Flu
Influenza commonly called the flu, is an infection of the nose, throat and lungs, which are part of the respiratory system, caused by the influenza viruses. Flu (influenza) is not the same as “stomach flu” viruses that cause diarrhea and vomiting.
There are 4 types of influenza viruses and they are constantly changing. They are:
- Type A flu virus – this is usually the more serious type. The influenza A virus is most likely to mutate into a new version that people are not resistant to. Influenza A viruses are divided into subtypes based on two proteins on the surface of the virus: hemagglutinin (H) and neuraminidase (N). There are 18 different hemagglutinin (H) subtypes and 11 different neuraminidase (N) subtypes (H1 through H18 and N1 through N11, respectively). While more than 130 influenza A subtype combinations have been identified in nature, primarily from wild birds, there are potentially many more influenza A subtype combinations given the propensity for virus “reassortment.” Reassortment is a process by which influenza viruses swap gene segments. Reassortment can occur when two influenza viruses infect a host at the same time and swap genetic information. Current subtypes of influenza A viruses that routinely circulate in people include A(H1N1) and A(H3N2). Influenza A subtypes can be further broken down into different genetic “clades” and “sub-clades” (Figure 1). Clades and sub-clades can be alternatively called “groups” and “sub-groups,” respectively. An influenza clade or group is a further subdivision of influenza viruses (beyond subtypes or lineages) based on the similarity of their HA gene sequences. The H1N1 (swine flu) strain is a type A virus, and flu pandemics in the past were type A viruses.
- Type B flu virus – this generally causes a less severe illness and is responsible for smaller outbreaks. It mainly affects young children. Influenza B viruses are not divided into subtypes, but instead are further classified into two lineages: B/Yamagata and B/Victoria. Similar to influenza A viruses, influenza B viruses can then be further classified into specific “clades” and “sub-clades”. Influenza B viruses generally change more slowly in terms of their genetic and antigenic properties than influenza A viruses, especially influenza A(H3N2) viruses. Influenza surveillance data from recent years shows co-circulation of influenza B viruses from both lineages in the United States and around the world. However, the proportion of influenza B viruses from each lineage that circulate can vary by geographic location and by season. In recent years, flu B/Yamagata viruses have circulated much less frequently in comparison to flu B/Victoria viruses globally.
- Type C flu virus – this usually causes a mild illness similar to the common cold.
- Type D flu virus – this primarily affect cattle and are not known to infect or cause illness in people.
Influenza A and B viruses cause seasonal epidemics of disease in people (known as flu season) almost every winter in the United States. Influenza A viruses are the only influenza viruses known to cause flu pandemics (i.e., global epidemics of flu disease). A pandemic can occur when a new and different influenza A virus emerges that infects people, has the ability to spread efficiently among people, and against which people have little or no immunity. Influenza C virus infections generally cause mild illness and are not thought to cause human epidemics. Influenza D viruses primarily affect cattle and are not known to infect or cause illness in people.
In addition to flu viruses, several other respiratory viruses also spread during flu season and can cause symptoms similar to those seen with flu infection. These respiratory viruses include rhinovirus (one cause of the “common cold”) and respiratory syncytial virus (RSV), which is the most common cause of severe respiratory illness in young children as well as a leading cause of death from respiratory illness in those aged 65 years and older. Other commonly circulating respiratory viruses include human parainfluenza viruses (HPIV), human metapneumovirus (HMPV), respiratory adenoviruses and human coronavirus.
At first, the flu may seem like a common cold with a runny nose, sneezing and sore throat. Colds usually develop slowly. But the flu tends to come on suddenly. And while a cold can be miserable, you usually feel much worse with the flu.
Flu symptoms come on very quickly and can include:
- sudden high temperature (fever)
- an aching body
- chills and sweats
- feeling tired or exhausted or weak
- dry, persistent cough
- sore throat
- eye pain
- headache
- difficulty sleeping
- loss of appetite
- vomiting and diarrhea or abdominal pain
- feeling sick and being sick
- shortness of breath
- runny or stuffy nose
The symptoms are similar for children, but they can also get pain in their ear and appear less active.
Cold and flu symptoms are similar, but flu tends to be more severe.
Most people with the flu get better on their own. But sometimes, influenza and its complications can be deadly. People at higher risk of developing flu complications include:
- Young children under age 2
- Adults older than age 65
- Residents of nursing homes and other long-term care facilities
- People who are pregnant or plan to be pregnant during flu season
- People with weakened immune systems
- American Indians or Alaska Natives
- People who have chronic illnesses, such as asthma, heart disease, kidney disease, liver disease and diabetes
- People with a body mass index (BMI) of 40 or higher
Most people who get the flu can treat themselves at home and often don’t need to see a health care provider. If you have flu, there are some things you can do to help get better more quickly:
- Do rest and sleep
- Do keep warm
- Do take acetaminophen (paracetamol) or ibuprofen to lower your temperature and treat aches and pains
- Do drink plenty of water to avoid dehydration (your urine should be light yellow or clear)
To help control the spread of influenza in your community, stay home and keep sick children home until the fever has been gone for 24 hours. Avoid being around other people until you’re feeling better, unless you’re getting medical care. If you do need to leave your home and get medical care, wear a face mask. Wash your hands often.
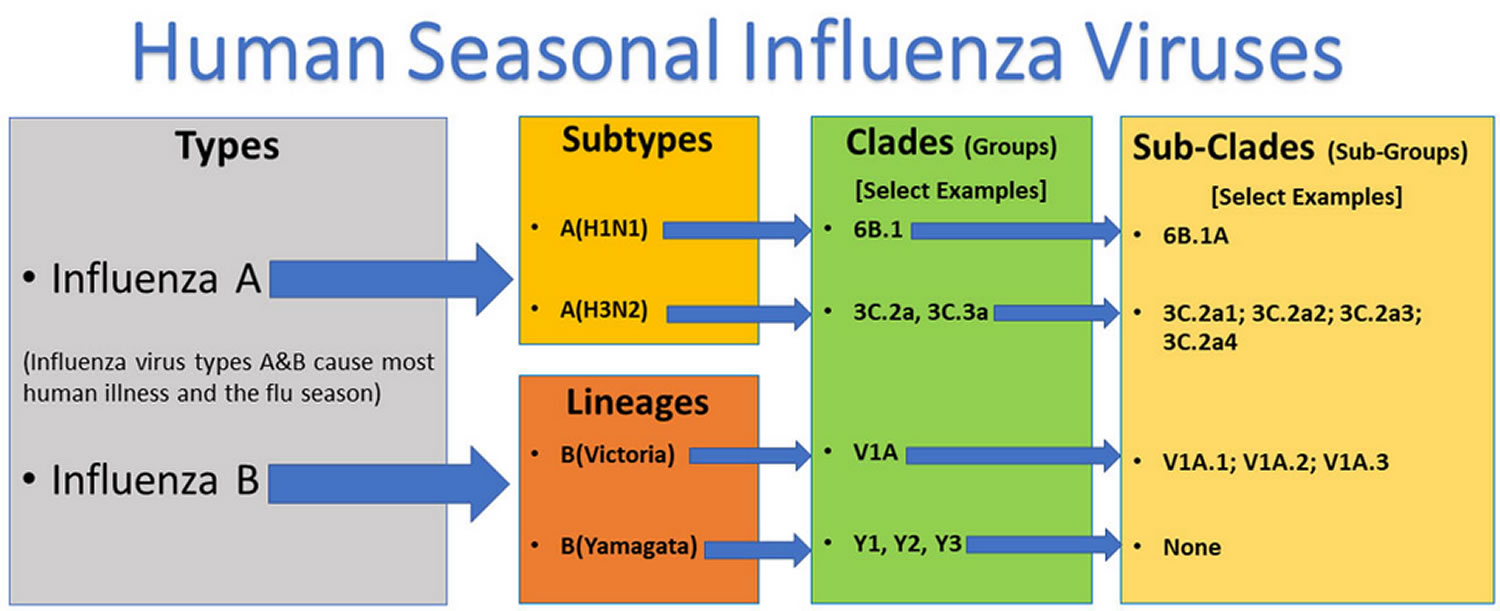
Figure 1. Influenza A and B viruses
Footnote: This graphic shows the two types of influenza viruses (A and B) that cause most human illness and that are responsible for flu seasons each year. Influenza A viruses are further classified into subtypes, while influenza B viruses are further classified into two lineages: B/Yamagata and B/Victoria. Both influenza A and B viruses can be further classified into clades and sub-clades (which are sometimes called groups and sub-groups.) Note that this graphic is an example, and currently circulating influenza clades and subclades may differ from those presented here.
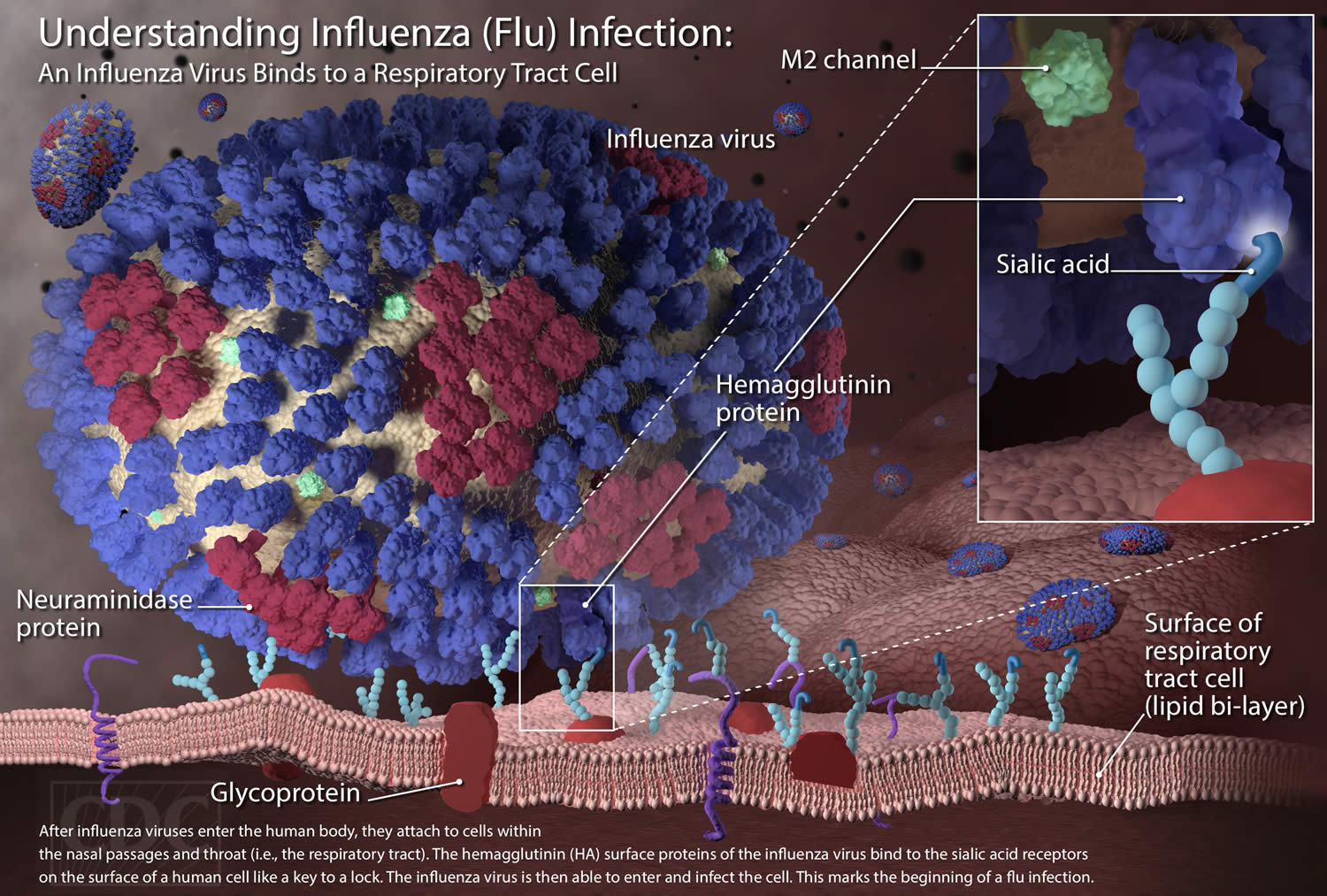
[Source 1 ]Figure 2. Influenza virus
Most people who get the flu can treat themselves at home and often don’t need to see a health care provider.
If you have flu symptoms and are at risk of complications, see your health care provider right away. Taking antiviral medication may shorten the length of your illness and help prevent more-serious problems.
See your doctor right away if you or your child have symptoms of flu and:
- you’re worried about your baby’s or child’s symptoms
- you’re 65 or over
- you’re pregnant
- you have a long-term medical condition – for example, diabetes or a condition that affects your heart, lungs, kidneys, brain or nerves
- you have a weakened immune system – for example, because of chemotherapy or HIV
- your symptoms do not improve after 7 days
If you have emergency symptoms of the flu, get medical care right away. For adults, emergency symptoms can include:
- Difficulty breathing or shortness of breath
- Chest pain
- Ongoing dizziness
- Seizures
- Worsening of existing medical conditions
- Severe weakness or muscle pain
Emergency symptoms in children can include:
- Difficulty breathing
- Pale, gray or blue-colored skin, lips or nail beds — depending on skin color
- Chest pain
- Dehydration
- Severe muscle pain
- Seizures
- Worsening of existing medical conditions
When is the flu season?
