What is ginseng tea
Ginseng applies to various herbs that commonly refers to species within the Panax genus in the family Araliaceae (Table 1). The two main species used in herbal medicines are Asian or Korean ginseng (Panax ginseng) and American ginseng (Panax quinquefolius). Asian ginseng (Panax ginseng C.A. Meyer) is one of several types of true ginseng; another is American ginseng (Panax quinquefolius). A herb called Siberian ginseng or eleuthero (Eleutherococcus senticosus) is not a true ginseng. Asian ginseng (Panax ginseng or Korean ginseng), which is native to China, Korea, and Russia, has been an important herbal remedy in traditional Chinese medicine for at least 2,000 years. The Panax genus consists of 17 species, but Asian or Korean ginseng (Panax ginseng), American ginseng (Panax quinquefolius), and Chinese ginseng (Panax notoginseng) are the species most commonly used as a functional food and medicine 1. Asian ginseng (Panax ginseng or Korean ginseng) has been reported to be the most effective in improving brain function, relieving pain and preventing tumors because it contains more types of ginsenoside and other compounds than American ginseng (Panax quinquefolius) and Panax notoginseng (Sanchi ginseng) 2. American ginseng (Panax quinquefolius), was valued by the American Indians long before the arrival of Europeans in the New World; American ginseng has been cultivated in North America for medicinal purposes since the eighteenth century 3. Other Panax species commonly used in herbal medicine are Japanese ginseng (Panax japonicus), Chinese ginseng (Panax notoginseng), Vietnamese ginseng (Panax vietnamensis), Omei ginseng (Panax omeiensis), Himalayan ginseng (Panax pseudoginseng), ginger ginseng (Panax zingiberensis), Pingpien ginseng (Panax stipuleanatus), dwarf ginseng (Panax trifolius), narrow-leaved pseudoginseng (Panax wangianus), feather-leaf bamboo ginseng (Panax bipinnatifidus), Panax variabilis, Panax sokpayensis, Panax assamicus, Panax shangianus, and Panax sinensis 4. Ginseng is generally classified in three different ways, depending on how it is processed: fresh ginseng (less than four years old); white ginseng (four to six years old and dried after peeling); and red ginseng (harvested when six years old, steamed and dried) 5 .
The genus Panax, first used by the Russian botanist, Carl A Meyer 6, is derived from the Greek pan, meaning “all”, and axos, meaning “medicine”, indicating that ginseng is a cure for all diseases 7. Due to the resemblance between the ginseng root and the human shape, the English name “ginseng” was introduced from the Chinese word “renshen” 8. Ginseng has been traditionally used as a panacea to promote longevity, and as a treatment for weakness and fatigue. It restores the balance between Yin and Yang, stabilizes the dynamic equilibrium of the five elements towards physiological processes, revitalizes and aids recovery from illness 9. Asian ginseng is one of several types of ginseng see Table 1 for more details. Ginseng is classified as fresh ginseng (raw ginseng less than four years old), white ginseng (four to six years old and dried after peeling), red ginseng (harvested when six years old, steamed and dried), fermented ginseng and cultured ginseng depending on how it is processed 10. Ginseng consists of 60% carbohydrate, 8–15% crude protein, 1–3% lipid, 4–6% ash, 3–7% crude saponin, and other chemicals, including phenolic and volatile compounds 11.
Ginseng that is produced in the United States and Canada (Panax quinquefolius) is particularly prized in Chinese societies 9. According to folk legend, American Ginseng root is more Yin (shadow, cold, negative, female). It is believed to promote Yin energy, clean excess Yang in the body, and restore the dynamic equilibrium of Yin and Yang 9. According to traditional Chinese medicine, for example, diabetes mellitus is defined as Yin deficiency (fatigue, weakness, lethargy, pale complexion), and American ginseng that is believed to promote Yin energy can be used to treat diabetes mellitus 12. The reason it has been claimed that American ginseng (Panax quinquefolius) promotes Yin (shadow, cold, negative, female) while East Asian ginseng (Panax ginseng) promotes Yang (sunshine, hot, positive, male) is that, according to traditional Korean medicine, things living in cold places are strong in Yang and vice versa, so that the two are balanced. Chinese/Korean ginseng grows in northeast China and Korea, the coldest area known to many Koreans in traditional times. Thus, ginseng from there is supposed to be very Yang. Originally, American ginseng (Panax quinquefolius) was imported into China via subtropical Guangzhou, the seaport next to Hong Kong, so traditional Chinese medicine practitioners believed that American ginseng must be good for Yin, because it came from a hot area. However they did not know that American ginseng can only grow in cold regions 9.
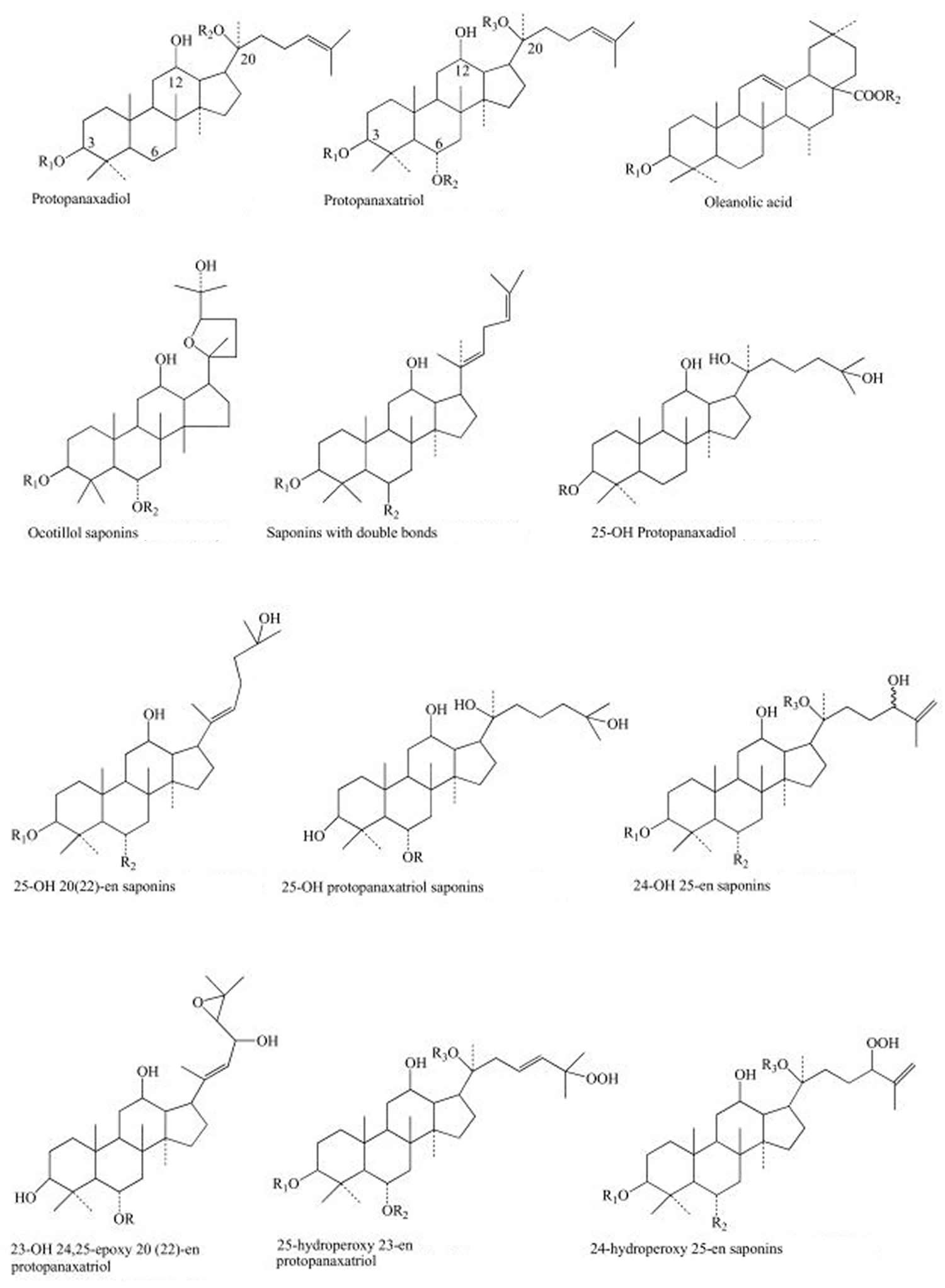
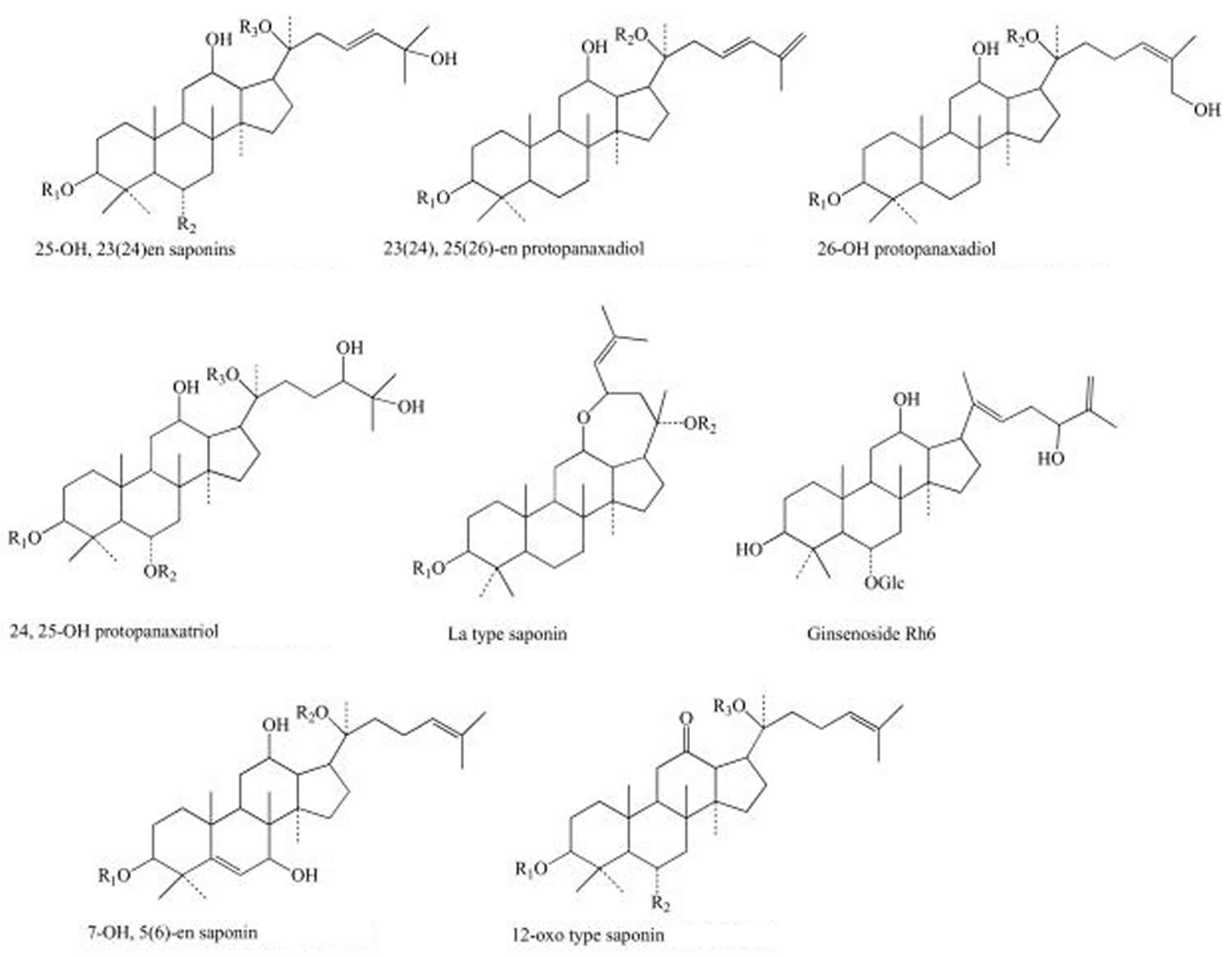
The main active ingredients of the ginseng (Panax genus) are the so-called “ginsenosides” or “panaxosides” (Figure 1), which belong to the chemical class of triterpene saponins that are thought to contribute to the herb’s claimed health-related properties (see Table 2 below). Other substances like some sugars and polysaccharides are also active agents (Table 3). Ginsenosides exhibit diverse biological activities, including anti-diabetic, anti-aging, anti-carcinogenic, anti-fatigue, anti-pyretic, and anti-stress activities, and are also known to promote DNA, RNA, and protein synthesis 13, 14, 15, 16, 17. Therefore, ginsenosides are recognized as a key index for quality evaluation of ginseng 3. The distribution of ginsenosides varies among different Panax species. Among ginsenosides isolated from ginseng plants to date, Rb1, Rb2, Rc, Rd, Re, Rf, and Rg1 typically constitute more than 90% of the total ginsenosides and are usually regarded as the major ginsenosides 1. The distribution of these major ginsenosides varies significantly due to environmental effects, including soil fertility, temperature, light, and humidity 18. Therefore, accurate determinations of the type and amount of ginsenosides in different Panax species are not only important for the pharmacological evaluation of various ginsengs, but also when assessing the quality of ginseng cultivated in different countries 3.
