Iridocorneal endothelial syndrome
Iridocorneal endothelial syndrome also known as ICE syndrome, is a rare eye condition that is characterized by proliferative and structural abnormalities of the corneal endothelium, progressive obstruction of the iridocorneal angle, and iris anomalies such as atrophy, correctopia, and polycoria 1. The consequences of these changes are corneal decompensation and secondary angle-closure glaucoma, which represent the most frequent causes of visual loss in these patients 1.
ICE syndrome is a group of conditions related to changes in corneal cells and the iris 2. Iridocorneal endothelial syndrome almost always involves cells moving from the cornea to the iris. Loss of cells from the cornea can cause corneal swelling, and the iris and pupil can become distorted. When the corneal cells move, they can block fluid from draining properly through the eye’s microscopic drainage channels. This blockage causes pressure in the eye to build, leading to glaucoma.
The three main features of ICE syndrome include 2:
- Swelling of the cornea (corneal edema)
- Iris atrophy
- Secondary angle-closure glaucoma
ICE syndrome is a group of disorders with three clinical variants 3:
- Chandler syndrome: This is the most common of the three sub-types, representing approximately 50% of all cases if ICE syndrome 4. Of the three, Chandler Syndrome typically presents with a greater degree of corneal pathology with associated corneal edema. The corneal edema can be microcystic, even with a normal intraocular pressure (this is common of all three variants). The iris findings are less common, and a majority of patients have no iris changes at all, making the diagnosis a challenge. The incidence of glaucoma is less severe than in the other two variants and sometimes the intraocular pressure could be normal.
- Essential / Progressive Iris Atrophy: The iris findings of this variant can be quite robust and progressive over time. Severe and progressive iris atrophy which can lead to abnormal position of the iris (Corectopia), irregular pupil shape, heterochromia, ectropion uveae and iris hole formation which is called pseudopolycoria. Pseudopolycoria can cause double vision because lights enter inside the eye through more than one pupil.
- Iris Nevus / Cogan-Reese syndrome: This variant of ICE syndrome is distinguished by its unique iris findings – diffuse or multiple nodules of iris nevus that spread all over the iris. The anterior surface of iris has tan pedunculated nodules or diffuse pigmented lesions 4. However, iris atrophy is uncommon with these particular patients.
Distinction can be made between the three clinical subtypes can be made based on unique characteristics on clinical exam. However, each can result in substantial visual impairment from glaucomatous optic neuropathy and / or corneal edema, making management a challenge.
ICE syndrome is considered sporadic in presentation, with no consistent association to other ocular or systemic disease, and familial cases have been very rare 3. ICE syndrome presents as a unilateral disease, more common in women, between the ages of 20 and 50 4. ICE syndrome must be considered within the differential diagnosis for any young to middle-aged patient presenting with unilateral glaucoma, corneal decompensation, and / or iris atrophy 3.
Given the progressive nature of ICE syndrome and the wide spectrum of clinical presentations it is important to establish the diagnosis from similar conditions. Posterior polymorphous dystrophy (PPD) may show similar clinical features such as iridocorneal adhesions, membranes, and ectropion uveae which are typically associated with ICE syndrome. This can at times complicate diagnosis. Posterior polymorphous dystrophy, in contrast to ICE syndrome, has a genetic component and is rarely progressive unlike ICE syndrome 5.
Specular microscopy can be used to differentiate between these two conditions 1. ICE cells are dark areas with a light central spot and a light peripheral zone. These are generally larger than normal endothelial cells, and occur in areas where the cornea appears to have a hammered silver appearance 5. These cells are regarded as pathognomonic of ICE syndrome and are termed “ICE cells” along with the tissue they form termed “ICE tissue” 6. Four basic patterns of ICE cells have been described by previous authors 7. More recent findings using in-vivo confocal microscopy have highlighted two main patterns of abnormal “epithelioid-like” endothelium, both characterized by marked hyperreflective nuclei and loss of regularity in cellular size and shape 8. Stromal nerve fibers in affected eyes were unusually thicker and distorted. It is suggested these signs can be examined to aid diagnosis, especially in edematous corneas 8.
There is no specific treatment for iridocorneal endothelial syndrome . The only treatment available is to treat glaucoma with eye drops, trabeculectomy and shunts. Treatment with eye drops is usually ineffective.