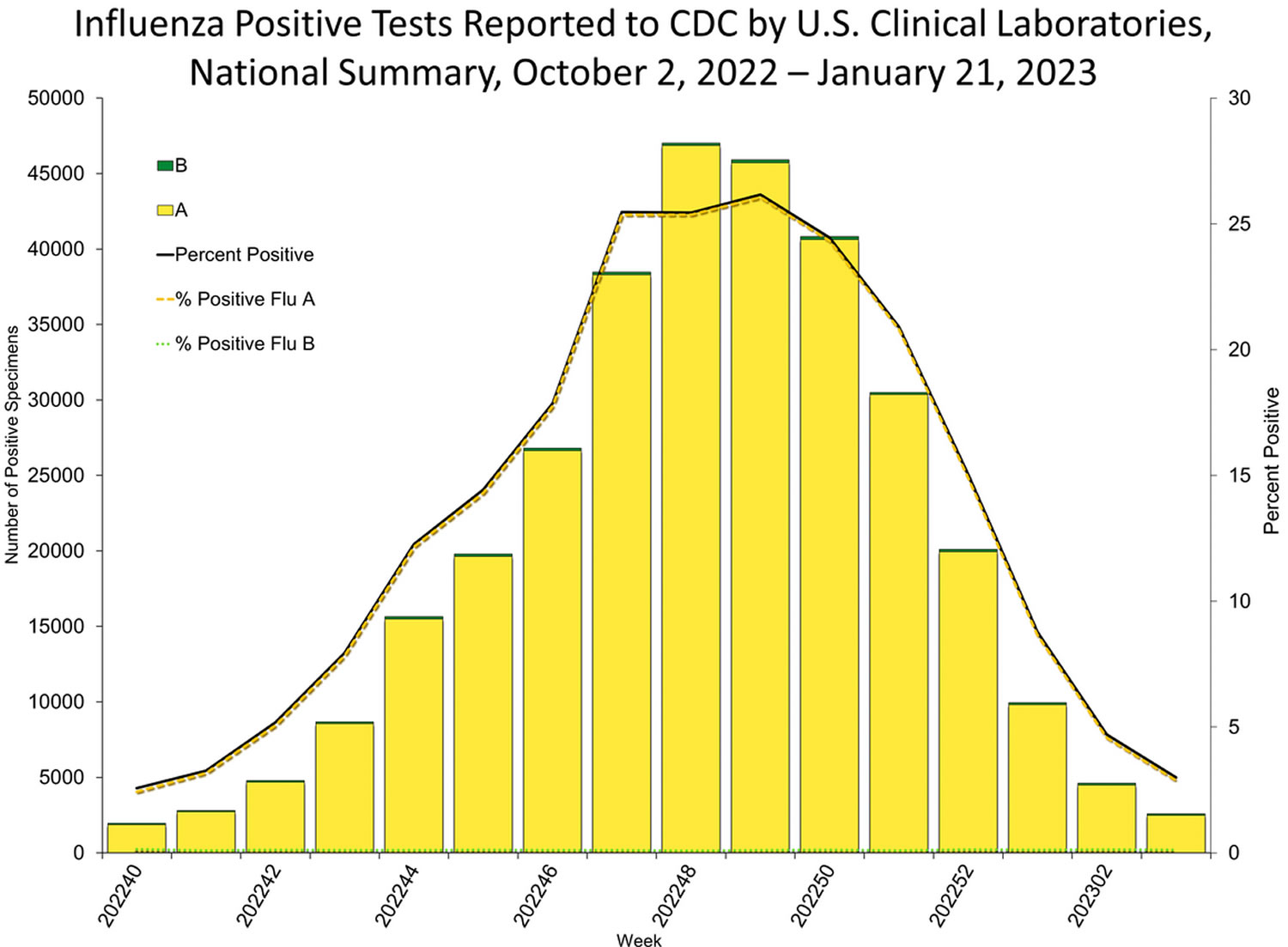
Seasonal influenza (flu) viruses are detected year-round in the United States, furthermore, flu viruses typically circulate in the United States during the fall and winter during what’s known as the “flu season” 2. The “peak month of flu activity” is the month with the highest percentage of respiratory specimens testing positive for influenza virus infection during that flu season. During the peak flu activity in the United States for 1982 to 2022 flu seasons, flu activity most often peaked in February (17 seasons), followed by December (7 seasons), January (6 seasons) and March (6 seasons) 2. However, the exact timing and duration of flu seasons varies, but in general flu activity often begins to increase in October. While influenza viruses spread all year-round, most of the time flu activity peaks between December and February, although significant activity can last as late as May 2. Since the start of the COVID-19 pandemic, the timing and duration of flu activity has been less predictable 2.
Figure 3. United States flu season
[Source 3 ]When are people with flu contagious?
Flu viruses can be detected in most infected persons beginning one day before symptoms develop and up to five to seven days after becoming sick. People with flu are most contagious in the first three to four days after their illness begins. However, infants and people with weakened immune systems who are infected with flu viruses may be contagious for longer than seven days.
Symptoms typically begin about two days (but can range from one to four days) after flu viruses infect a person’s respiratory tract. It is theoretically possible that before symptoms begin, an infected person can spread flu viruses to their close contacts. Some people can be infected with flu viruses and have no symptoms but may still be able to spread the virus to their close contacts.
How do I know if I have flu?
Your respiratory illness might be influenza (flu) if you have fever, cough, sore throat, runny or stuffy nose, body aches, headache, chills and/or fatigue. Some people may have vomiting and diarrhea, though this is more common in children. People may be sick with flu and have respiratory symptoms without a fever. Flu viruses usually cause the most illness during the colder months of the year. However, flu can also occur outside of the typical flu season. In addition, other viruses can also cause respiratory illness similar to flu. So, it is impossible to tell for sure if you have flu based on symptoms alone. If your doctor needs to know for sure whether you are sick with flu, there are laboratory tests that can be done.
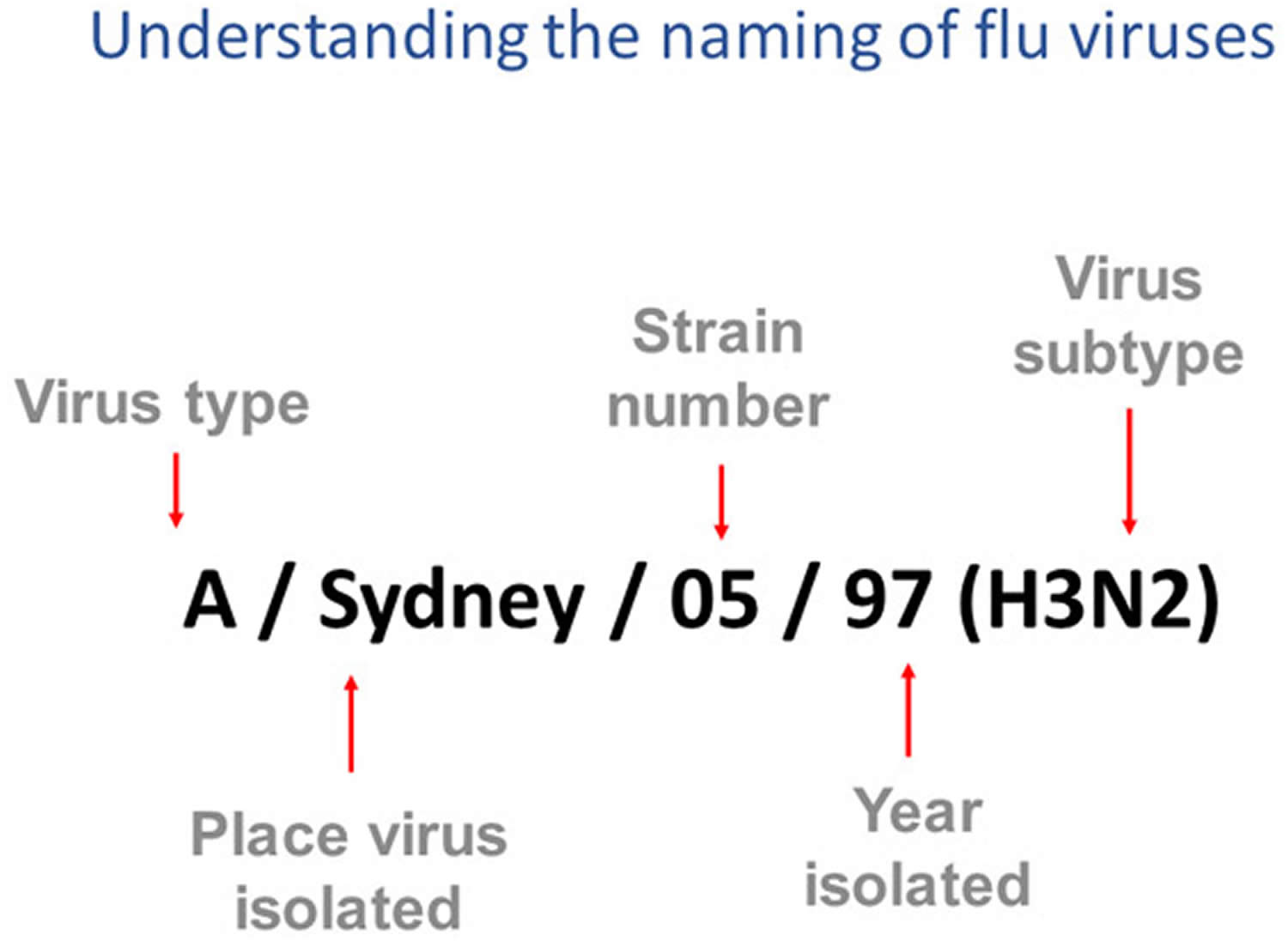
How influenza viruses are named
Influenza viruses names follow the internationally accepted World Health Organization (WHO) 1979 naming convention for influenza viruses 4.
The World Health Organization (WHO) 1979 naming convention uses the following components 4:
- The antigenic type (e.g., A, B, C, D)
- The host of origin (e.g., swine, equine, chicken, etc.). For human-origin viruses, no host of origin designation is given. Note the following examples:
- Duck example: avian influenza A(H1N1), A/duck/Alberta/35/76
- Human example: seasonal influenza A(H3N2), A/Perth/16/2019
- Geographical origin (e.g., Denver, Taiwan, etc.)
- Strain number (e.g., 7, 15, etc.)
- Year of collection (e.g., 57, 2009, etc.)
- For influenza A viruses, the hemagglutinin (H) and neuraminidase (N) antigen description are provided in parentheses (e.g., influenza A(H1N1) virus, influenza A(H5N1) virus)
- The 2009 pandemic virus was assigned a distinct name: A(H1N1)pdm09 to distinguish it from the seasonal influenza A(H1N1) viruses that circulated prior to the pandemic.
- When humans are infected with influenza viruses that normally circulate in swine (pigs), these viruses are call variant viruses and are designated with the letter “v” (e.g., an A(H3N2)v virus).
Figure 4. How influenza viruses are named
Footnotes: This image shows how influenza viruses are named. The name starts with the virus type, followed by the place the virus was isolated, followed by the virus strain number (often a sample identifier), the year isolated, and finally, the virus subtype.
[Source 1 ]Spanish flu
Spanish flu pandemic of 1918 sometimes referred to as the “Spanish flu” was caused by an influenza virus subtype H1N1, which spread to many parts of the world infecting an estimated half the world’s population at the time, and killing more than 675,000 people in the U.S. and more than 20 million people worldwide. A pandemic is an epidemic of disease that affects a wide geographic area 5, 6, 7, 8. The source of the 1918 H1N1 virus is unknown; avian and swine origins have been proposed 9, 10. An unusual characteristic of this virus was the high death rate it caused among healthy adults 15 to 34 years of age 6. The pandemic lowered the average life expectancy in the United States by more than 12 years 6. A comparable death rate has not been observed during any of the known flu seasons or pandemics that have occurred either prior to or following the 1918 pandemic 6.
The name “Spanish flu” does not mean that the epidemic started in Spain 11. Spain was neutral during the First World War and newspaper reports were not controlled by military censorship. Therefore, Spanish newspapers were only the first to report on the new disease 11. There are several theories about the origin of the “Spanish flu”: a) the origin could be in China and after in Philippines and the USA and the army in Europe. b) English soldiers in France in 1916, the disease soon spread to other neighboring countries (England, Italy, Spain) and to more distant ones (the USA) as a consequence of the displacement of the troops 12. c) the regular arrival of Chinese workers to Africa and Europe, throughout those years, could have been the origin of an earlier introduction (coinciding with the war). And this is a very plausible interpretation due to the circumstance that the Spanish Royal Family and the Spanish ministers suffered the flu, in the month of May 1918, and could contribute to this unjustified name 13.
Bird flu
Bird flu also called avian influenza or avian flu, refers to the disease caused by infection with avian (bird) influenza (flu) Type A viruses. These type A influenza viruses naturally spread among wild aquatic birds worldwide and can infect domestic poultry and other bird and animal species. Bird flu viruses do not normally infect humans. However, sporadic human infections with bird flu viruses have occurred.
Illness in humans from bird flu virus infections have ranged in severity from no symptoms or mild illness to
The reported signs and symptoms of bird flu virus infections in humans have ranged from no symptoms or mild illness such as eye redness (conjunctivitis) or mild flu-like upper respiratory symptoms, to severe disease such as pneumonia requiring hospitalization and included fever (temperature of 100ºF [37.8ºC] or greater) or feeling feverish (fever may not always be present), cough, sore throat, runny or stuff nose, muscle or body aches, headaches, fatigue, and shortness of breath or difficulty breathing. Less common signs and symptoms include diarrhea, nausea, vomiting, or seizures. A severe bird flu virus infections in humans can result in death.
