What is collagen
Collagen is the major insoluble fibrous protein in the extracellular matrix and in connective tissue 1. In fact, collagen is the single most abundant protein in humans, collagen comprises one-third of the total protein, accounts for three-quarters of the dry weight of skin, and is the most prevalent component of the extracellular matrix. Type I collagen is the predominant form accounting for up to 90% of all the collagen proteins 2. In the skin, collagen, in conjunction with other proteins, such as elastin, forms the basic flexible and pliable matrix that incorporates living dermal cells, blood vessels, sebaceous glands, and other components of the extracellular matrix (glycosaminoglycans, glycoproteins) 3. Collagen is a major structural protein, forming molecular cables that strengthen the tendons and resilient sheets that support the skin and internal organs. Bones and teeth are made by adding mineral crystals to collagen. Collagen provides structure to your bodies, protecting and supporting the softer tissues and connecting them with the skeleton. But, in spite of its critical function in the body, collagen is a relatively simple protein.
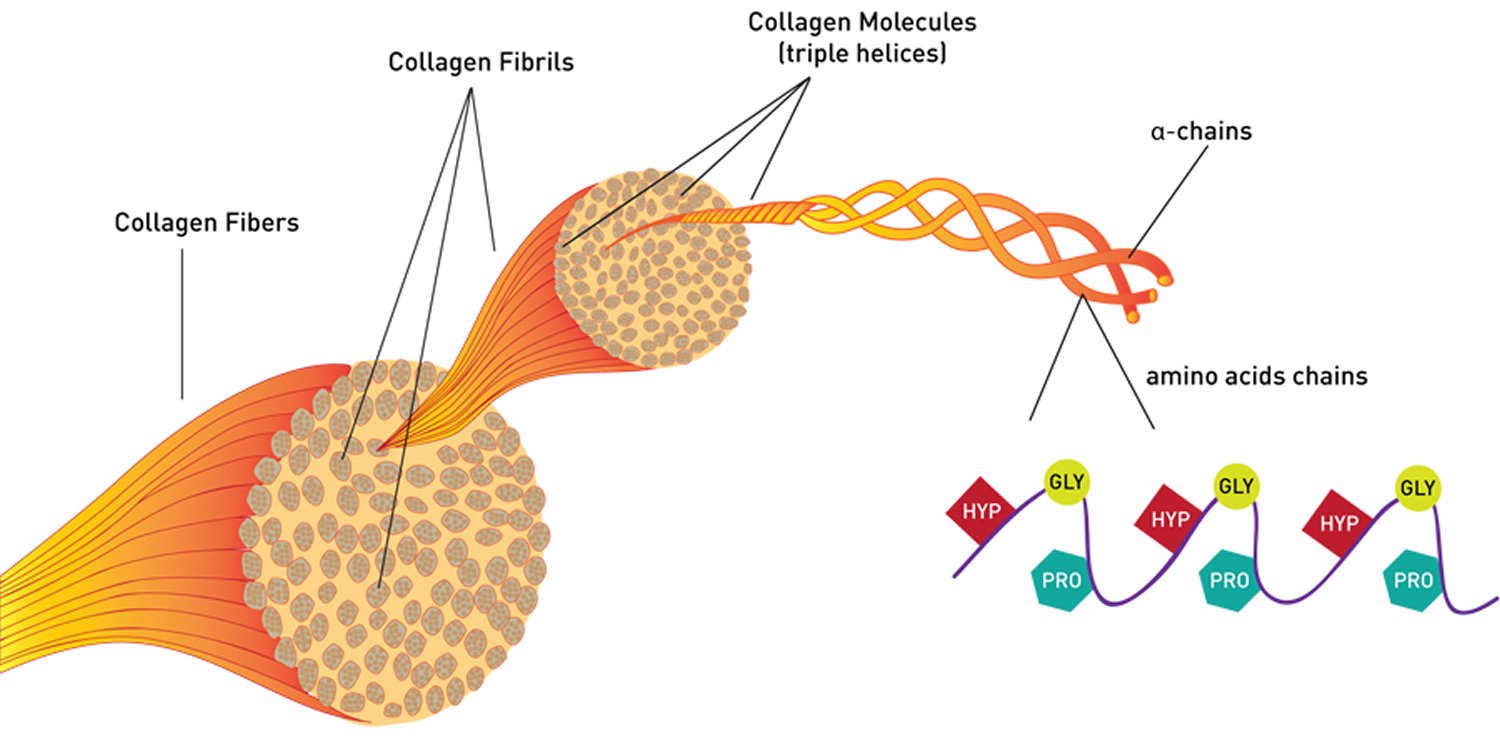
All collagens are composed of three polypeptide (alpha) α-chains, wound together in a tight triple helix configuration. The individual polypeptide chains of collagen each contain approximately 1000 amino acid residues. Because its abundance in tendon-rich tissue such as rat tail makes the fibrous type I collagen easy to isolate, it was the first to be characterized. Its fundamental structural unit is a long (300-nm), thin (1.5-nm-diameter) protein that consists of three coiled subunits: two α1(I) chains and one α2(I). Each chain contains precisely 1050 amino acids wound around one another in a characteristic right-handed triple helix (Figure 1). All collagens were eventually shown to contain three-stranded helical segments of similar structure; the unique properties of each type of collagen are due mainly to segments that interrupt the triple helix and that fold into other kinds of three-dimensional structures.
Collagen from livestock animals is a familiar ingredient for cooking. Like most proteins, when collagen is heated, it loses all of its structure. The triple helix unwinds and the chains separate. Then, when this denatured mass of tangled chains cools down, it soaks up all of the surrounding water like a sponge, forming gelatin.
The triple-helical structure of collagen arises from an unusual abundance of three amino acids: Glycine, Proline, and Hydroxyproline. These amino acids make up the characteristic repeating motif Gly-Pro-X, where X can be any amino acid 1. Each amino acid has a precise function. The side chain of glycine, an H atom, is the only one that can fit into the crowded center of a three-stranded helix. Hydrogen bonds linking the peptide bond NH of a glycine residue with a peptide carbonyl (C═O) group in an adjacent polypeptide help hold the three chains together. The fixed angle of the C – N peptidyl-proline or peptidyl-hydroxyproline bond enables each polypeptide chain to fold into a helix with a geometry such that three polypeptide chains can twist together to form a three-stranded helix. Interestingly, although the rigid peptidyl- proline linkages disrupt the packing of amino acids in an α helix, they stabilize the rigid three-stranded collagen helix.
Hydroxyproline, which is critical for collagen stability, is created by modifying normal proline amino acids after the collagen chain is built 4. The reaction requires vitamin C to assist in the addition of oxygen 4. Unfortunately, you cannot make vitamin C within your bodies, and if you don’t get enough in your diet, the results can be disastrous. Vitamin C deficiency slows the production of hydroxyproline and stops the construction of new collagen, ultimately causing scurvy. The symptoms of scurvy–loss of teeth and easy bruising–are caused by the lack of collagen to repair the wear-and-tear caused by everyday activities.
Figure 1. Collagen triple helix structure
Note: HYP= Hydroxyproline; GLY= Glycine; PRO= Proline. Every third amino acid residue is a Glycine residue, a small amino acid that fits perfectly inside the helix. Many of the remaining positions in the chain are filled by two amino acids: proline and a modified version of proline, hydroxyproline.