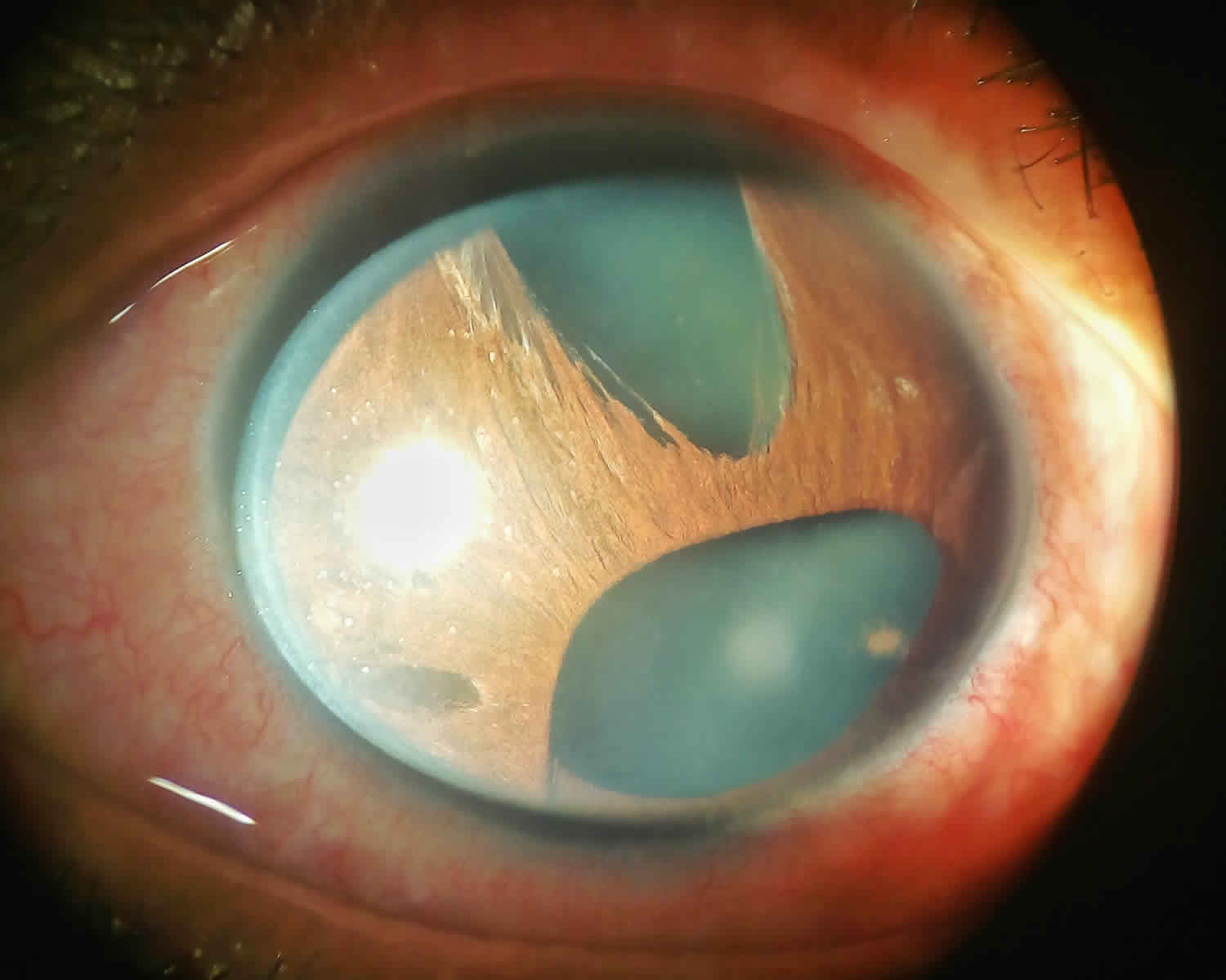
Figure 1. Iridocorneal endothelial syndrome
Footnote: (a) Iridocorneal endothelial syndrome, characterized by atrophy of the iris, multiple atrophic holes, and corectopia. (b) Cogan-Reese syndrome, characterized by iridocorneal adhesion, diffuse nevi, and ectropion uveae.
