Microsporidia
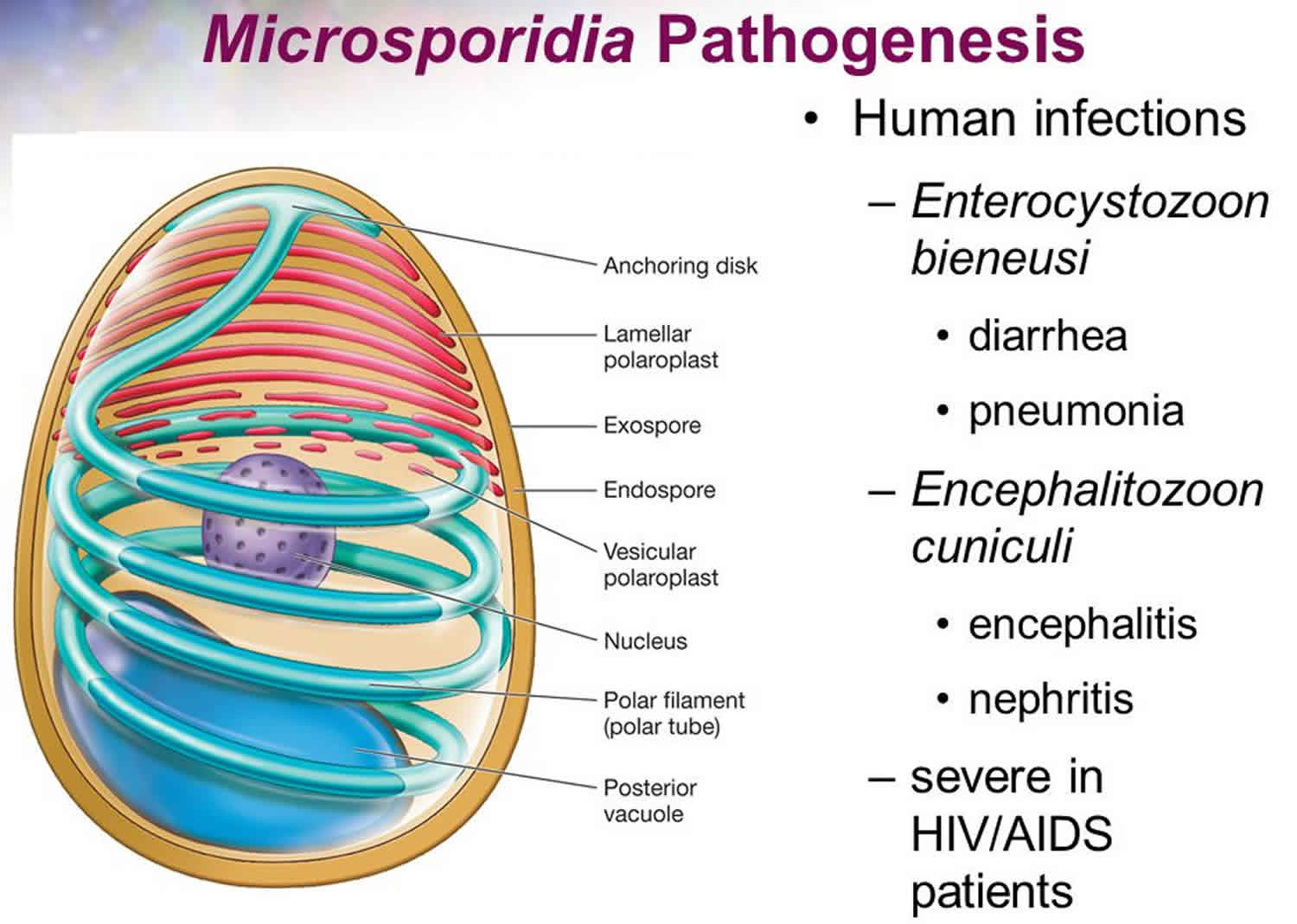
Microsporidia are an unusually large group of unique, eukaryotic, obligate, intracellular parasites closely related to fungi, although the nature of the relation to the kingdom Fungi is not clear 1. The taxonomic position of this group has been debated and revised repeatedly; historically, they were considered protozoa and often remain managed by diagnostic parasitology laboratories. Microsporidia are characterized by the production of resistant spores that vary in size (usually 1—4 µm for medically-important species). Microsporidia possess a unique organelle, the polar tubule or polar filament, which is coiled inside the spore as demonstrated by its ultrastructure. Microsporidia also possess degenerated mitochondria called mitosomes and lack a conventional Golgi apparatus.
