Intrauterine growth restriction
Intrauterine growth restriction also called IUGR, fetal growth restriction or Small-for-Gestational-Age (SGA), has been defined in several ways, but in general IUGR describes a condition in which the fetus exhibits poor growth in utero where the fetal weight is below the 10th percentile or less than 2 standard deviation for that population reference for gestational age as determined through an ultrasound 1, 2, 3, 4. This means that a baby with intrauterine growth restriction (IUGR) is smaller than 90% other babies of the same gestational age. Intrauterine growth restriction (IUGR) occurs when a baby does not grow well in the mother’s uterus (womb) because of problems with the placenta, the mother’s health, or birth defects. IUGR occurs when the fetus does not receive the necessary nutrients and oxygen needed for proper growth and development of organs and tissues. IUGR can begin at any time in pregnancy. Early-onset IUGR is often due to chromosomal abnormalities, maternal disease, or severe problems with the placenta. Late-onset growth restriction (after 32 weeks) is usually related to other problems.
Intrauterine growth restriction (IUGR) affects approximately 10% of pregnancies worldwide and is a leading cause of perinatal mortality and morbidity 5, 6, 7. Intrauterine growth restriction (IUGR) affects nearly 30%–50% of extremely preterm neonates 8.
Babies with intrauterine growth restriction (IUGR) may be born early or full-term; premature babies (born before 37 weeks of pregnancy) with IUGR may be very small and physically immature, and full-term (37 to 41 weeks) babies with IUGR may be physically mature but weak. Infants born after undiagnosed IUGR have 2–9 times higher risk of perinatal death and severe neurological complications to those diagnosed prenatally 9, 10.
Intrauterine growth restriction (IUGR) babies may appear physically and neurologically mature but are smaller than other babies of the same gestational age. Intrauterine growth restriction (IUGR) babies may be proportionately small (equally small all over) or they may be of normal length and size but have lower weight and body mass.
One of the most important things when diagnosing IUGR is to ensure accurate dating of the pregnancy. Gestational age can be calculated by using the first day of your last menstrual period (LMP) and also by early ultrasound calculations. Once gestational age has been established, the following methods can be used to diagnose IUGR:
- A fundal height that does not coincide with gestational age
- Measurements calculated in ultrasound are smaller than would be expected for the gestational age
- Abnormal findings discovered by a Doppler ultrasound
Despite new research, the optimal treatment for intrauterine growth restriction (IUGR) remains problematic. Up till now, no effective methods are available to reverse the pathological intrauterine condition or to accelerate the growth of fetuses in pregnancies complicated by IUGR 11. Most likely the treatment will depend on how far along you are in your pregnancy.
- If gestational age is 34 weeks or greater, health care providers may recommend being induced for early delivery.
- If gestational age is less than 34 weeks, health care providers will continue monitoring until 34 weeks or beyond. Fetal well-being and the amount of amniotic fluid will be monitored during this time.
- If either of these becomes a concern, then immediate delivery may be recommended. Depending on your health care provider, you will likely have appointments every 2 to 6 weeks until you deliver. If delivery is suggested prior to 34 weeks, your health care provider may perform an amniocentesis to help evaluate fetal lung maturity.
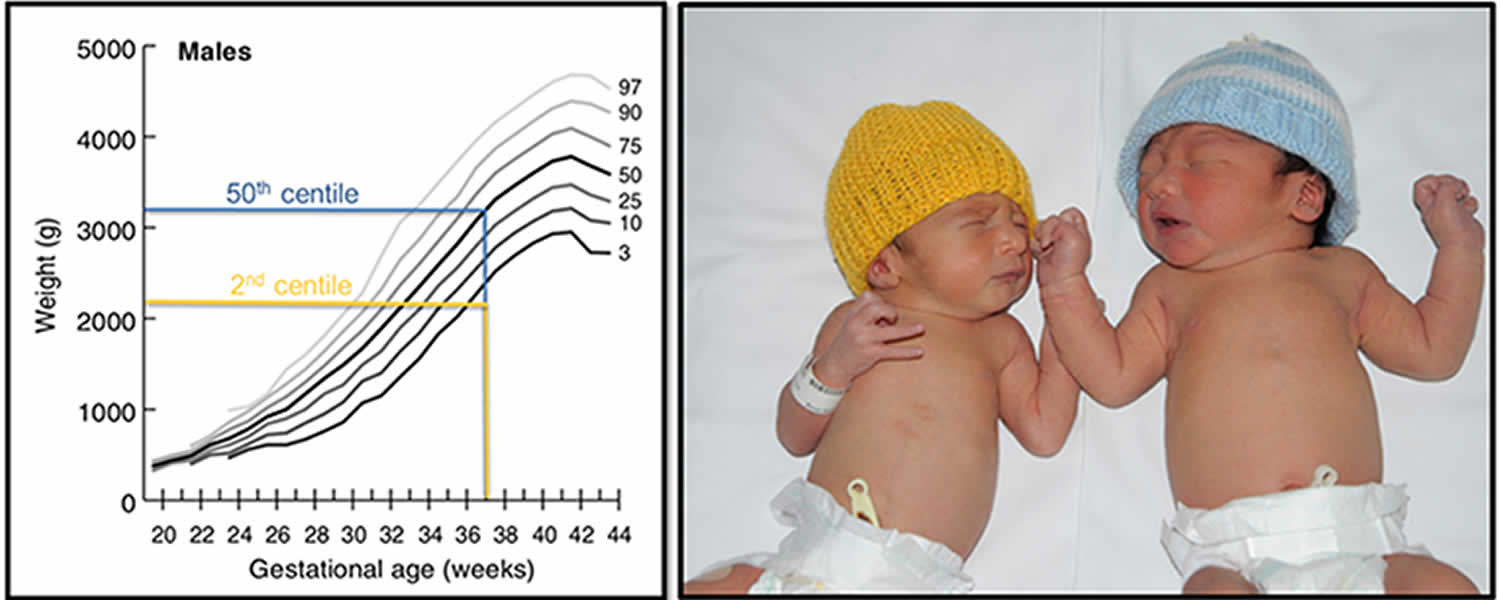
Figure 1. Intrauterine growth restriction (IUGR)
Footnote: Example of a IUGR (2nd centile weight for age, yellow), and an appropriately grown (50th centile weight for age, blue) infant born at 37 weeks gestation.
[Source 12 ]What are the risks to a baby born with IUGR?
- Increased risk for cesarean delivery
- Increased risk for hypoxia (lack of oxygen when the baby is born)
- Increased risk for meconium aspiration, which is when the baby swallows part of the first bowel movement. This can cause the alveoli to be over distended, a pneumothorax to occur, and/or the baby can develop bacterial pneumonia.