Major ginsenosides (including Rb1, Rb2, Rc, Rd, Re, Rf, and Rg1) are naturally present in most ginseng plants, however the distribution and concentrations of the major ginsenosides differ in each Panax species 3. Park et al. 19. reported that six major ginsenosides (Rb1, Rb2, Rc, Rd, Re, and Rg1) were found to comprise 90% of the total ginsenoside content of Panax ginseng, and Chen et al. 18 used the six ginsenosides to evaluate ginsenoside abundance in Panax ginseng from different regions. The major ginsenosides are also present in other ginseng species at varying concentrations. The Rb1:Rg1 ratio is commonly used to assess the differences between ginsengs: Rb1:Rg1 ratios between 1 and 3 are typical for Panax ginseng and Panax notoginseng, while ratios around 10 or greater are characteristic of American ginseng (Panax quinquefolius) 20. In addition, several researchers have identified potential marker ginsenosides that could discriminate among ginseng species: For example, Yang et al. 21 found that ginsenoside Rf and Rs1 were unique to Asian ginseng (Panax ginseng or Korean ginseng), whereas pseudoginsenoside-F11 (p-F11) was only present in American ginseng (Panax quinquefolius); four characteristic markers for Chinese ginseng (Panax notoginseng) (Ra3) and American ginseng (Panax quinquefolius) (Ro and p-F11) can be used to differentiate American ginseng and Chinese ginseng (Panax notoginseng); Rf, Re, Rg1, Rc, Rb2, and Rd are abundant in Asian ginseng (Panax ginseng or Korean ginseng); Re, Rb1, and Rd are rich in American ginseng (Panax quinquefolius); and noto-R1, Rg1, and Rd are plentiful in Chinese ginseng (Panax notoginseng) 22.
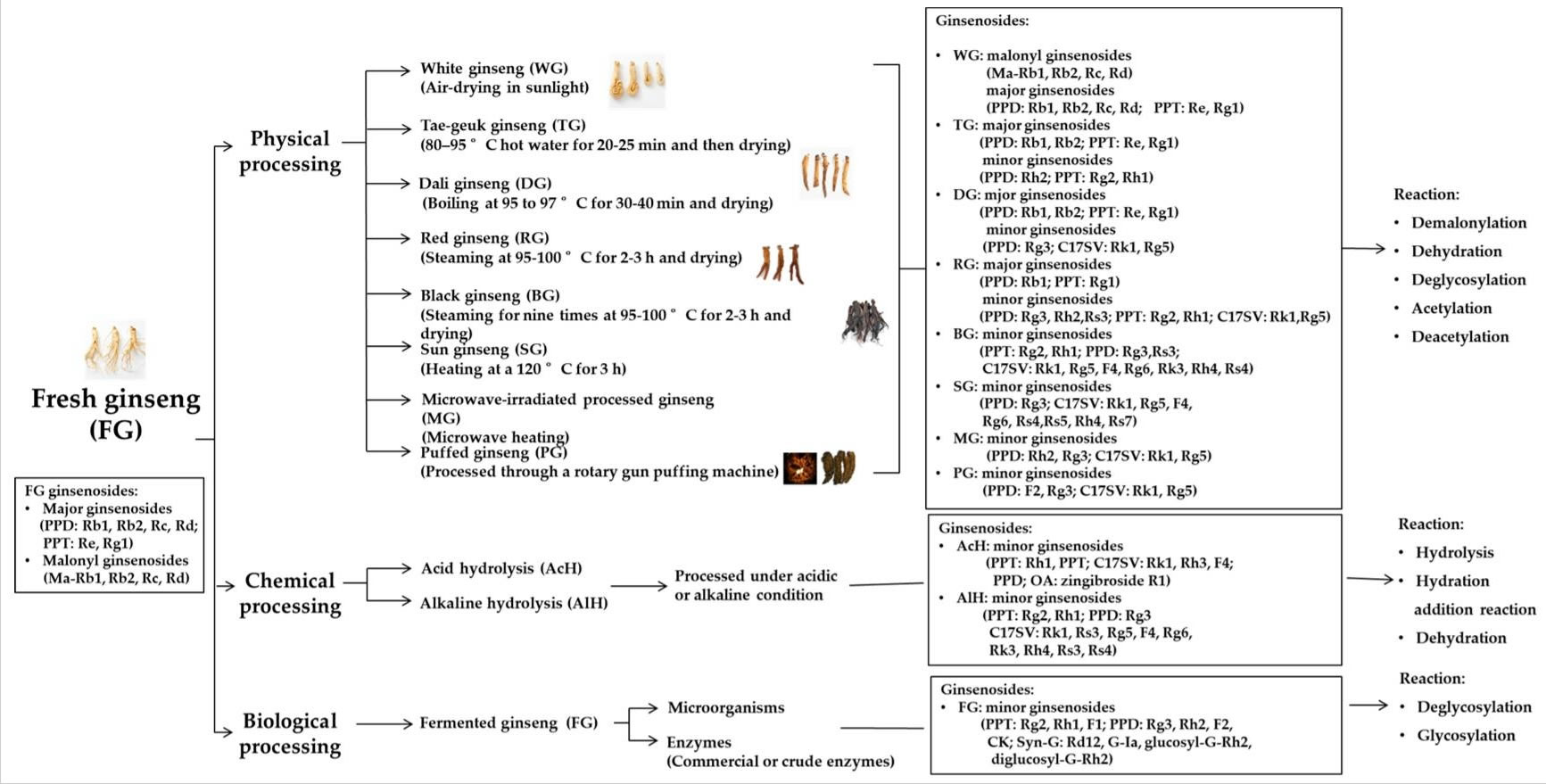
The chemical composition of ginseng is affected by the following factors: the species, the habitat in which it grows, the maturity of the plant and the geographical origin of cultivation. In addition, harvesting, storage and post-harvest processing can affect the bioactive compound of ginseng 23. The various ginseng processing technologies, such as physical, chemical, biological treatments, are outlined in Figure 2. Researchers have shown that the pharmaceutical and medicinal effects of ginseng can vary according to the species. Asian ginseng (Panax ginseng or Korean ginseng) has a ‘warm’ (Yang) property and is known to refill the ‘vital energy’, but American ginseng (Panax quinquefolius) has a ‘cooling’ (Yin) effect and is used mainly to reduce the ‘internal heat’ and uphold the secretion of body fluids 24. In addition, the distribution of the main bioactive compounds in ginseng-ginsenosides or ginseng saponins-varies with the species 25. When ginseng is used properly, it appears to be safe. In many countries, it is considered a supplement food, not a drug, and most documented side effects are related to inappropriate use. Nevertheless, reported side effects include hypertension, diarrhea, sleeplessness, mastalgia, skin eruptions and vaginal bleeding 26. Ginseng is generally safe, but it may affect glucose levels and also be unsafe for people with blood pressure disorders. Some experts recommend that women who are pregnant or breastfeeding not use ginseng.
Ginseng has been popularly referred to as an “adaptogen” in much of the alternative medicine literature. The term “adaptogen” implies a substance that increases resistance to physical, chemical, and biological stress and builds up general vitality including physical and mental capacity. Panax ginseng has been used for centuries as an important component of many Chinese prescriptions to treat disease and combat aging. Its medicinal efficacy was first documented in Shengnong Bencao Jing and was later summarised by Li Shizhen in Bencao Gangmu and Zhongyao Zhi (Chinese Materia Medica). More recently, ginseng has received much attention as a treatment claimed to enhance cognitive performance and well-being.
In traditional Chinese medicine, Asian ginseng was used as a tonic that was believed to replenish energy. Today, Asian ginseng (Panax ginseng or Korean ginseng) root is used as a dietary supplement to improve general well-being, physical stamina, and concentration; stimulate immune function; slow the aging process; and relieve various health problems such as respiratory disorders, cardiovascular disorders, depression, anxiety, erectile dysfunction, and menopausal hot flashes.
There have been many studies of Asian ginseng (Panax ginseng or Korean ginseng) in people, but few have been high quality. Results from a 2013 review of 65 randomized controlled trials suggest that Asian ginseng may help improve glucose metabolism and lower blood sugar 27. However, the scientists who published the review noted some issues with the studies they examined: that many were not high quality and that ginseng preparations were diverse 27. Therefore, our understanding of Asian ginseng’s health effects is limited. However, there’s currently no conclusive evidence supporting any health benefits of Asian ginseng 28.
The fresh ginseng root, after peeled, can be directly chewed, or soaked in various wines for a period of time before drink and chewing 9. Ginseng is most often available in dried form, either in whole or sliced form. Ginseng leaf, although not as highly prized, is also used; as with the root it is most often available in dried form 9. The correct and efficient cooking processes for the dried ginseng are as following: washing the dried roots with running water, immersing the dried roots in boiled water and maintaining the water hot for at least 3 hours or overnight, taking the soaked and hence soft roots out of water and slicing the roots with a knife as small as possible, and finally stewing the sliced roots slowly and gently in the water in a closed vessel before drinking the liquid 9. Ginseng is usually sliced and simmered in hot water to make a decoction. Ginseng ingredient may also be found in some popular energy drinks, ginseng tea varieties, or functional foods. Ginseng root is often sliced and steamed with chicken meat as a soup in China (including Taiwan), and Korea. Usually ginseng is used at subclinical doses for a short period and as such, it does not produce measurable medicinal effects.