Microsporidia are capable of infecting many species of animals. In humans, they can infect many organs, including the eye. They are widely distributed around the world and are considered an emerging cause of infectious disease, especially in immunocompromised patient populations.
Microsporidia are well-adapted pathogens and important agricultural parasites that infect honeybees, silkworms, and other insects 2. Microsporidia is also a parasite for fish, rodents, rabbits, primates, and humans. First identified in HIV-1 infection in the setting of chronic diarrhea, microsporidia has since been discovered to cause a variety of systemic and ocular infections in both immunocompromised and immunocompetent individuals. Microsporidia are now recognized as important pathogens of AIDS patients 3. However the implementation of effective anti-retroviral therapies has reduced the incidence in this group considerably.
Microsporidia are ubiquitous in the environment; they have evolved in a unique and sophisticated manner so that they not only survive in the surrounding environment but are also able to live inside other cells 4.
Classification of Microsporidia is based on microscopic morphology and ultrastructural spores characteristics. The host range in many cases is incongruent with the phylogenetic relationship that is further revealed by molecular methods through rRNA genetic sequencing which is currently used as the principle way of defining taxonomy 5.
To date, more than 1500 species belonging to over 200 genera have been described as parasites infecting a wide range of vertebrate and invertebrate hosts. There are at least 15 microsporidian species that have been identified as human pathogens; the vast majority of cases being caused by Enterocytozoon bieneusi, followed by some Encephalitozoon species (Encephalitozoon cuniculi, Encephalitozoon hellem, Encephalitozoon intestinalis (=Septata intestinalis)). Other less frequently reported agents include members of the genera Anncaliia (=Brachiola) (Anncaliia algerae, Anncaliia connori, Anncaliia vesicularum), Microsporidium (Microsporidium ceylonensis, Microsporidium africanum), Trachipleistophora (Trachipleistophora hominis, Trachipleistophora anthropophthera), Nosema ocularum, Pleistophora ronneafiei, Vittaforma corneae (=Nosema corneae), Tubulinosema acridophagus, and an unknown species likely belonging to Endoreticulatus 1.
Many domestic and wild animals may be naturally infected with various medically-important microsporidia. Enterocytozoon bieneusi is generally considered a human parasite, but has been detected in swine, primates, cattle, cats, dogs, and several other mammals. Some, but not all, of these animal-derived strains appear to represent zoonotic genotypes.
Encephalitozoon cuniculi is endemic in several captive and wild rabbit populations. It has also occasionally been found in domestic dogs, cats, foxes, captive monkeys, and mink. Birds, especially psittacines (parrots, parakeets, love birds, budgerigars, etc.), may represent reservoirs for Encephalitozoon hellem. Unlike the other two important members of the genus, Encephalitozoon intestinalis is only rarely identified in animals other than humans.
The host range of the other microsporidia known to infect humans is not as well known. No animal reservoir has been identified for Vittaforma cornea. Pleistophora spp. are found in fish and reptiles, but spore morphology in these species is inconsistent with that of the species implicated in human infections (Pleistophora ronneafiei). Tubulinosema acridophagus, Trachipleistophora spp., and Anncaliia algerae are related to known insect parasites, however, the significance of insects in transmission is unclear.
Microsporidia in humans
The clinical manifestations of microsporidiosis are very diverse, varying according to the causal species and route of infection. Disseminated infection can be fatal. Of all of the manifestations of microsporidiosis, Enterocytozoon bieneusi-associated diarrhea is the most common. Table 1 summarizes the typical sites of infection for various microsporidia species. Microsporidia infection in humans occurs worldwide with the highest prevalence in HIV infected individuals with diarrhea and less than 100 CD4+ T cells per mm³ blood. The majority of AIDS associated microsporidial infections are associated with Enterocytozoon bieneusi, which is linked too chronic diarrhea and wasting. Less than 50 CD4+ T cells per mm³ blood is associated with both Enterocytozoon bieneusi or Enterocytozoon intestinalis infections 6.
