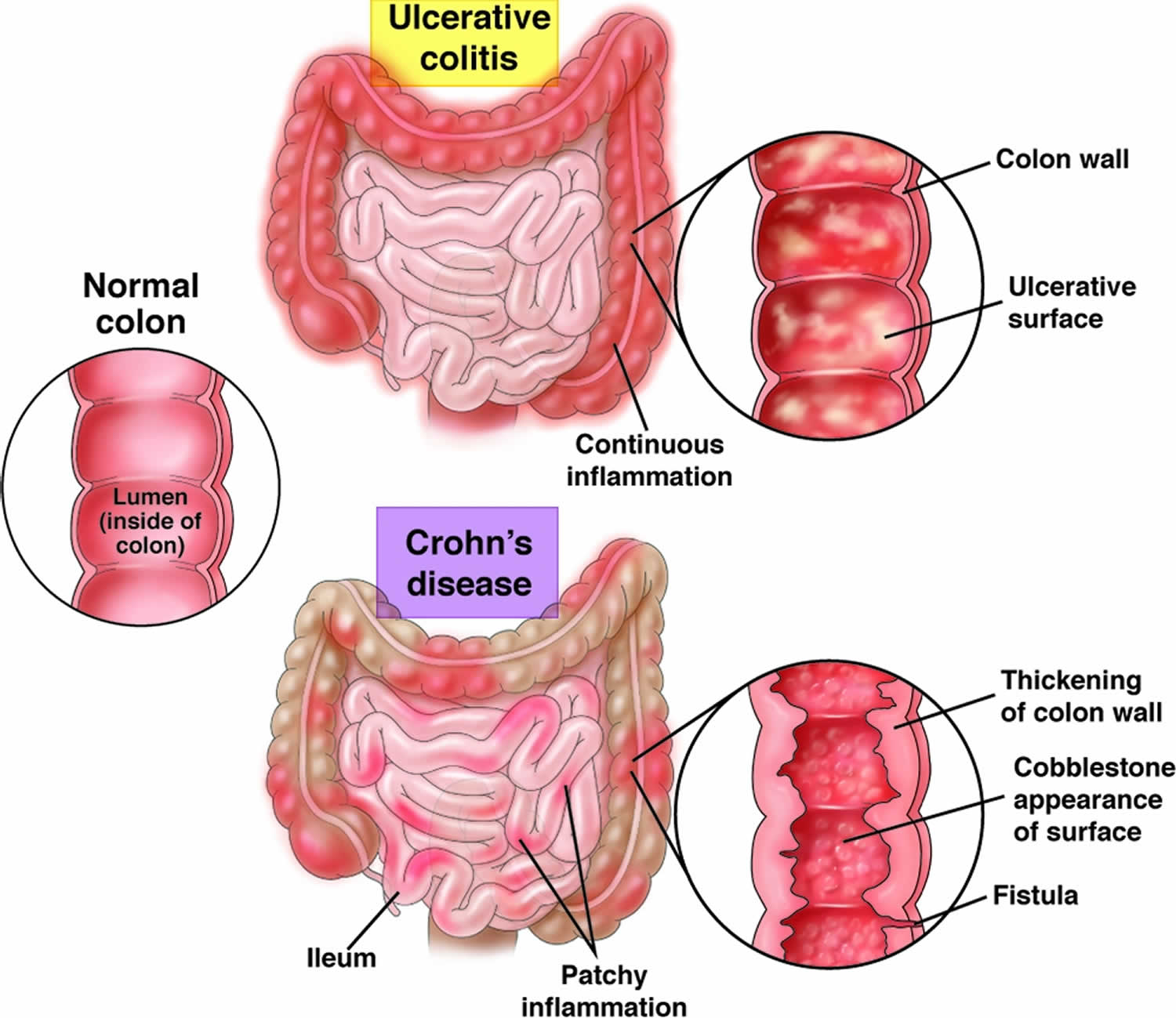
- Crohn’s disease can have “skip” areas that are normal. These normal areas lie in between areas that are affected. That is damaged areas appear in patches that are next to areas of healthy tissue.
- Inflammation may reach through the multiple layers of the walls of the gastrointestinal tract.
- Some people who have Crohn’s disease have severe symptoms. Others have symptoms that are not as severe. Some people who have the disease have long periods without symptoms, even without getting treatment. Others who have more severe symptoms will need long-term treatment or even surgery.
- Ulcerative colitis
- Ulcerative colitis occurs in the large intestine (colon) and the rectum.
- Ulcerative colitis is an IBD that causes your colon (large intestine) to become red and swollen. The redness and swelling can last for a few weeks or for several months. Ulcerative colitis always involves the last part of the colon (the rectum). It can go higher up in the colon, up to involving the whole colon.
- Ulcerative colitis never has the “skip” areas typical of Crohn’s disease. That is damaged areas are continuous (not patchy) – usually starting at the rectum and spreading further into the colon.
- Inflammation is present only in the innermost (deepest) layer of the lining of the colon.
- Symptoms may come and go. These occurrences are called flare-ups. Flare-ups can last many months and may come back at different times throughout your life.
Common symptoms of inflammatory bowel disease (both Crohn’s disease and ulcerative colitis) may include:
- Persistent diarrhea.
- Abdominal pain and cramping.
- Rectal bleeding or bloody stools.
- Reduced appetite.
- Unintended weight loss.
- Fatigue.
The exact cause of inflammatory bowel disease remains unknown. Previously, diet and stress were suspected, but now doctors know that these factors may aggravate but aren’t the cause of IBD. One possible cause is an immune system malfunction. When your immune system tries to fight off an invading virus or bacterium, an atypical immune response causes the immune system to attack the cells in your digestive tract, too. Several gene mutations have been associated with IBD. Heredity also seems to play a role in that IBD is more common in people who have family members with the disease. However, most people with IBD don’t have this family history.
To diagnose inflammatory bowel disease, your doctor will give you a physical exam and listen to you describe your symptoms. To help diagnose inflammatory bowel disease your doctor may order a number of tests, including blood tests and stool samples. Your doctor may also order one or more procedures to help them view your colon. Lower gastrointestinal (GI) endoscopy procedures include colonoscopy and flexible sigmoidoscopy. During these procedures, your doctor uses a narrow, flexible tube to look directly inside your large intestine. Upper gastrointestinal (GI) endoscopy allows a look at your stomach and small intestine for ulcers. For this type of endoscopy, you may swallow a small camera (called capsule endoscopy). If not, your doctor inserts a scope into your gastrointestinal tract through your mouth. Your doctor may also order other imaging tests such as X-rays, a CT scan, or an MRI.
Inflammatory bowel disease (IBD) is commonly treated with:
- Medications
- 5-aminosalicyclic acids.
- Immunomodulators.
- Corticosteroids.
- Biologics.
- In severe cases of inflammatory bowel disease, you may need to go to the hospital for intravenous (IV) fluids or surgery.
- Surgeries to remove damaged portions of the gastrointestinal tract.
Patients with inflammatory bowel disease (IBD) need lifelong follow-up for their disease. There is no particular diet or supplement that has been shown to delay or prevent the symptoms of the disease.
Figure 1. Inflammatory bowel disease (IBD)
What is microscopic colitis?
Microscopic colitis is an inflammatory bowel disease (IBD) 3 and is described by a clinicopathological triad characterized by a history of chronic or intermittent watery diarrhea, normal or almost normal endoscopic examination of the colon (e.g., with slight edema, erythema, and/or loss of vascular pattern, although rarely more significant macroscopic changes are reported, including pseudomembranes and ‘cat scratch changes’) 4, as well as a distinct histological pattern when examined under a microscope – hence the name microscopic colitis 5. Unlike the other types of inflammatory bowel disease (IBD), microscopic colitis does not increase your risk of developing colon cancer 6.
Microscopic colitis is divided into two subtypes: collagenous colitis and lymphocytic colitis. The two types cause different changes in colon tissue 7:
- Lymphocytic colitis, the colon lining contains more white blood cells than normal. The layer of collagen under the colon lining is normal or only slightly thicker than normal.
- Collagenous colitis, the layer of collagen under the colon lining is thicker than normal. The colon lining may also contain more white blood cells than normal.
Doctors call both types microscopic colitis, and they have the same symptoms and treatments.
Research suggests that, in the United States, about 700,000 people have microscopic colitis 8. Although most patients receive their diagnosis at an age of 60 or above, approximately 25% of patients get diagnosed before the age of 45 9. Microscopic colitis has even been reported in children, which, however, is very rare 10. A female preponderance exists in both collagenous and lymphocytic colitis, with a female-to-male ratio of 3.1 and 1.9, respectively 11.
To help diagnose microscopic colitis, your doctor will ask about your symptoms and medical history and will perform a physical exam. Your doctor may ask about factors that increase the risk of developing microscopic colitis, such as smoking or taking certain medicines.
Your doctor may order medical tests, such as blood and stool tests, to check for signs of conditions that cause symptoms similar to those of microscopic colitis. Conditions that cause similar symptoms include celiac disease, other types of inflammatory bowel disease (IBD), and infections.
To treat microscopic colitis, your doctor may recommend:
- quitting smoking, if you smoke
- changing any medicines you take that could be causing microscopic colitis or making your symptoms worse
- taking medicines to treat microscopic colitis
- changing what you eat and drink to help improve symptoms
Medicines most often treat microscopic colitis effectively. In rare cases, doctors may recommend surgery.
Inflammatory bowel disease causes
The exact cause of inflammatory bowel disease (IBD) remains unknown, but scientists believe that IBD occurs in genetically susceptible individuals after an inappropriate immune response to the intestinal flora 12. Normally, the immune system protects your body from infection. In people who have an inflammatory bowel disease, the immune system mistakes food, healthy bacteria, and other substances for an infection. This causes the immune system to attack the cells of the intestine, which leads to inflammation.
Many causes have been implicated but none is universally present in all patients. The one consistent feature of Crohn disease is that it has a strong link with tobacco. On the other hand, it appears that smoking protects against ulcerative colitis 12. The role of diet remains debatable 12.
Possible causes of IBD are:
- The immune system responds incorrectly to environmental triggers, such as a virus or bacteria, which causes inflammation of the gastrointestinal tract.
- There also appears to be a genetic component. Someone with a family history of inflammatory bowel disease is more likely to develop this inappropriate immune response.
The CARD15 gene has been associated with inflammatory bowel disease but because of its polymorphic features, it is not possible to determine which part of the gastrointestinal tract will be affected. The role of genes in ulcerative colitis is not as strong as in Crohn disease.
Risk factors for inflammatory bowel disease
- Age. Most people who develop IBD are diagnosed before they’re 30 years old. But some people don’t develop the disease until their 50s or 60s.
- Race or ethnicity. Although IBD is more common in white people, it can occur in any race. Cases are also increasing in other races and ethnicities.
- Family history. You’re at higher risk if you have a close relative — such as a parent, sibling or child — with the disease.
- Cigarette smoking. Cigarette smoking is the most important controllable risk factor for developing Crohn’s disease. Smoking may help prevent ulcerative colitis. However, its harm to overall health outweighs any benefit, and quitting smoking can improve the general health of your digestive tract, as well as provide many other health benefits.
- Nonsteroidal anti-inflammatory medications. These include ibuprofen (Advil, Motrin IB, others), naproxen sodium (Aleve), diclofenac sodium and others. These medications may increase the risk of developing IBD or worsen the disease in people who have IBD.
Inflammatory bowel disease prevention
At the present time because scientists don’t known what causes inflammatory bowel disease (IBD), IBD cannot be prevented. However, there are lifestyle changes you can make to minimize your symptoms. The best thing you can do is to take good care of yourself. It’s important to eat a healthy diet. Depending on your symptoms, your doctor may ask you to reduce the amount of fiber or dairy products in your diet. It also may be necessary to limit or avoid caffeine, alcohol, and carbonated beverages. In addition to eating well, you need to get enough rest and exercise regularly. It’s also important that you learn to manage the stress in your life. When you become overly upset by things that happen at home or at work, your intestinal problems can get worse.
Inflammatory bowel disease dietary guidelines
To help patients navigate their nutritional questions, the International Organization of Inflammatory Bowel Diseases recently reviewed the best current evidence to develop expert recommendations regarding dietary measures that might help to control and prevent relapse of inflammatory bowel disease 13. In particular, the International Organization of Inflammatory Bowel Diseases focused on the dietary components and additives that they felt were the most important to consider because they comprise a large proportion of the diets that inflammatory bowel disease patients may follow. Furthermore, the recent International Organization of Inflammatory Bowel Diseases guidelines are an excellent starting point for discussions between patients and their doctors about whether specific dietary changes might be helpful in reducing symptoms and risk of relapse of inflammatory bowel disease. However, all patients with inflammatory bowel disease (IBD) should work with their doctor or a nutritionist, who will conduct a nutritional assessment to check for malnutrition and provide advice to correct deficiencies if they are present.
The International Organization of Inflammatory Bowel Diseases recommendations were developed with the aim of reducing symptoms and inflammation 13. The ways in which altering the intake of particular foods may trigger or reduce inflammation are quite diverse, and the mechanisms are better understood for certain foods than others. For example, fruits and vegetables are generally higher in fiber, which is fermented by bacterial enzymes within the colon. This fermentation produces short-chain fatty acids (SCFAs) that provide beneficial effects to the cells lining the colon. Patients with active IBD have been observed to have decreased short-chain fatty acids, so increasing the intake of plant-based fiber may work, in part, by boosting the production of short-chain fatty acids. However, it is important to note disease-specific considerations that might be relevant to your particular situation. For example, about one-third of Crohn’s disease patients will develop an area of intestinal narrowing, called a stricture, within the first 10 years of diagnosis. Insoluble fiber can worsen symptoms and, in some cases, lead to intestinal blockage if a stricture is present. So, while increasing consumption of fruits and vegetable is generally beneficial for Crohn’s disease, patients with a stricture should limit their intake of insoluble fiber.
Table 1. The International Organization of Inflammatory Bowel Diseases Dietary guidelines
| Food | If you have Crohn’s disease | If you have ulcerative colitis |
| Fruits | increase intake | insufficient evidence |
| Vegetables | increase intake | insufficient evidence |
| Red/processed meat | insufficient evidence | decrease intake |
| Unpasteurized dairy products | best to avoid | best to avoid |
| Dietary fat | decrease intake of saturated fats and avoid trans fats | decrease consumption of myristic acid (palm, coconut, dairy fat), avoid trans fats, and increase intake of omega-3 (from marine fish but not dietary supplements) |
| Food additives | decrease intake of maltodextrin-containing foods | decrease intake of maltodextrin-containing foods |
| Thickeners | decrease intake of carboxymethylcellulose | decrease intake of carboxymethylcellulose |
| Carrageenan (a thickener extracted from seaweed) | decrease intake | decrease intake |
| Titanium dioxide (a food colorant and preservative) | decrease intake | decrease intake |
| Sulfites (flavor enhancer and preservative) | decrease intake | decrease intake |
What are specific diets for inflammatory bowel disease?
A number of specific diets have been explored for inflammatory bowel disease (IBD), including the Mediterranean diet, specific carbohydrate diet, Crohn’s disease exclusion diet, autoimmune protocol diet, and a diet low in fermentable oligo-, di-, monosaccharides, and polyols (FODMAPs). Although the International Organization of Inflammatory Bowel Diseases group initially set out to evaluate some of these diets, they did not find enough high-quality trials that specifically studied them 13. Therefore, they limited their recommendations to individual dietary components. Stronger recommendations may be possible once additional trials of these dietary patterns become available. For the time being, patients are to monitor for correlations of specific foods to their symptoms. In some cases, patients may explore some of these specific diets to see if they help.
Summary
There is scientific evidence that dietary factors may influence both the risk of developing inflammatory bowel disease and intestinal mucosal inflammation. However, there is lack of large prospective controlled trials to provide the dietary recommendations patients’ desire. Taken together, studies of exclusive enteral nutrition, exclusion diets, and semi-vegetarian diets suggest that minimizing exposure of the intestinal lumen to selected food items may prolong the remission state of patients with inflammatory bowel disease 14. Even less evidence exists for the efficacy of the specific carbohydrate diet, FODMAP, or Paleo diet. Furthermore, the practicality of maintaining these interventions over long periods of time is doubtful. Patient-targeted dietary recommendations focus on food restrictions and are highly conflicting. High quality dietary intervention studies are needed to facilitate creation of evidence-based dietary guidelines for patients with inflammatory bowel disease. At a practical level, adherence to defined diets may result in an unnecessary financial burden or reduction in overall caloric intake in patients who are already at risk for protein-calorie malnutrition.
Inflammatory bowel disease signs and symptoms
Inflammatory bowel disease symptoms vary, depending on the severity of inflammation and where it occurs. Symptoms may range from mild to severe. You are likely to have periods of active illness followed by periods of remission.
Common symptoms of inflammatory bowel disease (both Crohn’s disease and ulcerative colitis) may include:
- Persistent diarrhea.
- Abdominal pain and cramping.
- Rectal bleeding or bloody stools.
- Reduced appetite.
- Unintended weight loss.
- Fatigue.
Symptoms of Crohn’s disease
The symptoms of Crohn’s disease vary, depending on which part or parts of the gastrointestinal (GI) tract is/are affected. Common symptoms include:
- Diarrhea
- Stomach cramps
- Abdominal pain that comes and goes
- Blood in your stool
- Low appetite
- Unintended weight loss
Other less common symptoms may include fever, joint pain, eye problems, skin problems, and feeling tired (called fatigue). The symptoms of Crohn’s disease may be mild or severe. Symptoms may also come and go. They can start suddenly or gradually.
Symptoms of ulcerative colitis
Symptoms of ulcerative colitis vary. Symptoms depend on how severe your case is and how much of your large intestine is affected. Common symptoms include:
- Rectal pain or bleeding
- Frequent, small bowel movements
- Feeling an urgent need to have a bowel movement
- Diarrhea
- Blood in the stool
- Abdominal cramping and pain
- A strong feeling that you need to have a bowel movement, but not being able to do so (called tenesmus)
- Pain on the left side of the abdomen
- Unintended weight loss
- Fatigue
In most people who have ulcerative colitis, these symptoms tend to come and go. You may have periods where you have no symptoms, followed by periods where you do have symptoms.
Inflammatory bowel disease complications
Although inflammatory bowel disease usually isn’t fatal, it’s a serious disease that, in some cases, may cause life-threatening complications.
Complications of Crohn’s disease may include:
- Bowel obstruction. Crohn’s disease affects the full thickness of the intestinal wall. Over time, parts of the bowel can thicken and narrow, which may block the flow of digestive contents. You may require surgery to remove the diseased portion of your bowel.
- Malnutrition. Diarrhea, abdominal pain and cramping may make it difficult for you to eat or for your intestine to absorb enough nutrients to keep you nourished. It’s also common to develop anemia due to low iron or vitamin B-12 caused by the disease.
- Fistulas. Sometimes inflammation can extend completely through the intestinal wall, creating a fistula — an atypical connection between different body parts. Fistulas near or around the anal area (perianal fistula) are the most common kind. But they can also occur internally or toward the wall of the abdominal area. In some cases, a fistula may become infected and form an infected pocket of pus known as an abscess.
- Anal fissure. This is a small tear in the tissue that lines the anus or in the skin around the anus where infections can occur. It’s often associated with painful bowel movements and may lead to a perianal fistula.
Complications of ulcerative colitis may include:
- Toxic megacolon. Ulcerative colitis may cause the colon to rapidly widen and swell, a serious condition known as toxic megacolon.
- A hole in the colon (perforated colon). A perforated colon most commonly is caused by toxic megacolon, but it may also occur on its own.
The complications of inflammatory bowel disease (IBD) are divided into two categories, intestinal and extraintestinal.
Intestinal
- Hemorrhage (intestinal bleeding)
- Strictures (an area of narrowing in the intestines) causing bowel obstruction
- Colon perforation
- Anal fistulas
- Pelvic or perirectal abscesses
- Toxic megacolon
- Cholangiocarcinoma
- Colon cancer. Having ulcerative colitis or Crohn’s disease that affects most of your colon can increase your risk of colon cancer. Screening for cancer with a colonoscopy at regular intervals begins usually about 8 to 10 years after the diagnosis is made. Ask your doctor when and how frequently you need to have this test done.
Extra intestinal
- Osteoporosis
- Deep vein thrombosis (DVT)
- Anemia
- Gallstones
- Primary sclerosing cholangitis. In this rather uncommon condition seen in people with IBD, inflammation causes scarring within the bile ducts. This scarring eventually narrows the ducts, restricting bile flow. This can eventually cause liver damage.