There are lots of different strains of bird flu (avian flu) virus. Most of them don’t infect humans. But there are 4 avian flu strains that have caused concern in recent years:
- H5N1 (since 1997). The influenza A H5N1 subtype of the bird flu virus is very aggressive, and can cause serious infections in both birds and humans. Nearly 100 per cent of susceptible birds die from this infection. Since 2003, there have been more than 500 confirmed human cases of avian influenza, and more than 300 deaths from this disease. Human cases have been reported in Asia, Africa, the Middle East and Eastern Europe.
- H7N9 (since 2013). The influenza A H7N9 virus is difficult to detect in poultry because it causes few or no signs of disease in animals. Human cases of H7N9 avian influenza have been reported in China in 2013. To date, the confirmed number of cases is 132, with 37 deaths.
- H5N6 (since 2014)
- H5N8 (since 2016)
Although H5N1, H7N9 and H5N6 don’t infect people easily and aren’t usually spread from human to human, several people have been infected around the world, leading to a number of deaths.
The strain of bird flu presently affecting Asia is the H5N1 strain. This strain has killed more than 130 people in Indonesia, Vietnam, Cambodia, Thailand, Turkey, Azerbaijan, Eygpt, China, and Iraq since 2003. In February 2021 H5N8 was found to have infected a small number of people for the first time, in Russia.
Type A influenza viruses, which are the type of influenza viruses that cause bird flu, are able to change over time. There are 2 main ways that the virus can evolve and change, known as antigenic drift and antigenic shift.
- Antigenic drift. When influenza A viruses replicate, their genetic material changes slightly, so that new strains of the virus are constantly replacing older strains. This process, known as antigenic drift, causes only small changes in the genetic makeup of viruses.
- Antigenic shift. The virus can also mix its genetic material with other subtypes of the influenza A virus, resulting in a completely new and different subtype of virus from either of the original viruses. This is known as antigenic shift.
Scientists are worried because the H5N1 subtype of the bird flu virus is able to mutate rapidly, and can readily mix with viruses that infect other animal species. If one of the avian influenza viruses were to mix with the human flu virus through the process of antigenic shift, a highly infectious new virus could form, which could easily spread from one human to another.
The human population would not have any natural immunity to this entirely new viral subtype. Health officials are concerned that a virus that no-one had immunity to, which could be easily passed from one person to another, could result in an influenza pandemic (worldwide outbreak of disease), with high rates of illness and death.
There is currently no vaccine that protects against avian influenza.
How does bird flu virus infects humans?
Although avian (bird) influenza (flu) A viruses usually do not infect people, there have been some rare cases of human infection with these viruses. Asian lineage H7N9 and highly pathogenic avian influenza Asian lineage H5N1 viruses have been responsible for most human illness from bird flu viruses worldwide to date, including the most serious illnesses and illness with the highest mortality.
Infected birds (dead or alive) shed bird flu virus through their saliva, mucous and feces. Human infections with bird flu viruses can happen when virus gets into a person’s eyes, nose or mouth, or is inhaled. This can happen when virus is in the air (in droplets or possibly dust) and a person breathes it in, or possibly when a person touches something that has virus on it then touches their mouth, eyes or nose. Human infections with bird flu viruses have also occurred when killing or preparing infected poultry for cooking. Markets where live birds are sold can also be a source of bird flu. Avoid visiting these markets if you’re traveling to countries that have had an outbreak of bird flu.
Signs that a bird may be infected with bird flu include:
- sudden death
- swollen head
- closed and runny eyes
Human infections with bird flu viruses have occurred most often after unprotected contact with infected birds or surfaces contaminated with bird flu viruses. However, some infections have been identified where direct contact with infected birds or their environment was not known to have occurred.
You can’t catch bird flu through eating fully cooked poultry or eggs, even in areas with an outbreak of bird flu.
The spread of bird flu viruses from one infected person to a close contact is very rare, and when it has happened, it has only spread to a few people. However, because of the possibility that bird flu viruses could change and gain the ability to spread easily between people, monitoring for human infection and person-to-person spread is extremely important for public health.
Avian bird flu outbreak
Since late 2003, there have been several reported outbreaks of avian influenza or “bird flu” among domestic poultry and wild birds in Asia, Europe, the Middle East and Africa. These outbreaks are ongoing, and millions of birds have been culled in an attempt to eradicate the disease from domestic bird populations.
Avian influenza or “bird flu” viruses rarely infect people. Most previous bird flu virus infections in people have occurred following close, prolonged, and unprotected (e.g., no gloves or medical mask) contact with infected birds or environments contaminated by their saliva, mucous or poop. For any one person, the risk of infection depends on exposure and specifically on how close and how long the exposure is.
Identified avian bird flu outbreak (person-to-person spread of bird flu) 14:
- The first documented outbreak of human respiratory disease caused by avian influenza A (H5N1) viruses occurred in Hong Kong in 1997 15. In 1997, 18 human cases of bird influenza A (H5N1) illness occurred in Hong Kong, coincident with outbreaks of highly pathogenic avian influenza A (H5N1) among domestic poultry 16.
- In 2003, in the Netherlands, there was evidence of probable spread of H7N7 virus from two poultry workers to three family members. All three family members had conjunctivitis (pink eye) and one also had influenza (flu)-like illness 17.
- In 2004, in Thailand, there was evidence of probable person-to-person spread of H5N1 bird flu virus in a family. Spread of H5N1 bird flu was associated with prolonged, very close unprotected contact between an ill child with H5N1 virus infection and her mother and aunt while the child was hospitalized 18. Further spread did not occur.
- In 2005, in Indonesia, limited, non-sustained person-to-person spread of H5N1 bird flu virus could not be excluded among two groups of patients who had no known contact with poultry or other animals 19.
- In 2006, in Indonesia, limited, non-sustained person-to-person spread of H5N1 bird flu virus may have occurred among a family cluster of eight probable or confirmed H5N1 cases 20.
- In December 2007, in China, limited, non-sustained H5N1 bird flu virus spread likely occurred between a sick son and his father through prolonged very close unprotected exposure while the son was in the hospital 21.
- Also in 2007, in Pakistan, limited, non-sustained person-to-person H5N1 virus spread likely occurred among brothers 22.
- In 2013, person-to-person infections with (H7N9) bird flu virus were first reported in China 23. Yearly epidemics of person-to-person infections with H7N9 bird flu viruses in China driven mostly by contact with infected poultry at live poultry markets were reported for a number of years. A small percentage of reported cases were associated with possible limited, non-sustained person-to-person spread, mostly occurring in households between family members. However, limited, non-sustained spread of H7N9 bird flu has been reported in a few cases in hospitals 24, 25.
Bird flu symptoms in humans
The main symptoms of bird flu can appear very quickly and include:
- a very high temperature or feeling hot or shivery
- aching muscles
- headache
- a cough or shortness of breath
Other early symptoms may include:
- diarrhea
- sickness
- stomach pain
- chest pain
- bleeding from the nose and gums
- conjunctivitis
It usually takes 3 to 5 days for the first symptoms to appear after you’ve been infected. Within days of symptoms appearing, it’s possible to develop more severe complications such as pneumonia and acute respiratory distress syndrome (ARDS).
Getting treatment quickly, using antiviral medicine, may prevent complications and reduce the risk of developing severe illness.
Bird flu prevention
There is currently no vaccine that protects against avian influenza or bird flu.
If you’re visiting a foreign country that’s had a bird flu outbreak you should:
- wash your hands often with warm water and soap, especially before and after handling food, in particular raw poultry
- use different utensils for cooked and raw meat
- make sure meat is cooked until steaming hot
- avoid contact with live birds and poultry
What NOT to do:
- do not go near or touch bird droppings or sick or dead birds
- do not go to live animal markets or poultry farms
- do not bring any live birds or poultry back to the UK, including feathers
- do not eat undercooked or raw poultry or duck
- do not eat raw eggs
Bird flu diagnosis
Bird flu virus infection in people cannot be diagnosed by clinical signs and symptoms alone; laboratory testing is needed. Bird flu virus infection is usually diagnosed by collecting a swab from the upper respiratory tract (nose or throat) of the sick person. Testing is more accurate when the swab is collected during the first few days of illness.
For critically ill patients, collection and testing of lower respiratory tract specimens also may lead to diagnosis of bird flu virus infection. However, for some patients who are no longer very sick or who have fully recovered, it may be difficult to detect bird flu virus in a specimen.
Rapid influenza diagnostic tests and immunofluorescence assays are antigen detection tests that only identify whether an influenza A virus is detected and have unknown sensitivity and specificity to detect human infection with novel influenza A viruses in respiratory specimens. Some studies suggest that antigen detection tests have low sensitivity to detect highly pathogenic avian influenza A (H5N1) viruses. Therefore, negative results from either type of test do not exclude novel influenza A virus infection, especially in patients with signs and symptoms suggestive of influenza. A negative test result could be a false negative and should not be used as a final diagnostic test for influenza, including novel influenza A virus infection. These tests may yield a positive influenza A result for a specimen containing novel influenza A virus but cannot identify the subtype and cannot distinguish novel influenza A virus from seasonal influenza A virus infection. Therefore, testing by Real-Time RT-PCR (rRT-PCR) is recommended at state health laboratories for any patient with suspected novel influenza A virus infection.
Figure 5. Bird flu virus sample collection guideline
Bird flu treatment
If it’s thought you might have symptoms of bird flu you’ll be advised to stay at home, or you’ll be cared for in hospital in isolation from other patients. Empiric antiviral medicine such as oseltamivir (Tamiflu) or zanamivir (Relenza) should be started as soon as possible for all patients with possible infection with novel influenza A viruses with the potential to cause severe disease in humans. Antiviral medicines help reduce the severity of the condition, prevent complications and improve the chances of survival. They are also sometimes given to people who have been in close contact with infected birds, or those who have had contact with infected people, for example family or healthcare staff.
Choice of antiviral drug depends upon clinical severity. For outpatients with uncomplicated mild illness, treatment with a neuraminidase inhibitor (oral oseltamivir, inhaled zanamivir, IV peramivir) or oral baloxavir can be used 26. For outpatients with severe, progressive, or complicated illness, or for hospitalized patients, oral oseltamivir should be administered 26. For management of patients with laboratory-confirmed avian influenza A virus infection who are hospitalized with severe pneumonia, the CDC Influenza Division should be consulted.
Outpatients bird flu treatment guidelines 26:
- Initiation of antiviral treatment with a neuraminidase inhibitor is recommended as early as possible for symptomatic outpatients who are confirmed, probable, or suspected/cases under investigation of novel influenza A virus infection associated with severe human disease. Treatment is not currently recommended for uncomplicated illness in outpatients whose exposure criteria consists only of travel to an area (e.g. country, state/province, city, county) with human cases of novel influenza A virus infection associated with severe human disease, or where these viruses are known to be circulating in animals.
- For outpatients with severe, progressive, or complicated illness, oseltamivir treatment is recommended. For other outpatients with uncomplicated mild-to-moderate illness presenting within 2 days of illness onset, oral oseltamivir, inhaled zanamivir, IV peramivir, or oral baloxavir may be used.
- If more than 48 hours has elapsed since illness onset, oseltamivir treatment is recommended.
- For untreated outpatients who are confirmed cases, probable cases, or suspected/cases under investigation with uncomplicated disease in whom fever is absent and symptoms are nearly resolved, decisions to initiate antiviral treatment should be based on clinical judgment. Persons who are not treated with antiviral medications should be monitored for progression of illness.
- Recommended treatment duration for mild uncomplicated illness with a novel influenza A virus infection presenting within 2 days after illness onset is two doses per day of oral oseltamivir or inhaled zanamivir for 5 days, or a single dose of oral baloxavir, based on data for treatment of seasonal influenza.
- For IV peramivir, the recommendation for treatment of uncomplicated illness is one dose of IV peramivir for 1 day based on treatment of seasonal influenza.
- Inhaled zanamivir is not recommended for treatment of persons with underlying airway disease (e.g., asthma or chronic obstructive pulmonary disease).
Hospitalized patients bird flu treatment guidelines 26:
- Initiation of antiviral treatment with oral or enterically administered oseltamivir is recommended as soon as possible for hospitalized patients who are confirmed, probable, or suspected/cases under investigation of human infection with novel influenza A viruses associated with severe human disease, regardless of time since illness onset. Inhaled zanamivir is not recommended because of the lack of data for use in patients with severe seasonal influenza. There are insufficient data regarding the efficacy or effectiveness of intravenous (IV) peramivir for hospitalized seasonal influenza patients.
- Oral oseltamivir is approved by the FDA for treatment of acute uncomplicated influenza in persons ≥14 days old. Although use of oral oseltamivir for treatment of influenza in infants less than 14 days old is not part of the FDA-approved indication, it is recommended by the CDC and the American Academy of Pediatrics for treatment of influenza in patients of any age. Inhaled zanamivir is approved for treatment of acute uncomplicated influenza in persons aged 7 years and older. Clinicians may refer to the manufacturer’s package inserts for additional information regarding dosing, limitations of populations studied, contraindications, and adverse events.
- A randomized clinical trial of antiviral treatment of hospitalized patients with seasonal influenza reported that combination neuraminidase inhibitor and baloxavir treatment was not superior to neuraminidase inhibitor and placebo. No significant differences were found in the time to clinical improvement or other clinical outcomes, but the addition of baloxavir to a neuraminidase inhibitor significantly reduced duration of infectious viral shedding compared to neuraminidase inhibitor treatment alone 27.
- Antiviral treatment should not be delayed while waiting for laboratory testing results.
