What is Moringa oleifera
Moringa Oleifera also known as the moringa tree, the drumstick tree or the horseradish tree, is a small tree that is native to the foothills of the mighty Himalayan mountains in the northern part of India. For thousands of years, this small tree has been cultivated by different cultures throughout India and Africa because of its health benefits. However, it’s important to note that not all of the Moringa oleifera plant is safe, as toxic compounds have been found and that there are parts that can eventually harm people’s health 1. The most toxic part of Moringa oleifera is the bark and the root, and the safest part is the leaves; it is important to test their toxicity 2. Moringa oleifera leaf contains high amounts of crude protein, vitamin, mineral, and fatty acid. Moringa oleifera can provide 9 times more protein than yogurt, 17 times more calcium than milk, 7 times more vitamin C than oranges, 10 times more vitamin A than carrots, 25 times more iron than spinach, and 15 times more potassium than bananas 3. Given these excellent nutritional values, a daily intake of 10 g of Moringa oleifera leaf powder can help malnourished children to recover their body weights and enhance health indicators within a short time 4. Moreover, the consumption of Moringa oleifera leaf strengthens neural response, enhances immune functions, and improves health because of the large amounts of microelements and polyphenol antioxidants 5. Aside from promoting animal productivity and favorably influencing lipid composition, the potent antioxidant in Moringa oleifera leaf prevents meat products from deterioration 6. Studies have been carried out to evaluate the feeding effects of Moringa oleifera leaf meal on various animal species, including cattle 7, goats 8, chickens 9 and fish 10. All these studies concluded that Moringa oleifera leaf can be used as an alternative protein source for animal husbandry 11.
Beyond the uses of Moringa oleifera as a food and for human health, other possible uses exist. Moringa oleifera can be used as a natural plant growth enhancer; Moringa oleifera leaves are rich in zeatin (a plant hormone belong to the cytokinin group). Moringa oleifera leaf extracts can stimulate plant growth and increasing crop yield. Researches performed using a spray based on Moringa oleifera leaf extracts of wheat, maize and rice support the wide range of beneficial effect on crops 12. Moringa oleifera seed powder can be used for water purification, replacing dangerous and expensive chemicals such as aluminum sulfate 13. Interestingly, Moringa oleifera leaf extracts and also seed extracts show biopesticide activity, effective against larvae and adults of Trigoderma granarium and can reduce the incidence of fungi on groundnut seeds 12.
One of the interesting applications of Moringa oleifera seeds is their utilization as biomass for biodiesel production. Due to the increasing energy demand and environmental problems associated with fossil fuels, the improvement of alternative fuels and renewable sources of energy is required. Biodiesel can replace petroleum-derived oil (petrodiesel), without any sulphur or aromatic compound and with lower emission of monoxides, hydrocarbons and particulates. Furthermore, biodiesel can reduce dependence on imported fuels: a crucial problem in developing countries 14. Moringa oleifera seeds have an oil content of 30%–40%, with a high-quality fatty acid composition i.e., high oleic acid (>70%) 15. In addition they posses significant resistance to oxidative degradation. These proprieties make Moringa oil a good candidate to produce biodiesel after transesterificaton 16. Biswas and John 17, in a study conducted in Australia, report that approximately 3030 kg of oil are required to produce 1000 liters of biodiesel. Furthermore, an equivalent of 3.03 tonnes/hectare of oil seeds can be harvested from dry land, and 6.06 tonnes/hectare can be harvested from irrigated land. Since biodiesel production with Moringa oleifera seed oil is a second generation production (i.e., not in direct competition with existing farmland and with food crops) and as Moringa can grown on degraded land, studies suggest that Moringa biodiesel is an acceptable substitute to fossil fuels, even when compared against biodiesel derived from vegetable oil of other species 18.
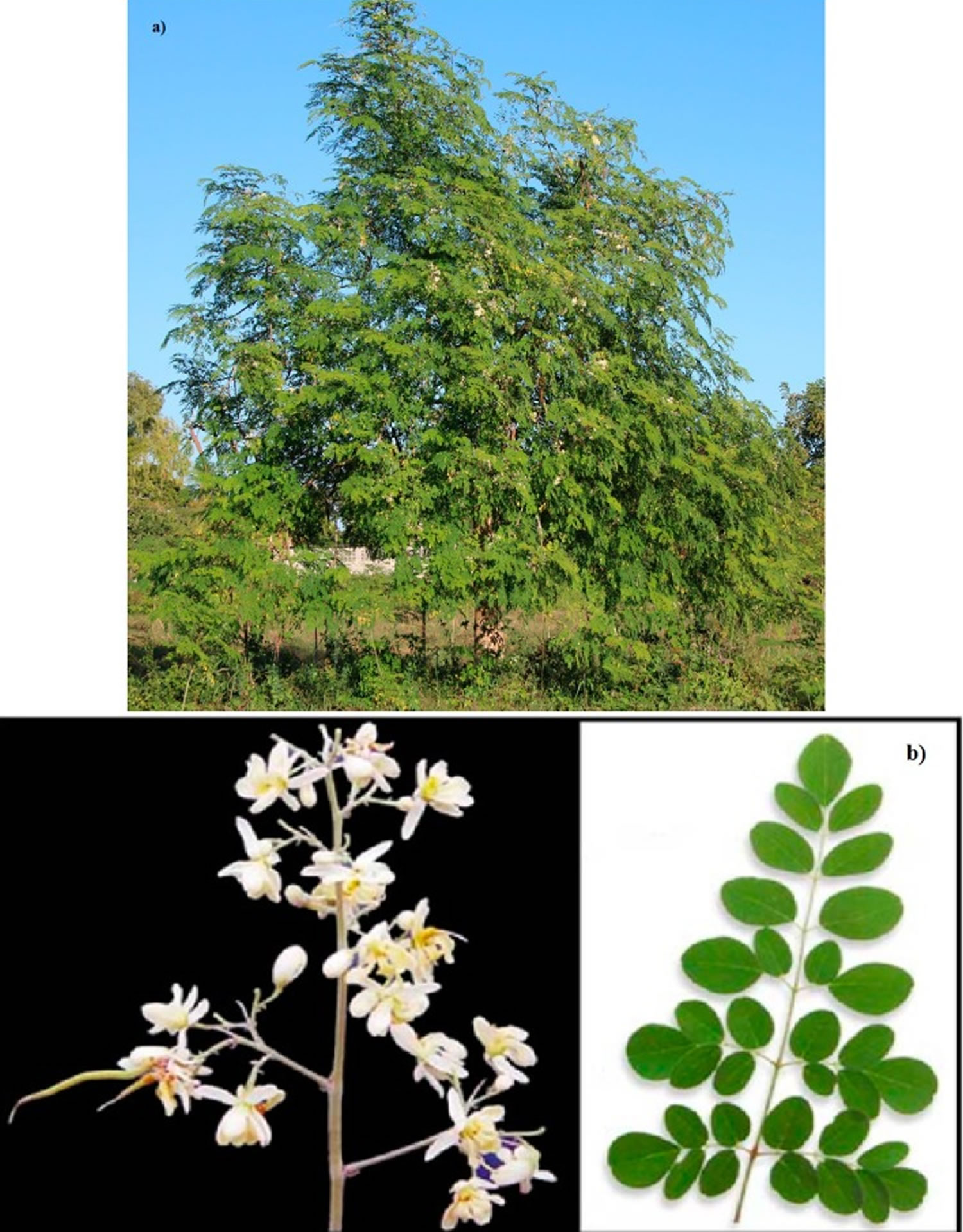
Figure 1. Moringa oleifera tree
Footnote: (a) A tree of Moringa oleifera; (b) Moringa flowers and leaves.
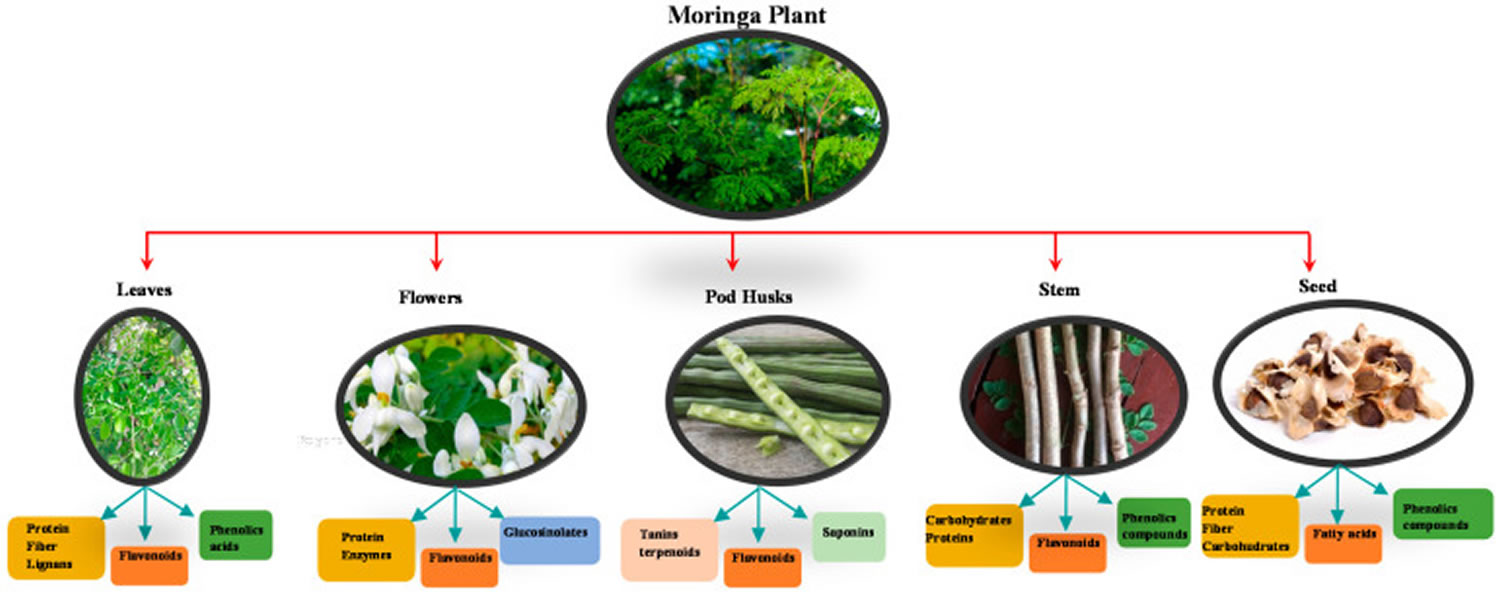
[Source 18 ]Figure 2. Moringa oleifera bioactive compounds
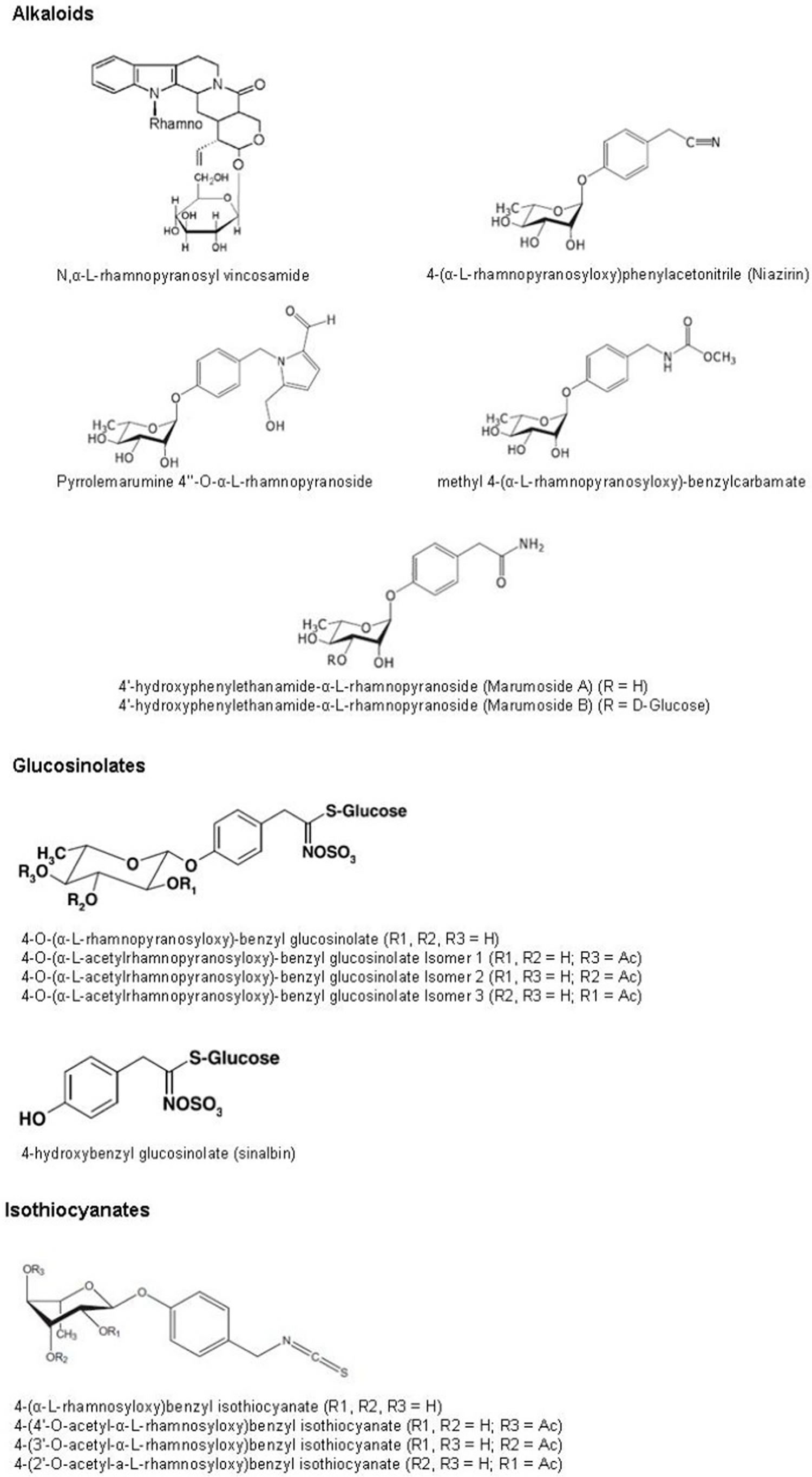
Figure 3. Moringa oleifera leaves bioactive compounds chemical structures
[Source 18 ]Moringa oleifera benefits
Moringa oleifera has several uses due to its composition. Moringa oleifera plants could be used for functional food and other industrial food applications 19. Moringa oleifera provides nutrients that benefit health, making it a key food for food security in areas with fewer economic resources 20. Moringa oleifera seed powder is used to purify water, eliminating a large amount of suspended material in rivers and turbid waters, making it a natural coagulant for water treatment. The oil from the Moringa oleifera seeds can be used as a fertiliser in plantations to encourage the growth of other species; it is also used for cosmetics such as soaps and perfumes 21 and even for the production of biodiesel 22. Moringa oleifera extracts can be used to produce zeatin effective for plant development, increasing crop yields 23. In addition to these applications, Moringa oleifera has been used in food, for example, in Mexico as an ingredient to partially replace fishmeal in tilapia feed, due to its protein and carbohydrate content 24.
Several bioactive compounds were recognized in the leaves of Moringa oleifera. They are grouped as vitamins, carotenoids, polyphenol, phenolic acids, flavonoids, alkaloids, glucosinolates, isothiocyanates, tannins, saponins and oxalates and phytates (see Figure 3 above). The amounts of different bioactive compounds found in Moringa oleifera leaves and reported in literature are summarized in Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9 and Table 10 below.
Moringa oleifera in food
The use of Moringa oleifera in food can be very beneficial; some researchers indicate that food products can be enriched with Moringa oleifera by providing vitamins, minerals, essential amino acids and oils in order to improve their nutritional value 19. Supplementation of Moringa oleifera powder in cereal porridge has been shown to improve the nutritional value by increasing the vitamin A content by up to 15 times. In the white maize variety, the protein content by fortification increased by 94% with 15% Moringa oleifera powder and for the yellow maize variety, the protein content increased by 44% 25. However, many studies have shown that products formulated with Moringa oleifera powder in high concentrations may be generally unacceptable to most consumers 25. Domenech et al. 26 reported that most Moringa oleifera is used almost exclusively in breads, biscuits and meat products, with its nutritional, technological and preservative purposes, respectively.
Moringa oleifera supplementation has a nutritional purpose, although it can provide other benefits to the product, such as improved digestibility 19, dough stability, antioxidant capacity, preservation, among other benefits associated with the plant. The use in bread and biscuits is a very useful strategy to make up for nutritional deficiencies, due to the high consumption of these foods. In addition, the food industry has tried to reduce the consumption of wheat flour in bakery products, in order to deliver foods that provide better nutritional characteristics, including reduced gluten, and for this, Moringa oleifera seems to be an option 27.
According to Ogunsina et al. 27, the incorporation of Moringa oleifera seed flour affects the organoleptic properties of different breads and biscuits; however, these differences are not significant when used in a ratio of 90% flour and 10% Moringa oleifera for bread and 80% flour and 20% Moringa oleifera for biscuits. Moreover, the taste was typical of Moringa oleifera seed, but acceptable in bread and the nutritional composition improved in both products, increasing the levels of protein, iron and calcium 27.
Chizoba et al. 28 also developed biscuits, substituting wheat flour with Moringa oleifera leaf flour; their results showed that the indicated proportion to maintain acceptable sensory characteristics and attributes is 90/10, that is to incorporate 10% of Moringa oleifera flour not exceeding 20% according to the authors 28.
The incorporation of Moringa oleifera seed meal in biscuits is reported to improve protein intake, increasing it by 45% to 90% if the addition of Moringa oleifera is 10% or 20% respectively. In the case of rice cakes, the addition of Moringa oleifera at 5% (freshly harvested) to 14% (dried) increases protein by approximately 26% 19.