- Aphthous ulcers
- Arthritis
- Iritis
- Uveitis
- Pyoderma gangrenosum
- Medication side effects. Certain medications for IBD are associated with a risk of infections. Some carry a small risk of developing certain cancers. Corticosteroids can be associated with a risk of osteoporosis, high blood pressure and other conditions.
- Severe dehydration. Excessive diarrhea can result in dehydration.
Inflammatory bowel disease diagnosis
To diagnose inflammatory bowel disease, your doctor will give you a physical exam and listen to you describe your symptoms. To help diagnose inflammatory bowel disease your doctor may order a number of tests, including blood tests and stool samples. Your doctor may also order one or more procedures to help them view your colon. Endoscopy evaluation with either esophagogastroduodenoscopy, colonoscopy, or both is essential to obtaining biopsies to confirm a diagnosis of IBD.
Diagnosing inflammatory bowel disease (IBD) requires a combination of clinical findings, inflammatory laboratory markers, imaging findings, and endoscopic biopsies. Blood findings include microcytic anemia, leukocytosis, and thrombocytosis, inflammatory markers such as the erythrocyte sedimentation rate (ESR), and high-sensitivity C-reactive protein (hsCRP) are commonly elevated 15, 16.
In some patients, the diagnosis may require ruling out parasitic diseases like giardia, amebiasis, strongyloides, and also tuberculosis.
Fecal calprotectin levels can be used as a marker for intestinal inflammation. Levels of perinuclear antineutrophilic cytoplasmic and anti-saccharomyces cerevisiae antibodies may be elevated in Crohn disease. Finally, stool studies must be done to rule out ova and parasitic organisms.
The abdominal x-ray can assess for the presence of free air, bowel obstruction, or toxic megacolon.
Barium studies are done to characterize the bowel disease; a lead pipe appearance indicates ulcerative colitis; sparing of the rectum is indicative of Crohn disease and thumb printing is indicative of mucosal inflammation. Further, the barium studies may reveal skip lesions and stricture formation in the ileum, which are indicative of Crohn disease.
Ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) have all been used in the diagnosis of IBD or to assess for complications. Ultrasound usage in trained individuals can evaluate the right lower quadrant for ileal disease. MRI can evaluate for rectal fistulas. Most commonly, CT is employed to evaluate for perforation or bowel obstruction. CT enterography can be helpful in assessing for strictures or in operative planning.
Lab tests
- Tests for anemia or infection. Your doctor may suggest blood tests to check for anemia — a condition in which there aren’t enough red blood cells to carry adequate oxygen to your tissues — or to check for signs of infection from bacteria or viruses.
- Stool studies. You may need to provide a stool sample so that your doctor can test for hidden (occult) blood or organisms, such as parasites, in your stool.
Endoscopic procedures
- Colonoscopy. This exam allows your provider to view your entire colon using a thin, flexible, lighted tube with a camera at the end. During the procedure, small samples of tissue (biopsy) may be taken for laboratory analysis. A biopsy is the way to make the diagnosis of IBD versus other forms of inflammation.
- Flexible sigmoidoscopy. Your provider uses a slender, flexible, lighted tube to examine the rectum and sigmoid, the last portion of your colon. If your colon is severely inflamed, your provider may perform this test instead of a full colonoscopy.
- Upper endoscopy. In this procedure, your provider uses a slender, flexible, lighted tube to examine the esophagus, stomach and first part of the small intestine (duodenum). While it is rare for these areas to be involved with Crohn’s disease, this test may be recommended if you are having nausea and vomiting, difficulty eating, or upper abdominal pain.
- Capsule endoscopy. This test is sometimes used to help diagnose Crohn’s disease involving your small intestine. You swallow a capsule that has a camera in it. The images are transmitted to a recorder you wear on your belt, after which the capsule exits your body painlessly in your stool. You may still need an endoscopy with a biopsy to confirm a diagnosis of Crohn’s disease. Capsule endoscopy should not be performed if a bowel obstruction is suspected.
- Balloon-assisted enteroscopy. For this test, a scope is used in conjunction with a device called an overtube. This enables the technician to look further into the small bowel where standard endoscopes don’t reach. This technique is useful when a capsule endoscopy shows abnormalities, but the diagnosis is still in question.
Imaging procedures
- X-ray. If you have severe symptoms, your provider may use a standard X-ray of your abdominal area to rule out serious complications, such as megacolon or a perforated colon.
- Computerized tomography (CT) scan. You may have a CT scan — a special X-ray technique that provides more detail than a standard X-ray does. This test looks at the entire bowel as well as at tissues outside the bowel. CT enterography is a special CT scan that provides better images of the small bowel. This test has replaced barium X-rays in most medical centers.
- Magnetic resonance imaging (MRI). An MRI scanner uses a magnetic field and radio waves to create detailed images of organs and tissues. An MRI is particularly useful for evaluating a fistula around the anal area (pelvic MRI) or the small intestine (MR enterography). Unlike CT, there is no radiation exposure with MRI.
Inflammatory bowel disease treatment
The goal of inflammatory bowel disease (IBD) treatment is to get rid of the inflammation that causes your signs and symptoms. Many types of medicine can help reduce inflammation. In the best cases, this may lead not only to symptom relief but also to long-term remission and reduced risks of complications. Talk to your doctor about anti-inflammatory drugs and drugs that suppress the immune system. Some medicines are needed only during flare-ups. You may need long-term medicines to suppress your immune system.
Depending on your symptoms, your doctor may also recommend these medicines and supplements:
- Antibiotic
- Anti-diarrheal
- Laxative
- Pain reliever
- Vitamin supplements
In severe cases of inflammatory bowel disease, you may need to go to the hospital for intravenous (IV) fluids or surgery.
During your treatment, you will most likely be treated by a team of doctors. This team may include your family physician, a gastroenterologist (a specialist in stomach and intestinal disorders), and, possibly, a surgeon.
Inflammatory bowel disease medicines
Many people with Crohn’s disease need medicines. Doctors prescribe medicines to reduce inflammation in the gastrointestinal tract and to help bring on and maintain remission—a time when your symptoms disappear. Which medicines your doctor prescribes will depend on your symptoms and whether you have Crohn’s disease or ulcerative colitis.
The first step in pharmacologic therapy for IBD is aminosalicylates. If the patient does not respond to an appropriate dose of aminosalicylates, the second step is the addition of corticosteroids, which tend to result in a significant decrease in inflammation. Once the response is seen, the dose can be tapered.
The immune-modifying agents (e.g Anti-TNF agents) are the step three drugs. These are used when the patient does not respond to corticosteroids, steroids are required for prolonged periods, or the steroids cannot be tapered down without recurrence of symptoms.
Lately, a step-down approach is being favored more for patients with high-risk or severe disease. This includes the early introduction of higher step medications such as anti-TNF agents with rapid de-escalation when the response is seen. Step down approach improves patient outcomes and prevents complications in patients with high-risk or severe disease.Step four includes clinical trial agents that tend to be disease-specific i.e., some work only for ulcerative colitis and others for Crohn disease. Examples of these experimental agents include thalidomide and interleukin (IL)-11 for Crohn disease, butyrate enema, nicotine patch, and heparin for ulcerative colitis. Multiple contraindications and serious side effects are associated with these experimental agents.
Anti-inflammatory drugs
Anti-inflammatory drugs are often the first step in the treatment of ulcerative colitis, typically for mild to moderate disease. Anti-inflammatories include aminosalicylates, such as mesalamine (Delzicol, Rowasa, others), balsalazide (Colazal) and olsalazine (Dipentum).
Time-limited courses of corticosteroids are also used to induce remission. In addition to being anti-inflammatory, steroids are immunosuppressing. Which medication you take depends on the area of your colon that’s affected.
Immune system suppressors
These drugs work in a variety of ways to suppress the immune response that releases inflammation-inducing chemicals into the body. When released, these chemicals can damage the lining of the digestive tract.
Some examples of immunosuppressant drugs include azathioprine (Azasan, Imuran), mercaptopurine (Purinethol, Purixan) and methotrexate (Trexall).
More recently, orally delivered agents also known as “small molecules” have become available for IBD treatment. These include tofacitinib (Xeljanz), upadacitinib (Rinvoq) and ozanimod (Zeposia).
The U.S. Food and Drug Administration (FDA) recently issued a warning about tofacitinib, stating that preliminary studies show an increased risk of serious heart-related problems and cancer from taking this drug. If you’re taking tofacitinib for ulcerative colitis, don’t stop taking the medication without first talking with your doctor.
Biologics
Biologics are a newer category of therapy in which therapy is directed toward neutralizing proteins in the body that are causing inflammation. Some are administered via intravenous (IV) infusions and others are injections you give yourself. Examples include infliximab (Remicade), adalimumab (Humira), golimumab (Simponi), certolizumab (Cimzia), vedolizumab (Entyvio), ustekinumab (Stelara), and risankizumab (Skyrizi).
Antibiotics
Antibiotics may be used in addition to other medications or when infection is a concern — in cases of perianal Crohn’s disease, for example. Frequently prescribed antibiotics include ciprofloxacin (Cipro) and metronidazole (Flagyl).
Other medications and supplements
In addition to controlling inflammation, some medications may help relieve your signs and symptoms, but always talk to your doctor before taking any over-the-counter medications. Depending on the severity of your IBD, your doctor may recommend one or more of the following:
- Anti-diarrheal medications. A fiber supplement — such as psyllium powder (Metamucil) or methylcellulose (Citrucel) — can help relieve mild to moderate diarrhea by adding bulk to your stool. For more severe diarrhea, loperamide (Imodium A-D) may be effective. These medications could be ineffective or detrimental in some people with strictures or certain infections. Please consult your doctor before taking these medications.
- Pain relievers. For mild pain, your doctor may recommend acetaminophen (Tylenol, others). However, ibuprofen (Advil, Motrin IB, others), naproxen sodium (Aleve) and diclofenac sodium likely will make your symptoms worse and can make your disease worse as well.
- Vitamins and supplements. If you’re not absorbing enough nutrients, your doctor may recommend vitamins and nutritional supplements.
Nutritional support
When weight loss is severe, your doctor may recommend a special diet given via a feeding tube (enteral nutrition) or nutrients injected into a vein (parenteral nutrition) to treat your IBD. This can improve your overall nutrition and allow the bowel to rest. Bowel rest can reduce inflammation in the short term.
If you have a stenosis or stricture in the bowel, your doctor may recommend a low-residue diet. This will help to minimize the chance that undigested food will get stuck in the narrowed part of the bowel and lead to a blockage.
Alternative medicine
Many people with digestive disorders have used some form of complementary and alternative medicine. However, there are few well-designed studies of the safety and effectiveness of complementary and alternative medicine.
Researchers suspect that adding more of the beneficial bacteria (probiotics) that are normally found in the digestive tract might help combat IBD. Although research is limited, there is some evidence that adding probiotics along with other medications may be helpful, but this has not been proved.
Surgery
If diet and lifestyle changes, drug therapy, or other treatments don’t relieve your IBD signs and symptoms, your provider may recommend surgery.
- Surgery for ulcerative colitis. Surgery involves removal of the entire colon and rectum and the production of an internal pouch attached to the anus that allows bowel movements without a bag. In some cases a pouch is not possible. Instead, surgeons create a permanent opening in your abdomen (ileal stoma) through which stool is passed for collection in an attached bag.
- Surgery for Crohn’s disease. Up to two-thirds of people with Crohn’s disease will require at least one surgery in their lifetime. However, surgery does not cure Crohn’s disease. During surgery, your surgeon removes a damaged portion of your digestive tract and then reconnects the healthy sections. Surgery may also be used to close fistulas and drain abscesses. The benefits of surgery for Crohn’s disease are usually temporary. Crohn’s disease often recurs, frequently near the reconnected tissue. The best approach is to follow surgery with medication to minimize the risk of recurrence.
Lifestyle and home remedies
Sometimes you may feel helpless when facing inflammatory bowel disease. But changes in your diet and lifestyle may help control your symptoms and lengthen the time between flare-ups.
There’s no firm evidence that what you eat actually causes inflammatory bowel disease. But certain foods and beverages can aggravate your signs and symptoms, especially during a flare-up.
It can be helpful to keep a food diary to keep track of what you’re eating, as well as how you feel. If you discover that some foods are causing your symptoms to flare, you can try eliminating those foods.
Here are some general dietary suggestions that may help you manage your condition:
- Limit dairy products. Many people with inflammatory bowel disease find that problems such as diarrhea, abdominal pain and gas improve by limiting or eliminating dairy products. You may be lactose intolerant — that is, your body can’t digest the milk sugar (lactose) in dairy foods. Using an enzyme product such as Lactaid may help as well.
- Eat small meals. You may find that you feel better eating five or six small meals a day rather than two or three larger ones.
- Drink plenty of liquids. Try to drink plenty of liquids daily. Water is best. Alcohol and beverages that contain caffeine stimulate your intestines and can make diarrhea worse, while carbonated drinks frequently produce gas.
- Consider multivitamins. Because Crohn’s disease can interfere with your ability to absorb nutrients and because your diet may be limited, multivitamin and mineral supplements are often helpful. Check with your doctor before taking any vitamins or supplements.
- Talk to a dietitian. If you begin to lose weight or your diet has become very limited, talk to a registered dietitian.
Quit smoking
Smoking increases your risk of developing Crohn’s disease, and once you have it, smoking can make it worse. People with Crohn’s disease who smoke are more likely to have relapses and need medications and repeat surgeries.
Smoking may help prevent ulcerative colitis. However, its harm to overall health outweighs any benefit, and quitting smoking can improve the general health of your digestive tract, as well as provide many other health benefits.
Stress management
The association of stress with Crohn’s disease is controversial, but many people who have the disease report symptom flares during high-stress periods. If you have trouble managing stress, try one of these strategies:
- Exercise. Even mild exercise can help reduce stress, relieve depression and normalize bowel function. Talk to your doctor about an exercise plan that’s right for you.
- Biofeedback. This stress-reduction technique may train you to reduce muscle tension and slow your heart rate with the help of a feedback machine. The goal is to help you enter a relaxed state so that you can cope more easily with stress.
- Regular relaxation and breathing exercises. One way to cope with stress is to regularly relax and use techniques such as deep, slow breathing to calm down.
Living with inflammatory bowel disease
If you have inflammatory bowel disease (IBD), you are at an increased risk of colon cancer. Talk to your doctor about when to start screening for colon cancer and how often to have screening.
Crohn’s disease and ulcerative colitis keep coming back and their symptoms can be unpredictable. This can cause patients who have these illnesses to become depressed.
Here are some things you can do:
- Be informed. One of the best ways to be more in control is to find out as much as possible about inflammatory bowel disease. Look for information from reputable sources such as the Crohn’s and Colitis Foundation (https://www.crohnscolitisfoundation.org).
- Join a support group. Although support groups aren’t for everyone, they can provide valuable information about your condition as well as emotional support. Group members frequently know about the latest medical treatments or integrative therapies. You may also find it reassuring to be among others with IBD.
- Talk to a therapist. Some people find it helpful to consult a mental health professional who’s familiar with inflammatory bowel disease and the emotional difficulties it can cause.
Inflammatory bowel disease prognosis
The prognosis for both ulcerative colitis and Crohn’s disease depends on the extent of the disease and treatment response 12. The stool markers lactoferrin and calprotectin are useful in determining postoperative recurrence of Crohn’s disease. Some evidence exists that these can also be used to predict future flares. However, patients with IBD tend to have much higher chances of dying compared to the general population 12. Causes of death include primary disease, infections, and respiratory illness. Heart disease is not a risk factor for death in IBD. Finally, psychological illness is very high in IBD patients and the quality of life is poor 12.
Continued surveillance for abnormal changes in the intestines is critical for long-standing ulcerative colitis patients due to the higher chance of developing bowel cancer. The cumulative risk of colorectal cancer is estimated to be as high as 30% for those with the disease of 30 years or more. The extraintestinal manifestation of primary sclerosing cholangitis leads to liver failure 17, 18.
Ulcerative colitis
Ulcerative colitis is a chronic (long lasting) disease that causes inflammation—irritation or swelling—and ulcers (sores) on the inner lining of your large intestine (colon) and rectum. Ulcerative colitis is a chronic inflammatory disease of the gastrointestinal (GI) tract, called inflammatory bowel disease (IBD) in which abnormal reactions of the immune system cause inflammation and ulcers on the inner lining of your large intestine. Ulcerative colitis is a chronic disease of multifactorial origin whose cause and pathogenesis are not yet fully understood 19. Crohn’s disease and microscopic colitis are other common types of inflammatory bowel disease (IBD). Inflammatory bowel disease (IBD) is more common in industrialized countries and in Western nations. The incidence is also increased in persons who live at higher latitudes 20. Worldwide and in less developed nations, ulcerative colitis is more common than Crohn’s disease, whereas in some studies of U.S. populations, the incidence of the two disorders is almost equal 20. Four percent of cases of inflammatory bowel disease (IBD) cannot be characterized definitively as either Crohn’s disease or ulcerative colitis; these patients are said to have indeterminate colitis if features are still indeterminate after colectomy histology is assessed 21. Inflammatory bowel disease-unclassified (IBD-U) is more common in children than adults 22. In a small proportion of ulcerative colitis patients their diagnosis is later changed to inflammatory bowel disease-unclassified (IBD-U) or Crohn’s disease 23.
Ulcerative colitis most often begins gradually and can become worse over time. However, it can also start suddenly. Symptoms can range from mild to severe. In between periods of flares—times when people have symptoms—most people have periods of remission—times when symptoms disappear. Periods of remission can last for weeks or years. Typically, patients with ulcerative colitis experience periods of relapse and remission. Up to 90% will have one or more relapses after the first attack, and early relapse or active disease in the first 2 years is associated with a worse disease course subsequently 24. The goal of treatment is to keep people in remission long term.
Ulcerative colitis may affect as many as 907,000 Americans 25. The annual incidence of ulcerative colitis in the United States is between 9 and 12 cases per 100,000 persons 20. Men and women are equally likely to be affected (in contrast with Crohn’s disease, which has a higher incidence in women), and most people are diagnosed in their mid-30s. The disease can occur at any age (including infants) and older men are more likely to be diagnosed than older women.
Ulcerative colitis can occur in people of any age. However, it is more likely to develop in people:
- between the ages of 15 and 30 26, although the disease may develop in people of any age 27
- older than 60 28
- who have a family member (a parent, sibling, or child) with inflammatory bowel disease (IBD)
- of Jewish descent 29
While ulcerative colitis tends to run in families, researchers have been unable to establish a clear pattern of inheritance. Studies show that up to 20 percent of people with ulcerative colitis will also have a close relative with the disease. The disease is more common among white people of European origin and among people of Jewish heritage.
Ulcerative colitis can be debilitating and can sometimes lead to life-threatening complications. While it has no known cure, treatment can greatly reduce signs and symptoms of the disease and even bring about long-term remission.
To diagnose ulcerative colitis, doctors review medical and family history, perform a physical exam, and order medical tests. Doctors may use blood tests, stool tests, and endoscopy of the large intestine to diagnose ulcerative colitis.
Common Extraintestinal Manifestations of Ulcerative Colitis 30.
Approximately one-third of patients with ulcerative colitis have extraintestinal manifestations, which may be present even when the disease is inactive.
- Arthritis (21%)
- Aphthous stomatitis (4%)
- Primary sclerosing cholangitis (4%)
- Uveitis (4%)
- Erythema nodosum (3%)
- Ankylosing spondylitis (2%)
- Pyoderma gangrenosum (2%)
- Psoriasis (1%)
Researchers have not found that specific foods cause ulcerative colitis symptoms, although healthier diets appear to be associated with less risk of developing inflammatory bowel disease (IBD). Researchers have not found that specific foods worsen ulcerative colitis. Talk with your doctor about any foods that seem to be related to your symptoms. Your doctor may suggest keeping a food diary to help identify foods that seem to make your symptoms worse.
Depending on your symptoms and the medicines you take, your doctor may recommend changes to your diet. Your doctor may also recommend dietary supplements. Most people with ulcerative colitis receive care from a gastroenterologist, a doctor who specializes in digestive diseases. The goal of care is to keep people in remission long term.
See your doctor if you experience a persistent change in your bowel habits or if you have signs and symptoms such as:
- Abdominal pain
- Blood in your stool
- Ongoing diarrhea that doesn’t respond to over-the-counter medications
- Diarrhea that awakens you from sleep
- An unexplained fever lasting more than a day or two
Although ulcerative colitis usually isn’t fatal, it’s a serious disease that, in some cases, may cause life-threatening complications.