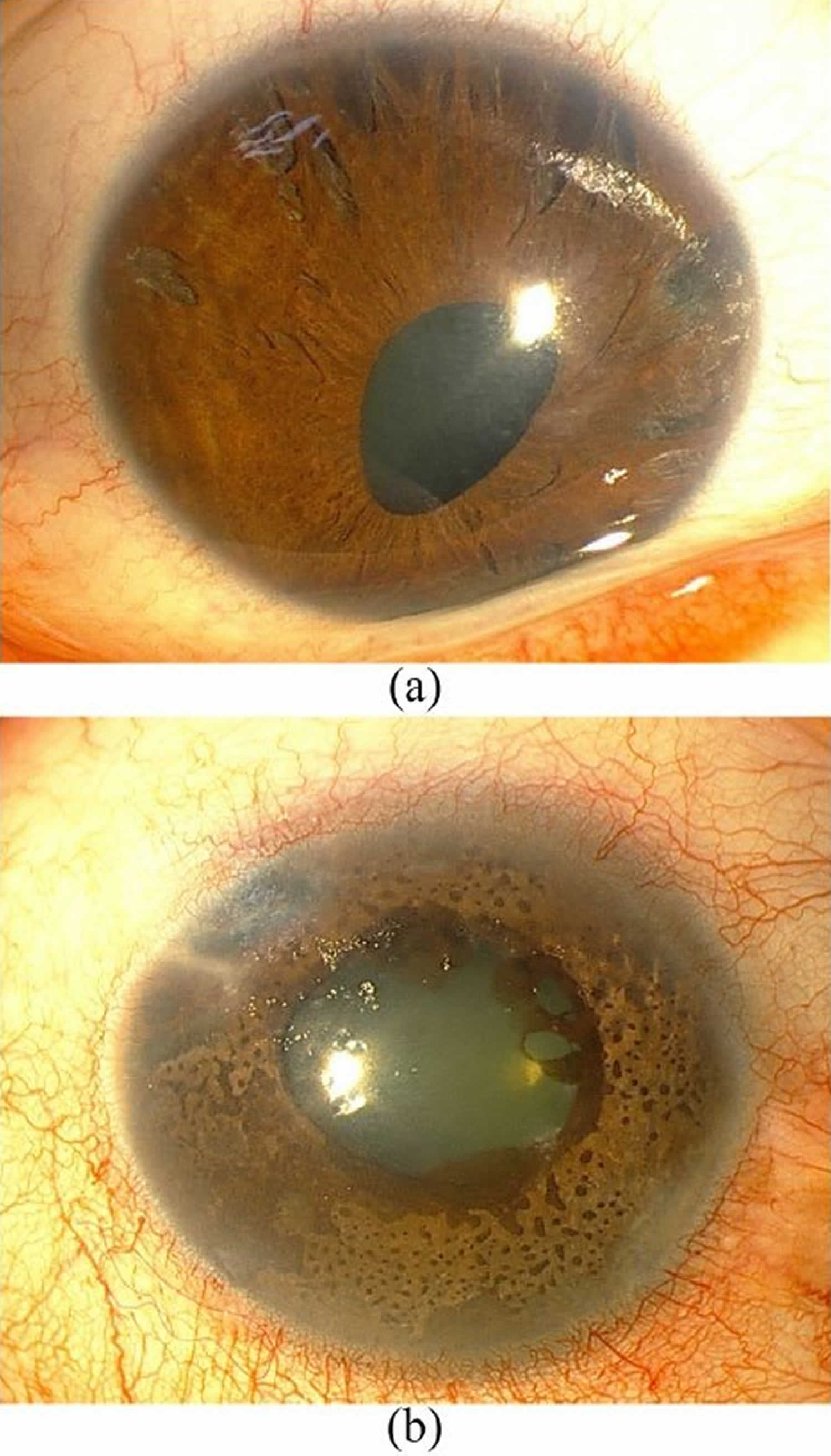
[Source 9 ]Figure 2. Essential Iris Atrophy (Progressive Iris Atrophy)
Footnote: Polycoria, iris hole formation, and corectopia visible on a slit-lamp photo of a patient with Essential / Progressive Iris Atrophy. The corneal graft and the edge of a superior conjunctival bleb, as this patient required both penetrating keratoplasty and trabeculectomy.