Rabie et al. 29 also made biscuits fortified with Moringa oleifera leaf powder and seed powder in different concentrations, ranging from 2.5% to 7.5%. Their results found that supplementation with Moringa oleifera leaf powder has a higher amount of protein, ash, crude fiber, dietary fibre and minerals, while seed powder is characterised by a higher content of fat, protein, dietary fibre and minerals, and when mixed, a higher amount of essential amino acids was achieved, and when compared to the control, the biscuits with Moringa oleifera incorporation showed a lower carbohydrate content.
In relation to the physical characteristics, there is an increase in weight with a reduction in the volume and diameter of the biscuits, concluding that the best concentration to improve the nutritional characteristics without altering their organoleptic characteristics was 5% for the incorporation of leaf and seed powder and 2.5% + 5% in the case of mixing leaf and seed powder respectively 29.
Most of the evidence related to the incorporation of Moringa oleifera in cakes is associated with the consumption of biscuits, and in all reports, the results are similar. Supplementation increases nutritional value but affects physical characteristics, decreasing volume and colour in some instances; this was also demonstrated by a study by Nutan Narwal et al. 30.
The same would be true for the preparation of brownies and cakes with wheat flour, according to Santos et al. 31, who incorporated 5% and 10% Moringa oleifera leaf flour in chocolate brownies, indicating that the samples improved the nutritional value by increasing the ash content with a lower lipid contribution in relation to the control. A feature not mentioned in previous studies with biscuits is that the brownie showed the acidity of the product. The researchers concluded that there is no major difference between 5% and 10%, as similar results were obtained in both cases 31.
Whereas, in the wheat flour cake, the value of protein, moisture, crude fiber, total ash increased, with a reduction in lipids and carbohydrates, concluding that the cake sample with the addition of 4 g of Moringa oleifera was the most acceptable in terms of colour, flavour, aroma and overall acceptability 32.
In the case of bakery products, the use of Moringa oleifera powder in wheat flour bread dough as in other products increases the nutritional value, the protein and crude fiber content of wheat bread flour enriched with 5% Moringa oleifera powder has been found to increase from approximately 17% to 54% and 56% to 88% respectively. It should be noted that this improvement is accompanied by poor sensory properties, such as crust and crumb colour, as well as product weight and height 19.
Specifically for the case of whole wheat bread, El-Gammal et al. 33 added Moringa oleifera in different concentrations (5%, 10%, 15% and 20%); the results obtained indicated that Moringa oleifera leaf powder contained high amounts of protein and crude fibre, in addition to some essential minerals such as calcium, magnesium, phosphorus and iron. When Moringa oleifera was added to the preparation of wholemeal sliced bread, the protein content increased to 21.85%, the ash content (5.21%) and carbohydrate content decreased by 59.34%, and the intake of magnesium, calcium and iron increased compared to the control.
In relation to the undesirable effects of Moringa oleifera in bread incorporation, it negatively affects farinograph and extensometer values. Although there was an improvement in nutritional value, the acceptability of all loaf bread samples decreased with increasing levels of Moringa oleifera powder concentration, especially the loaf bread with 15 and 20%.
On the other hand, there are modifications in texture, taste, chewiness, elasticity of all samples compared to the control, however the researchers conclude that the best concentration to add to sliced bread is 5 or 10%, thus obtaining an increase in nutritional value with acceptable sensory characteristics 33.
Similar results were obtained by Bolarinwa et al. 34, who added Moringa oleifera seed powder to bakery products, increasing the protein value from 8.55 to 13.46%, ash from 0.63 to 1.76%, lipids from 7.31 to 15.75%, fibre from 0.08 to 0.62%, vitamin A from 50 to 74%, with a reduction in moisture from 22.9% to 20.01% and carbohydrates from 57.68% to 46.73%, also highlighting the increase in calcium, iron, phosphorus, and potassium in all its breads. Sensory evaluation results indicated that bread enriched with 5% Moringa oleifera seeds was not significantly different from the 100% wheat flour control 34.
Finally, while Devisetti et al. 35 evaluated the effect of Moringa oleifera leaf flour in sandwiches reaching similar conclusions, the protein content in puffed sandwiches increased, presenting 21.6 g in 100 g of product; while dietary fibre was presented at 14.8 g per 100 g of product, there was also a reduction in fat content of 3.7 g per 100 g of product with a high presence of phenolic compounds and flavonoids. In relation to the sensory characteristics of the sandwiches, an acceptable result was obtained in terms of texture 35.
Figure 4. Moringa oleifera use in bakery industry
Table 1. Moringa oleifera in bakery products and its effect on product quality
| Bakery Products | Parts Used | Moringa oleifera Application/Concentration Used | Main Results/Conclusions | Reference |
| Cookies | Moringa oleifera leaves and seeds; and a combination of both. | 2.5%, 5%, 7.5% | Moringa oleifera raised the nutritional value highlighting the amount of protein, ash, fibre and minerals. In addition, it showed an increase in weight without increasing the volume of the cookies compared to the control. | 36 |
| Bread and cookies | Moringa oleifera seed flour. | 10%, 20%, 30% | Bread with 10% and cookies with 20% of Moringa oleifera seed flour respectively had more protein, iron and calcium. | 37 |
| Cookies | Moringa oleifera leaf flour | 10%, 20%, 30%, 50% | Incorporation at 10% of Moringa oleifera leaf flour showed better sensory attributes; however, acceptability decreased as Moringa oleifera levels increased. | 38 |
| Cookies | Moringa oleifera leaf flour | 5% | Moringa oleifera supplementation at 5% of Moringa oleifera leaf flour raised the nutritional value of proteins and ash, showing a lower content of carbohydrates. | 39 |
| Cookies | Moringa oleifera leaf flour | 0%, 10%, 20%, 30%, 50% | The best acceptability in wheat flour biscuits supplemented with Moringa oleifera was shown by the concentration at 10% of Moringa oleifera leaf flour | 40 |
| Brownie (cake) | Moringa oleifera leaf powder | 0%, 5%, 10% | It improved the physicochemical characteristics; and a higher ash content and lower lipid content was found, compared to the control sample. | 41 |
| Bread | Moringa oleifera leaf powder | 5%, 10%, 15%,20% | Supplementation of Moringa oleifera leaf powder in bread raised the nutritional characteristics of proteins, ash and minerals; however, the carbohydrate content decreased. The acceptability decreases as the incorporation of Moringa oleifera increases, so the greater acceptability is at 5% and 10% of leaf powder. | 42 |
| Bread | Moringa oleifera seed powder | 0–20% | The results showed an increase in the value of proteins, minerals, ash, lipids, and fiber; however, there was a decrease in the value of carbohydrates. There were no sensory differences with the control when incorporating 5% of Moringa oleifera seed flour. | 43 |
| Snack | Moringa oleifera leaf powder | 20% | Snacks increased their nutritional value in protein and fiber, showing a low amount of fat. Furthermore, flavonoids were found in the final product. | 44 |
| Rice crackers | Moringa oleifera leaves | 1%, 2%, 5% | 1% and 2% of Moringa oleifera leaves had higher levels of beta-carotene, vitamin C, and calcium than the control. Sensory scores were comparable to control even at the end of the storage test. | 45 |
| Bread | Moringa oleifera leaf powder | 0%, 1%, 2%, 3%, 4%, 5% | The nutritional composition of proteins, ash, fiber, minerals and β-carotene improved. Acceptability decreased when Moringa oleifera supplementation increased, affecting the bread physical and sensory attributes. | 40 |
Alkaloids
Alkaloids are a group of naturally occurring chemical compounds that contain mostly basic nitrogen atoms. This nitrogen may occur in the form of a primary amine (RNH2), a secondary amine (R2NH) or a tertiary amine (R3N). In addition to carbon, hydrogen and nitrogen, most alkaloids contain oxygen 46. Alkaloids are of particular interest thanks to their pharmacological properties. The presence of these compounds has been confirmed in Moringa oleifera leaves 47. Several of these compounds, such as N,α-l-rhamnopyranosyl vincosamide, 4-(α-l-rhamnopyranosyloxy) phenylacetonitrile (niazirin), pyrrolemarumine 4′′-O-α-l-rhamnopyranoside, 4′-hydroxy phenylethanamide-α-l-rhamnopyranoside (marumoside A) and its 3-O-β-d-glucopyranosyl-derivative (marumoside B) and methyl 4-(α-l-rhamnopyranosyloxy)-benzylcarbamate, have been isolated in Moringa oleifera leaves 48. However, their amounts in the Moringa oleifera leaves are still unknown.
Vitamins
Fresh leaves of Moringa oleifera are reported to contain 11,300–23,000 IU of vitamin A 49. Vitamin A plays key roles in many physiological processes such as vision, reproduction, embryonic growth and development, immune competence, cell differentiation, cell proliferation and apoptosis, maintenance of epithelial tissue, and brain function. Vitamin A deficiency is still prevalent in many developing countries, and considered responsible for child and maternal mortality 50.
Fresh Moringa oleifara leaves are also a good source of carotenoids with pro-vitamin A action. They contain 6.6–6.8 mg/100 g 51 of beta-carotene, greater that carrots, pumpkin and apricots (6.9, 3.6 and 2.2 mg/100 g, respectively).
Beta-carotene is more concentrated in the dried leaves, with amounts ranging from 17.6 to 39.6 mg/100 g of dry weight 52. This wide range may be explained by the different environmental conditions existing among different origin countries, genetic of the plant, drying method 53 and the different extraction and analysis methods employed as well. Freeze-drying seems to be the most conservative dehydration method. In freeze-drying leaves the β-carotene content is approximately 66 mg/100 g 54.
Moringa oleifera is an interesting source of vitamin C. Fresh leaves contain approximately 200 mg/100 g 55, greater than orange. These amounts are of particular interest, as the vitamin C intervenes in the synthesis and metabolism of many compounds, like tyrosine, folic acid and tryptophan, hydroxylation of glycine, proline, lysine carnitine and catecholamine. It facilitates the conversion of cholesterol into bile acids and hence lowers blood cholesterol levels and increases the absorption of iron in the gut by reducing ferric to ferrous state. Finally, it acts as antioxidant, protecting the body from various deleterious effects of free radicals, pollutants and toxins 56. However, being vitamin C sensitive to heat and oxygen, it is rapidly oxidized, so much so that its concentration in the Moringa oleifera dried leaves is lower than in the fresh leaves, dropping to 18.7 to 140 mg/100 g of dry weight 53.
Difference in (i) environmental conditions in the various origin countries; (ii) genetic of the plant; (iii) drying method 53 and (iv) different extraction and analysis methods, may explain the wide range of vitamin C content in Moringa leaves reported in literature. Freeze-drying seem to better preserve vitamin C from oxidation, so much so that greater amounts of this vitamin were found in leaves undergone to freeze-drying soon after the collection. In these latter, vitamin C concentration ranges between 271 and 920 mg/100 g of dry weight 54.
Moringa oleifera fresh leaves are a good source of vitamin E (in particular alpha-tocopherol) and contain approximately 9.0 mg/100 g 57 of this compound, similarly to nuts. Vitamin E acts mainly as liposoluble antioxidants, but it is also involved in the modulation of gene expression, inhibition of cell proliferation, platelet aggregation, monocyte adhesion and regulation of bone mass 58. Drying procedure determines a concentration of vitamin E up to values of 74.45–122.16 mg/100 g of dry weight 52.
Among vitamins of group B, only thiamine, riboflavin and niacin seem present in Moringa oleifera leaves. These vitamins mainly act as cofactors of many enzymes involved in the metabolism of nutrients and energy production, and their concentration in fresh leaves ranges between 0.06 and 0.6 mg/100 g, 0.05 and 0.17 mg/100 g and 0.8 and 0.82 mg/100 g for thiamine, riboflavin and niacin, respectively 59, similarly to fruits and vegetable. Only one study reported the contribution of vitamin B1, B2 and B3 of dried leaves of Moringa oleifera 60. Their concentrations were 2.85, 22.16 and 8.86 mg/100g of dry weight, respectively. However, the amount of riboflavin in dried leaves seems very high compared to that of fresh leaves. Further studies are needed to confirm these values. Finally, Girija et al. 61 showed an appreciable physiological availability of these three vitamins in leaves of Moringa oleifera (61.6%, 51.5% and 39.9%, respectively).
Scientists did not find studies about other vitamin of group B or vitamin D and K in Moringa oleifera leave; therefore further studies on this topic are needed.
Table 2. Vitamins content in Moringa oleifera leaves
| Bioactive Compound | Leaves | Value Found in Literature | Value Express as Dry Weight | Drying Method | Extractive Method | Analytical Method | Country | Reference |
| Vitamins | ||||||||
| Vitamin A | fresh | 11,300 IU | 45,200 IU | N/A | N/A | India | 55 | |
| fresh | 23,000 IU | 92,000 IU a | N/A | N/A | Brazil | 49 | ||
| Vitamin B1–Thiamine | fresh | 0.06 mg/100 g | 0.24 mg/100 g | N/A | N/A | India | 55 | |
| fresh | 0.21 mg/100 g | 0.84 mg/100 g | N/A | N/A | N/A | 60 | ||
| fresh | 0.6 mg/100 g | 2.58 mg/100 g | N/A | Microbiological method | India | 61 | ||
| dried | 2.64 mg/100 g | 2.85 mg/100 g | N/A | N/A | N/A | N/A | 60 | |
| Vitamin B2–Riboflavin | fresh | 0.05 mg/100 g | 0.20 mg/100 g | N/A | N/A | India | 55 | |
| fresh | 0.05 mg/100 g | 0.20 mg/100 g | N/A | N/A | N/A | 60 | ||
| fresh | 0.17 mg/100 g | 0.726 mg/100 g | N/A | Microbiological method | India | 61 | ||
| dried | 20.5 mg/100 g | 22.16 mg/100 g | N/A | N/A | N/A | N/A | 60 | |
| Vitamin B3–Niacin | fresh | 0.8 mg/100 g | 3.20 mg/100 g | N/A | N/A | India | 55 | |
| fresh | 0.8 mg/100 g | 3.20 mg/100 g | N/A | N/A | N/A | 60 | ||
| fresh | 0.82 mg/100 g | 3.5 mg/100 g | N/A | Microbiological method | India | 61 | ||
| dried | 8.2 mg/100 g | 8.86 mg/100 g | N/A | N/A | N/A | N/A | 60 | |
| Vitamin C–Ascorbic acid | fresh | 220 mg/100 g | 880 mg/100 g | N/A | N/A | India | 55 | |
| dried | 17.3 mg/100 g | 18.7 mg/100 g | N/A | N/A | N/A | N/A | 60 | |
| dried | 92 mg/100 g | 92 mg/100 g | Sun-drying for 4 days | N/A | AOAC 2004 | India | 53 | |
| 140 mg/100 g | 140 mg/100 g | Shadow-drying for 6 days | ||||||
| 56 mg/100 g | 56 mg/100 g | Oven-drying at 60 °C for 1 h | ||||||
| dried | 38.8 mg/100 g b | 38.8 mg/100 g b | Air-drying | Metaphosphoric acid | Indophenol titration | Pakistan | 62 | |
| freeze-dried | 271 mg/100 g | 271 mg/100 g | Freeze-drying | Deionized water | Colorimetric method | Florida, USA | 54 | |
| freeze-dried | 920 mg/100 g | 920 mg/100 g | Freeze-drying | 6% metaphosphoric acid | Titration against 2,6-dichlorophenolindophenol | Nicaragua | 63 | |
| 840 mg/100 g | 840 mg/100 g | India | ||||||
| 680 mg/100 g | 680 mg/100 g | Niger | ||||||
| Vitamin E–Tocopherol | fresh | 9.0 mg/100 g | 16.21 mg/100 g | N-hexane + ethyl acetate + BHT | Reverse-phase HPLC | Malaysia | 57 | |
| dried | 113 mg/100 g | 122.16 mg/100 g | N/A | N/A | N/A | N/A | 60 | |
| dried | 74.45 mg/100 g | 74.45 mg/100 g | Drying at 60 °C for 8 h | Microscale saponification and extraction with n-hexane | HPLC | Mexico | 59 | |
| dried | 77.0 mg/100 g | 85.08 mg/100 g | Air-dried under shade | N/A | HPLC Fluorescence | South Africa | 52 |
Abbreviations: a Obtained considering a moisture of 75%; b Mean value of samples collected in different seasons; N/A = Not available.
Table 3. Carotenoids content in Moringa oleifera leaves
| Bioactive Compound | Leaves | Value Found in Literature | Value Express as Dry Weight | Drying Method | Extractive Method | Analytical Method | Country | Reference |
| Carotenoids | ||||||||
| β-carotene | fresh | 6.63 mg/100 g | 33.48 mg/100 g | Acetone–n-hexane | HPLC | Taiwan | 51 | |
| fresh | 6.8 mg/100 g | 27.22 mg/100 g | N/A | N/A | N/A | 60 | ||
| dried | 36 mg/100 g | 36 mg/100 g | Sun-drying for 4 days | N/A | AOAC 2004 | India | 53 | |
| 39.6 mg/100 g | 39.6 mg/100 g | Shadow-drying for 6 days | ||||||
| 37.8 mg/100 g | 37.8 mg/100 g | Oven-drying at 60 °C for 1 h | ||||||
| dried | 16.3 mg/100 g | 17.62 mg/100 g | N/A | N/A | N/A | N/A | 60 | |
| dried | 18.5 mg/100 g | 20.44 mg/100 g | Air-dried under shade | N/A | HPLC | South Africa | 52 | |
| freeze-dried | 66 mg/100 g | 66 mg/100 g | Freeze-drying | Acetone | HPLC | Florida, USA | 54 | |
| Lutein | fresh | 6.94 mg/100 g | 35.05 mg/100 g | Acetone–n-hexane | HPLC | Taiwan | 51 | |
| freeze-dried | 102 mg/100 g | 102 mg/100 g | Freeze-drying | Acetone | HPLC | Florida, USA | 54 |
Abbreviations: N/A = Not available.
Polyphenols
Moringa oleifera dried leaves are a great source of polyphenols. Their concentrations range from 2090 to 12,200 mg gallic acid equivalent (GAE)/100 g of dry weight 64 (or 1600 to 3400 mg tannin acid equivalent (TAE)/100g of dry weight) 65. These amounts are greater than those found in fruits and vegetable 66. The different environmental conditions in the various origin countries, the harvesting season 62, the genetic of the plant, the drying method, the leaf maturity stage 47 and the extractive method used may explain such wide range of reported values. Principal polyphenol compounds in Moringa oleifera leaves are flavonoids and phenolic acids 67.