- Hypoglycemia (low blood sugar)
- Polycythemia (increased number of red blood cells)
- Hyperviscosity (decreased blood flow due to an increased number of red blood cells)
- Increased risk for motor and neurological disabilities
Types of intrauterine growth restriction (IUGR)
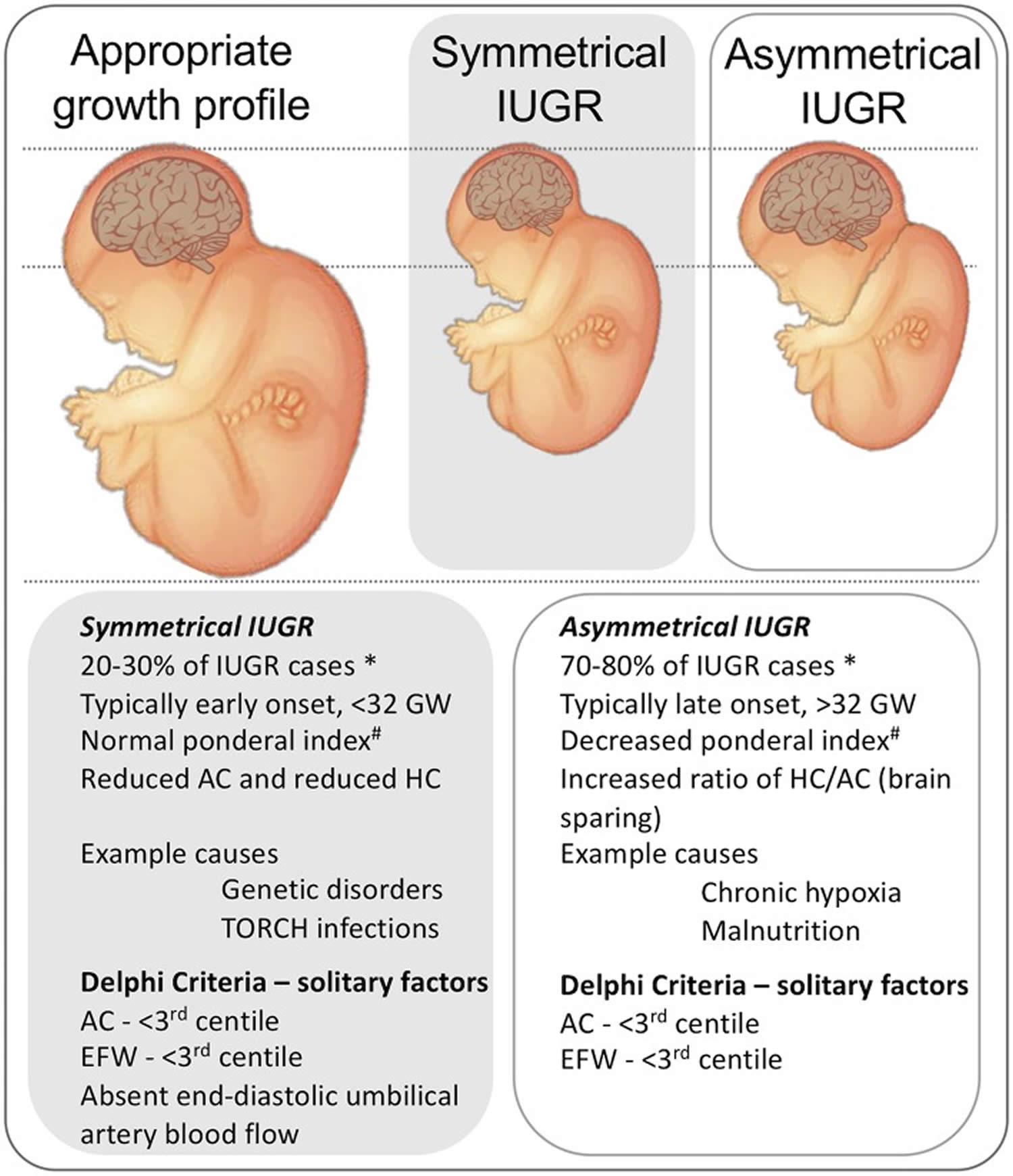
There are two different types of IUGR (Figure 2) 13, 14:
- Symmetric or primary IUGR is characterized by all internal organs being reduced in size. Symmetric IUGR accounts for 20% to 25% of all cases of IUGR. Symmetric IUGR is usually early onset with intrinsic genetic disorder or infections to fetus as the main causal factors. Ponderal index is normal, whereas biparietal diameter (BPD), femur length (FL), head and abdominal circumference (HC and AC) are proportionally reduced. Prognosis is poor.
- Asymmetric or secondary IUGR is characterized by the head and brain being normal in size, but the abdomen is smaller. Asymmetric IUGR incidence is 70–80%. Typically this is not evident until the third trimester. Ponderal index is low (< 3), whereas biparietal diameter (BPD), head circumference (HC) and femur length (FL) are normal. Abdominal circumference is decreased. Brain sparing growth is there, with advancing pregnancy, and features of malnutrition are exaggerated. Prognosis is better.
- Mixed IUGR. Initially symmetric but later on asymmetric. Etiology is mixed.
Footnotes:
- Ponderal index is an anthropometric measure of body mass index, defined as person’s weight in kilograms divided by the cube of height in meters (mass/height³). This is regarded as less satisfactory than the body mass index (BMI) where person’s weight in kilograms divided by the square of height in meters (mass/height²)
- Biparietal diameter (BPD) is one of the basic biometric parameters used to assess fetal size.
- Biparietal diameter (BPD) together with head circumference (HC), abdominal circumference (AC), and femur length (FL) are computed to produce an estimate of fetal weight. In the second trimester this may be extrapolated to an estimate of gestational age and an estimated due date (EDD).
- Head circumference (HC) is one of the basic biometric parameters used to assess fetal size. Head circumference (HC) together with biparietal diameter (BPD), abdominal circumference (AC), and femur length (FL) are computed to produce an estimate of fetal weight. In the second trimester, this may be extrapolated to an estimate of gestational age and an estimated due date (EDD).
Figure 2. Types of intrauterine growth restriction
Footnotes: Representation of the physical presentation of symmetrical and asymmetrical IUGR and a short list of clinical characteristics and causes. * note that incidence data are from high-resource settings. A third phenotype is proposed in low-resource settings, that includes characteristics of malnutrition and late gestation placental insufficiency 15, not shown. Delphi criteria from Gordijn et al. 16.
# Ponderal index is an anthropometric measure of body mass index, defined as person’s weight in kilograms divided by the cube of height in meters (mass/height³). This is regarded as less satisfactory than the body mass index (BMI) where person’s weight in kilograms divided by the square of height in meters (mass/height²)
Abbreviations: HC = head circumference; AC = Abdominal circumference; GW = gestational weeks; AC = abdominal circumference; EFW = Estimated fetal weight.
[Source 17 ]Asymmetrical intrauterine growth restriction
Asymmetric IUGR is the commonest IUGR with an incidence of 70–80%. Asymmetrical intrauterine growth restriction is a type of intrauterine growth restriction (IUGR) is characterized by the head and brain being normal in size due to the result of “brain sparing,” a process whereby brain growth is less affected than body growth due to the redistribution of cardiac output, but the abdomen is smaller. While brain sparing does not completely prevent the damaging effects of IUGR on brain development 18, 19, it is nonetheless associated with better neurological outcomes than when brain sparing does not occur 20. Typically asymmetric IUGR is not evident until the third trimester. Ponderal index is low (< 3), whereas biparietal diameter (BPD), head circumference (HC) and femur length (FL) are normal. Abdominal circumference is decreased. Brain sparing growth is there, with advancing pregnancy, and features of malnutrition are exaggerated. Prognosis is better.
