Male pattern baldness
Male-pattern baldness also known as ‘androgenetic alopecia’ or ‘androgenic alopecia‘ typically appears first at the hairline or top of the head. The typical pattern of male pattern baldness begins at the hairline. The hairline gradually moves backward (recedes) and forms an “M” shape. Eventually the hair becomes finer, shorter, and thinner, and creates a U-shaped (or horseshoe) pattern of hair around the sides of the head. It can progress to partial or complete baldness. Male-pattern baldness is the most common type of hair loss. Male-pattern baldness affects all men to some degree as they get older. Significant hair loss affects about one in five men (20%) in their 20s, about one in three men (30%) in their 30s and nearly half of men (40%) in their 40s. Male pattern baldness affects around 80% of men by the age of 80 years 1. For a few men, this process starts as early as the late teens. By the age of 60, most men have some degree of hair loss. Each strand of hair sits in a tiny hole in the skin called a hair follicle. In general, baldness occurs when the hair follicle shrinks over time, resulting in shorter and finer hair. Eventually, the hair follicle does not grow new hair. The hair follicles remain alive, which suggests that it is still possible to grow new hair.
Male pattern hair loss affects different populations at different rates, probably because of genetics. Up to half of male Caucasians will experience some degree of hair loss by age 50, and possibly as many as 80% by the age of 70 years, while other population groups such as Japanese and Chinese men are far less affected.
The severity of male pattern baldness can be classified in several ways. Norwood and Sinclair systems are shown below.
Male-pattern baldness is caused when hair follicles are oversensitive to the hormone dihydrotestosterone (DHT), produced by the male hormone, testosterone. Dihydrotestosterone (DHT) causes the follicles to shrink and eventually stop functioning. Both men and women produce this hormone in different amounts. DHT (dihydrotestosterone) attacks the hair follicle, causing it to shrink, finally causing the hair to fall out and not grow back and is implicated in male-pattern hair loss androgenetic alopecia pathophysiology 2. Hair follicle receptors are sensitive to DHT and thereby start the process of male or female pattern hair loss 3. Upon binding to androgen receptors in the hair follicle, dihydrotestosterone (DHT) promotes the shortening of the anagen phase and elongation of the telogen phase 4, resulting in enhanced apoptosis of hair cells and thus hair loss 5. A mouse-model study found that dihydrotestosterone (DHT) promoted premature hair regression, hair miniaturization, loss of hair density, and altered hair morphology in male mice, with partial reversal with an androgen receptor antagonist, bicalutamide 6.
The involvement of testosterone in balding has led to the myth that going bald is a sign of virility. But men with male-pattern baldness don’t have more male hormones than other men. Their hair follicles are simply more sensitive to the hormones.
Male pattern baldness is usually not a sign of an underlying medical disorder. Hair loss is usually permanent.
While hair loss is a normal part of the ageing process, for some men it can be distressing, particularly if it happens at an early age. Men losing their hair can feel less confident, less attractive and may think it makes them look older; some may feel depressed.
Some men find talking to a counselor helpful if they are feeling worried about their hair loss.
Male-pattern baldness is so called because it tends to follow a set pattern. The first stage is usually a receding hairline, followed by thinning of the hair on the crown and temples. When these two areas meet in the middle, it leaves a horseshoe shape of hair around the back and sides of the head. Eventually, some men go completely bald.
If you have inherited the genes responsible for male-pattern or female-pattern baldness there’s little you can do to prevent it from happening 7, 8, 9.
Treatments can slow down the process, but there’s no cure. The two most effective treatments for male-pattern baldness are medicines called minoxidil and finasteride. Side-effects are uncommon, but minoxidil can cause skin irritation or a rash in some men.
Current male-pattern baldness treatment options include:
- Hair replacement / transplantation
- Cosmetics
- Micropigmentation (tattoo) to resemble shaven scalp
- Hairpieces
- Minoxidil solution
- Finasteride tablets (type II 5-alpha reductase inhibitor)
- Dutasteride.
There is some evidence that ketoconazole shampoo may also be of benefit, perhaps because it is effective in seborrheic dermatitis and dandruff.