Figure 2. Overview of the collagen triple helix
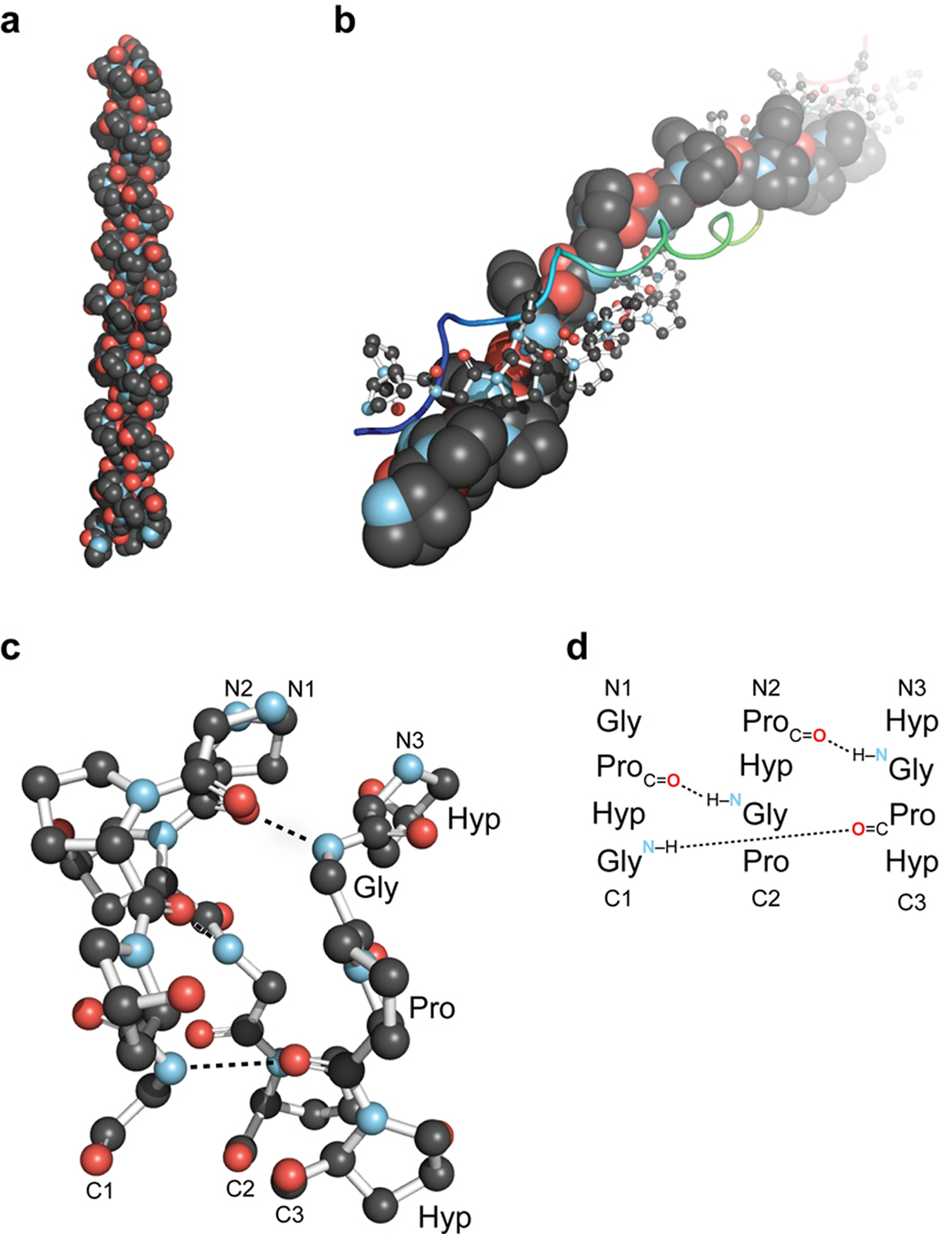
Note: (a) First high-resolution crystal structure of a collagen triple helix, formed from (ProHypGly)4–(ProHypAla)–(ProHypGly)5. (b) View down the axis of a (ProProGly)10 triple helix with the three strands depicted in space-filling, ball-and-stick, and ribbon representation. (c) Ball-and-stick image of a segment of collagen triple helix, highlighting the ladder of interstrand hydrogen bonds. (d) Stagger of the three strands in the segment in panel c.
Collagen Fibers Form by Lateral Interactions of Triple Helices
There are at least 16 types of collagen, but 80 – 90 percent of the collagen in the body consists of types I, II, and III (Table 1) 1. These collagen molecules pack together to form long thin fibers of similar structure. Type I collagen fibers have enormous tensile strength; that is, such collagen can be stretched without being broken. These fibers, roughly 50 nm in diameter and several micrometers long, are packed side-by-side in parallel bundles, called collagen fibers, in tendons, where they connect muscles with bones and must withstand enormous forces. Gram for gram, type I collagen is stronger than steel. Type IV, in contrast, forms a two-dimensional reticulum; several other types associate with fibril-type collagens, linking them to each other or to other matrix components. At one time it was thought that all collagens were secreted by fibroblasts in connective tissue, but we now know that numerous epithelial cells make certain types of collagens. The various collagens and the structures they form all serve the same purpose, to help tissues withstand stretching.
Many three-stranded type I collagen molecules pack together side-by-side, forming fibers with a diameter of 50 – 200 nm. In collagen fibers, adjacent collagen molecules are displaced from one another by 67 nm, about one-quarter of their length. This staggered array produces a striated effect that can be seen in electron micrographs of stained collagen fibrils; the characteristic pattern of bands is repeated about every 67 nm. The unique properties of the fibrous collagens — types I, II, III, and V — are due to the ability of the rodlike triple helices to form such side-by-side interactions.
