Ischemic preconditioning
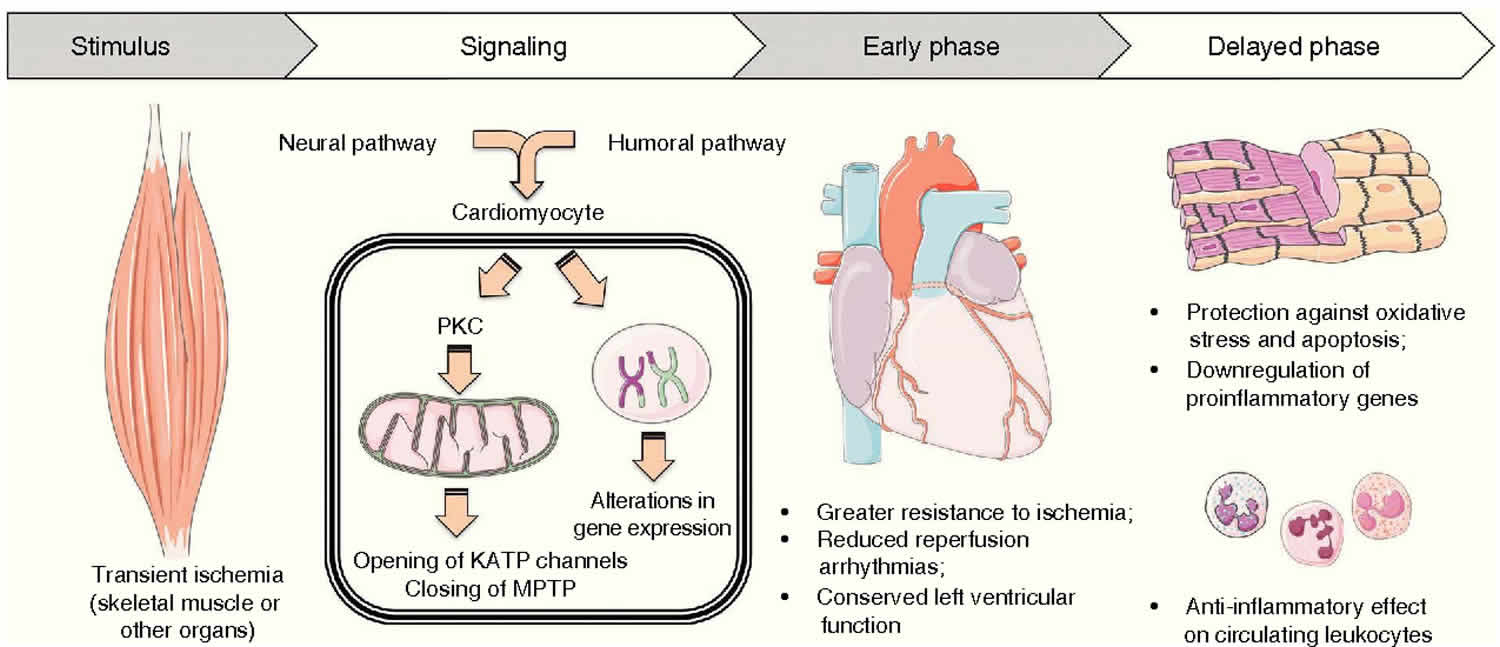
Ischemic preconditioning refers to the ability of short periods of ischemia to make the heart muscle (myocardium) more resistant to a subsequent ischemic insult 1. The term ischemic preconditioning was introduced for the first time in 1986 by Murry et al 2, who found in a canine model that 4 consecutive periods of coronary occlusion of 5 minutes were able to reduce the myocardial infarct size caused by a subsequent period of occlusion of 40 minutes by as much as 75%. In other word, if the blood supply to an organ or a tissue is halted for a short time (usually less than five minutes) and then restored two or more times so that blood flow is resumed the downstream cells of the tissue or the organ are robustly protected from a final ischemic insult when the blood supply is cut off entirely and permanently. In an experimental setting if the left anterior descending coronary artery of the animal is ligated the downstream cardiac cellular mass is infarcted and will be injured and then die. If, on the other hand the tissue is subjected to ischemic preconditioning the downstream cellular mass will sustain only minimal to modest damage. Ischemic preconditioning protects the tissue by initiating a cascade of biochemical events that allows for an up-regulation of the energetics of the tissue. Ischemic postconditioning, where a non-lethal ischemia-reperfusion is performed to the heart by interrupting the percutaneous coronary intervention (PCI)-induced reperfusion, delivers a similar outcome to ischemic preconditioning making it a better strategy to treat patients with acute myocardial infarction 3. Both ischemic preconditioning and ischemic postconditioning require interventional approaches, which limit application in clinical settings.
Ischemic preconditioning has been well studied and found to reduce ischemia-reperfusion associated damage to other organs including the lung 4, kidney 5, liver 6, skeletal muscle 7, intestine 8, brain 9 and improve post-operative recovery from cardiac surgeries 10. The potential clinical application of ischemic preconditioning is restricted to elective cardiac surgeries, where the timing of ischemic insult is well controlled. However, patients with acute myocardial infarction presented with blocked coronary arteries, making it impossible to precondition the heart 3.
Although ischemic preconditioning initially referred to the ability of short periods of ischemia to limit infarct size 2, some investigators extended this definition to include a beneficial effect on ischemia- and reperfusion-induced arrhythmias 11 and on myocardial stunning 12. It is questionable, however, whether the reduction in the incidence of arrhythmias by ischemic preconditioning is a result of a direct antiarrhythmic effect or a mere consequence of the delay of ischemic cell death 13. Regarding the beneficial effects of ischemic preconditioning on post-ischemic contractile dysfunction, Cohen et al 12 showed that preconditioning in rabbits can lead to enhanced recovery of contractile function of the myocardial region at risk. Also, in this case, the beneficial effects of preconditioning on acute recovery of contractile function might be a consequence of the delay of ischemic cell death; indeed, parameters of necrosis extent, ie, infarct size and enzyme leakage, correlate with the enhancement of functional recovery 12.
The chain of events which confers resistance to ischemia is only partially understood. Recently, Downey and coworkers 14 have developed the hypothesis that stimulation of a variety of G protein-coupled receptors results in the activation of protein kinase C. This, in turn, leads to the translocation of protein kinase C from the cytoplasm to the sarcolemma, where it phosphorylates a substrate protein (possibly the ATP-sensitive K+[KATP] channel), which confers resistance to ischemia.