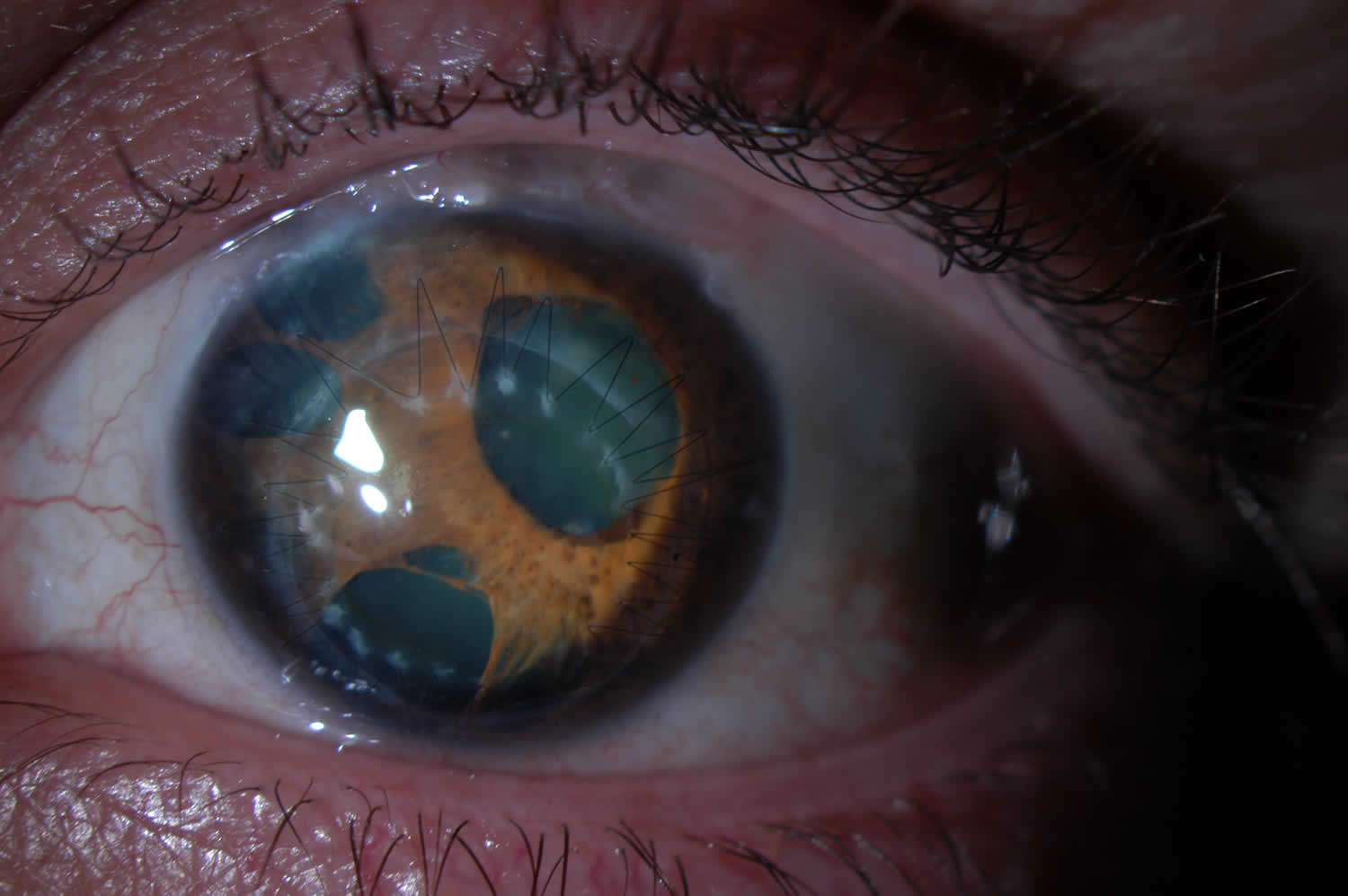
Figure 3. Iris Nevus (Cogan-Reese syndrome)
Footnote: Slit-lamp photo of a patient with Iris Nevus / Cogan-Reese sub-type of ICE syndrome. Note the pigmented pedunculated iris lesions that are characteristic of this disease.