Table 4. Polyphenols content in Moringa oleifera leaves
| Bioactive Compound | Leaves | Value Found in Literature | Value Express as Dry Weight | Drying Method | Extractive Method | Analytical Method | Country | Reference | |
| Polyphenols | |||||||||
| Total phenols | dried | 4581 mg GAE/100 g a | 4581 mg GAE/100 g a | Shade-drying | Water Soxhlet extraction for 18–20 h | Folin-Ciocalteau | India | 47 | |
| 3602 mg GAE/100 g b | 3602 mg GAE/100 g b | ||||||||
| dried | 3290 mg GAE/100 g | 3290 mg GAE/100 g | N/A | 50% MeOH | Folin-Ciocalteau | India | 68 | ||
| dried | 2090 mg GAE/100 g | 2090 mg GAE/100 g | N/A | 50% MeOH, 100% MeOH and water | Folin-Ciocalteau | India | 69 | ||
| dried | 10,504 mg GAE/100 g | 10,504 mg GAE/100 g | N/A | Water at 80 °C for 2 h | Folin-Ciocalteau | India | 64 | ||
| dried | 10,616 mg GAE/100 g c | 10,616 mg GAE/100 g c | Air-drying | 80% MeOH | Folin-Ciocalteau | Pakistan | 62 | ||
| dried | 10,300 mg GAE/100 g | 10,300 mg GAE/100 g | Air-drying | 100% MeOH | Extraction by shaker | Folin-Ciocalteau | Pakistan | 67 | |
| 12,200 mg GAE/100 g | 12,200 mg GAE/100 g | 80% MeOH | |||||||
| 9720 mg GAE/100 g | 9720 mg GAE/100 g | 100% EtOH | |||||||
| 11,600 mg GAE/100 g | 11,600 mg GAE/100 g | 80% EtOH | |||||||
| dried | 9630 mg GAE/100 g | 9630 mg GAE/100 g | Air-drying | 100% MeOH | Extraction by reflux | Folin-Ciocalteau | Pakistan | 67 | |
| 10,700 mg GAE/100 g | 10,700 mg GAE/100 g | 80% MeOH | |||||||
| 6160 mg GAE/100 g | 6160 mg GAE/100 g | 100% EtOH | |||||||
| 8210 mg GAE/100 g | 8210 mg GAE/100 g | 80% EtOH | |||||||
| dried | 2070 mg TAE/100 g | 2070 mg TAE/100 g | Air-drying | Acetone/Water (7:3) | Folin-Ciocalteau | India | 65 | ||
| dried | 1600 mg TEA/100 g d | 1600 mg TEA/100 g d | Air-drying | 80% EtOH | Folin-Ciocalteau | Nicaragua | 70 | ||
| 3400 mg TEA/100 g e | 3400 mg TEA/100 g e | ||||||||
| dried | 5350 mg CAE/100 g | 5350 mg CAE/100 g | Oven-drying at 60 °C for 24 h | Maceration with 70% EtOH | Folin-Ciocalteau | Thailand | 71 | ||
| 2930 mg CAE/100 g | 2930 mg CAE/100 g | Maceration with 50% EtOH | |||||||
| 3710 mg CAE/100 g | 3710 mg CAE/100 g | Percolation with 70% EtOH | |||||||
| 3280 mg CAE/100 g | 3280 mg CAE/100 g | Percolation with 50% EtOH | |||||||
| 4550 mg CAE/100 g | 4550 mg CAE/100 g | Soxhlet extraction with 70% EtOH | |||||||
| 4460 mg CAE/100 g | 4460 mg CAE/100 g | Soxhlet extraction with 50% EtOH | |||||||
| freeze-dried | 1535.6 mg GAE/100 g | 1535.6 mg GAE/100 g | Freeze-drying | 80% EtOH | Folin-Ciocalteau | Florida, USA | 54 | ||
Abbreviations: a Mature/old leaves; b Tender/young leaves; c Mean value of samples collected in different seasons; d Extracted leaves; e Unextracted leaves; N/A = Not available; GAE = Gallic acid equivalent; TAE = Tannin acid equivalent; CAE = Chlorogenic acid equivalent.
Phenolic acids
Phenolic acids are a sub-group of phenolic compounds derived from hydroxybenzoic acid and hydroxycinnamic acid, naturally present in plants. Thanks to their documented effects on human health, the contribution of food-supplied phenolic acids is a subject of increasing interest. In particular, these compounds are mainly studied for their documented antioxidant, anti-inflammatory, antimutagenic and anticancer properties 72. Particularly abundant in fruit and vegetables, phenolic acids were found in great amounts in Moringa oleifera leaves too. In dried leaves, gallic acid seems to be the most abundant, with a concentration of approximately 1.034 mg/g of dry weight 64, although Bajpai et al. 69 only found poorly detectable amounts. The concentration of chlorogenic and caffeic acids ranges from 0.018 to 0.489 mg/g of dry weight and not detected to 0.409 mg/g of dry weight, respectively 68. Lower, but appreciable, concentrations were found for ellagic and ferulic acids. Their concentrations range from not detected to 0.189 mg/g and 0.078 to 0.128 mg/g of dry weight, respectively 64. Some of these compounds were found more concentrated in freeze-dried leaves. Specifically, Zhang et al. 54, in Moringa oleifera leaves harvested in Florida and subsequently freeze-dried, found approximately 6.457 mg/g of dry weight of o-coumaric acid and 0.536 mg/g of dry weight of caffeic acid, while p-coumaric, synaptic, gentistic and syringic acids were found in poorly detectable amounts. Like for the flavonoids, the different environmental conditions, harvesting season, genetic of the plant, drying method, leaf maturity stage, extraction method used and the different sensitivity of the analytical methods may have contributed to the high inter-study variation in the concentrations of phenolic acids in Moringa oleifera leaves.
Table 5. Phenolic acids content in Moringa oleifera leaves
| Bioactive Compound | Leaves | Value Found in Literature | Value Express as Dry Weight | Drying Method | Extractive Method | Analytical Method | Country | Reference |
| Phenolic acids | ||||||||
| Caffeic acid | dried | ND | ND | N/A | 50% MeOH | HPLC and MS/MS | India | 68 |
| dried | 0.409 mg/g | 0.409 mg/g | N/A | 50% MeOH, 100% MeOH and water | HPLC | India | 69 | |
| freeze-dried | 0.536 mg/g | 0.536 mg/g | Freeze-drying | 80% EtOH | HPLC | Florida, USA | 54 | |
| Chlorogenic acid | dried | 0.018 mg/g | 0.018 mg/g | N/A | 50% MeOH | HPLC and MS/MS | India | 68 |
| dried | 0.489 mg/g | 0.489 mg/g | N/A | Water at 80 °C for 2 h | HPLC and MS/MS | India | 64 | |
| o-Coumaric acid | freeze-dried | 6.457 mg/g | 6.457 mg/g | Freeze-drying | 80% EtOH | HPLC | Florida, USA | 54 |
| p-Coumaric acid | freeze-dried | ND | ND | Freeze-drying | 80% EtOH | HPLC | Florida, USA | 54 |
| Ellagic acid | dried | ND | ND | N/A | 50% MeOH, 100% MeOH and water | HPLC | India | 69 |
| dried | 0.009 mg/g | 0.018 mg/g | N/A | 50% MeOH | HPLC and MS/MS | India | 68 | |
| dried | 0.189 mg/g | 0.189 mg/g | N/A | Water at 80 °C for 2 h | HPLC and MS/MS | India | 64 | |
| Ferulic acid | dried | 0.078 mg/g | 0.078 mg/g | N/A | 50% MeOH | HPLC and MS/MS | India | 68 |
| dried | 0.078 mg/g | 0.078 mg/g | N/A | 50% MeOH, 100% MeOH and water | HPLC | India | 69 | |
| dried | 0.128 mg/g | 0.128 mg/g | N/A | Water at 80°C for 2 h | HPLC and MS/MS | India | 64 | |
| Gallic acid | dried | ND | ND | N/A | 50% MeOH, 100% MeOH and water | HPLC | India | 69 |
| dried | 1.034 mg/g | 1.034 mg/g | N/A | 50% MeOH | HPLC and MS/MS | India | 68 | |
| dried | 1.034 mg/g | 1.034 mg/g | N/A | Water at 80 °C for 2 h | HPLC and MS/MS | India | 64 | |
| Gentistic acid | freeze-dried | ND | ND | Freeze-drying | 80% EtOH | HPLC | Florida, USA | 54 |
| Sinapic acid | freeze-dried | ND | ND | Freeze-drying | 80% EtOH | HPLC | Florida, USA | 54 |
| Syringic acid | freeze-dried | ND | ND | Freeze-drying | 80% EtOH | HPLC | Florida, USA | 54 |
Abbreviations: ND = Not detected; N/A = Not available.
Flavonoids
Flavonoids are a sub-group of polyphenolic compounds having a benzo-γ-pyrone structure and are ubiquitous in plants, as they are synthesized in response to microbial infections 73. Epidemiological studies have consistently shown that high intake of flavonoids has protective effects against many infectious (bacterial and viral diseases) and degenerative diseases such as cardiovascular diseases, cancers, and other age-related diseases 73. Moringa oleifera leaves are an interesting source of flavonoids compounds. Total flavonoids concentration in dried leaves ranges from 5.059 to 12.16 mg/g of dry weight 74, namely, close to or larger than that in many fruits and vegetable normally consumed 75. These values are the overall sum of the amounts of single flavonoids. However, some flavonoids were studied only by some authors and, therefore, these amounts may be inaccurate. The total concentration of flavonoids in freeze-dried leaves ranges from 21.0 to 61.62 mg rutein equivalent/g of dry weight 54. Myricetin, quercetin and kaempferol are the main flavonoids found in Moringa oleifera leaves. In dried leaves, myricetin concentration is approximately 5.804 mg/g of dry weight, while quercetin and kaempferol concentrations range from 0.207 to 7.57 mg/g of dry weight and not detectable amounts to 4.59 mg/g of dry weight, respectively 64. Higher amounts were found in freeze-dried leaves. In particular, quercitin and kaempferol concentrations range from 5.47 to 16.64 mg/g and 1.5 to 3.5 mg/g of dry weight, respectively 76. Isorhamnetin concentration in dried leaves is approximately 0.118 mg/g of dry weight 74, while, in freeze-dried leaves, its concentration is up to 7 times larger, ranging from 0.52 to 0.72 mg/g of dry weight 76. Other flavonoids, such as luteolin, apigenin, daidzein and genistein, were found in not detectable concentrations in Moringa oleifera leaves 74. However these compounds were investigated only in few studies and, therefore, further investigations are needed. In addition, in this case, the high inter-studies variations for these compounds may be explained taking into account different environmental conditions, harvesting season, genetic of the plant, drying method, leaf maturity stage, extraction method used and, finally, the different sensitivity of the analytical methods.
Table 6. Flavonoids content in Moringa oleifera leaves
| Bioactive Compound | Leaves | Value Found in Literature | Value Express as Dry Weight | Drying Method | Extractive Method | Analytical Method | Country | Reference | |
| Flavonoids | |||||||||
| Total flavonoids | dried | 1.29 mg/g a | 5.059 mg/g a | Vacuum-drying | MeOH + HCl + ascorbic acid | HPLC-DAD | Taiwan | 74 | |
| dried | 6.0 mg/g a,b | 6.0 mg/g a,b | Air-drying | 70% MeOH + 0.1% acetic acid | LC/MS | Ghana | 75 | ||
| 7.03 mg/g a,b | 7.03 mg/g a,b | Senegal | |||||||
| 12.16 mg/g a,b | 12.16 mg/g a,b | Zambia | |||||||
| dried | 31.28 mg QE/g | 31.28 mg QE/g | N/A | Water at 80 °C for 2 h | HPLC and MS/MS | India | 64 | ||
| dried | 27.0 mg QE/g c | 27.0 mg QE/g c | Shade-drying | Water Soxhlet extraction for 18–20 h | Colorimetric method | India | 47 | ||
| 15.0 mg QE/g d | 15.0 mg QE/g d | ||||||||
| dried | 96.12 mg ECE/100g e | 96.12 mg ECE/100 g e | Air-drying | 80% MeOH | Colorimetric method | Pakistan | 62 | ||
| dried | 60.6 mg CE/g | 60.6 mg CE/g | Air-drying | 100% MeOH | Extraction by shaker | Spectrophotometric method | Pakistan | 67 | |
| 86.6 mg CE/g | 86.6 mg CE/g | 80% MeOH | |||||||
| 53.3 mg CE/g | 53.3 mg CE/g | 100% EtOH | |||||||
| 62.1 mg CE/g | 62.1 mg CE/g | 80% EtOH | |||||||
| dried | 59.0 mg CE/g | 59.0 mg CE/g | Air-drying | 100% MeOH | Extraction by reflux | Spectrophotometric method | Pakistan | 67 | |
| 72.9 mg CE/g | 72.9 mg CE/g | 80% MeOH | |||||||
| 41.9 mg CE/g | 41.9 mg CE/g | 100% MeOH | |||||||
| 53.1 mg CE/g | 53.1 mg CE/g | 80% EtOH | |||||||
| dried | 25.1 mg IQE/g | 25.1 mg IQE/g | Oven-drying at 60° C for 24 h | Maceration with 70% EtOH | Colorimetric method | Thailand | 71 | ||
| 12.3 mg IQE/g | 12.3 mg IQE/g | Maceration with 50% EtOH | |||||||
| 18.0 mg IQE/g | 18.0 mg IQE/g | Percolation with 70% EtOH | |||||||
| 14.6 mg IQE/g | 14.6 mg IQE/g | Percolation with 50% EtOH | |||||||
| 24.5 mg IQE/g | 24.5 mg IQE/g | Soxhlet extraction with 70% EtOH | |||||||
| 12.7 mg IQE/g | 12.7 mg IQE/g | Soxhlet extraction with 50% EtOH | |||||||
| freeze-dried | 61.62 mg RE/g | 61.62 mg RE/g | Freeze-drying | 80% EtOH | Spectrophotometric method | Florida, USA | 54 | ||
| freeze-dried | 44.3 mg RE/g | 44.3 mg RE/g | Freeze-drying | 80% MeOH | Spectrophotometric method | Nicaragua India Niger | 77 | ||
| 21.0 mg RE/g | 21.0 mg RE/g | Nicaragua India Niger | |||||||
| 38.1 mg RE/g | 38.1 mg RE/g | Nicaragua India Niger | |||||||
| Apigenin | dried | ND | ND | N/A | MeOH + HCl + ascorbic acid | HPLC | Taiwan | 74 | |
| Daidzein | dried | ND | ND | N/A | 50% MeOH, 100% MeOH and water | HPLC | India | 69 | |
| Epicatechin | freeze-dried | 5.68 mg/g | 5.68 mg/g | Freeze-drying | 80% EtOH | HPLC | Florida, USA | 54 | |
| Genistein | dried | ND | ND | N/A | 50% MeOH, 100% MeOH and water | HPLC | India | 69 | |
| Isorhamnetin | dried | 0.03 mg/g | 0.118 mg/g | Vacuum-drying | MeOH + HCl + ascorbic acid | HPLC | Taiwan | 74 | |
| freeze-dried | 0.13 mg/g f | 0.52 mg/g f,g | Freeze-drying | 70% MeOH | HPLC-DAD-electrospray mass spectrometry | Ghana | 76 | ||
| 0.18 mg/g h | 0.72 mg/g g,h | ||||||||
| Kaempferol | dried | 0.04 mg/g | 0.04 mg/g | Air-drying | MeOH + 1% v/v HCl + TBHQ | HPLC | Pakistan | 78 | |
| dried | ND | ND | N/A | 50% MeOH | HPLC and MS/MS | India | 68 | ||
| dried | 2.360 mg/g | 2.360 mg/g | N/A | 50% MeOH, 100% MeOH and water | HPLC | India | 69 | ||
| dried | 0.198 mg/g | 0.198 mg/g | N/A | Water at 80°C for 2 h | HPLC and MS/MS | India | 64 | ||
| dried | 0.36 mg/g | 1.412 mg/g | Vacuum-drying | MeOH + HCl + 10 mg ascorbic acid | HPLC | Taiwan | 74 | ||
| dried | 0.8 mg/g | 0.8 mg/g | Air-drying | 70% MeOH + 0.1% acetic acid | LC/MS | Ghana | 75 | ||
| 1.23 mg/g | 1.23 mg/g | Senegal | |||||||
| 4.59 mg/g | 4.59 mg/g | Zambia | |||||||
| freeze-dried | 0.98 mg/g f | 3.92 mg/g f,g | Freeze-drying | 70% MeOH | HPLC-DAD-electrospray mass spectrometry | Ghana | 76 | ||
| 0.54 mg/g h | 2.16 mg/g g,h | ||||||||
| freeze-dried | 2.25 mg/g | 2.25 mg/g | Freeze-drying | 80% MeOH | HPLC-DAD | Nicaragua | 77 | ||
| 1.75 mg/g | 1.75 mg/g | India | |||||||
| 1.05 mg/g | 1.05 mg/g | Niger | |||||||
| freeze-dried | 2.9 mg/g d | 2.9 mg/g d | Freeze-drying | 70% MeOH | LC/MS | Malawi | 79 | ||
| 2.3 mg/g | 2.3 mg/g | Senegal | |||||||
| 3.5 mg/g | 3.5 mg/g | Nicaragua | |||||||
| 0.3 mg/g c | 0.3 mg/g c | ECHO | |||||||
| 0.16 mg/g d | 0.16 mg/g d | ECHO | |||||||
| Luteolin | dried | ND | ND | N/A | MeOH + HCl + ascorbic acid | HPLC | Taiwan | 74 | |
| Myricetin | dried | 5.804 mg/g | 5.804 mg/g | Air-drying | MeOH + 1% v/v HCl + TBHQ | HPLC | Pakistan | 78 | |
| Quercetin | dried | 0.281 mg/g | 0.281 mg/g | Air-drying | MeOH + 1% v/v HCl + TBHQ | HPLC | Pakistan | 78 | |
| dried | 0.207 mg/g | 0.207 mg/g | N/A | 50% MeOH | HPLC and MS/MS | India | 68 | ||
| dried | 0.207 mg/g | 0.207 mg/g | N/A | 50% MeOH, 100% MeOH and water | HPLC | India | 69 | ||
| dried | 0.807 mg/g | 0.807 mg/g | N/A | Water at 80 °C for 2 h | HPLC and MS/MS | India | 64 | ||
| dried | 0.90 mg/g | 3.529 mg/g | Vacuum-drying | MeOH + HCl + 10 mg ascorbic acid | HPLC | Taiwan | 74 | ||
| dried | 5.2 mg/g | 5.2 mg/g | Air-drying | 70% MeOH + 0.1% acetic acid | LC/MS | Ghana | 75 | ||
| 5.8 mg/g | 5.8 mg/g | Senegal | |||||||
| 7.57 mg/g | 7.57 mg/g | Zambia | |||||||
| freeze-dried | 3.21 mg/g f | 12.84 mg/g f,g | Freeze-drying | 70% MeOH | HPLC-DAD-electrospray mass spectrometry | Ghana | 76 | ||
| 4.16 mg/g h | 16.64 mg/g g,h | ||||||||
| freeze-dried | 9.26 mg/g | 9.26 mg/g | Freeze-drying | 80% MeOH | HPLC-DAD | Nicaragua | 77 | ||
| 6.34 mg/g | 6.34 mg/g | India | |||||||
| 7.70 mg/g | 7.70 mg/g | Niger | |||||||
| freeze-dried | 5.47 mg/g b | 5.47 mg/g b | Freeze-drying | 70% MeOH | LC/MS | Malawi | 79 | ||
| 9.1 mg/g | 9.1 mg/g | Senegal | |||||||
| 15.2 mg/g | 15.2 mg/g | Nicaragua | |||||||
| 0.58 mg/g c | 0.58 mg/g c | ECHO | |||||||
| 0.46 mg/g d | 0.46 mg/g d | ECHO | |||||||
| Rutin | dried | 0.390 mg/g | 0.390 mg/g | N/A | 50% MeOH, 100% MeOH and water | HPLC | India | 69 | |
| dried | ND | ND | N/A | 50% MeOH | HPLC and MS/MS | India | 68 | ||
| freeze-dried | 1.674 mg/g | 1.674 mg/g | Freeze-drying | 80% EtOH | HPLC | Florida, USA | 54 | ||
Abbreviations: a Obtained from the sum of single flavonoids measured; b Mean value of different samples; c Mature/old leaves; d Tender/young leaves; e Mean value of samples collected in different seasons; f Vegetative plants; g Obtained considering a moisture of 75%; h Flowering plants; ND = Not detected; N/A = Not available; QE = Quercetin equivalent; ECE = Epicatechine equivalent; CE = Catechin equivalent; IQE = Isoquercetin equivalent; RE = Rutein equivalent.