It is now well established that the protective effects of ischemic preconditioning are transient and last for <2 hours 14. However, a so-called second window of protection or delayed ischemic preconditioning has been shown in different species, occurring 24 hours after the preconditioning stimulus and lasting for about 48 hours 15. This time course is consistent with the concept that the second window of protection is mediated by the activation of genes encoding for cytoprotective proteins, such as heat shock proteins or antioxidant enzymes 15. Similar to the early phase of ischemic preconditioning, aside from a delayed anti-infarct effect, a delayed anti-arrhythmic effect following preconditioning has been reported 16. Furthermore, Bolli’s group 17 has recently described a preconditioning against myocardial stunning, independent of ischemic necrosis because the ischemic challenge used was insufficient to induce infarction.
Remote ischaemic preconditioning
In contrast to directly preconditioning the target organ, Przyklenk and Whittaker 18 in 1993 made the intriguing discovery that preconditioning the heart does not limit its efficacy to the perfused area of the coronary artery, but was extended to remote myocardial tissue. Similarly, Liauw et al 19. showed that skeletal muscle can be protected against ischemia-reperfusion injury by preconditioning the contralateral skeletal muscle. This discovery facilitated the extension of ischemic preconditioning techniques to protect other organs beyond the heart. This approach of remotely protecting a target organ through ischemic preconditioning is known as remote ischemic preconditioning 3. A major advance in myocardial remote ischemic preconditioning came with the use of skeletal muscle as the origin of remote ischemic preconditioning stimulus and brief ischemia-reperfusion injury produced with a tourniquet applied to one of the hind limbs of pig 20. This lead to a blood pressure measuring cuff around the arm to achieve the remote ischemic preconditioning stimulus making it possible to accommodate most of the clinical settings of acute ischemia-reperfusion injury. In a non-invasive approach, remote ischemic preconditioning has the capacity to protect the organ or tissue whether applied prior to ischemia-reperfusion (remote ischemic preconditioning), after ischemia but prior to reperfusion 21 or during reperfusion (remote ischemic postconditioning) 22. Pryds and colleagues 23 demonstrated the long term effect of remote ischemic preconditioning on heart failure patients and reported that though remote ischemic preconditioning does not improve left ventricular ejection fraction (LVEF) but reduces blood pressure and NT-proBNP in patients with compensated chronic ischemic heart failure and may reduce the risk of thrombosis by stimulating fibrinolysis 24. Table 1 summarizes the key clinical trials on the effect of remote ischemic preconditioning prior to coronary artery bypass graft (CABG) and percutaneous coronary intervention (PCI). Previous review papers by Hausenloy and Yellon in 2008 25 and Costa et al. in 2013 26 discussed the cardioprotective pathways induced by remote ischemic preconditioning.
The effect of remote ischemic preconditioning is not confined to one organ but impacts multiple organs. Similarly, different organs can be used as the remote ischemic preconditioning site. Table 2 summarizes the key findings on inter-organ preconditioning studies. Briefly, applying remote ischemic preconditioning stimulus to different organs has been shown to protect various target organs from ischemia-reperfusion injury. These protective effects include reduced infarct size, decrease arrhythmia, improved lung and liver function (Table 1).
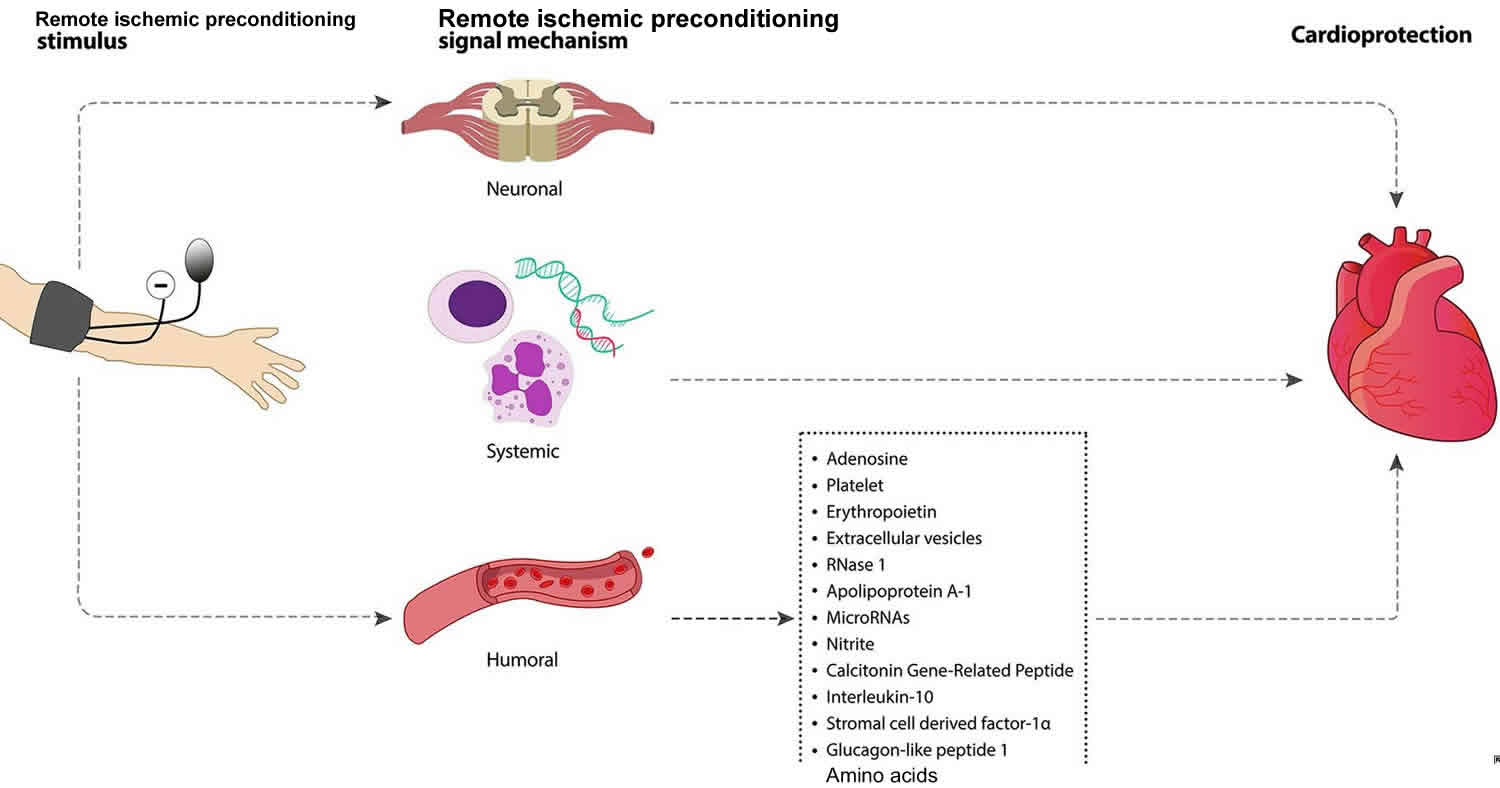
Figure 1. Remote ischemic preconditioning
Footnote: Signaling mechanisms underpinning remote ischemic preconditioning-induced cardioprotection. Intermittent limb ischemia and reperfusion confers cardioprotection through neuronal, systemic and humoral mechanism.