Types of ulcerative colitis
Doctors often classify ulcerative colitis according to its location. Types of ulcerative colitis include:
- Ulcerative proctitis. Inflammation is confined to the area closest to the anus (rectum), and rectal bleeding may be the only sign of the disease. This form of ulcerative colitis tends to be the mildest. For approximately 30% of all patients with ulcerative colitis, the illness begins as ulcerative proctitis. Because of its limited extent (usually less than the six inches of the rectum), ulcerative proctitis tends to be a milder form of ulcerative colitis. It is associated with fewer complications and offers a better outlook than more widespread disease.
- Proctosigmoiditis. Inflammation involves the rectum and sigmoid colon (lower end of the colon). Signs and symptoms include bloody diarrhea, abdominal cramps and pain, and an inability to move the bowels in spite of the urge to do so (tenesmus). Moderate pain on the lower left side of the abdomen may occur in active disease.
- Left-sided colitis. Inflammation extends from the rectum up through the sigmoid and descending colon. Signs and symptoms include bloody diarrhea, abdominal cramping and pain on the left side, and unintended weight loss.
- Pancolitis. Pancolitis often affects the entire colon and causes bouts of bloody diarrhea that may be severe, abdominal cramps and pain, fatigue, and significant weight loss. Potentially serious complications include massive bleeding and acute dilation of the colon (toxic megacolon), which may lead to an opening in the bowel wall. Serious complications may require surgery.
- Acute severe ulcerative colitis. This rare form of colitis affects the entire colon and causes severe pain, profuse diarrhea, bleeding, fever and inability to eat.
Ulcerative colitis causes
The exact cause of ulcerative colitis is unknown. Researchers believe the following factors may play a role in causing ulcerative colitis:
- Overactive intestinal immune system
Scientists believe one cause of ulcerative colitis may be an abnormal immune reaction in the intestine. Normally, the immune system protects the body from infection by identifying and destroying bacteria, viruses, and other potentially harmful foreign substances. Researchers believe bacteria or viruses can mistakenly trigger the immune system to attack the inner lining of the large intestine. This immune system response causes the inflammation, leading to symptoms.
- Genes
Ulcerative colitis sometimes runs in families. Research studies have shown that certain abnormal genes may appear in people with ulcerative colitis. Genetic factors have a role in ulcerative colitis. Having a sibling with ulcerative colitis increases your risk of developing the disease 4.6-fold and the relative risk of one monozygotic twin having the disease is 95 times higher if the other twin is affected 31. However, researchers have not been able to show a clear link between the abnormal genes and ulcerative colitis.
- Microbiome
The microbes in your digestive tract—including bacteria, viruses, and fungi—that help with digestion are called the microbiome. Studies have found differences between the microbiomes of people who have inflammatory bowel disease (IBD) and those who don’t. Researchers are still studying the relationship between the microbiome and inflammatory bowel disease (IBD).
- Environment
Some studies suggest that certain things in the environment may increase the chance of a person getting ulcerative colitis, although the overall chance is low. Nonsteroidal anti-inflammatory drugs 28, antibiotics 28 and oral contraceptives 32 may slightly increase the chance of developing ulcerative colitis. A diet high in refined sugar, fat, and meat increases the risk, whereas a diet rich in vegetables reduces the risk 20, 33. Infection with nontyphoid Salmonella or Campylobacter is associated with an eight to 10 times higher risk of developing ulcerative colitis in the following year 34. The risk diminishes with time, but is still present up to 10 years later. The intestinal bacterial flora (microbiome) of patients with inflammatory bowel disease has been shown to be markedly abnormal, but this finding has not yet led to therapeutic interventions 35.
The hygiene hypothesis states that excessive hygiene habits in Western industrialized nations prevent children from normal exposure to bacterial and helminthic antigens, thereby changing immune system responsiveness. Epidemiologic data provide indirect evidence for this theory, in the same way that they do for asthma and allergies 20.
Some people believe eating certain foods, stress, or emotional distress can cause ulcerative colitis. Emotional distress does not seem to cause ulcerative colitis. A few studies suggest that stress may increase a person’s chance of having a flare-up of ulcerative colitis. Also, some people may find that certain foods can trigger or worsen symptoms.
Researchers are still studying how people’s environments interact with genes, the immune system, and the microbiome to affect the chance of developing ulcerative colitis.
Risk factors for ulcerative colitis
Ulcerative colitis affects about the same number of women and men. Risk factors may include:
- Age. Ulcerative colitis usually begins before the age of 30. But, it can occur at any age, and some people may not develop the disease until after age 60.
- Race or ethnicity. Although whites have the highest risk of the disease, it can occur in any race. If you’re of Ashkenazi Jewish descent, your risk is even higher.
- Family history. You’re at higher risk if you have a close relative, such as a parent, sibling or child, with the disease.
Ulcerative colitis signs and symptoms
Symptoms of ulcerative colitis a person experiences can vary depending on the severity of the inflammation and where it occurs in the intestine. When symptoms first appear, most people with ulcerative colitis have mild to moderate symptoms and about 10 percent of people can have severe symptoms, such as frequent, bloody bowel movements (hematochezia); fevers; and severe abdominal cramping 28.
The most common signs and symptoms of ulcerative colitis are diarrhea with blood, mucus or pus and abdominal pain and cramping.
Other signs and symptoms include:
- an urgent need to have a bowel movement even though your bowel may be empty (tenesmus)
- inability to defecate despite urgency
- feeling tired
- nausea or loss of appetite
- weight loss
- fever
- anemia—a condition in which the body has fewer red blood cells than normal
- rectal pain
- rectal bleeding — passing small amount of blood with stool
- in children, failure to grow
Ulcerative colitis symptoms may cause some people to lose their appetite and eat less, and they may not get enough nutrients. In children, a lack of nutrients may play a role in problems with growth and development.
Less common symptoms include:
- joint pain or soreness
- eye irritation
- certain rashes.
Symptoms of ulcerative colitis may vary in severity. For example, mild symptoms may include having fewer than four bowel movements a day and sometimes passing blood with stool. Severe symptoms may include having more than six bowel movements a day and passing blood with stool most of the time. In extremely severe or fulminant ulcerative colitis, you may have more than 10 bloody bowel movements in a day 29.
Some symptoms are more likely to occur if ulcerative colitis is more severe or affects more of the large intestine. These symptoms include:
- fatigue, or feeling tired
- fever
- nausea or vomiting
- weight loss
You may have periods of remission—times when symptoms disappear—that can last for weeks or years. After a period of remission, you may have a relapse, or a return of symptoms.
Symptoms of ulcerative colitis flare-up
Some people may go for weeks or months with very mild symptoms, or none at all (known as remission), followed by periods where the symptoms are particularly troublesome (known as flare-ups or relapses).
During a ulcerative colitis flare-up, some people with ulcerative colitis also experience symptoms elsewhere in their body. For example, some people develop:
- painful and swollen joints (arthritis)
- mouth ulcers
- areas of painful, red and swollen skin
- irritated and red eyes
In severe cases, defined as having to empty your bowels six or more times a day, additional symptoms may include:
- shortness of breath
- a fast or irregular heartbeat
- a high temperature (fever)
- blood in your stools becoming more obvious
In most people, no specific trigger for flare-ups is identified, although a gut infection can occasionally be the cause. Stress is also thought to be a potential factor.
Ulcerative colitis complications
Possible complications of ulcerative colitis include:
- Severe bleeding
- A hole in the colon (perforated colon)
- Anemia, a condition in which you have fewer red blood cells than normal. Ulcerative colitis may lead to more than one type of anemia, including iron-deficiency anemia and anemia of inflammation or chronic disease.
- Severe dehydration
- Liver disease (rare)
- Primary sclerosing cholangitis
- Bone problems, because ulcerative colitis and corticosteroids used to treat the disease can affect your bones. Bone problems include low bone mass, such as osteopenia or osteoporosis.
- Inflammation of your skin, joints and eyes
- An increased risk of colon cancer, because patients with long-standing ulcerative colitis that involves a third or more of the colon are at increased risk and require closer screening.
- A rapidly swelling colon (toxic megacolon)
- Increased risk of blood clots in veins and arteries
- Problems with growth and development in children, such as gaining less weight than normal, slowed growth, short stature, or delayed puberty.
In some cases, ulcerative colitis may lead to serious complications that develop quickly and can be life-threatening. These complications require treatment at a hospital or emergency surgery. Serious complications include:
- Fulminant ulcerative colitis, which causes extremely severe symptoms, such as more than 10 bloody bowel movements in a day, often with fever, rapid heart rate, and severe anemia 27. People with fulminant ulcerative colitis have a higher chance of developing other complications, such as toxic megacolon and perforation.
- Perforation, or a hole in the wall of the large intestine.
- Severe rectal bleeding or passing a lot of blood from the rectum. In some cases, people with ulcerative colitis may have severe or heavy rectal bleeding that may require emergency surgery.
- Toxic megacolon, which occurs when inflammation spreads to the deep tissue layers of the large intestine, and the large intestine swells and stops working.
Severe ulcerative colitis or serious complications may lead to additional problems, such as severe anemia and dehydration. These problems may require treatment at a hospital with blood transfusions or intravenous (IV) fluids and electrolytes.
Some people with ulcerative colitis also have inflammation in parts of the body other than the large intestine, including the:
- joints, causing certain types of arthritis
- skin
- eyes
- liver and bile ducts, causing conditions such as primary sclerosing cholangitis
People with ulcerative colitis also have a higher risk of blood clots in their blood vessels.
Fertility
The chances of a woman with ulcerative colitis becoming pregnant aren’t usually affected by the condition. However, infertility can be a complication of surgery carried out to create an ileo-anal pouch.
This risk is much lower if you have surgery to divert the small intestine through an opening in your abdomen (an ileostomy).
Pregnancy
The majority of women with ulcerative colitis who decide to have children will have a normal pregnancy and a healthy baby.
However, if you’re pregnant or planning a pregnancy you should discuss it with your care team. If you become pregnant during a flare-up, or have a flare-up while pregnant, there’s a risk you could give birth early (premature birth) or have a baby with a low birthweight.
For this reason, doctors usually recommend trying to get ulcerative colitis under control before getting pregnant.
Most ulcerative colitis medications can be taken during pregnancy, including corticosteroids, most 5-ASAs and some types of immunosuppressant medication.
However, there are certain medications (such as some types of immunosuppressant) that may need to be avoided as they’re associated with an increased risk of birth defects.
In some cases, your doctors may advise you to take a medicine that isn’t normally recommended during pregnancy. This might happen if they think the risks of having a flare-up outweigh the risks associated with the medicine.
Osteoporosis
People with ulcerative colitis are at an increased risk of developing osteoporosis, when the bones become weak and are more likely to fracture.
This isn’t directly caused by ulcerative colitis, but can develop as a side effect of the prolonged use of corticosteroid medication. It can also be caused by the dietary changes someone with the condition may take – such as avoiding dairy products, if they believe it could be triggering their symptoms.
If you’re thought to be at risk of osteoporosis, the health of your bones will be regularly monitored. You may also be advised to take medication or supplements of vitamin D and calcium to strengthen your bones.
Primary sclerosing cholangitis
Primary sclerosing cholangitis, where the bile ducts become progressively inflamed and damaged over time, is a rare complication of ulcerative colitis. Bile ducts are small tubes used to transport bile (digestive juice) out of the liver and into the digestive system.
Primary sclerosing cholangitis doesn’t usually cause symptoms until it’s at an advanced stage. Symptoms can include:
- fatigue (extreme tiredness)
- diarrhea
- itchy skin
- weight loss
- chills
- a high temperature (fever)
- yellowing of the skin and the whites of the eyes (jaundice)
There’s currently no specific treatment for primary sclerosing cholangitis, although medications can be used to relieve some of the symptoms, such as itchy skin. In more severe cases, a liver transplant may be required.
Toxic megacolon
Toxic megacolon is a rare and serious complication of severe ulcerative colitis, where inflammation in the colon causes gas to become trapped, resulting in the colon becoming enlarged and swollen.
This is potentially very dangerous as it can cause the colon to rupture (split) and cause infection in the blood (septicaemia).
The symptoms of a toxic megacolon include:
- abdominal (tummy) pain
- a high temperature (fever)
- a rapid heart rate
Toxic megacolon can be treated with fluids, antibiotics and steroids given intravenously (directly into a vein). If medications don’t improve the conditions quickly then surgical removal of the colon (known as a colectomy) may be needed.
Treating symptoms of ulcerative colitis before they become severe can help prevent toxic megacolon.
Bowel cancer
People who have ulcerative colitis have an increased risk of developing bowel cancer (cancer of the colon, rectum or bowel), especially if the condition is severe or involves most of the colon. The longer you have ulcerative colitis, the greater the risk. People have a higher risk for developing colorectal cancer if ulcerative colitis affects more of their large intestine, is more severe, started at a younger age, or has been present for a longer time. People with ulcerative colitis also have a higher risk of developing colorectal cancer if they have primary sclerosing cholangitis or have a family history of colorectal cancer 27.
People with ulcerative colitis are often unaware they have bowel cancer as the initial symptoms of this type of cancer are similar. These include:
- blood in the stools
- diarrhea
- abdominal pain
Therefore, you’ll usually have regular check-ups to look for signs of bowel cancer from about 10 years after your symptoms first develop.
Check-ups will involve examining your bowel with a colonoscope – a long, flexible tube containing a camera – that’s inserted into your rectum. The frequency of the colonoscopy examinations will increase the longer you live with the condition, and will also depend on factors such as how severe your ulcerative colitis is and if you have a family history of bowel cancer. This can vary between every one to three years, starting 8 years after ulcerative colitis started 27. For people with ulcerative colitis and primary biliary cholangitis, doctors typically recommend colonoscopies every year, starting at diagnosis 27.
To reduce the risk of bowel cancer, it’s important to:
- eat a healthy, balanced diet including plenty of fresh fruit and vegetables
- take regular exercise
- maintain a healthy weight
- avoid alcohol and smoking
Taking aminosalicylates as prescribed can also help reduce your risk of bowel cancer.
Ulcerative colitis diagnosis
The diagnosis of ulcerative colitis cannot be established definitively by any single diagnostic study. Rather, it is made on the basis of an overall interpretation of the clinical signs, symptoms, laboratory tests, and endoscopic, histological, and radiological findings 36.
To diagnose ulcerative colitis, doctors review medical and family history, perform a physical exam, and order medical tests. Doctors may use blood tests, stool tests, and endoscopy of the large intestine to diagnose ulcerative colitis.
The onset of symptoms can be sudden or gradual 37. The presence of anemia, thrombocytosis (too many platelets in blood), or hypoalbuminemia (the level of albumin in the blood is abnormally low) may suggest inflammatory bowel disease, but most patients with ulcerative colitis will not have these abnormalities 38. C-reactive protein (CRP) level and erythrocyte sedimentation rate are relatively insensitive for detecting ulcerative colitis and should not be relied on to exclude inflammatory bowel disease. At the time of diagnosis, fewer than one-half of patients with ulcerative colitis have abnormal findings on these tests 39.
Endoscopic biopsy is used to confirm the diagnosis 40.
Elevated fecal calprotectin and lactoferrin levels have been proven sensitive for the detection of inflammatory bowel disease, but using these tests to exclude patients from endoscopic examination delays diagnosis in 6 to 8 percent of patients 39. Tests for perinuclear antineutrophil cytoplasmic antibodies and anti– Saccharomyces cerevisiae antibodies are often positive in patients with ulcerative colitis, and can help distinguish the condition from Crohn’s disease in those with indeterminate histology 39. Approximately one-third of patients with ulcerative colitis have extraintestinal manifestations, which may be present even when the disease is inactive.
A health care provider diagnoses ulcerative colitis with the following:
- Medical and family history. To help diagnose ulcerative colitis, your doctor will ask about your symptoms, your medical history, and any medicines you take. Your doctor will also ask about lifestyle factors, such as smoking, and about your family medical history.
- Physical exam. During a physical exam, your doctor may:
- check your blood pressure, heart rate, and temperature—if you have ulcerative colitis, doctors may use these measures, along with information about your symptoms and test results, to find out how severe the disease is
- use a stethoscope to listen to sounds within your abdomen
- press on your abdomen to feel for tenderness or masses
- The physical exam may also include a digital rectal exam to check for blood in your stool.
- Lab tests
- Endoscopies of the large intestine
- X-ray. If you have severe symptoms, your doctor may use a standard X-ray of your abdominal area to rule out serious complications, such as a perforated colon.
- CT scan. A CT scan of your abdomen or pelvis may be performed if your doctor suspects a complication from ulcerative colitis. A CT scan may also reveal how much of the colon is inflamed.
- Computerized tomography (CT) enterography and magnetic resonance (MR) enterography. Your doctor may recommend one of these noninvasive tests if he or she wants to exclude any inflammation in the small intestine. These tests are more sensitive for finding inflammation in the bowel than are conventional imaging tests. MR enterography is a radiation-free alternative.
The health care provider may perform a series of medical tests to rule out other bowel disorders, such as irritable bowel syndrome, Crohn’s disease, or celiac disease, that may cause symptoms similar to those of ulcerative colitis.
Lab Tests
A health care provider may order lab tests to help diagnose ulcerative colitis, including blood and stool tests.
- Blood tests. A blood test involves drawing blood at a health care provider’s office or a lab. A lab technologist will analyze the blood sample. A health care provider may use blood tests to look for:
- anemia
- inflammation or infection somewhere in the body
- markers that show ongoing inflammation
- low albumin, or protein—common in patients with severe ulcerative colitis
- Stool tests. A stool test is the analysis of a sample of stool. A health care provider will give the patient a container for catching and storing the stool at home. The patient returns the sample to the health care provider or to a lab. A lab technologist will analyze the stool sample. Health care providers commonly order stool tests to rule out other causes of gastrointestinal diseases, such as infections that could be causing your symptoms. Doctors may also use stool tests to check for signs of inflammation in the intestines.
Endoscopies of the Large Intestine
Endoscopies of the large intestine are the most accurate methods for diagnosing ulcerative colitis and ruling out other possible conditions, such as Crohn’s disease, diverticular disease, or cancer. Doctors also use endoscopy to find out how severe ulcerative colitis is and how much of the large intestine is affected. Endoscopies of the large intestine include:
- Colonoscopy. A colonoscopy can show irritated and swollen tissue, ulcers, and abnormal growths such as polyps––extra pieces of tissue that grow on the inner lining of the intestine. If the gastroenterologist suspects ulcerative colitis, he or she will biopsy the patient’s colon and rectum. A biopsy is a procedure that involves taking small pieces of tissue for examination by a pathologist under a microscope.
- Flexible sigmoidoscopy. Flexible sigmoidoscopy is a test that uses a flexible, narrow tube with a light and tiny camera on one end, called a sigmoidoscope or scope, to look inside the rectum, the sigmoid colon, and sometimes the descending colon. In most cases, a patient does not need anesthesia. The health care provider will look for signs of bowel diseases and conditions such as irritated and swollen tissue, ulcers, and polyps.
Ulcerative colitis treatment
Ulcerative colitis treatment usually involves either drug therapy or surgery. Each person experiences ulcerative colitis differently, and doctors recommend treatments based on how severe ulcerative colitis is and how much of the large intestine is affected. Doctors most often treat severe and fulminant ulcerative colitis in a hospital.
Several categories of drugs may be effective in treating ulcerative colitis. The type you take will depend on the severity of your condition. The drugs that work well for some people may not work for others, so it may take time to find a medication that helps you. In addition, because some drugs have serious side effects, you’ll need to weigh the benefits and risks of any treatment.