Glucosinolates and isothiocyanates
Glucosinolates are a group of secondary metabolites in plants. Structurally they are beta-S-glucosides of thio-oxime-O-sulfates and synthesized from amino acids. Appreciable amounts of these compounds were found in Moringa oleifera leaves. In particular, around 116 and 63 mg/g of dry weight in young and older leaves, respectively, are reported 80 These amounts are close to, and in some case larger than, those found in many cruciferous vegetables (e.g., broccoli, cabbage, radish), mainly sources of these compounds 81. 4-O-(α-l-rhamnopyranosyloxy)-benzyl glucosinolate has been identified as the dominant leaf glucosinolate of Moringa oleifera and is accompanied by lower levels of three isomeric 4-O-(α-l-acetylrhamnopyranosyloxy)-benzyl glucosinolates, which reflect the three position of the acetyl group at the rhamnose moiety of the molecule 80. The concentrations of these compounds seem affected by the physiological stage of the plant and by the maturity stage of the leaves. The concentration of 4-O-(α-l-rhamnopyranosyloxy)-benzyl glucosinolate ranges from 21.84 to 59.4 mg/g of dry weight, while the concentrations of the three isomer of 4-O-(α-l-acetylrhamnopyranosyloxy)-benzyl glucosinolates range from 2.16 to 5.0 mg/g of dry weight, 1.2 to 1.8 mg/g of dry weight and 12.76 to 50.2 mg/g of dry weight for isomer 1, 2 and 3, respectively 79. Amaglo et al. 76 report the presence of 4-hydroxybenzyl (sinalbin), with a concentration ranging between not detected and 2.36 mg/g of dry weight. Glucosinolates can be hydrolyzed by myrosinase to produce d-glucose and various other degradation products like isothiocyanates 82, which are also present in Moringa oleifera leaves 83. Both glucosinolates and isothiocyanates play an important role in health promoting and prevention of disease 84.
Table 7. Glucosinolates content in Moringa oleifera leaves
| Bioactive Compound | Leaves | Value Found in Literature | Value Express as Dry Weight | Drying Method | Extractive Method | Analytical Method | Country | Reference |
| Glucosinolates | ||||||||
| Benzyl | freeze-dried | ND a ND b | ND a ND b | Freeze-drying | 70% MeOH | HPLC-DAD-electrospray mass spectrometry | Ghana | 76 |
| freeze-dried | ND c ND d | ND c ND d | Freeze-drying | 70% MeOH | LC/MS | Many countries | 79 | |
| 4-hydroxybenzyl (sinalbin) | freeze-dried | 0.59 mg/g a ND b | 2.36 mg/g a,e ND b,e | Freeze-drying | 70% MeOH | HPLC-DAD-electrospray mass spectrometry | Ghana | 76 |
| 4-(α-L-rhamnopyranosyloxy)-benzyl | freeze-dried | 5.64 mg/g a | 22.56 mg/g a,e | Freeze-drying | 70% MeOH | HPLC-DAD-electrospray mass spectrometry | Ghana | 76 |
| 5.46 mg/g b | 21.84 mg/g b,e | |||||||
| freeze-dried | 33.9 mg/g c | 33.9 mg/g c | Freeze-drying | 70% MeOH | LC/MS | Many countries | 79 | |
| 59.4 mg/g d | 59.4 mg/g d | |||||||
| 4-O-(α-L-acetylrhamnopyranosyloxy)-benzyl isomer 1 | freeze-dried | 0.69 mg/g a | 2.76 mg/g a,e | Freeze-drying | 70% MeOH | HPLC-DAD-electrospray mass spectrometry | Ghana | 76 |
| 0.54 mg/g b | 2.16 mg/g b,e | |||||||
| freeze-dried | 2.9 mg/g c | 2.9 mg/g c | Freeze-drying | 70% MeOH | LC/MS | Many countries | 79 | |
| 5.0 mg/g d | 5.0 mg/g d | |||||||
| 4-O-(α-L-acetylrhamnopyranosyloxy)-benzyl isomer 2 | freeze-dried | 0.45 mg/g a | 1.80 mg/g a,e | Freeze-drying | 70% MeOH | HPLC-DAD-electrospray mass spectrometry | Ghana | 76 |
| 0.38 mg/g b | 1.52 mg/g b,e | |||||||
| freeze-dried | 1.2 mg/g c | 1.2 mg/g c | Freeze-drying | 70% MeOH | LC/MS | Many countries | 79 | |
| 1.5 mg/g d | 1.5 mg/g d | |||||||
| 4-O-(α-L-acetylrhamnopyranosyloxy)-benzyl isomer 3 | freeze-dried | 5.04 mg/g a | 20.16 mg/g a,e | Freeze-drying | 70% MeOH | HPLC-DAD-electrospray mass spectrometry | Ghana | 76 |
| 3.19 mg/g b | 12.76 mg/g b,e | |||||||
| freeze-dried | 17.4 mg/g c | 17.4 mg/g c | Freeze-drying | 70% MeOH | LC/MS | Many countries | 79 | |
| 50.2 mg/g d | 50.2 mg/g d |
Abbreviations: a Vegetative plants; b Flowering plants; c Mature/old leaves; d Tender/young leaves; e Obtained considering a moisture of 75%; ND = Not detected.
Tannins
Tannins are water-soluble phenolic compounds that bind to and precipitate alkaloids, gelatin and other proteins. They exhibit various biological properties: anti-cancer, antiatherosclerotic, anti-inflammatory, anti-hepatoxic, antibacterial and anti-HIV replication activity 85. Moringa oleifera leaves are an appreciable source of tannins. Their concentrations range between 13.2 and 20.6 gTAE/kg [68,69,76] in dried leaves and between 5.0 and 12.0 g tannin acid equivalent (TAE)/kg in freeze-dried leaves 86. These amounts are greater than concentrations found in nuts 87, similar to those found in some plants 88 and berries 89, but much lower compared to the concenctrations found in other medicinal plants 90.
Table 8. Tannins content in Moringa oleifera leaves
| Bioactive Compound | Leaves | Value Found in Literature | Value Express as Dry Weight | Drying Method | Extractive Method | Analytical Method | Country | Reference |
| Tannins | ||||||||
| Total tannins | dried | 13.2 gTAE/kg | 13.2 gTAE/kg | Air-drying | Acetone/Water (7:3) | Folin-Ciocalteau modified | India | 65 |
| dried | ND a 14.0 gTAE/kg b | ND a 14.0 gTAE/kg b | Air-drying | 80% EtOH | Folin-Ciocalteau modified | Nicaragua | 70 | |
| dried | 20.6 g/kg | 20.6 g/kg | Air-drying at 35 °C for 24 h | Double lipid extraction with n-hexane (1:5) | N/A | Brazil | 91 | |
| freeze-dried | 12 g/kg | 12 g/kg | Freeze-drying | 80% MeOH | Folin-Ciocalteau modified | Nicaragua | 92 | |
| freeze-dried | 5 g/kg | 5 g/kg | Freeze-drying | 80% EtOH | Folin-Ciocalteau modified | Niger | 86 | |
| Condensed tannins | dried | 1.05 gLE/kg | 1.05 gLE/kg | Air-drying | Acetone/Water (7:3) | Butanol–HCl–iron method | India | 65 |
| dried | 3.12 g/kg | 3.12 g/kg | Air-dried under shade | N/A | Butanol–HCl–iron method | South Africa | 52 |
Abbreviations: a Extracted leaves; b Unextracted leaves; TAE = Tannin acid equivalent; LE = Leucocyanidin equivalent; ND = Not detected; N/A = Not available.
Saponins
Saponins are a group of natural compounds that consist of an isoprenoidal-derived aglycone, designated genin or sapogenin, covalently linked to one or more sugar moieties 93. Even though some saponins have hemolytic side effects, they are studied for their anti-cancer properties 94. Moringa oleifera leaves are a good source of saponins. Their concentration in dried leaves is approximately 50 g diosgenin equivalent (DE)/kg of dry weight 70, while in freeze-dried leaves it ranges between 64 and 81 g diosgenin equivalent (DE)/kg of dry weight 86. These amounts are greater than the concentrations found in other plants 95, but slighty lower than ginseng root 96, one of the mainly source of these compounds.
Table 9. Saponins content in Moringa oleifera leaves
| Bioactive Compound | Leaves | Value Found in Literature | Value Express as Dry Weight | Drying Method | Extractive Method | Analytical Method | Country | Reference |
| Saponins | ||||||||
| Total saponins | dried | 2.0 g DE/kg a | 2.0 g DE/kg a | Air-drying | 80% EtOH | Spectrophotometric method | Nicaragua | 70 |
| 50.0 g DE/kg b | 50.0 g DE/kg b | |||||||
| freeze-dried | 81 g DE/kg | 81 g DE/kg | Freeze-drying | 80% MeOH | Spectrophotometric method | Nicaragua | 92 | |
| freeze-dried | 64 g DE/kg | 64 g DE/kg | Freeze-drying | 80% EtOH | Spectrophotometric method | Niger | 86 |
Abbreviations: a Extracted leaves; b Unextracted leaves; DE = Diosgenin equivalent.
Oxalates and phytates
Oxalates and phytates are anti-nutritional compounds as they bind minerals inhibiting the intestinal absorption. Moringa oleifera leaves present high contents of these compunds. Oxalates content of dried leaves range from 430 to 1050 mg/100 g of dry weight 91, similar to other plants rich in these compounds 97, while phytates concentration range from 25 to 31 g/kg of dry weight [69] in dried leaves and from 21 and 23 g/kg of dry weight in freeze-dried leaves 86. These amounts are greater than those found in legumes and cereals 98, but lower than brans 99.
Table 10. Oxalates and phytates content in Moringa oleifera leaves
| Bioactive Compound | Leaves | Value Found in Literature | Value Express as Dry Weight | Drying Method | Extractive Method | Analytical Method | Country | Reference |
| Oxalates and phytates | ||||||||
| Oxalates | dried | 430 mg/100 g | 430 mg/100 g | Sun-drying for 4 days | N/A | AOAC 2004 | India | 53 |
| 500 mg/100 g | 500 mg/100 g | Shadow-drying for 6 days | ||||||
| 450 mg/100 g | 450 mg/100 g | Oven-drying at 60 °C for 1 h | ||||||
| dried | 1050 mg/100 g | 1050 mg/100 g | Air-drying at 35 °C for 24 h | Double lipid extraction with n-hexane (1:5) | N/A | Brazil | 91 | |
| Phytates | dried | 25.0 g/kg a | 25.0 g/kg a | Air-drying | 3.5% HCl for 1 h | Colorimetric method | Nicaragua | 70 |
| 31.0 g/kg b | 31.0 g/kg b | |||||||
| freeze-dried | 21.0 g/kg | 21.0 g/kg | Freeze-drying | 3.5% HCl for 1 h | Colorimetric method | Nicaragua | 92 | |
| freeze-dried | 23.0 g/kg | 23.0 g/kg | Freeze-drying | 3.5% HCl for 1 h | Colorimetric method | Niger | 86 |
Abbreviations: a Extracted leaves; b Unextracted leaves; N/A = Not available.
Moringa oleifera traditional uses
All plant parts of Moringa oleifera are traditionally used for different purposes, but leaves are generally the most used 100. In particular, Moringa oleifera leaves are used in human and animal nutrition and in the traditional medicine. Moringa oleifera leaves are rich in protein, mineral, beta-carotene and antioxidant compounds, which are often lacking among the populations of underdeveloped or developing countries. Moringa oleifera leaves are added to food preparations as integrators of the diet. In traditional medicine, Moringa oleifera leaves are used to treat several ailments including malaria, typhoid fever, parasitic diseases, arthritis, swellings, cuts, diseases of the skin, genito-urinary ailments, hypertension and diabetes 18. Moringa oleifera leaves are also used to elicit lactation and boost the immune system (to treat HIV/AIDS related symptoms) 101, as well as cardiac stimulants and contraceptive remedy. One can directly consume either raw and dried leaves or the extract of an aqueous infusion.
Similarly, the use of Moringa oleifera seeds concerns both human nutrition and traditional medicine. Moringa oleifera tree barks are boiled in water and soaked in alcohol to obtained drinks and infusions that can be used to treat stomach ailments (ease stomach pain, ulcer and aiding digestion), poor vision, joint pain, diabetes, anemia and hypertension 102, toothache, hemorrhoids, uterine disorder 13. Moringa oleifera seeds are used to sediment impurities of water 13.
Moringa oleifera roots are soaked in water or alcohol and boiled with other herbs to obtained drinks and infusions as remedies for toothache, as anthelmintic and antiparalytic drugs and as sex enhancers 100.
Moringa oleifera flowers are used to produce aphrodisiac substances and to treat inflammations, muscle diseases, hysteria, tumors and enlargement of the spleen 101.
Moringa oleifera health benefits
Moringa oleifera has been used as a medicine in India since the 18th century BC 2. Traditional healers used different parts of the Moringa oleifera plant as traditional medicines. The medicinal uses are numerous and have long been recognised as an Ayurvedic and Unani system of medicine. Almost all parts of the Moringa oleifera plant: root, bark, gum, leaf, fruit (pods), flowers, seeds and seed oil, have been used to treat various diseases, like skin infections, swelling, anemia, asthma, bronchitis, diarrhea, headache, joint pain, rheumatism, gout, heart problems, fevers, digestive disorders, wounds, diabetes, conjunctivitis, hemorrhoids, goitre, earache, measles and smallpox in the indigenous system of medicine 103. Moringa oleifera leaf extract has been observed to decrease the conversion of T4 to T3 in female but not in male adult Swiss rats, therefore increasing the T4/T3 ratio, indicating a potential use for therapy of hyperthyroidism 104. Moringa oleifera is used as a galactogogue (help a breastfeeding mother to increase her breast milk supply) in Asia, particularly in the Philippines where it is called malunggay 105. Two small studies from the Philippines indicate that it might have some activity as a galactogogue in mothers of preterm infants 106, 107. Galactogogues should never replace evaluation and counseling on modifiable factors that affect milk production 108. Moringa oleifera leaves are widely used as a food and medicine in Asia and Africa and one small study found no adverse effects in nursing mothers who ingested Moringa leaves 106. No data exist on the safety of Moringa oleifera in nursing infants 109.
The bioactive compounds (Figure 3) present in Moringa oleifera confer properties associated with disease prevention and treatment, such as antimicrobial 110, anti-inflammatory 111, anticancer, antidiabetic, antioxidant, hepatoprotective and cardioprotective 112. Primary and secondary metabolites may also be involved in these applications. Primary metabolites are proteins, polysaccharides and lipids involved in physiological functions. Among them, polysaccharides and fibers are the main compounds showing positive effects on chronic diseases such as cancer, cardiovascular diseases, diabetes and obesity. On the other hand, secondary metabolites are minor molecules, such as phenolic compounds, halogenated compounds, sterols, terpenes and small peptides. Most of the phytochemicals reported in Moringa oleifera offer potential in the prevention and treatment of diseases.