[Source 3 ]Table 1. Key clinical trials of remote ischemic preconditioning
| First author | Nature of trial | Number of participants analyzed (Remote ischemic preconditioning / Control) | Remote ischemic preconditioning protocol | Cardioprotection |
|---|---|---|---|---|
| Coronary artery bypass graft | ||||
| Hong et al. 27 | Randomized control trial | 35/35 | 4 cycles of 5 min I/R on lower limb | Yes |
| Lucchinetti et al. 28 | Randomized control trial | 27/28 | 4 cycles of 5 min I/R of leg | No |
| Hausenloy et al. 29 | Randomized control trial | 27:30 | 3 cycles of 5 min I/R of right upper limb | Yes |
| Candilio et al. 30 | Randomized control trial | 89/89 | 2 cycles of simultaneous 5 min I/R on upper arm and upper thigh | Yes |
| Venugopal et al. 31 | Randomized control trial | 23/22 | 3 cycles of 5 min I/R of right forearm | Yes |
| Hausenloy et al. 32 | Multicenter randomized control trial | 801/811 | 4 cycles of 5 min I/R of upper arm | No |
| Krogstad et al. 33 | Randomized control trial | 45/47 | 3 cycles of 5 min I/R of upper arm | No |
| Hong et al. 34 | Randomized control trial | 644/636 | 4 cycles of 5 min I/R of upper limb as RIPC and 4 cycles of 5 min I/R of upper limb as RIPost | No |
| Meybohm et al. 35 | Multicenter randomized control trial | 692/693 | 4 cycles of 5 min I/R of upper arm | No |
| Percutaneous coronary intervention (PCI) | ||||
| Pryds et al.36 | Post-hoc analysis of randomized control trial | 166:167 | 4 cycles of 5 min I/R of upper arm | Yes |
| Sloth et al. 37 | Post-hoc analysis of randomized control trial | 71:68 | 4 cycles of 5 min I/R of upper arm | Yes |
| Pryds et al. 38 | Post-hoc analysis of randomized control trial | 71:68 | 4 cycles of 5 min I/R of upper arm | Yes |
| Botker et al. 39 | Randomized control trial | 126: 125 | 4 cycles of 5 min I/R of upper arm | Yes |
| Prasad et al. 40 | Randomized control trial | 47:48 | 3 cycles of 3 min I/R of upper arm | No |
| Verouhis et al. 41 | Randomized control trial | 60:55 | 1 cycle of 5 min I/R of left thigh before PCI and 4 cycles of 5 min I/R of left thigh post reperfusion | Neutral |
Table 2. Key studies on inter-organ ischemic preconditioning
| Study (remote ischemic preconditioning site) | Species | Target organ | Result |
|---|---|---|---|
| Renal | |||
| McClanahan et al. 42 | Rabbit | Heart | ↓Infarct size |
| Gho et al. 43 | Rat | Heart | ↓Infarct size |
| Verdouw et al. 44 | Pig | Heart | ↓Infarct size |
| Pell et al. 45 | Rabbit | Heart | ↓Infarct size |
| Takaoka et al. 46 | Rabbit | Heart | ↓Infarct size and improved myocardial energy metabolism |
| Diwan et al. 47 | Rat | Heart | Conferred cardioprotection by NFkB activation followed by opening of K(ATP) channels |
| Lang et al. 48 | Rat | Heart | ↓Infarct size |
| Singh et al. 49 | Rat | Heart | ↓Infarct size and proposed the involvement of angiotensin AT(1) receptors in renal preconditioning |
| Kant et al. 50 | Rat | Heart | Reduced myocardial injury through inhibition of hypoxia inducible factor-prolyl 4-hydroxylases |
| Small Intestine | |||
| Gho et al. 43 | Rat | Heart | ↓Infarct size |
| Verdouw et al. 51 | Pig | Heart | ↓Infarct size |
| Patel et al. 52 | Rat | Heart | ↓Infarct size |
| Heidbreder et al. 53 | Rat | Heart | ↓Infarct size and activated p38 MAPK, ERK ½ and JNK ½ selectively in the intestine but not in the heart |
| Liver | |||
| Ates et al. 54 | Rat | Kidney | Improved creatine clearance and improvement in hepatic histopathologic parameters |
| Brzozowski et al. 55 | Rat | Gut | Reduced gastric mucosa lesion |
| Brain | |||
| Tapuria et al. 56 | Rat | Liver | Improved hepatic microcirculation and reduced hepatic ischemia-reperfusion injury. |
| Hind Limb | |||
| Oxman et al. 57 | Rat | Heart | Decreased arrhythmias |
| Birnbaum et al. 58 | Rabbit | Heart | Reduced myocardial infarct size |
| Liauw et al. 19 | Rat | Thigh muscle | Reduced muscle necrosis |
| Kharbanda et al. 20 | Pig | Heart | Reduced MI size |
| Gunaydin et al. 59 | Human | Heart | Enhanced anaerobic glycolysis to protect heart |
| Xia et al. 60 | Sheep | Lung | Protected lung from repeated coronary artery occlusion (CAO) and reperfusion mimicking multi-vessel off-pump coronary artery bypass (OPCAB) revascularization and decreased pulmonary vascular resistance |
| Addison et al. 61 | Pig | Skeletal muscle | Protected global skeletal muscle against infarction |
| Kuntscher et al. 62 | Rat | Adipocutaneous flaps | Decreased flap necrosis |
| Kuntscher et al. 63 | Rat | Cremasteric muscle flaps | Decreased flap necrosis |
| Kuntscher et al. 64 | Rat | Epigastric adipocutaneous flaps | Decreased flap necrosis |
| Moses et al. 65 | Pig | Latissimus dorsi (LD) muscle flaps | Decreased flap infarction |
| Wang et al. 66 | Rat | Cremaster flap | Decreased flap necrosis |
| Harkin et al. 67 | Pig | Lung | Reduced acute remote lung damage against systemic inflammatory response from limb ischemia-reperfusion injury |
| Li et al. 68 | Mice | Heart | Protected LV function and reduced infarction size |
| Konstantinov et al. 69 | Pig | Heart | Reduced ischemia-reperfusion injury in the brain-dead donor heart following orthotopic heart transplantation. |
| Chen et al. 70 | Rat | Heart | Reduced infarction size |
| Chen et al. 71 | Rat | Heart | Reduced infarction size through free radical pathway |
| Luokogeorgakis et al. 72 | Human | Forearm | Preserved endothelial function in the forearm |
| Waldow et al. 73 | Pig | Lung | Protected lung function and reduced the plasma interleukin-1beta level |
| Kristiansen et al. 74 | Rat | Heart | Reduced myocardial infarction size through a mechanism involving mitochondrial K(ATP) channels and improved LV function during reperfusion |
| Zhang et al. 75 | Rat | Heart | Reduced infarction size and ischemia-reperfusion-induced plasma lactate dehydrogenase level |
| Dave et al. 76 | Rat | Heart | Increased neuroprotection from asphyxial cardiac arrest |
| Kanoria et al. 77 | Rabbit | Liver | Reduced liver ischemia-reperfusion injury and improved liver function |
| Lai et al. 78 | Rat | Liver | Remote ischemic preconditioning stimulated heme oxygenase-1 expression in liver tissue and associated with liver protection from ischemia-reperfusion injury |
| Cheung et al. 79 | Human | Heart | Postoperative improvement in lung function and reduction in plasma troponin-I level |
| Mudaliar et al. 80 | Rat | Heart | ↓ Infarct size through JAK-STAT pathway upregulation |
Abbreviations: CAO = coronary artery occlusion, OCABG = off-pump coronary artery bypass, LV = left ventricular
Remote ischemic preconditioning underlying mechanisms
The underlying mechanisms through which brief episodes of ischemia-reperfusion in an organ or tissue transduces a protective signal to a distant organ and renders it resistant to sustained ischemia-reperfusion injury is not fully understood. Some studies suggest there is similarity in the mechanistic process of direct preconditioning and remote ischemic preconditioning. Based on current knowledge, this can be divided into three major parts: (i) the humoral (ii) the neuronal pathway, and (iii) the systemic pathway (Figure 1). However, whether these pathways independently exert protective effect on the target organ or that crosstalk is involved is not well understood.