Low level laser therapy is of unproven benefit in pattern balding; one device has been approved by the FDA for marketing. Further studies are required to determine the magnitude of the benefit, if any.
Other treatments for hair loss include wigs, hair transplants and plastic surgery procedures, such as scalp reduction.
As a general rule, it’s easier to maintain existing hair than to regrow it, and once the hair follicle has stopped working it cannot be revived.
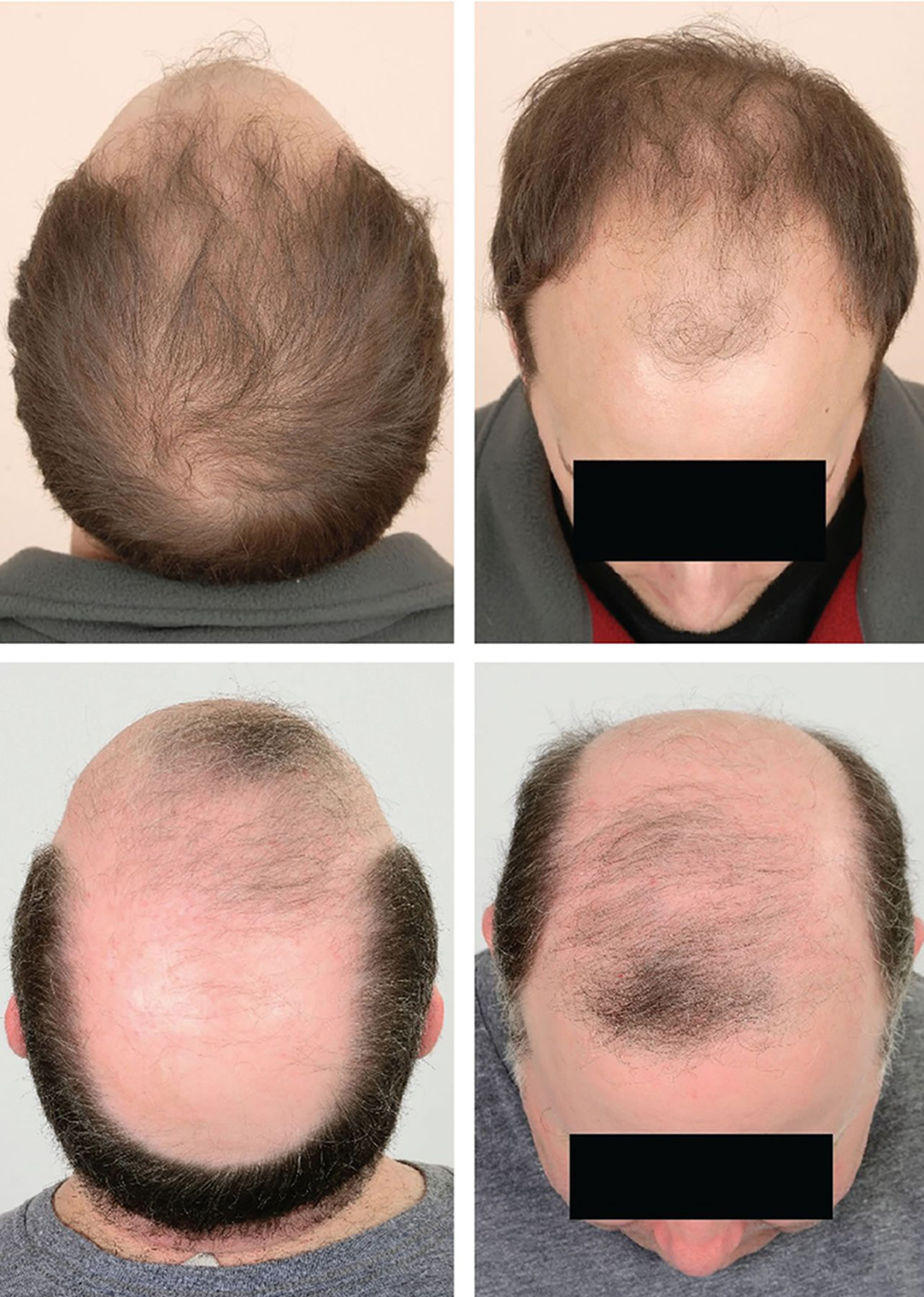
Figure 1. Male pattern hair loss
Figure 2. Male pattern baldness (androgenetic alopecia)
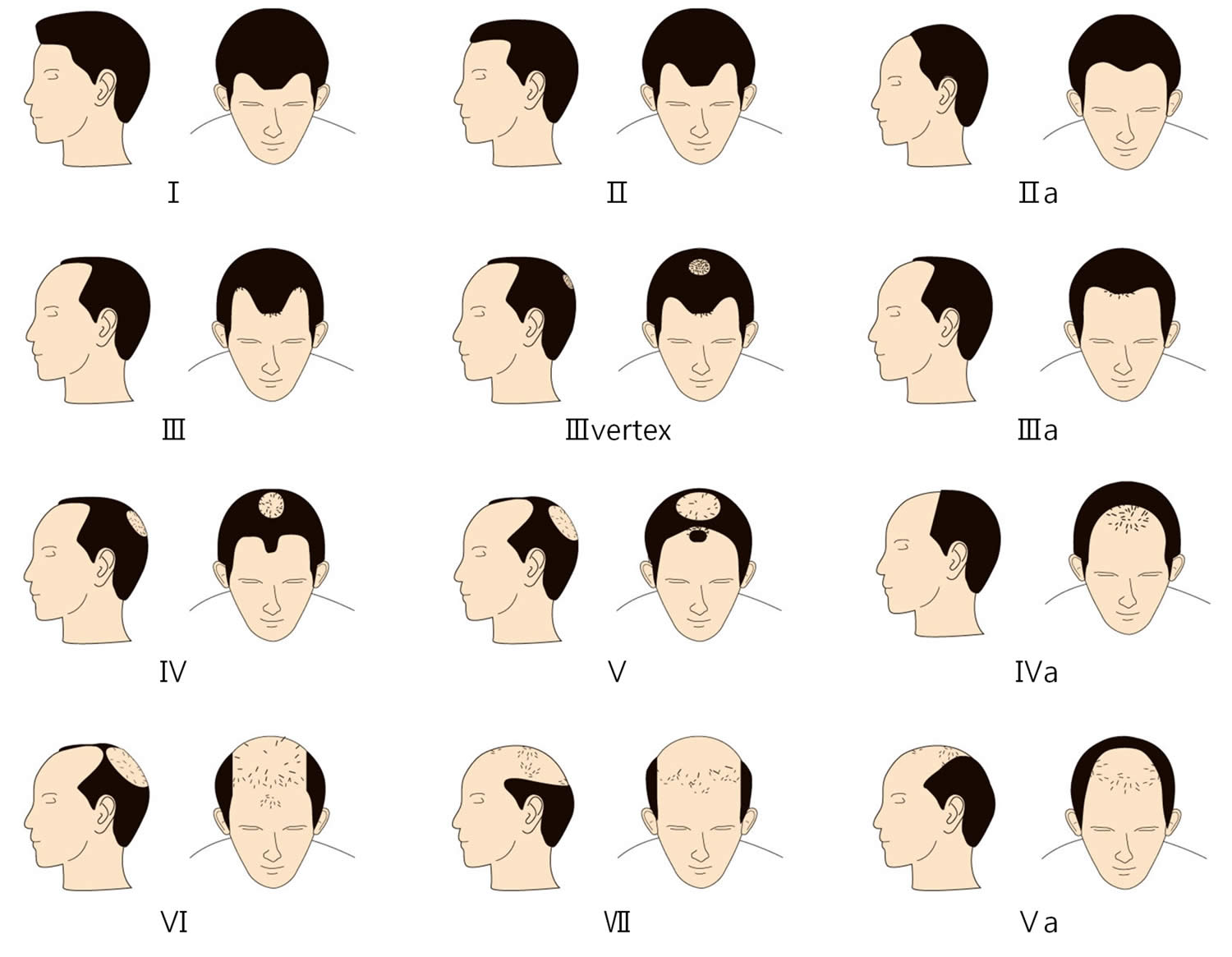
Figure 3. Norwood Classification male pattern baldness
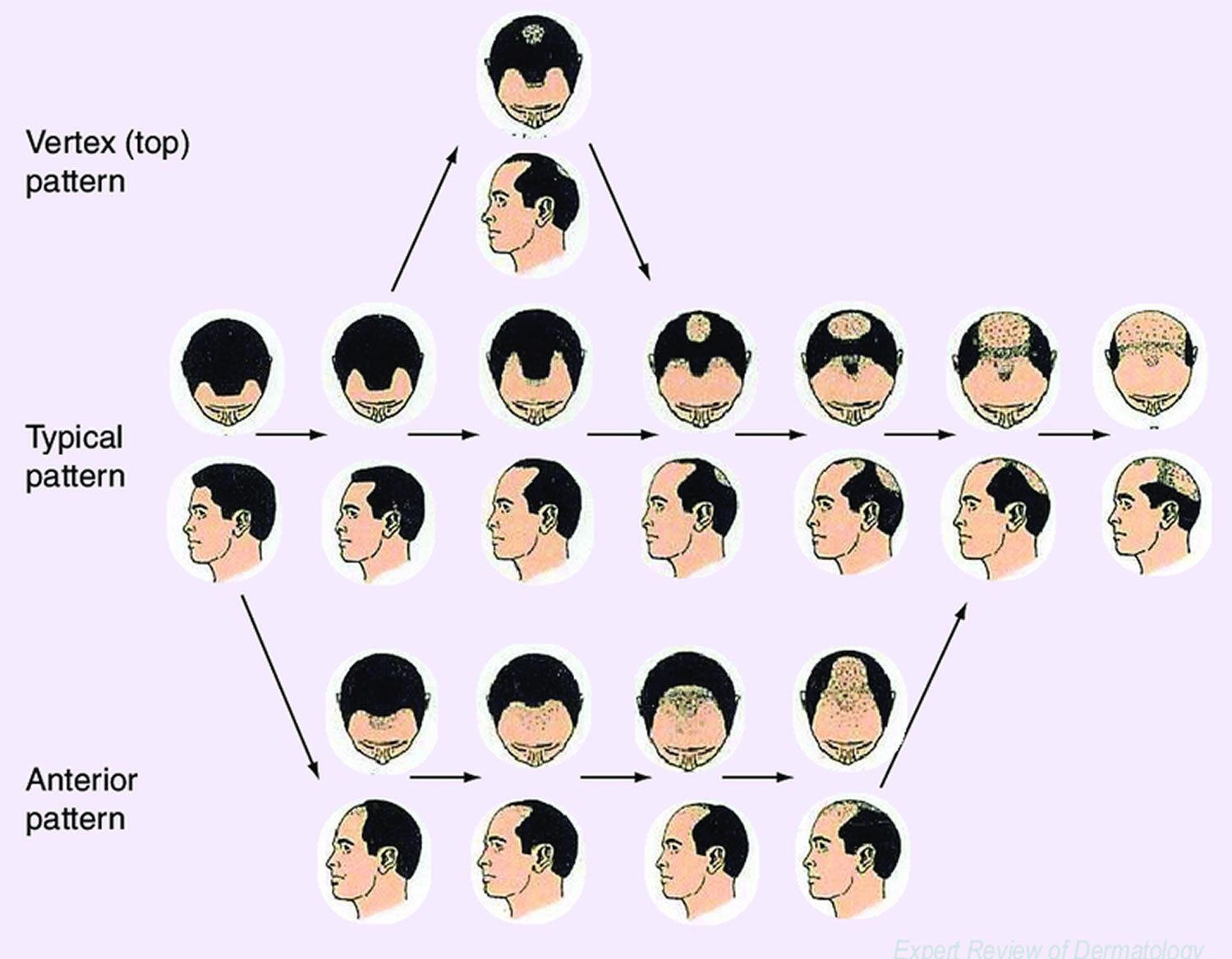
Figure 4. Hamilton scale for male androgenetic alopecia
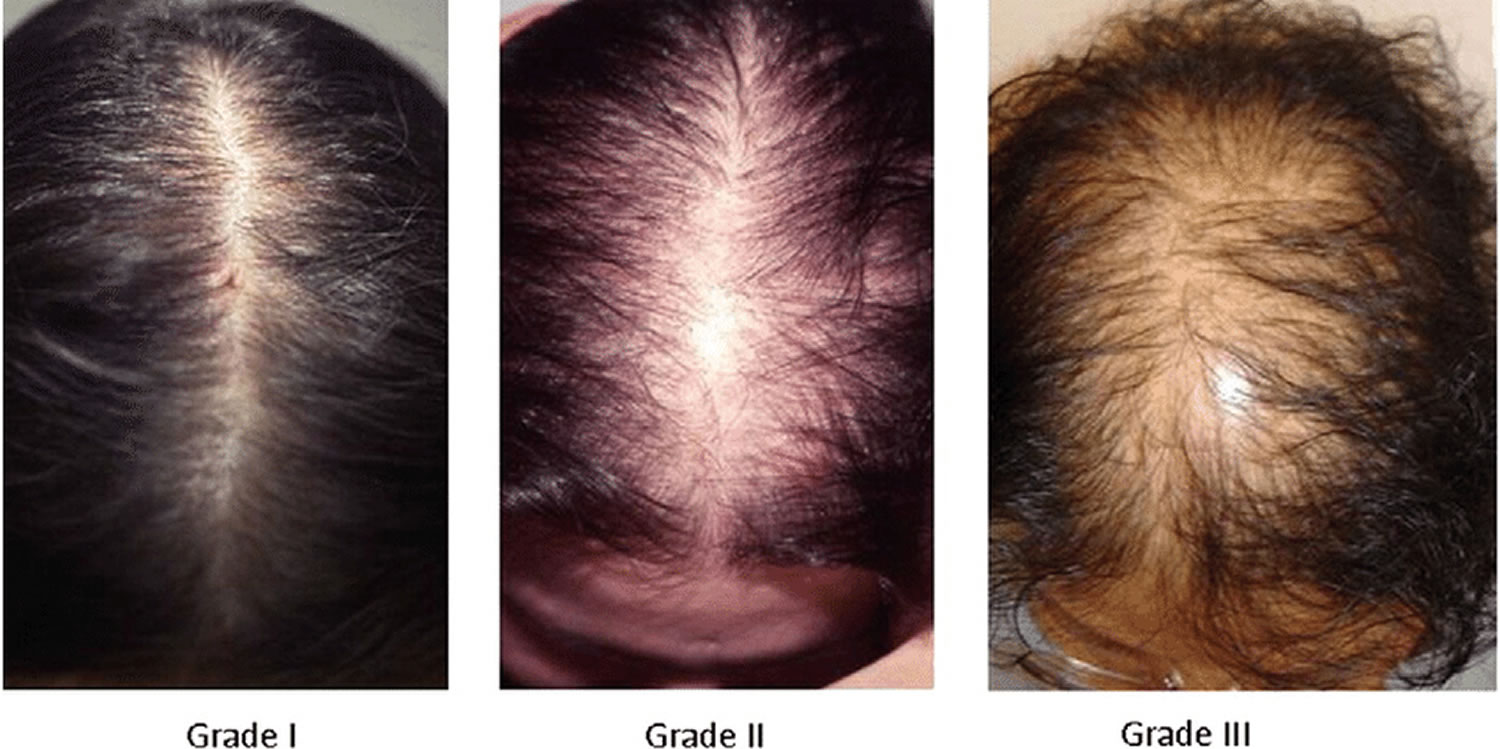
[Source 10 ]Figure 5. Ludwig classification for female pattern hair loss
Footnote: Ludwig classification for female pattern hair loss (androgenic alopecia in women). Grades 1, 2 and 3 (minimal, moderate, intense)
- Grade 1: Perceptible thinning of the hair on the crown, limited in the front by a line situated 1–3 cm behind the frontal hairline.
- Grade 2: Pronounced rarefaction of the hair on the crown, within the area seen in grade 1.
- Grade 3: Full baldness (total denudation) within the area seen in grades 1 and 2.
You should seek medical advice for hair loss if:
- you have recently started a new medicine;
- you are a woman and your hair loss is accompanied by excess growth of facial and body hair or you have acne;
- you have been diagnosed with (or think you may have) an autoimmune disorder such as systemic lupus erythematosis (SLE), nutritional deficiency or thyroid disease;
- you have been recently treated with chemotherapy or have used a hormonal medicine;
- the hair loss occurs in discrete patches;
- the hair loss is associated with scaling or inflammation of the scalp;
- you also have loss of body hair; or
- you are aware you have a compulsive hair-pulling habit.
How does your hair grow?
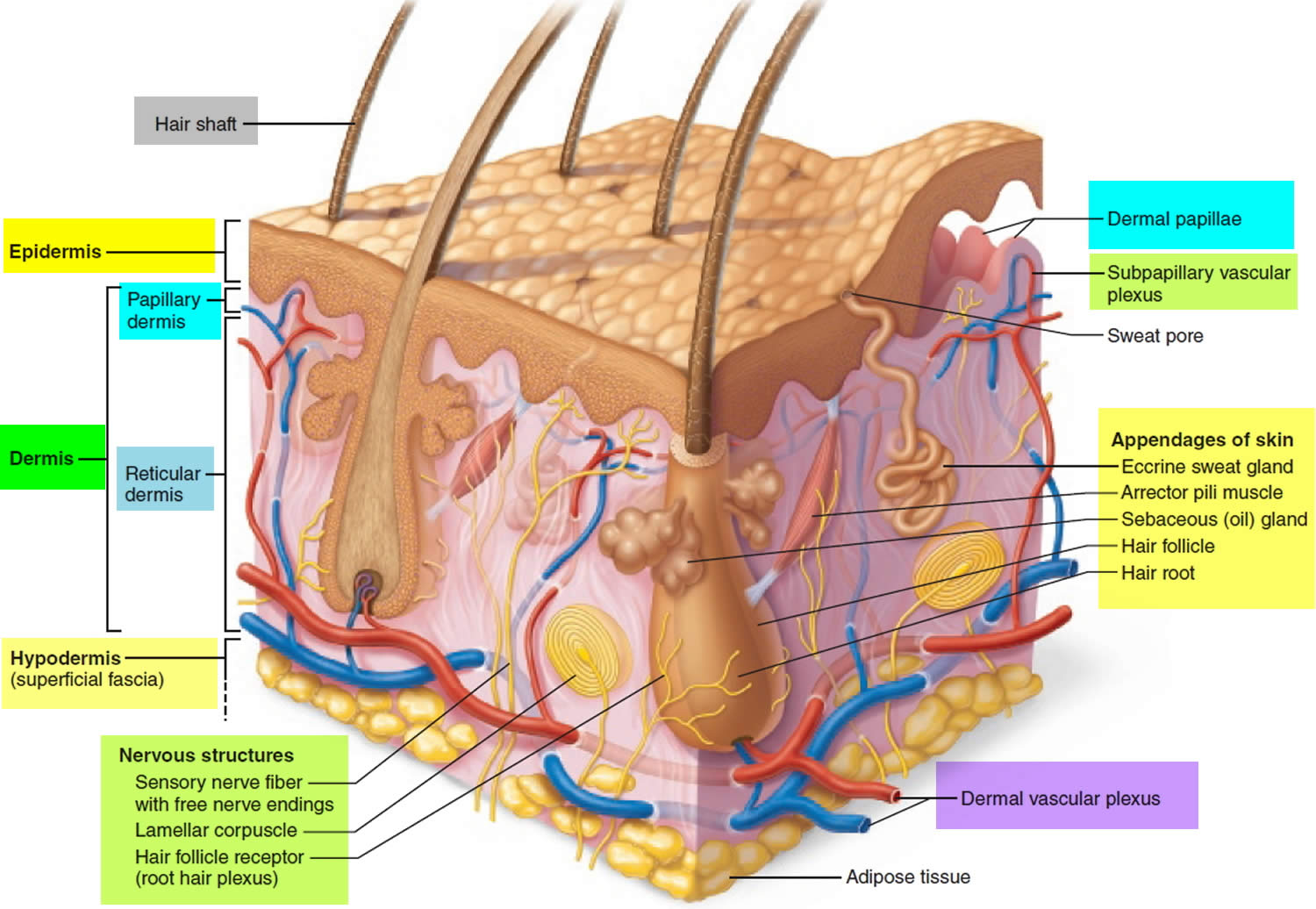
Hair is a slender filament of keratinized cells that grows from an oblique tube in the skin called a hair follicle. Each hair is composed of columns of dead, keratinized epidermal cells bonded together by extracellular proteins. The hair shaft is the superficial portion of the hair, which projects above the surface of the skin. The hair root is the portion of the hair deep to the shaft that penetrates into the dermis, and sometimes into the subcutaneous layer.
Hairs project beyond the surface of the skin almost everywhere except the sides and soles of the feet, the palms of the hands, the sides of the fingers and toes, the lips, and portions of the external genitalia. There are about 5 million hairs on the human body, and 98 percent of them are on the general body surface, not the head. Hairs are nonliving structures that form in organs called hair follicles.
The density of hair does not differ much from one person to another or even between the sexes; indeed, it is virtually the same in humans, chimpanzees, and gorillas. Differences in apparent hairiness are due mainly to differences in texture and pigmentation.
Types of Hairs
Hairs first appear after about three months of embryonic development. These hairs, collectively known as lanugo, are extremely fine and unpigmented. Most lanugo hairs are shed before birth.
The two types of hairs in the adult skin are vellus hairs and terminal hairs:
- Vellus hairs are the fine “peach fuzz” hairs found over much of the body surface.
- Terminal hairs are heavy, more deeply pigmented, and sometimes curly. The hairs on your head, including your eyebrows and eyelashes, are terminal hairs. After puberty, it also forms the armpit and pubic hair, male facial hair, and some of the hair on the trunk and limbs.
Hair follicles may alter the structure of the hairs they produce in response to circulating hormones.
Figure 5. Hair structure
Figure 6. Hair follicle
Structure of Hair Follicle
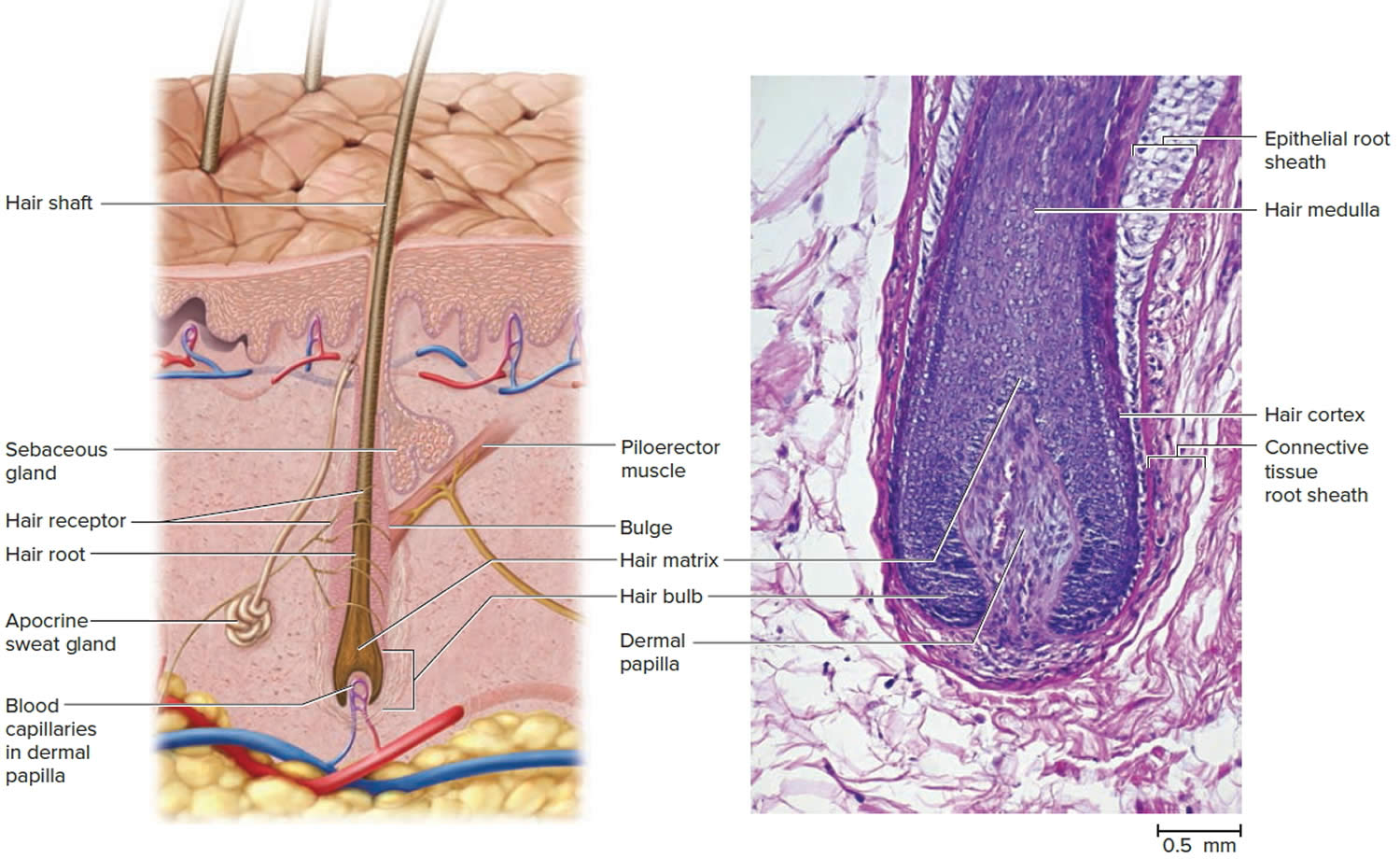
The portion of a hair above the skin is called the shaft, and all that beneath the surface is the root. The root penetrates deeply into the dermis or hypodermis and ends with a dilation called the hair bulb. The only living cells of a hair are in and near the hair bulb. The hair bulb grows around a bud of vascular connective tissue called the dermal papilla, which provides the hair with its sole source of nutrition. Immediately above the papilla is a region of mitotically active cells, the hair matrix, which is the hair’s growth center. All cells higher up are dead.