Table 1. Species of Ginseng belonging to the genus Panax
| English Name | Latin Name |
|---|---|
| Asian ginseng Asiatic ginseng Chinese ginseng Ginseng Korean ginseng Manchurian ginseng Oriental ginseng Red ginseng (steamed & dried peeled roots) White ginseng (sun-dried roots) | Panax ginseng |
| American ginseng Ginseng (USA) Wild American ginseng Occidental ginseng | Panax quinquefolius |
| Japanese ginseng | Panax japonicus |
| Narrow-leaved Japanese ginseng | Panax japonicus |
| Notoginseng Sanchi ginseng (USA) San-qi ginseng (USA) South China ginseng Tien-qi ginseng Yunnan ginseng | Panax notoginseng |
| False ginseng Nepal ginseng Himalayan ginseng Pseudoginseng | Panax pseudoginseng |
| Elegant pseudoginseng Pearl ginseng | Panax pseudoginseng |
| Pearl ginseng | Panax pseudoginseng |
| Pingpien ginseng | Panax stipuleanatus |
| Dwarf ginseng Groundnut (USA) | Panax trifolius |
| Bamboo ginseng Vietnamese ginseng | Panax vietnamensis |
| Narrow-leaved pseudoginseng | Panax wangianus |
| Ginger ginseng Ginger-like pseudo-ginseng | Panax zingiberensis |
Table 2. Ginsenosides types in various Panax species
| Common Name | Species | Geographical Distribution | Main Saponin Types | Distinctiveness | References |
|---|---|---|---|---|---|
| Korean ginseng | Panax ginseng | Asian countries | protopanaxatriol and protopanaxadiol | ginsenoside-Rf (protopanaxatriol) and ginsenoside-Rs1 (protopanaxadiol) | 18 |
| American ginseng | Panax quinquefolius | America | protopanaxatriol and protopanaxadiol | pseudoginsenoside-F11 (ocotillol) | 29 |
| Chinese (Sanchi) ginseng | Panax notoginseng | China | protopanaxatriol and protopanaxadiol | notoginsenoside–R1 (protopanaxatriol) | 21 |
| Japanese ginsengor Ye–sanchi | Panax japonicas | China and Japan | ocotillol | Yesanchinosides (ocotillol) | 30 |
| Vietnam ginseng | Panax vietnamensis | Vietnam | protopanaxatriol, protopanaxadiol, and ocotillol | majonoside–R2 (ocotillol) | 31 |
| Ginger ginseng or Myanmar ginseng | Panax zingiberensis | China | oleanolic acid and protopanaxatriol | – | 32 |
| Pingpien ginseng | Panax stipuleanatus | China and Vietnam | oleanolic acid | – | 33 |
| Feather-leaf bamboo ginseng | Panax bipinnatifidus | China, Eastern Himalayas, and Nepal | oleanolic acid | – | 34 |
| – | Panax sokpayensis | India | protopanaxatriol and protopanaxadiol | – | 34 |
| Omei ginseng | Panax omeiensis | China, Eastern Himalayas, and Nepal | – | – | 4 |
| Himalayan ginseng | Panax pseudoginseng | China, Eastern Himalayas, and Nepal | protopanaxatriol, protopanaxadiol, and oleanolic acid | 4 | |
| – | Panax assamicus | India and West Bengal | – | – | 35 |
| – | Panax shangianus | China | – | – | 4 |
| – | Panax sinensis | China | – | – | 4 |
| Dwarf ginseng | Panax trifolius | Ohio andPennsylvania | protopanaxatriol, protopanaxadiol, and oleanolic acid | – | 36 |
| – | Panax variabilis | China and India | – | – | 4 |
| Narrow-leaved pseudoginseng | Panax wangianus | China and India | – | – | 37 |
Table 3. Secondary metabolites produced by ginseng
| Compounds | Medicinal Properties | References |
|---|---|---|
| Ginsenoside | Anti-cancer, Anti-diabetes, Anti-inflammation, Hepatoprotection, Anti-aging, Anti-oxidative | 38 |
| Phytosterol (Stigmasterol and β-sterol) | Lower the cholesterol level | 39 |
| Sesquiterpenes (β-elemene and β-selinene) | – | 39 |
| Flavonoids (Kaempferol) | Anti-oxidant, Hepatoprotective, Anti-cancer, Anti-inflammatory, Anti-viral | 40 |
| Polyacetylenes (Panaxynol, Ginsenoyne A) | Possess Anti-tumor properties | 39 |
| Alkaloids (Fumarine, Girinimbin) | – | 39 |
| Phenolic Compounds (Elemicin, Dauricine, Maltol) | Anti-tumor, Anti-oxidant, Anti-inflammatory | 39 |
Figure 1. Main structures of ginsenosides
[Source 9 ]Figure 2. Various types of ginseng processed through different methods
Abbreviations: PPT = protopanaxatriol; PPD = protopanaxadiol; C17SCV = C17 side-chain varied; OA = oleanolic acid; Syn-G = synthetic ginsenoside; G = ginsenoside; Ma = malonyl.
[Source 3 ]Ginseng tea benefits
Ginsenosides or “panaxosides” are the most studied active components in ginseng 41. About 40 types of ginsenosides are contained in ginseng along with non-saponin compounds like acidic polysaccharides and polyacetylenes 42, 43. More than 10 phenolic compounds in fresh and/or processed ginseng have previously been reported as follows: salicylic acid, vanillic acid, ascorbic acid, p-coumaric acid, ferulic acid, caffeic acid, gentisic acid, p-hydroxybenzoic acid, maltol, cinnamic acid, protocatechuic acid, syringic acid, and quercetin 44. Among these phenolic compounds, ferulic acid is considered a phenolic compound with anticancer properties. Maltol, which is found in processed ginseng, shows strong scavenging activity against reactive oxygen species 45. Korean ginseng usually contains more phenolic compounds than Chinese ginseng; therefore, Korean ginseng has more health benefits than other ginseng species 46. This study 47 showed that chlorogenic acid, gentisic acid, p- and m-coumaric acid, and rutin were the main phenolic compounds in 3–6-yr-old ginseng fruit, leaves, and roots. In contrast, gallic acid, myricetin, and biochanin A were not found in 3–6-yr-old ginseng fruit, leaves, and roots. Much basic research on the range of efficacies of ginseng, including its immune-enhancing, anti-fatigue, and anti-cancer functions and improvements to cardiovascular function, is ongoing, along with numerous studies on its mechanisms 48.
In general, ginseng fruit and leaves are less attractive to consumers compared with the ginseng roots; in fact, the fruit and leaves are usually discarded 49. Therefore, information on the phenolic compounds present in the ginseng fruit or leaves is very limited to date. Traditionally, ginseng leaves have been used as a form of tea. In addition, the leaves of hydroponically grown ginseng have been recently used as salad 49. Furthermore, American ginseng berries have been reported to inhibit the growth of colorectal cancer both in vitro and in vivo 50.
Ginsenosides have been isolated and evaluated for pharmacological effects and on the basis of their chemical structure are classified into three groups: the Panaxadiol group (Rb1, Rb2, Rb3, Rc, etc.), the Panaxatriol group (Re, Rf, Rg1, Rg2, Rh1) and the oleanolic acid group (e.g. Ro) 51. These ginsenosides have varying concentrations in ginseng species as a result of the different processing methods that affect deacetylation of enzymes within the raw plant. It has been well documented that the polysaccharides contained within ginseng possess various antitumour activities, including preventive and inhibitory effects against tumours, as well as enhancing immunological functions 52.
Ginseng products are popularly referred to as “tonics,” a term that has been replaced by “adaptogen” in much of the alternative medicine literature. The term “adaptogen” connotes an agent that purportedly increases resistance to physical, chemical, and biological stress and builds up general vitality, including the physical and mental capacity for work. Both American and Panax (Asian) ginseng roots are taken orally as adaptogens, aphrodisiacs, nourishing stimulants, and in the treatment of sexual dysfunction in men 9. Panax ginseng (Asian or Korean ginseng) is used as a restorative medicine 53. Panax quinquefolius (American ginseng) is also used to treat thirst, fatigue, dryness of the mouth, the respiratory tract, and irritability 54.
What is ginseng tea good for?
Asian ginseng (Panax ginseng C.A. Meyer) is a common herb with many purported health benefits. However, there is no conclusive evidence supporting its use in the treatment of any particular disease. The therapeutic effects of ginseng are diverse, and the evidence for its efficacy in treating several conditions, such as cardiovascular disease 55, 56, neurological disorders 57, Alzheimer’s disease 58, common cold 59, antidiabetic effects 60, obesity and hyperlipidemia 61 and hypertension 62, 63, have been evaluated. Ginseng has also been used to improve general conditions relevant to quality of life and athletic performance in the healthy population 64.
Current evidence does not support the use of ginseng to treat cardiovascular risk factors 56. Some studies suggest a small reduction in blood pressure. Despite some evidence showing that ginseng lowers blood glucose and improves lipid profiles, well-designed, randomized, controlled trials evaluating its effects are lacking 56. A systematic review on ginseng as a treatment for Alzheimer’s disease, the evidence for ginseng as a treatment of Alzheimer’s disease is scarce and inconclusive and Further research is needed. 58. Athletes use ginseng for its alleged performance-enhancing attributes 64. However, many studies examining the pharmacological effects of ginseng on physical performance have not employed sound scientific design and methodology 64. This review authors concluded that many of the same methodological shortcomings observed in earlier studies persist 64. Enhanced physical performance after ginseng administration in well-designed investigations remains to be demonstrated 64. Currently, there is insufficient evidence to support the use of North American ginseng extracts in the prevention of common colds 59.
A 2013 systematic review 65 to evaluate randomized controlled trials evaluating Panax ginseng in patients with any type of disease or in healthy individuals. The authors assessed the quality of studies using the Cochrane risk of bias tool. Of the 475 potentially relevant studies, 65 met the inclusion criteria. These studies examined Panax ginseng’s effects on psychomotor performance (17 studies), physical performance (ten), circulatory system (eight), glucose metabolism (six), the respiratory system (five), erectile dysfunction (four), immunomodulation (four), quality of life/mood (four), antioxidant function (two), cancer (two), menopausal symptoms (two) and dry mouth (one). The risk of bias was unclear in most studies. The authors evaluated adverse events in 40 studies, with 135 minor events and no serious adverse events reported. Panax ginseng shows promising results for improving glucose metabolism and moderating the immune response 65. This may have implications for several diseases including type 2 diabetes and chronic respiratory conditions. Further studies are needed to explore Panax ginseng’s potential as an effective treatment for these and other health conditions.
Ginseng for cognition
Despite ginseng appearing to have some beneficial effects on cognition, behavior and quality of life in small trials 66, there is currently a lack of convincing evidence to show a cognitive enhancing effect of Panax ginseng in healthy participants and no high quality evidence about ginseng efficacy in patients with dementia 28. Randomized, double-blind, placebo-controlled, parallel group trials with large sample sizes are needed to further investigate the effect of ginseng on cognition in different populations, including dementia patients.
Ginseng and diabetes
Vuksan et al. 67 demonstrated that American ginseng (Panax quinquefolius Linn) reduced postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. In addition, Sotaniemi et al. 68 showed that treatment of newly diagnosed type 2 diabetes mellitus patients with ginseng (100 or 200 mg) or placebo for 8 weeks elevated mood, improved psychophysical performance, and reduced fasting blood glucose and body weight. The 200-mg dose of ginseng improved glycated hemoglobin, serum PIIINP, and physical activity. Placebo reduced body weight and altered the serum lipid profile but did not alter fasting blood glucose.
Sievenpiper’s study 69 used a randomized, double-blind, multiple-crossover, double-placebo controlled design. Each participant received 10 single-dose treatments in random order: 3 g of ground whole root of American (Panax quinquefolius L.), American-wild (wild Panax quinquefolius L), Asian (Panax ginseng C.A. Meyer), Asian-red (steam treated Panax ginseng C.A. Meyer), Vietnamese-wild (Panax vietnemensis), Siberian (Eleutherococcus senticosus), Japanese-ginseng (Panax Japonicus C.A. Meyer), and Sanchi (Panax notoginseng) ginsengs and two identical placebos. The two placebos were then mixed and treated as one for all comparisons to increase the probability of obtaining a true control for comparisons with the eight ginseng types. The studies showed decreasing, null and increasing effects of the 8 types of ginseng on acute postprandial glycemic indices in healthy humans. The experience with ginseng suggests that although reproducible efficacy may be achieved using an acute postprandial clinical screening model to select an efficacious ginseng batch, dose, and time of administration, there is a need to develop a basis for standardization that ties the composition of herbs to efficacy. In absence of such standardization, the use of herbs in diabetes must be approached cautiously.
