Lemierre syndrome
Lemierre syndrome is named after the French physician, Andre Lemierre, who in 1936 reported 20 cases of anaerobic septicemia originating from oropharyngeal infections 1. Lemierre syndrome is a rare complication of bacterial pharyngitis or tonsillitis and involves an extension of the infection (disseminated abscesses) into the lateral pharyngeal spaces of the neck with subsequent septic thrombophlebitis of the internal jugular vein(s). The predominant pathogen is a gram-negative anaerobic bacillus, Fusobacterium necrophorum 2. Lemierre syndrome is associated with anaerobic septicemia and death in young, healthy patients. Due to the high frequency of benign oropharyngeal infections in this population, the diagnosis of Lemierre syndrome is often elusive on initial presentation. This can result in treatment delays 3.
The diagnosis of Lemierre syndrome should be considered in young, otherwise healthy patients with an underlying oropharyngeal infection that present with a worsening clinical course and subsequent sepsis with evidence for metastatic septic emboli.
The highest reported incidence of Lemierre’s syndrome was prior to the antibiotic era. With the widespread use of penicillin in the 1960s and 1970s, the incidence of Lemierre syndrome declined sharply. Since the late 1970s, there has been a steady rise in reported cases which may be due to the decreased use of empiric antibiotics in oropharyngeal infections. It is still a rare syndrome with an estimated worldwide incidence of 1/1,000,000. Lemierre syndrome typically affects young, previously healthy, adolescents and young adults. The median age of patients with Lemierre syndrome is between 19 to 22 years of age depending on the study with a 2:1 male-to-female ratio. Approximately 90% of the patients who develop Lemierre syndrome are between 10 and 35 years of age.
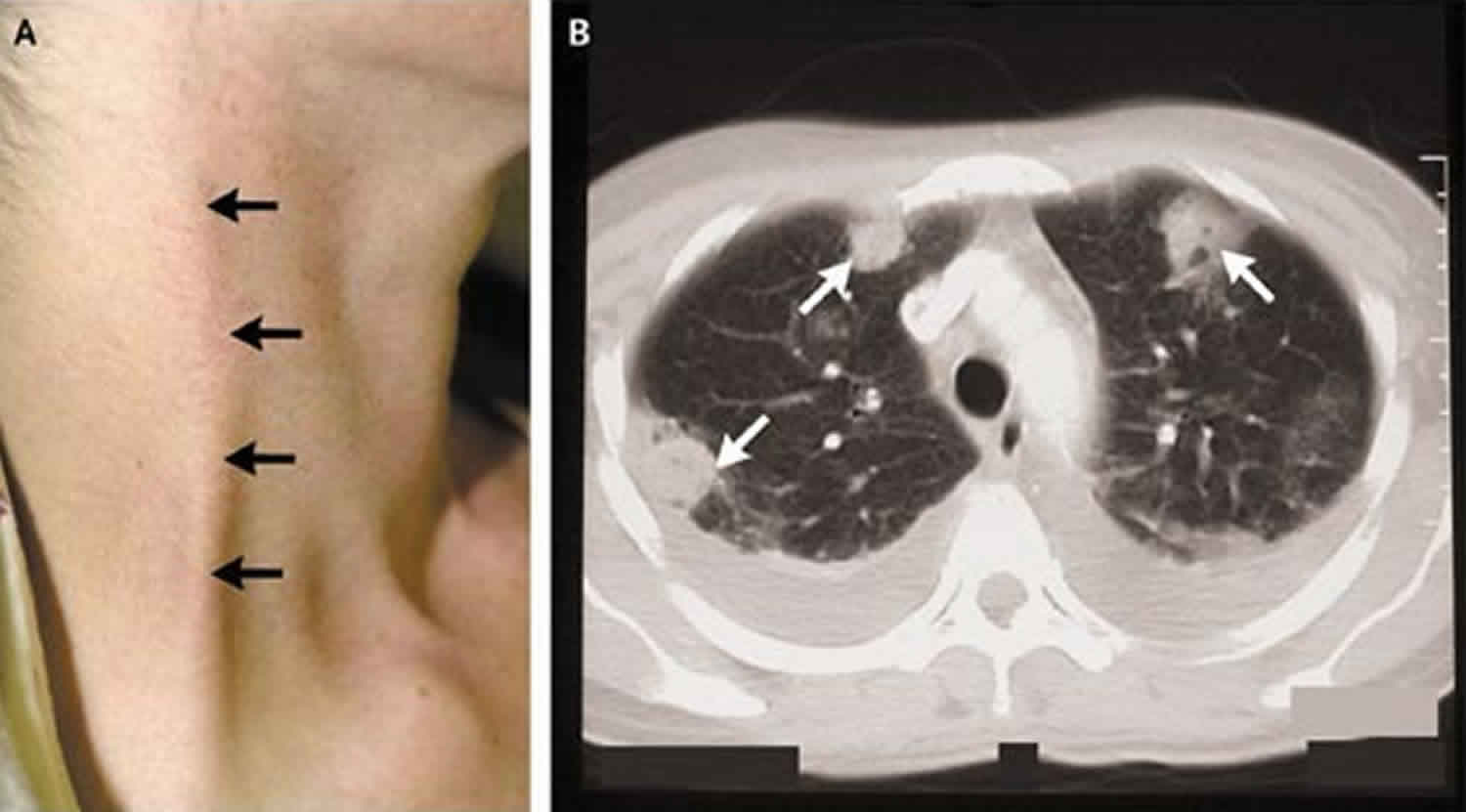
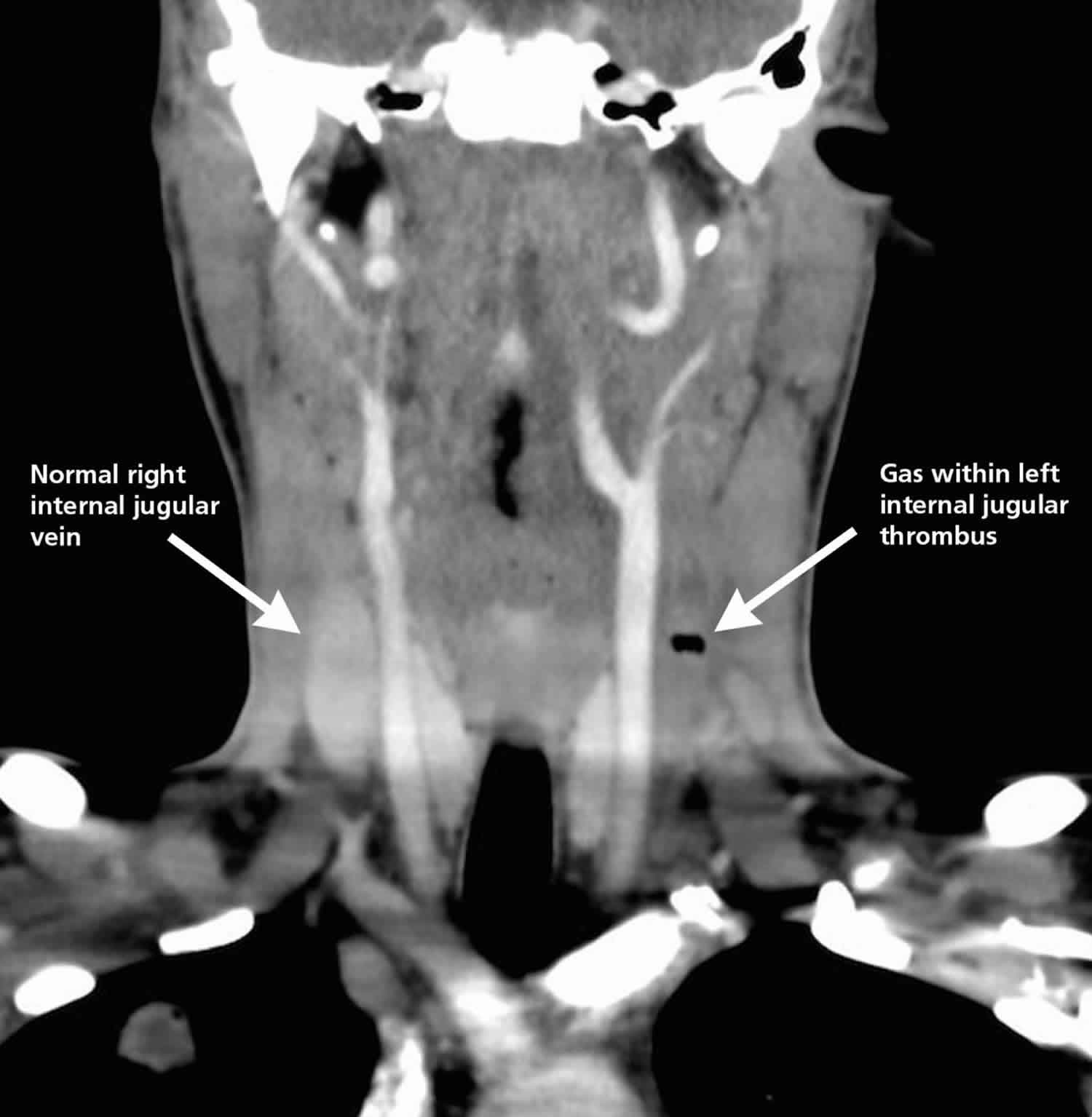
Figure 1. Lemierre syndrome
Footnote: Computed tomographic image of the neck of a 16-year-old boy showing left thrombosed internal jugular vein and gas within the thrombus.