Figure 7. Hair follicle and hair structure
Hair Structure
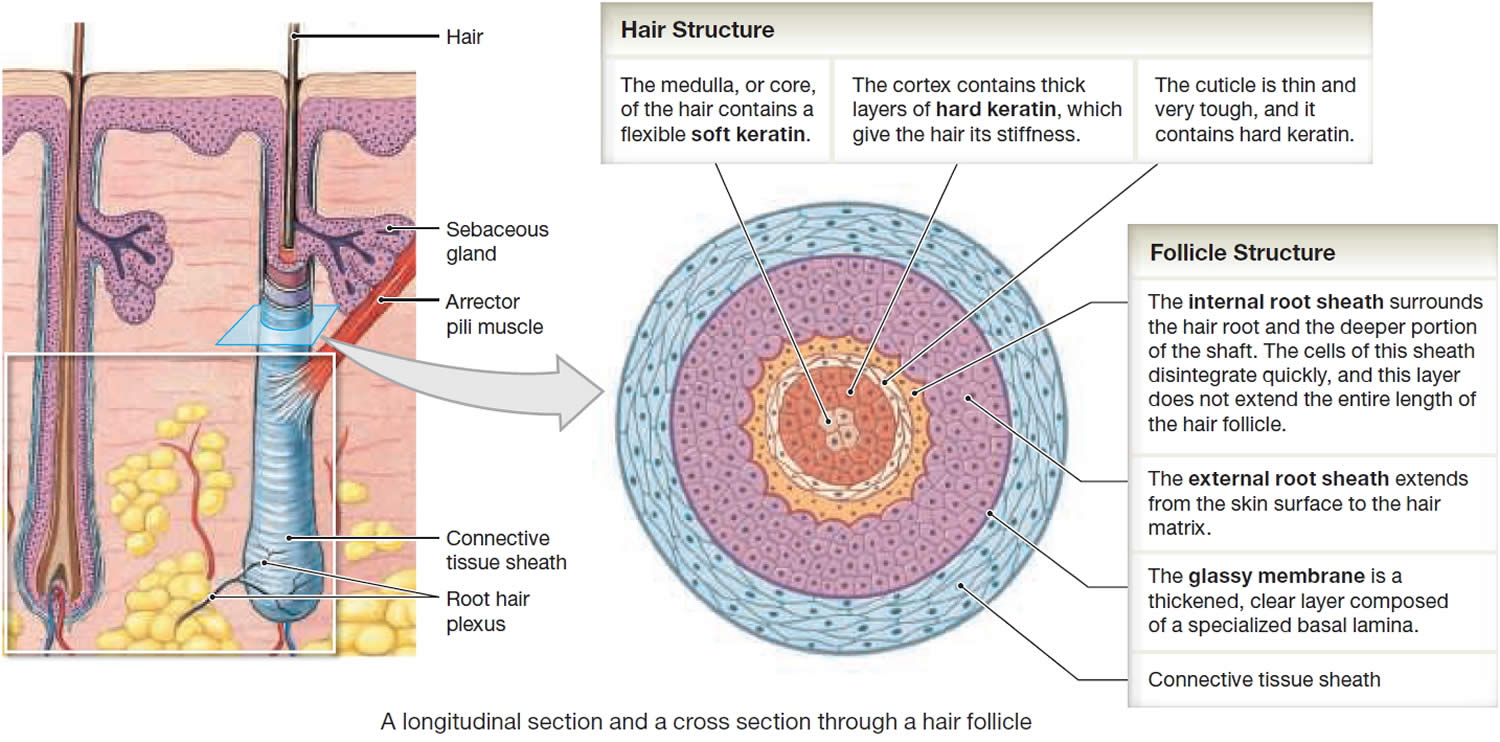
In cross section, a hair reveals up to three layers. From the inside out, these are the medulla, cortex, and cuticle.
The medulla is a core of loosely arranged cells and air spaces. It is most prominent in thick hairs such as those of the eyebrows, but narrower in hairs of medium thickness and absent from the thinnest hairs of the scalp and elsewhere.
The cortex constitutes most of the bulk of a hair. It consists of several layers of elongated keratinized cells that appear cuboidal to flattened in cross sections.
The cuticle is composed of multiple layers of very thin, scaly cells that overlap each other like roof shingles with their free edges directed upward.
Hair Follicle
Cells lining the hair follicle are like shingles facing in the opposite direction. They interlock with the scales of the hair cuticle and resist pulling on the hair. When a hair is pulled out, this layer of follicle cells comes with it.
The hair follicle is a diagonal tube that contains the hair root. It has two principal layers: an epithelial root sheath and a connective tissue root sheath. The epithelial root sheath is an extension of the epidermis; it consists of stratified squamous epithelium and lies immediately adjacent to the hair root. Toward the deep end of the follicle, it widens to form a bulge, a source of stem cells for follicle growth. The connective tissue root sheath, which is derived from the dermis and composed of collagenous connective tissue, surrounds the epithelial sheath and is somewhat denser than the adjacent dermis.
Associated with the hair follicle are nerve and muscle fibers. Nerve fibers called hair receptors entwine each hair follicle and respond to hair movements. You can feel their effect by carefully moving a single hair with a pin or by lightly running your finger over the hairs of your forearm without touching the skin.
Each hair has a piloerector muscle—also known as a pilomotor muscle or arrector pili—a bundle of smooth muscle cells extending from dermal collagen fibers to the connective tissue root sheath of the follicle. In response to cold, fear, touch, or other stimuli, the sympathetic nervous system stimulates the piloerector to contract, making the hair stand on end and wrinkling the skin in such areas as the scrotum and areola. In humans, it pulls the follicles into a vertical position and causes “goose bumps,” but serves no useful purpose.
Figure 8. Hair structure
Hair Production
Hair follicles extend deep into the dermis, often projecting into the underlying subcutaneous layer. The epithelium at the follicle base surrounds a small hair papilla, a peg of connective tissue containing capillaries and nerves. The hair bulb consists of epithelial cells that surround the papilla.
Hair production involves a specialized keratinization process. The hair matrix is the epithelial layer involved in hair production. When the superficial basal cells divide, they produce daughter cells that are pushed toward the surface as part of the developing hair. Most hairs have an inner medulla and an outer cortex. The medulla contains relatively soft and flexible soft keratin. Matrix cells closer to the edge of the developing hair form the relatively hard cortex. The cortex contains
hard keratin, which gives hair its stiffness. A single layer of dead, keratinized cells at the outer surface of the hair overlap and form the cuticle that coats the hair.
The hair root anchors the hair into the skin. The root begins at the hair bulb and extends distally to the point where the internal organization of the hair is complete, about halfway to the skin surface. The hair shaft extends from this halfway point to the skin surface, where we see the exposed hair tip.
The size, shape, and color of the hair shaft are highly variable.
Growth and Replacement of Hair
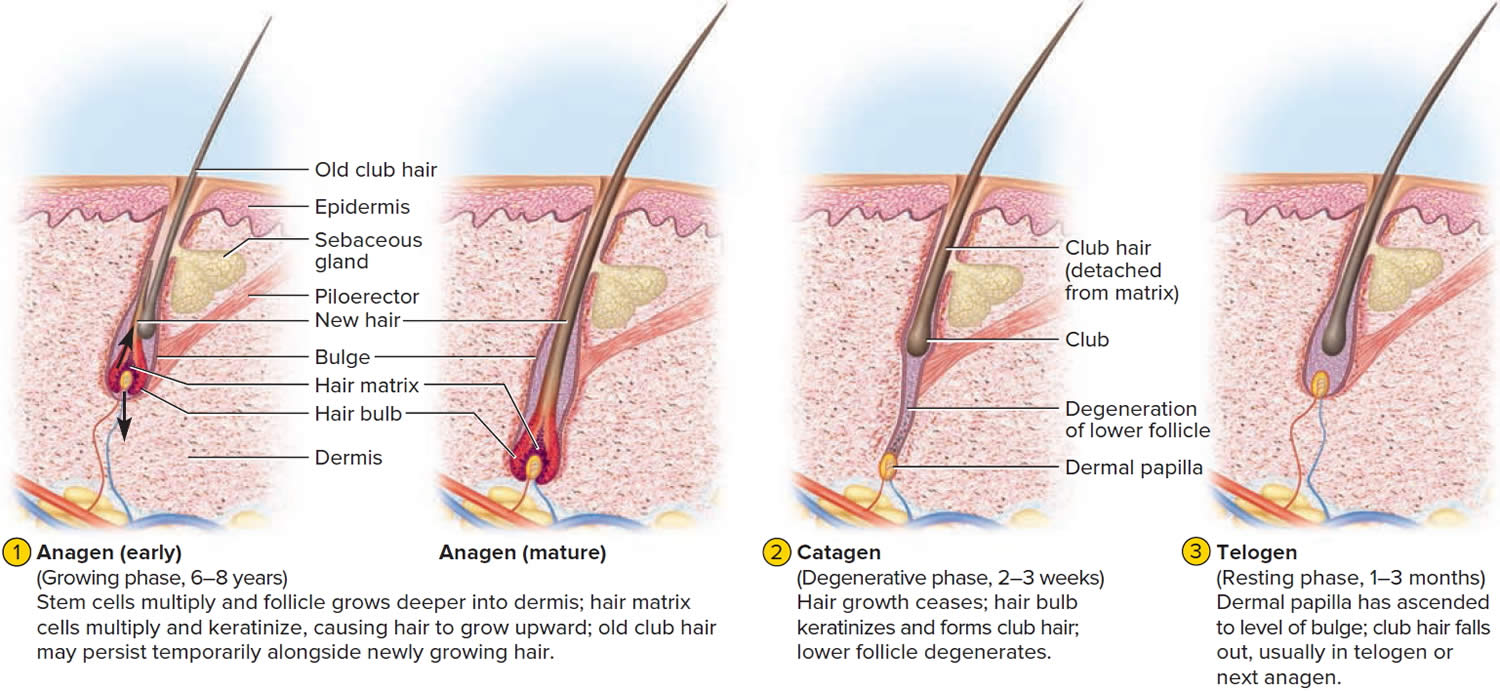
The human scalp contains about 100,000 hair follicles. These anchor the hair to the skin and contain the cells that produce new hairs. A hair in the scalp grows for two to five years, at a rate of around 0.33 mm/day (about 1/64 inch). Variations in hair growth rate and the duration of the hair growth cycle account for individual differences in uncut hair length. Hair grows in 3 developmental stages:
- Anagen. The anagen phase or actively hair growing phase starts the growing of new hair. The anagen phase is genetically determined and can vary from 2 to 6 years (the average is just under 3 years). Most hair follicles on the scalp are in the anagen phase.
- Catagen. The catagen phase is a transition stage (in-between phase) between the growing and resting phases and lasts 2-3 weeks. The catagen phase is when hair growth stops and the hair follicle shrinks. About 1–3% of hairs are in the catagen phase (in-between phase).
- Telogen. The telogen phase or resting phase is a mature hair with a root, which is held very loosely in the follicle. The telogen phase (resting phase) generally lasts about 4-5 months. Up to 10% of hairs in a normal scalp are in the telogen phase (resting phase). About 100 telogen hairs are lost from the human scalp each day.
Everyone is born with a fixed number of hair follicles on the scalp that produce hairs throughout life. Hair grows from the base of the follicle at a rate of about one centimetre a month for about three years. This growth phase is called anagen. After anagen, the hair dies (catagen hair) and no longer grows. It sits dormant in the follicle for a three-month phase called telogen. After telogen, the hair follicle undergoes another anagen phase to produce new hair that grows out of the same follicle. As it grows, the old telogen hair is dislodged or pushed out. The hair cycle continues throughout life.
At any given time, about 90% of the scalp follicles are in the Anagen stage. In this stage, stem cells from the bulge in the hair follicle multiply and travel downward, pushing the dermal papilla deeper into the skin and forming the epithelial root sheath. Root sheath cells directly above the papilla form the hair matrix. Here, sheath cells transform into hair cells, which synthesize keratin and then die as they are pushed upward away from the papilla. The new hair grows up the follicle, often alongside an old club hair left from the previous cycle.
Hair length depends on the duration of anagen stage. Short hairs (eyelashes, eyebrows, hair on arms and legs) have a short anagen phase of around one month. Anagen lasts up to 6 years or longer in scalp hair.
In the Catagen stage, mitosis in the hair matrix ceases and sheath cells below the bulge die. The follicle shrinks and the dermal papilla draws up toward the bulge. The base of the hair keratinizes into a hard club and the hair, now known as a club hair, loses its anchorage. Club hairs are easily pulled out by brushing the hair, and the hard club can be felt at the hair’s end. When the papilla reaches the bulge, the hair goes into a resting period called the Telogen stage. Eventually, anagen begins anew and the cycle repeats itself. A club hair may fall out during catagen or telogen, or as it is pushed out by the new hair in the next anagen phase.
You lose about 50 to 100 scalp hairs daily. In a young adult, scalp follicles typically spend 6 to 8 years in anagen, 2 to 3 weeks in catagen, and 1 to 3 months in telogen. Scalp hairs grow at a rate of about 1 mm per 3 days (10–18 cm/yr) in the anagen phase.
Hair grows fastest from adolescence until the 40s. After that, an increasing percentage of follicles are in the catagen and telogen phases rather than the growing anagen phase. Hair follicles also shrink and begin producing wispy vellus hairs instead of thicker terminal hairs. Thinning of the hair or baldness, is called alopecia. It occurs to some degree in both sexes and may be worsened by disease, poor nutrition, fever, emotional stress, radiation, or chemotherapy. In the great majority of cases, however, it is simply a matter of aging.
Pattern baldness is the condition in which hair is lost unevenly across the scalp rather than thinning uniformly. It results from a combination of genetic and hormonal influences. The relevant gene has two alleles: one for uniform hair growth and a baldness allele for patchy hair growth. The baldness allele is dominant in males and is expressed only in the presence of the high level of testosterone characteristic of men. In men who are either heterozygous or homozygous for the baldness allele, testosterone causes terminal hair to be replaced by vellus hair, beginning on top of the head and later the sides. In women, the baldness allele is recessive. Homozygous dominant and heterozygous women show normal hair distribution; only homozygous recessive women are at risk of pattern baldness. Even then, they exhibit the trait only if their testosterone levels are abnormally high for a woman (for example, because of a tumor of the adrenal gland, a woman’s principal source of testosterone). Such characteristics in which an allele is dominant in one sex and recessive in the other are called sex-influenced traits.
Excessive or undesirable hairiness in areas that are not usually hairy, especially in women and children, is called hirsutism. It tends to run in families and usually results from either masculinizing ovarian tumors or hypersecretion of testosterone by the adrenal cortex. It is often associated with menopause.
Contrary to popular misconceptions, hair and nails do not continue to grow after a person dies, cutting hair does not make it grow faster or thicker, and emotional stress cannot make the hair turn white overnight.