Ginseng as aphrodisiac
The effects of ginseng on the sexual performance appear to be mediated by the release and/or modification of release of nitric oxide (NO) from endothelial cells and perivascular nerves. Nitric oxide (NO) was named “The Molecule of the Year” in 1992 by the journal Science 70, but it took another 6 years for those who discovered it to win the 1998 Nobel Prize for Physiology and Medicine, including Dr. Robert Furchgott 71.
Ginseng has a long reputation in traditional Chinese medicine for treatment of sexual impotence. Recent studies in laboratory animals have shown that both Asian and American forms of ginseng enhance libido and copulatory performance 72. There is some evidence that ginsenosides can facilitate penile erection by directly inducing the vasodilatation and relaxation of penile corpus cavernosum. Treatment with American ginseng also affects the central nervous system and has been shown to significantly alter the activity of hypothalamic catecholamines involved in the facilitation of copulatory behavior and hormone secretion 73. Consistently with these findings, Choi et al. 74 confirmed in a clinical study the efficacy of Korean red ginseng for erectile dysfunction in 30 patients. In 2002, another double-blind, crossover study of Korean red ginseng’s effects on impotence reported that ginseng can be an effective alternative for treating male erectile dysfunction 75.
The effect of ginseng on the sexual performance may partially be due to changes in hormone secretion, and partially to its (or ginsenosides’) effects on the central nervous system, gonadal tissues, and production of nitric oxide (NO). Panax ginseng produced a dose-related increase in serum testosterone levels and American ginseng reduced the plasma level of prolactin hormone in rats 76. Testosterone might mediate the heightened copulatory behavior in ginseng-treated animals, while prolactin altered it. These results suggest that both ginseng species may have direct actions on the anterior pituitary gland and/or on the hypothalamic dopaminergic mechanisms 77. Recent findings that ginseng treatment decreased prolactin secretion also suggested a direct nitric oxide-mediated effect of ginseng at the level of the anterior pituitary 77. Thus, animal studies lend growing support for the use of ginseng in the treatment of sexual dysfunction and provide increasing evidence for a role of nitric oxide in the mechanism of ginsenoside action. Several recent studies have suggested that the antioxidant and organ-protective actions of ginseng are linked to enhance NO synthesis in endothelium of lung, heart, and kidney and in the corpus cavernosum 78. Enhanced production and function of NO synthase by ginseng thus could contribute to ginseng-associated vasodilatation and perhaps also to an aphrodisiac action of the root. Ginsenoside Re was demonstrated 79 to release NO via a membrane sex steroid receptors resulting in K(Ca) channel activation in vascular smooth muscle cells and promoting vasodilation. Korean ginseng is well known as a treatment for sexual dysfunction 80 because of its ‘Yang’ effect as mentioned above. Korean red ginseng is usually not peeled, but steamed, or heated, and subsequently dried. As a consequence, the process causes an increase in saponin content 80. Traditionally red ginseng has been used to restore and enhance normal well-being, and is often referred to as an adaptogenic. Kim et al. 81 recently showed that water extract of Korean red ginseng (1–20 mg/ml) produced relaxation response up to 85% in isolated female rabbit vaginal tissue in a dose-dependent manner. Similarly, Choi et al. 82 used male rabbit cavernosal muscle strips (precontracted with phenylephrine) to investigate effect of Korean red ginseng on penile erectile tissue and erectile response. After 3 months of oral administration of 50 mg/kg of Korean red ginseng to both rabbits and rats, relaxation effects were significant as measured in both the male rabbit cavernosal muscle strips and the cavernosal pressures after the stimulation of pelvic nerves innervating rat corpus cavernosum. These effects might be mediated partly through the NO pathway and hyperpolarization via Ca(2+)-activated K(+) channels, or mediated by endothelium-derived relaxing factor, which was later demonstrated to be NO 83 and peripheral neurophysiologic enhancement.
Ginseng for erectile dysfunction
Dietary supplements with ginseng, or ginseng alone, are widely used for a broad range of conditions, including erectile dysfunction 84.
According to a 2021 Cochrane Review 84, based on mostly low certainty evidence, ginseng may only have trivial effects on erectile function or satisfaction with intercourse compared to placebo (dummy drug) when assessed using two specialized questionnaires specially developed for this purpose. Ginseng may improve men’s self‐reported ability to have intercourse. When men were simply asked whether their erections improved (without using a specialized questionnaire), the results of this systematic review show that ginseng may improve the ability to have intercourse. Ginseng may have little to no effect on unwanted side effects. The review authors found no trial evidence comparing ginseng to other agents with a more established role in treating erectile dysfunction, such as phosphodiesterase‐5 inhibitors 84.
The certainty of evidence for most outcomes was low. This means that the true effect may be substantially different from what this review shows. No study reported quality of life as an outcome.
Cardiovascular effects of ginseng
Ginseng has been shown to produce a number of actions on the cardiovascular system. Intravenous administration of ginseng to anesthetized dogs resulted in reduction, followed by an increase in blood pressure, and transient vasodilatation 85. Recent studies found close relationship between ginseng’s effects and NO production pathway, including regulation of cGMP and cAMP levels [76]. For instance, in rats and rabbits, Lei and Chiou 86 and Kim et al. 87 found that extracts of Panax notoginseng decreased systemic blood pressure and ginsenosides exerted relaxing effects in rings of rat and rabbit aorta, respectively. This relaxing effect of ginseng and its active constituents on the cardiovascular system is partially due to the release of endothelial NO. Researchers have reported that chronic feeding of rabbits with ginsenosides may enhance indirectly vasodilatation by preventing NO degradation by oxygen radicals such as superoxide anions 88. Ginsenosides have depressant action on cardiomyocyte (heart muscle) contraction which may be mediated, in part, through increased NO production 89. Korean red ginseng can improve the vascular endothelial dysfunction in patients with hypertension possibly through increasing NO 90. In addition to endothelium-derived NO release, Li et al. 91 reported that ginsenoside-induced vasorelaxation involves Ca2+ activated K+ channels in vascular smooth muscle cells. It has also been reported that crude saponin fractions from Korean red ginseng enhanced cerebral blood flow in rats 92 and ginsenosides reduced plasma cholesterol levels and the formation of atheroma in the aorta of rabbits fed on a high cholesterol diet 88. This antiatherosclerotic action of ginseng components is apparently due to the correction in the balance between prostacyclin and thromboxane 93, inhibition of 5-hydroxytryptamine (5-HT) release, and adrenaline and thrombin-induced aggregation of platelets 94, regulation of cGMP and cAMP levels, and prolongation of the time interval between conversion of fibrinogen to fibrin 95. Also, ginsenosides have been shown to be relatively potent platelet activating factor antagonists 96.
In parallel with these findings, Nakajima et al. 97 concluded that red ginseng was found to promote the proliferation of vascular endothelial cells, to inhibit the production of endothelin (that is known to constrict blood vessels and result in raising blood pressure), and to increase the production of IL-1β, which suppresses the formation of thrombin in blood coagulation. In the same direction, Yuan et al. 98 used cultured human umbilical vein endothelial cells to conclude that panax quinquefolium extracts significantly decreased endothelin (an NO antagonist) concentration in a dose and time dependent manner after thrombin treatment. The role of ginseng in angiogenesis has also been reported. For instance, Kim et al. 99 demonstrated that water extract of Korean red ginseng stimulated in vitro and in vivo angiogenesis via activation of phosphorylation of ERK1/2, phosphatidylinositol 3-kinase (Akt), and endothelial nitric oxide synthase (eNOS) resulting in an increase in NO production 99. Ginsenoside Rg1 promoted functional neovascularization into a polymer scaffold in animal and tubulogenesis by endothelial cells in test tube study 100.
Ginseng and Parkinson’s disease
A number of studies have recently described the beneficial effect of ginseng and its main components, ginsenosides, on some neurodegenerative disease models. Special interest has been paid to Parkinson’s disease models either in animal or in test tube study. In an in animal model, Van Kampen et al. 101 reported that prolonged oral administration of ginseng extract G115 significantly protected against neurotoxic effects of parkinsonism inducing agents such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and its active metabolite 1-methyl-4-phenylpyridinium (MPP+) in rodents. The authors found that ginseng-treated animals sustained less damage and TH+ neuronal loss in substantia nigra pars compacta (SNpc) after MPP+ exposure. Likewise, reduction of TH immunoreactivity in the striatum was effectively diminished as a result of ginseng treatment compared to MPP+-exposed animals. Similarly, striatal dopamine transporter was significantly preserved due to ginseng treatment. In vitro studies showed that ginseng saponins enhanced neurite growth of dopaminergic SK-N-SH neuroblastoma cells 102.
Although the processes and mechanisms underlying the neuroprotective effects of ginseng upon dopaminergic neurons remain to be elucidated, several reports demonstrated the inhibition of ginseng on MPP+ uptake in dopaminergic neurons, the suppression of oxidative stress induced by auto-oxidation of dopamine, the attenuation of MPP+-induced apoptosis, and the potentiation of nerve growth factor. It has been shown that certain ginsenosides inhibit dopamine uptake into rat synaptosomes, and consequently, provide protection against MPP+ through blockade of its uptake by dopaminergic neurons 101. Ginsenoside Rg1 was shown to interrupt dopamine-induced elevation of reactive oxygen species (ROS) or NO generation in pheochromocytoma cells. Ginseng radix attenuated MPP+-induced apoptosis as it decreased the intensity of MPP+-induced DNA laddering in pheochromocytoma cells and ginsenoside Rg1 had protective effects against MPTP-induced apoptosis in the mouse substantia nigra. These anti-apoptotic effects of ginseng may be attributed to the enhanced expression of Bcl-2 and Bcl-xl, reduced expression of bax and nitric oxide synthase (NOS), and inhibited activation of caspase-3. Ginseng may also reverse the neurotoxic effects of MPP+ through elevation of NGF mRNA expression 101. In accordance, ginsenosides Rb1 and Rg1 elevated NGF mRNA expression in rat brain and potentiated NGF-induced neurite outgrowth in cell culture. Furthermore, it has been reported that ginsenosides Rb1, Rg1, Rc, and Re inhibited tyrosine hydroxylase activity and exhibited anti-dopaminergic action since they reduced the availability of dopamine at presynaptic dopamine receptors 103. Ginseng and its components prevent neuronal loss in amyotrophic lateral sclerosis models, and Ginseng radix has also been used for treatment of Alzheimer’s disease.
In addition to interactions of ginseng with the opioid systems, recent reports have demonstrated that the Korean ginseng product (ginseng total san, also called GTS) attenuates cocaine- or amphetamine-induced behavioral activity such as hyperactivity and conditioned place preference 103. Using real-time measurements of the extracellular DA concentrations in slices of rat brain nucleus accumbens, Nah et al. 104 showed the ginseng total san attenuated both the release enhancement and the rebound increase during cocaine application and withdrawal, respectively.
Ginseng and cancer
To date, clinical research results on ginseng are not conclusive enough to prove health claims associated with the herb and only a handful of large clinical trials on ginseng have been conducted. Some claims for health benefits are based only on studies conducted in animals 105. During active cancer therapy, ginseng should generally be evaluated in combination with chemotherapy and radiation. In this role, it acts as biological response modifiers and adaptogens to synergistically enhance efficacy of the conventional therapy 106. It can improve immune system activity of patients and their appetite, and functions as a supplementary agent of chemotherapy. For instance, Xing et al. 107 treated 35 rectal cancer patients with retention enema containing 85% ginsenoside for 4–6 h per day for 6–8 consecutive days before surgical operation. The control group (n=15) received retention enema containing saline in the same way. They reported that after ginsenoside treatment symptoms such as frequent defecation, hematochezia and tenesmus were palliated in most patients (25 out of 35), and abdominal pain was relieved in 7 patients with incomplete intestinal obstruction. Electron microscopic examination showed apoptosis in 23 treated patients. In comparison, the above-mentioned changes were not observed in the control group. Preclinical studies have showed some immune-stimulating activity of ginseng and ginsenosides 108.