The anti-inflammatory effect is due to the content of flavonoids, alkaloids, tannins and glycosides, among which quercetin appears to inhibit NF-KB activation, producing an anti-inflammatory effect 113. Other compounds with an anti-inflammatory effect include kaempferol derivatives, flavonol glycosides 114, aurantiamide acetate, 1,3-dibenzylurea 115, diterpenes, α- and β-amyrin 116, benzaldehyde 4-0-β-glucoside 114, β-sitosterol 117, rutin 118 and glucosinolate, mainly attributed to the glycosylate isothiocyanate, 4-(α-L-rhamnosyloxy) benzyl ITC, resulting from myrosinase 119. Moringa oleifera reduces inflammation by suppressing inflammatory enzymes and proteins in the body, and leaf concentrate can significantly reduce inflammation in cells 120.
The antimicrobial effect provided essential oils from the leaves and alcoholic extracts of the Moringa oleifera seeds. Martín et al. 39 demonstrated this activity of the leaf and leaves against dermatophytes such as Trichophyton rubrum and Trichophyton mentagrophytes. In addition to these compounds, other compounds have been found that also produce this effect, 4(βL-rhamnosyloxy) benzyl isocyanate or pterigospermine,4-(β-D-glucopyranosyl-1→4-β-l-ramnopyranosyloxy),benzyl thiocarboxamide,(-)-Catechin, phenylmethanamine, 4β-D-glucopyranosyl-1–>4 β-L-rhamnopyranosyloxy)-benzyl isocyanate, niazirine 115 and glucosinolate mainly attributed to the glycosylate isothiocyanate, 4-(α-L-rhamnosyloxy) benzyl ITC, resulting from myrosinase 119.
Phenolic compounds have been associated with the antimicrobial and antifungal activities of Moringa oleifera extracts 43, the Moringa oleifera leaves being the organs with the highest amount of these compounds. Regarding the antimicrobial effect of Moringa oleifera plants when included in food, Moringa Oleifera contributes to control the growth of undesirable microorganisms, due to low pH values and the presence of pterigospermin 44. The roots of Moringa oleifera have antibacterial properties and are described to be rich in antimicrobial agents. The bark extract has been found to have antifungal activities, while the juice of the bark and stem show an antibacterial effect against Staphylococcus aureus 121.
Studies have shown the anticarcinogenic effect of several compounds, namely glycosylated isothiocyanate, benzyl carbamate niazimycin and β-sitosterol, which have antitumour properties against lung, breast, skin, oesophageal and pancreatic cancer. These compounds are found in high concentrations in Moringa oleifera leaves and seeds 122. Moringa oleifera is rich in ascorbic acid, which provides an anti-diabetic effect by aiding insulin secretion, and another compound found in Moringa Oleifera that produces this effect is myricetin 123.
Antioxidants are popular because they scavenge free radicals that cause oxidative stress, cell damage and inflammation. Moringa oleifera contains antioxidants called flavonoids, polyphenols and ascorbic acid in the leaves, flowers and seeds 124. Studies have shown that the Moringa oleifera plant is rich in polyphenols, which gives it a high antioxidant capacity. The compounds in Moringa oleifera that provide this activity are feluric, gallic and ellagic acids, β-sitosterol, myricetin, niazimycin, niacimicin A and B, tocopherols: α-tocopherol, δ-tocopherol, γ-tocopherol, vanillin, kaempferol, quercetin, β-carotene (-)-catechin, astragalin and isoquercetin 2.
Moringa oleifera plays an important role in protecting the liver from damage, oxidation and toxicity due to the high concentrations of polyphenols in its leaves and flowers 2. Moringa oleifera oil can also restore liver enzymes to normal levels, reducing oxidative stress and increasing protein content in the liver. The flowers and roots of the Moringa oleifera plant contain a compound called quercetin, which is known to protect the liver 125. Other compounds contained in the plant with this activity are β-sitosterol, quercetin and some of its glycosides, rutin 126 and flavonoids, which also prevent lipid oxidation 42.
Moringa oleifera leaves and seeds have been found to help lower blood pressure; this is due to compounds called glycosides 125 and in the leaves it is also due to N-α-L-rhamnophyranosyl vincosamide 118. Moringa oleifera leaf extract has also been found to significantly reduce cholesterol levels due to the action of beta-sitosterol 125.
Analgesic, anti-inflammatory and antipyretic activities
Almost every part of Moringa oleifera has been found to exhibit analgesic activity in different animal models. Extract of Moringa oleifera leaves, seeds, and bark showed significant analgesic activity in both central (hot plate method) and peripheral models (acetic acid–induced writhing method) in a dose-dependent manner 127 and extracts of leaves exhibited analgesic potency similar to that of indomethacin 128 and antimigraine properties in a dose-dependent manner 129. Topical application showed efficacy against multiple sclerosis–induced neuropathic pain 130.
Anti-inflammatory activity of Moringa oleifera leaf extract has been observed in a carrageenan-induced paw edema model 131. Extracts of Moringa oleifera bark showed anti-inflammatory activity comparable to diclofenac in the same model. Anti-inflammatory properties of Moringa oleifera root have also been reported 132. Mechanism underlying the anti-inflammatory activity may be attributed to the regulation of neutrophils and c-Jun N-terminal kinase pathway 133 Active ingredients contributing to anti-inflammatory property are tannins, phenols, alkaloids, flavonoids, carotenoids, β-sitosterol, vanillin, hydroxymellein, moringine, moringinine, β-sitostenone, and 9-octadecenoic acid 134.
Moringa oleifera leaf extract showed significant antipyretic activity in a Brewer’s yeast–induced pyrexia model 135. Ethanol and ethyl acetate extracts of Moringa oleifera seeds also showed significant antipyretic activity 136.
Anticancer activity
Alcoholic and hydromethanolic extracts of Moringa oleifera leaves and fruits showed a significant growth delay in tumor kinetics in mouse melanoma tumor model studies 137. Extract of Moringa oleifera leaf also exhibited antiproliferative activity on A549 lung cells 138. Exploration of effects on prerequisites for cancer metastasis showed that the administration of Moringa oleifera leaf extract into chick chorioallantoic membrane led to an antiangiogenic effect, which was dose dependent, thereby showing their remarkable anticancer potential 139. Another study reported that Moringa oleifera pod extract suppressed azoxymethane and dextran sodium sulfate–induced colon destruction in male, Institute of Cancer Research mice 140. An extract of Moringa oleifera root and leaf showed a cytotoxic effect against breast cancer, hepatocarcinoma, and colorectal cancer cells in vitro and cisplatin-resistant ovarian cancer cells 141. Flower extract stimulated cell proliferation in normal cells but not in cancer cells, whereas leaf extract showed marked antitumor and hepatoprotective effects, these findings suggest the regenerative potential of Moringa oleifera besides its anticancer effects 142.
Phytoconstituents such as niazimicin, carbamates, thiocarbamate, nitrile glycosides and others such as quercetin and kaempferol are responsible for the anticancer activity of Moringa oleifera plant 143.
Antioxidant activity
Moringa oleifera fruits and leaves have antioxidant properties 144. Extract of Moringa oleifera leaf showed a concentration-dependent increase in glutathione level and a decrease in malondialdehyde level, fruit extract showed beneficial results in eliminating free radicals, extract of roots significantly reduced iron and FeSO4-induced microsomal lipid peroxidation in a dose-dependent manner 145. Pods were capable of scavenging peroxyl, superoxyl, and 2, 2-diphenyl-2-picryl hydrazyl (DPPH) radicals 146.
Besides displaying antioxidant activity, Moringa oleifera leaf extract also showed a dose-dependent nephroprotective action in an acetaminophen-induced nephrotoxicity model in male BALB/c rats 147. Triterpenoids, moringyne, monopalmitic and di-oleic triglyceride, campesterol, stigmasterol, β-sitosterol, avenasterol, vitamin A, and its precursor beta-carotene have been shown to contribute for antioxidant properties 148.
Neuropharmacological activity
Aqueous extract of leaves has shown protection against Alzheimer’s disease in a colchicine-induced Alzheimer’s model using behavioral testing (radial Y arm maze task) 149. It protected against Alzheimer’s disease by altering brain monoamine levels and electrical activity 150. Another study using toluene-ethyl acetate fraction of methanolic extract of Moringa oleifera leaf showed potent nootropic activity 151. Leaf extract contains vitamins C and E, which play a significant role in improving memory in patients with Alzheimer’s disease 152.
Anticonvulsant activity of Moringa oleifera leaves was shown in both pentylenetetrazole and maximum electric shock models using male albino mice 153. Aqueous extract of Moringa oleifera root suppressed penicillin-induced epileptic seizures in adult albino rats 154.
Ethanolic extract of Moringa oleifera leaves exhibited both central nervous system depressant and muscle relaxant activities in actophotometer and rotarod apparatuses, respectively 155 and also exhibited significant anxiolytic activity in staircase test and elevated plus maze test in a dose-dependent manner 156.
Effects on the reproductive system
Moringa oleifera leaf extract showed a significant increase in the weight of testis, seminal vesicle, epididymis, and a higher score for epididymal maturity and lumen formation along with an increase in seminiferous tubule diameter (all doses) 157.
Ethanolic extract of Moringa oleifera leaf protected prepubertal spermatogonial cells in Swiss male albino mice in cyclophosphamide-induced damage model; the possible underlying mechanism may be upregulation of expression of c-Kit and Oct4 transcripts independent of p53-mediated pathway 158.
The abortive effect of Moringa oleifera leaf extract on rats after treatment for 10 days after insemination has been reported 159. Moringa oleifera leaf extract showed a synergistic effect with estradiol and an inhibitory effect with progesterone 160. Fresh leaves of Moringa oleifera contain approximately 11,300–23,000 IU of vitamin A, which has a major role in various anatomical processes, such as reproduction, embryonic growth and development, immunity development, and cell differentiation 161.
Hepatoprotective activity
Extract of Moringa oleifera leaves has shown hepatoprotective effects against carbon tetrachloride and acetaminophen-induced liver toxicity in Sprague Dawley rats 162 and also hepatoprotective effect against antitubercular drugs and alloxan-induced liver damage in diabetic rats 163. Moringa oleifera plant-based diet for 21 days showed significant potential in attenuating hepatic injury 164. Alkaloids, quercetin, kaempferol, flavonoids, ascorbic acid, and benzylglucosinolate were found to be responsible for hepatoprotective activity 165.
Gastroprotective and anti-ulcer activities
Extract of Moringa oleifera leaves remarkably reduced ulcer index in ibuprofen-induced gastric ulcer model and in pyloric ligation test 166 and a significant reduction in cysteamine-induced duodenal ulcers and stress ulcers was also observed 167. Bisphenols and flavonoids could be contributing to this property 168.
Cardiovascular activity
Extract of Moringa oleifera leaf significantly reduced cholesterol levels and displayed a protective role on hyperlipidemia induced by iron deficiency in male Wistar rats 169. Antihypertensive effect of Moringa oleifera leaf extract on spontaneous hypertensive rats was shown, in addition to reduced chronotropic and inotropic effects in isolated frog hearts 170. Active constituents for hypotensive action are niazinin A, niazinin B, and niazimicin 171. Extract of Moringa oleifera leaves also showed cardioprotective effects against isoproterenol-induced myocardial infarction in male Wistar albino rats; the mechanism underlying this cardioprotective activity was found to be antioxidant effect, prevention of lipid peroxidation, and protection of histopathological and ultrastructural disturbances caused by isoproterenol 172.
A study was done of Moringa oleifera Lam. on various tissue systems and it showed reduction in inflammation and lipid accumulation 173.
Weight loss activity
Significant reduction in body mass index (BMI) was observed after oral treatment with Moringa oleifera leaf powder compared with that in obese control 174. Treatment of hypercholesterolemia rats with methanolic extract of Moringa oleifera leaf for 49 days showed a remarkable reduction in total cholesterol, triglycerides, and body weight, moreover, liver biomarkers, organ weight, and blood glucose levels were also decreased 175. Mechanisms include downregulation of mRNA expression of leptin and resistin and upregulation of adiponectin gene expression in obese rats 176.
Antiasthmatic activity
Extract of Moringa oleifera seeds showed protection against asthma as investigated in various models; the proposed mechanism for this effect was a direct bronchodilator effect combined with anti-inflammatory and antimicrobial actions 177 and inhibition of immediate hypersensitive reaction 178. Ethanol extract of Moringa oleifera seeds tested against ovalbumin-induced airway inflammation in guinea pigs showed a significant increase in respiratory parameters and reduction in interleukins in bronchoalveolar lavage 179.
Hematological activity
A randomized, double-blind, placebo-controlled study was carried out on women who were anemic with hemoglobin levels between 8 and 12g/dL and were treated with aqueous extract of Moringa oleifera leaf, the results showed an increase in mean hemoglobin and mean corpuscular hemoglobin concentration 180. Another study revealed that when Moringa oleifera was given to healthy human volunteers for 14 days, a significant improvement in platelet count was observed 181.
Anti-diabetic activity
Extract of Moringa oleifera leaf showed significant antihyperglycemic and hypoglycemic activity in normal and alloxan-induced diabetic rats 182. An elaborate study was performed to determine the effect of aqueous Moringa oleifera leaf extract on lipid profile, body weight, glucose, plasma insulin, homeostatic model assessment, and oral glucose tolerance test in insulin-resistant and type 1 diabetic rat models. Insulin resistant rats were fed a high-fructose diet, and type 1 diabetic rats were treated with Streptozotocin (55 mg/kg). Insulin-resistant rats showed an increase in hyperinsulinemia, hyperglycemia, and body weight, whereas Streptozotocin-induced diabetic rats showed hyperinsulinemia and hyperglycemia. Leaf extract administration for 60 days returned all the abnormal parameters to normal levels 183.
Furthermore, extract of Moringa oleifera leaf inhibited the formation of advanced glycation end products by reducing monosaccharide-induced protein glycation 184. Glucomoringin, phenols, flavonoids, quercetin-3-glucoside, fiber, and phenol have been reported to be responsible for antidiabetic activity 185.
Anti-kidney stone activity
Aqueous and alcoholic extracts of Moringa oleifera plant showed anti-urolithiatic activity in a hyperoxaluria-induced rat model 186 and in ethylene glycol–induced urolithiasis model 187.
Diuretic activity
Moringa oleifera leaves, flowers, seeds, roots, and bark extracts increased urine output in rats, extract of leaf showed a dose-dependent diuretic action greater than control but less than hydrochlorothiazide. Campesterol, stigmasterol, β-sitosterol, and avenasterol were responsible for this activity 188.
Anti-allergic activity
Ethanolic extract of Moringa oleifera seeds inhibited passive cutaneous anaphylaxis induced by anti-immunoglobulin G (IgG) antibody and histamine release from mast cells; the mechanism underlying this action could be membrane-stabilizing action 189 and also reduced scratching frequency in an Ovalbumin sensitization model 190.
Anthelmintic activity
Moringa oleifera plant showed potent anthelmintic activity, it took less time to paralyze Indian earthworm Pheretima posthuma 191. In ovicidal assay, ethanolic and aqueous extracts showed 95.89% and 81.72% egg hatch inhibition, respectively, and in larvicidal assay, they showed 56.94% and 92.50% efficacy, respectively 192.
Wound-healing activity
Extracts of Moringa oleifera leaf, dried pulp, and seeds showed a significant increase in hydroxyproline content, wound-closure rate, granuloma-breaking strength, and granuloma dry weight, and a decrease in scar area and skin-breaking strength in incision, excision, and dead space wound models in rats 193.
Studies conducted on the effect of wound healing of Moringa oleifera leaf extract in diabetic animals showed improved tissue regeneration, decreased wound size, downregulated inflammatory mediators, and upregulated vascular endothelial growth factor in wound tissues 194 and remarkable antiproliferative and anti-migratory effects on normal human dermal fibroblasts 195.
Anti-bacterial activity
Ethanolic extract of Moringa oleifera leaf showed antimicrobial activity against all the tested bacteria 196. Chloroform extract reported activity against pathogens such as Salmonella typhi, Pseudomonas aeruginosa, Escherichia coli, and Vibrio cholerae 197.
Ethanolic extracts of Moringa oleifera root and bark possessed antifungal activity against Aspergillus niger, Neurospora crassa, Rhizopus stolonifer, and Microsporum gypseum 198 and also showed inhibitory activity against Leishmania donovani 199. Many studies suggest that extracts of Moringa oleifera seeds could be a potential option to purify water sources as it inhibited bacterial growth in agar and nutrient medium 200.
Methanolic extract of Moringa oleifera leaves inhibited urinary tract pathogens, such as Staphylococcus aureus, Klebsiella pneumoniae, S. saprophyticus, and E. coli 201.
Flavonoids, tannins, steroids, alkaloids, saponins, benzyl isothiocyanate, and benzylglucosinolate were found to be responsible for antimicrobial activity 202, whereas pterygospermin was found to be responsible for antifungal activity 203.
Immunomodulatory activity
Methanolic extract of Moringa oleifera plant stimulated both humoral and cellular immune response 204. In addition, extract showed an increase in optical density and stimulation index, indicating splenocyte proliferation 205.
Antidiarrheal activity
Extract of Moringa oleifera seeds showed significant reduction in gastrointestinal motility and were found to be effective in castor oil induced diarrhoea in male Wister rats 206. Antidiarrheal activity can be attributed to phytochemical ingredients such as tannins, saponins, and flavonoids 207.
Miscellaneous effects
Moringa oleifera leaf extract exhibited a reduction in unwanted sebum secretions from sebaceous gland during winter in humans 208. A systematic review and meta-analysis have clearly accounted Moringa oleifera plant as a galactagogue (help a breastfeeding mother to increase her breast milk supply) 209. Methanolic extract of Moringa oleifera root showed local anesthetic action in frog and guinea pig models 210. Significant CYP3A4 inhibitory effects was exhibited by Moringa oleifera leaf extract 211. Thus, Moringa oleifera has a great potential for herb–drug interactions.