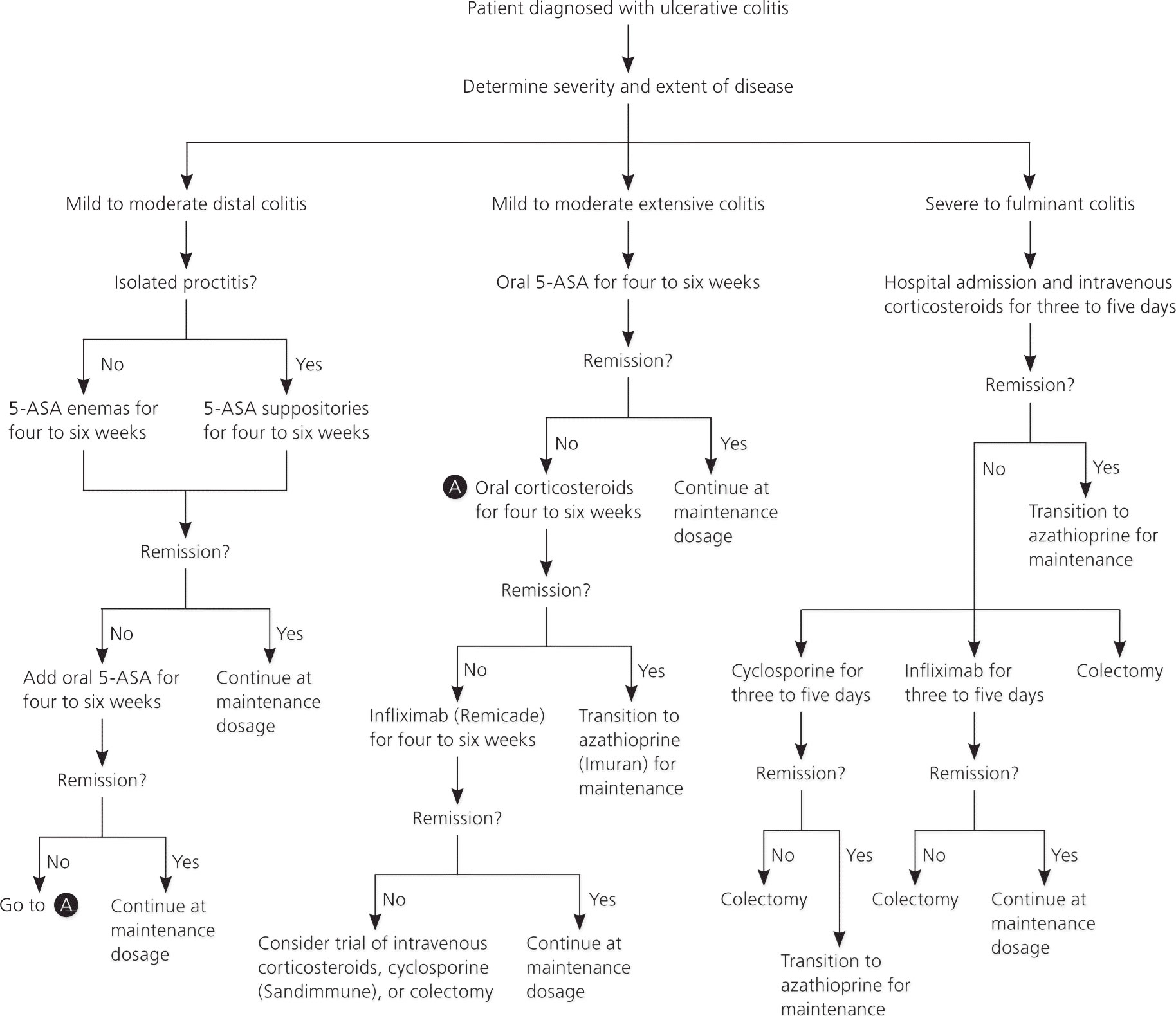
Figure 2. Ulcerative colitis treatment (recommended treatment approach)
[Source 41 ]Active disease
For active disease distal to the descending colon, topical 5-aminosalicylic acid (5-ASA), including suppository and enema formulations, is the preferred treatment 42. Topical 5-ASA is more effective than oral 5-ASA or topical corticosteroid foams or enemas. Suppositories are effective for proctitis, whereas enemas are effective for disease that reaches as proximally as the splenic flexure 42. Topical mesalamine enemas, a common formulation of 5-ASA, induce remission in 72 percent of cases of active, left-sided ulcerative colitis within four weeks 43. Corticosteroid foams are also effective, although less so than 5-ASA. However, they may be easier to administer and more comfortable to retain 42. Oral 5-ASA is effective and may be preferred by patients who have difficulty with irritation or retaining topical formulations.18 A combination of oral and topical 5-ASA is superior to either formulation alone and should be used when other treatments are ineffective 44.
Oral 5-aminosalicylic acid (5-ASA) is effective for mild to moderate active ulcerative colitis extending proximal to the sigmoid colon 45. If oral 5-ASA is ineffective, the addition of topical 5-ASA can be more effective than oral treatment alone 46. A short-term course of oral corticosteroids may be effective if the disease does not respond to combination 5-ASA therapy, or for patients in whom a more rapid response is desired 47. Infliximab (Remicade), an intravenously administered monoclonal antibody to tumor necrosis factor-α, is effective for corticosteroid-refractory disease 48. A recent meta-analysis of studies of azathioprine (Imuran) for treatment of active ulcerative colitis showed no statistically significant effect 49.
Patients with severe to fulminant disease can be divided into those who require urgent hospitalization and those who may receive a trial of outpatient treatment. Signs and symptoms that suggest the need for urgent hospitalization include severe pain, abdominal or colonic distension, gastrointestinal bleeding, and toxicity (e.g., fever, tachycardia, leukocytosis, anemia). In patients who do not require urgent hospitalization, a trial of maximal dosage combined oral/topical 5-ASA therapy is recommended in conjunction with oral corticosteroids. If symptoms do not improve and the patient still does not require urgent hospitalization, the addition of infliximab as outpatient therapy is another option 41.
For patients who do not improve on maximal outpatient medical management or who have signs of toxicity, hospital admission and administration of intravenous corticosteroids reduce morbidity and mortality 50. If intravenous corticosteroids are ineffective after three to five days, intravenous cyclosporine (Sandimmune) or infliximab increases remission rates 51.
For patients who do not improve with the addition of cyclosporine or infliximab, there is evidence that switching to the other agent may decrease the risk of colectomy; however, this is associated with an increased risk of infectious complications, and the decision should be individualized 51. For patients with severe acute or chronic colitis who do not improve with medical therapy, surgical proctocolectomy is the next step. Proctocolectomy with ileal pouch-anal anastomosis is most common; however, permanent ileostomy is also an option. Other indications for surgery are exsanguinating hemorrhage, perforation, or carcinoma 41. A recent systematic literature review showed that 12 months after surgery, health-related quality of life and health status are equivalent to that in the general population 52. Despite the potential benefits of surgery, complications are a concern. Colectomy is associated with a 54 percent reoperation rate for postsurgical complications 53. Pouchitis (inflammatory disease of the ileal pouchanal anastomosis pouch of unknown etiology) is also common 53.
Maintenance
Once remission is induced, the same agent is usually used to maintain remission. 5-ASA suppositories and enemas are effective for maintenance of distal disease 54. Oral 5-ASA is effective for extensive colitis.21 As with active disease, combined oral/topical therapy is more effective in maintaining remission than either agent alone 55. Corticosteroids are not effective in maintaining remission and have potentially serious adverse effects with long-term use 56. They should not be used for maintenance therapy. Azathioprine is an option for patients who require corticosteroids or cyclosporine for induction of remission or in whom remission is not adequately maintained with 5-ASA. However, it usually takes several months before it reaches full effect 49. For patients in whom remission was induced using infliximab, the same agent may be continued as maintenance therapy 48.
Ulcerative colitis medications
While no medication cures ulcerative colitis, many can reduce symptoms. Doctors prescribe medicines to reduce inflammation in the large intestine and to help bring on and maintain remission—a time when your symptoms disappear.
The goals of medication therapy are:
- inducing and maintaining remission
- improving the person’s quality of life
Many people with ulcerative colitis typically require medication therapy indefinitely (lifelong treatment), unless they have their colon and rectum surgically removed.
Which medicines your doctor prescribes will depend on how severe ulcerative colitis is. Ulcerative colitis medicines that reduce inflammation in the large intestine include:
- Aminosalicylates also called 5-aminosalicylic acid (5-ASA), which doctors prescribe to treat mild or moderate ulcerative colitis or to help people stay in remission.
- Corticosteroids also called steroids, which doctors prescribe to treat moderate to severe ulcerative colitis and to treat mild to moderate ulcerative colitis in people who don’t respond to aminosalicylates. Doctors typically don’t prescribe corticosteroids for long-term use or to maintain remission. Long-term use may cause serious side effects.
- Immunosuppressants, which doctors may prescribe to treat people with moderate to severe ulcerative colitis and help them stay in remission. Doctors may also prescribe immunosuppressants to treat severe ulcerative colitis in people who are hospitalized and don’t respond to other medicines.
- Biologics, which doctors prescribe to treat people with moderate to severe ulcerative colitis and help them stay in remission.
- A novel small molecule medicine (e.g., tofacitinib), which doctors may prescribe for adults with moderate to severe ulcerative colitis who don’t respond to other medicines or who have severe side effects with other medicines.
Table 2. Ulcerative Colitis medications
| Medication (form) | Dosage for active disease | Maintenance dosage | Adverse effects |
|---|---|---|---|
| Sulfasalazine (Azulfidine; oral) | 4 to 6 g per day in 4 divided doses | 2 to 4 g per day | Headache,† interstitial nephritis, nausea, vomiting |
| 5-Aminosalicylic acid (oral) | 2 to 4.8 g per day in 3 divided doses‡ | 1.2 to 2.4 g per day | Interstitial nephritis |
| 5-Aminosalicylic acid (suppository) | 1,000 mg once per day | 500 mg once or twice per day | Anal irritation, discomfort |
| 5-Aminosalicylic acid (enema) | 1 to 4 g per day | 2 to 4 g daily to every third day | Difficulty retaining, rectal irritation |
| Hydrocortisone (enema) | 100 mg | Not recommended | Difficulty retaining, rectal irritation |
| Hydrocortisone (10% foam) | 90 mg once or twice per day | Not recommended | Rectal irritation |
| Prednisone (oral) | 40 to 60 mg per day until clinical improvement, then taper by 5 to 10 mg per week | Not recommended | Adrenal suppression, bone disease,§ cataracts, cushingoid features, glaucoma, impaired wound healing, infections, metabolic abnormalities, peptic ulcers, psychiatric disturbances |
| Methylprednisolone (Solu-Medrol; IV) | 40 to 60 mg per day | Not recommended | |
| Infliximab (Remicade; IV) | 5 to 10 mg per kg on weeks 0, 2, and 6 | 5 to 10 mg per kg every 4 to 8 weeks | Increased risk of infection and lymphoma, infusion reaction |
| Azathioprine (Imuran; oral) | Not recommended | 1.5 to 2.5 mg per kg per day|| | Allergic reactions, bone marrow suppression, infection, pancreatitis |
| Cyclosporine (Sandimmune; IV) | 2 to 4 mg per kg per day | Not recommended | Infection, nephrotoxicity, seizures |
| Lactobacillus GG (oral) | Not recommended | 18 × 109 viable bacteria per day | Minimal |
| Escherichia coli Nissle 1917 (Mutaflor¶; oral) | Not recommended | 2.5 to 25 × 109 viable bacteria per day | Minimal |
Footnotes: Medications are listed in order of preferred use, with first-line therapies listed first.
IV = intravenous.
†—Because of sulfapyridine moiety, which is not present in 5-aminosalicylic acid.
‡—Dosage for the newer multimatrix formulation is 2.4 to 4.8 g once per day.
§—Guidelines on prevention of complications related to long-term glucocorticoid therapy are available from the American College of Rheumatology at http://www.rheumatology.org/practice/clinical/guidelines/osteoupdate.asp.
||—May take three to six months to achieve full effectiveness.
¶—Not available in the United States but can be shipped from Canada.
[Source 57 ]Anti-inflammatory drugs
Anti-inflammatory drugs are often the first step in the treatment of ulcerative colitis. They include:
- 5-aminosalicylates also called 5-aminosalicylic acid (5-ASA). Examples of this type of medication include sulfasalazine (Azulfidine), mesalamine (Asacol HD, Delzicol, others), balsalazide (Colazal) and olsalazine (Dipentum). Which one you take, and whether it is taken by mouth or as an enema or suppository, depends on the area of your colon that’s affected.
- Corticosteroids also known as steroids. These drugs, which include prednisone and hydrocortisone, are generally reserved for moderate to severe ulcerative colitis that doesn’t respond to other treatments. Due to the side effects, they are not usually given long term.
Immune system suppressors
These drugs also reduce inflammation, but they do so by suppressing the immune system response that starts the process of inflammation. For some people, a combination of these drugs works better than one drug alone.
Immunosuppressant drugs include:
- Azathioprine (Azasan, Imuran) and mercaptopurine (Purinethol, Purixan). These are the most widely used immunosuppressants for treatment of inflammatory bowel disease. Taking them requires that you follow up closely with your doctor and have your blood checked regularly to look for side effects, including effects on the liver and pancreas.
- Cyclosporine (Gengraf, Neoral, Sandimmune). This drug is normally reserved for people who haven’t responded well to other medications. Cyclosporine has the potential for serious side effects and is not for long-term use.
- Tofacitinib (Xeljanz). This is called a “small molecule” and works by stopping the process of inflammation. Tofacitinib is effective when other therapies don’t work. Main side effects include the increased risk of shingles infection and blood clots. The U.S. Food and Drug Administration (FDA) recently issued a warning about tofacitinib, stating that preliminary studies show an increased risk of serious heart-related problems and cancer from taking this drug. If you’re taking tofacitinib for ulcerative colitis, don’t stop taking the medication without first talking with your doctor.
Biologics
This class of therapies targets proteins made by the immune system. Types of biologics used to treat ulcerative colitis include:
- Infliximab (Remicade), adalimumab (Humira) and golimumab (Simponi). These drugs, called tumor necrosis factor (TNF) inhibitors, or biologics, work by neutralizing a protein produced by your immune system. They are for people with severe ulcerative colitis who don’t respond to or can’t tolerate other treatments.
- Vedolizumab (Entyvio). This medication was recently approved for treatment of ulcerative colitis for people who don’t respond to or can’t tolerate other treatments. It works by blocking inflammatory cells from getting to the site of inflammation.
- Ustekinumab (Stelara). This medication is approved for treatment of ulcerative colitis for people who don’t respond to or can’t tolerate other treatments. It works by blocking a different protein that causes inflammation.
Other medications
You may need additional medications to manage specific symptoms of ulcerative colitis. Always talk with your doctor before using over-the-counter medications.
He or she may recommend one or more of the following:
- Antibiotics. People with ulcerative colitis who run fevers will likely take antibiotics to help prevent or control infection.
- Anti-diarrheal medications. For severe diarrhea, loperamide (Imodium) may be effective. Use anti-diarrheal medications with great caution and after talking with your doctor, because they may increase the risk of toxic megacolon (enlarged colon).
- Pain relievers. For mild pain, your doctor may recommend acetaminophen (Tylenol, others) — but not ibuprofen (Advil, Motrin IB, others), naproxen sodium (Aleve), and diclofenac sodium (Voltaren), which can worsen symptoms and increase the severity of disease.
- Antispasmodics. Sometimes doctors will prescribe antispasmodic therapies to help with cramps.
- Iron supplements. If you have chronic intestinal bleeding, you may develop iron deficiency anemia and be given iron supplements.
Surgery
Surgery can often eliminate ulcerative colitis. But that usually means removing your entire colon and rectum (proctocolectomy).
In most cases, this involves a procedure called ileal pouch anal anastomosis (J-pouch surgery). This procedure eliminates the need to wear a bag to collect stool. Your surgeon constructs a pouch from the end of your small intestine. The pouch is then attached directly to your anus, allowing you to expel waste relatively normally.
In some cases a pouch is not possible. Instead, surgeons create a permanent opening in your abdomen (ileal stoma) through which stool is passed for collection in an attached bag.
Your doctor may recommend surgery if you have:
- colorectal cancer
- dysplasia, or precancerous cells that increase the risk for developing colorectal cancer
- complications that are life-threatening, such as severe rectal bleeding, toxic megacolon, or perforation of the large intestine
- symptoms that don’t improve or stop after treatment with medicines
- symptoms that only improve with continuous treatment with corticosteroids, which may cause serious side effects when used for a long time.
To treat ulcerative colitis, surgeons typically remove the colon and rectum and change how your body stores and passes stool. The most common types of surgery for ulcerative colitis are:
- ileoanal reservoir surgery. Surgeons create an internal reservoir, or pouch, from the end part of the small intestine, called the ileum. Surgeons attach the pouch to the anus. Ileoanal reservoir surgery most often requires two or three operations. After the operations, stool will collect in the internal pouch and pass through the anus during bowel movements.
- ileostomy. Surgeons attach the end of your ileum to an opening in your abdomen called a stoma. After an ileostomy, stool will pass through the stoma. You’ll use an ostomy pouch—a bag attached to the stoma and worn outside the body—to collect stool.
Surgery may be laparoscopic or open. In laparoscopic surgery, surgeons make small cuts in your abdomen and insert special tools to view, remove, or repair organs and tissues. In open surgery, surgeons make a larger cut to open your abdomen.
If you are considering surgery to treat ulcerative colitis, talk with your doctor or surgeon about what type of surgery might be right for you and the possible risks and benefits.
Treating symptoms and complications of ulcerative colitis
Doctors may recommend or prescribe other treatments for symptoms or complications of ulcerative colitis. Talk with your doctor before taking any over-the-counter medicines.
To treat mild pain, doctors may recommend acetaminophen (paracetamol) instead of nonsteroidal anti-inflammatory drugs (NSAIDs). People with ulcerative colitis should avoid taking NSAIDs for pain because these medicines can make symptoms worse. NSAIDs include aspirin, ibuprofen (Advil, Motrin IB, others), naproxen sodium (Aleve), diclofenac sodium (Voltaren) and others. While they do not cause ulcerative colitis, they can lead to inflammation of the bowel that makes ulcerative colitis worse.
To prevent or slow loss of bone mass and osteoporosis, doctors may recommend calcium and vitamin D supplements or medicines, if needed. For safety reasons, talk with your doctor before using dietary supplements or any other complementary or alternative medicines or practices.
Doctors most often treat severe complications in a hospital. Doctors may give:
- antibiotics, if severe ulcerative colitis or complications lead to infection
- blood transfusions to treat severe anemia
- IV fluids and electrolytes to prevent and treat dehydration
Doctors may treat life-threatening complications with surgery.
Cancer surveillance
Patients with ulcerative colitis have an increased risk of colorectal cancer. You will need more-frequent screening for colon cancer because of your increased risk. The recommended schedule will depend on the location of your disease and how long you have had it.
If your disease involves more than your rectum, you will require a surveillance colonoscopy every one to two years. Additionally, random biopsies spaced at regular intervals throughout the colon are recommended every one to two years during screening colonoscopies. Patients with proctitis or proctosigmoiditis do not seem to be at increased risk of cancer 41. You will need a surveillance colonoscopy beginning as soon as eight years after diagnosis if the majority of your colon is involved, or 15 years if only the left side of your colon is involved.
Foods to avoid with ulcerative colitis
Sometimes you may feel helpless when facing ulcerative colitis. But changes in your diet and lifestyle may help control your symptoms and lengthen the time between flare-ups. If you have ulcerative colitis, you should eat a healthy, well-balanced diet. Talk with your doctor or a registered dietitian about a healthy eating plan. Drink plenty of liquids. Try to drink plenty of liquids daily. Water is best. Alcohol and beverages that contain caffeine stimulate your intestines and can make diarrhea worse, while carbonated drinks frequently produce gas.
There’s no studies have suggested that diet can either cause or treat ulcerative colitis and there is no specific diet that patients with ulcerative colitis should follow, though it is advisable to eat a balanced diet 58. Likewise, there is no convincing evidence that ulcerative colitis results from food allergies. But certain foods and beverages can aggravate your signs and symptoms, especially during a flare-up.
It can be helpful to keep a food diary to keep track of what you’re eating, as well as how you feel. If you discover that some foods are causing your symptoms to flare, you can try eliminating them. Here are some suggestions that may help:
Foods to limit or avoid
- Limit dairy products. Many people with inflammatory bowel disease find that problems such as diarrhea, abdominal pain and gas improve by limiting or eliminating dairy products. You may be lactose intolerant — that is, your body can’t digest the milk sugar (lactose) in dairy foods. Using an enzyme product such as Lactaid may help as well.
- Limit fiber, if it’s a problem food. If you have inflammatory bowel disease, high-fiber foods, such as fresh fruits and vegetables and whole grains, may make your symptoms worse. If raw fruits and vegetables bother you, try steaming, baking or stewing them.
In general, you may have more problems with foods in the cabbage family, such as broccoli and cauliflower, and nuts, seeds, corn and popcorn. Avoid other problem foods. Spicy foods, alcohol and caffeine may make your signs and symptoms worse.