Humoral pathway
Multiple studies support the theory of blood borne mediators as a signal transduction mechanism and the requirement for a period of reperfusion to washout humoral factors generated by remote ischemic preconditioning 81. These protective substances circulate via the bloodstream and upon reaching the target organ bind to respective receptors and activate intracellular signaling pathways. Humoral pathway involvement in remote ischemic preconditioning was demonstrated by Konstantinov and colleagues 69. Denervated donor heart recipient pigs that underwent remote limb preconditioning showed significant reduction of myocardial infarction size, which provides evidence for the concept of humoral-mediated cardioprotection by remote ischemic preconditioning. Dickson and colleagues showed for the first time that remote ischemic preconditioning could elicit cross species protection 82. These studies explored transfusing blood from preconditioned rabbit hearts and kidneys to a non-preconditioned isolated rabbit heart and showed recovery of the heart from myocardial ischemia-reperfusion injury by reducing the infarct size. These authors also showed that coronary effluent from a preconditioned ex-vivo rabbit heart could potentiate the similar infarction limiting effect and improve left ventricle function 83. Shimizu et al. 84 reported similar cross species protection after using plasma dialysate from remote preconditioned rabbit and human blood to protect ex vivo rabbit heart from ischemia-reperfusion injury. These authors also confirmed that the transferrable factors are hydrophobic in nature and <15 kDa in size. Serejo et al. 85 provided evidence that the humoral factors released from the ischemic preconditioned heart were thermolabile, hydrophobic, >3.5 kDa and conferred cardioprotection via the activation of protein kinase C (PKC). Breivik et al. 86 also reported the presence of <30 kDa hydrophobic factors in the coronary ischemic preconditioning effluent, which can confer cardioprotection via the PI3K/AKT pathway. Interestingly, proteomic analysis of renal remote ischemic preconditioning conducted by Lang and colleagues 48 could not detect any cytoprotective factors larger than 8 kDa. The humoral factors responsible for the remote ischemic preconditioning effect on the target organs still remain unclear and investigation into the factors responsible continues. Identifying the potential humoral mediators of remote ischemic preconditioning could assist in confirming that the threshold for a remote ischemic preconditioning response has been achieved 87.
Neural pathway
A neural pathway is one that connects one part of the nervous system with another by way of axons. Evidence suggests that intact neural pathway is essential for the remote organ or tissue to convey protective signal to the target organ during the process of remote ischemic preconditioning. Denervation of the neural pathway in the remote organ abolishes remote ischemic preconditioning protection 88. In contrast Konstantinov and colleagues 69 show that denervation of the recipient donor heart does not eliminate the remote ischemic preconditioning-induced myocardial infarction size reduction effect. However, the exact role of the afferent and efferent component of the neural pathway is unclear. The involvement of the neural pathway in remote ischemic preconditioning-mediated cardioprotection was explored by Gho et al. 43 who demonstrated that transient occlusion of the anterior mesenteric artery can mediate cardioprotection, which can be abrogated by ganglionic blockers. This finding was supported with the proposition that remote ischemic preconditioning propels the production of autacoids such as adenosine, bradykinin, CGRP in the remote preconditioned organ, which stimulates afferent nerves and relays the neural signal to the myocardium via the efferent nerve fibers. Furthermore, Ding et al. 89 explored the role of renal nerve-mediated cardioprotection. They confirmed that renal nerve resection abolished renal preconditioning-induced cardioprotection. Liem et al. 90 provided confirmatory evidence implicating adenosine in a neural pathway of cardioprotection. They reported that adenosine released by the mesenteric artery during preconditioning reduced myocardial infarct size from 68% to 48%, a protective effect that was reversed by the ganglionic blocker hexamethonium. In addition, intramesenteric artery infusion of adenosine mimicked similar cardioprotection as mesenteric artery-induced preconditioning, which could be abolished by hexamethonium. From these findings, the investigators concluded that locally released adenosine during mesenteric artery preconditioning stimulates afferent nerves in the mesenteric bed which helps activate myocardial adenosine receptors. Dong et al. 91 demonstrated that dissecting the femoral nerve prior remote ischemic preconditioning does not protect the myocardium against ischemia-reperfusion injury and suggested that an intact neural pathway was required for the sensory afferent neural signaling from the preconditioned limb. A study carried out by Jones and colleagues 92 showed that instead of ischemic preconditioning, abdominal slit in mice activates the cardiac sensory and sympathetic nerves. This procedure elicits cardioprotection via bradykinin (a known hormone and neurotransmitter) release in the heart by the sympathetic nerves and bradykinin dependent activation of PKC-ε.