Iridocorneal endothelial syndrome causes
The true cause of ICE syndrome is not well understood 10. It has been theorized that an underlying viral infection with Herpes simplex virus (HSV) or Epstein-Barr virus (EBV) leads to a low grade inflammation at the level of the corneal endothelium, resulting in its unusual epithelial-like activity 4. Polymerase Chain Reaction (PCR) testing of corneal endothelial cells from ICE syndrome patients has been found to have high percentages of HSV DNA in comparison to normal controls 11.
Clinically, the corneal endothelium has been described to have a “hammered silver” or “beaten bronze” appearance in ICE syndrome patients, similar to corneal guttae seen in Fuchs Corneal Endothelial Dystrophy 4. On a pathological level, it is felt that the normal endothelial cells have been replaced with a more epithelial-like cell with migratory characteristics. Transmission and Scanning Electron Microscopic examination of these cells has demonstrated a population of well-differentiated cells with epithelial features such as desmosomes, tonofilaments, and microvilli 12. The altered endothelium migrates posteriorly, moving beyond Schwalbe’s line, onto the trabecular meshwork, and at times, onto peripheral iris. Contraction of this tissue within the angle and on the iris results in the high peripheral anterior synechiae and iris changes characteristic of ICE syndrome. Secondary angle-closure glaucoma is a consequence of high peripheral anterior synechiae, but can at times occur without overt synechiae because the advancing corneal endothelium can functionally close the angle without contraction 4. As a result, patients may initially present with what appears to be open-angle glaucoma because the fibrovascular membrane obstructing aqueous flow can be difficult to visualize with gonioscopy.
The corneal edema found in ICE syndrome patients is thought to be secondary to both elevated intraocular pressure (IOP) from secondary angle-closure glaucoma, and from subnormal pump function from the altered corneal endothelial cells 13. This can be found in any of the three clinical variants, but is more common in patients with Chandler syndrome.
Iridocorneal endothelial syndrome signs and symptoms
The initial presentation of ICE syndrome patients may be due to monocular pain (from corneal edema or elevated intraocular pressure from angle-closure [glaucoma]), blurry vision, or iris changes. Common signs and symptoms in ICE syndrome are glaucoma and corneal edema.
- Corneal edema: It occurs because these abnormal endothelial cells can’t regulate the flow of fluid in and out the cornea. Corneal edema cause blurred vision and halos on lights especially in the morning hours as the result of lid closure which prevents evaporation of tears and fluid from the cornea. Throughout the day, vision can improve a little because the cornea dehydrates with exposure to air.
- Glaucoma: Proliferation of these abnormal cells over the angle can cause the formation of anterior synechia. These synechia will block the drainage of aqueous humor outside the eye and lead to high intraocular pressure. This type of glaucoma is called angle closure glaucoma. Angle closure glaucoma can cause red eye, eye pain, and blurred vision.
Patients require a full ophthalmic work-up, with assessment of visual acuity and refractive error, intraocular pressure, and slit-lamp examination, dilated fundus examination, and gonioscopic examination. Close evaluation of the cornea should be done to assess for corneal edema and corneal endothelial irregularity (a ‘beaten bronze’ or ‘hammered silver’ appearance when viewed in specularly reflected light with the slit lamp) 14. Iris changes (such as heterochromia, ectropion uveae, corectopia, hole formation, and iris atrophy) may be evident on slit-lamp examination. Exam with gonioscopy may show high peripheral anterior synechiae (PAS) extending above Schwalbe line, which is pathognomonic for ICE syndrome 4.
Iridocorneal endothelial syndrome complications
Glaucomatous optic neuropathy
ICE syndrome can result in advanced glaucomatous optic neuropathy with extensive vision loss if left untreated. Glaucoma occurs in approximately 50% of patients with ICE syndrome and tends to be more severe in progressive iris atrophy and Cogan-Reese syndrome 4. Glaucoma is a result of trabecular meshwork obstruction from migrating dysfunctional corneal endothelial cells 14. High peripheral anterior synechiae (PAS) may develop within the angle after contraction of this membrane, making the diagnosis of secondary angle-closure glaucoma simple with gonioscopy. However, a ‘functional’ angle-closure (that can be confused with open-angle glaucoma) may exist because the endothelial cell membrane can advance without causing overt synechial formation 4.