Low-residue diet
Temporarily eating a low-residue or low-fiber diet can sometimes help improve symptoms of ulcerative colitis during a flare-up. These diets are designed to reduce the amount and frequency of the stools you pass.
Examples of foods that can be eaten as part of a low-residue diet include:
- white bread
- refined (non-wholegrain) breakfast cereals, such as cornflakes
- white rice, refined pasta and noodles
- cooked vegetables (but not the peel, seeds or stalks)
- lean meat and fish
- eggs
If you’re considering trying a low-residue diet, make sure you talk to your care team first.
Other dietary measures
- Eat small meals. You may find you feel better eating five or six small meals a day rather than two or three larger ones.
- Drink plenty of liquids. Try to drink plenty of fluids daily. Water is best. Alcohol and beverages that contain caffeine stimulate your intestines and can make diarrhea worse, while carbonated drinks frequently produce gas.
- Talk to a dietitian. If you begin to lose weight or your diet has become very limited, talk to a registered dietitian.
Ulcerative colitis diet
In studies involving people with chronic inflammatory bowel diseases (IBD) like Crohn’s disease and ulcerative colitis, the anti-Inflammatory diet (IBD-AID) has been used as an adjunct dietary therapy for the treatment of inflammatory bowel diseases (IBD) 59. The goal of the anti-Inflammatory diet (IBD-AID) is to assist with a decreased frequency and severity of flares, obtain and maintain remission in people with inflammatory bowel diseases (IBD). Dysbiosis, or altered bacterial flora, is one of the theories behind the development of anti-Inflammatory diet (IBD-AID), in that certain carbohydrates in the lumen of the gut provide pathogenic bacteria a substrate on which to proliferate 60, 61.
The anti-Inflammatory diet (IBD-AID) has five basic components:
- The first of which is the modification of certain carbohydrates, (including lactose, and refined or processed complex carbohydrates)
- The second places strong emphasis on the ingestion of pre- and probiotics (e.g.; soluble fiber, leeks, onions, and fermented foods) to help restore the balance of the intestinal flora 62, 63, 64
- The third distinguishes between saturated, trans, mono- and polyunsaturated fats 65, 66,
- The fourth encourages a review of the overall dietary pattern, detection of missing nutrients, and identification of intolerances.
- The last component modifies the textures of the foods (e.g.; blenderized, ground, or cooked) as needed (per patient symptomology) to improve absorption of nutrients and minimize intact fiber.
The phases indicated in Table 6 are examples of the modification of texture complexity, so that dietitian and patient can expand the diet as the patient’s tolerance and absorption improves. Some sensitivities common to many patients (not just those with IBD), are eased through supplementation of digestive enzymes or avoidance. A senior dietitian advised the patient and either family or spouse regarding the details of the diet during regular clinic visits. Patients taking supplements (probiotics, vitamin/minerals, omega-3 fatty acids) were advised to continue or discontinue, depending on the needs of the individual and the dietary intake 67.
The anti-Inflammatory diet (IBD-AID) consists of lean meats, poultry, fish, omega-3, eggs, particular sources of carbohydrate, select fruits and vegetables, nut and legume flours, limited aged cheeses (made with active cultures and enzymes), fresh cultured yogurt, kefir, miso and other cultured products (rich with certain probiotics) and honey. Prebiotics, in the form of soluble fiber (containing beta-glucans and inulin, such as bananas, oats, blended chicory root, and flax meal) are suggested. In addition, the patient is advised to begin at a texture phase of the diet matching with symptomology, starting with phase one if in an active flare. Many patients require foods to be softened and textures mechanically altered by pureeing the foods, and avoiding foods with stems and seeds when starting the diet (see phases 1–3 of Table 6), as intact fiber can be problematic for those with strictures and highly active mucosal inflammation. Some patients will require lifelong avoidance of intact fiber. Food irritants are not limited to intact fiber, but may include certain foods, processing agents and flavorings to which IBD patients may be reactive.
Table 3. The Anti-Inflammatory Diet (IBD-AID) Food Phase chart
| Phase type | Phase I | Phase II | Phase III | Phase IV |
|---|---|---|---|---|
| Soft, well-cooked or cooked then pureed foods, no seeds | Soft Textures: well-cooked or pureed foods, no seeds, choose floppy or tender foods | May still need to avoid stems, choose floppy greens or other greens depending on individual tolerance | If in remission with no strictures | |
| Vegetables | Butternut Squash, Pumpkin, Sweet Potatoes, Onions | Carrots, Zucchini, Eggplant, Peas, Snow peas, Spaghetti squash, Green beans, Yellow beans, Microgreens (2 week old baby greens), Watercress, Arugula, Fresh flat leaf parsley and cilantro, Seaweed, Algae | Butter lettuce, Baby spinach, Peeled cucumber, Olives, Leeks Bok Choy, Bamboo shoots, Collard greens, Beet greens, Sweet peppers, Kale, Fennel bulb | Artichokes, Asparagus, Tomatoes, Lettuce, Brussels sprouts, Beets, Cabbage, Kohlrabi, Rhubarb, Pickles, Spring onions, Water chestnuts, Celery, Celeriac, Cauliflower, Broccoli, Radish, Green pepper, Hot pepper |
| Pureed vegetables: Mushrooms, Phase II vegetables (pureed) | Pureed vegetables: all except cruciferous | Pureed vegetables: all from Phase IV, Kimchi | ||
| Fruits | Banana, Papaya, Avocado, Pawpaw | Watermelon (seedless), Mangoes, Honeydew, Cantaloupe, May need to be cooked: Peaches, Plums, Nectarines, Pears, (Phase III fruits are allowed if pureed and seeds are strained out) | Strawberries, Cranberries, Blueberries, Apricots, Cherries, Coconut, Lemons, Limes, Kiwi, Passion fruit, Blackberries, Raspberries, Pomegranate (May need to strain seeds from berries) | Grapes, Grapefruit, Oranges, Currants, Figs, Dates, Apples (best cooked), Pineapple, Prunes |
| Meats and fish | All fish (no bones), Sardines (small bones ok), Turkey and ground beef, Chicken, Eggs | Scallops | Lean cuts of Beef, Lamb, Duck, Goose | Shrimp, Prawns, Lobster |
| Non dairy unsweetened | Coconut milk, Almond milk, Oat milk, Soy milk | |||
| Dairy, unsweetened | Yogurt, Kefir | Farmers cheese (dry curd cottage cheese), Cheddar cheese | Aged cheeses | |
| Nuts/Oils/Legumes/Fats | Miso (refrigerated), Tofu, Olive oil, Canola oil, Flax oil, Hemp oil, Walnut oil, Coconut oil | Almond flour, Peanut flour, Soy flour, Sesame oil, Grapeseed oil, Walnut oil, Pureed nuts, Safflower oil, Sunflower oil | Whole nuts, Soybeans, Bean flours, Nut butters, Well-cooked lentils (pureed), Bean purees (e.g. hummus) | Whole beans and lentils |
| Grains | Ground flax or Chia Seeds (as tolerated) | Steel cut oats (well-cooked as oatmeal) | Rolled well-cooked oats | |
| Spices | Basil, Sage, Oregano, Salt, Nutmeg, Cumin, Cinnamon, Turmeric, Saffron, Mint, Bay leaves, Tamari (wheat free soy sauce), Fenugreek tea, Fennel tea, Vanilla | Dill, Thyme, Rosemary Tarragon, Cilantro, Basil, Parsley | Mint, Ginger, Garlic (minced), Paprika, Chives, Daikon, Mustard | Wasabi, Tamarind, Horseradish, Fenugreek, Fennel |
| Sweeteners | Stevia, Maple syrup, Honey (local), Unsweetened fruit juice | Lemon and lime juice | ||
| Misc. | Capsule or liquid supplements, Cocoa powder | Baking powder (no cornstarch), Baking soda, Unflavored gelatin | Ghee, Light mayonnaise, Vinegar | Ketchup (sugar free), Hot sauce (sugar free) |
Ulcerative colitis prognosis
Patients with ulcerative colitis do not appear to have an overall increased mortality compared with the general population, but are more likely to have a disability preventing them from working 68. At presentation, 30–60% of patients with ulcerative colitis have proctitis, 16–45% have left-sided colitis, and 14–35% have extensive pancolitis in population-based studies 69. Ulcerative colitis can progress proximally in 10–19% of patients after 5 years, and in up to 28% of patients at 10 years 69. Most patients with ulcerative colitis have a relapsing and remitting disease course with periodic flares 70. When ulcerative colitis flares are associated with proximal disease extension, patients are more likely to need immunosuppressants, biological drugs, or surgery 71. Age of onset appears to affect the disease course, since patients with disease onset after age 60 years tend to have milder disease compared with younger patients 72. Primary sclerosing cholangitis-associated ulcerative colitis could be a distinct phenotype, because it is more likely to be extensive, milder, and associated with rectal sparing and so-called backwash ileitis compared with patients with ulcerative colitis without primary sclerosing cholangitis 73. Risk factors for aggressive or complicated disease include a younger age at onset (<40 years), pancolitis, lack of endoscopic healing while in clinical remission, deep ulcerations, and high concentrations of perinuclear antineutrophil cytoplasmic antibodies 71. A small number of patients (5–10%) initially classified with ulcerative colitis might eventually have their diagnosis changed to Crohn’s disease 70. Patients with ulcerative colitis can develop structural and functional damage to the colon, including benign strictures, colonic dysmotility, and anorectal dysfunction 74. Patients with the disease are at increased risk of colorectal cancer, but over time this risk has decreased and might be approaching the general population; however, the risk remains elevated in certain populations, such as those with long duration of disease, primary sclerosing cholangitis, and uncontrolled inflammation 75. The risk for surgery in ulcerative colitis has decreased over past decades, but is still substantial with the chance of needing surgery at 5 years being 11.6%, and at 10 years being 15.6% 76. Risk factors for colectomy have been incorporated into a prediction model that includes age less than 40 years at diagnosis, extensive disease, need for systemic steroids, and elevated inflammatory markers 77.
Living with ulcerative colitis
Living with a long-term condition that is as unpredictable and potentially debilitating as ulcerative colitis can have a significant emotional impact.
In some cases, anxiety and stress caused by ulcerative colitis can lead to depression. Signs of depression include feeling very down, hopeless and no longer taking pleasure in activities you used to enjoy. If you think you might be depressed, contact your doctor for advice.
You may also find it useful to talk to others affected by ulcerative colitis, either face-to-face or via the internet. Crohn’s and Colitis Foundation 78 is a good resource, with details of local support groups and a large range of useful information on ulcerative colitis and related issues.
Although stress doesn’t cause inflammatory bowel disease, it can make your signs and symptoms worse and may trigger flare-ups.
Successfully managing stress levels may reduce the frequency of symptoms. The following advice may help:
To help control stress, try:
- Exercise. Even mild exercise can help reduce stress, relieve depression and normalize bowel function. Talk to your doctor about an exercise plan that’s right for you.
- Biofeedback. This stress-reduction technique helps you reduce muscle tension and slow your heart rate with the help of a feedback machine. The goal is to help you enter a relaxed state so that you can cope more easily with stress.
- Regular relaxation and breathing exercises. An effective way to cope with stress is to perform relaxation and breathing exercises. You can take classes in yoga and meditation or practice at home using books, online videos or DVDs.
Alternative medicine
Many people with digestive disorders have used some form of complementary and alternative therapy.
Some commonly used therapies include:
- Herbal and nutritional supplements. The majority of alternative therapies aren’t regulated by the FDA. Manufacturers can claim that their therapies are safe and effective but don’t need to prove it. What’s more, even natural herbs and supplements can have side effects and cause dangerous interactions. Tell your doctor if you decide to try any herbal supplement.
- Probiotics. Researchers suspect that adding more of the beneficial bacteria (probiotics) that are normally found in the digestive tract might help combat the disease 79. Lactobacillus GG and Escherichia coli Nissle 1917 (Mutaflor) have been shown to be as effective as 5-ASA for maintenance therapy 80, 81. Although research is limited, there is some evidence that adding probiotics along with other medications may be helpful, but this has not been proved.
- Fish oil. Fish oil acts as an anti-inflammatory, and there is some evidence that adding fish oil to aminosalicylates may be helpful, but this has not been proved.
- Aloe vera. Aloe vera gel may have an anti-inflammatory effect for people with ulcerative colitis, but it can also cause diarrhea.
- Acupuncture. Only one clinical trial 82 has been conducted regarding its benefit. The procedure involves the insertion of fine needles into the skin, which may stimulate the release of the body’s natural painkillers.
- Turmeric. Curcumin, a compound found in the spice turmeric, has been combined with standard ulcerative colitis therapies in clinical trials. There is some evidence of benefit, but more research is needed.
- Wheatgrass has also shown some effectiveness in reducing symptoms of active disease 83.
Crohn’s disease
Crohn’s disease is a chronic (long lasting), progressive, and disabling inflammatory bowel disease (IBD) 84. Ulcerative colitis and microscopic colitis are other common types of inflammatory bowel disease (IBD). Crohn’s disease causes inflammation and irritation in your digestive tract and can involve any site of your gastrointestinal tract from your mouth to your anus, and the inflammation caused by Crohn’s disease often spreads deep into the layers of affected bowel tissue. Most commonly, Crohn’s disease affects your small intestine and the beginning of your large intestine. Crohn’s disease sometimes may lead to life-threatening complications, such as bowel strictures, perforations, and fistula formation. Crohn’s disease causes inflammation of your digestive tract, which can lead to abdominal pain, severe diarrhea, fatigue, fever, weight loss, abdominal masses, anemia and malnutrition 85. Inflammation caused by Crohn’s disease can involve different areas of the digestive tract in different people. Extraintestinal manifestations of Crohn’s disease include osteoporosis, inflammatory arthropathies (inflammation of one or more joints, causing pain and stiffness), scleritis (inflammation involving the white part of the eye [sclera]), kidney stones (nephrolithiasis), gallstones (cholelithiasis) and erythema nodosum (an inflammatory disorder affecting subcutaneous fat, it presents as tender red bumps found symmetrically on the shins). Crohn’s disease most often begins gradually and can become worse over time. You may have periods of remission that can last for weeks or years.
Crohn’s disease affects about 500,000 Americans 86. Men and women appear to be affected equally. Symptoms usually start between the ages of 15-35 but can develop at any time during one’s lifetime. Studies show that, over time, Crohn’s disease has become more common in the United States and other parts of the world 87. It used to be thought that Crohn’s disease predominantly affects Caucasians in North America and Western Europe, however, experts now see individuals being diagnosed with Crohn’s disease in populations from South America, Africa and Asia where the disease was unheard of 20 years ago. Experts do not know the reason for this increase.
Crohn’s disease can run in families and having a sibling or another first degree relative afflicted with the disease can increase the risk of developing the disease by ten to fifteen times as compared to the general population. Children who have one parent with Crohn’s disease have approximately a 7-10% lifetime risk of developing this disease. If both parents have the disease then the lifetime risk increases to 35%. Some important genetic mutations have been identified in this disease including the NOD2/CARD 15 genes. However, more than 80% of patients with Crohn’s disease do not have a recognized genetic disposition. Given the complex genetic makeup the disease it is not inherited in the classic sense.
Smoking is an important controllable risk factor in Crohn’s disease. People who smoke with this disease tend to have more severe forms of the disease and are at higher risk of needing surgery. People who live in industrialized countries and urban areas are also at elevated risk of having the disease. Some other factors including the use of medications such as non-steroidal anti-inflammatory drugs (aspirin-like drugs) and particular infections have been theorized to exacerbate or cause the disease although none have been shown in a consistent fashion to be the cause.
If you have Crohn’s disease in your large intestine, you may be more likely to develop colon cancer 88. If you receive ongoing treatment for Crohn’s disease and stay in remission, you may reduce your chances of developing colon cancer 89. Talk with your doctor about how often you should get screened for colon cancer. Screening is testing for diseases when you have no symptoms. Screening for colon cancer can include colonoscopy with biopsies. Although screening does not reduce your chances of developing colon cancer, it may help to find cancer at an early stage and improve the chance of curing the cancer.
While there’s no known cure for Crohn’s disease, therapies can greatly reduce its signs and symptoms and even bring about long-term remission. With treatment, many people with Crohn’s disease are able to function well.
Types of Crohn’s disease
If you are diagnosed with Crohn’s disease, it’s important to know and understand which part of your GI tract is affected and how this may affect the symptoms and complications you experience.
The following are five types of Crohn’s disease, together with their presenting symptoms:
Ileocolitis
The most common form of Crohn’s, ileocolitis affects the end of the small intestine (the ileum) and the large intestine (the colon). Symptoms include diarrhea and cramping or pain in the right lower part or middle of the abdomen. This type is often accompanied by significant weight loss.
Ileitis
This type affects only the ileum. Symptoms are the same as ileocolitis. In severe cases, complications may include fistulas or inflammatory abscess in right lower quadrant of abdomen.
Gastroduodenal Crohn’s disease
This type affects the stomach and the beginning of the small intestine(the duodenum). Symptoms include loss of appetite, weight loss, nausea, and vomiting.
Jejunoileitis
This type is characterized by patchy areas of inflammation in the upper half of the small intestine (the jejunum). Symptoms include mild to intense abdominal pain and cramps following meals, as well as diarrhea. In severe cases or after prolonged periods, fistulas may form.
Crohn’s (granulomatous) colitis
This type affects the colon only. Symptoms include diarrhea, rectal bleeding, and disease around the anus (abscess, fistulas, ulcers). Skin lesions and joint pains are more common in this form of Crohn’s than in others.
Crohn’s disease causes
The exact cause of Crohn’s disease remains unknown. Previously, diet and stress were suspected, but now doctors know that these factors may aggravate but don’t cause Crohn’s disease. A number of factors, such as heredity and a malfunctioning immune system, likely play a role in its development.
Experts think the following factors may play a role in causing Crohn’s disease:
- Immune system. It’s possible that a virus or bacterium may trigger Crohn’s disease. When your immune system tries to fight off the invading microorganism, an abnormal immune response causes the immune system to attack the cells in the digestive tract, too. One cause of Crohn’s disease may be an autoimmune reaction when your immune system attacks healthy cells in your body. Experts think bacteria in your digestive tract can mistakenly trigger your immune system. This immune system response causes inflammation, leading to symptoms of Crohn’s disease.
- Heredity. Crohn’s disease is more common in people who have family members with the disease, so genes may play a role in making people more susceptible. Research has shown that if you have a parent or sibling with Crohn’s disease, you may be more likely to develop the disease. However, most people with Crohn’s disease don’t have a family history of the disease. Scientists continue to study the link between genes and Crohn’s disease.
Risk factors for Crohn’s disease
Some studies suggest that other factors may increase your chance of developing Crohn’s disease. Risk factors for Crohn’s disease may include:
- Age. Crohn’s disease can occur at any age, but you’re likely to develop the condition when you’re young. Most people who develop Crohn’s disease are diagnosed before they’re around 30 years old.
- Ethnicity. Although Crohn’s disease can affect any ethnic group, whites and people of Eastern European (Ashkenazi) Jewish descent have the highest risk. However, the incidence of Crohn’s disease is increasing among blacks who live in North America and the United Kingdom.
- Family history. You’re at higher risk if you have a close relative, such as a parent, sibling or child, with the disease. As many as 1 in 5 people with Crohn’s disease has a family member with the disease.
- Cigarette smoking. Cigarette smoking is the most important controllable risk factor for developing Crohn’s disease 90. Smoking also leads to more-severe disease and a greater risk of having surgery. If you smoke, it’s important to stop.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These include aspirin, ibuprofen (Advil, Motrin IB, others), naproxen sodium (Aleve), diclofenac sodium (Voltaren) and others. While they do not cause Crohn’s disease, they can lead to inflammation of the bowel that makes Crohn’s disease worse.
- Antibiotics and birth-control pills may slightly increase the chance of developing Crohn’s disease 32.
- A high-fat diet may also slightly increase your chance of getting Crohn’s disease 91.
- Where you live. If you live in an urban area or in an industrialized country, you’re more likely to develop Crohn’s disease. This suggests that environmental factors, including a diet high in fat or refined foods, may play a role in Crohn’s disease.
Crohn’s disease signs and symptoms
The main symptoms of Crohn’s disease are abdominal pain, diarrhea, and weight loss. It occurs mainly in the 10 to 20 years age group and lasts throughout a lifetime, as well as causes complications such as bowel stenosis, fistula, and perforation.
In some people with Crohn’s disease, only the last segment of the small intestine (ileum) is affected. In others, the disease is confined to the colon (part of the large intestine). The most common areas affected by Crohn’s disease are the last part of the small intestine and the colon.