Systemic pathway
Remote ischemic conditioning has been shown to provoke a systemic response by modulating inflammatory cells either post-transcriptionally or through transcriptional regulation 93. In contrast to the humoral pathway, the systemic pathway involves the inflammatory cells and provokes an inflammatory response to confer the remote ischemic preconditioning signal. Kharbanda et al. 94 previously showed that remote ischemic preconditioning reduced expression of neutrophil CD11b and platelet-neutrophil complexes in humans. In 2004, Konstantinov et al. 95 used microarray analysis of blood samples from healthy human volunteers subjected to forearm preconditioning to reveal that preconditioning suppressed genes regulating cytokine production, leukocyte chemotaxis, adhesion and migration, exocytosis, innate immunity, signaling pathways, and apoptosis, while up-regulating anti-inflammatory genes such as HSP-70 and calpastatin. Later, the same group provided evidence to show that remote ischemic preconditioning upregulated genes associated with growth and metabolism, DNA repair and redox regulation. ischemic preconditioning attenuated P-selectin expression in liver and prevented neutrophil infiltration in lung, stomach, pancreas, small intestine and colon via inhibition of systemic TNF-α production 96. In another study, Albrecht et al. 97 reported similar findings in human, showing that within the early phase of remote ischemic preconditioning, serum cytokines were upregulated. It may be that, cytokines function as both pro- and anti-inflammatory mediators in ischemic conditioning to prepare the target organ to mitigate the tissue damage. This group’s findings showed concurrent increase of IL-8, IL-1β, TNF-α and concurrent cardioprotection due to increased neutrophil infiltration after right atrial bypass surgery 97.
Summary
Remote ischemic preconditioning has provided an innovative non-invasive therapeutic strategy to prevent acute ischemia-reperfusion injury in susceptible organs and tissues with some variability. Non-invasive procedures such as using a blood pressure measuring cuff around the arm to achieve protection against ischemia-reperfusion injury has facilitated its translation from bench to bedside. Though there are several clinical trials that did not show beneficial effects of remote ischemic preconditioning, further mechanistic studies will help us understand the underlying cause of the failure of these studies. Optimal modality, site and duration of remote ischemic preconditioning remains unclear. remote ischemic preconditioning may nonetheless benefit children and adults undergoing certain elective surgeries where there is potential to improve clinical outcomes. Future insights into the control of circulating mediators of remote ischemic preconditioning, including transcriptional regulation and secretion into the bloodstream will assist the development of pharmacologic approaches stimulating protective signaling pathways in target organs.
References- Ischemic Preconditioning in Humans. Models, Mediators, and Clinical Relevance. Circulation. 1999;100:559-563. https://www.ahajournals.org/doi/pdf/10.1161/01.CIR.100.5.559
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation.1986; 74:1124–1136.
- Billah M, Ridiandries A, Allahwala U, et al. Circulating mediators of remote ischemic preconditioning: search for the missing link between non-lethal ischemia and cardioprotection. Oncotarget. 2019;10(2):216–244. Published 2019 Jan 4. doi:10.18632/oncotarget.26537 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6349428
- Soncul H, Oz E, Kalaycioglu S. Role of ischemic preconditioning on ischemia-reperfusion injury of the lung. Chest. 1999;115:1672–7.
- Timsit MO, Gadet R, Ben Abdennebi H, Codas R, Petruzzo P, Badet L. Renal ischemic preconditioning improves recovery of kidney function and decreases alpha-smooth muscle actin expression in a rat model. J Urol. 2008;180:388–91. doi: 10.1016/j.juro.2008.02.043
- Li JY, Gu X, Yin HZ, Zhou Y, Zhang WH, Qin YM. Protective effect of ischemic preconditioning on hepatic ischemia-reperfusion injury by advancing the expressive phase of survivin in rats. Hepatobiliary Pancreat Dis Int. 2008;7:615–20.
- Schoen M, Rotter R, Gierer P, Gradl G, Strauss U, Jonas L, Mittlmeier T, Vollmar B. Ischemic preconditioning prevents skeletal muscle tissue injury, but not nerve lesion upon tourniquet-induced ischemia. J Trauma. 2007;63:788–97. doi: 10.1097/01.ta.0000240440.85673.fc
- Erling Junior N, Montero EF, Sannomiya P, Poli-de-Figueiredo LF. Local and remote ischemic preconditioning protect against intestinal ischemic/reperfusion injury after supraceliac aortic clamping. Clinics (Sao Paulo) 2013;68:1548–54. doi: 10.6061/clinics/2013(12)12
- Shi S, Yang W, Tu X, Chen C, Wang C. Ischemic preconditioning reduces ischemic brain injury by suppressing nuclear factor kappa B expression and neuronal apoptosis. Neural Regen Res. 2013;8:633–8. doi: 10.3969/j.issn.1673-5374.2013.07.007
- Perrault LP, Menasche P, Bel A, de Chaumaray T, Peynet J, Mondry A, Olivero P, Emanoil-Ravier R, Moalic JM. Ischemic preconditioning in cardiac surgery: a word of caution. J Thorac Cardiovasc Surg. 1996;112:1378–86
- Shiki K, Hearse DJ. Preconditioning of ischemic myocardium: reperfusion-induced arrhythmias. Am J Physiol.1987; 253:H1470–1476.
- Cohen MV, Liu GS, Downey JM. Preconditioning causes improved wall motion as well as smaller infarcts after transient coronary occlusion in rabbits. Circulation.1991; 84:341–349.
- Yellon DM, Alkhulaifi AM, Pugsley WB. Preconditioning the human myocardium. Lancet.1993; 342:276–277.
- Downey JM, Cohen MV. Mechanisms of preconditioning: correlates and epiphenomena. In: Marber MS, Yellon DM, eds. Ischemia: Preconditioning and Adaptation. Oxford, UK: BIOS Scientific Publishers Limited; 1996:21–34.
- Yellon DM, Baxter GF. A. “second window of protection” or delayed preconditioning phenomenon: future horizons for myocardial protection? J Mol Cell Cardiol.1995; 27:1023–1034.
- Vegh A, Papp JG, Parrat JR. Prevention by dexamethasone of the marked antiarrhythmic effects of preconditioning induced 20 h after rapid cardiac pacing. Br J Pharmacol.1994; 113:1081–1082.
- Sun JZ, Tang XL, Knowlton AA, Park SW, Qiu Y, Bolli R. Late preconditioning against myocardial stunning: an endogenous protective mechanism that confers resistance to postischemic dysfunction 24 hours after brief ischemia in conscious pigs. J Clin Invest.1995; 95:388–403.
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87:893–9.
- Liauw SK, Rubin BB, Lindsay TF, Romaschin AD, Walker PM. Sequential ischemia/reperfusion results in contralateral skeletal muscle salvage. Am J Physiol. 1996;270:H1407–13.
- Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–3.