The recent studies have shown effects of ginsenosides on angiogenesis under many pathological conditions including tumor progression and cardiovascular dysfunctions 109. Angiogenesis in human body is regulated by two counteracting factors, angiogenic stimulators and inhibitors. Intriguingly, existing literature reports both wound-healing and antitumor effects of ginseng extract through opposing activities on the vascular system. The ‘Yin and Yang’ action of ginseng on angiomodulation is evidenced in parallel by the experimental data showing that the angiogenic effects of ginseng may be related to the compositional ratio of ginsenoside Rg1 to Rb1 110. Rb1 inhibits the earliest steps of angiogenesis and chemoinvasion of endothelial cells. By contrast, Rg1 promotes functional neovascularization in animal study and endothelial proliferation, chemoinvasion and tubulogenesis in test tube study, as well as the effects mediated through expression of nitric oxide synthase and phosphatidylinositol-3 kinase→Akt pathway. In addition, the anti-tumor and anti-angiogenic effects of ginsenosides (e.g. Rg3 and Rh2) have been demonstrated in various models of tumor and endothelial cells, indicating that different ginsenosides may have opposing activities in regulating the ‘Yin and Yang’ balance 111. Chronic inflammation is associated with a high cancer risk. Ginseng’s anti-inflammatory effect may help to prevent the progression of reactive oxygen species (ROS) related inflammation to cancer sequence 112.
Recent in test tube experimental studies have showed anti-cancer effects of various ginsenosides. However, we have to point out that these in vitro studies, although inexpensive to carry out, should only serve as an adequate screening mechanism, or help us understand the mechanisms of actions of ginsenosides. In addition, the concentrations of ginsenosides applied in some in vitro experiments are as high as 100 μM level in order to produce some biological effects. This fact suggests that the observed biological effects of ginsenosides be non-specific to a particular pathway. Therefore, we propose that when a difference arises between in test tube and in animal findings, the in animal results should always take precedence over in vitro studies.
Zhao et al. 113 recently characterized a new chemical entity 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, and found that this molecule exhibits apoptosis and cell cycle arrest in the G1 phase of many cancer cell lines including breast and prostate cancer lines at IC50 of low micromolar levels 114. Recently it has been shown that American ginseng can both prevent and treat mouse colitis 115, which is associated with a high colon cancer risk.
Ginsenosides could specifically induce apoptosis in HL-60 cells. The results provide an experimental basis for treating leukemia with ginsenosides as a supplementary agent in chemotherapy 116. Some ginsenosides have the effects to induce apoptosis in rectal cancer patients 107. Ginsenoside Rh2 induced apoptosis in various tumor cells by different pathways. Rh2 interfered with B-cell lymphocyte/leukemia-2 (Bcl-2) family proteins related apoptosis and activated protein kinase Caspase-3 to cause cell apoptosis 117. Rh2 induced apoptosis of rat C6Bu-1 glioma cells and human SK-N-BE(2) neuroblastoma cells through protein kinase C pathway 118. Ginsenoside Rh2 also induced apoptosis in human malignant melanoma, which was partially dependent on caspase-8 and caspase-3 119. Ginsenoside Rh2 induced apoptosis and inhibited cell growth in C6 glioma cells, human lung adenocarcinoma A549 cells, and various ovarian cancer cell lines 120. Mediating G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells appeared to be the molecular mechanisms of ginsenoside Rh2 121. Rh2 inhibited human hepatoma Bel-7404 cell lines via arresting cell cycle, up-regulating Bax protein expression and down-regulating mutated p53 protein expression 122. Rh2 inhibited the growth of MCF-7 cells by inducing p21 protein expression and reducing cyclin D levels. As a result, cyclin/Cdk complex kinase activity, pRb phosphorylation, and E2F release could be inhibited 123.
Rg3 is one of the most effective cytostasis ginsenosides separated from ginseng. Rg3 inhibits human prostate cancer cells and other androgen dependent cells from proliferating 124. The mechanisms of actions of Rg3 include (1) decreasing genetic expression of 5α-reductase; (2) inhibiting cell cycle evolution genes such as proliferating cell nuclear antigen gene and cell cycle protease D1 gene that would stop cells from proliferating; (3) increasing cyclinase suppressor gene such as p21and p27 so as to make cells settle down at G1 stage; (4) down-regulating Bcl-2 (the anti-induction apoptosis gene); and (5) activating caspase-3 (the induction apoptosis gene) to induce the cells to delete. Rg3 inhibited tumor cell proliferation and induced cell apoptosis in mice with induced liver cancer 125. In addition, Rg3 can affect the differential expression of cell signaling genes and other related genes in human lung adenocarcinoma cell line A549, and induce apoptosis in the A549 tumor cells and HUVEC 304 cell lines. It was showed that Rg3 and Rg5 had significant inhibition on benzo(a)pyrene-induced adenocarcinoma 126 and dimethylbenz(a)anthracene-induced lung tumor in mice 127. The ginsenosides had strong inhibitory effects on the development of rat mammary adenocarcinoma induced by methyl-N-nitrosourea and N-ethyl-N-nitrosourea administration, as well as on DMBA-induced uterine and vaginal tumors 128. Using athymic mice transplanted with ovarian SKOV-3 cancer cells, Xu et al. 129 showed that intraperitoneal injection of ginsenoside Rg3 alone, or Rg3 combined with cyclophosphamide to the mice for 10 days improved life quality and survival of the mice, and reduced tumor weight in the mice in comparison to the control untreated mice. Chen et al. 130 reported that Rg3 produced apoptosis in human bladder transitional cell carcinoma cell line EJ at IC50 125.5 μg/ml after 48 h of incubation. When treated with 150 μg/ml of Rg3 for 24 h and 48 h, the cells showed significant DNA ladders and apoptotic morphological characteristics including the condensed chromatin, the nuclear fragmentation, the apoptotic body and bright fluorescent granules as well as a higher caspase-3 expression. When the cells were treated with 75 μg/ml of Rg3 for 24 h and 48 h, or 150 μg/ml of Rg3 for 48 h, the percentage of cells in S phase and G2/M phase was increased, whereas the percentage of cells in G0-G1 was decreased.
Another ginsenoside, i.e., Compound K (or IH901), was shown to induce apoptosis in human hepatoblastoma HepG2 cells 131, and KMS-11 cells 132 through a cytochrome C-mediated activation of caspase-3 and caspase-8 proteases and inhibition of the fibroblast growth factor receptor 3 (FGFR3) expression 133. Incubation of archeo-marrow leukemic cells HL260 with Compound-K produced apoptosis in the cells in a concentration- and time-dependent manner with morphous changes in chromatic agglutination, atrophy, and nuclear fragmentation 134. Compound-K suppressed melanoma cell proliferation in BI6-BL6 mice by activating the protein kinase caspase-3 and releasing cytochrome C in mitochondria into cytoplasm 135. Western blot test revealed that Compound-K could elevate p27Kipl expression, degrade expression of c-Myc and cyclin D1, and induce cell LLC apoptosis through activating caspase-3 protein kinase at the same time 136. Recently, IH-901 has been reported to induce both G1 arrest and apoptosis, and the apoptosis could be inhibited by COX-2 induction 137.
Ginsenoside Rg1 138 has been reported to induce the apoptosis of melanoma cells implanted in mice. Inducing morphological change of the tumor cells seems to be the main mechanism of Rg1. Wakabayashi et al. 139 reported ginsenoside M, the intestinal bacterium metabolite of protopanoxadiol saponins, could induce apoptosis in melanoma cell B16-BL6 through up-regulating the expression p27kipl and down-regulating the expression of gene c-myc that promotes cell proliferation.
Park et al. 140 discovered that ginsenosides Rg3, Rg5, Rk1, Rs5 and Rs4 were toxic to the human hepatoma carcinoma cell Sk-Hep-1. Rg5 and Rs4 could induce apoptosis by selectively elevating protein levels of p53 and p21WAF (the inhibitor of periodicity-replying protein kinase) and down-regulating the activity of protein kinase in cell cycle E and A, 141.
Ginseng tea side effects
Short-term use of Asian ginseng (Panax ginseng, Korean ginseng) in recommended amounts appears to be safe for most people. However, questions have been raised about its long-term safety, and some experts recommend against its use by infants, children, and women who are pregnant or breastfeeding. Asian ginseng may be unsafe when taken orally during pregnancy. One of the chemicals in it has been found to cause birth defects in animals 142. Little is known about whether it’s safe to use Asian ginseng while breastfeeding 143. There are uncertainties about whether ginseng might interact with certain medications, such as calcium channel blockers and other high blood pressure medications, as well as statin medications and some antidepressants. Studies on the effect of Asian ginseng on the anticoagulant (blood thinner) warfarin (Coumadin) have had mixed results. If you’re taking medication, consult your health care provider before using Asian ginseng.
The most common side effects of ginseng are headaches, dizziness, trouble sleeping (insomnia), diarrhea, constipation, vomiting, gastric complaints, loss of appetite and dermatitis or eczema 144. Other reported side effects that occurred in some individuals include high or low blood pressure, increased heart rate, restlessness, anxiety, euphoria, nosebleed, breast pain and vaginal bleeding 9. Some evidence suggests that Asian ginseng might affect blood sugar and blood pressure. If you have diabetes or high blood pressure, consult your health care provider before using Asian ginseng. Siberian ginseng may cause nervousness and restlessness in some individuals. In rare cases, Siberian ginseng may cause mild diarrhea. Siberian ginseng is not recommended for individuals with very high blood pressure. It may cause insomnia in some people if taken too close to bedtime. If symptoms like breathing problems, tightness in the throat or chest, chest pain, skin hives, rash, or itchy or swollen skin develop, the use of Siberian ginseng should stop.
When used short-term as part of a specific multi-ingredient topical skin application, Asian ginseng is likely safe. Safety after prolonged repetitive topical use has not been determined.
American ginseng (Panax quinquefolius), although similar to its Asian ginseng (Panax ginseng), has a somewhat different profile of ginsenosides; therefore, generalizations about Asian ginseng should not be extended to American ginseng 142. There are fewer human clinical studies of American ginseng than Asian ginseng, although preclinical studies support a low potential for drug interactions. Two human trials have demonstrated no effect of American ginseng (Panax quinquefolius) on the human immunodeficiency virus (HIV) agents indinavir (Crixivan) and zidovudine (Retrovir) 145, 146. A single trial of American ginseng in healthy volunteers taking warfarin demonstrated a 0.2-point drop in INR 147. Patients taking warfarin should have their INR closely monitored or refrain from taking ginseng-containing supplements.
In mice, a lethal oral dose of purified ginseng was determined to be higher than 5 g/kg body weight, the highest dose that can be orally given to a mouse and considered good practice at the maximal dose volumes without violating animal welfare. That level is equivalent to about 0.4 g/kg of oral ginseng given to an adult man 148. In a 2-year human study, 14 out of a total of 133 subjects were reported to experience side effects attributed to long-term exposure of ginseng when consumed at levels up to 15 g/day 149. The long-term side effects of ginseng are characterized by hypertension, nervousness, sleeplessness, skin rash, diarrhea, confusion, depression, or depersonalization. The effects were described as “ginseng abuse syndrome”. This level of ginseng consumption far exceeds the recommended daily intake of 1–2 g/day of Asian ginseng, in which 4–5% are ginsenosides 150. Consuming six of 500-mg ginseng capsules daily was reported to produce side effects such as hypertension, gastrointestinal disturbances, insomnia and nervousness. The validity of these observations is difficult to evaluate quantitatively. Moreover, as is the case in many studies with ginseng, the ginsenoside content of ginseng consumed was not determined 151.