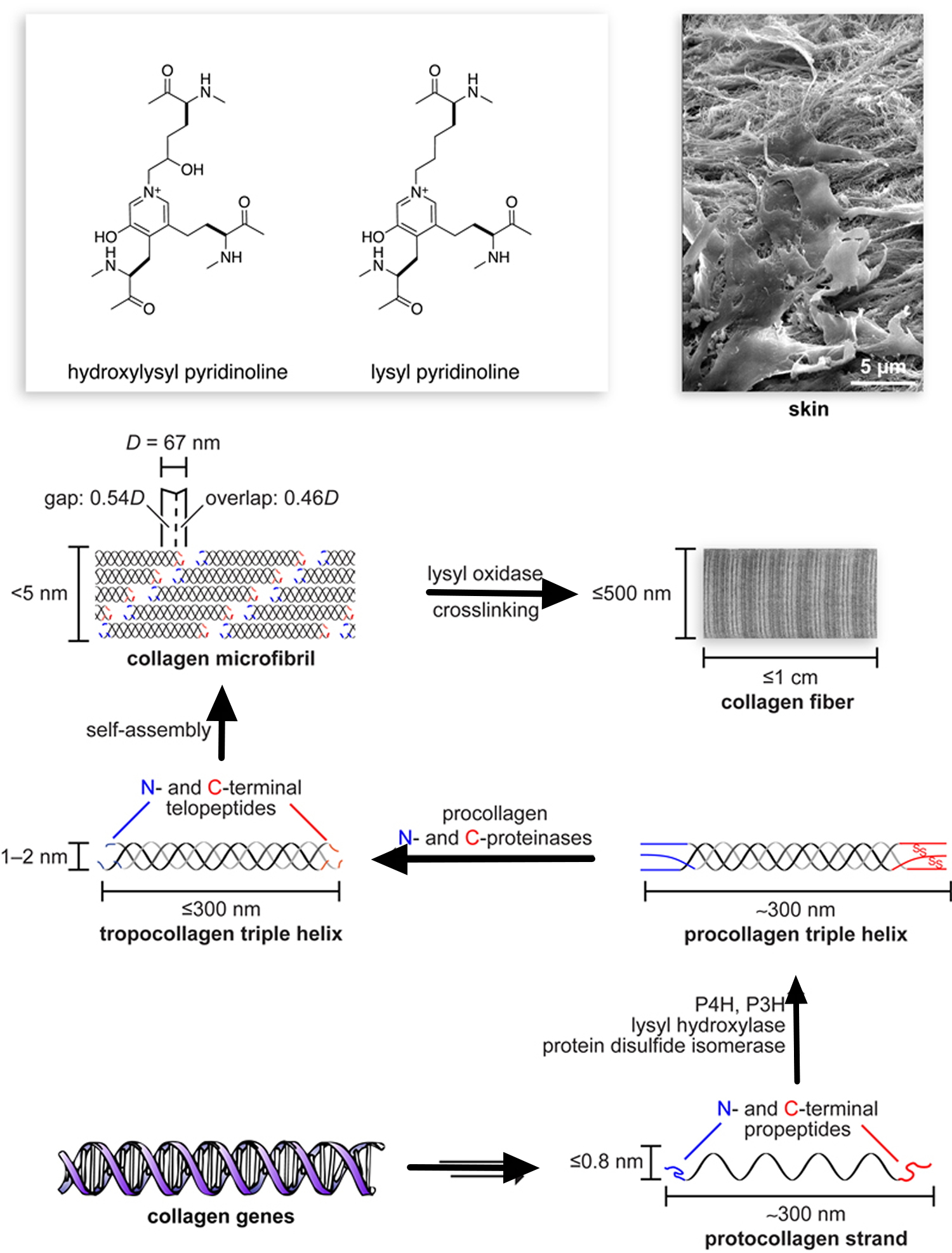
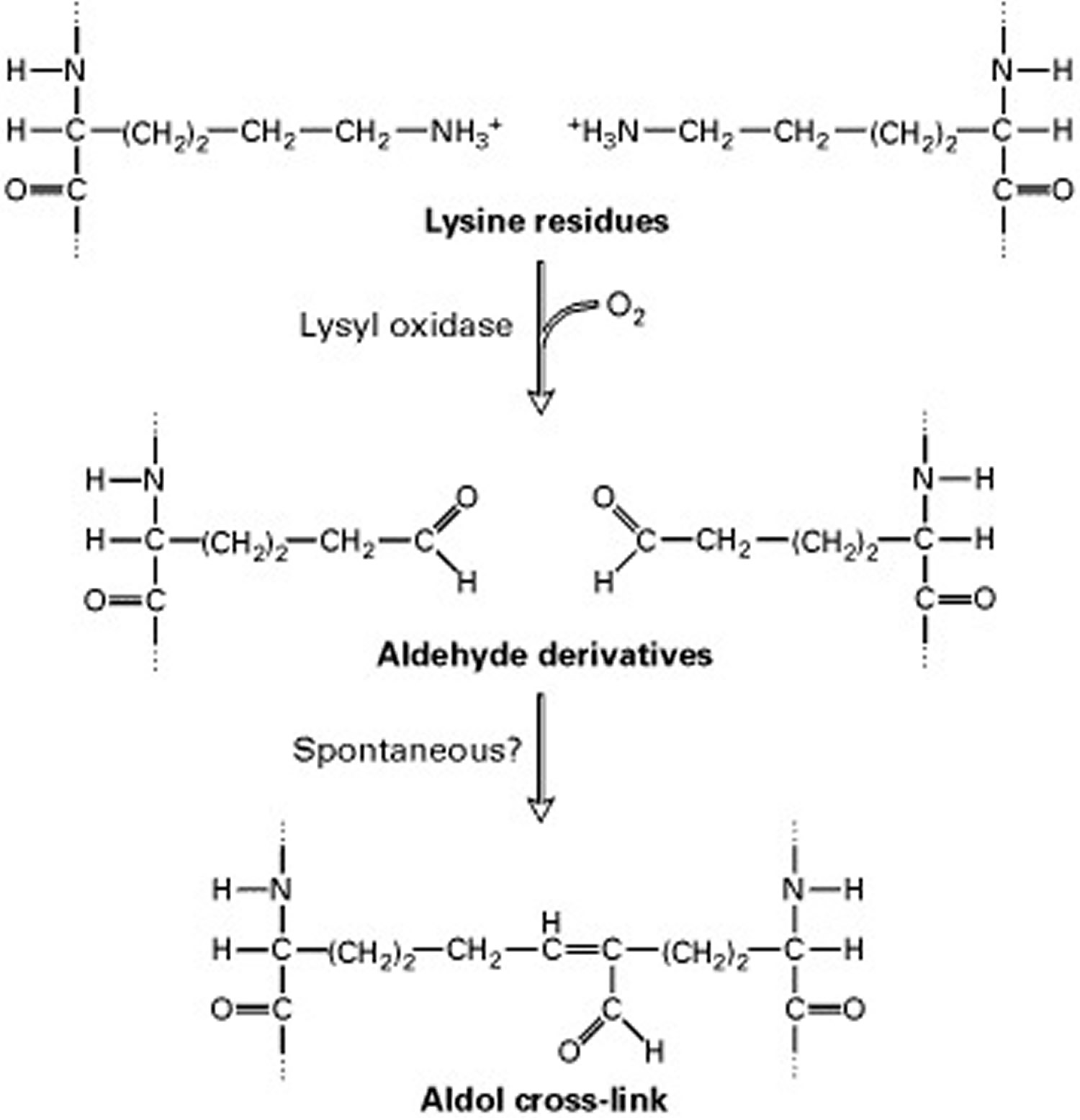
Short segments at either end of the collagen chains are of particular importance in the formation of collagen fibers. These segments do not assume the triple-helical conformation and contain the unusual amino acid hydroxylysine. Covalent aldol cross-links form between two lysine or hydroxylysine residues at the C-terminus of one collagen molecule with two similar residues at the N-terminus of an adjacent molecule. These cross-links stabilize the side-by-side packing of collagen molecules and generate a strong fiber.
Assembly of Collagen Fibers Begins in the endoplasmic reticulum and is completed outside the Cell
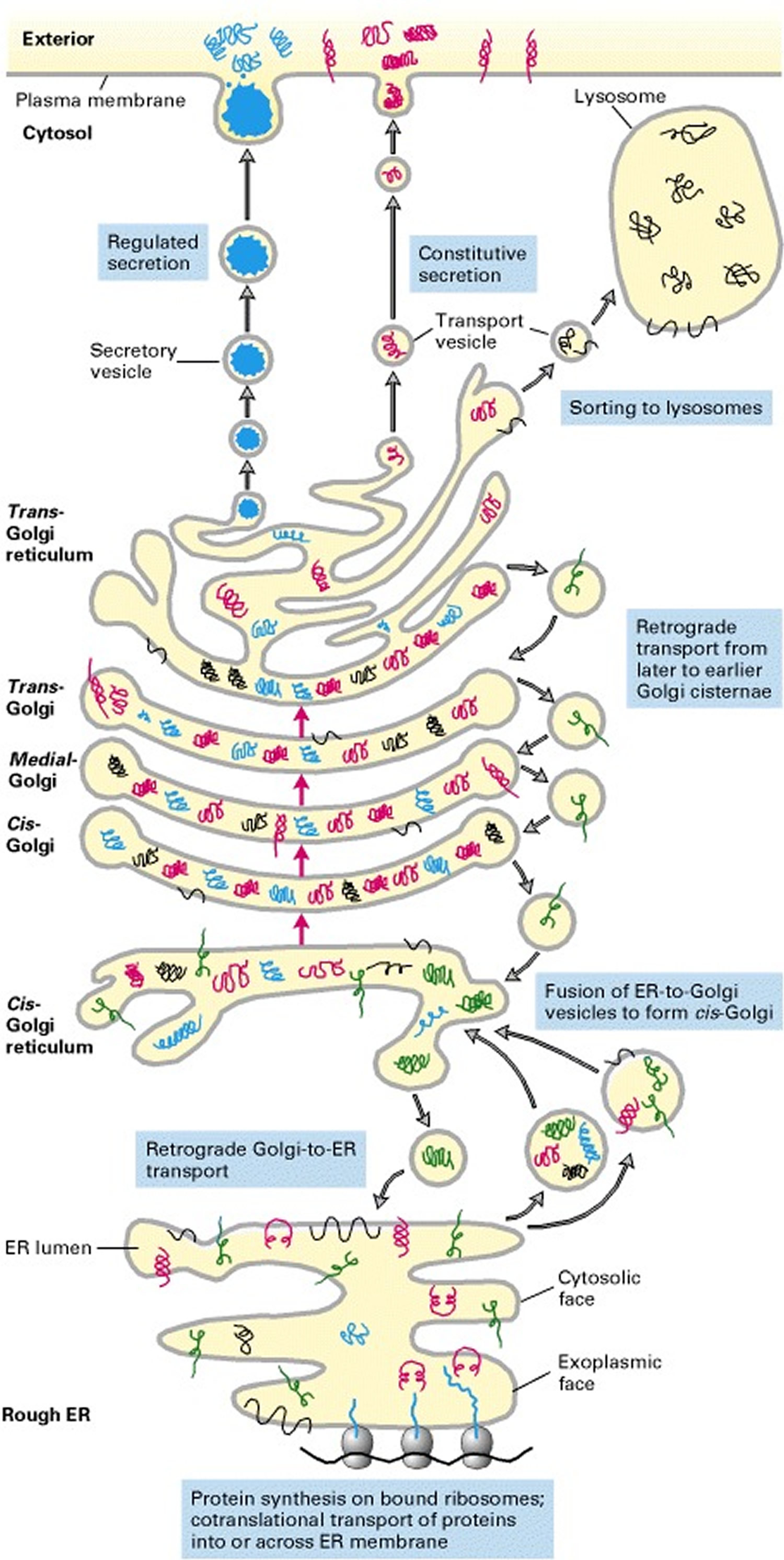
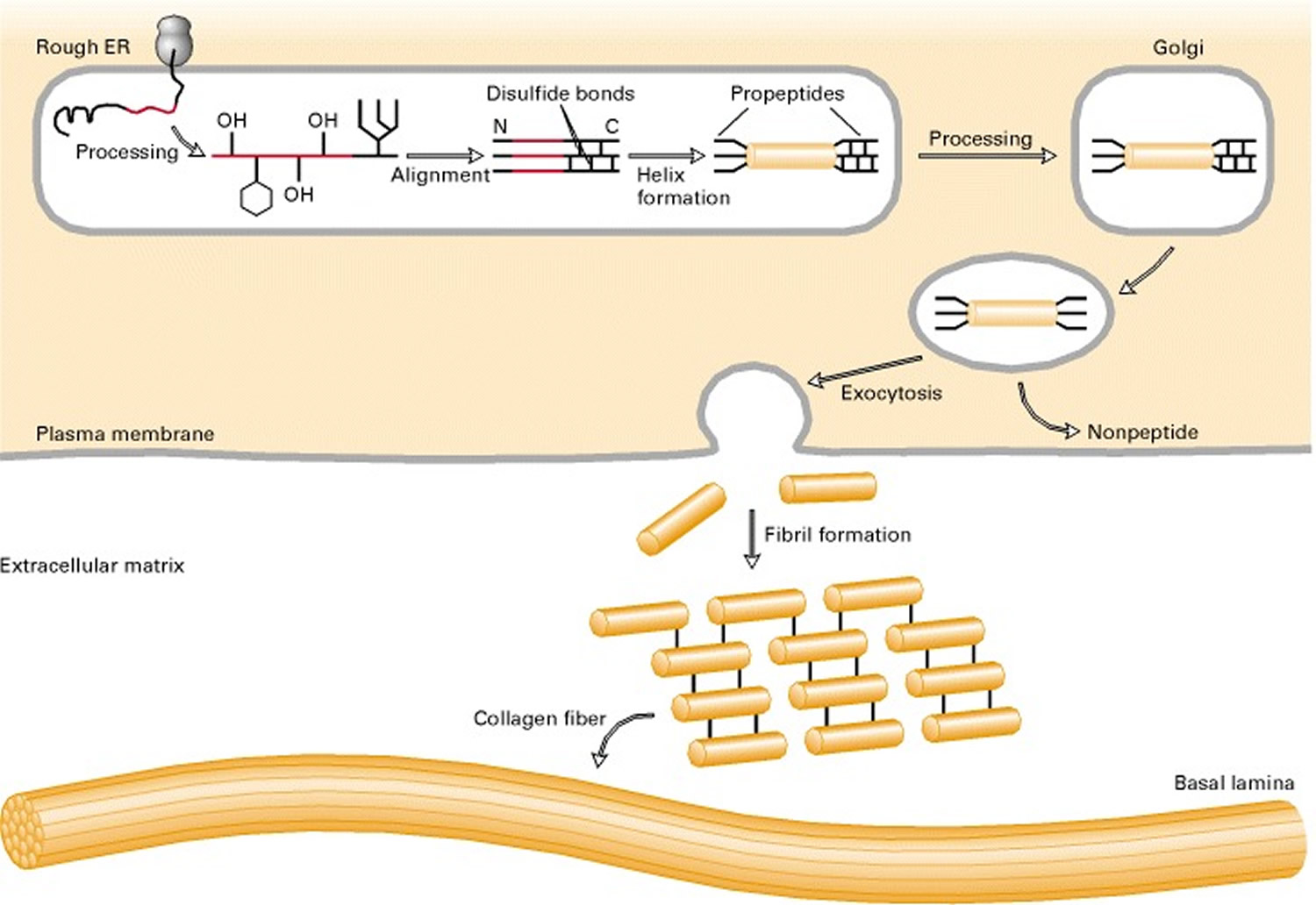
Collagen biosynthesis and assembly follows the normal pathway for a secreted protein (see Figure 3). The collagen chains are synthesized as longer precursors called procollagens; the growing peptide chains are co-translationally transported into the lumen of the rough endoplasmic reticulum (ER). In the ER, the procollagen chain undergoes a series of processing reactions (Figure 3). First, as with other secreted proteins, glycosylation of procollagen occurs in the rough ER and Golgi complex. Galactose and glucose residues are added to hydroxylysine residues, and long oligosaccharides are added to certain asparagine residues in the C-terminal propeptide, a segment at the C-terminus of a procollagen molecule that is absent from mature collagen. (The N-terminal end also has a propeptide.) In addition, specific proline and lysine residues in the middle of the chains are hydroxylated by membrane-bound hydroxylases. Lastly, intrachain disulfide bonds between the N- and C-terminal propeptide sequences align the three chains before the triple helix forms in the ER. The central portions of the chains zipper from C- to N-terminus to form the triple helix.