Signs and symptoms of Crohn’s disease can range from mild to severe. They usually develop gradually, but sometimes will come on suddenly, without warning. You may also have periods of time when you have no signs or symptoms (remission).
When the disease is active, signs and symptoms may include:
- Diarrhea
- Fever
- Fatigue
- Abdominal pain and cramping
- Blood in your stool
- Mouth sores
- Reduced appetite and weight loss
- Nausea
- Anemia
- Pain or drainage near or around the anus due to inflammation from a tunnel into the skin (fistula)
Other signs and symptoms:
People with severe Crohn’s disease also may experience:
- Inflammation of skin (skin changes that involve red, tender bumps under the skin), eyes (eye redness or pain) and joints (joint pain or soreness)
- Inflammation of the liver or bile ducts
- Delayed growth or sexual development, in children
Your symptoms may vary depending on the location and severity of your inflammation.
Some research suggests that stress, including the stress of living with Crohn’s disease, can make symptoms worse. Also, some people may find that certain foods can trigger or worsen their symptoms.
Crohn’s disease complications
Crohn’s disease may lead to one or more of the following complications:
- Bowel obstruction. Crohn’s disease affects the thickness of the intestinal wall. Over time, the thickened areas of your intestines can scar and narrow, which may block the flow of digestive contents. A partial or complete intestinal obstruction, also called a bowel blockage, can block the movement of food or stool through your intestines. You may require surgery to remove the diseased portion of your bowel.
- Ulcers. Chronic inflammation can lead to open sores (ulcers) anywhere in your digestive tract, including your mouth and anus, and in the genital area (perineum).
- Fistulas. Sometimes ulcers can extend completely through the intestinal wall, creating a fistula (a tunnel)— an abnormal connection between different body parts. Fistulas can develop between your intestine and skin on the outside of your body, or between your intestine and another organ. Fistulas near or around the anal area (perianal) are the most common kind. Fistulas may become infected.
When fistulas develop in the abdomen, food may bypass areas of the bowel that are necessary for absorption. Fistulas may occur between loops of bowel, into the bladder or vagina, or out through the skin, causing continuous drainage of bowel contents to your skin.
In some cases, a fistula may become infected and form an abscess, which can be life-threatening if not treated. Abscesses are painful, swollen, pus-filled pockets of infection.
- Anal fissure. This is a small tear in the tissue that lines your anus or in the skin around your anus where infections can occur. Anal fissure is often associated with painful bowel movements, itching, pain, or bleeding and may lead to a perianal fistula.
- Malnutrition. Malnutrition develops when your body does not get the right amount of vitamins, minerals, and nutrients it needs to maintain healthy tissues and organ function. Diarrhea, abdominal pain and cramping may make it difficult for you to eat or for your intestine to absorb enough nutrients to keep you nourished. It’s also common to develop anemia due to low iron or vitamin B-12 caused by Crohn’s disease.
- Colon cancer. Having Crohn’s disease that affects your colon increases your risk of colon cancer. General colon cancer screening guidelines for people without Crohn’s disease call for a colonoscopy every 10 years beginning at age 50. Ask your doctor whether you need to have this test done sooner and more frequently.
- Other health problems. Crohn’s disease can cause problems in other parts of the body. Among these problems are anemia, skin disorders, osteoporosis, arthritis, and gallbladder or liver disease.
- Inflammation in other areas of your body. You may have inflammation in your joints, eyes, and skin.
- Medication risks. Certain Crohn’s disease drugs that act by blocking functions of the immune system are associated with a small risk of developing cancers such as lymphoma and skin cancers. They also increase risk of infection.
Corticosteroids can be associated with a risk of osteoporosis, bone fractures, cataracts, glaucoma, diabetes and high blood pressure, among others. Work with your doctor to determine risks and benefits of medications.
Crohn’s disease diagnosis
Your doctor will likely diagnose Crohn’s disease only after ruling out other possible causes for your signs and symptoms. There is no one test to diagnose Crohn’s disease.
Your doctor will likely use a combination of tests to help confirm a diagnosis of Crohn’s disease, including:
Blood tests
- Tests for anemia or infection. Your doctor may suggest blood tests to check for anemia — a condition in which there aren’t enough red blood cells to carry adequate oxygen to your tissues — or to check for signs of infection. Expert guidelines do not currently recommend antibody or genetic testing for Crohn’s disease.
- Fecal occult blood test. You may need to provide a stool sample so that your doctor can test for hidden (occult) blood in your stool.
Crohn’s disease test
Intestinal endoscopies are the most accurate methods for diagnosing Crohn’s disease and ruling out other possible conditions, such as ulcerative colitis, diverticular disease, or cancer. Intestinal endoscopies include the following:
- Colonoscopy. This test allows your doctor to view your entire colon and the very end of your ileum (terminal ileum) using a thin, flexible, lighted tube with an attached camera on one end, called a colonoscope or endoscope. During the procedure, your doctor can also take small samples of tissue (biopsy) for laboratory analysis, which may help confirm a diagnosis. You won’t feel the biopsies. Clusters of inflammatory cells called granulomas, if present, help confirm the diagnosis of Crohn’s disease.
- Upper GI endoscopy and enteroscopy. In an upper GI endoscopy, your doctor uses an endoscope to see inside your upper digestive tract, also called your upper GI tract. A trained specialist performs the procedure at a hospital or an outpatient center. You should not eat or drink before the procedure. A health care professional will tell you how to prepare for an upper GI endoscopy. You most often receive a liquid anesthetic to numb your throat and a light sedative to help you stay relaxed and comfortable during the procedure. During the procedure, the doctor carefully feeds the endoscope down your esophagus and into your stomach and duodenum.
- During an enteroscopy, a doctor examines your small intestine with a special, longer endoscope using one of the following procedures:
- push enteroscopy, which uses a long endoscope to examine the upper portion of your small intestine
- single- or double-balloon enteroscopy, which uses small balloons to help move the endoscope into your small intestine
- spiral enteroscopy, which uses a tube attached to an endoscope that acts as a corkscrew to move the instrument into your small intestine.
- During an enteroscopy, a doctor examines your small intestine with a special, longer endoscope using one of the following procedures:
- Computerized tomography (CT). You may have a CT scan — a special X-ray technique that provides more detail than a standard X-ray does. This test looks at the entire bowel as well as at tissues outside the bowel. CT enterography is a special CT scan that provides better images of the small bowel. This test has replaced barium X-rays in many medical centers.
- Magnetic resonance imaging (MRI). An MRI scanner uses a magnetic field and radio waves to create detailed images of organs and tissues. MRI is particularly useful for evaluating a fistula around the anal area (pelvic MRI) or the small intestine (MR enterography).
- Capsule endoscopy. For this test, you swallow a capsule that has a camera in it. The camera takes pictures of your small intestine, which are transmitted to a recorder you wear on your belt. The images are then downloaded to a computer, displayed on a monitor and checked for signs of Crohn’s disease. The camera exits your body painlessly in your stool. You may still need endoscopy with biopsy to confirm the diagnosis of Crohn’s disease. The test begins in a doctor’s office, where you swallow the capsule. You can leave the doctor’s office during the test. As the capsule passes through your digestive tract, the camera will record and transmit images to a small receiver device that you wear. When the recording is done, your doctor downloads and reviews the images. The camera capsule leaves your body during a bowel movement, and you can safely flush it down the toilet.
- Balloon-assisted enteroscopy. For this test, a scope is used in conjunction with a device called an overtube. This enables the doctor to look further into the small bowel where standard endoscopes don’t reach. This technique is useful when capsule endoscopy shows abnormalities, but the diagnosis is still in question.
Crohn’s disease treatment
There is currently no cure for Crohn’s disease, and there is no one treatment that works for everyone. The goal of medical treatment is to reduce the inflammation that triggers your signs and symptoms. It is also to improve long-term prognosis by limiting complications. In the best cases, this may lead not only to symptom relief but also to long-term remission.
The treatment of patients with Crohn disease depends on disease severity, patient risk stratification, patient preference, and clinical factors, including age of onset and penetrating complications, and includes treatment with steroids, monoclonal antibody therapies, immunomodulators, and surgery 92.
Historically, treatment of Crohn’s disease has relied mainly on corticosteroids and immunosuppressive medication with thiopurines (azathioprine/6-mercaptopurine [6-MP]) or methotrexate (MTX) 93. Though the introduction of corticosteroids achieved reduction of mortality in Crohn’s disease patients, this treatment is associated with many well-known undesired adverse effects and therefore not useful for long-term treatment. Two decades ago, the treatment armamentarium was extended by infliximab, the first tumor necrosis factor (TNF)-inhibitor, especially for patients with severe disease course and those who were refractory or intolerant to immunosuppressives 94. However, therapy with TNF-inhibitors is still challenging as 20–40% of patients have primary nonresponse to the drug and 23–46% experience secondary loss of response over time 95. In addition, approximately 5% of patients suffer from anti-TNF induced psoriasis or lupus-like syndrome 96. Challenges also remain in treating elderlies who have significant comorbidities (e.g., heart failure) or patients with a history of malignancy.
Over the last couple of years, a number of new drugs have been developed and approved based either on inhibition of immune cell trafficking or on inhibition of cytokine signaling 97. Vedolizumab, a monoclonal antibody directed against α4β7 integrin, has been approved for the treatment of patients with moderate-to-severe ulcerative colitis or Crohn’s disease who have inadequate response, loss of response or intolerance to conventional therapy with corticosteroids, immunosuppressives, or anti-TNF therapy. It is effective in the induction and maintenance of remission in refractory and luminal Crohn’s disease 98. In real-world Crohn’s disease cohorts, clinical remission and response rates after induction therapy (usually evaluated at week 14) ranged from 24–36% to 49–64%, respectively 99. Vedolizumab prevents circulating immune cells from homing to the mucosa and is gut-selective through interactions with mucosal adhesion molecules. This may be a specific advantage in long-term safety and may also explain the longer period of time to induce remission (12–16 weeks), compared to TNF-inhibitors 100. Therefore, vedolizumab is not the ideal choice in patients with severe Crohn’s disease who are acutely ill where fast response to treatment is needed 93. In the latest European Crohn’s and Colitis Organisation (ECCO) guideline 101, the position of vedolizumab is adapted as it is now also clearly recommended to be used for induction and remission in patients with moderate to severe Crohn’s disease with inadequate response to conventional therapy with immunosuppressives. In other words, it is recommended as a first-line biological. However, the European Crohn’s and Colitis Organisation (ECCO) guideline does not discuss in detail which patients should be commenced on vedolizumab or anti-TNF inhibitors. According to some experts opinion, vedolizumab is suitable as a first choice biological in elderlies with comorbidities and elevated risk of infection and patients with history of malignancy, where safety is of particular concern 93.
Ustekinumab is a monoclonal IgG1 antibody against the p40 subunit of interleukin-12 (IL-12) and IL-23 that targets T-helper cell pathways which promote the accumulation of inflammatory cells within the intestine 102. It was approved for the treatment of patients with moderate-to-severe Crohn’s disease who have failed or were intolerant to treatment with corticosteroids, immunosuppressives, or anti-TNF therapy. Ustekinumab has shown to be effective in inducing and maintaining remission 103, with higher response rates in TNF-naïve than in TNF-experienced patients (54–58% vs. 34%, respectively) 104. In the updated ECCO guideline 101, ustekinumab has an expanded position within the approved biologicals as it can also be used as a first-line biological – such as vedolizumab. Again, the ECCO guideline does not give recommendation where to prefer ustekinumab over TNF-inhibitors or over vedolizumab as first-line option. According to some experts opinion, ustekinumab has its particular place as first-line biological, especially in patients with psoriasis and Crohn’s disease. Furthermore, this drug is a suitable option in patients who have developed severe TNF-induced psoriasiform disease 96.
However, at least to date, TNF-inhibitors are less expensive (especially since biosimilars are available), and the clinical experience with TNF-inhibitors is much greater compared to the new biologicals. The safety profile of both ustekinumab and vedolizumab seems favorable, but long-term safety still needs to be confirmed in postmarketing studies 93.
Crohn’s disease medications
Many people with Crohn’s disease need medicines. Which medicines your doctor prescribes will depend on your symptoms.
Although no medicine cures Crohn’s disease, many can reduce symptoms.
Anti-inflammatory drugs
Anti-inflammatory drugs are often the first step in the treatment of inflammatory bowel disease. They include:
- Corticosteroids also known as steroids. Corticosteroids such as prednisone, budesonide (Entocort EC), hydrocortisone and methylprednisolone can help reduce inflammation in your body, but they don’t work for everyone with Crohn’s disease. Doctors generally use them only if you don’t respond to other treatments. Budesonide is a steroid that is quickly metabolized by the liver thereby reducing corticosteroid-related side effects.
Corticosteroids may be used for short-term (three to four months) symptom improvement and to induce remission. Corticosteroids may also be used in combination with an immune system suppressor.
Side effects of corticosteroids include:
- acne
- bone mass loss
- high blood glucose
- high blood pressure
- a higher chance of developing infections
- mood swings
- weight gain
In most cases, doctors do not prescribe corticosteroids for long-term use.
- Oral 5-aminosalicylates also called 5-aminosalicylic acid (5-ASA). These drugs include sulfasalazine (Azulfidine), which contains sulfa, and mesalazine also known as mesalamine, balsalazide and olsalazine. Oral 5-aminosalicylates have been widely used in the past to control inflammation but now are generally considered of limited benefit. Some of the common side effects of aminosalicylates include: diarrhea, headaches, heartburn, nausea and vomiting, pain in your abdomen.
Immune system suppressors
These drugs also reduce inflammation, but they target your immune system, which produces the substances that cause inflammation. Doctors prescribe these medicines to help you go into remission or help you if you do not respond to other treatments. For some people, a combination of these drugs works better than one drug alone. Immunosuppressants can take several weeks to 3 months to start working. Immunosuppressant drugs include:
- Azathioprine (Azasan, Imuran) and 6-mercaptopurine (Purinethol, Purixan). These are the most widely used immunosuppressants for treatment of inflammatory bowel disease. Taking them requires that you follow up closely with your doctor and have your blood checked regularly to look for side effects, such as a lowered resistance to infection and inflammation of the liver. They may also cause nausea and vomiting.
- Infliximab (Remicade), adalimumab (Humira) and certolizumab pegol (Cimzia). These drugs, called TNF inhibitors or biologics, work by neutralizing an immune system protein known as tumor necrosis factor (TNF).
- Methotrexate (Trexall). This drug is sometimes used for people with Crohn’s disease who don’t respond well to other medications. You will need to be followed closely for side effects.
- Natalizumab (Tysabri) and vedolizumab (Entyvio). These drugs work by stopping certain immune cell molecules — integrins — from binding to other cells in your intestinal lining. Because natalizumab is associated with a rare but serious risk of progressive multifocal leukoencephalopathy — a brain disease that usually leads to death or severe disability — you must be enrolled in a special restricted distribution program to use it.
- Vedolizumab recently was approved for Crohn’s disease. It works like natalizumab but appears not to carry a risk of brain disease.
- Ustekinumab (Stelara). This drug is used to treat psoriasis. Studies have shown that it’s useful in treating Crohn’s disease as well and may be used when other medical treatments fail.
- Cyclosporine and cyclosporine (modified) are also sometimes used to treat Crohn’s disease. Doctors most often prescribe cyclosporine only if you have severe Crohn’s disease because of the medicine’s serious side effects. Talk with your doctor about the risks and benefits of cyclosporine.
Immunosuppressants may have the following side effects:
- a low white blood cell count, which can lead to a higher chance of infection
- feeling tired
- nausea and vomiting
- pancreatitis
Antibiotics
Antibiotics can reduce the amount of drainage and sometimes heal fistulas and abscesses in people with Crohn’s disease. Some researchers also think antibiotics help reduce harmful intestinal bacteria that may play a role in activating the intestinal immune system, leading to inflammation. Frequently prescribed antibiotics include ciprofloxacin (Cipro) and metronidazole (Flagyl).
Biologic therapies
Biologic therapies target proteins made by the immune system. Neutralizing these proteins decreases inflammation in the intestines. Biologic therapies work to help you go into remission, especially if you do not respond to other medicines. Biologic therapies include:
- Anti-tumor necrosis factor-alpha (anti-TNF-α) therapies, such as adalimumab, certolizumab and infliximab
- Anti-integrin therapies, such as natalizumab and vedolizumab
- Anti-interleukin-12 and interleukin-23 therapy, such as ustekinumab
Doctors most often give patients infliximab every 6 to 8 weeks at a hospital or an outpatient center. Side effects may include a toxic reaction to the medicine and a higher chance of developing infections, particularly tuberculosis.
Other medications
In addition to controlling inflammation, some medications may help relieve your signs and symptoms, but always talk to your doctor before taking any over-the-counter medications. Depending on the severity of your Crohn’s disease, your doctor may recommend one or more of the following:
- Anti-diarrheals. A fiber supplement, such as psyllium powder (Metamucil) or methylcellulose (Citrucel), can help relieve mild to moderate diarrhea by adding bulk to your stool. For more severe diarrhea, loperamide (Imodium A-D) may be effective. Loperamide to help slow or stop severe diarrhea. In most cases, people only take this medicine for short periods of time because it can increase the chance of developing megacolon.
- Pain relievers. For mild pain, your doctor may recommend acetaminophen (Tylenol, others). You should avoid using ibuprofen (Advil, Motrin IB, others), naproxen sodium (Aleve) and aspirin because these medicines can make your symptoms worse and can make your disease worse as well.
- Iron supplements. If you have chronic intestinal bleeding, you may develop iron deficiency anemia and need to take iron supplements.
- Vitamin B-12 shots. Crohn’s disease can cause vitamin B-12 deficiency. Vitamin B-12 helps prevent anemia, promotes normal growth and development, and is essential for proper nerve function.
- Calcium and vitamin D supplements. Crohn’s disease and steroids used to treat it can increase your risk of osteoporosis, so you may need to take a calcium supplement with added vitamin D.
Mild to Moderate Crohn’s disease
1. Sulfasalazine can be used to induce remission of mild colonic Crohn’s disease (quality of evidence, high; classification of recommendation, strong).
• Level of agreement: strongly agree 24%, agree 67%, uncertain 7%, disagree 2%, strongly disagree 0%
2. Although the efficacy of 5-aminosalicylic acid (5-ASA) for the remission induction of mild Crohn’s disease is limited, the use of this drug may be considered because of its fewer adverse effects and ease of administration (quality of evidence, high; classification of recommendation, weak).
• Level of agreement: strongly agree 20%, agree 72%, uncertain 8%, disagree 0%, strongly disagree 0%
3. Systemic corticosteroids are indicated for mild active Crohn’s disease that is refractory to 5-ASA (quality of evidence, high; classification of recommendation, strong).
• Level of agreement: strongly agree 36%, agree 53%, uncertain 9%, disagree 0%, strongly disagree 2%
4. Budesonide (9 mg/day) is preferred for induction therapy of mild to moderate Crohn’s disease confined to the terminal ileum or ileocecal area (quality of evidence, high; classification of recommendation, strong).
• Level of agreement: strongly agree 9%, agree 87%, uncertain 4%, disagree 0%, strongly disagree 0%
5. Systemic corticosteroid should be used if budesonide is not effective (quality of evidence, high; classification of recommendation, strong).
• Level of agreement: strongly agree 38%, agree 58%, uncertain 4%, disagree 0%, strongly disagree 0%
Moderate to Severe Crohn’s disease
6. Systemic corticosteroid (prednisolone 0.5 to 1 mg/kg/day or 40 to 60 mg/day) is the first-line induction therapy for moderate to severe Crohn’s disease (quality of evidence, moderate; classification of recommendation, strong).
• Level of agreement: strongly agree 38%, agree 60%, uncertain 2%, disagree 0%, strongly disagree 0%
7. Systemic corticosteroid should be reduced gradually according to disease severity and patient response, generally over 8 weeks (quality of evidence, low; classification of recommendation, strong).
• Level of agreement: strongly agree 39%, agree 61%, uncertain 0%, disagree 0%, strongly disagree 0%
8. Anti-TNF (anti-tumor necrosis factor) therapy is indicated if systemic corticosteroid therapy fails (quality of evidence, high; classification of recommendation, strong).
• Level of agreement: strongly agree 48%, agree 48%, uncertain 4%, disagree 0%, strongly disagree 0%
9. Thiopurine monotherapy is not recommended for induction therapy of moderate to severe Crohn’s disease (quality of evidence, moderate; classification of recommendation, weak).