Different causes of hair loss affect the hair follicles in different phases of growth. See below for the different types of hair loss.
Figure 9. Hair growth cycle
Male pattern baldness causes
Male pattern baldness also known as androgenic alopecia is related to your genes and male sex hormones. Testosterone is the most important androgen (male sex hormone) in men and is needed for normal reproductive and sexual function. Testosterone is important for the physical changes that happen during male puberty, such as development of the penis and testes, and for the features typical of adult men such as facial and body hair 12, 13. Testosterone also supports the behavioral aspects of mating effort, by promoting both libido and aggression in reproductive contexts 14, 15. Testosterone is produced by the male testes in men and to a lesser extent by the adrenal glands in both men and women 16. In the body, testosterone is converted to dihydrotestosterone (DHT) by an enzyme 5-alpha-reductase type 2 17. Dihydrotestosterone (DHT) acts on different organs in the body including the hair follicles and cells in the prostate.
Normally, in the hair cycle, 90 per cent of the hairs on a man’s scalp are in a growth phase (anagen). The other 10 per cent are mostly in a resting phase (telogen). Up to 100 telogen hairs fall out each day, and are replaced by the growth of new hairs.
Dihydrotestosterone (DHT) is the main hormone responsible for male pattern baldness in genetically susceptible individuals. In addition to the sexual development of males, DHT (dihydrotestosterone) promotes male-pattern hair loss and is implicated in male pattern boldness pathophysiology 2. Dihydrotestosterone (DHT) is the most potent hormone among the androgens [i.e, dehydroepiandrosterone (DHEA), androstenedione, testosterone, and dihydrotestosterone (DHT)] and is considered a pure androgen as it cannot be converted into estrogen 18. Dihydrotestosterone (DHT) causes hair loss by inducing a change in the hair follicles. Upon binding to androgen receptors in the hair follicle, DHT promotes the shortening of the anagen phase and elongation of the telogen phase 18, resulting in enhanced apoptosis of hair cells and thus hair loss 5. A mouse-model study found that DHT promoted premature hair regression, hair miniaturization, loss of hair density, and altered hair morphology in male mice, with partial reversal with an androgen receptor antagonist, bicalutamide 6. In addition, those with 5-alpha-reductase enzyme deficiencies are less likely to develop male-pattern hair loss androgenetic alopecia. The hairs produced by the follicles affected by dihydrotestosterone (DHT) become progressively smaller until eventually the follicles shrink completely and stop producing hair entirely. The role of dihydrotestosterone (DHT) in the promotion of transition to telogen and male-pattern hair loss androgenetic alopecia pathophysiology justifies the use of oral 5-alpha-reductase inhibitors, such as finasteride, in the management of hair loss 2.
Interestingly, however, the usefulness of collecting serum DHT levels in a routine hair loss work-up has been debated 2. A 2014 study 19 analyzed serum DHT concentrations among 19 women and 9 men with androgenetic alopecia, in addition to 17 healthy women and 4 healthy men without hair loss. Although increased serum DHT concentrations were observed in patients with androgenetic alopecia, as expected, increased serum DHT concentrations were also observed in the control group with no statistically significant difference between groups 19. In addition, the authors found no correlation between DHT concentrations and the progression of hair loss, although the study is limited by a small sample size. The authors concluded that rather than serum DHT concentration, the genetically determined sensitivity of hair follicles to DHT may mediate DHT-associated hair loss 20. However, these results are conflicting with another study including 178 patients with male pattern baldness and 61 healthy controls, which found a significantly greater level of DHT in male pattern baldness patients than normal controls 21. Yet, similarly to the prior study, authors found no significant difference in serum androgen levels based on hair loss severity 21.
Second is genetics, or the inheritance of genes from either the mother or father 22. Age added to genetics creates a time clock that signals the hair follicle to produce an enzyme named 5-alpha reductase. When testosterone is present in the hair follicle and it combines with the enzyme 5-alpha-reductase type 2, it produces dihydrotestosterone (DHT).
In some families there are genes passed on through the family that make men more likely to have androgenetic alopecia. In men with these genes, the hair follicles are more sensitive to dihydrotestosterone (DHT). This leads to hair follicle miniaturization (where the hairs growing from the follicles become thinner and shorter with each cycle of growth) at a younger age.
Scientists are still investigating why men with male pattern baldness inherit this sensitivity to dihydrotestosterone (DHT), but it causes changes in the hair follicle (called miniaturisation), which results in:
- fewer hairs emerging from each follicle;
- each hair becoming finer;
- the anagen growth phase of each hair gradually becoming shorter, so that each hair exists for a shorter time;
- the telogen hairs are shed faster; and
- the lag time between the end of the resting phase and the next growth phase increases.
Each strand of hair sits in a tiny hole (cavity) in the skin called a follicle. Generally, baldness occurs when the hair follicle shrinks over time, resulting in shorter and finer hair. Eventually, the follicle does not grow new hair.
So the hair becomes progressively shorter and finer, and can eventually stop growing altogether, resulting in baldness. The balding process is gradual and only hair on the scalp is affected. The follicles remain alive, which suggests that it is still possible to grow new hair.
It is your genes that will determine whether or not you will develop this type of hair loss and at what age it starts, as well as how extensive it will be and the pattern that the hair loss will follow. You can inherit male pattern hair loss from your mother or your father (or both).
Recently, poor sleep has been associated with increased risk and severity of alopecia subtypes, including alopecia areata and androgenetic alopecia 2. Conversely, those with alopecia have been found to exhibit reduced sleep quality compared to controls. A 2022 study 23 analyzed the prevalence of sleep abnormalities between 223 patients with male-pattern hair loss and 223 control subjects. The authors found a significant association between severe male-pattern hair loss and three sleep profiles: total sleep time less than or equal to six hours; a Pittsburgh Sleep Quality Index (PSQI) score greater than 5; and STOP-Bang score greater than or equal to 5 23. The STOP-Bang score specifically assesses signs of obstructive sleep apnea, and higher STOP-Bang and Pittsburgh Sleep Quality Index (PSQI) scores are negative findings, suggesting an association between sleep disturbances and male-pattern hair loss 23. Similarly, poor sleep habits is associated with increased severity of androgenetic alopecia 24. A similar study assessed the prevalence of sleep disturbances among 51 alopecia areata patients and 51 age- and sex-matched controls 25. As observed among individuals with male-pattern hair loss, the Pittsburgh Sleep Quality Index (PSQI) score was significantly greater among patients with alopecia areata compared to matched controls (7 ± 4.13 vs. 3.53 ± 1.96) 25. A greater number of alopecia areata patients depicted excess daytime sleepiness, measured with the Epworth Sleepiness Scale, than controls. Furthermore, sleep quality was worse among alopecia areata patients also suffering from anxiety or depression, thereby highlighting the importance of addressing both sleep quality and concomitant psychiatric distress in the management of alopecia areata 25. Furthermore, a 2018 study including 25,800 with diagnosed sleep disorders and 129,000 control subjects found those with sleep disorders to have a significantly greater risk for alopecia areata than controls 26. The authors found sleep disorders as an independent risk factor of alopecia areata 26.
Male pattern baldness genetics
Male pattern hair loss occurs in men who are genetically predisposed to be more sensitive to the effects of dihydrotestosterone (DHT). Researchers now believe that the condition can be inherited from either side of the family. There is a myth that hair loss is a genetic trait passed down from the mother’s side of the family. Genetics is the cause of male pattern hair loss, but a number of genes are responsible and they probably come from both parents. If there is a close relative with male pattern hair loss there is a higher risk of the condition.
Male pattern baldness prevention
There is no known prevention for male pattern baldness.
Male pattern baldness signs and symptoms
The typical sequence of hair loss in men with male pattern hair loss is loss of hair from the temples (sides of the head), which progresses to loss of hair from the front of the head. The hair loss is gradual and referred to as a receding hairline.
Over time, a bald spot may develop at the back or crown of the head, and, in some men, the entire top of the head may become bald over time, leaving a horseshoe-shaped rim of hair. And even that can be lost in some men.
Hair loss can start as early as puberty, although this is uncommon. Many men start to notice thinning of their hair in their late 20s or early 30s, and about 80 per cent of men are affected to some degree by the age of 70.
Some men find that their hair loss progresses quickly, losing much of their hair within 5 years. However, it is more common for hair loss to progress more slowly, usually over 15 to 25 years.
Male pattern baldness diagnosis
Classic male pattern baldness is usually diagnosed based on the appearance and pattern of the hair loss.
Hair loss may be due to other conditions. This may be true if hair loss occurs in patches, you shed a lot of hair, your hair breaks, or you have hair loss along with redness, scaling, pus, or pain.
A skin biopsy, blood tests, or other procedures may be needed to diagnose other disorders that cause hair loss.
Hair analysis is not accurate for diagnosing hair loss due to nutritional or similar disorders. But it may reveal substances such as arsenic or lead.
Before making a diagnosis, your doctor will likely give you a physical exam and ask about your diet, your hair care routine, and your medical and family history. You might also have tests, such as the following:
- Blood test for hematology, thyroid function and serology. This might help uncover medical conditions that can cause hair loss.
- Hair pull test. Your doctor gently pulls several dozen hairs to see how many come out. This helps determine the stage of the shedding process.
- Scalp biopsy. Your doctor scrapes samples from the skin or from a few hairs plucked from the scalp to examine the hair roots under a microscope. This can help determine whether an infection is causing hair loss.
- Wood lamp examination
- Swabs of pustules for bacterial and/or viral culture
- Skin scrapings and hair clippings for mycology
- Light microscopy. Your doctor uses a special instrument to examine hairs trimmed at their bases. Microscopy helps uncover possible disorders of the hair shaft.
Male pattern baldness treatment
Treatment is not necessary if you are comfortable with your appearance. Hair weaving, hairpieces, or change of hairstyle may disguise the hair loss. This is usually the least expensive and safest approach for male baldness.
Current male pattern baldness treatment options include:
- Hair replacement / transplantation. Hair transplants consist of removing tiny plugs of hair from areas where the hair is continuing to grow and placing them in areas that are balding. This can cause minor scarring and possibly, infection. The procedure usually requires multiple sessions and may be expensive.
- Cosmetics
- Micropigmentation (tattoo) to resemble shaven scalp
- Hairpieces
- Minoxidil (Rogaine) solution, a solution that is applied directly to the scalp to stimulate the hair follicles. It slows hair loss for many men, and some men grow new hair. Hair loss returns when you stop using this medicine.
- Finasteride tablets (Propecia, Proscar) (type II 5-alpha-reductase inhibitor), a pill that interferes with the production of a highly active form of testosterone that is linked to baldness. It slows hair loss. It works slightly better than minoxidil. Hair loss returns when you stop using this medicine.
- Dutasteride (type I and type II 5-alpha-reductase inhibitor). Dutasteride is similar to finasteride, but may be more effective.
Suturing hair pieces to the scalp is not recommended. It can result in scars, infections, and abscess of the scalp. The use of hair implants made of artificial fibers was banned by the FDA because of the high rate of infection.
Topical minoxidil (2% and 5%) and oral finasteride are the only treatments approved by the FDA for treatment of male pattern hair loss in men older than 18 years. Treatment with finasteride can cause decreased libido, impotence, and ejaculation disorders 27. These adverse effects often abate with continued treatment and happen in less than 2 percent of men younger than 40 years 28. Finasteride may also induce depression 29. Minoxidil is available over-the-counter, and it should be applied to the scalp and not the hair. Its mechanism of action is unclear. Some shedding during the first few months of treatment is common. The minoxidil 5% solution has not been shown to be consistently more effective than the 2% solution, and patients using the higher concentration had more adverse effects, including allergic contact dermatitis, dryness, and itching 30. These typically resolve after treatment is discontinued 31.
Several small studies have shown some increased effectiveness with combined minoxidil and finasteride treatment 32, 33. Starting treatment early can help maximize success, and patients can expect to see results after three to six months, although dense regrowth is not likely. Discontinuation of finasteride or minoxidil results in loss of any positive effects on hair growth within 12 and six months, respectively 34. When switching between treatment with finasteride and minoxidil, it is best to overlap treatments for three months to minimize hair loss 35.
A phase 2 randomized placebo-controlled study of dutasteride versus finasteride showed that the effect of dutasteride was dose dependent and 2.5mg of dutasteride was superior to 5mg finasteride in improving scalp hair growth in men between the ages of 21 and 45 years 36. It was also able to produce hair growth earlier than finasteride. This was evidenced by target area hair counts and clinical assessment at 12 and 24 weeks. In addition, a recent randomized, double blind, placebo-controlled study on the efficacy of dutasteride 0.5mg/day in identical twins demonstrated that dutasteride was able to significantly reduce hair loss progression in men with male pattern hair loss 37. A single case report showed improvement of hair loss with dutasteride 0.5mg in a woman who had failed to show any response to finasteride 38, 39.
In one phase 3 study dutasteride 0.5 mg daily showed significantly higher efficacy than placebo based on subject self-assessment and by investigator and panel photographic assessment 40. There was no major difference in adverse events between two groups the treatment and placebo groups. However, this study was limited to only 6 months. Another more recent phase 3 trial found that dutasteride 0.5 mg was statistically superior to finasteride 1 mg and placebo at 24 weeks 41.
There is some evidence that ketoconazole shampoo, an antifungal cortisol inhibitor shampoo, may also be of benefit, perhaps because it is effective in seborrheic dermatitis and dandruff 42, 43. A study compared shampoo containing 2% ketoconazole with unmedicated shampoo among 39 patients with male-pattern androgenetic alopecia 44. Medicated 2% ketoconazole shampoo increased hair density and the size and proportion of hair follicles residing in the anagen phase, both in isolation and in combination with minoxidil 44. Similarly, a 2007 study 45 including six patients with male-pattern androgenetic alopecia found hair regrowth with 2% ketoconazole topical lotion 46. Interestingly, one patient stopped using the lotion and depicted hair loss recurrence three months later, suggesting continual ketoconazole application is required for maintenance of hair regrowth. In addition, the authors found that ketoconazole may promote hair regrowth via both androgen-dependent and androgen-independent mechanisms 46. A 2019 study 47 compared the efficacy of 2% topical ketoconazole in comparison to 2% minoxidil among patients with female-pattern androgenetic alopecia. Whereas a significant difference between baseline and months 4 and 6 was observed among those receiving topical minoxidil, significant improvement with ketoconazole was observed only at month 6, suggesting delayed treatment efficacy with ketoconazole. However, whereas treatment-related side effects were reported among 55% of those receiving minoxidil, side effects were reported in only 10% receiving ketoconazole, and there was no difference in patient satisfaction between the groups 47. These studies highlight the potential therapeutic role of cortisol inhibition on hair regrowth in patients with both male and female-pattern androgenetic alopecia, although additional large, randomized controlled trials are needed to better assess efficacy.
Low-dose oral minoxidil (off label) can increase hair growth on the scalp, but may also result in generalized hypertrichosis and other adverse effects 48.