Figure 3. Collagen biosynthetic pathway
After processing and assembly of type I procollagen is completed, it is secreted into the extracellular space. During or following exocytosis, extracellular enzymes, the procollagen peptidases, remove the N-terminal and C-terminal propeptides. The resulting protein, often called tropocollagen (or simply collagen), consists almost entirely of a triple-stranded helix. Excision of both propeptides allows the collagen molecules to polymerize into normal fibrils in the extracellular space (see Figure 3). The potentially catastrophic assembly of fibrils within the cell does not occur both because the propeptides inhibit fibril formation and because lysyl oxidase, which catalyzes formation of reactive aldehydes, is an extracellular enzyme. As noted above, these aldehydes spontaneously form specific covalent cross-links between two triple-helical molecules, which stabilizes the staggered array characteristic of collagen molecules and contributes to fibril strength.
Post-translational modification of procollagen is crucial for the formation of mature collagen molecules and their assembly into collagen fibers. Defects in this process have serious consequences, as ancient mariners frequently experienced. For example, the activity of both prolyl hydroxylases requires an essential cofactor, ascorbic acid (vitamin C). In cells deprived of ascorbate, as in the disease scurvy, the procollagen chains are not hydroxylated sufficiently to form stable triple helices at normal body temperature, nor can they form normal fibers. Consequently, nonhydroxylated procollagen chains are degraded within the cell. Without the structural support of collagen, blood vessels, tendons, and skin become fragile. A supply of fresh fruit provides sufficient vitamin C to process procollagen properly.
Figure 4. Collagen fibers biosynthesis
Figure 5. Biosynthetic route to collagen fibers
Figure 6. The extracellular enzyme lysyl oxidase catalyzes formation of the aldehyde groups
Note: The side-by-side interactions of collagen helices are stabilized by an aldol cross-link between two lysine (or hydroxylysine) side chains.
Collagen Degradation
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases belonging to the metzincin superfamily. They participate in physiological (development and tissue repair) and pathological (tumorigenesis and metastasis) processes. Fibril-forming collagens I, II, and III are cleaved by MMP-1 (interstitial collagenase), MMP-8 (neutrophil collagenase), MMP-13 (collagenase 3) that generate three-quarter and one-quarter sized fragments, and by membrane-anchored MMP-14. MMP-2 is also able to cleave collagen I 5. Collagen II is a preferential substrate of MMP-13, whereas collagens I and III are preferentially cleaved by MMP-1 and MMP-8 5. Denatured collagens and collagen IV are degraded by MMP-2 and MMP-9 (also known as 72-kDa and 92-kDa gelatinases respectively). In contrast to the [α1 (I)]2α2(I) heterotrimer of collagen I, the [α2 (I)]3 homotrimer is not degraded by mammalian collagenases. This is because of resistance of the homotrimer to local triple helix unwinding by MMP-1 because it has higher triple helix stability near the MMP cleavage site 6. MMPs also contribute to the release of bioactive fragments or matricryptins such as endostatin and tumstatin from full-length collagens 7. Another group of enzymes, collectively called sheddases 8 releases the ectodomain of membrane collagens as soluble forms.
Collagens Form Diverse Structures
Collagens differ in their ability to form fibers and to organize the fibers into networks. We make many different kinds of collagen, which form long ropes and tough sheets that are used for structural support and as pathways for cellular movement during development. All contain a long stretch of triple helix connected to different types of ends. The simplest is merely a long triple helix, with blunt ends. These “type I” collagen molecules associate side-by-side, like fibers in a rope, to form tough fibrils. These fibers crisscross the space between nearly every one of our cells. Type II is the major collagen in cartilage. Its fibers are smaller in diameter than type I and are oriented randomly in the viscous proteoglycan matrix. Such rigid macromolecules impart a strength and compressibility to the matrix and allow it to resist large deformations in shape. This property allows joints to absorb shocks.
Type II fibers are cross-linked to proteoglycans in the matrix by type IX, a collagen of a different structure. Type IX collagen consists of two long triple helices connected by a flexible kink. The globular N-terminal domain extends from the composite fibrils, as does a heparan sulfate molecule, a type of large, highly charged polysaccharide (discussed later) that is linked to the α2(IX) chain at the flexible kink. These protruding nonhelical domains are thought to anchor the fibril to proteoglycans and other components of the matrix. The interrupted triple-helical structure of type IX collagen prevents it from assembling into fibrils; instead, these three collagens associate with fibrils formed from other collagen types and thus are called fibril-associated collagens (see Table 1).
A different collagen–“type IV”–forms the structural basis of basement membrane. Type IV collagen has a globular head at one end and an extra tail at the other. The heads bind strongly together, head-to-head, and four collagen molecules associate together through their tails, forming an X-shaped complex. Using these two types of interactions, type IV collagen forms an extended network, shown here in light blue. Two other molecules–cross-shaped laminin and long, snaky proteoglycans–fill in the spaces, forming a dense sheet.
Table 1. Major Collagen Molecules
| Type | Molecule Composition | Structural Features | Representative Tissues |
|---|---|---|---|
| Fibrillar Collagens | |||
| I | [α1(I)]2[α2(I)] | 300-nm-long fibrils | Skin, tendon, bone, ligaments, dentin, interstitial tissues |
| II | [α1(II)]3 | 300-nm-long fibrils | Cartilage, vitreous humor |
| III | [α1(III)]3 | 300-nm-long fibrils; often with type I | Skin, muscle, blood vessels |
| V | [α1(V)]3 | 390-nm-long fibrils with globular N-terminal domain; often with type I | Similar to type I; also cell cultures, fetal tissues |
| Fibril-Associated Collagens | |||
| VI | [α1(VI)][α2(VI)] | Lateral association with type I; periodic globular domains | Most interstitial tissues |
| IX | [α1(IX)][α2(IX)][α3(IX)] | Lateral association with type II; N-terminal globular domain; bound glycosaminoglycan | Cartilage, vitreous humor; |
| Sheet-Forming Collagens | |||
| IV | [α1(IV)]2[α2(IV)] | Two-dimensional network | All basal laminaes |
In many connective tissues, type VI collagen is bound to the sides of type I fibrils and may bind them together to form thicker collagen fibers. Type VI collagen is unusual in that the molecule consists of relatively short triple-helical regions about 60 nm long separated by globular domains about 40 nm long. Fibers of pure type VI collagen thus give the impression of beads on a string.