[Source 4 ]Lemierre syndrome cause
Lemierre syndrome originates as a complication of bacterial throat infections. The bacteria most commonly responsible is Fusobacterium necrophorum, an obligate anaerobic, gram-negative bacilli. Fusobacterium necrophorum and Fusobacterium nucleatum are the responsible for the majority of human fusobacterial infections. These bacteria can cause invasive disease secondary to multiple virulence factors including endotoxins and exotoxins. Other organisms such as Streptococcus species., Bacteroides species., Staphylococcus aureus, Klebsiella pneumoniae, and others have also been isolated in reported case studies 5.
Fusobacterium necrophorum is part of the normal bacterial flora in the pharynx, gastrointestinal tract, and female genital tract. In addition to primary infection, it is postulated that other causes of oropharyngeal infections can lead to conditions that are conducive to fusobacterial growth. These may be viral infections such as acute Epstein Barr, streptococcal infections, and others. Conditions conducive to anaerobic growth, like a peritonsillar abscess, allow growth and penetration of the surrounding tissues by Fusobacterium necrophorum. Extension of the infection from the oropharynx into the lateral pharyngeal space results in the seeding of the internal jugular vein, septic thrombophlebitis, and subsequent access to the venous system resulting in septicemia and septic emboli 6.
Liemierre syndrome begins as a localized oropharyngeal infection. It is not known definitively whether Fusobacterium necrophorum acts predominantly as a primary or secondary pathogen in the initial stages of Lemierre syndrome. In addition to direct infection, it is postulated that mucosal damage of the pharynx caused by other bacterial or viral infections leads to conditions conducive for a fusobacterial superinfection. The infection then spreads into the lateral pharyngeal space and soft tissues of the neck. Venous thrombosis is initiated locally in the peritonsillar veins and then extends to the internal jugular veins. Fusobacterium necrophorum has been shown to aggregate human platelets in vitro, subsequently precipitating intravascular coagulation. When combined with venous stasis from extrinsic compression and intrinsic vessel occlusion secondary to inflammation and edema, this leads to the development of septic thrombosis of the internal jugular vein. The release of septic emboli into the systemic circulation results in widespread dissemination of Fusobacterium necrophorum into the lung, pleura, joints, bones, muscles, spleen, liver, kidney, and other endpoints of circulation. Direct extension or propagation of thrombus results in central nervous system abscess formation as well as cavernous sinus thrombosis.
Lemierre’s syndrome symptoms
Lemierre’s should be suspected in patients with antecedent pharyngitis, septic pulmonary emboli, and persistent fever despite antimicrobial therapy.
- The septicemic illness typically begins 4–5 days after the onset of the sore throat, but the interval may be up to 12 days.
- Several features are highly suggestive of Lemierre’s syndrome including:
- Previously healthy young adult (mean age 20 years)
- History of sore throat in the preceding week
- High fever and rigors
- Signs of internal jugular venous thrombosis, presenting as neck swelling or cervical spine pain
- Dry cough
- Pleuritic pain and possible effusion
- Chest radiograph with nodular, cavitary lesions
- Metastatic abscess may occur, e.g. septic arthritis
The presentation and clinical progression of Lemierre’s disease can usually be divided into 3 main phases. These phases may also serve to diagnose Lemierre’s syndrome.
Oropharyngeal infection
The clinical features of Lemierre syndrome begin with an oropharyngeal infection with subsequent febrile episodes and rigors anywhere from 4 to 7 days after the initial illness. Early diagnosis can be challenging as the symptoms are non-specific and usually attributable to self-limited pharyngeal infections. Over two-thirds of patients recount a pharyngeal infection at the onset of the illness, but rarely the illness has been described in the setting of parotitis, otitis media, mastoiditis, sinusitis, and dental infections. Patients with confirmed Epstein-Barr infections may also develop Lemierre syndrome, and seropositive patients with mononucleosis can suffer diagnostic delays when Lemierre syndrome is not considered as an alternative diagnosis. The persistence of fever and worsening clinical status at 1 week can be an important clinical clue.
Infection extension to the paraphernal space of the neck with thrombophlebitis of the internal jugular vein
Neck tenderness and swelling may be an early clinical sign that pharyngitis has extended beyond the oropharynx. Unilateral tenderness and swelling at the mandibular angle, known as the “cord sign,” indicates internal jugular thrombosis, but is only present in 25% to 45% of cases. A focused exam of the neck including the suprasternal and supraclavicular regions for signs of cutaneous cellulitis should also be performed and can indicate an extension of the infection to cervical veins and the mediastinal structures.