Low-level laser therapy (LLLT) is of unproven benefit in male pattern balding; the Capillus® laser cap and Hairmax® Lasercomb/Laserband are two low‐level laser therapy (LLLT) devices have been approved by the FDA for the management of androgenetic alopecia 49, 50. Minimal side effects were reported. Small number of participants reported adverse events of acne, mild paresthesia such as burning sensation, dry skin, headache, and itch 51.
Light‐emitting diode (LED) devices. In contrast with low-level laser therapy (LLLT) that delivers a single, collimated wavelength of light, light‐emitting diode (LED) devices may emit a small band of wavelengths. In particular, an all‐LED device that delivers dual dark orange (620 nm) and red light (660 nm) (Revian Red) to promote blood flow, reduce inflammation, and inhibit DHT via 5-alpha-reductase downregulation 52. In a prospective, randomized, double‐blind, controlled study, 18 male pattern hair loss subjects were treated with Revian Red cap vs. 18 male pattern hair loss subjects were treated with a sham light device for 10 min daily for 16 weeks total 53. Preliminary photographic assessments revealed increased mean hair count in the active group as compared to placebo group. Specifically, active group participants demonstrated approximately 26.3 more hairs per cm² compared to the placebo group. Overall, literature has suggested light therapy to be a safe treatment modality for androgenic alopecia (androgenetic alopecia) in both male and female patients when used independently or in combination with topical/oral therapies 51, 54. Light therapy has an excellent side effect profile, and there are no contraindications for use, although caution may be taken when administering in patients with dysplastic lesions on the scalp 55.
Platelet-rich plasma injections are also under investigation 56. Platelet‐rich plasma treatment can be administered alone or in combination with other therapies for androgenetic alopecia, although better results are obtained if platelet‐rich plasma administration is used in association with topical (such as minoxidil) or oral therapies (finasteride) 56. Further studies are required to determine the magnitude of the benefit if any.
Platelet‐rich plasma is generally indicated for patients with early‐stage androgenic alopecia, as intact hair follicles are present and a more significant hair restorative effect can be achieved 57. During the procedure, approximately 10–30 mL of blood are drawn from the patient’s vein and centrifuged for 10 min in order to separate the plasma from red blood cells. The platelet‐rich plasma, containing numerous growth factors, is then injected into the deep dermis or subcutaneous tissue at a volume of 4–8 mL per session. Mild side effects include scalp pain, headache, and burning sensation, but these effects usually subside in 10–15 minutes post‐injection and do not warrant use of topical anesthesia or pain medications 58. Vibration or cool air is typically sufficient to alleviate any significant pain that a patient may feel from the treatment. Patients can resume regular activities immediately after treatment but should avoid strenuous physical activity 24 hour post‐treatment to allow for optimal absorption of platelet‐rich plasma into tissue.
Hausauer and Jones 59 conducted a single center, blinded, randomized controlled trial investigating the efficacy of two platelet‐rich plasma regimens in 40 androgenic alopecia subjects. Participants received either subdermal platelet‐rich plasma injections with 3 monthly sessions and booster 3 months later (group 1) or 2 sessions every 3 months (group 2). Folliscope hair count and shaft caliber, global photography, and patient satisfaction questionnaires were completed at baseline, 3‐month, and 6‐month visits. The authors reported statistically significant increases in hair count and shaft caliber in both groups at 6 months. Importantly, improvements occurred more rapidly and profoundly in group 1, indicating that platelet‐rich plasma injections should be administered first monthly 59. Alves and Grimalt 60 demonstrated significant differences in mean anagen hair and telogen hair count as well as telogen and overall hair density when compared to baseline. In a review of 16 studies comprising a total of 389 patients with androgenic alopecia, the majority demonstrated efficacy in promoting successful hair growth after 3–4 sessions on a monthly basis, followed by quarterly maintenance sessions 61.
Platelet‐rich plasma is not curative for hair loss and must be continued long term for hair sustenance. However, patient satisfaction is typically very high and 60–70% of patients continue to undergo maintenance treatments. Due to the relatively recent introduction of platelet‐rich plasma injections for androgenic alopecia, there are no long‐term studies evaluating its effectiveness. Additionally, it is difficult to compare the efficacy with other remedies due to the lack of standardization in regard to platelet‐rich plasma kits, treatment fractions, and regimens, including the use of newer multi‐needle injectors.
While platelet‐rich plasma injections are considered safe when performed by a trained medical provider, these treatments are not suitable for everyone. Platelet‐rich plasma may not be appropriate for those with a history of bleeding disorders, autoimmune disease, or active infection, or those currently taking an anticoagulant medication. Although the majority of patients seem to tolerate the pain associated with scalp injections, some patients may prefer to avoid it.
Finasteride
Finasteride (also known as Propecia®) is taken as a tablet and works by blocking the conversion of testosterone to DHT. The hair follicles are then not affected by DHT and can grow normally. About two in three men who take finasteride every day get some hair regrowth. One in three men may have no hair regrowth but most of these don’t have any further hair loss. Finasteride has no effect in about one in 100 men.
The usual dose of finasteride is one tablet daily, with regrowth or reduction of further hair loss visible after about 4 months. However, it may take 2 or more years before a noticeable effect is seen. Hair growth is usually greater over the crown than over the front areas of the scalp. Also, the effect of finasteride is only maintained while you continue to take the medicine – if you stop taking the tablets hair loss will resume, so ongoing treatment is needed for long-term benefit. Side-effects are uncommon, but about two in 100 men taking finasteride experience a lower libido (sex drive).
Finasteride (marketed as Proscar®) is taken in higher doses as a treatment for benign prostate enlargement.
Finasteride taken at these higher doses may raise a man’s chance of getting certain types of prostate cancer. However, the much lower dose used for hair loss is not known to have any effect on a man’s chance of developing prostate cancer.
If you have any concerns, speak to your doctor.
Be aware that finasteride does not lead to hair regrowth in all men who try it, although it does stop further hair loss in most men. In order to decide how effective the treatment is for you, it may be a good idea to photograph your scalp before treatment and again after 6-12 months. Finasteride is only available on prescription from your doctor.
Side effects of finasteride
Side effects of finasteride are uncommon and usually mild. Talk to your doctor about the risks of taking finasteride.
A small number of men report a decreased libido (sex drive) and problems with their erections (erectile dysfunction) are recognized side-effects of treatment with finasteride in men 18. Some small differences have been seen in the semen of males who take finasteride, such as low sperm counts 62. Sperm levels improved when the medication was stopped. Taking finasteride may increase the risk that you will develop high-grade prostate cancer (a type of prostate cancer that spreads and grows more quickly than other types of prostate cancer) or male breast cancer 63, 64.
In rare cases, gynecomastia (male breast enlargement) has occurred in men taking finasteride.
Although finasteride is generally well tolerated in a study of 3,200 men, including patients on therapy for up to two years. Recently many reports described adverse effects in men during finasteride treatment, such as sexual dysfunction and mood alteration 65, 66, 67, 68, 69. In addition, it has been also reported that persistent side effects may occur in some androgenetic alopecia patients. This condition, termed post-finasteride syndrome (PFS) represents a constellation of sexual, physical, and neurological symptoms that develop and persist during treatment and/or after finasteride discontinuation 70, 71, 72. Among the reported sexual and physical adverse effects associated with post-finasteride syndrome (PFS) are: loss of libido; erectile dysfunction; ejaculatory disorders; reduction in penis size; penile curvature; reduced sensation; decreased arousal and difficulty in achieving orgasm; gynecomastia; muscle atrophy; fatigue; and severely dry skin. Reported neurological (psychiatric) adverse events include: depression and anxiety; cognitive impairment; and suicidal ideation that are still present despite drug withdrawal 72. Furthermore, several case studies have linked finasteride with male infertility 73, 74, cataract and intraoperative floppy-iris syndrome 75, pseudoporphyria 76 and T cell–mediated acute localized exanthematous pustulosis 77. As a result, regulatory agencies in several countries generated warnings about finasteride (e.g., Swedish Medical Products Agency, the Medicines and Healthcare Products Regulatory Agency of UK and the U.S. Food and Drug Administration) required to include multiple persistent side effects within the finasteride labels 70.
Dutasteride
Dutasteride is the successor to finasteride acting as a second‐generation 5‐alpha‐reductase inhibitor and functioning as a selective competitive inhibitor of type 1 and type 2 isoenzymes of 5‐alpha‐reductase designed to decrease the production of dihydrotestosterone (DHT) 78, 79, 80, 81. 82. Dutasteride is reported to be three times more potent at inhibiting the type 1 5‐alpha‐reductase enzyme and 100 times more potent at inhibiting the type 2I 5‐alpha‐reductase enzyme than finasteride 83. Dutasteride comes in 2.5 and 5 mg doses, both of which have shown superior efficacy to finasteride 5 mg 36. Due to dutasteride’s large molecular size, it is difficult to formulate and deliver as a topical agent. However, its large size and fat soluble (lipophilic) nature contribute to it remaining on the scalp and preventing absorption into your body. If requested by clinicians, compounding pharmacies may formulate dutasteride topical solutions, although literature is sparse regarding its utility in treating androgenetic alopecia.
Dutasteride 0.5 mg/day can reduce dihydrotestosterone (DHT) serum levels by upwards of 90% in a dose dependent manner 36. By reducing dihydrotestosterone (DHT) levels in your scalp and your prostate gland, dutasteride decreases dihydrotestosterone (DHT) effects in your hair follicles and prostate gland, reversing the process of hair loss in men (androgenic alopecia) and reducing the size of your prostate in benign prostatic hyperplasia (BPH) 84.
Olszewska and Rudnicka 38 reported a case of a female patient with androgenetic alopecia who did not respond to minoxidil and initially benefited from finasteride. Given her persistent androgenetic alopecia, the patient was started on oral dutasteride. After 6 months of treatment, clinical and trichogram assessments revealed significant improvement in hair density 38. Several randomized, double‐blind, placebo‐controlled clinical studies have demonstrated dutasteride’s effectiveness for treating androgenetic alopecia 82, 85. Intradermal (injection into the skin) dutasteride was also reported in order to decrease the systemic side effects. Saceda‐Corralo et al. 86 administered 1 mL intradermal dutasteride 0.01% injections every 3 months for a total of three sessions to six subjects. Trichoscopy assessments revealed increased hair diameter and density, in addition to clinical improvement in androgenetic alopecia. There were no statistically significant differences in serum levels of total and free testosterone, 3 alpha androstanediol glucuronide, and dihydrotestosterone before and after treatment 86. Similar studies injecting dutasteride mesotherapy yielded promising results 87, 88, 89. Overall, oral dutasteride appears to be superior to the intradermal route. However, more studies are warranted 85.
Overall, dutasteride has shown superior efficacy both in blocking DHT and promoting hair growth compared to finasteride. In a study of 399 patients, dutasteride was found to block 98.4% of DHT, while finasteride blocked about 70% 83. In another study of 416 men between 21 and 45 years of age, dutasteride was found to produce better hair count results than finasteride over a period of 12–24 weeks 36. Despite the greater efficacy demonstrated by dutasteride, finasteride is still likely to be prescribed more often as a first‐line agent in treating male pattern baldness due to FDA approval and insurance coverage.
Side effects of dutasteride
Similar to finasteride, the side effects of oral dutasteride include decreased sex drive (libido), erectile dysfunction, and ejaculatory dysfunction 85.
Minoxidil
Minoxidil (also known as Rogaine®, Hair a-gain®, Hair Retreva®) is a lotion that is rubbed onto the head. Topical minoxidil is one of the only three FDA‐approved treatments for male and female pattern hair loss. Minoxidil is available in both 2% and 5% solutions and in foam preparation, so clinicians and patients have flexibility to select their preferred strength and formulation. The 5% solution has demonstrated greater efficacy than the 2% solution, and the 5% foam has shown equivalency to the 2% and 5% solutions depending on frequency of use 90, 91. Minoxidil foam is often more convenient to use, as it dries quicker and has less tendency to spread to the peripheral areas. Some patients report an unpleasant residue after applying the foam, in which case a solution formulation may be preferred. Minoxidil must be applied once or twice daily for full effect. If used properly, patients can expect to see hair growth within 4–8 months which stabilizes after 12–18 months 92. If a patient terminates treatment, progressive hair loss can be expected within 12–24 weeks 93.
Topical minoxidil was approved specifically for androgenic alopecia in 1988 as a first‐line treatment for men with mild‐to‐moderate androgenic alopecia 94, 95. About half of the men using minoxidil have a delay in further balding 96. About 15 in 100 men have hair regrowth, while hair loss continues in about one in three users. Minoxidil needs to be rubbed onto the scalp twice daily, and used for four months before results can be seen. There may actually be some hair loss at the beginning of treatment, as hair follicles in the resting phase are stimulated to move to the growth phase. Treatment needs to be ongoing for hair growth to continue; any hair that has regrown may fall out two months after treatment is stopped.
In a 1 year study of 904 males with androgenetic alopecia, 62% of the patients exhibited a significant decrease in the affected region of the scalp when treated with 5% topical minoxidil twice daily and 84.3% of patients reported hair regrowth of varying degrees 97. The 2% and 5% Minoxidil solutions have elicited a 70% greater improvement in mean hair density compared with placebo after 16 and 26 week treatment periods 98, 99, 100. In a randomized control trial of 278 patients treated with minoxidil, 45% demonstrated more hair regrowth when treated with 5% solution vs. 2% by 48 weeks of treatment 98.
Be aware that minoxidil is not effective for all men, and the amount of hair regrowth will vary among people. Some men experience hair regrowth while in others hair loss is just slowed down. If there is no noticeable effect after 6 months, it’s recommended that treatment is stopped.
Side effects associated with minoxidil
The most common side effects of minoxidil include a dry, red and itchy scalp. Higher-strength solutions are more likely to cause scalp irritation and excessive facial hair growth 101.
Bear in mind that minoxidil is also used in tablet form as a prescription medicine to treat high blood pressure, and there is a small chance that minoxidil solution could possibly affect your blood pressure and heart function. For this reason, minoxidil is generally only recommended for people who do not have heart or blood pressure problems.
Always follow the directions on the product, making sure you use minoxidil only when your scalp and hair are completely dry. Wash your hands after use.
Oral minoxidil
Although not FDA‐approved and not nearly as popular as finasteride, multiple studies were conducted to evaluate oral minoxidil for treating both male and female patients with androgenetic alopecia. Oral minoxidil is available as a 2.5 mg tablet, and it can be cut in halves or quarters to achieve optimal safe dosing for the treatment of androgenetic alopecia. Sinclair first reported the combination of oral minoxidil 0.25 mg and spironolactone 25 mg to be a safe and effective option in managing female pattern hair loss 102.