In humans, infection with this opportunistic pathogen was not highly recognized at the beginning of the century. In 1924, researchers first suggested that Microsporidia infected humans, and until 1985, only a few reports of human microsporidiosis were published. After this, Microsporidia became increasingly recognized worldwide as opportunistic infectious agents, and presently, Microsporidia are recognized as emerging organisms in many areas of both developed and developing countries 7. Microsporidia are widely distributed worldwide among children, travelers, organ recipients, elderly, patients with malignant disease and diabetes, and HIV patients 7. In developed countries, the occurrence of microsporidiosis in HIV patients has gradually decreased due to anti-retroviral therapy and better hygiene.
Among the nearly 1500 species described, only 17 are pathogenic to human, and some of them include Enterocytozoon bieneusi, Encephalitozoon, Anncaliia, Enterocytozoon, Tubulinosema, Microsporidium africanum, and Trachipleistophora hominis. In the majority of cases, Enterocytozoon bieneusi and Encephalitozoon intestinalis have been the 2 species detected most often in infected humans 8.
In developed countries, the prevalence rates for Microsporidia infection in HIV-seropositive persons with diarrhea range from 2% to 78% varying by degree of immunosuppression and treatment. In HIV-seropositive persons without diarrhea, infections range from 1.4% to 4.3% 9. In individuals not infected with HIV, the seroprevalence rates range from 1.3% to 22% among blood donors, pregnant women, slaughterhouse workers, and persons with unknown causes of diarrhea (possibly caused by Microsporidia infection) 10.
Table 1. Microsporidia species
| Microsporidia Species | Known sites of localization |
|---|---|
| Anncaliia algerae | Eyes, muscle |
| Anncaliia connori | Systemic |
| Anncaliia vesicularum | Muscle |
| Encephalitozoon cuniculi | Systemic |
| Encephalitozoon hellem | Eyes |
| Encephalitozoon intestinalis | Small intestine |
| Enterocytozoon bieneusi | Small intestine, biliary tree |
| Microsporidium spp. | Eyes |
| Nosema ocularum | Eyes |
| Pleistophora ronneafiei | Muscle |
| Trachipleistophora anthropopthera | Systemic |
| Trachipleistophora hominis | Eyes, muscle |
| Tubulinosema acridophagus | Systemic |
| Vittaforma corneae | Eyes |
Microsporidia transmission
Microsporidia disease transmission occurs mostly through food (foodborne disease) including the global food chain industry of fish and crustaceans (shrimp, lobster, clams, among others). Transmission also occurs through water including crop irrigation, seawater, drinking water, groundwater, wastewater, excreta in the environment, and sludge 12. Vertical transmission from mother to offspring has also been observed in rabbit, sheep, and non-human primates. Zoonotic transmission through animals acting as reservoirs has also been noted in some studies 13. Although rare, the transmission through fecal-oral and aerosols may also occur in human infection cases 14.