• Level of agreement: strongly agree 19%, agree 65%, uncertain 14%, disagree 2%, strongly disagree 0%
10. Anti-TNF agents may be used to induce remission of moderate to severe Crohn’s disease (quality of evidence, moderate; classification of recommendation, weak).
• Level of agreement: strongly agree 37%, agree 63%, uncertain 0%, disagree 0%, strongly disagree 0%
11. When anti-TNF is used for induction therapy of thiopurine-naïve patients, combined therapy with anti-TNF and thiopurine is more effective than anti-TNF alone (quality of evidence, moderate; classification of recommendation, weak).
• Level of agreement: strongly agree 34%, agree 62%, uncertain 2%, disagree 2%, strongly disagree 0%
12. Intramuscular methotrexate (MTX) may be used to induce remission for moderate to severe Crohn’s disease (quality of evidence, high; classification of recommendation, weak).
• Level of agreement: strongly agree 11%, agree 85%, uncertain 4%, disagree 0%, strongly disagree 0%
Crohn’s disease Refractory to Medical Treatment
13. Surgical treatment should be considered in cases that are refractory to medical therapy. Surgical decision making should be done with full communication with gastroenterologists, surgeons, and the patient (quality of evidence, very low; classification of recommendation, weak).
• Level of agreement: strongly agree 61%, agree 34%, uncertain 5%, disagree 0%, strongly disagree 0%
14. In case of primary nonresponse to anti-TNF, reevaluation of symptoms and change of treatment are necessary (quality of evidence, low; classification of recommendation, no specific recommendation).
• Level of agreement: strongly agree 42%, agree 58%, uncertain 0%, disagree 0%, strongly disagree 0%
15. Although testing of the serum anti-TNF trough level or antibodies to anti-TNF were reported to be useful for optimizing anti-TNF therapy or identifying cause of primary nonresponse or secondary loss of response, further study is required (quality of evidence, low; classification of recommendation, weak).
• Level of agreement: strongly agree 13%, agree 83%, uncertain 4%, disagree 0%, strongly disagree 0%
16. In patients who are intolerant or not responsive to one anti-TNF therapy, a different anti-TNF agent may be used (quality of evidence, infliximab [high], adalimumab [low]; classification of recommendation, infliximab [strong], adalimumab [weak]).
• Level of agreement: strongly agree 7%, agree 80%, uncertain 13%, disagree 0%, strongly disagree 0%
Bowel rest
If your Crohn’s disease symptoms are severe, you may need to rest your bowel for a few days to several weeks. Bowel rest involves drinking only certain liquids or not eating or drinking anything. During bowel rest, your doctor may:
- ask you to drink a liquid that contains nutrients
- give you a liquid that contains nutrients through a feeding tube inserted into your stomach or small intestine
- give you intravenous (IV) nutrition through a special tube inserted into a vein in your arm
You may stay in the hospital, or you may be able to receive the treatment at home. In most cases, your intestines will heal during bowel rest.
Surgery
Even with medicines, many people will need surgery to treat their Crohn’s disease. One study found that nearly 60 percent of people had surgery within 20 years of having Crohn’s disease 105. Although surgery will not cure Crohn’s disease, it can treat complications and improve symptoms. Doctors most often recommend surgery to treat:
- fistulas
- bleeding that is life threatening
- intestinal obstructions
- side effects from medicines when they threaten your health
- symptoms when medicines do not improve your condition
A surgeon can perform different types of operations to treat Crohn’s disease.
For any surgery, you will receive general anesthesia . You will most likely stay in the hospital for 3 to 7 days following the surgery. Full recovery may take 4 to 6 weeks.
Small bowel resection
Small bowel resection is surgery to remove part of your small intestine. When you have an intestinal obstruction or severe Crohn’s disease in your small intestine, a surgeon may need to remove that section of your intestine. The two types of small bowel resection are:
- laparoscopic—when a surgeon makes several small, half-inch incisions in your abdomen. The surgeon inserts a laparoscope—a thin tube with a tiny light and video camera on the end—through the small incisions. The camera sends a magnified image from inside your body to a video monitor, giving the surgeon a close-up view of your small intestine. While watching the monitor, the surgeon inserts tools through the small incisions and removes the diseased or blocked section of small intestine. The surgeon will reconnect the ends of your intestine.
- open surgery—when a surgeon makes one incision about 6 inches long in your abdomen. The surgeon will locate the diseased or blocked section of small intestine and remove or repair that section. The surgeon will reconnect the ends of your intestine.
Subtotal colectomy
A subtotal colectomy, also called a large bowel resection, is surgery to remove part of your large intestine. When you have an intestinal obstruction, a fistula, or severe Crohn’s disease in your large intestine, a surgeon may need to remove that section of intestine. A surgeon can perform a subtotal colectomy by:
- laparoscopic colectomy—when a surgeon makes several small, half-inch incisions in your abdomen. While watching the monitor, the surgeon removes the diseased or blocked section of your large intestine. The surgeon will reconnect the ends of your intestine.
- open surgery—when a surgeon makes one incision about 6 to 8 inches long in your abdomen. The surgeon will locate the diseased or blocked section of large intestine and remove that section. The surgeon will reconnect the ends of your intestine.
Proctocolectomy and ileostomy
A proctocolectomy is surgery to remove your entire colon and rectum. An ileostomy is a stoma, or opening in your abdomen, that a surgeon creates from a part of your ileum. The surgeon brings the end of your ileum through an opening in your abdomen and attaches it to your skin, creating an opening outside your body. The stoma is about three-quarters of an inch to a little less than 2 inches wide and is most often located in the lower part of your abdomen, just below the beltline.
A removable external collection pouch, called an ostomy pouch or ostomy appliance, connects to the stoma and collects stool outside your body. Stool passes through the stoma instead of passing through your anus. The stoma has no muscle, so it cannot control the flow of stool, and the flow occurs whenever occurs.
If you have this type of surgery, you will have the ileostomy for the rest of your life.
Crohn’s disease complications treatment
Your doctor may recommend treatments for the following complications of Crohn’s disease:
- Intestinal obstruction. A complete intestinal obstruction is life threatening. If you have a complete obstruction, you will need medical attention right away. Doctors often treat complete intestinal obstruction with surgery.
- Fistulas. How your doctor treats fistulas will depend on what type of fistulas you have and how severe they are. For some people, fistulas heal with medicine and diet changes, whereas other people will need to have surgery.
- Abscesses. Doctors prescribe antibiotics and drain abscesses. A doctor may drain an abscess with a needle inserted through your skin or with surgery.
- Anal fissures. Most anal fissures heal with medical treatment, including ointments, warm baths, and diet changes.
- Ulcers. In most cases, the treatment for Crohn’s disease will also treat your ulcers.
- Malnutrition. You may need IV fluids or feeding tubes to replace lost nutrients and fluids.
- Inflammation in other areas of your body. Your doctor can treat inflammation by changing your medicines or prescribing new medicines.
Nutrition therapy
Your doctor may recommend a special diet given via a feeding tube (enteral nutrition) or nutrients injected into a vein (parenteral nutrition) to treat your Crohn’s disease. This can improve your overall nutrition and allow the bowel to rest. Bowel rest can reduce inflammation in the short term.
Your doctor may use nutrition therapy short term and combine it with medications, such as immune system suppressors. Enteral and parenteral nutrition are typically used to get people healthier prior to surgery or when other medications fail to control symptoms.
Your doctor may also recommend a low residue or low-fiber diet to reduce the risk of intestinal blockage if you have a narrowed bowel (stricture). A low residue diet is designed to reduce the size and number of your stools.
Surgery
If diet and lifestyle changes, drug therapy, or other treatments don’t relieve your signs and symptoms, your doctor may recommend surgery. Nearly half of those with Crohn’s disease will require at least one surgery. However, surgery does not cure Crohn’s disease.
During surgery, your surgeon removes a damaged portion of your digestive tract and then reconnects the healthy sections. Surgery may also be used to close fistulas and drain abscesses.
The benefits of surgery for Crohn’s disease are usually temporary. The disease often recurs, frequently near the reconnected tissue. The best approach is to follow surgery with medication to minimize the risk of recurrence.
Crohn’s disease diet
Changing your diet can help reduce symptoms. Your doctor may recommend that you make changes to your diet such as:
- avoiding carbonated, or “fizzy,” drinks
- avoiding popcorn, vegetable skins, nuts, and other high-fiber foods
- drinking more liquids
- eating smaller meals more often
- keeping a food diary to help identify foods that cause problems
Depending on your symptoms or medicines, your doctor may recommend a specific diet, such as a diet that is:
- high calorie
- lactose free
- low fat
- low fiber
- low salt
Table 5. The Anti-Inflammatory Diet (IBD-AID) Food Phase chart
| Phase type | Phase I | Phase II | Phase III | Phase IV |
|---|---|---|---|---|
| Soft, well-cooked or cooked then pureed foods, no seeds | Soft Textures: well-cooked or pureed foods, no seeds, choose floppy or tender foods | May still need to avoid stems, choose floppy greens or other greens depending on individual tolerance | If in remission with no strictures | |
| Vegetables | Butternut Squash, Pumpkin, Sweet Potatoes, Onions | Carrots, Zucchini, Eggplant, Peas, Snow peas, Spaghetti squash, Green beans, Yellow beans, Microgreens (2 week old baby greens), Watercress, Arugula, Fresh flat leaf parsley and cilantro, Seaweed, Algae | Butter lettuce, Baby spinach, Peeled cucumber, Olives, Leeks Bok Choy, Bamboo shoots, Collard greens, Beet greens, Sweet peppers, Kale, Fennel bulb | Artichokes, Asparagus, Tomatoes, Lettuce, Brussels sprouts, Beets, Cabbage, Kohlrabi, Rhubarb, Pickles, Spring onions, Water chestnuts, Celery, Celeriac, Cauliflower, Broccoli, Radish, Green pepper, Hot pepper |
| Pureed vegetables: Mushrooms, Phase II vegetables (pureed) | Pureed vegetables: all except cruciferous | Pureed vegetables: all from Phase IV, Kimchi | ||
| Fruits | Banana, Papaya, Avocado, Pawpaw | Watermelon (seedless), Mangoes, Honeydew, Cantaloupe, May need to be cooked: Peaches, Plums, Nectarines, Pears, (Phase III fruits are allowed if pureed and seeds are strained out) | Strawberries, Cranberries, Blueberries, Apricots, Cherries, Coconut, Lemons, Limes, Kiwi, Passion fruit, Blackberries, Raspberries, Pomegranate (May need to strain seeds from berries) | Grapes, Grapefruit, Oranges, Currants, Figs, Dates, Apples (best cooked), Pineapple, Prunes |
| Meats and fish | All fish (no bones), Sardines (small bones ok), Turkey and ground beef, Chicken, Eggs | Scallops | Lean cuts of Beef, Lamb, Duck, Goose | Shrimp, Prawns, Lobster |
| Non dairy unsweetened | Coconut milk, Almond milk, Oat milk, Soy milk | |||
| Dairy, unsweetened | Yogurt, Kefir | Farmers cheese (dry curd cottage cheese), Cheddar cheese | Aged cheeses | |
| Nuts/Oils/Legumes/Fats | Miso (refrigerated), Tofu, Olive oil, Canola oil, Flax oil, Hemp oil, Walnut oil, Coconut oil | Almond flour, Peanut flour, Soy flour, Sesame oil, Grapeseed oil, Walnut oil, Pureed nuts, Safflower oil, Sunflower oil | Whole nuts, Soybeans, Bean flours, Nut butters, Well-cooked lentils (pureed), Bean purees (e.g. hummus) | Whole beans and lentils |
| Grains | Ground flax or Chia Seeds (as tolerated) | Steel cut oats (well-cooked as oatmeal) | Rolled well-cooked oats | |
| Spices | Basil, Sage, Oregano, Salt, Nutmeg, Cumin, Cinnamon, Turmeric, Saffron, Mint, Bay leaves, Tamari (wheat free soy sauce), Fenugreek tea, Fennel tea, Vanilla | Dill, Thyme, Rosemary Tarragon, Cilantro, Basil, Parsley | Mint, Ginger, Garlic (minced), Paprika, Chives, Daikon, Mustard | Wasabi, Tamarind, Horseradish, Fenugreek, Fennel |
| Sweeteners | Stevia, Maple syrup, Honey (local), Unsweetened fruit juice | Lemon and lime juice | ||
| Misc. | Capsule or liquid supplements, Cocoa powder | Baking powder (no cornstarch), Baking soda, Unflavored gelatin | Ghee, Light mayonnaise, Vinegar | Ketchup (sugar free), Hot sauce (sugar free) |
Talk with your doctor about specific dietary recommendations and changes. Your doctor may recommend nutritional supplements and vitamins if you do not absorb enough nutrients. For safety reasons, talk with your doctor before using dietary supplements, such as vitamins, or any complementary or alternative medicines or medical practices.
Patients have strong beliefs about the role of diet in the cause of inflammatory bowel disease (Crohn’s disease & ulcerative colitis) and in exacerbating or alleviating ongoing symptoms from inflammatory bowel disease 106. The rapid increase in the incidence and prevalence of inflammatory bowel disease in the past several decades strongly suggests an environmental trigger for inflammatory bowel disease, one of which may be dietary patterns. There are several pathways where diet may influence intestinal inflammation such as direct dietary antigens, altering the gut microbiome, and affecting gastrointestinal permeability. However, data that altering diet can change the natural history of inflammatory bowel disease are scarce and evidence based dietary guidelines for patients with inflammatory bowel disease are lacking. Patients therefore seek non-medical resources for dietary guidance such as patient support groups and unverified sources on the internet.
Various dietary components have been proposed to increase the risk of developing or exacerbating symptoms of inflammatory bowel disease. One of the first dietary components associated with developing inflammatory bowel disease was intake of sugar and refined carbohydrates 107. However, an ecological study in North America, Europe and Japan failed to show an association between refined sugar intake and Crohn’s disease incidence rates 108. There are consistent associations between both fatty acid and protein composition in the diet with the development of inflammatory bowel disease in both ecological and prospective cohort studies 109, 110. Furthermore, dietary fiber intake has been associated with a lower risk of developing Crohn’s disease, but not ulcerative colitis 111.
Two studies 112, 113 of patients who underwent ileocolonic resection provide the strongest evidence for the role of intestinal contents on the course of Crohn’s disease. Both studies demonstrated that recurrence of inflammation after ileal resection is dependent on exposure of the neo-terminal ileum to the fecal contents. Inflammation recurred within 8 days of exposure to the luminal contents 112, 113. However, the fecal stream is a complex mixture of bacteria, other microorganisms, digested food content, and the metabolic products of digestion of food components by the host and microbiota. This makes it very challenging to identify the components of the luminal content that drives the underlying inflammation. Furthermore, these components are not independent of each other.
There is a potential link between diet and the composition of the gut microbiome. Long term agrarian dietary patterns are associated with an enterotype characterized by Prevotella 114, a genera more commonly observed in people from rural Africa where inflammatory bowel disease and particularly Crohn’s disease is uncommon 115. Prevotella and related bacteria are efficient at fermenting dietary fiber, thereby leading to higher concentrations of short chain fatty acids (SCFA) 116 which may protect against bowel inflammation 117. In contrast, high fat diets, through dietary induced changes in the gut microbiota, may increase bowel permeability, a hallmark of Crohn’s disease 118. High fat diets also worsen dextran sodium sulfate induced colitis in mice, possibly by increasing colonic epithelial non-classical natural killer T cells and reducing regulatory T cells 119. In animal models, consumption of milk derived saturated fat alters bile acid composition, allowing for a bloom of sulfate-reducing bacteria, which in turn can produce greater amounts of the potentially mucosal toxic hydrogen sulfide 120. A major source of hydrogen sulfide (H2S) in the bowel is bacterial fermentation of sulfur amino acids which are found in high protein foods such as meats 121. Hydrogen sulfide has been proposed as contributing to bowel inflammation through a variety of mechanisms, including impaired utilization of short-chain fatty acids (SCFAs) and direct toxic effects 122. However, other research suggests that hydrogen sulfide (H2S) has anti-inflammatory properties and contributes to mucosal healing 123. These are but a few of the proposed mechanisms by which diet can affect the course of inflammatory bowel disease. Unfortunately, it is currently unknown whether these findings in animal models translate to humans with inflammatory bowel disease.
There are surprisingly few observational studies examining the association of diet with the natural history of inflammatory bowel disease. Jowett et al. 124 conducted a prospective study of patients with ulcerative colitis. Jowett observed that patients who reported higher levels of meat, eggs, protein and alcohol consumption were more likely to have a relapse of ulcerative colitis 124. Importantly, the association was much stronger for red and processed meats than for other meats and there was no association with fish consumption. Jowett hypothesized that these dietary patterns resulted in higher intestinal concentration of sulfate which in turn led to disease relapse. Another study found a correlation between sulfite consumption and endoscopic activity in ulcerative colitis 125.
References- Hanauer SB. Inflammatory bowel disease: epidemiology, pathogenesis, and therapeutic opportunities. Inflamm Bowel Dis. 2006 Jan;12 Suppl 1:S3-9. doi: 10.1097/01.mib.0000195385.19268.68
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years — United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65:1166–1169. DOI: http://dx.doi.org/10.15585/mmwr.mm6542a3
- Escudero-Hernández C, van Beelen Granlund A, Bruland T, Sandvik AK, Koch S, Østvik AE, Münch A. Transcriptomic profiling of collagenous colitis identifies hallmarks of nondestructive inflammatory bowel disease. Cellular and Molecular Gastroenterology and Hepatology. 2021;12:665–687. doi: 10.1016/j.jcmgh.2021.04.011
- Marlicz W, Skonieczna-Żydecka K, Yung DE, Loniewski I, Koulaouzidis A. Endoscopic findings and colonic perforation in microscopic colitis: A systematic review. Digestive and Liver Disease. 2004;49:1073–1085. doi: 10.1016/j.dld.2017.07.015
- Nielsen OH, Fernandez-Banares F, Sato T, Pardi DS. Microscopic colitis: Etiopathology, diagnosis, and rational management. Elife. 2022 Aug 1;11:e79397. doi: 10.7554/eLife.79397
- Definition & Facts of Microscopic Colitis. https://www.niddk.nih.gov/health-information/digestive-diseases/microscopic-colitis/definition-facts
- Vespa E, D’Amico F, Sollai M, Allocca M, Furfaro F, Zilli A, Dal Buono A, Gabbiadini R, Danese S, Fiorino G. Histological scores in patients with inflammatory bowel diseases: The state of the art. Journal of Clinical Medicine. 2022;11:939–952. doi: 10.3390/jcm11040939
- Gentile NM, Khanna S, Loftus EV Jr, Smyrk TC, Tremaine WJ, Harmsen WS, Zinsmeister AR, Kammer PP, Pardi DS. The epidemiology of microscopic colitis in Olmsted County from 2002 to 2010: a population-based study. Clin Gastroenterol Hepatol. 2014 May;12(5):838-42. doi: 10.1016/j.cgh.2013.09.066
- Bonderup OK, Wigh T, Nielsen GL, Pedersen L, Fenger-Grøn M. The epidemiology of microscopic colitis: A 10-year pathology-based nationwide Danish cohort study. Scandinavian Journal of Gastroenterology. 2015;50:393–398. doi: 10.3109/00365521.2014.940378
- Windon AL, Almazan E, Oliva-Hemker M, Hutchings D, Assarzadegan N, Salimian K, Montgomery EA, Voltaggio L. Lymphocytic and collagenous colitis in children and adolescents: Comprehensive clinicopathologic analysis with long-term follow-up. Human Pathology. 2020;106:13–22. doi: 10.1016/j.humpath.2020.09.011
- Tong J, Zheng Q, Zheng Q, Zhang C, Lo R, Shen J, Ran Z. Incidence, prevalence, and temporal trends of microscopic colitis: a systematic review and meta-analysis. The American Journal of Gastroenterology. 2015;110:265–276. doi: 10.1038/ajg.2014.431
- McDowell C, Farooq U, Haseeb M. Inflammatory Bowel Disease. [Updated 2022 Jun 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470312
- Levine A, Rhodes JM, Lindsay JO, Abreu MT, Kamm MA, Gibson PR, Gasche C, Silverberg MS, Mahadevan U, Boneh RS, Wine E, Damas OM, Syme G, Trakman GL, Yao CK, Stockhamer S, Hammami MB, Garces LC, Rogler G, Koutroubakis IE, Ananthakrishnan AN, McKeever L, Lewis JD. Dietary Guidance From the International Organization for the Study of Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2020 May;18(6):1381-1392. doi: 10.1016/j.cgh.2020.01.046
- Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006 Jun;4(6):744–53. https://www.ncbi.nlm.nih.gov/pubmed/16682258
- Lee JS, Kim ES, Moon W. Chronological Review of Endoscopic Indices in Inflammatory Bowel Disease. Clin Endosc. 2019 Mar;52(2):129-136. doi: 10.5946/ce.2018.042
- Dai C, Jiang M, Sun MJ. Fecal markers in the management of inflammatory bowel disease. Postgrad Med. 2018 Sep;130(7):597-606. doi: 10.1080/00325481.2018.1503919
- Kobayashi T, Hisamatsu T, Suzuki Y, Ogata H, Andoh A, Araki T, Hokari R, Iijima H, Ikeuchi H, Ishiguro Y, Kato S, Kunisaki R, Matsumoto T, Motoya S, Nagahori M, Nakamura S, Nakase H, Tsujikawa T, Sasaki M, Yokoyama K, Yoshimura N, Watanabe K, Katafuchi M, Watanabe M, Hibi T. Predicting outcomes to optimize disease management in inflammatory bowel disease in Japan: their differences and similarities to Western countries. Intest Res. 2018 Apr;16(2):168-177. doi: 10.5217/ir.2018.16.2.168
- Yang SK. How Does the Epidemiology of Inflammatory Bowel Disease Differ between East and West? A Korean Perspective. Inflamm Intest Dis. 2017 Nov;2(2):95-101. doi: 10.1159/000454712
- Kucharzik, T., Koletzko, S., Kannengiesser, K., & Dignass, A. (2020). Ulcerative Colitis-Diagnostic and Therapeutic Algorithms. Deutsches Arzteblatt international, 117(33-34), 564–574. https://doi.org/10.3238/arztebl.2020.0564
- Talley NJ, Abreu MT, Achkar JP, et al.; American College of Gastroenterology IBD Task Force. An evidence-based systematic review on medical therapies for inflammatory bowel disease. Am J Gastroenterol. 2011;106(suppl 1):S2–S25.