Several retrospective case series reported oral minoxidil to be an effective treatment for female androgenetic alopecia with favorable side effects 103, 104. Studies suggested that optimal safe doses range between 0.625 mg and 1.25 mg daily 105. Oral minoxidil has also shown equivalent effectiveness in women compared to the 5% topical formulation 106. Jimenez‐Cauhe et al. 48 conducted a retrospective review of 41 men diagnosed with androgenetic alopecia undergoing oral minoxidil 5 mg daily treatment. Side effects were detected in about 30% of the participants, but they were all tolerable 48. Another prospective study 107 using a 5 mg once daily regimen showed 100% improvement at week 12 and 24 with 43% patients achieving excellent improvement. Pirmez et al. 108 suggested that very low dose oral minoxidil (0.25 mg once daily) may be less effective in treating moderate androgenetic alopecia and higher dosage might be needed. However, the sample size was small.
Side effects oral minoxidil
Although it may be more convenient for patients to take the oral form of minoxidil, its systemic side effects such as increased heart rate, weight gain, hirsutism, hypertrichosis, and lower extremity edema make it unfavorable compared to topical minoxidil as a first‐line treatment 107. In a recent study of 1404 subjects 109, the most common side effect was noted to be hypertrichosis in about 15% of patients and the incidence of systemic adverse effects was noted in 1.7% of patients. Oral minoxidil’s side effects, however, are typically dose‐dependent and reversible with discontinuation of the drug. Rare side effects include pericardial effusion, congestive heart failure, and allergic reactions 107.
Hair transplantation
Hair transplant surgery is an option for men with hair loss that has not responded to other treatments. Hair transplantation involves taking tiny clusters, or plugs of hair (each containing up to 4 hairs) from areas where it continues to grow (usually the back of the head) and inserting them in bald or thinning areas. The procedures used to transplant hair are called micrografting or follicular unit transplantation. They can be expensive and painful and transplant sessions may be needed to achieve the desired effect and this can be expensive. Side effects such as skin infection and minor scarring are possible. However, results are usually good and are permanent. Choosing a surgeon with experience in this operation is recommended.
Procedures that involve transplanting body hair to the scalp or implanting artificial hair fibers may be used in some cases, although there is a higher rate of complications with artificial hair implants.
Talk to your doctor about whether these procedures would be suitable for you and about the risks and benefits of these treatments. Your doctor should be able to refer you to a dermatologist or hair transplant surgeon if hair transplant surgery is likely to be a suitable treatment for you.
Cosmetic treatments
Cosmetic treatment options include the following.
- Wearing a wig, hairpiece or toupee. Synthetic and real hair wigs are available.
- A special type of tattooing called scalp micropigmentation or dermatography can give the appearance of a shaved head of hair or thicker hair.
- Coloring the scalp with a camouflage dye may be recommended in some cases where the hair is thinning.
- The use of hair thickening fibers, such as natural keratin fiber, is a treatment that is inexpensive. It involves applying tiny, microfiber ‘hairs’ that bond to existing hair through static electricity to give the appearance of thicker hair.
Talk to your doctor about these options before deciding which, if any, may be suitable for you.
Wigs and hair pieces
Some affected individuals find wigs, toupees and even hair extensions can be very helpful in disguising androgenetic alopecia female. There are two types of postiche (false hairpiece) available to individuals; these can be either synthetic or made from real hair. Synthetic wigs and hairpieces, usually last about 6 to 9 months, are easy to wash and maintain, but can be susceptible to heat damage and may be hot to wear. Real hair wigs or hairpieces can look more natural, can be styled with low heat and are cooler to wear.
Skin camouflage
Spray preparations containing small pigmented fibers are available from the internet and may help to disguise the condition in some individuals. These preparations however, may wash away if the hair gets wet (i.e. rain, swimming, perspiration), and they only tend to last between brushing/shampooing.
Unproven treatments for hair loss
Remedies for hair loss which have not been shown or proven to be effective include:
- nutritional supplements;
- platelet-rich plasma injections – sometimes known as PRP rejuvenation;
- acupuncture;
- scalp massage; and
- laser treatment.
Discuss with your doctor any treatments that you are interested in trying. It’s important to discuss the risks and benefits before opting for any hair loss treatment options.
If you consult a hair loss clinic or alternative medicine practitioner about treatments for hair loss, make sure you understand exactly what treatments they offer and whether there is any proven scientific evidence for their effectiveness.
It’s always a good idea to check with your doctor before using any treatment, to avoid unwanted effects and spending time or money on a treatment that is unlikely to help.
Scalp massage
Developing hair follicles are surrounded by deep dermal vascular plexuses. Associated blood vessels function to supply nutrients to the developing hair follicle and foster waste elimination. As such, proper blood supply is necessary for effective hair follicle growth, further exemplified by the angiogenic properties of the anagen phase 110. A 2016 study 111 assessed the effect of a 4-minute standardized daily scalp massage for 24 weeks among nine healthy men. Authors found scalp massage to increase hair thickness, upregulate 2655 genes, and downregulate 2823 genes; hair cycle-related genes including NOGGIN, BMP4, SMAD4, and IL6ST were among those upregulated, and hair-loss related IL6 was among those downregulated 111. The authors thereby concluded that a standardized scalp massage and subsequent dermal papilla cellular stretching can increase hair thickness, mediated by changes in gene expression in dermal papilla cells 111.
In addition, of 327 survey respondents attempting standardized scalp massages following demonstration video, 68.9% reported hair loss stabilization or regrowth 112. Positive associations existed between self-reported hair changes and estimated daily minutes, months, and total standardized scalp massage effort. This study 112 is limited based on recall bias and reliance on patient adherence and technique, although it suggests promising therapeutic potential for standardized scalp massage, which functions to increase blood flow.
Low-level laser (light) therapy
Low-level laser (light) therapy refers to therapeutic exposure to low levels of red and near infrared light 113. Studies have demonstrated increased hair growth in mice with chemotherapy-induced alopecia and alopecia areata, in addition to both men and women human subjects. Proposed mechanisms of efficacy include stimulation of epidermal stem cells residing in the hair follicle bulge and promoting increased telogen to anagen phase transition 114. Interestingly, while minoxidil and finasteride are the only FDA-approved drugs for androgenetic alopecia, a 2017 study 54 found comparable efficacy among patients receiving low-level light therapy versus topical minoxidil among patients with female-pattern androgenetic alopecia. In addition, combination therapy resulted in the greatest patient satisfaction and lowest Ludwig classification scores of androgenetic alopecia.
A meta-analysis including eleven double-blinded randomized controlled trials found a significant increase in hair density among patients with androgenetic alopecia receiving low level light therapy compared to those in the placebo-controlled group 115. Low level light therapy was effective for men and women. Furthermore, a subgroup analysis observed a more significant increase in hair growth in those receiving low-frequency therapy than receiving high-frequency therapy 115. Despite the limitation of the heterogeneity of included trials, these results suggest low level laser (light) therapy to be a promising therapeutic strategy for androgenetic alopecia 115, although further research is necessary to determine the optimal wavelength and dosimetric parameters for hair growth 114.
Prostaglandins
Latanoprost is a prostaglandin F2 agonist and has been shown to have a direct effect on hair growth and pigmentation in eyelashes and hair around the eyes 116. Clinically used to treat glaucoma, latanoprost was found to affect the hair follicles in the telogen phase and cause them to move to the anagen phase; this was supported by the increased number and length of eyelashes seen in patients using latanoprost 116. Subsequently, the application of latanoprost for patients experiencing alopecia was assessed in clinical studies. One conducted in 2012 studied the effects of 0.1% latanoprost solution applied to the scalp for 24 weeks 117. Participants included 16 males with mild androgenetic alopecia and were instructed to apply placebo on one area of the scalp and the treatment on another area. The results indicated that the area of scalp receiving latanoprost had significantly improved hair density compared to placebo 117.
Another prostaglandin known as bimatoprost, a prostamide-F2 analog, was also found to have a positive effect on hair growth in human and mouse models 118. A study conducted in 2013 also found that bimatoprost, in both humans and mice, stimulated the anagen phase of hair follicles prompting an increase in hair length, i.e., promoting hair growth 118. The study also confirmed the presence of prostanoid receptors in human scalp hair follicles in vivo, opening the strong possibility that scalp follicles can also respond to bimatoprost in a similar fashion 118.
It is important to note, however, that not all prostaglandins induce hair growth 119. In a study analyzing individuals with androgenetic alopecia with a bald scalp versus a haired scalp, it was discovered that there was an elevated level of prostaglandin D2 synthase at the mRNA and protein levels in bald individuals 119. They were also found to have an elevated level of prostaglandin D2 (PGD2). When analyzing the level of prostaglandin D2 synthase presence through the various phases of hair follicular growth, it was found that the level steadily increased throughout the anagen phase with a peak in late anagen, at the time of transition to the catagen (breakdown) phase. Therefore, the study concluded that prostaglandin D2 (PGD2)’s hair loss effect represents a counterbalance to PGE2 and PGF2’s hair growth effects. In conclusion, prostaglandins are a promising treatment option for alopecia that require larger clinical studies; however, clinicians should be aware of which one to recommend for hair growth, as not all prostaglandins are alike 2.
Platelet rich plasma
Platelet rich plasma (PRP) has conventionally been used to supplement a patient’s endogenous platelet supply to promote increased healing 2. However, its prominent supply of growth factors has prompted assessment of platelet rich plasma for alopecia. Growth factors promote hair growth and increase the telogen to anagen transition. For example, a mice study found the fibroblast growth factor (FGF) induced the anagen phase and subsequently promoted hair growth 120. Growth factors prominently included in platelet rich plasma include platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor (IGF) and fibroblast growth factor (FGF) 121.
The growth factors of platelet-rich plasma stimulate the development of new follicles and neovascularization 122. Three meta-analyses have assessed the efficacy of platelet rich plasma injections compared to placebo control on the number of hairs per cm2 among patients with androgenetic alopecia. One meta-analysis involving 177 patients found a mean improvement of platelet rich plasma treatment compared to placebo of 17.9 123; a second meta-analysis with 262 androgenetic alopecia patients observed a mean difference of 38.8 124; and a third meta-analysis including studies with parallel or half-head design found a mean difference of 30.4 125.
Despite the efficacious results described by each meta-analysis for the use in androgenetic alopecia, gender differences have been observed. A 2020 meta-analysis 126 found that while platelet rich plasma significantly increased hair density and hair diameter from baseline in men, platelet rich plasma only increased hair diameter in women, in the absence of significantly increased hair density. Furthermore, hair density in men was only significantly increased by a double spin method, in contrast to a single spin method 126. The authors conclude that platelet rich plasma effectiveness may be improved via higher platelet concentrations 126. Ultimately, platelet rich plasma injections appear to have clinical efficacy in early studies although with slightly different effects in men vs. women. Future research is necessary to establish the optimal treatment protocol for both men and women with androgenetic alopecia. Also, the role of diet in the days prior to collection of the platelet rich plasma has not been assessed in conjunction with hair, although diet influences the quality of the platelet rich plasma 127.
Microneedling
Microneedling is a minimally invasive dermatologic procedure in which fine needles are rolled over the skin to puncture the stratum corneum. The physical trauma from needle penetration in microneedling induces a wound healing cascade with minimal damage to the epidermis stimulating collagen formation, neovascularization, and growth factor production in the treated areas 128. Microneedling enhances drug delivery in the treatment of androgenetic alopecia and female pattern hair loss and has been successfully paired with other hair growth-promoting therapies such as minoxidil, platelet-rich plasma, and topical steroidal medications 128.
Melatonin
Melatonin is known to have strong anti-oxidant properties and the ability to actively capture free radicals 129. Melatonin has been found to modulate hair growth, pigmentation, and molting in many species including humans 130. The topical application of the melatonin 0.1% solution was shown to significantly increase anagen hair in male and female androgenetic alopecia with good compliance in a controlled study 131.
Living with hair loss
Many studies have shown that male pattern boldness is not merely a cosmetic issue, but it also causes significant psychological distress. For some men, hair loss can be incredibly distressing and can be associated with low self-esteem, depression, introversion, and feelings of unattractiveness. Others might accept their hair loss a little more easily.
Hair loss is especially hard to live in a society that places great value on youthful appearance and attractiveness. Compared to unaffected men, those affected have a more negative body image and are less able to cope with daily functioning.
Some men find that hats and wigs can help. Hats whilst useful as cosmetic camouflages, also provide good sun protection to the scalp. Others find that synthetic ‘spray-on hair’ products are a clever way to disguise thinning hair.
References- Hamilton, J.B. (1951), PATTERNED LOSS OF HAIR IN MAN: TYPES AND INCIDENCE. Annals of the New York Academy of Sciences, 53: 708-728. https://doi.org/10.1111/j.1749-6632.1951.tb31971.x
- Natarelli N, Gahoonia N, Sivamani RK. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J Clin Med. 2023 Jan 23;12(3):893. doi: 10.3390/jcm12030893
- Girijala RL, Riahi RR, Cohen PR. Platelet-rich plasma for androgenic alopecia treatment: A comprehensive review. Dermatol Online J. 2018 Jul 15;24(7):13030/qt8s43026c https://doi.org/10.5070/D3247040910
- Thom E. Stress and the Hair Growth Cycle: Cortisol-Induced Hair Growth Disruption. J Drugs Dermatol. 2016 Aug 1;15(8):1001-4. https://jddonline.com/articles/stress-and-the-hair-growth-cycle-cortisol-induced-hair-growth-disruption-S1545961616P1001X/
- Bassino E, Gasparri F, Munaron L. Protective Role of Nutritional Plants Containing Flavonoids in Hair Follicle Disruption: A Review. Int J Mol Sci. 2020 Jan 14;21(2):523. doi: 10.3390/ijms21020523
- Fu D., Huang J., Li K., Chen Y., He Y., Sun Y., Guo Y., Du L., Qu Q., Miao Y., et al. Dihydrotestosterone-induced hair regrowth inhibition by activating androgen receptor in C57BL6 mice simulates androgenetic alopecia. Biomed. Pharmacother. 2021;137:111247. doi: 10.1016/j.biopha.2021.111247
- Helle Rexbye, Inge Petersen, Maria Iachina, Jakob Mortensen, Matt McGue, James W. Vaupel, Kaare Christensen, Hair Loss Among Elderly Men: Etiology and Impact on Perceived Age, The Journals of Gerontology: Series A, Volume 60, Issue 8, August 2005, Pages 1077–1082, https://doi.org/10.1093/gerona/60.8.1077
- Nyholt DR, Gillespie NA, Heath AC, Martin NG. Genetic Basis of Male Pattern Baldness. Journal of Investigative Dermatology. 2003;121(6):1561–4. 10.1111/j.1523-1747.2003.12615.x
- Richards JB, Yuan X, Geller F, Waterworth D, Bataille V, Glass D, Song K, Waeber G, Vollenweider P, Aben KK, Kiemeney LA, Walters B, Soranzo N, Thorsteinsdottir U, Kong A, Rafnar T, Deloukas P, Sulem P, Stefansson H, Stefansson K, Spector TD, Mooser V. Male-pattern baldness susceptibility locus at 20p11. Nat Genet. 2008 Nov;40(11):1282-4. doi: 10.1038/ng.255
- Yip, Leona & Sinclair, Rodney. (2006). Antiandrogen therapy for androgenetic alopecia. Expert Review of Dermatology. 1. 261-269. 10.1586/17469872.1.2.261
- Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977 Sep;97(3):247-54. https://doi.org/10.1111/j.1365-2133.1977.tb15179.x
- Weinbauer GF, Niehaus M, Nieschlag E. The role of testosterone in spermatogenesis. In: Nieschlag E, Behre HM, editors. Testosterone: Action, Deficiency, Substitution. Cambridge University Press; Cambridge: 2004. pp. 173–206.