In some places, several extracellular matrix components are organized into a basal lamina, a thin sheetlike structure. Type IV collagen forms the basic fibrous two-dimensional network of all basal laminae. Three type IV collagen chains form a 400-nm-long triple helix with large globular domains at the C-termini and smaller ones of unknown structure at the N-termini. The helical segment is unusual in that the Gly-X-Y sequences are interrupted about 24 times with segments that cannot form a triple helix; these nonhelical regions introduce flexibility into the molecule. Lateral association of the N-terminal regions of four type IV molecules yields a characteristic tetrameric unit that can be observed in the electron microscope. Triple-helical regions from several molecules then associate laterally, in a manner similar to fibril formation among fibrous collagens, to form branching strands of variable but thin diameters. These interactions, together with those between the C-terminal globular domains and the triple helices in adjacent type IV molecules, generate an irregular two-dimensional fibrous network.
Collagen supplements
Skin is the largest organ in the human body, and serves several important functions. As well as having a sensory role, it provides a physical barrier against environmental factors. The two main layers that make up the skin are the epidermis and the dermis. The former consists of cells, which are proliferating (basal), differentiated (keratinocytes), or squamous. The dermis, on the other hand, contains fibroblasts, which produce elastin and collagen, including type I and type III, among other extracellular matrix proteins 9. The major collagens in human skin are types I and III which account for approximately 80% and 10% of the total bulk of collagen, respectively 10. Embedded within the collagen fiber network are glycosaminoglycans, comprising mainly hyaluronic acid and dermatan sulfate. The molecular size of hyaluronic acid is quite important, as it affects the physicochemical properties of the skin, such as its ability to retain water, and its elasticity and viscosity. Hyaluronic acid consists of a helical chain that comprises alternating units of N-acetylglucosamine and glucuronic acid, and its average molecular weight has been shown to be in the range of 10–104 kDa 11.
The extracellular matrix accounts for almost 80% of the skin’s dry weight 12. For the skin to function normally and appear youthful, the structure of the dermal layer must be maintained because the dermis provides structural support to the epidermis, which carries the blood vessels and supplies the skin with important nutrients for its functioning. However, natural aging can affect the structural integrity of the dermis. In addition, areas of the body exposed to the sun are prone to photoaging. Both these types of aging can be exacerbated by diet. High sugar levels lead to development of advanced glycation end products due to a chemical reaction between glucose and the free amino groups in proteins. Advanced glycation end products remain in the tissue where they form because they cannot be degraded normally by enzymes. All these factors affect fibroblasts in the dermis by causing changes in their shape and in the amount and quality of elastin and type I collagen fiber production. Moreover, aged fibroblasts synthesize less collagen, both in vitro and in vivo, when compared with young adult fibroblasts 13. This results in visible signs of aging, which are usually most prominent on the face.
The photoaged dermis contains collagen fibers and elastin that are disorganized and abnormal in nature 14, 15. Recently, it has been shown that smoking alters the components of the dermis and leads to premature aging of components in the dermis.9,10 Stress also seems to affect the integrity of collagen in the skin. An important indicator of the harmful effects of chronic stress is dysregulation of the circadian cortisol/corticosterone rhythm 16 which is known to alter both the synthesis and degradation of collagen 17.
In order to be active in the deeper layers of the skin, hydrolyzed collagen has to cross the intestinal barrier and reach the bloodstream. It is worth noting that the rate of transport across the intestinal barrier could be a controlling step that affects the efficacy of these compounds in the skin. Therefore, it is imperative to demonstrate whether collagen peptides can be absorbed, and in what form and quantity, before speculating on their mechanism of action.
According to Richelle et. al 18 bioavailability is the amount of a nutritional bioactive compound that crosses the intestinal barrier to reach the bloodstream and is made available for either storage in the body (in this context, in the skin) or a metabolic process.
The first step in digestion consists of degradation of hydrolyzed collagen to form dipeptides and tripeptides or free amino acids. Several proteases (eg, pancreatic proteases, small intestinal brush-border proteases, peptidase) are involved in the degradation process.
Ingesting hydrolyzed collagen could be a useful strategy to counteract the changes associated with skin aging. An experiment by Iwai et. al 19 showed that a significant amount of hydrolyzed collagen derived from hydroxyproline appeared in the blood of healthy human volunteers who consumed hydrolyzed collagen from cartilage, chicken feet, and porcine skin after 12 hours of fasting. After intake of collagen, the amount of hydroxyproline-containing peptides in the blood increased, reaching a peak after 2 hours followed by a decrease to half the maximum level at 4 hours after ingestion. A small peptide, proline-hydroxyproline (Pro-Hyp), was found in the blood after ingestion of hydrolyzed collagen. It was found that the amount of Pro-Hyp present in human plasma was 25–60 nmol/mL after ingesting 9.4–23 g of hydrolyzed collagen 19. The higher levels of Pro-Hyp found in blood could be partly explained by the higher quantity of the Pro-Hyp sequence in collagen. Studies by Iwai et. al 19 suggest that Pro-Hyp can be considered an indigestible peptide as more than 75% of Pro-Hyp was shown to persist in the blood for 24 hours after in vitro reaction with human serum.
Peptides generated by hydrolysis of a large collagen molecule can have great benefits on health and can improve skin properties. Using “gut sac” experiments, Oesser et. al 20 investigated the molecular weight of hydrolyzed collagen absorbed in the intestinal tract. Techniques such as high performance liquid chromatography and sodium dodecyl sulfate polyacrylamide gel electrophoresis showed that peptides in the molecular weight range of 1–10 kDa may be absorbed.
Chen et. al 21 studied the effect of different concentrations of hydrolyzed collagen derived from fish on fibroblasts and keratinocytes. In particular, they looked at proliferation and collagen production. They found that a collagen concentration of 48–97 μg/mL resulted in optimal proliferation (191%) 16. Ohara et. al 22 conducted a single-blind, crossover study comparing the structure and quantity of food-derived gelatin hydrolysates in human blood from three sources of type I collagen. Five healthy male volunteers ingested type I gelatin hydrolysates from fish scales, fish skin, or porcine skin after 12 hours of fasting. It was found that about 30% of hydroxyproline-containing peptides were detected in blood even after a duration of 24 hours.
However, there is substantial evidence that peptides can be hydrolyzed in the gastrointestinal tract before they are absorbed, so that predominantly free amino acids can enter the circulation. Hydroxyproline is absorbed in two forms, ie, an amino acid form and a peptide form 22.
The mechanism of absorption across the intestine membrane has been extensively studied. Epithelial cells are important sites of absorption of numerous nutrients. There are three ways in which intestinal transport of oligopeptides can take place: PEPT1-mediated transport of dipeptides and tripeptides mediated by PEPT1 23; transport of macromolecules such as proteins via the transcytotic route 24; and transport for peptide absorption by the passive intracellular route 25. The complete role of these pathways in intestinal oligopeptide absorption is not yet fully understood.
Transcellular transport of these peptides across intestinal epithelial cells is a two-step mechanism, which involves transport across two separate membranes, ie, uptake of peptides by epithelial cells across the brush-border membrane and absorption into the bloodstream across the basolateral membrane 26. The first step is initiated by hydrogen ion-coupled peptide transporters, namely PEPT1 and PEPT2. PEPT1 functions as an enantioselective transporter of monovalent, polyvalent, and neutral charged peptides.25 It has been shown that collagen-derived peptides (Pro-Hyp and glycine-Pro-Hyp) 27 are absorbed via the PEPT1 transporter.
Distribution is usually defined as the process by which a compound reaches the target tissue through the blood circulation. Factors that can affect the rate of distribution are blood flow and the chemical features of a given compound, such as molecular size and polarity.
After ingestion, collagen peptides are digested and distributed throughout the body. Watanabe-Kamiyama et al 28 studied the distribution of collagen peptides to the skin and other tissues by means of an in vivo experiment in which 14C-labeled proline or collagen peptides were administered to Wistar rats. Radioactivity was measured in different tissues 0–6 hours after ingestion of the collagen peptides and for 14 days thereafter. The results were very promising in terms of residence time in the skin and showed that radioactivity remained in skin tissue at a high level for up to 14 days. This indicates the ability of collagen peptides to reach the dermis in the skin where their main benefit is observed.
Benefits of collagen
Skin anti-aging
In a study 29 on skin aging and 5,000 mg hydrolyzed collagen supplement with soluble hydrolyzed collagen type I obtained from the cartilage of fish, which contains low-molecular-weight hyaluronic acid, as well as several vitamins and minerals – citric acid, pyridoxine hydrochloride (vitamin B6), extract of black pepper (Piper nigrum), copper, borage seed (Borago officinalis) oil, glycerol, soy lecithin, biotin soybean polysaccharide, malic acid, ascorbic acid (vitamin C), hyaluronic acid, D-α-tocopherol (vitamin E), sucralose, N-acetylglucosamine, Stevia, zinc, and biotin. The 217 study volunteers (Caucasian women aged 23-69 years) were instructed to drink their food supplement once a day in the morning before breakfast. The study found that 15% of subjects taking 5,000 mg hydrolyzed collagen supplement had fewer facial lines and wrinkles after 60 days, even though they did not undergo any form of cosmetic procedure. An increased level of collagen in the dermis was detected in individual subjects after 12 weeks. Moreover, 32% had an improvement in the level of photoaging and 39% had less skin dryness 29. These subjects either had no form of cosmetic treatment or had only a localized procedure. However, before any recommendation can be derived from this study, a randomized placebo-controlled clinical trial is now required to confirm the true effect of hydrolyzed collagen supplement on skin aging (wrinkles, dryness, photoaging, elasticity) when compared with other cosmetic procedures.