- The standard dose of oseltamivir is 75 mg twice daily for 5 days. However, the optimal duration and dosing are uncertain for patients with severe disease. Avian influenza A (H5N1) and A(H7N9) viruses have been shown to be associated with higher virus levels and longer duration of viral replication (particularly in the lower respiratory tract) in hospitalized patients than with seasonal influenza A or B virus infection 28, 29. Pending further data, longer courses of treatment (e.g., 10 days) should be considered for severely ill hospitalized patients with novel influenza A virus infections.
- Clinical judgment and virologic testing of lower respiratory tract specimens by RT-PCR should guide decisions to consider treatment regimens longer than 5 days for hospitalized patients with severe and prolonged illness. For patients with lower respiratory tract disease, lower respiratory tract specimens such as bronchoalveolar lavage fluid or endotracheal aspirate are preferred; a combined nasal and oropharyngeal (throat) swab specimen may be collected if lower respiratory specimens are not available. Lower respiratory tract specimens may yield the diagnosis when testing of upper respiratory tract specimens produce negative results in hospitalized patients with severe disease. Multiple respiratory tract specimens collected on different days should be tested if novel influenza A virus infection associated with severe disease is suspected without another definitive diagnosis.
- Longer treatment regimens might be necessary in severely immunosuppressed persons (e.g., hematopoietic stem cell transplant recipients) who may have prolonged influenza viral replication. Such patients are at risk of developing antiviral-resistant virus infection with monotherapy. Although no data from clinical trials are available, combination treatment using antivirals with different mechanisms of action can be considered.
- Limited data do not suggest clinical benefit of higher antiviral dosing. A higher dose of oseltamivir has been recommended by some experts (e.g., 150 mg twice daily in adults with normal renal function instead of the standard 75 mg twice daily dose) for treatment of influenza in immunocompromised patients and in severely ill hospitalized patients 30. However, oral or enterically administered oseltamivir has been reported to be adequately absorbed in critically ill adults, with standard doses producing therapeutic blood levels 31. Although the higher dose was generally well-tolerated and not associated with severe adverse events, limited data suggest that higher dosing may not provide additional clinical benefit for seasonal influenza or for pandemic 2009 influenza A (H1N1) 32, 33. Studies indicate that the exposure to oseltamivir carboxylate (the active metabolite of oseltamivir) is similar between obese and non-obese subjects for both 75 mg and 150 mg doses given twice daily 34, 35.
- Limited data suggest that oseltamivir administered orally or by oro/naso gastric tube is well absorbed in critically ill influenza patients, including those in the intensive care unit, on continuous renal replacement therapy, and/or on extracorporeal membrane oxygenation 36, 37. However, for patients who cannot tolerate or absorb oral or enterically-administered oseltamivir because of suspected or known gastric stasis, malabsorption, or gastrointestinal bleeding, IV peramivir is an alternative.
- While studies have shown benefit of IV peramivir for treatment of uncomplicated influenza in outpatients 38, a randomized trial of treatment of seasonal influenza in hospitalized patients aged >6 years failed to demonstrate significant clinical benefit for intravenous peramivir plus standard of care compared with placebo plus standard of care. However, peramivir was generally safe and well tolerated at a dosage of 600 mg once daily (10 mg/kg once daily in children) for 5 days 39. If used to treat hospitalized patients with novel influenza A virus infection, this dose of IV peramivir is recommended for a minimum of 5 days (not a single dose as is recommended for outpatients with uncomplicated illness).
- Use of inhaled zanamivir in combination with oseltamivir or peramivir is not recommended, because of data suggesting that antagonism may occur when 2 neuraminidase inhibitors are given simultaneously compared to monotherapy.
- It is possible that some novel influenza A viruses might become resistant to oseltamivir and peramivir during antiviral treatment with one of these agents and remain susceptible to zanamivir 40, 41. If a hospitalized patient treated with oseltamivir and/or peramivir manifests progressive lower respiratory disease, the presence of a resistant virus should be considered. After consultation with CDC’s Influenza Division, investigation for antiviral resistance should be performed. Combination treatment with a neuraminidase inhibitor and baloxavir did not have clinical benefit compared with neuraminidase inhibitor and placebo in a randomized clinical trial in hospitalized patients with seasonal influenza, but the addition of baloxavir reduced duration of infectious viral shedding 27.
- Any questions regarding arranging testing for antiviral resistance, or regarding appropriate clinical management if antiviral resistance is a concern, should be directed to the CDC Influenza Division via the CDC Emergency Operations Center (770-488-7100).
Swine flu
Swine flu also known as H1N1 flu, is primarily caused by the type A H1N1 strain of the flu (influenza) virus that regularly causes outbreaks of influenza in pigs. Swine flu viruses can cause high levels of illness in pig herds, but cause few deaths in pigs. Swine influenza viruses can circulate among swine throughout the year, but most outbreaks occur during the late fall and winter months similar to outbreaks of seasonal influenza in humans. There are three main flu viruses that spread in U.S. pigs in recent years: swine triple reassortant (tr) H1N1, trH3N2 and trH1N2. These viruses are genetically different from the H1N1 and H3N2 viruses that commonly spread in people. People may have little to no immune protection against those flu viruses that spread in pigs, and human flu vaccines do not protect against the flu viruses that spread in pigs. With the exception of the 2009 H1N1 virus which started in Mexico, influenza viruses that circulate in swine are very different from influenza viruses that commonly circulate in people. In the spring of 2009, scientists recognized a particular strain of flu virus known as H1N1 42. This virus is a combination of viruses from pigs, birds and humans that causes disease in humans. During the 2009-10 flu season, H1N1 caused the respiratory infection in humans that was commonly referred to as swine flu. Because so many people around the world got sick, in 2009 the World Health Organization (WHO) declared the H1N1 flu to be a pandemic. In August 2010, WHO declared the pandemic over. After the pandemic was over, the H1N1 flu virus became one of the strains that cause seasonal flu.
Many people worldwide now have some immunity to pandemic (H1N1) influenza 2009 virus. However, the swine flu virus H1N1 is expected to continue to circulate as a seasonal influenza strain for years, and many people are still susceptible to infection. The pandemic (H1N1) influenza 2009 virus continues to pose a higher risk for severe illness in certain people, including pregnant women, young children and those with chronic health problems.
Swine flu viruses do not normally infect humans. However, sporadic human infections with influenza viruses that normally circulate in swine and not people have occurred. When this happens, these viruses are called “variant viruses.” They also can be denoted by adding the letter “v” to the end of the virus subtype designation. Human infections with H1N1v, H3N2v and H1N2v viruses have been detected in the United States.
Most commonly, human infections with variant viruses occur in people with exposure to infected pigs (e.g., children near pigs at a fair or workers in the swine industry). This is thought to happen mainly when an infected pig coughs or sneezes and droplets with influenza virus in them spread through the air. If these droplets land in your nose or mouth, or are inhaled, you can be infected. There also is some evidence that you might get infected by touching something that has virus on it and then touching your own mouth or nose. A third way to possibly get infected is to inhale particles containing influenza virus. Scientists aren’t really sure which of these ways of spread is the most common. Swine influenza has not been shown to be transmissible to people through eating properly handled and prepared pork (pig meat) or other products derived from pigs.
There have been documented cases of multiple people becoming sick after exposure to one or more infected pigs and also cases of limited spread of variant influenza viruses from person-to-person. The vast majority of human infections with variant influenza viruses do not result in person-to-person spread. However, each case of human infection with a swine influenza virus should be fully investigated to be sure that such viruses are not spreading in an efficient and ongoing way in humans and to limit further exposure of humans to infected animals if infected animals are identified.
Illness associated with variant virus infection has been mostly mild with symptoms similar to those of seasonal flu. Like seasonal flu, however, serious illness, resulting in hospitalization and death is possible. In 2012, for example, of 309 human infections with H3N2v, 16 people were hospitalized and one of these people died. Most of the people who were hospitalized and the person who died had one or more health or age factor that put them at high risk of serious flu-related complications. People at high risk of serious complications from seasonal influenza and H3N2v include children younger than 5, people with certain chronic conditions like asthma, diabetes, heart disease, weakened immune systems, pregnant women and people 65 years and older.
The flu vaccine can now help protect against the H1N1 swine flu. The H1N1 flu virus strain is included in the seasonal flu vaccine, including the vaccine for 2020-21.
It’s not necessary to see a doctor if you’re generally healthy and develop flu signs and symptoms, such as fever, cough and body aches. See your doctor, however, if you have flu symptoms and you’re pregnant or you have a chronic disease, such as asthma, emphysema, diabetes or a heart condition, because you have a higher risk of flu complications.
If you have flu symptoms and are at risk of complications, see your health care provider right away. Taking antiviral medication may shorten the length of your illness and help prevent more-serious problems.
See your doctor right away if you or your child have symptoms of flu and:
- you’re worried about your baby’s or child’s symptoms
- you’re 65 or over
- you’re pregnant
- you have a long-term medical condition – for example, diabetes or a condition that affects your heart, lungs, kidneys, brain or nerves
- you have a weakened immune system – for example, because of chemotherapy or HIV
- your symptoms do not improve after 7 days
If you have emergency signs and symptoms of the flu, get medical care right away. For adults, emergency signs and symptoms can include:
- Difficulty breathing or shortness of breath
- Chest pain
- Ongoing dizziness
- Seizures
- Worsening of existing medical conditions
- Severe weakness or muscle pain
Emergency signs and symptoms in children can include:
- Difficulty breathing
- Pale, gray or blue-colored skin, lips or nail beds — depending on skin color
- Chest pain
- Dehydration
- Severe muscle pain
- Seizures
- Worsening of existing medical conditions
Most people who get the flu can treat themselves at home and often don’t need to see a health care provider.
Swine flu symptoms in humans
The signs and symptoms of flu caused by the swine flu virus H1N1 are similar to those of infections caused by other flu strains and can include:
- Fever, but not always
- Chills
- Cough
- Sore throat
- Runny or stuffy nose
- Watery, red eyes
- Body aches
- Headache
- Fatigue
- Diarrhea
- Nausea and vomiting
Flu symptoms develop about one to three days after you’re exposed to the flu virus.
Swine flu complications
If you’re young and healthy, the flu usually isn’t serious. Although you may feel miserable while you have it, the flu usually goes away in a week or two with no lasting effects. But children and adults at high risk may develop complications that may include:
- Pneumonia
- Bronchitis
- Asthma flare-ups
- Heart problems
- Ear infections
- Acute respiratory distress syndrome (ARDS)
Pneumonia is one of the most serious complications. For older adults and people with a chronic illness, pneumonia can be deadly.
Swine flu causes
Influenza viruses such as swine flu H1N1 virus infects the cells that line your nose, throat and lungs. The swine flu H1N1 virus enters your body when you inhale contaminated droplets or transfer live virus from a contaminated surface to your eyes, nose or mouth.
You can’t catch swine flu from eating pork.
Risk factors for getting swine flu
If you live in or travel to an area where many people are infected with the swine flu H1N1 virus, you may be exposed to the virus.
Swine flu prevention
The best way to reduce your risk from seasonal flu and its potentially serious complications is to get vaccinated every year.
The U.S. Centers for Disease Control and Prevention (CDC) recommends annual flu vaccination for everyone age 6 months or older. Each year’s seasonal flu vaccine protects against the three or four influenza viruses that are expected to be the most common during that year’s flu season. The flu vaccine can reduce your risk of the flu and its severity and lower the risk of having serious illness from the flu and needing to stay in the hospital.
The influenza vaccine isn’t 100% effective, so it’s also important to take several measures to reduce the spread of infection, including:
- Wash your hands. Washing your hands often with soap and water for at least 20 seconds is an effective way to prevent many common infections. Or use alcohol-based hand sanitizers if soap and water aren’t available.
- Avoid touching your face. Avoid touching your eyes, nose and mouth.
- Cover your coughs and sneezes. Cough or sneeze into a tissue or your elbow. Then wash your hands.
- Clean surfaces. Regularly clean often-touched surfaces to prevent spread of infection from touching a surface with the virus on it and then your face.
- Avoid crowds. The flu spreads easily wherever people gather — in child care centers, schools, office buildings, auditoriums and public transportation. By avoiding crowds during peak flu season, you reduce your chances of infection.
Also avoid anyone who is sick. And if you’re sick, stay home for at least 24 hours after your fever is gone so that you lessen your chance of infecting others.
Swine flu diagnosis
Your doctor will conduct a physical exam, look for signs and symptoms of influenza, including swine flu (H1N1 flu), and possibly order a test that detects influenza viruses such as H1N1.
There are several tests used to diagnose influenza, but not everyone who has the flu needs to be tested. Your doctor may diagnose you with influenza based on your signs and symptoms. In most cases, knowing that someone has the flu doesn’t change the treatment plan. Doctors are more likely to use a test to diagnose flu if:
- You’re already in the hospital
- You’re at high risk of complications from the flu
- You live with someone who is at greater risk of flu complications
Your doctor may also use a test to determine whether a flu virus is the cause of your symptoms, or if you have or are showing signs of another problem besides the flu, such as:
- Heart problems, such as heart failure or an infection of the heart muscle
- Lung and breathing problems, such as asthma or pneumonia
- Brain and nervous system problems, such as encephalopathy or encephalitis
- Septic shock or organ failure
In some cases, your doctor may suggest that you be tested for influenza. He or she may use various tests to diagnose influenza. Polymerase chain reaction (PCR) testing is becoming more common in many hospitals and labs. This test may be done while you’re in your doctor’s office or in the hospital. PCR testing is more sensitive than other tests and may be able to identify the influenza strain.
Swine flu treatment
Most people with flu, including swine flu (H1N1 flu), require only symptom relief. Supportive care such as drinking liquids, taking pain relievers for fever and headache, and resting may be helpful. If you have a chronic respiratory disease, your doctor may prescribe additional medications to help relieve your symptoms.