Classically, in asymmetrical intrauterine growth restriction, there is relative preservation of the fetal brain (fetal head sparing theory), which is pathologically characterized by an increased brain-to-liver ratio (BLR) 21. This can also result in decreased fetal subcutaneous fat 22, 23. A rare paradoxical situation is with maternal cocaine use, where the head circumference (HC) is reduced out of proportion to other biometric parameters 24.
Brain sparing can be detected prenatally based on the Doppler pulsatility index (PI) in the middle cerebral artery (MCA); PI is reduced by the decreased cerebral resistance which allows a greater fraction of the cardiac output to perfuse the brain. However, this compensatory process of blood distribution can become “decompensatory” because the increase of brain blood flow and blood volume themselves become damaging 25. Understanding when and how the alteration of relative cerebral blood flow is switched from a compensatory to decompensatory response is clearly important for devising the most appropriate interventions and therapies. It is worth noting that brain sparing does not reduce the consequences of IUGR on later health, such as increased adiposity, diabetes, and cardiovascular risks, 26, 27.
Asymmetric IUGR causes
- Placental insufficiency: one of the commonest causes of asymmetrical IUGR
- Pre-eclampsia 23
Associations
- The incidence of concurrent karyotypic abnormalities is low or minimal, especially if asymmetrical IUGR is detected late in pregnancy 28.
- Syndromes that can give an asymmetrical IUGR picture include: Russell-Silver syndrome
Symmetrical intrauterine growth restriction
Symmetrical or primary IUGR is characterized by all internal organs being reduced in size. Both length and weight parameters are reduced. Symmetric IUGR accounts for 20% to 25% of all cases of IUGR. As a general rule, fetuses with symmetrical IUGR pattern may present at an earlier stage in gestation compared with the asymmetrical IUGR pattern. Symmetric IUGR is usually caused intrinsic genetic disorder or infections to fetus. It is also worth noting that mortality is higher in IUGR with symmetric growth even after adjusting for possible cofounding factors 18.
Symmetrical IUGR causes:
- Aneuploidic syndromes
- Triploidy. Triploidy is a rare lethal chromosomal (aneupliodic) abnormality caused by the presence of an entire extra chromosomal set.
- Trisomy 13
- Trisomy 18
- Fetal infections, e.g. toxoplasmosis, rubella cytomegalovirus (CMV), herpes simplex (HSV), and human immunodeficiency virus (HIV) [TORCH] 29
- Other
Ponderal index is normal, whereas biparietal diameter (BPD), femur length (FL), head and abdominal circumference (HC and AC) are proportionally reduced. Prognosis is poor.
Intrauterine growth restriction causes
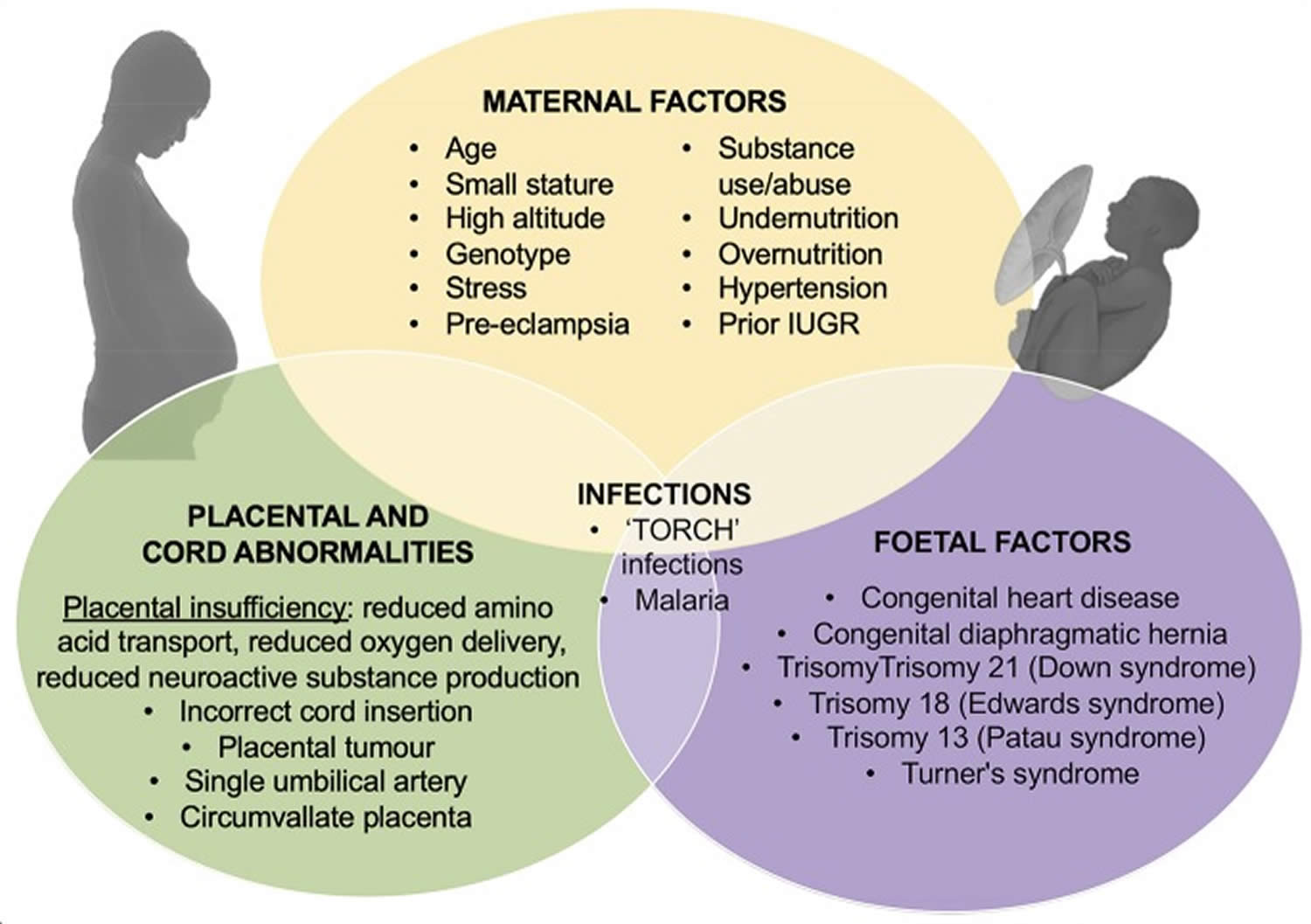
The primary cause of IUGR is widely considered to be placental insufficiency; i.e., inability of the placenta to adequately support fetal growth. However, the causes of placental insufficiency are many and over-lapping, and include constricted spiral arteries and increased coagulation leading to fetal hypoxia (as in maternal hypertension), and inappropriate substrate availability due to maternal under-nutrition or over-nutrition (see Figure 3) 31, 32. The leading causes of IUGR are maternal malnutrition and chronic maternal diseases, followed by placental insufficiency due to vascular or circulatory placental damage 33. Hypoxia, infections during pregnancy, fetal malformations, smoking, pollution, and chronic alcoholism are also causative factors, though less common 34, 35. It is thought that 40% of birth weight is ascribable to genetic factors and that the remaining 60% is due to fetal environmental exposures 1. Therefore, it is clear that poor macronutrient provision modified by micronutrient supply to the fetus is a critical component in the pathogenesis of IUGR. Nutrient provision is largely dependent on availability of nutrients to the fetus via maternal circulation, and as facilitated by placental transport 36.