The risk of interactions between ginseng and medications is believed to be low, but there are uncertainties about whether ginseng might interact with certain medications, such as the anticoagulant (blood thinner) warfarin (Coumadin) and phenelzine (a monoamine oxidase inhibitor) 152. If you’re taking medication, consult your health care provider before using Asian ginseng.
In conclusion, the most common side effects of ginseng resulted from its overdose are nervousness and excitability. These side effects usually decrease after the first few days. In inappropriate uses, the most common side-effects are the inability to sleep and high blood pressure. Other side-effects include nausea, diarrhea, euphoria, headaches, epistaxis, mastalgia, eruptions, and vaginal bleeding 153. Ginseng may lower levels of blood sugar; this effect may be seen more in people with diabetes. Therefore, diabetic patients who are taking anti-diabetic medicine should be cautious with Asian ginseng and monitor their blood sugar levels closely. Because ginseng has an estrogen-like effect, women who are pregnant or breastfeeding should take it at recommended doses. Occasionally, there have been reports of more serious side effects, such as asthma attacks, increased blood pressure, palpitations, and, in postmenopausal women, uterine bleeding 9.
References- Mohanan, P., Subramaniyam, S., Mathiyalagan, R., & Yang, D. C. (2018). Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. Journal of ginseng research, 42(2), 123–132. https://doi.org/10.1016/j.jgr.2017.01.008
- Mancuso C, Santangelo R. Panax ginseng and Panax quinquefolius: from pharmacology to toxicology. Food and Chemical Toxicology 2017;107:362-372.
- Piao, X. M., Huo, Y., Kang, J. P., Mathiyalagan, R., Zhang, H., Yang, D. U., Kim, M., Yang, D. C., Kang, S. C., & Wang, Y. P. (2020). Diversity of Ginsenoside Profiles Produced by Various Processing Technologies. Molecules (Basel, Switzerland), 25(19), 4390. https://doi.org/10.3390/molecules25194390
- Zhang, H., Abid, S., Ahn, J. C., Mathiyalagan, R., Kim, Y. J., Yang, D. C., & Wang, Y. (2020). Characteristics of Panax ginseng Cultivars in Korea and China. Molecules (Basel, Switzerland), 25(11), 2635. https://doi.org/10.3390/molecules25112635
- Jia L, Zuo T, Zhang C, Li W, Wang H, Hu Y, et al. Simultaneous profiling and holistic comparison of the metabolomes among the flower buds of Panax ginseng, Panax quinquefolius, and Panax notoginseng by UHPLC/IM-QTOF-HDMSE-based metabolomics analysis. Molecules 2019;24(11):E2188.
- Leung KW, Wong AS. Pharmacology of ginsenosides: a literature review. Chin Med. 2010 Jun 11;5:20. doi: 10.1186/1749-8546-5-20
- Cho C.-W., Kim Y.-C., Rhee Y.K., Lee Y.-C., Kim K.-T., Hong H.-D. Chemical composition characteristics of Korean straight ginseng products. J. Ethn. Foods. 2014;1:24–28. doi: 10.1016/j.jef.2014.11.007
- Chen X, Zhou H, Liu YB, Wang JF, Li H, Ung CY, Han LY, Cao ZW, Chen YZ. Database of traditional Chinese medicine and its application to studies of mechanism and to prescription validation. Br J Pharmacol. 2006 Dec;149(8):1092-103. doi: 10.1038/sj.bjp.0706945. Epub 2006 Nov 6. Erratum in: Br J Pharmacol. 2020 Dec;177(23):5434
- Jia L, Zhao Y, Liang X-J. Current Evaluation of the Millennium Phytomedicine— Ginseng (II): Collected Chemical Entities, Modern Pharmacology, and Clinical Applications Emanated from Traditional Chinese Medicine. Current medicinal chemistry. 2009;16(22):2924-2942. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2754208/
- Yun TK. Panax ginseng – a non-organ-specific cancer preventive?. Lancet Oncology 2001;2(1):49-55. https://www.ncbi.nlm.nih.gov/pubmed/11905620
- Kim K.Y., Shin J.K., Lee S.W., Yoon S.R., Chung H.S., Jeong Y.J., Choi M.S., Lee C.M., Moon K.D., Kwon J.H. Quality and functional properties of red ginseng prepared with different steaming time and drying methods. Kor J Food Sci Technol. 2007;39:494–499
- Covington MB. Traditional Chinese Medicine in the treatment of diabetes. Diabetes Spectrum. 2001;14:154–159.
- Han B.H., Park M.H., Han Y.N., Shin S.C. Studies on the antioxidant components of Korean ginseng (IV) Antifatigue active components. Yakhak Hoeji. 1984;28:231–235.
- Bhattacharya S., Mitra S. Anxiolytic activity of Panax ginseng roots: An experimental study. J. Ethnopharmacol. 1991;34:87–92. doi: 10.1016/0378-8741(91)90193-H
- Hu C., Kitts D.D. Free radical scavenging capacity as related to antioxidant activity and ginsenoside composition of Asian and North American ginseng extracts. J. Am. Oil Chem. Soc. 2001;78:249–255. doi: 10.1007/s11746-001-0253-8
- Angelova N., Kong H.W., Van Der Heijden R., Yang S.Y., Choi Y.H., Kim H.K., Wang M., Hankemeier T., Van Der Greef J., Xu G. Recent methodology in the phytochemical analysis of ginseng. Phytochem. Anal. 2008;19:2–16. doi: 10.1002/pca.1049
- Woo H.C., Shin B.K., Cho I., Koo H., Kim M., Han J. Anti-obesity effect of carbon dioxide supercritical fluid extracts of Panax ginseng CA Meyer. J. Korean Soc. Appl. Biol. Chem. 2011;54:738–743. doi: 10.1007/BF03253153
- Chen, W., Balan, P., & Popovich, D. G. (2019). Analysis of Ginsenoside Content (Panax ginseng) from Different Regions. Molecules (Basel, Switzerland), 24(19), 3491. https://doi.org/10.3390/molecules24193491
- Park, S. E., Na, C. S., Yoo, S. A., Seo, S. H., & Son, H. S. (2017). Biotransformation of major ginsenosides in ginsenoside model culture by lactic acid bacteria. Journal of ginseng research, 41(1), 36–42. https://doi.org/10.1016/j.jgr.2015.12.008
- Sun, S., Qi, L. W., Du, G. J., Mehendale, S. R., Wang, C. Z., & Yuan, C. S. (2011). Red notoginseng: higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food chemistry, 125(4), 1299–1305. https://doi.org/10.1016/j.foodchem.2010.10.049
- Yang, W., Qiao, X., Li, K., Fan, J., Bo, T., Guo, D. A., & Ye, M. (2016). Identification and differentiation of Panax ginseng, Panax quinquefolium, and Panax notoginseng by monitoring multiple diagnostic chemical markers. Acta pharmaceutica Sinica. B, 6(6), 568–575. https://doi.org/10.1016/j.apsb.2016.05.005
- Shahrajabian M.H., Sun W., Cheng Q. A review of ginseng species in different regions as a multipurpose herb in traditional Chinese medicine, modern herbology and pharmacological science. J. Med. Plant. Res. 2019;13:213–226.
- Qu C, Bai Y, Jin X, Wang Y, Zhang K, You J, et al. Study on ginsenosides in different parts and ages of Panax quinquefolius L. Food Chemistry 2009;115(1):340-6.
- Chan TW, But PP, Cheng SW, Kwok IM, Lau FW, Xu HX. Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS. Analytical Chemistry 2000;72(6):1281-7. https://www.ncbi.nlm.nih.gov/pubmed/10740871
- Cho IH, Lee HJ, Kim YS. Differences in the volatile compositions of ginseng species (Panax sp.). Journal of Agricultural and Food Chemistry 2012;60(31):7616-22. https://www.ncbi.nlm.nih.gov/pubmed/22804575
- Nocerino E, Amato M, Izzo AA. The aphrodisiac and adaptogenic properties of ginseng. Fitoterapia 2000;71(Suppl 1):S1-5. https://www.ncbi.nlm.nih.gov/pubmed/10930706
- Shergis, J.L., Zhang, A.L., Zhou, W. and Xue, C.C. (2013), Panax ginseng in Randomised Controlled Trials: A Systematic Review. Phytother. Res., 27: 949-965. https://doi.org/10.1002/ptr.4832
- Geng J, Dong J, Ni H, Lee MS, Wu T, Jiang K, Wang G, Zhou AL, Malouf R. Ginseng for cognition. Cochrane Database of Systematic Reviews 2010, Issue 12. Art. No.: CD007769. DOI: 10.1002/14651858.CD007769.pub2. http://cochranelibrary-wiley.com/doi/10.1002/14651858.CD007769.pub2/full
- Chen, W., Balan, P., & Popovich, D. G. (2020). Comparison of Ginsenoside Components of Various Tissues of New Zealand Forest-Grown Asian Ginseng (Panax Ginseng) and American Ginseng (Panax Quinquefolium L.). Biomolecules, 10(3), 372. https://doi.org/10.3390/biom10030372
- Zou K., Zhu S., Meselhy M.R., Tohda C., Cai S., Komatsu K. Dammarane-type saponins from Panax japonicus and their neurite outgrowth activity in SK-N-SH cells. J. Nat. Prod. 2002;65:1288–1292. doi: 10.1021/np0201117
- Van Le T.H., Lee S.Y., Kim T.R., Kim J.Y., Kwon S.W., Nguyen N.K., Park J.H., Nguyen M.D. Processed Vietnamese ginseng: Preliminary results in chemistry and biological activity. J. Ginseng Res. 2014;38:154–159. doi: 10.1016/j.jgr.2013.11.015
- Tang Q.Y., Chen G., Song W.L., Fan W., Wei K.H., He S.M., Zhang G.H., Tang J.R., Li Y., Lin Y. Transcriptome analysis of Panax zingiberensis identifies genes encoding oleanolic acid glucuronosyltransferase involved in the biosynthesis of oleanane-type ginsenosides. Planta. 2019;249:393–406. doi: 10.1007/s00425-018-2995-6
- Liang C., Ding Y., Nguyen H.T., Kim J.A., Boo H.J., Kang H.K., Nguyen M.C., Kim Y.H. Oleanane-type triterpenoids from Panax stipuleanatus and their anticancer activities. Bioorg. Med. Chem. Lett. 2010;20:7110–7115. doi: 10.1016/j.bmcl.2010.09.074
- Gurung B., Bhardwaj P.K., Rai A.K., Sahoo D. Major ginsenoside contents in rhizomes of Panax sokpayensis and Panax bipinnatifidus. Nat. Prod. Res. 2018;32:234–238. doi: 10.1080/14786419.2017.1343322
- Kharwanlang L., Das M.C., Kumaria S., Tandon P. Histological and SEM studies on somatic embryogenesis in rhizome-derived callus of Panax assamicus. Ban. Pharm. Innov. J. 2016;5:93–99.
- Yue P.Y.K., Mak N.K., Cheng Y.K., Leung K.W., Ng T.B., Fan D.T.P., Yeung H.W., Wong R.N.S. Pharmacogenomics and the Yin/Yang actions of ginseng: Anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin. Med. 2007;2:6. doi: 10.1186/1749-8546-2-6
- Nagulan V., Preeti A. A unique type of endosperm in Panax wangianus SC Sun. J. Plant. Dev. 2013;20:45–50.