Corneal edema and decompensation
Poor vision secondary to corneal endothelial cell dysfunction with resulting corneal decompensation and edema is common in patients with ICE syndrome. Mild cases can be managed with topical hypertonic saline drops and ointments. Severe cases may require corneal transplant surgery (Penetrating Keratoplasty (PKP) or Descemet’s Stripping Endothelial Keratoplasty (DSAEK or DSEK)). This complication has been found to be more profound in Chandler Syndrome.
Iris changes (atrophy, corectopia, polycoria, ectropion uveae)
Contraction of corneal endothelial cells that have advanced on to the iris can result in these degnerative changes. Patients with good visual potential may develop visual distortion and glare from these iris changes. This complication is most pronounced in Essential Iris Atrophy / Progressive Iris Atrophy.
Failed glaucoma surgery
Late failures have been reported with trabeculectomies secondary to advancing endothelialization of the fistula. In some cases these can be treated with a Nd:YAG laser procedure to re-open the fistula 14.
Iridocorneal endothelial syndrome diagnosis
Specular microscopy is an important diagnostic tool, as the corneal endothelium has a characteristic appearance in ICE syndrome patients. Asymmetric endothelial cell loss and atypical endothelial cell morphology is typically evident, which appears on a specular photomicrograph as dark, larger than normal endothelial cells, with a bright central spot 14. The endothelial cells have also been described to appear as dark areas with central highlights and light peripheral borders 13. These corneal endothelial cells are felt to be pathognomonic for ICE syndrome, and have hence been referred to as “ICE cells” when seen on specular photomicrographs 6. Resulting corneal edema can be quantified with a pachymeter at each visit.
Routine evaluation for glaucoma in these patients should be done by measuring intraocular pressure and evaluating the angle for peripheral anterior synechiae (PAS) with gonioscopy. Greater diagnostic evaluation for glaucoma can be accomplished with common testing devices utilized for any glaucoma patient. Stereo disc photographs and visual field analysis (Humphrey or Goldmann), along with optic nerve and nerve fiber layer assessment (heidelberg retinal tomogram or optical coherence tomography [OCT]), can all be implemented in the initial work-up and ongoing evaluation for glaucoma progression in these patients.
Anterior segment imaging in the form of ultrasound biomicroscopy or anterior segment optical coherence tomography (OCT) can be a useful tool for detecting peripheral anterior synechiae and iris atrophy more reliably than slit lamp microscopy and gonioscopy when corneal edema is present 15. Central anterior chamber depth is shown to be significantly less in patients with ICE syndrome (mean 2.25 (± SD 0.32) mm) than in normal subjects (2.76 (±0.32) mm) 15. In contrast, posterior polymorphous dystrophy vesicles appear as dark rings with distinct, scalloped edges surrounding a lighter center 5. With repeated specular microscopy examination over several years, the posterior polymorphous dystrophy cells showed no changes in configuration or migration. No accelerated endothelial cell loss occurred. In the ICE patients, normal endothelial cell density was lost, although the amount of ICE cells remained constant 5. Both conditions show multilayered endothelial cells and thickened Descemet’s membrane 16.
Corneas affected by ICE syndrome are said to exhibit extensive endothelial changes early in the course of the disease, before other manifestations are clinically apparent 17. Lymphocytes are often found in the endothelium, which may only be found early in the disease process 17. Epithelialization of the endothelial cell layer has been demonstrated using immunohistochemical studies 18, which results in cellular proliferation across the iridocorneal angle similar to that seen in epithelial downgrowth and posterior polymorphous endothelial dystrophy 18. Abnormal iris profiles have been reported using anterior segment OCT scanning 19.
The ICE cells that border normal endothelial cells are said to be in a static, immobile state. These cells are often damaged or necrotic at boundary zones, suggesting that ICE cells may have a toxic effect on normal neighboring endothelial cells 20. This may explain why corneal failure in this syndrome is often slowly progressive 21. Some evidence suggests that a subclinical form may exist in the contralateral eye 22, predisposing to shallow or closed anterior chamber angles, which should be examined for gonioscopically 15. Contralateral endothelial cells are not reduced, although normal hexagonal-shaped cells are reduced 23.