Some factors that may contribute to intrauterine growth restriction (IUGR) include the following
Maternal factors that may contribute to IUGR include the following:
- High blood pressure
- Preeclampsia
- Chronic kidney disease
- Advanced diabetes
- Heart or lung disease
- Malnutrition, anemia
- Sickle cell anemia and other hemoglobinopathies
- Autoimmune diseases: Antiphospholipid syndrome (APS), thrombophilia of pregnancy, connective tissue disorder
- Infection
- Substance use (alcohol, drugs). Fetal alcohol syndrome
- Cigarette smoking
Factors involving the uterus and placenta that may contribute to IUGR include the following:
- Decreased blood flow in the uterus and placenta (abnormal uteroplacental circulation)
- Placental abruption (placenta detaches from the uterus). Chronic low-grade abruption leading to multiple subchorionic hemorrhages, abruption, infarction, calcifications, villitis, chronic villitis of unknown etiology, hemorrhagic endovasculitis, avascular villi, syncytial knots
- Placenta previa (placenta attaches low in the uterus)
- Congenital infection, including: CMV, rubella, toxoplasmosis, malaria, congenital HIV infection, syphilis
- Weight of placenta (weight < 350 g at term)
- Abnormalities in trophoblastic invasion: preeclampsia and placenta accreta
- Tumors: chorioangiomas, placental hemangiomas, abnormal umbilical cord or abnormal umbilical cord insertion
- Placental genes: over-expression of IGFBP-3 and placenta IGF-2 and under-expression of placental IGF1 and epidermal growth factor (EGF) 37
Factors related to the developing baby (fetus):
- Multiple gestation (for example, twins or triplets)
- Intrauterine infections 38
- TORCH group:
- in utero toxoplasmosis infection / congenital toxoplasmosis infection:
- congenital cerebral toxoplasmosis
- in utero rubella infection
- in utero cytomegalovirus infection: most common in utero infection
- in utero herpes simplex virus (HSV) infection
- in utero toxoplasmosis infection / congenital toxoplasmosis infection:
- in utero syphilis infection
- in utero parvovirus B19 infection
- in utero varicella zoster virus (VZV) infection
- AIDS embryopathy / in utero HIV infection
- TORCH group:
- Birth defects, including: cardiac, e.g., Tetralogy of Fallot, transposition of the great vessels and gastroschisis
- Genetic abnormalities (chromosomal anomalies):
- Trisomy 13
- Trisomy 18
- Triploidy: IUGR is of early-onset
- Down syndrome: not a dominant feature
- Chromosome 4p deletion syndrome (Wolf-Hirschhorn syndrome)
- Chromosome 12p tetrasomy (Pallister-Killian syndrome)
- Turner’s syndrome,
- Ring chromosomes and uniparental disomy
- Constitutional small
- Genetic syndromes like, Rubenstein-Taybi syndrome, Russell-Silver syndrome, Seckel syndrome, Cornelia de Lange syndrome, Brachmann-de Lange syndrome, Bloom syndrome, Dubowitz syndrome, Johanson–Blizzard syndrome, Roberts syndrome, Fanconi syndrome and Mulibrey Nanism syndrome
- Fetal genetic factors: low nitric oxide, genetic deletion of IGF1, under-expression of N-terminal parathyroid hormone-related protein, high urinary protein S100B 37
- In utero substance exposure e.g. fetal hydantoin syndrome
Figure 3. Intrauterine growth restriction causes
Footnotes: Outline of the causes of IUGR including contributions from maternal, placental and umbilical cord, and fetal dysfunction or injury.
[Source 17 ]IUGR risk factors
Pregnancies that have any of the following conditions may be at a greater risk at developing IUGR:
- Maternal weight less than 100 pounds
- Poor nutrition during pregnancy
- Birth defects or chromosomal abnormalities
- Use of drugs, cigarettes, and/or alcohol
- Pregnancy-induced hypertension (gestational hypertension)
- Placental abnormalities
- Umbilical cord abnormalities
- Multiple pregnancies
- Gestational diabetes in the mother
- Low levels of amniotic fluid or oligohydramnios
Intrauterine growth restriction prevention
Prenatal care is important in all pregnancies, and especially to identify problems with fetal growth. Stopping smoking and use of substances such as drugs and alcohol are essential to a healthy pregnancy and can reduce the risk for sudden infant death syndrome (SIDS) and other sleep-related infant deaths. Eating a healthy diet in pregnancy may also help.
Intrauterine growth restriction symptoms
Babies with intrauterine growth restriction (IUGR) may have problems at birth including the following:
- Decreased oxygen levels
- Low Apgar scores (an assessment that helps identify babies with difficulty adapting after delivery)
- Meconium aspiration (inhalation of the first stools passed in utero) which can lead to difficulty breathing
- Hypoglycemia (low blood sugar)
- Difficulty maintaining normal body temperature
- Polycythemia (too many red blood cells)
After birth, IUGR infants are more likely to spend a significantly longer time in neonatal intensive care unit (NICU) compared to gestation age-matched infants 39.
Intrauterine growth restriction (IUGR) infants demonstrate elevated rates of intolerance to feeds/ milk, feeding difficulties and necrotizing enterocolitis 12. Necrotizing enterocolitis is predominantly seen in infants who are born preterm, but late preterm infants are more likely to develop necrotizing enterocolitis if they were growth restricted 40. It is likely that in-utero chronic fetal hypoxia and subsequent cardiovascular redistribution of blood flow away from the gastrointestinal tract contribute to immature gut development 41. Intrauterine growth restriction (IUGR) newborns, especially with abnormal flows in the umbilical artery prior to birth, are shown to have more feed intolerance when compared to their well-grown preterm counterparts 42. Superior mesenteric artery blood flows have been used as a marker for splanchnic perfusion in neonates and decreased flows correlate with feed intolerance 43. Application of near infra-red spectroscopy in the neonatal period as an assessment tool for monitoring gut perfusion can detect changes in splanchnic oxygen delivery, which may be reduced in intrauterine growth restriction (IUGR) infants and may predict feeding intolerance and development of necrotizing enterocolitis 44. Studies have shown that preterm intrauterine growth restriction (IUGR) infants do not tolerate enteral feeds in the first few days of life 45 but conversely there is evidence that delaying enteral feeds in preterm intrauterine growth restriction (IUGR) infants does not confer any protection against feed intolerance or necrotizing enterocolitis 46. In fact, it may delay establishment of feeds and increase length of stay in the neonatal unit 47.