Moringa oleifera side effects
Moringa oleifera is not entirely safe, as many studies have found various compounds that have been associated with major liver, kidney, hematological and other diseases 2. Roasted Moringa oleifera seeds contain potential mutagens such as 4-(α-lramnopyranosyloxy)-benzylglucosinolate, which increase the proportion of micronucleated polychromatophilic erythroblasts, indicative of some degree of genotoxicity 212. The leaf has a high concentration of saponins, which can be potentially harmful for vegetarians, as their consumption reduces the bioavailability of divalent and trivalent metals such as zinc and magnesium 213. Moringin alkaloids, spirochin and the phytochemical benzothiocyanate have been found in the root and bark, toxic substances that predominate in the root and bark; the leaf was therefore identified as the safest edible part 1.
It is also perceived that Moringa oleifera could adversely slow down the breaking down of substances in the liver 214. In that regard, Moringa oleifera could reduce the process of breaking down some medication in the liver. This could progress to cirrhosis and liver failure resulting in malnutrition and weight loss, as well as decreased cognitive function 215. In addition, Moringa oleifera has been noted to be a good regulator of insulin 216. Therefore, patients suffering from lack of insulin are bound to have adverse reductions in their sugar levels when using Moringa oleifera for medicinal purposes 214. It is hypothesised that it could decrease the blood sugar to even lower levels when used in combination with other modern medications 214.
A study by Barichella et al. 217 assessed the use, acceptability and safety of Moringa oleifera on children in Zambia. With regards to safety concerns, supplementation of 14 g per day of Moringa oleifera powder was deemed safe for children and adolescents both in the short and long term. Barichella et al. 218 also noted that mild nausea was reported in 20% of the children at various age groups when meals were supplemented with 20 g of Moringa oleifera daily. These side effects were deemed acceptable by the Ethics Committee 217. Overall, the findings of this study underscore the fact that despite the lack of safety information on the utility of Moringa oleifera, there are no scientifically proven side effects of Moringa oleifera to this date 218.
Moringa oleifera contraindications
Moringa oleifera contains harmful chemicals such as alkaloids and other phytotoxins, which when consumed in high doses have potentially nerve-paralysing properties and other adverse effects 219. Some of these phytochemicals include moringine, moringinine, estrogene, pectinesterase and phenols including tannin 220. There are also unconfirmed reports that Moringa oleifera stems and roots potentially contain harmful phytochemical constituents, especially to pregnant women. Specifically, it is suspected that these elements of Moringa oleifera contain phytochemicals which have a potential of facilitating uterus contraction, leading to miscarriages in pregnant women. It is also suspected that it has the ability to prevent implantation in women, hence it has to be avoided by those attempting to conceive 221. Some scientists suspect that the Moringa oleifera extracts from the roots have a potential of even causing paralysis and death. However, it is important to note that there are no major harmful effects of Moringa oleifera on humans that have been put forth by the scientific community to this date 218. Based on the studies, and ongoing research, that has been conducted to date on both humans and animals, no adverse effects have been noted from Moringa oleifera products 218. Although research is still ongoing, currently there are no scientifically confirmed toxic and harmful effects of Moringa oleifera extracts and products on both humans and animals.
Moringa oleifera summary
Moringa oleifera is one of the most studied and used plants. Its uses stretch from food and medicinal uses to water purification, biopesticide and production of biodiesel. Moringa oleifera leaves, the most used part of the plant, are rich in vitamins, carotenoids, polyphenol, phenolic acids, flavonoids, alkaloids, glucosinolates, isothiocyanates, tannins and saponins. In addition, even if leaves present high variation in the amounts of bioactive compounds as a result of the genetic characteristics of the plant, the environmental conditions to which the plant is subjected and the post-harvest treatments as well, they present greater amounts of these compounds than fruits, vegetables and other plants generally used in human nutrition 18. On the other hand, the high leaves content of oxalates and phytates could limit the intestinal adsorption of minerals. Therefore, this aspect should be taken in to account for future nutritional researches focused on using Moringa oleifera as minerals supplementation.
The high contribution in bioactive compounds may explain the pharmacological properties ascribed to Moringa oleifera leaves. Many test tube and animals studies have widely confirmed numerous pharmacological properties. However, few evidences on human beings are available. Therefore, it is too early to recommend Moringa oleifera leaves as medication in the prevention or treatment of diabetes, cardiovascular disease, dyslipidemia, cancer and infective diseases. Moreover, Moringa oleifera is not entirely safe, as many studies have found various compounds that have been associated with major liver, kidney, hematological and other diseases 2. Roasted Moringa oleifera seeds contain potential mutagens such as 4-(α-lramnopyranosyloxy)-benzylglucosinolate, which increase the proportion of micronucleated polychromatophilic erythroblasts, indicative of some degree of genotoxicity 212. The leaf has a high concentration of saponins, which can be potentially harmful for vegetarians, as their consumption reduces the bioavailability of divalent and trivalent metals such as zinc and magnesium 213. Moringin alkaloids, spirochin and the phytochemical benzothiocyanate have been found in the root and bark, toxic substances that predominate in the root and bark; the leaf was therefore identified as the safest edible part 1. Although research is still ongoing, currently there are no scientifically confirmed toxic and harmful effects of Moringa oleifera extracts and products on both humans and animals 218.
References- Martínez L., Bastida P., Castillo J., Ros G., Nieto G. Green alternatives to synthetic antioxidants, antimicrobials, nitrates, and nitrites in clean label Spanish Chorizo. Antioxidants. 2019;8:184. doi: 10.3390/antiox8060184
- Milla, P. G., Peñalver, R., & Nieto, G. (2021). Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants (Basel, Switzerland), 10(2), 318. https://doi.org/10.3390/plants10020318
- Gopalakrishnan L, Doriya K, Kumar DS. Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci Hum Welln. (2016) 5:49–56. doi: 10.1016/j.fshw.2016.04.001
- Zongo U, Zoungrana SL, Savadogo A, Traoré AS. Nutritional and clinical rehabilitation of severely malnourished children with Moringa oleifera Lam. leaf powder in Ouagadougou (Burkina Faso). Food Nutr Sci. (2013) 4:991–7. doi: 10.4236/fns.2013.49128
- Bamishaiye EI, Olayemi FF, Awagu EF, Bamshaiye OM. Proximate and phytochemical composition of Moringa oleifera leaves at three stages of maturation. Adv J Food Sci Tech. (2011) 3:233–7.
- Shah MA, Bosco S, Mir SA. Effect of Moringa oleifera leaf extract on the physicochemical properties of modified atmosphere packaged raw beef. Food Packaging Shelf. (2015) 3:31–8. doi: 10.1016/j.fpsl.2014.10.001
- Mendieta-Araica B, Sporndly R, Sanchez NR, Sporndly E. Moringa (Moringa oleifera) leaf meal as a source of protein in locally produced concentrates for dairy cows fed low protein diets in tropical areas. Livest Sci. (2011) 137:10–7. doi: 10.1016/j.livsci.2010.09.021
- Qwele K, Hugo A, Oyedemi SO, Moyo B, Masika PJ, Muchenje V. Chemical composition, fatty acid content and antioxidant potential of meat from goats supplemented with Moringa (Moringa oleifera) leaves, sun flower cake and grass hay. Meat Sci. (2013) 93:455–62. doi: 10.1016/j.meatsci.2012.11.009
- Wapi C, Nkukwana TT, Hoffman LC, Dzama K, Pieterse E, Mabusela T, et al. Physico-chemical shelf-life indicators of meat from broilers given Moringa oleifera leaf meal. S Afr J Anim Sci. (2013) 43:43–7. doi: 10.4314/sajas.v43i5.8
- Afuang W, Siddhuraju P, Becker K. Comparative nutritional evaluation of raw, methanol extracted residues and methanol extracts of Moringa (Moringa oleifera Lam) leaves on growth performance and feed utilization in Nile tilapia (Oreochromis niloticus L). Aquaculture Res. (2003) 34:1147–59. doi: 10.1046/j.1365-2109.2003.00920.x
- Current Status and Potential of Moringa oleifera Leaf as an Alternative Protein Source for Animal Feeds. Front. Vet. Sci., 26 February 2020. https://doi.org/10.3389/fvets.2020.00053
- Ashfaq M., Basra S.M., Ashfaq U. Moringa: A Miracle Plant for Agro-forestry. J. Agric. Soc. Sci. 2012;8:115–122.
- Popoola J.O., Obembe O.O. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J. Ethnopharmacol. 2013;150:682–691. doi: 10.1016/j.jep.2013.09.043
- Karmakar A., Karmakar S., Mukherjee S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010;101:7201–7210. doi: 10.1016/j.biortech.2010.04.079
- Rashid U., Anwar F., Moser B.R., Knothe G. Moringa oleifera oil: A possible source of biodiesel. Bioresour. Technol. 2008;99:8175–8179. doi: 10.1016/j.biortech.2008.03.066
- Ofor M.O., Nwufo M.I. The search for alternative energy sources: Jatropha and moringa seeds for biofuel production. J. Agric. Soc. Res. 2011;11:87–94.
- Biswas W.K., John M.B. Life Cycle Assessment of Biodiesel Production from Moringa oleifera Oilseeds. Curtin University of Technology, Centre of Excellence in Cleaner Production (COE); Perth, Australia: 2008.
- Leone, A., Spada, A., Battezzati, A., Schiraldi, A., Aristil, J., & Bertoli, S. (2015). Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview. International journal of molecular sciences, 16(6), 12791–12835. https://doi.org/10.3390/ijms160612791
- Oyeyinka A.T., Oyeyinka S.A. Moringa oleifera as a food fortificant: Recent trends and prospects. J. Saudi Soc. Agric. Sci. 2018;17:127–136. doi: 10.1016/j.jssas.2016.02.002
- Sagona W.C.J., Chirwa P.W., Sajidu S.M. The miracle mix of Moringa: Status of Moringa research and development in Malawi. S. Afr. J. Bot. 2020;129:138–145. doi: 10.1016/j.sajb.2019.03.021
- Folkard G., Sutherland J. Moringa oleifera un árbol con enormes potencialidades. Traducido De Agrofor. Today. 1996;8:5–8.
- Gómez V., Angulo J.O. Revisión Las Características Y Usosde la Planta Moringa Oleífera. Sist. Inf. Científica. 2014;22:309–330.
- Ashfaq M., Basra S.M.A., Ashfaq U. Moringa: A Miracle Plant of Agro-forestry. J. Agric. Soc. Sci. 2012 8:115–122.
- Rivas-Vega M.E., López-Pereira J.L., Miranda-Baeza A., Sandoval-Muy M.I. Sustitución parcial de harina de sardina con Moringa oleifera en alimentos balanceados para juveniles de tilapia (Oreochromismossambicus x Oreochromisniloticus) cultivada en agua de mar. BIOtecnia. 2012;14:3. doi: 10.18633/bt.v14i2.117
- Gómez A.V., Angulo K. Revision de las caracteristicas y usos de la planta moringa oleifera. Artic. De Revis. 2014;22:309–330.
- Asensi G.D., Villadiego A.M.D., Berruezo G.R. Moringa oleifera: Revisión sobre aplicaciones y usos en alimentos. Arch. Lat. De Nutr. 2017;67:86–97.
- Ogunsina B.S., Radha C., Indrani D. Quality characteristics of bread and cookies enriched with debittered Moringa oleifera seed flour. Int. J. Food Sci. Nutr. 2011;62:185–194. doi: 10.3109/09637486.2010.526928
- Chizoba N.N. Sensory Evaluation of Cookies Produced from Different Blends of Wheat and moringa Oleifera Leaf Flour. Int. J. Nutr. Food Sci. 2014;3:307. doi: 10.11648/j.ijnfs.20140304.21
- Rabie M.M., Faten I., Youssif Y., Ezz El-Ragal N. Effect of Moringa oleifera Leaves and Seeds Powder Supplementation on Quality Characteristics of Cookies. J. Food Dairy Sci. J. 2020;11:65–73. doi: 10.21608/jfds.2020.78888
- Nutan Narwal R.R. Nutritional evaluation and organoleptic acceptability of baked products based on blend flour (maize) Int. J. Curr. Res. 2017;9:52909–52912.
- Dos Santos A.F.R., Pontes E.D.S., de Araújo M.G.G., Melo P.C.M.F., Viera V.B., Jerônimo H.M.Â. Elaboração e caracterização física e físico-química de um brownie enriquecido com farinha da folha de Moringa (Moringa oleífera) Res. Soc. Dev. 2020;9:e101973927. doi: 10.33448/rsd-v9i7.3927
- Kolawole F., Balogun M., Opaleke D., Amali H. An Evaluation of Nutritional and Sensory Qualities of Wheat -Moringa Cake. Agrosearch. 2013;13:87. doi: 10.4314/agrosh.v13i1.9
- El-Gammal R., Ghoneim G., ElShehawy S. Effect of Moringa Leaves Powder (Moringa oleifera) on Some Chemical and Physical Properties of Pan Bread. J. Food Dairy Sci. 2016;7:307–314. doi: 10.21608/jfds.2016.46005
- Bolarinwa I.F., Aruna T.E., Raji A.O. Nutritive value and acceptability of bread fortified with moringa seed powder. J. Saudi Soc. Agric. Sci. 2019;18:195–200. doi: 10.1016/j.jssas.2017.05.002
- Devisetti R., Sreerama Y.N., Bhattacharya S. Processing effects on bioactive components and functional properties of moringa leaves: Development of a snack and quality evaluation. J. Food Sci. Technol. 2015;53:649–657. doi: 10.1007/s13197-015-1962-5
- Olson M.E., Fahey J.W. Moringa oleifera: Un árbol multiusos para las zonas tropicales secas. Rev. Mex. De Biodivers. 2011;82:1071–1082. doi: 10.22201/ib.20078706e.2011.4.678
- El-Massry F.H.M., Mossa M.E.M., Youssef S.M. Moringa oleifera Plant “Value and Utilization in Food Processing” J. Agric. Res. 2013;91:1597–1609.
- Timilsena Y.P., Vongsvivut J., Adhikari R., Adhikari B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017;228:394–402. doi: 10.1016/j.foodchem.2017.02.021
- Martín C., Martín G., García A., Fernández T., Hernandez E., Puls J. Potenciales aplicaciones de Moringa oleifera. Una revisión crítica. Pastos Forrajes. 2013;36:137–149.
- Sengev A.I., Abu J.O., Gernah D.I. Effect of Moringa oleifera Leaf Powder Supplementation on Some Quality Characteristics of Wheat Bread. Food Nutr. Sci. 2013;4:270–275.
- Castro-López C., Ventura-Sobrevilla J.M., González-Hernández M.D., Rojas R., Ascacio-Valdés J.A., Aguilar C.N., Martínez-Ávila G.C. Impact of extraction techniques on antioxidant capacities and phytochemical composition of polyphenol-rich extracts. Food Chem. 2017;237:1139–1148. doi: 10.1016/j.foodchem.2017.06.032
- Shah M.A., Bosco S.J.D., Mir S.A. Effect of Moringa oleifera leaf extract on the physicochemical properties of modified atmosphere packaged raw beef. Food Packag. Shelf Life. 2015;3:31–38. doi: 10.1016/j.fpsl.2014.10.001
- Tesfay S.Z., Magwaza L.S., Mbili N., Mditshwa A. Carboxyl methylcellulose (CMC) containing moringa plant extracts as new postharvest organic edible coating for Avocado (Persea americana Mill.) fruit. Scientia Horticulturae. 2017;226:201–207. doi: 10.1016/j.scienta.2017.08.047
- Jayawardana B.C., Liyanage R., Lalantha N., Iddamalgoda S., Weththasinghe P. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT Food Sci. Technol. 2015;64:1204–1208. doi: 10.1016/j.lwt.2015.07.028
- Manaois R., Morales A., Abilgos-ramos R. Acceptability, Shelf Life and Nutritional Quality of Moringa-Supplemented Rice Crackers. Philipp. J. Crop Sci. 2013;38:1–8.
- Cushnie T.P.T., Cushnie B., Lamb A.J. Alkaloids: An overview of their antibacterial, antibiotic-enhancing and antivirulence activities. Int. J. Antimicrob. Agents. 2014;44:377–386. doi: 10.1016/j.ijantimicag.2014.06.001
- Sreelatha S., Padma P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum. Nutr. 2009;64:303–311. doi: 10.1007/s11130-009-0141-0
- Panda S., Kar A., Sharma P., Sharma A. Cardioprotective potential of N,α-L-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorg. Med. Chem. Lett. 2013;23:959–962. doi: 10.1016/j.bmcl.2012.12.060
- Ferreira P.M.P., Farias D.F., Oliveira J.T.D.A., Carvalho A.D.F.U. Moringa oleifera: Bioactive compounds and nutritional potential. Rev. Nutr. 2008;21:431–437. doi: 10.1590/S1415-52732008000400007
- Alvarez R., Vaz B., Gronemeyer H., de Lera A.R. Functions, therapeutic applications, and synthesis of retinoids and carotenoids. Chem. Rev. 2014;114:1–125. doi: 10.1021/cr400126u
- Kidmose U., Yang R.Y., Thilsted S.H., Christensen L.P., Brandt K. Content of carotenoids in commonly consumed Asian vegetables and stability and extractability during frying. J. Food Comp. Anal. 2006;19:562–571. doi: 10.1016/j.jfca.2006.01.011
- Moyo B., Masika P.J., Hugo A., Muchenje V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr. J. Biotechnol. 2011;10:12925–12933.
- Joshi P., Mehta D. Effect of dehydration on the nutritive value of drumstick leaves. J. Metabolomics Syst. Biol. 2010;1:5–9.
- Zhang M., Hettiarachchy S.N., Horax R., Kannan A., Praisoody M.D.A., Muhundan A., Mallangi C.R. Phytochemicals, antioxidant and antimicrobial activity of Hibiscus sabdariffa, Centella asiatica, Moringa oleifera and Murraya koenigii leaves. J. Med. Plants Res. 2011;5:6672–6680.