Risk factors for microsporidia infection
Risk factors associated with microsporidia infection include male-male sexual encounters, intravenous drug use, exposure to swamp water or crop irrigation area, exposure to water with excreta, swimming pool and hot tubs use, or occupational contact with water 10.
Microsporidia life cycle
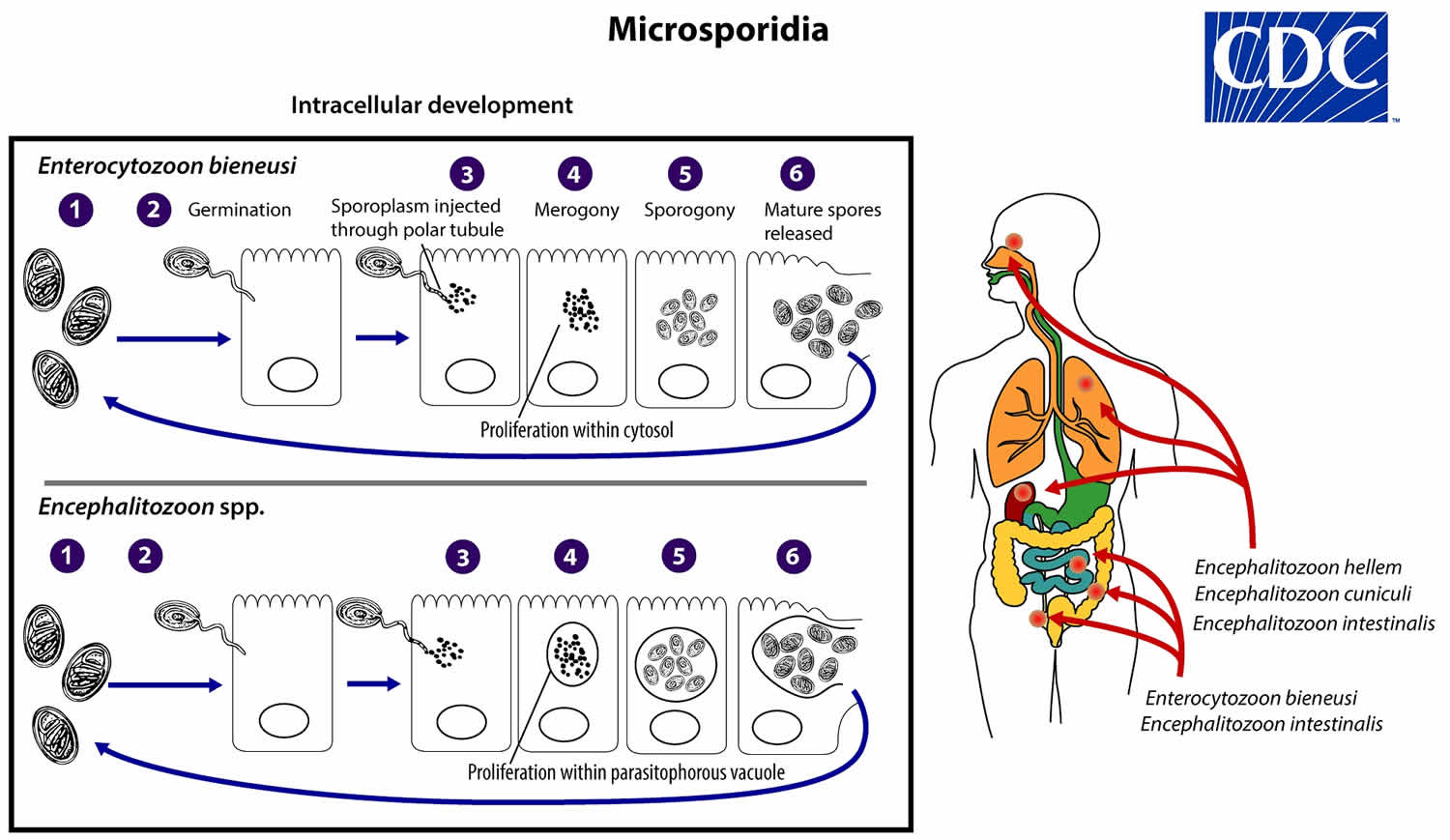
The life cycle of Microsporidia consists of 2 distinct stages which are merogony or the proliferating stage, and sporogony or infecting or mature stage 2. The infective form of microsporidia is the resistant spore, which can persist in the environment for months (number #1). The spore then germinates, rapidly everting its polar tubule which contacts the eukaryotic host cell membrane (number #2). The spore then injects the infective sporoplasm into the host cell through the polar tubule (number #3). Inside the cell, the sporoplasm enters the proliferative phase marked by extensive multiplication via merogony (binary fission or multiple fission), creating meronts (number #4). The location of this developmental stage within the host cell varies by genus; it can occur either in direct contact with the host cell cytosol (Enterocytozoon, Nosema), inside a parasitophorous vacuole of unknown origin (Encephalitozoon), in a parasite-secreted envelope (Pleistophora, Trachipleistophora), or surrounded by the host cell endoplasmic reticulum (Endoreticulatus, Vittaforma) (number #5). Following the proliferative phase, meronts undergo sporogony in which the thick spore wall and invasion apparatus develop, creating sporonts and eventually mature spores when all organelles are polarized. When the spores increase in number and completely fill the host cell cytoplasm, the cell membrane is disrupted and spores are released to the surroundings (number #6). These free mature spores can infect new cells thus continuing the cycle.
Mature spores of intestinal-localizing species may be shed in feces, although the route of transmission remains uncertain for many species. Exposure to spores in water or in soil appears to be a potentially major route, based on the finding of spores in these sources along with case histories. Enterocytozoon bieneusi and Vittaforma corneae (syn. Nosema corneum) have been identified in surface waters, and spores of Nosema sp. (and likely Anncaliia algerae) have been identified in ditch water. Cases of donor-derived microsporidiosis (Encephalitozoon cuniculi) following bone marrow, kidney, liver, and heart transplantation have been confirmed.