Antiviral drugs are sometimes prescribed within the first day or two of symptoms. They can reduce the severity of symptoms and possibly the risk of complications. The U.S. Food and Drug Administration has approved these four drugs:
- Oseltamivir (Tamiflu). Oseltamivir (Tamiflu) for oral administration is FDA-approved for early treatment of uncomplicated influenza in people two weeks and older, and for chemoprophylaxis to prevent influenza in people one year and older. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants younger than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year, is recommended by CDC and the American Academy of Pediatrics. If a child is younger than 3 months old, use of oseltamivir for chemoprophylaxis is not recommended unless the situation is judged critical, due to limited data in this age group.
- Zanamivir (Relenza). Zanamivir (Relenza) for oral inhalation through a device similar to an asthma inhaler is FDA-approved for early treatment of uncomplicated influenza in people 7 years and older and to prevent influenza in people 5 years and older. It is not recommended for use in people with underlying respiratory disease, including people with and lung disease.
- Peramivir (Rapivab). Peramivir (Rapivab) for intravenous administration is FDA-approved for early treatment of uncomplicated influenza in people 6 months and older.
- Baloxavir (Xofluza). Baloxavir marboxil (Xofluza) for oral administration is FDA-approved for early treatment of uncomplicated influenza in otherwise healthy non-high risk people 5 years to less than 12 years and for all persons 12 years and older, and for post-exposure prophylaxis of influenza in people 5 years and older. Baloxavir is not recommended for pregnant women, immunocompromised persons, breastfeeding mothers, outpatients with complicated or progressive illness, or hospitalized patients.
But flu viruses can develop resistance to these drugs.
To make development of resistance less likely and maintain supplies of these drugs for those who need them most, doctors reserve antivirals for people at high risk of complications and those who are in close contact with people who have high risk of complications.
People at higher risk of flu complications include people who:
- Are in a hospital, nursing home or other long-term care facility.
- Are younger than 5 years of age, particularly children younger than 2 years.
- Are 65 years old or older.
- Are pregnant or within two weeks of delivery, including women who have had pregnancy loss.
- Are younger than 19 years of age and are receiving long-term aspirin therapy. Using aspirin during a viral illness increases the risk of developing Reye’s syndrome, a rare but potentially life-threatening condition, in these individuals.
- Have a body mass index above 40, which is defined as morbid obesity.
- Have certain chronic medical conditions, such as asthma, emphysema, heart disease, diabetes, neuromuscular disease, or kidney, liver or blood disease.
- Are immunosuppressed due to certain medications or HIV.
- Are of American Indian or Alaska Native heritage.
Swine flu home remedies
If you develop any type of flu, these measures may help ease your symptoms:
- Drink plenty of liquids. Choose water, juice and warm soups to prevent dehydration.
- Rest. Get more sleep to help your immune system fight infection.
- Consider pain relievers. Use an over-the-counter pain reliever, such as acetaminophen (Tylenol, others) or ibuprofen (Advil, Motrin IB, others). Use caution when giving aspirin to children or teenagers. Though aspirin is approved for use in children older than age 3, children and teenagers recovering from chickenpox or flu-like symptoms should never take aspirin. This is because aspirin has been linked to Reye’s syndrome, a rare but potentially life-threatening condition, in such children.
If you have the flu, you can give it to others. Stay home for at least 24 hours after your fever is gone.
Flu symptoms
At first, the flu may seem like a common cold with a runny nose, sneezing and sore throat. Colds usually develop slowly. But the flu tends to come on suddenly. And while a cold can be miserable, you usually feel much worse with the flu.
Flu symptoms come on very quickly and can include:
- sudden high temperature (fever)
- an aching body
- chills and sweats
- feeling tired or exhausted or weak
- dry, persistent cough
- sore throat
- eye pain
- headache
- difficulty sleeping
- loss of appetite
- vomiting and diarrhea or abdominal pain
- feeling sick and being sick
- shortness of breath
- runny or stuffy nose
The symptoms are similar for children, but they can also get pain in their ear and appear less active.
Cold and flu symptoms are similar, but flu tends to be more severe.
Table 1. How to tell the difference between cold and flu symptoms
| Flu | Cold |
|---|---|
| Appears quickly within a few hours | Appears gradually |
| Affects more than just your nose and throat | Affects mainly your nose and throat |
| Makes you feel exhausted and too unwell to carry on as normal | Makes you feel unwell, but you still feel well enough to do your normal activities |
Flu complications
If you’re young and healthy, the flu usually isn’t serious. Although you may feel miserable while you have it, the flu usually goes away in a week or two with no lasting effects. But children and adults at high risk may develop complications that may include:
- Pneumonia
- Bronchitis
- Asthma flare-ups
- Heart problems
- Ear infections
- Acute respiratory distress syndrome (ARDS)
Pneumonia is one of the most serious complications. For older adults and people with a chronic illness, pneumonia can be deadly.
Sinus and ear infections are examples of moderate complications from flu, while pneumonia is a serious flu complication that can result from either influenza virus infection alone or from co-infection of flu virus and bacteria. Other possible serious complications triggered by flu can include inflammation of the heart (myocarditis), brain (encephalitis) or muscle (myositis, rhabdomyolysis) tissues, and multi-organ failure (for example, respiratory and kidney failure). Flu virus infection of the respiratory tract can trigger an extreme inflammatory response in the body and can lead to sepsis, the body’s life-threatening response to infection. Flu also can make chronic medical problems worse. For example, people with asthma may experience asthma attacks while they have the flu, and people with chronic heart disease may experience a worsening of this condition triggered by flu.
People at High Risk for Developing Flu-Related Complications
- Children younger than 5, but especially children younger than 2 years old
- Adults 65 years of age and older
- Pregnant women (and women up to two weeks postpartum)
- Residents of nursing homes and other long-term care facilities
- Also, American Indians and Alaska Natives
People who have medical conditions including:
- Asthma
- Neurological and neurodevelopmental conditions [including disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy (seizure disorders), stroke, intellectual disability, moderate to severe developmental delay, muscular dystrophy, or spinal cord injury].
- Chronic lung disease (such as chronic obstructive pulmonary disease [COPD] and cystic fibrosis)
- Heart disease (such as congenital heart disease, congestive heart failure and coronary artery disease)
- Blood disorders (such as sickle cell disease)
- Endocrine disorders (such as diabetes mellitus)
- Kidney disorders
- Liver disorders
- Metabolic disorders (such as inherited metabolic disorders and mitochondrial disorders)
- Weakened immune system due to disease or medication (such as people with HIV or AIDS, or cancer, or those on chronic steroids)
- People younger than 19 years of age who are receiving long-term aspirin therapy
- People with extreme obesity (body mass index [BMI] of 40 or more).
Note: There is no recommendation for pregnant women or people with pre-existing medical conditions to get special permission or written consent from their doctor or health care professional for influenza vaccination if they get vaccinated at a worksite clinic, pharmacy or other location outside of their physician’s office.
Influenza causes
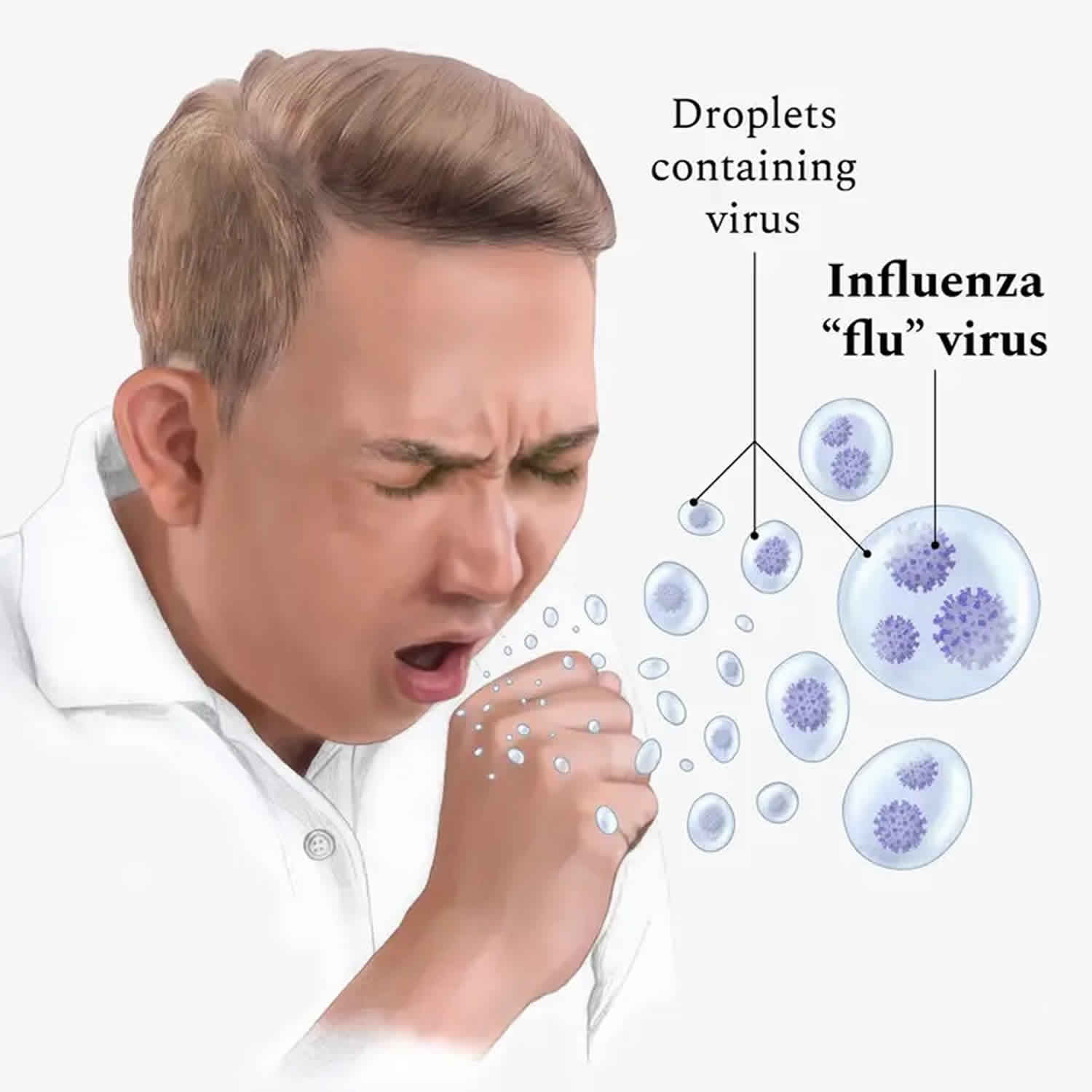
Influenza commonly called the flu, is an infection of the nose, throat and lungs, caused by the influenza viruses. Influenza viruses travel through the air in droplets when someone with the flu infection coughs, sneezes or talks. You can inhale the droplets directly. Or you can pick up the germs from an object such as a telephone or computer keyboard and then transfer them to your eyes, nose or mouth.
People with the influenza virus are likely contagious from about a day before symptoms appear until about four days after they start. Children and people with weakened immune systems may be contagious for a slightly longer time.
Influenza viruses are constantly changing, with new strains appearing regularly. If you’ve had influenza in the past, your body has already made antibodies to fight that specific strain of the virus. If future influenza viruses are similar to those you’ve encountered before, either by having the disease or by getting vaccinated, those antibodies may prevent infection or lessen its severity. But antibody levels may decline over time.
Also, antibodies against influenza viruses you’ve encountered in the past may not protect you from new influenza strains. New strains can be very different viruses from what you had before.
How flu spreads
People with flu can spread it to others. Most experts think that flu viruses spread mainly by droplets made when people with flu cough, sneeze, or talk. These droplets can land in the mouths or noses of people who are nearby (usually within about 6 feet away) or possibly be inhaled into the lungs. Less often, a person might get flu by touching a surface or object that has flu virus on it and then touching their own mouth, nose, or possibly their eyes.
Risk factors for getting the flu
Factors that may increase your risk of developing the flu or its complications include:
- Age. Seasonal influenza tends to have worse outcomes in children under age 2, and adults older than age 65.
- Living or working conditions. People who live or work in facilities with many other residents, such as nursing homes or military barracks, are more likely to develop the flu. People who are staying in the hospital also are at higher risk.
- Weakened immune system. Cancer treatments, anti-rejection medications, long-term use of steroids, organ transplant, blood cancer or HIV/AIDS can weaken the immune system. This can make it easier to catch the flu and may increase the risk of developing complications.
- Chronic illnesses. Chronic conditions may increase the risk of influenza complications. Examples include asthma and other lung diseases, diabetes, heart disease, nervous system diseases, metabolic disorders, problems with an airway, and kidney, liver or blood disease.
- Race. American Indians or Alaska Natives people may have an increased risk of influenza complications.
- Aspirin use under age 19. People who are younger than 19 years of age and receiving long-term aspirin therapy are at risk of developing Reye’s syndrome if infected with influenza.
- Pregnancy. Pregnant people are more likely to develop influenza complications, particularly in the second and third trimesters. This risk continues up to two weeks after the baby is born.
- Obesity. People with a body mass index (BMI) of 40 or higher have an increased risk of flu complications.
Flu prevention
The best way to reduce your risk from seasonal flu and its potentially serious complications is to get vaccinated every year.
The U.S. Centers for Disease Control and Prevention (CDC) recommends annual flu vaccination for everyone age 6 months or older. The flu vaccine can lower your risk of getting the flu. It also can lower the risk of having serious illness from the flu and needing to stay in the hospital.
The influenza vaccine isn’t 100% effective, so it’s also important to take several measures to reduce the spread of infection, including:
- Wash your hands. Washing your hands often with soap and water for at least 20 seconds is an effective way to prevent many common infections. Or use alcohol-based hand sanitizers if soap and water aren’t available.