- Ramachandran C., Peter K.V., Gopalakrishnan P.K. Drumstick (Moringa oleifera): A multipurpose Indian vegetable. Econ. Bot. 1980;34:276–283. doi: 10.1007/BF02858648
- Chambial S., Dwivedi S., Shukla K.K., John P.J., Sharma P. Vitamin C in disease prevention and cure: An overview. Indian J. Clin. Biochem. 2013;28:314–328. doi: 10.1007/s12291-013-0375-3
- Ching L.S., Mohamed S. Alpha-tocopherol content in 62 edible tropical plants. J. Agric. Food Chem. 2001;49:3101–3105. doi: 10.1021/jf000891u
- Borel P., Preveraud D., Desmarchelier C. Bioavailability of vitamin E in humans: An update. Nutr. Rev. 2013;71:319–331. doi: 10.1111/nure.12026
- Sanchez-Machado D.I., Lopez-Cervantes J., Vázquez N.J.R. High-performance liquid chromatography method to measure α-and γ-tocopherol in leaves, flowers and fresh beans from Moringa oleifera. J. Chromatogr. A. 2006;1105:111–114. doi: 10.1016/j.chroma.2005.07.048
- Price M.L. ECHO Technical Note. ECHO; Myers, FL, USA: 1985. The moringa tree.
- Girija V., Sharada D., Pushpamma P. Bioavailability of thiamine, riboflavin and niacin from commonly consumed green leafy vegetables in the rural areas of Andhra Pradesh in India. Int. J. Vitam. Nutr. Res. 1982;52:9–13.
- Iqbal S., Bhanger M.I. Effect of season and production location on antioxidant activity of Moringa oleifera leaves grown in Pakistan. J. Food Comp. Anal. 2006;19:544–551. doi: 10.1016/j.jfca.2005.05.001
- Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem. 2003 Apr 9;51(8):2144-55. doi: 10.1021/jf020444+
- Singh B.N., Singh B.R., Singh R.L., Prakash D., Dhakarey R., Upadhyay G., Singh H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009;47:1109–1116. doi: 10.1016/j.fct.2009.01.034
- Bhatta R., Saravanan M., Baruah L., Sampath K.T. Nutrient content, in vitro ruminal fermentation characteristics and methane reduction potential of tropical tannin-containing leaves. J. Sci. Food Agric. 2012;92:2929–2935. doi: 10.1002/jsfa.5703
- Fu L., Xu B.-T., Xu X.-R., Gan R.-Y., Zhang Y., Xia E.-Q., Li H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079
- Sultana B., Anwar F., Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–2180. doi: 10.3390/molecules14062167
- Prakash D., Suri S., Upadhyay G., Singh B.N. Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int. J. Food Sci. Nutr. 2007;58:18–28. doi: 10.1080/09637480601093269
- Bajpai M., Pande A., Tewari S.K., Prakash D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int. J. Food Sci. Nutr. 2005;56:287–291. doi: 10.1080/09637480500146606
- Makkar H.P.S., Becker K. Nutrional value and antinutritional components of whole and ethanol extracted Moringa oleifera Leaves. Anim. Feed Sci. Technol. 1996;63:211–228. doi: 10.1016/S0377-8401(96)01023-1
- Vongsak B., Sithisarn P., Mangmool S., Thongpraditchote S., Wongkrajang Y., Gritsanapan W. Maximizing total phenolics, total flavonoids contents and antioxidant activity of Moringa oleifera Leaf extract by the appropriate extraction method. Ind. Crop. Prod. 2013;44:566–571. doi: 10.1016/j.indcrop.2012.09.021
- Verma S., Singh A., Mishra A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013;35:473–485. doi: 10.1016/j.etap.2013.02.011
- Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750
- Yang R.Y., Yang R.Y., Lin S., Kuo G. Content and distribution of flavonoids among 91 edible plant species. Asia Pac. J. Clin. Nutr. 2008;17:275–279.
- Coppin J.P., Xu Y., Chen H., Pan M.H., Ho C.T., Juliani R., Simon J.E., Wu Q. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J. Funct. Foods. 2013;5:1892–1899. doi: 10.1016/j.jff.2013.09.010
- Amaglo N.K., Bennett R.N., Lo Curto R.B., Rosa E.A.S., Lo Turco V., Giuffrida A., Lo Curto A., Crea F., Timpo G.M. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010;122:1047–1054. doi: 10.1016/j.foodchem.2010.03.073
- Siddhuraju P., Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003;51:2144–2155. doi: 10.1021/jf020444+
- Sultana B., Anwar F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008;108:879–884. doi: 10.1016/j.foodchem.2007.11.053
- Bennett R.N., Mellon F.A., Foidl N., Pratt J.H., Dupont M.S., Perkins L., Kroon P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish Tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003;51:3546–3553. doi: 10.1021/jf0211480
- Forster N., Ulrichs C., Schreiner M., Muller C.T., Mewis I. Development of a reliable extraction and quantification method for glucosinolates in Moringa oleifera. Food Chem. 2015;166:456–464. doi: 10.1016/j.foodchem.2014.06.043
- Ciska E., Martyniak-Przybyszewska B., Kozlowska H. Content of glucosinolates in cruciferous vegetables grown at the same site for two years under different climatic conditions. J. Agric. Food Chem. 2000;48:2862–2867. doi: 10.1021/jf981373a
- Prakash D., Gupta C. Glucosinolates: The phytochemicals of nutraceutical importance. J. Complement. Integr. Med. 2012;9 doi: 10.1515/1553-3840.1611
- Waterman C., Rojas-Silva P., Tumer T., Kuhn P., Richard A.J., Wicks S., Stephens J.M., Wang Z., Mynatt R., Cefalu W., et al. Isothiocyanate-rich Moringa oleifera extract reduces weight gain, insulin resistance and hepatic gluconeogenesis in mice. Mol. Nutr. Food Res. 2015 doi: 10.1002/mnfr.201400679
- Dinkova-Kostova A.T., Kostov R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012;18:337–347. doi: 10.1016/j.molmed.2012.04.003
- Kancheva V.D., Kasaikina O.T. Bio-antioxidants-a chemical base of their antioxidant activity and beneficial effect on human health. Curr. Med. Chem. 2013;20:4784–805. doi: 10.2174/09298673113209990161
- Richter N., Siddhuraju P., Becker K. Evaluation of nutritional quality of moringa (Moringa oleifera Lam.) leaves as an alternative protein source for Nile tilapia (Oreochromis niloticus L.) Aquaculture. 2003;217:599–611. doi: 10.1016/S0044-8486(02)00497-0
- Venkatachalam M., Sathe S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006;54:4705–4714. doi: 10.1021/jf0606959
- Johns T., Mahunnah R.L., Sanaya P., Chapman L., Ticktin T. Saponins and phenolic content in plant dietary additives of a traditional subsistence community, the Batemi of Ngorongoro District, Tanzania. J. Ethnopharmacol. 1999;66:1–10. doi: 10.1016/S0378-8741(98)00179-2
- Kahkonen M.P., Hopia A.I., Heinonen M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001;49:4076–4082. doi: 10.1021/jf010152t
- Edeoga H.O., Okwu D.E., Mbaebie B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005;4:685–688. doi: 10.5897/AJB2005.000-312
- Teixeira E.M.B., Carvalho M.R.B., Neves V.A., Silva M.A., Arantes-Pereira L. Chemical characteristics and fractionation of proteins from Moringa oleifera Lam. leaves. Food Chem. 2014;147:51–54. doi: 10.1016/j.foodchem.2013.09.135
- Makkar H.P.S., Becker K. Nutrients and antiquality factors in different morphological parts of the Moringa oleifera tree. J. Agric. Sci. 1997;128:311–322. doi: 10.1017/S0021859697004292
- Augustin J.M., Kuzina V., Andersen S.B., Bak S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry. 2011;72:435–457. doi: 10.1016/j.phytochem.2011.01.015
- Tian X., Tang H., Lin H., Cheng G., Wang S., Zhang X. Saponins: The potential chemotherapeutic agents in pursuing new anti-glioblastoma drugs. Mini Rev Med Chem. 2013;13:1709–1724. doi: 10.2174/13895575113136660083
- Edeoga H.O., Okwu D.E., Mbaebie B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005;4:685–688. doi: 10.5897/AJB2005.000-3127
- Yun T.K., Lee Y.S., Kwon H.Y., Choi K.J. Saponin contents and anticarcinogenic effects of ginseng depending on types and ages in mice. Zhongguo Yao Li Xue Bao. 1996;17:293–298.
- Gupta S., Jyothi Lakshmi A., Manjunath M.N., Prakash J. Analysis of nutrient and antinutrient content of underutilized green leafy vegetables. LWT Food Sci. Technol. 2005;38:339–345. doi: 10.1016/j.lwt.2004.06.012
- Hídvégi M., Lásztity R. Phytic acid content of cereals and legumes and interaction with proteins. Chem. Eng. 2003;46:59–64.
- García-Estepa R.M., Guerra-Hernández E., García-Villanova B. Phytic acid content in milled cereal products and breads. Food Res. Int. 1999;32:217–221. doi: 10.1016/S0963-9969(99)00092-7
- Sivasankari B., Anandharaj M., Gunasekaran P. An ethnobotanical study of indigenous knowledge on medicinal plants used by the village peoples of Thoppampatti, Dindigul district, Tamilnadu, India. J. Ethnopharmacol. 2014;153:408–423. doi: 10.1016/j.jep.2014.02.040
- Yabesh J.E., Prabhu S., Vijayakumar S. An ethnobotanical study of medicinal plants used by traditional healers in silent valley of Kerala, India. J. Ethnopharmacol. 2014;154:774–789. doi: 10.1016/j.jep.2014.05.004
- Abe R., Ohtani K. An ethnobotanical study of medicinal plants and traditional therapies on Batan Island, the Philippines. J. Ethnopharmacol. 2013;145:554–565. doi: 10.1016/j.jep.2012.11.029
- Sanjay P., Dwivedi K. Shigru (Moringa Oleifera Lam.): A Critical Review. Int. J. Ayu. Pharm. Chem. 2015
- Kar A, Panda S, Bharti S. Relative efficacy of three medicinal plant extracts in the alteration of thyroid hormone concentrations in male mice. J. Ethnopharmacol. 81(2), 281–285 (2002).
- Mollik AH. Abstract. 1904;298 Plants from Sundarbans to the diet of lactating mothers during puerperium of Barguna district of Bangladesh. Pediatr Nephrol 2010;25.
- Estrella MC, Mantaring JB, David GZ, et al. A double-blind, randomized controlled trial on the use of malunggay (Moringa oleifera) for augmentation of the volume of breastmilk among non-nursing mothers of preterm infants. Philipp J Pediatr. 2000;49:3–6.
- Co MM, Hernandez EA, Co BG. A comparative study on the efficacy of the different galactogogues among mothers with lactational insufficiency. Presented at the American Academy of Pediatrics Section on Breastfeeding 2002:NCE. Abstract.
- Breastfeeding Challenges: ACOG Committee Opinion, Number 820. Obstet Gynecol. 2021 Feb 1;137(2):e42-e53. doi: 10.1097/AOG.0000000000004253
- Drugs and Lactation Database (LactMed) [Internet]. Bethesda (MD): National Library of Medicine (US); 2006-. Moringa. [Updated 2021 Feb 15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK501899
- Williams L.L. Moringa olefiera: Could this be an Answer to our Need for an Alternative to Fighting Drug-Resistance and Chronic Infections? Med. Aromat Plants. 2013;2:1–3. doi: 10.4172/2167-0412.1000e142
- Bhattacharya A., Tiwari P., Sahu P.K., Kumar S. A review of the phytochemical and pharmacological characteristics of moringa oleifera. J. Pharm. Bioallied Sci. 2018;10:181–191.
- Saini R.K., Sivanesan I., Keum Y.S. Phytochemicals of Moringa oleifera: A review of their nutritional, therapeutic and industrial significance. 3 Biotech. 2016;6 doi: 10.1007/s13205-016-0526-3
- Das N., Sikder K., Bhattacharjee S., Majumdar S.B., Ghosh S., Majumdar S., Dey S. Quercetin alleviates inflammation after short-term treatment in high-fat-fed mice. Food Funct. 2013;4:889–898. doi: 10.1039/c3fo30241e
- De Melo G.O., Malvar D.D., Vanderlinde F.A., Rocha F.F., Pires P.A., Costa E.A., de Matos L.G., Kaiser C.R., Costa S.S. Antinociceptive and anti-inflammatory kaempferol glycosides from Sedum dendroideum. J. Ethnopharmacol. 2009;124:228–232. doi: 10.1016/j.jep.2009.04.024
- Arora R., Malhotra P., Sharma A., Haniadka R., Yashawanth H.S., Baliga M.S. Bioactive Food as Interventions for Arthritis and Related Inflammatory Diseases. Elsevier Inc.; Amsterdam, The Netherlands: 2013. Medicinal Efficacy of Indian Herbal Remedies for the Treatment of Arthritis; pp. 601–617.
- Zhao S., Zhang D. Supercritical fluid extraction and characterisation of Moringa oleifera leaves oil. Sep. Purif. Technol. 2013;118:497–502. doi: 10.1016/j.seppur.2013.07.046
- Singh B.N., Singh B.R., Singh R.L., Prakash D., Dhakarey R., Upadhyay G., Singh H. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009;47:1109–1116. doi: 10.1016/j.fct.2009.01.034
- Panda S., Kar A., Sharma P., Sharma A. Cardioprotective potential of N,α-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorg. Med. Chem. Lett. 2013;23:959–962. doi: 10.1016/j.bmcl.2012.12.060
- Galuppo M., Nicola G., Iori R., Dell’Utri P., Bramanti P., Mazzon E. Antibacterial Activity of Glucomoringin Bioactivated with Myrosinase against Two Important Pathogens Affecting the Health of Long-Term Patients in Hospitals. Molecules. 2013;18:14340–14348. doi: 10.3390/molecules181114340
- Chaudhary K., Chaurasia S. Neutraceutical Properties of Moringa oleifera: A Review. Eur. J. Pharm. Med. Res. 2017;4:646–655.
- Khor K.Z., Lim V., Moses E.J., Abdul Samad N. The In Vitro and In Vivo Anticancer Properties of Moringa oleifera. Evid.-Based Complementary Altern. Med. 2018;2018:1071243. doi: 10.1155/2018/1071243
- Thapa K., Poudel M., Adhikari P. Moringa oleifera: A Review Article on Nutritional Properties and its Prospect in the Context of Nepal. Acta Sci. Agric. 2019;3:47–54. doi: 10.31080/ASAG.2019.03.0683
- Govardhan Singh R.S., Negi P.S., Radha C. Phenolic composition, antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J. Funct. Foods. 2013;5:1883–1891. doi: 10.1016/j.jff.2013.09.009
- Farooq F., Rai M., Tiwari A., Khan A.A., Farooq S. Medicinal properties of Moringa oleifera. J. Med. Plants Res. 2012 6:4368–4374.
- Fahey J. Moringa oleifera: A Review of the Medical Evidence for Its Nutritional, Therapeutic, and Prophylactic Properties. Part 1. Trees Life J. 2005:1–15. doi: 10.1201/9781420039078.ch12
- Mahajan S.G., Mehta A.A. Suppression of ovalbumin-induced Th2-driven airway inflammation by β-sitosterol in a guinea pig model of asthma. Eur. J. Pharmacol. 2011;650:458–464. doi: 10.1016/j.ejphar.2010.09.075
- Bhattacharya A, Agrawal D, Sahu PK, Kumar S, Mishra SS, Patnaik S. Analgesic effect of ethanolic leaf extract of Moringa oleifera on albino mice. Indian J Pain. 2014;28:89–94.
- Manaheji H, Jafari S, Jalal Z, Shamsali R, Reza T. Analgesic effects of methanolic extracts of the leaf or root of Moringa oleifera on complete Freund’s adjuvant-induced arthritis in rats. J Chin Integ Med. 2011;9:217–22.
- Kanchan PU, Vinod DR, Vijay BM. Antimigraine activity study of Moringa oleifera leaf juice. Int J Green Pharm. 2012;6:204–7.
- Jurairat K, Jintanaporn W, Supaporn M, Wipawee T, Cholathip T, Panakaporn W, et al. Moringa oleifera leaves extract attenuates neuropathic pain induced by chronic constriction injury. Am J Appl Sci. 2012;9:1182–7.
- Bhattacharya A, Agrawal D, Sahu PK, Swain TR, Kumar S, Mishra SS. Anti-inflammatory effect of ethanolic extract of Moringa oleifera leaves on albino rats. RJPBCS. 2014;5:540–4.
- Ezeamuzie IC, Ambakederemo AW, Shode FO, Ekwebelem SC. Anti-inflammatory effects of Moringa oleifera root extract. Int J Pharmacogn. 1996;34:207–12.
- McKnight M, Allen J, Waterman JD, Hurley S, Idassi J, Minor RC. Moringa tea blocks acute lung inflammation induced by swine confinement dust through a mechanism involving TNF-α expression, c-Jun N-terminal kinase activation and neutrophil regulation. Am J Immunol. 2014;10:73–87.
- Venkateshwara KN, Gopalakrishnan V, Loganathan V. Antiinflammatory action of Moringa oleifera Lam. Anc Sci Life. 1999;18:195–8.
- Bhattacharya A, Behera R, Agrawal D, Sahu PK, Kumar S, Mishra SS. Antipyretic effect of ethanolic extract of Moringa oleifera leaves on albino rats. Tanta Med J. 2014;42:74–8.
- Kumar S. Medicinal importance of Moringa oleifera: drumstick plant. Indian J Sci Res. 2017;16:129–32.
- Purwal L, Shrivastava V, Jain UK. Anti-tumour activity of crude extracts of leaves of Moringa oleifera (Moringaceae) Indian Drugs. 2010;47:31–4.
- Tiloke C, Phulukdaree A, Chuturgoon AA. The antiproliferative effect of Moringa oleifera crude aqueous leaf extract on cancerous human alveolar epithelial cells. BMC Complement Altern Med. 2013;13:226.
- Pachava VR, Krishnamurthy PT, Dahabal SP, Wadhwani A, Chinthamani PK. Anti-angiogenic potential of ethyl acetate extract of Moringa oleifera Lam leaves in chick chorioallantoic membrane (CAM) assay. J Nat Prod Plant Resource. 2017;7:18–22.
- Budda S, Butryee C, Tuntipopipat S, Rungsipipat A, Wangnaithum S, Lee JS, et al. Suppressive effects of Moringa oleifera Lam pod against mouse colon carcinogenesis induced by azoxymethane and dextran sodium sulfate. Asian Pac J Cancer Prev. 2011;12:3221–8.