Figure 1. Microsporidia life cycle
Microsporidia prevention
Like other pathogen and infection control, Microsporidia should be managed and prevented with improved hygiene. The improved regulation of water sources, as well as monitoring of excrement in soil and water, are important to lessen waterborne disease transmission. The tight regulation and hygiene improvement procedure in food chain production or industry, including breeding, processing, storing and packaging to control food contamination and pathogen spread, are an crucial.
Microsporidia symptoms
The clinical manifestation of microsporidia infection ranges from asymptomatic infection to symptomatic infection that includes diarrhea, myositis, keratitis, and bronchitis. Although rare, encephalitis may also occur 15. The median incubation time of the disease during foodborne transmission is between the date of the symptoms and onset of illness is about 7 days (range 3 to 15 days). In HIV-infected patients, microsporidia infection is recognized as an increasingly important cause of morbidity and is responsible for significant gastrointestinal (GI) and disseminated disease. On rare occasions, E. bieneusi causes pulmonary infections or infects the bile ducts leading to cholecystitis and cholangitis 10. Reports of ocular infections with microsporidia are rare but are more common in immunocompromised than in immunocompetent persons.
It remains unknown how much of the spore titer is required for infection in humans, but it appears to vary among the different subspecies. The spores that infect humans are usually 1 to 4 micrometers, and children with an asymptomatic picture appear to have 1.2 x 10 spores of Enterocytozoon bieneusi per gram of feces. Whereas, in HIV-infected patients, the concentration of Enterocytozoon bieneusi spores vary from 4.5 x 10 to 4.4 x 10 per milliliter of diarrheic feces, totaling 10 spores in 24 hours.
Table 2. Named human microsporidial pathogens and their clinical syndromes
| Microsporidia genus and species | Clinical syndromes |
|---|---|
| Pleistophora species | Myositis |
| Nosema connori | Disseminated infection |
| Nosema ocularum | Keratitis |
| Vittaforma corneae | Keratitis |
| Encephalitozoon cuniculi | Peritonitis, fulminant hepatitis, seizures, rhinosinusitis |
| Encephalitozoon hellem | Conjunctivitis, keratoconjunctivitis, bronchiolitis, pneumonia, rhinosinusitis, disseminated infection |
| Encephalitozoon intestinalis | Diarrhea, disseminated infection |
| Enterocytozoon bieneusi | Diarrhea, wasting syndrome, cholecystitis, cholangitis, bronchitis, pneumonia |
Microsporidia diagnosis
Microsporidia are difficult to detect because of their small size, their slowly infecting properties, and sometimes asymptomatic infection. Therefore, there are many undetermined cases of diarrhea worldwide. The organism needs to be visualized using special techniques involving special laboratory facilities and trained personnel to characterize the infection.
Detection of Microsporidia is based on stool examination through microscopic observation. However, the new staining methods including trichrome stain and fluorescence staining using optical brighteners can be useful. Microsporidia can be stained with Gram stain, carbol-fuchsin stain, and with silver stains 15.
Microscopy
Light microscopic examination of the stained clinical smears, especially the fecal samples, is an inexpensive method of diagnosing microsporidial infections even though it does not allow identification of microsporidia to the species level. The most widely used staining technique is the Chromotrope 2R method or its modifications. This technique stains the spore and the spore wall a bright pinkish red. Often, a darker-staining equatorial band is seen in the middle of the spore. This technique, however, is lengthy and time consuming and requires about 90 min. A recently developed “Quick-Hot Gram Chromotrope technique” however, cuts down the staining time to less than 10 min and provides a good differentiation from the lightly stained background fecal materials so that the spores stand out for easy visualization. The spores stain dark violet and the equatorial band is enhanced. In some cases dark staining Gram positive granules are also clearly seen. Chemofluorescent agents such as Calcofluor white are also useful in the quick identification of spores in fecal smears. The spores measure from 0.8 to 1.4 µm in the case of Enterocytozoon bieneusi, and 1.5 to 4 µm in Anncaliia algerae, Encephalitozoon spp., Vittaforma corneae, and Nosema spp.
Transmission electron microscopy is still the gold standard and is necessary for the identification of the microsporidian species, which is based on internal features of the spore such as the number of polar tubule coils. However, transmission electron microscopy is expensive, time consuming, and not feasible for routine diagnosis.