- Kaliraj L., Ahn J.C., Rupa E.J., Abid S., Lu J., Yang D.C. Synthesis of panos extract mediated ZnO nano-flowers as photocatalyst for industrial dye degradation by UV illumination. J. Photochem. Photobiol. B Biol. 2019;199:111588. doi: 10.1016/j.jphotobiol.2019.111588
- Park S., Park J., Kim H., Lee C., Lee H., Kang K.S., Kim C. Systems-level mechanisms of action of Panax ginseng: A network pharmacological approach. J. Ginseng Res. 2018;42:98–106. doi: 10.1016/j.jgr.2017.09.001
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013;2013:16. doi: 10.1155/2013/162750
- Kim Y-S, Woo J-Y, Han C-K, Chang I-M. Safety Analysis of Panax Ginseng in Randomized Clinical Trials: A Systematic Review. Adams JD, ed. Medicines. 2015;2(2):106-126. doi:10.3390/medicines2020106. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5533164/
- Sun Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng C.A. Meyer: An overview. Carbohydr. Polym. 2011;85:490–499. doi: 10.1016/j.carbpol.2011.03.033
- Christensen L.P. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv. Food Nutr. Res. 2009;55:1–99 https://www.ncbi.nlm.nih.gov/pubmed/18772102
- Kong Y.H., Lee Y.C., Choi S.Y. Neuroprotective and anti-inflammatory effects of phenolic compounds in Panax ginseng C.A. Meyer. J Ginseng Res. 2009;33:111–114
- Yang H.S. In vitro evaluation of the cytotoxicity of gallic acid and vitamin A. Kor J Oral Anatomy. 2003;27:83–92.
- Yoo R.S., Lee H.J., Byun S.Y. Differences in phenolic compounds between Korean ginseng and moutain ginseng. Kor Soc Biotechnol Bioeng J. 2000;15:120–124
- Chung I-M, Lim J-J, Ahn M-S, Jeong H-N, An T-J, Kim S-H. Comparative phenolic compound profiles and antioxidative activity of the fruit, leaves, and roots of Korean ginseng (Panax ginseng Meyer) according to cultivation years. Journal of Ginseng Research. 2016;40(1):68-75. doi:10.1016/j.jgr.2015.05.006. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4703808/
- Lee J., Zhao Y., Liang X.J. Current evaluation of the millennium phytomedicine—Ginseng (II): Collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr. Med. Chem. 2009;16:2924–2942 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2754208/
- Choi S.Y., Cho C.W., Lee Y., Kim S.S., Lee S.H., Kim K.T. Comparison of ginsenoside and phenolic ingredient contents in hydroponically-cultivated ginseng leaves, fruits, and roots. J Ginseng Res. 2012;36:425–429 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3659603/
- Xie J.T., Wang C.Z., Zhang B., Mehendale S.R., Li X., Sun S., Han A.H., Du W., He T.C., Yuan C.S. In vitro and in vivo anticancer effects of American ginseng berry: exploring representative compounds. Bio Pharma Bul. 2009;32:1552–1558 https://www.ncbi.nlm.nih.gov/pubmed/19721231
- Scholey A, Ossoukhova A, Owen L, Ibarra A, Pipingas A, He K, et al. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: an acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology 2010;212(3):345-56. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2952762/
- Zong A, Cao H, Wang F. Anticancer polysaccharides from natural resources: a review of recent research. Carbohydrate Polymers 2012;90(4):1395-410. https://www.ncbi.nlm.nih.gov/pubmed/22944395
- Wong AS, Che CM, Leung KW. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015 Feb;32(2):256-72. doi: 10.1039/c4np00080c
- Wang Y, Choi HK, Brinckmann JA, Jiang X, Huang L. Chemical analysis of Panax quinquefolius (North American ginseng): A review. J Chromatogr A. 2015 Dec 24;1426:1-15. doi: 10.1016/j.chroma.2015.11.012
- Lee CH, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. Journal of Ginseng Research 2014;38(3):161-166.
- Buettner C, Yeh GY, Phillips RS, Mittleman MA, Kaptchuk TJ. Systematic review of the effects of ginseng on cardiovascular risk factors. Annals of Pharmacotherapy 2006;40(1):83-95. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0023036/
- Ong WY, Farooqui T, Koh HL, Farooqui AA, Ling EA. Protective effects of ginseng on neurological disorders. Frontiers in Aging Neuroscience 2015;7:129.
- Lee MS, Yang EJ, Kim JI, Ernst E. Ginseng for cognitive function in Alzheimer’s disease: a systematic review. Journal of Alzheimer’s Disease 2009;18(2):339-44. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0028523/
- Seida JK, Durec T, Kuhle S. North American (Panax quinquefolius) and Asian ginseng (Panax ginseng) preparations for prevention of the common cold in healthy adults: a systematic review. https://www.hindawi.com/journals/ecam/2011/282151/
- Karmazyn M, Gan XT. Ginseng for the treatment of diabetes and diabetes-related cardiovascular complications: a discussion of the evidence. Canadian Journal of Physiology and Pharmacology 2019;97(4):265-276.
- Song M-Y, Kim BS, Kim H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. Journal of Ginseng Research 2014;38(2):106-15.
- Lee HW, Lim H-J, Jun JH, Choi J, Lee MS. Ginseng for treating hypertension: a systematic review and meta-analysis of double blind, randomized, placebo-controlled trials. Current Vascular Pharmacology 2017;15(6):549-556.
- Hur MH, Lee MS, Kim C, Ernst E. Ginseng for the treatment of hypertension: a systematic review. Journal of Ginseng Research 2010;34(4):342-7.
- Bahrke MS, Morgan WP, Stegner A. Is ginseng an ergogenic aid?. International Journal of Sport Nutrition and Exercise Metabolism 2009;19(3):298-322. https://www.ncbi.nlm.nih.gov/pubmed/19574616
- Shergis JL, Zhang AL, Zhou W, et al. Panax ginseng in randomised controlled trials: a systematic review. Phytotherapy Research. 2013;27(7):949-965 https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.4832
- Panax Ginseng Enhances Cognitive Performance in Alzheimer Disease. Alzheimer Disease & Associated Disorders: July-September 2008 – Volume 22 – Issue 3 – p 222-226. https://journals.lww.com/alzheimerjournal/Abstract/2008/07000/Panax_Ginseng_Enhances_Cognitive_Performance_in.5.aspx
- American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. Arch Intern Med. 2000 Apr 10; 160(7):1009-13. https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/485272
- Ginseng therapy in non-insulin-dependent diabetic patients. Sotaniemi EA, Haapakoski E, Rautio A. Diabetes Care. 1995 Oct; 18(10):1373-5. https://www.ncbi.nlm.nih.gov/pubmed/8721940/
- Decreasing, null and increasing effects of eight popular types of ginseng on acute postprandial glycemic indices in healthy humans: the role of ginsenosides. Sievenpiper JL, Arnason JT, Leiter LA, Vuksan V. J Am Coll Nutr. 2004 Jun; 23(3):248-58. https://www.ncbi.nlm.nih.gov/pubmed/15190050/
- Koshland DE., Jr The molecule of the year. Science. 1992;258:1861 https://www.ncbi.nlm.nih.gov/pubmed/1470903
- Jia L. Nitric oxide research and new drugs: Challenges and success. Commentary on the 1998 Nobel Prize in Medicine. Science (Chinese) 1999;51:49–52.
- Murphy LL, Lee TJ. Ginseng, sex behavior and nitric oxide. Ann NY Acad Sci. 2002;962:372–377.
- Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8
- Choi HK, Seong DH, Rha KH. Clinical efficacy of Korean red ginseng for erectile dysfunction. Int J Impot Res. 1995;7:181–186
- Hong B, Ji YH, Hong JH, Nam KY, Ahn TY. A double-blind crossover study evaluating the efficacy of korean red ginseng in patients with erectile dysfunction: a preliminary report. J Urol. 2002;168:2070–3. https://www.ncbi.nlm.nih.gov/pubmed/12394711
- Bahrke MS, Morgan WP. Evaluation of the ergogenic properties of ginseng. Sports Med. 1994;18:229–248
- Murphy LL, Lee TJ. Ginseng, sex behavior and nitric oxide. Ann NY Acad Sci. 2002;962:372–377
- Gillis CN. Panax ginseng pharmacology: a nitric oxide link? Biochem Pharmacol. 1997;54:1–8.
- Nakaya Y, Mawatari K, Takahashi A, Harada N, Hata A, Yasui S. The phytoestrogen ginsensoside Re activates potassium channels of vascular smooth muscle cells through PI3K/Akt and nitric oxide pathways. J Med Invest. 2007;54:381–384.
- Jang DJ, Lee MS, Shin BC, Lee YC, Ernst E. Red ginseng for treating erectile dysfunction: a systematic review. Br J Clin Pharmacol. 2008;66:444–450 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2561113/
- Kim SO, Kim MK, Lee HS, Park JK, Park K. The effect of Korean red ginseng extract on the relaxation response in isolated rabbit vaginal tissue and its mechanism. J Sex Med. 2008;5:2079–2084. https://www.ncbi.nlm.nih.gov/pubmed/18638003
- Choi YD, Rha KH, Choi HK. In vitro and in vivo experimental effect of Korean red ginseng on erection. J Urol. 1999;162:1508–1511
- Jia L. Nitric oxide research and new drugs: Challenges and success. Commentary on the 1998 Nobel Prize in Medicine. Science (Chinese) 1999;51:49–52
- Lee, H. W., Lee, M. S., Kim, T. H., Alraek, T., Zaslawski, C., Kim, J. W., & Moon, D. G. (2021). Ginseng for erectile dysfunction. The Cochrane database of systematic reviews, 4(4), CD012654. https://doi.org/10.1002/14651858.CD012654.pub2
- Wood WB, Roh BL, White RP. Cardiovascular actions of Panax ginseng in dogs. Jpn J Pharmacol. 1964;14:284–294
- Lei XL, Chiou GC. Cardiovascular pharmacology of Panax notoginseng. Am J Chin Med. 1986;14:145–152
- Kim ND, Kang SY, Schini VB. Ginsenosides evoke endothelium-dependent vascular relaxation in rat aorta. Gen Pharmacol. 1995;25:1071–1077
- Kang SY, Kim SH, Schini VB, Kim ND. Dietary ginsenosides improve endothelium-dependent relaxation in the thoracic aorta of hypercholesterolemic rabbit. Gen Pharmacol. 1995;26:483–487
- Scott GI, Colligan PB, Ren BH, Ren J. Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. Br J Pharmacol. 2001;134:1159–1165
- Sung J, Han KH, Zo JH, Park HJ, Kim CH, Oh BH. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med. 2000;28:205–216
- Li Z, Chen X, Niwa Y, Sakamoto S, Nakaya Y. Involvement of Ca2+-activated K+ channels in ginsenosides-induced aortic relaxation in rats. J Cardiovasc Pharmacol. 2001;37:41–47
- Kim CS, Park JB, Kim KJ, Chang SJ, Ryoo SW, Jeon BH. Effect of Korea red ginseng on cerebral blood flow and superoxide production. Acta Pharmacol Sin. 2002;23:1152–1156
- Shi L, Fan PS, Wu L, Fang JX, Han ZX. Effects of total saponins of Panax notoginseng on increasing PGI2 in carotid artery and decreasing TXA2 in blood platelets. Acta Pharmacol Sin. 1990;11:29–32
- Kimura Y, Okuda H, Arichi S. Effects of various ginseng saponins on 5-hydroxytryptamine release and aggregation in human platelets. J Pharm Pharmacol. 1988;40:838–843
- Park HJ, Lee JH, Song YB, Park KH. Effects of dietary supplementation of lipophilic fraction from Panax ginseng on cGMP and cAMP in rat platelets and on blood coagulation. Biol Pharm Bull. 1996;19:1434–1439