- Schmidt MR, Smerup M, Konstantinov IE, Shimizu M, Li J, Cheung M, White PA, Kristiansen SB, Sorensen K, Dzavik V, Redington AN, Kharbanda RK. Intermittent peripheral tissue ischemia during coronary ischemia reduces myocardial infarction through a KATP-dependent mechanism: first demonstration of remote ischemic perconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H1883–90. doi: 10.1152/ajpheart.00617.2006
- Kerendi F, Kin H, Halkos ME, Jiang R, Zatta AJ, Zhao ZQ, Guyton RA, Vinten-Johansen J. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100:404–12. doi: 10.1007/s00395-005-0539-2
- Pryds K, Nielsen RR, Jorsal A, Hansen MS, Ringgaard S, Refsgaard J, Kim WY, Petersen AK, Botker HE, Schmidt MR. Effect of long-term remote ischemic conditioning in patients with chronic ischemic heart failure. Basic Res Cardiol. 2017;112:67. doi: 10.1007/s00395-017-0658-6
- Pryds K, Kristiansen J, Neergaard-Petersen S, Nielsen RR, Schmidt MR, Refsgaard J, Kristensen SD, Botker HE, Hvas AM, Grove EL. Effect of long-term remote ischaemic conditioning on platelet function and fibrinolysis in patients with chronic ischaemic heart failure. Thromb Res. 2017;153:40–6. doi: 10.1016/j.thromres.2017.03.008
- Hausenloy DJ, Yellon DM. Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res. 2008;79:377–86. doi: 10.1093/cvr/cvn114
- Costa JF, Fontes-Carvalho R, Leite-Moreira AF. Myocardial remote ischemic preconditioning: from pathophysiology to clinical application. Rev Port Cardiol. 2013;32:893–904. doi: 10.1016/j.repc.2013.02.012
- Hong DM, Jeon Y, Lee CS, Kim HJ, Lee JM, Bahk JH, Kim KB, Hwang HY. Effects of remote ischemic preconditioning with postconditioning in patients undergoing off-pump coronary artery bypass surgery–randomized controlled trial. Circ J. 2012;76:884–90.
- Lucchinetti E, Bestmann L, Feng J, Freidank H, Clanachan AS, Finegan BA, Zaugg M. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on-pump coronary artery bypass graft surgery: lack of synergy or evidence of antagonism in cardioprotection? Anesthesiology. 2012;116:296–310. doi: 10.1097/ALN.0b013e318242349a
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, Ashley E, Vichare S, Di Salvo C, Kolvekar S, Hayward M, Keogh B, MacAllister RJ, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet. 2007;370:575–9. doi: 10.1016/S0140-6736(07)61296-3
- Candilio L, Malik A, Ariti C, Barnard M, Di Salvo C, Lawrence D, Hayward M, Yap J, Roberts N, Sheikh A, Kolvekar S, Hausenloy DJ, Yellon DM. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart. 2015;101:185–92. doi: 10.1136/heartjnl-2014-306178
- Venugopal V, Hausenloy DJ, Ludman A, Di Salvo C, Kolvekar S, Yap J, Lawrence D, Bognolo J, Yellon DM. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: a randomised controlled trial. Heart. 2009;95:1567–71. doi: 10.1136/hrt.2008.155770
- Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–17. doi: 10.1056/NEJMoa1413534
- Krogstad LE, Slagsvold KH, Wahba A. Remote ischemic preconditioning and incidence of postoperative atrial fibrillation. Scand Cardiovasc J. 2015;49:117–22. doi: 10.3109/14017431.2015.1010565
- Hong DM, Lee EH, Kim HJ, Min JJ, Chin JH, Choi DK, Bahk JH, Sim JY, Choi IC, Jeon Y. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote Ischaemic Preconditioning with Postconditioning Outcome Trial. Eur Heart J. 2014;35:176–83. doi: 10.1093/eurheartj/eht346
- Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–407. doi: 10.1056/NEJMoa1413579
- Pryds K, Terkelsen CJ, Sloth AD, Munk K, Nielsen SS, Schmidt MR, Bøtker HE, CONDI Investigators Remote ischaemic conditioning and healthcare system delay in patients with ST-segment elevation myocardial infarction. Heart. 2016;102:1023–8. doi: 10.1136/heartjnl-2015-308980
- Sloth AD, Schmidt MR, Munk K, Schmidt M, Pedersen L, Sørensen HT, Bøt ker HE, CONDI Investigators Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: post hoc subgroup analysis of a randomised controlled trial. BMJ Open. 2015;5:e006923. doi: 10.1136/bmjopen-2014-006923
- Pryds K, Bøttcher M, Sloth AD, Munk K, Rahbek Schmidt M, Bøtker HE, CONDI Investigators Influence of preinfarction angina and coronary collateral blood flow on the efficacy of remote ischaemic conditioning in patients with ST segment elevation myocardial infarction: post hoc subgroup analysis of a randomised controlled trial. BMJ Open. 2016;6:e013314. doi: 10.1136/bmjopen-2016-013314
- Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–34. doi: 10.1016/S0140-6736(09)62001-8
- Prasad A, Gossl M, Hoyt J, Lennon RJ, Polk L, Simari R, Holmes DR, Jr, Rihal CS, Lerman A. Remote ischemic preconditioning immediately before percutaneous coronary intervention does not impact myocardial necrosis, inflammatory response, and circulating endothelial progenitor cell counts: a single center randomized sham controlled trial. Catheter Cardiovasc Interv. 2013;81:930–6. doi: 10.1002/ccd.24443
- Verouhis D, Sorensson P, Gourine A, Henareh L, Persson J, Saleh N, Settergren M, Sundqvist M, Tornvall P, Witt N, Bohm F, Pernow J. Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J. 2016;181:66–73. doi: 10.1016/j.ahj.2016.08.004
- McClanahan TB, Nao BS, Woke LJ, Martin BJ, Mertz TE, Gallagher GP. Brief renal occlusion and reperfusion reduces myocardial infarct size in rabbits. FASEB J. 1993. p. 7.
- Gho BC, Schoemaker RG, van den Doel MA, Duncker DJ, Verdouw PD. Myocardial protection by brief ischemia in noncardiac tissue. Circulation. 1996;94:2193–200.
- Verdouw PD, Gho BC, Koning MM, Schoemaker RG, Duncker DJ. Cardioprotection by ischemic and nonischemic myocardial stress and ischemia in remote organs. Implications for the concept of ischemic preconditioning. Ann N Y Acad Sci. 1996;793:27–42.
- Pell TJ, Baxter GF, Yellon DM, Drew GM. Renal ischemia preconditions myocardium: role of adenosine receptors and ATP-sensitive potassium channels. Am J Physiol. 1998;275:H1542–7.
- Takaoka A, Nakae I, Mitsunami K, Yabe T, Morikawa S, Inubushi T, Kinoshita M. Renal ischemia/reperfusion remotely improves myocardial energy metabolism during myocardial ischemia via adenosine receptors in rabbits: effects of “remote preconditioning” J Am Coll Cardiol. 1999;33:556–64.