- Avoid touching your face. Avoid touching your eyes, nose and mouth.
- Cover your coughs and sneezes. Cough or sneeze into a tissue or your elbow. Then wash your hands.
- Clean surfaces. Regularly clean often-touched surfaces to prevent spread of infection from touching a surface with the virus on it and then your face.
- Avoid crowds. The flu spreads easily wherever people gather — in child care centers, schools, office buildings, auditoriums and public transportation. By avoiding crowds during peak flu season, you reduce your chances of infection.
Also avoid anyone who is sick. And if you’re sick, stay home for at least 24 hours after your fever is gone so that you lessen your chance of infecting others.
Flu vaccine
A flu vaccine also called flu shot that is given with a needle, usually in the arm. The seasonal flu shot causes antibodies to develop in your body about two weeks after you get it. These antibodies provide protection against infection with the flu viruses that are in the flu shot. There are many different flu viruses, and they are constantly changing. The composition of U.S. flu vaccines is reviewed annually and updated as needed. The seasonal flu shot protects against the four influenza viruses that research indicates will be most common during the season.
Seasonal influenza (flu) vaccines are designed to protect against the four main groups of flu type A and B viruses that research indicates are most likely to spread and cause illness among people during the upcoming flu season. All current U.S. flu vaccines protect against a flu A(H1) virus, a flu A(H3) virus, a flu B/Yamagata lineage virus and a flu B/Victoria lineage virus.
The recommendations for the 2022-2023 season include two updates compared with the recommended composition of last season’s U.S. flu vaccines. Both the influenza A(H3N2) and the influenza B (Victoria lineage) vaccine virus components were updated.
Current seasonal flu vaccines are formulated to protect against influenza viruses known to cause epidemics, including: one influenza A(H1N1) virus, one influenza A(H3N2) virus, one influenza B/Victoria lineage virus, and one influenza B/Yamagata lineage virus. Seasonal flu vaccines do not protect against influenza C or D viruses or against zoonotic (animal-origin) flu viruses that can cause human infections, such as variant or avian (bird) flu viruses. In addition, flu vaccines will NOT protect against infection and illness caused by other viruses that also can cause influenza-like symptoms. There are many other viruses besides influenza that can result in influenza-like illness (ILI) that spread during flu season.
This year’s seasonal flu shot provides protection against four influenza viruses expected to be the most common during this flu season. This year, the vaccine will be available as an injection and as a nasal spray. There will also be a high-dose flu vaccine for adults age 65 and older.
The nasal spray is approved for people between 2 and 49 years old. It isn’t recommended for some groups, such as:
- Children younger than age 2
- Adults age 50 and older
- Pregnant people
- Children between 2 and 17 years old who are taking aspirin or a salicylate-containing medication
- People with weakened immune systems
- Kids 2 to 4 years old who have had asthma or wheezing in the past 12 months
If you have an egg allergy, you can still get a flu vaccine.
People who can get the flu shot:
- Different flu shots are approved for people of different ages. Everyone should get a vaccine that is appropriate for their age.
- There are standard-dose inactivated flu vaccines that are approved for people as young as 6 months of age.
- Some vaccines are only approved for adults. For example, the recombinant flu vaccine is approved for people aged 18 years and older, and the adjuvanted and high-dose inactivated vaccines are approved for people 65 years and older.
- Pregnant people and people with certain chronic health conditions can get a flu shot.
- People with egg allergy can get a flu shot
People who SHOULD NOT get a flu shot include:
- Children younger than 6 months of age are too young to get a flu shot.
- People with severe, life-threatening allergies to any ingredient in a flu vaccine (other than egg proteins) should not get that vaccine. This might include gelatin, antibiotics, or other ingredients. See Special Considerations Regarding Egg Allergy for more information about egg allergies and flu vaccine.
- People who have had a severe allergic reaction to a dose of influenza vaccine should not get that flu vaccine again and might not be able to receive other influenza vaccines. If you have had a severe allergic reaction to an influenza vaccine in the past, it is important to talk with your health care provider to help determine whether vaccination is appropriate for you.
People who SHOULD NOT get a Nasal Spray Flu Vaccine:
- Children younger than 2 years of age.
- Adults 50 years of age and older.
- People who have had a severe or life-threatening allergic reaction to any ingredient in the nasal spray vaccine (other than egg proteins). See Special Considerations Regarding Egg Allergy for more information about egg allergies and flu vaccine.
- People who have had a severe allergic reaction to any flu vaccine.
- Children and adolescents 2 through 17 years of age who are receiving aspirin- or salicylate-containing medications.
- People with weakened immune systems (immunosuppression) due to any cause, including (but not limited to) immunosuppression from medications, congenital or acquired immune disorders, HIV infection, or asplenia.
- People who care for or are close contacts of severely immunocompromised persons who require a protected environment (or otherwise avoid contact with those persons for 7 days after getting the nasal spray vaccine).
- Pregnant people.
- Children 2 years through 4 years who have asthma or who have had a history of wheezing in the past 12 months.
- People with cerebrospinal fluid (CSF) leaks (communication and leakage of fluid between the space surrounding the brain and the nose, throat, ear, or any other place in the head).
- People with cochlear implants.
- People who have recently taken influenza antiviral drugs. This depends on the specific influenza antiviral medication that was taken, and how recently the last dose was taken.
People who should talk to their health care provider before getting a flu shot:
If you have one of the following conditions, talk with your health care provider. He or she can help decide whether vaccination is right for you, and select the best vaccine for your situation:
- If you have an allergy to eggs or any of the ingredients in the vaccine. Talk to your doctor about your allergy.
- Most flu shots and the nasal spray flu vaccine are manufactured using egg-based technology. Because of this, they contain a small amount of egg proteins, such as ovalbumin. However, studies that have examined the use of both the nasal spray vaccine and flu shots in egg-allergic and non-egg-allergic patients indicate that severe allergic reactions in people with egg allergies are unlikely 43. A recent CDC study found the rate of anaphylaxis after all vaccines is 1.31 per one million vaccine doses given 43.
- People with egg allergies no longer need to be observed for an allergic reaction for 30 minutes after receiving a flu vaccine 43. People with a history of egg allergy of any severity should receive any licensed, recommended, and age-appropriate influenza vaccine. Those who have a history of severe allergic reaction to egg (i.e., any symptom other than hives) should be vaccinated in an inpatient or outpatient medical setting (including but not necessarily limited to hospitals, clinics, health departments, and physician offices), under the supervision of a health care provider who is able to recognize and manage severe allergic conditions.
- If you are someone who has more serious reactions to eating eggs or egg-containing foods, like angioedema, respiratory distress, lightheadedness, or recurrent emesis; or who required epinephrine or another emergency medical intervention, you can get any licensed flu vaccine that is otherwise appropriate for your age and health status, but the vaccine should be given in an inpatient or outpatient medical setting (including but not necessarily limited to hospitals, clinics, health departments, and physician offices), under the supervision of a health care provider who is able to recognize and manage severe allergic conditions 43.
- If you ever had Guillain-Barré Syndrome (a severe paralyzing illness, also called GBS). Some people with a history of GBS should not get a flu vaccine. Talk to your doctor about your GBS history.
- If you had a severe allergic reaction to a previous dose of any other flu vaccine, talk to your health care provider.
- If you are not feeling well, talk to your doctor about your symptoms.
People who should talk to their health care provider before getting a Nasal Spray Flu Vaccine:
If you have one of the following conditions, talk with your health care provider. He or she can help decide whether vaccination is right for you, and select the best vaccine for your situation:
- People with asthma 5 years and older.
- People with other underlying medical conditions that can put them at higher risk of developing serious flu complications. These include conditions such as chronic lung diseases, heart disease (except isolated hypertension), kidney disease, liver disorders, neurologic and neuromuscular disorders, blood disorders, or metabolic disorders (such as diabetes).
- People with moderate or severe acute illness with or without fever.
- People with Guillain-Barré Syndrome after a previous dose of influenza vaccine.
Table 2. Influenza vaccines for 2022–23 influenza season in United States
| Trade name (manufacturer) | Presentations | Age indication | µg HA (IIV4s and RIV4) or virus count (LAIV4) for each vaccine virus (per dose) | Route | Mercury (from thimerosal, if present), µg/0.5 mL |
|---|---|---|---|---|---|
| Inactivated quadrivalent influenza vaccine (standard-dose, egg-based vaccines†) | |||||
| Afluria Quadrivalent (Seqirus) | 0.5-mL prefilled syringe § | ≥3 yrs§ | 15 µg/0.5 mL | Intramuscular ¶ | —** |
| 5.0-mL multidose vial § | ≥6 mos§ (needle/syringe) 18 through 64 yrs (jet injector) | 7.5 µg/0.25 mL 15 µg/0.5 mL | Intramuscular ¶ | 24.5 | |
| Fluarix Quadrivalent (GlaxoSmithKline) | 0.5-mL prefilled syringe | ≥6 mos | 15 µg/0.5 mL | Intramuscular ¶ | — |
| FluLaval Quadrivalent (GlaxoSmithKline) | 0.5-mL prefilled syringe | ≥6 mos | 15 µg/0.5 mL | Intramuscular ¶ | — |
| Fluzone Quadrivalent (Sanofi Pasteur) | 0.5-mL prefilled syringe †† | ≥6 mos†† | 15 µg/0.5 mL | Intramuscular ¶ | — |
| 0.5-mL single-dose vial †† | ≥6 mos†† | 15 µg/0.5 mL | Intramuscular ¶ | — | |
| 5.0-mL multidose vial †† | ≥6 mos†† | 15 µg/0.5 mL 7.5 µg/0.25 mL | Intramuscular ¶ | 25 | |
| Cell culture-based inactivated quadrivalent influenza vaccine (standard-dose, cell culture–based vaccine) | |||||
| Flucelvax Quadrivalent (Seqirus) | 0.5-mL prefilled syringe | ≥6 mos | 15 µg/0.5 mL | Intramuscular ¶ | — |
| 5.0-mL multidose vial | ≥6 mos | 15 µg/0.5 mL | Intramuscular ¶ | 25 | |
| High-Dose inactivated quadrivalent influenza vaccine (high-dose, egg-based vaccine†) | |||||
| Fluzone High-Dose Quadrivalent (Sanofi Pasteur) | 0.7-mL prefilled syringe | ≥65 yrs | 60 µg/0.7 mL | Intramuscular ¶ | — |
| Adjuvanted inactivated quadrivalent influenza vaccine (standard-dose, egg-based† vaccine with MF59 adjuvant). MF59 is an oil-in-water emulsion of squalene oil. Squalene, a naturally occurring substance found in humans, animals, and plants, is highly purified for the vaccine manufacturing process. | |||||
| Fluad Quadrivalent (Seqirus) | 0.5-mL prefilled syringe | ≥65 yrs | 15 µg/0.5 mL | Intramuscular ¶ | — |
| Recombinant quadrivalent influenza vaccine (recombinant HA vaccine) | |||||
| Flublok Quadrivalent (Sanofi Pasteur) | 0.5-mL prefilled syringe | ≥18 yrs | 45 µg/0.5 mL | Intramuscular ¶ | — |
| Live attenuated quadrivalent influenza vaccine (egg-based vaccine†) | |||||
| FluMist Quadrivalent (AstraZeneca) | 0.2-mL prefilled single-use intranasal sprayer | 2 through 49 yrs | 106.5–7.5 fluorescent focus units/0.2 mL | Intranasal | — |
Footnotes:
* Vaccination providers should consult FDA-approved prescribing information for 2022–23 influenza vaccines for the most complete and updated information, including but not limited to indications, contraindications, warnings, and precautions. Package inserts for U.S.-licensed vaccines are available at https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
† Although a history of severe allergic reaction (e.g., anaphylaxis) to egg is a labeled contraindication to the use of egg-based inactivated influenza quadrivalent vaccines and live attenuated influenza quadrivalent vaccine, Advisory Committee on Immunization Practices (ACIP) recommends that persons with a history of egg allergy may receive any licensed, recommended influenza vaccine that is otherwise appropriate for their age and health status. Those who report having had reactions to egg involving symptoms other than urticaria (e.g., angioedema or swelling, respiratory distress, lightheadedness, or recurrent emesis) or who required epinephrine or another emergency medical intervention should be vaccinated in an inpatient or outpatient medical setting (including but not necessarily limited to hospitals, clinics, health departments, and physician offices) supervised by a health care provider who is able to recognize and manage severe allergic reactions, if a vaccine other than cell culture-based inactivated influenza quadrivalent vaccine or recombinant influenza quadrivalent vaccine is used.
§ The approved dose volume for Afluria Quadrivalent is 0.25 mL for children aged 6 through 35 months and 0.5 mL for persons aged ≥3 years. However, 0.25-mL prefilled syringes are not expected to be available for the 2022–23 season. For children aged 6 through 35 months, a 0.25-mL dose must be obtained from a multidose vial.
¶ Intramuscular-administered influenza vaccines should be given by needle and syringe only, with the exception of the multidose vial presentation of Afluria Quadrivalent, which may alternatively be given by the PharmaJet Stratis jet injector for persons aged 18 through 64 years only. For adults and older children, the recommended site for intramuscular influenza vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. Additional specific guidance regarding site selection and needle length for intramuscular administration is available in the ACIP General Best Practice Guidelines for Immunization, available at https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html.
** Not applicable.
†† Fluzone Quadrivalent is currently approved for ages 6 through 35 months at either 0.25 mL or 0.5 mL per dose; however, 0.25-mL prefilled syringes are not expected to be available for the 2022–23 influenza season. If a prefilled syringe of Fluzone Quadrivalent is used for a child in this age group, the dose volume will be 0.5 mL per dose.