- Jung KY, Kim DS, Oh SR, Lee IS, Lee JJ, Park JD. Platelet activating factor antagonist activity of ginsenosides. Biol Pharm Bull. 1998;21:79–80
- Nakajima S, Uchiyama Y, Yoshida K, Mizukawa H, Haruki E. The effect of ginseng radius rubra on human vascular endothelial cells. Am J Chin Med. 1998;26:365–373
- Yuan CS, Attele AS, Wu JA, Lowell TK, Gu Z, Lin Y. Panax quinquefolium L. Inhibits thrombin-induced endothelin release in vitro. Am J Chin Med. 1999;27:331–338
- Kim YM, Namkoong S, Yun YG, Hong HD, Lee YC, Ha KS, Lee H, Kwon HJ, Kwon YG, Kim YM. Water extract of Korean red ginseng stimulates angiogenesis by activating the PI3K/Akt-dependent ERK1/2 and eNOS pathways in human umbilical vein endothelial cells. Biol Pharm Bull. 2007;30:1674–1679
- Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;7:1219–1225
- Van Kampen J, Robertson H, Hagg T, Drobitch R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp Neurol. 2003;184:21–29. https://www.ncbi.nlm.nih.gov/pubmed/14637121
- Tohda C, Matsumoto N, Zou K, Meselhy MR, Komatsu K. Axonal and dendritic extension by protopanaxadiol-type saponins from ginseng drugs in SK-N-SH cells. Jpn J Pharmacol. 2002;90:254–262. https://www.ncbi.nlm.nih.gov/pubmed/12499580
- Tsang D, Yeung HW, Tso WW, Peck H. Ginseng Saponins: influence on neurotransmitter uptake in rat brain synaptosomes. Planta Med. 1985;3:221–224
- Nah SY, Bhatia KS, Lyles J, Ellinwood EH, Lee TH. Effects of ginseng saponin on acute cocaine-induced alterations in evoked dopamine release and uptake in rat brain nucleus accumbens. Brain Res. 2009 doi: 10.1016/j.brainres.2008.10.064 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2667959/
- Shin HR, Kim JY, Yun TK, Morgan G, Vainio H. The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes Control. 2000;11:565–576 https://www.ncbi.nlm.nih.gov/pubmed/10880039
- Helms S. Cancer prevention and therapeutics: Panax ginseng. Altern Med Rev. 2004;9:259–74
- Xing JH, Chen YQ, Ji MX. Clinical study on effect of ginsenoside in inducing rectal cancer cell apoptosis. Chin J Integr Med. 2001;21:260–261 https://www.ncbi.nlm.nih.gov/pubmed/12577351
- Block KI, Mead MN. Immune system effects of echinacea, ginseng, and astragalus: a review. Integr Cancer Ther. 2003;2:247–67. https://www.ncbi.nlm.nih.gov/pubmed/15035888
- Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16:S28–S37. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3202208/
- Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RNS, Sasisekharan S, Fan TPD. Modulating Angiogenesis The Yin and the Yang in Ginseng. Circulation. 2004;110:1219–1225
- Yue PY, Mak NK, Cheng YK, Leung KW, Ng TB, Fan DT, Yeung HW, Wong RN. Pharmacogenomics and the Yin/Yang actions of ginseng: anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin Med. 2007;2:1–21. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1876803/
- Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137(1 Suppl):183S–185S. https://www.ncbi.nlm.nih.gov/pubmed/17182823
- Zhao Y, Wang W, Han L, Rayburn ER, Hill DL, Wang H, Zhang R. Isolation, structural determination, and evaluation of the biological activity of 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol [20(S)-25-OCH3-PPD], a novel natural product from Panax notoginseng. Med Chem. 2007;3:51–60
- Wang W, Wang H, Rayburn ER, Zhao Y, Hill DL, Zhang R. 20(S)-25-methoxyl-dammarane-3beta, 12beta, 20-triol, a novel natural product for prostate cancer therapy: activity in vitro and in vivo and mechanisms of action. Br J Cancer. 2008;98:792–802
- Jin Y, Kotakadi VS, Ying L, Hofseth AB, Cui X, Wood PA, Windust A, Matesic LE, Pena EA, Chiuzan C, Singh NP, Nagarkatti M, Nagarkatti PS, Wargovich MJ, Hofseth LJ. American ginseng suppresses inflammation and DNA damage associated with mouse colitis. Carcinogenesis. 2008;29:2351–9 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2639244/
- Niu YP, Gao R, Helen T. Study on induction of ginsenosides on HL-60 cell apoptosis. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22:450–452 https://www.ncbi.nlm.nih.gov/pubmed/12585194
- Jin YH, Yoo KJ, Lee YH. Caspase 3 mediated cleavage of p21WAF1/CIP1 associated with the cyclin A2cy2 clin2 dependent kinase 2 complex is a prerequisite for apoptosis in SK2HEP21 cells. J Biol Chem. 2000;275:30256–3026 http://www.jbc.org/content/275/39/30256.long
- Kim YS, Jin SH, Lee YH. Differential expression of protein kinase C subtypes during ginsenoside Rh2 induced apoptosis in SK2N2BE(2) and C6Bu21 cells. Arch Pharm Res. 2000;23:518–524
- Fei XF, Wang BX, Tashiro S, Li TJ, Ma JS, Ikejima T. Apoptotic effects of ginsenoside Rh2 on human malignant melanoma A375-S2 cells. Acta Pharmacol Sin. 2002;23:315–322
- Cheng CC, Yang SM, Huang CY, Chen JC, Chang WM, Hsu SL. Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemother Pharmacol. 2005;55:531–540
- Tran Q, Tezuka Y, Banskota AH, Tran QK. New spirostanol steroids and steroidal saponins from roots and rhizomes of Dracaena angustifolia and their antiproliferative activity. J Nat Prod. 2001;64:1127–1132.
- Jiang H, Fan GH. Efficacy of ginsenoside-Ph2 on proliferation and apoptosis for Bel-7404 cell line. Chin J Clin Oncol Rehabil. 2004;11:289–293.
- Oh M, Choi YH, Choi S. Anti-proliferating effects of ginsenoside Rh2 on MCF-7 human breast cancer cells. Int J Oncol. 1999;14:869–875
- Liu WK, Xu SX, Che CT. Anti-proliferative effect of ginseng saponins on human prostate cancer cell line. Life Sci. 2000;67:1297–1306
- Li X, Guan YS, Zhou XP. Anticarcinogenie effect of 20(R)-ginsenodide-Rg3 on induced hepatocellullar carcinoma in rats. J Sichuan Univ (Med Sci Edi) 2005;36:217–220.
- Yun TK, Lee YS, Lee YH. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J Korean Med Sci. 2001;16:S6–S18
- Shin HR, Kim JY, Yun TK, Morgan G, Vainio H. The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes Control. 2000;11:565–576
- Bespalov VG, Alexandrov VA, Limarenko AY. Chemoprevention of mammary, cervix and nervous system carcinogenesis in animals using cultured Panax ginseng drugs and preliminary clinical trials in patients with precancerous lesions of the esophagus and endometrium. J, Korean Med Sci. 2001;16:S42–S53.
- Xu TM, Xin Y, Cui MH, Jiang X, Gu LP. Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide on growth and angiogenesis of ovarian cancer. Chin Med J. 2007;120:584–588.
- Chen JX, Peng HM, Pu SP, Guo YP. Inducement effect of ginsenoside Rg3 on apoptosis of human bladder transitional cell carcinoma cell line EJ. Zhongguo Zhong Yao Za Zhi. 2007;32:1680–1684
- Oh S, Lee BH. A ginseng saponin metabolite-induced apoptosis in HepG2 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and Bid cleavage. Tox Appl Pharmacol. 2004;194(3):221–229.
- Choi HH, Jong HS, Jung H, Choi S. A novel ginseng saponin metabolite induces apoptosis and down-regulates fibroblast growth factor receptor 3 in myeloma cells. Int J Oncol. 2003;23:1087–1093
- Lee SJ, Ko WG, Kim JH. Induction of apoptosis by a novel intestinal metabolite of ginseng saponin via cytochrome c-mediated activation of caspase-3 protease. Biochem Pharmacol. 2000;60:677–685
- Suda K, Murakami K. Induction of apoptosis in Lewis lung carcinoma cells by an intestinal bacterial metabolite produced from orally administered ginseng protopanaxadiol saponins. J Trad Med. 2000;17:236–244.
- Hasegawa H, Uchiyama M. Antimetasatic efficacy of orally administered ginsenoside Rb1 in dependence no intestinal bacterial hydrolyzing potential and significance of treatment with an active bacterial metabolite. Planta Med. 1998;64:696–700
- Hideo H, Jong HS, Jae DH. Ginseng intestinal bacterial metabolite IH901 as a new antimetastatic agent. Arch Pharm Res. 1996;6:539–544
- Yim HW, Jong HS, Kim TY. Cyclooxygenase-2 inhibits novel ginseng metabolite-mediated apoptosis. Cancer Res. 2005;65:1952–1960
- Chen WZ, Ou YXN. Study on the mechanism of ginsenoside anticancer. Fujian J TCM. 2005;36:52–53.
- Wskabayashi C, Murakami K, Hasegava H. An intestinal bacterial metabolite of ginseng protopanaxadiol saponins has the ability to induce apoptosis in tumor cells. Biochem Biophys Res Commun. 1998;246:725–730
- Park IH, Piao LZ, Kwon SW. Cytotoxic dammarane glycosides from processed ginseng. Chem Pharm Bull. 2002;50:538–540
- Lee KY, Lee SK. Ginsenoside-Rg5 supresses cyclin E-dependent protein kinase activity via up-regulation of p21Waf1 with concomitant down-regulation of cdc25A in SK-HEP-1 Cells. Anticancer Res. 1997;17:1067–1072
- Common Herbal Dietary Supplement–Drug Interactions. Am Fam Physician. 2017 Jul 15;96(2):101-107. https://www.aafp.org/afp/2017/0715/p101.html
- Asian Ginseng. https://www.nccih.nih.gov/health/asian-ginseng
- Kim J, Chung SY, Park S, Park JH, Byun S, Hwang M, Oh DS, Choi H, Kim MY, Bu YC, Doré S, Whang WW, Kim H. Enhancing effect of HT008-1 on cognitive function and quality of life in cognitively declined healthy adults: A randomized, double-blind, placebo-controlled, trial. Pharmacology Biochemistry and Behavior 2008;90(4):517-24. https://www.ncbi.nlm.nih.gov/pubmed/18550156
- Andrade AS, Hendrix C, Parsons TL, et al. Pharmacokinetic and metabolic effects of American ginseng (Panax quinquefolius) in healthy volunteers receiving the HIV protease inhibitor indinavir. BMC Complement Altern Med. 2008;8:50.
- Lee LS, Wise SD, Chan C, Parsons TL, Flexner C, Lietman PS. Possible differential induction of phase 2 enzyme and antioxidant pathways by American ginseng, Panax quinquefolius. J Clin Pharmacol. 2008;48(5):599–609.
- Yuan CS, Wei G, Dey L, et al. Brief communication: American ginseng reduces warfarin’s effect in healthy patients: a randomized, controlled trial. Ann Intern Med. 2004;141(1):23–27.
- Liu XD, Jia L. The conduct of drug metabolism studies considered good practice (I): analytical systems and in vivo studies. Curr Drug Metab. 2007;8:815–821 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2758486/
- Siegel RK. Ginseng abuse syndrome-problems with the panacea. JAMA. 1979;241:1614–1615. https://jamanetwork.com/journals/jama/article-abstract/364391
- Klepser TB, Klepser ME. Unsafe herbal therapies. Am J Health System Pharm. 1999;56:125. http://www.ajhp.org/content/56/2/125.long
- Kitts DD, Hu C. Efficacy and safety of ginseng. Pub Health Nutrition. 2000;3:473–485 https://www.ncbi.nlm.nih.gov/pubmed/11276295
- Probable interaction between warfarin and ginseng. Janetzky K, Morreale AP. Am J Health Syst Pharm. 1997 Mar 15; 54(6):692-3. http://www.ajhp.org/content/54/6/692.long
- Panax ginseng: a systematic review of adverse effects and drug interactions. Coon JT, Ernst E. Drug Saf. 2002; 25(5):323-44. https://www.ncbi.nlm.nih.gov/pubmed/12020172/