Malnutrition and low birth weight puts intrauterine growth restriction (IUGR) infants at an increased risk of a number of transient neonatal morbidities including hypothermia, altered glucose metabolism (hypoglycemia, hyperglycemia), hypocalcemia, polycythemia, jaundice and sepsis 48. Increased risk of infection is also common, potentially related to depressed immunological state and competence 49. IUGR infants born preterm also have an increased risk of retinopathy of prematurity 50. IUGR is linked to altered nephrogenesis, due to suboptimal tubular development caused by intrauterine hypoxia 51, and in turn, urinary Cystatin-C excretion is increased in intrauterine growth restriction (IUGR) infants compared to appropriately-grown infants which is seen to reflect reduced renal volume 52. It is therefore suggested that increased secretion of Cystatin-C signifies nephron loss as a result of the negative impact of intrauterine growth restriction (IUGR) on kidney development. Factors involved in nephron loss may include intrauterine hypoxia, decreased antioxidant capacity, and altered levels of growth factors.
Table 1. Intrauterine growth restriction (IUGR) neonatal complications
| Cardiovascular morbidity | Respiratory morbidity | Neurological morbidity | Others | |
|---|---|---|---|---|
| Neonatal period |
|
|
|
|
| Long term impact |
|
|
|
|
Intrauterine growth restriction (IUGR) complications
Intrauterine growth restriction (IUGR) complications are many and may include the following:
Antepartum (before childbirth) complications:
- stillbirth
- iatrogenic prematurity (medically induced prematurity)
- placental abruption
- perinatal stroke
Intrapartum (during childbirth – a time period from the onset of labor through delivery of the placenta) complications:
- abnormal fetal status (fetal heart rate tracing)
- asphyxia
- emergency Cesarean section
- need for active neonatal resuscitation
- perinatal stroke
Neonatal (newborn infant under 28 days of age) complications:
- hypothermia
- hypoglycemia
- hypocalcemia
- polycythemia
- sepsis
- coagulopathy
- hepatocellular dysfunction
- respiratory distress syndrome
- necrotizing enterocolitis
- intraventricular hemorrhage, especially in premature IUGR neonates <750 g
- hypoxic-ischemic encephalopathy
Pediatric complications:
- Increased risk of:
- short stature
- cerebral palsy
- developmental delay
- behavioral and emotional problems
- lower IQ scores
- chronic lung disease
- future cardiovascular disease and hypertension
Intrauterine growth restriction diagnosis
The baby with intrauterine growth restriction (IUGR) is often identified before birth. During pregnancy, a baby’s size can be estimated in different ways. The height of the fundus (the top of a mother’s uterus) can be measured from the pubic bone. This measurement in centimeters usually corresponds with the number of weeks of pregnancy after the 20th week. If the measurement is low for the number of weeks, the baby may be smaller than expected.
Although many intrauterine growth restriction (IUGR) babies have low birthweight, they are not all premature and may not experience the problems of premature babies. Other intrauterine growth restriction (IUGR) babies, appear thin, pale, and with loose, dry skin. The umbilical cord is often thin, and dull-looking rather than shiny and fat.
Other diagnostic procedures may include the following:
- Ultrasound. Ultrasound (a test using sound waves to create a picture of internal structures) is a more accurate method of estimating fetal size. Measurements can be taken of the fetus’ head and abdomen and compared with a growth chart to estimate fetal weight. The fetal abdominal circumference is a helpful indicator of fetal nutrition.
- Doppler flow. Another way to interpret and diagnose IUGR during pregnancy is Doppler flow, which uses sound waves to measure blood flow. The sound of moving blood produces wave-forms that reflect the speed and amount of the blood as it moves through a blood vessel. Blood flow through blood vessels in both the fetal brain and the umbilical cord can be checked with Doppler flow studies.
- Mother’s weight gain. A mother’s weight gain can also indicate a baby’s size. Small maternal weight gains in pregnancy may correspond with a small baby
- Gestational assessment. Babies are weighed within the first few hours after birth. The weight is compared with the baby’s gestational age and recorded in the medical record. The birthweight must be compared to the gestational age. Some doctors use a formula for calculating a baby’s body mass to diagnose intrauterine growth restriction (IUGR).
Intrauterine growth restriction treatment
Specific treatment for intrauterine growth restriction (IUGR) will be determined by your baby’s doctor based on:
- Your baby’s gestational age, overall health, and medical history
- Extent of the condition
- Your baby’s tolerance for specific medications, procedures, or therapies
- Expectations for the course of the condition
- Your opinion or preference
Despite new research, there are currently no effective medical interventions for IUGR, management consists of close surveillance aimed at determining the most appropriate time for delivery. Up till now, no effective methods are available to reverse the pathological intrauterine condition or to accelerate the growth of fetuses in pregnancies complicated by IUGR 11. Most likely the treatment will depend on how far along you are in your pregnancy.
- If gestational age is 34 weeks or greater, health care providers may recommend being induced for early delivery.
- If gestational age is less than 34 weeks, health care providers will continue monitoring until 34 weeks or beyond. Fetal well-being and the amount of amniotic fluid will be monitored during this time.
- If either of these becomes a concern, then immediate delivery may be recommended. Depending on your health care provider, you will likely have appointments every 2 to 6 weeks until you deliver. If delivery is suggested prior to 34 weeks, your health care provider may perform an amniocentesis to help evaluate fetal lung maturity.
The STRIDER-UK study (multicentre, randomized, double-blind, 156 participants) tested the use of sildenafil in women with severe early-onset IUGR and found that treatment did not prolong pregnancy or cause any adverse effects, but did not improve pregnancy outcomes 53. However, this study is part of a more extensive international study, and in isolation, it does not have the power to adequately assess outcomes in this very high-risk cohort (45% of recruited infants in this study died) 54. A meta-analysis (nine studies, total of 576 treated patients) has shown that arginine supplementation increases gestational length and birth weight in IUGR pregnancies, except for infants born preterm (<32 weeks post-conceptional age) with severe IUGR 55; there were no reported side effects. The proposed mechanisms of action of arginine include the increased production of placental insulin that acts as a fetal trophic factor.
Creatine may also be a potential treatment for IUGR 56, 57, with recent studies showing a positive correlation of birth weight to placental creatine load 58, and the discovery that the human placenta expresses the enzymes to synthesize and transport creatine 59. Creatine is an energy substrate which protects ATP turnover during periods of oxidative stress 60 and as such may be a potential prophylactic treatment for IUGR outcomes 56, 57. Creatine readily crosses the placenta in humans and some other omnivores (but not in sheep, an herbivore), suggesting that maternal creatine supplementation could be used to increase placental creatine transfer and promote fetal growth in a hypoxic uterine environment. Supporting data include a number of pre-clinical studies 61, 62. Whilst there has been extensive animal research suggesting creatine’s potential to protect the fetus against periods of oxygen deprivation in several animal models, and there is a strong rationale for moving toward clinical trials for maternal creatine supplementation to reduce or prevent IUGR, no intervention studies have yet been undertaken in pregnant women. IUGR is also proposed to be a disorder of insulin-like growth factor-1 (IGF-1) deprivation. Of particular note is an extensive preclinical study of prenatal IGF-1 treatment in a sheep model of IUGR (41 controls, 66 IUGR + saline, 28 IUGR + IGF-1, powered for sex-specific analysis and long term follow-up) which show that prenatal IGF-1 treatment improves prenatal and postnatal indices of growth and biochemical dysfunction in IUGR 63, 64.