Another study conducted by Proksch et. al 30 involving 69 women aged 35-55 years to investigate the effect of daily collagen hydrolysate supplementation ( 2.5 g or 5 g of collagen hydrolysate) and cutaneous aging. After 8 weeks the investigators reported that skin elasticity in both collagen hydrolysate dosage groups was significantly improved in comparison with placebo. Skin moisture and evaporation were also positively influenced by treatment with collagen hydrolysate, but the results were not statistically significant.
Skin dermal filler
The appearance of wrinkles is important to people who want to prolong their youthful appearance. Some facial wrinkles have superficial and deep components, such as the nasolabial fold, crow’s feet around the eyes, forehead, and cheeks 31. Type III human collagen is becoming recognized as an important component of youthful skin. Type III collagen constitutes 50% of collagen in neonatal skin dermis but only 5% of collagen in aging adult skin. Collagen composition in the skin changes with age and facial rhytids, caused mainly by loss of collagen, especially type III. The ideal filler for soft tissue augmentation should be safe and effective; easy to obtain and administer; have a minimal risk for infection, extrusion, or migration; produce a minimal inflammatory reaction; last for an acceptable period of time 32; and be usable without pretesting in patients. It should also be cost effective, show consistency and reproducibility, and yield a highly acceptable result 32. The substance should be approved by the Food and Drug Administration 31.
Because collagen is immunologically benign, resistant to proteolysis, and a natural substrate for cell adhesion, collagen is the ideal biomaterial for soft tissue augmentation 33. Many collagen-based products are available to correct facial wrinkles, acne scars, and other small areas of skin defects.
Although bovine collagen preparations are of low immunogenicity, they are still foreign proteins. Their antigenicity is not low enough to escape an immunologic response in all patients. The major disadvantage of bovine collagen is a treatment-associated hypersensitivity reaction that consists of redness and swelling at the treatment site 31. These reactions occurred in 1.3 to 3% of patients treated with Zyderm I or II 34. These allergic reactions are believed to be an immunological response to bovine collagen. Therefore, 2 consecutive skin tests are recommended (6 and 2 weeks) prior to treatment 35. In addition, the risk of xenogenic transmission of bovine spongiform encephalopathy is not eliminated 36. In contrast, collagen products derived from human cells do not pose an allergy risk 37. Many investigators believe the use of human-based collagen will overcome this shortcoming 38.
Type III collagen induces tissue ingrowth and improves tissue repair, spatial organization, and neovascularization. Type III collagen is angiogenic and encourages neovessel formation and tissue regeneration. It has been shown in preclinical studies to induce fibrocytic ingrowth and neovascularization with no inflammation. Type III atelocollagen is naturally cross-linked by natural disulfide bridges that do not exist in type I atelocollagen.
For these reasons, using a dermal filler with a high type III collagen content may produce better results.
Type I and type III injectable human collagen was made from highly purified human collagen obtained from placentas according to a known method for collagen extraction and purification 39.
In this study 33 involving 123 Chinese subjects who were injected with type I and type III human collagen, which contains 50% type I and 50% type III collagen to treat wrinkles. The results of this clinical trial indicated that type I and type III injectable human collagen corrected small skin defects such as furrows with a satisfaction rate of 90% or higher. Type I and type III injectable human collagen is recommended for superficial furrows and scars. This is in agreement with a previous study 40. Patients may require retreatment 6 to 8 months after the first injection, 8 to 12 months after the second injection, and 12 to 18 months after the third injection, if not longer; many patients reported that collagen lasted 2 to 3 years after the first injection 33. There were no adverse or immune reactions were reported for these patients.
Injectable human collagen side effects: A slight redness and discomfort at or near the injection site were observed in 20 patients, with an incidence rate of 16.2%. This local reaction typically occurs after needle injection. The redness and discomfort usually resolve within 24 to 48 hours.
This incidence rate of 16.2% (out of 123 patients) included 18 cases of minor subcutaneous hematomas and 2 cases of subcutaneous hemorrhage at the site of injection. All of these responses (small hematomas and hemorrhages) were absorbed into the body and resolved naturally. One patient refused a second injection after the appearance of a subcutaneous hematoma and was removed from the study 33. No other side effects were observed. There were no differences in the tolerability of the first and subsequent injections.
Osteoarthritis of the knee
Arthritis afflicts approximately 43 million Americans or approximately 16.6% of the US population. The two most common types of arthritis are osteoarthritis and rheumatoid arthritis. Osteoarthritis of the knee and hip is a growing health concern and is the most common forms of arthritis 41. Pain and disease can range from very mild to very severe. Osteoarthritis can affect any joint, but it occurs most often in knees, hips, lower back and neck, small joints of the fingers and the bases of the thumb and big toe. Patients with osteoarthritis have pain that typically worsens with weight bearing, including walking and standing, and improves with rest 42. Other symptoms include morning stiffness and gelling of the involved joint after periods of inactivity. Currently, osteoarthritis affects nearly 27 million people in the United States, accounting for 25% of visits to primary care physicians, and half of all Non-Steroidal Anti-Inflammatory Drugs (NSAID) prescriptions 43. The diverse clinical patterns of osteoarthritis are observed in approximately 10% of people older than 60 years thus compromising the quality of life of millions of Americans. Current treatment of osteoarthritis includes exercise, heat/cold therapy, joint protection, weight loss, physiotherapy/occupational therapy and medications 44.
According to published research, orally administered collagen hydrolysate has been shown to be absorbed intestinally and to accumulate in cartilage. Collagen hydrolysate ingestion stimulates a statistically significant increase in synthesis of extracellular matrix macromolecules by chondrocytes ( compared with untreated controls). These findings suggest mechanisms that might help patients affected by joint disorders such as osteoarthritis 45. Four open-label and three double-blind studies were identified and reviewed; although many of these studies did not provide key information – such as the statistical significance of the findings – they showed collagen hydrolysate to be safe and to provide improvement in some measures of pain and function in some men and women with osteoarthritis or other arthritic conditions 45.
An emerging novel nutraceutical ingredient known as undenatured type II collagen derived from chicken sternum cartilage, has received considerable attention in the treatment of osteoarthritis. Type II collagen is the primary form of collagen contained in cartilage. Type II collagen extracts contain the amino acids found in the framework of human cartilage. In addition, these amino acids are required for the synthesis and repair of connective tissue throughout the body. These products reportedly aid in reducing the destruction of collagen within the body, may provide anti-inflammatory activity, and may improve joint flexibility 46. Previous studies have shown that undenatured type II collagen is effective in the treatment of rheumatoid arthritis 47, 48, and preliminary human 12 and animal 13 trials have shown it to be effective in treating osteoarthritis. Obese-arthritic dogs given 4 mg or 40 mg daily dose of undenatured type II collagen for 90 days showed significant declines in overall pain, pain during limb manipulation and lameness after physical exertion 49. Greater improvement was observed with the 40 mg dose. No adverse effects or significant changes in serum chemistry were noted. Following undenatured type II collagen withdrawal for a period of 30 days, all dogs experienced a relapse of overall pain, exercise-associated lameness and pain upon limb manipulation. Studies have also shown that small doses of orally administered undenatured type II chicken collagen inhibit killer T-cell attack 50. In this study 51, the efficacy of undenatured type II collagen was studied in 52 patients identified with moderate to severe osteoarthritis of the knee. taking a single oral daily dose of 40 mg undenatured type II collagen on an empty stomach prior to bedtime for 42 consecutive days, an average of 26% reduction of pain was noted in four of five subjects in the study. No side effects were associated with treatment. Moreover, treatment with undenatured type II collagen was found to be more effective in reducing the Western Ontario McMaster Osteoarthritis Index (WOMAC) scores by 33% as compared to 14% in glucosamine and chondroitin treated groups after 90 days. Similar results were observed for visual analog scale (VAS) scores. Although both the treatments reduced the visual analog scale (VAS) score, undenatured type II collagen was found to be more effective with 40% decrease after 90 days of treatment as compared to 15.4% in glucosamine and chondroitin treated groups. The Lequesne’s functional index was used to determine the effect of different treatments on pain during daily activities. Treatment with undenatured type II collagen reduced Lequesne’s functional index by 20.1% as compared to 5.9 % in glucosamine and chondroitin treated groups. Thus, undenatured type II collagen supplementation showed improvement in daily activities suggesting an improvement in overall quality of life in the patients receiving undenatured type II collagen 51. The precise biochemical mechanism involved in undenatured type II collagen induced pharmacological anti-arthritic effects in humans, dogs or horses is not clearly established.