Immunofluorescence Assays
Immunofluorescence Assays (IFA) are available for microsporidia using monoclonal and/or polyclonal antibodies. However, serological tests are unreliable in persons with HIV due to immune deficiency 9.
Molecular Methods (PCR)
The Centers for Disease Control and Prevention (CDC) offers molecular identification of Enterocytozoon bieneusi, Encephalitozoon intestinalis, Encephalitozoon hellem and Encephalitozoon cuniculi using species-specific polymerase chain reaction (PCR) assays. Molecular identification of other microsporidia species can be attempted using genera-specific primers and sequencing analysis on a case-by-case basis.
The molecular characterization including polymerase chain reaction (PCR) and nucleotide sequencing are promising highly sensitive assays with a sensitivity of more than 90% for a quick characterization of human-infected microsporidia. The array can simultaneously detect all 4 species of Microsporidia at a sensitivity of 10 spores per 100 microliters of fecal sample 15. Within hours, the clinician can interpret the result by isolating genetic/nucleic material of Microsporidia and amplifying the certain sequence enzymatically in thermal cyclic condition and then compare it to the control. To determine the genotype of microsporidia in the infecting host, the rRNA sequence is a gold standard for further characterization.
Microsporidia treatment
Frequently administered drugs for treating Microsporidia infection in animal and humans include albendazole and fumagillin. In the past, organic mercury compounds like Nosemack were also tested. However, Nosemack was less effective against the parasite and more toxic to agricultural bees than fumagillin. Fumagilin has amoebicidal properties and in vivo studies show that fumagillin inhibits Nosema apis development in the honeybee. Albendazole is effective and efficient against Encephalitozoon species infecting humans and animals but has variable effectiveness against Enterocytozoon bieneusi. Albendazole inhibits the tubulin polymerization and is also used as an anthelmintic and an anti-fungal agent 10.
In patients who are immunocompromised due to HIV, reconstitution of their immune status through antiretroviral medications has been associated with resolution of clinical symptoms 17. Aspartyl protease inhibitors of HIV have also been documented to directly inhibit the growth of Encephalitozoon intestinalis in tissue culture 18. Albendazole, which inhibits microtubule assembly, is also affective against several microsporidia, including Encephalitozoon spp. in systemic disease 19. Systemic antifungals have also been used to manage microsporidal infections in AIDS patients 20.
Fumagillin, an antibiotic and antiangiogenic agent derived from Aspergillus fumigatus, has been found to be effective against Encephalitozoon sp. and Enterocytozoon bieneusi. While toxic in systemic administration, topical use has been shown to be well tolerated and effective in treating keratoconjunctivitis 21. Topical fluoroquinolones (ciprofloxacin 0.3%, moxifloxacin 0.5%, gatifloxacin 0.5%, levofloxacin 0.5%, and norfloxacin 0.3%) as monotherapy or in combination with topical fumagillin and/or systemic albendazole have been studied for the treatment of microsporidial keratitis. Resolution occurred in 99% of cases with topical fluoroquinolone monotherapy 22.
References- Microsporidiosis. https://www.cdc.gov/dpdx/microsporidiosis/
- Faisal AF, Bokhari AA. Microsporidium. [Updated 2018 Dec 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2019 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK537166
- Fedorko, D. P., & Hijazi, Y. M. (1996). Application of Molecular Techniques to the Diagnosis of Microsporidial Infection. Emerging Infectious Diseases, 2(3), 183-191. https://dx.doi.org/10.3201/eid0203.960304
- Keeling PJ, Fast NM. Microsporidia: biology and evolution of highly reduced intracellular parasites. Annu. Rev. Microbiol. 2002;56:93-116.
- Adl SM, Simpson AG, Lane CE, Lukeš J, Bass D, Bowser SS, Brown MW, Burki F, Dunthorn M, Hampl V, Heiss A, Hoppenrath M, Lara E, Le Gall L, Lynn DH, McManus H, Mitchell EA, Mozley-Stanridge SE, Parfrey LW, Pawlowski J, Rueckert S, Shadwick L, Shadwick L, Schoch CL, Smirnov A, Spiegel FW. The revised classification of eukaryotes. J. Eukaryot. Microbiol. 2012 Sep;59(5):429-93.