- Diwan V, Kant R, Jaggi AS, Singh N, Singh D. Signal mechanism activated by erythropoietin preconditioning and remote renal preconditioning-induced cardioprotection. Mol Cell Biochem. 2008;315:195–201. doi: 10.1007/s11010-008-9808-3
- Lang SC, Elsasser A, Scheler C, Vetter S, Tiefenbacher CP, Kubler W, Katus HA, Vogt AM. Myocardial preconditioning and remote renal preconditioning–identifying a protective factor using proteomic methods? Basic Res Cardiol. 2006;101:149–58. doi: 10.1007/s00395-005-0565-0
- Singh D, Chopra K. Evidence of the role of angiotensin AT(1) receptors in remote renal preconditioning of myocardium. Methods Find Exp Clin Pharmacol. 2004;26:117–22.
- Kant R, Diwan V, Jaggi AS, Singh N, Singh D. Remote renal preconditioning-induced cardioprotection: a key role of hypoxia inducible factor-prolyl 4-hydroxylases. Mol Cell Biochem. 2008;312:25–31. doi: 10.1007/s11010-008-9717-5
- Verdouw PD, Gho BC, Koning MM, Schoemaker RG, Duncker DJ. Cardioprotection by ischemic and nonischemic myocardial stress and ischemia in remote organs. Implications for the concept of ischemic preconditioning. Ann N Y Acad Sci. 1996;793:27–42
- Patel HH, Moore J, Hsu AK, Gross GJ. Cardioprotection at a distance: mesenteric artery occlusion protects the myocardium via an opioid sensitive mechanism. J Mol Cell Cardiol. 2002;34:1317–23.
- Heidbreder M, Naumann A, Tempel K, Dominiak P, Dendorfer A. Remote vs. ischaemic preconditioning: the differential role of mitogen-activated protein kinase pathways. Cardiovasc Res. 2008;78:108–15. doi: 10.1093/cvr/cvm114
- Ates E, Genc E, Erkasap N, Erkasap S, Akman S, Firat P, Emre S, Kiper H. Renal protection by brief liver ischemia in rats. Transplantation. 2002;74:1247–51. doi: 10.1097/01.TP.0000032752.61372.36
- Brzozowski T, Konturek PC, Pajdo R, Kwiecien S, Sliwowski Z, Drozdowicz D, Ptak-Belowska A, Pawlik M, Konturek SJ, Pawlik WW, Hahn GG. Importance of brain-gut axis in the gastroprotection induced by gastric and remote preconditioning. J Physiol Pharmacol. 2004;55:165–77.
- Tapuria N, Junnarkar SP, Dutt N, Abu-Amara M, Fuller B, Seifalian AM, Davidson BR. Effect of remote ischemic preconditioning on hepatic microcirculation and function in a rat model of hepatic ischemia reperfusion injury. HPB (Oxford) 2009;11:108–17. doi: 10.1111/j.1477-2574.2009.00006.x
- Oxman T, Arad M, Klein R, Avazov N, Rabinowitz B. Limb ischemia preconditions the heart against reperfusion tachyarrhythmia. Am J Physiol. 1997;273:H1707–12.
- Birnbaum Y, Hale SL, Kloner RA. Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation. 1997;96:1641–6.
- Gunaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, Sancak B, Kanzik I, Karadenizli Y. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery? Pharmacol Res. 2000;41:493–6. doi: 10.1006/phrs.1999.0611
- Xia Z, Herijgers P, Nishida T, Ozaki S, Wouters P, Flameng W. Remote preconditioning lessens the deterioration of pulmonary function after repeated coronary artery occlusion and reperfusion in sheep. Can J Anaesth. 2003;50:481–8. doi: 10.1007/BF03021061
- Addison PD, Neligan PC, Ashrafpour H, Khan A, Zhong A, Moses M, Forrest CR, Pang CY. Noninvasive remote ischemic preconditioning for global protection of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol. 2003;285:H1435–43. doi: 10.1152/ajpheart.00106.2003
- Kuntscher MV, Kastell T, Engel H, Gebhard MM, Heitmann C, Germann G. Late remote ischemic preconditioning in rat muscle and adipocutaneous flap models. Ann Plast Surg. 2003;51:84–90. doi: 10.1097/01.SAP.0000054186.10681.E2
- Kuntscher MV, Kastell T, Sauerbier M, Nobiling R, Gebhard MM, Germann G. Acute remote ischemic preconditioning on a rat cremasteric muscle flap model. Microsurgery. 2002;22:221–6. doi: 10.1002/micr.10041
- Kuntscher MV, Schirmbeck EU, Menke H, Klar E, Gebhard MM, Germann G. Ischemic preconditioning by brief extremity ischemia before flap ischemia in a rat model. Plast Reconstr Surg. 2002;109:2398–404
- Moses MA, Addison PD, Neligan PC, Ashrafpour H, Huang N, Zair M, Rassuli A, Forrest CR, Grover GJ, Pang CY. Mitochondrial KATP channels in hindlimb remote ischemic preconditioning of skeletal muscle against infarction. Am J Physiol Heart Circ Physiol. 2005;288:H559–67. doi: 10.1152/ajpheart.00845.2004
- Wang WZ, Stepheson LL, Fang XH, Khiabani KT, Zamboni WA. Ischemic preconditioning-induced microvascular protection at a distance. J Reconstr Microsurg. 2004;20:175–81. doi: 10.1055/s-2004-820775
- Harkin DW, Barros D’Sa AA, McCallion K, Hoper M, Campbell FC. Ischemic preconditioning before lower limb ischemia–reperfusion protects against acute lung injury. J Vasc Surg. 2002;35:1264–73.
- Li G, Labruto F, Sirsjo A, Chen F, Vaage J, Valen G. Myocardial protection by remote preconditioning: the role of nuclear factor kappa-B p105 and inducible nitric oxide synthase. Eur J Cardiothorac Surg. 2004;26:968–73. doi: 10.1016/j.ejcts.2004.06.015
- Konstantinov IE, Li J, Cheung MM, Shimizu M, Stokoe J, Kharbanda RK, Redington AN. Remote ischemic preconditioning of the recipient reduces myocardial ischemia-reperfusion injury of the denervated donor heart via a Katp channel-dependent mechanism. Transplantation. 2005;79:1691–5.