Burns and wounds healing
Collagen, which is produced by fibroblasts, is the most abundant protein in the human body. A natural structural protein, collagen is involved in all 3 phases of the wound-healing cascade. It stimulates cellular migration and contributes to new tissue development 52. Because of their chemotactic properties on wound fibroblasts, collagen dressings encourage the deposition and organization of newly formed collagen, creating an environment that fosters healing. Collagen-based biomaterials stimulate and recruit specific cells, such as macrophages and fibroblasts, along the healing cascade to enhance and influence wound healing. These biomaterials can provide moisture or absorption, depending on the delivery system. Collagen dressings are easy to apply and remove and are conformable. Collagen dressings are usually formulated with bovine, avian, or porcine collagen. Oxidized regenerated cellulose, a plant-based material, has been combined with collagen to produce a dressing capable of binding to and protecting growth factors by binding and inactivating matrix metalloproteinases in the wound environment 53.
Films made from hydrolyzed collagen have been used as a tissue adhesive for suture replacement due to its chemical resemblance to connective tissue and its tissue fluid-binding properties. Moreover, it is biodegradable, non-toxic and readily absorbed; therefore, it does not impose a hindrance to the healing process 54.
The sponges made from pure collagen isolated from bovine skin are swollen at pH 3.0 and stabilized into the physical form of a sponge layer 55. In order to achieve highly resilient (elastic) activity and fluid-building capacity, collagen sponges have been combined with other materials like elastin, fibronectin or glycosaminoglycans 56. The starting material can be cross-linked with glutaraldehyde and subsequently co-polymerized with other polymers, such as polyhydroxyethylmethacrylate (PHEMA). The PHEMA chains, which are hydrophilic, keep the membranes wet and increase their tensile strength. This further affects the efficiency in the management of infected wounds and burns. Collagen sponges have been very useful in the treatment of severe burns and as a dressing for many types of wounds, such as pressure sores, donor sites, leg ulcers and decubitus ulcers, as well as for in vitro test systems 55. Collagen sponges have the ability to easily absorb large quantities of tissue exudates, smoothly adhere to the wet wound bed with preservation of low moist climate and also shield against mechanical harm and secondary bacterial infection. Coating of a collagen sponge with growth factor further facilitates dermal and epidermal wound healing 57. Collagen sponges are also used for delivery of steroids through topical applications, such as intravaginal delivery of lipophilic compounds including retinoic acid. Collagen-based sponges are inserted into a cervical cap made of hydrogel hypan to deliver all-trans-retinoic acid to patients with cervical dysplasia 58.
Collagen also has the ability of enhancing wound healing following dental therapy by clot formation and stabilization, neovascularization and epithelial cell rejuvenation thus acting as a natural hemostatic agent 59. Collagen also serves as a biologic scaffold for ingrowths of endothelial cells and progenitor cells from the periodontal ligament 60.
For oral applications, homogenized reconstituted collagen mixed with cell culture media has been used for endodontic repair 61.
Notably, collagen-based membranes have been widely used in periodontal and implant therapy as barriers that prevent the migration of epithelial cells and encourage wound repopulation by cells with regenerative potential 62.
Collagen side effects
Overall, possible side effects related to collagen supplement products were constipation and headaches (intermittently).
References- Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 22.3, Collagen: The Fibrous Proteins of the Matrix. Available from: https://www.ncbi.nlm.nih.gov/books/NBK21582/
- Abraham LC, Zuena E, Perez-Ramirez B, Kaplan DL. Guide to Collagen Characterization for Bio Studies. J. Biomed. Mat. Res. 2008;87B:264–285. https://www.ncbi.nlm.nih.gov/pubmed/18386843
- Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Water P. 4th ed. Garland Science; New York, NY: 2002. Molecular Biology of the Cell.
- Collagen. The RCSB PDB. http://pdb101.rcsb.org/motm/4
- Physiology and pathophysiology of matrix metalloproteases. Klein T, Bischoff R. Amino Acids. 2011 Jul; 41(2):271-90. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3102199/
- Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. Han S, Makareeva E, Kuznetsova NV, DeRidder AM, Sutter MB, Losert W, Phillips CL, Visse R, Nagase H, Leikin S. J Biol Chem. 2010 Jul 16; 285(29):22276-81. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2903388/
- Ricard-Blum S, Ballut L 2011. Matricryptins from collagens and proteoglycans. Front Biosci 16: 674–697. https://www.ncbi.nlm.nih.gov/pubmed/21196195
- Regulation of the proteolytic disintegrin metalloproteinases, the ‘Sheddases’. Murphy G. Semin Cell Dev Biol. 2009 Apr; 20(2):138-45. https://www.ncbi.nlm.nih.gov/pubmed/18840536/
- Champion RH, Rook A, Burton JL, Ebling FJG, Wilkinson DS. Textbook of Dermatology. Oxford, UK: Blackwell Scientific; 1992.
- Chung HJ, Uitto J. Type VII Collagen: The Anchoring Fibril Protein at Fault in Dystrophic Epidermolysis Bullosa. Dermatologic clinics. 2010;28(1):93-105. doi:10.1016/j.det.2009.10.011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2791403/
- Secondary structures in hyaluronan solutions: chemical and biological implications. Scott JE. Ciba Found Symp. 1989; 143:6-15; discussion 15-20, 281-5. https://www.ncbi.nlm.nih.gov/pubmed/2680349/
- Understanding premature skin aging. Uitto J. N Engl J Med. 1997 Nov 13; 337(20):1463-5. http://www.nejm.org/doi/full/10.1056/NEJM199711133372011
- Decreased collagen production in chronologically aged skin: roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Varani J, Dame MK, Rittie L, Fligiel SE, Kang S, Fisher GJ, Voorhees JJ. Am J Pathol. 2006 Jun; 168(6):1861-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1606623/
- Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, Ahmad H, Uitto J. Exp Dermatol. 2002 Oct; 11(5):398-405. https://www.ncbi.nlm.nih.gov/pubmed/12366692/
- Pathophysiology of premature skin aging induced by ultraviolet light. Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. N Engl J Med. 1997 Nov 13; 337(20):1419-28. http://www.nejm.org/doi/full/10.1056/NEJM199711133372003
- Diurnal cortisol rhythm as a predictor of breast cancer survival. Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. J Natl Cancer Inst. 2000 Jun 21; 92(12):994-1000. https://www.ncbi.nlm.nih.gov/pubmed/10861311/
- Genomic and nongenomic effects of glucocorticoids. Stahn C, Buttgereit F. Nat Clin Pract Rheumatol. 2008 Oct; 4(10):525-33. https://www.ncbi.nlm.nih.gov/pubmed/18762788/
- Skin bioavailability of dietary vitamin E, carotenoids, polyphenols, vitamin C, zinc and selenium. Richelle M, Sabatier M, Steiling H, Williamson G. Br J Nutr. 2006 Aug; 96(2):227-38. https://www.ncbi.nlm.nih.gov/pubmed/16923215/
- Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates. Iwai K, Hasegawa T, Taguchi Y, Morimatsu F, Sato K, Nakamura Y, Higashi A, Kido Y, Nakabo Y, Ohtsuki K. J Agric Food Chem. 2005 Aug 10; 53(16):6531-6. https://www.ncbi.nlm.nih.gov/pubmed/16076145/
- Oral administration of (14)C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). Oesser S, Adam M, Babel W, Seifert J. J Nutr. 1999 Oct; 129(10):1891-5. http://jn.nutrition.org/content/129/10/1891.long
- N-acetylglucosamine: production and applications. Chen JK, Shen CR, Liu CL. Mar Drugs. 2010 Sep 15; 8(9):2493-516. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2953398/
- Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. J Agric Food Chem. 2007 Feb 21; 55(4):1532-5. https://www.ncbi.nlm.nih.gov/pubmed/17253720/
- Molecular and integrative physiology of intestinal peptide transport. Daniel H. Annu Rev Physiol. 2004; 66():361-84. https://www.ncbi.nlm.nih.gov/pubmed/14977407/
- Adsorptive-mediated endocytosis of a basic peptide in enterocyte-like Caco-2 cells. Sai Y, Kajita M, Tamai I, Wakama J, Wakamiya T, Tsuji A. Am J Physiol. 1998 Sep; 275(3 Pt 1):G514-20. http://ajpgi.physiology.org/content/275/3/G514.long