- Morpeth, Susan C., and Nathan M. Thielman. “Diarrhea in Patients with AIDS.” Current Treatment Options in Gastroenterology, vol. 9, no. 1, 2006, pp. 23–37., doi:10.1007/s11938-006-0021-8.
- Didier ES, Weiss LM. Microsporidiosis: not just in AIDS patients. Curr. Opin. Infect. Dis. 2011 Oct;24(5):490-5.
- Lobo ML, Xiao L, Antunes F, Matos O. Microsporidia as emerging pathogens and the implication for public health: a 10-year study on HIV-positive and -negative patients. Int. J. Parasitol. 2012 Feb;42(2):197-205.
- Didier ES, Stovall ME, Green LC, Brindley PJ, Sestak K, Didier PJ. Epidemiology of microsporidiosis: sources and modes of transmission. Vet. Parasitol. 2004 Dec 09;126(1-2):145-66.
- Didier ES. Microsporidiosis: an emerging and opportunistic infection in humans and animals. Acta Trop. 2005 Apr;94(1):61-76.
- Han, B. and Weiss, L.M., 2017. Microsporidia: obligate intracellular pathogens within the fungal kingdom. Microbiology Spectrum, 5 (2).
- Stentiford GD, Becnel -J, Weiss LM, Keeling PJ, Didier ES, Williams BP, Bjornson S, Kent ML, Freeman MA, Brown MJF, Troemel ER, Roesel K, Sokolova Y, Snowden KF, Solter L. Microsporidia – Emergent Pathogens in the Global Food Chain. Trends Parasitol. 2016 Apr;32(4):336-348.
- Fiuza VRDS, Lopes CWG, Cosendey RIJ, de Oliveira FCR, Fayer R, Santín M. Zoonotic Enterocytozoon bieneusi genotypes found in brazilian sheep. Res. Vet. Sci. 2016 Aug;107:196-201.
- Didier PJ, Phillips JN, Kuebler DJ, Nasr M, Brindley PJ, Stovall ME, Bowers LC, Didier ES. Antimicrosporidial activities of fumagillin, TNP-470, ovalicin, and ovalicin derivatives in vitro and in vivo. Antimicrob. Agents Chemother. 2006 Jun;50(6):2146-55.
- Ghosh K, Weiss LM. Molecular diagnostic tests for microsporidia. Interdiscip Perspect Infect Dis. 2009;2009:926521.
- Weber R, Bryan RT, Schwartz DA, Owen RL. Human microsporidial infections. Clin Microbiol Rev. 1994;7(4):426–461. doi:10.1128/cmr.7.4.426 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC358336
- Didier, Elizabeth S, and Louis M Weiss. “Microsporidiosis: Current Status.” Current Opinion in Infectious Diseases, vol. 19, no. 5, 2006, pp. 485–492., doi:10.1097/01.qco.0000244055.46382.23.
- Menotti, J., et al. “Inhibitory Activity of Human Immunodeficiency Virus Aspartyl Protease Inhibitors against Encephalitozoon Intestinalis Evaluated by Cell Culture-Quantitative PCR Assay.” Antimicrobial Agents and Chemotherapy, vol. 49, no. 6, 2005, pp. 2362–2366., doi:10.1128/aac.49.6.2362-2366.2005.
- Tremoulet, Adriana H., et al. “Albendazole Therapy for Microsporidium Diarrhea in Immunocompetent Costa Rican Children.” The Pediatric Infectious Disease Journal, vol. 23, no. 10, 2004, pp. 915–918., doi:10.1097/01.inf.0000141724.06556.f9.
- Yee, Richard W., et al. “Resolution of Microsporidial Epithelial Keratopathy in a Patient with AIDS.” Ophthalmology, vol. 98, no. 2, 1991, pp. 196–201., doi:10.1016/s0161-6420(91)32331-5.
- Champion, L., et al. “Fumagillin for Treatment of Intestinal Microsporidiosis in Renal Transplant Recipients.” American Journal of Transplantation, vol. 10, no. 8, 2010, pp. 1925–1930., doi:10.1111/j.1600-6143.2010.03166.x.
- Loh, Raymond S., et al. “Emerging Prevalence of Microsporidial Keratitis in Singapore.” Ophthalmology, vol. 116, no. 12, 2009, pp. 2348–2353., doi:10.1016/j.ophtha.2009.05.004.