- Tremaine WJ. Review article: Indeterminate colitis–definition, diagnosis and management. Aliment Pharmacol Ther. 2007 Jan 1;25(1):13-7. doi: 10.1111/j.1365-2036.2006.03159.x
- Prenzel F, Uhlig HH. Frequency of indeterminate colitis in children and adults with IBD – a metaanalysis. J Crohns Colitis. 2009 Dec;3(4):277-81. doi: 10.1016/j.crohns.2009.07.001
- Romberg-Camps MJ, Dagnelie PC, Kester AD, Hesselink-van de Kruijs MA, Cilissen M, Engels LG, Van Deursen C, Hameeteman WH, Wolters FL, Russel MG, Stockbrügger RW. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009 Feb;104(2):371-83. doi: 10.1038/ajg.2008.38
- Höie O, Wolters F, Riis L, Aamodt G, Solberg C, Bernklev T, Odes S, Mouzas IA, Beltrami M, Langholz E, Stockbrügger R, Vatn M, Moum B; European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD). Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007 Aug;102(8):1692-701. doi: 10.1111/j.1572-0241.2007.01265.x
- Shivashankar, R., Tremaine, W. J., Harmsen, W. S., & Loftus, E. V., Jr (2017). Incidence and Prevalence of Crohn’s Disease and Ulcerative Colitis in Olmsted County, Minnesota From 1970 Through 2010. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 15(6), 857–863. https://doi.org/10.1016/j.cgh.2016.10.039
- Inflammatory bowel disease (IBD). Centers for Disease Control and Prevention www.cdc.gov/ibd
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG Clinical Guideline: Ulcerative Colitis in Adults. Am J Gastroenterol. 2019 Mar;114(3):384-413. doi: 10.14309/ajg.0000000000000152
- Shanahan F. Ulcerative colitis. In: Hawkey CJ, Bosch J, Richter JE, Garcia-Tsao G, Chan FKL, eds. Textbook of Clinical Gastroenterology and Hepatology. 2nd ed. Oxford: Wiley-Blackwell; 2012: 355–371.
- Hanauer SB, Podolsky DK. Chapter 71: Ulcerative colitis. In: Podolsky DK, Camilleri M, Fitz G, et al, eds. Yamada’s Textbook of Gastroenterology. 6th ed. John Wiley & Sons, Ltd; 2016:1378–1417.
- Vavricka SR, Brun L, Ballabeni P, et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011; 106(1):113.
- Bengtson MB, Aamodt G, Vatn MH, Harris JR. Concordance for IBD among twins compared to ordinary siblings—a Norwegian population-based study. J Crohns Colitis. 2010;4(3):312–318.
- Ko Y, Butcher R, Leong RW. Epidemiological studies of migration and environmental risk factors in the inflammatory bowel diseases. World Journal of Gastroenterology. 2014;20(5):1238–1247.
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–573.
- Jess T, Simonsen J, Nielsen NM, et al. Enteric Salmonella or Campylobacter infections and the risk of inflammatory bowel disease. Gut. 2011;60(3):318–324.
- Noor SO, Ridgway K, Scovell L, et al. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 2010;10:134.
- Kucharzik T, Dignass AU, Atreya R, Bokemeyer B, Esters P, Herrlinger K, Kannengießer K, Kienle P, Langhorst J, Lügering A, Schreiber S, Stallmach A, Stein J, Sturm A, Teich N, Siegmund B; Collaborators:. Aktualisierte S3-Leitlinie Colitis ulcerosa [August 2019 – AWMF-Registriernummer: 021-009]. Z Gastroenterol. 2019 Nov;57(11):1321-1405. German. doi: 10.1055/a-1015-7265. Epub 2019 Nov 18. Erratum in: Z Gastroenterol. 2019 Nov;57(11):e1.
- Sawczenko A, Sandhu BK. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch Dis Child. 2003;88(11):995–1000.
- Solem CA, Loftus EV Jr, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11(8):707–712.
- Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology. 2011;140(6):1817–1826.e2.
- Turkay C, Kasapoglu B. Noninvasive methods in evaluation of inflammatory bowel disease: where do we stand now? An update. Clinics (Sao Paulo). 2010;65(2):221–231.
- Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults. Am J Gastroenterol. 2010;105(3):501–523.
- Marshall JK, Thabane M, Steinhart AH, Newman JR, Anand A, Irvine EJ. Rectal 5-aminosalicylic acid for induction of remission in ulcerative colitis Cochrane Database Syst Rev. 2010;(1):CD004115.
- Cohen RD, Woseth DM, Thisted RA, Hanauer SB. A meta-analysis and overview of the literature on treatment options for left-sided ulcerative colitis and ulcerative proctitis. Am J Gastroenterol. 2000;95(5):1263–1276.
- Safdi M, DeMicco M, Sninsky C, et al. A double-blind comparison of oral versus rectal mesalamine versus combination therapy in the treatment of distal ulcerative colitis. Am J Gastroenterol. 1997;92(10):1867–1871.
- Ford AC, Achkar JP, Khan KJ, et al. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):601–616.
- Marteau P, Probert CS, Lindgren S, et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut. 2005;54(7):960–965.
- Ford AC, Bernstein CN, Khan KJ, et al. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):590–599.
- Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):644–659.
- Khan KJ, Dubinsky MC, Ford AC, Ullman TA, Talley NJ, Moayyedi P. Efficacy of immunosuppressive therapy for inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106(4):630–642.
- Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5(1):103–110.
- Leblanc S, Allez M, Seksik P, et al.; GETAID. Successive treatment with cyclosporine and infliximab in steroid-refractory ulcerative colitis. Am J Gastroenterol. 2011;106(4):771–777.
- Heikens JT, de Vries J, van Laarhoven CJ. Quality of life, health-related quality of life and health status in patients having restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis: a systematic review. Colorectal Dis. 2012;14(5):536–544.
- Loftus EV Jr, Delgado DJ, Friedman HS, Sandborn WJ. Colectomy and the incidence of postsurgical complications among ulcerative colitis patients with private health insurance in the United States. Am J Gastroenterol. 2008;103(7):1737–1745.
- Hanauer S, Good LI, Goodman MW, et al. Long-term use of mesalamine (Rowasa) suppositories in remission maintenance of ulcerative proctitis. Am J Gastroenterol. 2000;95(7):1749–1754.
- d’Albasio G, Pacini F, Camarri E, et al. Combined therapy with 5-ami-nosalicylic acid tablets and enemas for maintaining remission in ulcerative colitis: a randomized double-blind study. Am J Gastroenterol. 1997;92(7):1143–1147.
- Regueiro M, Loftus EV Jr, Steinhart AH, Cohen RD. Medical management of left-sided ulcerative colitis and ulcerative proctitis: critical evaluation of therapeutic trials. Inflamm Bowel Dis. 2006;12(10):979–994.
- Ulcerative Colitis. Am Fam Physician. 2013 May 15;87(10):699-705. https://www.aafp.org/afp/2013/0515/p699.html
- Ulcerative Colitis. http://patients.gi.org/topics/ulcerative-colitis/
- Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report . Nutrition Journal. 2014;13:5. doi:10.1186/1475-2891-13-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3896778/
- Vermeire S, Schreiber S, Sandborn WJ, Dubois C, Rutgeerts P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357–363. doi: 10.1016/j.cgh.2010.01.001. https://www.ncbi.nlm.nih.gov/pubmed/20096379
- Bosscher D, Breynaert A, Pieters L, Hermans N. Food-based strategies to modulate the composition of the intestinal microbiota and their associated health effects. J Physiol Pharmacol. 2009;60(Suppl 6):5–11. https://www.ncbi.nlm.nih.gov/pubmed/20224145
- Jia W, Whitehead RN, Griffiths L, et al. Is the abundance of Faecalibacterium prausnitzii relevant to Crohn’s disease? Ross F, ed. Fems Microbiology Letters. 2010;310(2):138-144. doi:10.1111/j.1574-6968.2010.02057.x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2962807/
- McFarland LV. Systematic review and meta-analysis of Saccharomyces boulardii in adult patients. World Journal of Gastroenterology : WJG. 2010;16(18):2202-2222. doi:10.3748/wjg.v16.i18.2202. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2868213/
- Lorea Baroja M, Kirjavainen PV, Hekmat S, Reid G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clinical and Experimental Immunology. 2007;149(3):470-479. doi:10.1111/j.1365-2249.2007.03434.x. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2219330/
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106:563–573. doi: 10.1038/ajg.2011.44. https://www.ncbi.nlm.nih.gov/pubmed/21468064
- Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutrition reviews. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. https://www.ncbi.nlm.nih.gov/pubmed/20500789
- Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutrition Journal. 2014;13:5. doi:10.1186/1475-2891-13-5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3896778/
- Gower-Rousseau C, Sarter H, Savoye G, Tavernier N, Fumery M, Sandborn WJ, Feagan BG, Duhamel A, Guillon-Dellac N, Colombel JF, Peyrin-Biroulet L; International Programme to Develop New Indexes for Crohn’s Disease (IPNIC) group; International Programme to Develop New Indexes for Crohn’s Disease (IPNIC) group. Validation of the Inflammatory Bowel Disease Disability Index in a population-based cohort. Gut. 2017 Apr;66(4):588-596. doi: 10.1136/gutjnl-2015-310151
- Magro F, Rodrigues A, Vieira AI, Portela F, Cremers I, Cotter J, Correia L, Duarte MA, Tavares ML, Lago P, Ministro P, Peixe P, Lopes S, Garcia EB. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis. 2012 Mar;18(3):573-83. doi: 10.1002/ibd.21815
- Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011 May;140(6):1785-94. doi: 10.1053/j.gastro.2011.01.055
- Reinisch W, Reinink AR, Higgins PD. Factors associated with poor outcomes in adults with newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2015 Apr;13(4):635-42. doi: 10.1016/j.cgh.2014.03.037
- Charpentier C, Salleron J, Savoye G, Fumery M, Merle V, Laberenne JE, Vasseur F, Dupas JL, Cortot A, Dauchet L, Peyrin-Biroulet L, Lerebours E, Colombel JF, Gower-Rousseau C. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut. 2014 Mar;63(3):423-32. doi: 10.1136/gutjnl-2012-303864
- Loftus, E. V., Jr, Harewood, G. C., Loftus, C. G., Tremaine, W. J., Harmsen, W. S., Zinsmeister, A. R., Jewell, D. A., & Sandborn, W. J. (2005). PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut, 54(1), 91–96. https://doi.org/10.1136/gut.2004.046615
- Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis. 2012 Jul;18(7):1356-63. doi: 10.1002/ibd.22839
- Choi, C. H., Rutter, M. D., Askari, A., Lee, G. H., Warusavitarne, J., Moorghen, M., Thomas-Gibson, S., Saunders, B. P., Graham, T. A., & Hart, A. L. (2015). Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. The American journal of gastroenterology, 110(7), 1022–1034. https://doi.org/10.1038/ajg.2015.65
- Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, Ghosh S, Wiebe S, Kaplan GG. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013 Nov;145(5):996-1006. doi: 10.1053/j.gastro.2013.07.041
- Solberg IC, Høivik ML, Cvancarova M, Moum B; IBSEN Study Group. Risk matrix model for prediction of colectomy in a population-based study of ulcerative colitis patients (the IBSEN study). Scand J Gastroenterol. 2015;50(12):1456-62. doi: 10.3109/00365521.2015.1064991
- Crohn’s & Colitis Foundation. http://www.crohnscolitisfoundation.org/what-are-crohns-and-colitis/what-is-ulcerative-colitis/
- Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2007;(4):CD005573.
- Zocco MA, dal Verme LZ, Cremonini F, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23(11):1567–1574.
- Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53(11):1617–1623.
- Joos S, Wildau N, Kohnen R, et al. Acupuncture and moxibustion in the treatment of ulcerative colitis: a randomized controlled study. Scand J Gastroenterol. 2006;41(9):1056–1063.
- Ben-Arye E, Goldin E, Wengrower D, Stamper A, Kohn R, Berry E. Wheat grass juice in the treatment of active distal ulcerative colitis: a randomized double-blind placebo-controlled trial. Scand J Gastroenterol. 2002;37(4):444–449.
- Park JJ, Yang S-K, Ye BD, et al. Second Korean guidelines for the management of Crohn’s disease. Intestinal Research. 2017;15(1):38-67. doi:10.5217/ir.2017.15.1.38. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5323307/
- Management of Crohn’s Disease in Adults. https://gi.org/guideline/management-of-crohn%E2%80%99s-disease-in-adults/
- Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured U.S. population. Digestive Diseases and Sciences. 2013;58:519–525.
- Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54.
- Definition & Facts for Crohn’s Disease. https://www.niddk.nih.gov/health-information/digestive-diseases/crohns-disease/definition-facts
- Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Current Opinion in Gastroenterology. 2013;29(4):357–362.
- Crohn Disease. https://emedicine.medscape.com/article/172940-overview#a4
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. American Journal of Gastroenterology. 2011;106(4):563–573.
- Cushing K, Higgins PDR. Management of Crohn Disease: A Review. JAMA. 2021;325(1):69–80. doi:10.1001/jama.2020.18936
- Sulz MC, Burri E, Michetti P, Rogler G, Peyrin-Biroulet L, Seibold F; on behalf of the Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Treatment Algorithms for Crohn’s Disease. Digestion. 2020;101 Suppl 1:43-57. doi: 10.1159/000506364
- Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013 Aug;369(8):754–62.
- Ben-Horin S, Kopylov U, Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun Rev. 2014 Jan;13(1):24–30.
- Tillack C, Ehmann LM, Friedrich M, Laubender RP, Papay P, Vogelsang H, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014 Apr;63(4):567–77.
- Nakase H. Optimizing the use of current treatments and emerging therapeutic approaches to achieve therapeutic success in patients with inflammatory bowel disease. Gut Liver. 2020 Jan;14(1):7–19.
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al.; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013 Aug;369(8):711–21.
- Kopylov U, Ron Y, Avni-Biron I, Koslowsky B, Waterman M, Daher S, et al. Efficacy and safety of vedolizumab for induction of remission in inflammatory bowel disease-the Israeli real-world experience. Inflamm Bowel Dis. 2017 Mar;23(3):404–8.
- Vermeire S, Colombel J-F, Feagan BG, Sandborn WJ, Sands BE, Danese S, et al. Long-term safety of vedolizumab in ulcerative colitis and Crohn’s disease: final results from the GEMINI LTS study. J Crohns Colitis. 2019;13(Suppl 1):S018–20.
- Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: medical Treatment. J Crohn’s Colitis. 2020 Jan;14(1):4–22.
- Sandborn WJ, Gasink C, Gao LL, Blank MA, Johanns J, Guzzo C, et al.; CERTIFI Study Group. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012 Oct;367(16):1519–28.
- MacDonald JK, Nguyen TM, Khanna R, Timmer A. Anti-IL-12/23p40 antibodies for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2016 Nov;11:CD007572.
- Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, et al.; UNITI–IM-UNITI Study Group. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N Engl J Med. 2016 Nov;375(20):1946–60.
- Peyrin-Biroulet L, Harmsen WS, Tremaine WJ, Zinsmeister AR, Sandborn WJ, Loftus EV. Surgery in a population-based cohort of Crohn’s disease from Olmsted County, Minnesota (1970–2004). American Journal of Gastroenterology. 2012;107(11):1639–1701.
- Hou JK, Lee D, Lewis J. Diet and Inflammatory Bowel Disease: Review of Patient-Targeted Recommendations. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(10):1592-1600. doi:10.1016/j.cgh.2013.09.063. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4021001/
- Mayberry JF, Rhodes J, Newcombe RG. Increased sugar consumption in Crohn’s disease. Digestion. 1980;20(5):323–6. https://www.ncbi.nlm.nih.gov/pubmed/7390057
- Sonnenberg A. Geographic and temporal variations of sugar and margarine consumption in relation to Crohn’s disease. Digestion. 1988;41(3):161–71. https://www.ncbi.nlm.nih.gov/pubmed/3265683
- Ananthakrishnan AN, Khalili H, Konijeti GG, et al. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut. 2013 Jul 4; Epub ahead of print. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3915038/
- John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol. 2010 May;22(5):602–6. https://www.ncbi.nlm.nih.gov/pubmed/20216220
- Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A Prospective Study of Long-term Intake of Dietary Fiber and Risk of Crohn’s Disease and Ulcerative Colitis. Gastroenterology. 2013 Aug 1; doi: 10.1053/j.gastro.2013.07.050. pii: S0016-5085(13)01140-2 Epub ahead of print. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3805714/
- Rutgeerts P, Goboes K, Peeters M, et al. Effect of faecal stream diversion on recurrence of Crohn’s disease in the neoterminal ileum. Lancet. 1991;338:771–4. https://www.ncbi.nlm.nih.gov/pubmed/1681159
- D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114:262–7. https://www.ncbi.nlm.nih.gov/pubmed/9453485
- Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3368382/
- De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2930426/
- Flint HJ, Bayer EA, Rincon MT, et al. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–31. https://www.ncbi.nlm.nih.gov/pubmed/18180751
- Scheppach W, Weiler F. The butyrate story: old wine in new bottles? Curr Opin Clin Nutr Metab Care. 2004;7:563–7. https://www.ncbi.nlm.nih.gov/pubmed/15295277
- Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2702831/
- Ma X, Torbenson M, Hamad AR, et al. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol. 2008;151:130–8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2276920/
- Sartor RB. Gut microbiota: Diet promotes dysbiosis and colitis in susceptible hosts. Nature Reviews Gastroenterology & Hepatology. 2012;9:561–2. https://www.nature.com/articles/nrgastro.2012.157
- Magee EA, Richardson CJ, Hughes R, et al. Contribution of dietary protein to sulfide production in the large intestine: an in vitro and a controlled feeding study in humans. Am J Clin Nutr. 2000;72:1488–94. http://ajcn.nutrition.org/content/72/6/1488.long
- Coffey JC, Docherty NG, O’Connell PR. Hydrogen sulphide: an increasing need for scientific equipoise. Gastroenterology. 2009;137:2181–2. author reply 2. https://www.ncbi.nlm.nih.gov/pubmed/19878749
- Wallace JL, Vong L, McKnight W, et al. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137:569–78. 78 e1. https://www.ncbi.nlm.nih.gov/pubmed/19375422
- Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study.[see comment] Gut. 2004;53:1479–84. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1774231/
- Magee EA, Edmond LM, Tasker SM, et al. Associations between diet and disease activity in ulcerative colitis patients using a novel method of data analysis. Nutr J. 2005;4:7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC549081/