- Abd-Rabou AA, Abdalla AM, Ali NA, Zoheir KM. Moringa oleifera root induces cancer apoptosis more effectively than leave nanocomposites and its free counterpart. Asian Pac J Cancer Prev. 2017;18:2141–9.
- Fernandes EE, Pulwale AV, Patil GA, Moghe AS. Probing regenerative potential of Moringa oleifera aqueous extracts using in vitro cellular assays. Pharmacognosy Res. 2016;8:231–7.
- Charoensin S. Antioxidant and anticancer activities of Moringa oleifera leaves. J Med Plants Res. 2014;8:318–25.
- Luqman S, Suchita S, Ritesh K, Anil KM, Debabrata C. Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vivo and in vitro . Evid Based Complement Alternat Med 2012. 2012:1–17.
- Satish A, Reddy PV, Sairam S, Ahmed F, Urooj A. Antioxidative effect and DNA protecting property of Moringa oleifera root extracts. J. Herbs Spices Med Plants. 2014;20:209–20.
- Paliwal R, Sharma V, Pracheta SS. Elucidation of free radical scavenging and antioxidant activity of aqueous and hydro-ethanolic extracts of Moringa oleifera pods. Res J Pharm Technol. 2011;4:566–71.
- He TB, Huang YP, Huang Y, Wang XJ, Hu JM, Sheng J. Structural elucidation and antioxidant activity of an arabinogalactan from the leaves of Moringa oleifera. Int J Biol Macromol. 2018;112:126–33.
- Stavros L, John T. Extraction and identification of natural antioxidants from the seeds of the Moringa oleifera tree variety of Malawi. J Am Oil Chem Soc. 2002;79:677–83.
- Ranira G, Rimi H, Kaushik R, Debajani G. Effect of Moringa oleifera in experimental model of Alzheimer’s disease: role of antioxidants. Ann Neurosci. 2005;12:33–6.
- Ganguly R, Guha D. Alteration of brain monoamines and EEG wave pattern in rat model of Alzheimer’s disease and protection by Moringa oleifera. Indian J Med Res. 2008;128:744–51.
- Mohan M, Kaul N, Punekar A, Girnar R, Junnare P, Patil L. Nootropic activity of Moringa oleifera leaves. J Nat Remedies. 2005;5:59–62.
- Akram M, Nawaz A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen Res. 2017;12:660–70.
- Amrutia JN, Lala M, Srinivasa U, Shabaraya AR, Semuel MR. Anticonvulsant activity of Moringa oleifera leaf. Int Res J Pharm. 2011;2:160–2.
- Fathima SN, Vasudevamurthy S, Rajkumar N. A review on phytoextracts with antiepileptic property. J Pharm Sci Res. 2015;7:994–1003.
- Kaur G, Invally M, Sanzagiri R, Buttar HS. Evaluation of the antidepressant activity of Moringa oleifera alone and in combination with fluoxetine. J Ayurveda Integr Med. 2015;6:273–9.
- Bhattacharya A, Santra S, Mahapatra S, Sahu PK, Agrawal D, Kumar S. Study of anxiolytic effect of ethanolic extract of drumstick tree leaves on albino mice in a basic neuropharmacology laboratory of a postgraduate teaching institute. J Health Res Rev. 2016;3:41–7.
- Cajuday LA, Pocsidio GL. Effects of Moringa oleifera Lam.(Moringaceae) on the reproduction of male mice (Musmusculus) J Med Plants Res. 2010;4:1115–21.
- Nayak G, Honguntikar SD, Kalthur SG, D’Souza AS, Mutalik S, Setty MM, et al. Ethanolic extract of Moringa oleifera Lam. leaves protect the pre-pubertal spermatogonial cells from cyclophosphamide-induced damage. J Ethnopharmacol. 2016;182:101–9.
- Nath D, Sethi N, Singh RK, Jain AK. Commonly used Indian abortifacient plants with special reference to their teratologic effects in rats. J Ethnopharmacol. 1992;36:147–54.
- Shukla S, Mathur R, Prakash AO. Histoarchitecture of the genital tract of ovariectomized rats treated with an aqueous extract of Moringa oleifera roots. J Ethnopharmacol. 1989;25:249–61.
- Vergara-Jimenez M, Almatrafi MM, Fernandez ML. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants. 2017;6:91.
- Das N, Kunal S, Santinath G, Bernard F, Sanjit D. Moringa oleifera Lam. ethanolic leaf extract prevents early liver injury and restores antioxidant status in mice fed with high-fat diet. Indian J Exp Biol. 2012;404:404–12.
- Omodanisi EI, Aboua YG, Chegou NN, Oguntibeju OO. Hepatoprotective, antihyperlipidemic, and anti-inflammatory activity of Moringa oleifera in diabetic-induced damage in male Wistar rats. Pharmacognosy Res. 2017;9:182–7.
- Eldaim MA, Elrasoul SA, Elaziz SA. An aqueous extract from Moringa oleifera leaves ameliorates hepatotoxicity in alloxan-induced diabetic rats. Biochem Cell Biol. 2017;95:524–30.
- Ruckmani K, Kavimani S, Anandan R, Jaykar B. Effect of Moringa oleifera Lam. on paracetamol induced hepatotoxicity. Indian J Pharm Sci. 1998;60:33–5.
- Dhimmar N, Patel NM, Gajera V, Lambole V. Pharmacological activities of Moringa oleifera: an overview. Res J Pharm Tech. 2015;8:476–80.
- Devaraj VC, Asad M, Prasad S. Effect of leaves and fruits of Moringa oleifera on gastric and duodenal ulcers. Pharm Biol. 2007;45:332–8.
- Debnath S, Guha D. Role of Moringa oleifera on enterochromaffin cell count and serotonin content of experimental ulcer model. Indian J Exp Biol. 2007;45:726–31.
- Ndong M, Uehara M, Katsumata S, Sato S, Suzuki K. Preventive effects of Moringa oleifera (Lam.) on hyperlipidemia and hepatocyte ultrastructural changes in iron deficient rats. Biosci Biotechnol Biochem. 2007;71:1826–33.
- Randriamboavonjy JI, Loirand G, Vaillant N, Lauzier B, Derbré S, Michalet S, et al. Cardiac protective effects of Moringa oleifera seeds in spontaneous hypertensive rats. Am J Hypertens. 2016;29:873–81.
- Gilani AH, Aftab K, Suria A, Siddiqui S, Salem R, Siddique BS, et al. Pharmacological studies on hypotensive and spamolytic activities of pure compounds from Moringa oleifera. Phytother Res. 1994;8:87–91.
- Nandave M, Ojha SK, Joshi S, Kumari S, Arya DS. Moringa oleifera leaf extract prevents isoproterenol-induced myocardial damage in rats: evidence for an antioxidant, antiperoxidative, and cardioprotective intervention. J Med Food. 2009;12:47–55.
- Barbagallo I, Distefano VA, Nicolosi D, Maravigna A, Lazzarino G, Rosa MD, et al. Moringa oleifera Lam. improves lipid metabolism during adipogenic differentiation of human stem cells. Eur Rev Med Pharmacol Sci. 2016;20:5223–32.
- Nahar S, Faisal FM, Iqbal J, Rahman MM, Yusuf MA. Antiobesity activity of Moringa oleifera leaves against high fat diet-induced obesity in rats. Int J Basic Clin Pharmacol. 2016;5:1263–8.
- Pare D, Hilou A, Ouedraogo N, Guenne S. Ethnobotanical study of medicinal plants used as anti-obesity remedies in the nomad and hunter communities of Burkina Faso. Medicines. 2016;3:9.
- Metwally FM, Rashad MH, Ahmed HH, Mahnoud AA, Raouf ER, Abdalla AM. Molecular mechanisms of the anti-obesity potential effect of Moringa oleifera in the experimental model. Asian Pac J Trop Biomed. 2017;7:214–21.
- Anita M, Babita A. Investigation into the mechanism of action of Moringa oleifera for its anti-asthmatic activity. Orient Pharm Exp Med. 2008;8:24–31.
- Goyal BR, Goyal RK, Mehta AA. Investigation into the mechanism of anti-asthmatic action of Moringa oleifera. J Diet Suppl. 2009;6:313–27.
- Mahajan SG, Mehta AA. Effect of Moringa oleifera Lam. seed extract on ovalbumin-induced airway inflammation in guinea pigs. Inhal Toxicol. 2008;20:897–909.
- Suzana D, Suyatna FD, Azizahwati, Andrajati R, Sari SP, Mun’im A. Effect of Moringa oleifera leaves extract against hematology and blood biochemical value of patients with iron deficiency anaemia. J Young Pharm. 2017;9:s79–84.
- Archibong AN, Nku CO, Ofem OE. Extract of Moringa oleifera attenuates hematological parameters following salt loading. MicroMedicine. 2017;5:24–30.
- Yassa HD, Tohamy AF. Extract of Moringa oleifera leaves ameliorates streptozotocin-induced diabetes mellitus in adult rats. Acta Histochem. 2014;116:844–54.
- Khan W, Parveen R, Chester K, Parveen S, Ahmad S. Hypoglycemic potential of aqueous extract of Moringa oleifera leaf and in vivo GC-MS metabolomics. Front Pharmacol. 2017;8:577.
- Nunthanawanich P, Sompong W, Sirikwanpong S, Mäkynen K, Adisakwattana S, Dahlan W, et al. Moringa oleifera aqueous leaf extract inhibits reducing monosaccharide-induced protein glycation and oxidation of bovine serum albumin. Springerplus. 2016;5:1098.
- Al-Malki AL, El Rabey HA. The antidiabetic effect of low doses of Moringa oleifera Lam. seeds on streptozotocin induced diabetes and diabetic nephropathy in male rats. Biomed Res Int 2015. 2015:381040.
- Karadi RV, Palkar MB, Gaviraj EN, Gadge NB, Mannur VS, Alagawadi KR. Antiurolithiatic property of Moringa oleifera root bark. Pharm Biol. 2008;46:861–5.
- Paikra BK, Dhongade HKJ, Gidwani B. Phytochemistry and pharmacology of Moringa oleifera Lam. J Pharmacopuncture. 2017;20:194–200.
- Caceres A, Saravia A, Rizzo S, Zabala L, Leon ED, Nave F. Pharmacologic properties of Moringa oleifera 1: preliminary screening for antimicrobial activity. J Ethnopharmacol. 1992;36:233–7.
- Mahajan SG, Mehta AA. Inhibitory action of ethanolic extract of seeds of Moringa oleifera Lam. on systemic and local anaphylaxis. j Immunotoxicol. 2007;4:287–94.
- Hagiwara A, Hidaka M, Takeda S, Yoshida H, Kai H, Sugita C, et al. Anti-allergic action of aqueous extract of Moringa oleifera Lam. leaves in mice. Eur J Med Plants. 2016;16:1–10.
- Rastogi T, Bhutda V, Moon K, Aswar PB, Khadabadi SS. Comparative study on the anthelmintic activity of Moringa oleifera and Vitex negundo. Asian J Res Chem. 2009;2:181–2.
- Cabardo DE, Jr, Portugaliza HP. Anthelmintic activity of Moringa oleifera seed aqueous and ethanolic extracts against Haemonchus contortus eggs and third stage larvae. Int J Vet Sci Med. 2017;5:30–4.
- Momoh MA, Salome CA, Onyishi IV. Natural healing compound for the treatment of excision and incision wound in rat’s model. Int J Pharm Sci Rev Res. 2013;22:1–5.
- Muhammad AA, Arulselvan P, Cheah PS, Abas F, Fakurazi S. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam. on experimentally induced diabetic animal model. Drug Des Devel Ther. 2016;10:1715–30.
- Gothai S, Arulselvan P, Tan WS, Fakurazi S. Wound healing properties of ethyl acetate fraction of Moringa oleifera in normal human dermal fibroblasts. J Intercult Ethnopharmacol. 2016;5:1–6.
- Patel P, Patel N, Patel D, Desai S, Meshram D. Phytochemical analysis and antifungal activity of Moringa oleifera. Int J Pharm Pharm Sci. 2014;6:144–7.
- Elangovan M, Rajalakshmi A, Jayachitra A, Mathi P, Bhogireddy N. Analysis of phytochemicals, antibacterial and antioxidant activities of Moringa oleifera Lam. leaf extract—an in vitro study. Int J Drug Dev Res. 2014;6:173–80.
- Zaffer M, Ganie SA, Gulia SS, Yadav SS, Singh R, Ganguly S. Antifungal efficacy of Moringa oleifera Lam. AJPCT. 2015;3:28–33.
- Ferreira PM, Carvalho AF, Farias DF, Cariolano NG, Melo VM, Queiroz MG, et al. Larvicidal activity of the water extract of Moringa oleifera seeds against Aedes aegypti and its toxicity upon laboratory animals. An Acad Bras Cienc. 2009;81:207–16.
- Dasgupta S, Gunda NSK, Mitra SK. Evaluation of antimicrobial activity of Moringa oleifera seed extract as a sustainable solution for portable water. RSC Advances. 2016;31:25918–26.
- Abdalla AM, Alwasilah HY, Mahjoub RA, Hind Ibrahim Mohammed HI, Yagoub M. Evaluation of antimicrobial activity of Moringa oleifera leaf extracts against pathogenic bacteria isolated from urinary tract infected patients. J Adv Lab Res Biol. 2016;7:47–51.
- Pinal P, Nivedita P, Dhara P, Sharav D, Dhananjay M. Phytochemical analysis and antifungal activity of Moringa oleifera. Int J Pharm Pharm Sci. 2014;6:144–7.
- Sudha P, Asdaq SM, Dhamingi SS, Chandrakala GK. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in animals. Indian J Physiol Pharmacol. 2010;54:133–40.
- Nfambi J, Bbosa GS, Sembajwe LF, Gakunga J, Kasolo JN. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. J Basic Clin Physiol Pharmacol. 2015;26:603–11.
- Jayanthi M, Garg SK, Yadav P, Bhatia AK, Goel A. Some newer marker phytoconstituents in methanolic extract of Moringa oleifera leaves and evaluation of its immunomodulatory and splenocytes proliferation potential in rats. Indian J Pharmacol. 2015;47:518–23.
- Choudhury S, Sharan L, Sinha MP. Anti-diarrhoeal potentiality of leaf extracts of Moringa oleifera. Br J Appl Sci Technol. 2013;3:1086–96.
- Lakshminarayana M, Shivkumar H, Rimaben P, Bhargava VK. Antidiarrhoeal activity of leaf extract of Moringa oleifera in experimentally induced diarrhoea in rats. Int J Phytomed. 2011;3:68–74.
- Ali A, Akhtar N, Khan MS, Khan MT, Ullah A, Shah MI. Effect of Moringa oleifera on undesirable skin sebum secretions of sebaceous glands observed during winter season in humans. Biomed Res. 2013;24:127–30.
- Raguindin PF, Dans LF, King JF. Moringa oleifera as a galactagogue. Breastfeed Med. 2014;9:323–4.
- Medhi HN, Khanikor LC, Lahon PM, Barua CC. Analgesic, anti-inflammatory and local anesthetic activity of Moringa in laboratory animals. Int J Pharmacogn. 1996;34:207–12.
- Monera TG, Wolfe AR, Maponga CC, Benet LZ, Guglielmo J. Moringa oleifera leaf extracts inhibit 6beta-hydroxylation of testosterone by CYP3A4. J Infect Dev Ctries. 2008;2:379–83.
- Asare G.A., Gyan B., Bugyei K., Adjei S., Mahama R., Addo P., Otu-Nyarko L., Wiredu E.K., Nyarko A. Toxicity potentials of the nutraceutical Moringa oleifera at supra-supplementation levels. J. Ethnopharmacol. 2012;139:265–272. doi: 10.1016/j.jep.2011.11.009
- Canett-Romero R., Arvayo-Mata K.L., Ruvalcaba-Garfias N.V. Aspectos Tóxicos Más Relevantes de Moringa oleífera oleífera y sus Posibles Daños. BIOtecnia. 2014;16:36. doi: 10.18633/bt.v16i2.45
- Sileshi T., Makonnen E., Debella A., Tesfaye B. Antihyperglycemic and subchronic toxicity study of Moringa stenopetala leaves in mice. J. Coast. Life Med. 2014;2:214–221.
- Tshabalala, T., Ncube, B., Madala, N. E., Nyakudya, T. T., Moyo, H. P., Sibanda, M., & Ndhlala, A. R. (2019). Scribbling the Cat: A Case of the “Miracle” Plant, Moringa oleifera. Plants (Basel, Switzerland), 8(11), 510. https://doi.org/10.3390/plants8110510
- Gholap S., Kar A. Hypoglycaemic effects of some plant extracts are possibly mediated through inhibition in corticosteroid concentration. Pharmazie. 2004;59:876–878.
- Barichella M., Pezzoli G., Faierman S.A., Raspini B., Rimoldi M., Cassani E., Bertoli S., Battezzati A., Leone A., Iorio L. Nutritional characterisation of Zambian Moringa oleifera: Acceptability and safety of short-term daily supplementation in a group of malnourished girls. Int. J. Food Sci. Nutr. 2019;70:107–115. doi: 10.1080/09637486.2018.1475550
- Stohs S.J., Hartman M.J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res. 2015;29:796–804. doi: 10.1002/ptr.5325
- Maizuwo A.I., Hassan A.S., Momoh H., Muhammad J.A. Phytochemical Constituents, Biological Activities, Therapeutic Potentials and Nutritional Values of Moringa oleifera (Zogale): A Review. J. Drug Des. Med. 2017;3:60.
- Annongu A., Karim O., Toye A., Sola-Ojo F., Kayode R., Badmos A., Alli O., Adeyemi K. Geo-Assessment of chemical composition and nutritional Evaluation of Moringa oleifera seeds in nutrition of Broilers. J. Agric. Sci. 2014;6:119. doi: 10.5539/jas.v6n4p119
- Dutta A.K. Moringa oleifera: A Review on its importance and medicinal applications in recent age. World J. Pharm. Pharm. Sci. 2017;6:1829–1843.