Intrauterine growth restriction prognosis
Intrauterine growth restriction (IUGR) affects approximately 10% of pregnancies worldwide and is a leading cause of perinatal mortality and morbidity 5, 6, 65, 66. IUGR fetuses are at increased risk of stillbirth, fetal compromise, early neonatal death, and neonatal morbidity 67. IUGR survivors are at increased risk of cardiovascular diseases and neurodevelopmental deficits, with lifelong neurological deficits ranging from behavioral and motor disabilities to cerebral palsy 68, 69, 70, 71. Underlying these functional impairments, IUGR also compromises brain development and is commonly associated with reduced myelination and decreased total brain cell number 72, 73.
The appropriate growth of the fetal brain is highly dependent on the availability of growth factors. Brain Derived Neurotrophic Factor (BDNF), a member of the neurotrophin family, is widely expressed in the developing fetal brain and plays a vital role in neuronal survival, neuronal differentiation, and synaptic plasticity 74. Brain Derived Neurotrophic Factor (BDNF) is downregulated in cord blood from IUGR neonates clinically 75 and lower levels of BDNF receptors have been demonstrated in cortical neurons of IUGR rats, leading to reduced cell viability and synaptic function 76.
Additionally, the IUGR neonates have been shown to be highly prone to developing metabolic syndrome (e.g., obesity and type 2 diabetes) due to the increasing hepatic gluconeogenic capacity and impairing beta-cell function 77, 78. Children with IUGR, especially if they achieve catch-up growth in childhood, as well as SGA subjects, are at a higher risk for long-term developmental consequences or developing diseases later in life such as short stature, hypertension, dyslipidemia, insulin resistance, and cardiovascular disease 79, 80, 81.
References- Devaskar SU, Chu A. Intrauterine Growth Restriction: Hungry for an Answer. Physiology (Bethesda). 2016 Mar;31(2):131-46. doi: 10.1152/physiol.00033.2015
- Unterscheider J, Daly S, Geary MP, Kennelly MM, et al. Optimizing the definition of intrauterine growth restriction: the multicenter prospective PORTO study editorial comment. Obstet Gynecol Surv. 2013;68:549–551. doi: 10.1097/ogx.0b013e3182a0597
- Shrivastava D, Master A. Fetal Growth Restriction. J Obstet Gynaecol India. 2020 Apr;70(2):103-110. doi: 10.1007/s13224-019-01278-4
- Mutamba AK, He X, Wang T. Therapeutic advances in overcoming intrauterine growth restriction induced metabolic syndrome. Front Pediatr. 2023 Jan 11;10:1040742. doi: 10.3389/fped.2022.1040742
- Nardozza LM, Caetano AC, Zamarian AC, Mazzola JB, Silva CP, Marçal VM, Lobo TF, Peixoto AB, Araujo Júnior E. Fetal growth restriction: current knowledge. Arch Gynecol Obstet. 2017 May;295(5):1061-1077. doi: 10.1007/s00404-017-4341-9
- Hung, T.-H., Hsieh, T.-T., Lo, L.-M., Chiu, T.-H., Hsieh, C.-C. and Hsu, J.-J. (2013), Risk factors and perinatal outcomes associated with idiopathic small for gestational age Taiwanese newborns. International Journal of Gynecology & Obstetrics, 122: 212-215. https://doi.org/10.1016/j.ijgo.2013.03.033
- Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J Matern Fetal Neonatal Med. 2013 Feb;26(3):222-5. doi: 10.3109/14767058.2012.715006
- Rosenberg A. The IUGR newborn. Semin Perinatol. 2008 Jun;32(3):219-24. doi: 10.1053/j.semperi.2007.11.003
- Evers AC, Nikkels PG, Brouwers HA, Boon J, Van Egmond-Linden A, Hart C, et al.. Substandard care in antepartum term stillbirths: prospective cohort study. Acta Obstet Gynecol Scand. (2011) 90:1416–22. 10.1111/j.1600-0412.2011.01251.x
- Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. (2013) 346:f108. 10.1136/bmj.f108
- Hung TH, Liu YC, Wu CH, Chen CC, Chao H, Yang FY, Chen SF. Antenatal low-intensity pulsed ultrasound reduces neurobehavioral deficits and brain injury following dexamethasone-induced intrauterine growth restriction. Brain Pathol. 2021 Nov;31(6):e12968. doi: 10.1111/bpa.12968
- Malhotra A, Allison BJ, Castillo-Melendez M, Jenkin G, Polglase GR, Miller SL. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front Endocrinol (Lausanne). 2019 Feb 7;10:55. doi: 10.3389/fendo.2019.00055
- Salam RA, Das JK, Bhutta ZA. Impact of intrauterine growth restriction on long-term health. Curr Opin Clin Nutr Metab Care. (2014) 17(3):249–54. 10.1097/MCO.0000000000000051
- Singh M. Disorders of weight and gestation. In: Singh M, editor. In care of the newborn. 5. New Delhi: Sagar Publications; 1999. pp. 224–245.
- Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin Med Insights Pediatr. (2016) 10:67–83. 10.4137/CMPed.S40070
- Gordijn SJ, Beune IM, Thilaganathan B, Papageorghiou A, Baschat AA, Baker PN, et al.. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstetr Gynecol. (2016) 48:333–9. 10.1002/uog.15884
- Fleiss B, Wong F, Brownfoot F, Shearer IK, Baud O, Walker DW, Gressens P, Tolcos M. Knowledge Gaps and Emerging Research Areas in Intrauterine Growth Restriction-Associated Brain Injury. Front Endocrinol (Lausanne). 2019 Mar 29;10:188. doi: 10.3389/fendo.2019.00188
- Flood K, Unterscheider J, Daly S, Geary MP, Kennelly MM, Mcauliffe FM, et al.. The role of brain sparing in the prediction of adverse outcomes in intrauterine growth restriction: results of the multicenter PORTO Study. Am J Obstet Gynecol. (2014) 211:288 e281–285. 10.1016/j.ajog.2014.05.008
- Beukers F, Aarnoudse-Moens CSH, Van Weissenbruch MM, Ganzevoort W, Van Goudoever JB, Van Wassenaer-Leemhuis AG. Fetal growth restriction with brain sparing: neurocognitive and behavioral outcomes at 12 years of age. J Pediatr. (2017) 188:103–9.e102. 10.1016/j.jpeds.2017.06.003
- Scherjon S, Briet J, Oosting H, Kok J. The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic signs of fetal brain-sparing. Pediatrics. (2000) 105:385–91. 10.1542/peds.105.2.385
- Rodeck, Charles H., Whittle, Martin J.. Fetal Medicine: Basic Science and Clinical Practice. (2009) ISBN: 9780443104084
- William W. Hay (Editor). Neonatal Nutrition and Metabolism. (2006) ISBN: 0521824559
- Werner O. Schmidt, Asim Kurjak. Color Doppler Sonography in Gynecology and Obstetrics. (2004) ISBN: 1588902560
- Little BB, Snell LM. Brain growth among fetuses exposed to cocaine in utero: asymmetrical growth retardation. Obstet Gynecol. 1991 Mar;77(3):361-4.