- Quantitative approaches to delineate paracellular diffusion in cultured epithelial cell monolayers. Adson A, Raub TJ, Burton PS, Barsuhn CL, Hilgers AR, Audus KL, Ho NF. J Pharm Sci. 1994 Nov; 83(11):1529-36. https://www.ncbi.nlm.nih.gov/pubmed/7891269/
- Role of amino acid transport and countertransport in nutrition and metabolism. Christensen HN. Physiol Rev. 1990 Jan; 70(1):43-77. http://physrev.physiology.org/content/70/1/43.long
- Transport of a tripeptide, Gly-Pro-Hyp, across the porcine intestinal brush-border membrane. Aito-Inoue M, Lackeyram D, Fan MZ, Sato K, Mine Y. J Pept Sci. 2007 Jul; 13(7):468-74. https://www.ncbi.nlm.nih.gov/pubmed/17554807/
- Absorption and effectiveness of orally administered low molecular weight collagen hydrolysate in rats. Watanabe-Kamiyama M, Shimizu M, Kamiyama S, Taguchi Y, Sone H, Morimatsu F, Shirakawa H, Furukawa Y, Komai M. J Agric Food Chem. 2010 Jan 27; 58(2):835-41. https://www.ncbi.nlm.nih.gov/pubmed/19957932/
- Borumand M, Sibilla S. Daily consumption of the collagen supplement Pure Gold Collagen® reduces visible signs of aging. Clinical Interventions in Aging. 2014;9:1747-1758. doi:10.2147/CIA.S65939. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4206255/
- Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study. Proksch E, Segger D, Degwert J, Schunck M, Zague V, Oesser S. Skin Pharmacol Physiol. 2014; 27(1):47-55. https://www.karger.com/Article/Abstract/351376
- Collagen substitutes: bovine collagen. Klein AW. Clin Plast Surg. 2001 Jan; 28(1):53-62. https://www.ncbi.nlm.nih.gov/pubmed/11248869/
- Facial soft-tissue augmentation with injectable autologous and allogeneic human tissue collagen matrix (autologen and dermalogen). Fagien S. Plast Reconstr Surg. 2000 Jan; 105(1):362-73; discussion 374-5. https://www.ncbi.nlm.nih.gov/pubmed/10627006/
- Liu B, Xu Z, Yu R, Wang J, Wang Z, Harrell CR. The Use of Type I and Type III Injectable Human Collagen for Dermal Fill: 10 Years of Clinical Experience in China. Seminars in Plastic Surgery. 2005;19(3):241-250. doi:10.1055/s-2005-919719. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2884804/
- Sclafani A P, Romo T., 3rd Injectable fillers for facial soft tissue enhancement. Facial Plast Surg. 2000;16:29–34. https://www.ncbi.nlm.nih.gov/pubmed/11802343
- A double-blind, clinical evaluation of facial augmentation treatments: a comparison of PRI 1, PRI 2, Zyplast and Perlane. Downie J, Mao Z, Rachel Lo TW, Barry S, Bock M, Siebert JP, Bowman A, Ayoub A. J Plast Reconstr Aesthet Surg. 2009 Dec; 62(12):1636-43. https://www.ncbi.nlm.nih.gov/pubmed/18986859/
- Lee JH, Choi YS, Kim SM, Kim YJ, Rhie JW, Jun YJ. Efficacy and Safety of Porcine Collagen Filler for Nasolabial Fold Correction in Asians: A Prospective Multicenter, 12 Months Follow-up Study. Journal of Korean Medical Science. 2014;29(Suppl 3):S217-S221. doi:10.3346/jkms.2014.29.S3.S217. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4248008/
- Liu B, Xu Z, Yu R. Experimental and clinical observation on wrinkle correction by medical cosmetic collagen injection. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1994;16:197–200. https://www.ncbi.nlm.nih.gov/pubmed/7805164
- Liu B, Spira M, Xu Z, Ozgentas E, Shenaq S M. A comparative study of gut suture, human amnion collagen, bovine skin collagen and Vicryl suture implants in rats. Chin Med Sci J. 1997;12:26–31. https://www.ncbi.nlm.nih.gov/pubmed/11243095
- A simple method to determine nanogram levels of 4-hydroxyproline in biological tissues. Jamall IS, Finelli VN, Que Hee SS. Anal Biochem. 1981 Mar 15; 112(1):70-5. https://www.ncbi.nlm.nih.gov/pubmed/7258630/
- [Experimental and clinical observation on wrinkle correction by medical cosmetic collagen injection]. Liu B, Xu Z, Yu R. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1994 Jun; 16(3):197-200. https://www.ncbi.nlm.nih.gov/pubmed/7805164/
- The effect of glucosamine supplementation on people experiencing regular knee pain. Braham R, Dawson B, Goodman C. Br J Sports Med. 2003 Feb; 37(1):45-9; discussion 49. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1724589/
- Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000 Sep; 43(9):1905-15. https://www.ncbi.nlm.nih.gov/pubmed/11014340/
- What is Osteoarthritis? Arthritis Foundation. http://www.arthritis.org/about-arthritis/types/osteoarthritis/what-is-osteoarthritis.php
- Osteoarthritis. Haq I, Murphy E, Dacre J. Postgrad Med J. 2003 Jul; 79(933):377-83. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1742743/
- Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders:a review of the literature. Current Medical Research and Opinion Vol. 22 , Iss. 11,2006. http://dx.doi.org/10.1185/030079906X148373
- Effects of orally administered undenatured type II collagen against arthritic inflammatory diseases: a mechanistic exploration. Bagchi D, Misner B, Bagchi M, Kothari SC, Downs BW, Fafard RD, Preuss HG. Int J Clin Pharmacol Res. 2002; 22(3-4):101-10. https://www.ncbi.nlm.nih.gov/pubmed/12837047/
- Treatment of rheumatoid arthritis with oral type II collagen. Results of a multicenter, double-blind, placebo-controlled trial. Barnett ML, Kremer JM, St Clair EW, Clegg DO, Furst D, Weisman M, Fletcher MJ, Chasan-Taber S, Finger E, Morales A, Le CH, Trentham DE. Arthritis Rheum. 1998 Feb; 41(2):290-7. https://www.ncbi.nlm.nih.gov/pubmed/9485087/
- Effects of oral administration of type II collagen on rheumatoid arthritis. Trentham DE, Dynesius-Trentham RA, Orav EJ, Combitchi D, Lorenzo C, Sewell KL, Hafler DA, Weiner HL. Science. 1993 Sep 24; 261(5129):1727-30. https://www.ncbi.nlm.nih.gov/pubmed/8378772/
- Therapeutic Efficacy and Safety of Undenatured Type II Collagen Singly or in Combination with Glucosamine and Chondroitin in Arthritic Dogs. D’Altilio M, Peal A, Alvey M, Simms C, Curtsinger A, Gupta RC, Canerdy TD, Goad JT, Bagchi M, Bagchi D. Toxicol Mech Methods. 2007; 17(4):189-96. https://www.ncbi.nlm.nih.gov/pubmed/20020968/
- Oral tolerance. Faria AM, Weiner HL. Immunol Rev. 2005 Aug; 206():232-59. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3076704/
- Crowley DC, Lau FC, Sharma P, et al. Safety and efficacy of undenatured type II collagen in the treatment of osteoarthritis of the knee: a clinical trial. International Journal of Medical Sciences. 2009;6(6):312-321. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2764342/
- Ayello E.A., Baranoski S., Kerstein M.D., Cuddigan J. Wound treatment options. In: Baranoski S., Ayello E.A., editors. Wound Care Essentials: Practice Principles. Lippincott Williams & Wilkins; Philadelphia, PA: 2003. p. 138.
- Mechanism of action of PROMOGRAN, a protease modulating matrix, for the treatment of diabetic foot ulcers. Cullen B, Smith R, McCulloch E, Silcock D, Morrison L. Wound Repair Regen. 2002 Jan-Feb; 10(1):16-25. https://www.ncbi.nlm.nih.gov/pubmed/11983003/
- Medical and surgical applications of collagen. Chvapil M, Kronenthal L, Van Winkle W Jr. Int Rev Connect Tissue Res. 1973; 6():1-61. https://www.ncbi.nlm.nih.gov/pubmed/4579316/
- Khan R, Khan MH. Use of collagen as a biomaterial: An update. Journal of Indian Society of Periodontology. 2013;17(4):539-542. doi:10.4103/0972-124X.118333. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3800424/
- Doillon CJ, Silver FH. Collagen based wound dressing effects of Hyaluronic acid and fibronectin on wound healing. Biomaterials. 1986;7:3–8. https://www.ncbi.nlm.nih.gov/pubmed/3955155
- Chvapil M, Kronenthal RL, Van Winkle W. Medical and surgical applications of collagen. Int Rev Connect Tissue Res. 1973;6:1–61. https://www.ncbi.nlm.nih.gov/pubmed/4579316
- Nair R, Sevukarajan M, Mohammed T, Badivaddin CK, Kumar A. Collagen based drug delivery systems: A Review. J Innov Trends Pharm Sci. 2010;1:288–304.
- Wikesjo UM, Nilveus RE, Selvig KA. Significance of early healing events on periodontal repair: A review. J Periodontol. 1992;63:158–65. https://www.ncbi.nlm.nih.gov/pubmed/1593409
- Prosthlewaite AE, Seyer JM, Kang AH. Chemostasis attraction of human fibroblast to Type I, II, and III collagens and collagen derived peptides. Proc Natl Acad Sci USA. 1978;75:870–5. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC411359/
- Bashutski JD, Wang HL. Periodontal and Endodontic Regeneration. J Endod. 2009;35:321–8. https://www.ncbi.nlm.nih.gov/pubmed/19249588
- Wang HL. Guided tissue regeneration. Dent Clin North Am. 1998;42:505–23. https://www.ncbi.nlm.nih.gov/pubmed/9700452