ICE syndrome treatment
The treatment of ICE syndrome, regardless of the variant, primarily revolves around the prevention of glaucomatous vision loss secondary to elevated intraocular pressure. Additionally, treatment of corneal edema and other corneal changes is vital to maintain high quality visual acuity. Both ocular hypertension and corneal decompensation can be addressed with medical and surgical treatment approaches.
Medical therapy
Topical medication is the first line therapy for patients with elevated intraocular pressure from secondary angle-closure glaucoma in the setting of ICE syndrome 10. More specifically, aqueous suppressants such as topical beta blockers, alpha agonists, and carbonic anhydrase inhibitors are typically used, rather than medications that would target the aqueous drainage sites of the eye (e.g. miotics). The role of prostaglandin analogs, which reduce intraocular pressure by enhancing uveoscleral outflow, remains unclear 4.
Corneal edema in ICE syndrome patients may be exacerbated by elevated IOP (intraocular pressure), and these corneal changes may benefit from the reduction of IOP by topical aqueous suppressants as well. Additionally, topical hypertonic saline solutions and gels can be utilized to improve corneal edema by dehydrating the cornea.
Surgical therapy
When medical therapy is unsuccessful at controling IOP, surgical therapy with a filtering procedure may be necessary. A trabeculectomy with antifibrotic agents (mitomycin-C or 5-fluorouracil) or a glaucoma drainage device (aqueous shunt) have been found to be effective in controling IOP in ICE syndrome patients 14. However, maintaining long-term success can be challenging, as the fistula can be obstructed with advancing abnormal corneal endothelial cells. Long-term surgical outcomes have been reported to be slightly better with glaucoma drainage implants (survival of 71% at 1 year, 71% at 3 years, and 53% at 5 years) versus trabeculectomy with antifibrotic agents (survival of 73% at 1 year, 44% at 3 years, and 29% at 5 years) 24. Regardless of the procedure, it has been noted that these patients typically require multiple surgeries to maintain stable IOP control 24. If surgical success is not obtained with a trabeculectomy or glaucoma drainage device, it may be necessary to treat patients with a ciliary body destruction procedure. Typically this is done with diode laser cyclophotocoagulation (diode CPC), and is reserved for intractable cases of glaucoma.
Corneal decompensation can similarly be treated with surgery when medical management fails. Penetrating keratoplasy (PKP) or endothelial keratoplasty (commonly descemet stripping endothelial keratoplasty [DSEK] or descemet stripping automated endothelial keratoplasty [DSAEK]) can be implemented to replace the abnormal corneal endothelial cells and improve corneal function 4. At times, both a filtering and corneal transplant procedure are necessary. Concomitant control of IOP is vital for maintenance of corneal graft clarity.
Iridocorneal endothelial syndrome prognosis
Prognosis for patients with ICE syndrome depends on whether the above complications develop. This is dependent on the timing of diagnosis within the disease course, and the success or failure of treatment. Many patients have subtle disease, and do quite well with intraocular pressure control (both topical and surgical). However, there are rare patients with aggressive disease who suffer from extensive vision loss from advanced glaucoma and / or corneal edema. The glaucoma tends to be more severe in progressive iris atrophy and Cogan-Reese syndrome 4. If surgical intervention is required for intraocular pressure control, the prognosis tends to be more guarded.
References- Iridocorneal endothelial syndrome: clinical perspectives. Clin Ophthalmol. 2018; 12: 657–664. doi: 10.2147/OPTH.S143132 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5898599
- What Is Iridocorneal Endothelial Syndrome (ICE)? https://www.aao.org/eye-health/diseases/iridocorneal-endothelial-syndrome-ice
- Iridocorneal Endothelial Syndrome and Secondary Glaucoma. https://eyewiki.org/Iridocorneal_Endothelial_Syndrome_and_Secondary_Glaucoma
- Glaucoma. Basic and Clinical Science Course (BCSC). American Academy of Ophthalmology, 2010-2011; pp142-144.
- Laganowski HC, Sherrard ES, Muir MG, Buckley RJ. Distinguishing features of the iridocorneal endothelial syndrome and posterior polymorphous dystrophy: value of endothelial specular microscopy. Br J Ophthalmol. 1991;75(4):212–216.