Septic emboli
Once Fusobacterium necrophorum has invaded the cervical veins, septic emboli occur. The most frequently affected organ is the lungs (85%), but joints, liver, kidney, brain, bones, heart and meninges can all be involved. Bacteremia is associated with fever, lethargy, or shock, as well as end-organ damage. Septic shock occurs in approximately 7% of cases. Acute respiratory distress syndrome requiring mechanical ventilation may affect up to 10% of patient presentations.
Lemierre’s syndrome diagnosis
The diagnosis of Lemierre syndrome is primarily clinical, but adjunctive testing can be helpful. Comprehensive laboratory evaluation is indicated in patients presenting with concern for sepsis or with systemic inflammatory response syndrome (SIRS) criteria. Some of the more frequent laboratory abnormalities include leukocytosis, mild to severe renal impairment, abnormal liver function tests including elevated bilirubin, thrombocytopenia, as well as other evidence of disseminated intravascular coagulation. Blood cultures should be collected, and growth typically occurs over 2 to 7 days and shows Fusobacterium species in over 70% of cases. Blood cultures may be negative in Lemierre syndrome due to the difficulties that can be associated with culturing anaerobic organisms.
The lungs are the most common site of metastatic infection, and imaging should include a chest radiograph to evaluate for septic emboli and other pulmonary complications including pulmonary effusions, pulmonary abscess, and empyema. Additional sites of bacterial metastasis result may result in septic arthritis, osteomyelitis, meningitis, pericarditis, and hepatic abscess. Other imaging modalities used to evaluate for septic thrombosis of the internal jugular vein may include ultrasound, CT of the neck with contrast, and magnetic resonance imaging (MRI). Magnetic Resonance Venography (MRV) has the highest sensitivity (97%) for detecting internal jugular thrombosis, while CT is usually the most available and most widely used clinically. Ultrasound can be a useful modality given its comparatively lower cost and lack of radiation risk, but the low echogenicity of fresh clots combined with anatomic constraints of the lower neck result in lower sensitivity for the detection of acute thrombosis.
Lemierre’s syndrome treatment
The mainstay of treatment for Lemierre syndrome is antibiotic therapy. A beta-lactamase resistant beta-lactam antibiotic is recommended as an empiric therapy due to case reports of treatment failures with penicillin secondary to beta-lactamase producing F. necrophorum. Antibiotics should be tailored to the culture results and susceptibility data when available. Alternative options include clindamycin or metronidazole for patients with significant clinical allergy to beta-lactams. Antibiotic therapy is continued for 6 weeks in most patients to achieve appropriate penetration into fibrin clots.
Surgical management may be necessary in cases of abscess formation, respiratory distress secondary to pulmonary thrombosis metastasis, and in patients with extension of thrombus into the mediastinum or cerebrum. Surgical incision and drainage of the abscess at affected sites may be indicated to control infection.
Anticoagulation therapy in Lemierre syndrome is controversial. Uncomplicated Lemierre syndrome without evidence of extensive clot burden resolves with appropriate antibiotic therapy and supportive care and does not require anticoagulation. There are no controlled trials to validate the practice, but anticoagulation is usually recommended when thrombus extends into the cerebral sinuses, for large or bilateral clot burden, or when a patient fails to improve in the first 72 hours with appropriate antibiotic and/or surgical therapy.
Lemierre’s syndrome prognosis
Advanced Lemierre syndrome is a life-threatening condition. Even with appropriate antibiotics and therapy, mortality has been reported to be between 5% and 18%. Hospital admission usually requires intensive care unit (ICU) status, and the average length of stay in the hospital is approximately 3 weeks. Septic emboli and end-organ effects can result in long-term morbidity.
References- Allen BW, Bentley TP. Lemierre Syndrome. [Updated 2019 May 14]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499846
- Lemierre’s Syndrome. N Engl J Med 2004; 350:e14 DOI: 10.1056/ENEJMicm030003
- Bonny V, Hourmant Y, Mirouse A, Valade S. A prostatic Lemierre syndrome. Int. J. Infect. Dis. 2019 Jul;84:73-74.
- Lemierre syndrome: early recognition and management. Chani Tromop-van Dalen, Ann-Marie Mekhail. CMAJ Nov 2015, 187 (16) 1229-1231; https://doi.org/10.1503/cmaj.150476
- Salami A, Assouan C, Garba I, Konan E. An unusual cause of Lemierre Syndrome. J Stomatol Oral Maxillofac Surg. 2019 Sep;120(4):358-360.
- Le C, Gennaro D, Marshall D, Alaev O, Bryan A, Gelfman A, Wang Z. Lemierre’s syndrome: One rare disease-Two case studies. J Clin Pharm Ther. 2019 Feb;44(1):122-124.