- Setchell JM, Smith T, Wickings EJ, Knapp LA. Social correlates of testosterone and ornamentation in male mandrills. Horm Behav. 2008 Aug;54(3):365-72. doi: 10.1016/j.yhbeh.2008.05.004
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30(3):319-45. doi: 10.1016/j.neubiorev.2004.12.007
- Isidori, A.M., Giannetta, E., Gianfrilli, D., Greco, E.A., Bonifacio, V., Aversa, A., Isidori, A., Fabbri, A. and Lenzi, A. (2005), Effects of testosterone on sexual function in men: results of a meta-analysis. Clinical Endocrinology, 63: 381-394. https://doi.org/10.1111/j.1365-2265.2005.02350.x
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Androgenic Steroids. [Updated 2020 May 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548931
- Paus R., Cotsarelis G. The Biology of Hair Follicles. N. Engl. J. Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706
- Kinter KJ, Anekar AA. Biochemistry, Dihydrotestosterone. [Updated 2022 Mar 9]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557634
- Urysiak-Czubatka I., Kmieć M.L., Broniarczyk-Dyła G. Assessment of the usefulness of dihydrotestosterone in the diagnostics of patients with androgenetic alopecia. Adv. Dermatol. Allergol. 2014;31:207–215. doi: 10.5114/pdia.2014.40925
- Kaufman K.D., Olsen E.A., Whiting D., Savin R., DeVillez R., Bergfeld W., Price V.H., Van Neste D., Roberts J.L., Hordinsky M., et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J. Am. Acad. Dermatol. 1998;39:578–589. doi: 10.1016/S0190-9622(98)70007-6
- Zhang Y., Xu J., Jing J., Wu X., Lv Z. Serum levels of androgen-associated hormones are correlated with curative effect in androgenic alopecia in young men. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018;24:7770–7777. doi: 10.12659/MSM.913116
- Hagenaars SP, Hill WD, Harris SE, Ritchie SJ, Davies G, Liewald DC, Gale CR, Porteous DJ, Deary IJ, Marioni RE. Genetic prediction of male pattern baldness. PLoS Genet. 2017 Feb 14;13(2):e1006594. doi: 10.1371/journal.pgen.1006594
- Liamsombut S., Pomsoong C., Kositkuljorn C., Leerunyakul K., Tantrakul V., Suchonwanit P. Sleep quality in men with androgenetic alopecia. Sleep Breath. 2022 doi: 10.1007/s11325-022-02618-x
- Yi Y., Qiu J., Jia J., Djakaya G.D.N., Li X., Fu J., Chen Y., Chen Q., Miao Y., Hu Z. Severity of androgenetic alopecia associated with poor sleeping habits and carnivorous eating and junk food consumption—A web-based investigation of male pattern hair loss in China. Dermatol. Ther. 2020;33:e13273. doi: 10.1111/dth.13273
- Shakoei S, Torabimirzaee A, Saffarian Z, Abedini R. Sleep disturbance in alopecia areata: A cross-sectional study. Health Sci Rep. 2022 Apr 1;5(3):e576. doi: 10.1002/hsr2.576
- Seo H.-M., Kim T.L., Kim J.S. The risk of alopecia areata and other related autoimmune diseases in patients with sleep disorders: A Korean population-based retrospective cohort study. Sleep. 2018;41:zsy111. doi: 10.1093/sleep/zsy111
- Leyden J, Dunlap F, Miller B, et al. Finasteride in the treatment of men with frontal male pattern hair loss. J Am Acad Dermatol. 1999;40(6 pt 1):930–937.
- Kaufman KD, Olsen EA, Whiting D, et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol. 1998;39(4 pt 1):578–589.
- Rahimi-Ardabili B, Pourandarjani R, Habibollahi P, Mualeki A. Finasteride induced depression: a prospective study. BMC Clin Pharmacol. 2006;6:7.
- Olsen EA, Dunlap FE, Funicella T, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47(3):377–385.
- Dawber RP, Rundegren J. Hypertrichosis in females applying minoxidil topical solution and in normal controls. J Eur Acad Dermatol Venereol. 2003;17(3):271–275.
- Diani AR, Mulholland MJ, Shull KL, et al. Hair growth effects of oral administration of finasteride, a steroid 5 alpha-reductase inhibitor, alone and in combination with topical minoxidil in the balding stumptail macaque. J Clin Endocrinol Metab. 1992;74(2):345–350.
- Khandpur S, Suman M, Reddy BS. Comparative efficacy of various treatment regimens for androgenetic alopecia in men. J Dermatol. 2002;29(8):489–498.
- Olsen EA, Weiner MS. Topical minoxidil in male pattern baldness: effects of discontinuation of treatment. J Am Acad Dermatol. 1987;17(1):97–101.
- Olsen EA. Female pattern hair loss and its relationship to permanent/cicatricial alopecia: a new perspective. J Investig Dermatol Symp Proc. 2005;10(3):217–221.
- Olsen EA, Hordinsky M, Whiting D, Stough D, Hobbs S, Ellis ML, Wilson T, Rittmaster RS; Dutasteride Alopecia Research Team. The importance of dual 5alpha-reductase inhibition in the treatment of male pattern hair loss: results of a randomized placebo-controlled study of dutasteride versus finasteride. J Am Acad Dermatol. 2006 Dec;55(6):1014-23. doi: 10.1016/j.jaad.2006.05.007
- Debruyne F, Barkin J, van Erps P, Reis M, Tammela TL, Roehrborn C; ARIA3001, ARIA3002 and ARIB3003 Study Investigators. Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004 Oct;46(4):488-94; discussion 495. doi: 10.1016/j.eururo.2004.05.008
- Olszewska M, Rudnicka L. Effective treatment of female androgenic alopecia with dutasteride. J Drugs Dermatol. 2005 Sep-Oct;4(5):637-40.
- Saceda-Corralo D, Moustafa F, Moreno-Arrones Ó, Jaén-Olasolo P, Vañó-Galván S, Camacho F. Mesotherapy With Dutasteride for Androgenetic Alopecia: A Retrospective Study in Real Clinical Practice. J Drugs Dermatol. 2022 Jul 1;21(7):742-747. https://jddonline.com/articles/mesotherapy-with-dutasteride-for-androgenetic-alopecia-a-retrospective-study-in-real-clinical-practice-S1545961622P0742X
- Eun HC, Kwon OS, Yeon JH, Shin HS, Kim BY, Ro BI, Cho HK, Sim WY, Lew BL, Lee WS, Park HY, Hong SP, Ji JH. Efficacy, safety, and tolerability of dutasteride 0.5 mg once daily in male patients with male pattern hair loss: a randomized, double-blind, placebo-controlled, phase III study. J Am Acad Dermatol. 2010 Aug;63(2):252-8. doi: 10.1016/j.jaad.2009.09.018
- Gubelin Harcha W, Barboza Martínez J, Tsai TF, Katsuoka K, Kawashima M, Tsuboi R, Barnes A, Ferron-Brady G, Chetty D. A randomized, active- and placebo-controlled study of the efficacy and safety of different doses of dutasteride versus placebo and finasteride in the treatment of male subjects with androgenetic alopecia. J Am Acad Dermatol. 2014 Mar;70(3):489-498.e3. doi: 10.1016/j.jaad.2013.10.049
- Inui S, Itami S. Reversal of androgenetic alopecia by topical ketoconzole: relevance of anti-androgenic activity. J Dermatol Sci. 2007 Jan;45(1):66-8. doi: 10.1016/j.jdermsci.2006.08.011
- Hugo Perez BS. Ketocazole as an adjunct to finasteride in the treatment of androgenetic alopecia in men. Med Hypotheses. 2004;62(1):112-5. doi: 10.1016/s0306-9877(03)00264-0
- Piérard-Franchimont C., De Doncker P., Cauwenbergh G., Piérard G.E. Ketoconazole shampoo: Effect of long-term use in androgenic alopecia. Dermatology. 1998;196:474–477. doi: 10.1159/000017954
- Inui S., Itami S. Reversal of androgenetic alopecia by topical ketoconzole: Relevance of anti-androgenic activity. J. Dermatol. Sci. 2007;45:66–68. doi: 10.1016/j.jdermsci.2006.08.011
- El-Garf A., Mohie M., Salah E. Trichogenic effect of topical ketoconazole versus minoxidil 2% in female pattern hair loss: A clinical and trichoscopic evaluation. Biomed. Dermatol. 2019;3:8. doi: 10.1186/s41702-019-0046-y
- Jimenez-Cauhe J, Saceda-Corralo D, Rodrigues-Barata R, Hermosa-Gelbard A, Moreno-Arrones OM, Fernandez-Nieto D, Vaño-Galvan S. Effectiveness and safety of low-dose oral minoxidil in male androgenetic alopecia. J Am Acad Dermatol. 2019 Aug;81(2):648-649. doi: 10.1016/j.jaad.2019.04.054
- Leavitt M, Charles G, Heyman E, Michaels D. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: A randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29(5):283-92. doi: 10.2165/00044011-200929050-00001
- Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J Am Acad Dermatol. 2017 Jul;77(1):136-141.e5. doi: 10.1016/j.jaad.2017.02.054
- Darwin E, Heyes A, Hirt PA, Wikramanayake TC, Jimenez JJ. Low-level laser therapy for the treatment of androgenic alopecia: a review. Lasers Med Sci. 2018 Feb;33(2):425-434. doi: 10.1007/s10103-017-2385-5
- RED light treatment for hair loss ‐ the science behind REVIAN RED. https://revian.com/how-it-works
- Kernel Networks Inc . Modulated light therapy in participants with pattern hair loss. Case Med Res. 2019. 10.31525/ct1-nct04019795
- Esmat, S.M., Hegazy, R.A., Gawdat, H.I., Abdel Hay, R.M., Allam, R.S., El Naggar, R. and Moneib, H. (2017), Low level light-minoxidil 5% combination versus either therapeutic modality alone in management of female patterned hair loss: A randomized controlled study. Lasers Surg. Med., 49: 835-843. https://doi.org/10.1002/lsm.22684
- Frigo L, Luppi JS, Favero GM, Maria DA, Penna SC, Bjordal JM, Bensadoun RJ, Lopes-Martins RA. The effect of low-level laser irradiation (In-Ga-Al-AsP – 660 nm) on melanoma in vitro and in vivo. BMC Cancer. 2009 Nov 20;9:404. doi: 10.1186/1471-2407-9-404
- Alves R, Grimalt R. Platelet-Rich Plasma and its Use for Cicatricial and Non-Cicatricial Alopecias: A Narrative Review. Dermatol Ther (Heidelb). 2020 Aug;10(4):623-633. doi: 10.1007/s13555-020-00408-5
- Nestor MS, Ablon G, Gade A, Han H, Fischer DL. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J Cosmet Dermatol. 2021 Dec;20(12):3759-3781. doi: 10.1111/jocd.14537
- Girijala RL, Riahi RR, Cohen PR. Platelet-rich plasma for androgenic alopecia treatment: A comprehensive review. Dermatol Online J. 2018 Jul 15;24(7):13030/qt8s43026c https://escholarship.org/uc/item/8s43026c
- Hausauer AK, Jones DH. Evaluating the Efficacy of Different Platelet-Rich Plasma Regimens for Management of Androgenetic Alopecia: A Single-Center, Blinded, Randomized Clinical Trial. Dermatol Surg. 2018 Sep;44(9):1191-1200. doi: 10.1097/DSS.0000000000001567
- Alves R, Grimalt R. Randomized Placebo-Controlled, Double-Blind, Half-Head Study to Assess the Efficacy of Platelet-Rich Plasma on the Treatment of Androgenetic Alopecia. Dermatol Surg. 2016 Apr;42(4):491-7. doi: 10.1097/DSS.0000000000000665
- Hesseler MJ, Shyam N. Platelet-Rich Plasma and Its Utilities in Alopecia: A Systematic Review. Dermatol Surg. 2020 Jan;46(1):93-102. doi: 10.1097/DSS.0000000000001965
- Laborde, E. and Brannigan, R.E. (2010), Effect of 1-mg Dose of Finasteride on Spermatogenesis and Pregnancy. Journal of Andrology, 31: e1-e2. https://doi.org/10.2164/jandrol.109.009381
- FDA Drug Safety Communication: 5-alpha reductase inhibitors (5-ARIs) may increase the risk of a more serious form of prostate cancer. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-5-alpha-reductase-inhibitors-5-aris-may-increase-risk-more-serious
- October – December 2009 | Potential Signals of Serious Risks/New Safety Information Identified by the Adverse Event Reporting System (AERS). https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/october-december-2009-potential-signals-serious-risksnew-safety-information-identified-adverse-event
- Baas W.R., Butcher M.J., Lwin A., Holland B., Herberts M., Clemons J., Delfino K., Althof S., Kohler T.S., McVary K.T. A review of the FAERS data on 5-alpha reductase inhibitors: implications for postfinasteride syndrome. Urology. 2018;120:143–149. doi: 10.1016/j.urology.2018.06.022
- Gupta A.K., Carviel J., MacLeod M.A., Shear N. Assessing finasteride-associated sexual dysfunction using the FAERS database. J. Eur. Acad. Dermatol. Venereol. 2017;31:1069–1075. doi: 10.1111/jdv.14223
- Tsunemi Y., Irisawa R., Yoshiie H., Brotherton B., Ito H., Tsuboi R., Kawashima M., Manyak M., Group A.R.I.S. Long-term safety and efficacy of dutasteride in the treatment of male patients with androgenetic alopecia. J. Dermatol. 2016;43:1051–1058. doi: 10.1111/1346-8138.13310
- Traish A.M., Melcangi R.C., Bortolato M., Garcia-Segura L.M., Zitzmann M. Adverse effects of 5alpha-reductase inhibitors: what do we know, don’t know, and need to know? Rev. Endocr. Metab. Disord. 2015;16:177–198. doi: 10.1007/s11154-015-9319-y
- Kaplan S.A., Chung D.E., Lee R.K., Scofield S., Te A.E. A 5-year retrospective analysis of 5alpha-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. Int. J. Clin. Pract. 2012;66:1052–1055. doi: 10.1111/j.1742-1241.2012.03010.x
- Diviccaro S, Melcangi RC, Giatti S. Post-finasteride syndrome: An emerging clinical problem. Neurobiol Stress. 2019 Dec 26;12:100209. doi: 10.1016/j.ynstr.2019.100209
- Traish A.M. The post-finasteride syndrome: clinical manifestation of drug-induced epigenetics due to endocrine disruption. Current Sexual Health Reports. 2018; 10: 88-103
- Traish AM. Post-finasteride syndrome: a surmountable challenge for clinicians. Fertil Steril. 2020 Jan;113(1):21-50. https://www.fertstert.org/article/S0015-0282(19)32599-3/fulltext
- Tu H.Y., Zini A. Finasteride-induced secondary infertility associated with sperm DNA damage. Fertil Steril. 2011; 95: 2125.e13-2125.e14
- Chiba K., Yamaguchi K., Li F., Ando M., Fujisawa M. Finasteride-associated male infertility. Fertil Steril. 2011; 95: 1786.e9-1786.e11
- Wong A.C., Mak S.T. Finasteride-associated cataract and intraoperative floppy-iris syndrome. J Cataract Refract Surg. 2011; 37: 1351-1354
- Santo Domingo D., Stevenson M.L., Auerbach J., Lerman J. Finasteride-induced pseudoporphyria. Arch Dermatol. 2011; 147: 747-748
- Tresch S, Cozzio A, Kamarashev J, Harr T, Schmid-Grendelmeier P, French LE, Feldmeyer L. T cell-mediated acute localized exanthematous pustulosis caused by finasteride. J Allergy Clin Immunol. 2012 Feb;129(2):589-94. doi: 10.1016/j.jaci.2011.07.033
- Manabe M., Tsuboi R., Itami S., Osada S.I., Amoh Y., Ito T., Inui S., Ueki R., Ohyama M., Kurata S., et al. Guidelines for the diagnosis and treatment of male-pattern and female-pattern hair loss, 2017 version. J. Dermatol. 2018;45:1031–1043. doi: 10.1111/1346-8138.14470
- Proaño B, Casani-Cubel J, Benlloch M, Rodriguez-Mateos A, Navarro-Illana E, Lajara-Romance JM, de la Rubia Ortí JE. Is Dutasteride a Therapeutic Alternative for Amyotrophic Lateral Sclerosis? Biomedicines. 2022 Aug 25;10(9):2084. doi: 10.3390/biomedicines10092084
- Melcangi R.C., Garcia-Segura L.M., Mensah-Nyagan A.G. Neuroactive Steroids: State of the Art and New Perspectives. Cell. Mol. Life Sci. 2008;65:777–797. doi: 10.1007/s00018-007-7403-5
- Rhie A, Son HY, Kwak SJ, Lee S, Kim DY, Lew BL, Sim WY, Seo JS, Kwon O, Kim JI, Jo SJ. Genetic variations associated with response to dutasteride in the treatment of male subjects with androgenetic alopecia. PLoS One. 2019 Sep 16;14(9):e0222533. doi: 10.1371/journal.pone.0222533
- Arif T, Dorjay K, Adil M, Sami M. Dutasteride in Androgenetic Alopecia: An Update. Curr Clin Pharmacol. 2017;12(1):31-35. doi: 10.2174/1574884712666170310111125
- Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α‐reductase inhibitor. J Clin Endocrinol Metab. 2004;89(5):2179‐2184. 10.1210/jc.2003-030330
- Nassar GN, Leslie SW. Physiology, Testosterone. [Updated 2023 Jan 2]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK526128
- Herz-Ruelas ME, Álvarez-Villalobos NA, Millán-Alanís JM, de León-Gutiérrez H, Ocampo-Garza SS, Gómez-Flores M, Grimalt R. Efficacy of Intralesional and Oral Dutasteride in the Treatment of Androgenetic Alopecia: A Systematic Review. Skin Appendage Disord. 2020 Nov;6(6):338-345. doi: 10.1159/000510697
- Saceda-Corralo D, Rodrigues-Barata AR, Vañó-Galván S, Jaén-Olasolo P. Mesotherapy with Dutasteride in the Treatment of Androgenetic Alopecia. Int J Trichology. 2017 Jul-Sep;9(3):143-145. doi: 10.4103/ijt.ijt_73_16
- Abdallah M, El‐Zawahry KA, Besar H. Mesotherapy using dutasteride‐containing solution in male pattern hair loss: a controlled pilot study. J Pan Arab Leag Dermatol. 2009;20:137‐145.