- Chen XG, Wu BY, Wang JK, Bai T. [Mechanism of the protective effects of noninvasive limbs preconditioning on myocardial ischemia-reperfusion injury]. [Article in Chinese] Chin Med J (Engl) 2005;118:1723–7
- Chen YS, Chien CT, Ma MC, Tseng YZ, Lin FY, Wang SS, Chen CF. Protection “outside the box” (skeletal remote preconditioning) in rat model is triggered by free radical pathway. J Surg Res. 2005;126:92–101. doi: 10.1016/j.jss.2005.01.007
- Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol. 2005;46:450–6. doi: 10.1016/j.jacc.2005.04.044
- Waldow T, Alexiou K, Witt W, Albrecht S, Wagner F, Knaut M, Matschke K. Protection against acute porcine lung ischemia/reperfusion injury by systemic preconditioning via hind limb ischemia. Transpl Int. 2005;18:198–205. doi: 10.1111/j.1432-2277.2004.00005.x
- Kristiansen SB, Henning O, Kharbanda RK, Nielsen-Kudsk JE, Schmidt MR, Redington AN, Nielsen TT, Botker HE. Remote preconditioning reduces ischemic injury in the explanted heart by a KATP channel-dependent mechanism. Am J Physiol Heart Circ Physiol. 2005;288:H1252–6. doi: 10.1152/ajpheart.00207.2004
- Zhang SZ, Wang NF, Xu J, Gao Q, Lin GH, Bruce IC, Xia Q. Kappa-opioid receptors mediate cardioprotection by remote preconditioning. Anesthesiology. 2006;105:550–6
- Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett. 2006;404:170–5. doi: 10.1016/j.neulet.2006.05.037
- Kanoria S, Jalan R, Davies NA, Seifalian AM, Williams R, Davidson BR. Remote ischaemic preconditioning of the hind limb reduces experimental liver warm ischaemia-reperfusion injury. Br J Surg. 2006;93:762–8. doi: 10.1002/bjs.5331
- Lai IR, Chang KJ, Chen CF, Tsai HW. Transient limb ischemia induces remote preconditioning in liver among rats: the protective role of heme oxygenase-1. Transplantation. 2006;81:1311–7. doi: 10.1097/01.tp.0000203555.14546.63
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol. 2006;47:2277–82. doi: 10.1016/j.jacc.2006.01.066
- Mudaliar H, Rayner B, Billah M, Kapoor N, Lay W, Dona A, Bhindi R. Remote ischemic preconditioning attenuates EGR-1 expression following myocardial ischemia reperfusion injury through activation of the JAK-STAT pathway. Int J Cardiol. 2017;228:729–41. doi: 10.1016/j.ijcard.2016.11.198
- Weinbrenner C, Nelles M, Herzog N, Sarvary L, Strasser RH. Remote preconditioning by infrarenal occlusion of the aorta protects the heart from infarction: a newly identified non-neuronal but PKC-dependent pathway. Cardiovasc Res. 2002;55:590–601.
- Dickson EW, Blehar DJ, Carraway RE, Heard SO, Steinberg G, Przyklenk K. Naloxone blocks transferred preconditioning in isolated rabbit hearts. J Mol Cell Cardiol. 2001;33:1751–6. doi: 10.1006/jmcc.2001.1436
- Dickson EW, Lorbar M, Porcaro WA, Fenton RA, Reinhardt CP, Gysembergh A, Przyklenk K. Rabbit heart can be “preconditioned” via transfer of coronary effluent. Am J Physiol. 1999;277:H2451–7.
- Shimizu M, Tropak M, Diaz RJ, Suto F, Surendra H, Kuzmin E, Li J, Gross G, Wilson GJ, Callahan J, Redington AN. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: evidence suggesting cross-species protection. Clin Sci (Lond) 2009;117:191–200. doi: 10.1042/CS20080523
- Serejo FC, Rodrigues LF, Jr, da Silva Tavares KC, de Carvalho AC, Nascimento JH. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J Cardiovasc Pharmacol. 2007;49:214–20. doi: 10.1097/FJC.0b013e3180325ad9
- Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011;106:135–45. doi: 10.1007/s00395-010-0133-0
- Chen G, Thakkar M, Robinson C, Dore S. Limb remote ischemic conditioning: mechanisms, anesthetics, and the potential for expanding therapeutic options. Front Neurol. 2018;9:40. doi: 10.3389/fneur.2018.00040
- Donato M, Buchholz B, Rodriguez M, Perez V, Inserte J, Garcia-Dorado D, Gelpi RJ. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp Physiol. 2013;98:425–34. doi: 10.1113/expphysiol.2012.066217
- Ding YF, Zhang MM, He RR. Role of renal nerve in cardioprotection provided by renal ischemic preconditioning in anesthetized rabbits. Sheng Li Xue Bao. 2001;53:7–12.
- Liem DA, Verdouw PD, Ploeg H, Kazim S, Duncker DJ. Sites of action of adenosine in interorgan preconditioning of the heart. Am J Physiol Heart Circ Physiol. 2002;283:H29–37. doi: 10.1152/ajpheart.01031.2001
- Dong JH, Liu YX, Ji ES, He RR. [Limb ischemic preconditioning reduces infarct size following myocardial ischemia-reperfusion in rats]. [Article in Chinese] Sheng Li Xue Bao. 2004;56:41–6.
- Jones WK, Fan GC, Liao S, Zhang JM, Wang Y, Weintraub NL, Kranias EG, Schultz JE, Lorenz J, Ren X. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation. 2009;120:S1–9. doi: 10.1161/CIRCULATIONAHA.108.843938
- Saxena P, Newman MA, Shehatha JS, Redington AN, Konstantinov IE. Remote ischemic conditioning: evolution of the concept, mechanisms, and clinical application. J Card Surg. 2010;25:127–34. doi: 10.1111/j.1540-8191.2009.00820.x
- Kharbanda RK, Peters M, Walton B, Kattenhorn M, Mullen M, Klein N, Vallance P, Deanfield J, MacAllister R. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation. 2001;103:1624–30.
- Konstantinov IE, Arab S, Kharbanda RK, Li J, Cheung MM, Cherepanov V, Downey GP, Liu PP, Cukerman E, Coles JG, Redington AN. The remote ischemic preconditioning stimulus modifies inflammatory gene expression in humans. Physiol Genomics. 2004;19:143–50. doi: 10.1152/physiolgenomics.00046.2004
- Peralta C, Fernandez L, Panes J, Prats N, Sans M, Pique JM, Gelpi E, Rosello-Catafau J. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology. 2001;33:100–13. doi: 10.1053/jhep.2001.20529
- Albrecht M, Zitta K, Bein B, Wennemuth G, Broch O, Renner J, Schuett T, Lauer F, Maahs D, Hummitzsch L, Cremer J, Zacharowski K, Meybohm P. Remote ischemic preconditioning regulates HIF-1alpha levels, apoptosis and inflammation in heart tissue of cardiosurgical patients: a pilot experimental study. Basic Res Cardiol. 2013;108:314. doi: 10.1007/s00395-012-0314-0