- Laganowski HC, Sherrard ES, Muir MG, and Buckley RJ. Distinguishing features of the iridocorneal endothelial syndrome and posterior polymorphous dystrophy: value of endothelial specular microscopy. Br J Ophthalmol. 1991 April; 75(4): 212–216.
- Sherrard ES, Frangoulis MA, Muir MG, Buckley RJ. The posterior surface of the cornea in the irido-corneal endothelial syndrome: a specular microscopical study. Trans Ophthalmol Soc U K. 1985;104(Pt 7):766–774.
- Le QH, Sun XH, Xu JJ. In-vivo confocal microscopy of iridocorneal endothelial syndrome. Int Ophthalmol. 2009;29(1):11–18.
- Feizi, Sepehr. (2018). Corneal endothelial cell dysfunction: etiologies and management. Therapeutic Advances in Ophthalmology. 10. 251584141881580. 10.1177/2515841418815802
- Doan A, Alward W: Iridocorneal Endothelial Syndrome (ICE) – essential iris atrophy : 63 year-old female with PAS, “iris mass”, corectopia, and increased IOP OS. http://www.eyerounds.org/cases/case14.htm
- Alvarado JA, Underwood JL, Green WR, et al. Detection of herpes simplex viral DNA in the iridocorneal endothelial syndrome. Arch Ophthalmol. 1994;112(12):1601–1609.
- Levy SG, Kirkness CM, Moss J, Ficker L, McCartney AC. The histopathology of the iridocorneal-endothelial syndrome. Cornea. 1996 Jan;15(1):46-54.
- External Disease and Cornea. Basic and Clinical Science Course (BCSC). American Academy of Ophthalmology, 2010-2011; pp344-345.
- Laganowski HC, Kerr Muir MG, Hitchings RA. Glaucoma and the iridocorneal endothelial syndrome. Arch Ophthalmol. 1992 Mar;110(3):346-50.
- Zhang M, Chen J, Liang L, Laties AM, Liu Z. Ultrasound biomicros-copy of Chinese eyes with iridocorneal endothelial syndrome. Br J Ophthalmol. 2006;90(1):64–69.
- Bromley JG, Randleman JB, Stone D, Stulting RD, Grossniklaus HE. Clinicopathologic findings in iridocorneal endothelial syndrome and posterior polymorphous membranous dystrophy after Descemet stripping automated endothelial keratoplasty. Cornea. 2012;31(9):1060–1064.
- Levy SG, Kirkness CM, Moss J, Ficker L, McCartney AC. On the pathology of the iridocorneal-endothelial syndrome: the ultrastructural appearances of “subtotal-ice” Eye (Lond) 1995;9(Pt 3):318–323.
- Hirst LW, Bancroft J, Yamauchi K, Green WR. Immunohistochemical pathology of the corneal endothelium in iridocorneal endothelial syndrome. Invest Ophthalmol Vis Sci. 1995;36(5):820–827.
- Holló G, Naghizadeh F. Optical coherence tomography characteristics of the iris in Cogan-Reese syndrome. Eur J Ophthalmol. 2014;24(5):797–799.
- Haemmerli G, Felix H. Shape and motility, two interdependent features. Scan Electron Microsc. 1982;(Pt 2):731–739.
- Bourne WM, Brubaker RF. Progression and regression of partial corneal involvement in the iridocorneal endothelial syndrome. Am J Ophthalmol. 1992;114(2):171–181.
- Malhotra C, Pandav SS, Gupta A, Jain AK. Phenotypic heterogeneity of corneal endothelium in iridocorneal endothelial syndrome by in vivo confocal microscopy. Cornea. 2014;33(6):634–637.
- Lucas-Glass TC, Baratz KH, Nelson LR, Hodge DO, Bourne WM. The contralateral corneal endothelium in the iridocorneal endothelial syndrome. Arch Ophthalmol. 1997;115(1):40–44.
- Doe EA, Budenz DL, Gedde SJ, Imami NR. Long-term surgical outcomes of patients with glaucoma secondary to the iridocorneal endothelial syndrome. Ophthalmology. 2001 Oct;108(10):1789-95.