- Moftah N, Moftah N, Abd‐Elaziz G, et al. Mesotherapy using dutasteride‐containing preparation in treatment of female pattern hair loss: photographic, morphometric and ultrustructural evaluation. J Eur Acad Dermatol Venereol. 2013;27(6):686‐693. 10.1111/j.1468-3083.2012.04535.x
- Sobhy N, Aly H, El Shafee A, El Deeb M. Evaluation of the effect of injection of dutasteride as mesotherapeutic tool in treatment of androgenetic alopecia in males. Our Derm Online. 2013;4(1):40‐45.
- Mirmirani P, Consolo M, Oyetakin-White P, Baron E, Leahy P, Karnik P. Similar response patterns to topical minoxidil foam 5% in frontal and vertex scalp of men with androgenetic alopecia: a microarray analysis. Br J Dermatol. 2015 Jun;172(6):1555-1561. doi: 10.1111/bjd.13399
- Blume-Peytavi U, Hillmann K, Dietz E, Canfield D, Garcia Bartels N. A randomized, single-blind trial of 5% minoxidil foam once daily versus 2% minoxidil solution twice daily in the treatment of androgenetic alopecia in women. J Am Acad Dermatol. 2011 Dec;65(6):1126-1134.e2. doi: 10.1016/j.jaad.2010.09.724
- Olsen EA, Weiner MS. Topical minoxidil in male pattern baldness: effects of discontinuation of treatment. J Am Acad Dermatol. 1987;17(1):97‐101. 10.1016/s0190-9622(87)70179-0
- Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012 May;6(2):130-6. doi: 10.2174/187221312800166859
- Cranwell W, Sinclair R. Male androgenetic alopecia. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. MDText.com, Inc.; 2016.
- Rossi, A., Anzalone, A., Fortuna, M.C., Caro, G., Garelli, V., Pranteda, G. and Carlesimo, M. (2016), Multi-therapies in androgenetic alopecia: review and clinical experiences. Dermatologic Therapy, 29: 424-432. https://doi.org/10.1111/dth.12390
- De Villez RL. Topical minoxidil therapy in hereditary androgenetic alopecia. Arch Dermatol. 1985 Feb;121(2):197-202. doi: 10.1001/archderm.121.2.197
- Rundegren J. A one‐year observational study with minoxidil 5% solution in Germany: results of independent efficacy evaluation by physicians and patients. J Am Acad Dermatol. 2004;50(3):P91.
- Olsen EA, Whiting D, Bergfeld W, Miller J, Hordinsky M, Wanser R, Zhang P, Kohut B. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2007 Nov;57(5):767-74. doi: 10.1016/j.jaad.2007.04.012
- Olsen EA, Dunlap FE, Funicella T, Koperski JA, Swinehart JM, Tschen EH, Trancik RJ. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002 Sep;47(3):377-85. doi: 10.1067/mjd.2002.124088
- R.S. Berger, J.L. Fu, K.A. Smiles, C.B. Turner, B.M. Schnell, K.M. Werchowski, K.M. Lammers, The effects of minoxidil, 1% pyrithione zinc and a combination of both on hair density: a randomized controlled trial, British Journal of Dermatology, Volume 149, Issue 2, 1 August 2003, Pages 354–362, https://doi.org/10.1046/j.1365-2133.2003.05435.x
- Ebner H, Müller E. Allergic contact dermatitis from minoxidil. Contact Dermat. 1995;32(5):316‐317. 10.1111/j.1600-0536.1995.tb00798.x
- Sinclair, R.D. (2018), Female pattern hair loss: a pilot study investigating combination therapy with low-dose oral minoxidil and spironolactone. Int J Dermatol, 57: 104-109. https://doi.org/10.1111/ijd.13838
- Beach RA. Case series of oral minoxidil for androgenetic and traction alopecia: Tolerability & the five C’s of oral therapy. Dermatol Ther. 2018 Nov;31(6):e12707. doi: 10.1111/dth.12707
- Vastarella, M, Cantelli, M, Patrì, A, Annunziata, MC, Nappa, P, Fabbrocini, G. Efficacy and safety of oral minoxidil in female androgenetic alopecia. Dermatologic Therapy. 2020; 33:e14234. https://doi.org/10.1111/dth.14234
- Randolph M, Tosti A. Oral minoxidil treatment for hair loss: a review of efficacy and safety. J Am Acad Dermatol. 2020;84(3):737‐746. 10.1016/j.jaad.2020.06.1009
- Ramos PM, Sinclair RD, Kasprzak M, Miot HA. Minoxidil 1 mg oral versus minoxidil 5% topical solution for the treatment of female-pattern hair loss: A randomized clinical trial. J Am Acad Dermatol. 2020 Jan;82(1):252-253. doi: 10.1016/j.jaad.2019.08.060
- Panchaprateep R, Lueangarun S. Efficacy and Safety of Oral Minoxidil 5 mg Once Daily in the Treatment of Male Patients with Androgenetic Alopecia: An Open-Label and Global Photographic Assessment. Dermatol Ther (Heidelb). 2020 Dec;10(6):1345-1357. doi: 10.1007/s13555-020-00448-x
- Pirmez R, Salas-Callo CI. Very-low-dose oral minoxidil in male androgenetic alopecia: A study with quantitative trichoscopic documentation. J Am Acad Dermatol. 2020 Jan;82(1):e21-e22. doi: 10.1016/j.jaad.2019.08.084
- Vañó-Galván S, Pirmez R, Hermosa-Gelbard A, Moreno-Arrones ÓM, Saceda-Corralo D, Rodrigues-Barata R, Jimenez-Cauhe J, Koh WL, Poa JE, Jerjen R, Trindade de Carvalho L, John JM, Salas-Callo CI, Vincenzi C, Yin L, Lo-Sicco K, Waskiel-Burnat A, Starace M, Zamorano JL, Jaén-Olasolo P, Piraccini BM, Rudnicka L, Shapiro J, Tosti A, Sinclair R, Bhoyrul B. Safety of low-dose oral minoxidil for hair loss: A multicenter study of 1404 patients. J Am Acad Dermatol. 2021 Jun;84(6):1644-1651. doi: 10.1016/j.jaad.2021.02.054
- Murphrey MB, Agarwal S, Zito PM. Anatomy, Hair. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513312
- Koyama T, Kobayashi K, Hama T, Murakami K, Ogawa R. Standardized Scalp Massage Results in Increased Hair Thickness by Inducing Stretching Forces to Dermal Papilla Cells in the Subcutaneous Tissue. Eplasty. 2016 Jan 25;16:e8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4740347
- English R.S., Barazesh J.M. Self-Assessments of Standardized Scalp Massages for Androgenic Alopecia: Survey Results. Dermatol. Ther. 2019;9:167–178. doi: 10.1007/s13555-019-0281-6
- Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013 Mar;32(1):41-52. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4126803
- Avci P, Gupta GK, Clark J, Wikonkal N, Hamblin MR. Low-level laser (light) therapy (LLLT) for treatment of hair loss. Lasers Surg Med. 2014 Feb;46(2):144-51. doi: 10.1002/lsm.22170
- Liu KH, Liu D, Chen YT, Chin SY. Comparative effectiveness of low-level laser therapy for adult androgenic alopecia: a system review and meta-analysis of randomized controlled trials. Lasers Med Sci. 2019 Aug;34(6):1063-1069. doi: 10.1007/s10103-019-02723-6
- Johnstone M.A., Albert D.M. Prostaglandin-induced hair growth. Surv. Ophthalmol. 2002;47:S185–S202. doi: 10.1016/S0039-6257(02)00307-7
- Blume-Peytavi U., Lönnfors S., Hillmann K., Bartels N.G. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J. Am. Acad. Dermatol. 2012;66:794–800. doi: 10.1016/j.jaad.2011.05.026
- Khidhir K.G., Woodward D.F., Farjo N.P., Farjo B.K., Tang E.S., Wang J.W., Picksley S.M., Randall V. The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013;27:557–567. doi: 10.1096/fj.12-218156
- Garza L.A., Liu Y., Yang Z., Alagesan B., Lawson J.A., Norberg S.M., Loy D.E., Zhao T., Blatt H.B., Stanton D.C., et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012;4:126ra34. doi: 10.1126/scitranslmed.3003122
- Lin W., Xiang L.-J., Shi H.-X., Zhang J., Jiang L., Cai P., Lin Z.-L., Lin B.-B., Huang Y., Zhang H.-L., et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. BioMed Res. Int. 2015;2015:730139. doi: 10.1155/2015/730139
- Pavlovic V., Ciric M., Jovanovic V., Stojanovic P. Platelet Rich Plasma: A short overview of certain bioactive components. Open Med. 2016;11:242–247. doi: 10.1515/med-2016-0048
- Stevens J., Khetarpal S. Platelet-rich plasma for androgenetic alopecia: A review of the literature and proposed treatment protocol. Int. J. Womens Dermatol. 2018;5:46–51. doi: 10.1016/j.ijwd.2018.08.004
- Giordano S., Romeo M., Lankinen P. Platelet-rich plasma for androgenetic alopecia: Does it work? Evidence from meta analysis. J. Cosmet. Dermatol. 2017;16:374–381. doi: 10.1111/jocd.12331
- Mao G., Zhang G., Fan W. Platelet-rich plasma for treating androgenic alopecia: A systematic review. Aesthetic Plast. Surg. 2019;43:1326–1336. doi: 10.1007/s00266-019-01391-9
- Dervishi G., Liu H., Peternel S., Labeit A., Peinemann F. Autologous platelet-rich plasma therapy for pattern hair loss: A systematic review. J. Cosmet. Dermatol. 2020;19:827–835. doi: 10.1111/jocd.13113
- Gupta A.K., Renaud H.J., Bamimore M. Platelet-rich plasma for androgenetic alopecia: Efficacy differences between men and women. Dermatol. Ther. 2020;33:e14143. doi: 10.1111/dth.14143
- Kuffler D.P. Variables affecting the potential efficacy of PRP in providing chronic pain relief. J. Pain Res. 2019;12:109–116. doi: 10.2147/JPR.S190065
- Shah KB, Shah AN, Solanki RB, Raval RC. A Comparative Study of Microneedling with Platelet-rich Plasma Plus Topical Minoxidil (5%) and Topical Minoxidil (5%) Alone in Androgenetic Alopecia. Int J Trichology. 2017 Jan-Mar;9(1):14-18. doi: 10.4103/ijt.ijt_75_16
- Fischer, T.W., Scholz, G., Knöll, B., Hipler, U.-C. and Elsner, P. (2001), Melatonin reduces UV-induced reactive oxygen species in a dose-dependent manner in IL-3-stimulated leukocytes. Journal of Pineal Research, 31: 39-45. https://doi.org/10.1034/j.1600-079X.2001.310106.x
- Singh M, Jadhav HR. Melatonin: functions and ligands. Drug Discov Today. 2014 Sep;19(9):1410-8. doi: 10.1016/j.drudis.2014.04.014
- Fischer TW, Trüeb RM, Hänggi G, Innocenti M, Elsner P. Topical melatonin for treatment of androgenetic alopecia. Int J Trichology. 2012 Oct;4(4):236-45. doi: 10.4103/0974-7753.111199