- Hernandez-Andrade E, Figueroa-Diesel H, Jansson T, Rangel-Nava H, Gratacos E. Changes in regional fetal cerebral blood flow perfusion in relation to hemodynamic deterioration in severely growth-restricted fetuses. Ultrasound Obstet Gynecol. (2008) 32:71–6. 10.1002/uog.5377
- Sehgal A, Doctor T, Menahem S. Cardiac function and arterial biophysical properties in small for gestational age infants: postnatal manifestations of fetal programming. J Pediatr. (2013) 163:1296–300. 10.1016/j.jpeds.2013.06.030
- Devaskar SU, Chu A. Intrauterine growth restriction: hungry for an answer. Physiology. (2016) 31:131–46. 10.1152/physiol.00033.2015
- Anandakumar, C., Chew, S., Wong, Y.C., Malarvishy, G., Po, L.U. and Ratnam, S.S. (1996), Early Asymmetric IUGR and Aneuploidy. Journal of Obstetrics and Gynaecology Research, 22: 365-370. https://doi.org/10.1111/j.1447-0756.1996.tb00990.x
- Eberhard Merz. Ultrasound in Obstetrics and Gynecology, Volume 1 Obstetrics. (2011) ISBN: 9783131614728
- Severe Symmetric Intrauterine Growth Retardation Associated with the Topical Use of Triamcinolone. Am J Obstet Gynecol. 1990;162(2):396-397. https://doi.org/10.1016/0002-9378(90)90394-M
- Vijayaselvi R, Cherian A. Risk assessment of intrauterine growth restriction. Curr Med Issues. (2017) 15:262–6. 10.4103/cmi.cmi_76_17
- Gaccioli F, Lager S. Placental nutrient transport and intrauterine growth restriction. Front Physiol. (2016) 7:40. 10.3389/fphys.2016.00040
- Brodsky D, Christou H. Current concepts in intrauterine growth restriction. J Intensive Care Med. 2004 Nov-Dec;19(6):307-19. doi: 10.1177/0885066604269663
- Burton GJ, Jauniaux E. Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol. 2018 Feb;218(2S):S745-S761. doi: 10.1016/j.ajog.2017.11.577
- Sharma D, Shastri S, Farahbakhsh N, Sharma P. Intrauterine growth restriction – part 1. J Matern Fetal Neonatal Med. 2016 Dec;29(24):3977-87. doi: 10.3109/14767058.2016.1152249
- Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci. 2014 Sep 12;15(9):16153-85. doi: 10.3390/ijms150916153
- Sharma D, Farahbakhsh N, Shastri S, Sharma P. Intrauterine growth restriction—part 2. J Matern Fetal Neonatal Med. 2016;29(24):4037–4048. doi: 10.3109/14767058.2016.1154525
- Stegmann BJ, Carey JC. TORCH Infections. Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes infections. Curr Womens Health Rep. 2002 Aug;2(4):253-8.
- Sasi A, Abraham V, Davies-Tuck M, Polglase GR, Jenkin G, Miller SL, et al.. Impact of intrauterine growth restriction on preterm lung disease. Acta Paediatr. (2015) 104:e552-6. 10.1111/apa.13220
- Gephart SM, Hanson CK. Preventing necrotizing enterocolitis with standardized feeding protocols: not only possible, but imperative. Adv Neonatal Care (2013) 13:48–54. 10.1097/ANC.0b013e31827ece0a
- Bozzetti V, Tagliabue PE. Enteral feeding of intrauterine growth restriction preterm infants: theoretical risks and practical implications. La Pediatria Medica e Chirurgica (2017) 39:160. 10.4081/pmc.2017.160
- Ahamed MF, Dar P, Vega M, Kim M, Gao Q, Havranek T. Early feeding tolerance in small for gestational age infants with normal versus abnormal antenatal Doppler characteristics. J Neonatal-Perinatal Med. (2017) 10:43–48. 10.3233/NPM-1682
- Bozzetti V, Paterlini G, De Lorenzo P, Gazzolo D, Valsecchi MG, Tagliabue PE. Impact of continuous vs bolus feeding on splanchnic perfusion in very low birth weight infants: a randomized trial. J Pediatr. (2016) 176:86–92.e2. 10.1016/j.jpeds.2016.05.031
- Fortune PM, Wagstaff M, Petros AJ. Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. (2001) 27:1401–7. 10.1007/s001340100994
- Kempley S, Gupta N, Linsell L, Dorling J, McCormick K, Mannix P, et al.. Feeding infants below 29 weeks’ gestation with abnormal antenatal Doppler: analysis from a randomised trial. Arch Dis Childhood. (2014) 99:F6–F11. 10.1136/archdischild-2013-304393
- Leaf A, Dorling J, Kempley S, McCormick K, Mannix P, Linsell L, et al. Early or delayed enteral feeding for preterm growth-restricted infants: a randomized trial. Pediatrics (2012) 129:e1260–8. 10.1542/peds.2011-2379
- Zecca E, Costa S, Barone G, Giordano L, Zecca C, Maggio L. Proactive enteral nutrition in moderately preterm small for gestational age infants: a randomized clinical trial. J Pediatr. (2014) 165:1135–1139.e1. 10.1016/j.jpeds.2014.08.065
- Sharma D, Shastri S, Sharma P. Intrauterine growth restriction: antenatal and postnatal aspects. clinical medicine insights. Pediatrics (2016) 10:67–83. 10.4137/CMPed.S40070
- Longo S, Borghesi A, Tzialla C, Stronati M. IUGR and infections. Early Hum Dev. (2014) 90(Suppl. 1):S42–4. 10.1016/S0378-3782(14)70014-3
- Lee JW, VanderVeen D, Allred EN, Leviton A, Dammann O. Prethreshold retinopathy in premature infants with intrauterine growth restriction. Acta Paediatr (2015) 104:27–31. 10.1111/apa.12799
- Aisa MC, Cappuccini B, Barbati A, Orlacchio A, Baglioni M, Di Renzo GC. Biochemical parameters of renal impairment/injury and surrogate markers of nephron number in intrauterine growth-restricted and preterm neonates at 30-40 days of postnatal corrected age. Pediatr Nephrol. (2016) 31:2277–87. 10.1007/s00467-016-3484-4
- Barbati A, Cappuccini B, Aisa MC, Grasselli C, Zamarra M, Bini V, et al.. Increased urinary cystatin-C levels correlate with reduced renal volumes in neonates with intrauterine growth restriction. Neonatology (2016) 109:154–60. 10.1159/000441273
- Sharp A, Cornforth C, Jackson R, Harrold J, Turner MA, Kenny LC, et al.. Maternal sildenafil for severe fetal growth restriction (STRIDER): a multicentre, randomised, placebo-controlled, double-blind trial. Lancet Child Adolesc Health. (2018) 2:93–102. 10.1016/S2352-4642(17)30173-6
- Smith GCS. The STRIDER trial: one step forward, one step back. Lancet Child Adolesc Health. (2018) 2:80–1. 10.1016/S2352-4642(17)30176-1