[Source 44 ]Influenza Vaccine Composition for 2022–23 United States
Vaccine strains for the 2022–23 influenza vaccines were selected by the Food and Drug Administration’s Vaccines and Related Biologic Products Advisory Committee based on WHO’s recommended Northern Hemisphere 2022–23 influenza vaccine composition 45.
- For the 2022-2023 flu season, there are three flu vaccines that are preferentially recommended for people 65 years and older. These are Fluzone High-Dose Quadrivalent vaccine, Flublok Quadrivalent recombinant flu vaccine and Fluad Quadrivalent adjuvanted flu vaccine.
- The recommended timing of flu shot is similar to last season. For most people who need only one dose for the season, September and October are generally good times to get vaccinated. Vaccination in July and August is not recommended for most adults but can be considered for some groups. While ideally it’s recommended to get vaccinated by the end of October, it’s important to know that vaccination after October can still provide protection during the peak of flu season.
- The age indication for the cell culture-based inactivated flu vaccine, Flucelvax Quadrivalent (ccIIV4), changed from 2 years and older to 6 months and older.
- Pre-filled Afluria Quadrivalent flu shots for children are not expected to be available this season. However, children can receive this vaccine from a multidose vial at the recommended dose.
The recommendations for egg-based and cell-based and recombinant flu vaccines are listed below 46:
- Egg-based vaccine composition recommendations
- an A/Victoria/2570/2019 (H1N1) pdm09-like virus;
- an A/Darwin/9/2021 (H3N2)-like virus (updated);
- a B/Austria/1359417/2021-like virus (B/Victoria lineage) (updated);
- a B/Phuket/3073/2013-like virus (B/Yamagata lineage)
- Cell- or recombinant-based vaccine composition recommendations
- an A/Wisconsin/588/2019 (H1N1) pdm09-like virus;
- an A/Darwin/6/2021 (H3N2)-like virus (updated);
- a B/Austria/1359417/2021-like virus (B/Victoria lineage) (updated);
- a B/Phuket/3073/2013-like virus (B/Yamagata lineage).
This recommendation is the same as the Southern Hemisphere flu vaccine recommendation.
How flu shot works?
The injected flu vaccine stimulates your body’s immune system to make antibodies to attack the flu virus. Antibodies are proteins that recognize and fight off germs, such as viruses, that have invaded your blood. If you’re exposed to the flu virus after you’ve had the flu vaccine, your immune system will recognize the virus and immediately produce antibodies to fight it.
It may take 10 to 14 days for your immunity to build up fully after you have had the flu shot.
You need to have a flu shot every year, as the antibodies that protect you from flu decline over time, and flu strains can also change from year to year.
Flu vaccines cause antibodies to develop in the body about two weeks after vaccination. These antibodies provide protection against infection with the viruses that are in the vaccine.
The seasonal flu vaccine protects against the influenza viruses that research indicates will be most common during the upcoming season. Traditional flu vaccines (called “trivalent” vaccines) are made to protect against three flu viruses; an influenza A (H1N1) virus, an influenza A (H3N2) virus, and an influenza B virus. There are also flu vaccines made to protect against four flu viruses (called “quadrivalent” vaccines). These vaccines protect against the same viruses as the trivalent vaccine and an additional B virus.
What is in the flu shot?
As there are lots of different flu vaccines produced each year, for more detailed information on ingredients ask your doctor or nurse for the patient information leaflet for the specific vaccine being offered.
Flu shot ingredients: for US-licensed flu vaccines are available at the U.S. Food and Drug Administration https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm
Flu shot side effects
Like any medical product, vaccines can cause side effects. Side effects of the flu vaccine are generally mild and go away on their own within a few days.
Common side effects from the flu shot include:
- Soreness, redness, and/or swelling from the shot
- Headache
- Fever
- Nausea
- Muscle aches
The flu shot, like other injections, can occasionally cause fainting.
Signs of a severe allergic reaction can include:
- Difficulty breathing
- Hoarseness or wheezing
- Swelling around the eyes or lips
- Hives
- Paleness
- Weakness
- A fast heart beat or dizziness
Life threatening allergic reactions to the flu shot are rare. These signs would most likely happen within a few minutes to a few hours after the vaccine is given. If you think it is a severe allergic reaction or other emergency that can’t wait, call your local emergency services number and get to the nearest hospital. Otherwise, call your doctor.
Afterward, the reaction should be reported to the Vaccine Adverse Event Reporting System. Your doctor might file this report, or you can do it yourself through the Vaccine Adverse Event Reporting System website (https://vaers.hhs.gov/) or by calling 1-800-822-7967.
Some studies have found a possible small association of injectable flu vaccine with Guillain-Barré syndrome (GBS). Overall, these studies estimated the risk for GBS after vaccination as fewer than 1 or 2 cases of GBS per one million people vaccinated. Other studies have not found any association. Guillain-Barré syndrome (GBS) also, rarely, occurs after flu illness. Even though Guillain-Barré syndrome (GBS) following flu illness is rare, Guillain-Barré syndrome (GBS) is more common following flu illness than following flu vaccination. GBS has not been associated with the nasal spray vaccine.
Are there some people who should not receive a flu vaccine?
CDC recommends everyone 6 months of age and older should receive an annual flu vaccination with rare exceptions. Individuals who can’t get the flu shot include 47:
- Children younger than 6 months, since they are too young to get a flu shot.
- Individuals with severe, life-threatening allergies to flu vaccine or any ingredient(s) in the vaccine.
Individuals should talk with their doctor before getting the flu shot if they:
- Have had a severe allergy to eggs or any of the ingredients in the vaccine.
- See Special Considerations Regarding Egg Allergy for more information about egg allergies and flu vaccine.
- Have had Guillain-Barré syndrome (GBS).
- Are not feeling well.
There are multiple flu vaccines available, and not all flu vaccines can be given to people of all ages. Talk to your doctor if you have any questions regarding which flu vaccine options are best for you and your family.
Can egg protein in flu vaccine cause allergic reactions in persons with a history of egg allergy?
Yes, allergic reactions can happen, but they occur very rarely with the flu vaccines available in the United States today 43. Occasional cases of anaphylaxis, a severe life-threatening reaction that involves multiple organ systems and can progress rapidly, in egg-allergic persons have been reported to the Vaccine Adverse Event Reporting System (VAERS) after administration of flu vaccine. Flu vaccines contain various components that may cause allergic reactions, including anaphylaxis. In a Vaccine Safety Datalink study, there were 10 cases of anaphylaxis after more than 7.4 million doses of inactivated flu vaccine, trivalent (IIV3) given without other vaccines, (rate of 1.35 per one million doses) 43. Most of these cases of anaphylaxis were not related to the egg protein present in the vaccine. CDC and the Advisory Committee on Immunization Practices continue to review available data regarding anaphylaxis cases following flu vaccines.
How effective are flu vaccines?
The CDC conducts studies each year to determine how well influenza (flu) vaccines protect against flu. While vaccine effectiveness can vary, recent studies show that flu vaccination reduces the risk of flu illness by between 40% and 60% among the overall population during seasons when most circulating flu viruses are well-matched to those used to make flu vaccines 48. In general, current flu vaccines tend to work better against influenza B and influenza A(H1N1) viruses and offer lower protection against influenza A(H3N2) viruses 48.
When should I get a flu shot?
You can get the flu shot each autumn when it becomes available, usually sometime in August through November.
- Optimally, vaccination should occur before onset of influenza activity in the community.
- Vaccination should be offered by end of October, if possible.
- Vaccination should be offered as long as influenza viruses are circulating and unexpired vaccine is available.
- Children aged 6 months through 8 years who require 2 doses should receive their first dose as soon as possible after vaccine becomes available, and the second dose ≥4 weeks later.
However, if flu shots are still available and you haven’t yet received a vaccination, you can still benefit from getting a flu shot in January or later. That’s because the flu season doesn’t usually peak until the winter.
Where to get flu shot?
Flu vaccines are offered in many locations, including doctor’s offices, clinics, health departments, pharmacies and college health centers, as well as by many employers, and even in some schools.
Even if you don’t have a regular doctor or nurse, you can get a flu vaccine somewhere else, like a health department, pharmacy, urgent care clinic, and often your school, college health center, or workplace.
The following Vaccine Locator (https://vaccinefinder.org/) is a useful tool for finding vaccine in your area.
How long does the flu shot last?
You need to have a flu shot every year, as the antibodies that protect you from flu decline over time, and flu strains can also change from year to year.
Why do I need a flu vaccine every year?
A flu vaccine is needed every season for two reasons. First, the body’s immune response from vaccination declines over time, so an annual vaccine is needed for optimal protection. Second, because flu viruses are constantly changing, the formulation of the flu vaccine is reviewed each year and sometimes updated to keep up with changing flu viruses. For the best protection, everyone 6 months and older should get vaccinated annually.
Can I get seasonal flu even though I got a flu vaccine this year?
Yes. There is still a possibility you could get the flu even if you got vaccinated. The ability of flu vaccine to protect a person depends on various factors, including the age and health status of the person being vaccinated, and also the similarity or “match” between the viruses used to make the vaccine and those circulating in the community. If the viruses in the vaccine and the influenza viruses circulating in the community are closely matched, vaccine effectiveness is higher. If they are not closely matched, vaccine effectiveness can be reduced. However, it’s important to remember that even when the viruses are not closely matched, the vaccine can still protect many people and prevent flu-related complications. Such protection is possible because antibodies made in response to the vaccine can provide some protection (called cross-protection) against different but related influenza viruses.
Does flu vaccine work right away?
No. It takes about two weeks after vaccination for antibodies to develop in the body and provide protection against influenza virus infection. That’s why it’s better to get vaccinated early in the fall, before the flu season really gets under way.
Can I get the flu from the flu vaccine?
No, the flu vaccine cannot cause flu. The vaccines either contain inactivated virus, meaning the viruses are no longer infectious, or a particle designed to look like a flu virus to your immune system. While the nasal spray flu vaccine does contain a live virus, the viruses are changed so that they cannot give you the flu.
Influenza diagnosis
Your doctor will conduct a physical exam, look for signs and symptoms of flu, and possibly order a test that detects influenza viruses. During times when flu is widespread, you may not need to be tested for it. Your health care provider may diagnose you based on your symptoms.
In some cases, your health care provider may suggest that you be tested for influenza. Your doctor may use many tests to diagnose flu. The most common are called “rapid influenza diagnostic tests (RIDTs).” Rapid influenza diagnostic tests work by detecting the parts of the virus (antigens) that stimulate an immune response. The rapid influenza diagnostic tests (RIDTs) can provide results within approximately 10-15 minutes but may not be as accurate as other flu tests. Therefore, you could still have flu, even though your rapid test result is negative. Other flu tests called “rapid molecular assays” detect genetic material of the flu virus. Rapid molecular assays produce results in 15-20 minutes and are more accurate than rapid influenza diagnostic tests (RIDTs).
In addition to rapid influenza diagnostic tests (RIDTs) and rapid molecular assays, there are several more accurate flu tests available that must be performed in specialized laboratories, such as hospital and public health laboratories. These tests include reverse transcription polymerase chain reaction (RT-PCR), viral culture, and immunofluorescence assays. All of these tests require that a health care provider swipe the inside of your nose or the back of your throat with a swab and then send the swab for testing. Results may take one to several hours. The reverse transcription polymerase chain reaction (RT-PCR) testing is more sensitive than other tests and may be able to identify the influenza strain.
Influenza treatment
Usually, you’ll need nothing more than rest and plenty of fluids to treat the flu. But if you have a severe infection or are at higher risk of complications, your health care provider may prescribe an antiviral medication to treat the flu. These drugs can include oseltamivir (Tamiflu), zanamivir (Relenza), peramivir (Rapivab) or baloxavir (Xofluza). These medications may shorten your illness by a day or so and help prevent serious complications.
- Oseltamivir (Tamiflu). Oseltamivir (Tamiflu) for oral administration is FDA-approved for early treatment of uncomplicated influenza in people two weeks and older, and for chemoprophylaxis to prevent influenza in people one year and older. Although not part of the FDA-approved indications, use of oral oseltamivir for treatment of influenza in infants younger than 14 days old, and for chemoprophylaxis in infants 3 months to 1 year, is recommended by CDC and the American Academy of Pediatrics. If a child is younger than 3 months old, use of oseltamivir for chemoprophylaxis is not recommended unless the situation is judged critical, due to limited data in this age group.
- Zanamivir (Relenza). Zanamivir (Relenza) for oral inhalation through a device similar to an asthma inhaler is FDA-approved for early treatment of uncomplicated influenza in people 7 years and older and to prevent influenza in people 5 years and older. It is not recommended for use in people with underlying respiratory disease, including people with and lung disease.
- Peramivir (Rapivab). Peramivir (Rapivab) for intravenous administration is FDA-approved for early treatment of uncomplicated influenza in people 6 months and older.
- Baloxavir (Xofluza). Baloxavir marboxil (Xofluza) for oral administration is FDA-approved for early treatment of uncomplicated influenza in otherwise healthy non-high risk people 5 years to less than 12 years and for all persons 12 years and older, and for post-exposure prophylaxis of influenza in people 5 years and older. Baloxavir is not recommended for pregnant women, immunocompromised persons, breastfeeding mothers, outpatients with complicated or progressive illness, or hospitalized patients.
Antiviral medication side effects may include nausea and vomiting. These side effects may be lessened if the medication is taken with food.
If you get sick with flu, influenza antiviral drugs may be a treatment option. Antiviral drugs work best when started early, such as one to two days after your flu symptoms begin.