- Chen J, Gong X, Chen P, Luo K, Zhang X. Effect of L-arginine and sildenafil citrate on intrauterine growth restriction fetuses: a meta-analysis. BMC Pregnancy Childbirth. (2016) 16:225. 10.1186/s12884-016-1009-6
- Dickinson H, Ellery S, Ireland Z, Larosa D, Snow R, Walker DW. Creatine supplementation during pregnancy: summary of experimental studies suggesting a treatment to improve fetal and neonatal morbidity and reduce mortality in high-risk human pregnancy. BMC Pregnancy Childbirth. (2014) 14:150. 10.1186/1471-2393-14-150
- Ellery SJ, Dickinson H, Mckenzie M, Walker DW. Dietary interventions designed to protect the perinatal brain from hypoxic-ischemic encephalopathy–Creatine prophylaxis and the need for multi-organ protection. Neurochem Int. (2016) 95:15–23. 10.1016/j.neuint.2015.11.002
- Dickinson H, Davies-Tuck M, Ellery SJ, Grieger JA, Wallace EM, Snow RJ, et al.. Maternal creatine in pregnancy: a retrospective cohort study. BJOG. (2016) 123:1830–8. 10.1111/1471-0528.14237
- Ellery SJ, Della Gatta PA, Bruce CR, Kowalski GM, Davies-Tuck M, Mockler JC, et al.. Creatine biosynthesis and transport by the term human placenta. Placenta. (2017) 52:86–93. 10.1016/j.placenta.2017.02.020
- Wallimann T, Tokarska-Schlattner M, Schlattner U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. (2011) 40:1271–96. 10.1007/s00726-011-0877-3
- Ireland Z, Castillo-Melendez M, Dickinson H, Snow R, Walker DW. A maternal diet supplemented with creatine from mid-pregnancy protects the newborn spiny mouse brain from birth hypoxia. Neuroscience. (2011) 194:372–9. 10.1016/j.neuroscience.2011.05.012
- Ireland Z, Dickinson H, Snow R, Walker DW. Maternal creatine: does it reach the fetus and improve survival after an acute hypoxic episode in the spiny mouse (Acomys cahirinus)? Am J Obstet Gynecol. (2008) 198:431 e1–6. 10.1016/j.ajog.2007.10.790
- Spiroski AM, Oliver MH, Jaquiery AL, Prickett TCR, Espiner EA, Harding JE, et al.. Postnatal effects of intrauterine treatment of the growth-restricted ovine fetus with intra-amniotic insulin-like growth factor-1. J Physiol. (2018) 596:5925–45. 10.1113/JP274999
- Wali JA, De Boo HA, Derraik JG, Phua HH, Oliver MH, Bloomfield FH, et al.. Weekly intra-amniotic IGF-1 treatment increases growth of growth-restricted ovine fetuses and up-regulates placental amino acid transporters. PLoS ONE. (2012) 7:e37899. 10.1371/journal.pone.0037899
- Guilbert JJ. The world health report 2002 – reducing risks, promoting healthy life. Educ Health. (2003) 16(2):230. 10.1080/1357628031000116808
- Maternal anthropometry and pregnancy outcomes. A WHO Collaborative Study. Bull World Health Organ. 1995;73 Suppl(Suppl):1-98. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2486648/pdf/bullwho00411-0007.pdf
- Unterscheider J, O’donoghue K, Daly S, Geary MP, Kennelly MM, Mcauliffe FM, et al.. Fetal growth restriction and the risk of perinatal mortality-case studies from the multicentre PORTO study. BMC Pregnancy Childbirth. (2014) 14:63. 10.1186/1471-2393-14-63
- van Wassenaer A. Neurodevelopmental consequences of being born SGA. Pediatr Endocrinol Rev. 2005 Mar;2(3):372-7.
- Brown AS, Wieben M, Murdock S, Chang J, Dizon MLV, St Pierre M, Chavez-Valdez R, Dorsky RI, Fung CM. Intrauterine Growth Restriction Causes Abnormal Embryonic Dentate Gyrus Neurogenesis in Mouse Offspring That Leads to Adult Learning and Memory Deficits. eNeuro. 2021 Oct 8;8(5):ENEURO.0062-21.2021. doi: 10.1523/ENEURO.0062-21.2021
- Leitner Y, Fattal-Valevski A, Geva R, Eshel R, Toledano-Alhadef H, Rotstein M, et al.. Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10-year prospective study. J Child Neurol. (2007) 22:580–7. 10.1177/0883073807302605
- Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. (1989) 298:564–7. 10.1136/bmj.298.6673.564
- Ruff CA, Faulkner SD, Rumajogee P, Beldick S, Foltz W, Corrigan J, Basilious A, Jiang S, Thiyagalingam S, Yager JY, Fehlings MG. The extent of intrauterine growth restriction determines the severity of cerebral injury and neurobehavioural deficits in rodents. PLoS One. 2017 Sep 21;12(9):e0184653. doi: 10.1371/journal.pone.0184653
- Samuelsen GB, Pakkenberg B, Bogdanović N, Gundersen HJ, Larsen JF, Graem N, Laursen H. Severe cell reduction in the future brain cortex in human growth-restricted fetuses and infants. Am J Obstet Gynecol. 2007 Jul;197(1):56.e1-7. doi: 10.1016/j.ajog.2007.02.011
- Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004 Jul;25(2):77-107. doi: 10.1016/j.yfrne.2004.04.001
- Ardiani Y, Defrin D, Yetti H. Differences in Brain-Derived Neurotrophic Factor and Matrix Metalloproteinase-9 between Appropriate Neonates between Normal Birth Weight and Intrauterine Growth Restriction. Open Access Maced J Med Sci. 2019 Mar 14;7(5):736-741. doi: 10.3889/oamjms.2019.159
- Ninomiya M, Numakawa T, Adachi N, Furuta M, Chiba S, Richards M, Shibata S, Kunugi H. Cortical neurons from intrauterine growth retardation rats exhibit lower response to neurotrophin BDNF. Neurosci Lett. 2010 May 31;476(2):104-9. doi: 10.1016/j.neulet.2010.03.082
- Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1297–R1305. doi: 10.1152/ajpregu.00494.2004
- Thorn SR, Regnault TR, Brown LD, Rozance PJ, Keng J, Roper M, Wilkening RB, Hay WW, Jr, Friedman JE. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150:3021–3030. doi: 10.1210/en.2008-1789
- Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. (2004) 58(2):114–5. 10.1136/jech.58.2.114
- Darendeliler F. IUGR: genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best Pract Res Clin Endocrinol Metab. (2019) 33(3):101260. 10.1016/j.beem.2019.01.001
- Barker DJ, Osmond C, Golding J, Kuh D, et al. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564