Check with your doctor promptly if you are at higher risk of serious flu complications and you get flu symptoms. People at higher risk of flu complications include young children, adults 65 years of age and older, pregnant people, and people with certain medical conditions such as asthma, diabetes and heart disease.
When treatment is started within 1-2 days after flu symptoms begin, influenza antiviral drugs can lessen symptoms and shorten the time you are sick by 1 or 2 days. They might also prevent some flu complications, like pneumonia. For people at higher risk of serious flu complications, treatment with influenza antiviral drugs can mean the difference between milder or more serious illness possibly resulting in a hospital stay.
Flu home remedies
If you do come down with the flu, these measures may help ease your symptoms:
- Drink plenty of liquids. Choose water, juice and warm soups to prevent dehydration.
- Rest. Get more sleep to help your immune system fight infection. You may need to change your activity level, depending on your symptoms.
- Consider pain relievers. Use acetaminophen (Tylenol, others) or ibuprofen (Advil, Motrin IB, others), to combat the achiness associated with influenza. Children and teens recovering from flu-like symptoms should never take aspirin because of the risk of Reye’s syndrome, a rare but potentially fatal condition.
To help control the spread of influenza in your community, stay home and keep sick children home until the fever has been gone for 24 hours. Avoid being around other people until you’re feeling better, unless you’re getting medical care. If you do need to leave your home and get medical care, wear a face mask. Wash your hands often.
References- Types of Influenza Viruses. https://www.cdc.gov/flu/about/viruses/types.htm
- Flu Season. https://www.cdc.gov/flu/about/season/flu-season.htm
- Weekly U.S. Influenza Surveillance Report. Updated January 27, 2023. https://www.cdc.gov/flu/weekly/index.htm
- A revision of the system of nomenclature for influenza viruses: a WHO Memorandum. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2395936/pdf/bullwho00427-0070.pdf
- Jester B, Uyeki TM, Jernigan DB, Tumpey TM. Historical and clinical aspects of the 1918 H1N1 pandemic in the United States. Virology. 2019 Jan 15;527:32-37. doi: 10.1016/j.virol.2018.10.019
- Jester B, Uyeki T, Jernigan D. Readiness for Responding to a Severe Pandemic 100 Years After 1918. Am J Epidemiol. 2018 Dec 1;187(12):2596-2602. doi: 10.1093/aje/kwy165
- Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918-1920 “Spanish” influenza pandemic. Bull Hist Med. 2002 Spring;76(1):105-15. doi: 10.1353/bhm.2002.0022
- W. Paul Glezen, Emerging Infections: Pandemic Influenza, Epidemiologic Reviews, Volume 18, Issue 1, 1996, Pages 64–76, https://doi.org/10.1093/oxfordjournals.epirev.a017917
- Worobey M, Han GZ, Rambaut A. Genesis and pathogenesis of the 1918 pandemic H1N1 influenza A virus. Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):8107-12. doi: 10.1073/pnas.1324197111
- Smith GJ, Bahl J, Vijaykrishna D, Zhang J, Poon LL, Chen H, Webster RG, Peiris JS, Guan Y. Dating the emergence of pandemic influenza viruses. Proc Natl Acad Sci U S A. 2009 Jul 14;106(28):11709-12. doi: 10.1073/pnas.0904991106
- Brüssow H. The beginning and ending of a respiratory viral pandemic-lessons from the Spanish flu. Microb Biotechnol. 2022 May;15(5):1301-1317. doi: 10.1111/1751-7915.14053
- Hannoun C. La grippe et ses virus Presses Universitatires de France; París: 1995.
- Eiros JM, Bachiller MR, Pérez A. La gripe de 1918. Centenario de una crisis sanitaria devastadora. Ed. Seqirus 2018.
- Past Examples of Possible Limited, Non-Sustained Person-to-Person Spread of Bird Flu. https://www.cdc.gov/flu/avianflu/h5n1-human-infections.htm
- Shortridge KF, Zhou NN, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti KG, Norwood M, Senne D, Sims L, Takada A, Webster RG. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998 Dec 20;252(2):331-42. doi: 10.1006/viro.1998.9488
- Carolyn Buxton Bridges, Jacqueline M. Katz, Wing Hong Seto, et al. Risk of Influenza A (H5N1) Infection among Health Care Workers Exposed to Patients with Influenza A (H5N1), Hong Kong, The Journal of Infectious Diseases, Volume 181, Issue 1, January 2000, Pages 344–348, https://doi.org/10.1086/315213
- Du Ry van Beest Holle M, Meijer A, Koopmans M, de Jager C M. Human-to-human transmission of avian influenza A/H7N7, The Netherlands, 2003. Euro Surveill. 2005;10(12):pii=584. https://doi.org/10.2807/esm.10.12.00584-en
- Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, Uiprasertkul M, Boonnak K, Pittayawonganon C, Cox NJ, Zaki SR, Thawatsupha P, Chittaganpitch M, Khontong R, Simmerman JM, Chunsutthiwat S. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med. 2005 Jan 27;352(4):333-40. doi: 10.1056/NEJMoa044021
- Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen, Hadisoedarsuno W, Purba W, Santoso H, Septiawati C, Tresnaningsih E, Heriyanto B, Yuwono D, Harun S, Soeroso S, Giriputra S, Blair PJ, Jeremijenko A, Kosasih H, Putnam SD, Samaan G, Silitonga M, Chan KH, Poon LL, Lim W, Klimov A, Lindstrom S, Guan Y, Donis R, Katz J, Cox N, Peiris M, Uyeki TM. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006 Nov 23;355(21):2186-94. doi: 10.1056/NEJMoa060930
- Avian influenza – situation in Indonesia – update 16. https://www.who.int/emergencies/disease-outbreak-news/item/2006_05_31-en
- Wang H, Feng Z, Shu Y, Yu H, Zhou L, Zu R, Huai Y, Dong J, Bao C, Wen L, Wang H, Yang P, Zhao W, Dong L, Zhou M, Liao Q, Yang H, Wang M, Lu X, Shi Z, Wang W, Gu L, Zhu F, Li Q, Yin W, Yang W, Li D, Uyeki TM, Wang Y. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 2008 Apr 26;371(9622):1427-34. doi: 10.1016/S0140-6736(08)60493-6
- WHO, Weekly Epidemiological Record, 2008. Human cases of avian influenza A (H5N1) in North-West Frontier Province, Pakistan, October-November 2007. Wkly Epidemiol Rec. 2008 Oct 3;83(40):359-64. English, French.
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013 May 16;368(20):1888-97. doi: 10.1056/NEJMoa1304459
- Xiang N, Li X, Ren R, et al. Assessing Change in Avian Influenza A(H7N9) Virus Infections During the Fourth Epidemic — China, September 2015–August 2016. MMWR Morb Mortal Wkly Rep 2016;65:1390–1394. DOI: http://dx.doi.org/10.15585/mmwr.mm6549a2
- Chen H, Liu S, Liu J, et al. Nosocomial Co-Transmission of Avian Influenza A(H7N9) and A(H1N1)pdm09 Viruses between 2 Patients with Hematologic Disorders. Emerg Infect Dis. 2016 Apr;22(4):598-607. doi: 10.3201/eid2204.151561
- Interim Guidance on the Use of Antiviral Medications for Treatment of Human Infections with Novel Influenza A Viruses Associated with Severe Human Disease. https://www.cdc.gov/flu/avianflu/novel-av-treatment-guidance.htm
- Kumar D, Ison MG, Mira JP, Welte T, Hwan Ha J, Hui DS, Zhong N, Saito T, Katugampola L, Collinson N, Williams S, Wildum S, Ackrill A, Clinch B, Lee N. Combining baloxavir marboxil with standard-of-care neuraminidase inhibitor in patients hospitalised with severe influenza (FLAGSTONE): a randomised, parallel-group, double-blind, placebo-controlled, superiority trial. Lancet Infect Dis. 2022 May;22(5):718-730. doi: 10.1016/S1473-3099(21)00469-2
- Chen Y, Liang W, Yang S, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013 Jun 1;381(9881):1916-25. doi: 10.1016/S0140-6736(13)60903-4
- Liang Yu, Zhaoming Wang, Yu Chen, et al. Clinical, Virological, and Histopathological Manifestations of Fatal Human Infections by Avian Influenza A(H7N9) Virus, Clinical Infectious Diseases, Volume 57, Issue 10, 15 November 2013, Pages 1449–1457, https://doi.org/10.1093/cid/cit541
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza AV, Abdel-Ghafar AN, Chotpitayasunondh T, et al. Update on avian influenza A (H5N1) virus infection in humans. The New England journal of medicine 2008; 358(3): 261-73.
- Ariano RE, Sitar DS, Zelenitsky SA, Zarychanski R, Pisipati A, Ahern S, Kanji S, Rello J, Kumar A. Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. CMAJ. 2010 Mar 9;182(4):357-63. doi: 10.1503/cmaj.092127
- N. Lee, D. S. C. Hui, Z. Zuo, K. L. K. Ngai, G. C. Y. Lui, S. K. Wo, W. W. S. Tam, M. C. W. Chan, B. C. K. Wong, R. Y. K. Wong, K. W. Choi, W. W. Y. Sin, E. L. Y. Lee, B. Tomlinson, F. G. Hayden, P. K. S. Chan, A Prospective Intervention Study on Higher-Dose Oseltamivir Treatment in Adults Hospitalized With Influenza A and B Infections, Clinical Infectious Diseases, Volume 57, Issue 11, 1 December 2013, Pages 1511–1519, https://doi.org/10.1093/cid/cit597
- South East Asia Infectious Disease Clinical Research Network. Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial. BMJ. 2013 May 30;346:f3039. doi: 10.1136/bmj.f3039
- Jittamala P, Pukrittayakamee S, Tarning J, Lindegardh N, Hanpithakpong W, Taylor WR, Lawpoolsri S, Charunwattana P, Panapipat S, White NJ, Day NP. Pharmacokinetics of orally administered oseltamivir in healthy obese and nonobese Thai subjects. Antimicrob Agents Chemother. 2014;58(3):1615-21. doi: 10.1128/AAC.01786-13
- Pai MP, Lodise TP Jr. Oseltamivir and oseltamivir carboxylate pharmacokinetics in obese adults: dose modification for weight is not necessary. Antimicrob Agents Chemother. 2011 Dec;55(12):5640-5. doi: 10.1128/AAC.00422-11
- Mulla H, Peek GJ, Harvey C, Westrope C, Kidy Z, Ramaiah R. Oseltamivir pharmacokinetics in critically ill adults receiving extracorporeal membrane oxygenation support. Anaesth Intensive Care. 2013 Jan;41(1):66-73. doi: 10.1177/0310057X1304100112
- Eyler RF, Heung M, Pleva M, Sowinski KM, Park PK, Napolitano LM, Mueller BA. Pharmacokinetics of oseltamivir and oseltamivir carboxylate in critically ill patients receiving continuous venovenous hemodialysis and/or extracorporeal membrane oxygenation. Pharmacotherapy. 2012 Dec;32(12):1061-9. doi: 10.1002/phar.1151
- Whitley R, Laughlin A, Carson S, Mitha E, Tellier G, Stich M, Elder J, Alexander WJ, Dobo S, Collis P, Sheridan WP. Single dose peramivir for the treatment of acute seasonal influenza: integrated analysis of efficacy and safety from two placebo-controlled trials. Antivir Ther. 2015;20(7):709-19. doi: 10.3851/IMP2874
- Menno D. de Jong, Michael G. Ison, Arnold S. Monto, Hristo Metev, Carol Clark, Brian O’Neil, Jenna Elder, Amy McCullough, Phil Collis, William P. Sheridan, Evaluation of Intravenous Peramivir for Treatment of Influenza in Hospitalized Patients, Clinical Infectious Diseases, Volume 59, Issue 12, 15 December 2014, Pages e172–e185, https://doi.org/10.1093/cid/ciu632
- Baek YH, Song MS, Lee EY, Kim YI, Kim EH, Park SJ, Park KJ, Kwon HI, Pascua PN, Lim GJ, Kim S, Yoon SW, Kim MH, Webby RJ, Choi YK. Profiling and characterization of influenza virus N1 strains potentially resistant to multiple neuraminidase inhibitors. J Virol. 2015 Jan;89(1):287-99. doi: 10.1128/JVI.02485-14
- Hai R, Schmolke M, Leyva-Grado VH, Thangavel RR, Margine I, Jaffe EL, Krammer F, Solórzano A, García-Sastre A, Palese P, Bouvier NM. Influenza A(H7N9) virus gains neuraminidase inhibitor resistance without loss of in vivo virulence or transmissibility. Nat Commun. 2013;4:2854. doi: 10.1038/ncomms3854
- Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, Miller MA. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009 Aug 13;361(7):674-9. doi: 10.1056/NEJMoa0904023
- Flu Vaccine and People with Egg Allergies. https://www.cdc.gov/flu/prevent/egg-allergies.htm
- TABLE. Influenza vaccines — United States, 2022–23 influenza season*. https://www.cdc.gov/flu/professionals/acip/2022-2023/acip-table.htm
- Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza Activity and Composition of the 2022–23 Influenza Vaccine — United States, 2021–22 Season. MMWR Morb Mortal Wkly Rep 2022;71:913–919. DOI: http://dx.doi.org/10.15585/mmwr.mm7129a1
- Frequently Asked Influenza (Flu) Questions: 2022-2023 Season. https://www.cdc.gov/flu/season/faq-flu-season-2022-2023.htm
- Flu Vaccine Safety Information. https://www.cdc.gov/flu/prevent/general.htm
- Vaccine Effectiveness: How Well Do Flu Vaccines Work? https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm